
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 11 September 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1403147
This article is part of the Research TopicTherapeutic Potential of Cannabinoids: From Health to DiseaseView all 5 articles
 Adriana Yndart Arias1,2*†
Adriana Yndart Arias1,2*† Kamila Vadell2†
Kamila Vadell2† Arti Vashist2
Arti Vashist2 Nagesh Kolishetti2
Nagesh Kolishetti2 Madepalli K. Lakshmana2
Madepalli K. Lakshmana2 Madhavan Nair2
Madhavan Nair2 Juan P. Liuzzi1
Juan P. Liuzzi1Background: Finding new strategies to treat cognitive disorders is a challenging task. Medication must defeat the blood–brain barrier. Cannabidiol (CBD), a non-intoxicating compound of the cannabis plant, has gained recognition as a nutraceutical for its potential effectiveness in treating anxiety, oxidative stress, convulsions, and inflammation. However, the dose, tolerable upper intake, formulation, administration routes, comorbidities, diet, and demographic factors to reverse cognitive impairments have not been completely explored. Trials using CBD as a primary intervention have been conducted to alleviate cognitive issues. This review evaluates the benefits of CBD supplementation, research design, formulations, and outcomes reported in randomized clinical trials.
Methods: An evidence-based systematic literature review was conducted using PUBMED and the Florida International University Research Library resources. Fourteen randomized trials were selected for review, and their designs and outcomes were compared conceptually and in the form of resume tables.
Results: CBD showed improvement in anxiety and cognitive impairments in 9 out of 16 analyzed trials. However, the variability could be justified due to the diversity of the trial designs, underpowered studies, assayed population, uncontrolled results for comorbidities, medications, severity of drug dependence, compliances, and adherences. Overall, oral single doses of 200 mg–1,500 mg or vaporized 13.75 mg of CBD were shown to be effective at treating anxiety and cognition with a good safety profile and no drug addiction behaviors. Conversely, results that did not have a significant effect on treating cognitive impairments can be explained by various factors such as THC or other abuse drugs masking effect, low dose, and unknown purity of CBD. Furthermore, CBD shows potential properties that can be tested in the future for Alzheimer’s disease.
Conclusion: As medical cannabis becomes more accessible, it is essential to understand whether medication rich in CBD exerts a beneficial effect on cognitive disorders. Our study concludes that CBD is a promising candidate for treating neurocognitive disorders; however, more studies are required to define CBD as a therapeutic candidate for managing cognitive disorders.
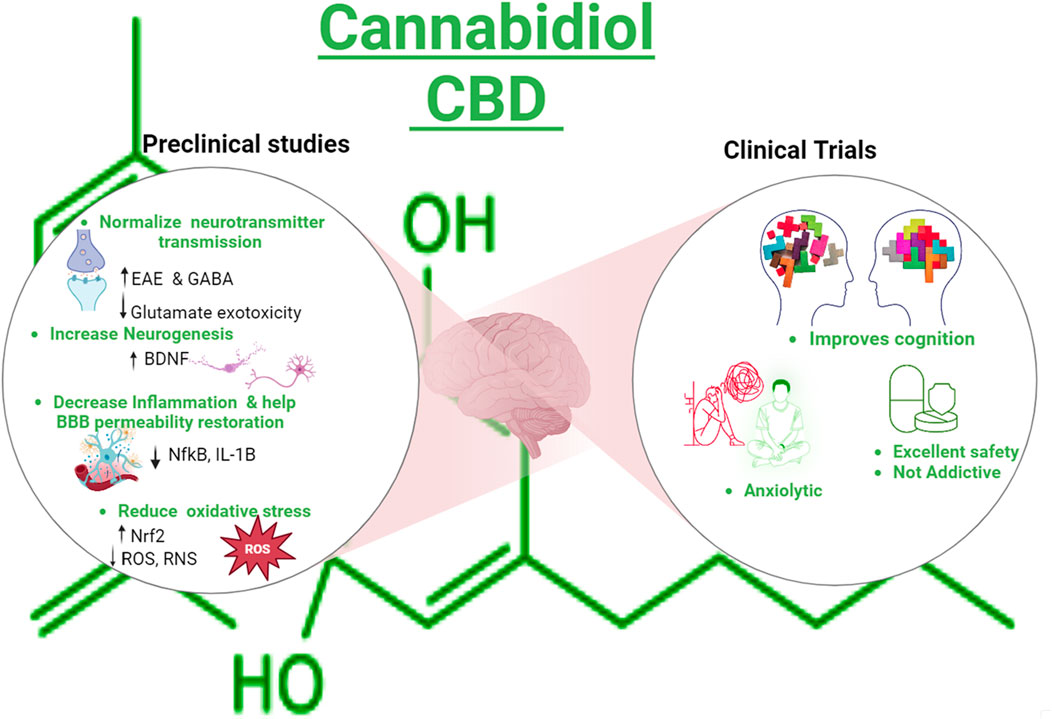
GRAPHICAL ABSTRACT | Created with Biorender.com.
