
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 17 May 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1396133
Objective: Growth differentiation factor 15 (GDF-15) is a stress-responsive cytokine that regulates myocardial injury, cardiac overloading pressure, and inflammation and is related to the risk of cardiovascular diseases and events. The current study aimed to investigate the correlation of GDF-15 levels with clinical features, biochemical indices, and especially the risk of cardiotoxicity in breast cancer patients receiving neoadjuvant dual anti-HER2 therapy.
Methods: A total of 103 HER2-positive breast cancer patients who underwent neoadjuvant dual anti-HER2 therapy (trastuzumab and pertuzumab plus chemotherapy) were included. Serum GDF-15 levels before neoadjuvant treatment were detected by enzyme-linked immunosorbent assay. Cardiotoxicity was evaluated during neoadjuvant therapy by referring to a decline of ≥10 percentage points in the left ventricular ejection fraction from baseline to an absolute level less than 50%.
Results: GDF-15 exhibited a skewed distribution, with a median level of 714 (range: 207–1805) pg/mL. GDF-15 was positively correlated with age (p = 0.037), diabetes (p = 0.036), and the N-terminal pro-brain natriuretic peptide level (p = 0.013) and positively correlated with the total cholesterol level (p = 0.086) and troponin T level (p = 0.082), but these correlations were not statistically significant. A total of 6.8% of patients experienced cardiotoxicity during neoadjuvant therapy. By comparison, the GDF-15 level was greater in patients who experienced cardiotoxicity than in those who did not (p = 0.008). A subsequent receiver operating characteristic curve revealed that GDF-15 predicted cardiotoxicity risk, with an area under the curve of 0.803 (95% CI: 0.664–0.939). After multivariate adjustment, GDF-15 independently predicted a greater risk of cardiotoxicity (p = 0.020).
Conclusion: GDF-15 is a candidate biomarker for increased risk of cardiotoxicity in breast cancer patients receiving neoadjuvant dual anti-HER2 therapy.
Breast cancer is the most prevalent cancer type in women and contributes to 2.26 million newly diagnosed cancer cases worldwide, 0.30 million newly diagnosed cancer cases in America, and 0.31 million newly diagnosed cancer cases in China (Sung et al., 2021; Siegel et al., 2023; Zheng et al., 2023). The treatment options and prognoses of different subtypes of breast cancer vary (Gradishar et al., 2022); among these subtypes, the human epidermal growth factor receptor 2 (HER2)-positive subtype accounts for nearly 15%–25% of all breast cancer cases and has a relatively poor prognosis (Orrantia-Borunda et al., 2022). Encouragingly, since the development of trastuzumab, the first approved anti-HER2 drug, the prognosis of HER2-positive breast cancer patients has greatly improved (Swain et al., 2023). Currently, dual anti-HER2 therapy can further improve the outcomes in HER2-positive breast cancer patients with acceptable tolerance; therefore, dual anti-HER2 therapy is currently recommended as a first-line treatment globally and in China (Swain et al., 2020; Giordano et al., 2022; Li and Jiang, 2022).
Cardiotoxicity is an unavoidable concern in breast cancer patients receiving anti-HER2 therapy (Lin et al., 2021), and a recent review revealed that approximately 3%–7% of breast cancer patients receiving anti-HER2 therapy experience cardiac dysfunction of some form (Jerusalem et al., 2019). However, due to the different definitions of cardiotoxicity applied in distinct studies and the varied follow-up durations, the incidence of anti-HER2 therapy-induced cardiotoxicity varies widely (Mantarro et al., 2016; Schneeweiss et al., 2018; Swain et al., 2018; Al-Saleh et al., 2022). Since most of the cardiotoxicity induced by anti-HER2 therapy is subclinical, the most commonly used definition of cardiotoxicity in this condition is a decline of ≥10 percentage points in the left ventricular ejection fraction (LVEF) relative to baseline, along with an absolute decrease to less than 50% (Al-Saleh et al., 2022; Lu et al., 2023).
Investigations of biomarkers that recognize the risk of cardiotoxicity early in breast cancer patients receiving anti-HER2 therapy are ongoing (Ponde et al., 2018; Gherghe et al., 2022). Growth differentiation factor 15 (GDF-15), a stress-responsive cytokine in the transforming growth factor (TGF) family, can regulate myocardial injury, the overloading status of cardiac pressure, and inflammatory infiltration (Adela and Banerjee, 2015; Pence, 2022); moreover, GDF-15 is correlated with the risk of cardiovascular diseases such as cardiac hypertrophy, acute coronary syndrome, and heart failure (Havranek and Marek, 2021; May et al., 2021; Sawalha et al., 2023) and is capable of predicting the risk of cardiovascular events and death (Li et al., 2022; Xie et al., 2022). Interestingly, a study reported that GDF-15 estimates the risk of cardiotoxicity in breast cancer patients receiving adjuvant therapy with trastuzumab, doxorubicin, and axanes (Putt et al., 2015). However, the correlation between GDF-15 levels and cardiotoxicity risk in patients receiving neoadjuvant anti-HER2 therapy, especially dual anti-HER2 therapy, is still unclear.
