- 1Animal Disease Research Unit, United States Department of Agriculture, Agricultural Research Service, WSU, Pullman, WA, United States
- 2Estación Experimental INTA Paraná Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Parana, Argentina
- 3Department of Veterinary Microbiology and Pathology, Washington State University, Pullman, WA, United States
- 4Program in Individualized Medicine, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, Washington State University, Pullman, United States
- 5Oregon Health and Science University, Portland, OR, United States
- 6VA Portland Healthcare System, Portland, OR, United States
Introduction: B. bovis is an apicomplexan parasite responsible for bovine babesiosis, a tick-borne disease with a worldwide impact. The disease remains inefficiently controlled, and few effective drugs, including imidocarb dipropionate (ID), are currently available in endemic areas. The objective of this study was to evaluate whether buparvaquone (BPQ), a drug currently used to treat cattle infected with the Babesia-related Theileria spp. parasites, could be active against Babesia parasites. Herein, we compared the effect of ID and BPQ on B. bovis growth in vitro erythrocyte culture.
Methods: We compared the effect of ID and BPQ on the culture-adapted Texas T2Bo strain of B. bovis. In vitro cultured parasites were incubated with ID and BPQ at two starting parasitemia levels (PPE), 0.2% and 1%. In vitro cultured parasites were treated with ID or BPQ at concentrations ranging from 10 to 300 nM, during 4 consecutive days. Parasitemia levels were daily evaluated using microscopic examination. Data was compared using the independent Student’s t-test.
Results and discussion: Both ID and BPQ significantly inhibited (p < 0.05) the growth of B. bovis, regardless of the initial parasitemia used. At 1% parasitemia, BPQ had lower calculated inhibitory concentration 50 (IC50: 50.01) values than ID (IC50: 117.3). No parasites were found in wells with 0.2% starting parasitemia, treated previously with 50 nM of BPQ or ID, after 2 days of culture without drugs. At 1% parasitemia, no parasite survival was detected at 150 nM of BPQ or 300 nM of ID, suggesting that both drugs acted as babesiacidals.
Conclusion: Overall, the data suggests that BPQ is effective against B. bovis and shows a residual effect that seems superior to ID, which is currently the first-line drug for treating bovine babesiosis globally.
1 Introduction
Bovine babesiosis is a tick-borne parasitic disease with global impact on all continents (Pipano and Hadani, 1984; Bock et al., 2004), especially in tropical and subtropical areas of the world (Jacob et al., 2020; Asrar et al., 2022). Six species of Babesia are known to cause disease in cattle; however, B. bovis, B. bigemina and B. divergens are the species with the highest impact on the cattle industry worldwide. B. bovis and B. bigemina cause high morbidity and mortality in tropical and semi-tropical regions. In contrast, B. divergens has zoonotic potential and is mainly prevalent in European countries (Zintl et al., 2003, Zintl et al., 2014).
B. bovis and B. bigemina are transovarially transmitted by the one-host cattle tick Rhipicephalus microplus and can cause acute and persistent disease in livestock (Smith et al., 2000). Acute babesiosis is especially problematic in adult or immunocompromised cattle (Azhar et al., 2023), which also cannot be vaccinated with current live attenuated vaccines. The high direct economic effects of bovine babesiosis are associated with mortalities that may be up to 70%–90% in susceptible cattle without treatment, and secondly, with costs derived from treatments and prevention (Pipano and Hadani, 1984; Almazan et al., 2018; Jacob et al., 2020)
In B. bovis infections, relatively low parasitemia occur (PPE< 0.5%) in the presence of severe clinical effects in the acute stage of the disease. In addition, cattle that survive acute infection remain persistently infected for life (Alvarez et al., 2020; Azhar et al., 2023). B. bigemina infections, in contrast, occur with much higher parasitemias (PPE 3%) that precede severe clinical manifestations (McCosker, 1981; Bock et al., 2004; Néo et al., 2016), and infected cattle are able, in some cases, to clear the parasites after the development of a shorter persistent stage of infection. However, a critical difference between B. bovis and B. bigemina is the relative predilection of the parasitized erythrocytes of B. bovis to adhere to microcirculatory endothelium and thus to accumulate and sequester in blood capillaries of many organs, including the brain, causing neurological syndrome (Kakoma and Ristic, 1984; Böse et al., 1995). Considering this parasite sequestration phenomenon, the disease usually progresses very fast to circulatory collapse, respiratory failure, coma, and death (Kakoma and Ristic, 1984), and thus B. bovis is considered the most pathogenic agent of bovine babesiosis.
Bovine babesiosis can be currently controlled by strategies targeting the tick vectors, the use of live vaccines, and babesiacidal drugs, such as diminazene aceturate, amicarbalide, and imidocarb (Todorovic et al., 1973; Vial and Gorenflot, 2006; Almazan et al., 2018; Alvarez et al., 2020; Azhar et al., 2023). However, current live vaccines are not fully safe and require a cold chain, among other limitations. The drugs against ticks for treatment are expensive and may lead to acaricide resistance, have toxic effects, and leave residues in the food chain. Thus, new approaches for controlling the disease are urgently needed (Pérez de León et al., 2010; Pérez de Leon et al., 2012; Almazan et al., 2018).
In addition, vaccination of less than year-old calves using live attenuated parasites, one of the main strategies for babesiosis control and prevention, needs to be integrated strategically to maintain enzootic stability in endemic areas (Smith et al., 2000; Asrar et al., 2022). Chemotherapy against bovine babesiosis is an important component in the control of the disease and several drugs and drug combinations have been reported to be effective against the parasite (Kuttler and Aliu, 1984; Silva et al., 2018; Silva et al., 2020; Li et al., 2021; Rizk et al., 2023). Treatment success is dependent on early diagnosis, the severity of the disease, the dosage used, the timing of treatment in the course of infection, and the length of time that the drug is present to affect the parasite (Mehlhorn, 2016). Large morphological Babesia species, such as B. bigemina, were shown to be more susceptible to antiprotozoal drugs than smaller species (such as B. bovis), but no treatment by itself can be relied upon to give a radical cure (Vial and Gorenflot, 2006; Mehlhorn, 2016).
Imidocarb dipropionate (ID) is considered the first-line treatment for bovine babesiosis and remains the most used chemotherapeutic measure worldwide. However, ID is only approved for Babesia treatment in horses (NADA 97-288) and dogs (NADA 141-071), but not for cattle in the US (CVM- FDA, 1997). This drug has a potent babesiacidal effect that consistently clears the parasites from the host (Lewis et al., 1981), and has been used as a prophylactic drug protecting against clinical diseases from 3 to 6 weeks. As the drug level decreases, it allows mild subclinical infection to occur, resulting in premunition and immunity (Kuttler, 1981; Kuttler et al., 1975; Todorovic et al., 1973), a feature that is especially important in endemic areas (Vial and Gorenflot, 2006). Clinical relapses or decreases in the efficacy of ID have been reported (Sears et al., 2020). The mechanism of action of ID is not clearly understood (Todorovic et al., 1973), but two possible modes of action have been proposed, including blocking inositol entry into Babesia-infected erythrocytes resulting in parasite starvation (McHardy et al., 1986), or binding to nuclear DNA in susceptible Babesia species causing nucleic acid damage, inhibiting cellular repair, and replication of the parasites (Baneth, 2018; Rizk et al., 2023).
