
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 23 July 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1399998
This article is part of the Research TopicThe role of validated tools, including pictorial aids, to support medication adherence and counsellingView all 8 articles
Objective: The objective of this research is to scrutinize adverse events (AEs) linked to Trifluridine/Tipiracil (TFTD/TPI), using data from the FDA Adverse Event Reporting System (FAERS) database.
Methods: The AEs data related to TFTD/TPI were collected from the fourth quarter of 2015 through the fourth quarter of 2023. After normalizing the data, multiple signal quantification techniques including Proportional Reporting Ratio (PRR), Reporting Odds Ratio (ROR), Bayesian approaches such as Bayesian Confidence Propagation Neural Network (BCPNN) and the Multi-item Gamma Poisson Shrinker (MGPS) were used for overall and subgroup analysis and visualization analyses were performed.
Results: From the FAERS database, we analyzed 13,520,073 reports, identifying 8,331 as primary suspect (PS) AEs for TFTD/TPI, occurring across 27 organ systems. The study retained 99 significant disproportionality Preferred Terms (PTs) across four algorithms and unveiled unexpected serious AEs such as iron deficiency and intestinal perforation, hepatic failure, cholangitis and so on. The median onset of TFTD/TPI-associated AEs was 44 days (IQR 20-97 days), with most occurring within the first 30 days of treatment.
Conclusion: This research uncovers critical new safety signals for TFTD/TPI, supporting its clinical monitoring and risk identification.
According to the latest data released by the WHO, the number of new cancer cases worldwide in 2022 will reach 20 million, with 9.7 million deaths. Among them, the incidence of colorectal cancer will be 1.9 million and the number of deaths will exceed 900,000, with the new incidence rate and mortality rate occupying the third and second places respectively (World Health Organization, 2024). Initially, 20% of colorectal cancer patients are diagnosed with metastatic disease, with a further 50% developing metastasis from locally advanced cancer (Ciardiello et al., 2022). The 5-year survival rate for metastatic colorectal cancer is under 20% (Biller and Schrag, 2021), highlighting the importance of non-surgical treatment strategies. Treatment varies by metastasis location and patient health, involving chemotherapy, radiation, surgery, and newer therapies like targeted and immunotherapy, aiming for a comprehensive approach to improve outcomes.
Advancements in sequencing technologies have refined metastatic colorectal cancer classification, supporting pharmacotherapy development. This progress has notably enhanced patient survival rates (Cann et al., 2023). Chemotherapy combinations, including fluorouracil, are fundamental in treating metastatic colorectal cancer (Glimelius et al., 2021). The long duration of treatment, significant tumor load, and genetic mutations can lead to fluorouracil resistance, a key challenge in managing advanced stages of the disease.
TAS102, or Trifluridine/Tipiracil (FTD/TPI), is a chemotherapy drug for colorectal cancer, akin to oral fluorouracil medications like capecitabine and S-1. It uniquely combines trifluridine, which inhibits tumor cell DNA replication, and tipiracil hydrochloride, enhancing trifluridine’s bioavailability by preventing its degradation. This combination effectively targets and kills tumor cells (Chen et al., 2016). A global Phase III clinical trial involving 800 patients with refractory metastatic colorectal cancer experienced significantly better disease control rates (44% vs. 16%) and longer survival (7.1 months vs. 5.3 months) with FTD/TPI compared to placebo, reducing the risk of death by 32% (Mayer et al., 2015). Based on the results of this study, Trifluridine/tipiracil was FDA approved in 2015 for metastatic colorectal cancer (mCRC) patients previously treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, and for those who have had or are unsuitable for anti-VEGF and anti-EGFR treatments (in RAS wild-type cases) (Bachet et al., 2020). Another Phase III clinical trial, exclusively involving Asian populations, indicated that the FTD/TPI group had a significantly lower risk of death compared to the placebo group (HR = 0.79, 95% CI: 0.62–0.99, p = 0.035), with a median overall survival of 7.8 months for FTD/TPI vs. 7.1 months for placebo. Pre-specified subgroup analysis of OS suggested a favorable trend in chemotherapy (FTD/TPI) effectiveness across all parameters except age, with similar rates of serious adverse events between both groups (Xu et al., 2018).
Although FTD/TPI has been widely used in fluoropyrimidine-resistant refractory colorectal cancer, reports of adverse events (AEs) have increased. The common adverse reactions and abnormal laboratory findings include anemia, neutropenia, fatigue, nausea, thrombocytopenia, decreased appetite, diarrhea, vomiting, abdominal pain, and fever (Mayer et al., 2015; Xu et al., 2018; Kröning et al., 2023). Studies have shown that Grade ≥3 neutropenia, after adjusting for age and the modified Glasgow prognostic score, is associated with longer survival periods (Watanabe et al., 2023). Anemia and neutropenia are more common among patients with renal impairment (Van Cutsem et al., 2022).
While there have been case reports, clinical trials, and meta-analyses on the efficacy and safety of FTD/TPI (Mohamed, 2023, Van Cutsem et al., 2022; Shitara et al., 2024), these studies were conducted under specific systems, with relatively limited sample sizes and specific inclusion criteria. Comprehensive safety data from large samples or real-world cohorts are currently lacking. Hence, employing data mining algorithms for pharmacovigilance analysis to assess the safety of FTD/TPI in real-world settings has become necessary.
The FDA Adverse Event Reporting System (FAERS) is a database that collects adverse event and medication error reports submitted to the FDA (Fusaroli et al., 2024). It is a tool for the FDA to monitor and improve the safety of drugs and therapeutic biological products. The data in FAERS support the FDA’s post-marketing safety surveillance program for drug and therapeutic biologic products, allowing for the identification of new safety information or trends. Healthcare professionals and consumers can report adverse events, which are then included in the database to aid in the evaluation of the risks and benefits of these products. The aim of this study is to evaluate and compare the safety profile of FTD/TPI by analyzing adverse events reported in the FAERS database related to FTD/TPI use.