Thus, this study aimed to investigate the correlation of GDF-15 levels with clinical features, biochemical indices, and especially its predictive value for the risk of cardiotoxicity in HER2-positive breast cancer patients receiving neoadjuvant dual anti-HER2 therapy.
A total of 103 patients with HER2-positive breast cancer who received neoadjuvant dual anti-HER2 therapy between January 2021 and September 2023 were included in this study. The inclusion criteria were as follows: 1) had a pathological diagnosis of breast cancer; 2) were aged greater than or equal to 18 years; 3) had confirmed HER2 positivity; 4) received neoadjuvant docetaxel, carboplatin, trastuzumab, and pertuzumab (TCbHP) or docetaxel, trastuzumab, and pertuzumab (THP) treatment; 5) had available and complete data on cardiotoxicity during neoadjuvant treatment; 6) had accessible serum samples before neoadjuvant treatment; and 7) had available and complete data on clinical characteristics before neoadjuvant treatment. The exclusion criteria were as follows: 1) had distal metastasis; 2) LVEF at baseline was less than 50%; 3) had uncontrolled hypertension or diabetes; 4) had preexisting cardiac diseases such as coronary artery disease and heart failure; 5) had undergone prior oncologic therapy; and 6) lactating or pregnant women. The ethics committee approved the study. The patients or their families provided written informed consent.
The following clinical characteristics were collected from the electronic medical systems for study analyses: 1) age; 2) menopausal status; 3) chronic diseases such as hypertension, hyperlipidemia, and diabetes; 4) disease-related characteristics such as the Eastern Cooperative Oncology Group Performance Status (ECOG PS), tumor node metastasis (TNM) stage, and the expression of human epidermal growth factor receptor 2 (HER2), hormone receptor (HR), and Ki67; 5) neoadjuvant treatment regimen; and 6) cardiovascular-related biochemical indices.
Patients received TCbHP or THP as neoadjuvant therapy based on their disease condition, willingness, and medical recommendation. Docetaxel (75 mg/m2) and carboplatin (area under the curve of 6 mg/mL/min) were administered intravenously every 3 weeks as part of the recommended TCbHP regimen. Docetaxel (80–100 mg/m2) was administered intravenously every 3 weeks as part of the recommended THP regimen. The loading dose of trastuzumab was 8 mg/kg, followed by intravenous administration of 6 mg/kg once every 3 weeks. Then, 840 mg of pertuzumab was used as the loading dose, and 420 mg was administered intravenously once every 3 weeks thereafter. The dose of each drug was adjusted according to the tolerance. Six cycles of neoadjuvant therapy were commonly administered (3 weeks per cycle).
Serum samples from patients collected before neoadjuvant treatment (at baseline) were used for study analyses, and serum GDF-15 levels were detected using a human GDF-15 enzyme-linked immunosorbent assay (ELISA) kit (Cat. No: E-EL-H0080, Elabscience Biotechnology Co., China).
The LVEF at baseline and after 2/4/6 cycles of treatment was collected. Specifically, the LVEF after cycles 1–3 was recorded as the LVEF after two cycles of treatment, the LVEF after cycles 3–5 was recorded as the LVEF after four cycles of treatment, and the LVEF after five or more cycles was recorded as the LVEF after six cycles of treatment. Then, cardiotoxicity was evaluated based on the LVEF data, which were defined as a decline of ≥10 percentage points in LVEF relative to the baseline, along with an absolute decrease to less than 50% (Al-Saleh et al., 2022; Lu et al., 2023).
For analyses regarding factors related to the risk of cardiotoxicity, GDF-15 was categorized into high and low using a cutoff value of 880.5 pg/mL (the best cutoff point, which was defined as the point on the ROC curve when the sum of sensitivity plus specificity reached the highest); age was categorized into elderly and nonelderly using a cutoff value of 60 years since people above 60 years of age were considered elderly people in China; tumor size was categorized into high and low using a cutoff value of 5 cm since it was an important boundary of tumor stage; Ki67 was categorized into high and low using a cutoff value of 30%, as commonly used in breast cancer studies; triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were categorized into abnormal and normal using cutoff values of 1.7, 5.2, 3.4, and 1.3 mmol/L, respectively, since they were the boundary settings in our hospital biochemical test; and TnT, NT-proBNP, and LVEF levels were categorized into high and low using cutoff values of their median values since their recognized cutoff values were not consistent in breast cancer patients.
SPSS v26.0 (IBM, United States) was used for statistical analysis. The Mann‒Whitney U test and repeated measures one-way analysis of variance were used for comparison; Spearman’s rank correlation test was used for correlations. Receiver operating characteristic (ROC) curves were used to assess the ability of GDF-15 to distinguish breast cancer patients who experienced cardiotoxicity from those who did not. Logistic regression analysis was performed to assess cardiotoxicity, and the forward stepwise method was used to screen the independent factors, which were further estimated for performance by ROC curve analysis. A p-value of less than 0.05 was considered statistically significant.
