
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 14 June 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1412938
Background: Capecitabine has been reported to be associated with severe gastrointestinal (GI) adverse drug reactions (gastrointestinal ulceration, haemorrhage, and obstruction). However, statistical correlations have not been demonstrated, and specific GI adverse drug reactions, such as GI obstruction, are not listed on its label.
Aim: We aimed to determine the associations between capecitabine and GI ulceration, haemorrhage, or obstruction among patients with breast cancer by examining data from the United States Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: We performed disproportionality analysis of GI ulceration, haemorrhage, and obstruction by evaluating the reporting odds ratio (ROR) and the information component (IC) with their 95% confidence intervals (CIs).
Results: We identified 279 patients with capecitabine-associated GI ulceration, haemorrhage, or obstruction reported between 1 January 2004 and 31 December 2020. One-fourth of the cases of GI ulceration, haemorrhage, or obstruction resulted in death. Capecitabine as a drug class had disproportionately high reporting rates for GI ulceration [ROR 1.94 (1.71–2.21); IC 0.80 (0.60–0.99)], haemorrhage [ROR 2.27 (1.86–2.76); IC 0.99 (0.69–1.28)], and obstruction [ROR 2.19 (1.63–2.95); IC 0.96 (0.51–1.40)].
Conclusion: Pharmacovigilance research on the FAERS has revealed a slight increase in reports of GI ulceration, haemorrhage, and obstruction in capecitabine users, which may cause serious or deadly consequences. In addition to the adverse reactions described in the package insert, close attention should be paid to GI obstruction to avoid discontinuation or life-threatening outcomes.
Intravenous 5-fluorouracil (5-FU) is an antimetabolite chemotherapeutic agent that requires hospitalization for constant infusion through a peripheral catheter or, if the venous condition is poor, a central catheter. By comparison, oral capecitabine, a fluoropyrimidine carbamate, is quickly absorbed through the intestine and converted to 5-FU by the enzyme thymidine phosphorylase, thereby inhibiting the ability of cancer cells to produce substances necessary for growth (Saif et al., 2008). Capecitabine, which is an effective and long-lasting treatment for colorectal and breast cancer, is utilized in mono- and combination therapy for metastatic cancer (Johnston and Kaye, 2001). It is also used in preoperative therapy, neoadjuvant therapy and postoperative or adjuvant treatment with radiotherapy (Chan and Chee, 2019).
As capecitabine has become more widely used, severe adverse events have been reported, including gastrointestinal (GI) toxicities such as GI haemorrhage (Zou et al., 2021; Hagiwara et al., 2022), GI ulceration (Saeki et al., 2005), and GI obstruction (Pow-Anpongkul et al., 2019). Importantly, GI obstruction is not mentioned on the package insert. Therefore, the incidence of GI toxicity might be underestimated in clinical practice. Given the severity of this GI toxicity and the site of capecitabine absorption, physicians need to be aware of the profiles of drug toxicity, especially in populations at high risk.
Since 1969, the United States Food and Drug Administration Adverse Event Reporting System (FAERS) has been compiling data concerning adverse event (AE) reports to support the FDA’s safety surveillance of postmarketing drugs. Safety data on drug usage can be evaluated through data mining of FAERS (Revol et al., 2018). Data mining algorithms are broadly adopted to detect signals such as drug-associated AEs quantitatively. Pharmacovigilance analysis through FAERS is the most common way to identify rare signals and has potential value in novel signal detection (Meng et al., 2021).
We aimed to determine the associations between capecitabine and GI ulceration, haemorrhage, and obstruction in patients with breast cancer by examining data from the FAERS.
This pharmacovigilance study was a retrospective disproportionality analysis of events in the FAERS database. We used OpenVigil to search for AE reports between 1 January 2004 and 31 December 2020. To avoid the impact of the primary disease on GI AEs, the AEs in this study were taken only from breast cancer patients. OpenVigil is a new publicly accessible pharmacovigilance tool for extracting, cleaning, mining, and dissecting data from FAERS using the interface of open-FDA (Bohm et al., 2012; Bohm et al., 2016).
