
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 24 July 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1403988
Background: Neuromuscular blocking agents (NMBAs) are primarily used during surgical procedures to facilitate endotracheal intubation and optimize surgical conditions. This study aimed to explore the adverse event signals of NMBAs, providing reference for clinical safety.
Methods: This study collected reports of atracurium, cisatracurium, rocuronium, and vecuronium as primary suspect drugs in The US Food and Drug Administration Adverse Event Reporting System (FAERS) from the first quarter of 2004 to the third quarter of 2023. The adverse events (AEs) reported in the study were retrieved based on the Preferred Terms (PTs) of the Medical Dictionary for Regulatory Activities. In addition, we conducted disproportionality analysis on relevant reports using the reporting odds ratio (ROR) method and Bayesian confidence propagation neural network (BCPNN) method. A positive signal was generated when both algorithms show an association between the target drug and the AE.
Results: A total of 11,518 NMBA-related AEs were reported in the FAERS database. The most AEs of rocuronium were collected. NMBA-related AEs involved 27 different system organs (SOCs), all of the four NMBAs had positive signals in “cardiac disorders,” “immune system disorders,” “respiratory, thoracic and mediastinal disorders” and “vascular disorders.” At the PTs level, a total of 523 effective AEs signals were obtained for the four NMBAs. AEs labled in the instructions such as anaphylaxis (include anaphylactic reaction and anaphylactic shock), bronchospasm, respiratory arrest and hypotension were detected positive signals among all NMBAs. In addition, we also found some new AEs, such as ventricular fibrillation for the four NMBAs, hyperglycaemia for atracurium, kounis syndrome and stress cardiomyopathy for rocuronium, hepatocellular injury for cisatracurium, hyperkalaemia for vecuronium. To further investigated the AEs associated with serious clinical outcomes, we found that cardiac arrest and anaphylaxis were the important risk factors for death due to NMBAs.
Conclusion: NMBA-related AEs have a significant potential to cause clinically severe consequences. Our study provides valuable references for the safety profile of NMBAs, and considering the limitations of the FAERS database, further clinical data are needed to validate the findings of this study.
Neuromuscular blocking agents (NMBAs) are a class of medications commonly used in anesthesia (McLean et al., 2015).They are used to facilitate airway management, improve surgical conditions, and insure immobility during critical points in an operation (Thilen et al., 2023). The NMBAs are administered over 100 million times annually to facilitate tracheal intubation (Macartney, 2013). Meta-analysis of clinical trials had shown that avoiding NMBAs during endotracheal intubation significantly increased the incidence of difficult intubation and discomfort (Lundstrøm et al., 2018). NMBAs are primarily categorized into two types: depolarizing NMBAs (e.g., succinylcholine) and non-depolarizing NMBAs (e.g., atracurium, cisatracurium, rocuronium, vecuronium). The ideal NMBA is considered to be a non-depolarizing agent that has a rapid onset of action, short duration of effect, and minimal side effects (Macartney, 2013). Atracurium, cisatracurium, rocuronium and vecuronium are frequently used non-depolarizing NMBAs in clinical practice. They act by competitively blocking acetylcholine receptors at the neuromuscular junction, producing prolonged muscle relaxation (Naguib et al., 2002). Cisatracurium is the stereoisomer of atracurium, and both belong to the benzylisoquinoline NMBAs. Compared to atracurium, cisatracurium has four times the potency on neuromuscular blockade (Kim et al., 2016). Atracurium and cisatracurium are the preferred drugs for continuous infusion. The latter, cisatracurium, is the NMBA of choice in the treatment of critically ill patients requiring neuromuscular blockade as it is unrelated to histamine release (Tsolaki et al., 2022). Rocuronium and vecuronium are both steroid NMBAs. Rocuronium has a rapid onset and intermediate duration of action, making it suitable for rapid sequence intubation (Tsolaki et al., 2022). In comparison, vecuronium has a slower onset but a similar duration of action to rocunorium (Engbaek et al., 1994).
The widespread use of NMBAs has raised concerns among clinicians and researchers regarding their related safety issues. During the process of general anesthesia, drug-related adverse events (AEs) can be severe and life-threatening, with NMBAs being the primary cause of these adverse events (Di Leo et al., 2018). Common AEs include anaphylaxis, hypotension, bronchospasm and bradycardia (Jick et al., 1989; Arnot-Smith et al., 2010; Petitpain et al., 2018). However, most of the evidence for NMBA-associated AEs came from case reports and clinical trials, with relatively small sample sizes. It is currently unclear whether NMBAs may cause other serious AEs. Thus, conducting comprehensive and large-scale study on AEs related to NMBAs is crucial. In this study, we collected AEs of NMBAs from the FDA Adverse Event Reporting System (FAERS), and assessed the potential relevance between NMBAs and AEs through risk factor analysis. The aim of this study is to investigate the overall safety profile of NMBAs and provide a reference for further identification of NMBA-related adverse events.
FAERS is a comprehensive and publicly available database that collects and stores information on adverse events and medication errors reported to the FDA. It serves as a valuable resource for monitoring the safety of pharmaceutical products, identifying potential risks, and facilitating post-marketing surveillance. AEs reported in FAERS include any untoward medical occurrence associated with the use of a medication, including both known and unknown side effects.
