- 1Graduate School, Heilongjiang University of Chinese Medicine, Harbin, China
- 2The Second Department of Surgery, The First Affiliated Hospital of Heilongjiang University of Traditional Chinese Medicine, Harbin, China
Increasing incidences of metastasis or recurrence (or both) in triple-negative breast cancer (TNBC) are a growing concern worldwide, as these events are intricately linked to higher mortality rates in patients with advanced breast cancer. Flavonoids possess several pharmaceutical advantages with multi-level, multi-target, and coordinated intervention abilities for treating TNBC, making them viable for preventing tumor growth and TNBC metastasis. This review focused on the primary mechanisms by which flavonoids from traditional Chinese medicine extracts inhibit TNBC, including apoptosis, blocking of cell cycle and movement, regulation of extracellular matrix degradation, promotion of anti-angiogenesis, inhibition of aerobic glycolysis, and improvement in tumor microenvironment. This review aims to improve the knowledge of flavonoids as a promising pharmacological intervention for patients with TNBC.
1 Introduction
Approximately 13% of women globally are diagnosed with breast cancer (Giaquinto et al., 2022). Triple-negative breast cancer (TNBC) is a kind of cancer where the estrogen receptor (ER), progesterone receptor, and human epidermal growth factor receptor 2 (HER-2) are not expressed. TNBC exhibits a low differentiation rate, high invasiveness, and high metastasis and recurrence (Lu et al., 2023). This type of breast cancer makes up 12% of all cases in the US and has a 5-year survival rate that varies from 8% to 16% (Howard and Olopade, 2021). The treatment options for TNBC include surgical intervention, radiation therapy, chemotherapy, targeted therapy, and immunotherapy. However, limited therapeutic strategies for TNBC due to a lack of effective biological targets and biomarkers, diverse molecular subtypes, and complex biological behaviors and clinical characteristics have made it a significant clinical challenge for years. Therefore, developing more efficient therapeutic drugs is necessary.
Flavonoids derived from TCM exhibit a diverse array of anti-tumor properties that can be combined with modern therapies to improve treatment efficacy and prevent the occurrence of breast cancer. Flavonoids extracted from TCM have wide-ranging effects linked to multiple cancer-related signaling pathways. This review briefly introduces flavonoids from TCM extracts, their targets, and potential mechanisms for TNBC.
2 Molecular pathogenesis of breast cancer
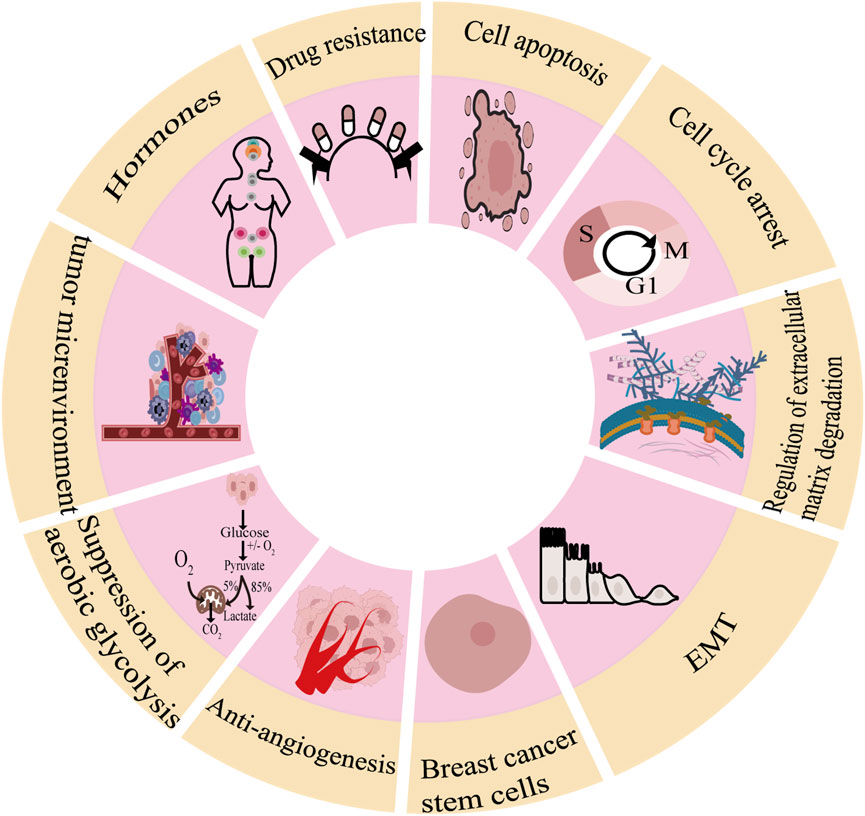
2.1 Breast cancer cell apoptosis
A disequilibrium between cellular division and cell death leads to uncontrolled proliferation of cancer cells. Apoptotic signaling pathways can be categorized into three distinct types: intrinsic, extrinsic, and endoplasmic reticulum pathways. Apoptosis evasion mechanisms can be roughly classified into three types: diminished caspase function, impaired death receptor signaling, and an altered balance of pro-apoptotic and anti-apoptotic proteins. This is related to the disruptions in the equilibrium of the Bcl-2 family, defects in the p53 tumor suppressor gene, anomalous expression of apoptosis protein inhibitors, decreased caspase activity, and impaired death receptor signaling (Singh and Lim, 2022).
2.2 Cell cycle arrest
Cell cycle dysregulation is the basis of uncontrolled cell proliferation. Cells that have lost the checkpoint mechanisms have highly unstable genomes. In breast cancer, cell cycle abnormalities are frequently observed, including the loss of Rb function, inhibitors, and increased abundance of cyclin D, cyclin E, and cyclin-dependent kinase (CDK). Cyclin D1 controls how cells move from the G1 phase, where they prepare for DNA replication, to the phase where they actually copy their DNA. This gene is also important for preventing human breast cancer cells from growing too much. There is a strong correlation between the elevated expression of CCND1, which codes for the protein Cyclin D1, and decreased survival rates (Aftab et al., 2021). Cyclin E serves as a potent prognostic indicator of breast cancer and plays a crucial role in determining tumor aggressiveness and predicting the recurrence of TNBC (Guerrero Llobet et al., 2020).
2.3 Regulation of extracellular matrix degradation
Breast cancer cells become potentially malignant after penetrating and dissolving the extracellular matrix (ECM), particularly the basement membrane. Epigenetic mechanisms are important for regulating EMT/ECM-related pathways. When cancer cells acquire mesenchymal features, it promotes the advancement of TNBC, and the process of DNA methylation and the action of enzymes that modify histones contribute to changes in ECM/EMT alterations in TNBC (Zolota et al., 2021). Matrix metalloproteinases (MMPs) are proteolytic enzymes containing active Zn2+. MMP9 degrades the ECM near tumors, which are intimately associated with tumor invasion and metastasis, performs the final degradation of collagen fibers, and removes malignant cells from the complicated network around them. Collagen is a significant metabolite of connective tissue that undergoes a two-step degradation process in mammary gland tissue. MMP-2 and MMP-9 specifically target the breakdown of denatured interstitial collagen or gelatin, as well as collagen types IV and V found in the basement membrane. MMP-1 is the sole MMP enzyme that can degrade all types of collagen in the mammary gland and is crucial in breaking down stromal fibers in various diseases (Argote Camacho et al., 2021). MMP9 is crucial for the development of the “metastatic niche” and controls the spread of cancer cells to the lungs when overexpressed. The coexpression of MMP-1, MMP-2, and MMP-9 may indicate an unfavorable prognosis in patients with breast cancer (Mohammadian et al., 2020; Jiang and Li, 2021).
2.4 Inhibition of the epithelial-mesenchymal transition (EMT)
Epithelial–mesenchymal transition (EMT) is a significant process that triggers tumor invasion and metastasis. Cancer metastasis can be divided into multiple phases, including in situ tumor growth, EMT, invasion, infiltration, survival in blood circulation, extravasation, dormancy, and metastatic growth (Fares et al., 2020). EMT involves the disruption of cell adhesions in cancer cells originating from epithelial tissue and the upregulation of particular metabolites in the constricted cytoskeleton, leading to a mesenchymal phenotype characterized by increased invasiveness and the migration of primary cancers. TNBC cells evolve from epithelial cells to hybrid epithelial/mesenchymal (E/M) and strong mesenchymal patterns during invasion. Similarly, during colony formation, TNBC cells transition from epithelial cells to a hybrid E/M state (Grasset et al., 2022). EMT is commonly identified by loss of cytokeratin and E-cadherin and gain of mesenchymal-associated molecules, N-cadherin, fibronectin, and vimentin. EMT involves many signaling pathways, including NF-κB, TGF-β, Akt, Wnt, Notch, PPARγ, and RAS, and is also affected by hypoxia and microRNA (miRNA) expression. The transcription factors TWIST, Snail, and Zeb1/Zeb2, as well as epigenetic regulators, miRNAs, and alternative splicing, are regulated by these signaling pathways during breast cancer growth.
2.5 Breast cancer stem cells
Breast cancer stem cells (BCSCs) are stem cells within a tumor that possess the ability to regenerate themselves and have the potential to develop into malignancies. The variation in tumors among different individuals can be ascribed to the inherent molecular subtypes of breast cancer, while the variation within a tumor can be elucidated by the concept of cancer stem cells, which primarily infiltrate the adjacent mesenchyme or enter the circulatory system through EMT during tumor metastasis. BCSCs undergo a mesenchymal-epithelial transition (MET) to form massive metastatic colonies in distant organs (Lu and Kang, 2019). BCSCs show higher metastatic potential due to the upregulation of proteins associated with cell metastasis and motility, as well as the downregulation of adhesion proteins. Tumorigenicity is demonstrated by the activation of many pathways associated with BCSCs. Furthermore, The ability of BCSCs to easily transition between EMT and MET is essential for promoting both EMT and metastasis in breast cancer. In addition, immunosuppressive cells are recruited by BCSCs to promote breast cancer progression. BCSCs maintain quiescent to prevent elimination by immune cells that are effective in their function, or they can establish a microenvironment that suppresses the immune system by attracting populations that inhibit immune detection (Tallerico et al., 2017). BCSCs of NTBC can be identified using specific markers, such as CD44, CD24, CD133, and aldehyde dehydrogenase (Brugnoli et al., 2019). They are also influenced by various signaling pathways, such as BMP2, Wnt, NF-κB, Notch, STAT3, and Hedgehog, which regulate their growth, survival, and migration.
2.6 Anti-angiogenesis
Angiogenesis provides nourishment and oxygen to tumor tissue and disseminates cancer cells via blood vessels. Tumor blood vessels exhibit structural instability and dysfunction, leading to inflammation and tissue fibrosis, DNA hypermethylation, genomic instability, transdifferentiation, immunosuppression, growth, invasion of tumor cells, and resistance to apoptosis (Martin et al., 2019). Stagnation of blood flow results in reduced vascular permeability and blood concentration, thereby reducing the pH in tissues and inducing hypoxia. Activated hypoxia-inducible transcription factors (HIFs) stimulate angiogenesis, leading to higher invasiveness and/or resistance to treatment (Kao et al., 2023). Furthermore, due to the widespread occurrence of vascular leakage in tumors, tumor cells invade the systemic circulation, leading to metastasis (Tomita et al., 2021). The dysregulation of tumor-associated angiogenesis is controlled by diverse molecular elements, including vascular endothelial growth factor (VEGF), TGF-β-1, Interleukin (IL)-8, CD34, CD31, Factor VIII, angiopoietin-1, angiopoietin-2, platelet-derived growth factor, and fibroblast growth factor (FGF)-2.
