- 1Cancer Minimally Invasive Therapies Centre, Guangdong Second Provincial General Hospital, Jinan University, Guangzhou, China
- 2Department of Cell Biology and Institute of Biomedicine, Guangdong Provincial Biotechnology and Engineering Technology Research Center, Guangdong Provincial Key Laboratory of Bioengineering Medicine, Genomic Medicine Engineering Research Center of Ministry of Education, MOE Key Laboratory of Tumor Molecular Biology, National Engineering Research Center of Genetic Medicine, State Key Laboratory of Bioactive Molecules and Druggability Assessment, College of Life Science and Technology, Jinan University, Guangzhou, China
- 3Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, The Second Affiliated Hospital, Institute of Neuroscience, Guangzhou Medical University, Guangzhou, China
- 4Department of Hepatobiliary Surgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Colorectal cancer is a common malignant tumor with high mortality, for which chemotherapy resistance is one of the main reasons. The high expression of ABCG2 in the cancer cells and expulsion of anticancer drugs directly cause multidrug resistance (MDR). Therefore, the development of new ABCG2 inhibitors that block the active causes of MDR may provide a strategy for the treatment of colorectal cancer. In this study, we find that dorsomorphin (also known as compound C or BML-275) potently inhibits the transporter activity of ABCG2, thereby preserving the chemotherapeutic agents mitoxantrone and doxorubicin to antagonize MDR in ABCG2-overexpressing colorectal cancer cells. Additionally, dorsomorphin does not alter ABCG2 protein expression. The results of molecular docking studies show that dorsomorphin is bound stably to the ABCG2-binding pocket, suggesting that dorsomorphin is a potent ABCG2 inhibitor that attenuates ABCG2-mediated MDR in colorectal cancer.
1 Introduction
Cancer multidrug resistance (MDR) refers to the resistance of cancer cells to various anticancer agents that are unrelated to structure and function, which reduces the effects of chemotherapy and is not conducive to the survival of cancer patients (Fan et al., 2023). An important reason for the development of MDR is ABCG2 overexpression in the cancer cells, which can excrete the anticancer drugs against the concentration gradient (Li et al., 2016). As one of the ATP-binding cassette transporters, ABCG2 is a transmembrane protein on the cell membrane that can obtain energy to expel substrates out of the cell through ATP hydrolysis (Eckenstaler and Benndorf, 2020). ABCG2 directly supports many anticancer drugs, such as mitoxantrone (Sugimoto et al., 2003), doxorubicin (Stacy et al., 2013), irinotecan (Nielsen et al., 2017), imatinib (Noguchi et al., 2009), dasatinib (Eadie et al., 2014), and erlotinib (Shi et al., 2007). Given the important role of ABCG2 in mediating MDR, effective ABCG2 inhibitors can help reverse MDR. Several research groups have discovered a series of ABCG2 inhibitors, such as AZ32 (Liu et al., 2021), AZ-628 (Wang J. Q. et al., 2020), febuxostat (Miyata et al., 2016), fumitremorgin C (Toyoda et al., 2019), GSK2606414 (Yu et al., 2023), KU55933 (Liu et al., 2022), MK-2206 (Gao et al., 2023), NVP-TAE684 (Wang J. et al., 2020), OTS964 (Yang et al., 2021), and VKIN-1 (Narayanan et al., 2021). However, there is no ABCG2 inhibitor that has been used successfully in clinical settings to reverse cancer MDR. Therefore, there is an urgent need to develop novel ABCG2 inhibitors.
In this study, we investigate the effects of dorsomorphin (also known as compound C or BML-275) on ABCG2 activity and ABCG2-mediated MDR in colorectal cancer. Colorectal cancer is one of the main types of fatal cancers (Ghasemian et al., 2023), and ABCG2 expression has been associated with tumor responses to irinotecan-based or FOLFOX therapy in metastatic colorectal cancer patients (Lin et al., 2013; Palshof et al., 2020). Our findings demonstrate that dorsomorphin is a potent ABCG2 inhibitor that attenuates ABCG2-mediated MDR in colorectal cancer.
