- 1Department of Pharmacy, The First Affiliated Hospital of Army Medical University (Third Military Medical University), Chongqing, China
- 2Department of Pharmacy, Nanan People’s Hospital of Chongqing, Chongqing, China
Background: Polatuzumab vedotin, the first FDA-approved antibody-drug conjugate (ADC) targeting CD79b, is utilized in the treatment of previously untreated diffuse large B-cell lymphoma (DLBCL) or high-grade B-cell lymphoma (HGBL), as well as relapsed or refractory (R/R) DLBCL. Despite its approval, concerns persist regarding the long-term safety profile of polatuzumab vedotin. This study aims to evaluate the adverse events (AEs) associated with polatuzumab vedotin since its approval in 2019, utilizing data mining strategies applied to the FDA Adverse Event Reporting System (FAERS).
Methods: Signal detection employed four methodologies, including reporting odds ratio (ROR), proportional reporting ratio (PRR), bayesian confidence propagation neural network (BCPNN), and multi-item gamma poisson shrinker (MGPS), to evaluate and quantify the signals of polatuzumab vedotin-associated AEs. Additionally, subgroup analyses based on patients age, gender, and fatal cases were conducted to investigate AEs occurrences in specific subpopulations.
Results: A total of 1,521 reports listing polatuzumab vedotin as a “principal suspect (PS)” drug were collected from the FAERS database. Through concurrent compliance with four algorithms, 19 significant Standardized MedDRA Query (SMQ) AEs and 92 significant Preferred Term (PT) AEs were detected. Subgroup analyses revealed a higher incidence of PTs in male patients compared to female patients, increased likelihood of polatuzumab vedotin-associated AEs in elder patients (>65 years), and AEs with a high risk of fatal cases include: blood lactate dehydrogenase increased, cytopenia, and hydronephrosis. The median time to AEs occurrence following polatuzumab vedotin initiation was 18.5 (5∼57.75) days, with 95% of AEs occurred within 162 days.
Conclusion: This study identified various AEs associated with polatuzumab vedotin, offering critical insights for clinical monitoring and risk identification in patients receiving polatuzumab vedotin therapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous and invasive tumor originating from mature B-cells, comprising 35%∼40% of all non-Hodgkin’s lymphoma (Testoni et al., 2015). Its incidence is on the rise annually (Cultrera and Dalia, 2012; Goy et al., 2019). Approximately 60% of DLBCL patients have received standard chemotherapy, however, 30%–40% of patients may encounter recurrence or progression to refractory DLBCL (Vodicka et al., 2022). Therefore, there is a critical need to explore novel drugs and treatment for recurrent or refractory DLBCL, with expanding therapeutic prospects through targeted therapy development. Some approved second-line or higher treatments include the combination therapy of polatuzumab vedotin, an antibody drug conjugate, in combination with Bendamustine (B) and Rituximab (R) (Sehn et al., 2022), and the combination of anti-CD19 monoclonal antibody tafasitamab with lenalidomide (Belada et al., 2023).
Polatuzumab vedotin is a novel humanized anti-CD79b antibody coupled with the anti-mitotic agent monomethylastatin E (MMAE) to form an antibody-drug conjugate (ADC). It was rapidly approved by the US Food and Drug Administration (FDA) on 10 June 2019, in combination with BR for adult patients with relapsed/refractory (R/R) DLBCL who received at least two prior treatments (Deeks, 2019). On 19 April 2023, the FDA extended its approval for adult patients with previously untreated DLBCL or high-grade B-cell lymphoma (HGBL) (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-polatuzumab-vedotin-piiq-previously-untreated-diffuse-large-b-cell-lymphoma-not). Polatuzumab vedotin combines advantages of immunotherapy and chemotherapy, with its target CD79b showing over 96% positivity in B-cell tumor cells and minimal expression in normal cells, enhancing targeting precision and reducing side-effects (Polson et al., 2007; Dornan et al., 2009). Additionally, the conjugated anti-microtubule agent MMAE exhibits potent cytotoxicity against lymphoma cells with higher selectivity and robust targeting, expanding its clinical application prospects.
With the widespread use of polatuzumab vedotin concerns about its safety have risen in recent years. The previous phase II and III clinical studies have identified the most common adverse events (AEs), including neutropenia, thrombocytopenia, anemia, hair loss, peripheral neuropathy, fatigue, diarrhea, fever, loss of appetite, and pneumonia (Terui et al., 2021; Sehn et al., 2022; Song et al., 2023). However, long-term safety and efficacy data primarily rely on case reports or clinical trials. It is necessary to perform comprehensive real-world safety assessments owing to limitations in sample sizes, selection criteria, follow-up duration, and AEs focused on specific systems. Given the widespread clinical application of polatuzumab vedotin and the lack of real-world AEs assessment, this pharmacovigilance analysis is essential in evaluating its safety and promoting rational prescription.
The FDA Adverse Event Reporting System (FAERS), a public pharmacovigilance database, facilitates post-market safety monitoring of drugs and therapeutic products (Vogel et al., 2020; Setyawan et al., 2021). This study aims to evaluate the safety of polatuzumab vedotin by analyzing post-marketing FAERS data to provide a comprehensive and valuable reference for its rational clinical application.
Materials and methods
Data sources and procedures
The FAERS database, a public database, is used by the FDA to gather all spontaneously reported AE reports. Data from the launch of polatuzumab vedotin (10 June 2019) to the third quarter of 2023 were downloaded from the FAERS database and imported into PostgreSQL for management. In this study, the basic information (including age, sex and weight), reporting country, type of reporter, indications, suspicious drugs, AEs, time of onset of AEs, date of occurrence of AEs and outcome of AEs were extracted for analysis.
In this study, “polatuzumab vedotin” and the trade name “POLIVY” were used to identify the related records of polatuzumab vedotin. To enhance accuracy, only cases in which the drug has the effect of “primary suspect” (PS) in AEs and the patients aged 18 or above were used. The preferred term (PT) in MedDRA terminology was used to encode every report in the FAERS database. We used PT to identify AEs. In addition, we also grouped AEs into the Standardized MedDRA Query (SMQ)to describe disease conditions.
Data mining algorithm
Based on a fourfold table, the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the Bayesian confidence propagation neural network (BCPNN), and the multi-item gamma Poisson shrinker (MGPS) were applied to detect an association between various polatuzumab vedotin regimens and AEs in accordance with the disproportionality analysis. The equations and criteria for the above four algorithms are shown in Supplementary Table S1.
The time to onset of AEs
Time to onset of AEs is calculated by subtracting the date of initiation of polatuzumab vedotin from the date of onset of AEs in the report. This study analyzed the time to AEs after polatuzumab vedotin use. The median and interquartile range were used to characterize the time to AEs, to compare the distribution of AEs by gender and age and the cumulative incidence of AEs.
Subgroup analysis
According to the patient’s age (18–65 years old, >65 years old), gender (male and female), and death cases, the subgroup analysis was conducted to investigate the occurrence of AEs in the subgroup.
Statistical analysis
The results were analyzed using descriptive statistics. Display the frequency (percentage) of categorical variables and continuous variables’ median (interquartile range). All data in this study were processed and analyzed using PostgreSQL (version 15.3), R software (version 4.3.1), and MedDRA (version 26.1).
