
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 26 June 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1409022
Introduction: To clarify the prevalence of adverse renal outcomes following targeted therapies in renal cell carcinoma (RCC).
Methods: A systematic search was performed in MEDLINE, EMBASE, and Cochrane Central Library. Studies that had reported adverse renal outcomes following targeted therapies in RCC were eligible. Outcomes included adverse renal outcomes defined as either renal dysfunction as evidenced by elevated serum creatinine levels or the diagnosis of acute kidney injury, or proteinuria as indicated by abnormal urine findings. The risk of bias was assessed according to Cochrane handbook guidelines. Publication bias was assessed using Funnel plot analysis and Egger Test.
Results: The occurrences of the examined outcomes, along with their corresponding 95% confidence intervals (CIs), were combined using a random-effects model. In all, 23 studies including 10 RCTs and 13 observational cohort studies were included. The pooled incidence of renal dysfunction and proteinuria following targeted therapies in RCC were 17% (95% CI: 12%–22%; I2 = 88.5%, p < 0.01) and 29% (95% CI: 21%–38%; I2 = 93.2%, p < 0.01), respectively. The pooled incidence of both types of adverse events varied substantially across different regimens. Occurrence is more often in polytherapy compared to monotherapy. The majority of adverse events were rated as CTCAE grades 1 or 2 events. Four studies were assessed as having low risk of bias.
Conclusion: Adverse renal outcomes reflected by renal dysfunction and proteinuria following targeted therapies in RCC are not uncommon and are more often observed in polytherapy compared to monotherapy. The majority of the adverse events were of mild severity.
Systematic Review Registration: Identifier CRD42023441979.
Renal cell carcinoma (RCC) ranks as the sixth most frequently diagnosed cancer in men and the tenth in women worldwide (Siegel et al., 2018). The incidence has been increasing, with up to 17% of patients had distant metastasis at the time of diagnosis (Capitanio and Montorsi, 2016; Capitanio et al., 2019). Notably, significant progress has been made in the treatment of RCC, particularly metastatic RCC, over the past decade, primarily through the development of targeted therapies based on biological pathway research (Capitanio and Montorsi, 2016). Targeted therapies have emerged as the current mainstays of care, demonstrating efficacy in achieving durable complete responses (Capitanio and Montorsi, 2016).
Despite of the substantial efficacy, targeted therapies in RCC are associated with various adverse events (AEs), among which fatigue, hypertension, gastrointestinal discomfort, dysphonia, and palmar-plantar erythrodysaesthesia are the most commonly reported (Ruiz et al., 2014; Krawczyk et al., 2023). In contrast to the high incidence in approximately one-third of the population, AEs in patients receiving targeted therapy for RCC are not given enough attention. A national survey in oncologists in the United Sates reported a although it is customary for oncologists to discuss adverse events with patients, less than half of the physicians proactively initiate these discussions (Ruiz et al., 2014).
Adverse renal outcomes are also observed following targeted treatment in RCC patients, including impaired renal function and proteinuria. Persistent presence of adverse renal events might lead to the discontinuation of targeted therapies. Therefore, understanding the overview of renal AEs following targeted therapies is helpful not only for consultation on clinical decision-making prior to the initiation of targeted therapies, but also for subsequent patient management. However, there is a lack of summary on the evidence regarding the frequency of adverse renal outcomes following targeted therapies in RCC in literature.
In light of this background, we undertook this comprehensive review and meta-analysis to clarify the incidence of unfavorable renal outcomes following targeted therapies in RCC in trial settings. Our objective was to enhance understanding of this subject matter and furnish substantiated evidence for clinical practice.
A comprehensive search was undertaken to identify relevant studies published until July 13th, 2023 in MEDLINE via PubMed, EMBASE via Ovid, and Cochrane Central Library via Ovid, adhering to the guidelines outlined in the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement (Liberati et al., 2009). The search utilized appropriate text terms related to the names and targeted molecules of commercially available pharmaceuticals for targeted therapies and renal cell carcinoma (see Supplementary Table S1). No restrictions were imposed on publication date or language. The search was prospectively registered on PROSPERO and amended (Identifier# CRD42023441979).
This systematic review considered studies that had reported targeted therapies in the context of renal cell carcinoma and adverse renal outcome following treatment as eligible. Adverse renal outcomes included renal dysfunction reflected by increased serum creatinine or definitions of renal failure and proteinuria. Both observational cohort studies and randomized controlled trials (RCTs) were included, without any restrictions on study population, type of targeted therapy, or targeted molecules of treatment.
The screening process was conducted independently by two reviewers (S.R. and S.Q.R.) using a standardized approach. The titles and abstracts of all retrieved records from the database search were meticulously examined. Exclusions were made for duplicates, pediatric studies, non-original studies (such as reviews, editorials, commentaries, guidelines, proceedings, and secondary analysis of published trials), case reports, study protocols, conference abstracts lacking sufficient information, in vitro studies, animal studies, studies unrelated to cancer, and cancer studies that had not reported kidney injury outcomes or any targeted therapy. Additionally, the reference lists of articles reviewed in their entirety were manually scrutinized to identify any relevant studies. Any discrepancy was adjudicated by a third reviewer (Y.L.F.).