2 Materials and methods
2.1 Reagents and cell culture
Dorsomorphin (#1219188-18-9), KU55933 (#587871-26-9), mitoxantrone (#70476-82-3), doxorubicin (#A603456-0025), cisplatin (#AA1A8019B), rhodamine 123 (#62669-70-9), and 3-(4,5-dimethylthiazol-yl)-2,5-diphenyl-tetrazolium bromide (MTT; #298-93-1) were procured from MREDA Technology Inc. (Beijing, China), TargetMol Chemicals Inc. (Shanghai, China), D&B Biotech Inc. (Shanghai, China), Sangon Biotech Inc. (Shanghai, China), Qilu Pharmaceutical Co. (Jinan, China), Sigma-Aldrich Trading Co. (Shanghai, China), and Yuanye Biotech Co. (Shanghai, China), respectively. Anti-ABCG2 antibody (#RLT0053) and anti-β-actin antibody (#SC-47778) were purchased from Ruiying Biotech (Wuxi, China) and Santa Cruz Biotech (Santa Cruz, California, United States), respectively. Human colorectal cancer cells S1-M1-80 vector with ABCG2 overexpression and S1-M1-80 sgABCG2 with ABCG2-knockout were established as reported previously (Liu et al., 2021) and cultured in Dulbecco’s modified Eagle’s medium (#C11995500BT) with 10% fetal bovine serum (#10270-106) from Thermo Fisher Scientific Inc. (Waltham, Massachusetts, United States) at 37°C in a humid atmosphere of 5% CO2.
2.2 Cytotoxicity assay
The cells were cultured in 96-well plates at 7 × 103 cells/well and treated with the indicated agents for 72 h. After incubating with 500 mg/mL MTT for another 4 h and discarding the solution in the wells, approximately 50 μL of dimethylsulfoxide (DMSO) was added to each well. The absorbance was then detected at 570 nm with a BioTek Synergy H1 microplate reader from Agilent Technologies Inc. (Santa Clara, California, United States). The Bliss method was used to calculate the 50% inhibitive concentration (IC50), as reported previously (Zhang et al., 2017).
2.3 Drug accumulation assay
The cells were cultured in 12-well plates at 5 × 104 cells/well and incubated with the indicated concentration of dorsomorphin or KU55933 for 1 h. After incubating with mitoxantrone, doxorubicin, or rhodamine 123 at a concentration of 10 μM for another 2 h, the images of the cells were acquired using the LSM900 confocal microscope from Carl Zeiss Inc. (Oberkohen, Germany). Next, the cells were collected and analyzed with a CytoFLEX flow cytometer from Beckman Coulter Inc. (Brea, California, United States), as reported previously (Liu et al., 2022).
2.4 Western blot
The cells were lysed with a lysis buffer (containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/mL phenylmethanesulfonyl fluoride, 0.03% aprotinin, and 1 µM sodium orthovanadate) at 4°C for 30 min. After centrifuging for 10 min at 1.4 × 104 g, the protein supernatants were collected and separated using 10% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin, the membranes were incubated with the specified primary antibodies and the corresponding horseradish-peroxidase-conjugated secondary antibodies. The signals were then acquired and examined using a ChemiDoc XRS chemiluminescent gel imaging system from Analytik Jena AG (Thuringia, Germany).
2.5 Docking analysis
The human ABCG2 protein crystal structure was archived from the Protein Data Bank (PDB ID: 6vxi). The molecular dockings of dorsomorphin and ABCG2 were analyzed using AutoDock Vina, and the data were visualized using PyMOL.
2.6 Statistical analysis
The significant differences were determined using Student’s t-test in GraphPad prism 8.3.0, and a p-value <0.05 was considered to be statistically significant.
3 Results
3.1 Dorsomorphin restores the chemosensitivity of colorectal cancer cells with ABCG2 overexpression
To investigate the effects of dorsomorphin (whose chemical structure is shown in Figure 1A) on colorectal cancer cells with ABCG2 overexpression, we first performed the MTT assay to assess the cytotoxicity of dorsomorphin in both the S1-M1-80 vector and S1-M1-80 sgABCG2 cells. Dorsomorphin at 1 μM did not show cytotoxicity in both types of cells (Figure 1B). Therefore, dorsomorphin was applied at concentrations of 0.3 μM and 1 μM to examine its sensitizing effects. As presented in Figures 1C, D and Table 1, compared with the known ABCG2 inhibitor KU55933, dorsomorphin relatively restores the chemosensitivity of the ABCG2 substrate mitoxantrone and doxorubicin in a dose-dependent manner only in the S1-M1-80 vector cells and not in the S1-M1-80 sgABCG2 cells. Both KU55933 and dorsomorphin are unable to restore the chemosensitivity of the non-ABCG2 substrate cisplatin in both types of cells. These results suggest that dorsomorphin can restore the chemosensitivity of colorectal cancer cells with ABCG2 overexpression.