2.7 Suppression of aerobic glycolysis
Abnormal metabolism is one of the most significant characteristics of malignancy. The Warburg effect, a feature of cancer cell energy metabolism, refers to the ability of tumor cells to utilize glycolytic products to synthesize their growth requirements under normoxic or hypoxic conditions. Despite the presence of sufficient oxygen, most tumor cells, including those in breast cancer, produce a substantial amount of energy through hyperglycolytic metabolism. Aerobic glycolysis closely regulates the proliferation and survival of breast cancer cells. Elevated levels of aerobic glycolysis hinder the effectiveness of cancer treatment and promote resistance to therapeutic agents. Aerobic glycolysis is assessed using fluorodeoxyglucose positron emission tomography (FDG-PET) and is used to monitor cancer recurrence and metastasis. The aerobic glycolytic pathway involves several key enzymes, such as hexokinase (HK), phosphofructokinase (PFK), pyruvate kinase, and glucose transporters. TNBC has distinct hypoxic characteristics and demonstrates abundant expression of HIF-1α (Liu et al., 2022). Other signaling pathways such as PI3K/Akt, mammalian target of rapamycin (mTOR), and AMP-activated protein kinase, along with transcription factors such as c-Myc, p53, and HIF-1 are also overexpressed in TNBC.
2.8 Improvement in tumor microenvironment
2.8.1 Tumor-associated macrophages
Tumor-associated macrophages (TAMs) are the primary immune cells in the microenvironment of breast tumors. These macrophages enhance the growth of breast tumors by inducing breast cancer stemness, controlling energy metabolism, stimulating angiogenesis, drug resistance and cancer cell metastasis, and supporting immune system suppression (Munir et al., 2021). These mechanisms include the secretion of inhibitory cytokines, promotion of regulatory T cells (Tregs), and reduction of effector functions of tumor-infiltrating lymphocytes. TAMs regulate PD-1/PD-L1 expression. TAMs demonstrate a significant level of cellular plasticity, and changes in the TME lead to the transformation of TAMs into M1 macrophages, which mediate anti-tumor immune responses (Huang et al., 2022).TNBC induces M2 macrophage polarization, which positively feedback promotes the malignant evolution of TNBC cells. M2 promotes the migration of TNBC cells and induces angiogenesis in TNBC. Reversing M2 polarization is considered a target for cancer treatment (Meng et al., 2022).
2.8.2 Chemokines
The TME comprises a heterogeneous combination of immune cells and soluble mediators, including chemokines, cytokines, and growth factors. These metabolites are found within or close to tumors. Chemokines function as immunological mediators, attracting and recruiting particular subsets of these cells into the TME and promoting tumor growth or regression. Some chemokines, such as CXCL9, CXCL10, and CCL16, can inhibit the growth of breast cancer cells. Other chemokines, such as CCL2, CCL5, CXCL8, and CXCL12, promote the growth of breast cancer (Masih et al., 2022).
2.8.3 Bone microenvironment
In the bone microenvironment, diverse cell types, including osteocytes, adipocytes, endothelial cells, and neural cells, are essential in maintaining bone homeostasis. The development of bone metastases is a selective and multistep process. The growth, dormancy, and metastasis in the bone microenvironment linked to breast cancer are influenced by various bone marrow environments, including the endosteal (comprising osteoblasts, osteoclasts, and adipocytes) and vascular niches. After tumor cells invade the bone, they rely on stromal cells to further their survival and proliferation. Therefore, the intricate equilibrium between bone resorption and generation is disturbed. Osteolytic lesions constitute most breast cancer metastases. Bone metastases from breast cancer are characterized by osteoclastic bone resorption. Osteoclasts modulate bone resorption and promote the activation of malignancy cells (Zarrer et al., 2020).
2.8.4 miRNAs
MicroRNAs (miRNAs) are a group of small molecule RNAs that are evolutionarily conserved and do not encode proteins. They are usually 21–23 nucleotides long and regulate the translational level of gene expression. miRNAs are abnormally expressed in various tumor types and are linked to several biological functions, including immunoregulation and cell metabolism. Therefore, they are used for cancer diagnosis, treatment, and prognosis. The primary oncogenic miRNAs in TNBC include miR-25-3p, miR-93, miR-21, and miR-455-3p. The primary miRNAs that suppress tumor growth in breast cancer are miR-29c, miR-30a-5p, miR-34a, miR-101, miR-130a, miR-134, miR-200a/b/c, miR-203, miR-206, miR-211, miR-223, miR-269-5p, miR-342-5p, miR-316-5p, miR-378, miR-384, miR-190-3p, miR-603, miR-613, miR-1296, and miR-4306 (Xu et al., 2020).
2.9 Hormones
Phytoestrogen are subtype of flavonoids and can mimic or induce estrogen-like responses. Their hydroxyl groups and phenolic rings, which are necessary for binding ERα and ERβ, make them similar to the most significant form of E2. Thus, ER, ERα, and ERβ can interact with the bioavailable phytoestrogens.In breast tissue, ERα activation promotes cell proliferation while ERβ decreases it and promotes apoptosis. In TNBC, the effects triggered by E2 are not only conducive to tumor growth but can also have anti-tumor properties, such as through the activation of ERβ. Decreased expression of mitochondrial ERβ led to a decline in mitochondrial activity in TNBC cells, promoting their growth through glycolysis and contributing to tumor advancement. Conversely, the enforced overexpression of mitochondrial ERβ reduced the proliferation of TNBC cells (Song et al., 2019). ERβ suppresses the growth of TNBC cell lines and also hinders their involvement in the formation of new blood vessels, invasion, and spread to other parts of the body. The ER-Beta agonist S-equol can effectively suppress the growth of TNBC by reducing the levels of Ki-67 (Lathrop et al., 2020).Chronic stress can contribute to the development of cancer. One of the most significant mechanisms is the continuous release of neurotransmitters caused by chronic stress, which ultimately leads to the activation of β2-adrenergic receptors (β2-AR) (Bernabé, 2021).
2.10 Drug resistance
