- 1Department of Clinical Pharmacy, Key Laboratory of Basic Pharmacology of Guizhou Province and School of Pharmacy, Zunyi Medical University, Zunyi, Guizhou, China
- 2Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, Guizhou, China
- 3The Key Laboratory of Clinical Pharmacy of Zunyi City, Zunyi Medical University, Zunyi, Guizhou, China
- 4Department of Pharmacy, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Amentoflavone (AME) is a flavonoid compound found in over 120 plants. Its extensive pharmacological activity for treating cardiocerebrovascular diseases and neurological disorders have attracted the attention of researchers in recent years. However, owing to the poor solubility and low bioavailability of AME, it has not been developed as a drug for treating these diseases. This review focuses on two aspects of AME: First, it provides a detailed summary and introduction to AME based on its chemical structure, physicochemical properties, plant sources, extraction and purification methods, administration systems, and pharmacokinetic properties. Second, it summarizes the effects of AME on cardiocerebrovascular diseases and neurological disorders, and its specific pharmacological mechanisms. This review aims to promote the use of AME for treating cardiocerebrovascular diseases and neurological disorders. AME exhibits multiple activities, indicating its potential as a natural drug for treating these diseases. Further studies on its pharmacokinetics and toxicology are required to ensure its safety and efficacy.
1 Introduction
Cardiocerebrovascular diseases refer to a group of conditions that affect the heart, brain, and other tissues. This category includes both cardiovascular and cerebrovascular diseases, as well as a variety of ischemic and hemorrhagic conditions. These diseases are precipitated by factors such as hyperlipidemia, thickened blood, atherosclerosis, and hypertension (Yan and Guo, 2022). Cardiovascular diseases are prevalent among individuals aged over 50 years, causing high morbidity and ranking first as a cause of death (Ma et al., 2021). In 2019, cardiovascular diseases accounted for approximately one-third of global deaths, with the highest number of deaths occurring in China (Roth et al., 2020). According to the 2022 China Cardiovascular Health and Disease Report, China currently has 13 million people with stroke, 11.39 million people with coronary heart disease, 8.9 million people with heart failure, 5 million people with cor pulmonale, 4.87 million people with atrial fibrillation, 2.5 million people with rheumatic heart disease, 2 million people with congenital heart disease, 45.3 million people with peripheral artery disease, and 245 million people with hypertension (The Writing Committee Of The Report On Cardivascular Health And Diseases and Hu, 2023). Neurological disorders are also widely prevalent. Neurological diseases were the second leading cause of death globally in 2015, resulting in approximately 9.4 million deaths, accounting for 16.8% of deaths. From 1990 to 2015, the number of deaths due to neurological diseases increased by 36.7% and disability-adjusted life years increased by 7.4% (Global Burden of Disease Study, 2013 Collaborators, 2015; Zhou et al., 2024). The high morbidity and mortality rates associated with these diseases urgently require effective treatment modalities to curb their progression (Ginkgo biloba L.). extract (GBE) has been approved for clinical use for treating various cardiovascular, metabolic, and neurodegenerative disorders (Peng et al., 2024). One of the critical components in GBE is flavonoids, which comprise up to 24% of its total content (Tao et al., 2022).
Amentoflavone (AME) is a flavonoid compound first isolated from the Selaginella plant (Okigawa et al., 1971). AME is one of the most common biflavonoid compounds found in Ginkgo biloba (Šamec et al., 2022). However, some studies suggest that the AME in Ginkgo biloba leaf extracts exhibits no biological activity and AME has been removed from the listing of the active components of such extracts (Xu et al., 2013). Recent research has demonstrated that AME has a wide range of pharmacological properties, including anti-inflammatory (Zhang and Wang, 2013), antioxidant (Li et al., 2020), anti-aging (Park and Kim, 2019), antibacterial (Bajpai et al., 2019), antiviral (Lee et al., 2023), anti-tumor (Qiu S. et al, 2021), antidepressant and anxiolytic (Ishola et al., 2012). AME also has beneficial effects on cardiocerebrovascular diseases and neurological diseases (Li et al., 2021; Saeedan et al., 2023; Sirimangkalakitti et al., 2019).
A bibliometric analysis was conducted on the literature pertaining to AME from 2014 to 2023, providing insights into the research advancements in the field on a global scale. The keyword “Amentoflavone” was queried in the Web of Science database, yielding a total of 394 research papers from 63 countries and 641 institutions, authored by 2,196 individuals. Figure 1A displays the countries with the highest number of publications, providing insight into the level of interest in AME among these nations. Analysis of the data indicates a consistent upward trend in publication output over the past decade, with the number of articles published in recent years significantly surpassing those published in 2014. This trend suggests sustained interest and advancement in the field of AME, as depicted in Figure 1B. Research on the role of AME in cardiocerebrovascular diseases and neurological disorders has gained increasing attention since 2015, emerging as a current research hotspot in the field. Furthermore, the impact of AME on other conditions such as tumors and diabetes should not be underestimated.
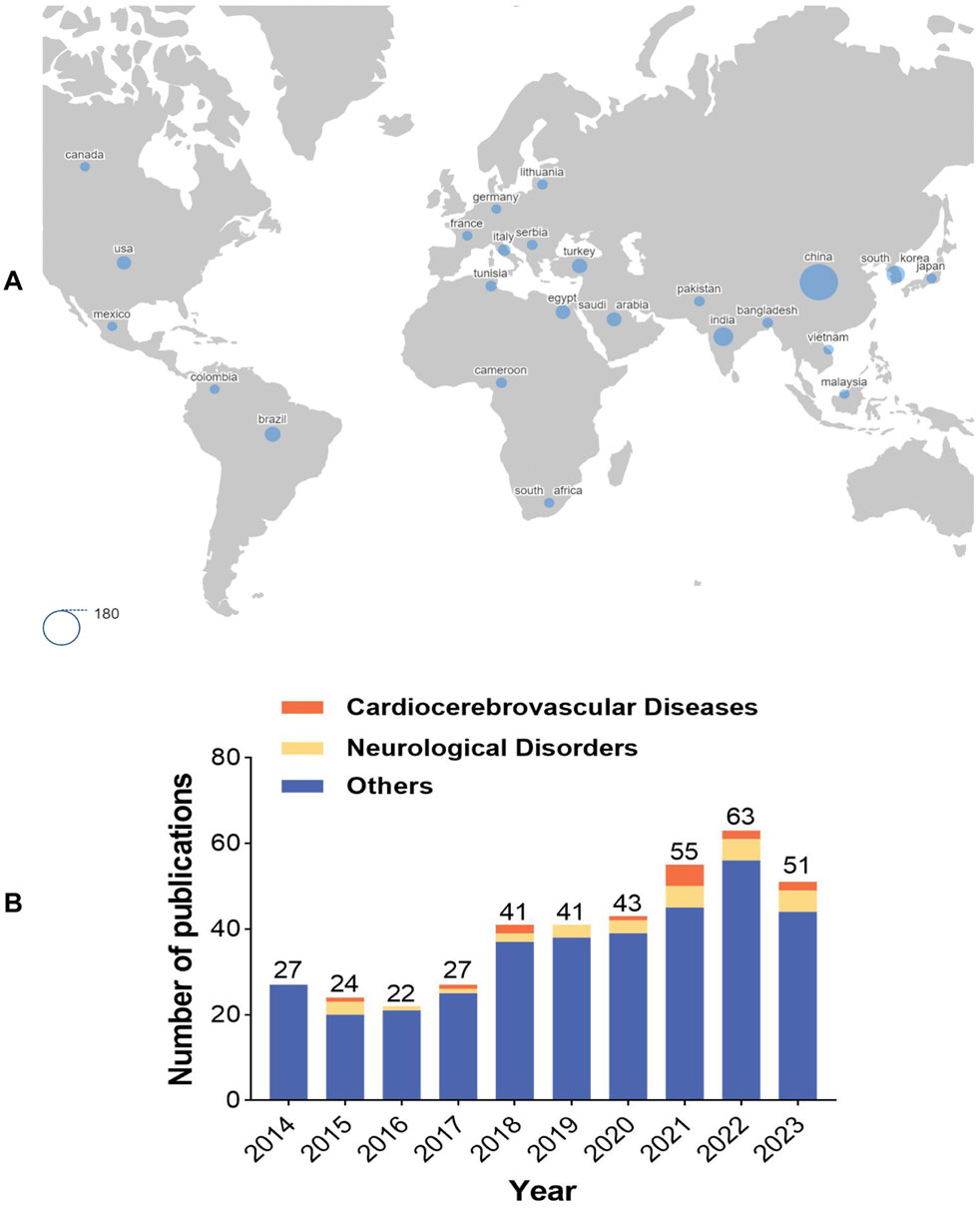
Figure 1. Bibliometric analysis of AME (A). Global distribution map of countries involved in AME. The larger the blue circle in the figure is, the greater the number of articles published by the corresponding country. The area of the circle in the lower left corner is equivalent to 180 articles. (B). Number of publications on AME in the past decade. Others represented other research fields of AME, with tumors and diabetes as the main research field.
This review provides a comprehensive summary of the chemical structure and physicochemical properties of AME, encompassing its botanical sources, extraction and purification techniques, and administration routes. Additionally, it examines the pharmacokinetic characteristics of AME, alongside its therapeutic effects on cardiocerebrovascular diseases and neurological disorders, elucidating the underlying pharmacological mechanisms. The objective of this review is to enhance the understanding of AME and its potential applications in the treatment of cardiocerebrovascular diseases and neurological disorders.
2 Background
2.1 Chemical structure and physicochemical properties of AME
AME is a biflavonoid compound with the chemical name 8-[5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one. It is an apigenin dimer, with multiple double bonds and hydroxyl groups in its molecular structure (Figure 2). The C2-C3 double bond is susceptible to hydrogenation, whereas the hydroxyl group is prone to substitution with methoxy groups. Therefore, many hydroxylated derivatives have an amentoflavone nucleus (Yu et al., 2017; Šamec et al., 2022; Xiao et al., 2018). It has been reported that the hydroxyl groups at positions C7 and C4''' are crucial for the anti-inflammatory activity of AME. Substitution of these hydroxyl groups with methoxy groups significantly reduces the anti-inflammatory efficacy (Mangmool et al., 2024). Additionally, the atropisomerism exhibited due to the C3’-C8” linkage in the structure of AME may affect its binding to targets, potentially influencing its biochemical activities (Bhattacharya and Mandal, 2024).
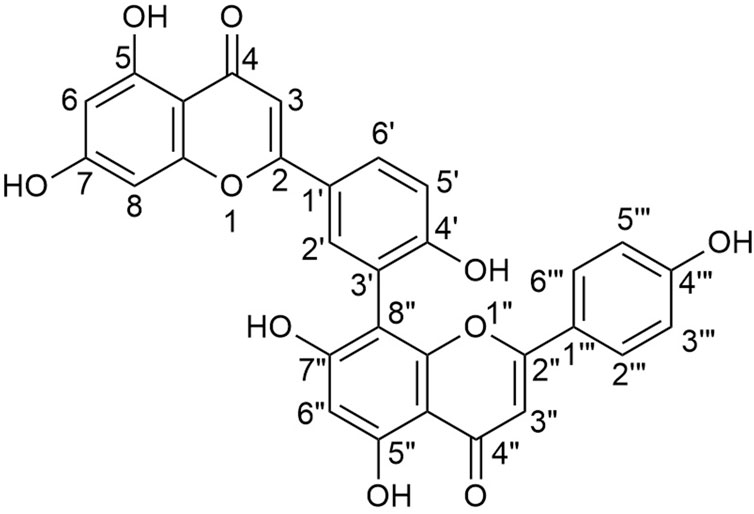
Figure 2. Chemical structure of amentoflavone (8-[5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one).
The molecular formula of AME is C30H18O10, the molecular weight is 538.46 g/mol, and the melting point is 300°C (Xiong et al., 2021). The cross-conjugated molecular structure has strong characteristic absorption of ultraviolet light (Li, 2011). AME is a planar molecule with a close arrangement between molecules and large intermolecular attraction; therefore, it is poorly soluble in water, but is easily soluble in organic solvents such as ethanol and dimethyl sulfoxide (Xiong et al., 2021). Among the various polymorphs of AME, the amorphous form exhibits higher dissolution rates and solubility, and demonstrates good physical stability during the dissolution process (Zhou et al., 2022). It has been reported that the monomer apigenin from AME can interrupt free radical chain reactions and reduce the photoxidation process by generating resonance-stabilized free radicals (Huvaere et al., 2012). The presence of catechol structures and a double bond adjacent to the carbonyl group, along with hydroxyl groups on a single benzene ring, plays a crucial role in effectively quenching singlet oxygen (Nagai et al., 2005). This indirectly suggests that AME may exhibit strong antioxidant and photostability properties.
2.2 AME sources, and methods of extraction, separation, and purification
2.2.1 Sources
AME is a bioactive compound present in numerous plant species. It was initially isolated from the leaves of (Selaginella tamariscina Maxim.), (Selaginella rupestris L.), and Ginkgo biloba. Subsequently, it has been extracted from over 120 plants species, including (Celaenodendron mexicanum L.), (Cupressus funebris Endl.), (Garcinia multiflora Bl.), and (Hypericum perforatum L.) (Xiong et al., 2021). The Selaginella genus, comprising 21 species, is the most prevalent source of amentoflavone. Other significant botanical families containing this compound include Cupressaceae, Euphorbiaceae, and Clusiaceae. Typically, AME is extracted from the leaves, aerial parts, and whole plants (Yu et al., 2017).
2.2.2 Extraction methods
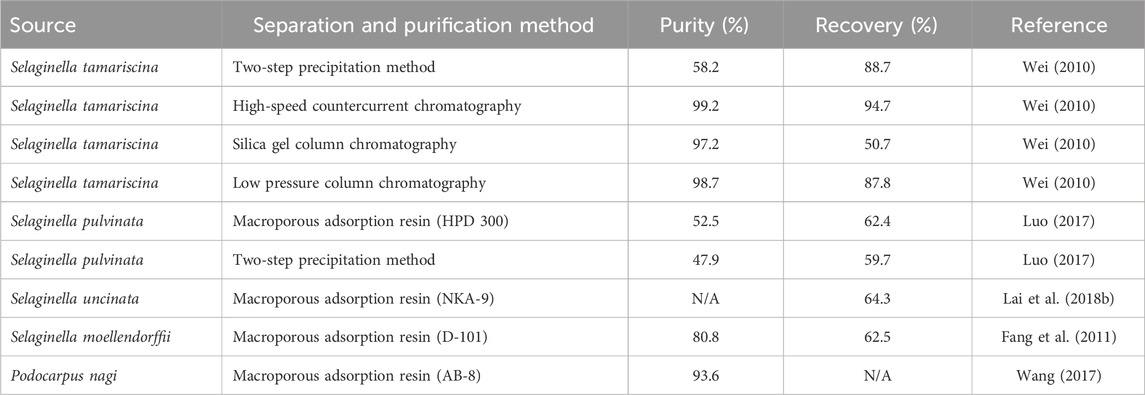
The principal methods for the extraction of AME include ultrasonic-assisted extraction, microwave-assisted extraction, organic solvent extraction, and semi-bionic extraction (Table 1). Each of these methodologies presents distinct advantages and disadvantages. For example, ultrasonic-assisted extraction offers benefits such as reduced extraction time, simplicity of operation, and high extraction efficiency. Nevertheless, its limited effective action area renders it unsuitable for industrial-scale production. Conversely, microwave-assisted extraction is noted for its straightforward operation, minimal byproduct formation, high extraction rates, and ease of product purification. However, it necessitates elevated extraction temperatures, which may compromise the integrity of active components. Organic solvent extraction is both cost-effective and straightforward; however, it suffers from low extraction efficiency, environmental pollution, and potential risks to human health make it less suitable (Li, 2011; Xu et al., 2021). Eutectic solvent (Liu et al., 2022) and infrared-assisted (Wang Y. et al., 2018) extraction techniques are gaining prominence in the extraction of AME due to their environmental sustainability, rapid processing times, and high efficiency.