In addition, ID may have toxic secondary effects in treated animals at therapeutic doses (Todorovic et al., 1973; Adams et al., 1980; Vial and Gorenflot, 2006) and is retained in edible tissues of cattle for prolonged periods after treatment (more than 7 months)(Coldham et al., 1994; Tang et al., 2023). These limitations suggest that, although effective as babesiacidal, ID is not an ideal drug for use in a Babesia control program due to its potential toxicity in animals and its persistence in the foodstuff derived from treated animals, and alternative new medicines are needed.
BPQ is a second-generation hydroxynaphthoquinone antiprotozoal drug originally developed as an anti-malarial compound (Hudson et al., 1985)that has also been studied in vivo and in vitro for the treatment of Theileria species in bovines(Wilkie et al., 1998; Muraguri et al., 2006; Carter, 2011; Bailey, 2013; Goud et al., 2021). BPQ is currently considered the drug of choice in endemic regions against theileriosis in cattle (Ibrahim et al., 2020). However, despite the similarities between Theileria and Babesia (both members of the order piroplasmida), we found only one previous report about the in vitro effect of BPQ on Babesia spp. parasites.
BPQ acts selectively against the parasite’s electron transport system at ubiquinone-cytochrome bc1 complex, leading to competitively inhibiting the parasite’s electron transport respiratory chain but not in the vertebrate host at the parasite inhibitory levels. This mechanism of action also prevents the parasite from taking up pre-formed pyrimidines to relieve the blockade, resulting in a lethal effect (Hudson et al., 1985; Fray and Pudney, 1992; Sharifiyazdi et al., 2012; Mhadhbi et al., 2015).
BPQ is registered in about 20 countries around the world for the treatment of East Coast fever and tropical theileriosis (Carter, 2011), but it is not registered in many others, such as the United States, European Union countries, and Australia, due to the persistence of chemical residues that could impact export markets (Müller et al., 2016), so maximum residue levels (MRL) for the drug have not been established. However, new analytical methods demonstrated in dairy cattle treated at a single time with 2.5 mg/kg of BPQ, that the drug could be detected (LOD; 0.005 mg/kg) in milk for at least 35 days post-treatment and in the liver and injection site to at least 328 days post-treatment (McDougall et al., 2016).Similar to ID, the efficacy of BPQ as an antiparasitic drug could be related to the persistence of the drug in cattle tissues for long periods of time.
Based on these preliminary observations and the fact that Theileria and Babesia are related intra-erythrocytic apicomplexan parasites of cattle, the aim of this study was to study the efficacy of BPQ on its ability to inhibit the in vitro growth of B. bovis, in comparison with ID.
Overall, the data emerging from this study indicates that BPQ is effective in controlling the growth of B. bovis in in vitro cultures, even at lower drug concentrations than ID, and supports further in vivo studies aimed at confirming the potential for the application of BPQ in babesiosis control programs.
2 Materials and methods
2.1 Chemical reagents
BPQ (98% pure) was obtained from Combi-Blocks, Inc. (San Diego, CA, United States). ID (VETRANAL™, Supelco® Buchs, Switzerland) was used as a positive control for the in vitro inhibition assays for B. bovis, using an identical protocol as BPQ described below. Purity of ID was determined to be >98% by proton-nuclear magnetic resonance spectroscopy and High-performance liquid chromatography (HPLC), according to the certificate of analysis.
BPQ and ID were diluted in 100% dimethyl sulfoxide (DMSO) to prepare stock solutions, which were kept at room temperature until use. Working solutions were freshly prepared in a parasite culture medium every test day before being added to the parasite cultures.
2.2 Parasites culture
B. bovis (Texas T2Bo strain: Goff et al., 1998) were grown in long-term microaerophilic stationary-phase cultures and incubated at 37°C in an atmospheric condition of 5% CO2, 5% O2, and 90% N2, as previously described (Levy and Ristic, 1980). B. bovis were cultured in 96-well-culture plates, in 180 μL per well of PL culture media (100 mL = pH 7.2; 29 mL F-12K Nutrient Mixture + L-glutamine Life Technologies, 29 mL Stable Cell IMDM Sigma Aldrich, 2 mL 0.5 M TAPSO Sigma Aldrich, 0.5 mL Antibiotic Antimycotic solution 100X Sigma Aldrich, 1 mM calcium chloride Sigma Aldrich, 100 μL Antioxidant Supplement 1000x Sigma Aldrich, 1 mL Insulin-Transferrin-Selenium-Ethanolamine 100x Sigma Aldrich, 1 mL 50% Glucose Teknova, 500 μL L-glutamine 200 mM GIBCO), supplemented with 40% bovine serum and contained a suspension of 10% cells volume of erythrocytes (RBCs).
2.3 In vitro growth of initial inhibitory assay
The in vitro inhibitory efficacies of BPQ and ID were evaluated against the growth of the T2Bo B. bovis strain, with a starting percentage of parasitized erythrocytes (PPE) of 0.2% and 1%. B. bovis were grown, as described above, in culture media containing different concentrations of ID and BPQ: 10, 25, 50, 100, 150, 200, and 300 nM, diluted in 100% DMSO. Parasites culture in the presence of DMSO (1 μL) and the absence of drug compounds were used as a positive control for parasite growth. Extra wells containing uninfected bovine RBC were prepared and used as negative controls. Parasite cultures were fed daily with fresh culture medium (180 μL/well) containing the respective drug concentration. The experiments were carried out in triplicate for each tested concentration and control, over 96 h (4 days). PPE was monitored daily by counting parasites in Hema 3 Stat Pack (Fisher Scientific) stained thin blood smears (GBS). Before the daily change of the media, the supernatant media (180 μL) of each well was extracted, and the bottom with the RBC layer was homogenized; 1 μL of sample was taken from each well to make a thin smear, and the number of infected red blood cells was counted by visual examination of 5000 erythrocytes in each slide. Morphological appearance was also recorded. Drug responsiveness of the parasites was measured as percentage parasitemia after every 24 h exposure to each concentration of drug.
2.4 Viability after treatment
At 96 h after the last treatment, fresh medium without drug was replaced in all the culture wells and 10 μL of fresh RBCs were added. The same procedure was done for the next 2 days to determine if the culture was viable and could continue growing in the absence of the drug. Quantitative and qualitative parasitemia was determined by microscopic examination of Hema 3 Stat Pack (Fisher Scientific) stained thin blood smears.
