- Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Gonadotrophin-releasing hormone analogs (GnRHas) play a significant role in addressing gynecological diseases, central precocious puberty, and cancer. However, ensuring the safety of GnRHas in real-world applications requires continuous vigilance. In light of this, we undertook a disproportionality analysis focused on adverse events (AEs) associated with GnRHas using data from both the FDA Adverse Event Reporting System (FAERS) and the Japanese Adverse Drug Event Report (JADER). We evaluated GnRHas-associated AEs and characterized the clinical priority of unlisted AEs caused by each GnRHa from the different databases.
Methods: In the disproportionality analysis, we applied two adjusted algorithms to identify signals related to GnRHas in the FAERS and JADER databases from 2004 to 2023. Additionally, we utilized the Statistical Analysis System (SAS, 9.4) to examine potential and high-aROR (adjusted reporting odds ratio) signals associated with GnRHas. We performed clinical priority assessment for suspicious PTs and an analysis of serious/non-serious outcomes. We also gathered information on the onset times of AEs linked with GnRHas from both databases.
Results: From January 2004 to September 2023, FAERS and JADER recorded a total of 50,360,413 and 1,440,200 AEs, respectively. Employing two algorithms, the suspicious preferred terms (PTs) related to leuprolide (Leu) were 562 potential PTs (44 unlisted in specifications), followed by goserelin (Gos) with 189 PTs (28 unlisted), triptorelin (Tri) with 172 PTs (28 unlisted), and Leu-JADER with 85 PTs (10 unlisted). At the same PT level, the differences in GnRHas between the two databases were observed, such as cardiac failure, diabetes mellitus, liver disorder, dementia, suicidal ideation, interstitial lung disease, urinary disorders, and hypertensive crisis. In an analysis of serious vs. non-serious outcomes, a total of 43 AEs of Leu were more likely to be reported as serious AEs with p < 0.05 (such as asthenia, urinary retention, diabetes mellitus, interstitial lung disease, gait disturbance, and so on), following by Tri (6 AEs), and Gos (4 AEs). Based on the clinical priority score, 41 PTs of Leu, 26 PTs of Tri, 24 PTs of Gos, and 8 PTs of Leu-JADER were graded as weak. There were 3 PTs of Leu, 2 PTs of Tri, 4 PTs of Gos, and 2 PTs of Leu-JADER that were graded as moderate. Notably, in the assessment of the relevant evidence, 2 PTs (loss of libido and urinary tract toxicity caused by Leu), 1 PT (electrolyte imbalance caused by Tri), and 2 PTs (anorexia and suicidal ideation caused by Gos) showed a strong level of evidence with “++.” The differences in the signal strength of the same PTs from two databases were also worth noting. Moreover, the median onset time for GnRHas (Leu, Tri, and Gos) was 23 days (0, 298), 22 days (0, 181), and 217 days (29, 706), respectively, as median (Q1, Q3).
Conclusion: An examination of two databases revealed suspicious AEs associated with GnRHas. Our study found potential new AE signals of GnRHas and supported continuous clinical monitoring, pharmacovigilance, regional differences, and further studies of GnRHas.
Introduction
Hormone-dependent cancers, such as breast and prostate cancers, constitute over 20% of all cancers globally and over 35% of cancers in women (Sung et al., 2021; Rižner and Romano, 2023). Imbalances at any level of the hypothalamic–pituitary–gonadal (HPG) axis may lead to various hormone-dependent diseases (HDDs), including precocious puberty, infertility, hormone-dependent cancers, polycystic ovarian syndrome, endometriosis, and uterine fibroids (Newton et al., 2018). Gonadotropin-releasing hormone analogs (GnRHas), synthetic derivatives of the hormone gonadorelin, stimulate the pituitary gland within the brain, promoting the production of reproductive hormones, including luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (Veth et al., 2023).
Hormone-dependent diseases also include noncancerous conditions such as precocious puberty, endometriosis, uterine fibroids, Cushing’s syndrome, and polycystic ovarian syndrome (PCOS) (Zondervan et al., 2020; Rižner and Romano, 2023). Although these conditions are non-life-threatening, they significantly impact quality of life. Hormonal therapy refers to both androgen deprivation therapy for prostate cancer and endocrine therapy for breast cancer (Okwuosa et al., 2021). Approximately 10% of women of reproductive age are affected by endometriosis. Additionally, central precocious puberty (CPP) is a prevalent pediatric hormone-dependent disorder (HDD) that can result in the premature activation of the hypothalamic–pituitary–gonadal (HPG) axis. It is noteworthy that approximately 90% of girls with CPP experience idiopathic central precocious puberty (ICPP) (Zondervan et al., 2020; Hou et al., 2024). CPP could affect the physical and psychological health of children. Since the 1980s, GnRHas, acting as inhibitors binding to the GnRH receptor in the anterior pituitary, have been extensively employed for the treatment of CPP (Carel et al., 2009). Furthermore, the combination of GnRHas with chemotherapy in premenopausal patients with breast cancer has been shown to decrease the risk of premature ovarian insufficiency, which also contributes to the restoration of ovarian function (Zong et al., 2022). However, the prolonged administration of GnRHas, including leuprorelin, triptorelin, goserelin, buserelin, and histrelin, leads to the suppression of gonadal function. Consequently, this influence extends to hormone levels within the HPG axis, including testosterone, estrogen, and progesterone (Harris et al., 2022; Raja et al., 2022). GnRHas also could result in a reduction in ovarian hyperstimulation syndrome and bone mineral density, like menopausal symptoms, since they momentarily halt the synthesis of reproductive hormones (Fatemi and Garcia-Velasco, 2015; Veth et al., 2023). A study examining bone mineral density (BMD) in short children undergoing growth hormone (GH) and GnRHas treatment revealed that 2 years of GnRHas supplementation, alongside GH treatment, had no adverse effects on BMD or body composition (Lem et al., 2013). In addition to hormone-related symptoms such as hot flushes, osteopenia, and reproductive system disorders, what other adverse events (AEs) could potentially be induced by GnRHas?
In a Cochrane meta-analysis, aside from the risk of hot flushes (RR 1.62; 95% CI 0.87–3.02), the use of GnRHas may also be associated with higher incidence of vasodilatation (RR 2.69; 95% CI 1.51–4.81), headache (RR 3.55; 95% CI 1.09–11.53), and sleep disturbances (RR 2.31; 95% CI 1.33–4.02) compared to placebo (Veth et al., 2023). In a phase-2 randomized clinical trial (RCT), the combination with GnRH analogs was found to significantly elevate the risk of erectile dysfunction (30.8%), decreased libido (42.3%), bone pain (23.1%), myalgia (17.3%), and sleeping disorders (7.7%) (Reinisch et al., 2021).
Recently, two large real-world spontaneous adverse event reporting databases—the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) and the Japanese Adverse Drug Event Report (JADER) —have collected abundant AEs reports from different cohorts (one mainly from America and one mainly from the Japan) (Neishi et al., 2023). These two databases are reported to have different features, such as FAERS having many non-serious AEs reported from non-health care professionals while JADER has many serious AEs many serious AEs reported by medical personnel (Nomura et al., 2015; Nagai and Ishikawa, 2021). The differences of reporting areas and reporters may result in different tendencies of these databases.
Methods
Data sources
We collected the data of all GnRHas (leuprorelin, triptorelin, goserelin, buserelin, and histrelin) from FAERS and JADER from January 2004 to September 2023. Medical personnel, consumers, manufacturers, and others submit AEs and updates to these databases as spontaneous report systems (Zou et al., 2023b). From JADER, this study defined AEs using the PTs from the standardized Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J 26.0). The data files of FAERS and JADER databases are available from their official websites (https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html; https://www.info.pmda.go.jp/fukusayoudb/CsvDownload).
Data extraction and descriptive analysis
We searched the disproportionality signals of GnRHas and their types in medical subject headings [MeSH] in Supplementary Table S1 from FAERS and JADER. We removed duplicate reports sharing the same identifier number from the demographic file and conducted an interlinkage of the reaction file with MedDRA using the PT code (Zou et al., 2023b). All data were imported into SAS software (v9.4), with the primary id as the primary link field (primary key) between different data files—patient demographic file [DEMO], drug file [DRUG], adverse events file [REAC], outcome file [OUTC], report source file [RPSR], drug therapy file [THER], and drug indication [INDI] (Shu et al., 2023a).
We screened cases by generic names and trade names (searching by MeSH in Supplementary Table S1) in the DRUG file and chose role_cod as the primary suspected (PS) in FAERS. The process of data mining is shown in Supplementary Figure S1. Because the quantity of some GnRHas (buserelin and histrelin) was too small, we ultimately only analyzed other GnRHas (leuprorelin, triptorelin, and goserelin). It is noteworthy that the serious outcomes included death, threats to life, hospitalization, disability, and other serious outcomes (Shu et al., 2022). We thus performed a statistical analysis of serious vs. non-serious (Burk et al., 2023).
