
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol. , 07 November 2024
Sec. Respiratory Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1420046
Voriconazole, a broad-spectrum antifungal agent, is considered the first-line treatment for invasive aspergillosis. In this article, we report three cases of patients who experienced visual disturbances and hallucinations following voriconazole therapy for invasive pulmonary aspergillosis. These symptoms appeared within 1 week after initiating voriconazole administration and resolved upon discontinuation or dose reduction of the drug. Considering the absence of any identifiable alternative cause and the temporal relationship with voriconazole initiation, these symptoms were attributed to the adverse effects of voriconazole. All three patients had trough concentrations exceeding 5 μg/mL at the time of adverse reactions, leading to subsequent therapeutic drug monitoring and dose adjustment. The clinical characteristics and management strategies of voriconazole-induced hallucinations and/or visual disturbances have been rarely reported previously. Therefore, our study reviewed and analyzed relevant case reports since 2014. This study highlights the importance of recognizing the potential risk of hallucinations and visual disturbances associated with voriconazole. Furthermore, our findings indicate that the route of voriconazole administration does not influence the frequency of these adverse events. Additionally, special attention should be given to monitoring adverse events related to voriconazole in Asian populations due to their higher prevalence of CYP2C19 poor metabolizers. In the event of adverse reactions to voriconazole, diligent monitoring of therapeutic drug levels and dosage adjustments is crucial. These clinical characteristics and management strategies offer advantages in terms of enhancing drug efficacy, ensuring treatment continuity, and minimizing the incidence of other severe adverse reactions.
Voriconazole (VRC), a second-generation azole-antifungal agent, is the recommended initial treatment for serious fungal infections, including invasive pulmonary aspergillosis (Veringa et al., 2023; Wang et al., 2022). VRC is associated with various side effects such as fever, rash, gastrointestinal symptoms, headache, and hepatotoxicity (Boyd et al., 2004; de Almeida Campos et al., 2023; Kinoshita et al., 2011; Mohammed et al., 2022). However, there has been limited research conducted on the clinical characteristics and management strategies for hallucinations and/or visual impairments induced by voriconazole. In this study, we report three cases of patients who experienced visual disturbances and hallucinations following VRC treatment for invasive pulmonary aspergillosis. Additionally, we performed a comprehensive review and analysis of relevant case reports from the past decade to investigate the clinical characteristics and management strategies associated with these adverse reactions.
The patient, an 88-year-old male (with unknown body weight due to being bedridden), was admitted to our hospital with a history of “repeated cough and expectoration for over 20 years”. Subsequent physical examination revealed bilateral moist rales in the lungs, along with fever, abdominal pain, chest pain, and fecal incontinence. This patient did not have any concurrent neurological disorders. Chest computed tomography (CT) showed emphysema and infectious lung lesions. Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine (CREA) were 21.3 IU/L, 25.5 IU/L, and 94.0 μmol/L, respectively. Therefore, the patient received piperacillin sodium and tazobactam sodium for injection (4.5 g q12 h) along with aminophylline injection. Subsequently, Aspergillus was identified in sputum culture leading to a diagnosis of pulmonary aspergillosis. VRC tablets were initiated at a dose of 300 mg q12 h for two doses followed by maintenance therapy at a dose of 200 mg q12 h from September 14 onwards. On day four of VRC treatment, the patient experienced hallucinations and visual disturbances characterized by perceiving strange shadows in front of him along with red-green discoloration and symptoms of gibberish speech. On September 17, 2023, the trough concentration of VRC was measured to be elevated at 11.55 μg/mL, which correlated with the observed adverse effects including hallucinations and visual disturbances. Consequently, we decided to discontinue VRC administration due to its supratherapeutic level causing these adverse effects which completely resolved by September 20th when the trough concentration decreased to approximately normal levels, measuring at around 5.22 μg/mL simultaneously. VRC treatment was resumed on September 21st at a reduced dose regimen (100 mg q12 h). The subsequent trough concentration measurement on September 25th indicated therapeutic levels within range at approximately 4.02 μg/mL without recurrence of any VRC-induced adverse effects (Figure 1).