The outcome in this systematic review was adverse renal outcomes following targeted therapies, defined as either renal dysfunction as evidenced by elevated serum creatinine levels or the diagnosis of acute kidney injury (AKI), acute renal failure (ARF), or renal failure, or proteinuria as indicated by abnormal urine findings. These outcomes were quantified using their incidences reported in each study cohort.
Two independent reviewers (S.R. and S.Q.R.) extracted data from eligible studies and compiled them into a shared document. Any discrepancy was resolved by the third reviewer (Y.L.F.).
The collected data included various elements such as the authors’ names, publication year, geographical location, total number of patients in the study population, specifics of the targeted therapies employed, and the occurrence of adverse renal outcomes following treatments. In the case of RCTs, the extracted data also encompassed the number of patients in both interventional and control groups, details regarding interventional and control treatments, and incidences of adverse renal outcomes observed within each respective group. Additional information regarding potential sources of heterogeneity, such as the demographic composition and average age of the study population, was also gathered for the purpose of conducting sensitivity analysis.
Two reviewers (S.R. and S.Q.R.) independently assessed the risk of bias of included studies using the Agency for Healthcare Research and Quality (AHRQ) tool (Chou et al., 2010). Any discrepancy was resolved by consensus.
Data analysis and synthesis were performed using Stata (version 14.0) and Review Manager (RevMan 5.2) software. Since we aimed to identify the pooled incidences of the outcomes, each study group in the RCTs was treated as an independent cohort, from which the incidences of adverse renal outcomes were collectively meta-analyzed along with those observed in the observational cohort studies. The occurrences of the examined outcomes, along with their corresponding 95% confidence intervals (CIs), were combined using a random-effects model. Additionally, subgroup analyses were performed based on the severity of adverse events evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) and classification of targeting agents. Due to substantial variations in targeted therapies across RCTs, it was not feasible to directly compare the study outcomes among different treatment regimens. Consequently, the targeted treatments were categorized based on their respective target molecules. The degree of statistical heterogeneity was assessed using the I2 statistic (Ioannidis, 2008). The pooled incidences of the study outcomes were classified as having low, moderate, and high statistical heterogeneity based on I2 values of <25%, between 26% and 75%, and >75%, respectively (Ioannidis, 2008). Publication bias was assessed using Funnel plot analysis and Egger Test. A two-sided p-value of < 0.05 was considered statistically significant.
A total of 2,141 records were initially identified through literature searching and after removing duplicates. 2002 records were excluded after screening the titles and abstracts, and another 115 publications were further excluded following full text review. Finally, 23 studies were incorporated into this systematic review and meta-analysis, including 10 RCT studies (Motzer et al., 2014; Motzer et al., 2015; Choueiri et al., 2016; Powles et al., 2016; Jonasch et al., 2017; Bergmann et al., 2020; Choueiri et al., 2022; Lee et al., 2022; Pal et al., 2022; Sheng et al., 2023) and 13 observational cohort studies (Hainsworth et al., 2010; Harzstark et al., 2011; Ryan et al., 2011; Molina et al., 2012; Guo et al., 2013; Hainsworth et al., 2013; Harshman et al., 2013; Molina et al., 2014; Motzer et al., 2016; Oudard et al., 2016; Oyama et al., 2017; Hutson et al., 2021; Pedersen et al., 2021) (see Figure 1).
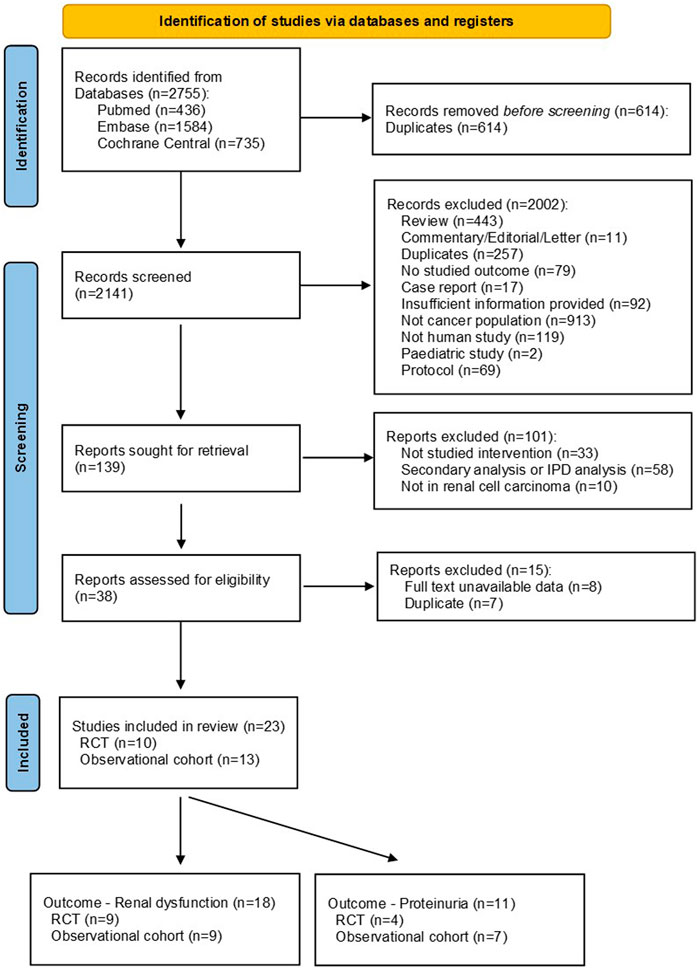
Figure 1. PRISMA flow chart for study selection. Abbreviations: IPD, individual patient data; RCT, randomized controlled trial.