Cannabidiol (CBD), a phytocannabinoid, is derived from the cannabis plant. Recently, CBD has gained significant attention due to its medical potential. It has anxiolytic, antioxidant, anti-inflammatory, antiemetic, and antipsychotic properties, making it a subject of interest in the scientific community (Mechoulam et al., 2002; Yndart Arias et al., 2023). In a recent review, we discussed the mechanism of action of CBD and its use in neurodegenerative diseases and preclinical studies related to literature (Bhunia et al., 2022). For instance, studies have shown that CBD enhances gamma-aminobutyric acid (GABA) synaptic transmission, a neurotransmitter associated with calming effects, and CBD consumption is related to the upregulation of the brain-derived neurotrophic factor (BDNF), indicating a potential to address anxiety-related conditions and cognitive issues (Augustin and Lovinger, 2022; Sales et al., 2019). In addition, preclinical evidence shows that CBD prevented the increase in blood–brain barrier (BBB) permeability in traumatic brain-injured mice, attenuating edema and neuroinflammation and helping recover neurological functions (Hampson et al., 1998; Prud’homme et al., 2015; Wang et al., 2022; Jiang et al., 2021). CBD counteracts the deficits of mRNA levels of AMPA receptor subunits (glutamate-gated ion channels), synaptophysin (SYP), DLG4, glial-cell-derived neurotrophic factor (GDNF), and BDNF in amyloid ß 1–42-induced mice (Chen et al., 2023). Similarly, TAU transgenic mice treated with CBD exhibited decreased anxiety and enhanced spatial reference memory impairment, which is partially explained by the fact that CBD is an agonist of the serotonin 1A receptor (5HT1A). Serotonin is a neurotransmitter depleted in depression and anxiety conditions (Chen et al., 2023). It has also been reported that CBD increases serotoninergic and glutamatergic transmission, modulating the serotonin receptor positively (Chen et al., 2023; Martinez Naya et al., 2023). Despite the promising properties, the Food Drug Administration (FDA) has not approved CBD as a food additive or supplement except for Epidiolex, a pharmaceutical and high-purity grade CBD oil used to treat epilepsy (Mechoulam et al., 2002; Karaźniewicz-Łada et al., 2021).
Of note, medications containing CBD can be administered through various routes, including oral, vaporized, intravenous, and intramuscular, and the administration route impacts the drug’s effectiveness. Absorption of orally administered CBD medications occurs in the small intestine, and its concentration diminishes before reaching systemic circulation (Kim and Jesus, 2023). In contrast, inhaled preparations bypass the small intestine and enter the systemic circulation, avoiding gastric degradation and requiring less CBD medication to obtain similar benefits. Therefore, the availability depends on the administration route: 11%–45% of the drug is available after inhaling, while 6% is available after the oral route (Chayasirisobhon, 2020). However, the entrance of drugs across the BBB (Kim and Jesus, 2023; Chayasirisobhon, 2020) depends on the lipid solubility, size, and charge of the substance. For example, CBD has a high lipophilicity grade, favoring the entrance to the brain (Chayasirisobhon, 2020). Each administration route has advantages and disadvantages, and the ideal route depends on the treatment’s specific target and end goals. In addition, CBD is hydroxylated by cytochrome P450 enzymes (CYP3A4 and CYP2C9) in the liver. Its plasma half-life varies from 18 h to 32 h, and it is excreted primarily in feces (Chayasirisobhon, 2020).
Cognition encompasses general mental processes critical to human functioning that include memory, attention, motor skills, language, and/or executive functioning (Bayne et al., 2019). Several neuropsychiatric conditions, including brain damage, stroke, degenerative dementia, amnestic populations, illnesses, experiences, trauma, or congenital abnormalities, can contribute to cognitive impairment (Bayne et al., 2019; Robbins, 2011). Moreover, anxiety can be closely intertwined with cognitive functioning. This primary emotional or primary response can significantly impact cognitive processes such as attention, memory, decision making, and problem solving (Hartley and Phelps, 2012; Alvi et al., 2022).
Because randomized controlled trials (RCT) are the most suitable method of answering questions about treatment (AND, 2022), we aimed to provide a comprehensive overview of clinical trial results examining the effects of CBD on cognitive functions. Our investigation entails healthy participants or those with a range of cognitive disorders, including anxiety, PTSD, and epilepsy. Furthermore, this review introduced an improvement in cognition associated with CBD interventions considering different formulations, administration doses, and routes while also exploring the role of an appropriate diet and demographic factors in enhancing CBD effects. Given the growing interest in CBD, the study aims to contribute valuable information to the relationship between CBD and cognitive function in a diverse population.
This review followed the five steps of the Academy of Dietetics and Nutrition for conducting an evidence-based review (AND, 2022). A systematic literature search used PubMed and the resources of the Florida International University Library to identify high-quality primary reports from clinical trials using CBD as an intervention to treat neurocognitive disorders.
The inclusion criteria for articles were primary reports of randomized, controlled, and uncontrolled clinical trials that included healthy participants or participants older than 12 years with cognitive disorders, sample size with more than four individuals per group, year range from 2013 to 2023, and language limited to English and Spanish. After examination, articles that did not meet the inclusion criteria were excluded (Table 1). Two searches were conducted. The keywords used for the first search were CBD, cognitive, dementia, not pediatric, not corticobasal, not schizophrenia, and not multiple sclerosis. The filters applied were as follows: Clinical Study, Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Randomized Controlled Trial, Humans, English, Spanish, Female, Male, Adult older than 19 years, and from 2013/1/1–2023/12/31. The final selection included 14 articles (Figure 1). The second search included dementia as a keyword, Cannabidiol AND Dementia NOT pediatric NOT pediatric NOT autism NOT corticobasal Filters: Clinical Trial, Clinical Trial, Phase I, Clinical Trial, Phase II, Clinical Trial, Phase III, Clinical Trial, Phase IV, Randomized Controlled Trial, in the last 10 years, Humans, English, Spanish, Female, Male, Adult: 19+ years. Articles that did not report CBD only as a treatment were excluded. The research question that guided the review was whether CBD administration could improve cognitive impairments in adults >12 years old. Graphical Abstract was created with Biorender.com/YXluZGEwMDFAZml1LmVkdQ==/YXluZGFydGFAZml1LmVkdQ==.
CBD is a compound that has gained popularity due to its promising effects. Cognitive impairments are on the rise, and finding alternative treatments is imperative. This article explored using CBD as an alternative to conventional cognition treatments. Understanding and treating cognitive impairments is crucial, as one in nine adults (11%) experience a subjective cognitive decline (CDC, 2024). Additionally, anxiety disorders affect 40 million adults (19.1% of the population aged 18 and older) every year (ADAA, 2024). With this prevalence of cognitive impairments, it is essential to develop effective alternative interventions. Therefore, exploring the effects of CBD on cognition will allow us to advance our understanding of its therapeutic potential. The summary of the included trials is described in Table 2.
