
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 18 June 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1406619
This article is part of the Research TopicPhytoconstituents as Adjuvant of Modern Synthetic Drugs: Therapeutic Opportunities and Challenges.View all 5 articles
 Deena Elsori1†
Deena Elsori1† Pratibha Pandey2†
Pratibha Pandey2† Seema Ramniwas3
Seema Ramniwas3 Rahul Kumar4
Rahul Kumar4 Sorabh Lakhanpal5
Sorabh Lakhanpal5 Safia Obaidur Rab6
Safia Obaidur Rab6 Samra Siddiqui7
Samra Siddiqui7 Ajay Singh8
Ajay Singh8 Mohd Saeed9*
Mohd Saeed9* Fahad Khan10*
Fahad Khan10*The bioactive compounds present in citrus fruits are gaining broader acceptance in oncology. Numerous studies have deciphered naringenin’s antioxidant and anticancer potential in human and animal studies. Naringenin (NGE) potentially suppresses cancer progression, thereby improving the health of cancer patients. The pleiotropic anticancer properties of naringenin include inhibition of the synthesis of growth factors and cytokines, inhibition of the cell cycle, and modification of several cellular signaling pathways. As an herbal remedy, naringenin has significant pharmacological properties, such as anti-inflammatory, antioxidant, neuroprotective, hepatoprotective, and anti-cancer activities. The inactivation of carcinogens following treatment with pure naringenin, naringenin-loaded nanoparticles, and naringenin combined with anti-cancer agents was demonstrated by data in vitro and in vivo studies. These studies included colon cancer, lung neoplasms, breast cancer, leukemia and lymphoma, pancreatic cancer, prostate tumors, oral squamous cell carcinoma, liver cancer, brain tumors, skin cancer, cervical and ovarian cancers, bladder neoplasms, gastric cancer, and osteosarcoma. The effects of naringenin on processes related to inflammation, apoptosis, proliferation, angiogenesis, metastasis, and invasion in breast cancer are covered in this narrative review, along with its potential to develop novel and secure anticancer medications.
Cancer is a serious and frequently fatal disease and one of the leading causes of death globally. It is a complex pathological condition that can be induced by oxidative stress, genetic mutations, uncontrolled cell proliferation due to defects in the cell cycle or apoptosis, exposure to harmful radiation, and pollution. Unfortunately, established anticancer treatments, such as chemotherapy and radiation therapy, pose severe side effects in patients with cancer. Lifestyle is one of the many factors affecting cancer incidence (Bakar et al., 2014; Martincorena and Campbell, 2015; Bhat et al., 2017; Hayes et al., 2020). Research indicates that diets high in plant-based and bioactive substances can reduce the incidence and spread of cancer. Novel medications and treatment technologies are currently being developed through creative research on cancer treatments (Roy et al., 2022). Bioactive compounds originating from plants have been the subject of an increasing number of recent studies aimed at discovering new therapeutic agents from natural products (Connor and Lee, 2010).
Increased fruit and berry intake, particularly citrus fruit intake, may help prevent cancer and slow its spread (Wallace et al., 2020; Akbari et al., 2022). Fruits contain polyphenolic flavonoid components, which are the primary active ingredients (Lima et al., 2014). Flavonoids have been shown to exhibit potent anticancer properties through their role as antioxidants; modulation of ROS-scavenging enzyme activity; upregulation of cell cycle arrest, autophagy, and apoptosis; and downregulation of inflammation, proliferation processes, and metastasis formation (Wei et al., 2018; Majidinia et al., 2019; Hazafa et al., 2020; Sun et al., 2023). Naturally occurring citrus flavonoid naringenin (NGE) possesses a wide range of pharmacological properties, including anti-inflammatory, antioxidant, anti-ulcer, anti-apoptotic, and anti-carcinogenic effects (Joshi et al., 2018; Wojnar et al., 2018; Bakar et al., 2019; Ucar and Goktas, 2023). Additionally, naringenin has been reported to increase cell apoptosis and growth arrest of tumor cells, including those that cause cervical, bladder, prostate, and breast cancers (Shi et al., 2021). To our best, very limited reviews have summarized the anticancer potential of naringenin against breast cancer, therefore, our research has been directed towards understanding the processes by which naringenin, either alone or in conjunction with other therapeutic drugs, influences the advancement of cancer. Additionally, we presented various nanoformulations that are employed for the regulated distribution of naringenin in malignancies.
We have included a literature review (approximately more than 500 manuscripts) of data from 2010 to 2024 from various sources including Google Scholar (https://scholar.google.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), and NCBI (https://www.ncbi.nlm.nih.gov/). We have also used in silico pathway databases and software, including PUBCHEM (https://pubchem.ncbi.nlm.nih.gov/) and SWISS-ADME (http://www.swissadme.ch/), to analyze the pharmacokinetic potential of naringenin as well as its crucial targets against several carcinomas.
Power and Tutin (1907) discovered naringenin (2, 3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one) as a flavanone (Zaidun et al., 2018). Dimethyl sulfoxide and ethanol are two chemical solvents that can dissolve naringenin, a hydrophobic molecule having a molecular weight of 272.25 g/mol (C15H12O5). It is a byproduct of the hydrolysis of naringenin or narirutin and is mostly found as an aglycone; however, it can also occur in glycosylated and neohesperidoside forms (Table 1). Oranges, tomatoes, lemons, and grapefruits are the primary producers of naringenin (Kiran et al., 2017). Naringenin is primarily transformed into its aglycone form, naringin, in the human body because of its limited absorption through the gastrointestinal system. Flavonoids are a 15-carbon skeleton, having two benzene rings connected by a three-carbon linking chain. Naringenin was more effective than naringin because of the steric barrier the two rhamnose units provided. The bitter flavor of grapefruit juice is attributed to NGE 7-O-neohesperidoside (Stabrauskiene et al., 2022).
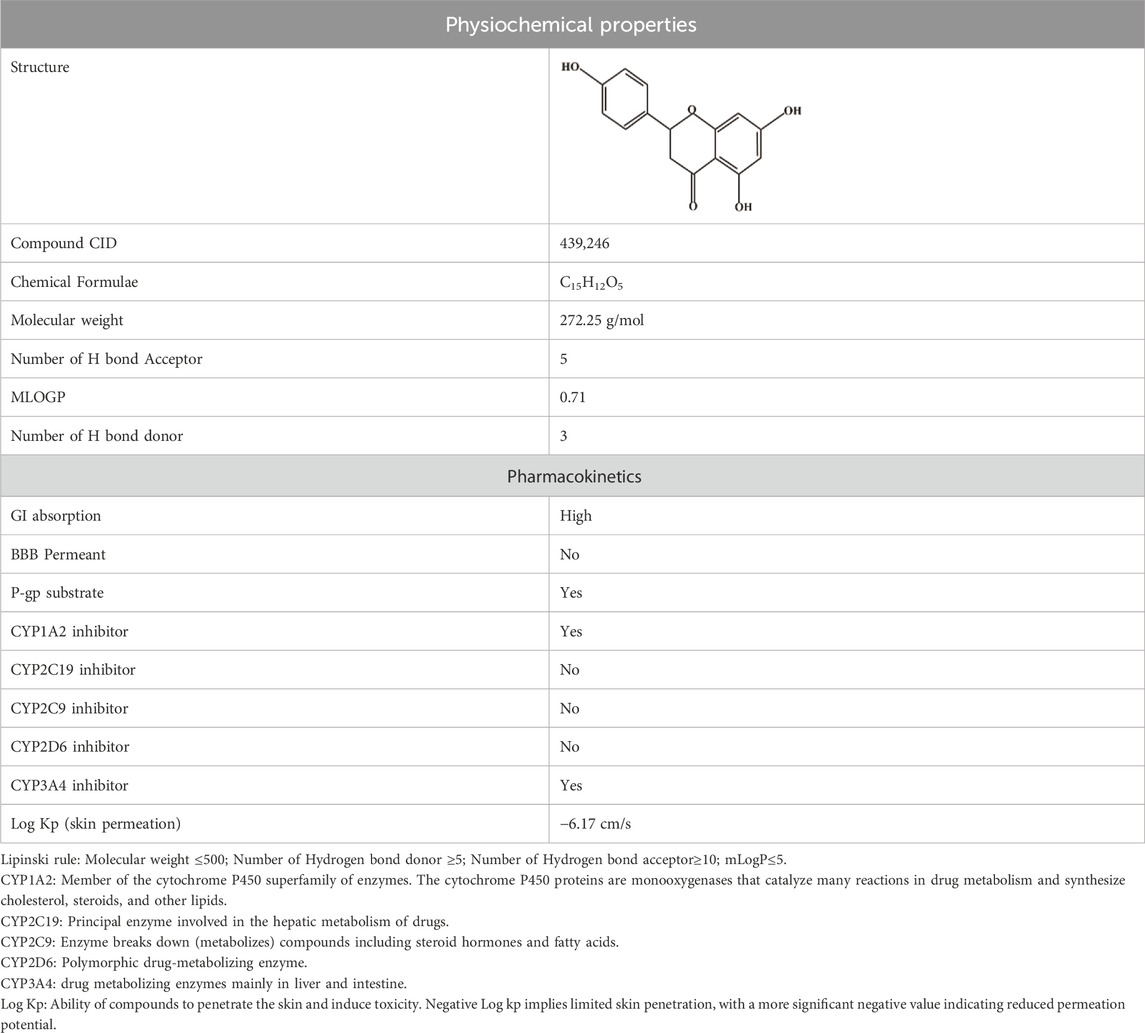
Table 1. Physicochemical descriptors and pharmacokinetic properties of naringenin (Source: PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/439246).