Results
Descriptive analysis
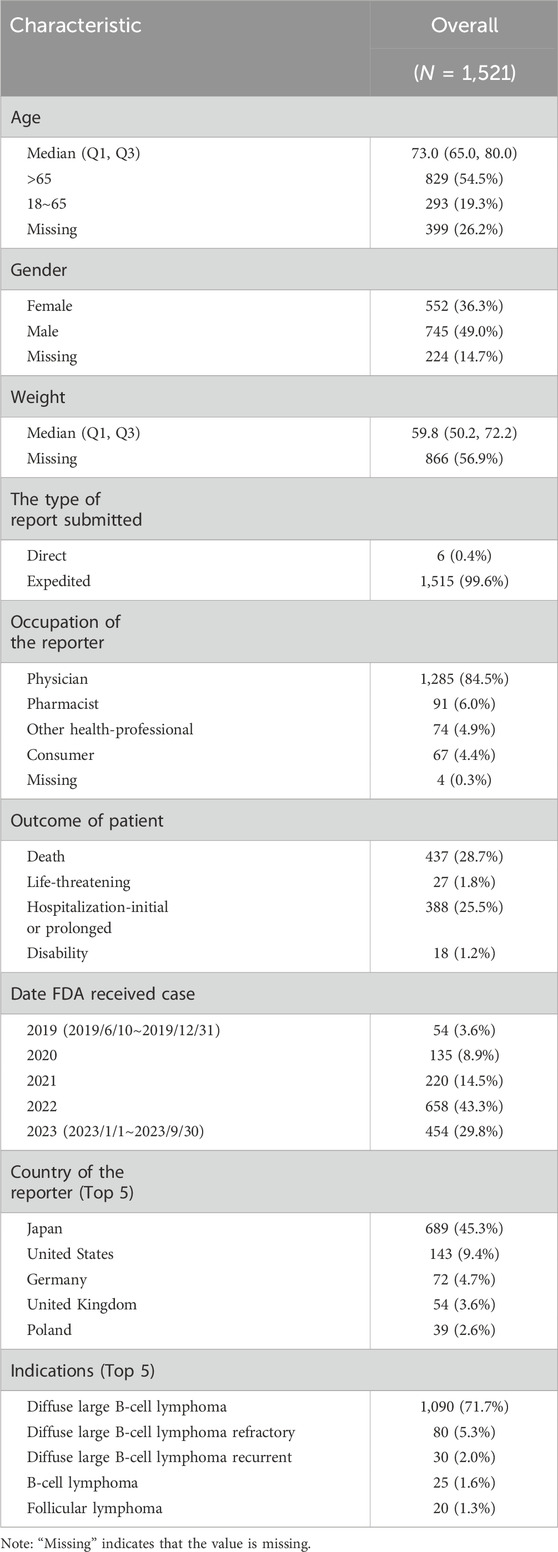
From 10 June 2019, to the third quarter of 2023, FAERS received 1,521 reports of AEs using polatuzumab vedotin. The median age of patients in the reports was 73 years old, with a median weight of 59.8 kg, male patients were the majority (49.0%). 99.6% were urgent reports, and medical authors reported 95.4%. The reporting countries are mainly Japan (45.3%), the United States (9.4%), and Germany (4.7%). The patient’s diagnosis is mainly diffuse large B-cell lymphoma (78.9%). The main accompanying medications are Rituximab (71.1%), Bendamustine (54.4%), and Cyclophosphamide (23.7%). The reported AE outcomes were mostly death (28.7%) and prolonged hospitalization (25.5%), as indicated in Table 1.
SMQ level signal detection
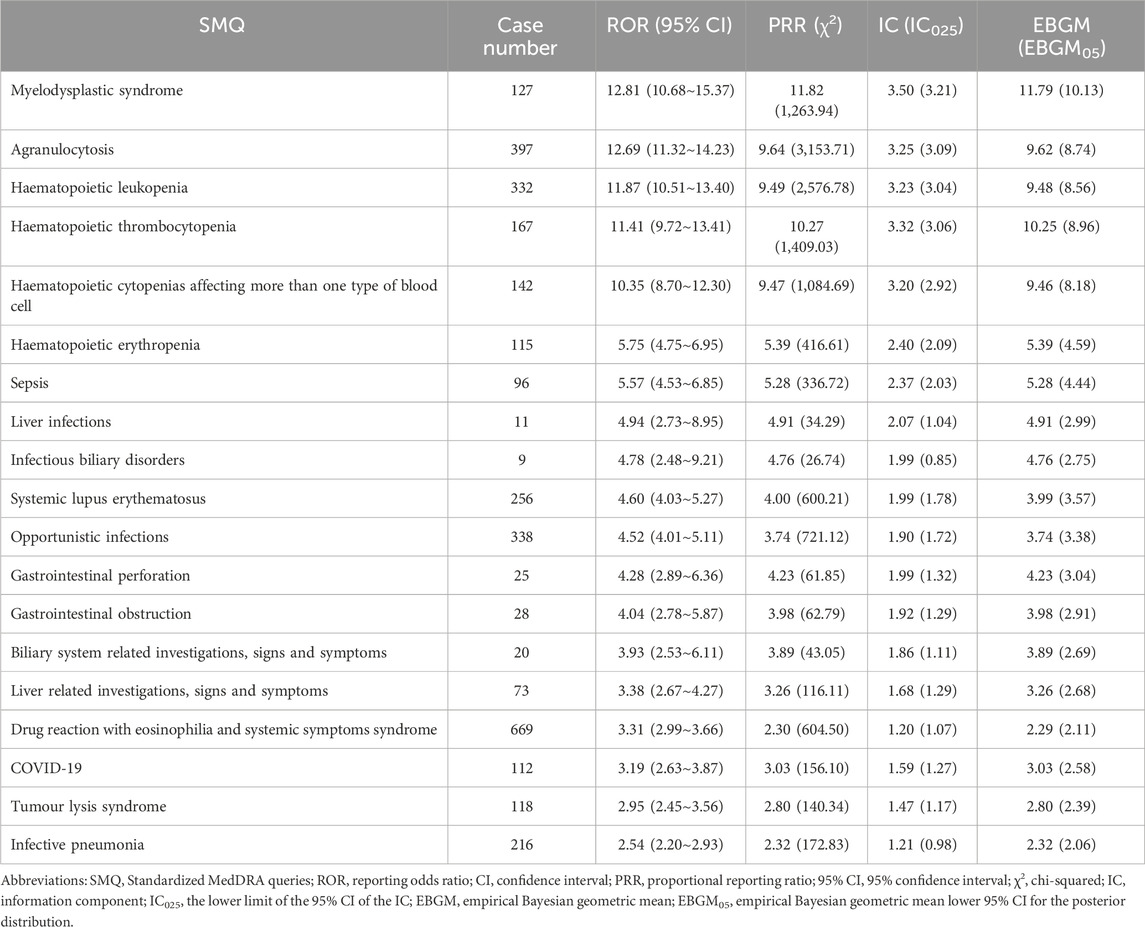
As demonstrated in Table 2, a total of 19 polatuzumab vedotin-related SMQ level AEs were detected after completing four algorithms simultaneously. It can be seen that the signal of myelodysplastic syndrome is the strongest (ROR = 12.81, PRR = 11.82, IC = 3.50, EBGM = 11.79), followed by agranulocytosis (ROR = 12.69, PRR = 9.64, IC = 3.25, EBGM = 9.62) and hematopoietic leukopenia (ROR = 11.87, PRR = 9.49, IC = 3.23, EBGM = 9.48). Other serious AEs include haematopoietic thrombocytopenia (ROR = 11.41, PRR = 10.27, IC = 3.32, EBGM = 10.25), haematopoietic cytopenias affecting more than one type of blood cell (ROR = 10.35, PRR = 9.47, IC = 3.20, EBGM = 9.46), haematopoietic erythropenia (ROR = 5.75, PRR = 5.39, IC = 2.40, EBGM = 5.39), and sepsis (ROR = 5.57, PRR = 5.28, IC = 2.37, EBGM = 5.28). Other rare signals include biliary system related investigations, signs and symptoms, liver related investigations, signs and symptoms, drug reaction with eosinophilia and systemic symptoms syndrome, COVID-19, tumor lysis syndrome, infective pneumonia, etc.