Five studies and 12 studies had only provided data on proteinuria (Hainsworth et al., 2010; Harshman et al., 2013; Molina et al., 2014; Motzer et al., 2015; Motzer et al., 2016) and renal dysfunction (Ryan et al., 2011; Molina et al., 2012; Guo et al., 2013; Motzer et al., 2014; Oudard et al., 2016; Powles et al., 2016; Jonasch et al., 2017; Oyama et al., 2017; Bergmann et al., 2020; Pedersen et al., 2021; Choueiri et al., 2022; Lee et al., 2022), respectively. Six studies had reported both outcomes (Harzstark et al., 2011; Hainsworth et al., 2013; Choueiri et al., 2016; Hutson et al., 2021; Pal et al., 2022; Sheng et al., 2023). The targeted therapies were classified into seven groups based on the targets of regimens, including mTOR inhibitors, Tyrosine kinase inhibitor (TKI), a combination of mTOR inhibitors and TKI, AKT inhibitors, and a combination of mTOR inhibitors with either PI3K inhibitors, GLS1 inhibitors and VEGF/HER2 inhibitors, among which the first three groups were the mainstays. Detailed characteristics of the included studies are shown in (Table 1).
The pooled incidence of renal dysfunction following targeted therapies was 17% (95% CI: 12%, 22%), with a high degree of heterogeneity observed among the studies (I2 = 88.5%, p < 0.01) (see Figure 2). Notably, the incidences of renal dysfunction varied substantially across different regimens of targeted therapy, ranging from 0.03% to 40% (heterogeneity between sub-groups: p < 0.01) (see Figure 3). Further analysis comparing the incidences of renal dysfunction among three regimens that had been reported in more than three studies revealed a range of 6%–20% (see Supplementary Figure S1). The pooled incidences of renal dysfunction events, categorized as CTCAE grade 1–2 and grade 3–4, were determined to be 15% (95% CI: 10%, 20%) and <1% (95% CI: 0, 1%), respectively (see Supplementary Figure S2).
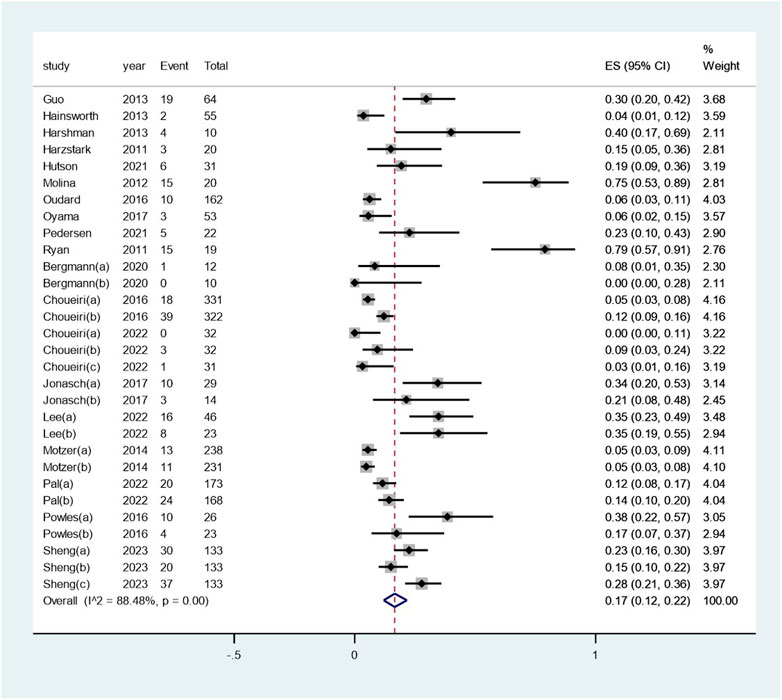
Figure 2. Aggregated occurrence rate of renal dysfunction subsequent to the administration of targeted therapies in renal cell carcinoma. Notes: The pooled incidence of renal dysfunction following targeted therapies was 17% (95% CI: 12%, 22%), with a high degree of heterogeneity observed among the studies (I2 = 88.5%, p < 0.01).
