
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Pharmacol., 05 August 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1418274
This article is a correction to:
Proteasome Inhibitor YSY01A Abrogates Constitutive STAT3 Signaling via Down-regulation of Gp130 and JAK2 in Human A549 Lung Cancer Cells
 Wei Huang1,2
Wei Huang1,2 Xia Yuan2
Xia Yuan2 Ting Sun2
Ting Sun2 Shujie Fan1
Shujie Fan1 Jun Wang1
Jun Wang1 Quan Zhou2
Quan Zhou2 Wei Guo2
Wei Guo2 Fuxiang Ran2
Fuxiang Ran2 Zemei Ge3
Zemei Ge3 Huayu Yang4
Huayu Yang4 Runtao Li3*
Runtao Li3* Jingrong Cui2*
Jingrong Cui2*A Corrigendum on
Proteasome inhibitor YSY01A abrogates constitutive STAT3 signaling via down-regulation of Gp130 and JAK2 in human A549 lung cancer cells
by Huang W, Yuan X, Sun T, Fan S, Wang J, Zhou Q, Guo W, Ran F, Ge Z, Yang H, Li R and Cui J (2017). Front. Pharmacol. 8:476. doi: 10.3389/fphar.2017.00476
In the published article, there was an error in Figures 3A, B and Figures 4A, C as published. The bands of some proteins shared the same control treated with DMSO vehicle. To avoid misunderstanding, Figures 3A, B and Figures 4A, C were replaced with the images from other repetitive experiments. The corrected Figures 3, 4 and their captions appear below.
In the published article, there was an error in the legends for Figure 3 and Figure 4 as published. The captions were changed to improve clarity. The corrected legends appear below.
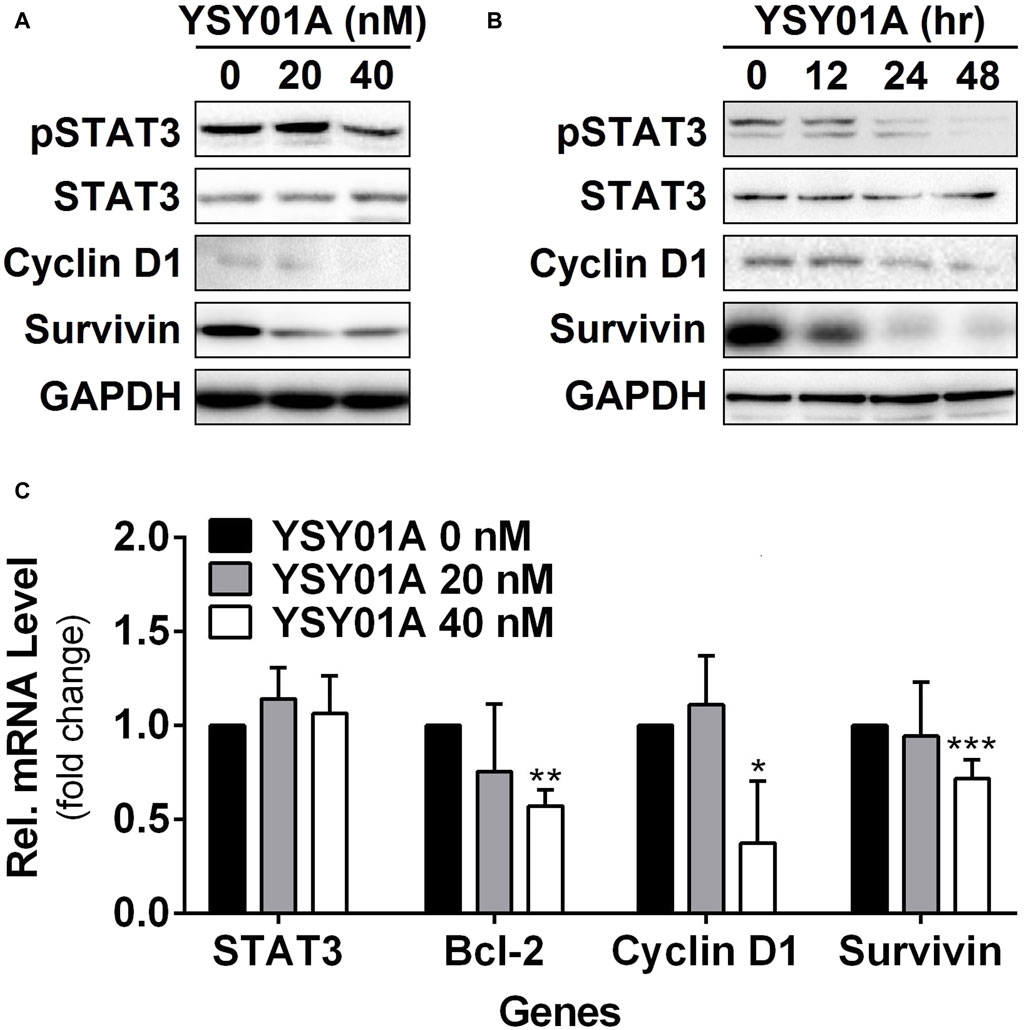
Figure 3. Effect of YSY01A on regulatory proteins important for cell proliferation and anti-apoptosis in A549 cells. (A) Expression levels of specific proteins important for cell proliferation and anti-apoptosis in a dose-response study. 24 h after seeding, A549 cells were exposed to 0 (vehicle control), 20 or 40 nM YSY01A for 48 h followed by sample collection and immunoblot analysis. GAPDH was used as a loading control. (B) Expression levels of specific proteins important for cell proliferation and anti-apoptosis in a time-course experiment. 24, 48 or 60 h after seeding, A549 cells were exposed to 40 nM YSY01A for 48, 24 or 12 h, respectively. Vehicle-treated cells were used as the 0-h control. At the end of study, all cell samples were harvested and subjected to immunoblot analysis of specific protein expression. GAPDH was used as a loading control. (C) Following the treatment as indicated, A549 cells were harvested for real-time qPCR analysis of selected genes. GAPDH was used as an internal control. (*p < 0.05, **p < 0.01, ***p < 0.001, by Student’s t-test as compared with vehicle control).