The FAERS database is updated quarterly. Each FAERS data package consists of several different files: Patient Demographic Information (DEMO), Drug Information (DRUG), adverse events (REAC), Patient outcomes (OUTC), Report Source information (RPSR), Drug Therapy Start and End Date (THER), and Drug indication information (INDI). Additionally, starting from the first quarter of 2019, DELETED data sheets were added to include revoked or withdrawn case data. We downloaded all reports from the first quarter of 2004 to the third quarter of 2023. Using Structured Query Language (SQL), we extracted and analyzed all reported AEs related to the targeted NMBAs (atracurium, cisatracurium, rocuronium and vecuronium). In order to enhance the accuracy and credibility of the results, we screened the role_code as “PS” (primary suspected) in the DRUG files, removed duplicate reports and those in the DELETED data sheets, and the remaining reports were included in the study, which we referred to as valid reports. Each report represents a unique patient, and it is important to note that a single patient may experience and report several different AEs. According to the FDA recommendations, we utilized MySQL 8.0 to correlate these subsets of the database and remove duplicate information. The deduplication process was performed based on the following criteria: when the CASEID (number for identifying a FAERS case) was the same, we selected the latest FDA_DT (the date when the FDA received the case), and when CASEID and FDA_DT were the same, we chose the record with the higher PRIMARYID (unique identifier for the FAERS report)
Due to the diverse backgrounds of reporters, the drug names and AEs names reported in the FAERS database are often inconsistent and non-standardized. To identify all the records of target drugs in FAERS, we extracted reports using the brand names, generic names, and chemical names of the target NMBA. Medical Dictionary for Regulatory Activities (MedDRA) is a standardized medical terminology that developed for recording and reporting of AE data (Brown, 2004; Singh, 2015). Its hierarchical structure consists of five levels, ranging from the lowest level terms (LLT) to system organ class (SOC). SOC is the highest level of terminology used for classifying AEs. The AEs in our collection were coded using the preferred term (PT) and then mapped to their corresponding SOC level in MedDRA (version 26.1). Serious clinical outcomes were classified as death, disability, hospitalization (initial or prolonged), life-threatening complications, required intervention to prevent permanent impairment or damage and other serious medical events.
In recent years, disproportionality analysis (Fusaroli et al., 2024) has emerged as an important approach for evaluating drug safety. To comprehensively assess the safety profile of the NMBAs, we conducted disproportionality analysis at both the SOC level and the PT level. The analysis at SOC level helps identify the potential association between NMBAs and specific systems or organs, providing a broader and more macro-level perspective. While the analysis at PT level is more specific, focusing on particular adverse event terms. It aids in discovering new adverse event signals and provides more detailed and specific information. Combining both analyses can lead to a comprehensive and systematic discovery of signals.
Two algorithms, including the reporting odds ratio (ROR) and Bayesian confidence propagation neural network (BCPNN), were used to detect whether NMBAs were significantly associated with AEs (Zhang et al., 2022; Wang et al., 2023). Because the number of AEs for a specific PT less than 3 cases may lead to false positive signals, they were not included in the analysis (Zhou et al., 2022). To further avoid false positive signals, a positive signal was generated only when both algorithms met the criteria. In the formulas below, the value a represents the number of reports that contain both the target drug and the target adverse event, b represents the number of reports that contain the target drug with other adverse events, c represents the number of reports that contain the target adverse event related to other drugs, d represents the number of reports that contain other drugs and other adverse events (Zhang et al., 2022). The specific formulae are as follows:
If the lower limit of 95% CI > 1 and a ≥ 3, it should be considered a positive signal.
If IC-2SD > 0, it should be defined a positive signal; Specifically, if 0 < IC- 2SD ≤ 1.5, it should be defined as a weak signal (+); if 1.5 < IC- 2SD ≤ 3, it should be defined as a medium signal (++); if IC-2SD > 3, it should be defined as a strong signal (+++).
During the study period from January 2004 to September 2023, FAERS received a total of 16, 961, 231 valid reports, among which 4,619 reports were identified with NMBAs as the primary suspected drug. The percentage of reports was highest for rocuronium (3,167 reports, 68.56%), followed by atracurium (506 reports, 10.95%), vecuronium (474 reports, 10.26%), and cisatracurium (472 reports, 10.22%). The clinical characteristics of the patients are summarized in Table 1.
The incidence of atracurium-related AEs appeared to be higher in females compared to males (52.77% vs. 42.09%). For cisatracurium, 21.61% of the reports lacked gender information, and among the reports that provided gender details, it seemed that males had higher occurrence in AEs than females (43.01% vs. 35.38%). For rocuronium and vecuronium, the occurrence rates of AEs are similar between males and females. From the perspective of age composition, patients were mainly between the ages of 18 and 59 among all NMBAs. Most of these reports were from the United States (31.83%), followed by France (14.42%), and Great Britain (11.21%). Physicians represented the primary source of reports (30.44%).
In total, 11,518 AEs were found to be related to NMBAs. Rocuronium was reported the most (7,476 AEs, 64.91%), followed by atracurium (1,702 AEs, 14.78%), vecuronium (1,340 AEs, 11.63%), and cisatracurium (1,000 AEs, 8.68%). These AEs were classified based on SOC of MedDRA, and a total of 27 SOCs were involved. The top five SOCs with the highest number of AEs among the four NMBAs were “general disorders and administration site conditions”, “respiratory, thoracic and mediastinal disorders,” “cardiac disorders,” “injury, poisoning and procedural complications” and “immune system disorders.” The details were described in Supplementary Figure S1.
In order to further understand the risk of NMBA-related adverse events at the SOC level, We compared the number of AEs of the four NMBAs with the number of AEs of the overall population at the same SOC level by disproportionality analysis. The larger the ROR and IC values were, the stronger the signal was (Jiang et al., 2022). As shown in Table 2, there were some differences in SOC involved in the four NMBAs. There were five positive signals for atracurium and rocuronium, six for cisatracurium and vecuronium. All four NMBAs had positive signals at SOC level in “cardiac disorders,” “immune system disorders,” “respiratory, thoracic and mediastinal disorders” and “vascular disorders.” In the aspect of “cardiac disorders,” all four NMBAs exhibited medium signals (++). In terms of “immune system disorders,” the signals of rocuronium were identified as strong signal (+++), atracurium and cisatracurium were medium signals (++), while vecuronium showed a weak signal (+). For “pregnancy, puerperium and perinatal conditions,” atracurium showed a weak signal (+), while the other three NMBAs showed no signal. For “hepatobiliary disorders,” cisatracurium showed a weak signal (+).