The characteristics of the 107 eligible HER2-positive patients with breast cancer are shown in Table 1. The age of the patients was 51.1 ± 10.7 years. A total of 27.2%, 11.7%, and 11.7% of patients had hypertension, hyperlipidemia, and diabetes, respectively. The levels of TG, TC, LDL-C, HDL-C, TnT, and NT-proBNP were 1.1 (0.8–1.4) mmol/L, 3.7 (3.3–4.4) mmol/L, 1.9 (1.6–2.4) mmol/L, 1.2 (1.0–1.2) mmol/L, 6.0 (3.0–10.0) pg/mL, and 123.0 (91.0–148.0) pg/mL, respectively. In addition, 76.7% of patients received TCbHP neoadjuvant therapy, and 23.3% received THP neoadjuvant therapy.
Generally, the GDF-15 level exhibited a skewed distribution in HER2-positive breast cancer patients (Figure 1). The median level of GDF-15 was 714.0 pg/mL; the minimum, 25th quantile, 75% quantile, and maximum levels of GDF-15 were 207.0, 495.0, 986.0, and 1,805.0 pg/mL, respectively.
GDF-15 levels were positively correlated with age (p = 0.037), diabetes (p = 0.036), and NT-proBNP levels (p = 0.013) in HER2-positive breast cancer patients and tended to be positively correlated with TC levels (p = 0.086) and TnT levels (p = 0.082), but the correlations were not statistically significant (Table 2). However, GDF-15 levels were not correlated with other clinical features, biochemical indices, or neoadjuvant therapy regimens.
The LVEF gradually decreased during the entire neoadjuvant therapy period (p < 0.001) (Figure 2A); in detail, the LVEF decreased to 62.4% ± 3.5%, 61.7% ± 4.7%, 61.3% ± 5.0%, and 60.4% ± 5.5% at baseline, after two cycles of treatment, after four cycles of treatment, and after six cycles of treatment, respectively. In addition, the baseline GDF-15 level was negatively correlated with the LVEF after four cycles of treatment (p = 0.048) and after six cycles of treatment (p = 0.012) (Figure 2B).
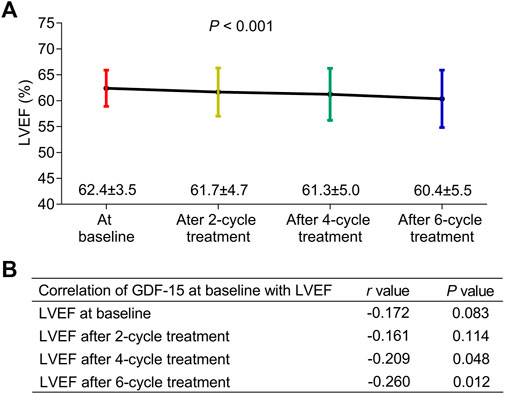
Figure 2. Correlation of GDF-15 levels with LEVF levels in HER2-positive breast cancer patients. The LVEF at the baseline, after two cycles of treatment, after four cycles of treatment, and after six cycles of treatment in HER2-positive breast cancer patients receiving neoadjuvant dual anti-HER2 therapy (A). Correlation of the GDF-15 level (at baseline) with the LVEF at the baseline, after two cycles of treatment, after four cycles of treatment, and after six cycles of treatment in those patients (B).
During the period of neoadjuvant dual anti-HER2 therapy, 6.8% of patients experienced a decline of ≥10 percentage points in LVEF relative to the baseline and an absolute decrease to less than 50% simultaneously, which was defined as cardiotoxicity (Figure 3A). By comparison, the GDF-15 level was much greater in patients who experienced cardiotoxicity (median, interquartile range (IQR): 1,048.0, 889.0–1,353.0 pg/mL) than in those who did not (median, IQR: 710.5, 482.8–940.8 pg/mL) (p = 0.008, Figure 3B). Subsequent ROC curve analysis revealed that the GDF-15 level could predict the risk of cardiotoxicity, with an area under the curve (AUC) of 0.803 (95% CI: 0.664–0.939) (Figure 3C). In particular, when choosing the best cutoff point (880.5 pg/mL), the sensitivity and specificity were 70.8% and 85.7%, respectively.
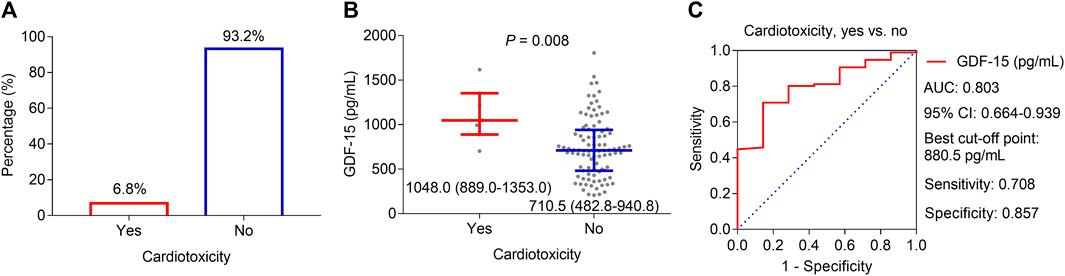
Figure 3. Correlation of GDF-15 levels with cardiotoxicity in HER2-positive breast cancer patients. Cardiotoxicity incidence in HER2-positive breast cancer patients receiving neoadjuvant dual anti-HER2 therapy (A). Difference in GDF-15 levels between patients who experienced cardiotoxicity and those who did not (B). ROC curve of the GDF-15 value for cardiotoxicity risk (C).