Standard chemotherapy remains the cornerstone of systemic therapy, but TNBC often becomes resistant to cytotoxic drugs (Ou et al., 2024). According to the National Comprehensive Cancer Network guidelines, anthracyclines, taxanes, anti-metabolites, and microtubule inhibitors are preferred for chemotherapy. TNBC drug resistance mechanisms include antioxidant activity, mediation of drug efflux, reduction of intracellular drug accumulation, mediation of intracellular detoxification of cytotoxic drugs, enhanced DNA repair, anti-apoptosis, anti-autophagy, metabolic reprogramming, EMT, TME heterogeneity, and immune evasion (Bai et al., 2021). One potential approach to enhance the effectiveness of therapies and minimize their adverse effects involves reversing medication resistance and enhancing sensitivity to chemotherapy. Therefore, it is crucial to choose medications that are less toxic and more effective for patients with cancer. Therefore, flavonoids have been investigated as potent chemosensitizers in conjunction with standard chemotherapeutic drugs.
3 Flavonoid
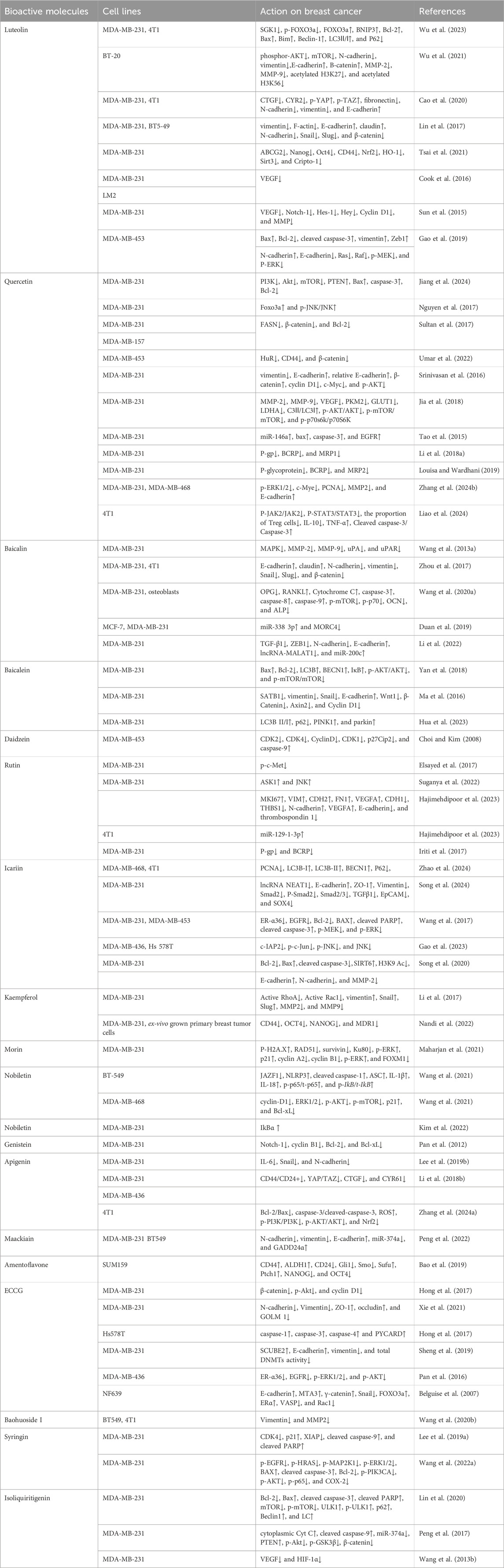
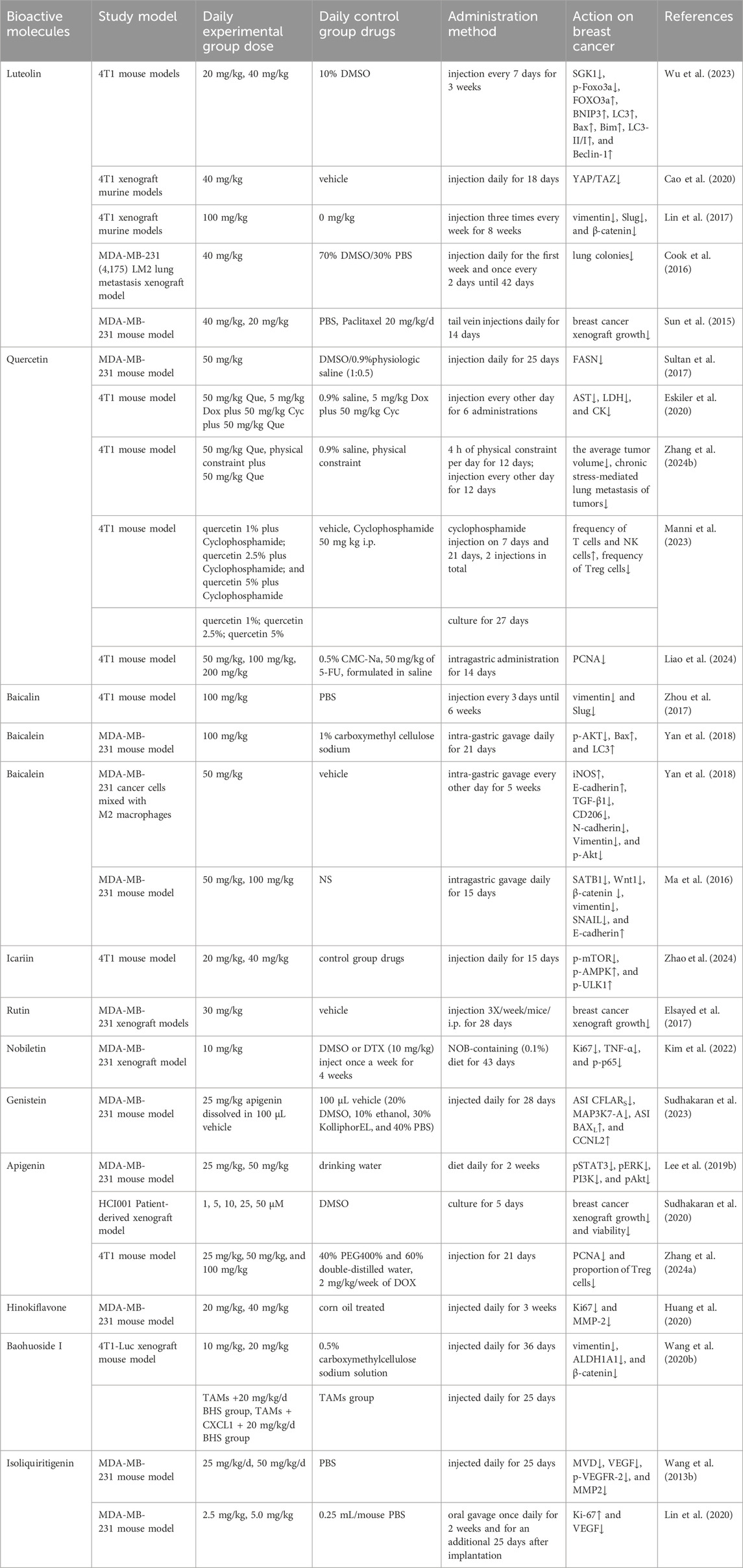
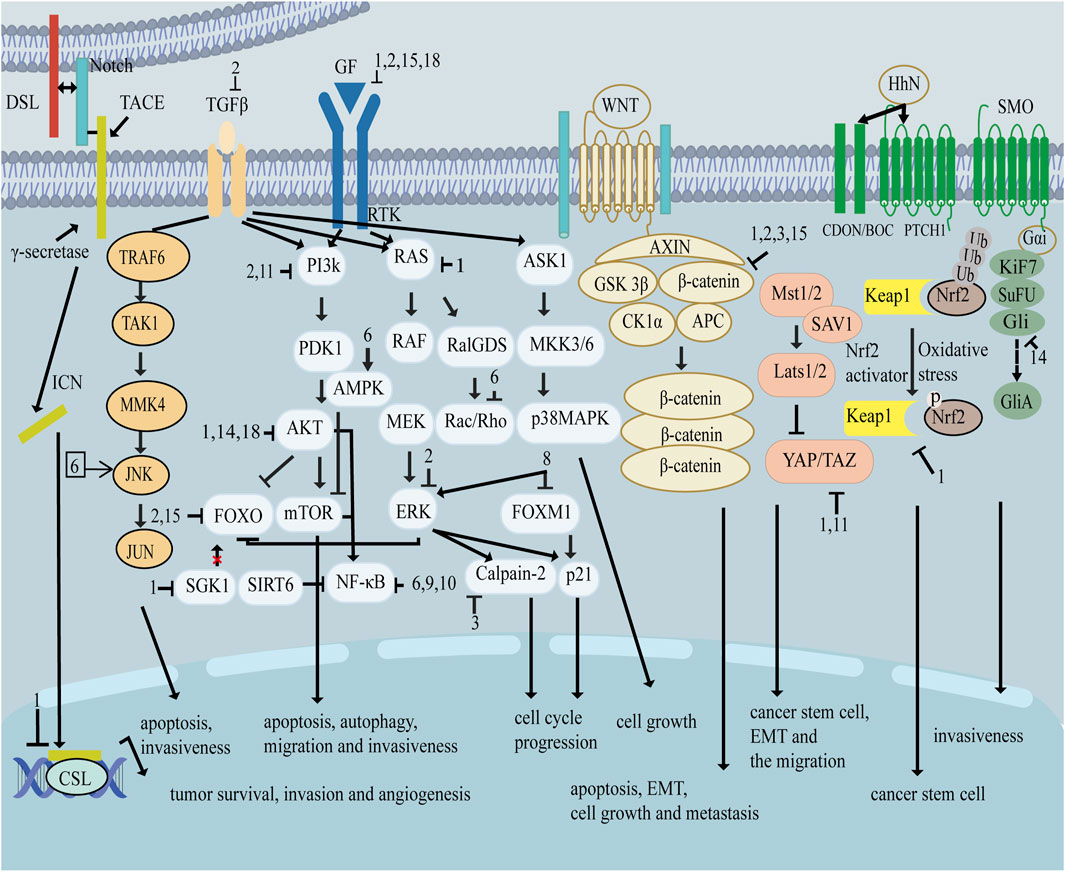
As shown in Figure 1, there are many therapeutic targets that show potential clinical utility in the treatment of TNBC. The following will introduce the inhibitory effects of specific drugs on TNBC. Figure 2 shows chemical structure of flavonoids. Tables 1, 2 provide a concise overview of the pertinent research findings from both in vivo and in vitro trials on various medications. Figure 3 summarizes the relevant pathways.
3.1 Luteolin
Luteolin is a flavonoid metabolite derived from the foliage and stems of mignonette plants. Additionally, it serves as a key metabolite of Lonicera japonica Thunb [Caprifoliaceae], Perilla frutescens (L.). Britton [Lamiaceae], and Chrysanthemum indicum L. [Asteraceae]. Luteolin possesses antioxidant, pro-oxidant, antibacterial, anti-inflammatory, and cancer-preventive properties (Rakoczy et al., 2023). The IC50 values of luteolin after 48 h of treatment were 39.31 μM for MDA-MB-231 cells and 63.06 μM for 4T1 cells.
Luteolin suppresses TNBC by inducing apoptosis and autophagy through the SGK1-FOXO3a-BNIP3 signaling pathway (Wu et al., 2023). AKT/mTOR-inducing H3K27Ac and H3K56Ac levels were decreased by luteolin, which in turn controlled MMP9 production, thereby suppressing the growth and metastasis of TNBC via the androgen receptors (Wu et al., 2021). Luteolin inhibits YAP/TAZ activity, suppressing EMT and the migration of TNBC (Cao et al., 2020), effectively inhibiting TNBC metastasis by reversing EMT through the suppression of β-catenin (Lin et al., 2017). Luteolin effectively reduced the characteristic features of breast cancer stemness by inhibiting the expressions regulated by Nrf2 (Tsai et al., 2021). Furthermore, luteolin inhibited the metastasis of MDA-MB-435 and MDA231-LM2-4175 cells to the lungs in vivo, and its capacity to prevent the generation of VEGF and block kinase domain receptor-mediated activity may contribute to its anti-metastatic effects (Cook et al., 2016). Luteolin effectively suppresses Notch signaling by modulating specific miRNAs involved in tumor growth. These include upregulated miR-34a, miR-139-5p, miR-181a, miR-224, and miR-246, and downregulated miR-155 (Sun et al., 2015). The anti-tumor effects of luteolin on TNBC cell growth and EMT may be attributed to the inhibition of Ras/Raf/MEK/ERK signaling, which is suppressed by miR-203 (Gao et al., 2019).
3.2 Quercetin
Quercetin is a flavonol metabolite obtained as a polyphenolic flavonoid found in various several Chinese herbal medicines, including Oldenlandia diffusa (Willd.) Roxb [Rubiaceae], Lobelia chinensis Lour. [Campanulaceae] Glehnia littoralis (A.Gray) F. Schmidt ex Miq. [Apiaceae], Ginkgo biloba L. [Ginkgoaceae], and others. Quercetin, a biologically active flavonoid, has demonstrated anti-diabetic, antioxidant, anti-cancer, and anti-aging characteristics (Wang G. et al., 2022). The IC50 values for MDA-MB-231 and MDA-MB-157 cells after 48 h of treatment were calculated to be 230 ± 3 μM and 415 ± 4 μM, respectively.
In TNBC, Quercetin suppresses breast cancer cell growth and survival by targeting the Akt/mTOR/PTEN signaling pathway (Jiang et al., 2024). Quercetin alters FOXO3a signaling and causes apoptosis and cell cycle arrest (Nguyen et al., 2017). By suppressing fatty acid synthase and β-catenin, quercetin induces apoptosis in TNBC cells (Sultan et al., 2017). Quercetin inhibits the progression and migration of TNBC cells by impairing HuR activity (Umar et al., 2022). Quercetin induces EMT by influencing the positioning of β-catenin within the cell nucleus and controlling the expression of genes targeted by β-catenin (Srinivasan et al., 2016). Quercetin controls the β-catenin signaling pathway and decreases the movement of TNBC cells. Quercetin hampers the growth of tumors, suppresses oncocytes proliferation, and induces tumor necrosis. Furthermore, it also inhibits cancer cell migration by suppressing glycolysis through the induction of autophagy mediated by the Akt-mTOR pathway (Jia et al., 2018). Quercetin inhibits the growth of human breast cancer cells by increasing miR-146a expression, followed by the induction of apoptosis via cystatinase-3 activation and the mitochondria-dependent pathway and inhibition of invasion via downregulation of epidermal growth factor receptor (EGFR) expression (Tao et al., 2015). Quercetin could inhibit chronic stress-induced ERK1/2 activity in TNBC cells, thereby weakening the potential for TNBC growth and metastasis (Zhang J. et al., 2024). Quercetin boosts the efficiency of doxorubicin in treating human breast cancer cells while minimizing its harmful side effects. This is achieved by decreasing the expression of efflux ABC transporters (Li S. et al., 2018). The efficacy of sorafenib is improved by quercetin, which reduces the levels of two drug efflux transporters: P-glycoprotein and BCRP (Louisa and Wardhani, 2019). Quercetin reduces the harmful effects of doxorubicin-cyclophosphamide treatment on the heart, enhancing its ability to treat TNBC. Quercetin may reduce the harmful effects of AC-induced heart damage by preventing the buildup of ROS and stimulating the ERK1/2 pathway in heart muscle cells. Thus, quercetin may augment the anti-cancer efficacy of AC by suppressing ROS accumulation and inhibiting the ERK1/2 pathway in TNBC cells (Zhang et al., 2022). Quercetin reverses talazopanib resistance in BRCA1-mutated TNBC cells by improving cytotoxicity and apoptosis (Eskiler et al., 2020). By modulating the IL-6/JAK2/STAT3 signaling pathway, quercetin reduces the number of Treg cells and activates the anti-tumor immune response (Liao et al., 2024). Cyclophosphamide and quercetin increased the overall occurrence of T cells and NK cells in the body, while decreasing the occurrence of Treg cells, which are associated with suppressing tumour growth (Manni et al., 2023).