PT level signal detection
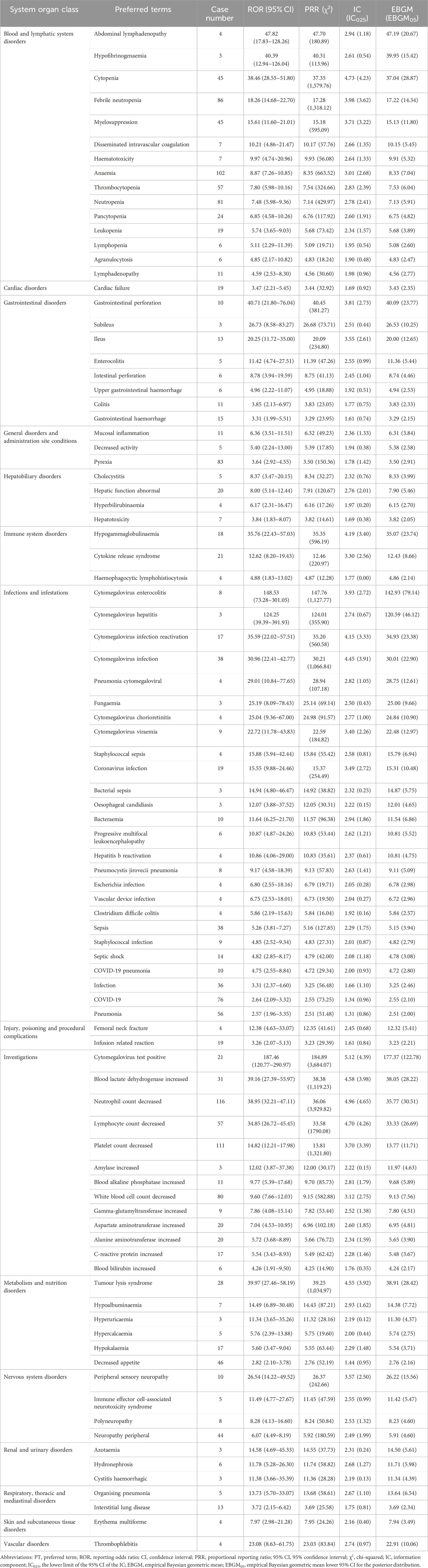
After complying with four algorithms simultaneously, 92 PT level AEs related to polatuzumab vedotin were detected, as shown in Table 3. The results revealed that 15 systems were involved in the AE signals associated with polatuzumab vedotin. The top five systemic organs (SOC) reported by polatuzumab vedotin-related AEs were blood and lymphatic system disorders (503 cases), investigations (502 cases), infections and infestations (394 cases), metabolism and nutrition disorders (106 cases), general disorders and administration site conditions (99 cases). Among them, blood and lymphatic system diseases involve 15 PT signals, mainly including anemia (102 cases), febrile neutrtropenia (86 cases), neutropenia (81 cases), thrombocytopenia (57 cases), and so on. Among the top 20 ranked PTs in the number of reports, neutrophil count decreased, anaemia, platelet count decreased, white blood cell count decreased, febrile neutropenia, pyrexia, neutropenia, thrombocytopenia, lymphocyte count decreased, pneumonia, decreased appetite, cytopenia, myelosuppression, cytomegalovirus infection, neuropathy peripheral, tumour lysis syndrome, sepsis and infection are AEs included in the polatuzumab vedotin manual; COVID-19 and blood lactate dehydrogenase increased is not explicitly indicated in the polatuzumab vedotin manual and deserves further evaluation.
Subgroup analysis
This study conducted a subgroup analysis of AEs caused by the use of polatuzumab vedotin in different genders and age groups. Additionally, an analysis was conducted on cases of AEs that resulted in death after taking polatuzumab vedotin. The results indicated that the order of signal intensity was consistent for each of the four signal detection methods, which involved 54 PTs in male patients and 49 PTs in female patients, with male patients having a higher number of PTs than female patients (Supplementary Table S2). Among the top 10 related AEs with ROR signal intensity, male patients are mainly involved in AEs after using polatuzumab vedotin, including neutral count decreased, lymphocyte count decreased, cytopia, cytomegalovirus infection, and blood lactate dehydrogenase increased. The main AEs in female patients using polatuzumab vedotin include neutrophil count decreased, lymphocyte count decreased, tumour lysis syndrome, cytomegalovirus infection, and cytomegalovirus test positive. The details are shown in Figure 1.
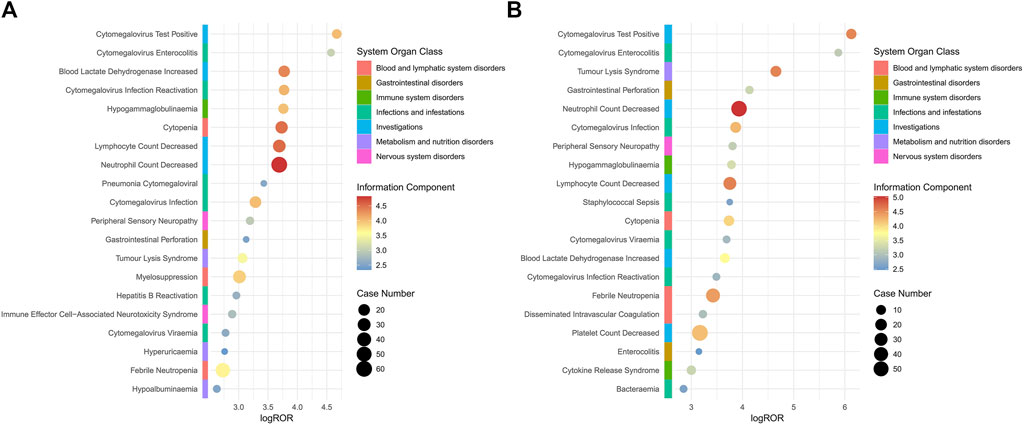
Figure 1. Subgroup analysis based on gender for polatuzumab vedotin related AEs. (A) Male group; (B) Female group. Abbreviations: ROR, reporting odds ratio; log ROR, logarithm of the reporting ROR; AEs, adverse events.
Age stratified analysis revealed that patients greater than 65 years of age were involved in 51 PTs and patients less than 65 years of age were involved in 36 PTs, and that individuals who were greater than 65 years of age were more likely to experience polatuzumab vedotin-related AEs, as depicted in Supplementary Table S3. The primary side effects of polatuzumab vedotin for patients over 65 years old include neutrophil count decreased, lymphocyte count decreased, cytomegalovirus infection, cytopenia, and cytomegalovirus test positive. The main AEs associated with the use of polatuzumab vedotin in patients under the age of 65 include neutral count decreased, lymphocyte count decreased, cytokine release syndrome, and blood lactate dehydrogenase increased. The details are shown in Figure 2.
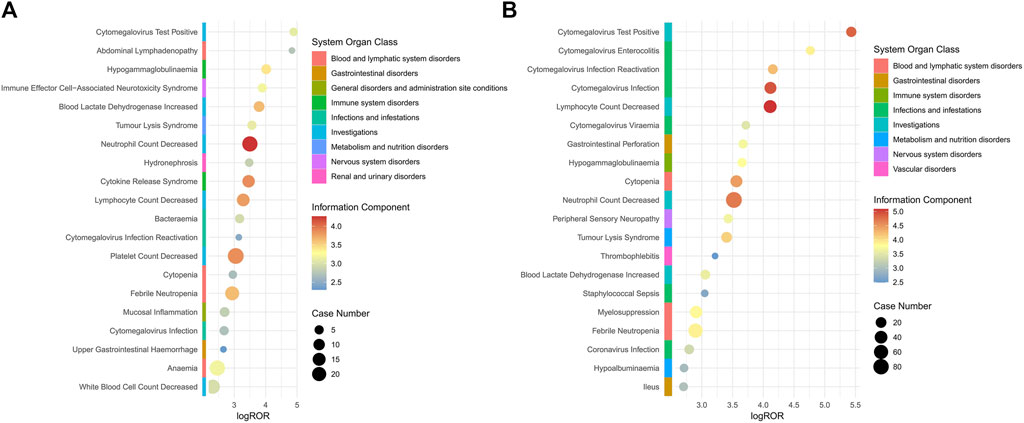
Figure 2. Subgroup analysis based on age for polatuzumab vedotin related AEs. (A) 18–65 years old (younger patients); (B) >65 years of old (elder patients). Abbreviations: ROR, reporting odds ratio; log ROR, logarithm of the reporting ROR; AEs, adverse events.
A total of 28 PTs were involved in death cases, as shown in Supplementary Table S4. High-risk AEs in fatal cases include blood lactate dehydrogenase increased, cytopenia, and hydronephrosis. It is recommended to enhance monitoring of such AEs when utilizing this drug. The study demonstrated that blood lactate dehydrogenase increased, hydronephrosis and hypogammaglobulinaemia were manifestations of PT signaling that were not mentioned in the drug instructions, as illustrated in Figure 3.