Figure 1. Dorsomorphin restores the chemosensitivity of colorectal cancer cells with ABCG2 overexpression. (A) Chemical structure of dorsomorphin. The cells were treated with the indicated agents for 72 h and examined via MTT assay. The representative cell survival curves are shown in (B–D).

Table 1. Summary of the IC50 values. The fold-reversal value was computed by dividing the IC50 of each drug in the S1-M1-80 vector or S1-M1-80 sgABCG2 cells in the absence and presence of inhibitors. *p < 0.05 and **p < 0.01 compared with the corresponding group.
3.2 Dorsomorphin increases the ABCG2 substrate levels in colorectal cancer cells with ABCG2 overexpression
To further explore whether dorsomorphin could directly suppress the transporter activity of ABCG2, we conducted drug accumulation experiments on the ABCG2 substrates mitoxantrone, doxorubicin, and rhodamine 123 with both the S1-M1-80 vector and S1-M1-80 sgABCG2 cells. As presented in Figure 2A–C, the levels of mitoxantrone, doxorubicin, and rhodamine 123 in the S1-M1-80 sgABCG2 cells are higher than those in the S1-M1-80 vector cells. Moreover, compared with KU55933, dorsomorphin relatively increases the levels of mitoxantrone, doxorubicin, and rhodamine 123 in a dose-dependent manner only in the S1-M1-80 vector cells and not in the S1-M1-80 sgABCG2 cells. These data indicate that dorsomorphin can increase the ABCG2 substrate levels in colorectal cancer cells with ABCG2 overexpression.
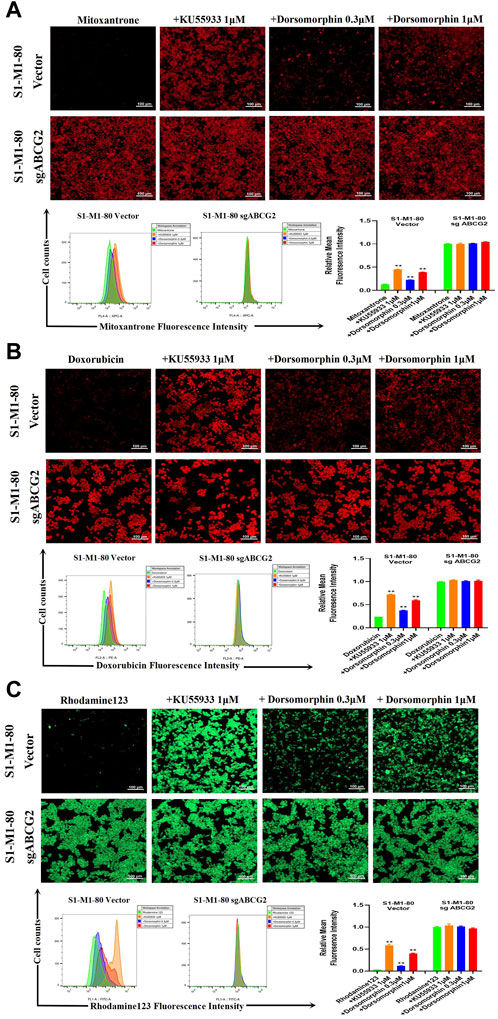
Figure 2. Dorsomorphin increases the ABCG2 substrate levels in colorectal cancer cells with ABCG2 overexpression. The cells were incubated with 10 μM (A) mitoxantrone, (B) doxorubicin, and (C) rhodamine 123 for 2 h after preincubation with dorsomorphin or KU55933 for 1 h and imaged with a confocal microscope, followed by quantification with a flow cytometer. **p < 0.01 compared with the corresponding group.
