- 1Department of Dermatology, The Union Hospital, Fujian Medical University, Fuzhou, Fujian, China
- 2Department of Dermatology, 900Th Hospital of Joint Logistics Support Force, Chinese People's Liberation Army, Fuzhou, Fujian, China
- 3Department of Dermatology, Fuzhou First General Hospital, Fuzhou, Fujian, China
Background: Tildrakizumab, the IL-23 inhibitor, is used to treat plaque psoriasis and psoriatic arthritis. Many studies have reported adverse drug reactions (ADRs) associated with Tildrakizumab.
Objective: The aim of this study was to describe ADRs associated with Tildrakizumab monotherapy by mining data from the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: The signals of Tildrakizumab-associated ADRs were quantified using disproportionality analyses such as the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multiitem gamma Poisson shrinker (MGPS) algorithms.
Results: A total of 10,530,937 reports of ADRs were collected from the FAERS database, of which 1,177 reports were identified with tildrakizumab as the “primary suspect (PS)”. Tildrakizumab-induced ADRs occurred against 27 system organ classes (SOCs). A total of 32 significant disproportionality Preferred Terms (PTs) conformed to the algorithms. Unexpected significant ADRs such as coronavirus infection, herpes simplex, diverticulitis, atrial fibrillation and aortic valve incompetence were also possible. The median time to onset of Tildrakizumab-associated ADRs was 194 days (interquartile range [IQR] 84–329 days), with the majority occurring, within the first 1 and 3 months after initiation of Tildrakizumab.
Conclusion: This study identified a potential signal for new ADRs with Tildrakizumab, which might provide important support for clinical monitoring and risk prediction.
1 Introduction
Psoriasis is a chronic inflammatory papulosquamous skin disease that affects approximately 60 million adults and children and is carries an increased risk of comorbidities, such as depression, psoriatic arthritis, cardiovascular disease, type 2 diabetes, obesity, and hypertension (Kaushik and Lebwohl, 2019; Ghoreschi, 2021; Sbidian et al., 2022). Common clinical types of psoriasis include psoriasis vulgaris and chronic plaque psoriasis. Other relatively rare types include guttate, erythrodermic, and pustular psoriasis. In Europe and North America, the prevalence of chronic plaque psoriasis is approximately 2%. Additionally, about 40% of individuals with pemphigus vulgaris develop chronic plaque psoriasis over time (Reich et al., 2017; Griffiths et al., 2021). Roughly 20% of patients with plaque psoriasis present with moderate to severe forms, affecting more than 10% of the body surface area or involving areas such as the scalp, face, joints, and nails (Narcisi et al., 2023). The pathogenesis of psoriasis is not fully understood, although immune dysregulation and genetic factors play a crucial role. Previous studies have highlighted the important role of the interleukin (IL)-23/IL-17 axis in the pathogenesis of psoriasis. This axis involves IL-23 secreted by macrophages and dendritic cells and IL-17 secreted by T-cells. It promotes epidermal proliferation, thickening of the stratum corneum, and affects angiogenesis leading to immune cell infiltration (Sabat et al., 2019).
IL-23 consists of the p40 and p19 subunits linked by a disulfide bond. It shares the p40 subunit with IL-12, and this subunit is also targeted in ustekinumab treatment. IL-23 promotes the expansion and differentiation of Th17 cells and the secretion of IL-17, which is mainly produced by activated T-cells. In addition, IL-23 upregulates CCL20, a chemokine that contributes to the movement of Th17 cells. IL-23 has a strong synergistic effect with TNF-α, IL-22, and IFN-γ, and is a key factor in the pathogenesis of arthritis and inflammatory bowel disease (Stritesky et al., 2008; Markham, 2018; Yang et al., 2021). Mild psoriasis is usually treated with steroids and vitamin D analogues, while moderate to severe psoriasis is treated with Phototherapy, systemic immunosuppressants, small molecules, and biologics (Mason et al., 2019; Ghoreschi et al., 2021). In some countries, concomitant use of biologics, oral medicine, or phototherapy may be considered for moderate-to-severe plaque psoriasis (Griffiths et al., 2021).
Tildrakizumab (TIL), is a high-affinity human IgG1/K monoclonal antibody, that selectively targets the p19 subunit of IL-23, thereby blocking both IL-23 activity and its receptor binding. In March 2018, the US Food and Drug Administration’s (FDA) approved to TIL for the treatment of moderate-to-severe chronic plaque psoriasis, either alone or in combination with systemic therapy or phototherapy (Galluzzo et al., 2022). The recommended dosage regimen for TIL administered via subcutaneous injection is 100 mg at week 0, week 4, and subsequently every 12 weeks thereafter (Pharma, 2022), and European countries also allow treatment with 200 mg in special circumstances, such as high disease burden or body weight ≥90 kg (Reich et al., 2020). A study involving 24 patients with moderate-to-severe plaque psoriasis showed that after treatment with TIL, 85.72% of patients achieved a PASI score of 75, and 80.9% had a PASI score of ≤3 at week 12, which suggests the efficacy of TIL for short-term treatment of chronic plaque psoriasis (Galán-Gutierrez et al., 2022). The large-scale randomized controlled phase III trials reSURFACE1 and reSURFACE2 (ClinicalTrials.gov identifiers: NCT01722331; NCT01729754) contributed significantly to the introduction of TIL, compared with placebo and etanercept5. The drug has shown significant efficacy in the treatment of chronic plaque psoriasis. This assertion is further supported by the study of Mastorino et al. (Mastorino et al., 2022). Additionally, TIL has shown efficacy in the treatment of psoriatic arthritis, nail psoriasis, and scalp psoriasis (Onuora, 2021; Bardazzi et al., 2023). However, common adverse drug reactions (ADRs) like nasopharyngitis, headache, upper respiratory tract infections, and psoriasis exacerbations should not be ignored during the course of medication. In addition, in the open-label, phase IV RIBUTE study, adult patients with moderate-to-severe psoriasis treated with TIL frequently developed neurological disorders such as headache, along with infections, infestations, and gastrointestinal disorders (Costanzo et al., 2023). Therefore, given that TIL has been on the market for 5-year, it is important to scrutinise its ADRs using data mining algorithms for pharmacovigilance. Furthermore, conducting short-term experimental studies and meta-analyses on the safety of the drug will also provide valuable insights.
The FDA Adverse Event Reporting System (FAERS) database, is the world’s largest repository of pharmacovigilance information and facilitates spontaneous reporting of ADRs and medication errors by various stakeholders including drug manufacturers, consumers, healthcare professionals, physicians, pharmacists, and others, stands as the world’s largest repository of pharmacovigilance information. It greatly facilitates the FDA’s comprehensive assessment of drug safety after a drug is marketed (Hosoya et al., 2021; Yin et al., 2022). Our findings have potential to provide physicians and health policymakers with meaningful insights in monitoring TIL-associated ADRs, thereby fostering rational use of clinical medications.
2 Method
2.1 Data sources and cleaning
We conducted a retrospective pharmacovigilance study using the FAERS database (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html) of patients using TIL from the first quarter (Q1) of 2018 to the fourth quarter (Q4) of 2023, since TIL was approved for use by the FDA in March 2018, FAERS covers seven areas: demographic and administrative information (DEMO), drug information (DRUG), adverse drug reaction information (REAC), patient outcome information (OUTC), reporting source information (RPSR), date of treatment initiation and date of end of reported medication (THER) and medication administration indications (INDI) (Yu et al., 2021; Zheng et al., 2023).