After closely analyzing all the results, CBD demonstrated improvement in anxiety and cognitive impairments in nine of 16 trials (Arkell et al., 2020; Berger et al., 2022; Bolsoni et al., 2022a; Bolsoni et al., 2022b; Grimm et al., 2018; Hurd et al., 2019; Lees et al., 2023; McCartney et al., 2022; McGuire et al., 2018). This limited consistency can be explained by various factors, including diversity of the assayed population, variability of trial designs, small sample size, underpowered studies, and uncontrolled results for comorbidities, medications, severity of dependence on drugs, etc. In addition, the variable fragile compliance and adherence monitoring in trials such as pill counting, checking the empty bottle, controlling puffs, plasma CBD, breathalyzer, daily records, different questionnaires, self-reporting, purity of formulation, and wide ranges of doses (200–1,500 mg) can account further for this inconsistency. Importantly, the CBD dose, formulation, method of administration, length of CBD treatment, and participants’ demographic characteristics and habits, such as BMI, age, race, food consumption pattern, hydration, physical activity, and more, may also account for the variability of results.
For instance, Berger et al. (2022) conducted a 12-week study intervention with a high-purity CBD combined with cognitive therapies. This trial investigated the effect of a dose escalation protocol from 200 mg/day to 800 mg/day of oral CBD on cognition. Thirty young participants with anxiety disorders who had previously failed to improve using standard treatments were included. CBD reduced the severity of the anxiety scale by 42.6% at week 12. In 12 participants, the reduction was 50%, while in 18 participants, the reduction was 33%. Additionally, depression symptom severity decreased by 29.9%, and social and occupational functioning increased by 11.3%. An enhancement of clinical global impression was observed at week 12 with respect to baseline in treated participants. These findings were not reproduced at the 6-month follow-up. To ensure treatment adherence, both plasma CBD and pill count were measured. The intention to treat analysis showed a potential decrease in the severity of anxiety at 12 weeks. CBD inhibits the cytochrome P450, which is an enzyme used to metabolize antidepressant drugs. In fact, participants taking antidepressants showed more adverse events (OR = 6.4; 95% CI, 1.16–35.44; p = 0.03) unrelated to plasma CBD (Spearman ρ = −0.03, p = 0.088) that were possibly produced by interferences of cannabidiol with antidepressant drug metabolization. Cannabidiol had an acceptable safety profile with no clinical changes in blood parameters. Several limitations were observed in this trial, such as the small sample size, which was not determined by power analysis, and results were not stratified per gender, education, marital status, or antidepressant drug (Nagendrababu et al., 2021). Moreover, the unblinded trial may have produced expectancy bias due to CBD marketing benefits and the fact that uncontrolled trial design prevents the determination of cause and effect. Dietary information, BMI, and recreational drugs were not recorded, which could influence the presented results (Chayasirisobhon, 2020; Robbins, 2011; Birnbaum et al., 2019).
Similarly, Bloomfield et al. (2022) conducted an RCT crossover study to investigate the behavioral and neural effects of 600 mg of oral CBD in 24 healthy participants. The study reported that CBD had an effect in increasing anxiety when pre- and post-treatment were assessed in the treated group. However, CBD had no effect on a range of emotional measures relative to placebo. There were no other differences between groups for anxiety, cognition, and physiological measures. Several limitations must be considered when interpreting these results. The study used two different surveys to measure anxiety at baseline and post-treatment; these surveys introduced variability into the trial, and two different sets of emotional faces were used to test the same outcome. Participants self-reported fasting conditions prior to the session, but no 24-h dietary recall was conducted. Participants were provided with a standardized meal and drinks. Furthermore, the capsules were not oil-based, and neuroimaging results were not analyzed with respect to groups. On a positive note, treatment and placebo groups were randomized and balanced for sex. Power was analyzed based on CBD acute effects, and participants underwent drug screening, breathalyzer tests, and pregnancy tests, if applicable.
Arkell et al. (2020) studied the effect of THC and CBD on driving as a model to measure cognition impairments in 22 healthy participants with previous cannabis experience. The trial was performed in four sessions within a month. Participants inhaled 13.75 mg of THC and/or CDB vaporized doses. Results showed that driving impairments were significant at 40–100 min (p < 0.001) post vaporization of THC and CBD in combination with THC. The CBD did not impair driving compared to placebo, while THC rated more impaired driving at 100 (p < 0.001) and 300 min (p = 0.008) than the placebo. Additionally, the group that combined THC/CBD showed higher driving impairment vs. the placebo at 100 min (p = 0.001) and 300 min, suggesting that the THC had predominantly impairment effects on cognition. When results were controlled for alcohol levels, CBD showed decreased driving deficiencies. This trial was not powered by the detection of CBD on driving, and dietary information was not recorded. Participants self-reported taking painkillers and/or medications that could have altered treatment metabolism. The dose used for CBD was lower than that used in previous trials. In the trial, participants used a breathalyzer for alcohol and a drug screener in urine tests at the beginning of a session, and standardized procedures to inhale drugs, drive, rest, and lunch were performed. The trial reported good adherence powered by inside treatments.
In a different study, McCartney et al. (2022) investigated the effects of CBD on driving performance over four sessions in 1 month involving 17 healthy participants. The placebo and CBD doses of 15 mg, 300 mg, and 1,500 mg were taken with a standardized breakfast and a light snack. Compared to the baseline, CBD 300 mg (p = 0.011) and CBD 1,500 mg (p = 0.007) improved in the divided attention task at the end of the trial. No other significant impairments or improvements were observed in cognitive and driving tasks across the CBD doses. Participants reported higher levels of anxiety on the placebo than on CBD doses. However, several limitations should be considered. The study required a 24-h diet recall, but the results were neither presented nor analyzed. The driving performance was measured on a stimulator, which may not fully replicate real-world conditions. The trial was underpowered, and the washout period between treatments was not sufficient to avoid carry-over effects, as detectable levels of CBD or metabolites were found in all participants. On the other hand, prior to sessions, breathalyzer, urine, and dehydration tests were performed, and participants had a standardized breakfast and snack. In addition, the exact formulation of CBD in medium-chain triglyceride oil was used throughout the study and participants and had no serious adverse events.
