
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 07 June 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1397029
Background: Tirzepatide, a glucose-dependent insulinotropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonist, is indicated for chronic weight management in adults with obesity or overweight as an adjunct to a reduced-calorie diet and increased physical activity. However, the safety profile of Tirzepatide-associated adverse events requires comprehensive evaluation.
Methods: The AE reports from the first quarter of 2022 to the third quarter of 2023 were selected by exploring the FDA Adverse Event Reporting System (FAERS) database. The new and unexpected potenial AE signals were detected using the disproportionality analysis, including reporting odds ratio(ROR), the proportional reporting ratio (PRR) the Bayesian confidence propagation neural network (BCPNN) and the empirical Bayes geometric mean(EBGM). Then the MedDRA was used to systematically classify the results.
Results: A total of 1,904,481 case reports were obtained from 2022Q2 to 2023Q3. Forty-sixth tirzepatide-induced ADRs at the preferred terms (PTs) level are associated with 8 system organ class In addition, this study uncovered multiple anticipated ADRs, such as gastrooesophageal reflux disease, dyspepsia, and vomiting, in line with the drug labels. Moreover, unexpected and significant ADRs at PTs level, such as incorrect dose administered, injection site haemorrhage, and increased appetite, were discovered and linked to Injury, poisoning, and procedural complications, General disorders and administration site conditions, and Metabolism and nutrition disorders at the System Organ Class level.
Conclusion: This study offered new perspectives on the monitoring, surveillance, and management of adverse drug reactions related to tirzepatide. The outcomes of severe adverse events and their respective detection signals, along with unexpected significant adverse event signals, are important to consider in efforts to enhance clinical medication safety when using tirzepatide.
The body mass index (BMI), calculated by dividing an individual’s weight in kilograms by their height in meters squared, is commonly used as a standard measure for defining obesity in populations. According to the World Health Organization guidelines, individuals are categorized as overweight (BMI 25-29.99) or obese (BMI ≥30). Furthermore, obesity can be classified into three categories: class I obesity (BMI 30-34.99), class II obesity (BMI 35-39.99), and class III obesity (BMI ≥40) (Elmaleh-Sachs et al., 2023). The prevalence of obesity has seen a consistent global increase since 1975, with rates rising from 3.2% to 10.8% in men and from 6.4% to 14.9% in women by 2014. Projections indicate that by 2025, around 18% of men and 21% of women worldwide will be classified as obese (NCD Risk Factor Collaboration NCD-RisC, 2016). Additionally, nearly 42% of American adults are affected by obesity, with associated links to conditions such as type 2 diabetes mellitus, hypertension, cardiovascular issues, sleep disorders, musculoskeletal problems, and premature mortality (Elmaleh-Sachs et al., 2023). The importance of implementing effective strategies in managing obesity is highlighted by these findings, as they demonstrate that achieving and maintaining a sustained weight loss of over 10% of total body weight can reduce the risk of severe health complications related to obesity and improve quality of life. The main challenge in obesity management is maintaining weight loss (Perdomo et al., 2023). New options for preventing complications of obesity are emerging through the development of innovative medications, supported by findings from trials on dietary weight loss and bariatric surgery (Drucker, 2024).
Tirzepatide is a single molecule that combines dual agonism of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors (Syed, 2022). The incretin system plays a crucial role in regulating postprandial metabolism through the action of two important incretin peptides, GIP and GLP-1, which activate their respective receptors on islet β cells and other tissues (Campbell et al., 2023). GIP and GLP-1 are crucial for controlling blood sugar levels and have a profound impact on the development of T2DM (Nauck and Müller, 2023). GIP is involved in nutrient and energy metabolism, while GLP-1 not only stimulates insulin secretion and decreases glucagon secretion but also contributes to the deceleration of gastric emptying, reduction of appetite, and enhancement of satiety. Tirzepatide was approved by the US Food and Drug Administration (FDA) for enhancing glycaemic control in adults diagnosed with T2DM, serving as a complement to dietary adjustments and physical activities in May 2022 (Syed, 2022). As a pioneering peptide that uniquely interacts with both GIP and GLP-1 incretin receptors, tirzepatide has demonstrated unparalleled outcomes in clinical studies, showcasing its ability to promote weight loss and enhance glucose control (Dahl et al., 2022; Jastreboff et al., 2022; Garvey et al., 2023). Moreover, the FDA approved tirzepatide as an adjunct to a reduced-calorie diet and increased physical activity for chronic weight management in adults with obesity or overweight who also have at least one weight-related comorbid condition, such as hypertension, dyslipidemia, type 2 diabetes mellitus, obstructive sleep apnea, or cardiovascular disease in November 2023.
In addition to promoting weight loss and improving blood sugar control, tirzepatide has been shown to reduce the risk of cardiovascular and diabetes-related events in obese patients, making it a highly recommended medication for weight management due to its significant efficacy (Hankosky et al., 2023; 2024; Xie et al., 2023). Tirzepatide has been widely used, but it has also been associated with adverse events. The most common ones are mild to moderate gastrointestinal issues such as nausea, diarrhea, constipation, and vomiting (Aronne et al., 2024). Additionally, decreased appetite and hypoglycemia related to tirzepatide have been reported (Ludvik et al., 2021). Patients receiving tirzepatide injections may also experience injection site reactions, hypersensitivity, headache, and nasopharyngitis. Moreover, pancreatitis has been observed in patients undergoing treatment with tirzepatide across multiple randomized clinical trials (Del Prato et al., 2021; Frías et al., 2021; Wadden et al., 2023).