All AE reports on FDA-approved capecitabine use that had the words “interacting,” “concomitant,” “primary suspect,” or “secondary suspect” were collected. All AEs in the FAERS are classified utilizing preferred terms (PTs) in the Medical Dictionary for Regulatory Activities (MedDRA, version 24.1). Based on our team’s previous experience with FAERS (Huang et al., 2022), we applied the definitions offered by MedDRA. The FAERS database collects information on AEs that are spontaneously reported by healthcare professionals, pharmaceutical manufacturers, patients, and others in different regions. Using MedDRA terms can overcome any interindividual inconsistencies in terminology by grouping like terms that are linked with an identical situation or AE of interest. Standardized MedDRA Queries (SMQs) are groupings of MedDRA terms broadly applied to FAERS research that can cover one defined medical situation or domain of interest (Meng et al., 2021). Disproportionality analysis was done to detect a relationship between exposure to capecitabine and the symptoms of GI ulceration, haemorrhage, or obstruction. The cases with any PT included in the broad scope of existing MedDRA SMQ terms (“Gastrointestinal ulceration,” “Gastrointestinal haemorrhage,” and “Gastrointestinal obstruction”) were chosen.
Disproportionality analysis was employed to evaluate whether the reported number of a particular drug–AE combination exceeded the anticipated number. The “anticipated” number was the number of a given AE caused by other drugs in the database (Bate and Evans, 2010). Disproportionality analyses can be split into frequentist and Bayesian methods. We employed the information component (IC) and the reporting odds ratio (ROR) as the statistical gauges, based on a two-by-two contingency table, and the whole database was used as the comparison group. IC, a statistical gauge of the Bayesian method, is a tool for detecting signals that has the ability to avoid false positives, especially in a small number of cases (van Puijenbroek et al., 2002; Harpaz et al., 2013). ROR, a statistical gauge of the frequentist method, is the pharmacovigilance equivalent of the odds ratio and has been validated (Bate et al., 2014). A signal was present when the number of AEs was ≥3, the lower limit of the ninety-five percent confidence interval was >1 (van Puijenbroek et al., 2002), and the lower limit of the ninety-five percent confidence interval (IC025) of the IC value was >0 (Bate et al., 1998). When a positive signal was detected, we used sensitivity analysis to verify that the signal was stable. The sensitivity analytical conditions were age 65 years or older and a reporting time between 1 January 2011 and 31 December 2020.
Microsoft Excel was used to perform descriptive analysis and assess the basic characteristics of the patients with capecitabine -associated GI ulceration, haemorrhage, and obstruction.
The search performed under the above conditions found 6,679 capecitabine -associated AEs in the FAERS (Table 1). Some 471 cases of GI ulceration, haemorrhage, and obstruction were detected, 94.90% of which occurred in female patients. The percentages of hospitalization, death, and life-threatening cases reached 52.23% (246/471), 25.05% (118/471), and 6.58% (31/471), respectively (Table 2).
IC and ROR were calculated to find the signal values of GI ulceration, haemorrhage, and obstruction associated with capecitabine in the FAERS database. Compared to the overall AEs in the database, capecitabine -associated “gastrointestinal ulceration” [ROR 1.94 (1.71–2.21); IC 0.80 (0.60–0.99)], “gastrointestinal haemorrhage” [ROR 2.27 (1.86–2.76); IC 0.99 (0.69–1.28)], and “gastrointestinal obstruction” [ROR 2.19 (1.63–2.95); IC 0.96 (0.51–1.40)] had significantly higher reporting rates (Table 3). When the criteria for sensitivity analysis were “age 65 years or older” and “reporting time between 1 January 2011, and 31 December 2020,” the reporting rates of “gastrointestinal ulcers,” “gastrointestinal haemorrhage,” and “gastrointestinal obstruction” were significantly higher than those for all AEs (Table 3).
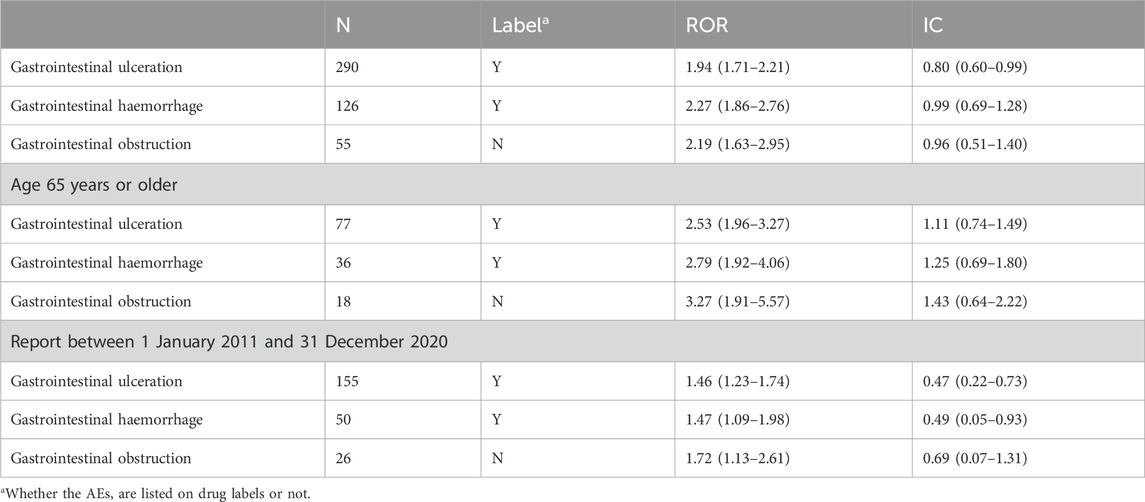
Table 3. Disproportionality analysis of capecitabine -associated GI ulceration, haemorrhage, and obstruction adverse events based on SMQ term.