2.5 Statistical analysis
Values of parasitemia were compared using independent Student’s t-test. The dose of a drug that produces 50% inhibition (IC50) relative to the control population, and the maximal (100%) inhibitory concentration (IC100) were estimated for BPQ and ID at concentrations ranging from 10 to 300 nM, at 24 h, 48 h, 72 h, and 96 h of incubation. Total inhibitory concentrations (IC100) were calculated as the doses of drug required to inhibit parasite growth to an identical level as found for non-infected erythrocytes (approximately 0.1%). The survival curves PPE 0.2% and 1% treated with imidocarb or buparvaquone were compared using Log-rank (Mantel-Cox) test. The level of significance was set at <0.05. GraphPad Prism ver. 7 software for Windows (Graphpad Software Inc., San Diego, CA, United States) was used for the statistical analysis.
3 Results
3.1 Comparative in vitro growth inhibitory effects of B. bovis by BPQ and ID
Parasite growth in vitro culture up to 96 h of incubation, starting with 0.2% PPE to BPQ and ID, are depicted in Figure 1 and Table 1. While BPQ appears to be non, or poorly effective, at 10 nM, ID is significantly more effective at this low concentration. In contrast, BPQ appears to be more effective than ID at all the other concentrations tested after 48 h in culture (Figure 1). Among all treatments applied, the differences in the median percentages of survival between BPQ and ID were statistically different (Table 1). However, while no parasite survival was found at 50 nM and above for BPQ, this is only the case for ID at 300 nM, suggesting that BPQ has higher efficacy than ID for eliminating the parasite under the experimental conditions tested.
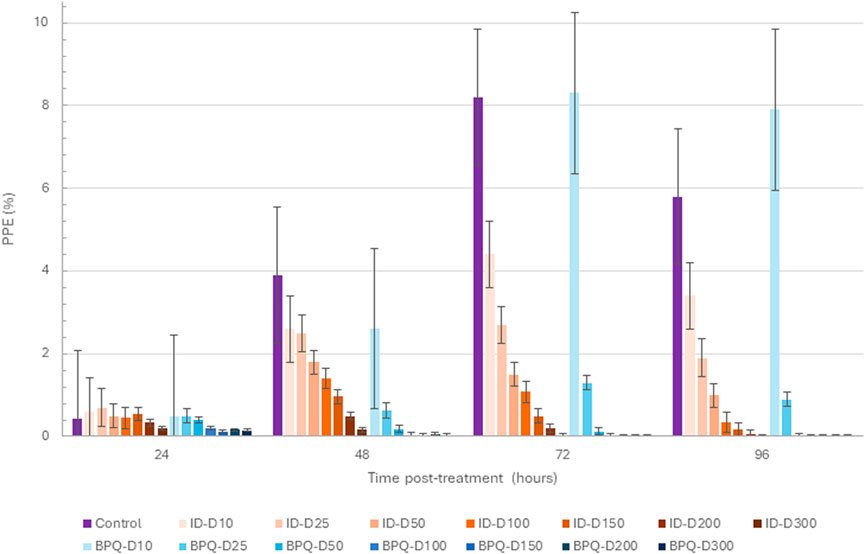
Figure 1. B. bovis T2Bo strain in vitro culture growth (starting PPE 0.2%) at 24, 48, 72, and 96 h in the control group, and after the addition of different concentrations of ID (orange gradients bars) and BPQ (blue gradient bars). Error bars indicate the standard error of the means for each BPQ and ID concentration (nM) tested.
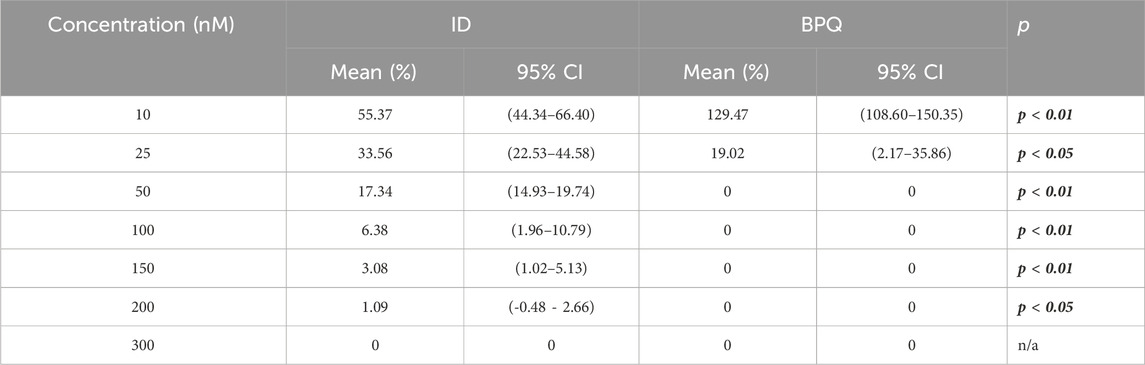
Table 1. Comparative survival (%) of in vitro B. bovis Texas strain culture at 0.2% PPE in the presence of different concentrations of buparvaquone and imidocarb at 96 h post-treatment.
The effect of ID and BPQ on parasite growth in cultures starting at 1% PPE are shown in Figure 2. The growth rate in ID-treated cultures gradually and consistently decreased over time in a dose-dependent manner. In contrast, parasites treated with 10 nM and 25 nM BPQ seem to grow at rates similar to those of the control non-treated parasites. However, the growth rate of the parasite decreases sharply at concentrations higher than 50 nM of BPQ. The parasitemia data presented in Table 2 consistently showed no significant differences in survival rate for parasites exposed to 10 or 25 nM BPQ, but significant differences at BPQ concentrations higher than 25 nM. Importantly, under these experimental conditions, no parasites were found at BPQ concentrations of 150 nM and above (Table 2). In contrast, full elimination of the parasite with ID was not achieved at any of the conditions tested herein.
