
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 15 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1400105
This article is part of the Research TopicTraditional Medicines and Natural Products for Gut-X Axis: Pharmacology, Toxicology and Microbiology in the Context of Drug Discovery and Herbal Medicine Use, volume IIView all 19 articles
Candida albicans: (C. albicans) is a prevalent opportunistic pathogen that can cause severe mucosal and systemic fungal infections, leading to high morbidity and mortality rates. Traditional chemical drug treatments for C. albicans infection have limitations, including the potential for the development of drug resistance. Essential oils, which are secondary metabolites extracted from plants, have gained significant attention due to their antibacterial activity and intestinal regulatory effects. It makes them an ideal focus for eco-friendly antifungal research. This review was aimed to comprehensively evaluate the research progress, mechanisms, and clinical application prospects of essential oils in treating C. albicans infections through their antibacterial and intestinal regulatory effects. We delve into how essential oils exert antibacterial effects against C. albicans infections through these effects and provide a comprehensive analysis of related experimental studies and clinical trials. Additionally, we offer insights into the future application prospects of essential oils in antifungal therapy, aiming to provide new ideas and methods for the development of safer and more effective antifungal drugs. Through a systematic literature review and data analysis, we hope to provide insights supporting the application of essential oils in antifungal therapy while also contributing to the research and development of natural medicines. In the face of increasingly severe fungal infections, essential oils might emerge as a potent method in our arsenal, aiding in the effective protection of human and animal health.
Candida albicans (C. albicans) is the most common and important type of Candida, belonging to the yeast genus. Its cells are round or oval in shape, typically ranging between 3 and 5 microns in diameter. It usually exists in the form of yeast and can form mycelium under appropriate conditions. Widely distributed in the natural environment, C. albicans is a common symbiotic fungus that often coexists with humans and animals as part of the normal microbial community (Moran et al., 2011). Infections caused by C. albicans occur when the body’s immune system is compromised, resulting in conditions such as oral candidiasis (Millsop and Fazel, 2016; Vila et al., 2020), vaginal candidiasis (Gonçalves et al., 2016; Rodríguez-Cerdeira et al., 2019), skin and nail candidiasis (Jautová et al., 2001; Espinosa-Hernández et al., 2020), and severe systemic fungal infections (Blanco and Garcia, 2008). Factors contributing to infection include a compromised immune system (e.g., HIV/AIDS, organ transplantation, chemotherapy), antibiotic usage, diabetes, pregnancy, and extended catheter or ventilator use. Oral candidiasis is a prevalent C. albicans infection, especially among young individuals and animals with weakened immune systems. It presents as white patches or spots on the oral mucosa and tongue, accompanied by symptoms like bad breath, loss of appetite, and difficulty eating. Vaginal candidiasis can affect the genitourinary system of animals, leading to symptoms such as urinary tract inflammation, frequent urination, urgency to urinate, hematuria, vaginal inflammation, and mastitis. Symptoms of skin and nail candidiasis may include redness, swelling, itching, desquamation, and ulcers at the affected site, causing discomfort and itching in animals. In some cases, C. albicans infections can progress to blood infections, resulting in severe symptoms like fever, weakness, loss of appetite, anemia, and organ failure, thereby posing a serious threat to the animal’s life and health (Miceli et al., 2011).
The main antifungal drugs used for treating infections of C. albicans are as follows: (1) Azole antifungal drugs (Polyazoles): Azole drugs serve as the initial choice for treating the infections caused by C. albicans. Common polyazole drugs such as Fluconazole, Itraconazole, and Posaconazole inhibit growth and replication by disrupting yeast ketones synthesized by the cell wall of C. albicans; (2) Polyene antifungal drugs (Polyketides): Polyenes target the cell membrane of C. albicans, leading to membrane rupture and cell death. Widely used polyene drugs include Neomycin B (Amphotericin B) and the liposomal form Amphotericin B lipid complex (ABLC), typically employed in severe infections of C. albicans; (3) Novel antifungal drugs: In recent years, several novel antifungal agents have emerged to address refractory or drug-resistant infections of C. albicans, including Isaviconazole and Voriconazole, offering a wider spectrum of antifungal activities suitable for complex infections of C. albicans (Kathiravan et al., 2012; Cowen et al., 2015; Houšť et al., 2020).
However, the use of antibiotics pose a number of challenges. The improper and overuse of antibiotics has resulted in an escalation of bacterial resistance to these drugs. Consequently, bacteria that could typically be effectively treated with antibiotics are becoming less sensitive to the drugs, leading to infections that are difficult to cure (Balkis et al., 2002). Antibiotics not only eliminate pathogenic bacteria but also disrupt the natural microbial community within the human body. This disruption can lead to an imbalance in the gut microbiota, heightening the risk of other infections, and may impact the immune system and overall health. Residues of antibiotics enter the environment, including soil and water bodies, exerting adverse effects on ecosystems. These residues may interact with environmental bacteria, fostering the development of drug resistance in bacteria, and potentially causing harmful effects on aquatic organisms and other members of the ecosystem. Therefore, the new environmentally friendly methods which can either substitute or decrease antibiotic usage are necessary.
Essential oil is a secondary metabolite usually extracted from flowers, leaves, roots, fruits, or bark of plants through distillation, cold pressing, or solvent extraction (Valdivieso-Ugarte et al., 2019; Spisni et al., 2020). Based on a strong aroma and active ingredients (Mancianti and Ebani, 2020), it is featured by antibacterial properties that can inhibit or eradicate various microorganisms, including bacteria, fungi, and viruses (Bakkali et al., 2008). The antibacterial efficacy of different essential oils varies, stemming from their unique chemical compositions. Numerous essential oils contain volatile compounds such as phenols, alcohols, esters, aldehydes, and ketones (Dhifi et al., 2016) capable of inhibiting or eradicating microorganisms. In addition, essential oil, as a natural product, is characterized by its safety, efficiency, absence of residue, lack of resistance, good tolerance within the animal body, and minimal toxic side effects, rendering it one of the most promising antibiotic alternatives. Unlike some chemical medications, the antibacterial activity of essential oils is the result of multiple components working together. This complex combination of ingredients makes it difficult for microbes to develop microbial resistance against essential oils, thereby reducing this risk. Beyond their antibacterial effects, some essential oils also offer gut-regulating effects. They aid in balancing intestinal flora, promoting the growth of beneficial bacteria, suppressing harmful bacterial proliferation, and thereby maintaining intestinal health. These attributes hold significant implications for the prevention and treatment of infections and diseases associated with the dysregulation of gut flora.
Essential oils typically consist of a variety of aldehydes, phenols, alcohols, and other chemical molecules. They are divided into two categories of compounds: terpenes (such as carvacrol and thymol) and phenylallens (such as cinnamaldehyde and eugenol), with terpenes being the predominant. Essential oil is hydrophobic and can penetrate the cell membrane of Gram-positive bacteria to enter the cell’s interior, exerting their antibacterial effect by disrupting enzyme production and protein denaturation (Pandey et al., 2017). Lipid soluble hydrophobic groups, such as hydroxyl and carbonyl groups in the structure of aldehyde phenols in essential oils, can interact with proteins within bacterial cell membranes, leading to structural and functional alterations in the membranes, membrane expansion and increased permeability, thereby eliciting antibacterial effects (Omonijo et al., 2018). The primary phenolic elements in essential oils, carvacrol, and thymol, which are isomers, are pivotal in modulating the balance of intestinal flora by changing the permeability of cell membrane and inhibiting the secretion of bacterial endotoxin.