Based on the AEs induced by all other drugs in the FAERS database, we further analyzed the PT signals for each NMBA to explore whether there was any association between NMBAs and PTs. According to the criteria of these two algorithms, we identified positive signals for atracurium, cisatracurium, rocuronium, vecuronium as 101, 61, 227, and 96, respectively. The most number of positive signals for the four NMBAs were focused on “respiratory, thoracic and mediastinal disorders,” followed by “injury, poisoning and procedural complications,” “cardiac disorders,” “Investigations,” “general disorders and administration site conditions.” Figure 1 provided an overview of the number of positive signals for the four NMBAs in the corresponding SOC.
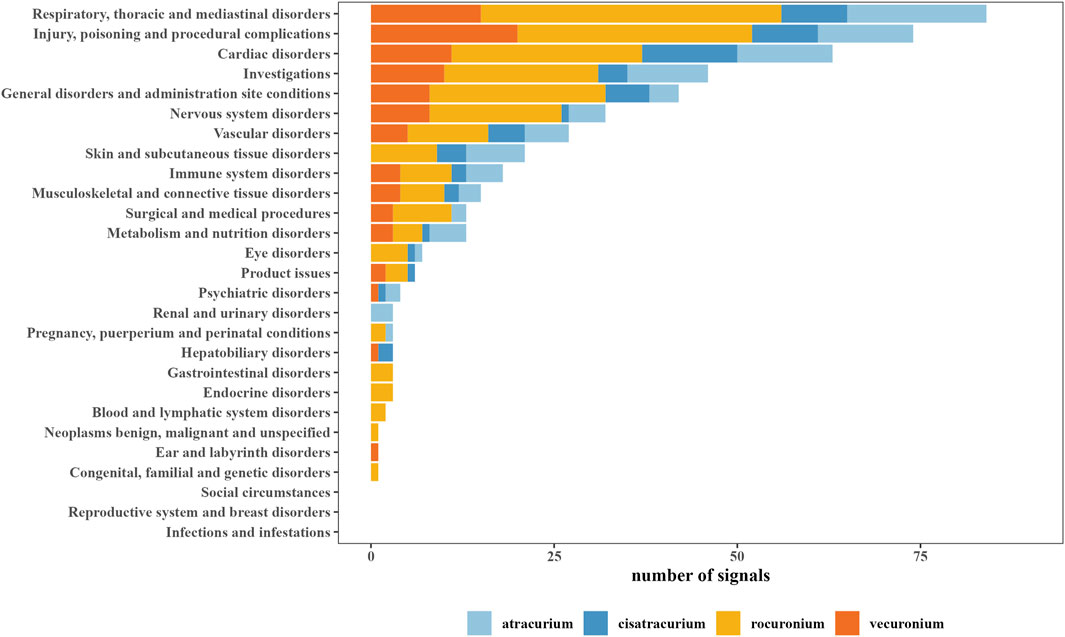
Figure 1. System organ class (SOC) of positive signals related to neuromuscular blocking agents (NMBAs).
The top 20 NMBA-related AEs were listed in Table 3. The PTs were arranged in ascending alphabetical order. Notably, anaphylactic reaction, anaphylactic shock, bronchospasm, bradycardia, cardiac arrest, hypotension, circulatory collapse, tachycardia and respiratory arrest were all identified positive signal for all NMBAs. Comparing the signal intensity of the four NMBAs, we found that the signal intensity of rocuronium was the strongest, while cisatracurium had the characteristic of having fewer signals, low signal strength, and overlapping signals with other NMBAs.
Comparing the detected positive signals with the AEs labeled in the instructions, 59, 36, 127, 43 unlabeled AEs were identified for atracurium, cisatracurium, rocuronium and vecuronium, respectively. Besides the AEs listed in Table 3, we also found other new AEs (Supplementary Table S1). For example, atracurium was identified to be associated with hyperglycaemia (IC-2SD = 2.64), ventricular fibrillation (IC-2SD = 2.00) and blood creatine phosphokinase increased (IC-2SD = 2.12), all of which showed medium signals (++). For cisatracurium, medium signals included ventricular fibrillation (IC-2SD = 2.15) and rhabdomyolysis (IC-2SD = 1.65), weak signals included hepatocellular injury (IC-2SD = 1.39). For rocuronium, kounis syndrome (IC-2SD = 3.60) was strong positive signal (+++), stress cardiomyopathy (IC-2SD = 2.56), ventricular fibrillation (IC-2SD = 2.72) and rhabdomyolysis (IC-2SD = 1.64) were medium signals (++). For vecuronium, ventricular fibrillation (IC-2SD = 2.26) was identified as medium signals (++), hyperkalaemia (IC-2SD = 1.39) and rhabdomyolysis (IC-2SD = 1.03) were identified as weak signals (+).