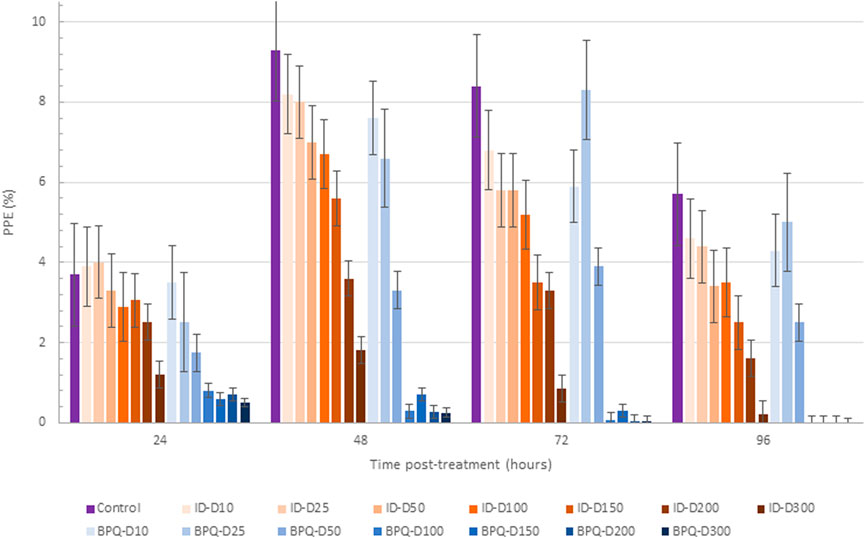
Figure 2. Babesia bovis T2Bo strain in vitro culture growth (starting PPE 1%) at 24, 48, 72, and 96 h in the control group, and after the addition of different concentrations (nM) of ID (ID: blue gradients bars) and BPQ (BPQ: orange gradient bars).
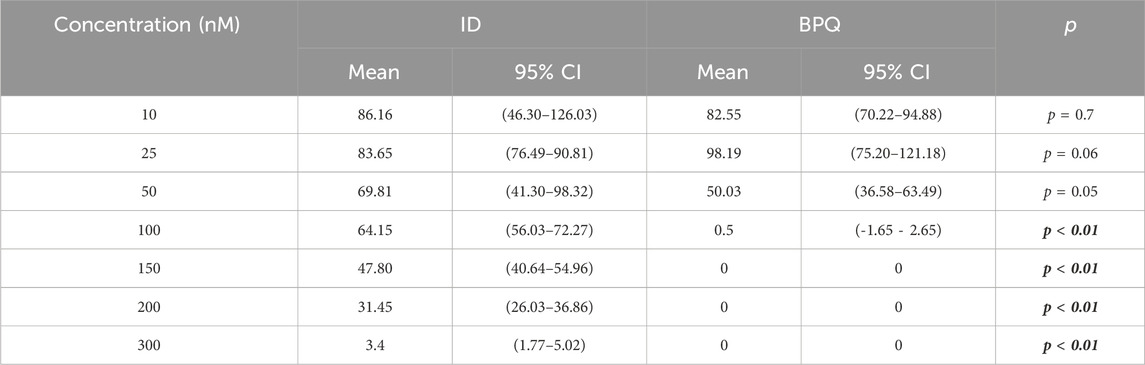
Table 2. Comparative survival of in vitro B. bovis Texas strain culture at 1% PPE in the presence of different concentrations of BPQ and ID at day four post-treatment.
Overall, in all cases, the final PPE across the range of drug concentrations tested reflected a dose and time-dependent effect for both drugs tested in these experiments. Also, BPQ appears to be more efficient in rendering non-viable parasites regardless of the initial PPE used in the cultures.
3.2 Drugs potency
The mean and range IC50 were calculated to compare drug potency on the growth of in vitro B. bovis T2Bo strain cultures. At 1% PPE, means and ranges IC50 were 50.01 (48.75–50.81) and 117.3 (77.35–140.2) for BPQ and ID, respectively. At 1%, BPQ showed statistically higher potency than ID (p < 0.05). At 0.2% PPE, it was not possible to fit a model to estimate the IC50 for BPQ, given the extraordinary lethality of BPQ under these experimental conditions; meanwhile, for ID, the mean and range IC50 was 12.99 nM (12.50–13.46), respectively.
3.3 Time of survival under treatment
At 0.2% PPE, BPQ was 100% lethal on the 4th day (96 h) in cultures treated with 50 nM, and at 3.5 days in culture media treated with concentrations between 100 and 300 nM (Table 1). These results were statistically different (p < 0.05) compared to ID (p < 0.01), which only demonstrated 100% lethality at the higher concentration of 300 nM at 96 h post-treatment (Table 1). Similar results were observed at 1% PPE, where BPQ was 100% lethal at 4 days in culture media treated with concentrations between 100 and 300 nM, and this was statistically different than media treated with ID (p < 0.01), in which 100% lethality could not be demonstrated, even with concentration doses of 300 nM (Table 2).
3.4 Parasite recrudescence after treatment
Considering 0.2% starting parasitemia treated previously with doses of 50 nM of BPQ and ID, parasites were no longer viable or capable of growth after 2 days of culture without drugs (Table 1). At 1% starting parasitemia, no parasite relapse was found in treatments at doses of 150 nM of BPQ and 300 nM of ID (Table 2).
Parasites treated with 10–300 nM have noticeable morphological differences compared to the non-treated control. Single trophozoite stages were bigger and rounded in shape, and paired pear-shaped stages appeared enlarged or amorphous. The remnants of parasites within the red blood cells appeared as small pycnotic dots (Figure 3).
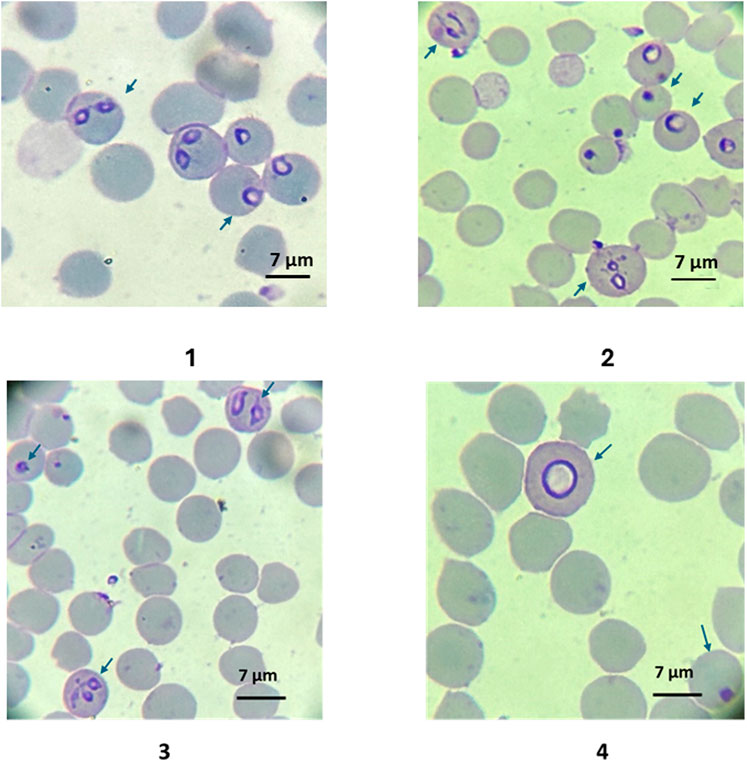
Figure 3. 1: Typical B. bovis blood stage morphologies (pear shaped merozoites and rounded trophozoites) of in vitro cultured parasites in control group at day 3; 2: Morphologies of B. bovis blood stage parasites (amorphous, dotted, and enlarged round forms) in cultures treated with ID 200 nM at day 3. Morphologic characteristics of in vitro cultured B. bovis treated with BPQ 200 nM on day 2 of culture. Dot-shaped parasites and amorphous pear-shaped stages within the red blood cells. 4. Detailed image of a large round shaped intraerythrocytic parasite. Slides stained with Hema 3 stain (100X). The arrows show the infected red blood cells.