Essential oils have antifungal properties and can inhibit or kill C. albicans. Ebani et al. studied the antibacterial activity of Litsea cubeba (Lour.) Pers. Essential oil, Origanum vulgare L. subsp. Hirtum essential oil, Origanum majorana L. essential oil, Thymus vulgaris L. essential oil, and their mixtures against pathogenic bacteria and C. albicans. They found that essential oil had different degrees of growth inhibition on the tested strains and C. albicans (Ebani et al., 2016). Hammer et al. found that concentrations of 0.25%–1.0% (v/v) of Melaleuca alternifolia (Maiden and Betche) Cheel oil and its components changed the permeability and fluidity of C. albicans (Hammer et al., 2004). Through a study on the antibacterial activity of M. alternifolia (Maiden and Betche) Cheel essential oil on 81 strains of C. albicans, it was found that the minimum inhibitory concentration for 90% of C. albicans was 0.25% (v/v) (Hammer et al., 1998). D'Auria et al. used Lavandula angustifolia Mill essential oil and its main components to inhibit C. albicans and found that the essential oil and its main components could inhibit the formation of bud tubes and mycelium extension of C. albicans, and had antibacterial and bactericidal activities as well (D’auria et al., 2005). Behmanesh et al. studied the inhibitory effect of L. angustifolia Mill essential oil on C. albicans in vitro and found that after 48 h of cultivation with L. angustifolia Mill essential oil, the number of fungal cells was low, indicating its antifungal effects (Behmanesh et al., 2015). Pinto et al. studied the components of Syzygium aromaticum (L.) Merr. and L.M.Perry essential oil and its antifungal activity and found that the essential oil and its main component eugenol could inhibit the formation of C. albicans bud tubes and had good antifungal activity (Pinto et al., 2009). Choonharuangdej et al. tested the bactericidal and inhibitory effects of Cinnamomum verum J. Presl essential oil and Cymbopogon citratus (DC.) Stapf essential oil on C. albicans in vitro and found that both essential oils could inhibit the formation of fungal biofilms (Choonharuangdej et al., 2021). Mat-Rani et al. studied the bactericidal effect of C. citratus (DC.) Stapf essential oil on C. albicans biofilm and found that 5% (v/v) essential oil could clear about 95% of the C. albicans biofilm, while 2.5% (v/v) essential oil had a better bactericidal effect on biofilm than a 20% (v/v) nystatin suspension. This indicates that C. citratus (DC.) Stapf essential oil has an obvious antifungal effect (Mat-Rani et al., 2021). Almeida et al. studied the effects of Cymbopogon winterianus Jowitt ex Bor and C. verum J. Presl essential oils on C. albicans biofilm and found that the minimum inhibitory concentration (MIC) of the two essential oils on C. albicans was 65 μg/mL and 250 μg/mL, respectively. Both oils significantly reduced the number of live bacteria and the biofilm area (Almeida et al., 2016). Banu et al. found that C. verum J. Presl essential oil destroyed the exopolysaccharide layer of Candida strains and inhibited the virulence of C. albicans (Banu et al., 2018). Filipowicz et al. studied the antifungal activity of Juniperus communis L. essential oil and found that it contained the highest concentration of (−)-α-pinene, p-cymene, and β-pinene, which had good antifungal properties. The MIC against C. albicans was 0.3 μg/mL (Filipowicz et al., 2003). Manoharan et al. studied the antibacterial activity of Cedrus deodara (Roxb. ex D. Don) G. Don essential oil on C. albicans and found that a 0.01% concentration of essential oil could reduce the biofilm formation of C. albicans by 87%. A 0.1% concentration of essential oil could completely stop the biofilm formation, indicating that C. deodara (Roxb. ex D. Don) G. Don essential oil has significant anti-C. albicans biofilm activity (Manoharan et al., 2017). Mahboubi et al. studied the chemical composition and antibacterial activity of Mentha piperita L. essential oil and found that it has a bactericidal effect on C. albicans (MIC = MLC = 0.125 μL/mL) (Mahboubi and Kazempour, 2014).
Essential oils usually contain dozens or even hundreds of chemical components. Their antibacterial activity is closely related to their chemical composition, especially some highly active chemical components. The antibacterial activity of essential oils primarily depends on their chemical functional groups. In 1996, Charai et al. reported that the activity of functional groups in plant essential oils was as follows: phenols (with the highest activity) > alcohols > aldehydes > ketones > esters > hydrocarbons (Charai et al., 1996). In 2003, Kalemba et al. summarized the antibacterial activity of functional groups in plant essential oils based on hundreds of previous studies as follows: phenols > cinnamaldehyde > alcohols > aldehydes = ketones > esters > hydrocarbons (Kalemba and Kunicka, 2003). Additionally, the same plant essential oils may show varying antibacterial and antifungal activities, suggesting that their diverse chemical components present in these oils contribute to different antibacterial and antifungal mechanisms. Table 1 presents essential oils against C. albicans, along with their main components.
The normal gut microbiota typically can suppress the proliferation of C. albicans. However, when the body’s immune system is compromised, antibiotics are used long-term, a poor diet is followed, or other diseases are present, the balance of gut microbiota may be disrupted. This disruption can result in an overgrowth of C. albicans, leading to conditions like Candida enteritis and related diseases. Several associations between C. albicans overgrowth and gut health issues include: (1) Candida Enteritis: This condition, marked by symptoms such as abdominal pain, diarrhea, indigestion, loss of appetite, weight loss, and inflammation, can negatively impact gut health (Rusu et al., 2020); (2) Leaky gut: Overgrowth of C. albicans may trigger “leaky gut”, characterized by damage and increased permeability of the intestinal mucosa, allowing harmful substances such as bacteria and toxins to enter the bloodstream, causing inflammation, immune system dysfunctions, and other health complications (Panpetch et al., 2020); (3) Effects on immune system: The overgrowth of C. albicans might disrupt immune system functions, interfering with immune cell activities and responses, subsequently elevating the risk of infections. Additionally, excessive growth of C. albicans can produce toxins that adversely affect the immune system (Tong and Tang, 2017); (4) Nutrient absorption problems: The overgrowth of C. albicans can impede the normal absorption of the intestine, affecting the nutritional status and overall health of the host (Martins et al., 2014).
Candida albicans infection may lead to an imbalance in the gut microbiota, which refers to a change in the proportion of beneficial and harmful bacteria in the microbiota. Under normal circumstances, the intestinal flora maintains a state of balance, with beneficial bacteria such as Lactobacillus and Bifidobacterium, inhibiting the growth of harmful bacteria, supporting the health of the intestinal mucosa, and providing nutritional support. However, C. albicans infection may result in an increase in harmful bacteria and a decrease in beneficial bacteria, potentially causing an imbalance in the flora. Given that C. albicans infection is a fungal infection, the number of fungi in the gut may increase significantly during infection, negatively impacting the diversity of flora, which signifies the richness and balance of various microorganisms in the flora (Cheng et al., 2023). Infection can cause alterations in the flora and an increase in dominant bacteria, ultimately lowering overall diversity (Wang et al., 2021). Furthermore, C. albicans infection may alter bacterial attachment and adhesion. The fungus can attach to the intestinal mucosa and form biofilms, creating a conducive environment for its growth and reproduction. This process may interfere with the ability of other bacteria to attach and adhere to the intestinal tract. The infection might also influence the intestinal immune system, potentially leading to an abnormal immune response triggering the activation of immune cells and an increase in inflammatory responses, further disrupting the balance of intestinal flora (Zeise et al., 2021). The impacts of C. albicans infection on intestinal flora are shown in Table 2.