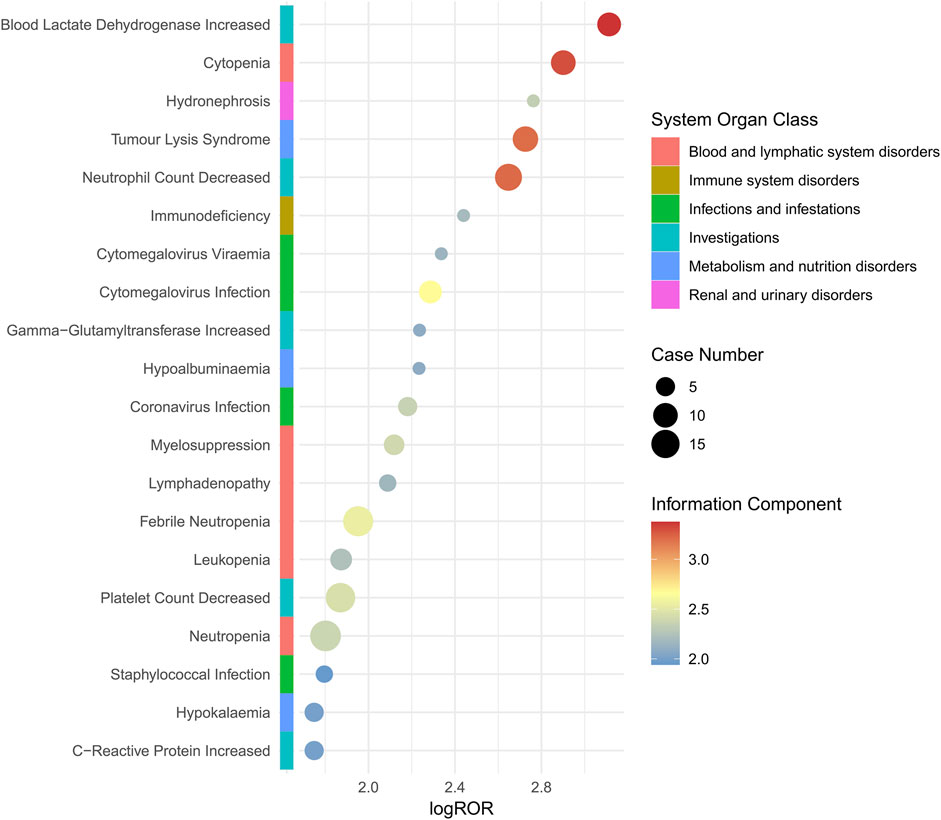
Figure 3. Subgroup analysis based on mortality cases for polatuzumab vedotin related AEs. Abbreviations: ROR, reporting odds ratio; log ROR, logarithm of the reporting ROR; AEs, adverse events.
Analysis of time to polatuzumab vedotin-induced AEs onset
The time of AEs was calculated by subtracting the date of AE occurrence in the report from the date when polatuzumab vedotin was first used in this study. It was found that the median time for AEs was 18.5 (5–57.75) days after initiation of treatment with polatuzumab vedotin, and 95% of cases experienced AEs within 162 days. Meanwhile, we examined the distribution of AEs that happened within 162 days of taking polatuzumab vedotin based on gender and age, and there was no statistically significant difference. Additional information can be found in Supplementary Table S5 and Figure 4.
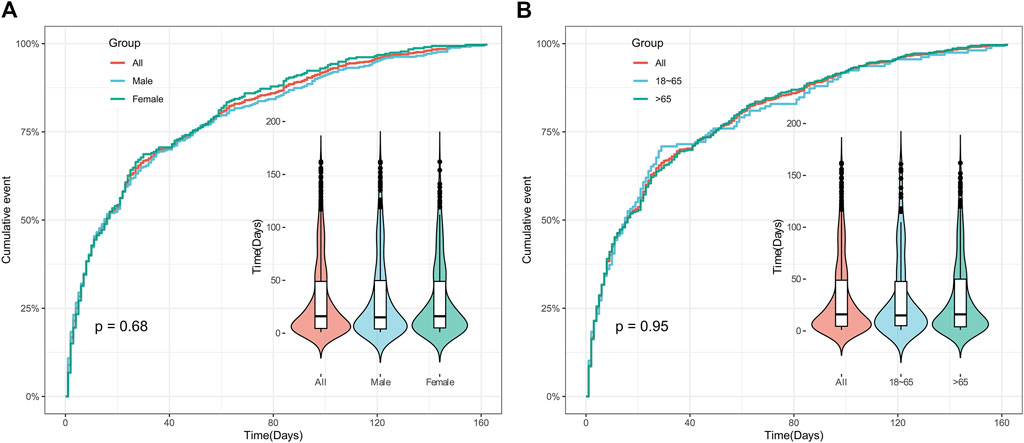
Figure 4. Time to onset of polatuzumab vedotin related AEs. (A) Accumulated incidence of AEs by gender within 162 days; (B) The cumulative incidence rate of AEs in different age groups within 162 days. Abbreviations: AEs, adverse events.
Discussion
In previous studies, research on polatuzumab vedotin has primarily focused on mechanisms, clinical trials, and literature analysis, with limited real-world post-marketing studies. Therefore, we utilized the FAERS database to comprehensively investigate the post-marketing AEs reports associated with polatuzumab vedotin to provide a basis for rational clinical use.
Our study revealed a higher rated of AEs reporting in men compared to women, potentially attributed to an increased male population diagnosed with DLBCL, consequently leading to more medication opportunities. Furthermore, our study suggested a high proportion of AEs in elderly patients (>65 years old), likely due to the increased DLBCL incidence with age, especially among individuals over 65 years old (Morgan et al., 1997; Morton et al., 2006; Hounsome et al., 2022). Elderly patients are also at higher risk of severe infections and exhibit decreased tissue/organ tolerance to drugs with age, rendering them more susceptible to AEs (Wildiers and de Glas, 2020; Thandra et al., 2021). With the expanding clinical use of polatuzumab vedotin, clinicians need to remain vigilant regarding associated AEs, particularly in elderly patients. Healthcare professionals accounted for approximately 95.4% of AE reports, with reporting primarily concentrated in Japan, the United States, and Germany, reflecting the frequent use of polatuzumab vedotin in these countries. The use of polatuzumab vedotin may lead to severe adverse events (SAEs), with approximately 54% of reported cases resulting in hospitalization or prolonged hospital stays. Although not all AEs may be directly caused by polatuzumab vedotin treatment, special attention must be paid to those associated with polatuzumab vedotin, especially SAEs.
In this study, 92 signals involving 15 SOC were mined, including blood and lymphatic system disorders, metabolism and nutrition disorders, general disorders and administration site conditions, gastrointestinal disorders, nervous system disorders, immune system disorders, hepatobiliary disorders, cardiac disorders, respiratory, thoracic, and mediastinal disorders, renal and urinary disorders, skin and subcutaneous tissue disorders, vascular disorders, etc.
High-frequency and high-signal-intensity SOCs were primarily observed in reactions such as blood and lymphatic system disorders, investigations, infections, and infestations, consistent with findings from a global randomized phase III study (Song et al., 2023). The blood systems with a higher frequency of occurrence in these systems include anaemia, febrile neutropenia, and neutropenia; investigations include neutrophil count decreased, platelet count decreased, and white blood cell count decreased; COVID-19, pneumonia, and cytomegalovirus infection have a higher frequency of occurrence in infections and infestations. These AEs are commonly reported in clinical studies and instruction manuals (Phillips et al., 2022; Sehn et al., 2022). Infusion-related reactions were observed in approximately one-third of patients across various systems, with antihistamines and antipyretics recommended prior to treatment to mitigate these reactions. Despite preoperative medication, a small percentage of patients experienced low-grade infusion reactions (Assi et al., 2021). Anemia was the most common AE in the blood system, often accompanied by grade 3 to 4 AEs such as neutropenia and leukopenia, consistent with previous research (Terui et al., 2021; Song et al., 2023). Additionally, peripheral neuropathy, usually dose and duration-dependent, is a common AEs to polatuzumab vedotin, with dosage adjustments and supportive therapy recommended for control (Tilly et al., 2019). Infections and infestations, particularly pneumonia, fungal pneumonia, and sepsis, are the leading causes of fatal AEs, emphasizing the importance of vigilant medication monitoring during treatment (Sehn et al., 2020).