ADRs in the FAERS database are coded according to Preferred Terms (PTs) in the Medical Dictionary for Regulatory Activities Code 24.0 (MedDRA), and multiple PTs can correspond to a System Organ Class (SOC) level (Tian et al., 2022; Wu et al., 2023). We used four algorithms to classify SOCs.We analysed SOC and PTs by four algorithms to assess the associations between drug-related ADRs and drugs. Only reports documenting TIL as “primary suspect (PS)” drug were included in our analyses. First, the FAERS database was searched using use brand names and generic names, including “TILDRAKIZUMAB”,“IIUMYA”, “SCH900222”, “SCH-900222”, “MK-3222”, “BLINDED TILDRAKIZUMAB 100 MG ML INJECTION”, “BLINDED TILDRAKIZUMAB INJECTION”, “BLINDED TILDRAKIZUMAB”, “TILDRAKIZUMAB ASMN”, “ILUMYA TILDRAKIZUMAB”, “ILUMYA TILDRAKIZUMAB ASMN” and “ILUMYA TILDRAKIZUMAB ASMN” as PT names and sorted them in order of CASEID, FDA_DT, and PRIMARYID, in that order Duplicate PRIMARYIDs were eliminated and the one with the largest PRIMARYID value, was taken to extract information about the patient’s relevant characteristics, and the information was integrated using Microsoft Excel software. In addition, non-drug related issues such as “product administration interrupted”, “product administration error”, “product distribution issue”, “product storage error”, “product dose omission issue”, “circumstance or information capable of leading to medication error”, “treatment noncompliance”, “needle issue”, “inappropriate schedule of product administration” etc., were also excluded from the PTs, and “product used for unknown indication” was excluded from the indications section.
2.2 Disproportionality analysis and signal detection
Disproportionality analysis refers to the assessment of spontaneously reported cases to detect signals in order to hypothesise a potential causal relationship between a specific drug and its ADRs, using all ADRs as a statistical background (Zou et al., 2023; Li et al., 2024). The detected signals indicate an association between medications and ADRs, The detected signals indicating an association between medications and ADRs can be quantitatively identified by two main statistical methods and four algorithms: 1). Frequentist: reporting odds ratios (ROR) and proportional reporting ratio (PRR); 2). Bayesian: Bayesian confidence propagation neural network (BCPNN) and multi-item gamma Poisson shrinker (MGPS). ROR and PRR have the advantages of early detection, high signal sensitivity, and simple calculation of the signal value, whereas BCPNN and MGPS are more conservative in terms of signal detection, with a high level of specificity, which reduces the generation of false positive signals (Chen et al., 2008; Noguchi et al., 2021). Among these four algorithms, ROR algorithm produces the highest number of signals with the strongest evidence, with a higher ROR ratios indicating a more robust signals (Sakaeda et al., 2013; Sakaeda et al., 2014). Therefore, we believe that signal detection should be based on the ROR algorithm as the minimum standard, when the more algorithms are satisfied, the higher the robustness and significance. Thus, we define a signal as positive if it meets the ROR algorithm criteria and significant if it meets the criteria of two or more algorithms. The criteria for the four algorithms were as follows: ROR required a number of reported cases (N) ≥ 3 and a lower limit of the 95% confidence interval (CI) > 1. PRR required a PRR ≥2, χ2 ≥ 4 and N ≥ 3. BCPNN requires IC025 (lower limit of the 95% CI for the IC) > 0, and MGPS requires an EBGM05 (lower limit of the 95% CI for the EBGM) > 2. Calculations for each algorithms were based on a 2x2 columnar table (Supplementary Table S1), and detailed algorithm and criteria are shown in Supplementary Table S2 ((Tian et al., 2022), (Fang et al., 2023)).
Time-to-event onset (TTO) indicates the time interval between the date of administration and the occurrence of ADRs. Reports containing input errors (ADRs occurring before administration) or inaccurate date input were excluded. We used the Kruskal–Wallis test to determine if there were differences in the median time of positive SOCs and PTs to observe the variations in different ADRs. Additionally, we conducted the Kolmogorov-Smirnov Goodness of Fit test and Weibull test on TTO (Quinn and Quinn, 2010; Hazra and Gogtay, 2016). A p-value >0.05 indicated that the data complied with the Weibull test in the Kolmogorov-Smirnov Goodness of Fit tests. The Weibull tests include shape parameter (β) and scale parameter (η), which can further explain the trend of ADRs and describe the characteristic time of occurrence for approximately 63.2% of ADRs. The flowchart is shown in Figure 1. All analyses were performed using R software version 4.2.2.
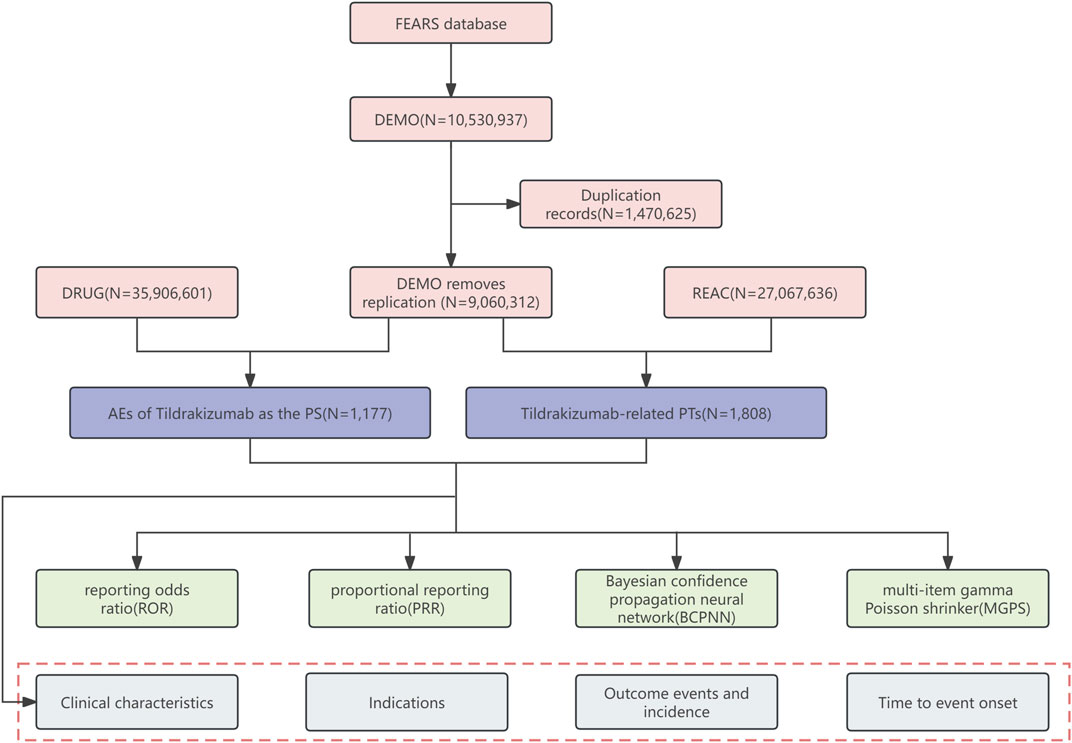
Figure 1. Flow diagram (DEMO demographic and administrative information, DRUG drug information, REAC preferred terminology for adverse event, PS primary suspect drug, PTs preferred terms).