2.2.3 Separation and purification methods
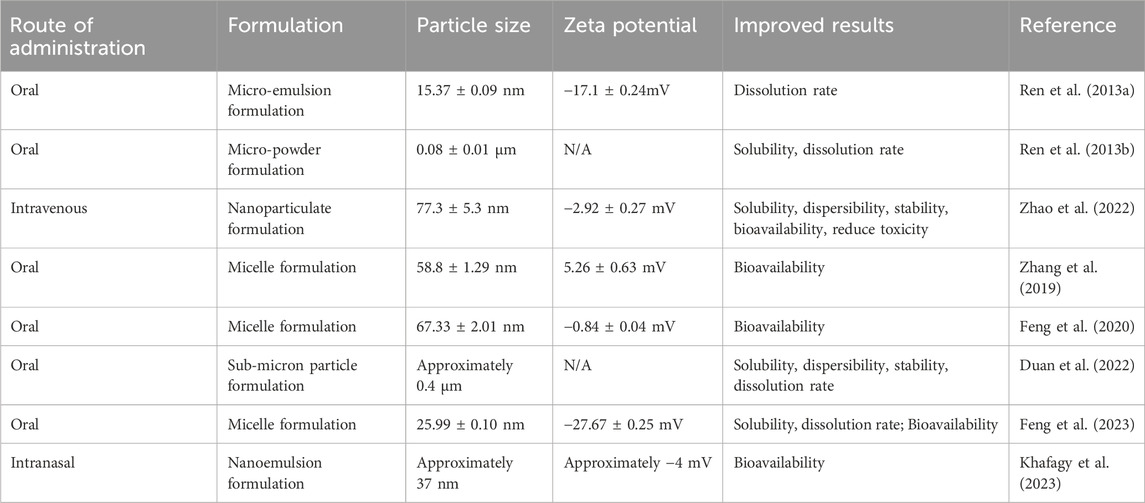
Currently, the primary methodologies for the separation and purification of AME are silica gel column chromatography, two-step precipitation, polyamide column chromatography, macroporous resin adsorption, and preparative high-performance liquid chromatography (HPLC) (Table 2). Researchers have been actively investigating enhanced techniques for separation and purification. Recently, flash chromatography has emerged as a novel method. Compared to traditional silica gel column chromatography, flash chromatography offers reduced the loading time, minimized dead adsorption, and improved efficiency and product purity. However, it exhibits lower separation capacity and is prone to interference from metal ions during elution. Macroporous resin adsorption offers several advantages, including rapid high-capacity and selective adsorption, as well as high elution efficiency, rendering it suitable for large-scale production. Nonetheless, its desorption efficiency remains suboptimal, and the purification rate is affected by the type of eluent and temperature (Xu et al., 2021). HPLC offers advantages including wide applicability, exceptional quantitative capabilities, and well-recognized methodologies. Nevertheless, it is marked by the necessity for extensive sample preparation, prolonged analysis durations, and expensive instrumentation (Vlasiou, 2023).
2.3 AME dosing forms
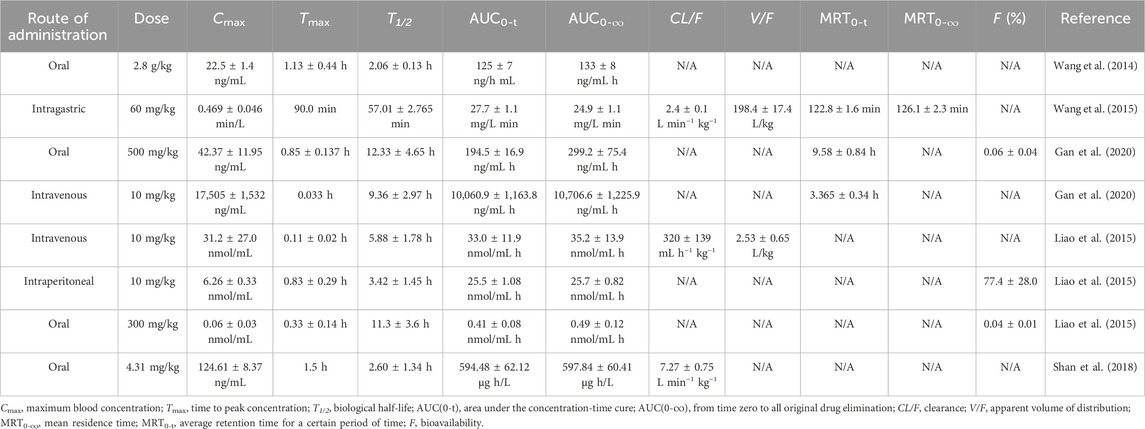
AME exhibits significant pharmaceutical potential and promise as a therapeutic agent (Xiong et al., 2021; Li et al., 2021). However, its poor water solubility constrains its release within the body, resulting in incomplete gastrointestinal absorption and low oral bioavailability, thereby limiting its pharmacological efficacy (Ren et al., 2013a). Following oral administration in rats, AME is dominantly distributed in the small intestine, stomach, liver, and large intestine, with minimal distribution to other tissues. To maximize the pharmacological efficacy of AME, it is imperative to enhance its solubility and bioavailability. Key strategies for improving drug solubility and bioavailability include modifying the delivery method, formulation (Table 3), and structural enhancement (Feng et al., 2020).
2.4 Pharmacokinetics of AME
Despite the multiple beneficial biological properties of AME, its pharmacokinetics remain inadequately characterized. A comprehensive understanding of AME’s pharmacokinetics is crucial for elucidating its in vivo mechanisms of action, characterizing its properties, and optimizing drug design and dosing, to maximize therapeutic efficacy. Insights into the fundamental pharmacokinetics of AME can be inferred from studies conducted in animal models (Table 4).
It has been demonstrated that AME is absorbed via passive diffusion in rats (Wei et al., 2017). Furthermore, a study employing the Caco-2 cell model indicated that AME exhibits intestinal absorption. The absorption may involve paracellular passive diffusion and clathrin-mediated endocytosis, while the efflux transporter appears to be uninvolved (Wang B. et al., 2020). The low bioavailability of AME is due to extensive glucuronidation catalyzed by uridine diphosphate glucuronosyltransferase 1 family, polypeptide A (UGT1A1) and UGT1A3 (Gan et al., 2020).
Another study conducted demonstrated that the peak blood concentration in rats was reached at 90 min following oral administration, with a volume of distribution (V/F) of 198.36 ± 17.422 L/kg. These findings suggest that AME is predominant distributed or sequestered in specific tissues and organs within the rat model. (Wang et al., 2015).
In vivo, 34 metabolites of AME were identified, while 24 metabolites were identified in vitro. In the in vivo study, all metabolites were distributed in feces, with three of the 34 metabolites were also detected in urine, and none were found in bile or plasma. In the in vitro study, 20 of the 24 metabolites were distributed in liver microsomes, and 17 of the 24 metabolites were associated with the intestinal microbiota. Of the total metabolites identified both in vivo and in vitro, 14 were classified as phase I metabolites and 26 as phase II metabolites. The primary metabolic pathways included oxidation, methylation, acetylation, and oxidation methylation (Feng, 2020). It has been reported that the bioactive form of AME is likely conjugated (Liao et al., 2015). Another study found that 90.7% of AME circulates as conjugated metabolites post-administration. In rats, 73.2% and 70.2% of AME in plasma were in conjugated form following intravenous and intraperitoneal injection, respectively (Yu et al., 2017).
The cumulative excretion rates of AME in feces and urine were 23.93% and 0.82%, respectively, indicating that fecal excretion is the predominant route of AME elimination from the body. This high rate of fecal excretion may contribute to the compound’s low bioavailability (Chen et al., 2022).
3 Biological activity of AME for treating cardiocerebrovascular and neurological diseases
Atherosclerosis is a main pathological basis for cardiovascular and cerebrovascular diseases (Dutta et al., 2023). The cerebrovascular system is closely related to the structure and function of brain tissue. Vascular factors are important in the development of neurological diseases (Iadecola, 2023; Smith et al., 2021). Therefore, this section discusses the effect of AME on cardiocerebrovascular and neurological diseases (Figure 3).
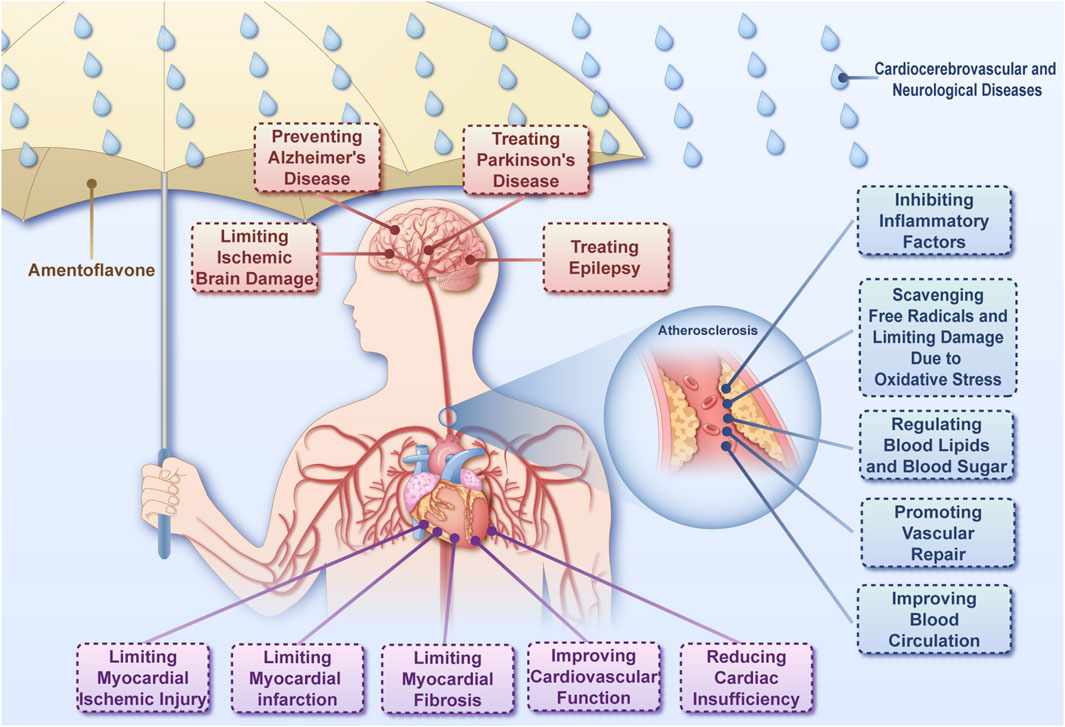
Figure 3. The ameliorative effect of amentoflavone on cardiocerebrovascular and neurological diseases Amentoflavone is capable of acting on cardiocerebrovascular diseases, neurological diseases through a variety of mechanisms.
3.1 Potential mechanisms of AME in atherosclerosis
Although there is currently no published literature regarding the use of AME in the treatment of atherosclerosis, its various potential mechanisms of action are promising. The process of atherosclerotic plaque formation can be divided into the following steps (Figure 4): (1) When the vascular wall is damaged, low-density lipoprotein (LDL) enters the intimal layer of the blood vessel through the gaps between endothelial cells (ECs), forming oxidized low-density lipoprotein (ox-LDL). Monocytes migrate into the intimal layer and are activated into macrophages. (2) Ox-LDL binds to receptors on the surface of macrophages, and cholesterol enters the macrophages, where it is esterified. When the intake, esterification, and release of cholesterol are out of equilibrium, intracellular lipid overload leads to the formation of macrophage-derived foam cells, creating fatty streaks in the lesions (Gui et al., 2022). (3) Ox-LDL induces changes in the phenotype of vascular smooth muscle cells (VSMCs) in the tunica media of the arterial wall, leading to abnormal proliferation and migration to the intimal layer. Subsequently, VSMCs engulf ox-LDL to form myogenic foam cells (Yang, 2023; He, 2013), which then form a fibrous plaque. (4) Necrosis and disintegration of macrophage-derived and myogenic foam cells lead to atherosclerotic plaque formation. Inflammatory cells secrete matrix metalloproteinases (MMPs) to break down collagen fibers in the extracellular matrix, thereby increasing plaque instability. Subsequently, the plaque ruptures, causing bleeding and thrombosis (Carracedo et al., 2019; Libby, 2021; Poznyak et al., 2020). Atherosclerosis is a chronic inflammatory disease involving various inflammatory, free radical, and oxidative stress-related injuries (Meng et al., 2024).
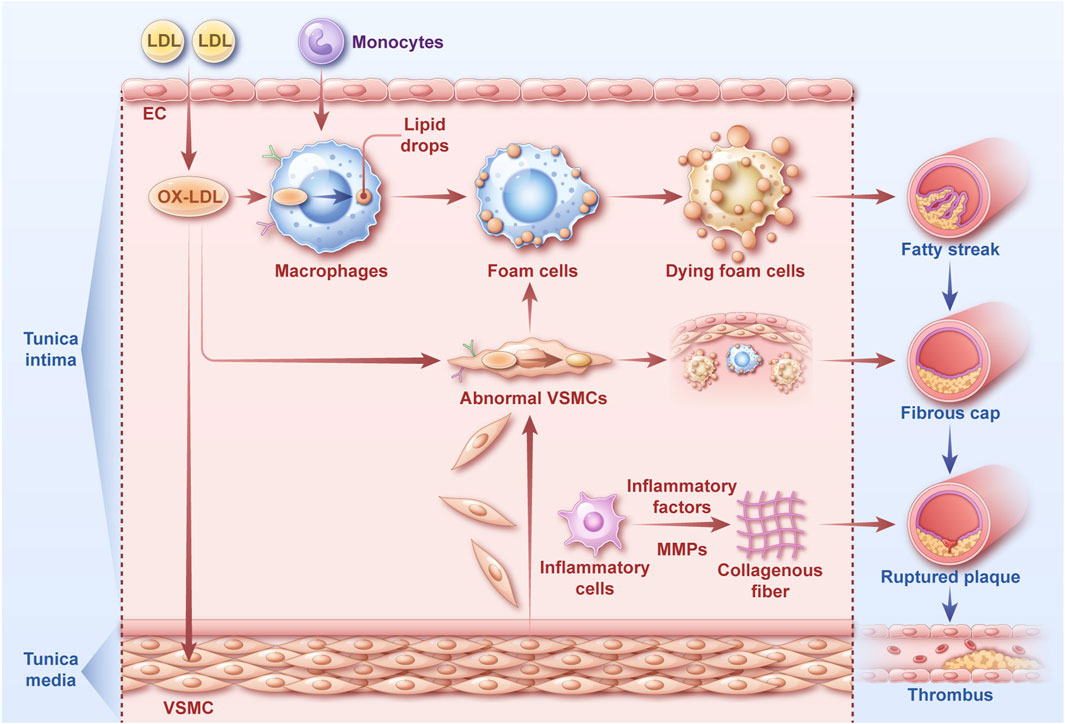
Figure 4. The atherosclerotic process The key steps of the atherosclerotic process can be divided into the following steps: Formation of oxidized low-density lipoprotein and activation of macrophages; Macrophage-derived foam cell formation; Abnormal VSMCs and myogenic foam cell formation; Formation and rupture of atherosclerotic plaque.