The top ten signals identified in this study, including blood alkaline phosphatase incremented, intrinsic performance, cholecystitis, and erythema multiform, were not explicitly mentioned in the polatuzumab vedotin instruction manual. High attention should be given to these emerging signals. Pneumocystis jirovecii pneumonia, polyeuropathy, hepatic function abnormal, haematotoxicity may be associated with the unspecific distribution of polatuzumab vedotin in multiple hyperperfusion organs, including the lungs, heart, liver, spleen, and kidneys, where ADC is used to decompose MMAE and other metabolites that contain MMAE (Yip et al., 2021). However, further research is necessary to determine the causal relationship between AEs and drugs. Anaemia, white blood cell count decreased, is one of the most common AEs of polatuzumab vedotin. Especially, anemia often leads to patient discontinuation or reduction of dosage (Terui et al., 2021).
The most common grade 3 AEs are those related to the blood and lymphatic system among DLBCL patients who receive polatuzumab vedotin treatment. The role of unbound MMAE in the cycle could be the cause of this effect (Camus and Tilly, 2021). Hematotoxicity is one of the most common dose-limiting adverse reactions in cancer treatment. Routine chemotherapy can be used to target blood cells that renew quickly, and this toxicity can result in a range of blood diseases (Haglund et al., 2010). Blood toxicity is a frequent AE of polatuzumab vedotin, and it includes anemia, thrombocytopenia, and neutropenia, all of which have been noted in the drug instructions. Our research results confirm this precisely. One of the most common and severe hematotoxic effects is anemia, which can cause polatuzumab vedotin treatment to be stopped and interfere with its effectiveness (Terui et al., 2021). Recombinant human erythropoietin (rhuEPO) is thought to be an effective method for treating anemia in cancer patients who undergo chemotherapy (Ozsahin et al., 2005). The majority of polatuzumab vedotin-induced neutropenia is characterized by laboratory abnormalities. In most cases, neutropenia is temporary, reversible, and does not result in treatment interruption. According to the published guidelines (Smith et al., 2006), restoring neutrophil count and continuing treatment can be achieved by administering growth factors and increasing the cycle duration to 28 days. Most clinical studies have reported neutropenia, mainly grade 3 or 4, which is similar to the situation observed in phase 1 studies (Palanca-Wessels et al., 2015) but do not lead to discontinuation of treatment in polatuzumab vedotin-R (Pola-R) patients (Morschhauser et al., 2019). However, severe neutropenia can occur in less than 10% of patients when B and polatuzumab vedotin are combined, which can result in treatment interruption. Therefore, it is necessary to conduct a close follow-up of patients who receive polatuzumab vedotin-BR (Pola-BR) (Lee et al., 2012). This study revealed that the Pola-BR group had a higher prevalence of grade 3 or greater leukopenia, but BR had a similar incidence of febrile neutropenia. The incidence of anemia and thrombocytopenia at all levels was higher in the Pola-BR group than in the BR group. Therefore, preventive treatment and monitoring must be provided by physicians prior to using polatuzumab vedotin to resolve AEs in most patients (Assi et al., 2021). To illustrate, granulocyte colony-stimulating factor and other preventive drugs can be utilized to treat neutropenia (Deeks, 2019).Additionally, we observed a significant increase in the signal intensity of AEs related to myelosuppression in our analysis, which is consistent with the signal intensity reported in the instructions and clinical safety data. Regular monitoring of blood and bone marrow examinations is necessary to prevent fatal AEs caused by myelosuppression, a common AE of anti-tumor drugs.
It’s noteworthy that polatuzumab vedotin is linked to infections and infestations, which include COVID-19, pneumonia, cytomegalovirus infection, and sepsis. Cancer patients are thought to be particularly vulnerable to novel coronavirus infections and more severe COVID-19 symptoms. Systemic immunosuppression may be responsible for this, both directly because of tumor growth and indirectly because of the effects of anti-cancer treatment (Liu et al., 2020). Cancer patients with COVID-19 may also experience various complications (Huang et al., 2020; Liang et al., 2020; Xu et al., 2020). Acute respiratory distress syndrome (28.6%), pulmonary embolism (7.1%), septic shock (3.6%), and acute myocardial infarction (3.6%) are the most common complications and causes of death among these patients. In a phase 1b/2 study of mosunetuzumab combined with polatuzumab vedotin for DLBCL, 3.3% of patients were diagnosed with severe COVID-19. COVID-19 had no specific antiviral treatment, and the treatment given or under study included non-invasive or invasive mechanical ventilation to prevent respiratory disorders (Ñamendys-Silva, 2020) drugs including corticosteroids (such as dexamethasone) (Ledford, 2020) antimalarial drugs (such as hydroxychloroquine) (Arshad et al., 2020) and antiviral drugs (such as lopinavir or ritonavir) (Rosa and Santos, 2020) Recovery plasma therapy (Piechotta et al., 2020) and anti-inflammatory antibodies (such as anti IL-6 receptor antibody tocilizumab) (Michot et al., 2020). The impact of pneumonia on cancer populations is particularly severe, with a higher incidence rate and mortality rate than any other infectious complications (Evans and Ost, 2015). In the 1b/2 phase GO29365 study, pneumonia accounted for 16% of grade 3 AEs in R/R DLBCL patients treated with polatuzumab vedotin. In order to prevent any more related infections, it is essential to receive appropriate antibiotic treatment and supportive care (Wang et al., 2022). Cytomegalovirus infection, which is a genetic product of human cytomegalovirus, is found in a variety of human malignant tumors and is typically connected to tumor cells and the tumor vascular system (Cobbs, 2019). Saburi et al. (2023) conducted research which revealed that 19.2% of R/R DLBCL patients with MYC translocation were diagnosed with cytomegalovirus reactivation when they received Pola-BR. Prophylactic treatment with acyclovir resulted in the absence of symptomatic diseases caused by cytomegalovirus in patients with cytomegalovirus reactivation. Patients who have solid tumors or hematological tumors are more prone to sepsis, and it is closely linked to the use of anti-tumor drugs, especially ADC type anti-tumor drugs (Xia et al., 2022). Cancer patients are at a higher risk of developing sepsis than the general population because of the suppression of inflammation and immune responses. The host’s immune response can be altered by cancer treatment, which can lead to increased susceptibility to infection (Williams et al., 2023). The risk of patient death can be increased by the rapid progression of sepsis into septic shock and multiple organ failure (Cecconi et al., 2018). Some patients in the Pola-BR group stopped treatment due to sepsis in a clinical study on its safety and effectiveness (Sehn et al., 2020). Therefore, clinical vigilance and immediate treatment are essential for managing sepsis patients (Huang et al., 2019).
It's worth noting that there is a discrepancy in reporting nervous system disorders, such as peripheral neuropathy, anxiety, peripheral sensory neuropathy, and so on. The occurrence may be caused by the action of unbound MMAE in the circulation, which may also be the cause of reducing or stopping treatment. Grade 1–2 peripheral neuritis was experienced by 27% of NHL patients when polatuzumab vedotin was administered as a single drug at 2.4 mg/kg, while grade 3–4 peripheral neuritis was experienced by 9%. The peripheral neuropathy that has been observed is mainly sensory and rarely encompasses motor events (Palanca-Wessels et al., 2015). Peripheral neuropathy patients experience a delay in dose before resuming treatment at a reduced dose until the neuropathy subsides. However, further investigation is needed to investigate the comprehensive impact of these measures on the reversibility of peripheral neuropathy (Palanca-Wessels et al., 2015) The Phase II study of polatuzumab vedotin combined with rituximab proved that neuropathy occurred in 40%, but only grade 1–2 toxicity was mentioned (Morschhauser et al., 2019). The cumulative risk of peripheral neuropathy may be higher in patients who have previously received treatment with Vinca alkaloids in these studies. To determine the correlation between conjugate exposure and treatment duration and the risk of peripheral neuropathy, Lu et al. (2017) developed an event time model using data from phase I and II studies, which supports limiting the dose of polatuzumab vedotin in combination with chemotherapy or pathway inhibitors to 1.8 mg/kg (Pfeifer et al., 2015). In the randomized GO29365 study (Sehn et al., 2020), when the polatuzumab vedotin dose was 1.8 mg/kg, 43.6% of patients in the Pola-BR group developed peripheral neuropathy. The reported events were limited to Grade 1 and Grade 2, and most of the symptoms were relieved after treatment.
