- 1Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong, Hong Kong SAR, China
- 3Key Laboratory of Glucolipid Metabolic Diseases of the Ministry of Education, Guangdong Pharmaceutical University, Guangzhou, China
- 4Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada
- 5Department of Cardiology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Editorial on the Research Topic
Clinical evidence for and advances in translational research on the classic formulas of traditional Chinese medicine
1 Introduction
Traditional Chinese medicine (TCM) is one of the ancient healing practices with a history of more than 2,500 years and unique theories that has been making great contributions to human healthcare. It includes Chinese herbal medicine, food therapy, acupuncture, moxibustion, massage (tuina), and physical exercises, among others (Tang et al., 2008), and is becoming increasingly popular in other Asian and Western countries. The classic formulas of TCM have clear verifiable scientific literature on traditional usage and are generally recognized to be derived from ancient medical books, such as Treatise on Febrile Diseases (伤寒论, Shang Han Lun) and Synopsis of the Golden Chamber (金匮要略, Jin Gui Yao Lue). TCM is also popular in Japan and Korea, so the classic formulas of TCM are also widely used in traditional Japanese Kampo medicine and traditional Korean medicine. TCM possesses the characteristics of fixed components (generally greater than or equal to two herbs) and clear curative effects with fewer adverse effects in clinical treatment. The classic formulas of TCM have made substantial contributions to the wellbeing of mankind, including viral diseases, cardiovascular and cerebrovascular diseases, digestive diseases, endocrine diseases, and tumors.
2 Clinical evidence for the classic formulas of TCM
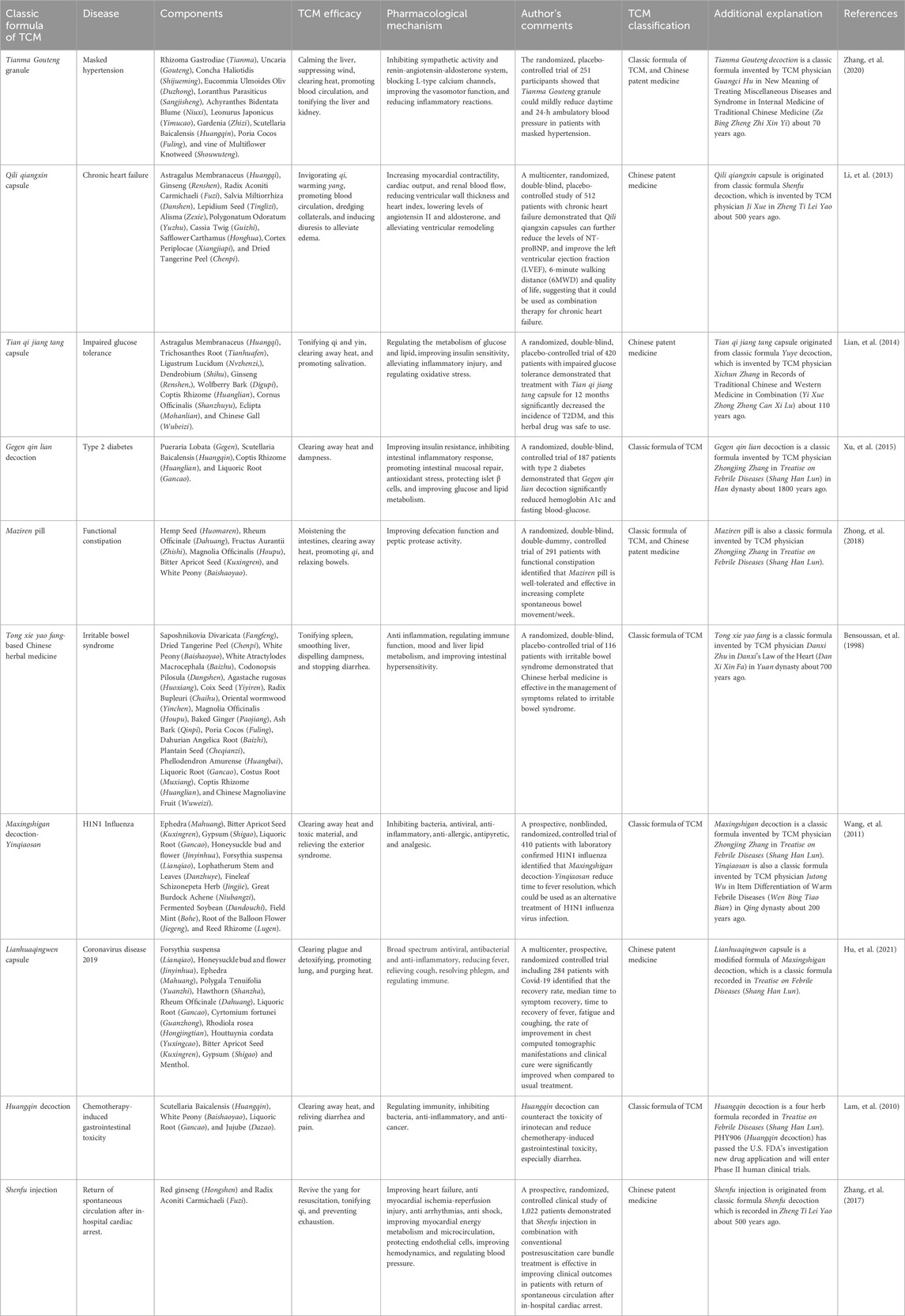
Currently, rigorous “totality of evidence” of the classic formulas of TCM that includes chemical standardization, biological assays, experimental studies, and clinical trials is receiving increasing attention from modern medicine. Several paradigms in the clinical and translational research efforts on the classic formulas of TCM, such as Tianma Gouteng decoction for hypertension (Zhang et al., 2020), Qili qiangxin capsule (Shenfu decoction) for chronic heart failure (Li et al., 2013), Tian qi jiang tang capsule (Yuye decoction) for impaired glucose tolerance (Lian et al., 2014), Gegenqinlian decoction for T2DM (Xu et al., 2015), Hemp seed pill for functional constipation (Zhong et