Data for pharmacovigilance research on FTD/TPI was downloaded and obtained from the FAERS database (https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers) covering the period from the fourth quarter of 2015 to the fourth quarter of 2023, focusing on the post-marketing environment of FTD/TPI.
The FAERS database aligns with the ICH’s International Safety Reporting Guidelines (ICH E2B), utilizing the MedDRA for coding adverse events. It comprises seven distinct data files: demographic and administrative information (DEMO), drug information (DRUG), adverse event coding (REAC), patient outcomes (OUTC), report sources (RPSR), therapy start and end dates for reported drugs (THER), and indications for drug administration (INDI). To eliminate duplicates, we prioritize reports based on the most recent FDA_DT per CASEID, or opt for the highest PRIMARYID in cases of identical CASEID and FDA_DT dates, ensuring data integrity and reliability (Zou et al., 2023). In the pharmaceutical documentation, the generic name (Trifluridine/Tipiracil), abbreviation (TAS102, FTD/TPI), and trade name (Lonsurf) are defined as the target search terms for the drug. Drugs identified in reports are categorized into primary suspect (PS), secondary suspect (SS), concomitant (C), and interacting (I) based on their relation to the event. During the study period, totally 13,520,073 reports of FTD/TPI were gained from FAERS database. 374,322 case reports of FTD/TPI as the primary suspect (PS) drug after the exclusion of duplicates, and 8,331 AEs were associated with FTD/TPI. AEs reported involving FTD/TPI were classified at both the system organ class (SOC) and preferred term (PT) levels based on the Medical Dictionary for Regulatory Activities (MedDRA, version 26.0). A comprehensive analysis of 21,345 terms associated with FTD/TPI identified them as preferred terms (PTs). The overall analysis process is shown in Figure 1.
Pharmacovigilance relies on disproportionality analysis, using methods like Proportional Reporting Ratio (PRR) and Reporting Odds Ratio (ROR) for signal detection, alongside Bayesian approaches such as Bayesian Confidence Propagation Neural Network (BCPNN) and the Multi-item Gamma Poisson Shrinker (MGPS) for a more robust analysis. PRR and ROR offer simplicity and are suited for small samples, respectively, but may face bias or complexity in calculations. BCPNN and MGPS provide a statistical foundation and handle sparse data efficiently, enhancing true signal detection. Combining these algorithms allows for a comprehensive evaluation of drug safety, with precise detection parameters outlined for clarity. This strategic approach facilitates the identification of reliable safety signals from the data. Four-compartment table of drugs and adverse reactions, utilized for evaluating the relationship between a particular medication and the incidence of a specific adverse event (Supplementary Table S1). For clarity and accuracy, the exact formulas and criteria used in this study were displayed in Supplementary Table S2.
The time to onset (TTO) of FTD/TPI induced AEs was defined as the time between START_DT (the date of FTD/TPI initiation in the THER file) and EVENT_DT (the date of onset of AEs in the DEMO file). Removed data inaccurate or missing dates entered and EVENT_DT earlier than START_DT.
Data were processed and visualized using R software (version 4.2.1) Microsoft EXCEL 2010, GraphPad Prism 8, “ggplot2”package.
Clinical characteristics of FTD/TPI-associated AEs are listed in Table 1.
Adverse events (AE) occurrence among males (57.10%) surpassed those in females (42.00%), indicating a higher prevalence of AEs in males patients. Besides, the report describes a slightly higher proportion of AEs seen the elderly patients (37.67% of patients >65 years old) compared to 18≥ and <65 years old (36.98%). We calculate the ratio of each specific serious outcome to the total number of serious outcome reports to ascertain its prevalence. The most common serious outcome was death, accounting for 42.02% of reports, likely linked to disease advancement from the tumor. Reports of hospitalizations and other serious outcomes constituted 21.77% and 10.19% respectively (Supplementary Figure S1A). From reported indications, the top ones were all related to colon and rectal cancer and their metastatic tumors (Supplementary Figure S1B). Consumers reported the largest number of adverse events at 42.50% (Supplementary Figure S1C). Geographically, America reported the highest percentage of 82.00%, followed by Canada, Japan, France, and Denmark with 7.50%, 2.50%, 1.20%, and 1.10% respectively (Supplementary Figure S1D). The reported year data showed a peak in case numbers, reaching 1526 in 2017, followed by a decline started in 2018, with figures then leveling off (Figure 2).
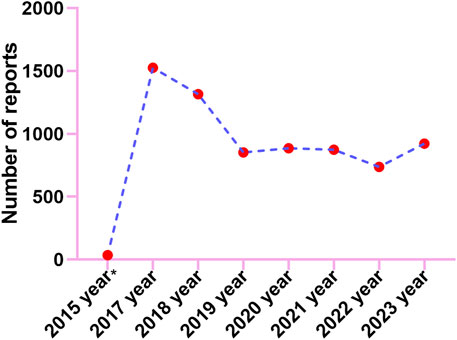
Figure 2. The annual distribution of TFTD/TPI related adverse events reported from 2015 (Q4) through 2023.