Unexpected and significant safety signals, such as immune system disorders, cardiac disorders, renal and urinary disorders, skin and subcutaneous tissue disorders, vascular disorders, were detected in our analysis. However, these AEs are not explicitly mentioned in the polatuzumab vedotin manual. The PT signals involved, in order of the number of cases, are cardiac failure, hypogammaglobulinaemia, femoral neck fracture, erythema multiform, thrombophlebitis, azotaemia. The newly emerging signals, particularly cardiac failure and hypogammaglobulinaemia, need to be given a great deal of attention. Cardiotoxicity is accompanied by a history of anti-tumor drug treatment, and whether it is traditional chemotherapy (Cadeddu Dessalvi et al., 2021), new targeted therapies (Tajiri et al., 2017) or immunotherapy (Knobloch et al., 2008), it may lead to cardiac related AEs. Myocardial infarction was observed in a clinical study of polatuzumab vedotin combined with immunochemotherapy for previously untreated DLBCL patients (Tilly et al., 2022). It is crucial to identify patients who are at risk of developing heart failure who are being treated with this treatment and it is recommended that cancer patients with cardiac risk factors undergo cardiac monitoring, which includes LVEF assessment (Ewer et al., 2021). The clinical manifestation of hypogammaglobulinemia is recurrent infections, with chronic upper and lower respiratory diseases being the most common. Reducing the severity of chronic upper and lower respiratory diseases, improving lung function and reaching normal IgG levels, and using immunoglobulin replacement therapy are currently acceptable treatment targets for patients with hypogammaglobulinemia (Long et al., 1987). To enhance the prognosis of the aforementioned AEs, early diagnosis, appropriate treatment, and prevention are considered important measures.
We conducted a subgroup analysis on gender to examine possible differences in the incidence of AE between populations of different genders. It was observed that male patients reported more PTs linked to polatuzumab vedotin than female patients, and the number of reported cases was higher than that for female patients. The reason may be that the incidence rate of DLBCL in men is higher than that of DLBCL in women (Wang, 2023). Male patients who use polatuzumab vedotin to some extent exceed female patients, and male patients report more AEs. This is consistent with our research findings on the reporting ratio of AEs. The male population made up 49% of the total reported cases among the 1,521 patients reported in Table 1, which is the reason for our hypothesis. Furthermore, the population that lacks gender is responsible for 14.7% of the total reported cases. Based on this, it is necessary to further track and report the occurrence of AEs in female patients after using this medication.
We further conducted an age-based subgroup analysis to examine whether age is a factor in the occurrence of polatuzumab vedotin-related AEs. The findings reveal that elderly people (≥65 years old) have a higher frequency of AEs. Compared to patients between 18 and 65, there has been a significant increase in the number of AE cases. The top three PT with ROR values detected in the two groups of people are not the same. The strongest signals detected in the population aged 65 and above are cytomegalovirus test positive, cytomegalovirus enterocolitis, and cytomegalovirus infection reactivation. The strongest signals detected in the population aged 65 and below are cytomegalovirus test positive, absolute lymphadenopathy, and hypogammaglobulinaemia. Therefore, there are certain differences in the occurrence of AEs among different age groups. The majority of AEs in elderly patients are due to infection, which may be related to the increased risk of severe infection (Thandra et al., 2021). Due to the fact that elderly patients often have complex/multiple comorbidities, doctors may need to be more cautious in their treatment of these patients. A phase 3 trial is currently being conducted to investigate the age adaptive combination of Pola-R-CHP and dose attenuated chemotherapy in the elderly patient population (ClinicalTrials.gov number, NCT04332822). In addition, Malecek et al. (2021) found that in young patients and patients without severe comorbidities, Pola-BR has the potential to serve as a bridge drug in more intensive treatment, with a high level of efficacy and tolerability.
To investigate the occurrence of AEs that are related to death cases, we conducted a subgroup analysis of cases where polatuzumab vedotin-induced AEs led to death. High-risk AEs in fatal cases include blood lactate dehydrogenase increased, cytopenia, and hydronephrosis. Dimou et al. (2021) studied that in the treatment of invasive B-cell lymphoma with Pola-BR combination therapy, 55% of patients experienced ≥ grade 3 AEs, mainly hematological AEs. Treatment interruption and death during treatment were mainly due to disease progression. These manifestations are frequently connected to the primary disease, or with disease progression or recurrence during the treatment process (Song et al., 2023). During treatment with polatuzumab vedotin, patients should receive close monitoring for the aforementioned AEs. In this research, it was discovered that PT signals that were not mentioned in the drug instructions were manifested as blood lactate dehydrogenase increased, hydronephrosis and hypogammaglobulinaemia. Furthermore, blood lactate dehydrogenase increased and hydronephrosishave a higher risk of mortality. This signal may be a new and important signal. When using drugs, it is necessary to strengthen the monitoring of biochemical indicators, kidneys, and gamma globulin to prevent the occurrence of malignant AEs that threaten patient safety.
In order to assess the time of occurrence of AEs to polatuzumab vedotin, we carried out an analysis on the time of occurrence of AEs to polatuzumab vedotin. The results indicated that the median time for detecting AEs with polatuzumab vedotin was 18.5 (5∼57.75) days, and most AEs occurred within 162 days, but AEs may still occur within 1 year (Walji and Assouline, 2020). As per these results, it is recommended that we pay special attention to AEs occurring in patients within the first month. Patient pain can be eased by the early identification of AEs caused by polatuzumab vedotin treatment (Lin et al., 2024), and longer follow-up periods will be required in future clinical studies to monitor AEs related to polatuzumab vedotin (Gouni et al., 2022).
Limitations
However, although data mining techniques have advantages in analysing clinical safety issues in real-world, there were also certain limitations. The FAERS database, as a voluntary, passive, and non-mandatory reporting system, faces inherent challenges. These include incompleteness, inaccuracy, inconsistency, and delay in reporting AEs. These limitations stem from various factors, primarily the lack of detailed patient characteristics, drug exposure information, and outcome details, such as the dose and duration of polatuzumab vedotin use, as well as whether patients received other treatment regimens and the sequence of medication. These factors may influence the associations observed and the study outcomes. Therefore, it is essential to carefully consider these limitations, particularly when interpreting the research results. To overcome these limitations and provide more robust insights, further prospective clinical studies are urgently needed. Additionally, the mechanisms underlying the association between polatuzumab vedotin use and AEs remain unclear, underscoring the need for fundamental research to address these uncertainties and advance our understanding of ADC drug targeted therapy. Finally, all signal detection results can only suggest that there is a statistical correlation; whether there is a real causal relationship still needs further evaluation and research.