Univariate logistic regression analyses revealed that high GDF-15 levels (p = 0.015) and diabetes (p = 0.018) were correlated with increased cardiotoxicity risk (Table 3) and high TnT levels (p = 0.081) and high NT-proBNP levels (p = 0.075) tended to be correlated with elevated cardiotoxicity risk but were not statistically significant. Multivariate logistic regression analyses further revealed that high GDF-15 levels (p = 0.020) and diabetes (p = 0.034) were independently correlated with increased cardiotoxicity risk; in addition, advanced age (p = 0.052) tended to be independently correlated with increased cardiotoxicity risk, but the correlation was not statistically significant. A predictive model involving high GDF-15 levels, diabetes status, and advanced age was constructed, and the ROC curve showed that the predictive model had good value in predicting the risk of cardiotoxicity, with an AUC of 0.885 (95% CI: 0.774–0.997) (Figure 4).
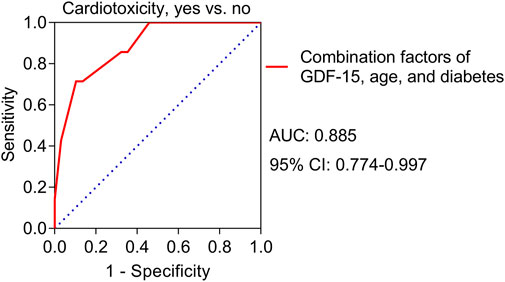
Figure 4. ROC curve for the value of the predictive model for cardiotoxicity risk in HER2-positive breast cancer patients receiving neoadjuvant dual anti-HER2 therapy.
Dual anti-HER2 therapy has improved the outcomes of HER2-positive breast cancer patients, but at the same time, it may increase the risk of treatment-induced cardiotoxicity to some extent (Swain et al., 2020; Huang et al., 2022). A previous study reported that 8.6% of HER2-positive breast cancer patients undergoing dual anti-HER2 therapy experienced cardiotoxicity (Huang et al., 2022), and another study reported that the rate of cardiotoxicity was 2.0%–6.5% under dual anti-HER2 therapy (Swain et al., 2018). Interestingly, a recent large-scale study involving 4,769 HER2-positive breast cancer patients revealed that 3.3% of patients experienced cardiotoxicity during dual anti-HER2 therapy (de Azambuja et al., 2023). The current study revealed that 6.8% of HER2-positive breast cancer patients experienced cardiotoxicity during neoadjuvant dual anti-HER2 therapy, which was within the range of cardiotoxicity reported in previous studies (Mantarro et al., 2016; Schneeweiss et al., 2018; Swain et al., 2018; Al-Saleh et al., 2022; Huang et al., 2022; de Azambuja et al., 2023). However, the rate of cardiotoxicity in the current study was slightly greater than the average reported by previous studies. Several explanations were provided, which were as follows (Sung et al., 2021): the baseline LVEF was relatively lower in the current study (Siegel et al., 2023); the definition of cardiotoxicity differed among different studies, and the current study applied the definition of cardiotoxicity as a decline of ≥10 percentage points in LVEF relative to the baseline, along with an absolute decrease to less than 50% (Al-Saleh et al., 2022; Lu et al., 2023), which was relatively loose; therefore, the rate of identified cardiotoxicity was relatively greater.
GDF-15 has been applied as a biomarker for predicting the risk of cardiovascular diseases and cardiovascular events (Adela and Banerjee, 2015; Havranek and Marek, 2021; May et al., 2021; Li et al., 2022; Pence, 2022; Xie et al., 2022; Sawalha et al., 2023). For instance, GDF-15 is upregulated in patients with coronary artery disease (CAD) compared to non-CAD controls, yielding a diagnostic value with an AUC of 0.9 for CAD (Hassanzadeh Daloee et al., 2021); moreover, GDF-15 is greater in heart failure patients with ischemic heart disease than in controls without ischemic heart disease (Elsewify et al., 2022). In addition, GDF-15 predicts the risk of major adverse cardiovascular events in patients with diabetes, as reported by a meta-analysis involving nearly twenty thousand patients (Xie et al., 2022). Furthermore, a very interesting meta-analysis reports that GDF-15 is consistently useful for prognostic prediction of the risk of cardiovascular death and hospitalization for heart failure among different types of atherosclerotic cardiovascular diseases (Kato et al., 2023).