3.1.1 Inhibiting inflammatory factors
When inflammation occurs in vivo, IκB kinase phosphorylates inhibitor of NF-κB (IκB) protein, and then IκB protein is separated from the p50 and p65 subunits of nuclear factor-kappa B (NF-κB), so that NF-κB is activated and enters the nucleus for gene transcription and expression of inflammatory factors, inflammatory mediators, chemokines, and adhesion factors (Guo et al., 2024). AME can protect vascular ECs through various mechanisms. AME increases the survival of human umbilical vein endothelial cells (HUVECs) induced by TNF-α in the S phase of cell proliferation. Disruption of the endothelin-1 (ET-1)/nitric oxide (NO) balance in the blood is an indicator of vascular endothelial damage. AME can increase the NO content of HUVECs, reduce the level of ET-1, inhibit the expression of adhesion factors vascular cell adhesion molecule-1 and E-selectin, and inflammatory factors interleukin IL-6 and IL-8, enhance the expression of the inhibitory protein IκBα activated by NF-κB, and reduce the expression of its transcription factor NF-κB in the nucleus, preventing further damage to the vascular endothelium (Zheng et al., 2013). AME downregulates the release of NO from mouse macrophages stimulated by lipopolysaccharide (LPS), and the levels of TNF-α and IL-1β induced by LPS and monosodium urate in THP-1 macrophages, via the NOD-like receptor thermal protein domain associated protein 3 (NLRP3)/apoptosis-associated speck-like protein/cysteinyl aspartate specific protease (caspase)-1 signaling pathway (Zhang X. et al., 2021). An experiment was conducted to examine the binding effects of 34 flavonoid products on the NLRP3 inflammasome, using CB-Dock molecular docking for binding predictions. It was found that AME has the strongest affinity for the NLRP3 inflammasome, surpassing that of its specific inhibitor (CY-09) (Fang H. Y. et al., 2023). Macrophage migration inhibitory factor (MIF) is a crucial pro-inflammatory mediator. AME has been reported to exhibit superior inhibitory activity against MIF compared to ISO-1, a well-established standard MIF inhibitor (Siddiqui et al., 2024).
During inflammation, arachidonic acid is converted to prostaglandin E−2 (PGE-2) by cyclooxygenase-2 (COX-2) through the action of cyclooxygenase-2, leading to pain and inflammation. At a concentration of 50 μM, AME can inhibit the activity and expression of COX-2 induced by TNF-α in cells, and upregulate the activity of peroxisome proliferator-activated receptor (PPAR) γ, blocking the degradation of IκBα, and inhibiting the translocation of NF-κB to inhibit the activation of the NF-κB signaling pathway (Banerjee et al., 2002). AME isolated from the root of (Prismatomeris glabra Mart.) exerts an anti-inflammatory effect by significantly reducing the production of TNF-α, IL-6, and PGE-2 in THP-1-derived macrophages in a dose-dependent manner (Alkadi et al., 2021). AME effectively suppresses the production of NO and PGE-2 in RAW264.7 macrophage cells stimulated by LPS. This inhibitory effect is achieved by inhibiting the kinase activity of extracellular signal-regulated kinase (ERK). Furthermore, AME also inhibits the expression of inflammation-related genes induced by LPS, including nitric oxide synthase (iNOS), COX-2, and TNF-α (Oh et al., 2013).
Macrophages of different polarization types are involved in distinct atherosclerosis development processes. Specifically, M1-type macrophages are primarily present in early plaques, associated with plaque formation. M2-type macrophages can exert anti-inflammatory effects, promoting the repair of atherosclerosis inflammation. PPARs are a class of ligand-activated transcription factors that play a crucial role in macrophage polarization, regulating macrophage metabolism, suppressing pro-inflammatory genes, and promoting the transformation of M2 macrophage phenotype (Zhuang, 2020). PPARγ, through its interaction with other transcription factors and the promoter regions of arginase 1 (Arg1), found in inflammatory zone (Fizz1), and chitinase-like protein 3 (Ym1) genes, promotes the expression of Arg1 and Fizz1 genes and regulates the M2 polarization level of macrophages. Macrophages lacking PPARγ exhibit significantly downregulated expression of Arg1. AME can inhibit the differentiation of THP-1-derived M0 cells toward M1 cells by activating PPAR-α/γ transcription factors, elevating the mRNA levels of TGF-β and IL-10, reducing TNF-α and IL-6 expression, and upregulating Arg1 and Fizz1 protein expression (Qiu F. et al., 2021). These findings suggest that AME might also reverse the transformation of M1 macrophages toward the M2 type, thereby exerting an anti-inflammatory effect.
3.1.2 Scavenging free radicals and limiting damage due to oxidative stress
NO produced iNOS in activated macrophages is one of the most important inflammatory mediators. iNOS-mediated NO production and the associated production of highly reactive free radicals such as peroxynitrite has a harmful effect (Wang Y. et al., 2020). Therefore, inhibiting NO may be a useful target for addressing oxidative stress.
In vitro antioxidant models have demonstrated that AME exhibits exceptional scavenging and antioxidant capabilities when eliminating (1,1-diphenyl-2 trinitrophenylhydrazine) DPPH free radicals, superoxide anions, and hydroxyl radicals. Additionally, AME possesses the ability to repair and protect DNA from oxidative damage in vitro. AME influences the generation and scavenging of OH free radicals, and eliminates free radicals by scavenging hydrogen ion and electron. Therefore AME can treat oxidative damage (Wang, 2013). AME can eliminate DPPH free radicals in a concentration-dependent manner and can alleviate the cell damage caused by ox-LDL in HUVECs. The mechanism might be due to the phenolic hydroxyl group in the molecular structure of AME, which can accept the electron transfer from lipid peroxidation free radicals, forming stable free radicals, thus preventing the damage of lipid peroxidation free radicals to vascular ECs (Xu et al., 2004). Advanced glycation end-products (AGEs) are associated with various diseases such as atherosclerosis and diabetes, leading to the production of reactive oxygen species (ROS), which subsequently activates the transcription factor NF-κB and is involved in various inflammatory diseases. Ferchichi et al. extracted various active components from plants, and showed that AME had the strongest in vitro ability to effectively resist AGEs and exhibits excellent scavenging capabilities (Ferchichi et al., 2012). Furthermore, AME can block the nuclear translocation of NF-κB p65, inhibit the phosphorylation of IκBα and formation of NO in macrophages induced by LPS in a concentration-dependent manner by blocking the degradation of IκBα to inhibit the formation and transcription activation of the iNOS gene induced by LPS (Woo et al., 2005).
AME reduces LPS-induced oxidative stress damage to HUVECs, increasing the activity of superoxide dismutase (SOD) and reducing the expression levels of NO and malondialdehyde (MDA). A multi-omics study found that glycine, argininosuccinic acid, putrescine, ornithine, spermidine, 5-oxoproline, and dihydrouracil are seven metabolites that might be related to the mechanism by which AME protects ECs (Yao et al., 2016).
The reduced form of thioredoxin (Trx) interacts with the N-terminus of apoptosis signal regulating kinase 1 (ASK1) both in vitro and in vivo, thereby inhibiting ASK1 activity. Under conditions of oxidative stress, the thiol group of the cysteine residue of Trx is oxidized to form intramolecular disulfide bonds, thereby activating ASK1 kinase activity. Therefore, in the complex of Trx with ASK1 protein, Trx1 and thioredoxin reductase (TrxR)-1 proteins are the key molecules regulating ROS-induced ASK1 activation. AME can regulate the ROS/ASK1/p38 mitogen-activated protein kinase (MAPK) pathway by inactivating ASK1 molecules, blocking p38 MAPK signaling, increasing the levels of thioredoxin Trx1 and reductase TrxR-1, and reducing ASK1 and p38 MAPK levels, thereby reducing oxidative stress damage to cells (Li et al., 2020). These studies demonstrate that AME can respond to oxidative stress through various mechanisms.
3.1.3 Regulating blood lipids and blood sugar
The fat accumulation index can serve as a predictive indicator of cardiometabolic diseases and stroke. The fat accumulation index is linearly related to the risk of atherosclerotic cardiovascular disease within 10 years and is an independent risk factor, indicating an integral relationship between body fat and atherosclerotic diseases (Liu et al., 2023). A randomized trial involving over 20 million participants demonstrated that blood lipid components such as LDL, apolipoprotein A, apolipoprotein B, and triacylglycerols can also affect the development of atherosclerosis (Ference et al., 2017). Therefore, controlling the level of blood lipids can alleviate and prevent atherosclerosis.
Obesity leads to the storage of excess energy in the form of triglycerides (TG) in adipose tissue. Dietary fat can lead to increased TG levels in the blood and obesity, whereas lipid absorption disorders can lead to hyperlipidemia and metabolic diseases. The cluster of differentiation 36 (CD36) protein primarily participates in the process of macrophage lipid uptake. When a large amount of ox-LDL appears in the vascular endothelial layer, the CD36 protein is activated to take up ox-LDL into the cell. After intake, ox-LDL is oxidized to new derivatives by linoleic acid, and these new derivatives can activate PPARγ, thereby promoting the increase in PPARγ protein expression as a transcription factor of CD36. Increased CD36 expression in turn promotes ox-LDL uptake. AME can reduce the uptake of ox-LDL by THP-1-derived macrophages through the CD36/PPARγ signaling pathway, thereby inhibiting foam formation induced by ox-LDL (Zhuang, 2020). Feeding AME to mice on a high-fat diet reduced the expression of the lipid absorption-related gene, CD36, by affecting plasma TG levels, thereby affecting the intestinal absorption of lipids (Lee et al., 2022). AME can reduce the body weight, total fat tissue, and serum TG content induced by a high-fat diet in a dose-dependent manner. AME has been demonstrated to reduce blood glucose levels and insulin resistance in rat models. Furthermore, AME influences various stages of 3T3-L1 adipocyte differentiation. Specifically, it modulates ROS production and inhibits the expression of the transcription factor CCAAT/enhancer-binding protein (C/EBP)-β during the mitotic clonal expansion (MCE) phase, thereby impacting MCE. Additionally, AME downregulates the expression of PPARγ and C/EBP-α during both the early and terminal differentiation phases, consequently affecting lipid droplet formation. (Chen et al., 2016).
Type 2 diabetes is intricately linked to atherosclerosis. Patients with diabetes often have disorders of lipid metabolism and insulin resistance syndrome. Elevation of blood glucose and lipid levels in patients with type 2 diabetes are risk factors for atherosclerosis, and are positively correlated with the extent of atherosclerosis lesions (Sun et al., 2023; Huang and Sun, 2023). High blood glucose levels disrupt ECs function, causing damage the vascular wall, thereby promoting atherosclerosis progression (Wei and Liang, 2021). AME significantly suppresses the elevation of blood glucose levels and reduces the Homeostatic Model Assessment of Insulin Resistance index. Furthermore, they demonstrated that AME also reduced lipid accumulation in the liver of high fructose and fat diet (HFFD)-fed rats and alleviated the damage caused by lipids to the liver (Qin et al., 2018). AME reduced the expression of genes related to fat production and upregulated the expression of genes related to insulin signaling transmission, thereby having anti-obesity and anti-hyperglycemic effects (Cho et al., 2021).
In vitro hypoglycemic effects of AME using HepG2 cell glucose models and insulin resistance models and found that AME significantly increased glucose metabolism of HepG2 cells and had a synergistic effect on the increased glucose consumption under insulin stimulation. In the insulin resistance HepG2 cell model induced by high insulin, AME also increased the glucose consumption of cells that developed insulin resistance (Zheng et al., 2008). These characteristics suggest that AME may serve as an insulin sensitizer, increasing cell glucose consumption and synergistically working with insulin to reduce blood glucose levels and reduces insulin resistance. In the insulin resistance HepG2 cell model induced by high glucose and high insulin, AME significantly increased the levels of rate-limiting enzymes for glucose oxidative decomposition, such as 6-phosphogluconate kinase, glucose kinase, and pyruvate kinase; reduced glucose synthesis by lowering glucose synthesis kinase-3-β (GSK-3-β) levels; and lowered phosphoenolpyruvate carboxy kinase and glucose-6-phosphate enzyme activity, thereby affecting the glucose biosynthesis pathway. They also demonstrated that AME increases phosphoinositide 3-kinase (PI3K) protein expression, which may improve insulin signal transduction disorders through the PI3K/protein kinase B (Akt) signaling pathway, thereby alleviating insulin resistance (Ke et al., 2013). In type 2 diabetes model (T2DM) rats, AME reduces the release of TNF-α, upregulates glucose transporter 2 expression, enhances the absorption and utilization of blood glucose by the liver and skeletal muscle, and reduces insulin resistance through the PI3K/Akt/mechanistic target of rapamycin (mTOR) and PPARγ signaling pathways (Zhang et al., 2019).
At a dose of 60 mg/kg AME can repair damaged pancreatic tissues in mice with diabetes and enhanced pancreatic islet β cell function (Zheng et al., 2008). The molecular structure of AME can stably bind to the structure of human islet amyloid polypeptide (hIAPP), thereby interfering with the peptide assembly and abnormal folding process, separating hIAPP fibrils into small oligomers and particles, and reducing the cytotoxicity induced by hIAPP oligomerization (Xu et al., 2022). These results indicate that AME can regulate blood lipids and blood glucose from multiple perspectives and different mechanisms and can alleviate various injuries.