3.3 Dorsomorphin does not alter the protein level of ABCG2 or the mode of binding with ABCG2
To test the effects of dorsomorphin on the protein level of ABCG2, we treated the S1-M1-80 vector cells with 1 μM dorsomorphin for 24, 48, and 72 h. The results of Western blotting show that dorsomorphin has no effect on the protein level of ABCG2 in the S1-M1-80 vector cells (Figure 3A). Next, we carried out a structure-based docking analysis to explore the binding of dorsomorphin with ABCG2. As shown in Figures 3B, C, dorsomorphin is located in the ABCG2-binding pocket, and the hydrophobic amino acid sites, such as Val-401, Asn-436, Phe-439, Ile-543, and Val-546, on ABCG2 engage in hydrophobic interactions to stabilize the binding conformation of dorsomorphin. Dorsomorphin is also observed to form intermolecular hydrogen bonds with Asn-436 of TM2 on ABCG2 and π-π bonds with Ph-439 of TM2 on ABCG2 to further stabilize its binding conformation.
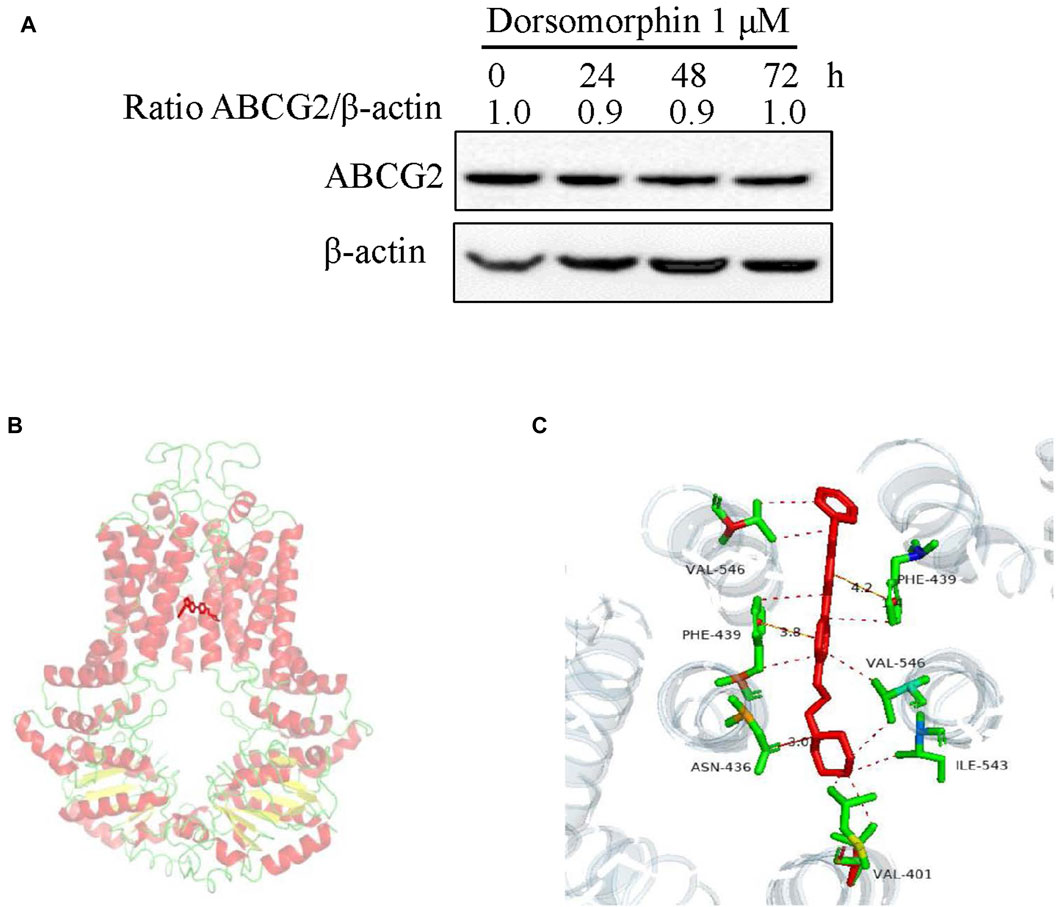
Figure 3. Dorsomorphin does not alter the protein level of ABCG2 or mode of binding with ABCG2. (A) ABCG2 expression levels in S1-M1-80 vector cells treated with 1 μM dorsomorphin for the indicated time points were measured via Western blot assay. (B) Optimal docked positions of dorsomorphin (red lines) within the binding pocket of human ABCG2 generated using AutoDock Vina. (C) Magnification of the highlighted area showing GSK2606414 interactions with the residues Val-401, Asn-436, Phe-439, Ile-543, and Val-546 of ABCG2.