Due to the widespread use of tirzepatide in clinical practice, it is possible that some serious adverse events have not been fully identified. Additionally, there is a significant lack of real-world data research on adverse drug reaction signals linked to tirzepatide. The FDA Adverse Event Reporting System (FAERS) database provides valuable information on adverse events associated with medications and biological products, making it a crucial tool for conducting pharmacovigilance analysis. Kumar’s team identified many of the adverse event signals of the drug data through disproportionate analysis methods, which were performed in the FAERS database (Sharma and Kumar, 2022; Jain et al., 2023; Sharma et al., 2023; Javed and Kumar, 2024). By analyzing case reports from the FAERS database, researchers can gain insights from real-world data and identify ADRs that may not be listed on the drug’s label (Singh and Kamath, 2021; Wu et al., 2022; Zhao et al., 2023; 2024). In this research, a disproportionality analysis was performed on adverse events associated with tirzepatide using the FAERS database to detecting potential signals. The objective of this research was to identify and characterize unforeseen ADRs not previously listed on the drug’s label, thereby enhancing awareness and prompting additional research into the medication’s safety.
The FAERS database, an open-access post-marketing safety surveillance database, was maintained by the FDA. This database collects information on adverse events and side effects related to various medical products, including prescription and over-the-counter drugs, biological products, medical devices, and dietary supplements.
In this study, The adverse events data related to tirzepatide/Zepbound from the FDA Adverse Event Reporting System (FAERS) were collected. The data underwent collection and preprocessing using SAS and MYSQL software to ensure accuracy and consistency. The collected data involving tirzepatide in FAERS has undergone the removal of duplicated case records through individual safety report (ISR) coding. Additionally, the drug names have been mapped to RxNorm concepts and adverse drug reaction (ADR) outcomes to Medical Dictionary for Regulatory Activities (MedDRA®) concepts (Liu et al., 2005). To assess the safety of tirzepatide in the post-marketing period, this study conducted queries in the FAERS database to extract all AEs reported between the 2022Q2 (after FDA approval of tirzepatide) and 2023Q3. The reported role of drugs in adverse events included categories such as primary suspect (PS), secondary suspect (SS), concomitant medications (C), and interacting drugs (I). Moreover, this study categorized serious clinical outcomes such as death, life-threatening, disability, hospitalization, congenital anomalies, and required intervention to prevent permanent impairment or damage. In addition to analyzing AEs, demographic factors such as gender and age, geographical locations of reported events by continent and country, as well as indications for tirzepatide use were also taken into consideration. These supplementary indicators provide a comprehensive overview of the safety profile and potential risks associated with tirzepatide in real-world post-marketing settings.
A disproportionality analysis is a crucial analytical tool within pharmacovigilance, essential for comparing the occurrence of adverse events specifically related to a study drug in comparison to all other drugs. In this study, four distinct algorithms were utilized to effectively measure the signals of tirzepatide-associated adverse events. These algorithms included the Reporting Odds Ratio (ROR), the Proportional Reporting Ratio (PRR), the Bayesian Confidence Propagation Neural Network (BCPNN), and the Empirical Bayesian Geometric Mean (EBGM) (Bate et al., 1998; Dumouchel, 1999; Evans et al., 2001; Rothman et al., 2004). The utilization of these algorithms were fundamental in successfully identifying and quantifying signals of tirzepatide-associated adverse events in the context of pharmacovigilance. To confirm the robustness of the ADRs findings, it was essential to align them with the stringent selection criteria of all four pharmacovigilance algorithms (ROR, PRR, BCPNN, and EBGM). The prerequisites were delineated in Supplementary Table S1 and the statistical algorthms including ROR, PRR, BCPNN, and EBGM are outlined below:
1) ROR Method
Positive signal detection criteria: the lower limit of 95% CI > 1, N ≥ 3;
2) PRR Method
Positive signal detection criteria: PRR ≥2, χ2 ≥ 4, N ≥ 3, and p < 0.05;
3) BCPNN Method
Positive signal detection criteria: IC025 > 0 (IC025 denotes the lower bound of 95% CI);
4) EBGM Method
Positive signal detection criteria: EBGM05 > 2 (EBGM05 denotes the lower bound of 95% CI).
A total of 1,904,481 case reports were obtained from the FAERS database over the span of the research period (from the 2022Q2 to 2023Q3). There were 20,043 case reports associated with tirzepatide as the primary suspected (PS). Additionally, the drug role counts of PS, SS, C, and I drug role are 20,043, 7780, 229, and 16 respectively. The detailed clinical features of events involving tirzepatide were outlined in Table 1.