Clinical prioritization of signals
According to the designated medical event (DME) and important medical event (IME) lists from the European Medicines Agency (EMA), we created a semiquantitative score method to rank the significant disproportionality PTs in order to display special AEs (Shu et al., 2023b). The significant disproportionate AEs with weak, moderate, or strong clinical priority depended on scores of 0 and 3, 4 and 6, or 7 and 9, respectively (Gatti et al., 2021; Shu et al., 2022).
Statistical analysis
The adjusted reporting odds ratio (aROR) and information component (IC) of Bayesian confidence propagation neural network (BCPNN), two of the algorithms used in the disproportionality analysis, were based on the 2 × 2 table calculation principle in Supplementary Table S1 (Nagai and Ishikawa, 2021; Zou et al., 2023a; Jain et al., 2023). For the sake of robustness, statistical shrinkage transformation was performed, with the calculation formulas for aROR and IC after transformation displayed in Supplementary Table S1 (Noren et al., 2013; Chen et al., 2021). We used the following statistical methods to observe the value of P: Pearson’s chi-squared (χ2) test, Fisher’s exact test, and Mann–Whitney U test (Sahai and Khurshid, 1995; Chau et al., 2017; Shi et al., 2023). The time-to-onset of adverse events used the formula Time-to-onset = Event onset date (EVENT_DT) –Therapy start date (START_DT), while we deleted missing or unreasonable data like the negative number of time-to-onset (24).
Results
Descriptive analysis
There were 50,360,413 AEs and 1,440,200 AEs reported in the FAERS and JADER databases from January 2004 to September 2023. After data mining and calculation in Supplementary Figure S1, 191,287 AEs of GnRHas were collected in our study, including leuprorelin (167,046), triptorelin (8,947), goserelin (12,042), leuprorelin (3,252 AEs from JADER), and goserelin (784 AEs from JADER). The characteristics of AEs, including age, sex, outcomes, indications, and the reports’ countries, are shown in Table 1. In terms of gender distribution, the proportions of male individuals were higher than female individuals for other GnRH analogs (Leu, Gos, Leu-JADER, and Gos-JADER) with percentages of 60.67% vs. 32.05%, 42.80% vs. 19.99%, 79.18% vs. 20.30%, and 76.40% vs. 23.34%, respectively. The reason for this phenomenon may be that those GnRHas (Leu, Gos, Leu-JADER, and Gos-JADER) were used more for prostate cancer. Regarding serious reports of each GnRHas (Leu, Tri, and Gos from FAERS), the serious proportion (52.59%, 56.86%, and 89.23%) was larger than the non-serious (47.41%, 43.14%, and 10.77%), especially Gos. Finally, suspicious PTs conforming to the two algorithms (N ≥ 3, aROR025 > 1 and IC025 > 0) are displayed in Supplementary Table S2, following by Leu (562 PTs and 44 in new), Gos (189 PTs and 28 in new), Tri (172 PTs and 28 in new), and Leu-JADER (85 PTs and 10 in new).
Signals of system organ class
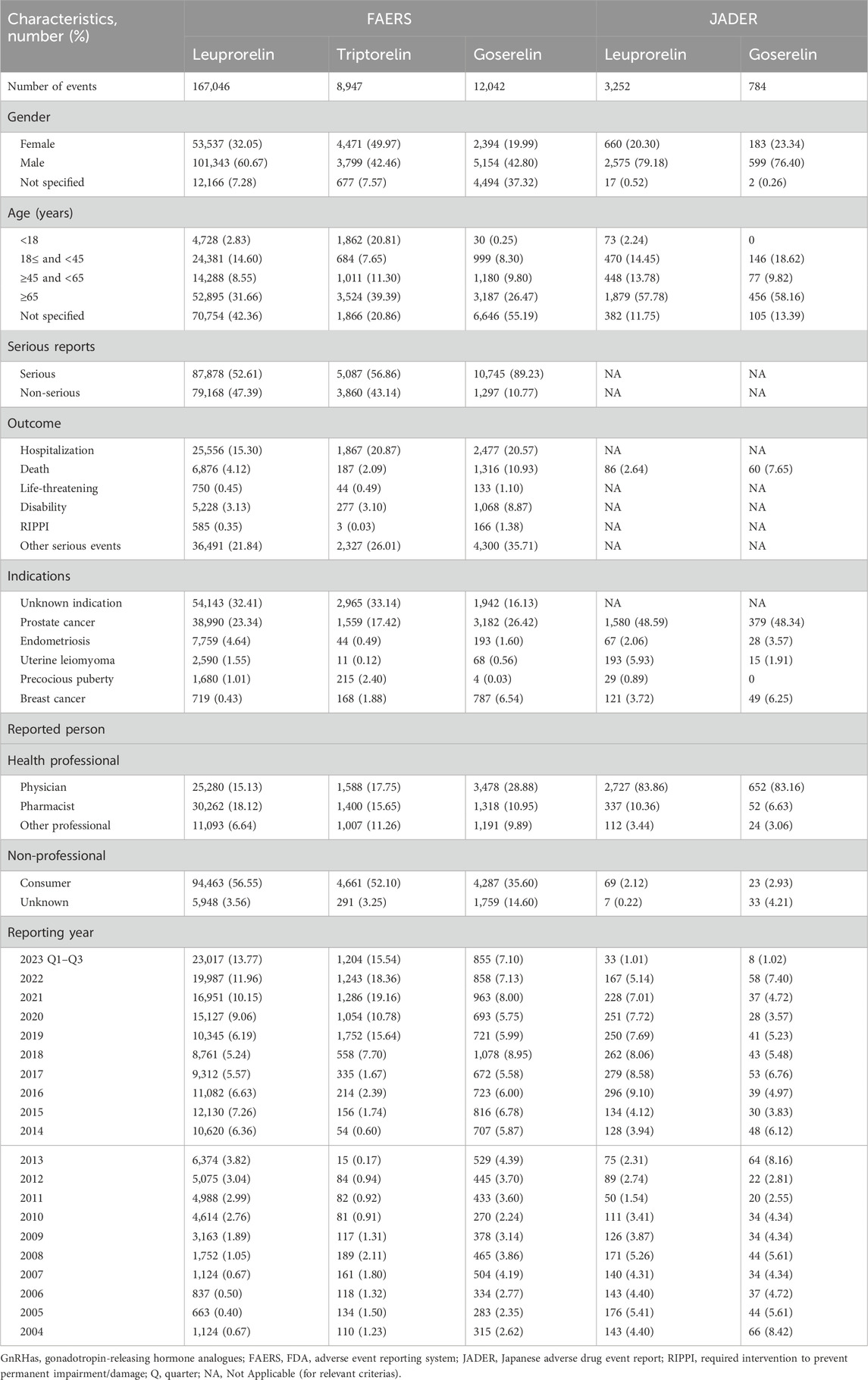
The system organ class (SOC) of all GnRHas, which conformed to the criterion (aROR025 > 1 or IC025 > 0), is showed in Figure 1. We detected that GnRHas-induced AEs occurred in 21 targeted organ systems, including Leu (9 SOCs), Tri (15 SOCs), Gos (8 SOCs), and Leu-JADER (11 SOCs). From Figure 1, we found that the SOC signals of Tri were the largest, including reproductive system disorders (aROR 8.44, 95% CI 7.68–9.29), product issues (aROR 6.97, 95% CI 6.41–7.59), endocrine disorders (aROR 4.46, 95% CI 3.35–5.94), social circumstances (aROR 3.72, 95% CI 2.76–5.02), injury conditions (aROR 2.14, 95% CI 2.02–2.27), psychiatric disorders (aROR 1.99, 95% CI 1.85–2.14), and skin disorders (aROR 1.17, 95% CI 1.06–1.29). Comparing FAERS and JADER, the SOC signals of Leu (FAERS) were more prominent in reproductive system disorders (aROR 5.28, 95% CI 5.07–5.30), product issues (aROR 4.32, 95% CI 4.24–4.41), vascular disorders (aROR 2.83, 95% CI 2.77–2.89), and renal/urinary disorders (aROR 1.05, 95% CI 1.02–1.09). Some SOC signals of Leu (JADER) demonstrated stronger signal values, including eye (aROR 2.53, 95% CI 1.66–3.85), metabolic (aROR 1.86, 95% CI 1.61–2.14), and cardiac disorders (aROR 1.48, 95% CI 1.25–1.76).