It is becoming more widely known that gut bacteria can further increase the bioavailability of flavonoids by metabolizing them into aromatic and phenolic ring fission catabolites. This metabolic pathway of naringenin biosynthesis consists of six sequential steps, each catalyzed by specific enzymes: chalcone synthase (the primary enzyme for naringenin synthesis), phenylalanine ammonia lyase (PAL), para-coumarate-CoA ligase, cinnamate 4-hydroxylase and its associated cytochrome P450 reductase, and chalcone isomerase. Naringenin is formed by the combination of para-coumaroyl-CoA and three malonyl-CoA units. Furthermore, the initial component for naringenin production is para-coumaroyl-CoA, obtained from phenylalanine through PAL deamination in dicotyledonous plants. The second compound is hydroxylated at carbon 4 by an enzyme called cinnamate-4-hydroxylase. It is then activated by a ligase that depends on CoA. This process occurs through the phenylpropanoid pathway, a common pathway for producing flavonoids and stilbenes. In addition, monocotyledonous plants can utilize tyrosine as a substrate to directly produce p-coumaric acid without requiring the activity of the cinnamate-4-hydroxylase enzyme (Salehi et al., 2019). Absorbed naringenin travels across the lumen of the small intestine and into the colon, where it is broken down into metabolites produced by gut microbiota, including phloroglucinol and HPPA (Ucar and Goktas, 2023).
Remarkably, phloroglucinol and HPPA were also detected in gastric samples, indicating the existence of microbes in the stomach that can metabolize naringenin. Naringenin-7-O-sulfate, free naringenin, naringenin-4-O glucuronide, Aperol, and flavone-derived alcohol (flavan-4-ol) are among the main metabolites identified in the liver, the primary organ for lipid and cholesterol metabolism (Memariani et al., 2021). Interestingly, different tissues had distinct dominant metabolites. For example, the major forms of naringenin glucuronides are found in the plasma, whereas free naringenin and naringenin 7-O-sulfate are found in the GI tract, liver, and other organs. This could be due to variations in the distribution of phase-II metabolizing enzymes across tissues.
In a randomized crossover trial, participants were given orange juice as well as the same batch of fresh oranges; however, following glucuronidase and sulfatase treatment, there was an average 1.7-fold increase in naringenin when consuming fresh oranges. This indicated significant differences in naringenin’s bioavailability from different intaking oranges forms (Yang et al., 2022). Because of the fiber matrix of oranges, which makes the juice more accessible due to mechanical processing, there may be changes in the bioavailability of naringenin among various intaking forms of oranges. Additionally, because citrus flavanones are found in the inner part of the peel, they are typically avoided while consuming oranges (Pandey et al., 2015). Flavanones, such as naringin and naringenin, which are not generally ingested from whole oranges, are added to the juice through commercial juicing, which permits the albedo to come into contact with orange juice bags (Kamgaing et al., 2017). The transformation of naringenin from a promising phytocompound into a practical medicinal drug encounters numerous obstacles. Naringenin has limited absorption through the oral route owing to its low solubility in water and significant metabolism during its first passage through the liver. This restricts their optimal concentration in the bloodstream (Joshi et al., 2018). Naringenin can serve as a substrate for P-glycoprotein, a transporter that actively removes it from cells, thereby decreasing its concentration within cells and diminishing its effectiveness as a therapeutic agent. Naringenin is rapidly metabolized in the liver, forming metabolites, such as naringenin glucuronide and naringenin sulfate. These metabolites may exhibit altered or diminished biological activity compared with the original substance (Shulman et al., 2011). Maintaining therapeutic levels of naringenin is difficult because of its rapid elimination and short half-life in the bloodstream. Therefore, it is crucial to develop formulations that enhance the solubility and stability of naringenin. Novel delivery strategies such as nanoparticles, liposomes, and micelles have been investigated to improve their bioavailability (Erlund, 2002).
Another obstacle is the attainment of a regulated and prolonged release pattern for naringenin, which ensures the maintenance of optimal therapeutic concentrations throughout the duration of treatment. It is essential to develop precise delivery methods that can route naringenin to specific tissues or cancer cells while reducing any unintended effects on healthy tissues and toxicity. Extensive research and clinical studies are necessary to determine the most effective dose schedule that maximizes therapeutic efficacy while reducing adverse effects. Thorough preclinical investigations are necessary to evaluate the prolonged safety, toxicity, and adverse effects of naringenin (Mir and Tiku, 2015). Subsequent clinical trials are required to determine the safety profile of this product in human subjects. The therapeutic efficacy and safety of naringenin can be influenced by genetic variations, food, and other environmental factors, leading to changes in metabolism and reactions among various populations (Adetunji et al., 2023). To overcome these challenges, a multidisciplinary strategy is necessary to convert naringenin into a therapeutic agent. This method involved the collaboration of chemists, pharmacologists, physicians, and regulatory specialists to develop successful techniques for optimizing naringenin.
The absorption of Naringenin occurs via both passive diffusion and active transport (Rani et al., 2016). Naringenin, a compound that is readily and swiftly assimilated, can be metabolized by entering the bloodstream (Arafah et al., 2020). Nevertheless, naringenin absorption through the oral cavity could be more efficient, resulting in a bioavailability of only 15%. This is because of the prolonged metabolism that occurs during the initial passage through the colon (Joshi et al., 2018; Arafah et al., 2020). Chabane et al., 2009 conducted a study on the permeability of Naringenin in human intestinal Caco-2 cells. The study found that Naringenin was partially absorbed through passive diffusion and its absorption was not influenced by pH. Furthermore, it was determined that the ATP-dependent transport substrate was promoted by multidrug resistance-associated protein (MRP1). Xu et al., 2009 conducted a study using a rat intestinal perfusion model to investigate the absorption of Naringenin. The results showed that the colon absorbed the largest amount (68%). In the same study, Naringenin exhibited an absorption rate of approximately 47% in the duodenum, 42% in the terminal ileum, and 39% in the jejunum. Kanaze et al., 2007 conducted a study to examine the pharmacokinetic parameters in humans following the administration of a 135 mg oral dosage of Naringenin. The researchers recorded a maximum concentration (Cmax) of 2009.51 ng/mL in 3.67 h after administration. The area under the concentration-time curve from 0 to infinity (AUC0−∞) was measured at 9424.52 ng h/mL. The elimination half-life was found to be 2.31 h, and its oral bioavailability was determined to be 5.81%.
Pharmacokinetic investigations of Naringenin, whether administered orally or intravenously, have indicated that its biological effects are attributed to its conjugated forms (Felgines et al., 2000; Wang et al., 2006). Naringenin mostly forms glucuronide and sulfate conjugates. Serum primarily contains naringenin glucuronides, but larger amounts of naringenin sulfates are found in tissues such as the liver, spleen, heart, and brain (Lin et al., 2014). Naringenin is present in higher amounts in tissues compared to plasma (Zeng et al., 2020). Furthermore, the presence of efflux transporters in cells does not affect naringenin (Joshi et al., 2018). The transport of Naringenin was investigated by Peng et al., 1998, who observed its uptake in the cerebral cortex and striatum, with a lower concentration in the latter. An in vitro model demonstrated that the apparent permeability of Naringenin ranges from 250 to 350 nm/s. Therefore, Naringenin demonstrated a significant level of permeability in both in situ BBB models and in vitro experiments. Thus, Naringenin has the potential to offer a significant level of neuroprotection to the central nervous system. The bioavailability of any substance is determined by its elimination process, which can occur through hepatic metabolism or excretion from the body, as well as by its permeability in the gastrointestinal system. The primary metabolites of flavanones identified thus far are glucuronide and sulfate conjugates (Felgines et al., 2000; El Mohsen et al., 2004; Wang et al., 2006).
After absorption, Naringenin undergoes a significant metabolic process called glucuronidation, resulting in the detection of 98% of Naringenin o β D glucuronide as a metabolite in the plasma. In addition, a significant concentration of naringenin glucuronide is present in the liver, kidney, heart, and brain. Owing to its increased polarity, naringenin glucuronide has a limited ability to penetrate tissues by crossing the lipophilic cell membrane. However, the tissue β-glucuronidase enzyme can help to break down this metabolite into free Naringenin, allowing it to be recirculated. Colonic bacterial microflora hydrolyzes Naringenin to produce 3 (4 hydroxyphenyl) propionic acid, which is readily absorbed in the gut (Day et al., 1998; Felgines et al., 2000; El Mohsen et al., 2004). Reports indicate that glucuronidation of Naringenin primarily occurs at the 7 and 4′ hydroxyl groups using the enzyme UDP-glucuronyltransferase. Therefore, sulfotransferases are involved in the O sulfation of Naringenin at the 7, 4′, or 5 hydroxyl groups (Justesen et al., 1998).