Intestinal flora refers to the microbial community in the human digestive system, playing a critical role in human health and immune function. Imbalances in intestinal flora are associated with the occurrence and development of various diseases. Essential oils are believed to contain diverse active ingredients that could potentially influence gut microbiota and health, but further research is necessary to understand these mechanisms. The compounds found in essential oils can act as prebiotics, providing nutrients and a growth-friendly environment for beneficial bacteria. These ingredients can be used by beneficial bacteria in the gut to promote their growth and reproduction. For example, dietary fiber, a common prebiotic, shares similarities with certain components in essential oils such as natural polysaccharides. Kondapalli et al. conducted oral tests of Ocimum sanctum L., Zingiber officinale Roscoe, and Piper nigrum L. essential oil on healthy rats and found that the three essential oils had high prebiotic potential and could promote the growth of beneficial intestinal bacteria (Lactobacillus and Bifidobacterium) (Kondapalli et al., 2022). Babu et al. found that O. sanctum L., Z. officinale Roscoe, and P. nigrum L. extracts contain high concentrations of polyphenols, which have a proliferation effect on some intestinal microbiota and have high probiotic potential (Babu et al., 2018). Phenolic compounds or polyphenols are widely existing secondary metabolites in the plant kingdom, which can maintain the balance of intestinal microorganisms by stimulating the growth of beneficial bacteria (such as Lactobacillus and Bifidobacterium) and inhibiting pathogenic bacteria, and play a prebiotic role, thus contributing to the maintenance of intestinal health (Dueñas et al., 2015). Oligosaccharides in plant extracts have a prebiotic effect and contribute to the growth of Lactobacillus and Bifidobacterium and the inhibition of Bacteroides (Markowiak and Śliżewska, 2017). Leong et al. studied the regulatory effects of Pogostemon cablin (Blanco) Benth essential oil on intestinal microbiota in mice and found that P. cablin (Blanco) Benth essential oil had significant prebiotic-likes effects (Leong et al., 2019). Some of the ingredients in essential oils have antibacterial activity that can inhibit the growth and reproduction of harmful gut bacteria, thereby mitigating their detrimental effects and preserving the balance of intestinal flora. For example, camellia alcohol in M. alternifolia (Maiden and Betche) Cheel essential oil has a broad spectrum of antibacterial activity, effectively restraining the proliferation of various harmful bacteria. Studies have demonstrated that Foeniculum vulgare Mill. Seed essential oil has significant inhibitory effects on Acinetobacter baumannii, Escherichia coli, and Staphylococcus aureus (Barrahi et al., 2020). Additionally, O. vulgare L. subsp. Hirtum, C. verum J. Presl, S. aromaticum (L.) Merr. and L.M.Perry, Thymus vulgaris L., and M. alternifolia (Maiden and Betche) Cheel essential oils have strong antibacterial effects against Salmonella enterica and Listeria monocytogenes (Mazzarrino et al., 2015). Cymbopogon schoenanthus (L.) Spreng. Essential oil has a good antibacterial effect on E. coli, Staphylococcus aureus, and methicillin-sensitive staphylococcus aureus (Hashim et al., 2017). Certain components within essential oils can regulate the acid-base balance of the intestine, influencing the microecological environment of the gut. Some beneficial bacteria thrive in acidic environments, while harmful bacteria prefer alkaline environments. By adjusting the pH of the gut, essential oils can create a favorable environment for the growth of beneficial bacteria and inhibit the proliferation of harmful bacteria. Giannenas et al. evaluated the effects of dietary supplementation of benzoic acid or thymol and its essential oil mixture on growth performance of Turkey, and found that essential oil mixture decreased the pH values of the caecal content, increased lactic acid bacteria, and decreased coliform group (Giannenas et al., 2014). Dibner et al. studied the nutrition and metabolism of organic acids on intestinal flora and found that organic acids can reduce the pH values of intestinal digesta and participate in antibacterial activity (Dibner and Buttin, 2002). Moreover, specific components in essential oils have anti-inflammatory properties that aid in promoting intestinal health by reducing inflammatory responses. Ocimum basilicum L. essential oil, for instance, has been shown to mitigate tissue damage and myeloperoxidase (MPO) activity caused by colitis (Rashidian et al., 2016). Foeniculum vulgare Mill. Essential oil has been found to reduce histological lesions from colitis and impact the expression of MPO, tumor necrosis factor α, and nuclear factor-κB mucosal mRNA levels (Rezayat et al., 2018). Furthermore, some essential oils show positive effects on the growth and activity of beneficial bacteria, leading to an increase in beneficial bacteria populations while decreasing harmful ones. This reduction in intestinal oxidative stress is crucial for maintaining intestinal health. Research indicates that the number of Lactobacillus jejuni increased, while the number of Enterococcus and E. coli decreased in piglets treated with a carvhol-thymol mixture (Wei et al., 2017). Similarly, the administration of carvall essential oil to broilers showed a reduction in Salmonella and E. coli populations in their intestines (Liu et al., 2018). Sahoo et al. added Curcuma longa L. and Z. officinale Roscoe to the diets of broilers, it was observed that the growth of several pathogenic bacteria in the chickens’ intestines was restricted, ultimately contributing to balanced intestinal microflora and improved feed utilization (Sahoo et al., 2019). The regulatory effects of essential oils on gut health are shown in Supplementary Table S1.
The primary applications of essential oils in infections of C. albicans mainly involve the treatment of oral and skin infections. Here are the main applications of essential oils in these areas are as follows:
(1) Oral infection: (Ⅰ) M. alternifolia (Maiden and Betche) Cheel oil: M. alternifolia (Maiden and Betche) Cheel oil can be used as an oral mouthwash to treat oral infections of C. albicans. Its antifungal and antibacterial properties can reduce the growth and spread of C. albicans; (Ⅱ) S. aromaticum (L.); Merr. and L.M.Perry essential oil: S. aromaticum (L.) Merr. and L.M.Perry essential oil is effective in managing oral infections of C. albicans due to its potent antifungal and antibacterial effects.
(2) Skin infection: (Ⅰ) M. alternifolia (Maiden and Betche) Cheel oil: M. alternifolia (Maiden and Betche) Cheel oil can be topically applied to address skin infections of C. albicans such as candida dermatitis. It can be directly applied to affected areas, possessing antifungal and antibacterial effects; (Ⅱ) L. angustifolia Mill. Essential oil: L. angustifolia Mill. Essential oil is effective in treating skin infections of C. albicans. With its antifungal and anti-inflammatory properties, it can alleviate itching and reduce inflammatory responses; (Ⅲ) C. verum J. Presl essential oil: C. verum J. Presl essential oil can be topically used to manage skin infections of C. albicans. Despite its antifungal and antibacterial activity, caution should be exercised to prevent skin irritation; (Ⅳ) Other essential oils: F. vulgare Mill. Essential oil, Salvia rosmarinus Spenn. Essential oil, Cupressus funebris Endl. Essential oil, C. citratus (DC.) Stapf essential oil, and Eucalyptus robusta Sm. Essential oil are also beneficial for the topical treatment of skin infections of C. albicans. They possess antifungal and antibacterial properties that can relieve symptoms and aid in wound healing.
It's important to note that the application of essential oils should follow the appropriate dilution ratio and application method. In cases of oral infections, essential oils can be diluted and used as a mouthwash. Regarding skin infections, essential oils should be mixed with a carrier oil and gently applied to the affected area. It is best to perform a skin sensitivity test before usage and it is essential to adhere to the recommended guidelines for application. Although essential oils show some potential in the treatment of infections of C. albicans, further research is necessary to validate their effectiveness and safety. The mechanism diagrams of the Intestinal regulation of essential oils and Clinical application are shown in Figure 1.
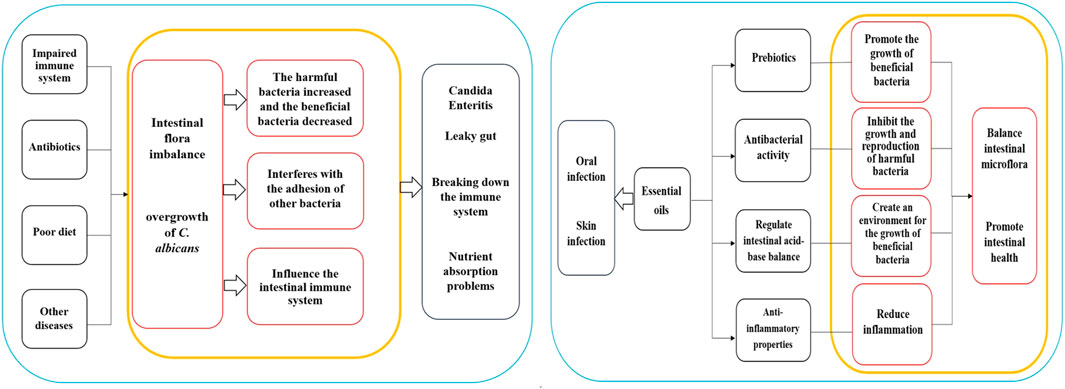
Figure 1. Mechanism diagrams of the Intestinal regulation of essential oils and Clinical application.