4 Discussion
A main challenge for the chemotherapeutic control of bovine babesiosis is the limited number of drugs that are effective against Babesia parasites, with most of them not registered in many countries or approved for specific implementation by the national regulatory agencies (Azhar et al., 2023). In addition, several reports show variable efficacy to ID or clinical relapses against certain species of Babesia (Pudney and Gray, 1997; Kumar et al., 2003; Checa et al., 2017). This poses a serious problem, considering that ID is considered as the drug-of-choice against bovine babesiosis. Therefore, further studies should be conducted to identify new compounds with effective babesiacidal properties. We pose hereby that buparvaquone (BPQ), a theilericidal drug, is a viable Babesiacidal that can be an alternative to ID. Importantly, BPQ was previously shown to be effective against B. bovis and B. bigemina in in vitro testing and nontoxic for vertebrates (McHardy, 1990; McHardy et al., 2010; Müller and Hemphill, 2016; Checa et al., 2017; Isik et al., 2018; Rufener et al., 2018) at the treatment doses used, environmentally safe, and already approved for treatment of bovine theileriosis in several countries.
In this study, we compared the efficacy of ID and BPQ using in vitro B. bovis culture. These are the second in vitro results reported on the effects of BPQ against B. bovis (Nugraha et al., 2019). ID is relatively effective to treat cattle babesiosis, but it is far from an ideal drug due to the aforementioned severe side effects in cattle (Todorovic et al., 1973; Adams et al., 1980; Vial and Gorenflot, 2006). In addition, ID leaves undesirable long-term residues in food (Coldham et al., 1994; Tang et al., 2023). Even more, although ID drug resistance has not been officially reported yet in B. bovis, it is likely that resistance will develop over time. On the other hand, BPQ has been approved for use in cattle against Theileria parasites in several countries and has a better safety and tolerance record compared to ID in cattle and other animal species (McHardy, 1990; Kumar et al., 2003; Müller et al., 2016; Checa et al., 2017; Isik et al., 2018; Tahir et al., 2020), making this drug, if effective, a viable therapeutic option for treating B. bovis infections.
The efficacy in vitro of ID and BPQ against B. bovis was also addressed in previous studies. The DL50 of ID on B. bovis T2Bo was found hereby to be between 45,55 nM–50 nM (around 1.59 × 10−5 mg/mL to 1.74 × 10−5 mg/mL), at 48–96 h post-treatment respectively. These values were lower than those reported by Brasseur et al. (1998) on B. divergens (human and bovine isolates in vitro cultures), who reported a DL50 of 77 (2.7 × 10−5 mg/mL) and 97 nM (3.4 × 10-5 mg/mL), respectively (Brasseur et al., 1998), using a tritiated hypoxanthine incorporation assay to evaluate the PPE at 48 h post-treatment. Although the IC50 values obtained with the tritiated hypoxanthine incorporation assay were higher than those obtained by microscopic examination, these differences were found not to be statistically significative.
Also, the effect of Imidocarb was also tested in other Babesia-related species. A study performed by Hines, in Theileria equi, with a starting PPE of approximately 0.5% and using flow cytometry to evaluate parasite infection of erythrocytes, reported and IC50 of 24 nM, which is higher than the IC50 values for ID found for B. bovis in the current study (13 nM). Interestingly, in the same study, complete growth inhibition was achieved when the PPE remained at the starting level of 0.3%–0.5% after 72 h, indicating no active parasite growth. In addition, Hines et al. did not determine the IC90 at 72 h post-treatment because, even at the maximum drug concentration (775 nM), the PPE still reached 1.2%, reflecting a lack of complete growth inhibition for this T. equi strain (Hines et al., 2015). In our B. bovis study, the parasites were no longer viable or capable to growth after 2 days of culture without ID upon treatment at 300 nM for 96 h (4 days)
On the other hand, we demonstrated hereby that B. bovis T2B strain parasites were not able to growth after 96 h of culture, with the addition of 100 nM, 150 nM, 200 nM, and 300 nM of BPQ, regardless of their initial PPE. However, at 0.2% initial parasitemia, 100% lethality was seen with BPQ at lower doses (50 nM). Importantly, no surviving parasites were detected after 2 days of culture without the drug in cultures derived from the wells treated previously with BPQ from concentrations 50 nM at 0.2% PPE, and from 150 nM at 1% PPE. Interestingly, a previous report on the effect of BPQ on B. bovis (Nugraha et al., 2019), reported IC50s for BPQ of 135+/−41 nM after 96 h of B. bovis culture at 1% starting PEE and 488+/−30 nM for B. bigemina; their reported IC50 for B. bovis was twice the IC50 obtained in our study (IC50: 50.01 (48.75–50.81) (Nugraha et al., 2019). However, the Nugraha et al. study uses distinct experimental conditions (including the method for parasite quantification), and B. bovis strain, precluding thorough comparisons with the current study.
Overall, the findings in this study suggest that BPQ represents a promising candidate as a new babesiacidal agent. This is supported by several lines of evidence in our study: 1] At 0.2% and 1% PPE, BPQ was 100% cidal at 4 days (96 hs) in cultures treated with concentrations between 100 and 300 nM; 2] No parasite growth was found after 2 days of incubation without replacing the drug during daily media changes at doses 150 nM; 3] The performance of BPQ was superior to ID, which could not attain 100% lethality even at 300 nM after 4 days of treatment; and 4] At higher PPE (1%), BPQ potency (IC50) 50.01 (ranging from 48.75 to 50.81) was about twice the potency of ID 117.3 (ranging between 77.35–140.2). Furthermore, regardless of the potency comparison of drugs, the calculated IC50 for BPQ against B. bovis was two times lower than ID, and much lower than the values previously reported for ID (Rodriguez and Trees, 1996) and also BPQ (Nugraha et al., 2019). A residual effect against parasites was seen after treatment with BPQ and ID. Post-antibiotic effect (PAE) is described for some antimicrobial agents, as the suppression of bacterial growth following a limited period of exposure to an antibiotic and removal of the compound (Proma et al., 2020), and it has been proposed as an explanation for the success of intermittent dosing regimens. Depending on the drug concentration and susceptibility of the target organisms, PAE may last for several hours post-administration (Zhanel et al., 1991). Applying this concept to apicomplexan parasites, these observations suggest that both BPQ and ID can have a persistent effect comparable to the PAE described for some antibiotics. The exact reason explaining this finding remains to be determined. It is possible to speculate that the extent of the damage or metabolic change precludes parasite recovery after 4 days of exposure to the drug. The clinical impact of this observation also deserves further investigation. In this study, we found that the inhibitory effect of both drugs was time and dose-dependent since the PPE decreased upon longer exposures, and no growth of parasites was observed with increased concentrations of drugs. Dose and time-dependence drug results agree with previous studies on B. bovis testing endochin-like quinolones, tulathromycin drugs (Silva et al., 2018; Silva et al., 2020), and artemisinin (Mazuz et al., 2013). In contrast, the results were independent of the starting PPE in both previous studies. A dose-dependent effect was observed in Theileria exposed to ID (Hines et al., 2015).