Regarding drug-induced cardiotoxicity in breast cancer patients, a study revealed that GDF-15 can predict the risk of cardiotoxicity in breast cancer patients receiving adjuvant trastuzumab-based anti-HER2 therapy (Putt et al., 2015). However, the predictive value of GDF-15 levels for the risk of cardiotoxicity induced by neoadjuvant dual anti-HER2 therapy has not been reported in HER2-positive breast cancer patients, which is clinically important. Our current study was built on the existing studies that report the value of GDF-15 quantification in predicting cardiotoxicity risk in breast cancer patients receiving trastuzumab-based treatment (Ky et al., 2014; Putt et al., 2015; Yu et al., 2018; Kirkham et al., 2022). The differences of our study from the mentioned studies included the following (Sung et al., 2021): they focused on trastuzumab-based treatment or doxorubicin-induced cardiotoxicity, while we focused on dual anti-HER2 therapy (Siegel et al., 2023); they focused on the adjuvant settings, while we focused on the neoadjuvant setting.
The present study revealed that in HER2-positive breast cancer patients receiving neoadjuvant dual anti-HER2 therapy, the GDF-15 level was greater in patients who experienced cardiotoxicity than in those who did not. Subsequent ROC curve analysis revealed that the GDF-15 level predicted the risk of cardiotoxicity, with an AUC of 0.803 (95% CI: 0.664–0.939), which is an inspiring finding. Furthermore, after adjustment by multivariate analysis, the GDF-15 level still independently estimated the risk of cardiotoxicity in those patients. These results indicated that GDF-15 could serve as a candidate biomarker for increased risk of cardiotoxicity induced by neoadjuvant dual anti-HER2 therapy. The explanations might be the close relationship of GDF-15 with myocardial injury, cardiac overloading pressure status, inflammation, etc. (Adela and Banerjee, 2015; Pence, 2022). Furthermore, the current study revealed that GDF-15 levels were positively correlated with age, diabetes status, and NT-proBNP levels in HER2-positive breast cancer patients, which might explain the predictive value of GDF-15 for cardiotoxicity risk to some extent. It is noteworthy that GDF-15 is an established biomarker of diabetes and cardiovascular disease. Therefore, it is uncertain if the association between baseline GDF-15 and the risk of cardiotoxicity would remain statistically significant when analyzing a cohort that does not have any pre-existing cardio-metabolic conditions. To investigate this issue, we screened out the patients without hypertension, hyperlipidemia, or diabetes in this current study and then analyzed the correlation between GDF-15 and cardiotoxicity. However, in the patients without hypertension, hyperlipidemia, or diabetes, the cardiotoxicity only occurred in one patient (1.6%); therefore, it cannot be analyzed. The one patient who experienced cardiotoxicity had a GDF-15 level of 889 pg/mL; the number was higher than the median level 703.5 pg/mL of those without cardiotoxicity in the cohort without hypertension, hyperlipidemia, or diabetes. These indicate that the control of risk factors (such as hypertension and diabetes) is sub-optimal for cardiotoxicity prevention during breast cancer treatment (Kaneko et al., 2023).
Unavoidably, some limitations existed in the current study. First, GDF-15 levels were detected at a single time point (at baseline, which was the time before initiation of neoadjuvant dual anti-HER therapy), and the variation in GDF-15 levels during or after neoadjuvant treatment was not investigated. Second, the source of blood GDF-15 was not investigated. Third, the relation between GDF-15 and dual anti-HER2 plus endocrine therapy-induced cardiotoxicity was not investigated in this study. Fourth, this was a single-center study, which might have biases; thus, further validation by a multiple-center study is needed. Fifth, the sample size was not large enough; moreover, a validation cohort in the future would be more desirable to make a firm conclusion.