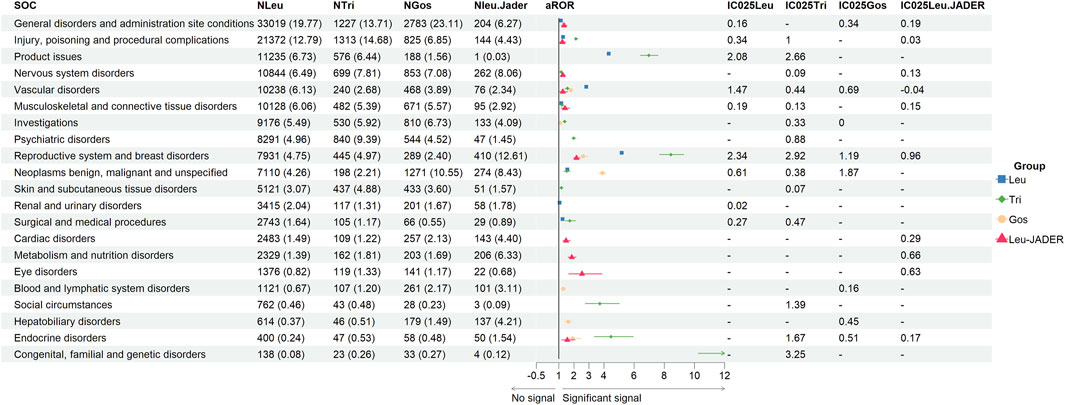
Figure 1. Signal strength of adverse events of GnRHas at the SOC level from FAERS database. -, showed a negative signal (aROR025 ≤ 1 and IC025 ≤ 0); GnRHas, gonadotropin-releasing hormone analogs; SOC, system organ class; FAERS, FDA Adverse Event Reporting System; N, number of target adverse events; Leu, leuprolide; Tir, triptorelin; Gos, goserelin; aROR, adjusted reporting odds ratio; IC025, adjusted lower limit of 95% confidence interval of the information component of BCPNN; BCPNN, Bayesian confidence propagation neural network.
Signals of same preferred terms from FAERS
Figure 2 showed the same PTs (84 PTs from 15 SOCs) of GnRHas (Leu, Tri, and Gos) from FAERS, comparing the statistical significance of their signal strength by Pearson χ2 test. To facilitate analysis of this data, the negative signals were marked “-”. In blood system disorders, Leu and Gos showed stronger PTs signals, including retroperitoneal lymphadenopathy, neutropenia, and lymphadenopathy. In cardiac disorders, suspected PT of cardiac failure (Gos) detected the stronger signal (aROR 1.73, 95% CI 1.20–2.51). In the PT of death, the order of signal from strong to weak is Gos (aROR 7.90, 95% CI 7.46–8.37), Leu (aROR 2.98, 95% CI 2.91–3.06), and Tri (aROR 1.51, 95% CI 1.31–1.75). In hepatobiliary disorders, Leu and Gos showed stronger PTs signals than hepatitis fulminant, jaundice, and liver disorder. In musculoskeletal and connective tissue disorders, partial PTs only showed Leu having positive signals as back pain, extreme pain, muscle spasms, myalgia, and fibromyalgia. Moreover, two PTs (polyarthritis and polymyositis) were observed to be significantly associated with Tri.
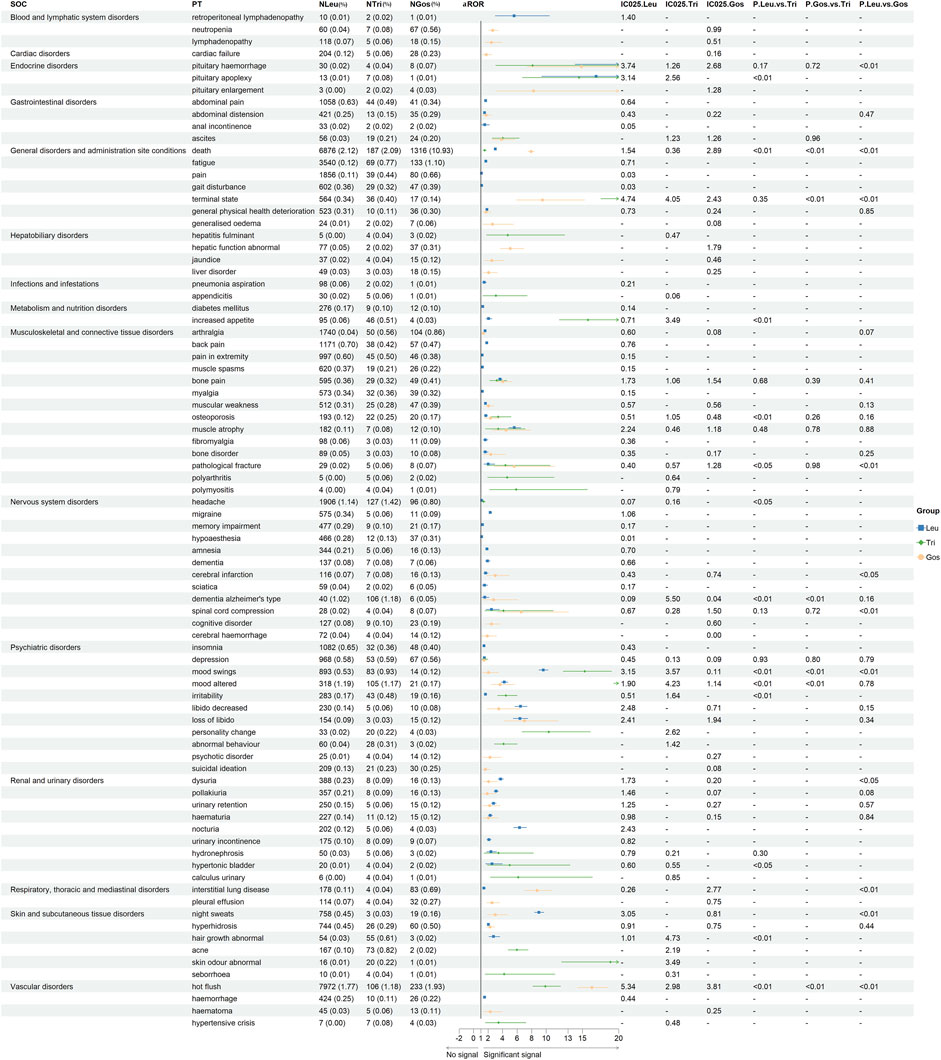
Figure 2. Signal strength of the same adverse events of GnRHas at the PT level from FAERS database. -, showed a negative signal (aROR025 ≤ 1 or IC025 ≤ 0); p < 0.05, showed the significant difference by Pearson χ2 test. GnRHas, gonadotropin-releasing hormone analogs; SOC, system organ class; PT, preferred term; FAERS, FDA Adverse Event Reporting System; N, cases of target adverse events; Leu, leuprolide; Tir, triptorelin; Gos, goserelin; aROR, adjusted reporting odds ratio; IC025, adjusted lower limit of 95% confidence interval of the information component of BCPNN; BCPNN, Bayesian confidence propagation neural network.
In metabolic and nutrition disorders, only Leu showed a positive signal for diabetes mellitus (aROR 1.26, 95% CI 1.12–1.42). In the nervous system and psychiatric disorders, seven PTs showed a unique and positive signal for Leu involving migraine, memory impairment, hypoaesthesia, amnesia, dementia, sciatica, and insomnia. In renal and urinary disorders, the dysuria signal of Leu was larger than that of Gos (aROR 3.72 vs. 2.06, p < 0.05). In interstitial lung disease and pleural effusion, Gos (aROR 8.75 and 2.54) showed the largest signals. In skin disorders, Tri detected the largest signals, including abnormal hair growth, acne, abnormal skin odor, and seborrhea. Moreover, hot flush of different GnRHas also showed different aROR values, and Leu had the highest value of IC025 (5.34), followed by Gos (IC025, 3.81) and Tri (IC025, 2.98).
Serious vs. non-serious cases from FAERS
As shown in Table 2, we performed an analysis of serious vs. non-serious cases of each GnRHa (leuprorelin, triptorelin, and goserelin from FAERS) with the same PTs. There were statistically significant differences in gender, age (69 vs. 61 years; p < 0.001, 63 vs. 9 years; p < 0.001; 70 vs. 59 years; p < 0.001) and body weight (79 vs. 73 kg; p < 0.001, 70 vs. 78 years; p = 0.003; 63 vs. 75 years; p < 0.001) between severe and non-severe cases of GnRHas-associated AEs from FAERS. A total of 43 AEs of Leu were more likely to be reported as serious AEs with p < 0.05 (such as asthenia, urinary retention, diabetes mellitus, interstitial lung disease, gait disturbance, and so on), following by Tri (6 AEs), and Gos (4 AEs). Back pain was reported as non-serious AEs with p > 0.05, as Leu (χ2 = 0.013, p = 0.91), Tri (χ2 = 2.080, p = 0.15), and Gos (χ2 = 0.635, p = 0.43). For Lue, 16 AEs showed non-serious events with p > 0.05, including back pain, bone pain, memory impairment, muscle atrophy, musculoskeletal pain, lower abdominal pain, abscess, groin pain, osteopenia, micturition urgency, sciatica, apathy, decreased proctalgia, exercise tolerance, increased fat tissue, and aggression. Like Leu, it was noteworthy that all outcomes for AEs of cerebral infarction (N = 116), urinary tract obstruction (N = 69), marasmus (N = 57), urinary bladder hemorrhage (N = 53), hydronephrosis (N = 50), aortic aneurysm (N = 38), and pituitary hemorrhage (N = 30) were severe cases.