However, although studying the potency of drugs using an in vitro approach may be a rationale predictor of their therapeutic potential in vivo, the approach has several limitations, including differences among laboratories and parasite strains (Alzan et al., 2022). Many other host-related factors, such as immune response, drug metabolism and pharmacokinetic behavior, do not play a role in in vitro settings (Sim and Kauser, 2016), making in vivo testing a requirement for drug validation.
In summary, the results presented here, through the in vitro B. bovis culture evaluation of Buparvaquone and Imidocab, demonstrate an overall higher efficacy of BPQ at inhibiting B. bovis, than ID. Taking together previous in vivo safety and efficacy studies performed in other apicomplexan parasites that can infect animal species and humans, and the data generated hereby, BPQ emerges as an optimal candidate for in vivo evaluation as an alternative drug for the treatment of bovine babesiosis caused by B. bovis. Future work will address the potential of BPQ as a babesiacidal drug in in vivo studies.
5 Conclusion
The data collected in an in vitro testing model using cultured parasites suggests that BPQ is effective against B. bovis, displaying a residual effect that seems superior to ID, which is currently the first-line drug for treating bovine babesiosis globally. However, the potential of BPQ as a babesiacidal drug remains to be assessed using an in in vivo infected bovine model.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the IACUC University of Idaho #2020-42, bovine and equine bleeding. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
NC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing, Software. PL: Methodology, Writing–review and editing, Supervision. NV: Supervision, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Software. JD: Conceptualization, Supervision, Writing–review and editing, Resources, Validation. MR: Conceptualization, Supervision, Validation, Writing–review and editing. RB: Supervision, Validation, Writing–review and editing. JL: Supervision, Writing–review and editing. MU: Supervision, Writing–review and editing. CS: Supervision, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported the United States Department of Agriculture (ARS-USDA CRIS 2090- 32000-040-000-D), This project was also supported with funds from the United States Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Program Award number i01 BX003312 (MR). MR is a recipient of a VA Research Career Scientist Award (14S-RCS001). Research reported in this publication was also supported by the US National Institutes of Health under award numbers R01AI100569 and R01AI141412 (MR) and by the U.S. Department of Defense Peer Reviewed Medical Research Program (PR181134) (MR). This work was also funded by VA Merit Review Award BX004522 to JD from the US Department of Veterans Affairs Biomedical Laboratory Research and Development.
Acknowledgments
NC is a fellow of the Research Participation Program administered by the Oak Ridge Institute for Science and Technology (ORISE).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, L., Corrier, D., and Williams, J. (1980). A study of the toxicity of imidocarb dipropionate in cattle. Res. Vet. Sci. 28, 172–177. doi:10.1016/s0034-5288(18)32741-3
Almazan, C., Tipacamu, G. A., Rodriguez, S., Mosqueda, J., and Perez De Leon, A. (2018). Immunological control of ticks and tick-borne diseases that impact cattle health and production. Front. Biosci. 23, 1535–1551. doi:10.2741/4659
Alvarez, J. A., Rojas, C., and Figueroa, J. V. (2020). An overview of current knowledge on in vitro Babesia cultivation for production of live attenuated vaccines for bovine babesiosis in Mexico. Front. Vet. Sci. 7 (364), 364–412. doi:10.3389/fvets.2020.00364
Alzan, H. F., Bastos, R. G., Laughery, J. M., Scoles, G. A., Ueti, M. W., Johnson, W. C., et al. (2022). A culture-adapted strain of Babesia bovis has reduced subpopulation complexity and is unable to complete its natural life cycle in ticks. Front. Cell Infect. Microbiol. 12, 827347. doi:10.3389/fcimb.2022.827347
Asrar, R., Farhan, H. R., Sultan, D., Ahmad, M., Hassan, S., Kalim, F., et al. (2022). Review Article Bovine Babesiosis; Review on its global prevalence and anticipatory control for one health. Cont. Vet. J. 2, 42–49.
Azhar, M., Gadahi, J. A., Bhutto, B., Tunio, S., Vistro, W. A., Tunio, H., et al. (2023). Babesiosis: current status and future perspectives in Pakistan and chemotherapy used in livestock and pet animals. Heliyon 9, E17172. doi:10.1016/j.heliyon.2023.e17172
Bailey, G. (2013). Buparvaquone tissue residue study. Final Report. Australia: NSW Department of Primary Industries, 1–152.
Bock, R., Jackson, L., De Vos, A., and Jorgensen, W. (2004). Babesiosis of cattle. Parasitology 129, S247–S269. doi:10.1017/S0031182004005190
Böse, R., Jorgensen, W. K., Dalgliesh, R. J., Friedhoff, K. T., and de Vos, A. J. (1995). Current state and future trends in the diagnosis of babesiosis. Vet. Parasitol. 57, 61–74. doi:10.1016/0304-4017(94)03111-9
Brasseur, P., Lecoublet, S., Kapel, N., Favennec, L., and Ballet, J. J. (1998). In vitro evaluation of drug susceptibilities of Babesia divergens isolates. Antimicrob. Agents Chemother. 42, 818–820. doi:10.1128/AAC.42.4.818
Carter, P. (2011). “Animal Health Assessment of the efficacy of Buparvaquone for the treatment of “benign” bovine theileriosis,” in Tick fever centre department of employment, economic development and innovation, 1–12.
Checa, R., Montoya, A., Ortega, N., González-Fraga, J. L., Bartolomé, A., Gálvez, R., et al. (2017). Efficacy, safety and tolerance of imidocarb dipropionate versus atovaquone or buparvaquone plus azithromycin used to treat sick dogs naturally infected with the Babesia microti-like piroplasm. Parasit. Vectors 10, 145–214. doi:10.1186/S13071-017-2049-0
Coldham, N. G., Moore, A. S., Sivapathasundaram, S., and Sauer, M. J. (1994). Imidocarb depletion from cattle liver and mechanism of retention in isolated bovine hepatocytes. Analyst 119, 2549–2552. doi:10.1039/an9941902549
Fray, M., and Pudney, M. (1992). Site of action of the antimalarial hydroxynaphthoquinone,2- [trans-4-(40chlorophenyl)cyclohexyl]-3-hydroxy-1,4,-naphthoquinone (566c80). Biochem. Pharmacol. 40, 914–919. doi:10.1016/0006-2952(92)90213-3
Goud, S. K., Vijayakumar, K., Davis, J., Tresamol, P. V., Ravishankar, C., and Devada, K. (2021). Efficacy of different treatment regimens against Oriental Theileriosis in naturally infected cattle. Indian J. Vet. Med. 40, 14–19.