As environmental regulations become more stringent, there is a growing consensus on the need to reduce and replace resistance. People are growing more health-conscious and environmentally aware, leading to a surge in interest in promoting natural and eco-friendly plant essential oils as a burgeoning research Frontier and focal point of study. Presently, numerous scholars both domestically and internationally have conducted extensive research on essential oils, focusing particularly on their physical and chemical properties, biological activities, mechanisms of action, etc. However, the majority of studies regarding the antibacterial activity of essential oils are conducted based on the MIC of these oils against bacteria or fungi. There is no systematic and thorough exploration into the antibacterial kinetics and mechanisms related to essential oils, indicating considerable groundwork to be undertaken. Specifically, understanding the mechanisms behind the antibacterial and antifungal effects of essential oils, exploring potential synergistic antibacterial properties among different oils, and investigating the relationship between chemical components require further investigation.
Essential oils represent a promising biological resource sourced from a wide variety of origins, known for their low toxicity and minimal side effects. The future industrialization of essential oils should not be confined to specific sectors such as traditional medicine, food, or daily necessities. Instead, it should incorporate a multidisciplinary, multi-directional, and versatile approach into its development. With the continuous advancement of various separation and detection technologies, the composition, structure, and functions of plant essential oils are becoming increasingly elucidated, leading to expanded applications and developmental opportunities in areas such as medicine, healthcare products, disease and pest prevention, food, and environmental protection (Rath, 2007; Pavela and Benelli, 2016).
Essential oils have demonstrated their ability to combat the infections of C. albicans through various antibacterial mechanisms and by regulating intestinal flora. Further research and clinical trials on essential oils are imperative to comprehensively assess their efficacy and safety as a viable treatment approach for the infections caused by C. albicans.
GH: Investigation, Writing–original draft. TH: Investigation, Methodology, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the GuangDong Basic and Applied Basic Research Foundation (Grant No. SL2022A04J01333), Xingning Meat Pigeon Industrial Park Research Foundation (Grant No. D122222G903) and Key Realm R&D Program of GuangDong Province (Grant No. 2020B0202080002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1400105/full#supplementary-material
Abo Ghanima, M. M., Elsadek, M. F., Taha, A. E., Abd El-Hack, M. E., Alagawany, M., Ahmed, B. M., et al. (2020). Effect of housing system and rosemary and cinnamon essential oils on layers performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals 10, 245. doi:10.3390/ani10020245
Abouelezz, K., Abou-Hadied, M., Yuan, J., Elokil, A. A., Wang, G., Wang, S., et al. (2019). Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal 13, 2216–2222. doi:10.1017/S1751731119000508
Acar, Ü., Kesbiç, O. S., Yılmaz, S., İnanan, B. E., Zemheri-Navruz, F., Terzi, F., et al. (2021). Effects of essential oil derived from the bitter orange (Citrus aurantium) on growth performance, histology and gene expression levels in common carp juveniles (Cyprinus carpio). Animals 11, 1431. doi:10.3390/ani11051431
Al-Maharik, N., Jaradat, N., Hawash, M., Al-Lahham, S., Qadi, M., Shoman, I., et al. (2022). Chemical composition, antioxidant, antimicrobial and anti-proliferative activities of essential oils of rosmarinus officinalis from five different sites in Palestine. Separations 9, 339. doi:10.3390/separations9110339
Almeida, L., de, F. D. de, Paula, J. F. de, Almeida, R. V. D. de, Williams, D. W., Hebling, J., et al. (2016). Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. Acta Odontol. Scand. 74, 393–398. doi:10.3109/00016357.2016.1166261
Babu, K. N., Hemalatha, R., Satyanarayana, U., Shujauddin, M., Himaja, N., Bhaskarachary, K., et al. (2018). Phytochemicals, polyphenols, prebiotic effect of Ocimum sanctum, Zingiber officinale, Piper nigrum extracts. J. Herb. Med. 13, 42–51. doi:10.1016/j.hermed.2018.05.001
Bakkali, F., Averbeck, S., Averbeck, D., and Idaomar, M. (2008). Biological effects of essential oils–a review. Food Chem. Toxicol. 46, 446–475. doi:10.1016/j.fct.2007.09.106
Balkis, M. M., Leidich, S. D., Mukherjee, P. K., and Ghannoum, M. A. (2002). Mechanisms of fungal resistance: an overview. Drugs 62, 1025–1040. doi:10.2165/00003495-200262070-00004
Banu, S. F., Rubini, D., Shanmugavelan, P., Murugan, R., Gowrishankar, S., Pandian, S. K., et al. (2018). Effects of patchouli and cinnamon essential oils on biofilm and hyphae formation by Candida species. J. de Mycol. medicale 28, 332–339. doi:10.1016/j.mycmed.2018.02.012
Barbarestani, S. Y., Jazi, V., Mohebodini, H., Ashayerizadeh, A., Shabani, A., and Toghyani, M. (2020). Effects of dietary lavender essential oil on growth performance, intestinal function, and antioxidant status of broiler chickens. Livest. Sci. 233, 103958. doi:10.1016/j.livsci.2020.103958
Barrahi, M., Esmail, A., Elhartiti, H., Chahboun, N., Benali, A., Amiyare, R., et al. (2020). Chemical composition and evaluation of antibacterial activity of fennel (Foeniculum vulgare Mill) seed essential oil against some pathogenic bacterial strains. Casp. J. Environ. Sci. 18, 295–307. doi:10.22124/cjes.2020.4276
Behmanesh, F., Pasha, H., Sefidgar, A. A., Taghizadeh, M., Moghadamnia, A. A., Adib Rad, H., et al. (2015). Antifungal effect of lavender essential oil (Lavandula angustifolia) and clotrimazole on Candida albicans: an in vitro study. Scientifica 2015, 261397. doi:10.1155/2015/261397
Bertolini, M., Ranjan, A., Thompson, A., Diaz, P. I., Sobue, T., Maas, K., et al. (2019). Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection. PLoS Pathog. 15, e1007717. doi:10.1371/journal.ppat.1007717
Blanco, J. L., and Garcia, M. E. (2008). Immune response to fungal infections. Veterinary Immunol. Immunopathol. 125, 47–70. doi:10.1016/j.vetimm.2008.04.020
Charai, M., Mosaddak, M., and Faid, M. (1996). Chemical composition and antimicrobial activities of two aromatic plants: Origanum majorana L. and O. compactum Benth. J. Essent. Oil Res. 8, 657–664. doi:10.1080/10412905.1996.9701036
Chen, Y., Wang, J., Yu, L., Xu, T., and Zhu, N. (2020). Microbiota and metabolome responses in the cecum and serum of broiler chickens fed with plant essential oils or virginiamycin. Sci. Rep. 10, 5382. doi:10.1038/s41598-020-60135-x
Cheng, T., Xu, C., Wu, D., Yan, G., Wang, C., Wang, T., et al. (2023). Sodium houttuyfonate derived from Houttuynia cordata Thunb improves intestinal malfunction via maintaining gut microflora stability in Candida albicans overgrowth aggravated ulcerative colitis. Food and Funct. 14, 1072–1086. doi:10.1039/d2fo02369e