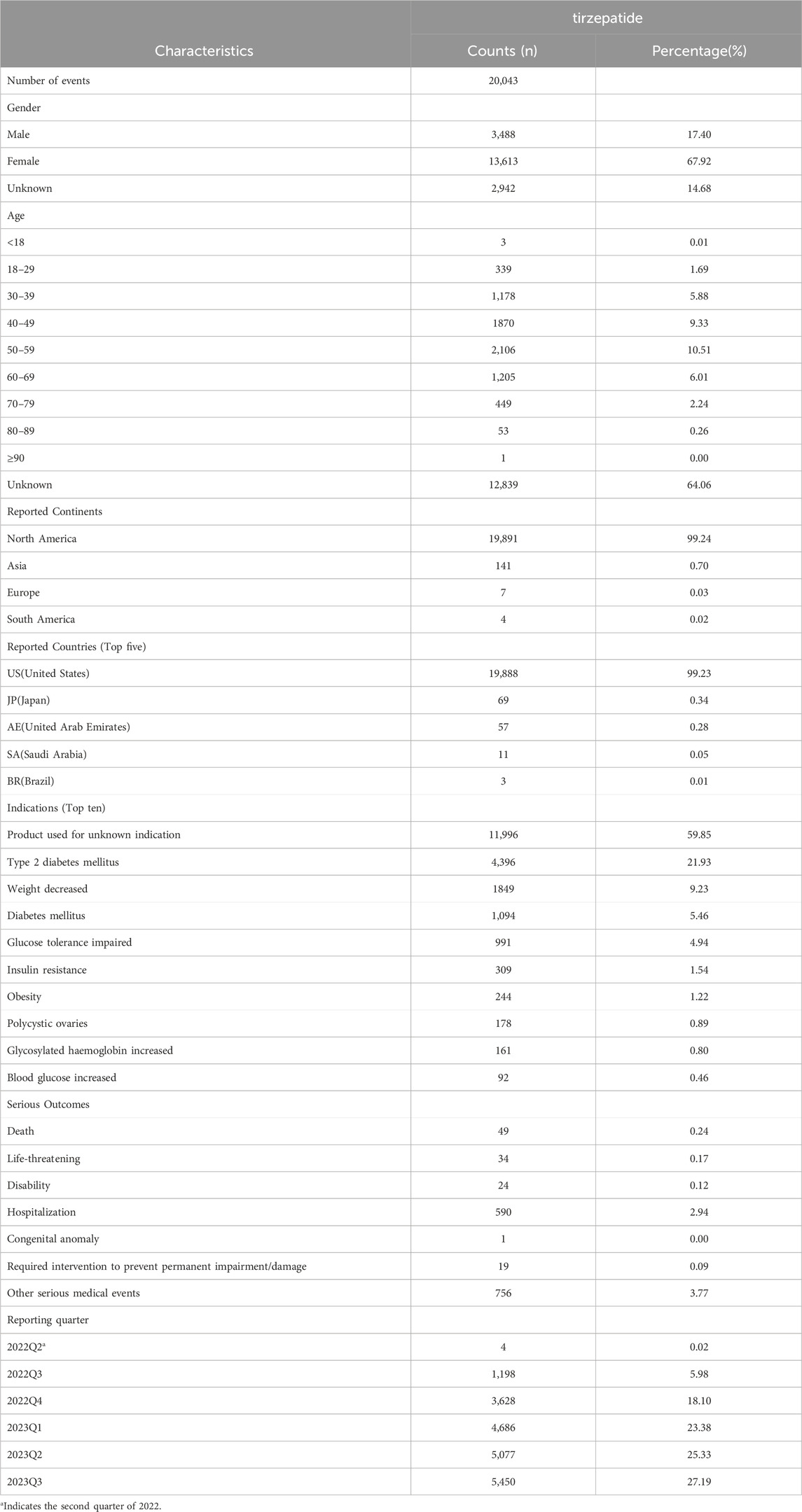
Table 1. Clinical characteristics of reports associated with tirzepatide from the FAERS database (2022Q2 to 2023Q3).
Regarding the case reports that documented all AEs, females (67.92%) accounted for a larger proportion than males (17.40%). Among the cases where the age of the patient was recorded, individuals aged 50 to 59 were more likely to experience AEs than other age groups, accounting for 10.51% (2,106) of cases. The majority of adverse event reports associated with tirzepatide originated from North America (99.24%), with a smaller number from Asia (0.70%), Europe (0.03%), and South America (0.02%). In terms of usage, the United States (19,888, 99.23%), Japan (69, 0.34%), United Arab Emirates (57, 0.28%), Saudi Arabia (11, 0.05%), and Brazil (3, 0.01%) were the top five countries. The most common indications for AEs related to tirzepatide were product used for unknown indication (11,996, 59.85%), type 2 diabetes mellitus (4396, 21.93%), weight decreased (1849, 9.23%), diabetes mellitus (1,094, 5.46%), and glucose tolerance impaired (991, 4.94%). Among the recorded AEs, the most common serious outcome was hospitalization (590, 2.94%), followed by death (49, 0.24%), life-threatening (34, 0.17%), disability (24, 0.12%), required intervention to prevent permanent impairment or damage (19, 0.09%), and congenital anomaly (1, 0.00%). With the exception of 0.02% reported in the second quarter of 2022, the reporting times for these adverse events were most frequently in the 2023Q3 (5,450, 27.19%), followed by the 2023Q2 (5,077, 25.33%), 2023Q1 (4,686, 23.38%), 2022Q4 (3,628, 18.10%), and 2022Q3 (1,198, 5.98%), respectively.