3 Results
3.1 Baseline characteristics
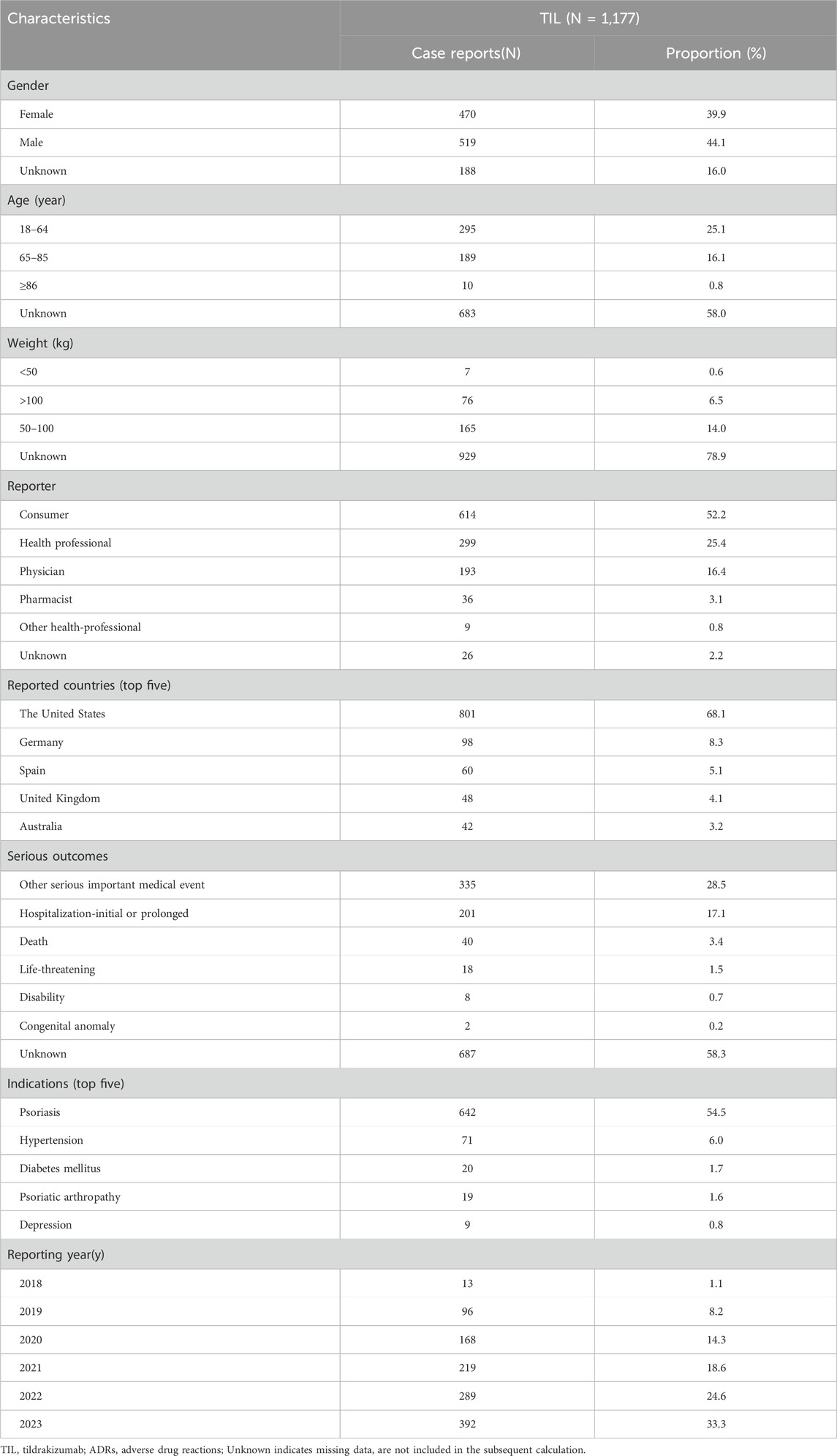
From the first quarter (Q1) of 2018 to the fourth quarter (Q4) of 2023, a total of 1,177 reports of TIL as the primary suspect drug were identified in the FAERS database, excluding duplicate reports (Table 1). A higher proportion of reports were made by males (44.1%) than females (39.9%), and the missing data rate was 16.0%. Patients aged 18–64 years accounted for 25.1% of the total number of reports, and patients weighing between 50–100 kg accounted for 14.0%. The majority of ADR reports came from consumers (52.2%) and health professionals (25.4%). Geographically, the United States had the highest number of ADR reports (68.1%), followed by Germany (8.3%), Spain (5.1%), the United Kingdom (4.1%), and Australia (3.2%). Countries in Asia and Africa contributed to Asian and African countries accounted for a smaller proportion of the reporting population, as shown in Figure 2. Serious ADRs-related to TIL mainly included other significant medical events (28.5%) and hospitalization—initial or prolonged (17.1%), with 40 (3.4%) deaths, 18 (1.5%) life-threatening injuries and in 8 (0.7%) disabilities.
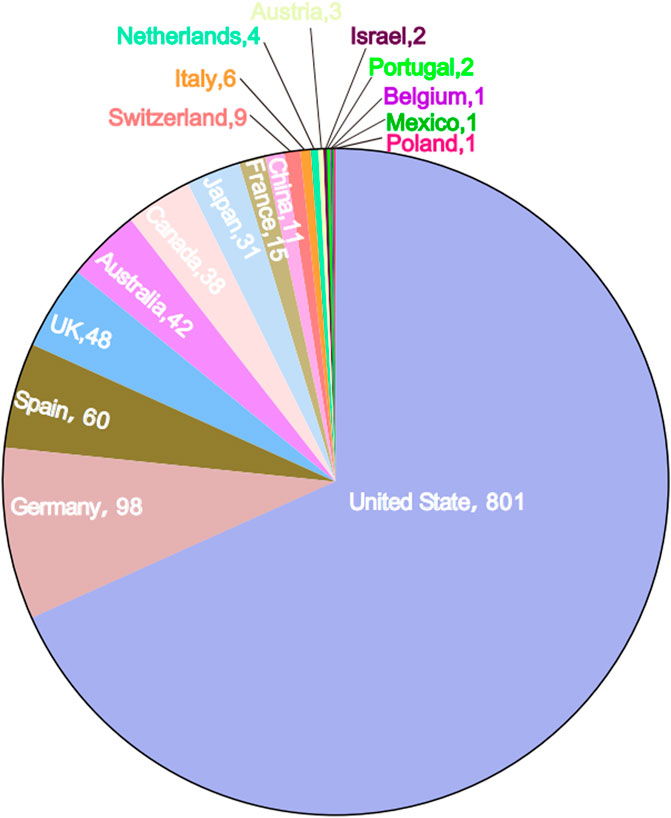
Figure 2. Number of Tildrakizumab-related ADR reports by country in the data table. The name of the country and the number of cases report are indicated.
3.2 Disproportionality analysis
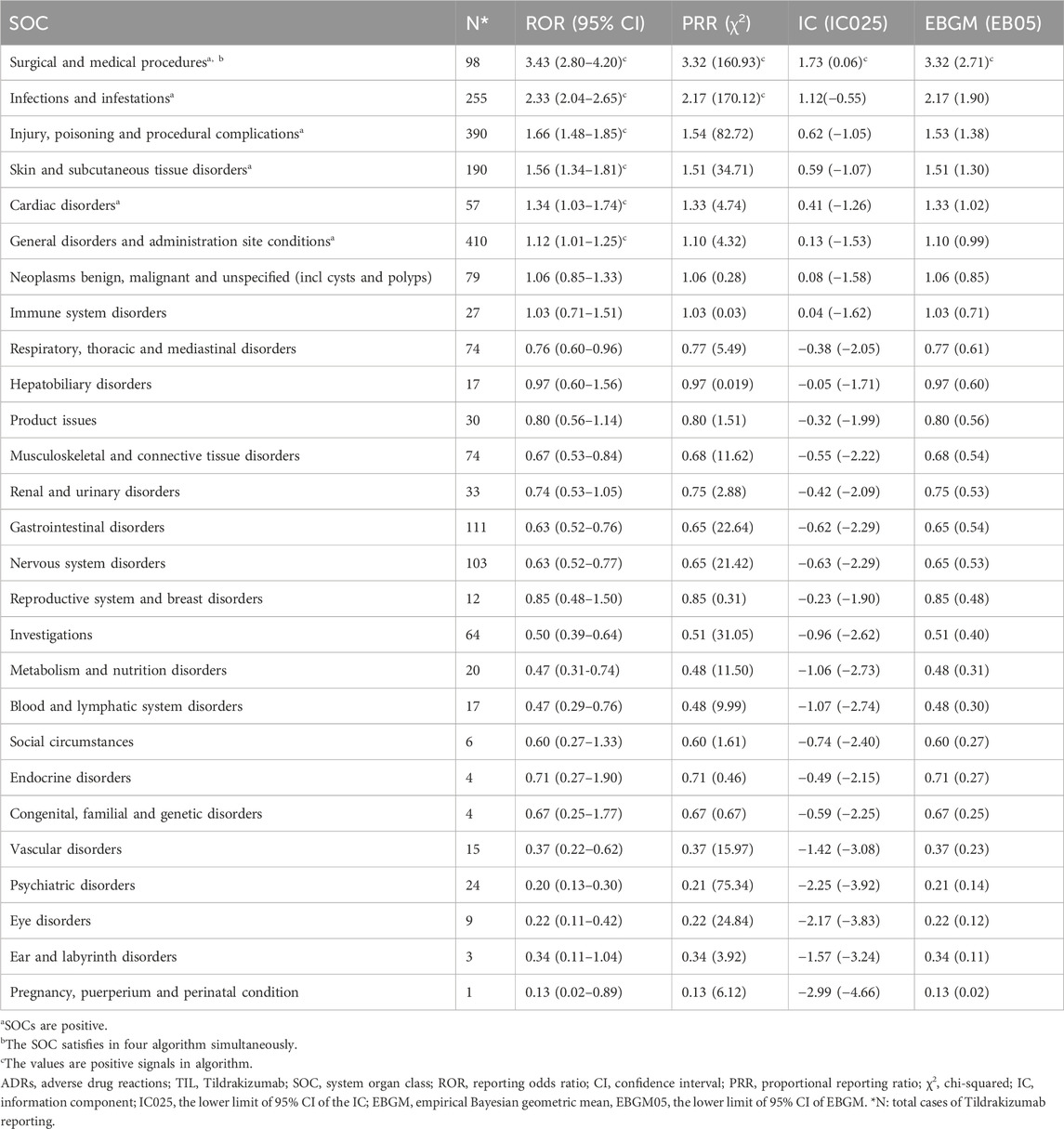
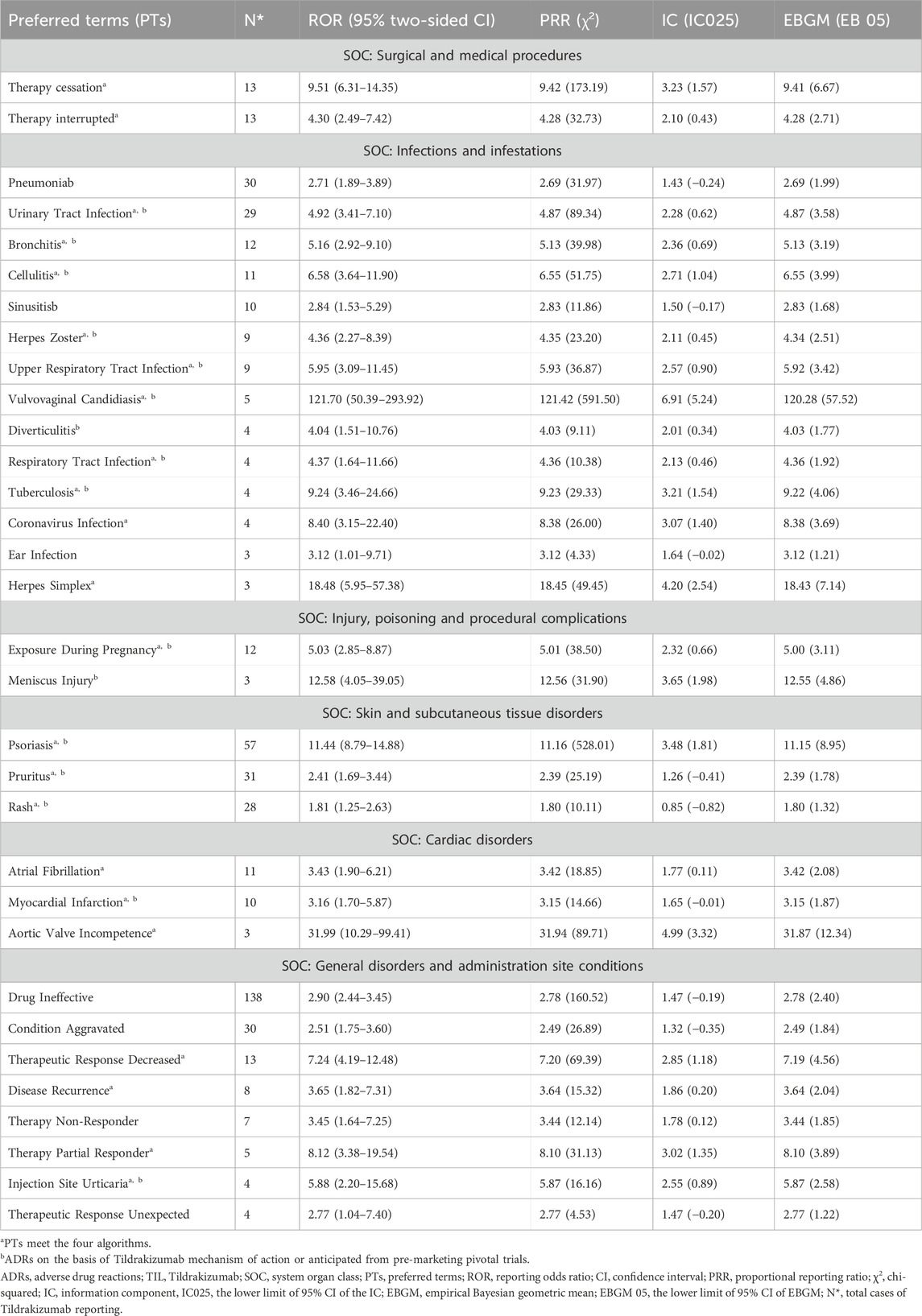
Disproportionality analyses of ADRs with TIL were initially performed at the SOC level. We identified 27 organ systems affected by TIL-related ADRs (Table 2). “Surgical and medical procedures” satisfied all four algorithms and has the highest values for each. This SOC reports the least amount of medical expertise required. “Infections and infestations” met the ROR and PRR algorithms, indicating high sensitivity and early adverse reactions