Choonharuangdej, S., Srithavaj, T., and Thummawanit, S. (2021). Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: an in vitro study. J. Prosthet. Dent. 125, 707. e1–e707.e6. doi:10.1016/j.prosdent.2020.12.017
Chowdhury, S., Mandal, G. P., Patra, A. K., Kumar, P., Samanta, I., Pradhan, S., et al. (2018). Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Animal Feed Sci. Technol. 236, 39–47. doi:10.1016/j.anifeedsci.2017.12.003
Cid-Chevecich, C., Müller-Sepúlveda, A., Jara, J. A., López-Muñoz, R., Santander, R., Budini, M., et al. (2022). Origanum vulgare L. essential oil inhibits virulence patterns of Candida spp. and potentiates the effects of fluconazole and nystatin in vitro. BMC Complementary Med. Ther. 22, 39. doi:10.1186/s12906-022-03518-z
Cowen, L. E., Sanglard, D., Howard, S. J., Rogers, P. D., and Perlin, D. S. (2015). Mechanisms of antifungal drug resistance. Cold Spring Harb. Perspect. Med. 5, a019752. doi:10.1101/cshperspect.a019752
D’auria, F. D., Tecca, M., Strippoli, V., Salvatore, G., Battinelli, L., and Mazzanti, G. (2005). Antifungal activity of Lavandula angustifolia essential oil against Candida albicans yeast and mycelial form. Med. Mycol. 43, 391–396. doi:10.1080/13693780400004810
Dhifi, W., Bellili, S., Jazi, S., Bahloul, N., and Mnif, W. (2016). Essential oils’ chemical characterization and investigation of some biological activities: a critical review. Medicines 3, 25. doi:10.3390/medicines3040025
Dibner, J. J., and Buttin, P. (2002). Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J. Appl. Poult. Res. 11, 453–463. doi:10.1093/japr/11.4.453
Dueñas, M., Muñoz-González, I., Cueva, C., Jiménez-Girón, A., Sánchez-Patán, F., Santos-Buelga, C., et al. (2015). A survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 850902. doi:10.1155/2015/850902
Ebani, V. V., Nardoni, S., Bertelloni, F., Giovanelli, S., Rocchigiani, G., Pistelli, L., et al. (2016). Antibacterial and antifungal activity of essential oils against some pathogenic bacteria and yeasts shed from poultry. Flavour Fragr. J. 31, 302–309. doi:10.1002/ffj.3318
Eckstein, M.-T., Moreno-Velásquez, S. D., and Pérez, J. C. (2020). Gut bacteria shape intestinal microhabitats occupied by the fungus Candida albicans. Curr. Biol. 30, 4799–4807. e4. doi:10.1016/j.cub.2020.09.027
Espinosa-Hernández, V. M., Morales-Pineda, V., and Martínez-Herrera, E. (2020). Skin infections caused by emerging Candida species. Curr. Fungal Infect. Rep. 14, 99–105. doi:10.1007/s12281-020-00380-9
Essid, R., Ayed, A., Djebali, K., Saad, H., Srasra, M., Othmani, Y., et al. (2023). Anti-Candida and anti-leishmanial activities of encapsulated Cinnamomum verum essential oil in chitosan nanoparticles. Molecules 28, 5681. doi:10.3390/molecules28155681
Feng, J., Lu, M., Wang, J., Zhang, H., Qiu, K., Qi, G., et al. (2021). Dietary oregano essential oil supplementation improves intestinal functions and alters gut microbiota in late-phase laying hens. J. Animal Sci. Biotechnol. 12, 72–15. doi:10.1186/s40104-021-00600-3
Filipowicz, N., Kamiński, M., Kurlenda, J., Asztemborska, M., and Ochocka, J. R. (2003). Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytotherapy Res. 17, 227–231. doi:10.1002/ptr.1110
Gao, S., Liu, G., Li, J., Chen, J., Li, L., Li, Z., et al. (2020). Antimicrobial activity of lemongrass essential oil (Cymbopogon flexuosus) and its active component citral against dual-species biofilms of Staphylococcus aureus and Candida species. Front. Cell. Infect. Microbiol. 10, 603858. doi:10.3389/fcimb.2020.603858
Ge, C., Luo, X., Wu, L., Lv, Y., Hu, Z., Yu, D., et al. (2023). Plant essential oils improve growth performance by increasing antioxidative capacity, enhancing intestinal barrier function, and modulating gut microbiota in Muscovy ducks. Poult. Sci. 102, 102813. doi:10.1016/j.psj.2023.102813
Giannenas, I., Papaneophytou, C. P., Tsalie, E., Pappas, I., Triantafillou, E., Tontis, D., et al. (2014). Dietary supplementation of benzoic acid and essential oil compounds affects buffering capacity of the feeds, performance of Turkey poults and their antioxidant status, pH in the digestive tract, intestinal microbiota and morphology. Asian-Australasian J. Animal Sci. 27, 225–236. doi:10.5713/ajas.2013.13376
Gonçalves, B., Ferreira, C., Alves, C. T., Henriques, M., Azeredo, J., and Silva, S. (2016). Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 42, 905–927. doi:10.3109/1040841X.2015.1091805
Gutierrez, D., Weinstock, A., Antharam, V. C., Gu, H., Jasbi, P., Shi, X., et al. (2020). Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to Candida albicans colonization in the gastrointestinal tract. FEMS Microbiol. Ecol. 96, fiz187. doi:10.1093/femsec/fiz187
Hall, H. N., Wilkinson, D. J., and Le Bon, M. (2021). Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim. microbiome 3, 2–17. doi:10.1186/s42523-020-00064-2
Hammer, K. A., Carson, C. F., and Riley, T. V. (1998). In-vitro activity of essential oils, in particular Melaleuca alternifolia (tea tree) oil and tea tree oil products, against Candida spp. J. Antimicrob. Chemother. 42, 591–595. doi:10.1093/jac/42.5.591
Hammer, K. A., Carson, C. F., and Riley, T. V. (2004). Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 53, 1081–1085. doi:10.1093/jac/dkh243
Hashim, G. M., Almasaudi, S. B., Azhar, E., Al Jaouni, S. K., and Harakeh, S. (2017). Biological activity of Cymbopogon schoenanthus essential oil. Saudi J. Biol. Sci. 24, 1458–1464. doi:10.1016/j.sjbs.2016.06.001
Hazrati, S., Rezaeipour, V., and Asadzadeh, S. (2020). Effects of phytogenic feed additives, probiotic and mannan-oligosaccharides on performance, blood metabolites, meat quality, intestinal morphology, and microbial population of Japanese quail. Br. Poult. Sci. 61, 132–139. doi:10.1080/00071668.2019.1686122
Hekmatpanah, A., Sharifzadeh, A., Shokri, H., and Nikaein, D. (2022). Efficacy of Syzygium aromaticum essential oil on the growth and enzymatic activity of pathogenic Candida albicans strains. Curr. Med. Mycol. 8, 12–19. doi:10.18502/cmm.8.1.9209
Hossrini, R. M., Teymouri, F., Arjomandzadegan, M., and Zolfaghari, M. R. (2022). Effects of zataria multiflora, Mentha longifolia, and Origanum vulgare plant essential oils on the inhibition of Candida albicans. Herb. Med. J. (Herb Med J) 7, 103–109. doi:10.22087/hmj.v7i3.1023
Houšť, J., Spížek, J., and Havlíček, V. (2020). Antifungal drugs. Metabolites 10, 106. doi:10.3390/metabo10030106
Hu, W., Huang, L., Zhou, Z., Yin, L., and Tang, J. (2022). Diallyl Disulfide (DADS) ameliorates intestinal candida albicans infection by modulating the gut microbiota and metabolites and providing intestinal protection in mice. Front. Cell. Infect. Microbiol. 11, 743454. doi:10.3389/fcimb.2021.743454
Hu, W., Xu, D., Zhou, Z., Zhu, J., Wang, D., and Tang, J. (2021). Alterations in the gut microbiota and metabolic profiles coincide with intestinal damage in mice with a bloodborne Candida albicans infection. Microb. Pathog. 154, 104826. doi:10.1016/j.micpath.2021.104826