This study found that capecitabine may cause GI ulceration, haemorrhage, and obstruction. Gastrointestinal obstruction was not mentioned in the package insert and can be considered a new potential adverse effect with value in guiding clinical care.
Early studies revealed that capecitabine causes mitotic arrest of crypt cells in the G2 phase, impairing their migration and differentiation into mature enterocytes (Ikuno et al., 1995). In addition, fluoropyrimidines may increase the gene expression of inflammatory cytokines and reduce the expression of colonic aquaporin channels through neutrophilic inflammation (Sakai et al., 2013). Both pathways induce intestinal inflammation by inducing epithelial cell loss secondary to acute mucosal injury. Pete Pow-Anpongkul reported (Pow-Anpongkul et al., 2019) on a 60-year-old man with colorectal cancer who presented with abdominal pain, bloating, and vomiting after two cycles of capecitabine. Abdominal CT showed thickening of the small bowel wall involving the distal duodenum and jejunum with dilated gastric and proximal small bowel loops, consistent with the diagnosis of ileus. However, endoscopy revealed severe inflammation from the distal duodenum to the proximal jejunum. Mild enteritis may be characterized by abdominal pain and diarrhoea (Gómez-Escudero and Remes-Troche, 2021; Trontzas et al., 2023), while severe enteritis may lead to intestinal ulceration and obstruction (Ali and Robles, 2021; Hamdeh et al., 2021; Sasaki et al., 2022).
Fluoropyrimidines are directly toxic to the endothelium through the increased generation of reactive oxygen species. This toxicity can cause vasospasm or thrombosis through the release of sequestered vasoactive substances (Focaccetti et al., 2015). If mesenteric blood vessels are embolized, the intestinal blood circulation will be impaired, and the intestinal tube will lose its peristaltic ability, thereby leading to intestinal vascular obstruction; this phenomenon explains the intestinal obstruction caused by capecitabine (Stancu et al., 2024). Obstruction might also be due to patient factors. Capecitabine is converted to 5-FU in the body, and most 5-FU is catabolized by dihydropyrimidine dehydrogenase (DPD) (Matsusaka and Lenz, 2015). DPD deficiency is reported in roughly 5%–9% of patients, so administering fluoropyrimidines in the context of depressed enzyme activity can be fatal (Saif and Diasio, 2006; Wormann et al., 2020). We speculate that DPD deficiency aggravates the gastrointestinal toxicity caused by capecitabine and increases the likelihood of gastrointestinal haemorrhage, ulceration, and obstruction. Of course, a combination of factors could lead to GI obstruction in patients taking capecitabine.
Recommendations are in place for routine screening for the four most common DPD variants before initiating treatment with capecitabine (Iacovelli et al., 2014). For patients with DPD deficiency, a Japanese study showed that the initial administration of a small amount of capecitabine followed by a gradual dose increase could eventually increase the DPD protein level up to 12-fold before chemotherapy. This safe administration method for patients with DPD deficiency is worth exploring in clinical practice (Yoshida et al., 2015).
Another approach that deserves attention is probiotics. Probiotics may have antitumour effects, particularly against colorectal and breast cancer (de Moreno de LeBlanc et al., 2007; Osterlund et al., 2007), and are more suitable for patients with breast or colorectal cancer who are treated with capecitabine. Recent studies on inflammatory bowel disease have revealed a pivotal therapeutic and anti-inflammatory role for probiotics (Sartor and Muehlbauer, 2007). Potential mechanisms of probiotic action include competitive inhibition of pathogen binding to intestinal epithelial cells, inhibition of pro-inflammatory cytokines, induction of anti-inflammatory cytokines, production of antimicrobial metabolites, dialogue with the epithelium, and immune modulation (de Moreno de LeBlanc et al., 2007; Osterlund et al., 2007). Probiotics may play a preventive role in ileitis-induced obstruction.