In an attempt to clarify the differential effects of the most popular types of cannabinoids in healthy populations, Grimm et al. (2018) investigated whether CBD or THC-enhanced neuronal pathways are associated with neuropsychiatric conditions and cognition improvement. Sixteen healthy participants took a single dose of placebo, or 10 mg THC, or 600 mg CBD. CBD increased frontostriatal connectivity compared to THC and placebo. This brain region is involved in learning, language, reward, motor, and addiction. However, the increase in brain connectivity did not correlate with changes in cognitive tests, and there were no significant effects on anxiety, positive and negative effect, dissociative symptom scales, and subjective valence and arousal ratings for THC vs. placebo or CBD vs. placebo. Some limitations should be considered. This trial did not show demographics, BMI, comorbidities, or baseline characteristics with respect to anxiety and perception related to cognition, and neither did the results stratify this variance. Additionally, results and symptoms were self-reported, and power was based on within-subject, with no information presented about whether it reached 80%. All participants were men, and no information on CBD purity, brand, or carrier vehicle was reported. Furthermore, the trial did not report the possible consumption or experience with THC, CBD, or any illicit drug, and different doses were used for both cannabinoids. On a positive note, participants had their plasma CBD and THC levels measured throughout the study, and participants were excluded from analysis if no detectable plasma concentrations were found. Participants received standardized meals prior to treatment.
Haney et al. (2016) obtained different outcomes. Haney et al. (2016) assessed the subjective, cognitive, reinforcing, and physiological effects of consuming CBD prior to smoking cannabis. The study included 31 participants, and 200 mg, 400 mg, and 800 mg of CBD were provided before participants smoked a cannabis cigarette with inactive and active concentrations of THC. CBD did not show significance in the subjective, reinforcing, or cardiovascular effects and did not reduce the reinforcing or positive subjective effects of the two active cannabis concentrations used. Throughout the study, participants were allowed to smoke nicotine and purchase three additional cannabis puffs, and CBD was well-tolerated and produced no significant psychoactive or cardiovascular effects relative to the placebo. However, 24-h dietary recall, power calculation, and dose effects were not recorded. In addition, plasma CBD was only measured in the last session.
Partial benefits were found in the first trial conducted by Bolsoni et al. (2022a); however, the findings changed when results were stratified by type of trauma (Bolsoni et al., 2022b). Bolsoni et al. (2022a) found that CBD had no significant effects on anxiety, discomfort, sedation, salivary cortisol, heart rate, and diastolic blood pressure. In this trial, a single dose of 300 mg of high-purity CBD or a placebo was administered to 33 patients suffering from post-traumatic stress disorder. CBD helped decrease cognitive impairment associated with trauma recall, and this effect persisted over time (1.5 h p = 0.03 and 1 week p = 0.04 post treatment). This study has several limitations to consider. Treatment was administered only once. The study did not include a 24-h dietary recall, drug screening, or other covariates that could potentially affect the efficacy of CBD. Moreover, the study had a small sample size and did not stratify CBD results based on participants’ comorbidities, but groups were matched by sex, age, BMI, and severity of PTSD symptoms. In a separate article, Bolsoni et al. (2022b) assessed whether CBD attenuates anxiety in 33 patients with post-traumatic stress disorder in a 2-week study where CBD 300 mg or placebo was administrated once. PTSD trauma was divided into sexual and nonsexual trauma. CBD lowered anxiety in nonsexual (p = 0.035) vs. sexual trauma. Additionally, CBD decreased anxiety related to nonsexual trauma (p = 0.033) and cognitive impairment (p = 0.008) after treatment compared to placebo. It is important to consider that participants in the sexual trauma group were younger; therefore, the traumatic event occurred more recently. Participants had no urine or breathalyzer test before treatment. Furthermore, comorbidities were not specified, and neither 24-h dietary recall nor medications that could impact CBD metabolism were recorded. On the other hand, groups were randomized by sex, age, BMI, and trauma severity, and CBD had a high purity.
Despite the positive findings mentioned in previous trials with CBD, Flores et al. (2023) and Birnbaum et al. (2019) did not find a significant enhancement in cognition or inflammation. Flores et al. (2023) assessed the effectiveness of CBD on aerobic and anerobic fitness, physical activity, mental health and wellbeing, and inflammation in 48 healthy participants. It was an 8-week study where participants consumed 50 mg of CBD or 225 mg of MCT oil daily. CBD did not improve cognitive function, well-being aspect, or inflammation markers (C-reactive protein (CRP), interleukin-1 and 6, tumor necrosis factor, claudin 3, and myoglobulin). The study reported relaxed methods of compliance. The authors did not refer to how consumption of CBD was tracked, recorded daily, or remembered. Moreover, no blood or urine CBD or THC tests were performed, and the calculated power of the study was based on the reduction of CRP in active adults. Dietary recall was not recorded, and surveys for cognitive function did not target attention, working memory, and executive functions.
Birnbaum et al. (2019) evaluated the kinetics of CBD administered with and without a high-fat meal in eight adults with refractory epilepsy. CBD was administered in the fed stage with a high-fat breakfast and in the fasting stage with water. Higher plasma CBD levels were seen in the fed stage (126 ng/mL fed vs. 9 ng/mL fasting). There were no significant changes in cognitive test scores, adverse events, or changes in seizures between the fasting and feeding stages assessed using self-reports. The study reached 80% power, but the results were not stratified. No 24-h dietary recall was collected, and more studies are required to determine drug efficacy vs. food.