3,904 patients had experienced serious clinical outcomes, indicating that NMBAs may have potentially dangerous attributes. For outcome, since a patient could experience multiple outcomes, the total sum of percentages may be greater than 100%, but this is not contradictory. As shown in Figure 2A, atracurium had the highest reported rates of death, hospitalization (initial or prolonged) and life-threatening complications, with rates of 11.26%, 34.78%, and 46.84%, respectively, while rocuronium had the lowest percentage of death (6.32%), vecuronium had the lowest percentage of hospitalization (initial or prolonged) and life-threatening (21.31% and 24.47%, respectively). A total of 341 patients reported death outcome, in order to further explore the AEs leading to death, we assessed the mortality caused by different AEs of the four drugs based on the number of deaths reports. Among them, cardiac arrest and anaphylaxis were the main risk factors for death due to atracurium (Figure 2B), cisatracurium (Figure 2C), rocuronium (Figure 2D) and vecuronium (Figure 2E).
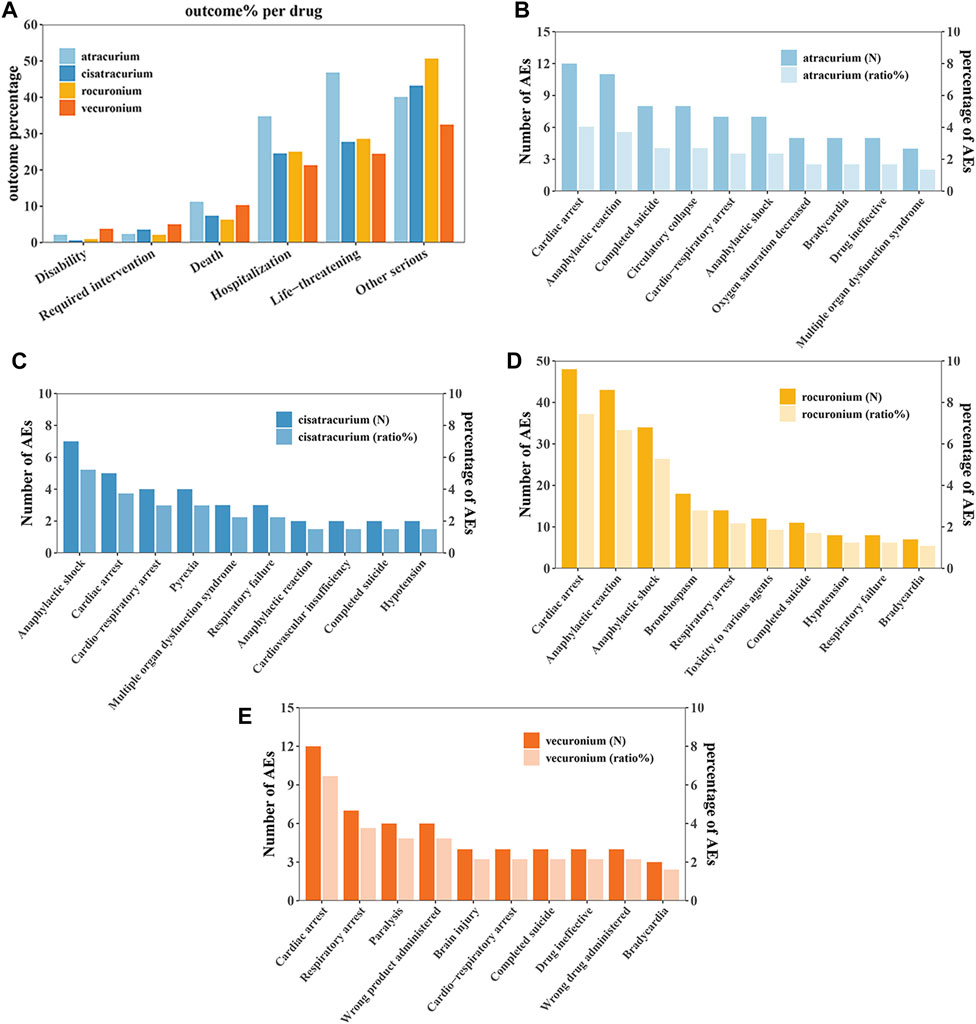
Figure 2. Outcomes of NMBA-related adverse events. (A) Outcomes for adverse events associated with Neuromuscular blocking agents (NMBAs). (B) The top 10 AEs leading to death for atracurium. (C) The top 10 AEs leading to death for cisatracurium. (D) The top 10 AEs leading to death for rocuronium. (E) The top 10 AEs leading to death for vecuronium.
Although NMBAs have been used for a long time in clinical practice, there is still a lack of big data research to evaluate post-marketing AEs of NMBAs. As far as we know, this is the first study on the safety of NMBAs based on FAERS. In this study, we used two statistical methods (ROR and BCPNN) to mine the potential AEs signals of NMBAs in the FAERS database. These two methods can validate each other and quantitatively reflect the correlation between target drugs and AEs. The AEs labled in the instructions (such as anaphylaxis, bronchospasm, respiratory arrest and hypotension) were detected in above, indicating that the AEs signal mining based on the FAERS system was reliable and predictable.
At the level of SOC, the large-scale comparison of NMBAs was carried out, and it demonstrated that the four NMBAs all had positive signals in “cardiac disorders,” “immune system disorders,” “respiratory, thoracic and mediastinal disorders,” and “vascular disorders.” Cisatracurium was the only drug associated with “hepatobiliary disorders,” which has not been reported before. Atracurium showed positive signal in “pregnancy, puerperium and perinatal conditions,” the PTs includes neonatal disorder. A study has found that due to the low degree of placental transfer of atracurium, no health issues were observed in the newborns of pregnant women after they were administered atracurium as a muscle relaxant (Flynn et al., 1984). Greco et al., (1995) found that during intrauterine surgery, the use of atracurium on the fetus did not result in side effects related to paralysis at birth and during the 2-year follow-up period. Our results were inconsistent with these studies, and the possible reasons are as follows: First, the current data on the safety of atracurium during pregnancy were limited, and the sample sizes of existing studies were relatively small, whereas our methodology encompassed a larger sample size. Second, AEs related to newborns may be distributed across different PTs/SOCs, which may lead to bias in the results. Consequently, further research, including well-designed clinical trials, is necessary to provide a stronger understanding of the safety of atracurium use in pregnant women and their offspring.