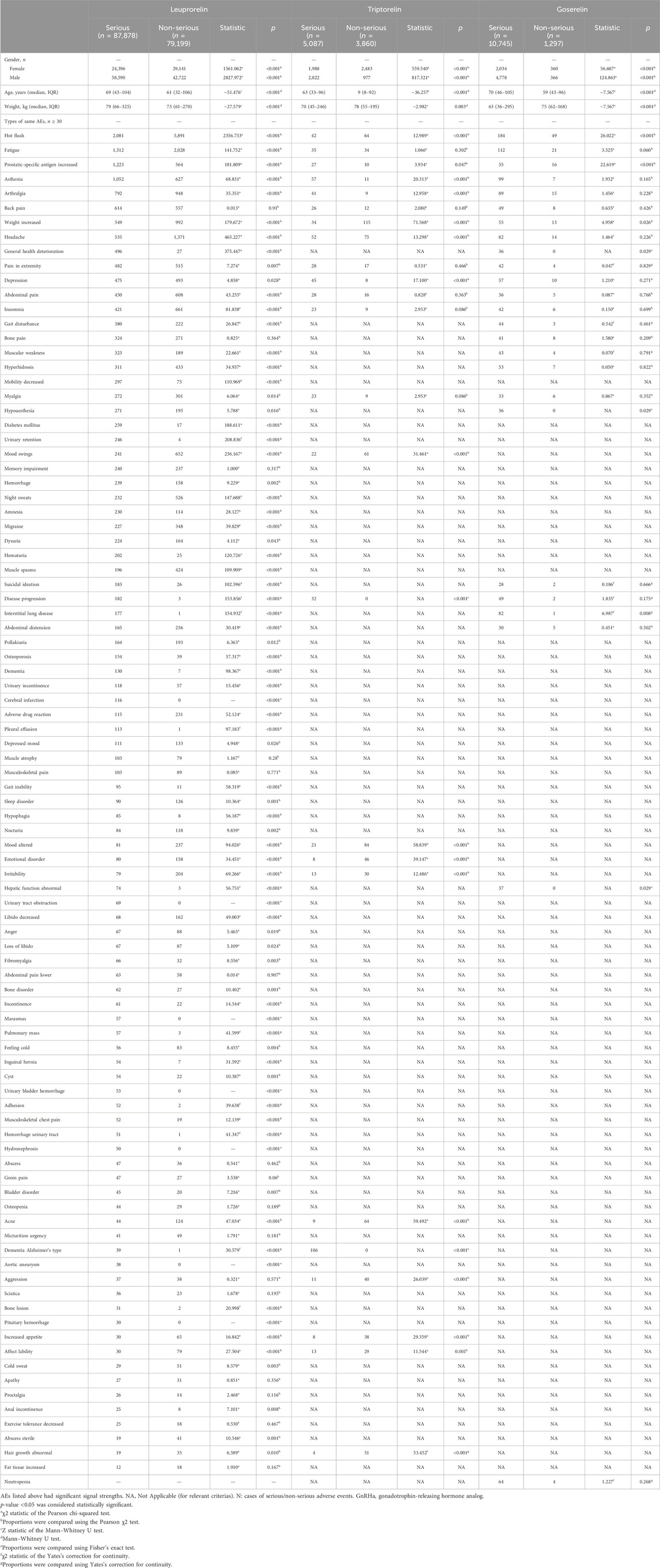
Table 2. Differences in clinical characteristics of serious and non-serious reports of each GnRHa (n ≥ 30).
Sensitivity analyses of GnRHas from FAERS
We performed sensitivity analyses (excluding all concomitant medications) of GnRHas from FAERS in Supplementary Table S3. Out of total 312 PTs, 34 PTs showed the significant differences (p < 0.05), such as dysuria, pollakiuria, urinary retention, hematuria, and nocturia. Overall, other results were largely similar and consistent with the analysis that included all concomitant medications based on the aROR for GnRHas.
Signals of same preferred terms from JADER
We collected the same PTs of Leu and Gos from JADER (Figure 3). From the same 36 PTs, we found ten with p < 0.05, including death (aROR, 4.03 vs. 0.81; p < 0.001), interstitial lung disease (aROR, 3.18 vs. 4.25; p = 0.009), injection site hematoma (aROR, 2.53 vs. 35.43; p < 0.001), hyperglycemia (aROR, 2.20 vs. 4.86; p = 0.040), abdominal wall hematoma (aROR, 1.70 vs. 14.36; p < 0.001), abnormal hepatic function (aROR, 1.31 vs. 2.41; p = 0.007), intra-abdominal hemorrhage (aROR, 0.91 vs. 10.97; p < 0.001), liver disorder (aROR, 0.87 vs. 2.31; p = 0.002), pure red cell aplasia (aROR, 0.80 vs. 4.20; p = 0.025), and lung disorder (aROR, 0.55 vs. 6.01; p < 0.001).
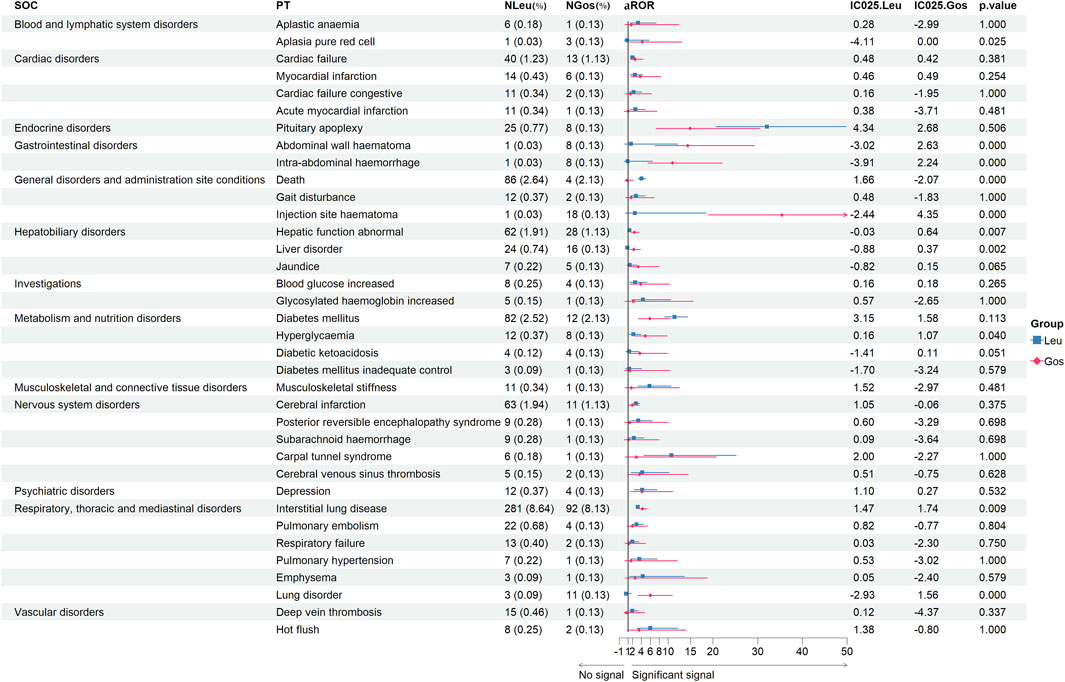
Figure 3. Signal strength of the same adverse events of leuprolide and goserelin at the PT level from JADER databases. -, showed a negative signal (aROR025 ≤ 1, and IC025 ≤ 0); p < 0.05, showed significant difference by Pearson χ2 test; IC025 > 0 and aROR025 > 1, showed a suspicious signal. PT, preferred term; JADER, Japanese Adverse Drug Event Report; SOC, system organ class; JADER, Japanese Adverse Drug Event Report; Leu, leuprolide; Gos, goserelin; N, number of target adverse events; aROR, adjusted reporting odds ratio; IC025, adjusted lower limit of 95% confidence interval of the information component of BCPNN; BCPNN, Bayesian confidence propagation neural network.