4 Discussion
Dorsomorphin, initially named as compound C, was earlier identified as an AMP-activated protein kinase (AMPK; Ki = 109 nM) inhibitor but did not obviously suppress the activities of structurally related kinases, including JAK3, PKA, PKCθ, SYK, and ZAPK (Zhou et al., 2001). Dorsomorphin was also observed to inhibit the bone morphogenetic protein (BMP) type I receptors activin receptor-like kinase (ALK) 2, ALK3, and ALK6, thereby suppressing BMP-induced SMAD1/5/8 phosphorylation and transcription of the target genes in zebrafish (Yu et al., 2008). This compound was named dorsomorphin owing to its ability to induce dorsoventral patterning defects that usually occur in BMP-pathway-mutant zebrafish embryos (Yu et al., 2008). In a profiling study of dorsomorphin against 70 human kinases, dorsomorphin at 1 μM suppressed the activities of 10 out of 70 kinases more powerfully than it suppressed 73% of the AMPK activity, including (from the strongest to weakest) MELK1, PHK, DYRK3, ERK8, DYRK1A, MNK1, Lck, DYRK2, Src, and HIPK2 (Bain et al., 2007). In another profiling study of dorsomorphin against 123 human kinases, dorsomorphin at 1 μM suppressed the activities of 31 out of 123 kinases more powerfully than it suppressed 50% of the AMPK activity, including (from the strongest to weakest) VEGFR, RIPK2, ERK8, GCK, CLK2, DYRK1A, PHK, ABL, CAMKKβ, CK1, NUAK1, MELK, PRK2, YES1, Lck, EPHB2, IRAK4, TrkA, HIPK2, MINK1, IRR, EPHB4, Src, EPHA2, MLK3, FGFR1, DYRK3, EPHB3, CK2, ALK3, and MARK3 (Vogt et al., 2011). In the present study, we found that dorsomorphin at 0.3 μM can inhibit the ABCG2 transporter activity, thereby preserving the chemotherapeutic agents mitoxantrone and doxorubicin to antagonize MDR in ABCG2-overexpressing colorectal cancer cells. Additionally, dorsomorphin does not alter ABCG2 protein expression. The results of molecular docking show that dorsomorphin is bound stably to the ABCG2-binding pockets; therefore, dorsomorphin is a multitarget agent.
The anticancer effects of dorsomorphin have been explored extensively. Dorsomorphin causes cell-cycle arrest at the G2/M phase and apoptosis in glioma cells through AMPK-dependent and -independent mechanisms (Vucicevic et al., 2009). Another study has shown that the dorsomorphin AMPK-independent mode induces G2/M cell-cycle arrest, autophagy, and necroptosis through activation of the calpain/cathepsin pathway and inhibition of AKT/mTORC1/C2 in glioma cells (Liu et al., 2014). Additionally, the dorsomorphin AMPK-independent mode induces apoptosis through increased ceramide production in breast cancer cells (Jin et al., 2009). Dorsomorphin also causes G2/M cell-cycle arrest and growth inhibition by inducing autophagy and apoptosis in human colorectal cancer cells (Yang et al., 2012). Moreover, dorsomorphin inhibits cell growth and migration by interfering with the Akt/mTOR/Wnt pathways in colon cancer cells (Ghanaatgar-Kasbi et al., 2019). The dorsomorphin AMPK-independent mode enhances TRAIL-induced apoptosis through reactive-oxygen-species-mediated decreases of c-FLIPL and Mcl-1 in human renal cancer cells (Jang et al., 2010). Dorsomorphin also AMPK-independently causes cancer cell apoptosis and enhances the sensitivity of cancer cells to both HSP90 and proteasome inhibitors through downregulation of the nuclear heat shock factor 1 (Li et al., 2019). Dorsomorphin sensitizes multiple acute leukemia cells to BH3 mimetic-induced cell death by decreasing the phosphorylation of BAD at Ser75 and Ser99 to enhance BAD translocation to the mitochondria and inhibit BCLXL (Jia et al., 2024). In the present work, our data show that dorsomorphin can restore the sensitivity of mitoxantrone and doxorubicin in colorectal cancer cells with ABCG2 overexpression in vitro. However, the effects of dorsomorphin in vivo need to be explored in the future. In conclusion, dorsomorphin is a potent ABCG2 inhibitor that can attenuate ABCG2-mediated MDR in colorectal cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