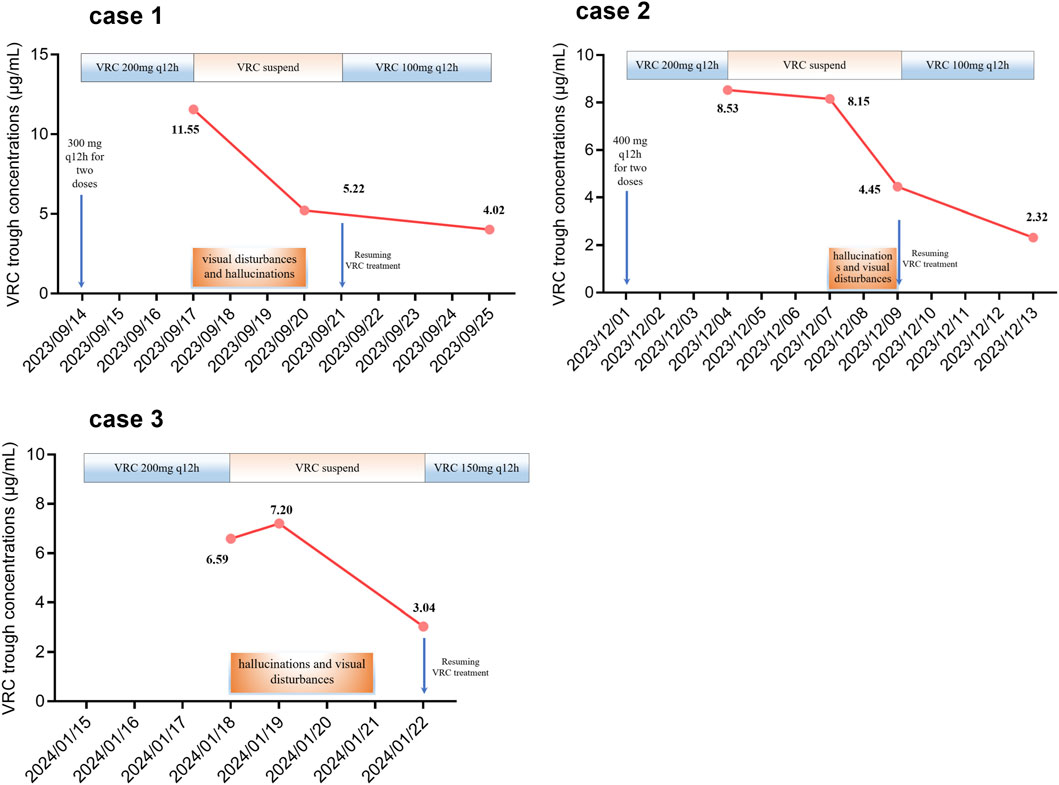
Figure 1. Alterations in the trough concentration of voriconazole and the occurrence of visual disturbances and hallucinations.
An 85-year-old male (with unknown body weight due to being bedridden) with a medical history of COPD, bronchiectasis, diabetes, and coronary heart disease presented to our hospital. The patient has no prior neurological disorders. Subsequent tests revealed bilateral lower leg edema and bilateral moist rales in the lungs. A combination of acute exacerbation of COPD (AECOPD) and respiratory infection leading to respiratory failure was considered. Serum levels of ALT, AST, and CREA were 5.6 IU/L, 15.5 IU/L, and 157.0 μmol/L, respectively. Consequently, spironolactone tablets, meropenem for injection, and moxifloxacin injection were prescribed. Subsequently, the presence of serum galactomannan (GM) confirmed the diagnosis of pulmonary aspergillosis. VRC tablets were administered at a dose of 400 mg q12 h for two doses followed by 200 mg q12 h starting from December 1, 2023. On day four of VRC treatment, the trough concentration of VRC was observed to be elevated at 8.53 μg/mL. Subsequently, VRC administration was discontinued while the trough concentration levels were monitored. The patient experienced hallucinations and visual disturbances on December 7 which resolved by December 9 without intervention. The trough concentrations of VRC measured on December 7 and December 9 were 8.15 μg/mL and 4.45 μg/mL respectively. VRC treatment resumed on December 9 with a reduced dosage regimen (100 mg q12 h po). On December 13th, the trough concentration level reached a value of 2.32 μg/mL without any recurrence of adverse effects associated with VRC (Figure 1).