Huong, L. T., Chau, D. T. M., An, N. T. G., Dai, D. N., Giwa-Ajeniya, A. O., and Ogunwande, I. A. (2024). Essential oils of Lauraceae: antimicrobial activity and constituents of essential oil from two Machilus species from Vietnam. J. Essent. Oil Bear. Plants 27, 177–187. doi:10.1080/0972060X.2023.2291455
Hurtado, R., Peltroche, N., Mauricio, F., Gallo, W., Alvítez-Temoche, D., Vilchez, L., et al. (2020). Antifungal efficacy of four different concentrations of the essential oil of Cinnamomum zeylanicum (canela) against Candida albicans: an in vitro study. J. Int. Soc. Prev. Community Dent. 10, 724–730. doi:10.4103/jispcd.JISPCD_251_20
Imbabi, T., Sabeq, I., Osman, A., Mahmoud, K., Amer, S. A., Hassan, A. M., et al. (2021). Impact of fennel essential oil as an antibiotic alternative in rabbit diet on antioxidant enzymes levels, growth performance, and meat quality. Antioxidants 10, 1797. doi:10.3390/antiox10111797
Jautová, J., Virágová, S., Ondraŝoviĉ, M., and Holoda, E. (2001). Incidence of Candida species isolated from human skin and nails: a survey. Folia Microbiol. 46, 333–337. doi:10.1007/BF02815623
Kalemba, D., and Kunicka, A. (2003). Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 10, 813–829. doi:10.2174/0929867033457719
Kaskatepe, B., Aslan Erdem, S., Ozturk, S., Safi Oz, Z., Subasi, E., Koyuncu, M., et al. (2022). Antifungal and anti-virulent activity of Origanum majorana L. Essential oil on Candida albicans and in vivo toxicity in the Galleria mellonella larval model. Molecules 27, 663. doi:10.3390/molecules27030663
Kathiravan, M. K., Salake, A. B., Chothe, A. S., Dudhe, P. B., Watode, R. P., Mukta, M. S., et al. (2012). The biology and chemistry of antifungal agents: a review. Bioorg. Med. Chem. 20, 5678–5698. doi:10.1016/j.bmc.2012.04.045
Khoshbakhtt, S., Taliei, F., Bezdi, K. G., and Gharakhlo, S. (2023). Anti-fungal effects of essential oil and nano-emulsion of some medicinal plants on the human pathogenic fungus Candida albicansAntifungal potential of essential oil nanoemulsions: antifungal potential of essential oil nanoemulsions. Iran. J. Pharm. Sci. 19, 37–49. doi:10.22037/ijps.v19.42201
Kırkpınar, F., Ünlü, H. B., and Özdemir, G. (2011). Effects of oregano and garlic essential oils on performance, carcase, organ and blood characteristics and intestinal microflora of broilers. Livest. Sci. 137, 219–225. doi:10.1016/j.livsci.2010.11.010
Kondapalli, N. B., Hemalatha, R., Uppala, S., Yathapu, S. R., Mohammed, S., Venkata Surekha, M., et al. (2022). Ocimum sanctum, Zingiber officinale, and Piper nigrum extracts and their effects on gut microbiota modulations (prebiotic potential), basal inflammatory markers and lipid levels: oral supplementation study in healthy rats. Pharm. Biol. 60, 437–450. doi:10.1080/13880209.2022.2033797
Leong, W., Huang, G., Khan, I., Xia, W., Li, X., Su, Z., et al. (2019). Patchouli essential oil and its derived compounds revealed prebiotic-like effects in C57BL/6J mice. Front. Pharmacol. 10, 1229. doi:10.3389/fphar.2019.01229
Li, A., Ni, W., Zhang, Q., Li, Y., Zhang, X., Wu, H., et al. (2020a). Effect of cinnamon essential oil on gut microbiota in the mouse model of dextran sodium sulfate-induced colitis. Microbiol. Immunol. 64, 23–32. doi:10.1111/1348-0421.12749
Li, C., Niu, J., Liu, Y., Li, F., and Liu, L. (2021). The effects of oregano essential oil on production performance and intestinal barrier function in growing Hyla rabbits. Italian J. Animal Sci. 20, 2165–2173. doi:10.1080/1828051x.2021.2005471
Li, M., Li, C., Wu, X., Chen, T., Ren, L., Xu, B., et al. (2020b). Microbiota-driven interleukin-17 production provides immune protection against invasive candidiasis. Crit. Care 24, 268. doi:10.1186/s13054-020-02977-5
Li, P., Piao, X., Ru, Y., Han, X., Xue, L., and Zhang, H. (2012a). Effects of adding essential oil to the diet of weaned pigs on performance, nutrient utilization, immune response and intestinal health. Asian-Australasian J. Animal Sci. 25, 1617–1626. doi:10.5713/ajas.2012.12292
Li, S. Y., Ru, Y. J., Liu, M., Xu, B., Péron, A., and Shi, X. G. (2012b). The effect of essential oils on performance, immunity and gut microbial population in weaner pigs. Livest. Sci. 145, 119–123. doi:10.1016/j.livsci.2012.01.005
Liu, S., Song, M., Yun, W., Lee, J., Lee, C., Kwak, W., et al. (2018). Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. animal physiology animal Nutr. 102, 1257–1265. doi:10.1111/jpn.12944
Magouz, F. I., Amer, A. A., Faisal, A., Sewilam, H., Aboelenin, S. M., and Dawood, M. A. (2022). The effects of dietary oregano essential oil on the growth performance, intestinal health, immune, and antioxidative responses of Nile tilapia under acute heat stress. Aquaculture 548, 737632. doi:10.1016/j.aquaculture.2021.737632
Mahboubi, M., and Kazempour, N. (2014). Chemical composition and antimicrobial activity of peppermint (Mentha piperita L.) Essential oil. Songklanakarin J. Sci. Technol. 36, 83–87.
Mancianti, F., and Ebani, V. V. (2020). Biological activity of essential oils. Molecules 25, 678. doi:10.3390/molecules25030678
Manoharan, R. K., Lee, J.-H., and Lee, J. (2017). Antibiofilm and antihyphal activities of cedar leaf essential oil, camphor, and fenchone derivatives against Candida albicans. Front. Microbiol. 8, 1476. doi:10.3389/fmicb.2017.01476
Marino, A., Nostro, A., Mandras, N., Roana, J., Ginestra, G., Miceli, N., et al. (2020). Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complementary Med. Ther. 20, 89. doi:10.1186/s12906-020-2877-x
Markowiak, P., and Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9, 1021. doi:10.3390/nu9091021
Martins, N., Ferreira, I. C., Barros, L., Silva, S., and Henriques, M. (2014). Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 177, 223–240. doi:10.1007/s11046-014-9749-1
Mason, K. L., Erb Downward, J. R., Mason, K. D., Falkowski, N. R., Eaton, K. A., Kao, J. Y., et al. (2012). Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect. Immun. 80, 3371–3380. doi:10.1128/IAI.00449-12
Mat-Rani, S., Chotprasert, N., Srimaneekarn, N., and Choonharuangdej, S. (2021). Fungicidal effect of lemongrass essential oil on Candida albicans biofilm pre-established on maxillofacial silicone specimens. J. Int. Soc. Prev. Community Dent. 11, 525–530. doi:10.4103/jispcd.JISPCD_63_21
Mazzarrino, G., Paparella, A., Chaves-López, C., Faberi, A., Sergi, M., Sigismondi, C., et al. (2015). Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food control. 50, 794–803. doi:10.1016/j.foodcont.2014.10.029
Mendonça, S. C., Aazza, S., Carvalho, A. A. de, Silva, D. M. da, Oliveira, N., de, M. S., et al. (2023). Biological screening of herbal extracts and essential oil from Plectranthus species: α-amylase and 5-lipoxygenase inhibition and antioxidant and anti-Candida potentials. Braz. J. Pharm. Sci. 59, e21117. doi:10.1590/s2175-97902023e21117
Miceli, M. H., Díaz, J. A., and Lee, S. A. (2011). Emerging opportunistic yeast infections. Lancet Infect. Dis. 11, 142–151. doi:10.1016/S1473-3099(10)70218-8
Millsop, J. W., and Fazel, N. (2016). Oral candidiasis. Clin. dermatology 34, 487–494. doi:10.1016/j.clindermatol.2016.02.022