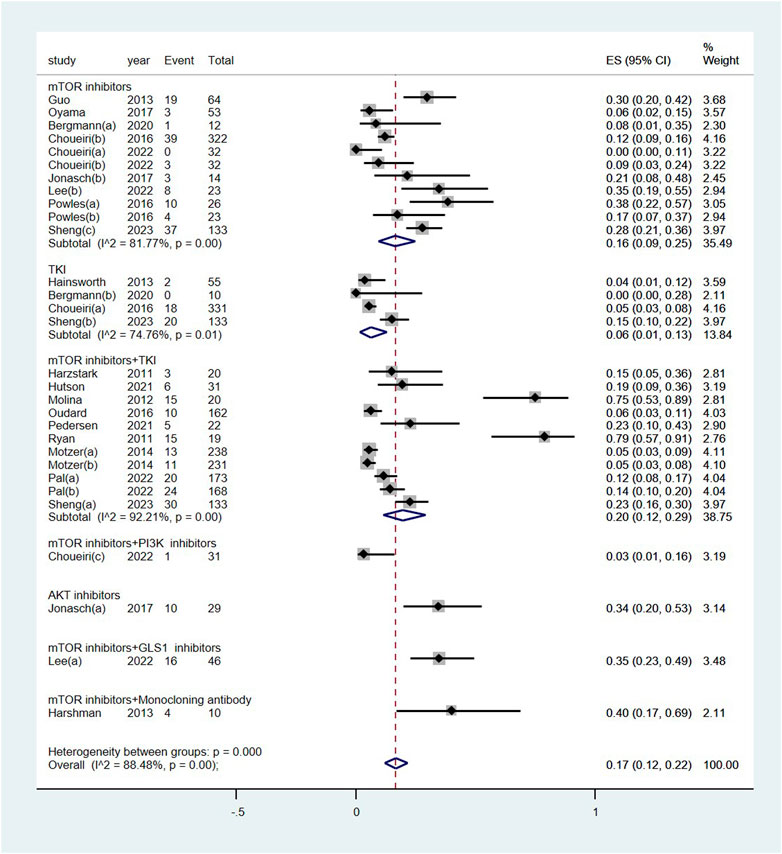
Figure 3. Subgroup examination of renal dysfunction following targeted therapies in renal cell carcinoma, categorized according to the classification of targeted agents. Notes: The targeted therapies are classified into seven subgroups based on their targets.
The pooled incidence of proteinuria subsequent to the administration of targeted therapy was 29% (95% CI: 21%, 38%), with a high degree of heterogeneity observed among the studies (I2 = 93.2%, p < 0.01) (see Figure 4). The pooled incidence of proteinuria following targeted therapy substantially ranged from 19% (95% CI: 4%, 42%) after mTOR inhibitor treatment to 48% (95% CI: 37%, 59%) after a combination of mTOR inhibitor and monoclonal antibody treatment (heterogeneity between sub-groups: p = 0.017) (see Figure 5). The pooled incidences of proteinuria events, categorized as CTCAE grade 1–2 and grade 3–4, were deemed to be 21% (95% CI: 15%, 28%) and 7% (95% CI:3%, 11%), respectively (see Supplementary Figure S3).
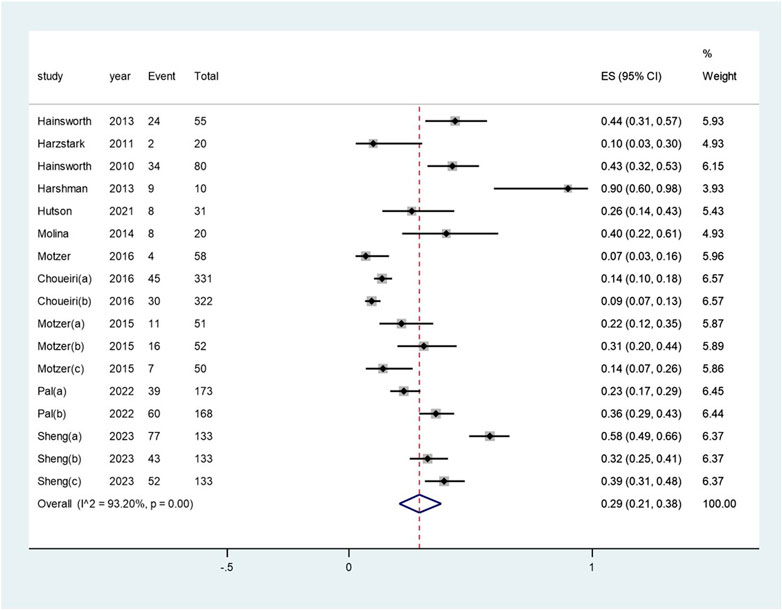
Figure 4. Aggregated occurrence rate of proteinuria subsequent to the administration of targeted therapies in renal cell carcinoma. Notes: The pooled incidence of proteinuria was 28% (95% CI: 2o%, 36%), with a high degree of heterogeneity observed among the studies (I2 = 92.9%, p < 0.01).
