- 1Department of Pharmacy, Affiliated Children’s Hospital of Jiangnan University, Jiangsu University, Wuxi, China
- 2Department of Burns and Plastic Surgery, Affiliated Children’s Hospital of Jiangnan University, Jiangsu University, Wuxi, China
- 2Department of Gastroenterology, Affiliated Children’s Hospital of Jiangnan University, Jiangsu University, Wuxi, China
Background: H. pylori (Helicobacter pylori) infections typically occur in early childhood. Although the prevalence of H. pylori in children is lower than that in adults, the eradication rate of this infection in children is relatively low because of resistance. In this study, we analyzed personalized treatment strategies to achieve treatment goals based on H. pylori resistance characteristics. This retrospective single-center study was conducted between January 2019 and December 2022 and enrolled 1,587 children who presented with upper gastrointestinal symptoms and underwent endoscopy. H. pylori culturing and antimicrobial susceptibility testing were performed.
Results: Culture-positive results for H. pylori were obtained in 535 children. The resistance rates to clarithromycin (CLA), metronidazole (MET), and levofloxacin (LEV) were 39.8%, 78.1%, and 20.2%, respectively. None of the isolates were resistant to tetracycline (TET), amoxicillin (AMO), or furazolidone (FZD). Double resistance rates to CLA + MET, CLA + LEV, and MET + LEV were 19.1%, 3.0%, and 5.8%, respectively. Notably, triple-resistant to CLA + MET + LEV was 9.7%. Based on susceptibility tests, individualized triple therapy [proton pump inhibitor (PPI) +AMO + CLA/MET] was selected for 380 children with H. pylori sensitive to MET and/or CLA. In 155 children resistant to CLA and MET, bismuth-based quadruple therapy was recommended; for unable to receive bismuth, concomitant therapy was recommended for 14 children (<8 years of age); triple therapy with TET was recommended for 141 children (>8 years of age), with 43 children (>14 years of age) requiring FZD rather than TET.
Conclusion: Resistance to H. pylori in Chinese children was relatively poor. Personalized therapy regimens should be based on susceptibility tests and avoided factors associated with treatment failure.
1 Introduction
Helicobacter pylori (Helicobacter pylori), identified by Warren and Marshall over 40 years ago, remains a common chronic infectious disease worldwide (Seo et al., 2020). The overall global prevalence of H. pylori is predicted to exceed 50% (Katelaris et al., 2023). With recent socioeconomic development and environmental advances, the prevalence of H. pylori has markedly declined (Peleteiro et al., 2014). Its prevalence in China decreased from 58.3% between 1983 and 1994 to 40% between 2015 and 2019 (Ren et al., 2022). Similar trends were observed in Japan, with an adult infection rate of 72.7% in 1974, which decreased to 40% by 2014 (Ueda et al., 2014). In Nepal, The prevalence of H. pylori infection in children has also decreased from 39% before 2000 to 26% in 2010 (Mehata et al., 2021). Moreover, H. pylori infection rates vary among children of different ages and increase with age (Borka et al., 2022), although this trend is not observed in adults (Ren et al., 2022).
Most H. pylori infections are acquired during early childhood (Okuda et al., 2019). Long-term infection may lead to serious diseases, such as cancer and peptic ulcers. Consensus guidelines state that adults with H. pylori infection should initiate eradication therapy (Romano et al., 2022; Garcés-Duran et al., 2023). In contrast, unlike adults, children with H. pylori infection rarely develop severe complications, and early childhood infection may lead to later immune benefits (Melby et al., 2020; Martin-Nuñez et al., 2021). Therefore, “test to treat” strategies are not applicable to children (Malfertheiner et al., 2017; Ding et al., 2022), and the decision to initiate H. pylori eradication treatment in children requires caution.