to TIL. The remaining four SOCs met only the ROR algorithm criteria, as shown in bold in Table 2. The lack of positive signals in PRR, BCPNN, and EBGM suggests that these signals are more ambiguous and less specific in the TIL user group. Table 3 shows 32 clearly disproportionate PTs, of 21 PTs met all four algorithms simultaneously (Figure 3). Previous studies on TIL have shown that upper respiratory tract infection (PT: 170715085), respiratory tract infection (PT: 228041992), injection site urticaria (PT: 163513461), psoriasis (PT: 172351161), pruritus (PT: 175510751), rash (PT: 157440671), and PTs such as myocardial infarction (PT: 222780722) were consistent with the results of clinical trials (Galluzzo et al., 2022). Some of these PTs showed high RORs, including vulvovaginal candidiasis (ROR = 121.70, PT:213490533), herpes simplex (ROR = 18.48, PT: 201335143), and aortic valve incompetence (ROR = 31.99, PT: 203349819). After further grouping by gender, we observed that diminished response to treatment, urinary tract infection, bronchitis and psoriasis were more common in females, whereas drug ineffectiveness, aggravated condition recurrence, therapy non-response, cellulitis, upper respiratory tract infection, pruritus, and therapy cessation were more prevalent in males (Figure 4; Supplementary Table S3). Although the p values were not significant, more cases in the male and female subgroup may be needed to show differences. Further analysis will be necessary as more reports are submitted.
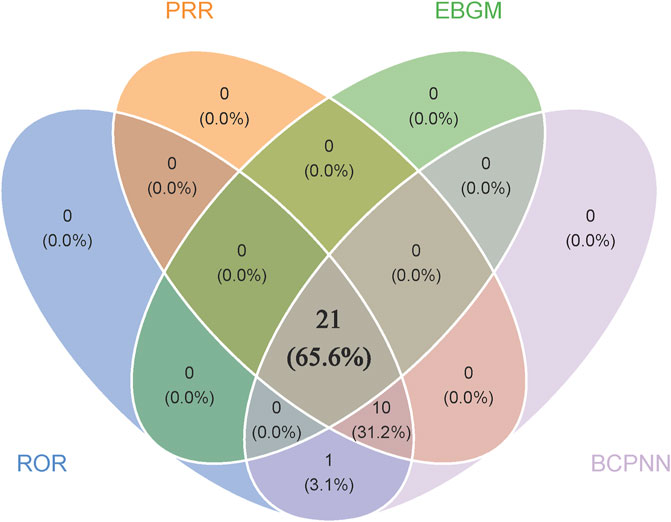
Figure 3. Venn that 21 PTs satisfy all four algorithms simultaneously. Blue module represents ROR, orange module represents PRR, green module represents EBGM and purple module represents BCPNN.
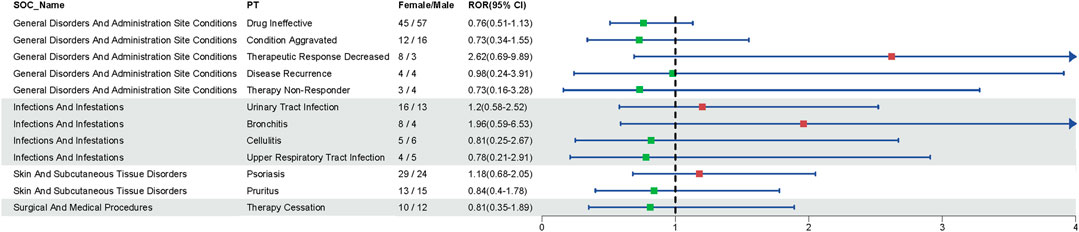
Figure 4. Gender-related ADRs. Reporting odds ratio (ROR) and 95% CI for all significant gender-related ADRs.Green and red dots represent the ROR.