Signal strengths of reports of tirzepatide at the SOC level are described in Table 2. The top three SOCs ranked by case numbers were injury, poisoning and procedural complication (case numbers: 10,634, ROR (95% CI) = 12.69 (12.34–13.05), PRR (95% CI) = 6.49 (6.4–6.58), IC (IC025) = 2.62 (2.54), EBGM (EBGM05) = 6.13 (5.96)), general disorders and administration site conditions (case numbers: 7,851, ROR (95% CI) = 7.62 (7.41–7.85), PRR (95%CI) = 5.03 (4.94–5.12), IC (IC025) = 2.27 (2.18), EBGM (EBGM05) = 4.82 (4.69)), and gastrointestinal disorders (case numbers: 5,759, ROR (95% CI) = 7.79 (7.55–8.04), PRR (95% CI) = 5.84 (5.71–5.98), IC (IC025) = 2.47 (2.37), EBGM (EBGM05) = 5.56 (5.39)).
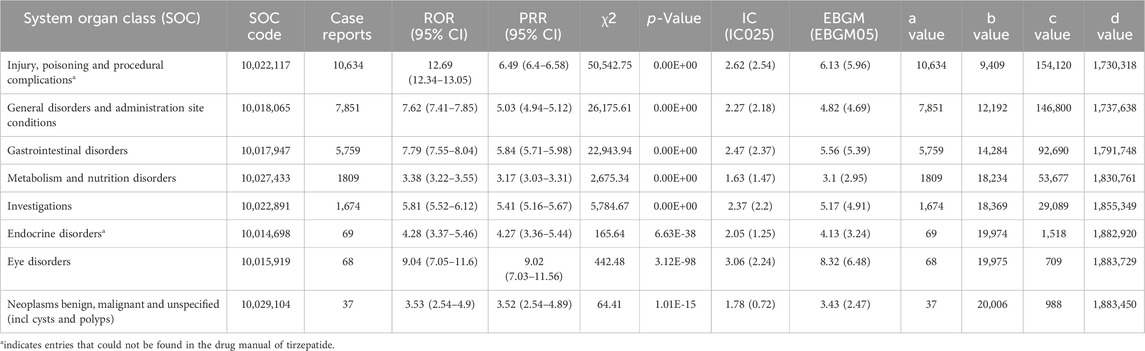
Table 2. Signal strength of reports associated with tirzepatide at the System Organ Class (SOC) level in the FAERS database.
Remarkably, two SOC terms were not mentioned in the drug label of tirzepatide, including injury, poisoning and procedural complication (case numbers: 10,634, ROR (95% CI) = 12.69 (12.34–13.05), PRR (95% CI) = 6.49 (6.4–6.58), IC (IC025) = 2.62 (2.54), EBGM (EBGM05) = 6.13 (5.96)), and endocrine disorders (case numbers: 69, ROR (95% CI) = 4.28 (3.37–5.46), PRR (95%CI) = 4.27 (3.36–5.44), IC (IC025) = 2.05 (1.25), EBGM (EBGM05) = 4.13 (3.24)).
There were a total of 46 ADRs terms associated with eight SOCs. As shown in Table 3, the top five strongest signal ADRs, ranked by the EBGM05 at the PTs level, included gastrooesophageal reflux disease (case numbers: 207, EBGM05 = 24.04), dyspepsia (case numbers: 409, EBGM05 = 23.54), incorrect dose administered (case numbers: 5,752, EBGM05 = 21.71), vomiting (case numbers: 970, EBGM05 = 19.3), and blood glucose abnormal (case numbers: 183, EBGM05 = 14.14)). Moreover, the top five PTs for tirzepatide based on case numbers were incorrect dose administered (case numbers: 5,752, EBGM05 = 21.71), off label use (case numbers: 3,101, EBGM05 = 2.36), injection site pain (case numbers: 2,742, EBGM05 = 9.16), nausea (case numbers: 2,456, EBGM05 = 3.62), and injection site haemorrhage (case numbers: 1,256, EBGM05 = 13.86).