Hines, S. A., Ramsay, J. D., Kappmeyer, L. S., Lau, A. O. T., Ojo, K. K., Van Voorhis, W. C., et al. (2015). Theileria equi isolates vary in susceptibility to imidocarb dipropionate but demonstrate uniform in vitro susceptibility to a bumped kinase inhibitor. Parasit. Vectors 8, 33–11. doi:10.1186/s13071-014-0611-6
Hudson, A. T., Randall, A. W., Fry, M., Ginger, C. D., Hill, B., Latter, V. S., et al. (1985). Novel anti-malarial hydroxynaphthoquinones with potent broad spectrum anti-protozoal activity. Parasitology 90, 45–55. doi:10.1017/s0031182000049003
Isik, N., Derinbay Ekici, O., Ilhan, C., and Coskun, D. (2018). Safety of antitheilerial drug buparvaquone in rams. Acta Sci. Veterinariae 46, 1547. doi:10.22456/1679-9216.86688
Jacob, S. S., Sengupta, P. P., Paramanandham, K., Suresh, K. P., Chamuah, J. K., Rudramurthy, G. R., et al. (2020). Bovine babesiosis: an insight into the global perspective on the disease distribution by systematic review and meta-analysis. Vet. Parasitol. 283, 109136. doi:10.1016/J.VETPAR.2020.109136
Kakoma, I., and Ristic, M. (1984). “Pathogenesis of babesiosis,” in Malaria and babesiosis. Editors M. Ristic, P. Ambroise-Thomas, and J. P. Kreier (New York, NY, USA: Academic Press), 99–118. doi:10.1201/9781351070027
Kumar, S., Gupta, A. K., Pal, Y., and Dwivedi, S. K. (2003). In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. J. Vet. Med. Sci. 65, 1171–1177. doi:10.1292/jvms.65.1171
Kuttler, K. L., and Aliu, Y. O. (1984). “Chemotherapy of babesiosis,” in Malaria and babesiosis. Editors M. Ristic, P. Ambroise-Thomas, and J. P. Kreier (New York, NY, USA: Academic Press), 151–172. doi:10.1007/978-94-009-6042-8_11
Levy, M. G., and Ristic, M. (1980). Babesia bovis: continuous cultivation in a microaerophilous stationary phase culture. Science 207, 1218–1220. doi:10.1126/science.7355284
Li, Y., Rizk, M. A., Galon, E. M., Liu, M., Li, J., Ringo, A. E., et al. (2021). Discovering the potent inhibitors against Babesia bovis in vitro and Babesia microti in vivo by repurposing the natural product compounds. Front. Vet. Sci. 8, 762107. doi:10.3389/fvets.2021.762107
Mazuz, M. L., Golenser, J., Fish, L., Haynes, R. K., Wollkomirsky, R., Leibovich, B., et al. (2013). Artemisone inhibits in vitro and in vivo propagation of Babesia bovis and B. bigemina parasites. Exp. Parasitol. 135, 690–694. doi:10.1016/j.exppara.2013.10.006
McCosker, P. J. (1981). “The global importance of babesiosis,” in Babesiosis. Editors M. Ristic, and J. P. Kreier (New York, NY, USA: Academic Press), 1–24.
McDougall, S., Hillerton, J. E., and Pegram, D. (2016). Concentrations of buparvaquone in milk and tissue of dairy cows. N. Z. Vet. J. 64, 318–323. doi:10.1080/00480169.2016.1204960
McHardy, N. (1990). “Butalex (buparvaquone): a new therapeutic for theilerosis,” in Recent developments on Theileria annulata. Workshop held at ilrad nairobi, Kenya (Nairobi, Kenya: ILRAD), 59–66.
McHardy, N., Smith, R., and Brady, P. D. (2010). Use of buparvaquone for controlling mites parasitising honeybees. Eur. Pat. Specif., 2–23. doi:10.1016/j.vetpar.2006.02.024
Mehlhorn, H. (2016). “Babesiacidal drugs,” in Encyclopedia of parasitology (Berlin, Heidelberg: Springer), 1–11. doi:10.1007/978-3-642-27769-6_338-2
Mhadhbi, M., Chaouch, M., Ajroud, K., Darghouth, M. A., and BenAbderrazak, S. (2015). Sequence polymorphism of cytochrome b gene in Theileria annulata Tunisian isolates and its association with buparvaquone treatment failure. PLoS One 10, 01296788–e129711. doi:10.1371/JOURNAL.PONE.0129678
Müller, J., Aguado-Martínez, A., Manser, V., Wong, H. N., Haynes, R. K., and Hemphill, A. (2016). Repurposing of antiparasitic drugs: the hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Vet. Res. 47, 32–38. doi:10.1186/s13567-016-0317-1
Müller, J., and Hemphill, A. (2016). Drug target identification in protozoan parasites. Expert Opin. Drug Discov. 11, 815–824. doi:10.1080/17460441.2016.1195945
Muraguri, G. R., Ngumi, P. N., Wesonga, D., Ndungu, S. G., Wanjohi, J. M., Bang, K., et al. (2006). Clinical efficacy and plasma concentrations of two formulations of buparvaquone in cattle infected with East Coast fever (Theileria parva infection). Res. Vet. Sci. 81, 119–126. doi:10.1016/j.rvsc.2005.09.012
Néo, T. A., Giglioti, R., Obregón, D., Bilhassi, T. B., Oliveira, H. N., Machado, R. Z., et al. (2016). Detection of <i&gt;Babesia bovis&lt;/i&gt; and <i&gt;Babesia bigemina&lt;/i&gt; in Water Buffaloes (<i&gt;Bubalus bubalis&lt;/i&gt;) in Endemic Areas of S&#227;o Paulo State, Brazil. Open J. Vet. Med. 6, 75–84. doi:10.4236/ojvm.2016.65009
Nugraha, A. B., Tuvshintulga, B., Guswanto, A., Tayebwa, D. S., Rizk, M. A., Gantuya, S., et al. (2019). Screening the medicines for malaria venture pathogen box against piroplasm parasites. Int. J. Parasitol. Drugs Drug Resist 10, 84–90. doi:10.1016/j.ijpddr.2019.06.004
Pérez de León, A. A., Strickman, D. A., Knowles, D. P., Fish, D., Thacker, E., de la Fuente, J., et al. (2010). One Health approach to identify research needs in bovine and human babesioses: workshop report. Parasit. Vectors 3, 36. doi:10.1186/1756-3305-3-36