Clinical prioritization of suspicious PTs
We performed a clinical prioritization of the suspicious PTs (unlisted in instructions) of four GnRHas (Leu, Tri, Gos, and Leu-JADER) (Table 3). Of the 110 unlisted PTs (from Leu, Tri, Gos, and Leu-JADER), 43 with significant signals were categorized as IMEs, with only three representing DMEs: hepatitis fulminant (aROR 4.71, 95% CI 1.76–12.55, from Tri), hepatic necrosis (aROR 3.44, 95% CI 1.29–9.19, from Gos), and aplastic anemia (aROR 3.23, 95% CI 1.45–7.23, from Leu-JADER). Based on the clinical priority score, 41 PTs (Leu), 26 PTs (Tri), 24 PTs (Gos), and 8 PTs (Leu-JADER) were graded as weak. Three PTs (Leu), 2 PTs (Tri), 4 PTs (Gos), and 2 PTs (Leu-JADER) were graded as moderate. Notably, in assessing the relevant evidence, two PTs (loss of libido and urinary tract toxicity caused by Leu), one PT (electrolyte imbalance caused by Tri), and two PTs (anorexia and suicidal ideation caused by Gos) showed a strong level of evidence with “++.”
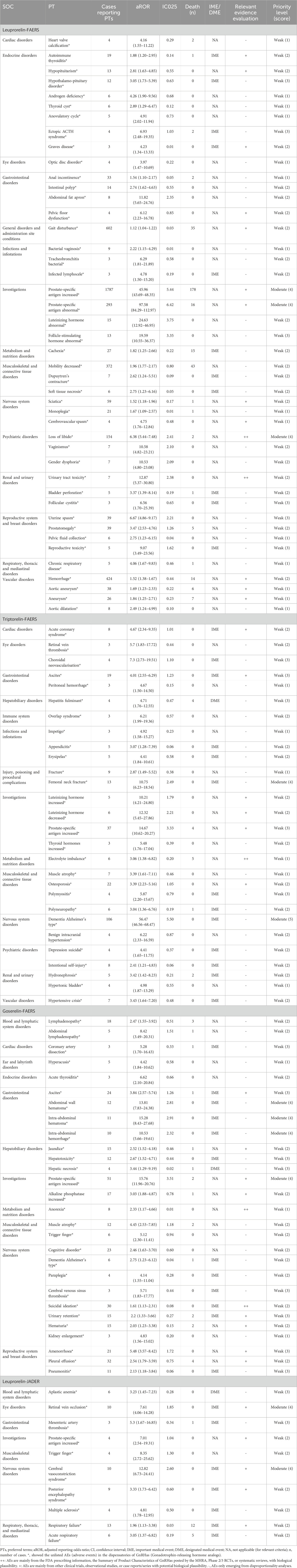
Table 3. Signal strength and clinical priority assessing results of suspicious PTs (unlisted in instructions).
Signal profiles of different GnRHas (top 30 of IC025)
If data mining was conducted only for the same PTs, some important signals might be ignored. Therefore, the signal spectrum of each GnRHa is shown in Figure 4, where the top 30 of the 95% confidence intervals (95%CI) of IC (IC025) is regarded as an indicator; whole signals of different GnRHas are displayed in Supplementary Table S2. If IC025 > 3.0, there is a strong signal; 1.5 < IC025 ≤ 3.0, indicates a medium intensity signal; 0 < IC025 ≤ 1.5, indicates a weak intensity signal (Li Sun et al., 2019; Zou et al., 2023b).
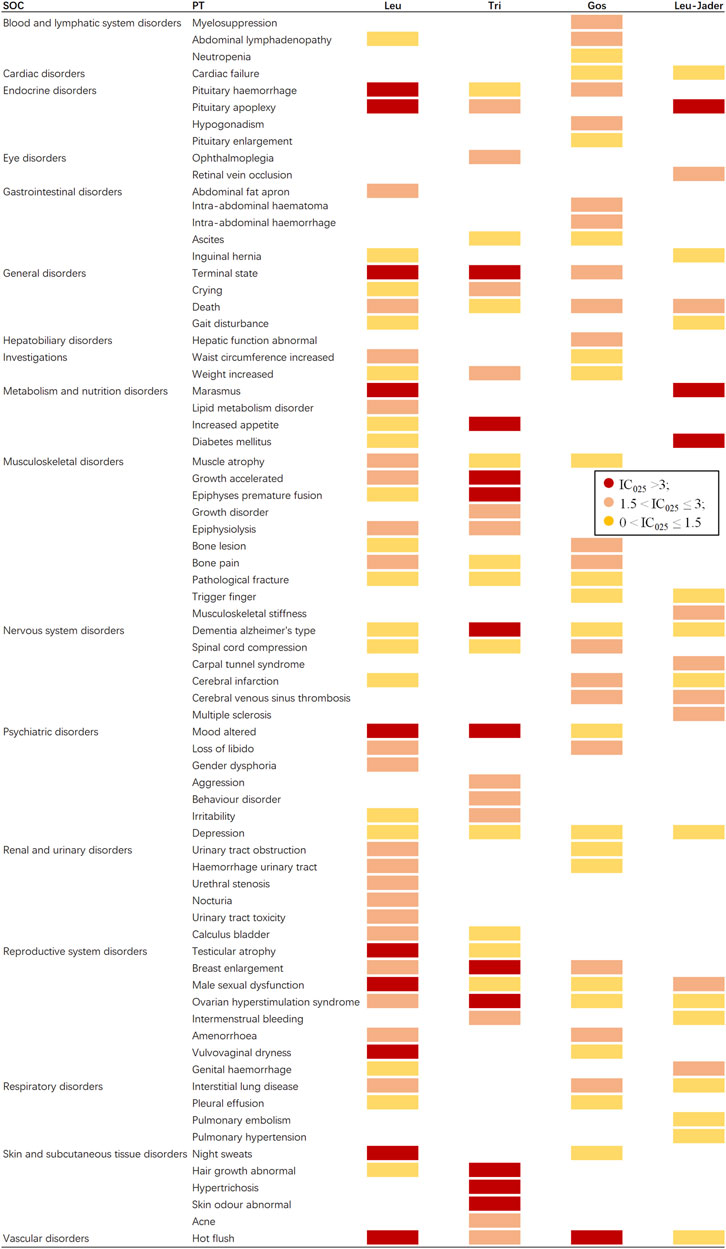
Figure 4. Signal profiles of different GnRHas from FAERS and JADER databases (top 30 based on IC025 of each GnRHas). GnRHas, gonadotropin-releasing hormone analogs; FAERS, FDA Adverse Event Reporting System; JADER, Japanese Adverse Drug Event Report; SOC, system organ class; PT, preferred term; Leu, leuprolide; Tir, triptorelin; Gos, goserelin; IC025, adjusted the lower limit of 95% confidence interval of the information component of BCPNN; BCPNN, Bayesian confidence propagation neural network. IC025 > 3.0 indicates a strong signal; 1.5 < IC025 ≤ 3.0 indicates a medium-intensity signal; 0 < IC025 ≤ 1.5 indicates a weak-intensity signal.
As shown in Figure 4 (excluding PTs caused by indications, operations, and unqualified products), Tri and Leu (FAERS) presented the greatest spectrum (IC025 > 3.0), with a total of 11 and 10 strong GnRHas-induced signals (12 and 20 medium signals) detected, ranging from endocrine disorders to vascular disorders, following by Gos (one strong and 18 medium signals) and Leu-JADER (three strong and nine medium signals). In Figure 4, the suspicious PTs of Leu were obviously concentrated in endocrine disorders (pituitary hemorrhage [IC025 3.74] and pituitary apoplexy [IC025 3.14]), metabolism and nutrition disorders (marasmus [IC025 3.14], lipid metabolism disorder [IC025 2.63], increased appetite [IC025 2.53], and diabetes mellitus [IC025 1.42]), renal and urinary disorders (urinary tract obstruction [IC025 2.91], urinary tract hemorrhage [IC025 2.32], urethral stenosis [IC025 2.51], nocturia [IC025 2.42], urinary tract toxicity [IC025 2.38], and calculus bladder [IC025 1.97]). Interestingly, from FAERS, some suspicious PTs of Tri differed from Leu (FAERS) in nervous system disorders (Alzheimer’s type dementia [IC025 5.50 vs. 2.09]), musculoskeletal disorders (epiphyses premature fusion [IC025 4.89 vs. 0.93]), and skin disorders (abnormal hair growth [IC025 4.73 vs. 1.01], hypertrichosis [IC025 3.63], abnormal skin odor [IC025 3.49], and acne [IC025 2.19]). Notably, hot flush was the only PT significantly related to all GnRHas from FAERS with markedly strong intensity.
Onset time of GnRHas-related AEs
Because the effective onset-time of Leu-JADER was too short to calculate, we only detected the GnRHas (Leu, Tri, and Gos) from the FAERS database. We collected the onset times of GnRHas-associated AEs from FAERS (Supplementary Figure S2). The onset times of adverse events of Leu and Tri were highly similar. The median onset time of GnRHas (Leu, Tri, and Gos) was 23 days (0, 298), 22 days (0, 181), and 217 days (29, 706), as median (Q1, Q3).