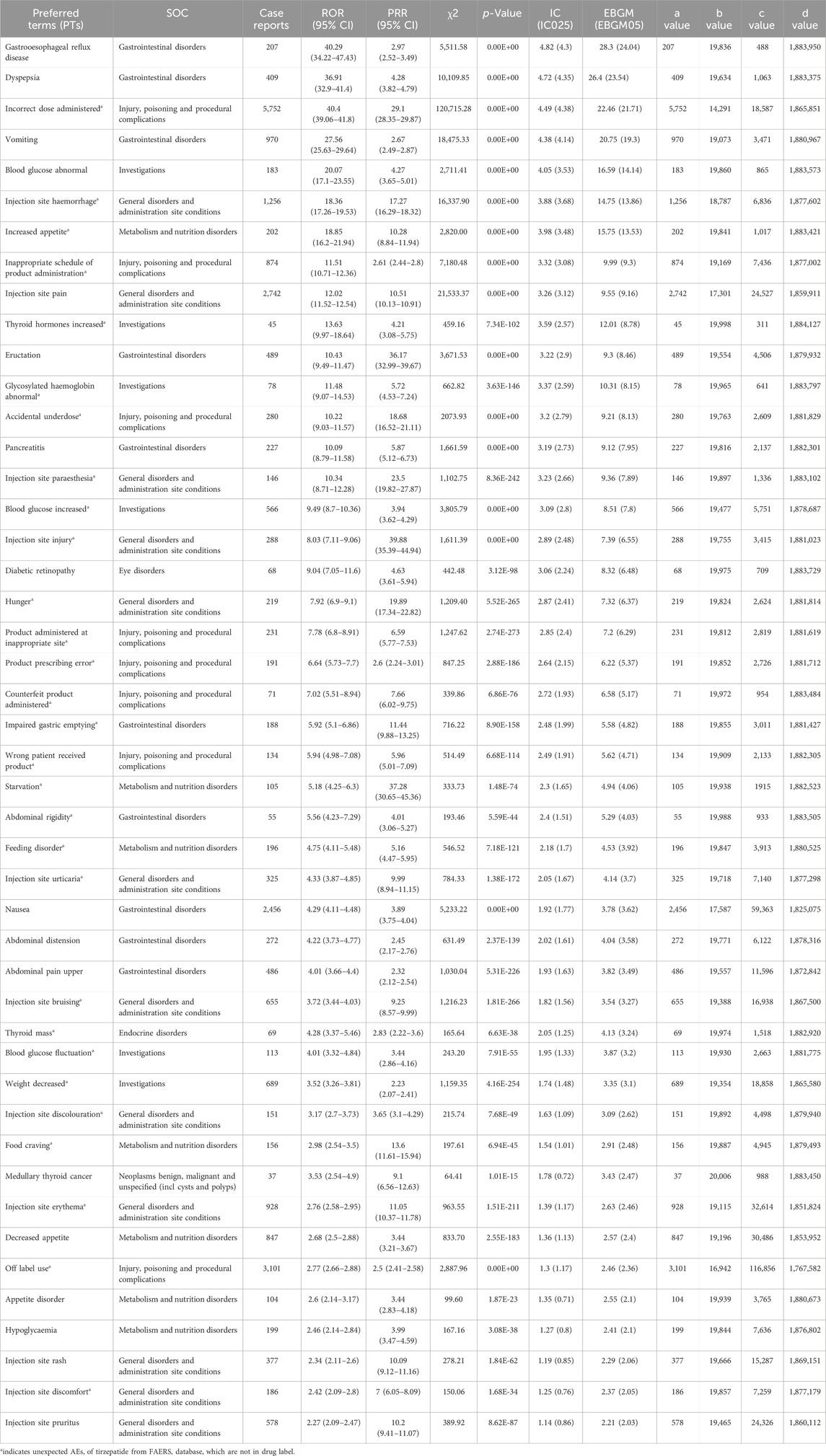
Table 3. Signal strength of reports associated with tirzepatide at the Preferred Terms (PTs) level in the FAERS database.
Among the unexpected significant ADRs revealed, the top five strongest signal ADRs ranked by the EBGM05 were incorrect dose administered (case numbers: 5752, EBGM05 = 21.71), injection site haemorrhage (case numbers: 1256, EBGM05 = 13.86), increased appetite (case numbers: 202, EBGM05 = 13.53), inappropriate schedule of product administration (case numbers: 874, EBGM05 = 9.3), and thyroid hormones increased (case numbers: 45, EBGM05 = 8.78). Additionally, the top five PTs for these unexpected significant ADRs based on case numbers were incorrect dose administered (case numbers: 5,752, EBGM05 = 21.71), off label use (case numbers: 3,101, EBGM05 = 2.36), injection site haemorrhage (case numbers: 1256, EBGM05 = 13.86), injection site erythema (case numbers: 928, EBGM05 = 2.46), and inappropriate schedule of product administration (case numbers: 874, EBGM05 = 9.3).
This study conducted a thorough analysis of adverse drug reactions (ADRs) associated with tirzepatide using data from the FAERS database. By employing various statistical methods such as ROR, PRR, BCPNN, and EBGM, this study examined 1,904,481 case reports and identified 20,043 cases of suspected ADRs linked to tirzepatide. There was a significant predominance of female subjects, making up almost two-thirds (67.92%), while male subjects accounted for a smaller proportion of 17.40%. This observation is consistent with findings from two prominent epidemiological studies that have shown a higher prevalence of obesity among adult women compared to adult men in the United States (Abarca-Gómez et al., 2017; Phelps et al., 2024). Furthermore, among the recorded cases, individuals aged 50-59 exhibited a higher likelihood of experiencing adverse events, comprising 10.51% (2,106) of the total cases. This was followed by the 40-49 age group with 9.33% (1,870) of cases, and the 60-69 age group with 6.01% (1,205). These results underscore the importance of closely monitoring the safety of tirzepatide in middle-aged and elderly patients.
As shown in Table 1, it presented the prevalent indications mentioned in relevant case studies of tirzepatide, encompassing type 2 diabetes mellitus, weight loss, impaired glucose tolerance, insulin resistance, obesity, increased glycosylated hemoglobin, and elevated blood glucose levels. These indications were consistent with the prescribed applications of tirzepatide. Notably, polycystic ovary syndrome was mentioned as an indication in 178 case reports. Polycystic ovary syndrome, known as PCOS, is a prevalent endocrine-metabolic condition among women in their reproductive years, affecting approximately 15% of the female population. Characterized by insulin resistance, PCOS is frequently linked to obesity and type 2 diabetes. Women diagnosed with polycystic ovarian syndrome commonly exhibit a heightened risk for developing metabolic syndrome and potential cardiovascular health complications in the long run (Engmann et al., 2017; Arya et al., 2021). Furthermore, findings from a 10-year case-control study revealed that the onset of diabetes tends to occur a decade earlier in females with PCOS compared to individuals without the condition (Ng et al., 2019). Based on the aforementioned evidence, it is reasonable to consider using tirzepatide for the treatment of PCOS. Among these reported events, hospitalization emerged as the most frequent serious outcome. Furthermore, tirzepatide is linked to various serious outcomes such as death, life-threatening conditions, disability, interventions to prevent permanent damage, and congenital anomalies. The findings of this study underscore the significance of fortifying early warning systems and implementing vigilant monitoring for ADRs linked to tirzepatide.