Overall, GDF-15 serves as a candidate biomarker for predicting an increased risk of cardiotoxicity in breast cancer patients receiving neoadjuvant dual anti-HER2 therapy. However, more evidence is needed for validation to make a final conclusion.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of the Affiliated Hospital of Chengde Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CT: writing–original draft, methodology, investigation, and formal analysis. HZ: writing–original draft, methodology, formal analysis, and data curation. JL: writing–review and editing, methodology, investigation, formal analysis, and data curation. MX: writing–original draft, visualization, investigation, formal analysis, and data curation. LM: writing–review and editing, validation, supervision, resources, funding acquisition, and conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical Science Research Project of Hebei Province in 2022 (No. 20220414).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adela, R., and Banerjee, S. K. (2015). GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J. Diabetes Res. 2015, 490842. Epub 2015/08/15. PubMed PMID: 26273671; PubMed Central PMCID: PMCPMC4530250. doi:10.1155/2015/490842
Al-Saleh, K., Abdel-Warith, A., Alghamdi, M., Aldiab, A., Ali, A., Alsaeed, E. F., et al. (2022). Incidence of trastuzumab-induced cardiotoxicity and impact of body mass index in patients with breast cancer: results from a Saudi tertiary cancer center. Mol. Clin. Oncol. 16 (4), 78. Epub 2022/03/08. PubMed PMID: 35251629; PubMed Central PMCID: PMCPMC8892432. doi:10.3892/mco.2022.2511
de Azambuja, E., Agostinetto, E., Procter, M., Eiger, D., Ponde, N., Guillaume, S., et al. (2023). Cardiac safety of dual anti-HER2 blockade with pertuzumab plus trastuzumab in early HER2-positive breast cancer in the APHINITY trial. ESMO Open 8 (1), 100772. Epub 2023/01/22. PubMed PMID: 36681013; PubMed Central PMCID: PMCPMC10044361. doi:10.1016/j.esmoop.2022.100772
Elsewify, W. A. E., Ashry, M. A., Elsaied, A. A., Hassan, M. H., Ahmed, M. A., and Mahmoud, H. E. M. (2022). Validity of B-type natriuretic peptide, growth differentiation factor 15, and high-sensitivity troponin I levels in ischemic heart failure. Clin. Lab. 68 (5). Epub 2022/05/11. PubMed PMID: 35536060. doi:10.7754/Clin.Lab.2021.210751
Gherghe, M., Lazar, A. M., Mutuleanu, M. D., Bordea, C. I., Ionescu, S., Mihaila, R. I., et al. (2022). Evaluating cardiotoxicity in breast cancer patients treated with HER2 inhibitors: could a combination of radionuclide ventriculography and cardiac biomarkers predict the cardiac impact? Cancers (Basel) 15 (1), 207. Epub 2023/01/09. PubMed PMID: 36612202; PubMed Central PMCID: PMCPMC9818586. doi:10.3390/cancers15010207
Giordano, S. H., Franzoi, M. A. B., Temin, S., Anders, C. K., Chandarlapaty, S., Crews, J. R., et al. (2022). Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol. 40 (23), 2612–2635. Epub 2022/06/01. PubMed PMID: 35640077. doi:10.1200/JCO.22.00519
Gradishar, W. J., Moran, M. S., Abraham, J., Aft, R., Agnese, D., Allison, K. H., et al. (2022). Breast cancer, version 3.2022, NCCN clinical practice guidelines in Oncology. J. Natl. Compr. Canc Netw. 20 (6), 691–722. Epub 2022/06/18. PubMed PMID: 35714673. doi:10.6004/jnccn.2022.0030
Hassanzadeh Daloee, S., Nakhaei, N., Hassanzadeh Daloee, M., Mahmoodi, M., and Barzegar-Amini, M. (2021). Evaluation of growth differentiation factor-15 in patients with or without coronary artery disease. Acta Biomed. 92 (2), e2021051. Epub 2021/05/15. PubMed PMID: 33988174; PubMed Central PMCID: PMCPMC8182609 consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article. doi:10.23750/abm.v92i2.9267
Havranek, S., and Marek, J. (2021). Biomarker GDF-15 in cardiology. Vnitr Lek. 67 (E-3), 11–14. Epub 2021/06/27. PubMed PMID: 34171946. doi:10.36290/vnl.2021.045
Huang, P., Huang, J. H., Zheng, Y. B., Cao, W. M., Shao, X. Y., Chen, J. Q., et al. (2022). Cardiac safety in breast cancer patients receiving pegylated liposome doxorubicin sequential anti-HER2 monoclonal antibody therapy. Front. Pharmacol. 13, 883600. Epub 2022/08/23. PubMed PMID: 35991878; PubMed Central PMCID: PMCPMC9386561. doi:10.3389/fphar.2022.883600
Jerusalem, G., Lancellotti, P., and Kim, S. B. (2019). HER2+ breast cancer treatment and cardiotoxicity: monitoring and management. Breast Cancer Res. Treat. 177 (2), 237–250. Epub 2019/06/06. PubMed PMID: 31165940; PubMed Central PMCID: PMCPMC6661020. doi:10.1007/s10549-019-05303-y