This study excavated signs of AEs that were not labeled in the instructions, such as ventricular fibrillation for the four NMBAs, hyperglycaemia for atracurium, kounis syndrome and stress cardiomyopathy for rocuronium, hepatocellular injury for cisatracurium, hyperkalaemia for vecuronium. Some researchers believe that the mechanism behind rocuronium-induced kounis syndrome involves complex immune responses and allergic processes. Rocuronium triggers the activation of mast cells, which can selectively release mediators such as interleukin-1, serotonin and leukotrienes, thereby promoting coronary artery inflammation (Fagley et al., 2009; Del Val Villanueva et al., 2018). Regarding other new AEs, since there are currently no relevant reports, the mechanisms are unclear, and thus a definitive conclusion cannot be drawn and further validation requires.
Currently, the focus of research on AEs associated with NMBAs has been primarily on IgE-mediated anaphylaxis (Baldo et al., 2009; Petitpain et al., 2018). The majority of reports on the incidence of anaphylaxis originate in Australia (Fisher et al., 1993), France (Laxenaire, 1993), the United Kingdom (Clarke et al., 1993) and New Zealand (Galletly et al., 1985). Anaphylaxis accounted for the largest proportion of NMBAs adverse event reports in the FAERS database. Compared with other NMBAs, our results show that rocuronium has the highest number of reported anaphylaxis, and previous clinical studies had also found that rocuronium were markedly more involved in perioperative anaphylaxis than the other available NMBAs. A regulatory information had already been sent to French anesthetists to communicate about the results of a survey revealing a higher frequency of anaphylaxis with rocuronium than with other NMBAs (Mertes et al., 2003). Sadleir et al., (2013) also highlighted the high incidence of rocuronium induced anaphylaxis in a 10-year survey performed in a specialized diagnostic center. They found that rocuronium was responsible for 56% of cases of NMBA anaphylaxis and had a higher rate of IgE-mediated anaphylaxis compared with vecuronium. Petitpain et al., (2018) recently reported that rocuronium has an anaphylaxis incidence rate 11-fold higher than atracurium and 37-fold higher than cisatracurium, they also found that the annual incidence rates of rocuronium showed increasing trend. However, the debate on the AEs of NMBAs is still controversial. Some reports suggest a higher frequency of AEs involving rocuronium (Sadleir et al., 2013; Petitpain et al., 2018), whereas others consider that the incidence reflect market use (Rose et al., 2001; Watkins, 2001). In the FAERS database, the AEs of rocuronium were much more than other NMBAs. This variation may be due to poor reporting and/or reporting bias, which means that clinicians reported the AEs of rocuronium and ignored other NMBAs adverse events. There is also a possibility that, as reported in most clinical studies, the incidence of rocuronium adverse events is higher than other NMBAs.
NMBAs can affect the cardiovascular system through a variety of mechanisms, such as causing autonomic nerve balance disorders, releasing histamine or causing anaphylaxis (Hameedullah et al., 1997). Individuals receiving NMBAs can be expected to represent critically ill patients. These patients may have higher risk for cardiovascular adverse events as over half of the patients were already receiving vasopressors or inotropic drugs prior to initiation of NMBAs (VanderWeide et al., 2017). It has been reported that rocuronium mainly causes tachycardia and rarely causes bradycardia (Harvey et al., 1999). However, we had detected strong positive signal of bradycardia in rocuronium. In addition, we also found that these four NMBAs were associated with other cardiovascular adverse events, such as hypotension, cardiac arrest, circulatory collapse and ventricular fibrillation. It has been proposed that hypotension resulted by atracurium may be associated with histamine release (Hosking et al., 1988; Murray et al., 2016), and several studies have demonstrated that the combined use of an H1 and H2 antagonist can attenuate this response (Scott et al., 1985; Hosking et al., 1988; Murray et al., 2016). However, cisatracurium, rocuronium, and vecuronium do not release histamine (Murray et al., 2016), but clinical studies (Hameedullah et al., 1997; Jeong et al., 2010; VanderWeide et al., 2017) and our results have shown that these three drugs are also associated with hypotension, suggesting that the three NMBAs may cause hypotension through other mechanisms.
Bronchospasm is an adverse event associated with NMBAs, which is documented in the instructions or confirmed in previous clinical trials (Woods et al., 1985; O’Callaghan et al., 1986; Heier et al., 2000; Wang et al., 2021). However, fewer cases were reported and the reporting time was earlier. We found that these four NMBAs had strong positive signals of bronchospasm, suggesting a high risk of NMBA-induced bronchospasm. Our study reminds anesthetists to pay attention to bronchospasm caused by NMBAs and avoid serious respiratory complications such as respiratory failure and respiratory arrest.
As shown in the results above, atracurium showed the the highest reported rates of death, hospitalization (initial or prolonged), and life-threatening complications. Besides the histamine release caused by atracurium, we believe that it is also related to the use of its antagonists. Currently, the available antagonists for NMBAs include neostigmine and sugammadex. Neostigmine can be used to reverse any non-depolarizing NMBAs, while sugammadex can only be used to reverse rocuronium and vecuronium (Thilen et al., 2023). Compared with sugammadex, neostigmine cannot reverse deep neuromuscular blockade, which means that if deep blockade occurs after the use of atracurium, neostigmine cannot be given until it returns to shallow blockade. However, if deep neuromuscular blockade occurs after the use of rocuronium or vecuronium, sugammadex can be selected to rapidly reverse it (Thilen et al., 2023).