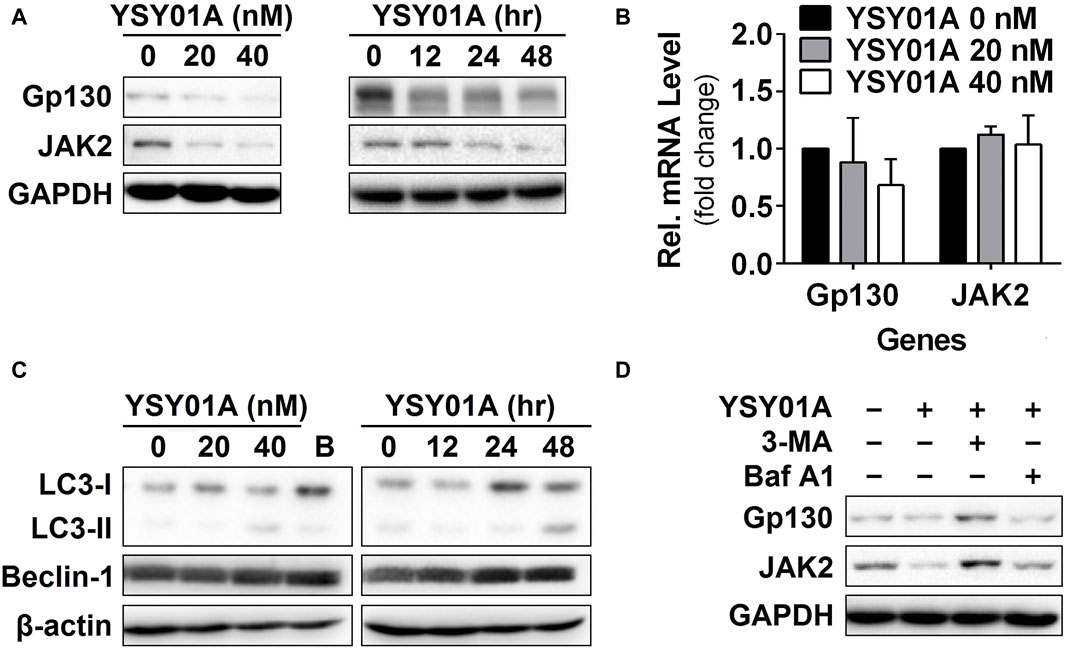
Figure 4. Gp130 and JAK2 are degraded by the autophagy lysosome pathway. (A) For the dose-response study, 24 h after seeding, A549 cells were exposed to 0 (vehicle control), 20 or 40 nM YSY01A for 48 h followed by sample collection and immunoblot analysis. GAPDH was used as a loading control. For the time-course experiment, 24, 48 or 60 h after seeding, A549 cells were exposed to 40 nM YSY01A for 48, 24 or 12 h, respectively. Vehicle-treated cells were used as the 0-h control. At the end of study, all cell samples were harvested and subjected to immunoblot analysis of specific protein expression. GAPDH was used as a loading control. (B) cDNA from A549 cells treated with 0 (vehicle control), 20 or 40 nM YSY01A for 48 h was evaluated for mRNA expression of gp130 and JAK2. Data are relative to GAPDH and normalized to cells treated with vehicle control. (C) Effects of YSY01A and bortezomib on LC3 and beclin 1, two markers of autophagy. B: bortezomib. (D) In the presence of proteasome inhibition, A549 cells were co-treated with 5 mM 3-methyladenine (3-MA) or 100 nM Baflomycin A1 (Baf A1) for 48 h. Whole cell lysates were evaluated for gp130, JAK2, and GAPDH by immunoblot analysis.
The authors apologize for these errors and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Keywords: proteasome inhibitor, YSY01A, STAT3 signaling, protein degradation, non-small cell lung carcinoma
Citation: Huang W, Yuan X, Sun T, Fan S, Wang J, Zhou Q, Guo W, Ran F, Ge Z, Yang H, Li R and Cui J (2024) Corrigendum: Proteasome inhibitor YSY01A abrogates constitutive STAT3 signaling via down-regulation of Gp130 and JAK2 in human A549 lung cancer cells. Front. Pharmacol. 15:1418274. doi: 10.3389/fphar.2024.1418274
Received: 16 April 2024; Accepted: 03 July 2024;
Published: 05 August 2024.
Edited and reviewed by:
Apurva Srivastava, National Cancer Institute at Frederick (NIH), United StatesCopyright © 2024 Huang, Yuan, Sun, Fan, Wang, Zhou, Guo, Ran, Ge, Yang, Li and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingrong Cui, anJjdWlAYmptdS5lZHUuY24=; Runtao Li, bGlydEBiam11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.