Kaneko, H., Yano, Y., Lee, H., Lee, H. H., Okada, A., Suzuki, Y., et al. (2023). Blood pressure classification using the 2017 ACC/AHA guideline and heart failure in patients with cancer. J. Clin. Oncol. 41 (5), 980–990. Epub 2022/09/09. PubMed PMID: 36075006. doi:10.1200/JCO.22.00083
Kato, E. T., Morrow, D. A., Guo, J., Berg, D. D., Blazing, M. A., Bohula, E. A., et al. (2023). Growth differentiation factor 15 and cardiovascular risk: individual patient meta-analysis. Eur. Heart J. 44 (4), 293–300. Epub 2022/10/29. PubMed PMID: 36303404; PubMed Central PMCID: PMCPMC10066747. doi:10.1093/eurheartj/ehac577
Kirkham, A. A., Pituskin, E., Thompson, R. B., Mackey, J. R., Koshman, S. L., Jassal, D., et al. (2022). Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: a secondary analysis of the MANTICORE trial. Eur. Heart J. Cardiovasc Pharmacother. 8 (2), 130–139. Epub 2021/02/20. PubMed PMID: 33605416; PubMed Central PMCID: PMCPMC8847070. doi:10.1093/ehjcvp/pvab016
Ky, B., Putt, M., Sawaya, H., French, B., Januzzi, J. L., Sebag, I. A., et al. (2014). Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 63 (8), 809–816. Epub 2013/12/03. PubMed PMID: 24291281; PubMed Central PMCID: PMCPMC4286181. doi:10.1016/j.jacc.2013.10.061
Li, J., and Jiang, Z. (2022). Chinese society of clinical Oncology breast cancer (CSCO BC) guidelines in 2022: stratification and classification. Cancer Biol. Med. 19 (6), 769–773. Epub 2022/06/29. PubMed PMID: 35765123; PubMed Central PMCID: PMCPMC9257320. doi:10.20892/j.issn.2095-3941.2022.0277
Li, T., Chen, Y., Ye, T., Zheng, L., Chen, L., Fan, Y., et al. (2022). Association of growth differentiation factor-15 level with adverse outcomes in patients with stable coronary artery disease: a meta-analysis. Atheroscler. Plus 47, 1–7. Epub 2021/12/06. PubMed PMID: 36643602; PubMed Central PMCID: PMCPMC9833259. doi:10.1016/j.athplu.2021.11.003
Lin, M., Xiong, W., Wang, S., Li, Y., Hou, C., Li, C., et al. (2021). The research progress of trastuzumab-induced cardiotoxicity in HER-2-positive breast cancer treatment. Front. Cardiovasc Med. 8, 821663. Epub 2022/02/01. PubMed PMID: 35097033; PubMed Central PMCID: PMCPMC8789882. doi:10.3389/fcvm.2021.821663
Lu, H., Yan, H., Liao, S., Deng, J., Zhang, J., Yao, F., et al. (2023). Efficacy, cardiotoxicity and factors affecting pathologic complete response of neoadjuvant chemotherapy with anthracycline-containing verses anthracycline-free regimens plus dual HER2 blockade for HER2-positive early-stage breast cancer: a retrospective study. Transl. Cancer Res. 12 (6), 1490–1502. Epub 2023/07/12. PubMed PMID: 37434677; PubMed Central PMCID: PMCPMC10331454. doi:10.21037/tcr-22-2547
Mantarro, S., Rossi, M., Bonifazi, M., D'Amico, R., Blandizzi, C., La Vecchia, C., et al. (2016). Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg. Med. 11 (1), 123–140. Epub 2015/12/30. PubMed PMID: 26712595. doi:10.1007/s11739-015-1362-x
May, B. M., Pimentel, M., Zimerman, L. I., and Rohde, L. E. (2021). GDF-15 as a biomarker in cardiovascular disease. Arq. Bras. Cardiol. 116 (3), 494–500. Epub 2021/02/11. PubMed PMID: 33566936; PubMed Central PMCID: PMCPMC8159541. doi:10.36660/abc.20200426
Orrantia-Borunda, E., Anchondo-Nunez, P., Acuna-Aguilar, L. E., Gomez-Valles, F. O., and Ramirez-Valdespino, C. A. (2022). “Subtypes of breast cancer,” in Breast cancer. Editor H. N. Mayrovitz (Brisbane (AU): Exon Publications).
Pence, B. D. (2022). Growth differentiation factor-15 in immunity and aging. Front. Aging 3, 837575. Epub 2022/07/14. PubMed PMID: 35821815; PubMed Central PMCID: PMCPMC9261309. doi:10.3389/fragi.2022.837575
Ponde, N., Bradbury, I., Lambertini, M., Ewer, M., Campbell, C., Ameels, H., et al. (2018). Cardiac biomarkers for early detection and prediction of trastuzumab and/or lapatinib-induced cardiotoxicity in patients with HER2-positive early-stage breast cancer: a NeoALTTO sub-study (BIG 1-06). Breast Cancer Res. Treat. 168 (3), 631–638. Epub 2017/12/28. PubMed PMID: 29280043; PubMed Central PMCID: PMCPMC5843537. doi:10.1007/s10549-017-4628-3
Putt, M., Hahn, V. S., Januzzi, J. L., Sawaya, H., Sebag, I. A., Plana, J. C., et al. (2015). Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin. Chem. 61 (9), 1164–1172. Epub 2015/07/30. PubMed PMID: 26220066; PubMed Central PMCID: PMCPMC4667170. doi:10.1373/clinchem.2015.241232
Sawalha, K., Norgard, N. B., Drees, B. M., and Lopez-Candales, A. (2023). Growth differentiation factor 15 (GDF-15), a new biomarker in heart failure management. Curr. Heart Fail Rep. 20 (4), 287–299. Epub 2023/06/08. PubMed PMID: 37289373. doi:10.1007/s11897-023-00610-4
Schneeweiss, A., Chia, S., Hickish, T., Harvey, V., Eniu, A., Waldron-Lynch, M., et al. (2018). Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 89, 27–35. Epub 2017/12/11. PubMed PMID: 29223479. doi:10.1016/j.ejca.2017.10.021