Prior to absorption in the cecum, Naringenin undergoes hydrolysis by beta-glucosidase in the small intestine (Yao et al., 2004). Naringenin undergoes further metabolism by intestinal bacterial microflora, resulting in the production of p-hydroxybenzoic acid, p-hydroxyphenylpropionic acid, and p-coumaric acid. These metabolites can be detected in both plasma and urine (Erlund et al., 2001). Flavonoids are mostly eliminated through two primary pathways: biliary and urinary excretion. The biliary excretion of metabolites (conjugates) results in their reabsorption into the enterohepatic circulation, which prolongs the half-life of the elimination phase (Ma et al., 2006). Naringenin 7-glucuronide, naringenin 7-sulfate 4′-glucuronide, and naringenin 7-glucuronide 4′-sulfate are excreted by bile, while naringenin 4′-glucuronide, naringenin 7-glucuronide, and naringenin 7,4′-disulfate are excreted through urine (Rani et al., 2016). The urine excretion rate of Naringenin varies from 7% to 23% (Joshi et al., 2018).Aging affects pharmacokinetic processes’ absorption, distribution, metabolism, and excretion phases (Sera and McPherson, 2012; Schlender et al., 2016). Aging specifically affects intestinal surface area, splanchnic blood flow, cytochrome P450 enzymatic activity, and the timing of stomach emptying and peristalsis. In terms of naringenin absorption, sex-related variations exist between rats and humans. Naringenin pharmacokinetics was also found to differ significantly based on sex in adult humans and rats (Bai et al., 2020). Notably, naringenin pharmacokinetics vary depending on the species. For instance, naringenin metabolism in the human gut was found to differ from that in rats and dogs, resulting in a notable lag time in human plasma following the oral administration of naringenin (Rebello et al., 2020). The rationale behind these variations is that they stem from variations in the body weight or body surface area of the animal species, as well as variations in metabolic processes and blood flow rate.
Variations in naringenin metabolism between species have also been reported (Isobe et al., 2018). For instance, only glucuronidation and sulfation conjugation occur in humans, whereas methylation, glucuronidation, and sulfation conjugation of metabolites are observed in rats (and dogs). Understanding the differences in naringenin and its pharmacokinetic properties among species is crucial because it facilitates the application of findings from research on non-human animals, such as rats and dogs, to human health (Burkina et al., 2016). Citrus flavanones have interesting effects on gut bacteria’s growth and gene expression and increase the number of beneficial bacteria that produce short-chain fatty acids (SCFAs). SCFAs have been demonstrated to lower inflammation, enhance the function of the intestinal barrier, and exert chemopreventive effects on colonocytes. Naringenin markedly boosted the growth and gene expression of Bifidobacterium catenulatum when incubated with it. These genes are involved in molecular transport, DNA repair, and cellular metabolism (de Souza et al., 2019).
It has been demonstrated that naringenin inhibits cancer initiation, growth, and spread by altering several aberrant signaling pathways linked to inflammation, autophagy, apoptosis, proliferation, angiogenesis, invasion, and metastasis (Ponte et al., 2021). Flavonoids have anticancer properties, including apoptosis induction and cell proliferation inhibition. These effects may be due to antioxidant activity, increased carcinogen detoxification, decreased metabolic activity, inhibition of DNA adduct synthesis, and inactivation of oncogenes (Zughaibi et al., 2021). By modifying many signaling pathways, such as the Wnt/β-catenin pathway activation and EGF and TGF-β pathway blockage, naringenin prevents the proliferation and spread of cancer cells (Martínez-Rodríguez et al., 2020). Figure 1 shows the molecular processes associated with the anticancer efficacy of naringenin in breast cancer (Zhao et al., 2019).
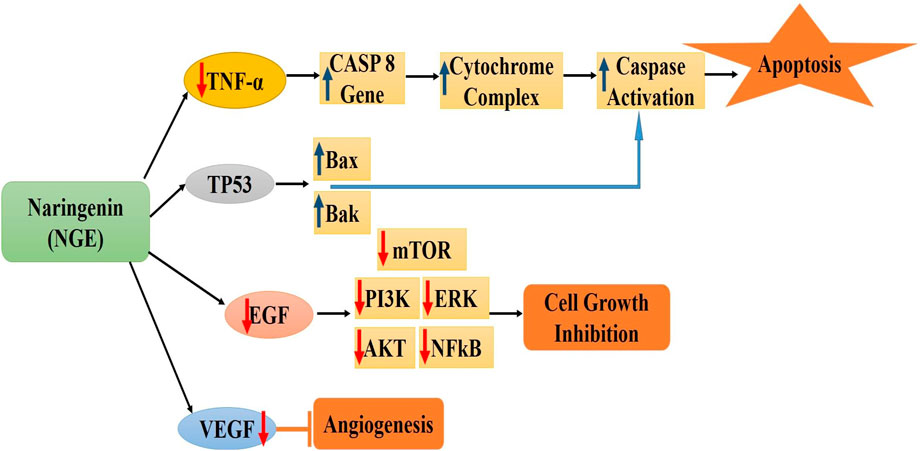
Figure 1. Molecular mechanisms associated with the anticancer efficacy of naringenin in breast cancer [Figures are drawn originally using MS-Powerpoint] *CASP8: Protein Coding gene; TP53: tumor protein p53; Bax and Bak: pro-apoptotic members of the Bcl-2 family; EGF: Epidermal growth factor; mTOR: mammalian target of rapamycin; ERK: Extracellular signal-regulated kinase; AKT: serine/threonine-specific protein kinase; NFkB: Nuclear factor kappa-light-chain-enhancer of activated B cells; VEGF: Vascular endothelial growth factor.
Critical side effects, multidrug resistance, and unsuccessful medical operations are potential consequences of breast cancer therapies such as surgery, chemotherapy, radiation, and hormone therapy (van den Boogaard et al., 2022). Numerous studies have demonstrated the ability of naringenin to suppress aggressive breast tumors (Table 2). Naturally derived NGE decreases pulmonary tumor metastasis and the amount of TGF-β1 secreted by breast cancer cells (Zhang et al., 2016). It is hypothesized that naringenin possesses a high affinity for estrogen receptors (ER), namely, ER-α. Studies on triple-negative breast cancer cell lines have demonstrated that naringenin can cytotoxically reduce cell proliferation and induce cell cycle arrest in the G0/G1 phase. Apoptosis induction was evidenced by a decreased number of viable cells, growth arrest (G0/G1 phase) and nuclear condensation (Li et al., 2013; Zhao et al., 2019).
Furthermore, morphological examination demonstrated that treated rats had a decreased tumor volume and a lower incidence of adenocarcinoma in the mammary gland (Mani et al., 2018). Additionally, naringenin has been shown to exhibit antioxidant effects in the tumor environment by increasing the concentrations of glutathione S-transferase, vitamin C, vitamin E, and glutathione reductase, and by suppressing the production of superoxide dismutase, catalase, thiobarbituric acid reactive substances, and nitrate (Motallebi et al., 2022). 7-O-butyl naringenin (BN), a chemically produced derivative of naringenin, has shown significant anti-proliferative chemotherapeutic potential in MCF-7 human breast cancer cells. Dose-dependent approach of BN inhibition of MCF-7 cell proliferation led to an increase in the sub-G1 phase cell population. Intracellular reactive oxygen species (ROS) were generated by BN and mitigated by pretreatment with N-acetylcysteine (NAC) (Yousuf et al., 2022). Additionally, BN increased the phosphorylation of p38, c-Jun, and stress-activated protein kinase/c-Jun NH4-terminal kinase 1/2 (SAPK/JNK1/2). However, in cells treated with BN, there was a decrease in the phosphorylation of extracellular-regulated kinase 1/2. The cytotoxicity of BN in MCF-7 cells is mediated by the activation of the p38, SAPK/JNK1/2, and c-Jun signaling pathways, in addition to the production of ROS (Park et al., 2010).
Naringenin (ethanol extract of Thymus vulgaris) inhibits the growth of human colorectal and breast cancer cells in a manner that depends on both dose and time. This is achieved through cell cycle arrest at the S and G2/M phases, followed by an increase in apoptotic cell death. Moreover, naringenin modulates the expression of genes that control apoptosis and the cell cycle by upregulating cell cycle regulators, caspases, Bak, apoptosis-inducing factor (AIF), and Bax in breast and colorectal cancer cells. Furthermore, treatment with naringenin led to downregulation of cyclin-dependent kinases, Bcl2, X-linked inhibitor of apoptosis protein (x-IAP), and c-IAP-2. By contrast, it reduced the expression levels of NF-κB, p65, pAkt, PI3K, and pIκBα, which are factors involved in cell survival. Furthermore, NGE increases the susceptibility of breast and colorectal cancer cells to medications that act on DNA (Abaza et al., 2015).
Naringenin exhibits both antiestrogenic and estrogenic properties. Naringenin (estrogen agonist) in T47D-KBluc breast cancer cells demonstrated estrogenic efficacy in estrogen-deficient conditions and anti-estrogenic efficacy in estrogen-rich conditions. Effect of naringenin, 17-estradiol (E2), and genistein on ER activity in T47D-KBluc and ER-negative MDA-MB-231 cells was investigated. Naringenin was reported as a partial agonist and functioning as a competitive antagonist in the presence of E2 or genistein (full agonist) and inefficient ER antagonist (Kim and Park, 2013). The ability of naringenin to inhibit excessive estrogen activity and increase insufficient estrogen activity may contribute to its clinical value. Naringenin has been suggested to be a novel selective estrogen receptor modulator that may be an effective treatment for illnesses associated with sex hormones (Kim and Park, 2013).