In this research, tirzepatide was found to be linked with 46 ADRs at the PTs level across 8 distinct organ systems. Within the eight SOCs associated with tirzepatide, the category of general disorders and administration site conditions encompasses 12 ADRs, representing the highest number of ADRs among all SOCs. Apart from hunger, these ADRs principally pertain to injection site reactions, which numerous clinical trials have reported (Frías et al., 2021; Ludvik et al., 2021; Rosenstock et al., 2021; Wadden et al., 2023). These ADRs pertaining to injection sites included documented events such as injection site pain, rash, and pruritus, as described in the product instructions. Additionally, ADRs such as injection site paresthesia, discoloration, and erythema have also been observed, despite not being explicitly detailed in the instructions. The incidence of such events is strongly correlated with the weekly subcutaneous administration of tirzepatide via a single-dose pen. Simultaneously, the gastrointestinal disorders category comprises 10 ADRs, constituting the second-largest grouping of ADRs among all SOCs. Gastrooesophageal reflux disease (case numbers: 207, EBGM05 = 24.04) and dyspepsia (case numbers: 409, EBGM05 = 23.54) emerged as the top two PTs linked to tirzepatideb according to the EBGM05 rankings. Furthermore, ADRs associated with gastrointestinal disorders, such as vomiting, eructation, nausea, and upper abdominal pain, have been noted in several randomized clinical trials (Frias et al., 2020; Del Prato et al., 2021; Wadden et al., 2023). In this research, pancreatitis was identified using four distinct algorithms during the signal detection process at the PT level. Importantly, tirzepatide has not been evaluated in individuals with a history of pancreatitis, leaving it unclear whether such a history places patients at an increased risk of developing pancreatitis while on tirzepatide. Consequently, it is imperative for physicians to inform patients about the potential risk of pancreatitis associated with this medication. Doctors should advise patients to cease tirzepatide immediately and seek medical attention if they experience symptoms indicative of pancreatitis, such as severe abdominal pain radiating to the back, or vomiting. During the study, two unforeseen adverse drug reactions (ADRs), impaired gastric emptying and abdominal rigidity, were identified, although they were not included in the medication’s prescribing information. There might have a correlation exists between abdominal rigidity and tirzepatide-induced pancreatitis. This discovery underscores the necessity for heightened surveillance of ADRs, especially in patient groups receiving concurrent treatment with drugs that suppress gastrointestinal motility or provoke abdominal muscle contractions.
In accordance with literature on two randomized, open-label, parallel-group, phase 3 trials, this study discovered that patients with type 2 diabetes mellitus who use tirzepatide may be at risk for developing diabetic retinopathy (Del Prato et al., 2021; Ludvik et al., 2021). The rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Therefore, patients with a history of diabetic retinopathy should be closely monitored for any progression of the condition. Additionally, regarding ADRs related to metabolism and nutritional disorders, the tirzepatide instructions indicate potential ADRs such as decreased appetite, appetite disorder, and hypoglycemia. Nevertheless, this study also uncovered unexpected ADRs including increased appetite, food craving, starvation, and feeding disorder. It is evident that the ADRs associated with increased appetite and food cravings are in stark contrast to those characterized by decreased appetite and appetite disorders. Furthermore, there may be a potential connection between starvation and hypoglycemia. Healthcare professionals should maintain vigilance, as tirzepatide has the potential to lower blood glucose levels and precipitate hypoglycemia during diagnosis and therapy. It is imperative for physicians to advise patients of the hypoglycemic risk and to educate them regarding its indicators and manifestations. For those with diabetes mellitus, it is crucial to monitor blood glucose levels pre- and post-initiation of tirzepatide therapy. The hypoglycemic risk can be mitigated by decreasing the dosage of sulfonylurea or insulin when used in conjunction. In addition, during the signal detection at the PTs level related to the SOC of investigations, several ADRs were identified. The ADRs comprised an increase in blood glucose levels, abnormal blood glucose levels, fluctuations in blood glucose levels, and abnormalities in glycosylated hemoglobin levels. This finding highlights the importance of daily monitoring of blood glucose and related indicators in patients. Moreover, the increased ADR of thyroid hormones prompts us to consider the potential impact of tirzepatide on human hormone levels and endocrine organs and systems, including the thyroid gland.