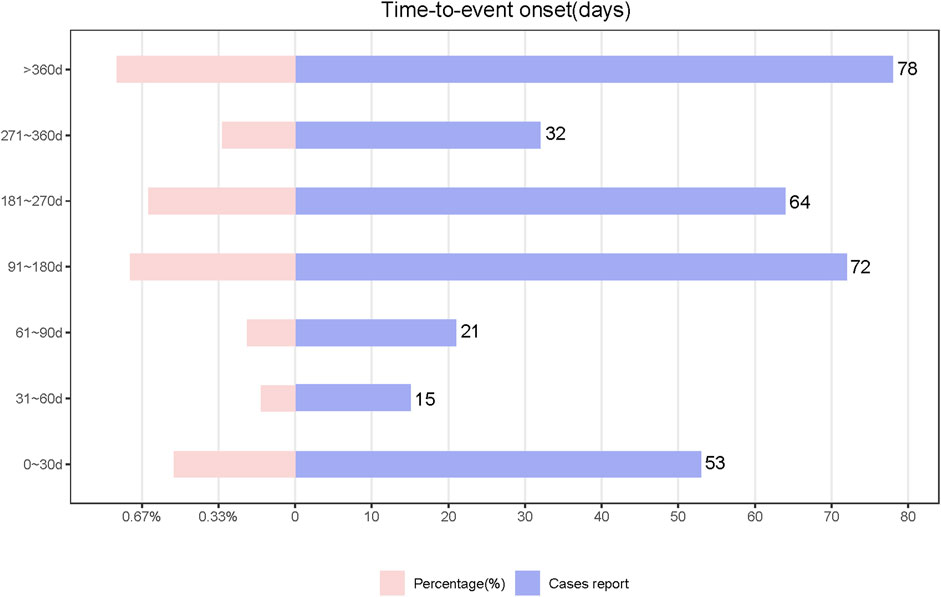
3.3 Time-to-event onset analysis
We analyzed TIL-induced 335 ADRs using the TTO reported after excluding missing data and reporting errors (Figure 5). The median time to onset was 194 days, with the IQR range from 84 to 329 days. The number of ADR reports was higher in the first month after administration (n = 53, percentage = 15.82%). The growth rate of the ADRs reports in the second and third months was −71.7% and 40%, respectively. The number of ADRs decreased each quarter over time with the following numbers and growth rates: N = 89; N = 72, growth rate = −19.1%; N = 64, growth rate = −11.1%; N = 32, growth rate = −50%. After 1 year of TIL treatment, the incidence of ADRs was 23.28% (n = 78). Analyzed by the Kolmogorov-Smirnov Goodness of Fit test (p = 0.93) and Weibull test (Supplementary Table S4), β = 0.89 (95% CI:CI:0.80–0.97),andη = 269.56 (95% CI:231.36–307.76), indicating that the trend of ADRs was towards the early type. This implies that the risk of ADRs increases after medication and then decreases over time, which is also consistent with the trend in growth rates. Whereas about 63.2% of ADRs occurred within 269.56 days, which suggests that monitoring of TIL ADRs should be long term. Top 5 SOCs with earliest onset to ADRs after medication were observed for hepatobiliary disorders (median = 53), eye disorders (median = 60.5), gastrointestinal disorders (median = 86), immune system disorders (median = 99), and respiratory, thoracic, and mediastinal disorders (median = 112), as shown in Figure 6A, which all occurred 2–4 months after dosing. We analyzed PTs within six groups of SOCs with positive signals, and the Kruskal–Wallis test determined that there was no significant differences in the timing within the groups (Figure 6B).
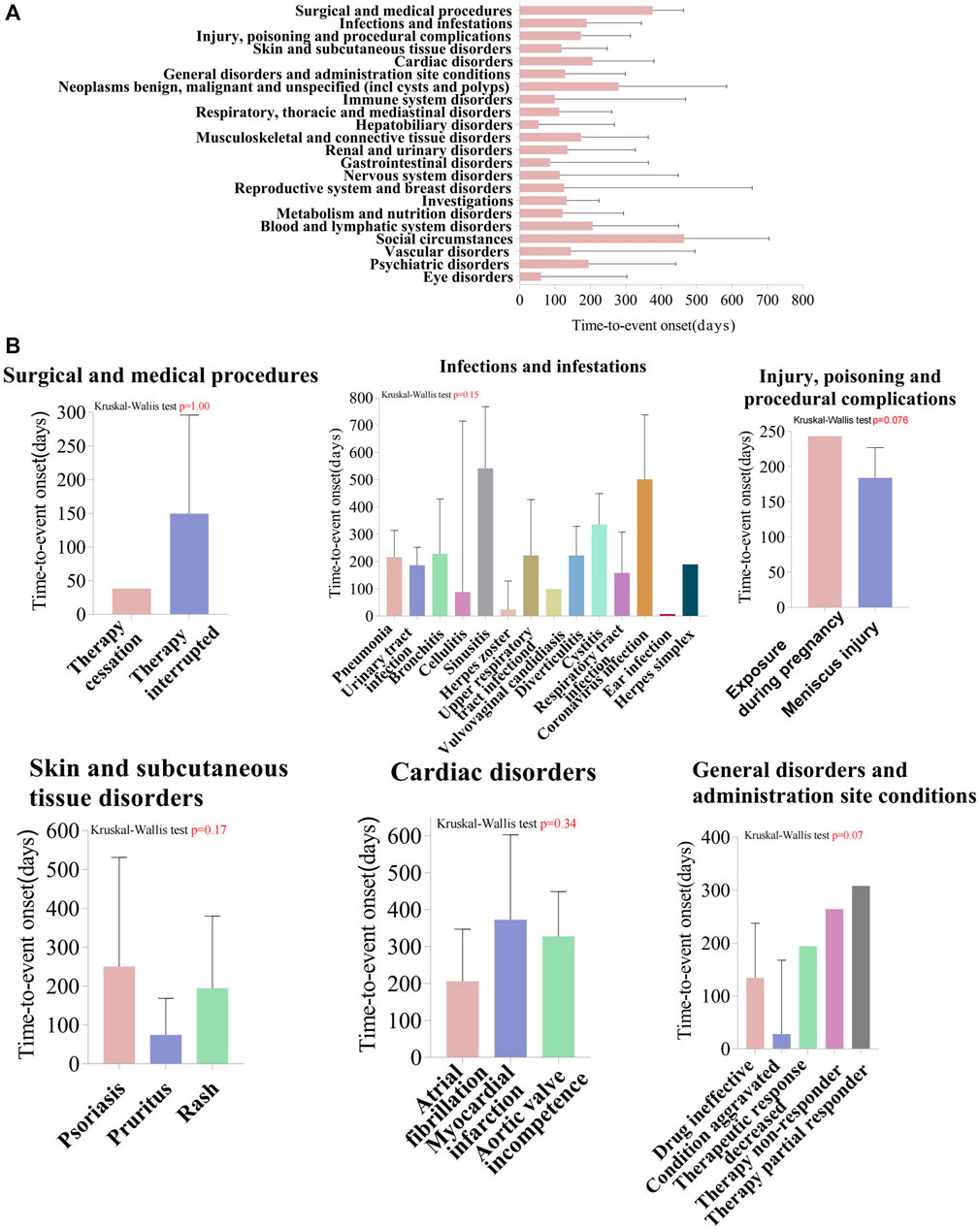
Figure 6. Time-to-event onset (TTO) analysis of ADRs at the SOC and PT levels. (A) Box plot of the TTO at the SOC level for Tildrakizumab. The blue box represents the median, while the black bar indicates the interquartile range. (B) Comparing the TTO among PTs within the SOC level, using Kruskal–Wallis test to detect whether the medians within each group are significantly different from each other. The box represents the median, while the black bar indicates the interquartile range. SOC, System Organ Class; PTs, preferred terms.