3.3 Baicalin
Baicalin is the primary metabolite of Scutellaria baicalensis Georgi [Lamiaceae], Oroxylum indicum (L.) Kurz [Bignoniaceae], and Pinellia ternata (Thunb.) Makino [Araceae] has diverse effects, including anti-cancer, anti-inflammatory, anti-apoptotic, and antibacterial properties (Bajek-Bil et al., 2023). The IC50 values of baicalein in MDA-MB-231 cells were 60.12 μM at 24 h, 27.98 μM at 48 h, and 19.01 μM at 72 h. Baicalin suppresses tumor growth in MDA-MB-231 cells by decreasing the expression of MMP-2, MMP-9, uPAR, and uPA by disrupting the p38MAPK signaling pathway (Wang X.-F. et al., 2013). Furthermore, baicalin targets β-catenin signaling to reverse EMT, thereby inhibiting the metastasis of breast cancer cells (Zhou et al., 2017). Baicalin substantially inhibited the proliferation of bone metastases, reduced bone degradation, diminished osteoclastogenesis of osteoclast progenitors, and inhibited the growth of metastasized MDA-MB-231 cells, thus maintaining bone mass (Wang B. et al., 2020). Baicalin reduces the viability, motility, and invasion of breast cancer cells by modulating MORC4 and miR-338-3p (Wang B. et al., 2020). Baicalin notably reduces breast cancer cell survivability, mobility, and invasiveness by controlling the TGF-β/lncRNA-MALAT1/miR-200c pathway (Li et al., 2022). Furthermore, Baicalein triggered programmed cell death and self-degradation in triple-negative breast cancer cells by blocking the PI3K/AKT pathway (Yan et al., 2018). Baicalein suppressed fibronectin-induced EMT by reducing calpain-2 activation and upregulation (Chen et al., 2019). Baicalein inhibited EMT in breast cancer by regulating the polarization of TAMs (Zhao et al., 2018). Baicalein may inhibit EMT, which is linked to the downregulation of the Wnt/β-catenin pathway and SATB1 to decrease breast cancer metastasis (Ma et al., 2016). By blocking the G-protein-coupled receptor 30 pathway, baicalein prevents 17-β-estradiol from causing BC cells to migrate, adhere, or invade (Shang et al., 2015). Baicalein decreased CDK1 activity through autophagy, increasing MDA-MB-231 cells’ susceptibility to doxorubicin (Hua et al., 2023).
3.4 Daidzein
Daidzein, an isoflavone, possesses significant nutritional value and is primarily derived from soy plants Cyathula officinalis K.C.Kuan [Amaranthaceae] and Corethrodendron multijugum (Maxim.) B.H.Choi and H.Ohashi [Fabaceae], and Spatholobus suberectus Dunn [Fabaceae]. Daidzein has biphasic effects on breast cancer cell proliferation and ERα expression, with either stimulatory or inhibitory effects. It exhibits pharmacological and therapeutic characteristics, including cholesterol-lowering, cardiovascular function improvement, anti-tumor, anti-fibrotic, and anti-diabetic properties (Ubaid et al., 2023). Daidzein’s anti-cancer properties in TNBC involve inducing cell cycle arrest, particularly at the G1 and G2/M phases (Choi and Kim, 2008).
3.5 Rutin
Rutin is a flavonoid that is derived from many plants, including Artemisia argyi H.Lév. and Vaniot [Asteraceae], Podophyllum versipelle Hance [Berberidaceae], Ginkgo biloba L. [Ginkgoaceae], and others. Rutin possesses a wide range of therapeutic properties, such as anti-allergic, antioxidant, anti-inflammatory, anti-cancer, and anti-diabetic effects (Yong et al., 2020). For a 24-h period, the rutin IC50 values for MDA-MB-231 cells were 40 ± 1.0 µM. Rutin acts as a c-Met inhibitor that inhibits TNBC cell proliferation, migration, and invasion (Elsayed et al., 2017), and its therapy in TNBC induces ER stress (Suganya et al., 2022). Rutin enhances the growth, migration, and pro-angiogenic functions of TNBC cell lines (Hajimehdipoor et al., 2023). Rutin can enhance the upregulation of miR-129-1-3p in 4T1 cells. By regulating the expression of GRIN2D, Calm1, and CaMKIIδ, miR-129-1-3p reduces calcium overcharge and downstream Ca2+ signaling in 4T1 cells, thereby contributing to the inhibition of breast tumor cell proliferation and metastasis (Li et al., 2021). Rutin and doxorubicin inhibit cell proliferation by arresting the cell cycle at the G2/M phase and inducing apoptosis through ER stress in MDA-MB-231 cells (Suganya et al., 2022). Rutin efficiently arrests the cell cycle in chemoresistant TNBC cells by inhibiting P-gp and BCRP pumps, reversing multidrug resistance, and restoring sensitivity to cyclophosphamide (Iriti et al., 2017).
3.6 Icariin
Icariin, a flavonoid extracted from Epimedium sagittatum (Siebold and Zucc.) Maxim. [Berberidaceae] has various beneficial properties, including anti-inflammatory, antioxidant, antidepressant, and aphrodisiac effects (Liu et al., 2023). Icariin triggers autophagy to hinder the progression of TNBC by stimulating the AMPK/mTOR/ULK1 signaling pathway (Zhao et al., 2024). By altering the lncRNA NEAT1/TGFβ/SMAD2 Signaling Pathway, Icariin regulates EMT and stem cell-like characteristics in breast cancer (Song et al., 2024). Icariin triggered cellular apoptosis and broke the positive regulatory loop between ER-α36 and EGFR in TNBC cells, resulting in a reduction of cell growth promoted by E2 in TNBC (Wang et al., 2017). It induces apoptosis by increasing ROS levels and suppressing the invasion of TNBC cells through the JNK/c-Jun signaling channel (Gao et al., 2023). Icariin induces apoptosis and inhibits migration of TNBC through the SIRT6/NF-κB signaling pathway. Icariin exhibits tumor growth inhibition and anti-lung metastasis effects in tumor animal models of MDA-MB-231 and 4T1 cells via the immunosuppressive microenvironment of the tumor (Song et al., 2020).
3.7 Kaempferol
Kaempferol is found in high levels in tea, Paeonia lactiflora Pall [Paeoniaceae], Platycladus orientalis (L.) Franco [Cupressaceae] and several other sources. It possesses notable antibacterial, antifungal, anti-cancer, antioxidant, antiprotozoal, and anti-inflammatory properties (Periferakis et al., 2022). Low-dose kaempferol inhibited TNBC cell migration and encroachment by plugging the RhoA and Rac1 signaling channels, whereas HER2 overexpression rescued both cell migration and RhoA and Rac1 activation in kaempferol-treated MDA-MB-231 cells (Li et al., 2017). The combined anticancer effect of kaempferol and verapamil is strengthened by deregulation the CD44-NANOG-MDR1-associated chemoresistance pathway in breast cancer stem cells (Nandi et al., 2022).
3.8 Morin
Morin, a widely recognized flavonoid derived from plants in the Moraceae family and the leaves of Maclura tricuspidata Carrière [Moraceae], has anti-inflammatory, anti-oxidant, anti-diabetic, anti-tumor, anti-hypertensive, antibacterial, and neuroprotective properties (Maharjan et al., 2021). MDA-MB-231 cell death triggered by morin results from prolonged interruption of the cell cycle, which occurs due to the activation of ERK and suppression of FOXM1 signaling pathways, leading to the stimulation of p21 expression. Morin suppresses FOXM1 and attenuates EGFR/STAT3 signaling pathways to sensitize TNBC cells to doxorubicin cytotoxicity (Maharjan et al., 2023).
3.9 Nobiletin
Nobiletin, a flavonoid extracted from Citrus reticulata Blanco [Rutaceae], exhibits many positive effects, including neuroprotection, cardiovascular protection, anti-metabolic disorder prevention, anti-cancer, anti-inflammatory, and antioxidant properties (Arshad et al., 2024). Nobiletin induces apoptosis and pyroptosis of TNBC cells via miR-200b/JAZF1/NF-κB axis (Wang et al., 2021). Nobiletin exhibits anti-cancer effects in TNBC cells via inducing apoptosis through Bcl-xL and causing cell cycle arrest in the G0/G1 phase (Chen et al., 2014). The anti-cancer effectiveness was improved by activating retinoic acid receptor-related orphan receptors with nobiletin. This enhancement is achieved by suppressing the IκB/NF-κB signaling pathway in TNBC. The concurrent use of nobiletin with either docetaxel or carboplatin effectively suppresses the proliferation of TNBC cells (Kim et al., 2022).
3.10 Genistein
Genistein is a prevalent isoflavone present in soy products. Genistein demonstrates anti-inflammatory, antioxidant, antibacterial, and antiviral properties. It impacts angiogenesis and estrogen synthesis and has pharmacological effects on diabetes and lipid metabolism (Sharifi-Rad et al., 2021). Eisenstein effectively attenuated complications in TNBC lacking ERs across all doses (Malik et al., 2023). Genistein suppresses the growth of TNBC cells by reducing the activity of NF-κB through the Notch-1 pathway (Pan et al., 2012). Genistein can effectively suppress the growth of TNBC cells by intricately modulating the cell cycle and the response to DNA damage (Fang et al., 2016). Genistein can potentially prevent and reverse AHR-dependent BRCA1 hypermethylation and restore ERα-mediated responsiveness. This makes TNBC more sensitive to estrogen therapy (Donovan et al., 2019).
3.11 Apigenin
Apigenin is found in several medicinal plants, such as Plantago indica L. [Plantaginaceae] and Lobelia chinensis Lour. [Campanulaceae], and Mentha canadensis L. [Lamiaceae]. Apigenin exhibits anti-tumor, cardioprotective, neuroprotective, and anti-inflammatory effects (Salehi et al., 2019). The IC50 values of apigenin in MDA-MB-231 and MDA-MB-436 cells were around 33 and 30 μM, respectively, after 72 h. Apigenin alters transcriptome-wide TNBC-specific alternative splicing, specifically in TNBC, leading to apoptosis and tumor growth inhibition (Sudhakaran et al., 2023). Apigenin inhibited the invasion of xenograft tumors derived from MDA-MB-231 by decreasing the IL-6-associated downstream signaling cascade (Lee H. H. et al., 2019). Apigenin inhibits the activation of the PI3K/Akt channels and the activity of integrin β4, resulting in a decrease in the metastasis of tumor cells to the lungs in nude mice and the occurrence of spontaneous intravasation and organ metastasis in chick embryos (Lee et al., 2008). Apigenin inhibits YAP/TAZ activity in TNBC cells and suppresses the stem cell-like properties of apigenin in TNBC cells, which is partially mediated by disturbing the YAP/TAZ-TEAD protein-protein interaction (Li Y.-W. et al., 2018). By concentrating on hnRNPA2, apigenin modulated the activity of ABCC4 and ABCG2 drug expulsion transporters and increased the sensitivity of TNBC spheroids to DOX-induced apoptosis (Sudhakaran et al., 2020). Apigenin boosts the suppressive impact of cisplatin on telomerase activity in TNBC cells (A.Aziz et al., 2017). Apigenin may induce apoptosis in breast cancer cells by activating the PI3K/AKT/Nrf2 pathway. Additionally, it can enhance the tumor immune microenvironment in mice with breast tumors, leading to the suppression of breast cancer growth (Zhang C. et al., 2024).