3.1.4 Promoting vascular repair
Endothelial growth factor (VEGF) is a key factor in the early formation of blood vessels and a highly selective mitogen for ECs, promoting the proliferation and migration of ECs to promote angiogenesis (Wiszniak and Schwarz, 2021). AME promotes the proliferation of HUVECs in the mitotic S phase in a dose-dependent manner, and upregulates the expression of VEGF, indicating that it plays a role in repairing vascular endothelial cell damage (Zheng et al., 2011). However, under pathological conditions, VEGF increases the instability of atherosclerotic plaques by promoting angiogenesis and inflammatory infiltration, leading to plaque shedding (Qin et al., 2021). Using two different experimental models, it was found that AME can specifically bind to members of the VEGF family, VEGF-A and placental growth factor-1 (PIGF-1), preventing them from further binding to their receptors and inhibiting endothelial cell migration and capillary-like tube production induced by VEGF-A and PIGF-1, thereby inhibiting the growth and formation of vessels (Tarallo et al., 2011). The abnormal proliferation and migration of VSMCs can lead to vascular lesions, which are the hallmark of atherosclerosis, vascular intimal hyperplasia, and arterial stenosis. AME can inhibit the ox-LDL-induced transformation of VSMCs to foam cells through the CD-36/PPARγ signaling pathway, and can inhibit VSMC migration, thereby promoting vascular repair (Zhuang, 2020).
3.1.5 Improving blood circulation
The vasodilator effect of AME may be associated with the muscarinic receptor, the β-adrenergic receptor, and the endothelium-derived vasodilator factor. NO is produced by the catalytic action of iNOS on L-arginine. The vasodilator effect of NO is mediated through the increase of guanosine 3′,5′-cyclic monophosphate (cGMP) levels in smooth muscle. When the vascular endothelium experiences dysfunction, the release of NO decreases, and the vasoconstriction produced by directly activating vascular smooth muscle further reduces the production of NO (Borow et al., 2015). AME has a significant vasodilator effect; however, after injury to the vascular endothelium of injured rats, its vasodilator effect is inhibited, suggesting that AME may act on the vascular endothelium. The vasodilator effect of AME is also inhibited by NO inhibitors in intact vascular endothelium but is not affected by the addition of propranolol hydrochloride and atropine, suggesting that AME may produce a vasodilator effect by affecting the release of NO from the vascular endothelium (Xun and Yin, 2009). iNOS inhibitors can block the vasodilator effect of AME. Guanylate cyclase inhibitors also block the vasodilator effect of AME. Based on a series of experiments, they concluded that AME activates the Ca2+-dependent K+ channel of ECs, affecting the NO-cGMP signaling pathway, thereby relaxing the vascular smooth muscle and causing vasodilation (Kang et al., 2004).
After the rupture of atherosclerotic plaques, the activation of platelets and thrombin promotes thrombus formation, leading to vascular blockage and interruption of circulation (Ahmed et al., 2020). Thrombin is a serine protease that plays a significant role in the coagulation cascade, thrombus formation, and platelet activation (Brummel et al., 2002). Therefore, drugs that act on thrombin can alleviate disease progression caused by atherosclerosis. AME in (St. John’s Wort Diosc.) can inhibit the activity of human thrombin in a dose-dependent manner (Wei et al., 2019). The study compared the effects of 16 major components in Ginkgo biloba on thrombin and found that AME has a high affinity for human thrombin and is a strong human thrombin inhibitor. Subsequent molecular docking experiments revealed that it can primarily bind to key amino acids in the active site of thrombin through salt bridges and hydrogen bonds (Chen et al., 2019). Using an acute rat blood stasis model, it was demonstrated that AME can reduce plasma fibrinogen levels to prolong coagulation time, thereby improving blood circulation (Xi et al., 2020). Furthermore, AME has been shown to inhibit platelet aggregation induced by adenosine diphosphate and arachidonic acid, but has no effect on platelet aggregation induced by thrombin (Zhang et al., 2018). The mechanism underlying these effects warrant further study. These results suggest that AME could serve as a natural drug for targeting thrombin and platelets.
3.2 Effect of AME on cardiocerebrovascular diseases
3.2.1 Limiting myocardial ischemic injury, myocardial infarction and myocardial fibrosis
Myocardial ischemic injury can lead to myocardial infarction, and myocardial fibrosis may occur after infarction, a process that further affects heart function. If left untreated, myocardial ischemia can lead to damage of the heart, ultimately increasing the risk of developing myocardial infarction over time. Patients who have had a myocardial ischemia often have persistently elevated TNF-α levels. (Ridker et al., 2000). Overexpression of TNF-α, IL-1β, and IL-6 can amplify the harmful effects of inflammation by inducing cell apoptosis. Therefore, inhibiting these inflammatory factors is a promising strategy to prevent the escalation of myocardial ischemic injury. (Kumari et al., 2024).
Ischemic reperfusion injury experiments in mice, have shown that AME significantly reduces the levels of serum myocardial enzymes, specifically lactate dehydrogenase and creatine kinase MS isoenzyme, indicating the protective effects of AME on H9c2 myocardial cells. AME was also shown to significantly reduce the levels of IL-1β, IL-6, and TNF-α in cell supernatants, inhibiting cell apoptosis after myocardial ischemia-reperfusion injury in rats and reducing the size of the myocardial infarction area (Li et al., 2021). It was discovered that AME improved myocardial ischemia-reperfusion injury by modulating the PI3K/Akt-NF-κB signaling pathway, enhancing the phosphorylation of Akt and inhibiting the phosphorylation of NF-κB, reducing cardiac cell apoptosis, and lowering the release of associated inflammatory factors (Li, 2020). In the myocardial infarction model, AME can reduce the values of left ventricular end-systolic diameter and left ventricular end-diastolic diameter, and increase the value of left ventricular ejection fraction, indicating that AME can effectively improve the cardiac function of rats after myocardial infarction. AME can also reduce the expression of carboxy terminal peptide of type I procollagen and the amino terminal peptide of type III procollagen in rats after myocardial infarction, indicating that AME can inhibit myocardial fibrosis after myocardial infarction. The mechanism may involve downregulation of matrix metalloproteinase MMP-2 and transforming growth factor TGF-β1 expression (Chen et al., 2023).
3.2.2 Reducing cardiac insufficiency
AME can improve adriamycin (DOX)-induced cardiac dysfunction and reduce myocardial injury. Furthermore, AME can significantly reduce the expression of cell pyroptosis-related proteins, such as NLRP3, cleaved caspase-1, and cleaved gasdermin D, thereby inhibiting myocardial cell pyroptosis without affecting the expression of apoptosis-related proteins. The primary mechanism of AME action is through the inhibition of the stimulator of interferon genes (STING)/NLRP3 inflammasome signaling pathway (Fang G. et al., 2023). The effect of AME on DOX-induced cardiotoxicity was investigated from several perspectives, including pathological characterization, antioxidant stress, mitochondrial function recovery, anti-inflammatory effects, and apoptosis inhibition. It was found that AME increases heart weight to reverse DOX-induced heart atrophy, ameliorates oxidative stress-induced cardiac injury by reducing MDA, inhibits NADPH oxidase (NOX) expression, increases SOD levels, upregulates the expression of myocardial mitochondrial-related genes nuclear respiratory factor-1 (NRF-1) and mitochondrial transcription factor A (TFAM) to address mitochondrial dysfunction, decreases IL-6 and NF-κB expression to exert anti-inflammatory effects, upregulates heat shock protein 27 (HSP-27) and downregulates fas ligand (Fasl) expression to influence the process of cell apoptosis (Alherz et al., 2022). Through comparing effects of different dietary supplements on rat atria, it was discovered that AME and quercetin might be the pharmacologically active components of GBE in terms of its positive inotropic and chronotropic effects. Moreover, 10–50 μg/mL of AME can significantly increase the heart rate of rat atria without altering myocardial contractility (Kubota et al., 2002). However, other studies have shown that AME can inhibit the activity of cAMP diesterase, thereby enhancing myocardial contractility and dilating peripheral vessels, further promoting blood flow in the body and reducing pressure on the heart and vessels (Saponara and Bosisio, 1998). This suggests that AME is a potential drug for the treatment of heart failure.
3.2.3 Improving cardiovascular function
It was discovered that AME exerts a protective effect on cardiovascular dysfunction through several mechanisms: (1) Ultrasonic electrocardiographic evaluations have shown that AME can inhibit the increase in left ventricular internal diameter and the thickness of the posterior wall during diastole, reduce left ventricular mass, alter the ejection fraction and relative wall thickness, and inhibit the increase in cardiac stiffness and left ventricular wet weight induced by an HFFD. (2) In settings of oxidative stress, AME can modify the levels of oxidative stress markers such as thiobarbituric acid reactive substances, glutathione (GSH), SOD, catalase (CAT) in plasma, and can inhibit the increase in NOX in the heart, thereby reducing the degree of oxidative stress-induced cardiac injury. (3) In rats fed a HFFD, AME inhibits the increased expression of angiotensin (Ang) II receptors, angiotensin-1A receptor and the decreased expression of angiotensin type 2 receptors in the renin-angiotensin system and can significantly inhibit the increase in blood pressure. (4) AME inhibits phenylephrine-induced aortic vasoconstriction and increased acetylcholine-induced vascular relaxation. The mechanism of action of AME may involve the regulation of NOX, thereby modulating angiotensin II cell signaling and oxidative stress (Qin et al., 2018).
3.2.4 Limiting ischemic brain damage
Ischemic stroke is one of the most common brain diseases, accounting for 85% of cerebrovascular diseases (Oliveira et al., 2023). Research has shown that AME can protect the brain from hypoxic-ischemic injury from multiple perspectives. First, administration of AME to rats with hypoxic-ischemic brain injury reduced brain tissue damage in the forebrain by 50%. Second, in vitro experiments showed that AME exhibits excellent neuroprotective effects against DNA damage, mitochondrial damage, and NO-induced injury. In vivo experiments demonstrated that AME inhibits caspase 3-induced cell pyroptosis in a dose-dependent manner, decreases the expression of iNOS and COX-2, and suppresses the production of inflammatory factors such as IL-1β and TNF-α stimulated by LPS, thereby reducing the damage caused to microglia by these inflammatory factors (Shin et al., 2006). In a study, a model of left common carotid artery occlusion stroke to investigate the protective effects of AME on cerebral ischemia/reperfusion injury in rats. The findings indicated that after ischemia/reperfusion injury, AME significantly reduced the neurological deficit scores, improved motor coordination ability and spontaneous activity, reduced the levels of TNF-α, IL-1β, and IL-6, inhibited the NF-κB signaling pathway, reduced caspase-3 to block cell apoptosis, increased the levels of TNF receptor-associated factor family member-associated NF-κB activator-binding kinase 1 and interferon β, reduced MDA levels, and increased GSH and CAT levels in the brain. The mechanism of AME may be mediated by high mobility group box protein B1 (HMGB1) through the toll-like receptor-4 (TLR4)/NF-κB signaling pathway (Saeedan et al., 2023). These experimental phenomena and mechanisms indicate that AME has a good protective effect on ischemic brain injury.
3.3 Effect of AME on neurological diseases
3.3.1 Preventing Alzheimer’s disease
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by the accumulation of β-amyloid (Aβ) peptides, neurofibrillary tangles formed by hyperphosphorylated tau proteins, abnormal oxidative stress damage, inflammatory responses, and neurotransmitter disorders in affected brain regions (Breijyeh and Karaman, 2020). It is commonly treated with acetylcholinesterase (AChE) inhibitors and N-methyl-D-aspartate receptor antagonists. Although these medications can alleviate clinical manifestations, they do not reverse cognitive impairment, and are often accompanied by common side effects, including gastrointestinal symptoms, confusion, dizziness, and headaches. (Chin et al., 2022).
Overproduction of Aβ leads to the formation of neurofibrillary plaques in the brain, which subsequently accumulate in the blood vessels to cause cerebral amyloid angiopathy (Han et al., 2022). Therefore, inhibiting the production of Aβ and promoting its clearance are crucial for improving AD. In a study, researchers used the fluorescent dye thiazine 1,3,5-tetracarboxylic acid to investigate the inhibitory effect of various flavonoid compounds on Aβ aggregation and the structure-activity relationship of promoting Aβ fibril disaggregation. The results indicated that AME could inhibit the formation of Aβ1-42 fibrils, had a better affinity for Aβ1-42 fibrils than for Aβ1-40 fibrils. The hydrophilic group of AME was the key group for its function. Furthermore, among flavonoid compounds with different structures, AME with four hydroxyl groups could most effectively inhibit Aβ fibrillation and promote the disaggregation of pre-formed Aβ fibrils (Choi et al., 2020). Another study showed that increasing the number of methoxy groups on the AME parent structure weakened the inhibitory activity of AME on Aβ40, and the connection of single bonds also affected the inhibitory activity (Sirimangkalakitti et al., 2019). A study elucidated how AME inhibits the aggregation of Aβ42 peptide at the atomic level. AME bound to aromatic residues in the N-terminal of Aβ42 peptide, forming a stable π–π interaction, which destabilized the fibril structure. Subsequently, AME utilized its hydrogen bond donor/acceptor specificity to disrupt the hydrogen bond potential of the fibril peptide backbone, thereby disrupting the fibril structure and promoting Aβ fibril disaggregation (Windsor et al., 2021).
A study explained the anti-AD function of AME from the perspective of receptor-mediated endocytosis for Aβ clearance. AME can significantly increase Aβ uptake by mouse N2a neural cells through class A scavenger receptors, and then enter the lysosome within the cell, where it is degraded by relevant enzymes without causing cytotoxicity. The hydroxyl group in the molecular structure of AME enhances Aβ uptake, whereas the substitution of methyl groups decreases its uptake effect (Han et al., 2022). Another study identified AME from a library of polyphenolic compounds that can increase the activity and expression of neuropeptidase, an Aβ degradation enzyme, thereby delaying the progression of AD. Among them, the double-bond chemical structure in the C ring of the AME structure can significantly enhance neuropeptidase activity (Hori et al., 2023).
Research indicated that among various flavonoid compounds, AME exhibited the strongest neuroprotective effect, significantly inhibiting ROS-induced oxidative stress damage in SHSY5Y neuroblastoma cells. AME reduced DNA damage induced by etoposide DNA damage, whereas other flavonoid compounds increased cell death caused by DNA damage. Moreover, at a concentration of 2 μM, AME was most effective at reducing cytotoxicity induced by the Aβ25-35 peptide in rat PC12 cells, indicating the therapeutic potential of AME in neurodegenerative diseases (Kang et al., 2005). Futher studies found that AME could differentially protect SH-SY5Y neural cells from various cytotoxic factors such as hydrogen peroxide, okadiac acid, and Aβ25-35. Specifically, AME was able to inhibit okadiac acid-induced tau protein hyperphosphorylation, restore mitochondrial membrane potential to inhibit cell apoptosis, and protect microtubule structure and mitochondrial function. Furthermore, AME exhibited strong antioxidant capabilities against mitochondrial dysfunction and ROS-induced oxidative stress damage. AME also bound to β-secretase to inhibit its activity, thereby preventing the degradation of Aβ protein precursor to produce large amounts of Aβ (Zhang, 2014). In models of AD, AME could activate nuclear factor erythroid 2-related factor-2 (Nrf2) through affecting the adenosine monophosphate-activated protein kinase (AMPK)/GSK-3-β signaling pathway to exert antioxidant stress effects, ameliorating Aβ-induced neural function deficits and neuronal apoptosis (Chen et al., 2018). Although studies have shown that GSK-3-β is involved in the regulation of Nrf2 by AME, further verification is required to determine the mechanism by which AME affects GSK-3-β signaling-mediated tau phosphorylation. Tt was demonstrated through multiple experiments that AME, as a bifunctional chelator, can bind to various Aβ aggregates with high affinity and reduce their induced cytotoxicity. Additionally, AME can effectively chelate with Cu2+ and exhibit strong antioxidant activity, thereby preventing the formation of soluble Aβ oligomers and ROS-induced by metal ions in the brain (Sun et al., 2020).