In addition to considering the drug’s efficacy, it is also important to investigate whether a drug has addictive properties. Schoedel et al. (2018) conducted a study to investigate the abuse potential of high-purity CBD in 41 healthy recreational polydrug users in a crossover trial. Participants were administered different CBD doses of 750 mg, 1,500 mg, and 4,500 mg while fasting. The study reported that CBD did not lead to drug addiction or adverse events, and all CBD doses demonstrated significantly lower drug-liking visual analog scale (VAS) scores than positive controls. Furthermore, CBD had no significant effect on cognitive or motor assessments compared to placebo. Several limitations should be considered. Most participants were White and men, limiting the generalizability of the results. The use of subjective measures, such as VAS, might introduce bias. The study relied on self-reporting, and no drug screening or dietary recall was recorded. CBD was administered during fasting and in a single dose, which may not reflect real-life conditions and results. Additionally, the study results were not stratified to account for the use of depressants, opioids, and derivatives among the polydrugs used. On a positive note, the study had a positive control group that served to validate the experimental procedure, and all drugs were administered within the testing center. Furthermore, the study had more than 80% power based on a 15-point difference in Emax scores on a drug-liking VAS of CBD and anxiolytic drugs.
Based on these results and the presence of cognitive issues in the drug-addicted population, Lees et al. (2023), Hurd et al. (2019), and Rizkallah et al. (2022) conducted clinical trials to investigate whether CBD could diminish the craving for illicit drugs and cognition problems. Lees et al. (2023) investigated whether CBD attenuates cognitive disorders produced by cannabis use disorders (CUD) in 70 participants over a 4-week study. Placebo and CBD doses of 400 mg and 800 mg were administered twice a day throughout the length of the study. CBD demonstrated improvement in the recall task at week 4 compared to baseline, and performance in the backward digit span, a type of cognitive test, increased in the 800 mg of CBD-treated group. However, no significant effects of CBD were observed on other secondary cognitive outcomes compared to placebo. At the end of the study, all participants reported reduced cannabis use. The treatment adherence was measured by diary cards and the return of the pill box, with urine tests performed at baseline, week 4, and week 12. The study was divided into two stages: stage 1 was dedicated to investigating which CBD dose was most effective in reducing cannabis use, and stage 2 further expanded on the effects of the chosen effective doses. Limitations such as instructed time of day to ingest capsules, dietary information, and BMI were not recorded. In addition, due to continued exposure to the cognitive tasks and environment through the trial, participants might have experienced lower anxiety and scored higher. In the same way, Hurd et al. (2019) investigated the potential short and acute effects of CBD in reducing cue-induced craving and anxiety in 42 heroin patients. Participants took the placebo or Epidiolex containing CBD 400 mg or 800 mg for 3 consecutive days. After assessing patients, no significant changes in cognitive performance between baseline and end of study were found. Both CBD dosages were effective in reducing cue-induced cravings for heroin and anxiety, and the 800 mg/d dosage showed the best outcome. This study has several limitations, such as no power calculation and lack of dietary recall, and not all participants satisfied the inclusion criterion of abstaining from heroin for at least 1 month. Furthermore, no drug abuse test was conducted during the trial after the initial test at the beginning, and results were not extrapolated for participants’ conditions, such as HIV and hepatitis C virus, which could affect the interpretation of results. Craving and anxiety outcomes were subjectively measured. The sample size was small, with high diversity in the BMI ranges and mostly male participants. Lastly, Rizkallah et al. (2022) tested whether CBD was effective at improving cognitive function in individuals with cocaine use disorder in a 3-month study. Participants took oral CBD 800 mg or placebo daily for 92 days. CBD was not effective at improving cognitive function, and there were no significant differences in pattern recognition memory, stop signal task, and Cambridge gambling task compared to placebo. Participants were excluded from the study if they had additional substance use disorders. The study had no control group, power calculation, or 24-h recall recorded. Additionally, participants’ compliance and treatment adherence were not measured or recorded, and the trial had high attrition rates of 28% in Phase I and 22% in Phase II.
A systematic review and meta-analysis using Bayesian analysis on preclinical studies found that preexisting anxiety conditions in animals predicted more significant effects of CBD than on unconditioned anxiety (Kwee et al., 2023). Likewise, Berger et al. (2022) obtained satisfactory outcomes for the reduction of anxiety and cognitive improvement by CBD when treating patients with preexisting anxiety and resistance compared to standard-of-care treatments. Bolsoni et al. (2022b) conducted a secondary analysis from a previous trial (Bolsoni et al., 2022a) and elucidated that CBD not only improved cognition but also protected against anxiety when a nonsexual trauma was established and recalled (Bolsoni et al., 2022b). In contrast to previous results, Bloomfield et al. (2022) did not obtain significant results on cognitive measures or modulation of experimentally induced anxiety by CBD in healthy patients with low anxiety levels.
Cannabis use disorders and associated cognitive issues led us to four studies that met our search criteria. Among them, Arkell et al. (2020), McCartney et al. (2022), and Lees et al. (2023) reported improvement in cognition functions, while Flores et al. (2023) did not. This divergence in findings underscores the complexity of the topic and the need for further research. Note that doses of more than 300 mg for oral administration consistently led to better outcomes. Flores et al. (2023) used doses as low as 50 mg/day for 8 weeks, while McCartney et al. (2022) and Lees et al. (2023) achieved expected results with doses of more than 300 mg once a week or twice daily, respectively. Interestingly, Arkell et al. (2020) obtained their results using only 13.75 mg/week primarily through an inhaled preparation that offered a higher percentage of CBD availability and rapid distribution to the brain with no reported serious adverse events.