X-PL: investigation, methodology, project administration, writing–original draft, and writing–review and editing. L-QC: investigation, project administration, validation, writing–original draft, and writing–review and editing. Z-ZY: methodology, project administration, software, writing–original draft, and writing–review and editing. KH: formal analysis, resources, and writing–review and editing. P-BD: investigation, validation, and writing–review and editing. J-SL: data curation, formal analysis, and writing–review and editing. Y-YS: methodology, validation, and writing–review and editing. Z-BS: formal analysis, software, and writing–review and editing. Z-MY: funding acquisition, resources, and writing–review and editing. ZS: conceptualization, funding acquisition, resources, supervision, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the financial support from the National Natural Science Foundation of China (No. 82272996 to ZS), the Science and Technology Program of Guangzhou (No. 202206010081 to ZS), and the Foundation of Guangzhou Municipal Science and Technology Bureau (No. 202002020088 to Z-MY).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1393693/full#supplementary-material
References
Bain, J., Plater, L., Elliott, M., Shpiro, N., Hastie, C. J., McLauchlan, H., et al. (2007). The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408 (3), 297–315. doi:10.1042/bj20070797
Eadie, L. N., Hughes, T. P., and White, D. L. (2014). Interaction of the efflux transporters ABCB1 and ABCG2 with imatinib, nilotinib, and dasatinib. Clin. Pharmacol. Ther. 95 (3), 294–306. doi:10.1038/clpt.2013.208
Eckenstaler, R., and Benndorf, R. A. (2020). 3D structure of the transporter ABCG2-What's new? Br. J. Pharmacol. 177 (7), 1485–1496. doi:10.1111/bph.14991
Fan, J., To, K. K. W., Chen, Z. S., and Fu, L. (2023). ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist Updat 66, 100905. doi:10.1016/j.drup.2022.100905
Gao, H. L., Cui, Q., Wang, J. Q., Ashby, C. R., Chen, Y., Shen, Z. X., et al. (2023). The AKT inhibitor, MK-2206, attenuates ABCG2-mediated drug resistance in lung and colon cancer cells. Front. Pharmacol. 14, 1235285. doi:10.3389/fphar.2023.1235285
Ghanaatgar-Kasbi, S., Amerizadeh, F., Rahmani, F., Hassanian, S. M., Khazaei, M., Ferns, G. A., et al. (2019). AMP-kinase inhibitor dorsomorphin reduces the proliferation and migration behavior of colorectal cancer cells by targeting the AKT/mTOR pathway. IUBMB Life 71 (12), 1929–1936. doi:10.1002/iub.2136
Ghasemian, A., Omear, H. A., Mansoori, Y., Mansouri, P., Deng, X., Darbeheshti, F., et al. (2023). Long non-coding RNAs and JAK/STAT signaling pathway regulation in colorectal cancer development. Front. Genet. 14, 1297093. doi:10.3389/fgene.2023.1297093
Jang, J. H., Lee, T. J., Yang, E. S., Min do, S., Kim, Y. H., Kim, S. H., et al. (2010). Compound C sensitizes Caki renal cancer cells to TRAIL-induced apoptosis through reactive oxygen species-mediated down-regulation of c-FLIPL and Mcl-1. Exp. Cell Res. 316 (13), 2194–2203. doi:10.1016/j.yexcr.2010.04.028
Jia, J., Ji, W., Saliba, A. N., Csizmar, C. M., Ye, K., Hu, L., et al. (2024). AMPK inhibition sensitizes acute leukemia cells to BH3 mimetic-induced cell death. Cell Death Differ. 31, 405–416. doi:10.1038/s41418-024-01283-9