Patients should be adequately educated on medication safety. Intestinal obstruction is a surgical emergency in the abdomen. If early detection and timely treatment are performed, satisfactory clinical results can be achieved. Otherwise, the treatment will possibly cause delay, and in addition, complications can occur, such as abdominal infection and septic shock which can be fatal. Therefore, medical staff should fully inform patients who take capecitabine about the health issues reported for this medication.
This research has some limitations 1) Overreporting, missing figures, and underreporting were unavoidable owing to the voluntary and retrospective nature of FAERS reporting (Michel et al., 2017). 2) Considering the possibility of confounding elements, including concomitant drugs or potential illnesses, causal correlations cannot be definitively inferred from our results. 3) The IC or ROR does not indicate an increased risk of AE occurrence but rather an increased risk of AE reports (Raschi et al., 2020). 4) Due to database limitations, we do not have a clear picture of whether patients were taking capecitabine in combination with radiotherapy and whether there were gene variants in the DPYD gene. It is not possible to determine whether these factors are associated with severe gastrointestinal toxicity. 5) The only disease examined in this study was breast cancer, and although the positive signal was obtained, it does not indicate that the relevant results may occur in other diseases. The above limitations also lead to the difference between the incidence of the data collected in this study and the drug label. The nature of FAERS weakens the conclusions that can be drawn. Nevertheless, this research offers a pivotal foundation for hypothesis generation (but not testing) and emphasizes possible patient health problems worthy of thorough exploration.
Despite these limitations, our research is the first to recognize the link between capecitabine and GI ulceration, haemorrhage, and obstruction by analysing the world’s most extensive database of postmarketing drugs. Our findings will contribute to the safe utilization of capecitabine. To further study whether capecitabine causes gastrointestinal toxicity in patients with colorectal cancer, we retrospectively analysed data from three medical institutions that have given patients capecitabine monotherapy after colorectal cancer surgery over the past 10 years. The investigation is ongoing, and we will publish our findings in due course. In addition, more prospective and preclinical epidemiological research is needed to verify our findings and conclusions.
By searching the FAERS database, we found an adverse reaction, GI obstruction, that was not listed on the capecitabine drug label. The present pharmacovigilance research revealed a mild increase in reports of GI ulceration, haemorrhage, and obstruction, which might trigger severe and even fatal outcomes, among capecitabine users. Further in-depth epidemiological research is needed to validate any causal relationships.
Significant increases were observed in the reported rates of “gastrointestinal ulceration,” “gastrointestinal haemorrhage,” and “gastrointestinal obstruction” associated with capecitabine. GI obstruction was found to be a positive adverse reaction signal not recorded in the package insert.
Although no clinical trials or observational studies have confirmed these signals, capecitabine’s medication guidance should be reinforced to warn of GI toxicity, including GI ulceration, haemorrhage, and obstruction.