An 74-year-old male (with unknown body weight due to being bedridden) was admitted to our hospital due to a history of recurrent cough. Digital X-ray photography (DX) revealed scattered speckled, flaky, and cable-like high-density shadows in the left middle and lower lung fields. The patient has no history of neurological disorders. Consequently, a diagnosis of chronic obstructive pulmonary disorder (COPD) with chronic inflammation of the left lung was considered. Additionally, the patient’s lung CT indicated bronchiectasis accompanied by infection. Serum levels of ALT, AST, and CREA were 26.6 IU/L, 20.9 IU/L, and 53.0 μmol/L, respectively. Consequently, methylprednisolone sodium succinate for injection, piperacillin sodium and tazobactam sodium for injection, and ambroxol hydrochloride injection were prescribed. Subsequently, the patient was found to be positive for Influenza A, and thus oseltamivir phosphate capsules were added. Specific IgG for Aspergillus fumigatus and positive GM levels were detected in the patient’s serum. Additionally, sputum culture confirmed the presence of Aspergillus fumigatus and Aspergillus flavus. Pulmonary aspergillosis was therefore diagnosed. VRC treatment at a dose of 200 mg q12 h commenced on January 15, 2024. On day 4 of VRC treatment, the patient experienced hallucinations and visual disturbances characterized by blurry vision and floating in front of his eyes. These symptoms appeared suddenly. Simultaneously, the trough concentration of VRC measured at 6.59 μg/mL raised concerns regarding drug-induced hallucinations and visual disturbances. Consequently, VRC administration was suspended while monitoring its trough concentration. Complete resolution of hallucinations and visual disorders occurred on January 21, 2024. The trough concentrations of VRC were 7.20 μg/mL on January 19th and decreased to 3.04 μg/mL on January 22nd respectively. VRC treatment resumed at a reduced dosage of 150 mg q12 h starting from January 22nd without recurrence of adverse effects induced by VRC (Figure 1).
We assessed our patient’s score on the Naranjo Scale (Naranjo et al., 1981). The three cases we report had respective weighted scores of 8, 5, and 5 on the Naranjo Scale, indicating that hallucinations and visual disturbances were probably caused by VRC rather than other medications. Then, we conducted an extensive search in both English and Chinese databases, including PubMed, Embase, Web of Science, Wanfang Data and the China National Knowledge Infrastructure (CNKI), for relevant literature published within the past 10 years using search terms such as “voriconazole”, “hallucinations”, “visual disturbances”, and “visual toxicity”. A comprehensive literature review was performed, which included a summary of eight studies investigating hallucinations and/or visual disturbances associated with VRC (Table 1) (Bayhan et al., 2016; Cao and Chen, 2020; Jansen et al., 2017; Jiang J., 2020; Kato et al., 2016; Shan and Rao, 2017; Zhang et al., 2021; Zheng et al., 2021). A total of fourteen patients were reported across the eight studies that were included. Of these, oral dosage form was administered to eight patients (57.1%), injectable dosage form to five patients (35.7%), and the mode of administration for one patient remained unknown (7.2%). Zonios et al. (2008) observed that hallucinations associated with the intravenous formulation vanish when VRC is administered orally. However, all three cases of VRC-induced visual disturbances and hallucinations reported in this article were orally administered. Therefore, we speculate that the route of VRC administration does not affect the frequency of hallucinations and visual disturbances.
Although the optimal therapeutic concentration of VRC remains unknown, it is generally recommended to fall within the range of 0.5–5.0 μg/mL (Chen et al., 2018). However, it is currently unclear whether there are variations in the concentration range of voriconazole in the blood of elderly individuals. A positive correlation has been identified between the concentration of VRC in the plasma and visual disturbances induced by the drug (Tan et al., 2006). Specifically, the odds ratio for visual disturbances increased by 4.7% for every 1 μg/mL increase in plasma VRC concentration. Furthermore, a notable discrepancy is apparent in the mean plasma VRC concentration (2.52 μg/mL) among 78 patients who did not experience hallucinations versus the 4.53 μg/mL level observed in 14 of 16 patients who reported hallucinations (Zonios et al., 2014). It was discovered that the individuals with plasma VRC concentrations >5 μg/mL [10/31 subjects (32%)] had a higher incidence of neurotoxic adverse effects, specifically visual and auditory hallucinations, compared to those with concentrations ≤5 μg/mL [2/170 patients (1.2%)] (Dolton et al., 2012). Of note, a study revealed a strong association between VRC-induced hallucinations and visual impairment (Imataki et al., 2008).