An eradication rate of 90% is required for H. pylori to prevent antibiotic resistance and reduce risks associated with rescue treatment (Graham and Fischbach, 2010; Alfaro et al., 2023). In the 1990s, triple therapy with a proton pump inhibitor (PPI) + amoxicillin (AMO) + clarithromycin (CLA) was used to achieve an eradication rate of 98%; however, this rate recently decreased to 70%, particularly in children (Malfertheiner et al., 2007; Agudo et al., 2010). Various factors lead to eradication failure, such as a high bacterial load, inappropriate treatment or antibiotic concentrations, host mucosal immunity, and extragastric sources of bacteria (Moghadam et al., 2021). Unlike in adults, personalized treatment is crucial for children because age groups limit the flexibility of antibiotic choices. In 2016, the European and North American Societies of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN, NASPGHAN) recommended that tailored therapy for naïve patients should be based on antimicrobial susceptibility (Jones et al., 2017). Even using these measures, recent susceptibility-guided therapy studies reported that the eradication rate has not reached 90% (Luo et al., 2020; Van Thieu et al., 2021). Hence, apart from individualized antibiotics, the treatment regimen for H. pylori must be improved, and personalized decisions should be made regarding other drugs and treatment measures.
In this study, we designed individualized treatment strategies to achieve treatment goals based on the resistance characteristics of H. pylori in children from different regions. The tailored design not only includes the selection of antibacterial drugs based on antimicrobial susceptibility tests, but also includes PPI optimization based on the characteristics of the Chinese population, compliance improvement, and probiotic combination. This study provides a foundation for further clinical research and treatment decisions.
2 Materials and methods
2.1 Patients and ethical considerations
In this retrospective study, we evaluated 1,587 children who presented with upper gastrointestinal symptoms and underwent gastrointestinal endoscopy at the Gastroenterology Clinic of the Affiliated Children’s Hospital of Jiangnan University between January 2019 and December 2022. Exclusion criteria were as follows: patients under 1 year old; those who used antibiotics, bismuth agents, or acid-inhibitory drugs in the previous month; and those with other serious diseases. For children who underwent endoscopy more than once, only the first positive test result was included. This study was approved by the Ethics Committee of the Affiliated Children’s Hospital of Jiangnan University (Approval Number: WXCH 2023-04-070). Informed consent was obtained from the children’s guardians before gastroscopy.
2.2 Helicobacter pylori culture
Gastric mucosa samples were collected from within 5 cm of the curvature of the pylorus antrum, transferred into sterile vials containing brain heart infusion broth (Oxoid, Basingstoke, UK) supplemented with 20% glycerol, and stored at 4 °C. The samples were transferred to Zhiyuan Medical Inspection Institute for H. pylori culture and antibiotic susceptibility testing. H. pylori test results from this company have also been reported in other Chinese studies (Shu et al., 2022). The homogenates of the gastric mucosa samples were inoculated on a plate containing 5% fresh fibrous defibrinated sheep blood and maintained at 37 °C under micro-aerobic conditions (5% O2, 10% CO2, and 85% N2). Colony growth was observed after 48–72 h. Suspected bacterial colonies were stained for microscopic examination. Gram-negative bacteria were observed under a microscope, those with a curved or gull-shaped morphology and positive activity testing (including oxidase, catalase, and urease) were determined to be H. pylori-positive. If no suspicious colonies were found, the cultivation time was extended to 7 days. If any strain was found, it was determined to be H. pylori-positive through microscopic examination.
2.3 Antimicrobial susceptibility testing
We performed sensitivity testing of H. pylori to six antibiotics [CLA, AMO, tetracycline (TET), furazolidone (FZD), metronidazole (MET), and levofloxacin (LEV)] using the agar dilution method. According to a 2.0 McFarland standard, each suspension (2 µL) was inoculated onto an antibiotic plate containing 5% defibrillated sheep blood and maintained at 37 °C in a tri-gas incubator in a microaerobic environment for 3 days. Drug sensitivity was evaluated based on the growth status of the colonies at the inoculation site. Resistance to CLA, AMO, FZD, MET, LEV, and TET was defined as minimal inhibitory concentrations of >1, >2, >2, >8, >2, and >2 μg/mL, respectively, according to the Clinical and Laboratory Standards Institute document M100-S18 and other previously published data (Peretz et al., 2014; Shu et al., 2022). The standard strain of H. pylori ATCC43504 (NCTC11637) was used as a control, and each experiment was repeated twice in parallel. We retrospectively analyzed the test results of these children at that time.