Conclusion
Based on FAERS database, our study investigated and analyzed AE signals associated with polatuzumab vedotin to enhance clinical medication safety. The AEs identified in this study largely align with those documented in the drug’s manual. Additionally, we uncovered AEs that were not previously listed, including cardiac failure, hypogammaglobulinaemia, femoral neck fracture, erythema multiform, etc. These findings supplement the limited sample size of pre-market clinical studies. However, we have not yet explored the correlation between polatuzumab vedotin-related AEs and the drug itself, underscoring the need to augment safety information. Further research is still warranted to elucidate the relationship between novel polatuzumab vedotin-related AEs and the drug and to refine polatuzumab vedotin’s safety profile. Comprehensive understanding of polatuzumab vedotin AEs is crucial for mitigating usage risks, promptly identifying AEs, and appropriately managing them to optimize patient treatment outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
DL: Investigation, Writing–original draft. WM: Data curation, Writing–original draft. BH: Formal Analysis, Writing–original draft. XL: Writing–original draft. QZ: Writing–review and editing. LZ: Data curation, Writing–review and editing. JH: Investigation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This study was performed using the FAERS database that was provided by the FDA. The information, the results, or interpretation of the current study do not represent any opinion of the FDA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1405023/full#supplementary-material
References
Arshad, S., Kilgore, P., Chaudhry, Z. S., Jacobsen, G., Wang, D. D., Huitsing, K., et al. (2020). Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. IJID official Publ.97, 396–403. doi:10.1016/j.ijid.2020.06.099
Assi, R., Masri, N., Dalle, I. A., El-Cheikh, J., Ghanem, H., and Bazarbachi, A. (2021). Polatuzumab vedotin: current role and future applications in the treatment of patients with diffuse large B-cell lymphoma. Clin. Hematol. Int. 3, 21–26. doi:10.2991/chi.k.210305.001
Belada, D., Kopeckova, K., Bergua Burgues, J. M., Stevens, D., André, M., Persona, E. P., et al. (2023). Safety and efficacy of tafasitamab with or without lenalidomide added to first-line R-CHOP for DLBCL: the phase 1b First-MIND study. Blood 142, 1348–1358. doi:10.1182/blood.2023020637
Cadeddu Dessalvi, C., Deidda, M., Noto, A., Madeddu, C., Cugusi, L., Santoro, C., et al. (2021). Antioxidant approach as a cardioprotective strategy in chemotherapy-induced cardiotoxicity. Antioxid. Redox Signal. 34, 572–588. doi:10.1089/ars.2020.8055
Camus, V., and Tilly, H. (2021). Polatuzumab vedotin, an anti-CD79b antibody-drug conjugate for the treatment of relapsed/refractory diffuse large B-cell lymphoma. Future Oncol. Lond. Engl. 17, 127–135. doi:10.2217/fon-2020-0675
Cecconi, M., Evans, L., Levy, M., and Rhodes, A. (2018). Sepsis and septic shock. Lancet 392, 75–87. doi:10.1016/S0140-6736(18)30696-2
Cobbs, C. (2019). Cytomegalovirus is a tumor-associated virus: armed and dangerous. Curr. Opin. Virol. 39, 49–59. doi:10.1016/j.coviro.2019.08.003
Cultrera, J. L., and Dalia, S. M. (2012). Diffuse large B-cell lymphoma: current strategies and future directions. Cancer control. 19, 204–213. doi:10.1177/107327481201900305
Deeks, E. D. (2019). Polatuzumab vedotin: first global approval. Drugs 79, 1467–1475. doi:10.1007/s40265-019-01175-0
Dimou, M., Papageorgiou, S. G., Stavroyianni, N., Katodritou, E., Tsirogianni, M., Kalpadakis, C., et al. (2021). Real-life experience with the combination of polatuzumab vedotin, rituximab, and bendamustine in aggressive B-cell lymphomas. Hematol. Oncol. 39, 336–348. doi:10.1002/hon.2842
Dornan, D., Bennett, F., Chen, Y., Dennis, M., Eaton, D., Elkins, K., et al. (2009). Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 114, 2721–2729. doi:10.1182/blood-2009-02-205500
Evans, S. E., and Ost, D. E. (2015). Pneumonia in the neutropenic cancer patient. Curr. Opin. Pulm. Med. 21, 260–271. doi:10.1097/MCP.0000000000000156
Ewer, M. S., Tekumalla, S. H., Walding, A., and Atuah, K. N. (2021). Cardiac safety of osimertinib: a review of data. J. Clin. Oncol. official J. Am. Soc.39, 328–337. doi:10.1200/JCO.20.01171
Gouni, S., Rosenthal, A. C., Crombie, J. L., Ip, A., Kamdar, M. K., Hess, B., et al. (2022). A multicenter retrospective study of polatuzumab vedotin in patients with large B-cell lymphoma after CAR T-cell therapy. Blood Adv. 6, 2757–2762. doi:10.1182/bloodadvances.2021006801
Goy, A., Ramchandren, R., Ghosh, N., Munoz, J., Morgan, D. S., Dang, N. H., et al. (2019). Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood 134, 1024–1036. doi:10.1182/blood.2018891598
Haglund, C., Aleskog, A., Håkansson, L. D., Höglund, M., Jacobsson, S., Larsson, R., et al. (2010). The FMCA-GM assays, high throughput non-clonogenic alternatives to CFU-GM in preclinical hematotoxicity testing. Toxicol. Lett. 194, 102–107. doi:10.1016/j.toxlet.2010.02.006
Hounsome, L., Eyre, T. A., Ireland, R., Hodson, A., Walewska, R., Ardeshna, K., et al. (2022). Diffuse large B cell lymphoma (DLBCL) in patients older than 65 years: analysis of 3 year Real World data of practice patterns and outcomes in England. Br. J. Cancer 126, 134–143. doi:10.1038/s41416-021-01525-4
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi:10.1016/S0140-6736(20)30183-5
Huang, M., Cai, S., and Su, J. (2019). The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 20, 5376. doi:10.3390/ijms20215376
Knobloch, K., Tepe, J., Lichtinghagen, R., Luck, H. J., and Vogt, P. M. (2008). Simultaneous hemodynamic and serological cardiotoxicity monitoring during immunotherapy with trastuzumab. Int. J. Cardiol. 125, 113–115. doi:10.1016/j.ijcard.2007.01.010
Ledford, H. (2020). Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature 582, 469. doi:10.1038/d41586-020-01824-5
Lee, L., Crump, M., Khor, S., Hoch, J. S., Luo, J., Bremner, K., et al. (2012). Impact of rituximab on treatment outcomes of patients with diffuse large b-cell lymphoma: a population-based analysis. Br. J. Haematol. 158, 481–488. doi:10.1111/j.1365-2141.2012.09177.x
Liang, W., Guan, W., Chen, R., Wang, W., Li, J., Xu, K., et al. (2020). Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21, 335–337. doi:10.1016/S1470-2045(20)30096-6
Lin, W., Xu, J., Liao, Y., Lin, X., Yang, J., and Zhuang, W. (2024). Assessing safety concerns of interstitial lung disease associated with antibody-drug conjugates: a real-world pharmacovigilance evaluation of the FDA adverse event reporting system. Int. J. Clin. Pharm. 46, 614–622. doi:10.1007/s11096-023-01673-y
Liu, C., Zhao, Y., Okwan-Duodu, D., Basho, R., and Cui, X. (2020). COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol. Med. 17, 519–527. doi:10.20892/j.issn.2095-3941.2020.0289
Long, A. A., Denburg, J. A., and Dent, P. B. (1987). Hypogammaglobulinemia: therapeutic rationale. CMAJ Can. Med. Assoc. J. = J. de l’Association medicale 137, 793–797.