According to a systematic review and meta-analysis of 1,158 patients, VRC increased the risk of neurotoxicity when administered at a trough concentration exceeding 4.0 μg/mL (Jin et al., 2016). In the present study, we observed hallucinations and visual disturbances in three patients with a trough concentration of VRC >5 μg/mL (Figure 1). Consequently, we postulate that an elevated plasma concentration of VRC may be associated with an increased susceptibility to hallucinations and visual disturbances. However, a retrospective analysis of 103 patients revealed no statistically significant difference in plasma VRC concentrations between individuals with and without hallucinations (Sakurada et al., 2016). Further investigations are warranted to determine whether the occurrence of visual disturbances and hallucinations depends on VRC concentration.
The hepatic enzyme CYP2C19 plays a crucial role in the metabolism of VRC, with genetic polymorphisms significantly influencing its metabolic process and leading to substantial interindividual variations in plasma concentrations of this drug (Lamoureux et al., 2016). The most prevalent loss-of-function allele for CYP2C19 is *2, with frequencies of approximately 15% among Caucasians and Africans, and 29%–35% among Asians. Poor metabolizers of CYP2C19 account for around 2%–5% in Caucasians and Africans, while reaching approximately 15% in Asians (Scott et al., 2011). Among the reviewed articles, there were a total of 14 patients from Japan (6 cases, 42.9%), China (6 cases, 42.9%), Turkey (1 case, 7.1%), and the USA (1 case, 7.1%). Notably, all three documented cases of VRC -induced adverse reactions occurred within the Chinese population. Therefore, it is essential to pay further attention to adverse reactions associated with VRC use in Asians and consider implementing therapeutic drug monitoring as well as CYP2C19 genotyping specifically within Asian populations.
The management of pulmonary aspergillosis typically requires long-term treatment, and abruptly discontinuing or altering medications may potentially impact the efficacy of antifungal therapy to some extent. Moreover, compared to alternative antifungal agents, VRC is a more favorable choice for outpatients undergoing persistent treatment due to its dosage form and price. Among the 14 patients reviewed in this study, when adverse reactions such as visual impairment and hallucination occurred due to VRC, four patients (28.6%) discontinued the medication, three patients (21.4%) reduced the dosage, two patients (14.3%) maintained the original regimen (One patient exhibited a trough concentration of 3.79 for VRC while no data was available for the other patient), and five patients (35.7%) switched to alternative antifungal drugs (e.g., fluconazole, itraconazole, micafungin or caspofungin). Furthermore, in the three cases of VRC -induced visual disturbances and hallucinations documented in this study, all patients exhibited a trough concentration exceeding 5 μg/mL. Subsequently, therapeutic drug monitoring was implemented and subsequently resulted in dose reduction leading to resolution of these adverse reactions. Hence, we suggest that the implementation of therapeutic drug monitoring in conjunction with careful dosage adjustment represents a beneficial strategy for alleviating the visual disturbances and hallucinations induced by VRC.
This study has the following limitations. Firstly, it is constrained in estimating event incidence as it based on available cases and literature reviews. Future research will require a greater reliance on real-world data and individual case reports to further elucidate the clinical features of visual disturbances induced by voriconazole. Secondly, the search was limited to electronic databases, resulting in the unavailability of full texts for some studies, potentially introducing bias in selection and information. Furthermore, the included cases were from various clinical settings with incomplete crucial information. Discrepancies in diagnostic methods, medical protocols, and reporting standards may have influenced the data, implying inherent bias in this article. Thirdly, these three cases lack some clinical information, such as weight data and CYP2C19 genetic polymorphism. Increasing evidence implicates that the visual disturbances and hallucinations induced by VRC may be associated with individualized factors, such as CYP2C19 genetic polymorphism and drug sensitivity. Additionally, patient 3 was not administered the initial loading dose but was directly given the maintenance dose. Nevertheless, this study conducted a preliminary investigation into the clinical features of visual disturbances and hallucinations caused by VRC, aiming to enhance understanding and management of the adverse reactions associated with VRC.