Autophagy can degrade and recycle proteins produced by misfolding and damaged organelles, thereby maintaining protein homeostasis. The activation of autophagy in cells can effectively eliminate the accumulation of Aβ, thereby reducing the progression of AD (Zhang Z. et al., 2021). AME can improve the memory and cognitive impairment induced by Aβ25-35 in the brains of mice and alleviate inflammation, oxidative stress, and the immune response in the hippocampus. Additionally, it can enhance autophagy by binding to multiple amino acid residues of the mTOR protein, thereby inhibiting further phosphorylation of mTOR and exerting a neuroprotective effect against AD (Cao et al., 2021). Experiments were conducted on neuronal cell damage induced by Aβ1-42. It was found that AME can improve neuronal dysfunction induced by Aβ1-42 in rats and inhibit NLRP3 inflammasome-induced pyroptosis by regulating AMPK/GSK-3-β signaling, thereby ameliorating the neurotoxicity caused by Aβ1-42 in AD (Zhao et al., 2019).
Using the scopolamine-induced dementia mouse model, it was discovered that oral administration of AME can inhibit the activity of AChE and increase acetylcholine levels. Furthermore, it can improve the oxidative stress damage induced by scopolamine in mice, mainly by reducing MDA levels and increasing GSH activity, thereby ameliorating cognitive impairment (Ishola et al., 2013a). Addtionally, a study collected 50 flavonoid compounds with therapeutic potential for AD. Molecular docking experiments were conducted with the human α7 nicotinic acetylcholine receptor (α7nACHR) and found that after binding with AME, it exhibited the best affinity and good stability parameters (Singh et al., 2023). However, the effectiveness of AME as an α7nACHR agonist for treating AD requires further verification.
3.3.2 Treating Parkinson’s disease
In Parkinson’s disease (PD), melanin is released as neurons degenerate. Subsequently, it is recognized and ingested by microglia, leading to inflammatory damage. In patients with PD, the breakdown of dopaminergic neurons can be attributed to chronic neuroinflammation, which leads to the production and accumulation of Lewy bodies in the compact region of the substantia nigra in the brain. The main pathological features of PD are damage to dopaminergic neurons in the midbrain substantia nigra and the establishment of diffuse Lewy bodies, which subsequently lead to motor neuron dysfunction. Therefore, taking measures to inhibit neuroinflammation may be beneficial for alleviating PD clinical manifestations (Sharma et al., 2023).
Microglia, analogous to macrophages within the brain, can be activated by various stimuli to release a range of inflammatory mediators, thereby initiating an inflammatory response that may result in neural damage (Cai et al., 2022). AME has been shown to improve motor dysfunction and anxiety-like and depressive-like behaviors in the LPS-induced PD rat model. Mechanistic studies revealed that AME effectively inhibits the expression of pro-inflammatory factors (TNF-α, IL-1β, IL-6, iNOS, COX-2) and enhances the expression of anti-inflammatory factors (IL-4, TGF-β, Arg-1, CD206), indicating that AME promotes the polarization of microglia towards the M2 anti-inflammatory phenotype. Furthermore, AME significantly ameliorates the reduction in tyrosine hydroxylase-positive neurons and the increase in α-synuclein expression observed in the model, suggesting that AME exerts neuroprotective effects in the LPS-induced PD model (Liu et al., 2024). It has been discovered that after LPS stimulation2 of rat astrocytoma cells (C6), AME significantly inhibited the production of nitrites, ROS, MDA, and TNF-α, upregulated GSH levels, and reduced intracellular oxidative stress (Ishola et al., 2013b). Activated ERK1/2 promotes cell death due to oxidative toxicity, but AME can alleviate oxidative stress damage caused by glutamate and ROS in HT22 neuronal cells by maintaining the activity of antioxidant enzymes and inhibiting ERK1/2 activity (Jeong et al., 2014). Molecular docking studies have revealed that AME exhibits strong binding affinity with the glutathione peroxidase 4 (GPX4) protein, which is involved in ferroptosis (Xiong et al., 2024). It was reported that AME can inhibit inflammation induced by ferroptosis in HT22 cells triggered by homocysteine. However, it is important to note that AME treatment results in decreased expression levels of both solute carrier family 7 member 11 (SLC7A11) and GPX4. Given that the SLC7A11/GPX4 signaling pathway requires the upregulation of these proteins to effectively suppress ferroptosis, the observed reduction in their expression following AME treatment may affect its efficacy in preventing ferroptosis (Wang et al., 2024).
In vivo and in vitro PD model studies demonstrated that AME can significantly reduce the expression of glial fibrillary acidic protein and lba1 markers in glial cells under inflammatory conditions, decrease the activation of caspase-3 and p21, and reduce the expression levels of IL-1β and iNOS and increase the Bcl-2/Bax ratio, through the PI3K/Akt and ERK signaling pathways. In this study, in a vitro mouse model induced by methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), AME was able to protect dopaminergic neurons and reduce striatal fiber loss (Cao et al., 2017). These findings suggest that AME can exert beneficial effects on dopaminergic neurons and glial cells, thereby ameliorating the clinical manifestations of PD. Furthermore, studies have shown a deregulated angiotensin-converting enzyme (ACE)/Ang II/angiotensin II receptor-1 (AT1R) axis is activated at the onset of PD, leading to free radical damage, cell apoptosis, and neuronal disruption. AME can bind to the mitochondrial assembly receptor (MASR) protein and activate the ACE2/Ang (1–7)/MASR, thereby neutralizing the neurodegenerative changes triggered by the ACE/Ang II/AT1R axis (Bhadauriya et al., 2023).
3.3.3 Treating epilepsy
The development of inflammation is closely related to the onset of epilepsy and its clinical progression. Brain inflammation can promote neuronal excitability, reducing the seizure threshold, thereby triggering seizures. Therefore, anti-inflammatory therapy can be used to prevent and treat seizures (Alsaegh et al., 2021). Studies found that AME improve pentetrazole-induced cognitive dysfunction by inhibiting the NLRP3 inflammasome, reducing the susceptibility to seizures and apoptosis in hippocampal neurons of mice. In this study, AME can also inhibit the mRNA expression of NLRP3, ASC, and caspase-1 in BV2 microglia induced by LPS, reducing the expression of inflammatory factors IL-1β, IL-18, and TNF-α. However, in the absence of inflammation, administration of AME to mice did not affect the expression of these proteins and inflammatory factors (Rong et al., 2019). Research indicated that AME can inhibit the activation and nuclear translocation of NF-κB p65 subunit in mice, thereby inhibiting the NF-κB signaling pathway to reduce the injury of hippocampal CA1 neural cells. AME can also reduce the production of inflammatory mediators NO and PGE-2, and inflammatory factors IL-6 and IL-β in neural cells. Furthermore, histological analysis found that AME can protect neurons after status epilepticus, inhibit the excessive discharge of hippocampal neurons, and shorten the duration of seizures (Zhang et al., 2015). These results indicate that AME has a good protective effect on hippocampal neuronal injury caused by epilepsy and has good anti-inflammatory effects. A study employed various computational methods and found that AME exhibits superior affinity for α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and voltage-gated sodium ion channels (VGSC) receptors compared to phenytoin. Additionally, physicochemical and pharmacokinetic studies indicate that AME is suitable for oral administration, demonstrating favorable intestinal permeability and the ability to cross the blood-brain barrier, with no significant risk of toxicity (Salaria et al., 2024). These findings suggest that AME is a promising candidate for further research as a potential treatment for epilepsy.
4 Conclusion and future perspective
AME has been reported in multiple studies as the primary active pharmacological component from its plant sources, with a wide range of pharmacological effects (Yu et al., 2017; Li et al., 2021; Zhang et al., 2015). Due to the unique chemical structure of AME, its biological activity can be significantly influenced by multiple factors. Further studies could elucidate how various factors, such as photooxidation, affect the structural stability of AME. Additionally, pharmacokinetic studies have demonstrated that AME undergoes rapid metabolism in the body, resulting in low bioavailability. Therefore, modifying the natural structure or developing suitable drug formulations of AME is crucial for improving its therapeutic properties and enhancing its bioavailability.
The anti-inflammatory, antioxidant, and lipid-lowering effects of AME are notable, and recent studies have demonstrated its application in various cardiac and neurological diseases, suggesting that it may have significant anti-atherosclerotic effects. However, most of the current data on AME are derived from in vitro cell experiments, with limited in vivo animal testing. More comprehensive animal experiments are required to validate its pharmacological activity. One study has found that AME may have hepatotoxic and nephrotoxic effects (Li D et al., 2019). This warrants further investigation.
AME exhibits significant therapeutic effects on various cardiocerebrovascular diseases and neurological disorders, potentially functioning through multiple mechanisms. All these disease processes are accompanied by inflammatory responses, which are triggered by multiple factors. AME can affect multiple inflammatory mediators and mechanisms, suggesting that it has considerable potential for treating these diseases. However, the specific mechanisms of action involved in the effects of AME on inflammatory diseases require further research. Hemodynamic factors such as hypertension cannot be ignored in the development of cardiocerebrovascular; however, the mechanisms and targets by which AME counters the effects of these factors remain unclear. Currently, there remains controversy regarding whether AME can exert a therapeutic effect on AD. Furthermore, the specific mechanisms by which AME influences the cellular uptake and clearance of Aβ also require further validation research.
In summary, AME exhibits multiple activities, indicating its potential as a natural drug for treating cardiocerebrovascular diseases and neurological disorders. Further studies on its pharmacokinetics and toxicology are required to ensure its safety and efficacy.
Author contributions
HZ: Writing–original draft. Y-MB: Writing–original draft. D-ML: Writing–original draft. GW: Writing–review and editing. JG: Funding acquisition, Writing–review and editing. LZ: Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82360786), the Technology Research and Development Program of Guizhou Province (Qian Science Combination Basis -ZK [2022]- General 625), the Science and Technology Program of Zunyi City (zun shi kehe zi [2020] 71),Science and Technology Support Project of the Department of Science and Technology in Guizhou Province [Qian Ke He support S 2020 (2319)].
Acknowledgments
We thank Home for Researchers FigDraw team for the mechanism mapping service (www.home-for-researchers.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, M. U., Kaneva, V., Loyau, S., Nechipurenko, D., Receveur, N., Le Bris, M., et al. (2020). Pharmacological blockade of glycoprotein VI promotes thrombus disaggregation in the absence of thrombin. Arterioscler. Thromb. Vasc. Biol. 40 (9), 2127–2142. doi:10.1161/ATVBAHA.120.314301
Alherz, F. A., El-Masry, T. A., Negm, W. A., and El-Kadem, A. H. (2022). Potential cardioprotective effects of Amentoflavone in doxorubicin-induced cardiotoxicity in mice. Biomed. Pharmacother. 154, 113643. doi:10.1016/j.biopha.2022.113643
Alkadi, K. A. A., Ashraf, K., Adam, A., Shah, S. A. A., Taha, M., Hasan, M. H., et al. (2021). In vitro cytotoxicity and anti-inflammatory cytokinine activity study of three isolated novel compounds of Prismatomeris glabra. J. Pharm. Bioallied Sci. 13 (1), 116–122. doi:10.4103/jpbs.JPBS_279_19
Alsaegh, H. Z., Eweis, H. S. A. E-K., and Kamel, F. O. (2021). Pathophysiological cascade of events leading to epilepsy: role of inflammation. J. Pharm. Res. Int. 32 (48), 74–84. doi:10.9734/jpri/2020/v32i4831127
Bajpai, V. K., Park, I., Lee, J., Shukla, S., Nile, S. H., Chun, H. S., et al. (2019). Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 271, 239–247. doi:10.1016/j.foodchem.2018.07.159
Banerjee, T., Valacchi, G., Ziboh, V. A., and van der Vliet, A. (2002). Inhibition of TNFalpha-induced cyclooxygenase-2 expression by amentoflavone through suppression of NF-kappaB activation in A549 cells. Mol. Cell Biochem. 238 (1-2), 105–110. doi:10.1023/a:1019963222510
Bhadauriya, P., Varshney, V., and Goyal, A. (2023). Molecular docking-based identification of potential natural neuroprotective molecules for Parkinson's disease. Chem. Biodivers. 20 (10), e202300979. doi:10.1002/cbdv.202300979
Bhattacharya, P., and Mandal, A. (2024). Identification of amentoflavone as a potent SARS-CoV-2 Mpro inhibitor: a combination of computational studies and in vitro biological evaluation. J. Biomol. Struct. Dyn. 1, 1–19. doi:10.1080/07391102.2024.2304676
Borow, K. M., Nelson, J. R., and Mason, R. P. (2015). Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis 242 (1), 357–366. doi:10.1016/j.atherosclerosis.2015.07.035
Breijyeh, Z., and Karaman, R. (2020). Comprehensive review on Alzheimer's disease: causes and treatment. Molecules 25 (24), 5789. doi:10.3390/molecules25245789
Brummel, K. E., Paradis, S. G., Butenas, S., and Mann, K. G. (2002). Thrombin functions during tissue factor-induced blood coagulation. Blood 100 (1), 148–152. doi:10.1182/blood.v100.1.148
Cai, Y., Liu, J., Wang, B., Sun, M., and Yang, H. (2022). Microglia in the neuroinflammatory pathogenesis of Alzheimer's disease and related therapeutic targets. Front. Immunol. 13, 856376. doi:10.3389/fimmu.2022.856376
Cao, B., Zeng, M., Zhang, Q., Zhang, B., Cao, Y., Wu, Y., et al. (2021). Amentoflavone ameliorates memory deficits and abnormal autophagy in aβ25-35-induced mice by mTOR signaling. Neurochem. Res. 46 (4), 921–934. doi:10.1007/s11064-020-03223-8
Cao, Q., Qin, L., Huang, F., Wang, X., Yang, L., Shi, H., et al. (2017). Amentoflavone protects dopaminergic neurons in MPTP-induced Parkinson's disease model mice through PI3K/Akt and ERK signaling pathways. Toxicol. Appl. Pharmacol. 319, 80–90. doi:10.1016/j.taap.2017.01.019
Carracedo, M., Artiach, G., Arnardottir, H., and Bäck, M. (2019). The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 41 (6), 757–766. doi:10.1007/s00281-019-00767-y
Chen, B., Xu, D., Li, Z., Jing, Y., Lin, L., Li, S., et al. (2022). Tissue distribution, excretion, and interaction with human serum albumin of total bioflavonoid extract from Selaginella doederleinii. Front. Pharmacol. 13, 849110. doi:10.3389/fphar.2022.849110
Chen, C., L, i B., Cheng, G., Yang, X., Zhao, N., and Shi, R. (2018). Amentoflavone ameliorates aβ1-42-induced memory deficits and oxidative stress in cellular and rat model. Neurochem. Res. 43 (4), 857–868. doi:10.1007/s11064-018-2489-8
Chen, G., Han, Y., He, W., and Liang, F. (2016). Amentoflavone protects against high fat-induced metabolic dysfunction: possible role of the regulation of adipogenic differentiation. Int. J. Mol. Med. 38 (6), 1759–1767. doi:10.3892/ijmm.2016.2772
Chen, T. R., Wei, L. H., Guan, X. Q., Huang, C., Liu, Z. Y., Wang, F. J., et al. (2019). Biflavones from Ginkgo biloba as inhibitors of human thrombin. Bioorg Chem. 92, 103199. doi:10.1016/j.bioorg.2019.103199
Chen, W. M., Chen, J. M., and Jian, M. H. (2023). Effect of amentoflavone on myocardial fibrosis in rats with acute myocardial infarction. J. Zunyi Med. Univ. 46 (07), 646–651+656. doi:10.14169/j.cnki.zunyixuebao.2023.0103
Chin, E., Jaqua, E., Safaeipour, M., and Ladue, T. (2022). Conventional versus new treatment: comparing the effects of acetylcholinesterase inhibitors and N-Methyl-D-Aspartate receptor antagonist with aducanumab. Cureus 14 (11), e31065. doi:10.7759/cureus.31065
Cho, S., Lee, H., Han, J., Lee, H., Kattia, R. O., Nelson, Z. V., et al. (2021). Viburnum stellato-Tomentosum extract suppresses obesity and hyperglycemia through regulation of lipid metabolism in high-fat diet-fed mice. Molecules 26 (4), 1052. doi:10.3390/molecules26041052
Choi, E. Y., Kang, S. S., Lee, S. K., and Han, B. H. (2020). Polyphenolic biflavonoids inhibit amyloid-beta fibrillation and disaggregate preformed amyloid-beta fibrils. Biomol. Ther. Seoul. 28 (2), 145–151. doi:10.4062/biomolther.2019.113
Duan, S., Jia, J. F., Hong, B., Zhou, J., Zhang, Y., Ge, F., et al. (2022). Assessment of amentoflavone loaded sub-micron particle preparation using supercritical antisolvent for its antitumor activity. Curr. Drug Deliv. 19 (1), 41–48. doi:10.2174/1567201818666210810142750
Dutta, S., Singhal, A. K., Suryan, V., and Chandra, N. C. (2023). Obesity: an impact with cardiovascular and cerebrovascular diseases. Ind. J. Clin. Biochem. 39, 168–178. doi:10.1007/s12291-023-01157-w
Fang, G., Li, X., Yang, F., Huang, T., Qiu, C., Peng, K., et al. (2023b). Amentoflavone mitigates doxorubicin-induced cardiotoxicity by suppressing cardiomyocyte pyroptosis and inflammation through inhibition of the STING/NLRP3 signalling pathway. Phytomedicine 117, 154922. doi:10.1016/j.phymed.2023.154922
Fang, H. Y., Zhao, X. N., Zhang, M., Ma, Y. Y., Huang, J. L., and Zhou, P. (2023a). Beneficial effects of flavonoids on cardiovascular diseases by influencing NLRP3 inflammasome. Inflammopharmacology 31 (4), 1715–1729. doi:10.1007/s10787-023-01249-2
Fang, H. Y., Zhuang, R. X., Wang, F. G., Xi, J. J., Zang, H. Q., and Miao, L. B. (2011). Separation and purification of total flavonoids from Selaginella moellendorfii hieron by macroporous resin and detected by HPLC. Chin. Arch. Tradit. Chin. Med. 29 (12), 2796–2798. doi:10.13193/j.archtcm.2011.12.198.fanghy.052
Feng, X. (2020). Study on metabolism of amentoflavone and isoginkgetin in vitro and in vivo preparation and evaluation of their nanomicelles. Shijiazhuang (Hebei): Hebei Medical University. dissertation.