2.4 Statistics
We performed statistical analysis using the SPSS statistical software package version 26.0 (SPSS, Inc., Chicago, IL, USA). The outcome variable was the frequency count and expressed as the rate (%) of resistance to the antibiotics. Chi-squared (χ2) test was used to investigate associations between sex, age, and antibiotic resistance among pediatric patients. Statistical significance was set at p < 0.05.
3 Results
3.1 Epidemiology and resistance of Helicobacter pylori in Chinese children
Gastric biopsies were collected from 1,587 children and adolescents for H. pylori culture. In total, 535 (33.7%) patients with H. pylori were enrolled, including 294 (55.0%) boys and 241 (45.0%) girls, with no difference in H. pylori prevalence between sexes (Table 1). The infection rate of H. pylori in Chinese children varied slightly among the three age groups, in descending order: 13–18 years, 7–12 years, and 1–6 years (p < 0.05) (Table 1).
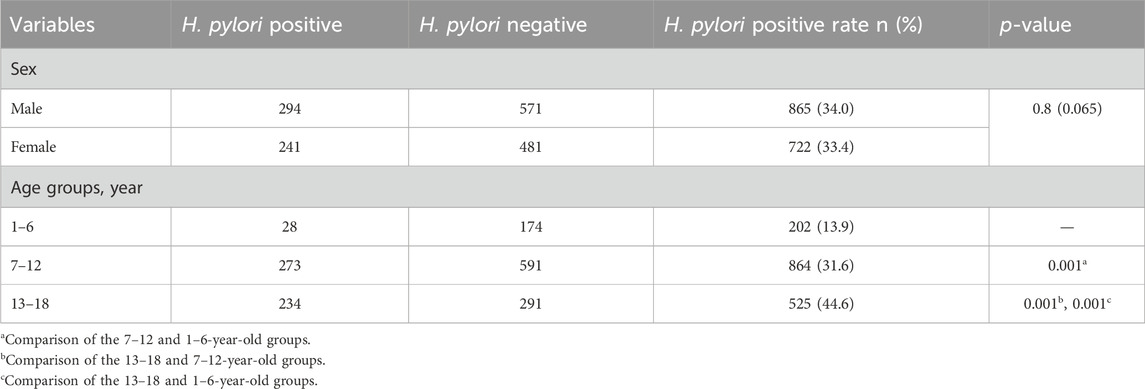
Table 1. Demographic characteristics of the patients and Helicobacter pylori culture of 1,587 samples obtained from patients from 2019 to 2022.
Of the 535 H. pylori-positive patients, 9.5% (51/535) were not resistant to any of antibiotics. The total resistance rates of H. pylori to CLA, MET, and LEV were 39.8% (213/535), 78.1% (418/535), and 20.2% (108/535), respectively (Table 2). None of the strains were resistant to AMO, FZD, or TET. Additionally, 52.7% (282/535) of the strains were resistant to CLA, MET, or LEV, and 37.8% (202/535) were resistant to more than one antibiotic; 27.9% (149/535) were double-resistant, and 9.9% (53/535) were triple-resistant. Notably, double resistance rates to CLA + MET, CLA + LEV, and MET + LEV were 19.1%, 3.0%, and 5.8%, respectively (Table 2). Moreover, only 60 children infected with H. pylori were sensitive to MET and CLA. Sex was not associated with antibiotic resistance. Considering the low infection rate of H. pylori in children under the age of 6 years, the one to six and 7–12 years groups were merged into a 1–12 years group for age stratification to analyze resistance to various antibiotics. Our results indicate that age is associated with resistance to MET and LEV, but not to CLA (Table 3). The MET resistance rate increased with age, whereas LEV resistance rate decreased.