4 Disscusion
TIL is approved for the treatment of moderate to severe plaque psoriasis in the United States, European Union, Australia, and Japan (Lebwohl et al., 2021). As an IL-23 antagonist, it was approved for chronic plaque psoriasis in 2018. We observed a significant increase in ADRs from an initial 13 to 392 cases reported in 2023 over 5 years. We consider that the notable rise in ADRs during this period to the authorisation of TIL, which led to an increase in the number of prescriptions and an expanded of the user base. Based on the FAERS spontaneous reporting system, we collected a total of 1,177 post-marketing-induced ADRs for TIL, the highest number of TIL ADR reports collected to date. ADRs can lead to discontinuation of medication or non-adherece by the patient, which can compromise effective disease control. Therefore, a thorough understanding of the side effects of TIL is essential for patient management (Kolli et al., 2019). The age of onset of psoriasis is centred on 16-22 and 57–60 years, and is common in Caucasians and high-income countries. This is consistent with the reported age distribution (18–64 years) and the reporting areas mainly in Caucasian developed countries (Di Lernia and Goldust, 2018; Griffiths et al., 2021). Previous studies have shown that TIL is more effective in patients with lower body weight (Papp et al., 2019). Based on the data collected, patients weighing less than 50 kg also had the lowest rate of ADRs. In our findings, although there was no significant gender difference in the incidence of psoriasis, the proportion of ADRs was slightly higher in male patients than in females (Griffiths et al., 2021).
Although health professionals comprised 25.4% of the reporting population, the proportion was higher among non-professional consumers (52.2%). Considering the phenomenon that “surgical and medical procedures” have the highest SOC signal intensity, the increase in detection of ADRs may be due to the simplicity of these procedures, such as therapy cessation and therapy interruption, leading to more frequent reporting by patients without relevant medical expertise. This indicates the need for enhanced educational interventions for FAERS reporters to improve reporting accuracy and reporting rate (Cervantes-Arellano et al., 2024). Meanwhile, We know that treatment is often stopped or interrupted in the real world for a variety of reasons. Interruption of TIL treatment was also modelled in the previous reSURFACE one trial, which showed a decrease in disease control after interruption. However, approximately 85% of patients who relapsed and resumed TIL achieved PASI 75, which provides an important guide to restoring confidence in treatment for patients who interrupt therapy (Kimball et al., 2020).
Due to their high targeting of inflammation, biologics paradoxically lead to an enhanced immune response, which is particularly evident with anti-IL-23p19 and anti-IL-17 drugs (Griffiths et al., 2021). In addition, the use of TIL reduces the production of psoriasis-associated lymphocyte cytokines (e.g., IL-17), which normally induce the production of antimicrobial peptides (e.g.,β-defensin and S100A7) against fungi, thereby reducing fungal resistance (Ghoreschi et al., 2021). In addition to infections mentioned in relevant trials and manufacturer’s documents, such as upper respiratory tract infection, respiratory tract infection, tuberculosis, cellulitis, diverticulitis, pneumonia, urinary tract infection, bronchitis, herpes zoster, vulvovaginal candidiasis (Thaci et al., 2021; Egeberg et al., 2023), coronavirus infection (ROR = 8.40) and herpes simplex (ROR = 18.48) also exhibit significant signals, which have not been emphasized in previous RCT studies or retrospective cases. Clinicians who are treating plaque-type psoriasis with TIL in patients with plaque psoriasis should monitor the incidence of these infections and be aware of gender differences in incidence. IL-23 antagonists act on similar inflammatory pathways as IL-17 antagonists. Given that IL-17 antagonists exacerbate inflammatory bowel disease (IBD) in the real world, relevant studies have begun to examine whether the use of TIL is associated with the development or exacerbation of IBD. However, the phase 2b (P05495, NCT01225731) and two phase 3 (reSURFACE 1; reSURFACE 2) trial studies found that TIL did not induce or exacerbate IBD in patients with psoriasis (Gooderham et al., 2019). Moreover, in a pooled analysis after 148 weeks of reSURFACE one and reSURFACE, the incidence of Crohn’s disease (CD) was 0.05 events per 100 person-years (Reich et al., 2020), further confirming the accuracy of previous studies. Supplementary Table S5 showed diarrhoea (N = 29, ROR: 1.29, 95% CI: 0.89–1.86), IBD (N = 2, ROR: 11.26, 95% CI: 2.81–45.08), and CD (N = 1, ROR: 0.42, 95% CI: 0.06–2.98), none of which showed a positive signal. Furthermore, IL-23 antagonists have shown good potential in the treatment of IBD, as evidenced by the fact that Ustekinumab has been approved by the FDA for the treatment of CD. However, the relationship between TIL and IBD needs to be confirmed by further studies.
We observed that common adverse events such as nasopharyngitis, diarrhoea and headache, which have been documented in previous trials and various retrospective case analyses, did not show a positive signal in disproportionality analysis (Egeberg et al., 2023). This suggests that these ADRs are quite common in the FAERS database, preventing the manifestation of disproportionality, and therefore remain unnoticed in disproportionality analyses. This is known as the masking effect or cloaking effect, and also affects BCPNN and EBGM(30). Accurate assessment of TIL-associated ADRs requires a combination of extensive experimental data and real-world analyses.
Significant signals are observed with “Exposure during pregnancy”. Subcutaneous administration of 100 mg/kg to pregnant monkeys did not produce treatment-related effects on the developing fetuses (Santostefano et al., 2019). Post hoc analyses of nine clinical trials of TIL did not identify any birth defects or abnormalities in the infants. The rate of miscarriage (14%) was similar to that of the general population (12%–15%) (Haycraft et al., 2020). IgG1 can cross the placental barrier efficiently by Fc receptor (FcRn)-mediated transport from 13th week of pregnancy (Palmeira et al., 2012). The known half-life of TIL is 23 days (Pharma, 2022), suggesting that it has the potential to cross the placental barrier. Given the significant signals calculated to result from exposure to TIL during pregnancy and the limited data available on the use of TIL in pregnant women, we agree with Haycraft K et al. that caution should be exercised in the use of TIL in women of childbearing potential (Haycraft et al., 2020). Skin adverse reactions such as psoriasis, pruritus, rash, and injection site urticaria have also been observed in previous studies, which is consistent with our findings. It is well known that patients with psoriasis are more prone to cardiovascular disease (Ozden et al., 2016), so cardiac disorder is also a noteworthy SOC. In this study, they also showed strong signals including atrial fibrillation, myocardial infarction, and aortic valve incompetence. During the 5-year treatment follow-up, the common cardiac disorder was acute myocardial infarction, which led to one fatal case (Macaluso et al., 2019). However, based on our findings, atrial fibrillation and aortic valve incompetence are also noteworthy. No significant positive signals were observed for neoplasms, benign tumours, malignant tumours and tumours of unknown origin (including cysts and polyps), which is consistent with the previously low incidence of neoplasms.
Although we performed a detailed analysis of TIL-associated ADRs in FAERS, the study still has some omissions due to the characteristics of the FAERS data itself. Firstly, the absence of prescribing information for TIL, including information on drug dosage and administration, may have led to discrepancies in ADRs; secondly, some ADRs may have been reported by non-healthcare professionals, leading to possible inaccuracies in the characterisation of ADRs; thirdly, the FAERS database comprises mainly data from Western countries, with less self-reported data from Third World countries. Fourthly, data sources that use joint analysis of multiple databases, such as the British Association of Dermatologists Biologics and Immunomodulators and Germany’s PsoBest, were not included. Finally, each method for calculating ADRs has its own advantages and disadvantages. Although ROR is the most common and straightforward FAERS-based analysis (Rana et al., 2021), its performance, along with that of PRR, declines in the presence of the innocent bystander effect (i.e., concomitant use of a specific drug with the drug that caused the ADR, leading to incorrect attribution of the ADR to the specific drug) (Dijkstra et al., 2020). On the other hand, BCPNN and MGPS tend to be overly conservative and may ignore ADRs (Noguchi et al., 2021). Nonetheless, FAERS remains the largest ADR database in the world. It is crucial to understand that signal detection should not be interpreted as a direct causal relationship, but rather the association between drugs and ADRs should be analysed to provide real-world evidence. Therefore, it is important to use this database to assess the relationship between TIL and its ADRs to provide a more comprehensive perspective on TIL vigilance analyses.