There is limited data on fatalities specifically attributed to NMBAs in published studies. Cardiac arrest and anaphylaxis have been shown to be the main risk factors for death due to NMBAs. Apart from atracurium, the instructions for the other three NMBAs do not mention cardiac arrest, and there is almost no literature reporting NMBA-related cardiac arrest. Anaphylaxis are likely to cause serious consequences. Reitter et al., (2014) had found that despite aggressive use of epinephrine and fluid therapy, approximately 4% of cases of NMBAs anaphylaxis still result in fatalities. Petitpain et al., (2018) suggested use skin test to avoid anaphylaxis. Taken together, for patients using NMBAs, anesthetists should be vigilant about anaphylaxis and the patient’s cardiac system, maintain surveillance throughout the treatment process to ensure the best outcome.
It is worth noting that our study has some limitations. First, the FAERS database relies on voluntary reporting, which may lead to underreporting or reporting biases. Second, spontaneous reporting systems are usually considered inappropriate for the assessment of adverse drug reaction rates. Third, our study spans nearly 20 years, during which clinical practices, NMBA usage patterns, and reporting practices may have changed, potentially affecting the comparability of AEs among the four NMBAs. Fourth, it is difficult to control confounding factors, such as comorbidities, drug combinations, and other factors that may affect AEs. Therefore, establishing clear causal relationship between NMBAs and AEs is restricted, and the disproportionality analysis can only provide statistical associations. Our research is an important step in identifying potential safety signals, and further experimental studies are needed to validate these results. Despite these limitations, the FAERS database remains a valuable resource for pharmacovigilance and identifying potential safety concerns. Several advantages can be identified. We utilized a large-scale publicly accessible pharmacovigilance database that accepts reports from various locations globally, thus supporting the generalizability of the results (Raschi et al., 2022; Cecco et al., 2024) and helping accumulate knowledge about the safety of NMBAs in an unselected populations, which is still a topic lacking research. In summary, potential AEs signals associated with NMBAs were systematically identified, which can provide valuable evidence for further research and clinical use.
We reviewed the safety profiles of atracurium, cisatracurium, rocuronium, and vecuronium based on AEs submitted to the FAERS database from the first quarter of 2004 to the third quarter of 2023. According to the analysis results, AEs of NMBAs occurred in multiple organs and tissues, including the cardiac, immune, respiratory and vascular systems. NMBAs have different safety profiles, which may lead to serious adverse events, resulting in hospitalization or death. In order to ensure the safety of patients and reduce the risk of adverse events, anesthetists should be aware of these differences and adjust treatment regimens according to different patients. Although several post-marketing safety signals that were unlabled in the instructions were found, it is necessary to conduct prospective clinical trials to confirm these findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
LL: Data curation, Investigation, Methodology, Software, Writing–original draft, Writing–review and editing. QX: Data curation, Writing–original draft, Writing–review and editing. YL: Data curation, Visualization, Writing–original draft, Writing–review and editing. LP: Formal Analysis, Writing–original draft, Writing–review and editing. ZC: Data curation, Writing–original draft, Writing–review and editing. YL: Writing–original draft, Writing–review and editing.
The authors declare that no financial support was received for the research, authorship, and publication of this article.
The authors would like to thank the FAERS database for its free open source data. We acknowledge all the persons reporting AEs in FAERS in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1403988/full#supplementary-material
Arnot-Smith, J., and Smith, A. F. (2010). Patient safety incidents involving neuromuscular blockade: analysis of the UK national reporting and learning system data from 2006 to 2008. Anaesthesia 65 (11), 1106–1113. doi:10.1111/j.1365-2044.2010.06509.x
Baldo, B. A., Fisher, M. M., and Pham, N. H. (2009). On the origin and specificity of antibodies to neuromuscular blocking (muscle relaxant) drugs: an immunochemical perspective. Clin. Exp. Allergy 39 (3), 325–344. doi:10.1111/j.1365-2222.2008.03171.x
Brown, E. G. (2004). Using MedDRA: implications for risk management. Drug Saf. 27 (8), 591–602. doi:10.2165/00002018-200427080-00010
Cecco, S., Puligheddu, S., Fusaroli, M., Gerratana, L., Yan, M., Zamagni, C., et al. (2024). Emerging toxicities of antibody-drug conjugates for breast cancer: clinical prioritization of adverse events from the FDA adverse event reporting system. Target Oncol. 19 (3), 435–445. doi:10.1007/s11523-024-01058-9