More studies on capecitabine are needed to characterize its GI ulceration, haemorrhage, and obstruction risk factors and outcomes.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
YW: Data curation, Methodology, Writing–original draft. LM: Data curation, Resources, Writing–review and editing. XL: Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is supported by grants from the Joint Project of Chongqing Health Commission and Science and Technology Bureau (Grant No. 2022QNXM011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ali, P. M., and Robles, M. S.De (2021). Chemoimmunotherapy-related enteritis resulting in a mechanical small bowel obstruction – a case report. Int. J. Surg. Case Rep. 79, 131–134. doi:10.1016/j.ijscr.2020.12.096
Bate, A., and Evans, S. J. W. (2010). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 18 (6), 427–436. doi:10.1002/pds.1742
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54 (4), 315–321. doi:10.1007/s002280050466
Bate, A., Pariente, A., Hauben, M., and Bégaud, B. (2014). Quantitative signal detection and analysis in pharmacovigilance. Mann’s Pharmacovigil., 331–354. doi:10.1002/9781118820186.ch20
Bohm, R., Hocker, J., Cascorbi, I., and Herdegen, T. (2012). OpenVigil—free eyeballs on AERS pharmacovigilance data. Nat. Biotechnol. 30 (2), 137–138. doi:10.1038/nbt.2113
Bohm, R., von Hehn, L., Herdegen, T., Klein, H. J., Bruhn, O., Petri, H., et al. (2016). OpenVigil FDA–inspection of US American adverse drug events pharmacovigilance data and novel clinical applications. PloS One 11 (6), e0157753. doi:10.1371/journal.pone.0157753
Chan, G. H., and Chee, C. E. (2019). Making sense of adjuvant chemotherapy in colorectal cancer. J. Gastrointest. Oncol. 10 (6), 1183–1192. doi:10.21037/jgo.2019.06.03
de Moreno de LeBlanc, A., Matar, C., and Perdigón, G. (2007). The application of probiotics in cancer. Br. J. Nutr. 98 (1), S105–S110. doi:10.1017/S0007114507839602
Focaccetti, C., Bruno, A., Magnani, E., Bartolini, D., Principi, E., Dallaglio, K., et al. (2015). Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One 10 (2), e0115686. doi:10.1371/journal.pone.0115686
Gómez-Escudero, O., and Remes-Troche, J. M. (2021). Approach to the adult patient with chronic diarrhea: a literature review. Rev. Gastroenterol. México 86, 387–402. doi:10.1016/j.rgmxen.2021.08.007
Hagiwara, Y., Yamamoto, Y., Inagaki, Y., Tomisaki, R., Tsuji, M., Fukuda, S., et al. (2022). Severe gastrointestinal disorder due to capecitabine associated with dihydropyrimidine dehydrogenase deficiency: a case report and literature review. Intern Med. 61 (16), 2449–2455. doi:10.2169/internalmedicine.8636-21
Hamdeh, S., Micic, D., and Hanauer, S. (2021). Review article: drug-induced small bowel injury. Aliment. Pharmacol. Ther. 00, 1370–1388. doi:10.1111/apt.16642
Harpaz, R., DuMouchel, W., LePendu, P., Bauer-Mehren, A., Ryan, P., and Shah, N. H. (2013). Performance of pharmacovigilance signal-detection algorithms for the FDA adverse event reporting system. Clin. Pharmacol. Ther. 93 (6), 539–546. doi:10.1038/clpt.2013.24
Huang, J., Zhao, Y., Cao, Y., Zhang, Q., and Ran, D. (2022). Anaplastic lymphoma kinase tyrosine kinase inhibitors associated gastrointestinal obstruction, perforation, and ulceration: an analysis of the FDA adverse event reporting system database (FAERS). Int. J. Clin. Pharm. 44 (4), 993–1003. doi:10.1007/s11096-022-01425-4
Iacovelli, R., Pietrantonio, F., Palazzo, A., Maggi, C., Ricchini, F., de Braud, F., et al. (2014). Incidence and relative risk of grade 3 and 4 diarrhoea in patients treated with capecitabine or 5-fluorouracil: a meta-analysis of published trials. Br. J. Clin. Pharmacol. 78, 1228–1237. doi:10.1111/bcp.12449
Ikuno, N., Soda, H., Watanabe, M., and Oka, M. (1995). Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. Natl. Cancer Inst. 87, 1876–1883. doi:10.1093/jnci/87.24.1876
Johnston, P., and Kaye, S. (2001). Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs 12 (8), 639–646. doi:10.1097/00001813-200109000-00001
Matsusaka, S., and Lenz, H. J. (2015). Pharmacogenomics of fluorouracil-based chemotherapy toxicity. Expert Opin. Drug Metab. Toxicol. 11, 811–821. doi:10.1517/17425255.2015.1027684
Meng, L., Yang, B., Qiu, F., Jia, Y., Sun, S., Yang, J., et al. (2021). Lung cancer adverse events reports for angiotensin-converting enzyme inhibitors: data mining of the fda adverse event reporting system database. Front. Med. (Lausanne) 8, 594043. doi:10.3389/fmed.2021.594043