Pérez de Leon, A. A., Teel, P. D., Auclair, A. N., Messenger, M. T., Guerrero, F. D., Schuster, G., et al. (2012). Integrated strategy for sustainable cattle fever tick eradication in USA is required to mitigate the impact of global change. Front. Physiol. 3, 195–217. doi:10.3389/fphys.2012.00195
Pipano, E., and Hadani, A. (1984). “Control of bovine babesiosis,” in Malaria and babesiosis. Editors M. Ristic, P. Ambroise-Thomas, and J. P. Kreier (New York, NY, USA: Academic Press), 263–303. doi:10.1007/978-94-009-6042-8_15
Proma, F. H., Shourav, M. K., and Choi, J. (2020). Post-antibiotic effect of ampicillin and levofloxacin to Escherichia coli and Staphylococcus aureus based on microscopic imaging analysis. Antibiotics 9, 458–513. doi:10.3390/antibiotics9080458
Pudney, M., and Gray, J. S. (1997). Therapeutic efficacy of atovaquone against the bovine intraerythrocytic parasite, Babesia divergens. J. Parasitol. 83, 307–310. doi:10.2307/3284461
Rizk, M. A., El-Sayed, S. A. E.-S., and Igarashi, I. (2023). Diminazene aceturate and imidocarb dipropionate-based combination therapy for babesiosis – a new paradigm. Ticks Tick. Borne Dis. 14, 102145. doi:10.1016/J.TTBDIS.2023.102145
Rodriguez, R. I., and Trees, A. J. (1996). In vitro responsiveness of Babesia bovis to imidocarb dipropionate and the selection of a drug-adapted line. Vet. Parasitol. 62, 35–41. doi:10.1016/0304-4017(95)00850-0
Rufener, R., Dick, L., D’Ascoli, L., Ritler, D., Hizem, A., Wells, T. N. C., et al. (2018). Repurposing of an old drug: in vitro and in vivo efficacies of buparvaquone against Echinococcus multilocularis. Int. J. Parasitol. Drugs Drug Resist 8, 440–450. doi:10.1016/j.ijpddr.2018.10.011
Sears, K., Knowles, D., Dinkel, K., Mshelia, P. W., Onzere, C., Silva, M., et al. (2020). Imidocarb dipropionate lacks efficacy against Theileria haneyi and fails to consistently clear Theileria equi in horses co-infected with T. haneyi. Pathogens 9, 1035–1112. doi:10.3390/pathogens9121035
Sharifiyazdi, H., Namazi, F., Oryan, A., Shahriari, R., and Razavi, M. (2012). Point mutations in the Theileria annulata cytochrome b gene is associated with buparvaquone treatment failure. Vet. Parasitol. 187, 431–435. doi:10.1016/j.vetpar.2012.01.016
Silva, M. G., Bastos, R. G., Stone Doggett, J., Riscoe, M. K., Pou, S., Winter, R., et al. (2020). Endochin-like quinolone-300 and ELQ-316 inhibit Babesia bovis, B. bigemina, B. caballi and Theileria equi. Parasit. Vectors 13, 606–611. doi:10.1186/s13071-020-04487-3
Silva, M. G., Villarino, N. F., Knowles, D. P., and Suarez, C. E. (2018). Assessment of Draxxin® (tulathromycin) as an inhibitor of in vitro growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist 8, 265–270. doi:10.1016/J.IJPDDR.2018.04.004
Sim, D. S., and Kauser, K. (2016). In vivo target validation using biological molecules in drug development. Handb. Exp. Pharmacol. 232, 59–70. doi:10.1007/164_2015_17
Smith, R. D., Evans, D. E., Martins, J. R., Ceresér, V. H., Correa, B. L., Cardozo, C. P. H., et al. (2000). Babesiosis (Babesia bovis) stability in unstable environments. Ann. N. Y. Acad. Sci. 916, 510–520. doi:10.1111/J.1749-6632.2000.TB05330.X
Tang, Y., Yu, N., Liu, C., Han, M., Wang, H., Chen, X., et al. (2023). Residue depletion of imidocarb in bovine tissues by UPLC-MS/MS. Animals 13, 104–113. doi:10.3390/ani13010104
Todorovic, R. A., Viscaino, O. G., Gonzalez, E. F., and Adams, L. G. (1973). Chemoprophylaxis (imidocarb) against Babesia bigemina and Babesia argentina infections. Am. J. Vet. Res. 34, 1153–1161.
Vial, H. J., and Gorenflot, A. (2006). Chemotherapy against babesiosis. Vet. Parasitol. 138, 147–160. doi:10.1016/j.vetpar.2006.01.048
Wilkie, G. M., Kirvar, E., Thomas, E. M., Sparagano, O., and Brown, C. G. D. (1998). Stage-specific activity in vitro on the Theileria infection process of serum from calves treated prophylactically with buparvaquone. Vet. Parasitol. 80, 127–136. doi:10.1016/s0304-4017(98)00204-0
Keywords: Babesia bovis, buparvaquone, imidocarb, in vitro cultured parasites, efficacy
Citation: Cardillo NM, Lacy PA, Villarino NF, Doggett JS, Riscoe MK, Bastos RG, Laughery JM, Ueti MW and Suarez CE (2024) Comparative efficacy of buparvaquone and imidocarb in inhibiting the in vitro growth of Babesia bovis. Front. Pharmacol. 15:1407548. doi: 10.3389/fphar.2024.1407548
Received: 26 March 2024; Accepted: 17 April 2024;
Published: 01 May 2024.
Edited by:
Ali A. Rabaan, Johns Hopkins Aramco Healthcare (JHAH), Saudi ArabiaReviewed by:
Exequiel Oscar Jesus Porta, University College London, United KingdomLuís Adriano Anholeto, Acadia University, Canada
Copyright © 2024 Cardillo, Lacy, Villarino, Doggett, Riscoe, Bastos, Laughery, Ueti and Suarez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlos E. Suarez, c3VhcmV6QHdzdS5lZHUsIA==Y2FybG9zLnN1YXJlekB1c2RhLmdvdg==; Natalia M. Cardillo, bmF0YWxpYS5jYXJkaWxsb0B3c3UuZWR1
 Natalia M. Cardillo
Natalia M. Cardillo Paul A. Lacy1
Paul A. Lacy1 Nicolas F. Villarino
Nicolas F. Villarino J. Stone Doggett
J. Stone Doggett Michael K. Riscoe
Michael K. Riscoe Reginaldo G. Bastos
Reginaldo G. Bastos Jacob M. Laughery
Jacob M. Laughery Massaro W. Ueti
Massaro W. Ueti Carlos E. Suarez
Carlos E. Suarez