Clarke, R. S., and Watkins, J. (1993). Drugs responsible for anaphylactoid reactions in anaesthesia in the United Kingdom. Ann. Fr. Anesth. Reanim. 12 (2), 105–108. doi:10.1016/s0750-7658(05)81017-2
Del Val Villanueva, B., Telletxea Benguria, S., González-Larrabe, I., and Suárez Romay, J. M. (2018). Kounys syndrome after rocuronium administration. Rev. Esp. Anestesiol. Reanim. Engl. Ed. 65 (6), 343–346. doi:10.1016/j.redar.2017.12.009
Di Leo, E., Delle Donne, P., Calogiuri, G. F., Macchia, L., and Nettis, E. (2018). Focus on the agents most frequently responsible for perioperative anaphylaxis. Clin. Mol. Allergy 16 (16), 16. doi:10.1186/s12948-018-0094-7
Engbaek, J., Roed, J., Hangaard, N., and Viby-Mogensen, J. (1994). The agreement between adductor pollicis mechanomyogram and first dorsal interosseous electromyogram. A pharmacodynamic study of rocuronium and vecuronium. Acta Anaesthesiol. Scand. 38 (8), 869–878. doi:10.1111/j.1399-6576.1994.tb04020.x
Fagley, R. E., Woodbury, A., Visuara, A., and Wall, M. (2009). Rocuronium-induced coronary vasospasm--Kounis syndrome. Int. J. Cardiol. 137 (2), e29–e32. doi:10.1016/j.ijcard.2008.05.052
Fisher, M. M., and Baldo, B. A. (1993). The incidence and clinical features of anaphylactic reactions during anesthesia in Australia. Ann. Fr. Anesth. Reanim. 12 (2), 97–104. doi:10.1016/s0750-7658(05)81016-0
Flynn, P. J., Frank, M., and Hughes, R. (1984). Use of atracurium in caesarean section. Br. J. Anaesth. 56 (6), 599–605. doi:10.1093/bja/56.6.599
Fusaroli, M., Salvo, F., Begaud, B., Alshammari, T. M., Bate, A., Battini, V., et al. (2024). The REporting of A disproportionality analysis for DrUg safety signal detection using individual case safety reports in PharmacoVigilance (READUS-PV): explanation and elaboration. Drug Saf. 47 (6), 585–599. doi:10.1007/s40264-024-01423-7
Galletly, D. C., and Treuren, B. C. (1985). Anaphylactoid reactions during anaesthesia. Seven years’ experience of intradermal testing. Anaesthesia 40 (4), 329–333. doi:10.1111/j.1365-2044.1985.tb10785.x
Greco, P., Dardes, N., Fiore, G., Vimercati, A., Sasanelli, B., Loverro, G., et al. (1995). Pharmacologic induction of fetal immobilization for prenatal diagnostic-therapeutic procedures. Minerva Ginecol. 47 (5), 207–210.
Hameedullah, H., and Khan, F. A. (1997). Incidence of intra-operative bradycardia. Comparison of atracurium and vecuronium in gynaecological surgery. Anaesthesia 52 (12), 1221–1224. doi:10.1111/j.1365-2044.1997.235-az0367.x
Harvey, A., Anderson, L., and Broome, I. J. (1999). A comparison of the effect of rocuronium and vecuronium on heart rate during gynaecological laparoscopy. Anaesthesia 54 (12), 1212–1216. doi:10.1046/j.1365-2044.1999.01076.x
Heier, T., and Guttormsen, A. B. (2000). Anaphylactic reactions during induction of anaesthesia using rocuronium for muscle relaxation: a report including 3 cases. Acta Anaesthesiol. Scand. 44 (7), 775–781. doi:10.1034/j.1399-6576.2000.440702.x
Hosking, M. P., Lennon, R. L., and Gronert, G. A. (1988). Combined H1 and H2 receptor blockade attenuates the cardiovascular effects of high-dose atracurium for rapid sequence endotracheal intubation. Anesth. Analg. 67 (11), 1089–1092. doi:10.1213/00000539-198867110-00012
Jeong, W. J., Kim, W. Y., Son, J. H., Lee, Y. S., Kim, J. H., and Park, Y. C. (2010). Anaphylaxis with angioedema by rocuronium during induction of general anesthesia -A case report. Korean J. Anesthesiol. 58 (4), 391–395. doi:10.4097/kjae.2010.58.4.391
Jiang, T., Su, H., Li, Y., Wu, Y., Ming, Y., Li, C., et al. (2022). Post-marketing safety of immunomodulatory drugs in multiple myeloma: a pharmacovigilance investigation based on the FDA adverse event reporting system. Front. Pharmacol. 13, 989032. doi:10.3389/fphar.2022.989032
Jick, H., Andrews, E. B., Tilson, H. H., Pfanschmidt, M., Branche, C., Walker, A. M., et al. (1989). Atracurium--a post-marketing surveillance study: methods and U.S. experience. Br. J. Anaesth. 62 (6), 590–595. doi:10.1093/bja/62.6.590
Kim, J. H., Lee, Y. C., Lee, S. I., Park, S. Y., Choi, S. R., Lee, J. H., et al. (2016). Effective doses of cisatracurium in the adult and the elderly. Korean J. Anesthesiol. 69 (5), 453–459. doi:10.4097/kjae.2016.69.5.453
Laxenaire, M. C. (1993). Drugs and other agents involved in anaphylactic shock occurring during anaesthesia. A French multicenter epidemiological inquiry. Ann. Fr. Anesth. Reanim. 12 (2), 91–96. doi:10.1016/s0750-7658(05)81015-9
Lundstrøm, L. H., Duez, C. H. V., Nørskov, A. K., Rosenstock, C. V., Thomsen, J. L., Møller, A. M., et al. (2018). Effects of avoidance or use of neuromuscular blocking agents on outcomes in tracheal intubation: a Cochrane systematic review. Br. J. Anaesth. 120 (6), 1381–1393. doi:10.1016/j.bja.2017.11.106
Macartney, D. H. (2013). Cucurbit[n]uril type hosts for the reversal of steroidal neuromuscular blocking agents. Future Med. Chem. 5 (17), 2075–2089. doi:10.4155/fmc.13.164
Mclean, D. J., Diaz-Gil, D., Farhan, H. N., Ladha, K. S., Kurth, T., and Eikermann, M. (2015). Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology 122 (6), 1201–1213. doi:10.1097/aln.0000000000000674