Respecting other types of non-cannabinoid drugs, such as opioids and cocaine, three trials (Hurd et al., 2019; Schoedel et al., 2018; Rizkallah et al., 2022) explored the use of CBD as an anxiolytic, cognitive improvement agent, and for the reduction of drug craving (Hurd et al., 2019). None of them showed the expected results for cognition (Hurd et al., 2019), even when they included variable oral doses such as 750/1500/4,500 mg as single doses (Schoedel et al., 2018), 400/800 mg for three consecutive days, or 800 mg/day/14weeks (Rizkallah et al., 2022). At the same time, the anxiolytic action of CBD was achieved in opioid users, while Rizkallah et al. (2022) did not assess anxiety in cocaine-user participants. CBD reduced drug cue cravings in opioid users. Results in this work were not extrapolated to the type of abused drugs that affect our analysis for generalization.
Grimm et al. (2018) was the only included study that used healthy human subjects and reported an increase of frontostriatal connectivity as an indirect measure of cognition improvement in healthy individuals led by CBD treatment.
McGuire et al. (2018) and Boggs et al. (2018) studied the effect of CBD on the improvement of cognitive issues associated with schizophrenia and obtained different results. McGuire et al. (2018) found that CBD decreased positive psychotic symptoms and improved cognitive performance and level of functioning after the administration of 1,000 mg/day of oral CBD for 8 weeks in adults aged 18–65 with schizophrenia. These findings are in contrast to the outcomes reported by Boggs et al. (2018), who gave 600 mg of CBD daily for 6 weeks without interrupting the antipsychotic medication. The differences in findings could be influenced by variable doses used, sample size, length of the trials, and the masking effects of drugs of abuse included in the outcomes of McGuire et al. (2018) and not in the Boggs et al. (2018) trial. In addition, both teams assessed self-report adherences; one of them used overweight participants, and the results were not extrapolated to the type of medication used. It is important to highlight that even in schizophrenia patients, CBD showed a safe and tolerable profile, with no adverse events and no worsening of psychosis or mood or suicidality.
Overall, oral doses of 300 mg–1,500 mg or unique vaporized 13.75 mg of CBD in different administration schemes were effective at treating anxiety and cognition (Arkell et al., 2020; Berger et al., 2022; Bolsoni et al., 2022a; Bolsoni et al., 2022b; Grimm et al., 2018; Hurd et al., 2019; Lees et al., 2023; McCartney et al., 2022; McGuire et al., 2018). The improvements were seen in participants with post-traumatic stress disorders, anxiety, drug of abuse (heroin, cannabis), schizophrenia, and healthy subjects. In contrast, seven trial results did not show a significant effect on treating cognitive impairments, which can be explained by various factors such as the presence of THC or other abuse drugs that can interfere with the CBD effect, low dose, and unknown purity of CBD (Birnbaum et al., 2019; Bloomfield et al., 2022; Haney et al., 2016; Flores et al., 2023; Schoedel et al., 2018; Rizkallah et al., 2022; Boggs et al., 2018). Overall, CBD showed a safe profile with scarce or no serious adverse events and no drug addiction behavior (Arkell et al., 2020; Berger et al., 2022; Bolsoni et al., 2022a; Grimm et al., 2018; Hurd et al., 2019; Lees et al., 2023; McCartney et al., 2022; McGuire et al., 2018; Birnbaum et al., 2019; Bloomfield et al., 2022; Haney et al., 2016; Flores et al., 2023; Schoedel et al., 2018; Rizkallah et al., 2022; Boggs et al., 2018). Furthermore, CBD shows potential properties that can be tested in Alzheimer’s disease treatment, and no reported clinical trial uses CBD as a primary intervention for the improvement of cognitive disorders associated with Alzheimer’s disease.
Despite CBD’s established safety profile, a significant need for more investigation remains. This includes elucidating the optimal dose, formulation, treatment length, effective administration route, and associated diet for achieving cognitive improvement through CBD treatment. The potential of CBD in Alzheimer’s disease treatment is promising, but it requires the active involvement of the medical community and researchers. Notably, no clinical trial has yet utilized CBD as a primary intervention for Alzheimer’s disease improvement.
In summary, although many of the studies support the notion that CBD is a promising compound for treating cognitive disorders, there is insufficient evidence to safely conclude that CBD is a good candidate for this purpose. Of 16 studied trials, nine significantly showed improvement in cognitive issues over a range of CBD doses. However, the absence of a general pattern makes it difficult to generate a common recommendation, and more trials are needed for conclusive evidence of CBD’s beneficial role on cognition.
This review may help summarize published work related to CBD as a candidate for cognitive dysfunction and anxiety treatment in healthy and non-healthy participants, inclusively to reduce craving for addictive drugs. Furthermore, it can open avenues for new trial designs and applications in other neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, as a research priority.
AYA: visualization, resources, methodology, investigation, formal analysis, conceptualization, writing–review and editing, and writing–original draft. KV: writing–review and editing and writing–original draft. AV: writing–review and editing and resources. NK: writing–review and editing and resources. ML: writing–review and editing and resources. MN: writing–review and editing, supervision, resources, project administration, and methodology. JL: writing–review and editing, writing–original draft, supervision, resources, methodology, investigation, data curation, and conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Institute of Health (R01DA052271). We thanks FIU-University Graduate School for the support of publication fee of this article. Arti Vashist thanks the support from Young Investigator Pilot Award (YIPA) by AIDS and Cancer Specimen Resources sponsored by the National Cancer Institute (NCI).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AIMS, abnormal involuntary movements scale; AE, adverse events; AUC, Area under the curve; BACS, brief assessment of cognition in schizophrenia; BAS, Barnes akathisia scale; BMI, body mass index; BP, blood pressure; CBT, cognitive behavioral therapy; CGI, clinical global impressions scale; CLTS, controls; DAT, divided attention task; DBP, diastolic blood pressure; DSS, dissociative symptoms scale; GAF, global assessment of functioning scale; HR, heart rate; ITT analysis, intention to treat analysis; MCT, medium-chain triglyceride; PANAS, positive and negative affect schedule; PANSS, positive and negative symptom scale; pCBD plasma CBD concentration; PP, participants; PTSD, post-traumatic stress disorders; RBC, red blood cells; SAS, Simpson Angus scale; SBP, systolic blood pressure; SC, salivary cortisol; STAI, state anxiety inventory; VAMS, visual analog mood scale; WBC, white blood cells.