3.12 Maackiain
Maackiain is a flavonoid with several functions and is extracted from many Chinese herbs, including Spatholobus suberectus Dunn [Fabaceae] and Sophora flavescens Aiton [Fabaceae]. Maackiain exhibits many pharmacologic activities, including anti-cancer, anti-allergic, and anti-inflammatory effects (Huh et al., 2020). Maackiain regulates the miR-374a/GADD45A axis to suppress the beginning and marching of TNBC (Peng et al., 2022).
3.13 Hinokiflavone
Hinokiflavone is natural metabolites in several plants, such as Platycladus orientalis (L.) Franco [Cupressaceae], Selaginella moellendorffii Hieron [Selaginellaceae], and Toxicodendron succedaneum (L.). Kuntze [Anacardiaceae] exhibits various pharmacological effects, including anti-inflammatory, antiprotozoal, antioxidant, and anti-cancer effects (Goossens et al., 2021). Hinokiflavone inhibits the transformation of TNBC cells and leads to apoptosis via the EMT signaling pathway (Huang et al., 2020).
3.14 Amentoflavone
Amentoflavone is a natural biflavonoid found in several plants, such as Ginkgo biloba L. [Ginkgoaceae] and Platycladus orientalis (L.) Franco [Cupressaceae]. It exhibits anti-inflammatory, musculoskeletal protection, anti-microorganism, anti-oxidation, metabolic regulation, neuroprotection, radioprotection, antidepressant, and anti-carcinogenic effects (Xiong et al., 2021). Amentoflavone inhibits TNFα-induced Gli1 activation in breast tumor cells, resulting in reduced invasiveness of human breast cancer cells by interceding AKT/mTOR/S6K1 signaling and blocking the migration and invasiveness of MDA-MB-231 cells (Qiu et al., 2021). Amentoflavone suppresses the development of tumorspheres in SUM159 BCSCs by regulating the Hedgehog/Gli1 signaling pathway (Bao et al., 2019).
3.15 Epigallocatechin-3-gallate
Epigallocatechin-3-gallate (EGCG) is a polyphenolic metabolite extracted from traditional Chinese medicines such as Ginkgo biloba L. [Ginkgoaceae], Eriobotrya japonica (Thunb.) Lindl. [Rosaceae], and Phyllanthus emblica L. [Phyllanthaceae]. EGCG shows anti-fibrotic, anti-inflammatory, pro-apoptotic, and anti-tumorous effects (Mokra et al., 2022). EGCG suppresses the proliferation of MDA-MB-231 breast cancer cells by deactivating the β-catenin signaling system (Hong et al., 2017). By blocking VEGF expression, EGCG prevents human breast cancer cells from proliferating, migrating, and invading (Braicu et al., 2013). EGCG suppresses the migration of MDA-MB-231 cells by inhibiting GOLM1 via the HGF/HGFR/AKT/GSK-3β/β-catenin/c-Myc signaling pathway (Xie et al., 2021).EGCG induces cell death and apoptosis in TNBC Hs578 by activating the anti-apoptotic genes BAG3, XIAP, and RIPK2(Braicu et al., 2013). EGCG reduced SCUBE2 methylation status by reducing the expression and activity of DNA methyltransferase, thereby inhibiting cell migration and invasion (Sheng et al., 2019). By downregulating the positive feedback loop between ER-α36 and EGFR, EGCG effectively blocks the growth of both ER-negative stem cells and ER-negative progenitor cells. EGCG also stimulates FOXO3a, which in turn activates ERα and reverses the invasiveness of breast cancer cells. Furthermore, EGCG treatment induces the expression of constitutively active FOXO3a, which counteracts the transforming growth factor-β1-induced invasive phenotype and promotes an epithelial phenotype that depends on ERα expression and signaling (Belguise et al., 2007; Zhang et al., 2009). ECCG-induced growth suppression of ER-negative breast cancer stem and progenitor cells mediated by ERa36 (Pan et al., 2016).
3.16 Baohuoside I
Baohuoside I (BHS) is a flavonoid derived from the plant Epimedium sagittatum (Siebold and Zucc.) Maxim. [Berberidaceae] and has several pharmacological activities, including anti-osteoporotic and anti-tumor effects, enhancement of cognitive function, protection against cerebral ischemia-reperfusion injury, and neuroprotection (Agrawal et al., 2024). BHS effectively inhibits breast cancer metastasis and the activation of TAMs/CXCL1 in mouse breast cancer xenografts as well as in a zebrafish model of breast cancer xenotransplantation (Wang S. et al., 2020).
3.17 Syringoside
Syringoside is the primary active metabolite in Eleutherococcus senticosus (Rupr. and Maxim.) Maxim. [Araliaceae]. Syringoside has also been extracted from Isatis tinctoria L. [Brassicaceae], Glehnia littoralis (A.Gray) F.Schmidt ex Miq. [Apiaceae], and Cirsium japonicum DC. [Asteraceae]. It is primarily used as an anti-inflammatory agent. Syringin induces oxidative stress and inhibits TNBC growth (Lee C.-H. et al., 2019). Syringin inhibits the growth and migratory capabilities of TNBC cells while inducing apoptosis via the PI3K-AKT-PTGS2 and EGFR-RAS-RAF-MEK-ERK signaling pathways (Wang F. et al., 2022).
3.18 Isoliquiritigenin
Isoliquiritigenin, a flavonoid obtained from the plant Glycyrrhiza glabra L. [Fabaceae], has many pharmacological properties such as anticancer, antiaging, antioxidative, anti-inflammatory, and anti-diabetic effects (Zhao et al., 2019). Isoliquiritigenin efficiently suppress the growth of TNBC cells by inducing apoptotic cell death and promoting the accumulation of p62, which in turn stimulated autophagy-mediated apoptosis (Lin et al., 2020). Isoliquiritigenin suppresses the formation of new blood vessels in TNBC by targeting the VEGF/VEGFR-2 signaling pathway (Wang Z. et al., 2013). Isoliquiritigenin has the ability to regulate the miR-374a/PTEN/Akt pathway, which leads to the inhibition of breast cancer tumor growth and spread (Peng et al., 2017). Isoliquiritigenin derivative also had a more significant inhibitory effect on breast cancer cell viability, especially on MDA-MB-231 (Peng et al., 2020).
4 Conclusion
Treating TNBC is challenging because it has few effective therapeutic options. Recent studies have shown the anti-tumor properties of several natural metabolites, including flavonoids. There are many types of flavonoids, and how to screen and summarize the most effective ingredients from a large number of compounds remains a challenge. In addition, although the research on flavonoids has made certain progress, there is still a lack of a mature and unified framework to guide its application in clinical treatment. In this case, the concept of holism and treatment based on pattern differentiation of traditional Chinese medicine provide a new direction to explore the potential of flavonoids in tumor treatment. Based on the investigation of literature and ancient books, starting from ethnic folk medicines, clinical prescriptions and experience prescriptions, the selection is made with reference to the medicinal properties and efficacy of the drugs. The medicinal properties represented by the four qi and five flavors, meridians, ascending and descending, floating and sinking, etc., are theories for studying the properties and application rules of traditional Chinese medicine. Linking the efficacy of drugs with modern pharmacological mechanisms is the focus of future research. Various traditional Chinese medicines with functions such as nourishing the body and eliminating evil, clearing heat and detoxifying, promoting blood circulation and removing blood stasis have been proven to have direct or indirect anti-tumor effects.
This review provides a thorough overview of the various mechanisms by which flavonoids can effectively treat TNBC, including regulating cell proliferation and cell cycle, inhibiting cell invasion and metastasis, overcoming drug resistance, inducing apoptosis and autophagy, and inhibiting angiogenesis. Additionally, this review delves into the specific effects of different flavonoid metabolites or treating TNBC. Flavonoids are natural substances with anti-cancer activities. When combined with TCM, they can improve their efficacy and treatment outcomes. However, the bioavailability of flavonoids is low, their dosage is difficult to control, and are also potentially toxic. It is challenging to find definitive and universally applicable mechanisms of action in mechanism research. The structure-activity relationship should be conducted to design and optimize compounds. Clinical and basic research need to be closely integrated and verified to ensure clinical safety and effectiveness. The anti-cancer properties of flavonoids have opened new avenues for pharmacological intervention in TNBC.
Author contributions
SW: Writing–review and editing, Writing–original draft. KW: Writing–review and editing. CL: Writing–review and editing, Supervision. JC: Writing–review and editing, Supervision. XK: Writing–review and editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1411059/full#supplementary-material
References
A.Aziz, N., Froemming, G., Sheikh Abdul Kadir, S. H., and Ibahim, M. (2017). Apigenin increases cisplatin inhibitory effects on the telomerase activity of triple negative breast cancer cells. J. Teknol. 80. doi:10.11113/jt.v80.10378
Aftab, A., Khan, R., Shah, W., Azhar, M., Unar, A., Jafar Hussain, H. M., et al. (2021). Computational analysis of Cyclin D1 gene SNPs and association with breast cancer. Biosci. Rep. 41, BSR20202269. doi:10.1042/BSR20202269
Agrawal, N., Goyal, D., and Bansal, D. (2024). A comprehensive review on chemistry and contribution of Chinese herb Epimedium brevicornum Maxim in medicine. Curr. Tradit. Med. 10, 102–114. doi:10.2174/2215083810666230607151656
Argote Camacho, A. X., González Ramírez, A. R., Pérez Alonso, A. J., Rejón García, J. D., Olivares Urbano, M. A., Torné Poyatos, P., et al. (2021). Metalloproteinases 1 and 3 as potential biomarkers in breast cancer development. Int. J. Mol. Sci. 22, 9012. doi:10.3390/ijms22169012
Arshad, R., Masood, S., Sharif, H. R., Goksen, G., Xu, B., and Faisal Manzoor, M. (2024). New insights into health-promoting effects of nobiletin from Citrus fruits. Food Rev. Int. 40, 1276–1295. doi:10.1080/87559129.2023.2212047
Bai, X., Ni, J., Beretov, J., Graham, P., and Li, Y. (2021). Triple-negative breast cancer therapeutic resistance: where is the Achilles’ heel? Cancer Lett. 497, 100–111. doi:10.1016/j.canlet.2020.10.016
Bajek-Bil, A., Chmiel, M., Włoch, A., and Stompor-Gorący, M. (2023). Baicalin-Current trends in detection methods and health-promoting properties. Pharm. (Basel) 16, 570. doi:10.3390/ph16040570
Bao, C., Chen, J., Kim, J. T., Qiu, S., Cho, J. S., and Lee, H. J. (2019). Amentoflavone inhibits tumorsphere formation by regulating the Hedgehog/Gli1 signaling pathway in SUM159 breast cancer stem cells. J. Funct. Foods 61, 103501. doi:10.1016/j.jff.2019.103501
Belguise, K., Guo, S., and Sonenshein, G. E. (2007). Activation of FOXO3a by the green tea polyphenol epigallocatechin-3-gallate induces estrogen receptor alpha expression reversing invasive phenotype of breast cancer cells. Cancer Res. 67, 5763–5770. doi:10.1158/0008-5472.CAN-06-4327
Bernabé, D. G. (2021). Catecholamines mediate psychologic stress–induced cancer progression. Cancer Res. 81, 5144–5146. doi:10.1158/0008-5472.CAN-21-3077
Braicu, C., Gherman, C. D., Irimie, A., and Berindan-Neagoe, I. (2013). Epigallocatechin-3-Gallate (EGCG) inhibits cell proliferation and migratory behaviour of triple negative breast cancer cells. J. Nanosci. Nanotechnol. 13, 632–637. doi:10.1166/jnn.2013.6882
Brugnoli, F., Grassilli, S., Al-Qassab, Y., Capitani, S., and Bertagnolo, V. (2019). CD133 in breast cancer cells: more than a stem cell marker. J. Oncol. 2019, 7512632. doi:10.1155/2019/7512632
Cao, D., Zhu, G.-Y., Lu, Y., Yang, A., Chen, D., Huang, H.-J., et al. (2020). Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 129, 110462. doi:10.1016/j.biopha.2020.110462
Chen, C., Ono, M., Takeshima, M., and Nakano, S. (2014). Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Res. 34, 1785–1792.