Naringenin has demonstrated encouraging anti-metastatic characteristics in preclinical investigations (Memariani et al., 2021), suggesting its potential importance in breast cancer treatment. Naringenin affects multiple signaling pathways associated with cancer metastasis (Chen et al., 2019). For example, it hinders the PI3K/Akt and MAPK/ERK pathways, which play vital roles in cell survival, growth, and movement (Lim et al., 2017). Blocking of these pathways can result in decreased cell movement and invasiveness. In addition, naringenin regulates the NF-κB signaling system, which is linked to inflammation and metastasis, thereby enhancing its anti-metastatic properties. Naringenin has demonstrated the ability to impede the movement and infiltration of breast cancer cell lines (Sudhakaran et al., 2019). Naringenin reduces the metastatic efficacy of breast cancer cells by EMT suppression. SNS (Si-Ni-San) effectively suppressed the metastasis of breast cancer in mice exposed to chronic psychological stress (Zhang et al., 2023). A pharmacokinetic investigation demonstrated that naringenin had the most significant absorption in the hepatic tissue. Subsequent experiments conducted in living organisms and laboratory settings showed that naringenin effectively hinders the growth and spread of breast cancer caused by stress Zhang et al. (2023). This is achieved by enhancing the breakdown of estradiol through the FXR/EST signaling pathway Zhang et al. (2023).
Naringenin decreased the survival rate of MDA-MB-231 cells by causing cell cycle arrest at the G2 phase. The administration of naringenin not only affected the stage of cell cycle arrest but also triggered apoptosis in a manner that was dependent on the dosage. Treatment with naringenin also led to a substantial rise in the activity of caspase-3 and caspase-9 (p < 0.001). The findings of the current investigation indicate that naringenin has a suppressive impact on MDA-MB-231 cells by triggering apoptosis and restraining the activities of caspase-3 and -9 (Wang et al., 2019). Naringenin inhibits the movement of TGF-β1 from the trans-Golgi network by reducing PKC activity, leading to a decrease in the release of TGF-β1 from breast cancer cells. This discovery implies that naringenin could be a promising therapeutic option for disorders associated with TGF-β1. Naringenin demonstrated suppression of pulmonary metastasis in both 4T1/TGF-β1 tumors and 4T1/RFP cancers, leading to enhanced survival of the mice (Zhang et al., 2016).
Naringenin suppressed the rate of cell growth in a manner that was dependent on both the dosage and the duration of treatment. The AO/EB staining technique detected morphological alterations that suggest apoptotic cell death. The Annexin V/PI staining experiment demonstrated a higher proportion of apoptotic cells as the medication dosage rose. The medication naringenin was found to have the ability to induce apoptosis through the activation of caspases. Flow cytometric studies indicated cell cycle arrest, specifically at the G2/M phase of the cell cycle. The administration of the naringenin medication significantly minimized both cell migration and cell invasion propensity of MDA-MB-231 cells, as demonstrated by Qi et al. (2021).
Oral administration of naringenin dramatically reduced the number of metastatic tumor cells in the lungs and prolonged the lifespan of mice that had their tumors removed (Qin et al., 2011). Flow cytometry study demonstrated that T cells exhibited heightened antitumor efficacy in mice treated with naringenin, characterized by an elevated proportion of T cells producing IFN-γ and IL-2 (Noori et al., 2022). Additional in vitro investigations have shown that alleviating immunosuppression induced by regulatory T cells may be the underlying mechanism by which naringenin inhibits metastasis (Hermawan et al., 2021). The results suggest that orally administered naringenin can suppress the growth of metastases following surgery by modulating the host’s immune response (Hermawan et al., 2021). Therefore, according to Qin et al. (2011), naringenin has the potential to serve as a beneficial supplementary treatment for breast cancer patients after surgery.
In different areas of clinical practice, combination therapy, which combines many therapeutic strategies, usually yields quicker and more significant outcomes than monotherapy. Cancer is a multifactorial illness, so combination treatment aimed at many molecular targets may prove beneficial (Bhatia and Das, 2020). Recently, several pharmacological approaches to cancer treatment have been based on combination therapies (drug-food therapy and multi-drug therapy) using dietary supplements, immunotherapy, and natural products (Mokhtari et al., 2017; Guardascione and Tofoli, 2020; Sauter, 2020). Numerous treatments have targeted the estrogen receptor (ER), and more than 60% of breast tumors are ER-positive. The phosphorylation and binding of estrogens activate the ER (Prabhu et al., 2014). Although effective, anti-estrogen treatments such as tamoxifen do not specifically target the growth factor that promotes ER phosphorylation (Chang, 2012). Breast cancer cells exhibit activation of other proliferation pathways, such as the PI3K and MAPK pathways, which are linked to poor prognosis. Therefore, targeting several cellular proliferation and survival pathways at the beginning of treatment is essential to develop more effective treatments. Naringenin inhibited the MAPK and PI3K pathways (Lim et al., 2017; Cheng et al., 2020). The serum (Naringenin or Tamoxifen used charcoal-stripped serum) removed estrogen and other components. In MCF-7 breast cancer cells, combination therapy using NGE and tamoxifen was more effective than either drug alone (Hatkevich et al., 2014; Xu et al., 2018). It also significantly reduced the proliferation and viability of the cells.
Additionally, when using a combination therapy, lower concentrations of both drugs are needed to provide the same effects on viability and proliferation. In MCF-7 cells, naringenin may localize ERα to the cytoplasm and block PI3K and MAPK pathways (Ramos et al., 2017). These investigations have further helped researchers to understand the molecular processes underlying breast cancer cell division and apoptosis.
An additional investigation of combination therapy for breast cancer agents was conducted by Hatkevich et al. (2014), utilizing NGE in conjunction with the well-known medication tamoxifen. By downregulating the expression of MMP-9 and MMP-2, the combination of NGE and tamoxifen in MCF-7 cancer cells demonstrated greater efficacy with reduced cell proliferation when compared to either monotherapy. Additionally, lower quantities of both substances are needed to provide similar therapeutic effects on viability and proliferation. In addition to inducing cell death, combination treatment increases ROS generation and controls the expression of mitochondrial apoptotic proteins (Hatkevich et al., 2014). Thorough mechanistic analyses have demonstrated that tamoxifen monotherapy decreases ERα66 and GPR30 expression. However, when NGE and tamoxifen were combined, they reduced the expression of ERα66 and GPR30 and increased ERβ and ERα36, which was not the case with either monotherapy (Xu et al., 2018). The details of naringenin in breast cancer combination therapy with other agents have been summarized in Table 3.
A new avenue for physicians to employ combination therapy to combat tumors resistant to monotherapy has been made possible by numerous studies conducted on various cancer cell lines that have shown the beneficial effects of this treatment. In a different research, Naringenin was combined with daunomycin, a therapeutically utilized medication, to overcome MCF-7 breast cancer cells’ resistance to it and increase its efficacy. Daunomycin accumulation was higher in MCF-7 (sensitive) cells than in MCF-7/ADR (resistant) cells, whereas daunomycin efflux was higher in MCF-7/ADR cells than in MCF-7 (sensitive) cells. The overexpression of P-gp in resistant cells was demonstrated by a 22-fold increase in the daunomycin IC50 value in MCF-7/ADR cells compared to MCF-7 (sensitive) cells. The IC50 values dramatically lowered when 50 μM NGE and 0.5 μM daunomycin were combined, indicating that naringenin effectively reduced daunomycin efflux in MCF-7/ADR and increased MCF-7/ADR’s resistance to daunomycin (Mir and Tiku, 2015).
Nanoparticles have several features, including thermal conductivity, a wide melting point range, and light absorption, and have been increasingly popular in the past few decades in various fields, including medical imaging and treatment tactics (Parvanian et al., 2017). Nanoscale platforms can be used as drug delivery systems to deliver medications or agents to specific tissues in a regulated manner. Additionally, as demonstrated for naringenin, nanocarriers can increase various plant-based compounds’ bioavailability and water solubility (Gera et al., 2017). Naringenin has been encapsulated in multiple nanoformulations, including micelles, hydrogels, carbon-based nanocarriers, and polymeric and lipid-based nanoparticles for treating inflammatory disorders, liver and brain diseases, and cancer therapy (Table 4 and Figure 2).
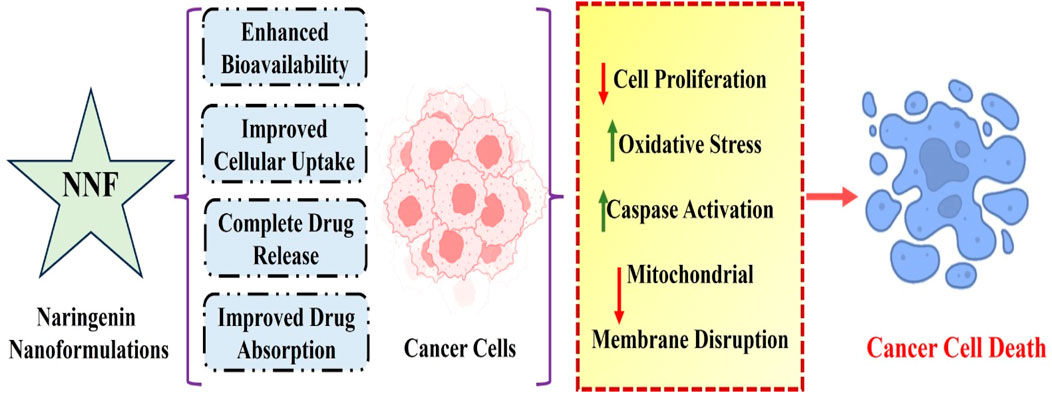
Figure 2. Molecular mechanisms of naringenin-based nanoformulations against breast cancer. Overall, these results highlight the enormous potential of innovative naringenin nanoformulations as effective medical delivery methods for breast cancer treatment and imply that naringeninencapsulated nanoparticles are more potent in breast cancer therapy than the solely given phytochemical agent.