The analysis revealed that AEs resulted from FTD/TPI usage impacted 27 System Organ Classes (SOCs). Figure 3 displays the frequency of adverse events (AEs) by SOCs, succinctly showing their occurrence across different organ systems. Table 2 presents the signal intensities of FTD/TPI associated AEs across different SOC. Within this study, various SOCs were deemed significant based on their fulfillment of one or more criteria from the four indices utilized for the analysis. Several vital SOCs included: General disorders and administration site conditions (N = 7038, ROR [2.2, 95% CI 2.20-2.33]), Gastrointestinal disorders (N = 3911, ROR [2.49, 95% CI 2.41-2.58]), Investigations (N = 1676, ROR [1.38, 95% CI 1.31-1.45]), Metabolism and nutrition disorders (N = 1012, ROR [2.41, 95% CI 2.27-2.57]), Blood and lymphatic system disorders (N = 935, ROR [2.76, 95% CI 2.59-2.95]), Surgical and medical procedures (N = 502, ROR [1.72, 95% CI 1.57-1.87]), Hepatobiliary disorders (N = 286, ROR [1.66, 95% CI 1.48-1.87]). The analysis underscores the primary organ systems affected by FTD/TPI related AEs, pinpointing the need for enhanced scrutiny and research in these areas to better understand and mitigate these effects.
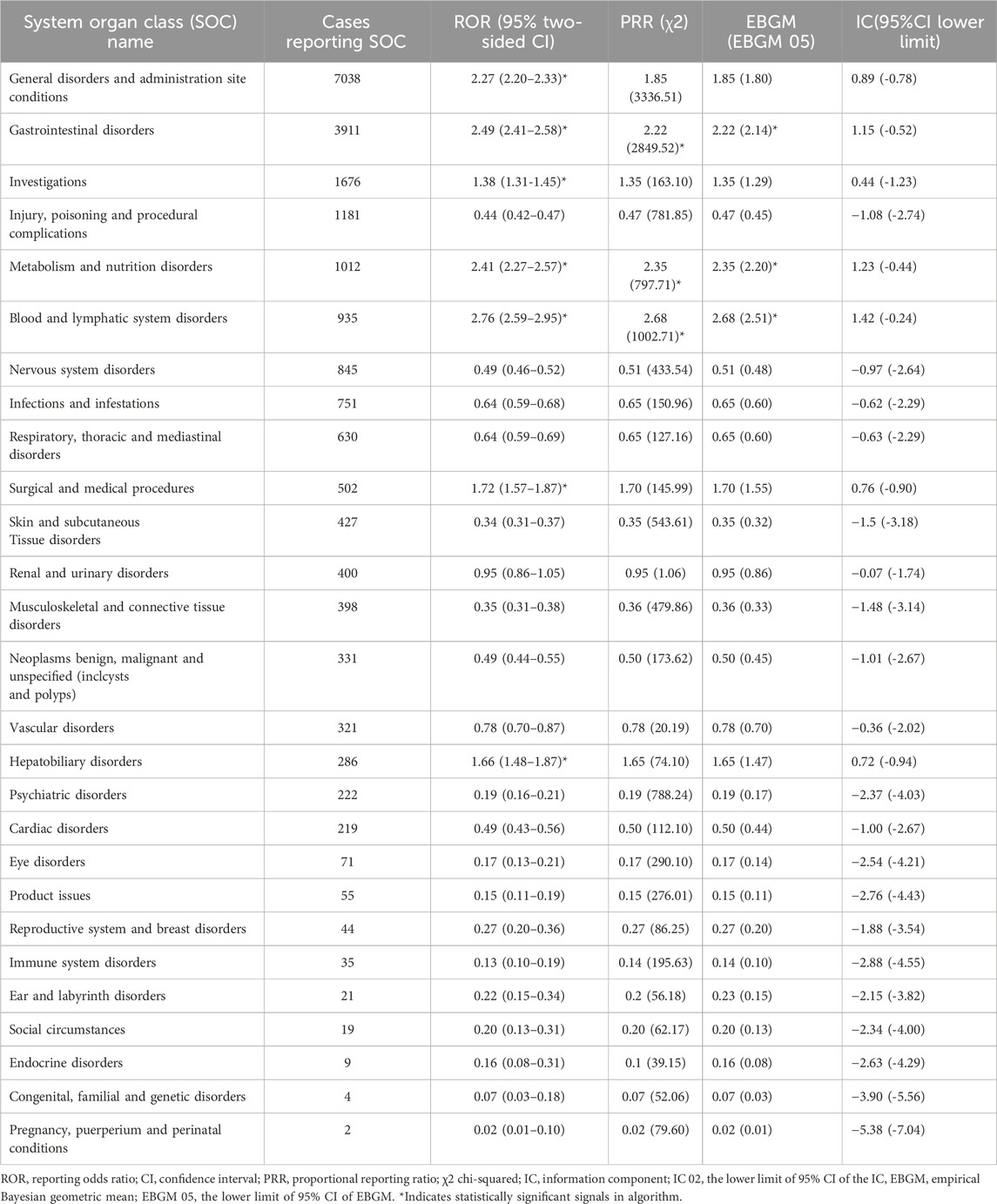
Table 2. Signal strength of reports of Trifluridine/Tipiracil at the System Organ Class (SOC) level in FAERS database.
The combined use of four algorithms successfully pinpointed 99 AEs attributed to FTD/TPI, spanning across 16 SOCs, detailed in Supplementary Table S3. Table 3 offers an overview of reported Preferred Terms (PTs) that occurred at least 10 times, featuring 50PTs across 11 SOCs. Figure 4 displays the top 20 PTs along with their respective SOCs. The three leading PTs are Death, Disease Progression, and Fatigue, all categorized under General Disorders and Administration Site Conditions. In the blood and lymphatic system disorders, gastrointestinal disorders, and general disorders and administration Site Conditions, our findings were largely consistent with previously reported PTs from previous studies. Critically, our data mining revealed several significant AEs not listed in FTD/TPI product labeling, such as iron deficiency, intestinal obstruction, ascites, small intestinal obstruction, ileus, intestinal perforation, intra-abdominal fluid collection, obstruction, hepatic failure, cholangitis, product dose omission in error, stoma site haemorrhage, carcinoembryonic antigen increased, blood iron decreased, blood magnesium decreased, dehydration, product distribution issue, product packaging quantity issue, hydronephrosis, ureteric obstruction, transfusion, stent placement and radiotherapy. More AEs were identified in our analysis, which emphasize and strengthen the overall understanding of the safety of FTD/TPI.