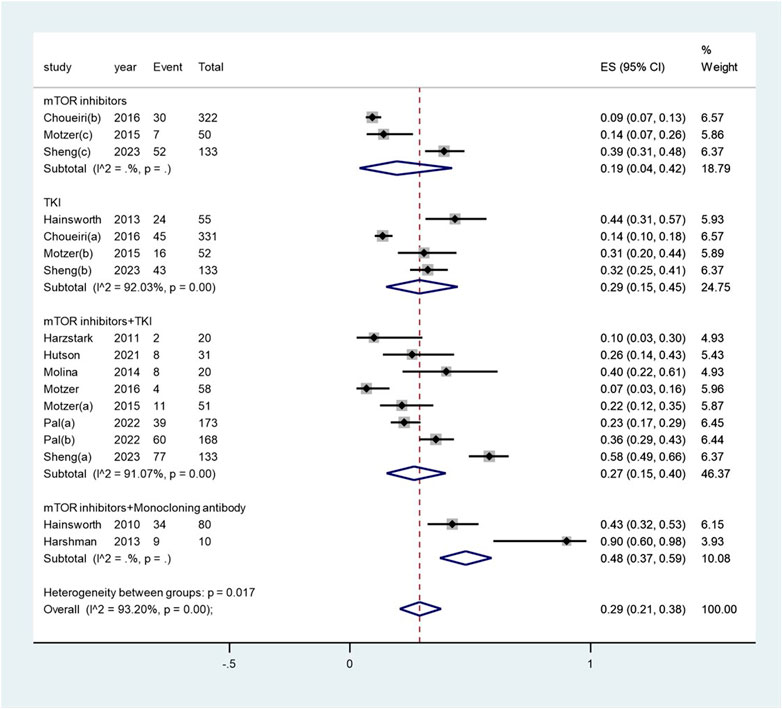
Figure 5. Subgroup examination of proteinuria following targeted therapies in renal cell carcinoma, categorized according to the classification of targeted agents.
The evaluation based on the AHRQ tool identified six, eight, and nine studies with low, unclear, and high risk of bias, respectively (Supplementary Figure S4).
The funnel plot revealed symmetry on visual inspection, suggesting the absence of publication bias (Supplementary Figure S5). This observation was supported by the results of the Egger test (p = 0.10, Supplementary Figure S6).
Our findings suggested adverse renal outcomes are rarely reported in targeted therapy in RCC, not being a focused topic in clinical trials or pharmacovigilance; however, the incidence is not low. The pooled occurrence rates of renal dysfunction measured by elevated serum creatinine or diagnosis of AKI or ARF and proteinuria were up to 17% and 29%, respectively. Renal dysfunction was more commonly observed in the mTOR inhibitors and TKIs polytherapy compared to either monotherapy, whereas proteinuria occurred at a similar rate in the combination of mTOR inhibitors and TKI compared to the TKI monotherapy. The majority of the adverse events were CTCAE grades 1 or 2 events. Nearly one-fourth of the included studies in this meta-analysis were rated as having low risk of bias.
Although kidney related adverse events are not the leading adverse events in studies on targeted therapy in RCC (Di Lorenzo et al., 2011), the findings of this study and previous studies consistently suggest these events are not uncommon (Ruiz et al., 2014; Krawczyk et al., 2023; Semenescu et al., 2023). The reported rate of proteinuria is higher than renal function impairment. The occurrence of impaired renal function might be underestimated due to the fact that AKI defined using the creatinine-based criteria is often left undiagnosed, particularly when the increase of creatinine achieves the diagnostic criteria (i.e., 26.5 μmol/L) yet the highest level of creatinine is still below the upper limit. Pharmacovigilance studies on check-point inhibitors (CIs), which belong to another important class of therapies for RCCs, have reported high prevalence of AKI following CI treatments (Hu et al., 2021; Zhu et al., 2022), which is also more commonly seen in polytherapy (Hu et al., 2021). Therefore, adverse renal outcomes are worthy noted in applying targeted therapies in RCC patients and should be closely monitored.
Despite of the relatively high occurrence, the severity of renal adverse events in this clinical setting is not alerting. Our findings showed most of the adverse events are grades 1 or 2, not necessitating drug treatment. This is consistent with our observations in clinical practice, in which the elevated serum creatinine level following targeted therapies rarely exceed 150 μmol/L in most patients with previously normal renal function. Although the elevation of creatinine exhibits a clear relationship with the timing of targeted therapies, the treatment is mainly close monitoring and supportive regimens, such as sufficient uptake of fluid and controlling risk factors for kidney injury (Ruiz et al., 2014). In most cases, the creatinine stops to increase, sometimes gradually decreases, even to normal range. Renal replacement therapy is extremely rare. The results of this meta-analysis provide evidence for this management strategy of close follow up of targeted therapies in the RCC population.