Mirzaii, M., Yaeghoobi, M., Afzali, M., Amirkhalili, N., Mahmoodi, M., and Sajirani, E. B. (2021). Antifungal activities of quince seed mucilage hydrogel decorated with essential oils of Nigella sativa, Citrus sinensis and Cinnamon verum. Iran. J. Microbiol. 13, 352–359. doi:10.18502/ijm.v13i3.6398
Moran, G., Coleman, D., and Sullivan, D. (2011). An introduction to the medically important Candida species. Candida candidiasis, 9–25. doi:10.1128/9781555817176.ch2
Nguyen-Ngoc, H., Giang, L. D., Tran-Trung, H., Thang, T. D., Tuan, N. H., Nguyen, D. K., et al. (2024). Chemical constituents and in vitro antimicrobial activity of rhizome essential oils of Zingiber densissimum S.Q.Tong and Y.M.Xia and Kaempferia laotica Gagnep. growing wild in Vietnam. J. Essent. Oil Bear. Plants 27, 73–81. doi:10.1080/0972060X.2024.2307905
Noumi, E., Alshammari, G. S., Zmantar, T., Bazaid, A. S., Alabbosh, K. F., Elasbali, A. M., et al. (2022). Antibiofilm potential and exoenzyme inhibition by Elattaria cardamomum essential oil in Candida spp. strains. Strains. Life 12, 1756. doi:10.3390/life12111756
Oliveira, D. P. de, Frota, L. S., Barbosa, S. I., Lopes, F. F., Neto, J. R., Marinho, M. M., et al. (2024). CG-MS, radical scavenging, acetylcholinesterase inhibition, antifungal and molecular docking studies of essential oil from the leaves of tapirira guianensis aubl. Archives Curr. Res. Int. 24, 80–96. doi:10.9734/acri/2024/v24i2635
Omonijo, F. A., Ni, L., Gong, J., Wang, Q., Lahaye, L., and Yang, C. (2018). Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 4, 126–136. doi:10.1016/j.aninu.2017.09.001
Pan, C.-H., Lo, H.-J., Yan, J.-Y., Hsiao, Y.-J., Hsueh, J.-W., Lin, D.-W., et al. (2021). Candida albicans colonizes and disseminates to the gastrointestinal tract in the presence of the microbiota in a severe combined immunodeficient mouse model. Front. Microbiol. 11, 619878. doi:10.3389/fmicb.2020.619878
Pandey, A. K., Kumar, P., Singh, P., Tripathi, N. N., and Bajpai, V. K. (2017). Essential oils: sources of antimicrobials and food preservatives. Front. Microbiol. 7, 2161. doi:10.3389/fmicb.2016.02161
Panpetch, W., Hiengrach, P., Nilgate, S., Tumwasorn, S., Somboonna, N., Wilantho, A., et al. (2020). Additional Candida albicans administration enhances the severity of dextran sulfate solution induced colitis mouse model through leaky gut-enhanced systemic inflammation and gut-dysbiosis but attenuated by Lactobacillus rhamnosus L34. Gut microbes 11, 465–480. doi:10.1080/19490976.2019.1662712
Panpetch, W., Kullapanich, C., Dang, C. P., Visitchanakun, P., Saisorn, W., Wongphoom, J., et al. (2021). Candida administration worsens uremia-induced gut leakage in bilateral nephrectomy mice, an impact of gut fungi and organismal molecules in uremia. Msystems 6, e01187. doi:10.1128/msystems.01187-20
Pavela, R., and Benelli, G. (2016). Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends plant Sci. 21, 1000–1007. doi:10.1016/j.tplants.2016.10.005
Pinto, E., Vale-Silva, L., Cavaleiro, C., and Salgueiro, L. (2009). Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 58, 1454–1462. doi:10.1099/jmm.0.010538-0
Qu, S.-S., Zhang, Y., Ren, J.-N., Yang, S.-Z., Li, X., Fan, G., et al. (2023). Effect of different ways of ingesting orange essential oil on blood immune index and intestinal microflora in mice. J. Sci. Food Agric. 103, 380–388. doi:10.1002/jsfa.12152
Rajali, A., Zain, N. M., Amran, N. A., and Azmi, N. H. E. M. (2023). Antifungal efficacy of Ocimum basilicum essential oil in tissue conditioner against Candida albicans: an in vitro study. Contemp. Clin. Dent. 14, 115–122. doi:10.4103/ccd.ccd_654_21
Rashidian, A., Roohi, P., Mehrzadi, S., Ghannadi, A. R., and Minaiyan, M. (2016). Protective effect of Ocimum basilicum essential oil against acetic acid–induced colitis in rats. J. evidence-based complementary Altern. Med. 21, NP36–NP42. doi:10.1177/2156587215616550
Rath, C. C. (2007). Prospects and challenges of essential oils as natural food preservatives—a review. Food 1, 172–180.
Rezayat, S. M., Dehpour, A.-R., Motamed, S. M., Yazdanparast, M., Chamanara, M., Sahebgharani, M., et al. (2018). Foeniculum vulgare essential oil ameliorates acetic acid-induced colitis in rats through the inhibition of NF-kB pathway. Inflammopharmacology 26, 851–859. doi:10.1007/s10787-017-0409-1
Rhimi, W., Mohammed, M. A., Zarea, A. A., Greco, G., Tempesta, M., Otranto, D., et al. (2022). Antifungal, antioxidant and antibiofilm activities of essential oils of cymbopogon spp. Antibiotics 11, 829. doi:10.3390/antibiotics11060829
Rodríguez-Cerdeira, C., Gregorio, M. C., Molares-Vila, A., López-Barcenas, A., Fabbrocini, G., Bardhi, B., et al. (2019). Biofilms and vulvovaginal candidiasis. Colloids Surfaces B Biointerfaces 174, 110–125. doi:10.1016/j.colsurfb.2018.11.011
Ruan, D., Fan, Q., Fouad, A. M., Sun, Y., Huang, S., Wu, A., et al. (2021). Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity, and intestinal microbiota in yellow-feathered chickens. J. Animal Sci. 99, skab033. doi:10.1093/jas/skab033
Rusu, A. V., Penedo, B. A., Schwarze, A.-K., and Trif, M. (2020). “The influence of Candida spp. in intestinal microbiota; diet therapy, the emerging conditions related to candida in athletes and elderly people,” in Update in geriatrics. (IntechOpen).
Ruzauskas, M., Bartkiene, E., Stankevicius, A., Bernatoniene, J., Zadeike, D., Lele, V., et al. (2020). The influence of essential oils on gut microbial profiles in pigs. Animals 10, 1734. doi:10.3390/ani10101734
Sadeghi, N., Sadeghi, H., Mohan, D. N., Sepahvand, A., Alizadeh, A., and Garavand, S. (2023). Chemical composition, anti-fungal and cytotoxic effects of Ferula macrecolea essential oil against Candida albicans resistant and sensitive strains. J. Herbmed Pharmacol. 12, 228–232. doi:10.34172/jhp.2023.24
Sahoo, N., Mishra, S., Swain, R., Acharya, A., Pattnaik, S., Sethy, K., et al. (2019). Effect of turmeric and ginger supplementation on immunity, antioxidant, liver enzyme activity, gut bacterial load and histopathology of broilers. Indian J. Anim. Sci. 9, 774–779. doi:10.56093/ijans.v89i7.92046
Shahina, Z., Molaeitabari, A., Sultana, T., and Dahms, T. E. S. (2022). Cinnamon leaf and clove essential oils are potent inhibitors of Candida albicans virulence traits. Microorganisms 10, 1989. doi:10.3390/microorganisms10101989
Shao, Y., Peng, Q., Wu, Y., Peng, C., Wang, S., Zou, L., et al. (2023). The effect of an essential oil blend on growth performance, intestinal health, and microbiota in early-weaned piglets. Nutrients 15, 450. doi:10.3390/nu15020450
Sidiropoulou, E., Skoufos, I., Marugan-Hernandez, V., Giannenas, I., Bonos, E., Aguiar-Martins, K., et al. (2020). In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front. Veterinary Sci. 7, 420. doi:10.3389/fvets.2020.00420
Soltani, F. Z., Meddah, B., Chelli, N., Tir Touil, A., and Sonnet, P. (2023). Atriplex halimus L. And Centaurium erythraea rafn. Essential oils: the phytochemical profile, antimicrobial and antioxidant properties. Agric. Conspec. Sci. 88, 215–223.