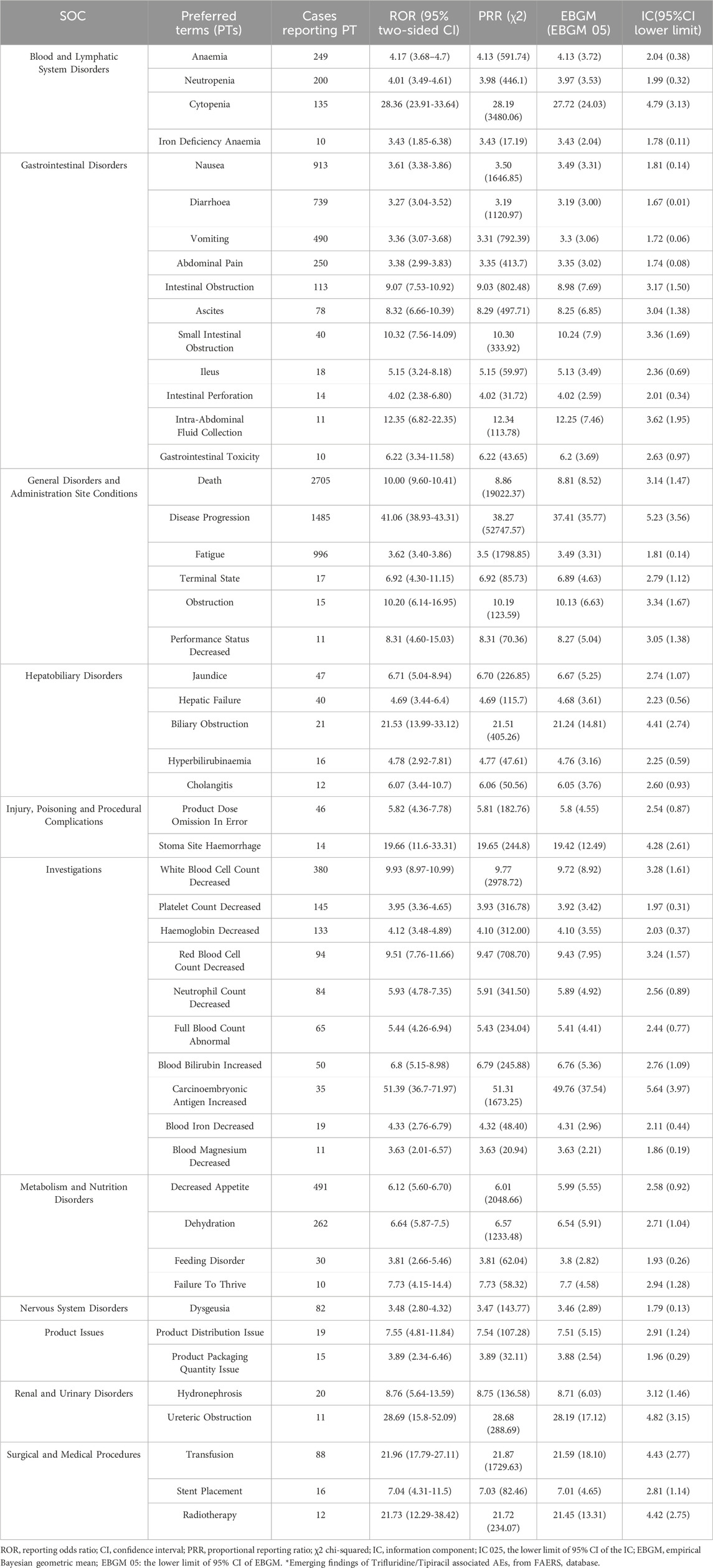
Table 3. Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database.
In the analysis of the two age subgroups, 18-65 years and over 65 years (<18 years subgroup excluded due to under-reporting of cases), Vomiting, Abdominal Pain, Platelet Count Decreased, and Intestinal Obstruction were notably prevalent in the younger cohort, while Malaise and Haemoglobin Decreased were more common in the older group (Supplementary Tables S4, S5). Together, such information would be critical for more refined clinical management, guiding clinical decision makers to tailor treatments to the characteristics of specific subgroups.
We analyzed the top 15 Preferred Terms (PTs) for both male and female subgroups and found that the first nine PTs were identical in both groups. However, Anaemia, Constipation, Neutropenia, Malaise, and Platelet Count Decreased were more common in males, while Dehydration, Weight Decreased, and Alopecia were ranked higher in females (Supplementary Tables S6, S7).
The database provided detailed information on the timing of AEs linked to FTD/TPI use. Among all reports, 2,487 offered detailed and accurate timelines for when these incidents occurred. The AEs had a median onset time of 44 days, with an IQR of 20–97 days Figure 5 illustrates that a significant portion of adverse events (AEs), specifically 953 (38.33%), occurred within the first month of starting FTD/TPI, according to the AE onset time distribution. The time to treatment greater than 1 year was the lowest reported rate. The lowest rates were reported for treatment durations greater than 1 year (N = 80, 3.22%). There was no pattern in the reported rates for the remaining dosing times.
Earlier research on FTD/TPI largely targeted its mechanism, clinical trials, and literature reviews, with minimal focus on contemporary real-world studies. To the best of our knowledge, this research utilizing the FAERS database to explore FTD/TPI related AEs represents the broadest pharmacovigilance study to date. It delivers an in-depth and systematic examination of global reports on adverse events associated with FTD/TPI as recorded in FAERS. The objective is to unearth novel and significant adverse reactions, aid in updating the product’s summary of characteristics, and support evidence-based clinical usage.