Feng, X., Chen, Y., Li, L., Zhang, Y., Zhang, L., and Zhang, Z. (2020). Preparation, evaluation and metabolites study in rats of novel amentoflavone-loaded TPGS/soluplus mixed nanomicelles. Drug Deliv. 27 (1), 137–150. doi:10.1080/10717544.2019.1709920
Feng, Y., Wang, J., Zhang, S., Li, Y., Wang, B., Zhang, J., et al. (2023). Preparation of amentoflavone-loaded DSPE-PEG (2000) micelles with improved bioavailability and in vitro antitumor efficacy. Biomed. Chromatogr. 37 (9), e5690. doi:10.1002/bmc.5690
Ferchichi, L., Derbré, S., Mahmood, K., Touré, K., Guilet, D., Litaudon, M., et al. (2012). Bioguided fractionation and isolation of natural inhibitors of advanced glycation end-products (AGEs) from Calophyllum flavoramulum. Phytochemistry 78, 98–106. doi:10.1016/j.phytochem.2012.02.006
Ference, B. A., Ginsberg, H. N., Graham, I., Ray, K. K., Packard, C. J., Bruckert, E., et al. (2017). Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 38 (32), 2459–2472. doi:10.1093/eurheartj/ehx144
Gan, L., Ma, J., You, G., Mai, J., Wang, Z., Yang, R., et al. (2020). Glucuronidation and its effect on the bioactivity of amentoflavone, a biflavonoid from Ginkgo biloba leaves. J. Pharm. Pharmacol. 72 (12), 1840–1853. doi:10.1111/jphp.13247
Global Burden of Disease Study 2013 Collaborators (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386 (9995), 743–800. doi:10.1016/S0140-6736(15)60692-4
Gui, Y., Zheng, H., and Cao, R. Y. (2022). Foam cells in atherosclerosis: novel insights into its origins, consequences, and molecular mechanisms. Front. Cardiovasc Med. 9, 845942. doi:10.3389/fcvm.2022.845942
Guo, Q., Jin, Y., Chen, X., Ye, X., Shen, X., Lin, M., et al. (2024). NF-κB in biology and targeted therapy: new insights and translational implications. Signal Transduct. Target Ther. 9 (1), 53. doi:10.1038/s41392-024-01757-9
Han, B. H., Cofell, B., Everhart, E., Humpal, C., Kang, S. S., Lee, S. K., et al. (2022). Amentoflavone promotes cellular uptake and degradation of amyloid-beta in neuronal cells. Int. J. Mol. Sci. 23 (11), 5885. doi:10.3390/ijms23115885
He, C. (2013). The mechanism of Mfn2 Gene to promote cholesterol effulux in vascular smooth muscle cell-derived foam cells. Wuhan (Hubei): Huazhong University of Science and Technology. dissertation.
Hori, Y., Watanabe, K., Yassen, A. S. A., Shirotani, K., Tanaka, T., and Iwata, N. (2023). Enhancement of neprilysin activity by natural polyphenolic compounds and their derivatives in cultured neuroglioma cells. Biol. Pharm. Bull. 46 (3), 446–454. doi:10.1248/bpb.b22-00833
Huang, Q. Z., and Sun, J. (2023). Correlation analysis of blood glucose and lipid levels with lower extremity vascular disease in patients with type 2 diabetes mellitus. Mod. Med. Health Res. Electron J. 7 (18), 16–18. doi:10.3969/j.issn.2096-3718.2023.18.006
Huvaere, K., Sinnaeve, B., Van Bocxlaer, J., and Skibsted, L. H. (2012). Flavonoid deactivation of excited state flavins: reaction monitoring by mass spectrometry. J. Agric. Food Chem. 60 (36), 9261–9272. doi:10.1021/jf301823h
Iadecola, C. (2023). The pathobiology of vascular dementia. Neuron 80 (4), 844–866. doi:10.1016/j.neuron.2013.10.008
Ishola, I. O., Chatterjee, M., Tota, S., Tadigopulla, N., Adeyemi, O. O., Palit, G., et al. (2012). Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol. Biochem. Behav. 103 (2), 322–331. doi:10.1016/j.pbb.2012.08.017
Ishola, I. O., Chaturvedi, J. P., Rai, S., Rajasekar, N., Adeyemi, O. O., Shukla, R., et al. (2013b). Evaluation of amentoflavone isolated from Cnestis ferruginea Vahl ex DC (Connaraceae) on production of inflammatory mediators in LPS stimulated rat astrocytoma cell line (C6) and THP-1 cells. J. Ethnopharmacol. 146 (2), 440–448. doi:10.1016/j.jep.2012.12.015
Ishola, I. O., Tota, S., Adeyemi, O. O., Agbaje, E. O., Narender, T., and Shukla, R. (2013a). Protective effect of Cnestis ferruginea and its active constituent on scopolamine-induced memory impairment in mice: a behavioral and biochemical study. Pharm. Biol. 51 (7), 825–835. doi:10.3109/13880209.2013.767360
Jeong, E. J., Hwang, L., Lee, M., Lee, K. Y., Ahn, M. J., and Sung, S. H. (2014). Neuroprotective biflavonoids of Chamaecyparis obtusa leaves against glutamate-induced oxidative stress in HT22 hippocampal cells. Food Chem. Toxicol. 64, 397–402. doi:10.1016/j.fct.2013.12.003
Jiang, Y., Wang, S., Yu, M., Wu, D., Lei, J., Li, W., et al. (2020). Ultrasonic-assisted ionic liquid extraction of two biflavonoids from Selaginella tamariscina. ACS Omega 5 (51), 33113–33124. doi:10.1021/acsomega.0c04723
Kang, D. G., Yin, M. H., Oh, H., Lee, D. H., and Lee, H. S. (2004). Vasorelaxation by amentoflavone isolated from Selaginella tamariscina. Planta Med. 70 (8), 718–722. doi:10.1055/s-2004-827201
Kang, S. S., Lee, J. Y., Choi, Y. K., Song, S. S., Kim, J. S., Jeon, S. J., et al. (2005). Neuroprotective effects of naturally occurring biflavonoids. Bioorg Med. Chem. Lett. 15 (15), 3588–3591. doi:10.1016/j.bmcl.2005.05.078
Ke, Y. Y., Yuan, P. P., Zhou, Y. Y., Zhang, X., Li, M., Wang, S., et al. (2013). “The intervention experimental study of total flavonoids in Selaginella tamariscina (Beauv.) Spring and Amentotaxus Biflavone in HepG2 cells of insulin resistance,” in 2013 China Pharmaceutical Conference and the 13th Chinese pharmacist weekly treatise, 1382–1391.
Khafagy, E. S., Soliman, G. A., Shahba, A. A., Aldawsari, M. F., Alharthy, K. M., Abdel-Kader, M. S., et al. (2023). Brain targeting by intranasal drug delivery: effect of different formulations of the biflavone “cupressuflavone” from juniperus sabina L. On the motor activity of rats. Molecules 28 (3), 1354. doi:10.3390/molecules28031354
Kubota, Y., Umegaki, K., Tanaka, N., Mizuno, H., Nakamura, K., Kunitomo, M., et al. (2002). Safety of dietary supplements: chronotropic and inotropic effects on isolated rat atria. Biol. Pharm. Bull. 25 (2), 197–200. doi:10.1248/bpb.25.197
Kumari, S., Dhapola, R., Sharma, P., Nagar, P., Medhi, B., and HariKrishnaReddy, D. (2024). The impact of cytokines in neuroinflammation-mediated stroke. Cytokine Growth Factor Rev. 78, 105–119. doi:10.1016/j.cytogfr.2024.06.002
Lai, H. F., Huang, X. X., and Shi, Z. L. (2018a). Optimization of ultrasonic-assisted extraction of amentoflavone from Selaginella uncinate (Desv.)spring using response surface methodology. Food Res. Dev. 39 (13), 47–51+224. doi:10.3969/j.issn.1005-6521.2018.13.009
Lai, H. F., Pan, L. W., Lv, G. M., and Huang, X. X. (2018b). Purification of amentoflavone from Selaginella uncinate (Desv.)spring by macroporous resin. Jiangsu Agric. Sci. 46 (07), 201–204. doi:10.15889/j.issn.1002-1302.2018.07.050
Lee, H., Cho, S., Kim, S. Y., Ju, J., Lee, S. W., Choi, S., et al. (2022). Amentoflavone-enriched Selaginella rossii warb. Suppresses body weight and hyperglycemia by inhibiting intestinal lipid absorption in mice fed a high-fat diet. Life (Basel) 12 (4), 472. doi:10.3390/life12040472
Lee, M. M., Cho, W. K., Cha, M. H., Yim, N. H., Yang, H. J., and Ma, J. Y. (2023). The antiviral activity of Thuja orientalis folium against Influenza A virus. Virus Res. 335, 199199. doi:10.1016/j.virusres.2023.199199
Li, D. (2020). Protective effect of amentoflavone against myocardial ischemia-reperfusion injury in rats. Zunyi (Guizhou): ZunYi Medical University. dissertation.
Li, W. W., Li, D., Qin, Y., Sun, C. X., Wang, Y. L., Gao, L., et al. (2021). Cardioprotective effects of Amentoflavone by suppression of apoptosis and inflammation on an in vitro and vivo model of myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 101 (Pt B), 108296. doi:10.1016/j.intimp.2021.108296
Li, X. (2011). Extraction, characteriazation of Bi-flavonoid from Selaginella doederleinii and its interaction with bovine serum albumin. Changsha (Hunan): Central South University. dissertation.
Li, Y. L., Chen, X., Niu, S. Q., Zhou, H. Y., and Li, Q. S. (2020). Protective antioxidant effects of amentoflavone and total flavonoids from Hedyotis diffusa on H2O2-induced HL-O2 cells through ASK1/p38 MAPK pathway. Chem. Biodivers. 17 (7), e2000251. doi:10.1002/cbdv.202000251
Liao, S., Ren, Q., Yang, C., Zhang, T., Li, J., Wang, X., et al. (2015). Liquid chromatography-tandem mass spectrometry determination and pharmacokinetic analysis of amentoflavone and its conjugated metabolites in rats. J. Agric. Food Chem. 63 (7), 1957–1966. doi:10.1021/jf5019615
Libby, P. (2021). Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. 117 (13), 2525–2536. doi:10.1093/cvr/cvab303
Li, D., Sun, C., Yang, J., Ma, X., Jiang, Y., Qiu, S., et al. (2019). Ionic liquid-microwave-based extraction of biflavonoids from Selaginella sinensis. Molecules 24 (13), 2507. doi:10.3390/molecules24132507
Liu, C., Li, W. W., Lei, J., Li, G., Lu, D. M., Xu, D. J., et al. (2022). Deep eutectic solvents extraction and optimization of amentoflavone from Selaginella moellendorffii. Sci. Technol. Food Ind. 43 (16), 176–184. doi:10.13386/j.issn1002-0306.2021100160
Liu, T. T., Qian, H., Li, Y. P., Wu, W. W., Min, X. W., Yang, H. D., et al. (2023). Study on the correlation between lipid accumulation product and risk levels of atherosclerotic cardiovascular disease. Chin. Med. Her. 20 (23), 77–80. doi:10.20047/j.issn1673-7210.2023.23.16
Liu, Z., Ma, H., Sun, T., Wang, X. Y., and Kong, M. Z. (2024). Neuroprotective role of amentoflavone on LPS-induced Parkinson's disease animal model via inhibition of microglia-mediated inflammation. J. Biol. Regul. Homeost. Agents 38 (1), 705–718. doi:10.23812/j.biol.regul.homeost.agents.20243801.58
Li, Y. Y., Lu, X. Y., Sun, J. L., Wang, Q. Q., Zhang, Y. D., Zhang, J. B., et al. (2019). Potential hepatic and renal toxicity induced by the biflavonoids from Ginkgo biloba. Chin. J. Nat. Med. 17 (9), 672–681. doi:10.1016/S1875-5364(19)30081-0
Luo, S. (2017). Study on the comprehensive quality control mode and preliminary activity of selaginella pulvinate. Hangzhou (Zhejiang): Zhejiang University of Technology. dissertation.