Despite the advantages associated with conducting large-scale population studies and employing data mining techniques in this research endeavor, it is imperative to acknowledge several limitations. Firstly, the FAERS database, being an open-access spontaneous reporting system, is susceptible to the upload of incomplete and inaccurate information by various submitters. This lack of standardization in data quality introduces potential biases into the analysis. Additionally, controlling for confounding variables such as dosage, duration of use, comorbidities, and concurrent drug therapies, which may influence adverse events (AEs), poses a significant challenge. Moreover, due to the absence of comprehensive data on the total number of patients using tirzepatide in real-world settings, it is not feasible to accurately calculate the true incidence rates of each AE. Furthermore, this study’s inability to establish a causal relationship between tirzepatide and adverse drug reactions (ADRs) is noteworthy. Disproportionality analysis, while indicating the strength of a signal, merely offers statistical significance without quantifying risk or causality. Nonetheless, the wealth of international records available supports this study’s capacity to quantify potential risks associated with tirzepatide. However, it is essential to recognize that the true risk of these ADRs can only be determined through prospective studies. Therefore, while the research contributes valuable insights, further investigation is warranted to comprehensively understand the risks associated with tirzepatide usage.
The study utilized data from the FDA Adverse Event Reporting System (FAERS) to investigate the potential association between tirzepatide and adverse drug reactions (ADRs). The analysis identified several previously unrecognized ADRs, including elevated thyroid hormones, abdominal rigidity, and feeding disorders, that may manifest in patients using tirzepatide. It should remain vigilant for these newly identified ADRs that may be associated with tirzepatide. In summary, the findings of this study contribute valuable empirical evidence to the understanding of tirzepatide’s safety profile following its release to the market.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
LL: Data curation, Methodology, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
I wish to express my gratitude to my wife, Junyu Chen, for her unwavering support and encouragement.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1397029/full#supplementary-material
Abarca-Gómez, L., Abdeen, Z. A., Hamid, Z. A., Abu-Rmeileh, N. M., Acosta-Cazares, B., Acuin, C., et al. (2017). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390, 2627–2642. doi:10.1016/S0140-6736(17)32129-3
Aronne, L. J., Sattar, N., Horn, D. B., Bays, H. E., Wharton, S., Lin, W.-Y., et al. (2024). Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 331, 38–48. doi:10.1001/jama.2023.24945
Arya, S., Hansen, K. R., Peck, J. D., and Wild, R. A.National Institute of Child Health and Human Development Reproductive Medicine Network (2021). Metabolic syndrome in obesity: treatment success and adverse pregnancy outcomes with ovulation induction in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 225, 280.e1–280.e11. doi:10.1016/j.ajog.2021.03.048
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. doi:10.1007/s002280050466
Campbell, J. E., Müller, T. D., Finan, B., DiMarchi, R. D., Tschöp, M. H., and D’Alessio, D. A. (2023). GIPR/GLP-1R dual agonist therapies for diabetes and weight loss—chemistry, physiology, and clinical applications. Cell Metab. 35, 1519–1529. doi:10.1016/j.cmet.2023.07.010
Dahl, D., Onishi, Y., Norwood, P., Huh, R., Bray, R., Patel, H., et al. (2022). Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA 327, 534–545. doi:10.1001/jama.2022.0078
Del Prato, S., Kahn, S. E., Pavo, I., Weerakkody, G. J., Yang, Z., Doupis, J., et al. (2021). Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet 398, 1811–1824. doi:10.1016/S0140-6736(21)02188-7
Drucker, D. J. (2024). Prevention of cardiorenal complications in people with type 2 diabetes and obesity. Cell Metab. 36, 338–353. doi:10.1016/j.cmet.2023.12.018
Dumouchel, W. (1999). Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Stat. 53, 177–190. doi:10.1080/00031305.1999.10474456
Elmaleh-Sachs, A., Schwartz, J. L., Bramante, C. T., Nicklas, J. M., Gudzune, K. A., and Jay, M. (2023). Obesity management in adults: a review. JAMA 330, 2000–2015. doi:10.1001/jama.2023.19897
Engmann, L., Jin, S., Sun, F., Legro, R. S., Polotsky, A. J., Hansen, K. R., et al. (2017). Racial and ethnic differences in the polycystic ovary syndrome metabolic phenotype. Am. J. Obstet. Gynecol. 216, 493.e1–493.e13. doi:10.1016/j.ajog.2017.01.003
Evans, S. J. W., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidem. Drug Safe. 10, 483–486. doi:10.1002/pds.677
Frías, J. P., Davies, M. J., Rosenstock, J., Pérez Manghi, F. C., Fernández Landó, L., Bergman, B. K., et al. (2021). Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515. doi:10.1056/NEJMoa2107519
Frias, J. P., Nauck, M. A., Van, J., Benson, C., Bray, R., Cui, X., et al. (2020). Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: a 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes. Metab. 22, 938–946. doi:10.1111/dom.13979
Garvey, W. T., Frias, J. P., Jastreboff, A. M., le Roux, C. W., Sattar, N., Aizenberg, D., et al. (2023). Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402, 613–626. doi:10.1016/S0140-6736(23)01200-X