Following the baseline profile information, men experienced adverse reactions to FTD/TPI more frequently (57.10%) than women (42.00%), possibly due to the higher incidence of colorectal cancer in males, which may lead to increased medication usage. This aligns with the epidemiology of colorectal cancer (Kocarnik et al., 2022). In addition, we found that more AEs were noted in the over 65 age group (37.67%) compared to 18-65 age group (36.90%). This trend underscores the age-specific vulnerability and the critical period for heightened surveillance and management of AEs in the elderly demographic of colorectal cancer patients. One issue we have to note is that death was categorized as the most severe AEs in the report on outcomes, with a percentage of 42.02%. Although this may be due to the condition’s severity or its progression, given FTD/TPI’s use for fluoropyrimidine-resistant metastatic colorectal cancer, the significance of such a high rate of AEs cannot be overlooked. Attention is particularly warranted for those in late-stage or with compromised health, concerning the adjustment of TFTD/TPI dosage. It is notable that a substantial proportion (42.50%) of adverse reaction reports for TFTD/TPI were submitted by consumers, not healthcare professionals. This trend could indicate a higher propensity among patients to report side effects directly or suggest underreporting by healthcare workers. Given that a vast majority of these reports come from the U.S. (82.00%), it may also highlight specific regional or cultural reporting habits. Further analysis is necessary to ascertain if regional or cultural factors significantly influence this reporting pattern.
Disproportionality analysis identified significant AEs associated with TFTD/TPI across various SOCs, such as general disorders and administration site conditions, gastrointestinal disorders, investigations, metabolism and nutrition disorders, blood and lymphatic system disorders, surgical and medical procedures, hepatobiliary disorders (Figure 2). This highlights the need for careful monitoring in these areas during TFTD/TPI treatment. Aside from surgical and medical procedures, several SOCs are frequently reported in clinical trials (Zaniboni et al., 2021; Taieb et al., 2023). This study identified positive signals for specific adverse reactions, such as myelosuppression, gastrointestinal toxicity, systemic weakness/fatigue, decreased appetite, and increased blood bilirubin, all mentioned in drug labels, thus reinforcing the reliability of our findings. However, it also revealed that certain AEs within some SOCs, such as obstruction, decreased blood iron, decreased blood magnesium, dehydration, feeding disorder, failure to thrive, need for transfusion or stent placement, radiotherapy, biliary obstruction, hyperbilirubinemia, and cholangitis, are not listed on the drug’s label.
Within the SOC of General disorders and administration site conditions, notable associations were observed with death (n = 2705), disease progression (n = 1485), and fatigue (n = 996), highlighting these as significant adverse events in the treatment context. Currently, there are no studies explicitly indicating that the use of TFTD/TPI increases patient mortality. This includes two major clinical trials where, despite 5 and 1 reported deaths in the TFTD/TPI groups respectively, the adverse events leading to death were not considered related to the study drug (Mayer et al., 2015; Xu et al., 2018). WJOG14520G is a study on the combination of FTD/TPI and Bevacizumab for treating mCRC in frail patients, with a median age of 79 years in the cohort, 65% of patients aged ≥75 years, 26% having severe comorbidities, and 20% with poor performance status. The study observed no treatment-related deaths (Kito et al., 2024). Other research has indicated that disease progression is the most common cause reported in fatal adverse events among patients (Kröning et al., 2023). It might be unreasonable to judge solely by signals whether TFTD/TPI causes tumor metastasis and progression, as previously discussed. Nonetheless, clinicians should still discern if patient death or disease progression is related to TFTD/TPI use. Despite this, other AEs induced by TFTD/TPI treatment deserve attention since they significantly exceed those caused by placebo and/or best supportive care (Huang et al., 2024). The incidence of overall AEs is higher with combination therapy compared to using TFTD/TPI alone (Kagawa et al., 2023). Numerous studies have shown that neutropenia, anemia, thrombocytopenia, fatigue, nausea, decreased appetite, diarrhea, vomiting, and abdominal pain are among the most common adverse reactions or laboratory abnormalities associated with TFTD/TPI(Prager et al., 2023; Taieb et al., 2023), consistent with our observations. Neutropenia is the most common hematological adverse reaction, yet studies suggest that neutropenia serves as a useful prognostic indicator for the efficacy of TFTD/TPI treatment (Nose et al., 2020; Yoshino et al., 2020; Domínguez Senín et al., 2023). Additionally, we identified other signals, such as intestinal obstruction and perforation, not specifically linked to TFTD/TPI use alone. However, combination treatments like TFTD/TPI with Bevacizumab, commonly used for metastatic colorectal cancer, may contribute (Kagawa et al., 2023; Prager et al., 2023; Kuboki et al., 2024). Reports exist of Bevacizumab causing gastrointestinal perforation (Qi et al., 2014; Wichelmann et al., 2021) and obstruction (Zaniboni et al., 2021). When treating both gastrointestinal and non-gastrointestinal cancers. Patients with a history of chemotherapy for gynecological cancer who use Bevacizumab have an increased risk of gastrointestinal perforation, especially those with more than three chemotherapy cycles and a history of intestinal resection, facing even higher risks of perforation (Matsumiya et al., 2020). Another meta-analysis on the increased risk of gastrointestinal perforation in cancer patients treated with Bevacizumab showed a significantly higher risk in colorectal cancer patients (Qi et al., 2014). Clinicians should be aware of the potential increased risk of gastrointestinal perforation in patients treated with TFTD/TPI and Bevacizumab for colorectal cancer and recommend close monitoring. Additionally, we reported other adverse events related to obstruction including ureteral obstruction and hydronephrosis.
We conducted subgroup analyses based on age and gender for reported adverse reactions. The top 20 adverse reactions reported were 3,892 in the <65 years group and 4,588 in the ≥65 years group, with the same first two adverse reactions in both groups, death and disease progression. However, vomiting, decreased platelet count, and intestinal obstruction were more reported in the <65 group, while malaise and decreased haemoglobin were more prevalent in the ≥65 group.