Jin, J., Mullen, T. D., Hou, Q., Bielawski, J., Bielawska, A., Zhang, X., et al. (2009). AMPK inhibitor Compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J. Lipid Res. 50 (12), 2389–2397. doi:10.1194/jlr.M900119-JLR200
Li, N., Wang, T., Li, Z., Ye, X., Deng, B., Zhuo, S., et al. (2019). Dorsomorphin induces cancer cell apoptosis and sensitizes cancer cells to HSP90 and proteasome inhibitors by reducing nuclear heat shock factor 1 levels. Cancer Biol. Med. 16 (2), 220–233. doi:10.20892/j.issn.2095-3941.2018.0235
Li, W., Zhang, H., Assaraf, Y. G., Zhao, K., Xu, X., Xie, J., et al. (2016). Overcoming ABC transporter-mediated multidrug resistance: molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat 27, 14–29. doi:10.1016/j.drup.2016.05.001
Lin, P. C., Lin, H. H., Lin, J. K., Lin, C. C., Yang, S. H., Li, A. F., et al. (2013). Expression of ABCG2 associated with tumor response in metastatic colorectal cancer patients receiving first-line FOLFOX therapy--preliminary evidence. Int. J. Biol. Markers 28 (2), 182–186. doi:10.5301/jbm.5000004
Liu, K., Chen, Y., Shi, X. B., Xing, Z. H., He, Z. J., Wang, S. T., et al. (2022). Inhibiting the activity of ABCG2 by KU55933 in colorectal cancer. Recent Pat. Anticancer Drug Discov. 17 (4), 387–395. doi:10.2174/1574892817666220112100036
Liu, K., Li, Y. C., Chen, Y., Shi, X. B., Xing, Z. H., He, Z. J., et al. (2021). AZ32 reverses ABCG2-mediated multidrug resistance in colorectal cancer. Front. Oncol. 11, 680663. doi:10.3389/fonc.2021.680663
Liu, X., Chhipa, R. R., Nakano, I., and Dasgupta, B. (2014). The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Mol. Cancer Ther. 13 (3), 596–605. doi:10.1158/1535-7163.Mct-13-0579
Miyata, H., Takada, T., Toyoda, Y., Matsuo, H., Ichida, K., and Suzuki, H. (2016). Identification of febuxostat as a new strong ABCG2 inhibitor: potential applications and risks in clinical situations. Front. Pharmacol. 7, 518. doi:10.3389/fphar.2016.00518
Narayanan, S., Fan, Y. F., Gujarati, N. A., Teng, Q. X., Wang, J. Q., Cai, C. Y., et al. (2021). VKNG-1 antagonizes ABCG2-mediated multidrug resistance via p-AKT and bcl-2 pathway in colon cancer: in vitro and in vivo study. Cancers (Basel) 13 (18), 4675. doi:10.3390/cancers13184675
Nielsen, D. L., Palshof, J. A., Brunner, N., Stenvang, J., and Viuff, B. M. (2017). Implications of ABCG2 expression on irinotecan treatment of colorectal cancer patients: a review. Int. J. Mol. Sci. 18 (9), 1926. doi:10.3390/ijms18091926
Noguchi, K., Katayama, K., Mitsuhashi, J., and Sugimoto, Y. (2009). Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv. Drug Deliv. Rev. 61 (1), 26–33. doi:10.1016/j.addr.2008.07.003
Palshof, J. A., Cederbye, C. N., Høgdall, E. V. S., Poulsen, T. S., Linnemann, D., Nygaard, S. B., et al. (2020). ABCG2 protein levels and association to response to first-line irinotecan-based therapy for patients with metastatic colorectal cancer. Int. J. Mol. Sci. 21 (14), 5027. doi:10.3390/ijms21145027
Shi, Z., Peng, X. X., Kim, I. W., Shukla, S., Si, Q. S., Robey, R. W., et al. (2007). Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 67 (22), 11012–11020. doi:10.1158/0008-5472.Can-07-2686
Stacy, A. E., Jansson, P. J., and Richardson, D. R. (2013). Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 84 (5), 655–669. doi:10.1124/mol.113.088609
Sugimoto, Y., Tsukahara, S., Imai, Y., Sugimoto, Y., Ueda, K., and Tsuruo, T. (2003). Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol. Cancer Ther. 2 (1), 105–112.