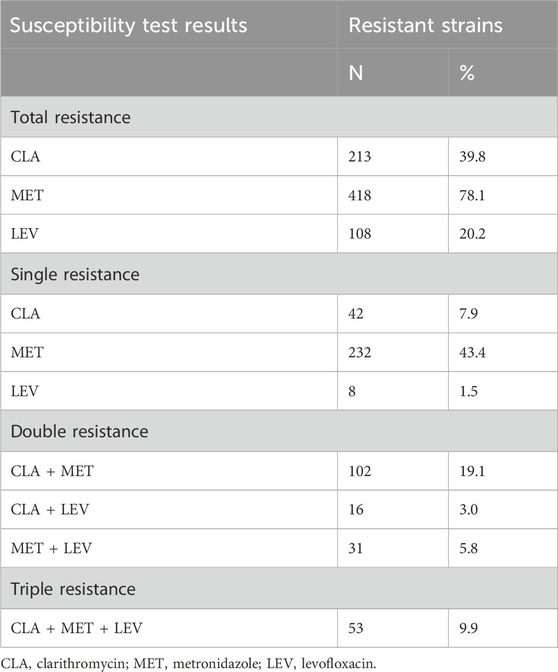
Table 2. Results of antibiotics susceptibility tests of 535 Helicobacter pylori strains isolated from 2019 to 2022.
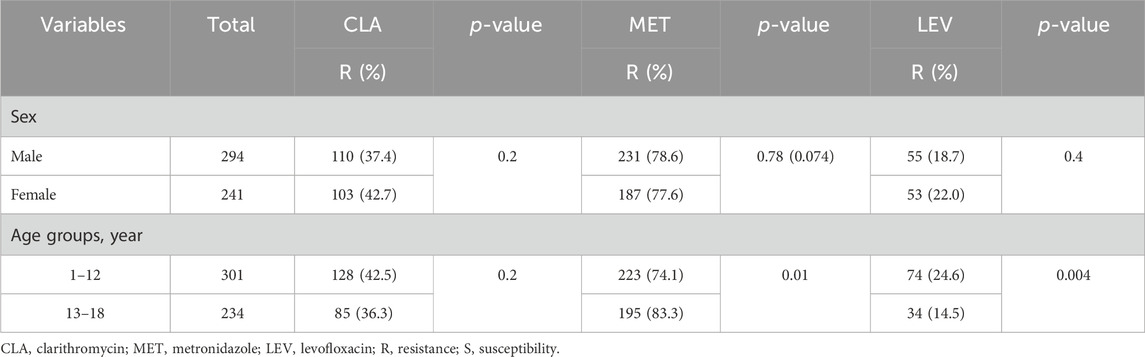
Table 3. Factors associated with Helicobacter pylori resistance to CLA, MTZ, and LEV in pediatric patients.
4 Discussion
4.1 Antibiotic resistance in Helicobacter pylori
In this study, we analyzed the current resistance of H. pylori in children in the local area. Our study showed that approximately 33.7% of children are infected with H. pylori, which is not substantially different from the global infection rate in children (Seo et al., 2020). We cited an overall resistance rate to CLA (39.8%), MET (78.1%) and LEV (20.2%) with 535 H. pylori pediatric strains isolated in Southeast China. In our study, the resistance rate of LEV is 20.2%, which is lower than that of CLA and MET. However, as quinolone antibiotic, LEV can cause arthropathy and bone/cartilage disease in some species of animals. Its safety for children has not been established, and it is forbidden to use under 18 years old in China. In our research, 19.1% of children with H. pylori infection were resistant to CLA and MET.
Antimicrobial resistance is the most common cause of treatment failure in H. pylori infection. The MET resistance rate of H. pylori ranges from 10.1% to 91% in pediatric patients (Borka et al., 2023). Children in East and Southeast Asia and Africa exhibit high resistance to MET (Borka et al., 2023). We demonstrated that MET resistance among children was 78.1%, which was much higher than the regional MET resistance rate reported in Jiangxi province (42%) (Liu et al., 2019) and Taiwan (26.8%) (Lu et al., 2019); however, these results were comparable to the reported rates of 75.2% in Zhejiang province (Li et al., 2017) and 71.3% in southeast China (Hu et al., 2018). Moreover, resistance to MET in this study markedly increased with age. Benefiting from its restricted use, the MET resistance rate was not high in Japan. In triple therapy, the eradication rate in children using MET was over 95% (Malfertheiner et al., 2017; Ding et al., 2022). However, the results of our study and those of other studies on Chinese children showed high MET resistance, although MET use in standard triple therapy is not applicable in Chinese children (Li et al., 2017; Hu et al., 2018).