Hankosky, E. R., Wang, H., Neff, L. M., Kan, H., Wang, F., Ahmad, N. N., et al. (2023). Tirzepatide reduces the predicted risk of developing type 2 diabetes in people with obesity or overweight: post hoc analysis of the SURMOUNT-1 trial. Diabetes Obes. Metab. 25, 3748–3756. doi:10.1111/dom.15269
Hankosky, E. R., Wang, H., Neff, L. M., Kan, H., Wang, F., Ahmad, N. N., et al. (2024). Tirzepatide reduces the predicted risk of atherosclerotic cardiovascular disease and improves cardiometabolic risk factors in adults with obesity or overweight: SURMOUNT-1 post hoc analysis. Diabetes Obes. Metab. 26, 319–328. doi:10.1111/dom.15318
Jain, D., Sharma, G., and Kumar, A. (2023). Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs). Expert Opin. Drug Saf. 22, 741–752. doi:10.1080/14740338.2023.2189698
Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., et al. (2022). Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216. doi:10.1056/NEJMoa2206038
Javed, F., and Kumar, A. (2024). Identification of signal of clindamycin associated renal failure acute: a disproportionality analysis. CDS 19, 123–128. doi:10.2174/1574886318666230228142856
Ludvik, B., Giorgino, F., Jódar, E., Frias, J. P., Fernández Landó, L., Brown, K., et al. (2021). Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet 398, 583–598. doi:10.1016/S0140-6736(21)01443-4
Nauck, M. A., and Müller, T. D. (2023). Incretin hormones and type 2 diabetes. Diabetologia 66, 1780–1795. doi:10.1007/s00125-023-05956-x
NCD Risk Factor Collaboration (NCD-RisC) (2016). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387, 1377–1396. doi:10.1016/S0140-6736(16)30054-X
Ng, N. Y. H., Jiang, G., Cheung, L. P., Zhang, Y., Tam, C. H. T., Luk, A. O. Y., et al. (2019). Progression of glucose intolerance and cardiometabolic risk factors over a decade in Chinese women with polycystic ovary syndrome: a case-control study. PLoS Med. 16, e1002953. doi:10.1371/journal.pmed.1002953
Perdomo, C. M., Cohen, R. V., Sumithran, P., Clément, K., and Frühbeck, G. (2023). Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 401, 1116–1130. doi:10.1016/S0140-6736(22)02403-5
Phelps, N. H., Singleton, R. K., Zhou, B., Heap, R. A., Mishra, A., Bennett, J. E., et al. (2024). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 0, 1027–1050. doi:10.1016/S0140-6736(23)02750-2
Rosenstock, J., Wysham, C., Frías, J. P., Kaneko, S., Lee, C. J., Fernández Landó, L., et al. (2021). Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155. doi:10.1016/S0140-6736(21)01324-6
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidem. Drug Safe. 13, 519–523. doi:10.1002/pds.1001
Sharma, A., and Kumar, A. (2022). Identification of novel signal of clobazam-associated drug reaction with eosinophilia and systemic symptoms syndrome: a disproportionality analysis. Acta Neurol. Scand. 146, 623–627. doi:10.1111/ane.13690
Sharma, A., Roy, S., Sharma, R., and Kumar, A. (2023). Association of antiviral drugs and their possible mechanisms with DRESS syndrome using data mining algorithms. J. Med. Virol. 95, e28671. doi:10.1002/jmv.28671
Singh, A., and Kamath, A. (2021). Assessment of adverse events associated with remdesivir use for coronavirus disease 2019 using real-world data. Expert Opin. Drug Saf. 20, 1559–1564. doi:10.1080/14740338.2021.1962846
Syed, Y. Y. (2022). Tirzepatide: first approval. Drugs 82, 1213–1220. doi:10.1007/s40265-022-01746-8
Wadden, T. A., Chao, A. M., Machineni, S., Kushner, R., Ard, J., Srivastava, G., et al. (2023). Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT-3 phase 3 trial. Nat. Med. 29, 2909–2918. doi:10.1038/s41591-023-02597-w
Wu, T., Zhang, Y., Shi, Y., Yu, K., Zhao, M., Liu, S., et al. (2022). Safety of glucagon-like peptide-1 receptor agonists: a real-world study based on the us FDA adverse event reporting system database. Clin. Drug Invest. 42, 965–975. doi:10.1007/s40261-022-01202-1
Xie, Z., Hu, J., Gu, H., Li, M., and Chen, J. (2023). Comparison of the efficacy and safety of 10 glucagon-like peptide-1 receptor agonists as add-on to metformin in patients with type 2 diabetes: a systematic review. Front. Endocrinol. 14, 1244432. doi:10.3389/fendo.2023.1244432
Zhao, B., Fu, Y., Cui, S., Chen, X., Liu, S., and Luo, L. (2024). A real-world disproportionality analysis of Everolimus: data mining of the public version of FDA adverse event reporting system. Front. Pharmacol. 15, 1333662. doi:10.3389/fphar.2024.1333662
Zhao, B., Zhang, X., Chen, M., and Wang, Y. (2023). A real-world data analysis of acetylsalicylic acid in FDA Adverse Event Reporting System (FAERS) database. Expert Opin. Drug Metab. Toxicol. 19, 381–387. doi:10.1080/17425255.2023.2235267
Keywords: tirzepatide, real-world data analysis, FDA adverse events reporting system, signal detection, pharmacovigilance, obesity
Citation: Liu L (2024) A real-world data analysis of tirzepatide in the FDA adverse event reporting system (FAERS) database. Front. Pharmacol. 15:1397029. doi: 10.3389/fphar.2024.1397029
Received: 06 March 2024; Accepted: 22 May 2024;
Published: 07 June 2024.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Mahmathi Karuppannan, MARA University of Technology, MalaysiaCopyright © 2024 Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyuan Liu, anh4ZmxseUAxNjMuY29t
†ORCID: Liyuan Liu, orcid.org/0000-0001-9748-546X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.