Discussion
To the best of our knowledge, we were the first to mine GnRHas data from the FAERS and JADER databases. Juan Tamargo et al. achieved greater understanding of the differences in the genetic variants of drug-metabolizing enzymes (DMEs) and transporters that determine the differences in the exposure, efficacy, and safety of cardiovascular drugs between races/ethnicities(Tamargo et al., 2022). Our study showed that the most commonly reported and newest signals of GnRHas were found at the SOC and PT levels in two databases.
In our disproportionality analysis, many organs or tissues could have been under the influence of GnRHas at SOC levels. Tri (FAERS) seemed to have the broadest and strongest signals of SOC, such as for reproductive system disorders (ROR 8.44, IC025 2.92), product issues (aROR 6.97, IC025 2.67), endocrine disorders (aROR 4.46, IC025 1.67), psychiatric disorders (aROR 1.99, IC025 0.88), social circumstances (aROR 3.72, IC025 1.39), and skin disorders (aROR 1.17, IC025 0.07). Unlike JAERS, Leu from JADER had also the broadest aROR of SOC, although many of the values are not the largest and had the characteristic SOCs, such as cardiac disorders (aROR 1.48, IC025 0.29) and eye disorders (aROR 2.53, IC025 0.63). The reason for this might be that most reports were by medical personnel in Japan and most by public in the United States (Table 1). For example, for hot flush in Figure 4, three GnRHas (Leu, Tri, and Gos from FAERS) showed strong signals (IC025, 5.34, 2.98, and 3.81) but Leu (JADER) showed a weak signal (IC025, 1.38).
Furthermore, we found that some PTs might result in serious outcomes, with statistically significant differences observed between severe and non-severe cases (p < 0.001) in different GnRHas, such as hot flush, osteoporosis, and dementia. A prospective, single-center, single-arm, open label study of the long-term use of Tri showed an increased risk of developing osteopenia after 12 months of treatment (Alshehre et al., 2020). A 4-year interventional case–control study reported a case of metatarsal fracture (Dotremont et al., 2023). Similarly, we also found the clinical priority for femoral neck fracture caused by Tri (aROR 10.75, 95% CI 6.23–18.54) was moderate.
It is noteworthy that the aROR of dementia (Alzheimer’s type, n = 106) was 56.47 (95%, 46.56–68.47) with IC025 (5.50, a strong signal) caused by Tri. The clinical priority of the PT showed a moderate (Zondervan et al., 2020) signal. A cohort study of 23,651 patients with newly diagnosed prostate cancer showed the use of antiandrogen monotherapy was associated with an increased risk of dementia or Alzheimer’s disease (AD) (Huang et al., 2020).
Interestingly, even for the same PTs, the aROR values of different GnRHas were quite different. In Figures 2 and 3, only positive signals showed in the forest plot. A meta-analysis of the use of GnRHas for the treatment of endometriosis revealed that the most frequently reported adverse events included vaginal dryness, hot flushes, headaches, muscle cramps (myalgia), sleep disturbance (insomnia), altered libido, weight gain, bone loss, and acne (Veth et al., 2023; Zheng et al., 2023).
A clinical study of leuprolide acetate 6-month depot showed that eight subjects in the study encountered AEs that were associated with diabetes mellitus (n = 2), elevations in serum glucose (n = 5), or hypoglycemia (n = 1) (Spitz et al., 2012). Another study involving girls with idiopathic CPP treated with GnRHas also demonstrated a deterioration in glucose metabolism during GnRHas treatment, with complete restoration observed afterward; this effect was independent of pre-treatment body mass index (BMI) (Bruzzi et al., 2022). Coincidentally, we found that only leuprolide had a positive signal for diabetes mellitus (aROR 1.26, 95% CI 1.12–1.42, IC025 0.14) in FAERS. Interestingly, in Supplementary Table S2, compared with FAERS, leuprolide (JADER) had stronger signals in metabolism disorders such as increased blood glucose (aROR 2.59 vs. no-significant), increased blood triglycerides (aROR 3.48 vs. no-significant), diabetes mellitus (aROR 11.43 vs. 1.26), and marasmus (aROR 45.03 vs. 14.58). An observational study of 37,443 population-based men showed that androgen deprivation therapy with GnRHa was associated with an increased risk of diabetes (Keating et al., 2010). In a mechanistic study of fat accumulation, Dr Hefeng Huang found that FSH downregulated aquaporin 7 (AQP7) expression and glycerol efflux function in mature adipocytes of post-menopausal women and ovariectomized (OVX) mice (Chen et al., 2020).
However, although Leu had the broadest positive signals in same PTs (Figure 4), Tri had the greatest number of strong signals (aROR ≥ 10) for the following PTs: Alzheimer’s type dementia (aROR 56.47, 95% CI 46.56–68.47), abnormal hair growth (aROR 36.21, 95% CI 27.75–47.27), terminal state (aROR 24.37, 95% CI 17.55–33.84), altered mood (aROR 23.41, 95% CI 19.31–28.39), abnormal skin odor (aROR 18.86, 95% CI 12.14–29.28), increased appetite (aROR 15.77, 95% CI 11.8–21.08), mood swings (ROR 15.31, 95% CI 12.33–19.01), pituitary apoplexy (aROR 14.54, 95% CI 6.72–31.45), and personality change (aROR 10.36, 95% CI 6.68–16.08).
Hormone therapy was not perfect: Wang et al. (2023) discovered that androgen deprivation therapy (ADT), including GnRHas, induced SPP1+ myofibroblastic cancer-associated fibroblasts (myCAFs), which proved to be crucial stromal constituents that drove the development of castration-resistant prostate cancer (CRPC). In ovarian hyperstimulation syndrome (OHSS), a rare but potentially life-threatening condition (for every 55 patients with a long GnRHa protocol, one would be hospitalized because of OHSS), some evidences indicates that the routine use of GnRH-ant instead of GnRHa during ovarian stimulation drastically reduces the relative risk of OHSS (Kolibianakis et al., 2006; Al-Inany et al., 2007; Griesinger, 2010).
Extended periods of high hormone concentration might potentially lead to hormone-related adverse events (AEs). The reason for the difference might be the different signaling pathways, structure, and dosage form of GnRHas. Robert P Millar et al. (2008) found that the effects of various GnRHas were notably distinct in relation to their impact on the pituitary gonadotrope, as they engage different signaling pathways (Millar et al., 2008). The GnRH receptor can adopt different conformations, each exhibiting varying selectivity for GnRHas and intracellular signaling protein complexes (Millar et al., 2008).
Even if both GnRHas acted on the reproductive system (Supplementary Table S2), their effects were different. In the reproductive system, prostatic GnRH- receptors appeared to be different from the pituitary, endometrium, and ovary, which showed a lower binding affinity for GnRH and its analogs (Limonta et al., 1992; Dondi et al., 1994; Casati et al., 2023). Thus, the adverse effects of GnRHa on women and men might be different. However, it is worth noting that when concomitant medications were removed in a sensitivity analysis, the aROR of GnRHa from FAERS were largely in keeping with the results from the primary analysis (Supplementary Table S3).
Finally, we collected the onset time of GnRHas in Supplementary Figure S2. The onset-time curves of adverse events caused by Leu and Tri were highly similar, as median (Q1, Q3) (23 days [0, 298] and 22 days [0, 181]). The results might be caused by the similar protein structure of Leu and Tri, which were derived from the native GnRH by substitution of a L-glycine in position 6 (Warner et al., 1983; Lahlou, 2005).
Our study also had certain limitations. First, because of the concomitant medications, missing, incomplete and repetitive reports, disproportionality analysis alone cannot prove causation or measure incidence, and specific limitations, including confounding, reporting bias and efforts to mitigate them. Second, the number of total cases had large differences between the two databases. JADER is a database limited to individual case reports, which reports serious cases individually, while FAERS also includes periodic reports that collectively report non-serious cases collected over a period of time (Noguchi et al., 2021). However, we applied the statistical shrinkage transformation for the sake of robustness to minimize this bias as much as possible. Third, in this study, we did not conduct sensitivity analyses on the influence of the indications of GnRHas. Notably, even if the number of FAERS reports is limited to data from Japan and the United States, the available Japanese data account for less than 5% of the total United States data (Nawa et al., 2021).
Due to the distinct signaling pathways, further investigation into GnRH analogs (GnRHas) held significant value for the fields of pharmacovigilance and pharmacodynamics, especially diverse racial populations. We anticipated that our analysis, utilizing spontaneous adverse drug event report databases, will serve as a valuable contribution to future studies in this domain.
Conclusion
This is the first study to analyze GnRHas from the FAERS and JADER databases comprehensively and systematically. The continuous monitoring of drug safety profiles in real-world scenarios is imperative. Moreover, our study provided the differences of GnRHas from FAERS and JADER.