Chen, Y., Chen, L., Hong, D., Chen, Z., Zhang, J., Fu, L., et al. (2019). Baicalein inhibits fibronectin-induced epithelial-mesenchymal transition by decreasing activation and upregulation of calpain-2. Cell Death Dis. 10, 341. doi:10.1038/s41419-019-1572-7
Choi, E. J., and Kim, G.-H. (2008). Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine 15, 683–690. doi:10.1016/j.phymed.2008.04.006
Cook, M., Liang, Y., Besch-Williford, C., and Hyder, S. (2016). Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. BCTT 9, 9–19. doi:10.2147/BCTT.S124860
Donovan, M. G., Selmin, O. I., Doetschman, T. C., and Romagnolo, D. F. (2019). Epigenetic activation of BRCA1 by genistein in vivo and triple negative breast cancer cells linked to antagonism toward aryl hydrocarbon receptor. Nutrients 11, 2559. doi:10.3390/nu11112559
Duan, X., Guo, G., Pei, X., Wang, X., Li, L., Xiong, Y., et al. (2019). Baicalin inhibits cell viability, migration and invasion in breast cancer by regulating miR-338-3p and MORC4. Onco Targets Ther. 12, 11183–11193. doi:10.2147/OTT.S217101
Elsayed, H. E., Ebrahim, H. Y., Mohyeldin, M. M., Siddique, A. B., Kamal, A. M., Haggag, E. G., et al. (2017). Rutin as A Novel c-met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer 69, 1256–1271. doi:10.1080/01635581.2017.1367936
Eskiler, G. G., Çeçener, G., Egeli, U., and Tunca, B. (2020). Reversal effect of quercetin on talazoparib resistance in BRCA1 mutant triple negative breast cancer. Eur. Res. J. 6, 19–25. doi:10.18621/eurj.454176
Fang, Y., Zhang, Q., Wang, X., Yang, X., Wang, X., Huang, Z., et al. (2016). Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int. J. Oncol. 48, 1016–1028. doi:10.3892/ijo.2016.3327
Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A., and Fares, Y. (2020). Molecular principles of metastasis: a hallmark of cancer revisited. Sig Transduct. Target Ther. 5, 28–17. doi:10.1038/s41392-020-0134-x
Gao, G., Ge, R., Li, Y., and Liu, S. (2019). Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells, Nanomedicine, Biotechnol. 47, 3265–3271. doi:10.1080/21691401.2019.1646749
Gao, S., Zhang, X., Liu, J., Ji, F., Zhang, Z., Meng, Q., et al. (2023). Icariin induces triple-negative breast cancer cell apoptosis and suppresses invasion by inhibiting the JNK/c-Jun signaling pathway. DDDT 17, 821–836. doi:10.2147/DDDT.S398887
Giaquinto, A. N., Sung, H., Miller, K. D., Kramer, J. L., Newman, L. A., Minihan, A., et al. (2022). Breast cancer statistics, 2022. CA A Cancer J. Clin. 72, 524–541. doi:10.3322/caac.21754
Goossens, J.-F., Goossens, L., and Bailly, C. (2021). Hinokiflavone and related C–O–C-type biflavonoids as anti-cancer compounds: properties and mechanism of action. Nat. Prod. Bioprospect 11, 365–377. doi:10.1007/s13659-021-00298-w
Grasset, E. M., Dunworth, M., Sharma, G., Loth, M., Tandurella, J., Cimino-Mathews, A., et al. (2022). Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci. Transl. Med. 14, eabn7571. doi:10.1126/scitranslmed.abn7571
Guerrero Llobet, S., van der Vegt, B., Jongeneel, E., Bense, R. D., Zwager, M. C., Schröder, C. P., et al. (2020). Cyclin E expression is associated with high levels of replication stress in triple-negative breast cancer. npj Breast Cancer 6, 40–13. doi:10.1038/s41523-020-00181-w
Hajimehdipoor, H., Tahmasvand, Z., Nejad, F. G., Maresca, M., and Rajabi, S. (2023). Rutin promotes proliferation and orchestrates epithelial–mesenchymal transition and angiogenesis in MCF-7 and MDA-MB-231 breast cancer cells. Nutrients 15, 2884. doi:10.3390/nu15132884
Hong, O.-Y., Noh, E.-M., Jang, H.-Y., Lee, Y.-R., Lee, B. K., Jung, S. H., et al. (2017). Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 14, 441–446. doi:10.3892/ol.2017.6108
Howard, F. M., and Olopade, O. I. (2021). Epidemiology of triple-negative breast cancer: a review. Cancer J. 27, 8–16. doi:10.1097/PPO.0000000000000500
Hua, F., Xiao, Y.-Y., Qu, X.-H., Li, S.-S., Zhang, K., Zhou, C., et al. (2023). Baicalein sensitizes triple negative breast cancer MDA-MB-231 cells to doxorubicin via autophagy-mediated down-regulation of CDK1. Mol. Cell Biochem. 478, 1519–1531. doi:10.1007/s11010-022-04597-9
Huang, W., Liu, C., Liu, F., Liu, Z., Lai, G., and Yi, J. (2020). Hinokiflavone induces apoptosis and inhibits migration of breast cancer cells via EMT signalling pathway. Cell Biochem. and Funct. 38, 249–256. doi:10.1002/cbf.3443
Huang, X., Cao, J., and Zu, X. (2022). Tumor-associated macrophages: an important player in breast cancer progression. Thorac. Cancer 13, 269–276. doi:10.1111/1759-7714.14268
Huh, J.-W., Lee, J.-H., Jeon, E., Ryu, H. W., Oh, S.-R., Ahn, K.-S., et al. (2020). Maackiain, a compound derived from Sophora flavescens, increases IL-1β production by amplifying nigericin-mediated inflammasome activation. FEBS Open Bio 10, 1482–1491. doi:10.1002/2211-5463.12899
Iriti, M., Kubina, R., Cochis, A., Sorrentino, R., Varoni, E. M., Kabała-Dzik, A., et al. (2017). Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytotherapy Res. 31, 1529–1538. doi:10.1002/ptr.5878
Jia, L., Huang, S., Yin, X., Zan, Y., Guo, Y., and Han, L. (2018). Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 208, 123–130. doi:10.1016/j.lfs.2018.07.027
Jiang, H., and Li, H. (2021). Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: a systematic review and meta-analysis. BMC Cancer 21, 149. doi:10.1186/s12885-021-07860-2
Jiang, J., Yang, Y., Wang, F., Mao, W., Wang, Z., and Liu, Z. (2024). Quercetin inhibits breast cancer cell proliferation and survival by targeting Akt/mTOR/PTEN signaling pathway. Chem. Biol. and Drug Des. 103, e14557. doi:10.1111/cbdd.14557
Kao, T.-W., Bai, G.-H., Wang, T.-L., Shih, I.-M., Chuang, C.-M., Lo, C.-L., et al. (2023). Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance. J. Exp. Clin. Cancer Res. 42, 171. doi:10.1186/s13046-023-02724-y
Kim, E., Kim, Y.-J., Ji, Z., Kang, J. M., Wirianto, M., Paudel, K. R., et al. (2022). ROR activation by Nobiletin enhances antitumor efficacy via suppression of IκB/NF-κB signaling in triple-negative breast cancer. Cell Death Dis. 13, 374–415. doi:10.1038/s41419-022-04826-5
Lathrop, K. I., Kaklamani, V. G., Brenner, A. J., Li, R., Nazarullah, A., Hackman, S., et al. (2020). Novel estrogen receptor beta agonist S-equol decreases tumor proliferation in patients with triple negative breast cancer (TNBC). JCO 38, 560. doi:10.1200/JCO.2020.38.15_suppl.560
Lee, C.-H., Huang, C.-W., Chang, P.-C., Shiau, J.-P., Lin, I.-P., Lin, M.-Y., et al. (2019a). Reactive oxygen species mediate the chemopreventive effects of syringin in breast cancer cells. Phytomedicine 61, 152844. doi:10.1016/j.phymed.2019.152844
Lee, H. H., Jung, J., Moon, A., Kang, H., and Cho, H. (2019b). Antitumor and anti-invasive effect of apigenin on human breast carcinoma through suppression of IL-6 expression. Int. J. Mol. Sci. 20, 3143. doi:10.3390/ijms20133143
Lee, W.-J., Chen, W.-K., Wang, C.-J., Lin, W.-L., and Tseng, T.-H. (2008). Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 226, 178–191. doi:10.1016/j.taap.2007.09.013
Li, J., Liu, H., Lin, Q., Chen, H., Liu, L., Liao, H., et al. (2022). Baicalin suppresses the migration and invasion of breast cancer cells via the TGF-β/lncRNA-MALAT1/miR-200c signaling pathway. Med. Baltim. 101, e29328. doi:10.1097/MD.0000000000029328
Li, Q., Xu, D., Gu, Z., Li, T., Huang, P., and Ren, L. (2021). Rutin restrains the growth and metastasis of mouse breast cancer cells by regulating the microRNA-129-1-3p-mediated calcium signaling pathway. J Biochem. and Mol. Tox 35, e22794. doi:10.1002/jbt.22794
Li, S., Yan, T., Deng, R., Jiang, X., Xiong, H., Wang, Y., et al. (2017). Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. OTT 10, 4809–4819. doi:10.2147/OTT.S140886
Li, S., Yuan, S., Zhao, Q., Wang, B., Wang, X., and Li, K. (2018a). Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomed. and Pharmacother. 100, 441–447. doi:10.1016/j.biopha.2018.02.055
Li, Y.-W., Xu, J., Zhu, G.-Y., Huang, Z.-J., Lu, Y., Li, X.-Q., et al. (2018b). Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 4, 105. doi:10.1038/s41420-018-0124-8
Liao, Y., Xie, X., Zhang, C., Zhong, H., Shan, L., Yu, P., et al. (2024). Quercetin exerts anti-tumor immune mechanism by regulating IL-6/JAK2/STAT3 signaling pathway to deplete Treg cells. Toxicon 243, 107747. doi:10.1016/j.toxicon.2024.107747
Lin, D., Kuang, G., Wan, J., Zhang, X., Li, H., Gong, X., et al. (2017). Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 37, 895–902. doi:10.3892/or.2016.5311
Lin, P.-H., Chiang, Y.-F., Shieh, T.-M., Chen, H.-Y., Shih, C.-K., Wang, T.-H., et al. (2020). Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants 9, 228. doi:10.3390/antiox9030228
Liu, F.-Y., Ding, D.-N., Wang, Y.-R., Liu, S.-X., Peng, C., Shen, F., et al. (2023). Icariin as a potential anticancer agent: a review of its biological effects on various cancers. Front. Pharmacol. 14, 1216363. doi:10.3389/fphar.2023.1216363