Mertes, P. M., Laxenaire, M. C., and Alla, F.Groupe d’Etudes des Réactions Anaphylactoïdes Peranesthésiques. (2003). Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999-2000. Anesthesiology 99 (3), 536–545. doi:10.1097/00000542-200309000-00007
Murray, M. J., Deblock, H., Erstad, B., Gray, A., Jacobi, J., Jordan, C., et al. (2016). Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit. Care Med. 44 (11), 2079–2103. doi:10.1097/ccm.0000000000002027
Naguib, M., Flood, P., Mcardle, J. J., and Brenner, H. R. (2002). Advances in neurobiology of the neuromuscular junction: implications for the anesthesiologist. Anesthesiology 96 (1), 202–231. doi:10.1097/00000542-200201000-00035
O’callaghan, A. C., Scadding, G., and Watkins, J. (1986). Bronchospasm following the use of vecuronium. Anaesthesia 41 (9), 940–942. doi:10.1111/j.1365-2044.1986.tb12921.x
Petitpain, N., Argoullon, L., Masmoudi, K., Fedrizzi, S., Cottin, J., Latarche, C., et al. (2018). Neuromuscular blocking agents induced anaphylaxis: results and trends of a French pharmacovigilance survey from 2000 to 2012. Allergy 73 (11), 2224–2233. doi:10.1111/all.13456
Raschi, E., Fusaroli, M., Gatti, M., Caraceni, P., Poluzzi, E., and De Ponti, F. (2022). Liver injury with nintedanib: a pharmacovigilance-pharmacokinetic appraisal. Pharm. (Basel) 15 (5), 645. doi:10.3390/ph15050645
Reitter, M., Petitpain, N., Latarche, C., Cottin, J., Massy, N., Demoly, P., et al. (2014). Fatal anaphylaxis with neuromuscular blocking agents: a risk factor and management analysis. Allergy 69 (7), 954–959. doi:10.1111/all.12426
Rose, M., and Fisher, M. (2001). Rocuronium: high risk for anaphylaxis? Br. J. Anaesth. 86 (5), 678–682. doi:10.1093/bja/86.5.678
Sadleir, P. H., Clarke, R. C., Bunning, D. L., and Platt, P. R. (2013). Anaphylaxis to neuromuscular blocking drugs: incidence and cross-reactivity in Western Australia from 2002 to 2011. Br. J. Anaesth. 110 (6), 981–987. doi:10.1093/bja/aes506
Scott, R. P., Savarese, J. J., Basta, S. J., Sunder, N., Ali, H. H., Gargarian, M., et al. (1985). Atracurium: clinical strategies for preventing histamine release and attenuating the haemodynamic response. Br. J. Anaesth. 57 (6), 550–553. doi:10.1093/bja/57.6.550
Singh, J. (2015). International conference on harmonization of technical requirements for registration of pharmaceuticals for human use. J. Pharmacol. Pharmacother. 6 (3), 185–187. doi:10.4103/0976-500x.162004
Thilen, S. R., Weigel, W. A., Todd, M. M., Dutton, R. P., Lien, C. A., Grant, S. A., et al. (2023). 2023 American society of anesthesiologists practice guidelines for monitoring and antagonism of neuromuscular blockade: a report by the American society of anesthesiologists task force on neuromuscular blockade. Anesthesiology 138 (1), 13–41. doi:10.1097/aln.0000000000004379
Tsolaki, V., Zakynthinos, G. E., Papadonta, M. E., Bardaka, F., Fotakopoulos, G., Pantazopoulos, I., et al. (2022). Neuromuscular blockade in the pre- and COVID-19 ARDS patients. J. Pers. Med. 12 (9), 1538. doi:10.3390/jpm12091538
Vanderweide, L. A., Abdel-Rasoul, M., and Gerlach, A. T. (2017). The Incidence of hypotension with continuous infusion atracurium compared to cisatracurium in the Intensive Care Unit. Int. J. Crit. Illn. Inj. Sci. 7 (2), 113–118. doi:10.4103/ijciis.Ijciis_35_16
Wang, N., Zhang, Y., Hu, Y., Yang, Q., and Su, Z. (2021). Serious bronchospasm induced by cisatracurium besylate: a case report. Med. Baltim. 100 (15), e25516. doi:10.1097/md.0000000000025516
Wang, W., Guan, X., Wang, S., Shi, L., Zhu, Y., Hua, P., et al. (2023). Epirubicin and gait apraxia: a real-world data analysis of the FDA adverse event reporting system database. Front. Pharmacol. 14, 1249845. doi:10.3389/fphar.2023.1249845
Watkins, J. (2001). Incidence of UK reactions involving rocuronium may simply reflect market use. Br. J. Anaesth. 87 (3), 522.
Woods, I., Morris, P., and Meakin, G. (1985). Severe bronchospasm following the use of atracurium in children. Anaesthesia 40 (2), 207–208. doi:10.1111/j.1365-2044.1985.tb10733.x
Zhang, L., Mao, W., Li, X., Wang, X., Liu, J., Hu, S., et al. (2022). Analysis of acute pancreatitis associated with SGLT-2 inhibitors and predictive factors of the death risk: based on food and drug administration adverse event report system database. Front. Pharmacol. 13, 977582. doi:10.3389/fphar.2022.977582
Keywords: neuromuscular blocking agents, adverse events, FAERS, disproportionality analysis, safety
Citation: Li L, Xu Q, Liu Y, Pang L, Cui Z and Lu Y (2024) Adverse events related to neuromuscular blocking agents: a disproportionality analysis of the FDA adverse event reporting system. Front. Pharmacol. 15:1403988. doi: 10.3389/fphar.2024.1403988
Received: 20 March 2024; Accepted: 11 July 2024;
Published: 24 July 2024.
Edited by:
Anick Bérard, Montreal University, CanadaReviewed by:
Ki Tae Jung, Chosun University, Republic of KoreaCopyright © 2024 Li, Xu, Liu, Pang, Cui and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Lu, MTU0MDQ2MjA5QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.