Michel, C., Scosyrev, E., Petrin, M., and Schmouder, R. (2017). Can disproportionality analysis of post-marketing case reports be used for comparison of drug safety profiles? Clin. Drug Investig. 37 (5), 415–422. doi:10.1007/s40261-017-0503-6
Osterlund, P., Ruotsalainen, T., Korpela, R., Saxelin, M., Ollus, A., Valta, P., et al. (2007). Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. Br. J. Cancer 97, 1028–1034. doi:10.1038/sj.bjc.6603990
Pow-Anpongkul, P., Chu, P. G., and Kidambi, T. D. (2019). Capecitabine-induced enteritis leading to small bowel obstruction. Gastroenterology 156 (5), e8–e9. doi:10.1053/j.gastro.2018.11.076
Raschi, E., Gatti, M., Gelsomino, F., Ardizzoni, A., Poluzzi, E., and De Ponti, F. (2020). Lessons to be learnt from real-world studies on immune-related adverse events with check-point inhibitors: a clinical perspective from pharmacovigilance. Target Oncol. 15, 449–466. doi:10.1007/s11523-020-00738-6
Revol, B., Jullian-Desayes, I., Tamisier, R., Puel, V., Mallaret, M., Joyeux-Faure, M., et al. (2018). Ticagrelor and central sleep apnea. J. Am. Coll. Cardiol. 71 (20), 2378–2379. doi:10.1016/j.jacc.2018.03.447
Saeki, T., Takashima, S., Terashima, M., Satoh, A., Toi, M., Osaki, A., et al. (2005). A Japanese phase I study of continuous oral capecitabine in patients with malignant solid tumors. Int. J. Clin. Oncol. 10 (1), 51–57. doi:10.1007/s10147-004-0460-y
Saif, M. W., and Diasio, R. (2006). Is capecitabine safe in patients with gastrointestinal cancer and dihydropyrimidine dehydrogenase deficiency? Clin. Colorectal Cancer 5, 359–362. doi:10.3816/CCC.2006.n.007
Saif, M. W., Katirtzoglou, N. A., and Syrigos, K. N. (2008). Capecitabine: an overview of the side effects and their management. Anticancer Drugs 19 (5), 447–464. doi:10.1097/CAD.0b013e3282f945aa
Sakai, H., Sagara, A., Matsumoto, K., Hasegawa, S., Sato, K., Nishizaki, M., et al. (2013). 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS One 8, e54788. doi:10.1371/journal.pone.0054788
Sartor, R. B., and Muehlbauer, M. (2007). Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr. Gastroenterol. Rep. 9, 497–507. doi:10.1007/s11894-007-0066-4
Sasaki, Y., Abe, Y., Mizumoto, N., Nomura, E., and Ueno, Y. (2022). Small bowel endoscopic features of eosinophilic gastroenteritis. Diagn. (Basel) 13 (1), 113. doi:10.3390/diagnostics13010113
Stancu, B., Chira, A., Coman, H. F., Mihaileanu, F. V., Ciocan, R., Gherman, C. D., et al. (2024). Intestinal obstruction as initial presentation of idiopathic portal and mesenteric venous thrombosis: diagnosis, management, and literature review. Diagn. (Basel) 14 (3), 304. doi:10.3390/diagnostics14030304
Trontzas, I. P., Rapti, V. E., Syrigos, N. K., Gomatou, G., Lagou, S., Kanellis, G., et al. (2023). Capecitabine-associated enterocolitis: narrative literature review of a rare adverse event and a case presentation. J. Chemother. 35 (1), 63–71. doi:10.1080/1120009X.2021.2025316
van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. G. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11 (1), 3–10. doi:10.1002/pds.668
Wormann, B., Bokemeyer, C., Burmeister, T., Köhne, C. H., Schwab, M., Arnold, D., et al. (2020). Dihydropyrimidine dehydrogenase testing prior to treatment with 5-fluorouracil, capecitabine, and tegafur: a consensus paper. Oncol. Res. Treat. 43, 628–636. doi:10.1159/000510258
Yoshida, Y., Ogura, K., Hiratsuka, A., Aisu, N., Yamada, T., Kojima, D., et al. (2015). 5-fluorouracil chemotherapy for dihydropyrimidine dehydrogenase-deficient patients: potential of the dose-escalation method. Anticancer Res. 35 (9), 4881–4887.
Keywords: adverse drug reactions, capecitabine, FAERS, gastrointestinal ulceration, gastrointestinal haemorrhage, gastrointestinal obstructions
Citation: Wang Y, Meng L and Liu X (2024) Capecitabine-associated gastrointestinal ulceration, haemorrhage, and obstruction: a pharmacovigilance analysis based on the FAERS. Front. Pharmacol. 15:1412938. doi: 10.3389/fphar.2024.1412938
Received: 06 April 2024; Accepted: 03 June 2024;
Published: 14 June 2024.
Edited by:
Meghana V. Trivedi, University of Houston, United StatesReviewed by:
Erika Brown, University of Houston, United StatesCopyright © 2024 Wang, Meng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Liu, MjAwNTI2MDdAcXEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.