Lu, D., Gillespie, W. R., Girish, S., Agarwal, P., Li, C., Hirata, J., et al. (2017). Time-to-Event analysis of polatuzumab vedotin-induced peripheral neuropathy to assist in the comparison of clinical dosing regimens. CPT Pharmacometrics Syst. Pharmacol. 6, 401–408. doi:10.1002/psp4.12192
Malecek, M. K., Watkins, M. P., and Bartlett, N. L. (2021). Polatuzumab vedotin for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma. Expert Opin. Biol. Ther. 21, 831–839. doi:10.1080/14712598.2020.1777979
Michot, J. M., Albiges, L., Chaput, N., Saada, V., Pommeret, F., Griscelli, F., et al. (2020). Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann. Oncol. official J. Eur. Soc. Med. 31, 961–964. doi:10.1016/j.annonc.2020.03.300
Morgan, G., Vornanen, M., Puitinen, J., Naukkarinen, A., Brincker, H., Olsen, J., et al. (1997). Changing trends in the incidence of non-Hodgkin’s lymphoma in Europe. Ann. Oncol. 8 (Suppl. 2), 49–54. doi:10.1093/annonc/8.suppl_2.s49
Morschhauser, F., Flinn, I. W., Advani, R., Sehn, L. H., Diefenbach, C., Kolibaba, K., et al. (2019). Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet. Haematol. 6, e254–e265. doi:10.1016/S2352-3026(19)30026-2
Morton, L. M., Wang, S. S., Devesa, S. S., Hartge, P., Weisenburger, D. D., and Linet, M. S. (2006). Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 107, 265–276. doi:10.1182/blood-2005-06-2508
Ñamendys-Silva, S. A. (2020). Respiratory support for patients with COVID-19 infection. Lancet Respir. Med. 8, e18. doi:10.1016/S2213-2600(20)30110-7
Ozsahin, M., Azria, D., Beer, K., Mirimanoff, R. O., and Zouhair, A. (2005). External radiotherapy and anaemia treatment: state of the art. Swiss Med. Wkly. 135, 4–10. doi:10.2005/01/smw-10805
Palanca-Wessels, M. C., Czuczman, M., Salles, G., Assouline, S., Sehn, L. H., Flinn, I., et al. (2015). Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 16, 704–715. doi:10.1016/S1470-2045(15)70128-2
Pfeifer, M., Zheng, B., Erdmann, T., Koeppen, H., McCord, R., Grau, M., et al. (2015). Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 29, 1578–1586. doi:10.1038/leu.2015.48
Phillips, T., Brunvand, M., Chen, A. I., Essell, J., Chiappella, A., Diefenbach, C., et al. (2022). Safety and efficacy of polatuzumab vedotin + obinutuzumab for relapsed/refractory non-Hodgkin lymphomas: a phase IB/II study. Am. J. Hematol. 97, E24–E27. doi:10.1002/ajh.26400
Piechotta, V., Chai, K. L., Valk, S. J., Doree, C., Monsef, I., Wood, E. M., et al. (2020). Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane database Syst. Rev. 7, CD013600. doi:10.1002/14651858.CD013600.pub2
Polson, A. G., Yu, S. F., Elkins, K., Zheng, B., Clark, S., Ingle, G. S., et al. (2007). Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood 110, 616–623. doi:10.1182/blood-2007-01-066704
Rosa, S., and Santos, W. C. (2020). Clinical trials on drug repositioning for COVID-19 treatment. Revista Panam. de salud publica = Pan Am. J. public health 44, e40. doi:10.26633/RPSP.2020.40
Saburi, M., Sakata, M., Kodama, Y., Uraisami, K., Takata, H., Miyazaki, Y., et al. (2023). Poor clinical outcome of relapsed/refractory diffuse large B-cell lymphoma with MYC translocation treated with polatuzumab vedotin, bendamustine, and rituximab. J. Clin. Exp. Hematop 63, 201–204. doi:10.3960/jslrt.23017
Sehn, L. H., Herrera, A. F., Flowers, C. R., Kamdar, M. K., McMillan, A., Hertzberg, M., et al. (2020). Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 38, 155–165. doi:10.1200/JCO.19.00172
Sehn, L. H., Hertzberg, M., Opat, S., Herrera, A. F., Assouline, S., Flowers, C. R., et al. (2022). Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 6, 533–543. doi:10.1182/bloodadvances.2021005794
Setyawan, J., Azimi, N., Strand, V., Yarur, A., and Fridman, M. (2021). Reporting of thromboembolic events with JAK inhibitors: analysis of the FAERS database 2010-2019. Drug Saf. 44, 889–897. doi:10.1007/s40264-021-01082-y
Smith, T. J., Khatcheressian, J., Lyman, G. H., Ozer, H., Armitage, J. O., Balducci, L., et al. (2006). 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J. Clin. Oncol. 24, 3187–3205. doi:10.1200/JCO.2006.06.4451
Song, Y., Tilly, H., Rai, S., Zhang, H., Jin, J., Goto, H., et al. (2023). Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial. Blood 141, 1971–1981. doi:10.1182/blood.2022017734
Tajiri, K., Aonuma, K., and Sekine, I. (2017). Cardiovascular toxic effects of targeted cancer therapy. Jpn. J. Clin. Oncol. 47, 779–785. doi:10.1093/jjco/hyx071
Terui, Y., Rai, S., Izutsu, K., Yamaguchi, M., Takizawa, J., Kuroda, J., et al. (2021). A phase 2 study of polatuzumab vedotin + bendamustine + rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 112, 2845–2854. doi:10.1111/cas.14937
Testoni, M., Zucca, E., Young, K. H., and Bertoni, F. (2015). Genetic lesions in diffuse large B-cell lymphomas. Ann. Oncol. 26 (6), 1069–1080. doi:10.1093/annonc/mdv019
Thandra, K. C., Barsouk, A., Saginala, K., Padala, S. A., Barsouk, A., and Rawla, P. (2021). Epidemiology of non-hodgkin’s lymphoma. Med. Sci. Basel, Switz. 9, 5. doi:10.3390/medsci9010005
Tilly, H., Morschhauser, F., Bartlett, N. L., Mehta, A., Salles, G., Haioun, C., et al. (2019). Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. Lancet Oncol. 20, 998–1010. doi:10.1016/S1470-2045(19)30091-9
Tilly, H., Morschhauser, F., Sehn, L. H., Friedberg, J. W., Trněný, M., Sharman, J. P., et al. (2022). Polatuzumab vedotin in previously untreated diffuse large B-cell lymphoma. N. Engl. J. Med. 386, 351–363. doi:10.1056/NEJMoa2115304
Vodicka, P., Klener, P., and Trneny, M. (2022). Diffuse large B-cell lymphoma (DLBCL): early patient management and emerging treatment options. Onco Targets Ther. 15, 1481–1501. doi:10.2147/OTT.S326632
Vogel, U., van Stekelenborg, J., Dreyfus, B., Garg, A., Habib, M., Hosain, R., et al. (2020). Investigating overlap in signals from EVDAS, FAERS, and VigiBase®. Drug Saf. 43, 351–362. doi:10.1007/s40264-019-00899-y
Walji, M., and Assouline, S. (2020). An evaluation of polatuzumab vedotin for the treatment of patients with diffuse large B-cell lymphoma. Expert Rev. Hematol. 13, 933–942. doi:10.1080/17474086.2020.1795828
Wang, S. S. (2023). Epidemiology and etiology of diffuse large B-cell lymphoma. Semin. Hematol. 60, 255–266. doi:10.1053/j.seminhematol.2023.11.004
Wang, Y. W., Tsai, X. C., Hou, H. A., Tien, F. M., Liu, J. H., Chou, W. C., et al. (2022). Polatuzumab vedotin-based salvage immunochemotherapy as third-line or beyond treatment for patients with diffuse large B-cell lymphoma: a real-world experience. Ann. Hematol. 101, 349–358. doi:10.1007/s00277-021-04711-9
Wildiers, H., and de Glas, N. A. (2020). Anticancer drugs are not well tolerated in all older patients with cancer. Lancet Healthy Longev. 1, e43–e47. doi:10.1016/S2666-7568(20)30001-5
Williams, J. C., Ford, M. L., and Coopersmith, C. M. (2023). Cancer and sepsis. Clin. Sci. Lond. Engl. 1979 137, 881–893. doi:10.1042/CS20220713
Xia, S., Zhao, Y. C., Guo, L., Gong, H., Wang, Y. K., Ma, R., et al. (2022). Do antibody-drug conjugates increase the risk of sepsis in cancer patients? A pharmacovigilance study. Front. Pharmacol. 13, 967017. doi:10.3389/fphar.2022.967017
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8, 420–422. doi:10.1016/S2213-2600(20)30076-X
Keywords: polatuzumab vedotin, FAERS, pharmacovigilance, adverse events, antibody-drug conjugate
Citation: Liu D, Mao W, Hu B, Li X, Zhao Q, Zhang L and Hu J (2024) A real-world pharmacovigilance study of polatuzumab vedotin based on the FDA adverse event reporting system (FAERS). Front. Pharmacol. 15:1405023. doi: 10.3389/fphar.2024.1405023
Received: 22 March 2024; Accepted: 07 June 2024;
Published: 25 June 2024.
Edited by:
Jan Willem Van Der Laan, Medicines Evaluation Board, NetherlandsReviewed by:
Marcel Kwa, Medicines Evaluation Board, NetherlandsTongyi Men, Qianfoshan Hospital Affiliated to Shandong University, China
Copyright © 2024 Liu, Mao, Hu, Li, Zhao, Zhang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Zhang, MTEyNjYyMTY4NEBxcS5jb20=; Jing Hu, MTgyMTk3OTdAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Dan Liu
Dan Liu Wei Mao
Wei Mao Bin Hu1†
Bin Hu1† Xingxing Li
Xingxing Li Lin Zhang
Lin Zhang Jing Hu
Jing Hu