As a macrolide antibiotic, CLA is stable and available in the low-pH environment of the stomach. Recently, the CLA resistance rate has steadily increased in some regions and countries (Abadi, 2017), which may be related to the widespread use of macrocyclic antibiotics in children with respiratory infections. Eradication rates in areas with high CLA resistance are lower than those in areas with low CLA resistance (Liang et al., 2012). In a study performed in Wenzhou, Zhejiang province, China, when CLA resistance reached 26.12%, the efficacies of triple therapy and bismuth-containing quadruple therapy based on the antibiotic susceptibility test were 67.32% and 68.49%, respectively (Pan et al., 2020). Studies in Vietnam revealed increased resistance to CLA, with rates of 50.9% in 2006 (Le et al., 2022), 84.6% in 2012 (Le et al., 2022), 92.1% in 2021 (Van Thieu et al., 2021), and 81% in 2022 (Borka et al., 2023). The success rate of treatment based on antimicrobial testing is 75% in Vietnam (Nguyen et al., 2012). Nevertheless, in our study, CLA resistance among 535 H. pylori strains was as high as 39.8%, which was much higher than the reported rate of 16.4% from 2009 to 2015 in Zhejiang, of our neighbored city (Li et al., 2017). A similar trend of CLA resistance has also emerged in Europe, where they have implemented the intervention program of macrolide drugs, particularly for respiratory tract infections in children (Megraud et al., 2021). Therefore, we need to pay attention to the rational use of macrolide antibiotics in children to reduce drug resistance.
The resistance of H. pylori in Chinese children is critical. Therefore, individualized treatment based on a susceptibility test may be challenging for the doctor.
4.2 Analysis of Helicobacter pylori in Chinese children based on antimicrobial susceptibility testing
4.2.1 Sensitivity to CLA and/or MET
Age restrictions exist for certain antibacterial agents, therefore, antibacterial agents for H. pylori eradication in children include AMO, CLA, MET, FZD, and TET. Currently, standard triple, sequential, concomitant, and bismuth-based quadruple therapies are recommended to eradicate H. pylori. A study of Chinese children showed that the eradication rates of standard triple, sequential, concomitant, and bismuth-based quadruple therapy were 74.1%, 69.5%, 84.6%, and 89.8%, respectively (Zhou et al., 2020). In the previous study, bismuth-based therapy, but not sequential or concomitant therapy, was superior to triple therapy (Zhou et al., 2020). However, the authors did not consider antimicrobial susceptibility or adherence. Children susceptible to CLA and MET received either 10-day sequential or triple therapy, whereas those resistant to CLA or MET received 10-day triple therapy (Kotilea et al., 2017). The eradication rates of sequential treatment, triple therapy with CLA, and triple therapy with MET were 92%, 77%, and 70%, respectively (Kotilea et al., 2017). Furthermore, eradication was achieved in 86.8% and 92.3% of children who received at least 90% of the prescription drugs in triple therapy containing CLA or MET, respectively (Kotilea et al., 2017). Similar findings were reported in children from Japan, emphasizing that triple therapy, with good adherence to the prescribed drugs, can achieve a primary eradication rate of 97.7%, according to the antimicrobial susceptibility test (Miyata et al., 2021). Currently, most countries consider personalized triple therapy as the preferred treatment for children infected with sensitive H. pylori (Jones et al., 2017; Kato et al., 2020; Subspecialty Group of Gastroenterology et al., 2023). ESPGHAN/NASPGHAN also recommends sequential therapy for children sensitive to both MET and CLA (Jones et al., 2017). According to Schwarzer et al. (2016), the eradication of sequential therapy in no resistance and single resistance were 85.8% and 72.6%, respectively, based on the use of esomeprazole and high compliance. However, sequential therapy for pediatric patients involves exposure to three antibacterial drugs, and the medication regimen for sequential therapy is complex, which can affect children’s treatment compliance. Therefore, most current guidelines do not recommend sequential therapy as first- or second-line therapy, particularly for adults (Chey et al., 2017; Malfertheiner et al., 2017; Kato et al., 2019; Kato et al., 2020; Romano et al., 2022). Notably, children with single resistance have a higher risk of treatment failure than those susceptible to CLA and MET (Le et al., 2023). The eradication with single resistance is usually below 90% (Schwarzer et al., 2016; Le et al., 2023). This may be because the resistant site is omitted during sampling, and thus does not represent the actual resistance.