Ma, Y. Q., Lan, Y. M., and Zhang, Z. L. (2021). Research progress of correlation between intestinal microecolofy and cardiovascular and cerebrovasculr disease. J. Northwest Minzu Univ. Nat. Sci. Ed. 42 (04), 21–27. doi:10.14084/j.cnki.cn62-1188/n.2021.04.007
Mangmool, S., Duangrat, R., Rujirayunyong, T., and Anantachoke, N. (2024). Anti-inflammatory effects of the Thai herbal remedy Yataprasen and biflavonoids isolated from Putranjiva roxburghii in RAW264.7 macrophages. J. Ethnopharmacol. 327, 117997. doi:10.1016/j.jep.2024.117997
Meng, Q. W., Liu, H. J., Yi, H. R., and Liu, Q. B. (2024). Mechanisms of NLRP3 inflammasome in atherosclerosis and advances in targeted inflammatory therapy. Zhongguo dong mai ying hua za zhi 32 (01), 79–86. doi:10.20039/j.cnki.1007-3949.2024.01.011
Nagai, S., Ohara, K., and Mukai, K. (2005). Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. J. Phys. Chem. B 109 (9), 4234–4240. doi:10.1021/jp0451389
Oh, J., Rho, H. S., Yang, Y., Yoon, J. Y., Lee, J., Hong, Y. D., et al. (2013). Extracellular signal-regulated kinase is a direct target of the anti-inflammatory compound amentoflavone derived from Torreya nucifera. Mediat. Inflamm. 2013, 761506. doi:10.1155/2013/761506
Okigawa, M., Hwa, C. W., Kawano, N., and Rahman, W. (1971). Biflavones in selaginella species. Phytochemistry 10, 3286–3287. doi:10.1016/S0031-9422(00)97392-8
Oliveira, A. R., Jesus, P. A. P., Bulhões, F. V., Martins Netto, E., Oliveira Filho, J., Roever, L., et al. (2023). Morbimortality and determinants of reperfusion in ischemic stroke. Rev. Assoc. Medica Bras. (1992) 70 (1), e20230472. doi:10.1590/1806-9282.20230472
Park, H. J., and Kim, M. M. (2019). Amentoflavone induces autophagy and modulates p53. Cell J. 21 (1), 27–34. doi:10.22074/cellj.2019.5717
Peng, Y., Chen, Q., Xue, Y. H., Jin, H., Liu, S., Du, M. Q., et al. (2024). Ginkgo biloba and its chemical components in the management of Alzheimer's disease. Am. J. Chin. Med. 52 (3), 625–666. doi:10.1142/S0192415X24500277
Poznyak, A., Grechko, A. V., Poggio, P., Myasoedova, V. A., Alfieri, V., and Orekhov, A. N. (2020). The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 21 (5), 1835. doi:10.3390/ijms21051835
Qin, L., Zhao, Y., Zhang, B., and Li, Y. (2018). Amentoflavone improves cardiovascular dysfunction and metabolic abnormalities in high fructose and fat diet-fed rats. Food Funct. 9 (1), 243–252. doi:10.1039/c7fo01095h
Qin, T., Huang, Z. H., and Liu, Y. (2021). Research progress of vascular endothelial growth factor and atherosclerotic plaque. Chin. Youjiang Med. J. 49 (06), 465–468. doi:10.3969/j.issn.1003-1383.2021.06.012
Qiu, F., Zhang, L., Zheng, J., Cao, L., Zhang, Z., and Deng, Y. (2021b). Amentoflavone inhibits M1 polarization of THP-1-derived foam cells by activating PPAR- α/γ. J. South. Med. Univ. 41 (3), 344–351. doi:10.12122/j.issn.1673-4254.2021.03.05
Qiu, S., Zhou, Y., Kim, J. T., Bao, C., Lee, H. J., and Chen, J. (2021a). Amentoflavone inhibits tumor necrosis factor-α-induced migration and invasion through AKT/mTOR/S6k1/hedgehog signaling in human breast cancer. Food Funct. 12 (20), 10196–10209. doi:10.1039/d1fo01085a
Ren, Q. X., Wang, Y. N., Qu, X. Y., Zhen, B. Q., Hong, T., and Zhou, Z. (2013a). Preparation and in vitro evaluation of self-microemulsifying drug delivery system containing amentoflavone. Chin. J. Exp. Tradit. Med. Formulae 19 (24), 5–9. doi:10.11653/syfj2013240005
Ren, Q. X., Zhou, Z., and Wang, Q. S. (2013b). Preparation and analytical characterization of micronized amentoflavone by antisolvent freeze-drying method. J. Int. Pharm. Res. 40 (02), 237–241. doi:10.13220/j.cnki.jipr.2013.02.009
Ridker, P. M., Rifai, N., Pfeffer, M., Sacks, F., Lepage, S., and Braunwald, E. (2000). Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101 (18), 2149–2153. doi:10.1161/01.cir.101.18.2149
Rong, S., Wan, D., Fan, Y., Liu, S., Sun, K., Huo, J., et al. (2019). Amentoflavone affects epileptogenesis and exerts neuroprotective effects by inhibiting NLRP3 inflammasome. Front. Pharmacol. 10, 856. doi:10.3389/fphar.2019.00856
Roth, G. A., Mensah, G. A., and Fuster, V. (2020). The global burden of cardiovascular diseases and risks: a compass for global action. J. Am. Coll. Cardiol. 76 (25), 2980–2981. doi:10.1016/j.jacc.2020.11.021
Ruan, X., Yan, L. Y., Li, X. X., Liu, B., Zhang, H., and Wang, Q. (2014). Optimization of process parameters of extraction of amentoflavone, quercetin and ginkgetin from Taxus chinensis using supercritical-CO2 fluid extraction. Molecules 19 (11), 17682–17696. doi:10.3390/molecules191117682
Saeedan, A. S., Abdel-Rahman, R. F., Soliman, G. A., Ogaly, H. A., and Abdel-Kader, M. S. (2023). Amentoflavone attenuates oxidative stress and neuroinflammation induced by cerebral ischemia/reperfusion in rats by targeting HMGB1-mediated TLR4/NF-κB signaling pathway. Saudi Pharm. J. 31 (11), 101798. doi:10.1016/j.jsps.2023.101798
Salaria, P., Subrahmanyeswara Rao, N. N., Dhameliya, T. M., and Amarendar Reddy, M. (2024). In silico investigation of potential phytoconstituents against ligand- and voltage-gated ion channels as antiepileptic agents. 3 Biotech. 14 (4), 99. doi:10.1007/s13205-024-03948-1
Šamec, D., Karalija, E., Dahija, S., and Hassan, S. T. S. (2022). Biflavonoids: important contributions to the health benefits of Ginkgo (Ginkgo biloba L.). Plants (Basel) 11 (10), 1381. doi:10.3390/plants11101381
Saponara, R., and Bosisio, E. (1998). Inhibition of cAMP-phosphodiesterase by biflavones of Ginkgo biloba in rat adipose tissue. J. Nat. Prod. 61 (11), 1386–1387. doi:10.1021/np970569m
Shan, C. X., Guo, S. C., Yu, S., Shan, M. Q., Li, S. F. Y., Chai, C., et al. (2018). Simultaneous determination of quercitrin, afzelin, amentoflavone, hinokiflavone in rat plasma by UFLC-MS-MS and its application to the pharmacokinetics of Platycladus orientalis leaves extract. J. Chromatogr. Sci. 56 (10), 895–902. doi:10.1093/chromsci/bmy066
Sharma, P., Kishore, A., De, I., Negi, S., Kumar, G., Bhardwaj, S., et al. (2023). Mitigating neuroinflammation in Parkinson's disease: exploring the role of proinflammatory cytokines and the potential of phytochemicals as natural therapeutics. Neurochem. Int. 170, 105604. doi:10.1016/j.neuint.2023.105604
Shin, D. H., Bae, Y. C., Kim-Han, J. S., Lee, J. H., Choi, I. Y., Son, K. H., et al. (2006). Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J. Neurochem. 96 (2), 561–572. doi:10.1111/j.1471-4159.2005.03582.x
Siddiqui, A. R., Mushtaq, M., Sardar, M., Atta, L., Nur-E-Alam, M., Ahmad, A., et al. (2024). Mechanistic insight into the mode of inhibition of dietary flavonoids; targeting macrophage migration inhibitory factor. Front. Mol. Biosci. 11, 1414572. doi:10.3389/fmolb.2024.1414572
Singh, S., Goyal, A., and Agrawal, N. (2023). Molecular docking and dynamic simulation to identify α7nAChR binding affinity of flavonoids for the treatment of alzheimer’s disease. Chem. Biodivers. 20 (7), e202300306. doi:10.1002/cbdv.202300306
Sirimangkalakitti, N., Juliawaty, L. D., Hakim, E. H., Waliana, I., Saito, N., Koyama, K., et al. (2019). Naturally occurring biflavonoids with amyloid β aggregation inhibitory activity for development of anti-Alzheimer agents. Bioorg Med. Chem. Lett. 29 (15), 1994–1997. doi:10.1016/j.bmcl.2019.05.020
Smith, E. E., Duchesne, S., Gao, F., Saad, F., Whitehead, V., McCreary, C. R., et al. (2021). Vascular contributions to neurodegeneration: protocol of the COMPASS-ND study. Can. J. Neurol. Sci. 48 (6), 799–806. doi:10.1017/cjn.2021.19
Sun, B. R., Wang, Z., and Lin, Z. Y. (2023). Risk factors of carotid atherosclerosis in elderly patients with type 2 diabetes mellitus complicated with coronary heart disease. Jilin Med. J. 44 (05), 1183–1185.
Sun, L., Sharma, A. K., Han, B. H., and Mirica, L. M. (2020). Amentoflavone: a bifunctional metal chelator that controls the formation of neurotoxic soluble Aβ42 oligomers. ACS Chem. Neurosci. 11 (17), 2741–2752. doi:10.1021/acschemneuro.0c00376
Tao, Y., Zhu, F., Pan, M., Liu, Q., and Wang, P. (2022). Pharmacokinetic, metabolism, and metabolomic strategies provide deep insight into the underlying mechanism of Ginkgo biloba flavonoids in the treatment of cardiovascular disease. Front. Nutr. 9, 857370. doi:10.3389/fnut.2022.857370
Tarallo, V., Lepore, L., Marcellini, M., Dal Piaz, F., Tudisco, L., Ponticelli, S., et al. (2011). The biflavonoid amentoflavone inhibits neovascularization preventing the activity of proangiogenic vascular endothelial growth factors. J. Biol. Chem. 286 (22), 19641–19651. doi:10.1074/jbc.M110.186239
The Writing Committee Of The Report On Cardivascular Health And Diseases Hu, S. S. (2023). Report on cardiovascular health and diseases in China 2021: an updated summary. J. Geriatr. Cardiol. 20 (6), 399–430. doi:10.26599/1671-5411.2023.06.001
Vlasiou, M. C. (2023). Cheese and milk adulteration: detection with spectroscopic techniques and HPLC: advantages and disadvantages. Dairy 4 (3), 509–514. doi:10.3390/dairy4030034
Wang, B., Lu, Y., Wang, R., Liu, S., Hu, X., and Wang, H. (2020a). Transport and metabolic profiling studies of amentoflavone in Caco-2 cells by UHPLC-ESI-MS/MS and UHPLC-ESI-Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 189, 113441. doi:10.1016/j.jpba.2020.113441
Wang, G., Li, D., Jiang, F. Q., Xie, X. Y., and He, Y. Q. (2018b). Optimization of microwave-assisted ionic liquid extraction of amentoflvone from Selaginella doederleinii Hieron. Chin. Tradit. Pat. Med. 40 (08), 1851–1855. doi:10.3969/j.issn.1001-1528.2018.08.040
Wang, G., Tian, Y. B., Jiang, Y. M., He, Q., Yuan, S. M., and Wei, W. (2018c). Optimization of microwave extraction of amentoflavone from Selaginella doederleinii by response surface method. Sci. Technol. Food Ind. 39 (07), 146–151. doi:10.13386/j.issn1002-0306.2018.07.027
Wang, H. (2017). Study on extraction, isolation, characterization and anti-oxidant activities of flavonoids from Podocarpus nagi. Jishou (Hunan): Jishou University. dissertation.
Wang, J. Q., Li, M. M., Fan, B., Cui, W. Y., Wang, Q., Lu, C., et al. (2023). Research progress on extraction, separation and biological activity of natural Bi-flavonoids. Food Nutr. China, 1–8. doi:10.19870/j.cnki.11-3716/ts.20231018.001
Wang, L. (2013). Protective effect against hydroxyl radical-induced DNA damage and antioxidant mechanism of amentoflavone. Guangzhou (Guangdong): Guangzhou University of Chinese Medicine. dissertation.
Wang, X., Zhao, X., Gu, L., Lv, C., He, B., Liu, Z., et al. (2014). Simultaneous determination of five free and total flavonoids in rat plasma by ultra HPLC-MS/MS and its application to a comparative pharmacokinetic study in normal and hyperlipidemic rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 953-954, 1–10. doi:10.1016/j.jchromb.2014.01.042
Wang, Y., Chen, L. Z., Duan, H. T., Wang, L. P., Xu, C., Ling, L., et al. (2018a). Determination of content of amentoflavone in Selaginella tamariscina by infrared-assisted extraction method coupled with HPLC. Chin. J. Clin. Pharm. 27 (03), 164–166. doi:10.19577/j.1007-4406.2018.03.005
Wang, Y., Wang, K., and Fu, J. (2020b). HDAC6 mediates macrophage iNOS expression and excessive nitric oxide production in the blood during endotoxemia. Front. Immunol. 11, 1893. doi:10.3389/fimmu.2020.01893
Wang, Y. Z., Zhang, M., Liu, Y., Zhao, H. L., and Zhen, X. K. (2015). Pharmacokinetics study on amentoflavone in rats. Chin. Tradit. Pat. Med. 37 (11), 2397–2401. doi:10.3969/j.issn.1001-1528.2015.11.013
Wang, Z., Wang, B., and Jin, X. (2024). Amentoflavone attenuates homocysteine-induced neuronal ferroptosis-mediated inflammatory response: involvement of the SLC7A11/GPX4 axis activation. Brain Res. Bull. 215, 111005. doi:10.1016/j.brainresbull.2024.111005
Wei, L. N. (2010). Study on the separation and purification of amentoflavone from selaginella tamariscina (beauv.) spring. Beijing (Beijing): Beijing University of Chemical Technology. dissertation.