Data availability statement
Publicly available datasets were analyzed in this study. These data can be found at: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html and https://www.info.pmda.go.jp/fukusayoudb/CsvDownload.
Author contributions
SZ: writing–review and editing, writing–original draft, software, methodology, and formal analysis. MO: writing–review and editing, investigation, funding acquisition, and data curation. YZ: writing–original draft, visualization, validation, supervision, and resources. QC: writing–original draft, visualization, resources, and data curation. XS: writing–review and editing, software, and formal analysis. MS: writing–original draft, writing–review and editing, project administration, funding acquisition, and conceptualization.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1392914/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Process of searching GnRHas-associated adverse events from the FAERS and JADER databases. GnRHas, gonadotropin-releasing hormone analogs; FAERS, FDA Adverse Event Reporting System; JADER, Japanese Adverse Drug Event Report.
SUPPLEMENTARY FIGURE 2 | Time to onset of different GnRHas-related AEs from the FAERS databases (Unit: days). GnRHas, gonadotropin-releasing hormone analogs; AEs, adverse events; FAERS, FDA Adverse Event Reporting System; Leu, leuprolide; Tir, triptorelin; Gos, goserelin. The median onset time of GnRHas (Leu, Tri, and Gos) was 23 days (0, 298), 22 days (0, 181), and 217 days (29, 706), as median (Q1, Q3).
References
Al-Inany, H. G., Abou-Setta, A. M., and Aboulghar, M. (2007). Gonadotrophin-releasing hormone antagonists for assisted conception: a Cochrane review. Reprod. Biomed. online 14 (5), 640–649. doi:10.1016/S1472-6483(10)61059-0
Alshehre, S. M., Duffy, S., Jones, G., Ledger, W. L., and Metwally, M. (2020). A prospective, single-centre, single-arm, open label study of the long term use of a gonadotropin releasing hormone agonist (Triptorelin SR, 11.25 mg) in combination with Tibolone add-back therapy in the management of chronic cyclical pelvic pain. Reproductive Biol. Endocrinol. RB&E 18 (1), 28. doi:10.1186/s12958-020-00586-z
Bruzzi, P., Valeri, L., Sandoni, M., Madeo, S. F., Predieri, B., Lucaccioni, L., et al. (2022). The impact of BMI on long-term anthropometric and metabolic outcomes in girls with idiopathic central precocious puberty treated with GnRHas. Front. Endocrinol. 13, 1006680. doi:10.3389/fendo.2022.1006680
Burk, B. G., DiGiacomo, T., Polancich, S., Pruett, B. S., Sivaraman, S., and Birur, B. (2023). Antipsychotics and obsessive-compulsive disorder/obsessive-compulsive symptoms: a pharmacovigilance study of the FDA adverse event reporting system. Acta psychiatr. Scand. 148 (1), 32–46. doi:10.1111/acps.13567
Carel, J. C., Eugster, E. A., Rogol, A., Ghizzoni, L., Palmert, M. R., Antoniazzi, F., et al. (2009). Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 123 (4), e752–e762. doi:10.1542/peds.2008-1783
Casati, L., Ciceri, S., Maggi, R., and Bottai, D. (2023). Physiological and pharmacological overview of the gonadotropin releasing hormone. Biochem. Pharmacol. 212, 115553. doi:10.1016/j.bcp.2023.115553
Chau, A., Bibbo, C., Huang, C. C., Elterman, K. G., Cappiello, E. C., Robinson, J. N., et al. (2017). Dural puncture epidural technique improves labor analgesia quality with fewer side effects compared with epidural and combined spinal epidural techniques: a randomized clinical trial. Anesth. Analg. 124 (2), 560–569. doi:10.1213/ane.0000000000001798
Chen, C., Chen, T., Liang, J., Guo, X., Xu, J., Zheng, Y., et al. (2021). Cardiotoxicity induced by immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019 based on FAERS. Front. Pharmacol. 12, 616505. doi:10.3389/fphar.2021.616505
Chen, L., Chen, H., Liu, X., Li, J., Gao, Q., Shi, S., et al. (2020). AQP7 mediates post-menopausal lipogenesis in adipocytes through FSH-induced transcriptional crosstalk with AP-1 sites. Reprod. Biomed. online 41 (6), 1122–1132. doi:10.1016/j.rbmo.2020.08.008
Dondi, D., Limonta, P., Moretti, R. M., Marelli, M. M., Garattini, E., and Motta, M. (1994). Antiproliferative effects of luteinizing hormone-releasing hormone (LHRH) agonists on human androgen-independent prostate cancer cell line DU 145: evidence for an autocrine-inhibitory LHRH loop. Cancer Res. 54 (15), 4091–4095.
Dotremont, H., France, A., Heinrichs, C., Tenoutasse, S., Brachet, C., Cools, M., et al. (2023). Efficacy and safety of a 4-year combination therapy of growth hormone and gonadotropin-releasing hormone analogue in pubertal girls with short predicted adult height. Front. Endocrinol. 14, 1113750. doi:10.3389/fendo.2023.1113750
Fatemi, H. M., and Garcia-Velasco, J. (2015). Avoiding ovarian hyperstimulation syndrome with the use of gonadotropin-releasing hormone agonist trigger. Fertil. Steril. 103 (4), 870–873. doi:10.1016/j.fertnstert.2015.02.004
Gatti, M., Antonazzo, I. C., Diemberger, I., De Ponti, F., and Raschi, E. (2021). Adverse events with sacubitril/valsartan in the real world: emerging signals to target preventive strategies from the FDA adverse event reporting system. Eur. J. Prev. Cardiol. 28 (9), 983–989. doi:10.1177/2047487320915663
Griesinger, G. (2010). Ovarian hyperstimulation syndrome prevention strategies: use of gonadotropin-releasing hormone antagonists. Seminars reproductive Med. 28 (6), 493–499. doi:10.1055/s-0030-1265676
Harris, A. E., Metzler, V. M., Lothion-Roy, J., Varun, D., Woodcock, C. L., Haigh, D. B., et al. (2022). Exploring anti-androgen therapies in hormone dependent prostate cancer and new therapeutic routes for castration resistant prostate cancer. Front. Endocrinol. 13, 1006101. doi:10.3389/fendo.2022.1006101
Hou, L., Ying, Y., Wu, W., Ye, F., Zhang, C., and Luo, X. (2024). The effect of GnRHa treatment on body mass index in central precocious puberty: a systematic review and meta-analysis. Horm. Res. Paediatr., 1–14. doi:10.1159/000535132
Huang, W. K., Liu, C. H., Pang, S. T., Liu, J. R., Chang, J. W., Liaw, C. C., et al. (2020). Type of androgen deprivation therapy and risk of dementia among patients with prostate cancer in taiwan. JAMA Netw. open 3 (8), e2015189. doi:10.1001/jamanetworkopen.2020.15189
Jain, D., Sharma, G., and Kumar, A. (2023). Adverse effects of proton pump inhibitors (PPIs) on the renal system using data mining algorithms (DMAs). Expert Opin. drug Saf. 22 (8), 741–752. doi:10.1080/14740338.2023.2189698
Keating, N. L., O'Malley, A. J., Freedland, S. J., and Smith, M. R. (2010). Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J. Natl. Cancer Inst. 102 (1), 39–46. doi:10.1093/jnci/djp404
Kolibianakis, E. M., Collins, J., Tarlatzis, B. C., Devroey, P., Diedrich, K., and Griesinger, G. (2006). Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used? A systematic review and meta-analysis. Hum. Reprod. Update 12 (6), 651–671. doi:10.1093/humupd/dml038
Lahlou, N. (2005). Pharmacokinetics and pharmacodynamics of triptorelin. Ann. d'urologie 39 (Suppl. 3), S78–S84. doi:10.1016/s0003-4401(05)80013-0
Lem, A. J., van der Kaay, D. C., and Hokken-Koelega, A. C. (2013). Bone mineral density and body composition in short children born SGA during growth hormone and gonadotropin releasing hormone analog treatment. J. Clin. Endocrinol. metabolism 98 (1), 77–86. doi:10.1210/jc.2012-2492
Limonta, P., Dondi, D., Moretti, R. M., Maggi, R., and Motta, M. (1992). Antiproliferative effects of luteinizing hormone-releasing hormone agonists on the human prostatic cancer cell line LNCaP. J. Clin. Endocrinol. metabolism 75 (1), 207–212. doi:10.1210/jcem.75.1.1320049