Spisni, E., Petrocelli, G., Imbesi, V., Spigarelli, R., Azzinnari, D., Donati Sarti, M., et al. (2020). Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: implications in colonic pathophysiology. Int. J. Mol. Sci. 21, 4152. doi:10.3390/ijms21114152
Sun, J., Cheng, Z., Zhao, Y., Wang, Y., Wang, H., and Ren, Z. (2022). Influence of increasing levels of oregano essential oil on intestinal morphology, intestinal flora and performance of Sewa sheep. Italian J. Animal Sci. 21, 463–472. doi:10.1080/1828051x.2022.2048208
Tadić, V., Božović, M., Sapienza, F., Astolfi, R., Mladenović, M., Zaka, M. C., et al. (2023). Chemical composition and anti-Candida activity of Mentha suaveolens ehrh. Essential oils obtained by different distillation processes. Molecules 28, 6934. doi:10.3390/molecules28196934
Tchinang, F. T. K., Ndoyé Foé, F. M.-C., Keumoe, R., Zeuko’o, E. M., Fekam, F. B., and Etoa, F.-X. (2023). In vitro anti-yeast activity, kinetics and mechanism of action of essential oils from two cameroonian medicinal plants. BMC Complementary Med. Ther. 23, 115. doi:10.1186/s12906-022-03827-3
Tiihonen, K., Kettunen, H., Bento, M. H. L., Saarinen, M., Lahtinen, S., Ouwehand, A. C., et al. (2010). The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 51, 381–392. doi:10.1080/00071668.2010.496446
Tomičić, Z., Tomičić, R., Možina, S. S., Bucar, F., Turek, I., and Raspor, P. (2022). Antifungal and anti-adhesion activity of plant extracts and essential oils against Candida spp. and Pichia spp. J. Food Nutr. Res. 61.
Tong, Y., and Tang, J. (2017). Candida albicans infection and intestinal immunity. Microbiol. Res. 198, 27–35. doi:10.1016/j.micres.2017.02.002
Tran, H. N., Graham, L., and Adukwu, E. C. (2020). In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris. Appl. Microbiol. Biotechnol. 104, 8911–8924. doi:10.1007/s00253-020-10829-z
Valdivieso-Ugarte, M., Gomez-Llorente, C., Plaza-Díaz, J., and Gil, Á. (2019). Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: a systematic review. Nutrients 11, 2786. doi:10.3390/nu11112786
Vila, T., Sultan, A. S., Montelongo-Jauregui, D., and Jabra-Rizk, M. A. (2020). Oral candidiasis: a disease of opportunity. J. Fungi 6, 15. doi:10.3390/jof6010015
Wang, F., Ye, Y., Xin, C., Liu, F., Zhao, C., Xiang, L., et al. (2021). Candida albicans triggers qualitative and temporal responses in gut bacteria. J. Med. Mycol. 31, 101164. doi:10.1016/j.mycmed.2021.101164
Wang, X., Wu, S., Li, L., Yan, Z., Jin, Y., Hu, H., et al. (2023). Controllable synthesis, formation mechanism, and photocatalytic activity of tellurium with various nanostructures. Mycology 15, 1–13. doi:10.3390/mi15010001
Wang, Z., Yin, L., Qi, Y., Zhang, J., Zhu, H., and Tang, J. (2022). Intestinal flora-derived kynurenic acid protects against intestinal damage caused by Candida albicans infection via activation of aryl hydrocarbon receptor. Front. Microbiol. 13, 934786. doi:10.3389/fmicb.2022.934786
Wei, H.-K., Xue, H.-X., Zhou, Z. X., and Peng, J. (2017). A carvacrol–thymol blend decreased intestinal oxidative stress and influenced selected microbes without changing the messenger RNA levels of tight junction proteins in jejunal mucosa of weaning piglets. Animal 11, 193–201. doi:10.1017/S1751731116001397
Weng, Y.-X., Wang, H.-C., Chu, Y.-L., Wu, Y.-Z., Liao, J.-A., and Su, Z.-Y. (2024). Essential oil from Citrus depressa peel exhibits antimicrobial, antioxidant and cancer chemopreventive effects. J. Sci. Food Agric. n/a 104, 3982–3991. doi:10.1002/jsfa.13280
Wijesinghe, G. K., de Oliveira, T. R., Maia, F. C., De Feiria, S. B., Barbosa, J. P., Joia, F., et al. (2021). Efficacy of true cinnamon (Cinnamomum verum) leaf essential oil as a therapeutic alternative for Candida biofilm infections. Iran. J. basic Med. Sci. 24, 787–795. doi:10.22038/ijbms.2021.53981.12138
Wijesinghe, G. K., Maia, F. C., de Oliveira, T. R., de Feiria, S. N. B., Joia, F., Barbosa, J. P., et al. (2020) “Effect of Cinnamomum verum leaf essential oil on virulence factors of Candida species and determination of the in-vivo toxicity with Galleria mellonella model. Mem. Inst. Oswaldo Cruz 115, e200349. doi:10.1590/0074-02760200349
Xu, Y. T., Liu, L. I., Long, S. F., Pan, L., and Piao, X. S. (2018). Effect of organic acids and essential oils on performance, intestinal health and digestive enzyme activities of weaned pigs. Animal Feed Sci. Technol. 235, 110–119. doi:10.1016/j.anifeedsci.2017.10.012
Yaldiz, B., Saglam-Metiner, P., Cakmak, B., Kaya, E., Deliogullari, B., and Yesil-Celiktas, O. (2022). Essential oil and supercritical carbon dioxide extract of grapefruit peels formulated for Candida albicans infections: evaluation by an in vitro model to study fungal–host interactions. ACS omega 7, 37427–37435. doi:10.1021/acsomega.2c04189
Yan, J.-Y., Lin, T.-H., Jong, Y.-T., Hsueh, J.-W., Wu, S.-H., Lo, H.-J., et al. (2024). Microbiota signatures associated with invasive Candida albicans infection in the gastrointestinal tract of immunodeficient mice. Front. Cell. Infect. Microbiol. 13, 1278600. doi:10.3389/fcimb.2023.1278600
Yang, C., Zhang, L., Cao, G., Feng, J., Yue, M., Xu, Y., et al. (2019). Effects of dietary supplementation with essential oils and organic acids on the growth performance, immune system, fecal volatile fatty acids, and microflora community in weaned piglets. J. animal Sci. 97, 133–143. doi:10.1093/jas/sky426
Youssef, I. M., Männer, K., and Zentek, J. (2021). Effect of essential oils or saponins alone or in combination on productive performance, intestinal morphology and digestive enzymes’ activity of broiler chickens. J. Animal Physiology Animal Nutr. 105, 99–107. doi:10.1111/jpn.13431
Zeise, K. D., Woods, R. J., and Huffnagle, G. B. (2021). Interplay between Candida albicans and lactic acid bacteria in the gastrointestinal tract: impact on colonization resistance, microbial carriage, opportunistic infection, and host immunity. Clin. Microbiol. Rev. 34, e0032320. doi:10.1128/CMR.00323-20
Zhang, Q., Zhang, J., Zhang, Y., Sui, Y., Du, Y., Yang, L., et al. (2024). Antifungal and anti-biofilm activities of patchouli alcohol against Candida albicans. Int. J. Med. Microbiol. 314, 151596. doi:10.1016/j.ijmm.2023.151596
Zhang, R., Wang, X. W., Liu, L. L., Cao, Y. C., and Zhu, H. (2020). Dietary oregano essential oil improved the immune response, activity of digestive enzymes, and intestinal microbiota of the koi carp, Cyprinus carpio. Aquaculture 518, 734781. doi:10.1016/j.aquaculture.2019.734781
Zou, Y., Xiang, Q., Wang, J., Peng, J., and Wei, H. (2016). Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. BioMed Res. Int. 2016, 5436738. doi:10.1155/2016/5436738
Keywords: essential oils, Candida albicans, antimicrobial, gut bacteria, intestinal regulation
Citation: Hou G-w and Huang T (2024) Essential oils as promising treatments for treating Candida albicans infections: research progress, mechanisms, and clinical applications. Front. Pharmacol. 15:1400105. doi: 10.3389/fphar.2024.1400105
Received: 13 March 2024; Accepted: 18 April 2024;
Published: 15 May 2024.
Edited by:
Yi Wu, Nanjing Agricultural University, ChinaReviewed by:
Ranran Hou, Qingdao Agricultural University, ChinaCopyright © 2024 Hou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Huang, aHRqaW5hbkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.