al., 2018), Tong xie yao fang-based Chinese herbal medicine for irritable bowel syndrome (Bensoussan et al., 1998), Maxingshigan decoction-Yinqiaosan for H1N1 influenza virus (Wang et al., 2011), Maxingshigan decoction for COVID-19 (Hu et al., 2021), PHY906 (Huangqin decoction) for chemotherapy-induced gastrointestinal toxicity (Lam et al., 2010), and Shenfu injection for patients with return of spontaneous circulation after in-hospital cardiac arrest (Zhang et al., 2017), highlight the tremendous progress and advances in this field (as shown in Table 1). Furthermore, large amounts of clinical evidence have provided some biological functions and potential mechanisms of the classic formulas of TCM. These suggest that the classic formulas of TCM could be considered effective complementary and alternative approaches in the future.
3 Classic formulas of TCM-oriented new drug development model
Owing to challenges in modern drug research and development such as improving investments and preventing the decline of drug approval, scientists have focused their attention on natural herbs and the classic formulas of TCM to create promising drugs and drug candidates. Derivatives from natural products have been widely used as cornerstones in Western medicine, such as aspirin, digitalis, and paclitaxel. Recently, natural herbs and the classic formulas of TCM-oriented new drug development model have achieved significant breakthroughs, including artemisinin and dihydroartemisinin for malaria (Tu, 2011), arsenic trioxide for acute promyelocytic leukemia (Soignet et al., 1998), tripterygium glycosides for active rheumatoid arthritis (Lv et al., 2015), red yeast rice for hyperlipidemia (Zhao et al., 2004), and berberine for hyperlipidemia and diabetes (Kong et al., 2004; Zhang et al., 2008); these can help avoid blind screening while reducing the cost and time required for drug development.
4 Advances in translational research on the classic formulas of TCM
Given the difficulty of retrieving original literature in Chinese as well as the poor methodological quality of the original clinical trials, such as high risk of bias, unclear reporting of outcome measures, lack of preregistration in an international database, lack of high-level clinical recommendation evidence, and insufficient data reports on the toxicology, pharmacological effects, and adverse effects, several classic formulas of TCM have been regarded as a “mystery” to modern science. Accordingly, more clinical and experimental data are warranted to verify their application values.