Wei, B. Y., and Liang, M. H. (2021). Research progress of mechanism of diabetes melitus causes atherosclerosis. Chin. J. Cardiovasc Rehabil. Med. 30 (01), 85–87. doi:10.3969/j.issn.1008-0074.2021.01.22
Wei, D. D., Chen, X., Zhao, J. J., Hu, X. L., Xiong, F., and Wang, H. (2017). Studies on the intestinal absorption kinetics of amentoflavone in rats. Pharm. Clin. Res. 25 (04), 282–285. doi:10.13664/j.cnki.pcr.2017.04.002
Wei, L. H., Chen, T. R., Fang, H. B., Jin, Q., Zhang, S. J., Hou, J., et al. (2019). Natural constituents of St. John's Wort inhibit the proteolytic activity of human thrombin. Int. J. Biol. Macromol. 134, 622–630. doi:10.1016/j.ijbiomac.2019.04.181
Windsor, P. K., Plassmeyer, S. P., Mattock, D. S., Bradfield, J. C., Choi, E. Y., Miller, B. R., et al. (2021). Biflavonoid-induced disruption of hydrogen bonds leads to amyloid-β disaggregation. Int. J. Mol. Sci. 22 (6), 2888. doi:10.3390/ijms22062888
Wiszniak, S., and Schwarz, Q. (2021). Exploring the intracrine functions of VEGF-A. Biomolecules 11 (1), 128. doi:10.3390/biom11010128
Woo, E. R., Lee, J. Y., Cho, I. J., Kim, S. G., and Kang, K. W. (2005). Amentoflavone inhibits the induction of nitric oxide synthase by inhibiting NF-kappaB activation in macrophages. Pharmacol. Res. 51 (6), 539–546. doi:10.1016/j.phrs.2005.02.002
Xi, C. C., Gu, W., and Zhou, L. L. (2020). The isolation, identification, antioxidation and anticoagulation activity of selaginellin and amentoflavone from selaginella tamariscina (beauv.) Spring. Acta Medica Mediterr. 36, 2697–2706. doi:10.19193/0393-6384_2020_4_414
Xiao, L., Chen, Y., Zhang, F., Long, X. Y., Nie, J., and Huang, Z. J. (2018). Chemical constituents of biflavonoids from Selaginella uncinata. J. Pharm. Anal. 38 (12), 2093–2103. doi:10.16155/j.0254-1793.2018.12.06
Xiong, L., Liu, Y., Wang, Y., Zhao, H., Song, X., Fan, W., et al. (2024). The protective effect of Lonicera japonica Thunb. against lipopolysaccharide-induced acute lung injury in mice: modulation of inflammation, oxidative stress, and ferroptosis. J. Ethnopharmacol. 331, 118333. doi:10.1016/j.jep.2024.118333
Xiong, X., Tang, N., Lai, X., Zhang, J., Wen, W., Li, X., et al. (2021). Insights into amentoflavone: a natural multifunctional biflavonoid. Front. Pharmacol. 12, 768708. doi:10.3389/fphar.2021.768708
Xu, F., Li, J., Mao, Y., Xu, X. J., and He, J. H. (2013). Ginkgo biloba leaf extract research progress. Food Res. Dev. 16, 124–127. doi:10.3969/j.issn.1005-6521.2013.16.035
Xu, H., Li, R. H., Xia, Y. S., and Yang, X. Q. (2021). Research status and prospect of extraction and purification methods of flavonoids. Appl. Chem. Ind. 50 (06), 1677–1682. doi:10.16581/j.cnki.issn1671-3206.2021.06.010
Xu, J., Wang, Y., Zheng, T., Huo, Y., and Du, W. (2022). Biflavones inhibit the fibrillation and cytotoxicity of the human islet amyloid polypeptide. J. Mater Chem. B 10 (24), 4650–4661. doi:10.1039/d2tb00230b
Xu, Z., Jia, S. J., Tan, G. S., and Li, Y. J. (2004). Study on pharmacological activity of biflavones from Selaginella pulvinata (hook. Et grev.) Maxim. Chin. J. Mod. Med. 14, 88–89+100.
Xun, L., and Yin, M. H. (2009). Experiment study on vasodilative effects of amentoflavone ethylacetate extract of Selaginella tamariscina. J. Med. Sci. Yanbian Univ. 32 (04), 246–248. doi:10.16068/j.1000-1824.2009.04.011
Yan, X. D., and Guo, M. L. (2022). Research progress of the effect of flavonoids on cardiovascular and cerebrovascular ischemic diseases. J. Pharm. Pract. Serv. 40 (02), 97–102. doi:10.12206/j.issn.1006-0111.202111059
Yang, Y. T. (2023). The effect of EQST on atherosclerosis formation in mice and its mechanism. Guilin (Guangxi): Guangxi Normal University. dissertation.
Yao, W., Li, H., Liu, Q., Gao, Y., Dai, J., Bao, B., et al. (2016). Cellular metabolomics revealed the cytoprotection of amentoflavone, a natural compound, in lipopolysaccharide-induced injury of human umbilical vein endothelial cells. Int. J. Mol. Sci. 17 (9), 1514. doi:10.3390/ijms17091514
Yu, S., Yan, H., Zhang, L., Shan, M., Chen, P., Ding, A., et al. (2017). A review on the phytochemistry, pharmacology, and pharmacokinetics of amentoflavone, a naturally-occurring biflavonoid. Molecules 22 (2), 299. doi:10.3390/molecules22020299
Zhang, J., Zhou, J., Zhang, T., Niu, Z., Wang, J., Guo, J., et al. (2019). Facile fabrication of an amentoflavone-loaded micelle system for oral delivery to improve bioavailability and hypoglycemic effects in KKAy mice. ACS Appl. Mater Interfaces 11 (13), 12904–12913. doi:10.1021/acsami.9b03275
Zhang, L. J. (2014). The effects and potential mechanism of total flavonoids of Selaginella Pulvinata and amentoflavone against cognitive impairment. Changchun (Jiling): Jiling University. dissertation.
Zhang, Q., Wang, Y. L., Gao, D., Cai, L., Yang, Y. Y., Hu, Y. J., et al. (2018). Comparing coagulation activity of Selaginella tamariscina before and after stir-frying process and determining the possible active constituents based on compositional variation. Pharm. Biol. 56 (1), 67–75. doi:10.1080/13880209.2017.1421673
Zhang, X., Liu, Y., Deng, G., Huang, B., Kai, G., Chen, K., et al. (2021a). A purified biflavonoid extract from Selaginella moellendorffii alleviates gout arthritis via NLRP3/ASC/Caspase-1 Axis suppression. Front. Pharmacol. 12, 676297. doi:10.3389/fphar.2021.676297
Zhang, Z., Sun, T., Niu, J. G., He, Z. Q., Liu, Y., and Wang, F. (2015). Amentoflavone protects hippocampal neurons: anti-inflammatory, antioxidative, and antiapoptotic effects. Neural Regen. Res. 10 (7), 1125–1133. doi:10.4103/1673-5374.160109
Zhang, Z., and Wang, F. (2013). Progress in research of the biological activity of amentoflavone. Chin. J. New Drugs. 22 (23), 2775–2778.
Zhang, Z., Yang, X., Song, Y. Q., and Tu, J. (2021b). Autophagy in Alzheimer's disease pathogenesis: therapeutic potential and future perspectives. Ageing Res. Rev. 72, 101464. doi:10.1016/j.arr.2021.101464
Zhao, F., Qian, Y., Li, H., Yang, Y., Wang, J., Yu, W., et al. (2022). Amentoflavone-loaded nanoparticles enhanced chemotherapy efficacy by inhibition of AKR1B10. Nanotechnology 33 (38), 385101. doi:10.1088/1361-6528/ac7810
Zhao, N., Sun, C., Zheng, M., Liu, S., and Shi, R. (2019). Amentoflavone suppresses amyloid β1-42 neurotoxicity in Alzheimer's disease through the inhibition of pyroptosis. Life Sci. 239, 117043. doi:10.1016/j.lfs.2019.117043
Zheng, X. K., Ling, T. L., Wang, X. L., Liu, C. X., Liu, Y. Y., and Feng, W. S. (2011). Effects of total flavonoids and amentoflavone isolated from Selaginella tamariscina on human umbilical vein endothelial cells proliferation and VEGF expreesion. Chin. Pharm. J. 46 (13), 998–1002.
Zheng, X. K., Liu, C. X., Zhai, Y. Y., Li, L. L., Wang, X. L., and Feng, W. S. (2013). Protection effect of amentoflavone in Selaginella tamariscina against TNF-α-induced vascular injure of endothelial cells. Yao Xue Xue Bao 48 (09), 1503–1509. doi:10.16438/j.0513-4870.2013.09.014
Zheng, X. K., Wei, Y., Feng, W. S., Li, Y. J., and Zhao, X. M. (2008). “Study on hypoglycemic activity of amentoflavone in vitro,” in Communication between biochemistry and molecular biology of traditional Chinese medicine, 182–187.
Zhou, L. N., Wang, R., Wang, J. J., Zhao, K., Zhang, T., Hu, X. L., et al. (2022). Characterization, solubility and stability of amentoflavone polymorphs. J. Mol. Struct. 1262, 133101. doi:10.1016/j.molstruc.2022.133101
Zhou, Y., Yang, Z., Guo, Z., Kang, Y., and Zhou, K. J. (2024). Research progress on the mechanism of Tianma Gouteng decoction in preventing and treating nervous system diseases. Glob. Tradit. Chin. Med. 17 (01), 157–165. doi:10.3969/j.issn.1674-1749.2024.01.031
Zhuang, J. L. (2020). Effects and mechanisms of amentoflavone on lipid accumulation and cell migration. Guangzhou (Guangdong): Guangzhou University of Chinese Medicine. dissertation.
Glossary
AME Amentoflavone
AGEs Advanced glycation end-products
ASK1 Apoptosis signal regulating kinase 1
Akt Protein kinase B
AD Alzheimer’s disease
Aβ β-amyloid
AChE Acetylcholinesterase
Ang Angiotensin
ACE Angiotensin-converting enzyme
AMPK Adenosine monophosphate-activated protein kinase
AT1R Angiotensin II receptor-1
Arg1 Arginase 1
AUC(0-t) Area under the concentration-time cure
AUC(0-∞) From time zero to all original drug elimination
AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
α7nACHR α7 nicotinic acetylcholine receptor
Cmax Maximum blood concentration
CL/F Clearance
COX-2 Cyclooxygenase-2
CD36 Cluster of differentiation 36
C/EBP Transcription factor CCAAT enhancer binding protein
CAT Catalase
cGMP Guanosine 3’,5’-cyclic monophosphate
caspase Cysteinyl aspartate specific protease
DPPH 1,1-diphenyl-2 trinitrophenylhydrazine
DOX Adriamycin
ECs Endothelial cells
ET-1 Endothelin-1
ERK Extracellular signal-regulated kinase
Fasl Fas ligand
F Bioavailability
Fizz1 Found in inflammatory zone
GSK-3-β Glucose synthesis kinase-3-β
GBE Ginkgo biloba extract
GSH Glutathione
GPX4 Glutathione peroxidase 4
HMGB1 High mobility group box protein B1
HSP-27 Heat shock protein 27
HFFD High fructose and fat diet
HPLC High-performance liquid chromatography
HUVECs Human umbilical vein endothelial cells
hIAPP Human islet amyloid polypeptide
IL Interleukin
IκB Inhibitor of NF-κB
iNOS Nitric oxide synthase
LPS Lipopolysaccharide
LDL Low-density lipoprotein
MRT0-∞ Mean residence time
MRT0-t Average retention time for a certain period of time
MMPs Matrix metalloproteinases
MCE Mitotic clonal expansion phase
MDA Malondialdehyde
MAPK Mitogen-activated protein kinase
mTOR Mechanistic target of rapamycin
MPTP Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
MASR Mitochondrial assembly receptor
MIF Macrophage migration inhibitory factor
NLRP3 NOD-like receptor thermal protein domain associated protein 3
NOX NADPH oxidase
NF-κB Nuclear factor-kappa B
NO Nitric oxide
Nrf2 Nuclear factor erythroid 2-related factor-2
NRF-1 Nuclear respiratory factor-1
ox-LDL Oxidized low-density lipoprotein
PGE-2 Prostaglandin E-2
PPAR Peroxisome proliferator-activated receptor
PI3K Phosphoinositide 3-kinase
PD Parkinson’s disease
PIGF-1 Placental growth factor-1
ROS Reactive oxygen species
SOD Superoxide dismutase
STING Stimulator of interferon genes
SLC7A11 Solute carrier family 7 member 11
TG Triglycerides
Trx Thioredoxin
TrxR-1 Thioredoxin reductase-1
Tmax Time to peak concentration
T1/2 Biological half-life
T2DM Type 2 diabetes model
TNF Tumor necrosis factor
TGF Transforming growth factor
TLR4 Toll-like receptor-4
TFAM Mitochondrial transcription factor
UGT1A Uridine diphosphate glucuronosyltransferase 1 family, polypeptide A
V/F Apparent distribution volume
VSMCs Vascular smooth muscle cells
VEGF Endothelial growth factor
VGSC Voltage-gated sodium ion channels
Ym1 Chitinase-like protein 3
Keywords: amentoflavone, cardiovascular diseases, cerebrovascular diseases, neurological diseases, pharmacological effects, anti-inflammatory agents, Chinese herbal medicine
Citation: Zhang H, Ban Y-m, Li D-m, Wang G, Gu J and Zhu L (2024) Amentoflavone for treating cardiocerebrovascular diseases and neurological disorders. Front. Pharmacol. 15:1406510. doi: 10.3389/fphar.2024.1406510
Received: 25 March 2024; Accepted: 07 November 2024;
Published: 18 November 2024.
Edited by:
Yusof Kamisah, Universiti Kebangaan Malaysia, MalaysiaReviewed by:
Baskaran Stephen Inbaraj, Fu Jen Catholic University, TaiwanPrabuddha Bhattacharya, West Bengal State University, India
Copyright © 2024 Zhang, Ban, Li, Wang, Gu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhu, emh1bGVpbWFpbEB6bXUuZWR1LmNu; emh1bGVpbGVpQGxpdmUuY24=
 Hang Zhang1,2,3
Hang Zhang1,2,3 Gang Wang
Gang Wang Juan Gu
Juan Gu Lei Zhu
Lei Zhu