The occurrence of adverse renal outcomes subsequent to targeted therapies in RCC results from a combination of various factors. The underlying reasons are multifaceted in pathophysiological and clinical perspectives. Firstly, targeted agents, such as sorafenib and sunitinib in the included trials in this meta-analysis have been reported to cause thrombotic microangiopathy (Garcia and Atallah, 2017; Genest et al., 2023), which is an important cause of proteinuria and acute decline in renal function. Secondly, the gastrointestinal discomfort including diarrhea and vomiting can cause dehydration, reducing the kidney perfusion, thus increasing the risk of pre-renal kidney injury (Di Lorenzo et al., 2011). Thirdly, the toxicity of targeted agents is also an important source of injury in this setting. Renal biopsy studies indicated AKI in CIs treatment is most commonly induced by acute tubulointerstitial nephritis, either alone or accompanied by other renal lesions including acute tubular injury or glomerular lesions (Cortazar et al., 2016; Moss and Perazella, 2022). However, there is a lack of renal biopsy study on pathological manifestation of kidney injury in targeted therapies in RCC, most likely because these adverse events were not severe enough for conducting invasive kidney biopsy. With the advances of pharmacological research, the underlying mechanisms of targeted agents for kidney injury might also evolve (Moss and Perazella, 2022). Fourthly, common risk factors for kidney injury, for example, the diabetes, hypertension, senior age, and the use of NSAIDs, proton pump inhibitors, and ACEi/ARBs are frequently observed in cancer patients and can all increase the risk of renal dysfunction (Chen et al., 2023). The exploration of potential reasons also reminds us the importance to manage risk factors when prescribe targeted therapies in RCC patients, take necessary measures to alleviate the hazard effects of damaging factors, and pay attention to close monitoring.
To our best acknowledgment, this is the first systematic review and meta-analysis on the occurrence of adverse renal outcomes following targeted therapies in RCC. Our results benefited from a comprehensive literature search and unbiased comparisons in RCTs. There are still some limitations worth mentioning. First, the results were limited by the reporting in the included studies. Although the funnel plot analysis did not suggest the existence of publication bias, as mentioned earlier, since mild elevation in serum creatinine and proteinuria might be asymptomatic thus being left undiagnosed, the reported incidence might have been underestimated. Second, there are variations on definitions of renal dysfunction and proteinuria across the studies. Some studies even just simply reported the adverse renal events as increased creatinine or proteinuria without details in the definitions. The variations might have been an important source of the observed high heterogeneity and must be considered in the interpretation of the results. Third, the proportion of included studies with low risk of bias was only one-fourth in this meta-analysis, precluding from making robust conclusions. Future studies covering renal adverse outcomes following targeted therapies in RCC will help us gain more insight into the appropriate clinical management approaches.
In summary, the results indicate that adverse renal outcomes including renal dysfunction and proteinuria are not infrequent in RCC patients receiving targeted therapies, particularly in cases of polytherapy as opposed to monotherapy. The majority of these adverse events were of mild severity. The results remind us to take appropriate measures to mitigate risk factors for renal injury and closely monitor the outcome of adverse events in this population and are awaiting confirmation with real-world clinical data.
SoR: Investigation, Methodology, Writing–original draft, Writing–review and editing. XC: Software, Writing–original draft. YZ: Formal Analysis, Investigation, Writing–original draft. TC: Data curation, Writing–original draft. XH: Project administration, Validation, Visualization, Writing–review and editing. YF: Conceptualization, Resources, Supervision, Writing–original draft, Writing–review and editing. ShR: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1409022/full#supplementary-material
Bergmann, L., Grünwald, V., Maute, L., Grimm, M. O., Weikert, S., Schleicher, J., et al. (2020). A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR central European society for anticancer drug research-EWIV and the interdisciplinary working group on renal cell cancer (IAGN) of the German cancer society. Oncol. Res. Treat. 43 (7-8), 333–339. doi:10.1159/000508450
Capitanio, U., Bensalah, K., Bex, A., Boorjian, S. A., Bray, F., Coleman, J., et al. (2019). Epidemiology of renal cell carcinoma. Eur. Urol. 75 (1), 74–84. doi:10.1016/j.eururo.2018.08.036