Toyoda, Y., Takada, T., and Suzuki, H. (2019). Inhibitors of human ABCG2: from technical background to recent updates with clinical implications. Front. Pharmacol. 10, 208. doi:10.3389/fphar.2019.00208
Vogt, J., Traynor, R., and Sapkota, G. P. (2011). The specificities of small molecule inhibitors of the TGFß and BMP pathways. Cell Signal 23 (11), 1831–1842. doi:10.1016/j.cellsig.2011.06.019
Vucicevic, L., Misirkic, M., Janjetovic, K., Harhaji-Trajkovic, L., Prica, M., Stevanovic, D., et al. (2009). AMP-activated protein kinase-dependent and -independent mechanisms underlying in vitro antiglioma action of compound C. Biochem. Pharmacol. 77 (11), 1684–1693. doi:10.1016/j.bcp.2009.03.005
Wang, J., Wang, J. Q., Cai, C. Y., Cui, Q., Yang, Y., Wu, Z. X., et al. (2020a). Reversal effect of ALK inhibitor NVP-TAE684 on ABCG2-overexpressing cancer cells. Front. Oncol. 10, 228. doi:10.3389/fonc.2020.00228
Wang, J. Q., Teng, Q. X., Lei, Z. N., Ji, N., Cui, Q., Fu, H., et al. (2020b). Reversal of cancer multidrug resistance (MDR) mediated by ATP-binding cassette transporter G2 (ABCG2) by AZ-628, a RAF kinase inhibitor. Front. Cell Dev. Biol. 8, 601400. doi:10.3389/fcell.2020.601400
Yang, W. L., Perillo, W., Liou, D., Marambaud, P., and Wang, P. (2012). AMPK inhibitor compound C suppresses cell proliferation by induction of apoptosis and autophagy in human colorectal cancer cells. J. Surg. Oncol. 106 (6), 680–688. doi:10.1002/jso.23184
Yang, Y., Wu, Z. X., Wang, J. Q., Teng, Q. X., Lei, Z. N., Lusvarghi, S., et al. (2021). OTS964, a TOPK inhibitor, is susceptible to ABCG2-mediated drug resistance. Front. Pharmacol. 12, 620874. doi:10.3389/fphar.2021.620874
Yu, P. B., Hong, C. C., Sachidanandan, C., Babitt, J. L., Deng, D. Y., Hoyng, S. A., et al. (2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4 (1), 33–41. doi:10.1038/nchembio.2007.54
Yu, Z. Z., Xu, B. Q., Wang, Y. Y., Zhang, P. W., Shu, Y. B., and Shi, Z. (2023). GSK2606414 sensitizes ABCG2-overexpressing multidrug-resistant colorectal cancer cells to chemotherapeutic drugs. Biomedicines 11 (11), 3103. doi:10.3390/biomedicines11113103
Zhang, W. J., Li, Y., Wei, M. N., Chen, Y., Qiu, J. G., Jiang, Q. W., et al. (2017). Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 386, 100–109. doi:10.1016/j.canlet.2016.11.011
Keywords: dorsomorphin, chemosensitivity, ABCG2, multidrug resistance, colorectal cancer
Citation: Li X-P, Cao L-Q, Yu Z-Z, He K, Ding P-B, Li J-S, Shan Y-Y, Su Y-B, Yuan Z-M and Shi Z (2024) Dorsomorphin attenuates ABCG2-mediated multidrug resistance in colorectal cancer. Front. Pharmacol. 15:1393693. doi: 10.3389/fphar.2024.1393693
Received: 29 February 2024; Accepted: 25 April 2024;
Published: 24 May 2024.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Changliang Shan, Nankai University, ChinaXiaofang Guo, University of South Florida, United States
Copyright © 2024 Li, Cao, Yu, He, Ding, Li, Shan, Su, Yuan and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Shi, dHNoaXpoaUBqbnUuZWR1LmNu
†These authors have contributed equally to this work
 Xiao-Peng Li
Xiao-Peng Li Liang-Qi Cao3†
Liang-Qi Cao3† Ke He
Ke He Zhong-Min Yuan
Zhong-Min Yuan Zhi Shi
Zhi Shi