Nanoformulations of naringenin have numerous benefits compared with traditional formulations, effectively overcoming the limitations associated with its bioavailability, stability, and therapeutic effectiveness. Nanoformulations augment the surface area of naringenin particles, thus boosting their solubility in biological fluids and facilitating absorption. Nanoparticles enhance the absorption of naringenin in the gastrointestinal tract, thereby circumventing the solubility limitations of conventional formulations (Smruthi et al., 2022). Naringenin is shielded from enzymatic breakdown and acidic conditions in the stomach by encapsulating it in nanoparticles, enhancing stability and bioavailability (Chaurasia et al., 2017). Nanoformulations can be engineered to achieve a sustained release of naringenin, ensuring consistent therapeutic concentrations in the bloodstream for an extended duration and minimizing the need for frequent administration. Nanoformulations can utilize the enhanced permeability and retention (EPR) effect, whereby nanoparticles preferentially accumulate in tumor tissues due to their porous blood vessels, augmenting the concentration of naringenin at the tumor site (Paun et al., 2024). Nanoparticles can be used to overcome efflux systems, such as P-glycoprotein, which decreases the amount of naringenin inside cells, increasing its therapeutic effectiveness (Bhia et al., 2021). Nanoformulations can simultaneously encapsulate naringenin with other therapeutic compounds, enabling combination therapies that target numerous pathways simultaneously. It has the potential to enhance the efficacy of anti-cancer treatments. Nanoformulations can be designed to extend the duration of naringenin in the bloodstream, thereby improving its pharmacokinetic characteristics and ensuring long-lasting therapeutic benefits (Deshmukh et al., 2023). Nanoformulations can enhance the safety of naringenin by delivering it directly to specific tissues or cells, which reduces its overall exposure in the body and decreases the associated toxicity. This targeted delivery approach minimizes the side effects and improves the safety profile of naringenin (Sharma et al., 2022). Ultimately, nanoformulations have significant potential for addressing the drawbacks of traditional naringenin formulations by improving their solubility, stability, bioavailability, and targeted administration. Further investigation and advancement in this field will be crucial for ultimately harnessing the therapeutic capabilities of naringenin in clinical settings.
pH- and thermo-sensitive PHEMA-nanoparticles incorporating naringenin were formulated to increase cytotoxic efficacy against MCF-7 cancer cells (Yildirim et al., 2023). The maximum release of naringenin from NPs occurred at 41°C, pH 6.0. NGE-SNPs significantly decreased the viability of the cells compared to free naringenin. The NPs induced early apoptosis and increased the percentage of cells in the G1 phase of the cell cycle in a dose-dependent manner. Rajamani et al. conducted an additional experimental study to examine the effects of d-alpha tocopheryl poly (ethylene glycol) 1000 succinate (TPGS)-decorated naringenin nanoparticles on MCF-7 breast cancer cells and mouse models of cancer. These findings demonstrated the antitumor effects of naringenin nanoparticles through increased ROS levels, GSH attenuation, and caspase-3 activation, which ultimately induced apoptosis (Rajamani et al., 2018).
Naringenin has also been encapsulated using chitosan and dextran sulfate to enhance its medicinal qualities. The cytotoxic effects of spherical chitosan dextran sulfate naringenin nanoparticles on the MCF-7 breast cancer cell line were assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay after a 24-h incubation period. The results showed that chitosan-dextran sulfate-NGE released 80% of the free NGE in a controlled manner and exhibited significant cytotoxic action after 36 h of treatment. Therefore, the chitosan-dextran-sulfate- NGE nanocarrier is an appropriate drug delivery strategy for NGE and other hydrophobic substances (Muralidharan and Shanmugam, 2021).
The naringenin-cell penetrating peptide-galactose nanoparticles (NCG) demonstrated targeted delivery to the liver and enhanced intestinal barrier permeability in both cell and zebrafish xenotransplantation models. In addition, NCG demonstrated liver-specific targeting and enterohepatic circulation in mice breast cancer xenografts after being given orally. The cancer inhibitory effectiveness of NCG was greater than that of both NGE and the positive control tamoxifen. This was accompanied by increased hepatic EST expression and decreased estradiol levels in the liver, blood, and tumor tissue (Zhao et al., 2024).
Naringenin has been demonstrated to have the combined effect of inhibiting the growth of tumor cells and accelerating apoptotic cell death by activating signaling molecules and pro-apoptotic pathways in a range of distinct carcinomas. Naringenin can modulate numerous signal transduction pathways and be a suitable candidate for combinatorial therapies. There are insufficient in vivo studies and clinical trials, even though most cancer cell line investigations have highlighted naringenin as a viable option for treating many cancer types. Combinatorial studies of naringenin with several chemotherapeutic agents have displayed significant anticancer potential against breast cancer. Naringenin may function as a strong chemosensitizer, enhancing the cytotoxic effect of existing anticancer medications and destroying drug-resistant cancer cells. This review concludes that naringenin can reduce carcinogenesis through pleiotropic processes such as antioxidative, apoptotic-inducing ROS generation, and cell cycle arrest. However, further research is needed to elucidate the enhanced therapeutic potential of naringenin for better management of breast carcinoma.
DE: Writing–review and editing, Writing–original draft, Visualization, Validation, Formal Analysis. PP: Writing–review and editing, Writing–original draft, Visualization, Validation, Formal Analysis, Conceptualization. SR: Data curation, Supervision, Formal Analysis, Validation, Resources, Visualization, Writing–review and editing. RK: Writing–review and editing, Visualization, Validation, Resources, Investigation, Formal Analysis. SL: Writing–review and editing, Visualization, Supervision, Investigation, Formal Analysis. SR: Writing–review and editing, Visualization, Supervision, Project administration, Investigation, Formal Analysis. SS: Writing–review and editing, Visualization, Validation, Investigation, Formal Analysis. AS: Writing–review and editing, Visualization, Validation, Project administration, Investigation, Formal Analysis. MS: Writing–review and editing, Visualization, Validation, Supervision, Project administration, Conceptualization. FK: Writing–review and editing, Visualization, Validation, Supervision, Project administration, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are thankful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/44/45.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NGE, Naringenin; HPPA, 3-(4-hydroxyphenyl) propionic acid; SCFAs, Short-chain fatty acids; BN, 7-O-butyl Naringenin; ROS, Reactive oxygen species; NAC, N-acetylcysteine; SAPK/JNK1/2, stress-activated protein kinase/c-Jun NH4-terminal kinase 1/2; MCF-7, Human breast cancer cell line with estrogen, progesterone, and glucocorticoid receptors; AIF, Apoptosis-inducing factor; x-IAP, X-linked inhibitor of apoptosis protein; c-IAP-2, Cellular inhibitor of apoptosis 2; pIκBα, Phospho-inhibitory subunit of NF Kappa B alpha; T47D-KBluc breast cancer cells, Epithelial cell line isolated from a pleural effusion of a patient with ductal carcinoma of the mammary gland; MDA-MB-231, Late-stage breast cancer cells isolated at M D Anderson from a pleural effusion of a patient with invasive ductal carcinoma; MMP-2, Matrix metalloproteinase-2; MMP-9, Matrix Metalloproteinase-9; FKBPs, cis-trans prolyl isomerase protein; NR3C1, Nuclear receptor subfamily 3 group C member 1; NRF2, Nuclear factor erythroid 2-related factor 2; BPA, Bisphenol A; MNU, Methylnitrosourea; GSH, γ-l-glutamyl-l-cysteinyl-glycine (Glutathione); TPGS, Tocopheryl polyethylene glycol succinate; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide.