ADAA (2024). Anxiety disorders - facts and statistics. Available at: https://adaa.org/understanding-anxiety/facts-statistics.
Alvi, T., Kumar, D., and Tabak, B. A. (2022). Social anxiety and behavioral assessments of social cognition: a systematic review. J. Affect. Disord. 311, 17–30. doi:10.1016/j.jad.2022.04.130
Arkell, T. R., Vinckenbosch, F., Kevin, R. C., Theunissen, E. L., McGregor, I. S., and Ramaekers, J. G. (2020). Effect of cannabidiol and Δ9-tetrahydrocannabinol on driving performance: a randomized clinical trial. Jama 324 (21), 2177–2186. doi:10.1001/jama.2020.21218
Augustin, S. M., and Lovinger, D. M. (2022). Synaptic changes induced by cannabinoid drugs and cannabis use disorder. Neurobiol. Dis. 167, 105670. doi:10.1016/j.nbd.2022.105670
Bayne, T., Brainard, D., Byrne, R. W., Chittka, L., Clayton, N., Heyes, C., et al. (2019). What is cognition? Curr. Biol. CB 29 (13), R608–r615. doi:10.1016/j.cub.2019.05.044
Berger, M., Li, E., Rice, S., Davey, C. G., Ratheesh, A., Adams, S., et al. (2022). Cannabidiol for treatment-Resistant anxiety disorders in young People: an open-Label trial. J. Clin. psychiatry 83 (5), 21m14130. doi:10.4088/JCP.21m14130
Bhunia, S., Kolishetti, N., Arias, A. Y., Vashist, A., and Nair, M. (2022). Cannabidiol for neurodegenerative disorders: a comprehensive review. Front. Pharmacol. 13, 989717. doi:10.3389/fphar.2022.989717
Birnbaum, A. K., Karanam, A., Marino, S. E., Barkley, C. M., Remmel, R. P., Roslawski, M., et al. (2019). Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 60 (8), 1586–1592. doi:10.1111/epi.16093
Bloomfield, M. A. P., Yamamori, Y., Hindocha, C., Jones, A. P. M., Yim, J. L. L., Walker, H. R., et al. (2022). The acute effects of cannabidiol on emotional processing and anxiety: a neurocognitive imaging study. Psychopharmacology 239 (5), 1539–1549. doi:10.1007/s00213-022-06070-3
Boggs, D. L., Surti, T., Gupta, A., Gupta, S., Niciu, M., Pittman, B., et al. (2018). The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 235 (7), 1923–1932. doi:10.1007/s00213-018-4885-9
Bolsoni, L. M., Crippa, J. A. S., Hallak, J. E. C., Guimarães, F. S., and Zuardi, A. W. (2022a). Effects of cannabidiol on symptoms induced by the recall of traumatic events in patients with posttraumatic stress disorder. Psychopharmacology 239 (5), 1499–1507. doi:10.1007/s00213-021-06043-y
Bolsoni, L. M., Crippa, J. A. S., Hallak, J. E. C., Guimarães, F. S., and Zuardi, A. W. (2022b). The anxiolytic effect of cannabidiol depends on the nature of the trauma when patients with post-traumatic stress disorder recall their trigger event. Rev. Bras. Psiquiatr. Sao Paulo, Braz. 44 (3), 298–307. doi:10.1590/1516-4446-2021-2317
CDC (2024). Source: division of population health, N. C. F. C. D. P. A. H. P. Subjective cognitive decline — a public health issue. Available at: https://www.cdc.gov/aging/data/subjective-cognitive-decline-brief.html (Accessed May 17, 2024).
Chayasirisobhon, S. (2020). Mechanisms of action and pharmacokinetics of cannabis. Perm. J. 25, 1–3. doi:10.7812/TPP/19.200
Chen, L., Sun, Y., Li, J., Liu, S., Ding, H., Wang, G., et al. (2023). Assessing cannabidiol as a therapeutic agent for preventing and alleviating Alzheimer's disease neurodegeneration. Cells 12 (23), 2672. doi:10.3390/cells12232672
Flores, V. A., Kisiolek, J. N., Ramani, A., Townsend, R., Rodriguez, E., Butler, B., et al. (2023). Effects of oral cannabidiol on health and fitness in healthy adults: an 8-week randomized trial. Nutrients 15 (12), 2664. doi:10.3390/nu15122664
Grimm, O., Löffler, M., Kamping, S., Hartmann, A., Rohleder, C., Leweke, M., et al. (2018). Probing the endocannabinoid system in healthy volunteers: cannabidiol alters fronto-striatal resting-state connectivity. Eur. Neuropsychopharmacol. 28 (7), 841–849. doi:10.1016/j.euroneuro.2018.04.004
Hampson, A. J., Grimaldi, M., Axelrod, J., and Wink, D. (1998). Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. U. S. A. 95 (14), 8268–8273. doi:10.1073/pnas.95.14.8268
Haney, M., Malcolm, R. J., Babalonis, S., Nuzzo, P. A., Cooper, Z. D., Bedi, G., et al. (2016). Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 41 (8), 1974–1982. doi:10.1038/npp.2015.367
Hartley, C. A., and Phelps, E. A. (2012). Anxiety and decision-making. Biol. psychiatry 72 (2), 113–118. doi:10.1016/j.biopsych.2011.12.027
Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., et al. (2019). Cannabidiol for the reduction of cue-induced craving and anxiety in drug-Abstinent individuals with heroin Use disorder: a double-blind randomized placebo-controlled trial. Am. J. psychiatry 176 (11), 911–922. doi:10.1176/appi.ajp.2019.18101191
Jiang, H., Li, H., Cao, Y., Zhang, R., Zhou, L., Zhou, Y., et al. (2021). Effects of cannabinoid (CBD) on blood brain barrier permeability after brain injury in rats. Brain Res. 1768, 147586. doi:10.1016/j.brainres.2021.147586
Karaźniewicz-Łada, M., Główka, A. K., Mikulska, A. A., and Główka, F. K. (2021). Pharmacokinetic drug-drug Interactions among Antiepileptic drugs, including CBD, drugs used to treat COVID-19 and Nutrients. Int. J. Mol. Sci. 22 (17), 9582. doi:10.3390/ijms22179582
Kim, J., and Jesus, O. D. (2023). “Medication routes of administration,” in StatPearls (Treasure Island (FL): StatPearls Publishing). Available at: https://www.ncbi.nlm.nih.gov/books/NBK568677/.