Siegel, R. L., Miller, K. D., Wagle, N. S., and Jemal, A. (2023). Cancer statistics, 2023. CA Cancer J. Clin. 73 (1), 17–48. Epub 2023/01/13. PubMed PMID: 36633525. doi:10.3322/caac.21763
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. Epub 2021/02/05. PubMed PMID: 33538338. doi:10.3322/caac.21660
Swain, S. M., Ewer, M. S., Viale, G., Delaloge, S., Ferrero, J. M., Verrill, M., et al. (2018). Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 29 (3), 646–653. Epub 2017/12/19. PubMed PMID: 29253081; PubMed Central PMCID: PMCPMC5888999. doi:10.1093/annonc/mdx773
Swain, S. M., Miles, D., Kim, S. B., Im, Y. H., Im, S. A., Semiglazov, V., et al. (2020). Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21 (4), 519–530. Epub 2020/03/17. PubMed PMID: 32171426. doi:10.1016/S1470-2045(19)30863-0
Swain, S. M., Shastry, M., and Hamilton, E. (2023). Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 22 (2), 101–126. Epub 2022/11/08. PubMed PMID: 36344672; PubMed Central PMCID: PMCPMC9640784 funding from the following: AbbVie, Acerta Pharma, Accutar Biotechnology, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Artios, AtlasMedx, Bliss BioPharmaceuticals, Cascadian Therapeutics, Clovis, Compugen, Cullen-Florentine, Curis, Dana Farber Cancer Institute, Duality Biologics, eFFECTOR Therapeutics, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, Karyopharm, Leap Therapeutics, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merus, Millennium, Molecular Templates, Myraid Genetic Laboratories, Nucana, Olema, OncoMed, Onconova Therapeutics, ORIC Pharmaceuticals, Orinove, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Radius Health, Regeneron, Repertoire Immune Medicine, Rgenix, Sermonix Pharmaceuticals, Shattuck Labs, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Vincerx Pharma, Zenith Epigenetics and Zymeworks. E.H.'s institution has received consulting fees from the following: Arcus, Eisai, Greenwich Lifesciences, H3 Biomedicine, iTeos, Janssen, Loxo, Orum Therapeutics, Propella Therapeutics and Puma Biotechnology; and her institution has received research funding and consulting fees from the following: Arvinas, Black Diamond, Boehringer Ingelheim, CytomX, Dantari, Deciphera, Lilly, Merck, Mersana, Novartis, Pfizer, Relay Therapeutics, Roche/Genentech, SeaGen, Silverback. S.M.S. reports grants or contracts from Genentech/Roche, Kailos, Genetics, BCRF; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Genentech/Roche, Daiichi Sankyo; support for attending meetings and/or travel from Genentech/Roche (travel 11/2019), Daiichi Sankyo (travel 9/2022) and Sanofi (travel 9/2022); participation on a Data Safety Monitoring Board for AstraZeneca; participation in an advisory board for AstraZeneca, Daiichi Sankyo, Exact Sciences, Biotheranostics, Natera, Merck, Silverback Therapeutics, Athenex, Lilly, Aventis; and participation in a Scientific Advisory Board for Inivata. S.M.S. reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: NSABP Vice Chairman; CCF, ASCO Director; and third party writing support from Genentech/Roche and AstraZeneca. doi:10.1038/s41573-022-00579-0
Xie, S., Li, Q., Luk, A. O. Y., Lan, H. Y., Chan, P. K. S., Bayes-Genis, A., et al. (2022). Major adverse cardiovascular events and mortality prediction by circulating GDF-15 in patients with type 2 diabetes: a systematic review and meta-analysis. Biomolecules 12 (7), 934. Epub 2022/07/28. PubMed PMID: 35883490; PubMed Central PMCID: PMCPMC9312922. doi:10.3390/biom12070934
Yu, L. R., Cao, Z., Makhoul, I., Daniels, J. R., Klimberg, S., Wei, J. Y., et al. (2018). Immune response proteins as predictive biomarkers of doxorubicin-induced cardiotoxicity in breast cancer patients. Exp. Biol. Med. (Maywood) 243 (3), 248–255. Epub 2017/12/12. PubMed PMID: 29224368; PubMed Central PMCID: PMCPMC5813873. doi:10.1177/1535370217746383
Keywords: GDF-15, biomarker, breast cancer, dual anti-HER2, cardiotoxicity
Citation: Tian C, Zhang H, Liu J, Xu M and Ma L (2024) GDF-15 is a potential candidate biomarker for an elevated risk of cardiotoxicity in breast cancer patients receiving neoadjuvant dual anti-HER2 therapy. Front. Pharmacol. 15:1396133. doi: 10.3389/fphar.2024.1396133
Received: 05 March 2024; Accepted: 23 April 2024;
Published: 17 May 2024.
Edited by:
Onur Bender, Ankara University, TürkiyeReviewed by:
Abeer M. Mahmoud, University of Illinois Chicago, United StatesCopyright © 2024 Tian, Zhang, Liu, Xu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihui Ma, bWFsaDc5MDkwOUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.