Liu, Q., Guan, C., Liu, C., Li, H., Wu, J., and Sun, C. (2022). Targeting hypoxia-inducible factor-1alpha: a new strategy for triple-negative breast cancer therapy. Biomed. and Pharmacother. 156, 113861. doi:10.1016/j.biopha.2022.113861
Louisa, M., and Wardhani, B. W. (2019). Quercetin improves the efficacy of sorafenib in triple negative breast cancer cells through the modulation of drug efflux transporters expressions. Int. J. Appl. Pharm. 11, 129–134. doi:10.22159/ijap.2019.v11s6.33576
Lu, B., Natarajan, E., Balaji Raghavendran, H. R., and Markandan, U. D. (2023). Molecular classification, treatment, and genetic biomarkers in triple-negative breast cancer: a review. Technol. Cancer Res. Treat. 22, 15330338221145246. doi:10.1177/15330338221145246
Lu, W., and Kang, Y. (2019). Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 49, 361–374. doi:10.1016/j.devcel.2019.04.010
Ma, X.-C., Yan, W., Dai, Z., Gao, X., Ma, Y., Xu, Q., et al. (2016). Baicalein suppresses metastasis of breast cancer cells by inhibiting EMT via downregulation of SATB1 and Wnt/β-catenin pathway. DDDT 1419, 1419–1441. doi:10.2147/DDDT.S102541
Maharjan, S., Kwon, Y.-S., Lee, M.-G., Lee, K.-S., and Nam, K.-S. (2021). Cell cycle arrest-mediated cell death by morin in MDA-MB-231 triple-negative breast cancer cells. Pharmacol. Rep. 73, 1315–1327. doi:10.1007/s43440-021-00272-w
Maharjan, S., Lee, M.-G., Kim, S.-Y., Lee, K.-S., and Nam, K.-S. (2023). Morin sensitizes MDA-MB-231 triple-negative breast cancer cells to doxorubicin cytotoxicity by suppressing FOXM1 and attenuating EGFR/STAT3 signaling pathways. Pharmaceuticals 16, 672. doi:10.3390/ph16050672
Malik, P., Singh, R., Kumar, M., Malik, A., and Mukherjee, T. K. (2023). Understanding the phytoestrogen genistein actions on breast cancer: insights on estrogen receptor equivalence, pleiotropic essence and emerging paradigms in bioavailability modulation. Curr. Top. Med. Chem. 23, 1395–1413. doi:10.2174/1568026623666230103163023
Manni, A., Sun, Y.-W., Schell, T. D., Lutsiv, T., Thompson, H., Chen, K.-M., et al. (2023). Complementarity between microbiome and immunity may account for the potentiating effect of quercetin on the antitumor action of cyclophosphamide in a triple-negative breast cancer model. Pharm. (Basel) 16, 1422. doi:10.3390/ph16101422
Martin, J. D., Seano, G., and Jain, R. K. (2019). Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 81, 505–534. doi:10.1146/annurev-physiol-020518-114700
Masih, M., Agarwal, S., Kaur, R., and Gautam, P. K. (2022). Role of chemokines in breast cancer. Cytokine 155, 155909. doi:10.1016/j.cyto.2022.155909
Meng, Z., Zhang, R., Wu, X., Zhang, M., and Jin, T. (2022). PD-L1 mediates triple-negative breast cancer evolution via the regulation of TAM/M2 polarization. Int. J. Oncol. 61, 150. doi:10.3892/ijo.2022.5440
Mohammadian, H., Sharifi, R., Rezanezhad Amirdehi, S., Taheri, E., and Babazadeh Bedoustani, A. (2020). Matrix metalloproteinase MMP1 and MMP9 genes expression in breast cancer tissue. Gene Rep. 21, 100906. doi:10.1016/j.genrep.2020.100906
Mokra, D., Adamcakova, J., and Mokry, J. (2022). Green tea polyphenol (-)-Epigallocatechin-3-Gallate (EGCG): a time for a new player in the treatment of respiratory diseases? Antioxidants (Basel) 11, 1566. doi:10.3390/antiox11081566
Munir, M. T., Kay, M. K., Kang, M. H., Rahman, M. M., Al-Harrasi, A., Choudhury, M., et al. (2021). Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int. J. Mol. Sci. 22, 6526. doi:10.3390/ijms22126526
Nandi, S. K., Roychowdhury, T., Chattopadhyay, S., Basu, S., Chatterjee, K., Choudhury, P., et al. (2022). Deregulation of the CD44-NANOG-MDR1 associated chemoresistance pathways of breast cancer stem cells potentiates the anti-cancer effect of Kaempferol in synergism with Verapamil. Toxicol. Appl. Pharmacol. 437, 115887. doi:10.1016/j.taap.2022.115887
Nguyen, L. T., Lee, Y.-H., Sharma, A. R., Park, J.-B., Jagga, S., Sharma, G., et al. (2017). Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. physiology and Pharmacol. 21, 205–213. doi:10.4196/kjpp.2017.21.2.205
Ou, X., Tan, Y., Xie, J., Yuan, J., Deng, X., Shao, R., et al. (2024). Methylation of GPRC5A promotes liver metastasis and docetaxel resistance through activating mTOR signaling pathway in triple negative breast cancer. Drug Resist Updat 73, 101063. doi:10.1016/j.drup.2024.101063
Pan, H., Zhou, W., He, W., Liu, X., Ding, Q., Ling, L., et al. (2012). Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 30, 337–343. doi:10.3892/ijmm.2012.990
Pan, X., Zhao, B., Song, Z., Han, S., and Wang, M. (2016). Estrogen receptor-α36 is involved in epigallocatechin-3-gallate induced growth inhibition of ER-negative breast cancer stem/progenitor cells. J. Pharmacol. Sci. 130, 85–93. doi:10.1016/j.jphs.2015.12.003
Peng, F., Tang, H., Liu, P., Shen, J., Guan, X., Xie, X., et al. (2017). Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci. Rep. 7, 9022. doi:10.1038/s41598-017-08422-y
Peng, F., Wang, L., Xiong, L., Tang, H., Du, J., and Peng, C. (2022). Maackiain modulates miR-374a/gadd45a Axis to inhibit triple-negative breast cancer initiation and progression. Front. Pharmacol. 13, 806869. doi:10.3389/fphar.2022.806869
Peng, F., Xiong, L., Xie, X., Tang, H., Huang, R., and Peng, C. (2020). Isoliquiritigenin derivative regulates miR-374a/BAX axis to suppress triple-negative breast cancer tumorigenesis and development. Front. Pharmacol. 11, 378. doi:10.3389/fphar.2020.00378
Periferakis, A., Periferakis, K., Badarau, I. A., Petran, E. M., Popa, D. C., Caruntu, A., et al. (2022). Kaempferol: antimicrobial properties, sources, clinical, and traditional applications. Int. J. Mol. Sci. 23, 15054. doi:10.3390/ijms232315054
Qiu, S., Zhou, Y., Kim, J. T., Bao, C., Lee, H. J., and Chen, J. (2021). Amentoflavone inhibits tumor necrosis factor-α-induced migration and invasion through AKT/mTOR/S6k1/hedgehog signaling in human breast cancer. Food Funct. 12, 10196–10209. doi:10.1039/d1fo01085a
Rakoczy, K., Kaczor, J., Sołtyk, A., Szymańska, N., Stecko, J., Sleziak, J., et al. (2023). Application of luteolin in neoplasms and nonneoplastic diseases. Int. J. Mol. Sci. 24, 15995. doi:10.3390/ijms242115995
Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20, 1305. doi:10.3390/ijms20061305
Shang, D., Li, Z., Zhu, Z., Chen, H., Zhao, L., Wang, X., et al. (2015). Baicalein suppresses 17-β-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol. Rep. 33, 2077–2085. doi:10.3892/or.2015.3786
Sharifi-Rad, J., Quispe, C., Imran, M., Rauf, A., Nadeem, M., Gondal, T. A., et al. (2021). Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid. Med. Cell Longev. 2021, 3268136. doi:10.1155/2021/3268136
Sheng, J., Shi, W., Guo, H., Long, W., Wang, Y., Qi, J., et al. (2019). The inhibitory effect of (−)-Epigallocatechin-3-Gallate on breast cancer progression via reducing SCUBE2 methylation and DNMT activity. Molecules 24, 2899. doi:10.3390/molecules24162899
Singh, P., and Lim, B. (2022). Targeting apoptosis in cancer. Curr. Oncol. Rep. 24, 273–284. doi:10.1007/s11912-022-01199-y
Song, B., Wei, F., Peng, J., Wei, X., Liu, M., Nie, Z., et al. (2024). Icariin regulates EMT and stem cell-like character in breast cancer through modulating lncRNA NEAT1/tgfβ/SMAD2 signaling pathway. Biol. Pharm. Bull. 47, 399–410. doi:10.1248/bpb.b23-00668
Song, I.-S., Jeong, Y. J., Jeong, S. H., Kim, J. E., Han, J., Kim, T.-H., et al. (2019). Modulation of mitochondrial ERβ expression inhibits triple-negative breast cancer tumor progression by activating mitochondrial function. Cell Physiol. Biochem. 52, 468–485. doi:10.33594/000000034
Song, L., Chen, X., Mi, L., Liu, C., Zhu, S., Yang, T., et al. (2020). Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 111, 4242–4256. doi:10.1111/cas.14648
Srinivasan, A., Thangavel, C., Liu, Y., Shoyele, S., Den, R. B., Selvakumar, P., et al. (2016). Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 55, 743–756. doi:10.1002/mc.22318
Sudhakaran, M., Navarrete, T. G., Mejía-Guerra, K., Mukundi, E., Eubank, T. D., Grotewold, E., et al. (2023). Transcriptome reprogramming through alternative splicing triggered by apigenin drives cell death in triple-negative breast cancer. Cell Death Dis. 14, 824–910. doi:10.1038/s41419-023-06342-6
Sudhakaran, M., Parra, M. R., Stoub, H., Gallo, K. A., and Doseff, A. I. (2020). Apigenin by targeting hnRNPA2 sensitizes triple-negative breast cancer spheroids to doxorubicin-induced apoptosis and regulates expression of ABCC4 and ABCG2 drug efflux transporters. Biochem. Pharmacol. 182, 114259. doi:10.1016/j.bcp.2020.114259
Suganya, K., Poornima, A., Sumathi, S., Chigurupati, S., Alyamani, N. M., Ghazi Felemban, S., et al. (2022). Rutin induces endoplasmic reticulum stress-associated apoptosis in human triple-negative breast carcinoma MDA-MB-231 cells – in vitro and in silico docking studies. Arabian J. Chem. 15, 104021. doi:10.1016/j.arabjc.2022.104021
Sultan, A. S., Khalil, M. I., Sami, B. M., Alkhuriji, A. F., and Sadek, O. (2017). Quercetin induces apoptosis in triple-negative breast cancer cells via inhibiting fatty acid synthase and β-catenin. Int. J. Clin. Exp. Pathol. 10, 156–172.