Li Sun, S. S. T. W., Li, J., Jiyun, L., and Jingsheng, L. (2019). Parallel ADR detection based on spark and BCPNN. Tsinghua Sci. Technol. 24 (2), 195–206. doi:10.26599/tst.2018.9010074
Millar, R. P., Pawson, A. J., Morgan, K., Rissman, E. F., and Lu, Z. L. (2008). Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front. Neuroendocrinol. 29 (1), 17–35. doi:10.1016/j.yfrne.2007.06.002
Nagai, J., and Ishikawa, Y. (2021). Analysis of anticholinergic adverse effects using two large databases: the US food and drug administration adverse event reporting system database and the Japanese adverse drug event report database. PloS one 16 (12), e0260980. doi:10.1371/journal.pone.0260980
Nawa, H., Niimura, T., Hamano, H., Yagi, K., Goda, M., Zamami, Y., et al. (2021). Evaluation of potential complications of interstitial lung disease associated with antiandrogens using data from databases reporting spontaneous adverse effects. Front. Pharmacol. 12, 655605. doi:10.3389/fphar.2021.655605
Neishi, M., Hamano, H., Niimura, T., Denda, M., Yagi, K., Miyata, K., et al. (2023). Structural characterization of the optical isomers esomeprazole and omeprazole using the JADER and FAERS databases. Toxicol. Appl. Pharmacol. 475, 116632. doi:10.1016/j.taap.2023.116632
Newton, C. L., Riekert, C., and Millar, R. P. (2018). Gonadotropin-releasing hormone analog therapeutics. Minerva Ginecol. 70 (5), 497–515. doi:10.23736/s0026-4784.18.04316-2
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform 22 (6), bbab347. doi:10.1093/bib/bbab347
Nomura, K., Takahashi, K., Hinomura, Y., Kawaguchi, G., Matsushita, Y., Marui, H., et al. (2015). Effect of database profile variation on drug safety assessment: an analysis of spontaneous adverse event reports of Japanese cases. Drug Des. Devel Ther. 9, 3031–3041. doi:10.2147/dddt.S81998
Noren, G. N., Hopstadius, J., and Bate, A. (2013). Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. methods Med. Res. 22 (1), 57–69. doi:10.1177/0962280211403604
Okwuosa, T. M., Morgans, A., Rhee, J. W., Reding, K. W., Maliski, S., Plana, J. C., et al. (2021). Impact of hormonal therapies for treatment of hormone-dependent cancers (breast and prostate) on the cardiovascular system: effects and modifications: a scientific statement from the American heart association. Circulation Genomic Precis. Med. 14 (3), e000082. doi:10.1161/hcg.0000000000000082
Raja, T., Sud, R., Addla, S., Sarkar, K. K., Sridhar, P. S., Talreja, V., et al. (2022). Gonadotropin-releasing hormone agonists in prostate cancer: a comparative review of efficacy and safety. Indian J. cancer 59 (Suppl. ment), S142–s159. doi:10.4103/ijc.IJC_65_21
Reinisch, M., Seiler, S., Hauzenberger, T., Kamischke, A., Schmatloch, S., Strittmatter, H. J., et al. (2021). Efficacy of endocrine therapy for the treatment of breast cancer in men: results from the MALE phase 2 randomized clinical trial. JAMA Oncol. 7 (4), 565–572. doi:10.1001/jamaoncol.2020.7442
Rižner, T. L., and Romano, A. (2023). Targeting the formation of estrogens for treatment of hormone dependent diseases-current status. Front. Pharmacol. 14, 1155558. doi:10.3389/fphar.2023.1155558
Sahai, H., and Khurshid, A. (1995). On analysis of epidemiological data involving a 2 x 2 contingency table: an overview of Fisher's exact test and Yates' correction for continuity. J. Biopharm. statistics 5 (1), 43–70. doi:10.1080/10543409508835098
Shi, X., Cheng, Q., Zhao, Y. Z., Zou, S. P., and Sun, M. H. (2023). A real-world pharmacovigilance study of abaloparatide based on the FDA Adverse Event Reporting System (FAERS). Osteoporos. Int. 34 (12), 2047–2058. doi:10.1007/s00198-023-06877-6
Shu, Y., Chen, J., Ding, Y., and Zhang, Q. (2023b). Adverse events with risankizumab in the real world: postmarketing pharmacovigilance assessment of the FDA adverse event reporting system. Front. Immunol. 14, 1169735. doi:10.3389/fimmu.2023.1169735
Shu, Y., He, X., Wu, P., Liu, Y., Ding, Y., and Zhang, Q. (2022). Gastrointestinal adverse events associated with semaglutide: a pharmacovigilance study based on FDA adverse event reporting system. Front. public health 10, 996179. doi:10.3389/fpubh.2022.996179
Shu, Y., Wang, L., Ding, Y., and Zhang, Q. (2023a). Disproportionality analysis of abemaciclib in the FDA adverse event reporting system: a real-world post-marketing pharmacovigilance assessment. Drug Saf. 46 (9), 881–895. doi:10.1007/s40264-023-01334-z
Spitz, A., Young, J. M., Larsen, L., Mattia-Goldberg, C., Donnelly, J., and Chwalisz, K. (2012). Efficacy and safety of leuprolide acetate 6-month depot for suppression of testosterone in patients with prostate cancer. Prostate cancer prostatic Dis. 15 (1), 93–99. doi:10.1038/pcan.2011.50
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Tamargo, J., Kaski, J. C., Kimura, T., Barton, J. C., Yamamoto, K., Komiyama, M., et al. (2022). Racial and ethnic differences in pharmacotherapy to prevent coronary artery disease and thrombotic events. Eur. heart J. Cardiovasc. Pharmacother. 8 (7), 738–751. doi:10.1093/ehjcvp/pvac040
Veth, V. B., van de Kar, M. M., Duffy, J. M., van Wely, M., Mijatovic, V., and Maas, J. W. (2023). Gonadotropin-releasing hormone analogues for endometriosis. Cochrane database Syst. Rev. 6 (6), Cd014788. doi:10.1002/14651858.CD014788.pub2
Wang, H., Li, N., Liu, Q., Guo, J., Pan, Q., Cheng, B., et al. (2023). Antiandrogen treatment induces stromal cell reprogramming to promote castration resistance in prostate cancer. Cancer Cell 41 (7), 1345–1362.e9. doi:10.1016/j.ccell.2023.05.016
Warner, B., Worgul, T. J., Drago, J., Demers, L., Dufau, M., Max, D., et al. (1983). Effect of very high dose D-leucine6-gonadotropin-releasing hormone proethylamide on the hypothalamic-pituitary testicular axis in patients with prostatic cancer. J. Clin. investigation 71 (6), 1842–1853. doi:10.1172/jci110940
Zheng, Y., Ma, R., Xu, H., Wang, L., Zhang, L., Mao, H., et al. (2023). Efficacy and safety of different subsequent therapies after fertility preserving surgery for endometriosis: a systematic review and network meta-analysis. Medicine 102 (31), e34496. doi:10.1097/md.0000000000034496
Zondervan, K. T., Becker, C. M., and Missmer, S. A. (2020). Endometriosis. N. Engl. J. Med. 382 (13), 1244–1256. doi:10.1056/NEJMra1810764
Zong, X., Yu, Y., Yang, H., Chen, W., Ding, X., Liu, S., et al. (2022). Effects of gonadotropin-releasing hormone analogs on ovarian function against chemotherapy-induced gonadotoxic effects in premenopausal women with breast cancer in China: a randomized clinical trial. JAMA Oncol. 8 (2), 252–258. doi:10.1001/jamaoncol.2021.6214
Zou, S. P., Yang, H. Y., Ouyang, M., Cheng, Q., Shi, X., and Sun, M. H. (2023a). Post-marketing safety of anti-IL-5 monoclonal antibodies (mAbs): an analysis of the FDA Adverse Event Reporting System (FAERS). Expert Opin. drug Saf. 23, 353–362. doi:10.1080/14740338.2023.2251382
Keywords: GnRHas, FAERS, JADER, data mining, adverse events
Citation: Zou S, Ouyang M, Zhao Y, Cheng Q, Shi X and Sun M (2024) A disproportionality analysis of adverse events caused by GnRHas from the FAERS and JADER databases. Front. Pharmacol. 15:1392914. doi: 10.3389/fphar.2024.1392914
Received: 28 February 2024; Accepted: 30 April 2024;
Published: 04 July 2024.
Edited by:
Yanwu Xu, Baidu, ChinaReviewed by:
Anoop Kumar, Delhi Pharmaceutical Sciences and Research University, IndiaChunmei Ji, Nanjing Medical University, China
Yoshihiro Noguchi, Gifu Pharmaceutical University, Japan
Copyright © 2024 Zou, Ouyang, Zhao, Cheng, Shi and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Sun, c21oMDA3dGpAMTYzLmNvbQ==
 Shupeng Zou
Shupeng Zou Mengling Ouyang
Mengling Ouyang Yazheng Zhao
Yazheng Zhao Xuan Shi
Xuan Shi Minghui Sun
Minghui Sun