A total of 43 papers were received under this Research Topic, out of which eight papers were accepted for publication. The original research articles and reviews in this Research Topic cover a wide range of medical conditions, including essential hypertension, contrast-induced nephropathy after percutaneous coronary intervention, hepatocellular carcinoma, lung cancer, doxorubicin-induced cardiotoxicity, DNCB-induced atopic-dermatitis-like skin lesions, and heat stroke.
Two papers elaborate on the clinical applications of the classic formulas of TCM for cardiovascular diseases. Lin et al. provide clinical evidence on the Qiangli Dingxuan tablet, which is widely used in traditional Chinese patent medicine for the treatment of essential hypertension. Fu et al. evaluated the clinical improvement of contrast-induced nephropathy after percutaneous coronary intervention with the compound Danshen dripping pills, which could be used to relieve angina pectoris.
Three papers clarify the clinical efficacies and mechanisms of the classic formulas of TCM for tumor-based diseases. Luo et al. tested the protective effects of the Jianpi Huayu decoction for hepatocellular carcinoma. Shahid et al. investigated the effectiveness and mechanisms of the medicinal mushroom Ganoderma lucidum for lung tumorigenesis induced by the carcinogens in tobacco smoke. Wang et al. addressed the effects of the active ingredients in Salvia miltiorrhiza on doxorubicin-induced cardiotoxicity.
Additionally, Zhao et al. examined the therapeutic effects of the Fangji Dihuang formulation, a classic formula of TCM first described by Zhongjing Zhang (150–219 A.D.), for DNCB-induced atopic-dermatitis-like skin lesions. The remaining two articles focus on research into heat stroke and the quality markers of the classic formulas of TCM.
5 Conclusion
In recent times, steadily increasing amounts of evidence have shown that the classic formulas of TCM have important clinical value in the treatment of various conditions, including chronic and infectious diseases. Findings under this Research Topic can significantly promote comprehension of the therapeutic efficacies and potential protective mechanisms of the classic formulas of TCM. Thus, this Research Topic encompasses exploration of new clinical evidence and novel approaches in the application of the classic formulas of TCM to the treatment of various diseases, paving the evidence-based path for the future.
Author contributions
PW: writing–original draft, and review and editing. WC-sC: writing–review and editing. DY: writing–review and editing. YZ: writing–review and editing. XX: writing–original draft, and review and editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Central High Level Traditional Chinese Medicine Hospital Clinical Research and Achievement Transformation Ability Enhancement Project (Nos. HLCMHPP2023081 and CZ30981), National Natural Science Foundation of China (No. 82174101), Beijing-Tianjin-Hebei Basic Research Cooperation Special Project (No. J230037), Joint Research and Development Project of China Science and Technology Development Center for Chinese Medicine (No. CXZH202301), Young Elite Scientists Sponsorship Program by CACM (No. 2019-QNRC2-A02), Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (No. CI 2021A03804), and Fundamental Research Funds for the Central Public Welfare Research Institutes (Nos. ZZ14-YQ-023, ZZ11-073, and ZXKT21017).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
References
Bensoussan, A., Talley, N. J., Hing, M., Menzies, R., Guo, A., and Ngu, M. (1998). Treatment of irritable bowel syndrome with Chinese herbal medicine: a randomized controlled trial. JAMA 280 (18), 1585–1589. doi:10.1001/jama.280.18.1585
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2021). Efficacy and Safety of Lianhuaqingwen Capsules, a repurposed Chinese Herb, in Patients with Coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. PHYTOMEDICINE 94, 153242. doi:10.1016/j.phymed.2020.153242