Kwee, C. M. B., Leen, N. A., Van der Kamp, R. C., Van Lissa, C. J., Cath, D. C., Groenink, L., et al. (2023). Anxiolytic effects of endocannabinoid enhancing compounds: a systematic review and meta-analysis. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 72, 79–94. doi:10.1016/j.euroneuro.2023.04.001
Lees, R., Hines, L. A., Hindocha, C., Baio, G., Shaban, N. D. C., Stothart, G., et al. (2023). Effect of four-week cannabidiol treatment on cognitive function: secondary outcomes from a randomised clinical trial for the treatment of cannabis use disorder. Psychopharmacology 240 (2), 337–346. doi:10.1007/s00213-022-06303-5
Martinez Naya, N., Kelly, J., Corna, G., Golino, M., Abbate, A., and Toldo, S. (2023). Molecular and Cellular mechanisms of action of cannabidiol. Mol. Basel, Switz. 28 (16), 5980. doi:10.3390/molecules28165980
McCartney, D., Suraev, A. S., Doohan, P. T., Irwin, C., Kevin, R. C., Grunstein, R. R., et al. (2022). Effects of cannabidiol on simulated driving and cognitive performance: a dose-ranging randomised controlled trial. J. Psychopharmacol. Oxf. Engl. 36 (12), 1338–1349. doi:10.1177/02698811221095356
McGuire, P., Robson, P., Cubala, W. J., Vasile, D., Morrison, P. D., Barron, R., et al. (2018). Cannabidiol (CBD) as an Adjunctive Therapy in schizophrenia: a Multicenter randomized controlled trial. Am. J. psychiatry 175 (3), 225–231. doi:10.1176/appi.ajp.2017.17030325
Mechoulam, R., Parker, L. A., and Gallily, R. (2002). Cannabidiol: an overview of some pharmacological aspects. J. Clin. Pharmacol. 42 (S1), 11S–19S. doi:10.1002/j.1552-4604.2002.tb05998.x
Nagendrababu, V., Duncan, H. F., Pulikkotil, S. J., and Dummer, P. M. H. (2021). Glossary for randomized clinical trials. Int. Endod. J. 54 (3), 354–365. doi:10.1111/iej.13434
Prud'homme, M., Cata, R., and Jutras-Aswad, D. (2015). Cannabidiol as an intervention for addictive behaviors: a systematic review of the evidence. Subst. abuse Res. Treat. 9, 33–38. doi:10.4137/SART.S25081
Rizkallah, E., Mongeau-Pérusse, V., Lamanuzzi, L., Castenada-Ouellet, S., Stip, E., Juteau, L. C., et al. (2022). Cannabidiol effects on cognition in individuals with cocaine use disorder: exploratory results from a randomized controlled trial. Pharmacol. Biochem. Behav. 216, 173376. doi:10.1016/j.pbb.2022.173376
Robbins, T. W. (2011). Cognition: the ultimate brain function. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 36 (1), 1–2. doi:10.1038/npp.2010.171
Sales, A. J., Fogaça, M. V., Sartim, A. G., Pereira, V. S., Wegener, G., Guimarães, F. S., et al. (2019). Cannabidiol induces rapid and sustained antidepressant-like effects through increased BDNF signaling and synaptogenesis in the prefrontal cortex. Mol. Neurobiol. 56 (2), 1070–1081. doi:10.1007/s12035-018-1143-4
Schoedel, K. A., Szeto, I., Setnik, B., Sellers, E. M., Levy-Cooperman, N., Mills, C., et al. (2018). Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy and Behav. E&B 88, 162–171. doi:10.1016/j.yebeh.2018.07.027
Wang, L., Wu, X., Yang, G., Hu, N., Zhao, Z., Zhao, L., et al. (2022). Cannabidiol alleviates the damage to Dopaminergic Neurons in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-induced Parkinson's disease mice via Regulating neuronal Apoptosis and neuroinflammation. Neuroscience 498, 64–72. doi:10.1016/j.neuroscience.2022.06.036
Keywords: CBD, cannabidiol, cognition, clinical trial, drug of abuse, anxiety, Alzheimer’s disease, neurocognitive disorders
Citation: Yndart Arias A, Vadell K, Vashist A, Kolishetti N, Lakshmana MK, Nair M and Liuzzi JP (2024) Cannabidiol, a plant-derived compound, is an emerging strategy for treating cognitive impairments: comprehensive review of randomized trials. Front. Pharmacol. 15:1403147. doi: 10.3389/fphar.2024.1403147
Received: 18 March 2024; Accepted: 19 August 2024;
Published: 11 September 2024.
Edited by:
Xu Qin, Huazhong University of Science and Technology, ChinaReviewed by:
Antoni Pastor, Hospital del Mar Medical Research Institute (IMIM), SpainCopyright © 2024 Yndart Arias, Vadell, Vashist, Kolishetti, Lakshmana, Nair and Liuzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Yndart Arias, YXluZGFydGFAZml1LmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.