Abaza, M. S. I., Orabi, K. Y., Al-Quattan, E., and Al-Attiyah, R. A. J. (2015). Growth inhibitory and chemo-sensitization effects of Naringenin, a natural flavanone purified from Thymus vulgaris, on human breast and colorectal cancer. Cancer Cell Int. 15, 46–19. doi:10.1186/s12935-015-0194-0
Adetunji, J. A., Fasae, K. D., Awe, A. I., Paimo, O. K., Adegoke, A. M., Akintunde, J. K., et al. (2023). The protective roles of citrus flavonoids, naringenin, and naringin on endothelial cell dysfunction in diseases. Heliyon 9, e17166. doi:10.1016/j.heliyon.2023.e17166
Akbari, B., Baghaei-Yazdi, N., Bahmaie, M., and Mahdavi Abhari, F. (2022). The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 48 (3), 611–633. doi:10.1002/biof.1831
Arafah, A., Rehman, M. U., Mir, T. M., Wali, A. F., Ali, R., Qamar, W., et al. (2020). Multi-therapeutic potential of naringenin (4′, 5, 7-trihydroxyflavonone): experimental evidence and mechanisms. Plants 9 (12), 1784. doi:10.3390/plants9121784
Askar, M. A., El Shawi, O. E., Mansour, N. A., and Hanafy, A. M. (2021). Breast cancer suppression by curcumin-Naringenin-magnetic-nano-particles: in vitro and in vivo studies. Tumor Biol. 43 (1), 225–247. doi:10.3233/TUB-211506
Bai, Y., Peng, W., Yang, C., Zou, W., Liu, M., Wu, H., et al. (2020). Pharmacokinetics and metabolism of naringin and active metabolite naringenin in rats, dogs, humans, and the differences between species. Front. Pharmacol. 11, 364. doi:10.3389/fphar.2020.00364
Bakar, E., Ulucam, E., Cerkezkayabekir, A., Sanal, F., and Inan, M. (2019). Investigation of the effects of naringin on intestinal ischemia reperfusion model at the ultrastructural and biochemical level. Biomed. Pharmacother. 109, 345–350. doi:10.1016/j.biopha.2018.10.045
Baskar, R., Dai, J., Wenlong, N., Yeo, R., and Yeoh, K. W. (2014). Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 1, 24. doi:10.3389/fmolb.2014.00024
Bhat, S. A., Hassan, T., Majid, S., Ashraf, R., and Kuchy, S. (2017). MicroRNAs and its emerging role as breast cancer diagnostic marker- A review. Cancer Clin. Res. Rep. 1 (3), 1–8. doi:10.1016/j.abst.2019.05.001
Bhatia, K., and Das, A. (2020). Combinatorial drug therapy in cancer-New insights. Life Sci. 258, 118134. doi:10.1016/j.lfs.2020.118134
Bhia, M., Motallebi, M., Abadi, B., Zarepour, A., Pereira-Silva, M., Saremnejad, F., et al. (2021). Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 13 (2), 291. doi:10.3390/pharmaceutics13020291
Bulzomi, P., Bolli, A., Galluzzo, P., Acconcia, F., Ascenzi, P., and Marino, M. (2012). The Naringenin-induced proapoptotic effect in breast cancer cell lines holds out against a high bisphenol a background. Iubmb Life 64 (8), 690–696. doi:10.1002/iub.1049
Burkina, V., Zlabek, V., Halsne, R., Ropstad, E., and Zamaratskaia, G. (2016). In vitro effects of the citrus flavonoids diosmin, naringenin and naringin on the hepatic drug-metabolizing CYP3A enzyme in human, pig, mouse and fish. Biochem. Pharmacol. 110, 109–116. doi:10.1016/j.bcp.2016.04.011
Chabane, M. N., Ahmad, A. A., Peluso, J., Muller, C. D., and Ubeaud-Séquier, G. (2009). Quercetin and naringenin transport across human intestinal Caco-2 cells. J. Pharm. Pharmacol. 61 (11), 1473–1483. doi:10.1211/jpp/61.11.0006
Chang, M. (2012). Tamoxifen resistance in breast cancer. Biomol. Ther. 20 (3), 256–267. doi:10.4062/biomolther.2012.20.3.256
Chaurasia, S., Patel, R. R., Vure, P., and Mishra, B. (2017). Oral naringenin nanocarriers: fabrication, optimization, pharmacokinetic and chemotherapeutic efficacy assessments. Nanomedicine 12 (11), 1243–1260. doi:10.2217/nnm-2016-0436
Chen, Y. Y., Chang, Y. M., Wang, K. Y., Chen, P. N., Hseu, Y. C., Chen, K. M., et al. (2019). Naringenin inhibited migration and invasion of glioblastoma cells through multiple mechanisms. Environ. Toxicol. 34 (3), 233–239. doi:10.1002/tox.22677
Cheng, H., Jiang, X., Zhang, Q., Ma, J., Cheng, R., Yong, H., et al. (2020). Naringin inhibits colorectal cancer cell growth by repressing the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 19 (6), 3798–3804. doi:10.3892/etm.2020.8649
Connor, J., and Lee, S. (2010). in Bioactive compounds and cancer. Editors J. A. Milner, and D. F. Romagnolo (NewYork, NY, USA: Humana Press).
Day, A. J., DuPont, M. S., Ridley, S., Rhodes, M., Rhodes, M. J., Morgan, M. R., et al. (1998). Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 436 (1), 71–75. doi:10.1016/s0014-5793(98)01101-6
Deshmukh, R., Prajapati, M., and Harwansh, R. K. (2023). Recent advances and prospects in naringin nanocarrier drug delivery system for cancer management. J. Drug Deliv. Sci. Technol. 91, 105182. doi:10.1016/j.jddst.2023.105182
de Souza, E. L., de Albuquerque, T. M. R., Dos Santos, A. S., Massa, N. M. L., and de Brito Alves, J. L. (2019). Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities–A review. Crit. Rev. food Sci. Nutr. 59 (10), 1645–1659. doi:10.1080/10408398.2018.1425285
Eanes, L., and Patel, Y. M. (2016). Inhibition of the MAPK pathway alone is insufficient to account for all of the cytotoxic effects of Naringenin in MCF-7 breast cancer cells. Biochim. open 3, 64–71. doi:10.1016/j.biopen.2016.09.004
El Mohsen, M. A., Marks, J., Kuhnle, G., Rice-Evans, C., Moore, K., Gibson, G., et al. (2004). The differential tissue distribution of the citrus flavanone naringenin following gastric instillation. Free Radic. Res. 38 (12), 1329–1340. doi:10.1080/10715760400017293
Erlund, I. (2002). Chemical analysis and pharmacokinetics of the flavonoids quercetin, hesperetin and naringenin in humans.
Erlund, I., Meririnne, E., Alfthan, G., and Aro, A. (2001). Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 131 (2), 235–241. doi:10.1093/jn/131.2.235
Felgines, C., Texier, O., Morand, C., Manach, C., Scalbert, A., Régerat, F., et al. (2000). Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiology-Gastrointestinal Liver Physiology 279 (6), G1148–G1154. doi:10.1152/ajpgi.2000.279.6.G1148
Gera, S., Talluri, S., Rangaraj, N., and Sampathi, S. (2017). Formulation and evaluation of Naringenin nanosuspensions for bioavailability enhancement. Aaps Pharmscitech 18, 3151–3162. doi:10.1208/s12249-017-0790-5
Guardascione, M., and Toffoli, G. (2020). Immune checkpoint inhibitors as monotherapy or within a combinatorial strategy in advanced hepatocellular carcinoma. Int. J. Mol. Sci. 21 (17), 6302. doi:10.3390/ijms21176302
Hatkevich, T., Ramos, J., Santos-Sanchez, I., and Patel, Y. M. (2014). A Naringenin–tamoxifen combination impairs cell proliferation and survival of MCF-7 breast cancer cells. Exp. Cell Res. 327 (2), 331–339. doi:10.1016/j.yexcr.2014.05.017
Hayes, J. D., Dinkova-Kostova, A. T., and Tew, K. D. (2020). Oxidative stress in cancer. Cancer Cell 38 (2), 167–197. doi:10.1016/j.ccell.2020.06.001
Hazafa, A., Rehman, K. U., Jahan, N., and Jabeen, Z. (2020). The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. cancer 72 (3), 386–397. doi:10.1080/01635581.2019.1637006
Hermawan, A., Ikawati, M., Jenie, R. I., Khumaira, A., Putri, H., Nurhayati, I. P., et al. (2021). Identification of potential therapeutic target of naringenin in breast cancer stem cells inhibition by bioinformatics and in vitro studies. Saudi Pharm. J. 29 (1), 12–26. doi:10.1016/j.jsps.2020.12.002
Isobe, T., Ohkawara, S., Ochi, S., Tanaka-Kagawa, T., Jinno, H., and Hanioka, N. (2018). Naringenin glucuronidation in liver and intestine microsomes of humans, monkeys, rats, and mice. Food Chem. Toxicol. 111, 417–422. doi:10.1016/j.fct.2017.11.057
Jalalpour Choupanan, M., Shahbazi, S., and Reiisi, S. (2023). Naringenin in combination with quercetin/fisetin shows synergistic anti-proliferative and migration reduction effects in breast cancer cell lines. Mol. Biol. Rep. 50 (9), 7489–7500. doi:10.1007/s11033-023-08664-2
Joshi, R., Kulkarni, Y. A., and Wairkar, S. (2018). Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: an update. Life Sci. 215, 43–56. doi:10.1016/j.lfs.2018.10.066
Justesen, U., Knuthsen, P., and Leth, T. (1998). Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 799 (1-2), 101–110. doi:10.1016/s0021-9673(97)01061-3
Kamgaing, T., Doungmo, G., Melataguia Tchieno, F. M., Gouoko Kouonang, J. J., and Mbadcam, K. J. (2017). Kinetic and isotherm studies of bisphenol a adsorption onto orange albedo (Citrus sinensis): sorption mechanisms based on the main albedo components vitamin C, flavones glycosides and carotenoids. J. Environ. Sci. Health, Part A 52 (8), 757–769. doi:10.1080/10934529.2017.1303315
Kanaze, F. I., Bounartzi, M. I., Georgarakis, M., and Niopas, I. (2007). Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 61 (4), 472–477. doi:10.1038/sj.ejcn.1602543
Ke, J. Y., Banh, T., Hsiao, Y. H., Cole, R. M., Straka, S. R., Yee, L. D., et al. (2017). Citrus flavonoid Naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. food Res. 61 (9), 1600934. doi:10.1002/mnfr.201600934
Kim, S., and Park, T. I. (2013). Naringenin: a partial agonist on estrogen receptor in T47D-KBluc breast cancer cells. Int. J. Clin. Exp. Med. 6 (10), 890–899.
Kiran, S. D. V. S., Rohini, P., and Bhagyasree, P. (2017). Flavonoid: a review on naringenin. J. Pharmacogn. Phytochemistry 6 (5), 2778–2783.