In summary, the occurrence of visual disturbances and hallucinations induced by VRC is frequently observed in clinical settings. Patients undergoing VRC treatment should remain vigilant for potential symptoms such as blurred vision, altered color perception, visual and auditory hallucinations, among others. If any of these symptoms manifest during VRC administration, therapeutic drug monitoring is recommended along with appropriate dosage adjustments if necessary. Regular follow-up assessments should be conducted throughout the treatment period. Additionally, clinicians should promptly identify visual disturbances and hallucinations caused by VRC to enable timely intervention.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Xiangtan Central Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The blood samples of patients come from the normal course of treatment, and this article only collects some data during the treatment of patients. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL: Conceptualization, Data curation, Funding acquisition, Writing–original draft. YH: Data curation, Writing–review and editing. XL: Supervision, Writing–review and editing. DW: Visualization, Writing–review and editing. YH: Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the research project of Health Commission of Hunan Province (No. D202313018969) and the research project of Chinese Medical Association (No. Z-2021-46-2101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bayhan, G. I., Garipardic, M., Karaman, K., and Akbayram, S. (2016). Voriconazole-associated visual disturbances and hallucinations. Cutan. Ocul. Toxicol. 35 (1), 80–82. doi:10.3109/15569527.2015.1020544
Boyd, A. E., Modi, S., Howard, S. J., Moore, C. B., Keevil, B. G., and Denning, D. W. (2004). Adverse reactions to voriconazole. Clin. Infect. Dis. 39 (8), 1241–1244. doi:10.1086/424662
Cao, J., and Chen, X. (2020). Analysis and treatment of visual abnormality caused by voriconazole gene polymorphism: a case report. Cent. South Pharm. 18 (7), 1257–1258. doi:10.7539/j.issn.1672-2981.2020.07.038
Chen, K., Zhang, X., Ke, X., Du, G., Yang, K., and Zhai, S. (2018). Individualized medication of voriconazole: a practice guideline of the division of therapeutic drug monitoring, Chinese pharmacological society. Ther. Drug Monit. 40 (6), 663–674. doi:10.1097/ftd.0000000000000561
de Almeida Campos, L., Fin, M. T., Santos, K. S., de Lima Gualque, M. W., Freire Cabral, A. K. L., Khalil, N. M., et al. (2023). Nanotechnology-based approaches for voriconazole delivery applied to invasive fungal infections. Pharmaceutics 15 (1), 266. doi:10.3390/pharmaceutics15010266
Dolton, M. J., Ray, J. E., Chen, S. C., Ng, K., Pont, L. G., and McLachlan, A. J. (2012). Multicenter study of voriconazole pharmacokinetics and therapeutic drug monitoring. Antimicrob. Agents Chemother. 56 (9), 4793–4799. doi:10.1128/aac.00626-12
Imataki, O., Ohnishi, H., Kitanaka, A., Kubota, Y., Ishida, T., and Tanaka, T. (2008). Visual disturbance comorbid with hallucination caused by voriconazole in the Japanese population. Int. J. Hematol. 88 (1), 3–6. doi:10.1007/s12185-008-0114-3
Jansen, J. W., Sen, S. K., and Moenster, R. P. (2017). Elevated voriconazole level associated with hallucinations and suicidal ideation: a case report. Open Forum Infect. Dis. 4 (1), ofw215. doi:10.1093/ofid/ofw215
Jiang, J. (2020). Voriconazole induced hallucination: a case report. Her. Med. 39 (1), 120–121. doi:10.3870/j.issn.1004-0781.2020.01.026
Jin, H., Wang, T., Falcione, B. A., Olsen, K. M., Chen, K., Tang, H., et al. (2016). Trough concentration of voriconazole and its relationship with efficacy and safety: a systematic review and meta-analysis. J. Antimicrob. Chemother. 71 (7), 1772–1785. doi:10.1093/jac/dkw045
Kato, H., Hagihara, M., Hamada, Y., Koizumi, Y., Nishiyama, N., Yamagishi, Y., et al. (2016). Visual disturbance or central symptom like hallucination in patients treated voriconazole: report of six cases. Jpn. J. Antibiot. 69 (3), 143–150.