Subgroup analysis by gender revealed that males reported a higher total of the top 20 adverse reactions (5999 items) compared to females (4839 items). However, the names of the specific AEs within the top 10 rankings were the same for both groups.
The EROTAS-R study on TAS-102’s effects and risk factors in metastatic CRC patients found that gender and age are not predictors of adverse event (AE) occurrence (Yoshida et al., 2023). Another study analyzing risk factors for nausea and vomiting in metastatic colorectal cancer patients treated with FTD/TPI and Bevacizumab also concluded that age and gender are not risk factors for these conditions (Prejac et al., 2024). An American study on the safety of FTD/TPI in elderly and younger patients with metastatic colorectal cancer showed consistent results between the two age groups. Both the elderly (≥65 years old) and younger (<65 years old) subgroups experienced similar proportions of Grade≥3 AEs (Mayer et al., 2018). The TERRA study indicated that for patients under 65, the risk of death in the FTD/TPI group was 0.90 (95% CI 0.69-1.18) compared to the placebo group, while for older patients, the risk was significantly lower at 0.45 (95% CI 0.28-0.74) (Xu et al., 2018). A multicenter retrospective study involving an elderly patient subgroup analysis showed no difference in common hematological and gastrointestinal adverse reactions between the <70 and ≥70 older age groups. However, neutropenia, febrile neutropenia, and asthenia were significantly higher in the group aged ≥75 compared to those <75 years old (Conti et al., 2023). Further findings show no disparity in overall age-related adverse event (AE) rates between FTD/TPI and placebo groups. Patients over 65 tend to report more grade ≥3 and severe AEs than their younger counterparts. The group aged 75 and above showed a marginally higher occurrence of treatment-related AEs compared to those aged 65-74 (91.7% vs 83.6%), yet the incidence of Grade ≥3 AEs was nearly identical (75% vs 74.8%), with severe AEs reported at 33.3% and 30.8%, respectively (Victorino et al., 2023). The PRECONNECT study, a multicenter clinical trial on the safety and efficacy of FTD/TPI, found that the incidence of grade ≥3 treatment-related adverse reactions was 79.6% in patients over 70 years old and 72.5% in those aged 70 or younger (Bachet et al., 2020). There are variations in the results across reports. Overall, our study results offer insights into side effects related to gender and age. Although these findings require further validation, they provide improved guidance for drug monitoring. We should also emphasize the significance of sociodemographic factors such as age and gender to enhance the safe use of FTD/TPI.
The time-to-onset analysis indicated that adverse events associated with TFTD/TPI typically emerged 44 days post-treatment initiation, with a significant number of cases (n = 953, 38.33%) occurring within the first month of TFTD/TPI use. This underscores the critical period shortly after treatment commencement for monitoring adverse reactions.
While the study benefits from large-scale real-world data and advanced analytics, limitations exist due to reliance on voluntary reporting, potentially leading to bias and incomplete data. For example, reports from consumers might lack the reliability and detail of those from healthcare professionals, and there may be a reporting bias towards regions with more frequent reporting (Jiang et al., 2024). Given TFTD/TPI’s extended market presence, including its availability as a generic drug in some countries, which may alter absorption rates and its safety profile, the accurate incidence of AEs cannot be ascertained solely from FAERS data. Disproportionality analysis, while highlighting signal strength statistically, does not measure risk or establish causality, offering only a signal estimation (Zou et al., 2024). Future clinical research is essential to establish a definitive cause-and-effect link. Despite its limitations, our findings serve as a crucial resource for healthcare practitioners to enhance patient follow-up and vigilance regarding TFTD/TPI-related adverse reactions.
Our pharmacovigilance analysis of the FAERS database provides a comprehensive and systematic overview of TFTD/TPI’s safety signals and timing of AEs. We’ve identified both new and unexpected significant AEs like iron deficiency, intestinal obstruction, and ureteric obstruction, alongside common AEs such as myelosuppression and gastrointestinal toxicity. Continuous monitoring and risk identification for these AEs across all populations are recommended. Yet, cohort studies and long-term clinical research are necessary to validate these findings and further elucidate TFTD/TPI’s safety profile.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethical approval was not required in accordance with local legislations and institutional requirements because publicly available anonymous data were analyzed in this study.
YH: Writing–original draft, Funding acquisition, Writing–review and editing. YD: Data curation, Formal Analysis, Writing–review and editing. ZQ: Formal Analysis, Writing–review and editing. CZ: Investigation, Writing–review and editing. JW: Methodology, Writing–review and editing. TL: Investigation, Writing–review and editing. TXL: Data curation, Funding acquisition, Writing–review and editing. MD: Conceptualization, Funding acquisition, Resources, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (No.82160588), Intra-Hospital Research Fund Project of Gansu Provincial Hospital (NO. 22GSSYB-8), Science and Technology Planning Project of Guilin (NO. 20220139-7-11).
We gratefully acknowledge all who were involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1399998/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Clinical characteristics of FTD/TPI-associated AEs.
SUPPLEMENTARY TABLE S1 | Four-compartment table of drugs and adverse reactions.
SUPPLEMENTARY TABLE S2 | Four algorithmic formulas and criteria.
SUPPLEMENTARY TABLE S3 | Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database.
SUPPLEMENTARY TABLE S4 | Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database (<65 years old).
SUPPLEMENTARY TABLE S5 | Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database (≥65years old).
SUPPLEMENTARY TABLE S6 | Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database (male).
SUPPLEMENTARY TABLE S7 | Signal strength of reports of Trifluridine/Tipiracil at the Preferred Terms (PTs) level in FAERS database (female).