Li, H., Yang, B., Huang, J., Xiang, T., Yin, X., Wan, J., et al. (2013). Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol. Lett. 220 (3), 219–228. doi:10.1016/j.toxlet.2013.05.006
Lim, W., Park, S., Bazer, F. W., and Song, G. (2017). Naringenin-induced apoptotic cell death in prostate cancer cells is mediated via the PI3K/AKT and MAPK signaling pathways. J. Cell. Biochem. 118 (5), 1118–1131. doi:10.1002/jcb.25729
Lima, G. P. P., Vianello, F., Corrêa, C. R., Campos, R. A. D. S., and Borguini, M. G. (2014). Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 05, 1065–1082. doi:10.4236/fns.2014.511117
Lin, S. P., Hou, Y. C., Tsai, S. Y., Wang, M. J., and Chao, P. D. L. (2014). Tissue distribution of naringenin conjugated metabolites following repeated dosing of naringin to rats. Biomedicine 4 (3), 16. doi:10.7603/s40681-014-0016-z
Ma, Y., Li, P., Chen, D., Fang, T., Li, H., and Su, W. (2006). LC/MS/MS quantitation assay for pharmacokinetics of naringenin and double peaks phenomenon in rats plasma. Int. J. Pharm. 307 (2), 292–299. doi:10.1016/j.ijpharm.2005.10.018
Majidinia, M., Bishayee, A., and Yousefi, B. (2019). Polyphenols: major regulators of key components of DNA damage response in cancer. DNA repair 82, 102679. doi:10.1016/j.dnarep.2019.102679
Mani, G., Arumugam, M., Maril, A., and Devaki, T. (2018). Naringenin attenuates DMBA-induced mammary carcinogenesis in rats via regulating the oxidative stress and antioxidants status. J Chem Pharmaceu Res 10, 0975–7384.
Martincorena, I., and Campbell, P. J. (2015). Somatic mutation in cancer and normal cells. Science 349 (6255), 1483–1489. doi:10.1126/science.aab4082
Martínez-Rodríguez, O. P., Thompson-Bonilla, M. D. R., and Jaramillo-Flores, M. E. (2020). Association between obesity and breast cancer: molecular bases and the effect of flavonoids in signaling pathways. Crit. Rev. Food Sci. Nutr. 60 (22), 3770–3792. doi:10.1080/10408398.2019.1708262
Memariani, Z., Abbas, S. Q., Ul Hassan, S. S., Ahmadi, A., and Chabra, A. (2021). Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 171, 105264. doi:10.1016/j.phrs.2020.105264
Mir, I. A., and Tiku, A. B. (2015). Chemopreventive and therapeutic potential of “Naringenin,” a flavanone present in citrus fruits. Nutr. cancer 67 (1), 27–42. doi:10.1080/01635581.2015.976320
Mokhtari, R. B., Homayouni, T. S., Baluch, N., Morgatskaya, E., Kumar, S., Das, B., et al. (2017). Combination therapy in combating cancer. Oncotarget 8 (23), 38022–38043. doi:10.18632/oncotarget.16723
Motallebi, M., Bhia, M., Rajani, H. F., Bhia, I., Tabarraei, H., Mohammadkhani, N., et al. (2022). Naringenin: a potential flavonoid phytochemical for cancer therapy. Life Sci. 305, 120752. doi:10.1016/j.lfs.2022.120752
Muralidharan, S., and Shanmugam, K. (2021). Synthesis and characterization of Naringenin-loaded chitosan-dextran sulfate nanocarrier. J. Pharm. Innovation 16, 269–278. doi:10.1007/s12247-020-09444-2
Noori, S., Nourbakhsh, M., Imani, H., Deravi, N., Salehi, N., and Abdolvahabi, Z. (2022). Naringenin and cryptotanshinone shift the immune response towards Th1 and modulate T regulatory cells via JAK2/STAT3 pathway in breast cancer. BMC Complementary Med. Ther. 22 (1), 145–211. doi:10.1186/s12906-022-03625-x
Noori, S., Tavirani, M. R., Deravi, N., Rabbani, M. I. M., and Zarghi, A. (2020). Naringenin enhances the anti-cancer effect of cyclophosphamide against MDA-MB-231 breast cancer cells via targeting the STAT3 signaling pathway. Iran. J. Pharm. Res. IJPR 19 (3), 122–133. doi:10.22037/ijpr.2020.113103.14112
Pandey, R. P., Gurung, R. B., and Sohng, J. K. (2015). “Dietary sources, bioavailability and biological activities of Naringenin and its derivatives,” in Apigenin and Naringenin: natural sources, pharmacology and role in cancer prevention (New York: Nova Science Publishers), 151–172.
Park, J. H., Lee, J. W., Paik, H. D., Cho, S. G., Nah, S. Y., Park, Y. S., et al. (2010). Cytotoxic effects of 7-O-butyl Naringenin on human breast cancer MCF-7 cells. Food Sci. Biotechnol. 19, 717–724. doi:10.1007/s10068-010-0101-3
Parvanian, S., Mostafavi, S. M., and Aghashiri, M. (2017). Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens. Bio-Sensing Res. 13, 81–87. doi:10.1016/j.sbsr.2016.08.002
Pateliya, B., Burade, V., and Goswami, S. (2021). Combining Naringenin and metformin with doxorubicin enhances anticancer activity against triple-negative breast cancer in vitro and in vivo. Eur. J. Pharmacol. 891, 173725. doi:10.1016/j.ejphar.2020.173725
Paun, R. A., Jurchuk, S., and Tabrizian, M. (2024). A landscape of recent advances in lipid nanoparticles and their translational potential for the treatment of solid tumors. Bioeng. Transl. Med. 9 (2), e10601. doi:10.1002/btm2.10601
Peng, H. W., Cheng, F. C., Huang, Y. T., Chen, C. F., and Tsai, T. H. (1998). Determination of naringenin and its glucuronide conjugate in rat plasma and brain tissue by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 714 (2), 369–374. doi:10.1016/s0378-4347(98)00204-7
Ponte, L. G. S., Pavan, I. C. B., Mancini, M. C. S., da Silva, L. G. S., Morelli, A. P., Severino, M. B., et al. (2021). The hallmarks of flavonoids in cancer. Molecules 26 (7), 2029. doi:10.3390/molecules26072029
Prabhu, J. S., Korlimarla, A., Desai, K., Alexander, A., Raghavan, R., Anupama, C. E., et al. (2014). A majority of low (1-10%) ER positive breast cancers behave like hormone receptor negative tumors. J. Cancer 5 (2), 156–165. doi:10.7150/jca.7668
Qi, Z., Kong, S., Zhao, S., and Tang, Q. (2021). Naringenin inhibits human breast cancer cells (MDA-MB-231) by inducing programmed cell death, caspase stimulation, G2/M phase cell cycle arrest and suppresses cancer metastasis. Cell. Mol. Biol. (Noisy-le-Grand, France) 67 (2), 8–13. doi:10.14715/cmb/2021.67.2.2
Qin, L., Jin, L., Lu, L., Lu, X., Zhang, C., Zhang, F., et al. (2011). Naringenin reduces lung metastasis in a breast cancer resection model. Protein & Cell 2 (6), 507–516. doi:10.1007/s13238-011-1056-8
Rajamani, S., Radhakrishnan, A., Sengodan, T., and Thangavelu, S. (2018). Augmented anticancer activity of Naringenin-loaded TPGS polymeric nanosuspension for drug resistive MCF-7 human breast cancer cells. Drug Dev. industrial Pharm. 44 (11), 1752–1761. doi:10.1080/03639045.2018.1496445
Ramos, J., Hatkevich, T., Eanes, L., Santos-Sanchez, I., and Patel, Y. M. (2017). “Naringenin inhibits proliferation and survival of tamoxifen-resistant breast cancer cells,” in Breast cancer-from biology to medicine (London, UK: IntechOpen).