Kong, W., Wei, J., Abidi, P., Lin, M., Inaba, S., Li, C., et al. (2004). Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10 (12), 1344–1351. doi:10.1038/nm1135
Lam, W., Bussom, S., Guan, F., Jiang, Z., Zhang, W., Gullen, E. A., et al. (2010). The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med. 2 (45), 45ra59–59. doi:10.1126/scitranslmed.3001270
Li, X., Zhang, J., Huang, J., Ma, A., Yang, J., Li, W., et al. (2013). A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J. Am. Coll. Cardiol. 62 (12), 1065–1072. doi:10.1016/j.jacc.2013.05.035
Lian, F., Li, G., Chen, X., Wang, X., Piao, C., Wang, J., et al. (2014). Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J. Clin. Endocrinol. Metab. 99 (2), 648–655. doi:10.1210/jc.2013-3276
Lv, Q. W., Zhang, W., Shi, Q., Zheng, W. J., Li, X., Chen, H., et al. (2015). Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann. Rheum. Dis. 74 (6), 1078–1086. doi:10.1136/annrheumdis-2013-204807
Soignet, S. L., Maslak, P., Wang, Z. G., Jhanwar, S., Calleja, E., Dardashti, L. J., et al. (1998). Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339 (19), 1341–1348. doi:10.1056/NEJM199811053391901
Tang, J. L., Liu, B. Y., and Ma, K. W. (2008). Traditional Chinese medicine. Lancet 372 (9654), 1938–1940. doi:10.1016/S0140-6736(08)61354-9
Tu, Y. (2011). The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 17 (10), 1217–1220. doi:10.1038/nm.2471
Wang, C., Cao, B., Liu, Q. Q., Zou, Z. Q., Liang, Z. A., Gu, L., et al. (2011). Oseltamivir compared with the Chinese traditional therapy Maxingshigan–Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern Med. 155 (4), 217–225. doi:10.7326/0003-4819-155-4-201108160-00005
Xu, J., Lian, F., Zhao, L., Zhao, Y., Chen, X., Zhang, X., et al. (2015). Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 9 (3), 552–562. doi:10.1038/ismej.2014.177
Zhang, D. Y., Cheng, Y. B., Guo, Q. H., Shan, X. L., Wei, F. F., Lu, F., et al. (2020). Treatment of masked hypertension with a Chinese herbal formula: a randomized, placebo-controlled trial. CIRCULATION 142 (19), 1821–1830. doi:10.1161/CIRCULATIONAHA.120.046685
Zhang, Q., Li, C., Shao, F., Zhao, L., Wang, M., and Fang, Y. (2017). Efficacy and safety of combination therapy of Shenfu injection and postresuscitation bundle in patients with return of spontaneous circulation after in-hospital cardiac arrest: a randomized, assessor-blinded, controlled trial. Crit. Care Med. 45 (10), 1587–1595. doi:10.1097/CCM.0000000000002570
Zhang, Y., Li, X., Zou, D., Liu, W., Yang, J., Zhu, N., et al. (2008). Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 93 (7), 2559–2565. doi:10.1210/jc.2007-2404
Zhao, S. P., Liu, L., Cheng, Y. C., Shishehbor, M. H., Liu, M. H., Peng, D. Q., et al. (2004). Xuezhikang, an extract of cholestin, protects endothelial function through antiinflammatory and lipid-lowering mechanisms in patients with coronary heart disease. Circulation 110 (8), 915–920. doi:10.1161/01.CIR.0000139985.81163.CE
Keywords: traditional Chinese medicine, classic formulas, Chinese herbal medicine, translational research, evidence-based medicine
Citation: Wang P, Cho WC-s, Ye D, Zhang Y and Xiong X (2024) Editorial: Clinical evidence for and advances in translational research on the classic formulas of traditional Chinese medicine. Front. Pharmacol. 15:1392930. doi: 10.3389/fphar.2024.1392930
Received: 28 February 2024; Accepted: 13 March 2024;
Published: 15 April 2024.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Luca Rastrelli, University of Salerno, ItalyCopyright © 2024 Wang, Cho, Ye, Zhang and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingjiang Xiong, eGlvbmd4aW5namlhbmd0Y21AMTYzLmNvbQ==
 Pengqian Wang
Pengqian Wang William Chi-shing Cho
William Chi-shing Cho Dewei Ye
Dewei Ye Yuqing Zhang4
Yuqing Zhang4 Xingjiang Xiong
Xingjiang Xiong