Sun, D.-W., Zhang, H.-D., Mao, L., Mao, C.-F., Chen, W., Cui, M., et al. (2015). Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing Notch signaling and regulating MiRNAs. Cell Physiol. Biochem. 37, 1693–1711. doi:10.1159/000438535
Tallerico, R., Conti, L., Lanzardo, S., Sottile, R., Garofalo, C., Wagner, A. K., et al. (2017). NK cells control breast cancer and related cancer stem cell hematological spread. OncoImmunology 6, e1284718. doi:10.1080/2162402X.2017.1284718
Tao, S., He, H., and Chen, Q. (2015). Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell Biochem. 402, 93–100. doi:10.1007/s11010-014-2317-7
Tomita, T., Kato, M., and Hiratsuka, S. (2021). Regulation of vascular permeability in cancer metastasis. Cancer Sci. 112, 2966–2974. doi:10.1111/cas.14942
Tsai, K.-J., Tsai, H.-Y., Tsai, C.-C., Chen, T.-Y., Hsieh, T.-H., Chen, C.-L., et al. (2021). Luteolin inhibits breast cancer stemness and enhances chemosensitivity through the nrf2-mediated pathway. Molecules 26, 6452. doi:10.3390/molecules26216452
Ubaid, M., Salauddin, , Shadani, M. A., Kawish, S. M., Albratty, M., Makeen, H. A., et al. (2023). Daidzein from dietary supplement to a drug candidate: an evaluation of potential. ACS Omega 8, 32271–32293. doi:10.1021/acsomega.3c03741
Umar, S. M., Patra, S., Kashyap, A., Dev, J. R. A., Kumar, L., and Prasad, C. P. (2022). Quercetin impairs HuR-driven progression and migration of triple negative breast cancer (TNBC) cells. Nutr. Cancer 74, 1497–1510. doi:10.1080/01635581.2021.1952628
Wang, B., Huang, T., Fang, Q., Zhang, X., Yuan, J., Li, M., et al. (2020a). Bone-protective and anti-tumor effect of baicalin in osteotropic breast cancer via induction of apoptosis. Breast Cancer Res. Treat. 184, 711–721. doi:10.1007/s10549-020-05904-y
Wang, F., Yuan, C., Liu, B., Yang, Y.-F., and Wu, H.-Z. (2022a). Syringin exerts anti-breast cancer effects through PI3K-AKT and EGFR-RAS-RAF pathways. J. Transl. Med. 20, 310. doi:10.1186/s12967-022-03504-6
Wang, G., Wang, Y., Yao, L., Gu, W., Zhao, S., Shen, Z., et al. (2022b). Pharmacological activity of quercetin: an updated review. Evidence-Based Complementary Altern. Med. 2022, 3997190–3997212. doi:10.1155/2022/3997190
Wang, J.-G., Jian, W.-J., Li, Y., and Zhang, J. (2021). Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. Kaohsiung J. Med. Sci. 37, 572–582. doi:10.1002/kjm2.12371
Wang, S., Wang, N., Huang, X., Yang, B., Zheng, Y., Zhang, J., et al. (2020b). Baohuoside i suppresses breast cancer metastasis by downregulating the tumor-associated macrophages/C-X-C motif chemokine ligand 1 pathway. Phytomedicine 78, 153331. doi:10.1016/j.phymed.2020.153331
Wang, X., Zheng, N., Dong, J., Wang, X., Liu, L., and Huang, J. (2017). Estrogen receptor-α36 is involved in icaritin induced growth inhibition of triple-negative breast cancer cells. J. Steroid Biochem. Mol. Biol. 171, 318–327. doi:10.1016/j.jsbmb.2017.05.009
Wang, X.-F., Zhou, Q.-M., Du, J., Zhang, H., Lu, Y.-Y., and Su, S.-B. (2013a). Baicalin suppresses migration, invasion and metastasis of breast cancer via p38MAPK signaling pathway. Anticancer Agents Med. Chem. 13, 923–931. doi:10.2174/18715206113139990143
Wang, Z., Wang, N., Han, S., Wang, D., Mo, S., Yu, L., et al. (2013b). Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PloS one 8, e68566. doi:10.1371/journal.pone.0068566
Wu, H.-T., Lin, J., Liu, Y.-E., Chen, H.-F., Hsu, K.-W., Lin, S.-H., et al. (2021). Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 81, 153437. doi:10.1016/j.phymed.2020.153437
Wu, L., Lin, Y., Gao, S., Wang, Y., Pan, H., Wang, Z., et al. (2023). Luteolin inhibits triple-negative breast cancer by inducing apoptosis and autophagy through SGK1-FOXO3a-BNIP3 signaling. Front. Pharmacol. 14, 1200843. doi:10.3389/fphar.2023.1200843
Xie, L., Yi, J., Song, Y., Zhao, M., Fan, L., and Zhao, L. (2021). Suppression of GOLM1 by EGCG through HGF/HGFR/AKT/GSK-3β/β-catenin/c-Myc signaling pathway inhibits cell migration of MDA-MB-231. Food Chem. Toxicol. 157, 112574. doi:10.1016/j.fct.2021.112574
Xiong, X., Tang, N., Lai, X., Zhang, J., Wen, W., Li, X., et al. (2021). Insights into amentoflavone: a natural multifunctional biflavonoid. Front. Pharmacol. 12, 768708. doi:10.3389/fphar.2021.768708
Xu, J., Wu, K., Jia, Q., and Ding, X. (2020). Roles of miRNA and lncRNA in triple-negative breast cancer. J. Zhejiang Univ. Sci. B 21, 673–689. doi:10.1631/jzus.B1900709
Yan, W., Ma, X., Zhao, X., and Zhang, S. (2018). Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Dev. Ther. 12, 3961–3972. doi:10.2147/DDDT.S181939
Yong, D. O. C., Saker, S. R., Chellappan, D. K., Madheswaran, T., Panneerselvam, J., Choudhury, H., et al. (2020). Molecular and immunological mechanisms underlying the various pharmacological properties of the potent bioflavonoid, rutin. Endocr. Metab. Immune Disord. Drug Targets 20, 1590–1596. doi:10.2174/1871530320666200503053846
Zarrer, J., Haider, M.-T., Smit, D. J., and Taipaleenmäki, H. (2020). Pathological crosstalk between metastatic breast cancer cells and the bone microenvironment. Biomolecules 10, 337. doi:10.3390/biom10020337
Zhang, C., Liao, Y., Li, T., Zhong, H., Shan, L., Yu, P., et al. (2024a). Apigenin promotes apoptosis of 4T1 cells through PI3K/AKT/Nrf2 pathway and improves tumor immune microenvironment in vivo. Toxicol. Res. 13, tfae011. doi:10.1093/toxres/tfae011
Zhang, J., Teng, F., Wu, T., Li, S., and Li, K. (2024b). Quercetin inhibits chronic stress-mediated progression of triple-negative breast cancer by blocking β2-AR/ERK1/2 pathway. Biomed. and Pharmacother. 177, 116985. doi:10.1016/j.biopha.2024.116985
Zhang, P., Zhang, J., Zhao, L., Li, S., and Li, K. (2022). Quercetin attenuates the cardiotoxicity of doxorubicin–cyclophosphamide regimen and potentiates its chemotherapeutic effect against triple-negative breast cancer. Phytotherapy Res. 36, 551–561. doi:10.1002/ptr.7342
Zhang, Y., Han, G., Fan, B., Zhou, Y., Zhou, X., Wei, L., et al. (2009). Green tea (-)-epigallocatechin-3-gallate down-regulates VASP expression and inhibits breast cancer cell migration and invasion by attenuating Rac1 activity. Eur. J. Pharmacol. 606, 172–179. doi:10.1016/j.ejphar.2008.12.033
Zhao, M., Xu, P., Shi, W., Wang, J., Wang, T., and Li, P. (2024). Icariin exerts anti-tumor activity by inducing autophagy via AMPK/mTOR/ULK1 pathway in triple-negative breast cancer. Cancer Cell Int. 24, 74. doi:10.1186/s12935-024-03266-9
Zhao, T.-T., Xu, Y.-Q., Hu, H.-M., Gong, H.-B., and Zhu, H.-L. (2019). Isoliquiritigenin (ISL) and its formulations: potential antitumor agents. Curr. Med. Chem. 26, 6786–6796. doi:10.2174/0929867325666181112091700
Zhao, X., Qu, J., Liu, X., Wang, J., Ma, X., Zhao, X., et al. (2018). Baicalein suppress EMT of breast cancer by mediating tumor-associated macrophages polarization. Am. J. Cancer Res. 8, 1528–1540.
Zhou, T., Zhang, A., Kuang, G., Gong, X., Jiang, R., Lin, D., et al. (2017). Baicalin inhibits the metastasis of highly aggressive breast cancer cells by reversing epithelial-to-mesenchymal transition by targeting β-catenin signaling. Oncol. Rep. 38, 3599–3607. doi:10.3892/or.2017.6011
Keywords: triple-negative breast cancer, flavonoids, traditional Chinese medicine, breast cancer, pharmacology
Citation: Wang S, Wang K, Li C, Chen J and Kong X (2024) Role of flavonoids in inhibiting triple-negative breast cancer. Front. Pharmacol. 15:1411059. doi: 10.3389/fphar.2024.1411059
Received: 02 April 2024; Accepted: 13 August 2024;
Published: 27 August 2024.
Edited by:
Tsong-Long Hwang, Chang Gung University of Science and Technology, TaiwanReviewed by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaKunming Qin, Jiangsu Ocean University, China
Copyright © 2024 Wang, Wang, Li, Chen and Kong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kuanyu Wang, d2FuZ2t1YW55dV8xOTY0QDE2My5jb20=
 Shuai Wang
Shuai Wang Kuanyu Wang
Kuanyu Wang Cheng Li2
Cheng Li2