Rani, N., Bharti, S., Krishnamurthy, B., Bhatia, J., Sharma, C., Amjad Kamal, M., et al. (2016). Pharmacological properties and therapeutic potential of naringenin: a citrus flavonoid of pharmaceutical promise. Curr. Pharm. Des. 22 (28), 4341–4359. doi:10.2174/1381612822666160530150936
Rebello, C. J., Beyl, R. A., Lertora, J. J., Greenway, F. L., Ravussin, E., Ribnicky, D. M., et al. (2020). Safety and pharmacokinetics of Naringenin: a randomized, controlled, single-ascending-dose clinical trial. Diabetes, Obes. Metabolism 22 (1), 91–98. doi:10.1111/dom.13868
Rhman, M. A., DevNGEain, N., Khan, R., and Owira, P. M. (2022). Synergism potentiates oxidative antiproliferative effects of naringenin and quercetin in MCF-7 breast cancer cells. Nutrients 14 (16), 3437. doi:10.3390/nu14163437
Roy, A., Datta, S., Bhatia, K. S., Jha, P., and Prasad, R. (2022). Role of plant derived bioactive compounds against cancer. South Afr. J. Bot. 149, 1017–1028. doi:10.1016/j.sajb.2021.10.015
Salehi, B., Fokou, P. V. T., Sharifi-Rad, M., Zucca, P., Pezzani, R., Martins, N., et al. (2019). The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals 12 (1), 11. doi:10.3390/ph12010011
Sandhu, P. S., Kumar, R., Beg, S., Jain, S., Kushwah, V., Katare, O. P., et al. (2017). Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and Naringenin: systematic approach for improved breast cancer therapeutics. Nanomedicine Nanotechnol. Biol. Med. 13 (5), 1703–1713. doi:10.1016/j.nano.2017.03.003
Sauter, E. R. (2020). Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 13 (3), 265–285. doi:10.1080/17512433.2020.1738218
Schlender, J. F., Meyer, M., Thelen, K., Krauss, M., Willmann, S., Eissing, T., et al. (2016). Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin. Pharmacokinet. 55, 1573–1589. doi:10.1007/s40262-016-0422-3
Sera, L. C., and McPherson, M. L. (2012). Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin. geriatric Med. 28 (2), 273–286. doi:10.1016/j.cger.2012.01.007
Sharma, S., Hafeez, A., and Usmani, S. A. (2022). Nanoformulation approaches of naringenin-an updated review on leveraging pharmaceutical and preclinical attributes from the bioactive. J. Drug Deliv. Sci. Technol. 76, 103724. doi:10.1016/j.jddst.2022.103724
Shi, X., Luo, X., Chen, T., Guo, W., Liang, C., Tang, S., et al. (2021). Naringenin inhibits migration, invasion, induces apoptosis in human lung cancer cells and arrests tumour progression in vitro. J. Cell. Mol. Med. 25 (5), 2563–2571. doi:10.1111/jcmm.16226
Shulman, M., Cohen, M., Soto-Gutierrez, A., Yagi, H., Wang, H., Goldwasser, J., et al. (2011). Enhancement of naringenin bioavailability by complexation with hydroxypropyl-β-cyclodextrin. [corrected]. PloS one 6 (4), e18033. doi:10.1371/journal.pone.0018033
Smruthi, M. R., Nallamuthu, I., and Anand, T. (2022). A comparative study of optimized naringenin nanoformulations using nano-carriers (PLA/PVA and zein/pectin) for improvement of bioavailability. Food Chem. 369, 130950. doi:10.1016/j.foodchem.2021.130950
Stabrauskiene, J., Kopustinskiene, D. M., Lazauskas, R., and Bernatoniene, J. (2022). Naringin and naringenin: their mechanisms of action and the potential anticancer activities. Biomedicines 10 (7), 1686. doi:10.3390/biomedicines10071686
Sudhakaran, M., Sardesai, S., and Doseff, A. I. (2019). Flavonoids: new frontier for immuno-regulation and breast cancer control. Antioxidants 8 (4), 103. doi:10.3390/antiox8040103
Sun, Q., Tao, Q., Ming, T., Tang, S., Zhao, H., Liu, M., et al. (2023). Berberine is a suppressor of Hedgehog signaling cascade in colorectal cancer. Phytomedicine 114, 154792. doi:10.1016/j.phymed.2023.154792
Sun, Y., and Gu, J. (2015). Study on effect of Naringenin in inhibiting migration and invasion of breast cancer cells and its molecular mechanism. Zhongguo Zhong yao za zhi= Zhongguo zhongyao zazhi= China J. Chin. materia medica 40 (6), 1144–1150.
Uçar, K., and Göktaş, Z. (2023). Biological activities of naringenin: a narrative review based on in vitro and in vivo studies. Nutr. Res. 119, 43–55. doi:10.1016/j.nutres.2023.08.006
van den Boogaard, W. M., Komninos, D. S., and Vermeij, W. P. (2022). Chemotherapy side-effects: not all DNA damage is equal. Cancers 14 (3), 627. doi:10.3390/cancers14030627
Wallace, T. C., Bailey, R. L., Blumberg, J. B., Burton-Freeman, B., Chen, C. O., Crowe-White, K. M., et al. (2020). Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. food Sci. Nutr. 60 (13), 2174–2211. doi:10.1080/10408398.2019.1632258
Wang, M. J., Chao, P. D., Hou, Y. C., Hsiu, S. L., Wen, K. C., and Tsai, S. Y. (2006). Pharmacokinetics and conjugation metabolism of naringin and naringenin in rats after single dose and multiple dose administrations. J. Food Drug Analysis 14 (3), 4. doi:10.38212/2224-6614.2468
Wang, R., Wang, J., Dong, T., Shen, J., Gao, X., and Zhou, J. (2019). Naringenin has a chemoprotective effect in MDA-MB-231 breast cancer cells via inhibition of caspase-3 and -9 activities. Oncol. Lett. 17 (1), 1217–1222. doi:10.3892/ol.2018.9704
Wei, S., Sun, T., Du, J., Zhang, B., Xiang, D., and Li, W. (2018). Xanthohumol, a prenylated flavonoid from Hops, exerts anticancer effects against gastric cancer in vitro. Oncol. Rep. 40 (6), 3213–3222. doi:10.3892/or.2018.6723
Wojnar, W., Zych, M., and Kaczmarczyk-Sedlak, I. (2018). Antioxidative effect of flavonoid naringenin in the lenses of type 1 diabetic rats. Biomed. Pharmacother. 108, 974–984. doi:10.1016/j.biopha.2018.09.092
Xiong, H., Chen, Z., Lin, B., Xie, B., Liu, X., Chen, C., et al. (2022). Naringenin regulates FKBP4/NR3C1/NRF2 axis in autophagy and proliferation of breast cancer and differentiation and maturation of dendritic cell. Front. Immunol. 12, 745111. doi:10.3389/fimmu.2021.745111
Xu, H., Kulkarni, K. H., Singh, R., Yang, Z., Wang, S. W., Tam, V. H., et al. (2009). Disposition of naringenin via glucuronidation pathway is affected by compensating efflux transporters of hydrophilic glucuronides. Mol. Pharm. 6 (6), 1703–1715. doi:10.1021/mp900013d
Xu, Z., Huang, B., Liu, J., Wu, X., Luo, N., Wang, X., et al. (2018). Combinatorial anti-proliferative effects of tamoxifen and Naringenin: the role of four estrogen receptor subtypes. Toxicology 410, 231–246. doi:10.1016/j.tox.2018.08.013
Yang, Y., Trevethan, M., Wang, S., and Zhao, L. (2022). Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: an update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 104, 108967. doi:10.1016/j.jnutbio.2022.108967
Yao, L. H., Jiang, Y. M., Shi, J., Tomas-Barberan, F. A., Datta, N., Singanusong, R., et al. (2004). Flavonoids in food and their health benefits. Plant foods Hum. Nutr. 59, 113–122. doi:10.1007/s11130-004-0049-7
Yıldırım, M., Acet, Ö., Yetkin, D., Acet, B. Ö., Karakoc, V., and Odabası, M. (2022). Anti-cancer activity of Naringenin loaded smart polymeric nanoparticles in breast cancer. J. Drug Deliv. Sci. Technol. 74, 103552. doi:10.1016/j.jddst.2022.103552
Yıldırım, M., Sessevmez, M., Poyraz, S., and Düzgüneş, N. (2023). Recent strategies for cancer therapy: polymer nanoparticles carrying medicinally important phytochemicals and their cellular targets. Pharmaceutics 15 (11), 2566. doi:10.3390/pharmaceutics15112566
Yousuf, M., Shamsi, A., Khan, S., Khan, P., Shahwan, M., Elasbali, A. M., et al. (2022). Naringenin as a potential inhibitor of human cyclin-dependent kinase 6: molecular and structural insights into anti-cancer therapeutics. Int. J. Biol. Macromol. 213, 944–954. doi:10.1016/j.ijbiomac.2022.06.013
Zaidun, N. H., Thent, Z. C., and Abd Latiff, A. (2018). Combating oxidative stress disorders with citrus flavonoid: naringenin. Life Sci. 208, 111–122. doi:10.1016/j.lfs.2018.07.017
Zeng, X., Yao, H., Zheng, Y., He, Y., He, Y., Rao, H., et al. (2020). Tissue distribution of naringin and derived metabolites in rats after a single oral administration. J. Chromatogr. B 1136, 121846. doi:10.1016/j.jchromb.2019.121846
Zhang, F., Dong, W., Zeng, W., Zhang, L., Zhang, C., Qiu, Y., et al. (2016). Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast cancer Res. BCR 18 (1), 38. doi:10.1186/s13058-016-0698-0
Zhang, J., Wang, N., Zheng, Y., Yang, B., Wang, S., Wang, X., et al. (2023). Naringenin in Si-Ni-San formula inhibits chronic psychological stress-induced breast cancer growth and metastasis by modulating estrogen metabolism through FXR/EST pathway. J. Adv. Res. 47, 189–207. doi:10.1016/j.jare.2022.06.006
Zhao, Y., Tan, H., Zhang, J., Zhan, D., Yang, B., Hong, S., et al. (2024). Developing liver-targeted naringenin nanoparticles for breast cancer endocrine therapy by promoting estrogen metabolism. J. nanobiotechnology 22 (1), 122. doi:10.1186/s12951-024-02356-0
Zhao, Z., Jin, G., Ge, Y., and Guo, Z. (2019). Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 27, 1021–1036. doi:10.1007/s10787-018-00556-3
Keywords: cancer therapeutics, naringenin, phytochemicals, breast cancer, nanoformulations
Citation: Elsori D, Pandey P, Ramniwas S, Kumar R, Lakhanpal S, Rab SO, Siddiqui S, Singh A, Saeed M and Khan F (2024) Naringenin as potent anticancer phytocompound in breast carcinoma: from mechanistic approach to nanoformulations based therapeutics. Front. Pharmacol. 15:1406619. doi: 10.3389/fphar.2024.1406619
Received: 25 March 2024; Accepted: 29 May 2024;
Published: 18 June 2024.
Edited by:
Subhash Chander, Amity University, IndiaReviewed by:
Shatadal Ghosh, University of North Carolina at Chapel Hill, United StatesCopyright © 2024 Elsori, Pandey, Ramniwas, Kumar, Lakhanpal, Rab, Siddiqui, Singh, Saeed and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd Saeed, bW8uc2FlZWRAdW9oLmVkdS5zYQ==; Fahad Khan, ZmFoYWRpbnRlZ3JhbGlhbkBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.