Kinoshita, J., Iwata, N., Ohba, M., Kimotsuki, T., and Yasuda, M. (2011). Mechanism of voriconazole-induced transient visual disturbance: reversible dysfunction of retinal ON-bipolar cells in monkeys. Invest. Ophthalmol. Vis. Sci. 52 (8), 5058–5063. doi:10.1167/iovs.11-7183
Lamoureux, F., Duflot, T., Woillard, J. B., Metsu, D., Pereira, T., Compagnon, P., et al. (2016). Impact of CYP2C19 genetic polymorphisms on voriconazole dosing and exposure in adult patients with invasive fungal infections. Int. J. Antimicrob. Agents 47 (2), 124–131. doi:10.1016/j.ijantimicag.2015.12.003
Mohammed, Y., Abousamra, A., Abdeldayem, A. A. I., Zafar, M., and Muhammad, T. (2022). Voriconazole-induced cholestatic hepatotoxicity in an immune competent patient. Cureus 14 (1), e21346. doi:10.7759/cureus.21346
Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 30 (2), 239–245. doi:10.1038/clpt.1981.154
Sakurada, H., Yasuhara, K., Kato, K., Asano, S., Yoshida, M., Yamamura, M., et al. (2016). An investigation of visual hallucinations associated with voriconazole administration to patients with hematological malignancies. Pharmazie 71 (11), 660–664. doi:10.1691/ph.2016.6725
Scott, S. A., Sangkuhl, K., Gardner, E. E., Stein, C. M., Hulot, J. S., Johnson, J. A., et al. (2011). Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 90 (2), 328–332. doi:10.1038/clpt.2011.132
Shan, W., and Rao, Y. (2017). Voriconazole injection induced phantom in patient with pulmonary infection opsia: a case report. Chin. Hosp. Pharm. J. 37 (8), 781–782. doi:10.13286/j.cnki.chinhosppharma-cyj.2017.08.25
Tan, K., Brayshaw, N., Tomaszewski, K., Troke, P., and Wood, N. (2006). Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46 (2), 235–243. doi:10.1177/0091270005283837
Veringa, A., Brüggemann, R. J., Span, L. F. R., Biemond, B. J., de Boer, M. G. J., van den Heuvel, E. R., et al. (2023). Therapeutic drug monitoring-guided treatment versus standard dosing of voriconazole for invasive aspergillosis in haematological patients: a multicentre, prospective, cluster randomised, crossover clinical trial. Int. J. Antimicrob. Agents 61 (2), 106711. doi:10.1016/j.ijantimicag.2023.106711
Wang, T., Miao, L., Shao, H., Wei, X., Yan, M., Zuo, X., et al. (2022). Voriconazole therapeutic drug monitoring and hepatotoxicity in critically ill patients: a nationwide multi-centre retrospective study. Int. J. Antimicrob. Agents 60 (5-6), 106692. doi:10.1016/j.ijantimicag.2022.106692
Zhang, X., Yang, H., Guo, X., and Xie, P. (2021). Adverse reactions of visual disturbances and hallucinations induced by voriconazole tablets in two cases and related literature review. Chin. J. Pharmacovigil. 18 (6), 588–591. doi:10.19803/j.1672-8629.2021.06.20
Zheng, R., Li, Y., Guo, C., Pei, Y., Ke, Z., and Huang, L. (2021). Voriconazole induced hallucinations and visual disturbances in a female child: a case report and literature review. Front. Pediatr. 9, 655327. doi:10.3389/fped.2021.655327
Zonios, D., Yamazaki, H., Murayama, N., Natarajan, V., Palmore, T., Childs, R., et al. (2014). Voriconazole metabolism, toxicity, and the effect of cytochrome P450 2C19 genotype. J. Infect. Dis. 209 (12), 1941–1948. doi:10.1093/infdis/jiu017
Keywords: voriconazole, adverse reactions, CYP2C19 genotyping, clinical characteristics, management strategies
Citation: Liu Y, Huang Y, Liu X, Wang D and Hu Y (2024) Characteristics of voriconazole-induced visual disturbances and hallucinations: case reports and literature review. Front. Pharmacol. 15:1420046. doi: 10.3389/fphar.2024.1420046
Received: 19 April 2024; Accepted: 23 October 2024;
Published: 07 November 2024.
Edited by:
Wagdy Mohamed Eldehna, Kafrelsheikh University, EgyptCopyright © 2024 Liu, Huang, Liu, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixiang Hu, eWl4aWFuZ2h1QGhudS5lZHUuY24=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.