Capitanio, U., and Montorsi, F. (2016). Renal cancer. Lancet London, Engl. 387 (10021), 894–906. doi:10.1016/S0140-6736(15)00046-X
Chen, P., Zhu, J., Xu, Y., Huang, Q., Su, J., Gao, Z., et al. (2023). Risk factors of immune checkpoint inhibitor-associated acute kidney injury: evidence from clinical studies and FDA pharmacovigilance database. BMC Nephrol. 24 (1), 107. doi:10.1186/s12882-023-03171-9
Chou, R., Aronson, N., Atkins, D., Ismaila, A. S., Santaguida, P., Smith, D. H., et al. (2010). AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health-care program. J. Clin. Epidemiol. 63 (5), 502–512. doi:10.1016/j.jclinepi.2008.06.007
Choueiri, T. K., Escudier, B., Powles, T., Tannir, N. M., Mainwaring, P. N., Rini, B. I., et al. (2016). Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 17 (7), 917–927. doi:10.1016/S1470-2045(16)30107-3
Choueiri, T. K., Porta, C., Suarez, C., Hainsworth, J., Voog, E., Duran, I., et al. (2022). Randomized phase II trial of sapanisertib ± TAK-117 vs. Everolimus in patients with advanced renal cell carcinoma after VEGF-targeted therapy. Oncol. 27 (12), 1048–1057. doi:10.1093/oncolo/oyac192
Cortazar, F. B., Marrone, K. A., Troxell, M. L., Ralto, K. M., Hoenig, M. P., Brahmer, J. R., et al. (2016). Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 90 (3), 638–647. doi:10.1016/j.kint.2016.04.008
Di Lorenzo, G., Porta, C., Bellmunt, J., Sternberg, C., Kirkali, Z., Staehler, M., et al. (2011). Toxicities of targeted therapy and their management in kidney cancer. Eur. Urol. 59 (4), 526–540. doi:10.1016/j.eururo.2011.01.002
Garcia, G., and Atallah, J. P. (2017). Antineoplastic agents and thrombotic microangiopathy. J. Oncol. Pharm. Pract. 23 (2), 135–142. doi:10.1177/1078155216628324
Genest, D. S., Patriquin, C. J., Licht, C., John, R., and Reich, H. N. (2023). Renal thrombotic microangiopathy: a review. Am. J. Kidney Dis. 81 (5), 591–605. doi:10.1053/j.ajkd.2022.10.014
Guo, J., Huang, Y., Zhang, X., Zhou, F., Sun, Y., Qin, S., et al. (2013). Safety and efficacy of everolimus in Chinese patients with metastatic renal cell carcinoma resistant to vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy: an open-label phase 1b study. BMC cancer 13, 136. doi:10.1186/1471-2407-13-136
Hainsworth, J. D., Rubin, M. S., Arrowsmith, E. R., Khatcheressian, J., Crane, E. J., and Franco, L. A. (2013). Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clin. Genitourin. cancer 11 (3), 270–275. doi:10.1016/j.clgc.2013.04.006
Hainsworth, J. D., Spigel, D. R., Burris, H. A., Waterhouse, D., Clark, B. L., and Whorf, R. (2010). Phase II trial of bevacizumab and everolimus in patients with advanced renal cell carcinoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 28 (13), 2131–2136. doi:10.1200/JCO.2009.26.3152
Harshman, L. C., Barbeau, S., McMillian, A., and Srinivas, S. (2013). A phase II study of bevacizumab and everolimus as treatment for refractory metastatic renal cell carcinoma. Clin. Genitourin. cancer 11 (2), 100–106. doi:10.1016/j.clgc.2012.12.002
Harzstark, A. L., Small, E. J., Weinberg, V. K., Sun, J., Ryan, C. J., Lin, A. M., et al. (2011). A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer 117 (18), 4194–4200. doi:10.1002/cncr.25931
Hu, F., Zhai, Y., Yuan, L., Liang, J., Xu, J., Guo, X., et al. (2021). Renal toxicities in immune checkpoint inhibitors with or without chemotherapy: an observational, retrospective, pharmacovigilance study leveraging US FARES database. Cancer Med. 10 (24), 8754–8762. doi:10.1002/cam4.4343
Hutson, T. E., Michaelson, M. D., Kuzel, T. M., Agarwal, N., Molina, A. M., Hsieh, J. J., et al. (2021). A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur. Urol. 80 (2), 162–170. doi:10.1016/j.eururo.2021.03.015
Ioannidis, J. P. (2008). Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 14 (5), 951–957. doi:10.1111/j.1365-2753.2008.00986.x
Jonasch, E., Hasanov, E., Corn, P. G., Moss, T., Shaw, K. R., Stovall, S., et al. (2017). A randomized phase 2 study of MK-2206 versus everolimus in refractory renal cell carcinoma. Ann. Oncol. official J. Eur. Soc. Med. Oncol. 28 (4), 804–808. doi:10.1093/annonc/mdw676
Krawczyk, K., Sladowska, K., Holko, P., and Kawalec, P. (2023). Comparative safety of tyrosine kinase inhibitors in the treatment of metastatic renal cell carcinoma: a systematic review and network meta-analysis. Front. Pharmacol. 14, 1223929. doi:10.3389/fphar.2023.1223929
Lee, C. H., Motzer, R., Emamekhoo, H., Matrana, M., Percent, I., Hsieh, J. J., et al. (2022). Telaglenastat plus everolimus in advanced renal cell carcinoma: a randomized, double-blinded, placebo-controlled, phase II entrata trial. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 28 (15), 3248–3255. doi:10.1158/1078-0432.CCR-22-0061
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339, b2700. doi:10.1136/bmj.b2700
Molina, A. M., Feldman, D. R., Voss, M. H., Ginsberg, M. S., Baum, M. S., Brocks, D. R., et al. (2012). Phase 1 trial of everolimus plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 118 (7), 1868–1876. doi:10.1002/cncr.26429