Bachet, J. B., Wyrwicz, L., Price, T., Cremolini, C., Phelip, J. M., Portales, F., et al. (2020). Safety, efficacy and patient-reported outcomes with trifluridine/tipiracil in pretreated metastatic colorectal cancer: results of the PRECONNECT study. ESMO Open 5, e000698. doi:10.1136/esmoopen-2020-000698
Biller, L. H., and Schrag, D. (2021). Diagnosis and treatment of metastatic colorectal cancer: a review. Jama 325, 669–685. doi:10.1001/jama.2021.0106
Cann, C. G., Lapelusa, M. B., Cimino, S. K., and Eng, C. (2023). Molecular and genetic targets within metastatic colorectal cancer and associated novel treatment advancements. Front. Oncol. 13, 1176950. doi:10.3389/fonc.2023.1176950
Chen, J., Han, M., and Saif, M. W. (2016). TAS-102 an emerging oral fluoropyrimidine. Anticancer Res. 36, 21–26.
Ciardiello, F., Ciardiello, D., Martini, G., Napolitano, S., Tabernero, J., and Cervantes, A. (2022). Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J. Clin. 72, 372–401. doi:10.3322/caac.21728
Conti, M., Bolzacchini, E., Luchena, G., Bertu, L., Tagliabue, P., Aglione, S., et al. (2023). Tas-102 for refractory metastatic colorectal cancer: a multicenter retrospective cohort study. Cancers (Basel) 15, 3465–3470. doi:10.3390/cancers15133465
Domínguez senín, L., Rodriguez Garcés, M. Y., Aviñó Tarazona, V., Amor Urbano, M., Santos-Rubio, M. D., and Bayo Calero, J. (2023). Analysis of neutropenia as a predictive factor of the efficacy of trifluridine-tipiracil treatment. Int. J. Clin. Pharmacol. Ther. 61, 346–353. doi:10.5414/CP204410
Fusaroli, M., Giunchi, V., Battini, V., Puligheddu, S., Khouri, C., Carnovale, C., et al. (2024). Enhancing transparency in defining studied drugs: the open-source living DiAna dictionary for standardizing drug names in the FAERS. Drug Saf. 47, 271–284. doi:10.1007/s40264-023-01391-4
Glimelius, B., Stintzing, S., Marshall, J., Yoshino, T., and De Gramont, A. (2021). Metastatic colorectal cancer: advances in the folate-fluoropyrimidine chemotherapy backbone. Cancer Treat. Rev. 98, 102218. doi:10.1016/j.ctrv.2021.102218
Huang, F., Yang, H., Bao, W., Bin, Y., Zhou, S., Wang, M., et al. (2024). Efficacy and safety of trifluridine/tipiracil (TAS-102) in patients with metastatic colorectal cancer: a systematic review and meta-analysis. Clin. Transl. Oncol. 26, 468–476. doi:10.1007/s12094-023-03268-5
Jiang, Y., Zhou, L., Shen, Y., Zhou, Q., Ji, Y., and Zhu, H. (2024). Safety assessment of Brexpiprazole: real-world adverse event analysis from the FAERS database. J. Affect Disord. 346, 223–229. doi:10.1016/j.jad.2023.11.025
Kagawa, Y., Shinozaki, E., Okude, R., Tone, T., Kunitomi, Y., and Nakashima, M. (2023). Real-world evidence of trifluridine/tipiracil plus bevacizumab in metastatic colorectal cancer using an administrative claims database in Japan. ESMO Open 8, 101614. doi:10.1016/j.esmoop.2023.101614
Kito, Y., Kawakami, H., Mitani, S., Nishina, S., Matsumoto, T., Tsuzuki, T., et al. (2024). Trifluridine/tipiracil plus bevacizumab for vulnerable patients with pretreated metastatic colorectal cancer: a retrospective study (WJOG14520G). Oncologist 29, e330–e336. doi:10.1093/oncolo/oyad296
Kocarnik, J. M., Compton, K., Dean, F. E., Fu, W., Gaw, B. L., Harvey, J. D., et al. (2022). Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 8, 420–444. doi:10.1001/jamaoncol.2021.6987
Kröning, H., Göhler, T., Decker, T., Grundeis, M., Kojouharoff, G., Lipke, J., et al. (2023). Effectiveness, safety and quality of life of trifluridine/tipiracil in pretreated patients with metastatic colorectal cancer: real-world data from the noninterventional TACTIC study in Germany. Int. J. Cancer 153, 1227–1240. doi:10.1002/ijc.34603
Kuboki, Y., Terazawa, T., Masuishi, T., Nakamura, M., Watanabe, J., Ojima, H., et al. (2024). Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine-irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br. J. Cancer 128, 1897–1905. doi:10.1038/s41416-023-02212-2
Matsumiya, H., Todo, Y., Yamazaki, H., Shimada, C., Minobe, S., and Kato, H. (2020). Bevacizumab-related gastrointestinal perforation in patients with three or more prior chemotherapy regimens: a real-world experience. Taiwan J. Obstet. Gynecol. 59, 377–380. doi:10.1016/j.tjog.2020.03.007
Mayer, R. J., Hochster, H. S., Cohen, S. J., Winkler, R., Makris, L., and Grothey, A. (2018). Safety of trifluridine/tipiracil in an open-label expanded-access program in elderly and younger patients with metastatic colorectal cancer. Cancer Chemother. Pharmacol. 82, 961–969. doi:10.1007/s00280-018-3686-5
Mayer, R. J., Van Cutsem, E., Falcone, A., Yoshino, T., Garcia-Carbonero, R., Mizunuma, N., et al. (2015). Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 372, 1909–1919. doi:10.1056/NEJMoa1414325
Mohamed, E. L. B. (2023). Case report: long-term survival in a patient with metastatic colorectal cancer treated with trifluridine/tipiracil in the third-line setting. Front. Oncol. 13, 1112224. doi:10.3389/fonc.2023.1112224
Nose, Y., Kagawa, Y., Hata, T., Mori, R., Kawai, K., Naito, A., et al. (2020). Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study. Cancer Chemother. Pharmacol. 86, 427–433. doi:10.1007/s00280-020-04129-6
World Health Organization (2024). “Global cancer burden growing, amidst mounting need for services,” in International Agency for Research on Cancer. Available: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (Accessed February 1, 2024).