5 Conclusion
Our study used the data mining algorithms ROR, PRR, IC and EBGM to detect ADR signals in long-term treatment with TIL, and identified a number of unreported ADRs associated with TIL, which could be beneficial for future risk detection and clinical use of the drug.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JL: Conceptualization, Data curation, Methodology, Visualization, Writing–original draft, Writing–review and editing. XC: Conceptualization, Data curation, Methodology, Writing–original draft, Writing–review and editing. ML: Conceptualization, Data curation, Methodology, Writing–review and editing. QZ: Visualization, Writing–review and editing. HZ: Visualization, Writing–review and editing. NC: Validation, Writing–review and editing. YZ: Validation, Writing–review and editing. YH: Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. National Natural Science Foundation of China (8220120391), Fujian Province Natural Science Foundation (2022J01745), Fujian Provincial Health Technology Project (2020GGA030), Scientific Research Project of Union Hospital Affiliated to Fujian Medical University (2020XH003) and Excellent Young Scholars Cultivation Project of Fujian Medical University Union Hospital (2022XH027).
Acknowledgments
We would like to express our sincere gratitude to all staff of FAERS that completed the valid collections that made up the current analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1420478/full#supplementary-material
Abbreviations
TIL, Tildrakizumab; IL-23, Interleukin-23; IL-17, Interleukin-17; ADRs, Adverse Drug Reactions; PTs, Preferred Terms; SOC, System Organ Class; FDA, Food and Drug Administration; FAERS, FDA Adverse Event Reporting Database; TTO, Time-to-event onset; ROR, Reporting Odds Ratios; IC, Confidence Interval; IBD, Inflammatory Bowel Disease; CD, Crohn’s disease.
References
Bardazzi, F., Viviani, F., Piraccini, B. M., Lasagni, C., Bigi, L., Manfredini, M., et al. (2023). Tildrakizumab in complex psoriatic patients: an experience in emilia-romagna (Italy). J. Cutan. Med. Surg. 27 (2), 126–132. doi:10.1177/12034754231155889
Cervantes-Arellano, M. J., Castelán-Martínez, O. D., Marín-Campos, Y., Chávez-Pacheco, J. L., Morales-Ríos, O., and Ubaldo-Reyes, L. M. (2024). Educational interventions in pharmacovigilance to improve the knowledge, attitude and the report of adverse drug reactions in healthcare professionals: systematic Review and Meta-analysis. Daru 32 (1), 421–434. doi:10.1007/s40199-024-00508-z
Chen, Y., Guo, J. J., Steinbuch, M., Lin, X., Buncher, C. R., and Patel, N. C. (2008). Comparison of sensitivity and timing of early signal detection of four frequently used signal detection methods. Pharm. Med. 22 (6), 359–365. doi:10.1007/bf03256733
Costanzo, A., Llamas-Velasco, M., Fabbrocini, G., Cuccia, A., Rivera-Diaz, R., Gaarn Du Jardin, K., et al. (2023). Tildrakizumab improves high burden skin symptoms, impaired sleep and quality of life of moderate-to-severe plaque psoriasis patients in conditions close to clinical practice. J. Eur. Acad. Dermatol Venereol. 37 (10), 2004–2015. doi:10.1111/jdv.19229
Dijkstra, L., Garling, M., Foraita, R., and Pigeot, I. (2020). Adverse drug reaction or innocent bystander? A systematic comparison of statistical discovery methods for spontaneous reporting systems. Pharmacoepidemiol Drug Saf. 29 (4), 396–403. doi:10.1002/pds.4970
Di Lernia, V., and Goldust, M. (2018). An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin. Biol. Ther. 18 (8), 897–903. doi:10.1080/14712598.2018.1504016
Egeberg, A., Jullien, D., Gaarn Du Jardin, K., and Thaçi, D. (2023). Five-year safety of tildrakizumab in patients with moderate-to-severe psoriasis from two phase 3 trials (reSURFACE 1 and reSURFACE 2): number needed to harm for occurrence of adverse events of special interest. J. Dermatol. Treat. 34 (1), 2220447. doi:10.1080/09546634.2023.2220447
Fang, Z., Xu, Z., Zhu, W., Yu, M., and Ji, C. (2023). A real-world disproportionality analysis of apalutamide: data mining of the FDA adverse event reporting system. Front. Pharmacol. 14, 1101861. doi:10.3389/fphar.2023.1101861
Galán-Gutierrez, M., Rodriguez-Fernandez Freire, L., and Ruiz-Villaverde, R. (2022). Tildrakizumab: short-term efficacy and safety in real clinical practice. Int. J. Dermatol 61 (9), e355–e357. doi:10.1111/ijd.15840
Galluzzo, M., Chiricozzi, A., Cinotti, E., Brunasso, G., Congedo, M., Esposito, M., et al. (2022). Tildrakizumab for treatment of moderate to severe psoriasis: an expert opinion of efficacy, safety, and use in special populations. Expert Opin. Biol. Ther. 22 (3), 367–376. doi:10.1080/14712598.2022.1988566
Ghoreschi, K., Balato, A., Enerbäck, C., and Sabat, R. (2021). Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet 397 (10275), 754–766. doi:10.1016/S0140-6736(21)00184-7
Gooderham, M., Elewski, B. E., Pariser, D. M., Sofen, H., Mendelsohn, A. M., Rozzo, S. J., et al. (2019). Incidence of serious gastrointestinal events among tildrakizumab-treated patients with psoriasis: letter to the Editor. J. Eur. Acad. Dermatol Venereol. 33 (10), e350–e352. doi:10.1111/jdv.15643
Griffiths, C. E. M., Armstrong, A. W., Gudjonsson, J. E., and Barker, JNWN (2021). Psoriasis. Lancet. 397 (10281), 1301–1315. doi:10.1016/S0140-6736(20)32549-6
Haycraft, K., DiRuggiero, D., Rozzo, S. J., Mendelsohn, A. M., and Bhutani, T. (2020). Outcomes of pregnancies from the tildrakizumab phase I-III clinical development programme. Br. J. Dermatol 183 (1), 184–186. doi:10.1111/bjd.18897
Hazra, A., and Gogtay, N. (2016). Biostatistics series module 3: comparing groups: numerical variables. Indian J. Dermatol 61 (3), 251–260. doi:10.4103/0019-5154.182416
Hosoya, R., Ishii-Nozawa, R., Kurosaki, K., and Uesawa, Y. (2021). Analysis of factors associated with hiccups using the FAERS database. Pharm. (Basel) 15 (1), 27. doi:10.3390/ph15010027
Kaushik, S. B., and Lebwohl, M. G. (2019). Psoriasis: which therapy for which patient: psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol 80 (1), 27–40. doi:10.1016/j.jaad.2018.06.057
Kimball, A. B., Papp, K. A., Reich, K., Gooderham, M., Li, Q., Cichanowitz, N., et al. (2020). Efficacy and safety of tildrakizumab for plaque psoriasis with continuous dosing, treatment interruption, dose adjustments and switching from etanercept: results from phase III studies. Br. J. Dermatol 182 (6), 1359–1368. doi:10.1111/bjd.18484
Kolli, S. S., Kepley, A. L., Cline, A., and Feldman, S. R. (2019). A safety review of recent advancements in the treatment of psoriasis: analysis of clinical trial safety data. Expert Opin. Drug Saf. 18 (6), 523–536. doi:10.1080/14740338.2019.1614561
Lebwohl, M. G., Leonardi, C. L., Mehta, N. N., Gottlieb, A. B., Mendelsohn, A. M., Parno, J., et al. (2021). Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J. Am. Acad. Dermatol 84 (2), 398–407. doi:10.1016/j.jaad.2020.09.047
Li, D., Wang, H., Qin, C., Du, D., Wang, Y., Du, Q., et al. (2024). Drug-induced acute pancreatitis: a real-world pharmacovigilance study using the FDA adverse event reporting system database. Clin. Pharmacol. Ther. 115 (3), 535–544. doi:10.1002/cpt.3139