Molina, A. M., Hutson, T. E., Larkin, J., Gold, A. M., Wood, K., Carter, D., et al. (2014). A phase 1b clinical trial of the multi-targeted tyrosine kinase inhibitor lenvatinib (E7080) in combination with everolimus for treatment of metastatic renal cell carcinoma (RCC). Cancer Chemother. Pharmacol. 73 (1), 181–189. doi:10.1007/s00280-013-2339-y
Moss, E. M., and Perazella, M. A. (2022). The role of kidney biopsy in immune checkpoint inhibitor nephrotoxicity. Front. Med. (Lausanne) 9, 964335. doi:10.3389/fmed.2022.964335
Motzer, R. J., Alyasova, A., Ye, D., Karpenko, A., Li, H., Alekseev, B., et al. (2016). Phase II trial of second-line everolimus in patients with metastatic renal cell carcinoma (RECORD-4). Ann. Oncol. official J. Eur. Soc. Med. Oncol. 27 (3), 441–448. doi:10.1093/annonc/mdv612
Motzer, R. J., Barrios, C. H., Kim, T. M., Falcon, S., Cosgriff, T., Harker, W. G., et al. (2014). Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 32 (25), 2765–2772. doi:10.1200/JCO.2013.54.6911
Motzer, R. J., Hutson, T. E., Glen, H., Michaelson, M. D., Molina, A., Eisen, T., et al. (2015). Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 16 (15), 1473–1482. doi:10.1016/S1470-2045(15)00290-9
Oudard, S., Joly, F., Geoffrois, L., Laguerre, B., Houede, N., Barthelemy, P., et al. (2016). Clinical benefit of everolimus as second-line therapy in metastatic renal cell carcinoma: the French retrospective SECTOR study. Clin. Genitourin. cancer 14 (6), e595–e607. doi:10.1016/j.clgc.2016.04.019
Oyama, M., Sugiyama, T., Nozawa, M., Fujimoto, K., Kishida, T., Kimura, G., et al. (2017). Efficacy and safety of sequential use of everolimus in Japanese patients with advanced renal cell carcinoma after failure of first-line treatment with vascular endothelial growth factor receptor tyrosine kinase inhibitor: a multicenter phase II clinical trial. Jpn. J. Clin. Oncol. 47 (6), 551–559. doi:10.1093/jjco/hyw194
Pal, S. K., Puente, J., Heng, D. Y. C., Glen, H., Koralewski, P., Stroyakovskiy, D., et al. (2022). Assessing the safety and efficacy of two starting doses of lenvatinib plus everolimus in patients with renal cell carcinoma: a randomized phase 2 trial. Eur. Urol. 82 (3), 283–292. doi:10.1016/j.eururo.2021.12.024
Pedersen, K. S., Grierson, P. M., Picus, J., Lockhart, A. C., Roth, B. J., Liu, J., et al. (2021). Vorolanib (X-82), an oral anti-VEGFR/PDGFR/CSF1R tyrosine kinase inhibitor, with everolimus in solid tumors: results of a phase I study. Investig. new drugs 39 (5), 1298–1305. doi:10.1007/s10637-021-01093-7
Powles, T., Wheater, M., Din, O., Geldart, T., Boleti, E., Stockdale, A., et al. (2016). A randomised phase 2 study of AZD2014 versus everolimus in patients with VEGF-refractory metastatic clear cell renal cancer. Eur. Urol. 69 (3), 450–456. doi:10.1016/j.eururo.2015.08.035
Ruiz, J. N., Belum, V. R., Creel, P., Cohn, A., Ewer, M., and Lacouture, M. E. (2014). Current practices in the management of adverse events associated with targeted therapies for advanced renal cell carcinoma: a national survey of oncologists. Clin. Genitourin. cancer 12 (5), 341–347. doi:10.1016/j.clgc.2014.04.001
Ryan, C. W., Vuky, J., Chan, J. S., Chen, Z., Beer, T. M., and Nauman, D. (2011). A phase II study of everolimus in combination with imatinib for previously treated advanced renal carcinoma. Investig. new drugs 29 (2), 374–379. doi:10.1007/s10637-009-9365-y
Semenescu, L. E., Kamel, A., Ciubotaru, V., Baez-Rodriguez, S. M., Furtos, M., Costachi, A., et al. (2023). An overview of systemic targeted therapy in renal cell carcinoma, with a focus on metastatic renal cell carcinoma and brain metastases. Curr. Issues Mol. Biol. 45 (9), 7680–7704. doi:10.3390/cimb45090485
Sheng, X., Ye, D., Zhou, A., Yao, X., Luo, H., He, Z., et al. (2023). Efficacy and safety of vorolanib plus everolimus in metastatic renal cell carcinoma: a three-arm, randomised, double-blind, multicentre phase III study (CONCEPT). Eur. J. cancer (Oxford, Engl. 1990) 178, 205–215. doi:10.1016/j.ejca.2022.10.025
Siegel, R. L., Miller, K. D., and Jemal, A. (2018). Cancer statistics, 2018. CA Cancer J. Clin. 68 (1), 7–30. doi:10.3322/caac.21442
Keywords: renal dysfunction, proteinuria, targeted therapy, renal cell carcinoma, systematic review, meta-analysis
Citation: Ren S, Chen X, Zheng Y, Chen T, Hu X, Feng Y and Ren S (2024) Adverse renal outcomes following targeted therapies in renal cell carcinoma: a systematic review and meta-analysis. Front. Pharmacol. 15:1409022. doi: 10.3389/fphar.2024.1409022
Received: 29 March 2024; Accepted: 31 May 2024;
Published: 26 June 2024.
Edited by:
Zhijie Xu, Central South University, ChinaReviewed by:
Hashem Obaid Alsaab, Taif University, Saudi ArabiaCopyright © 2024 Ren, Chen, Zheng, Chen, Hu, Feng and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shangqing Ren, cnNxMDUxNkAxNjMuY29t; Yunlin Feng, ZmVuZ3l1bmxpbkBtZWQudWVzdGMuZWR1LmNu; Xu Hu, c2N1aHV4dUBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.