Prager, G. W., Taieb, J., Fakih, M., Ciardiello, F., Van Cutsem, E., Elez, E., et al. (2023). Trifluridine-tipiracil and bevacizumab in refractory metastatic colorectal cancer. N. Engl. J. Med. 388, 1657–1667. doi:10.1056/NEJMoa2214963
Prejac, J., Omrčen, T., Radić, J., Vrdoljak, E., FröBE, A., and Pleština, S. (2024). Predicting trifluridine/tipiracil treatment outcomes in refractory metastatic colorectal cancer patients: a multicenter exploratory analysis. Oncology 102, 217–227. doi:10.1159/000533567
Qi, W. X., Shen, Z., Tang, L. N., and Yao, Y. (2014). Bevacizumab increases the risk of gastrointestinal perforation in cancer patients: a meta-analysis with a focus on different subgroups. Eur. J. Clin. Pharmacol. 70, 893–906. doi:10.1007/s00228-014-1687-9
Shitara, K., Falcone, A., Fakih, M. G., George, B., Sundar, R., Ranjan, S., et al. (2024). Efficacy and safety of trifluridine/tipiracil-containing combinations in colorectal cancer and other advanced solid tumors: a systematic review. Oncologist 29, e601–e615. doi:10.1093/oncolo/oyae007
Taieb, J., Price, T., Vidot, L., Chevallier, B., Wyrwicz, L., and Bachet, J. B. (2023). Safety and efficacy of trifluridine/tipiracil in previously treated metastatic colorectal cancer: final results from the phase IIIb single-arm PRECONNECT study by duration of therapy. BMC Cancer 23, 94. doi:10.1186/s12885-022-10489-4
Van Cutsem, E., Hochster, H., Shitara, K., Mayer, R., Ohtsu, A., Falcone, A., et al. (2022). Pooled safety analysis from phase III studies of trifluridine/tipiracil in patients with metastatic gastric or gastroesophageal junction cancer and metastatic colorectal cancer. ESMO Open 7, 100633. doi:10.1016/j.esmoop.2022.100633
Victorino, A., Meton, F., Mardegan, L., Festa, J., Piranda, D. N., and Araujo, K. B. (2023). Trifluridine/tipiracil (FTD/TPI) and regorafenib in older patients with metastatic colorectal cancer. J. Geriatr. Oncol. 14, 101477. doi:10.1016/j.jgo.2023.101477
Watanabe, D., Fujii, H., Ohata, K., Iihara, H., Makiyama, A., Kobayashi, R., et al. (2023). Prognostic impact of severe neutropenia in colorectal cancer patients treated with TAS-102 and bevacizumab, addressing immortal-time bias. BMC Cancer 23, 1078. doi:10.1186/s12885-023-11618-3
Wichelmann, T. A., Abdulmujeeb, S., and Ehrenpreis, E. D. (2021). Bevacizumab and gastrointestinal perforations: a review from the FDA adverse event reporting system (FAERS) database. Aliment. Pharmacol. Ther. 54, 1290–1297. doi:10.1111/apt.16601
Xu, J., Kim, T. W., Shen, L., Sriuranpong, V., Pan, H., Xu, R., et al. (2018). Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in asian patients with previously treated metastatic colorectal cancer: the TERRA study. J. Clin. Oncol. 36, 350–358. doi:10.1200/JCO.2017.74.3245
Yoshida, N., Kuriu, Y., Ikeda, J., Kudou, M., Kirishima, T., Okayama, T., et al. (2023). Effects and risk factors of TAS-102 in real-world patients with metastatic colorectal cancer, EROTAS-R study. Int. J. Clin. Oncol. 28, 1378–1387. doi:10.1007/s10147-023-02389-9
Yoshino, T., Cleary, J. M., Van Cutsem, E., Mayer, R. J., Ohtsu, A., Shinozaki, E., et al. (2020). Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann. Oncol. 31, 88–95. doi:10.1016/j.annonc.2019.10.005
Zaniboni, A., Barone, C. A., Banzi, M. C., Bergamo, F., Blasi, L., Bordonaro, R., et al. (2021). Italian results of the PRECONNECT study: safety and efficacy of trifluridine/tipiracil in metastatic colorectal cancer. Future Oncol. 17, 2315–2324. doi:10.2217/fon-2020-1278
Zou, F., Cui, Z., Lou, S., Ou, Y., Zhu, C., Shu, C., et al. (2024). Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database. Front. Pharmacol. 15, 1338902. doi:10.3389/fphar.2024.1338902
Keywords: FAERS database, trifluridine/tipiracil, adverse events, disproportionality analysis, pharmacovigilance
Citation: Hu Y, Du Y, Qiu Z, Zhu C, Wang J, Liang T, Liu T and Da M (2024) Signal mining and analysis of trifluridine/tipiracil adverse events based on real-world data from the FAERS database. Front. Pharmacol. 15:1399998. doi: 10.3389/fphar.2024.1399998
Received: 12 March 2024; Accepted: 26 June 2024;
Published: 23 July 2024.
Edited by:
Piotr Merks, Cardinal Stefan Wyszyński University, PolandReviewed by:
Claudiu Morgovan, Lucian Blaga University of Sibiu, RomaniaCopyright © 2024 Hu, Du, Qiu, Zhu, Wang, Liang, Liu and Da. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianxiang Liu, dGlhbnhpYW5nbGl1bGl1QDE2My5jb20=; Mingxu Da, bGR5eV9kYW14QGx6dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.