Macaluso, F. S., Orlando, A., and Cottone, M. (2019). Anti-interleukin-12 and anti-interleukin-23 agents in Crohn’s disease. Expert Opin. Biol. Ther. 19 (2), 89–98. doi:10.1080/14712598.2019.1561850
Markham, A. (2018). Tildrakizumab: first global approval. Drugs 78 (8), 845–849. doi:10.1007/s40265-018-0917-3
Mason, K. J., Williams, S., Yiu, Z. Z. N., McElhone, K., Ashcroft, D. M., Kleyn, C. E., et al. (2019). Persistence and effectiveness of nonbiologic systemic therapies for moderate-to-severe psoriasis in adults: a systematic review. Br. J. Dermatol 181 (2), 256–264. doi:10.1111/bjd.17625
Mastorino, L., Cariti, C., Susca, S., Sciamarrelli, N., Borriello, S., Ortoncelli, M., et al. (2022). Tildrakizumab in real-life shows good efficacy in moderate-to-severe psoriasis regardless of previous use of biologic drugs and joint involvement. Dermatol Ther. 35 (11), e15818. doi:10.1111/dth.15818
Narcisi, A., Valenti, M., Gargiulo, L., Ibba, L., Amoruso, F., Argenziano, G., et al. (2023). Real-life effectiveness of tildrakizumab in chronic plaque psoriasis: a 52-week multicentre retrospective study-IL PSO (Italian landscape psoriasis). J. Eur. Acad. Dermatol Venereol. 37 (1), 93–103. doi:10.1111/jdv.18594
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform 22 (6), bbab347. doi:10.1093/bib/bbab347
Onuora, S. (2021). Tildrakizumab shows promise in phase IIb study. Nat. Rev. Rheumatol. 17 (7), 378. doi:10.1038/s41584-021-00646-7
Ozden, H. K., Polat, M., Ozturk, S., and Bugdayci, G. (2016). Assessment of subclinical cardiac damage in chronic plaque psoriasis patients: a case control study. Arch. Med. Sci. Atheroscler. Dis. 1 (1), e126–e132. doi:10.5114/amsad.2016.64165
Palmeira, P., Quinello, C., Silveira-Lessa, A. L., Zago, C. A., and Carneiro-Sampaio, M. (2012). IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 985646. doi:10.1155/2012/985646
Papp, K. A., Reich, K., Blauvelt, A., Kimball, A. B., Gooderham, M., Tyring, S. K., et al. (2019). Efficacy of tildrakizumab for moderate-to-severe plaque psoriasis: pooled analysis of three randomized controlled trials at weeks 12 and 28. J. Eur. Acad. Dermatol Venereol. 33 (6), 1098–1106. doi:10.1111/jdv.15400
Pharma, S. (2022). Sun pharma announces U.S. FDA approval of ILUMYATM (tildrakizumab-asmn) for the treatment of moderate-to-severe plaque psoriasis. Available at: https://www.prnewswire.com/news-releases/sun-pharma-announces-us-fda-approval-of-ilumya-tildrakizumab-asmn-for-the-treatment-of-moderate-to-severe-plaque-psoriasis-300617454.html.
Quinn, J. B., and Quinn, G. D. (2010). A practical and systematic review of Weibull statistics for reporting strengths of dental materials, Dent. Mater., 26, 135–147. doi:10.1016/j.dental.2009.09.006
Rana, P., Aleo, M. D., Wen, X., and Kogut, S. (2021). Hepatotoxicity reports in the FDA adverse event reporting system database: a comparison of drugs that cause injury via mitochondrial or other mechanisms. Acta Pharm. Sin. B 11 (12), 3857–3868. doi:10.1016/j.apsb.2021.05.028
Reich, K., Papp, K. A., Blauvelt, A., Tyring, S. K., Sinclair, R., Thaçi, D., et al. (2017). Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 390 (10091), 276–288. doi:10.1016/S0140-6736(17)31279-5
Reich, K., Warren, R. B., Iversen, L., Puig, L., Pau-Charles, I., Igarashi, A., et al. (2020). Long-term efficacy and safety of tildrakizumab for moderate-to-severe psoriasis: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2) through 148 weeks. Br. J. Dermatol 182 (3), 605–617. doi:10.1111/bjd.18232
Sabat, R., Wolk, K., Loyal, L., Döcke, W. D., and Ghoreschi, K. (2019). T cell pathology in skin inflammation. Semin. Immunopathol. 41 (3), 359–377. doi:10.1007/s00281-019-00742-7
Sakaeda, T., Kadoyama, K., Minami, K., and Okuno, Y. (2014). Commonality of drug-associated adverse events detected by 4 commonly used data mining algorithms. Int. J. Med. Sci. 11 (5), 461–465. doi:10.7150/ijms.7967
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Santostefano, M., Herzyk, D., Montgomery, D., and Wolf, J. (2019). Nonclinical safety of tildrakizumab, a humanized anti-IL-23p19 monoclonal antibody, in nonhuman primates. Regul. Toxicol. Pharmacol. 108, 104476. doi:10.1016/j.yrtph.2019.104476
Sbidian, E., Chaimani, A., Garcia-Doval, I., Doney, L., Dressler, C., Hua, C., et al. (2022). Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst. Rev. 5 (5), CD011535. doi:10.1002/14651858.CD011535.pub5
Stritesky, G. L., Yeh, N., and Kaplan, M. H. (2008). IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 181 (9), 5948–5955. doi:10.4049/jimmunol.181.9.5948
Thaci, D., Piaserico, S., Warren, R. B., Gupta, A. K., Cantrell, W., Draelos, Z., et al. (2021). Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br. J. Dermatol 185 (2), 323–334. doi:10.1111/bjd.19866
Tian, X., Chen, L., Gai, D., He, S., Jiang, X., and Zhang, N. (2022). Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS. Front. Pharmacol. 13, 851246. doi:10.3389/fphar.2022.851246
Wu, X. P., Lu, X. K., Wang, Z. T., Huang, L., Cai, R. W., Yu, H. M., et al. (2023). Post-marketing safety concerns with upadacitinib: a disproportionality analysis of the FDA adverse event reporting system. Expert Opin. Drug Saf. 22 (10), 975–984. doi:10.1080/14740338.2023.2223952
Yang, K., Oak, A. S. W., and Elewski, B. E. (2021). Use of IL-23 inhibitors for the treatment of plaque psoriasis and psoriatic arthritis: a comprehensive review. Am. J. Clin. Dermatol 22 (2), 173–192. doi:10.1007/s40257-020-00578-0
Yin, Y., Shu, Y., Zhu, J., Li, F., and Li, J. (2022). A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci. Rep. 12 (1), 19555. doi:10.1038/s41598-022-23834-1
Yu, R. J., Krantz, M. S., Phillips, E. J., and Stone, C. A. (2021). Emerging causes of drug-induced anaphylaxis: a review of anaphylaxis-associated reports in the FDA adverse event reporting system (FAERS). J. Allergy Clin. Immunol. Pract. 9 (2), 819–829.e2. doi:10.1016/j.jaip.2020.09.021
Zheng, Y., Guo, X., Chen, C., Chi, L., Guo, Z., Liang, J., et al. (2023). Toxicity signals associated with secukinumab: a pharmacovigilance study based on the United States Food and drug administration adverse event reporting system database. Br. J. Clin. Pharmacol. 89 (2), 865–873. doi:10.1111/bcp.15535
Keywords: plaque psoriasis, tildrakizumab, adverse drug reactions, disproportionality analysis, FAERS
Citation: Lin J, Chen X, Luo M, Zhuo Q, Zhang H, Chen N, Zhuo Y and Han Y (2024) Safety of tildrakizumab: a disproportionality analysis based on the FDA adverse event reporting system (FAERS) database from 2018–2023. Front. Pharmacol. 15:1420478. doi: 10.3389/fphar.2024.1420478
Received: 20 April 2024; Accepted: 13 June 2024;
Published: 10 July 2024.
Edited by:
Tomoya Tachi, Nagoya City University, JapanReviewed by:
Yoshihiro Noguchi, Gifu Pharmaceutical University, JapanMoetaza M. Soliman, Mansoura University, Egypt
Copyright © 2024 Lin, Chen, Luo, Zhuo, Zhang, Chen, Zhuo and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Han, ZHJfaGFueXVlQDEyNi5jb20=
†These authors have contributed equally to this work
 Jinger Lin
Jinger Lin Xiangqi Chen2†
Xiangqi Chen2† Min Luo
Min Luo Yue Han
Yue Han