Based on these findings and considering the resistance to CLA and MET in Chinese children, PPI + AMO + CLA should be selected for H. pylori-infected children who are sensitive to MET and CLA, and medication guidance should be provided to improve compliance and to ensure that more than 90% of prescriptions are followed. For who are sensitive to MET or CLA, the eradication rate is reduced, and it is possible to consider initiating bismuth-based quadruple therapy recommended by ESPGHAN/NASPGHAN (Jones et al., 2017).
4.2.2 Resistance to CLA and MET
According to the ESPGHAN/NASPGHAN guidelines, triple therapy with PPI-AMO at high doses for 14 days or concomitant therapy, or bismuth-based quadruple therapy is recommended for patients who are resistant to CLA and MET. However, research on the use of high-dose AMO in children is limited. The eradication rate achieved using a combination of esomeprazole, high-dose AMO, and MET in children with dual resistance did not exceed 75% (Schwarzer et al., 2011), even when using esomeprazole, which is not strongly influenced by CYP2C19 (Schwarzer et al., 2011). A similar conclusion was found in another study conducted in Vietnam; for children showing resistance to MET and CLA, the eradication rate of using high-dose AMO therapy was only 32.6%, which was lower than that obtained using the standard dose of AMO (60.6%, p = 0.015) (Van Thieu et al., 2021; Ding et al., 2022). In these studies, high-dose AMO had a poor effect on children with multi-antibiotic-resistant H. pylori.
Combination and sequential therapies have the same drawbacks, exposing the three antimicrobial drugs and affecting treatment compliance. Combination therapy does not have a superior eradication rate and, therefore, is not recommended when other treatment regimens are available. Bismuth compounds may play two main roles: heavy metals possess antimicrobial properties and interfere with H. pylori attachment when these electron-dense bodies are deposited on the cell surface (Pacifico et al., 2012), and bismuth compounds have a synergistic effect with antibiotics in overcoming multi-antibiotic resistance (Ko et al., 2019).
In China, TET and FZD are recommended for treating H. pylori infections in children. We detected no strains resistant to TET or FZD. The resistance rate of Chinese children to these two antibiotics is low. Therefore, TET and FZD can be chosen as substitutes for CLA and MET, respectively, to overcome dual resistance. However, because of the genotoxic and carcinogenic effects of FZD and tooth calcification caused by TET, these drugs are prescribed for children older than eight and 14 years, respectively. However, there are age limitations for the administration of TET and FZD, which have insufficient efficacy in treating H. pylori infection in children.
ESPGHAN/NASPGHAN guidelines recommend alternative drugs for children with AMO allergies. Limiting antibiotic use leads to lower antibiotic resistance development. Therefore, the selection of new antibiotics to overcome antibiotic resistance may lead to antibiotic resistance. Most clinical physicians and patients evade bismuth-containing therapy because of adverse reactions. The bismuth compounds currently used are insoluble inorganic salts with a systemic absorption of <0.5%, and the low dose of bismuth compounds used to eradicate H. pylori typically does not cause neurotoxicity (Alkim et al., 2017).
Hence, for children infected with dual-resistant H. pylori, bismuth-based quadruple therapy is preferred. If bismuth compounds are not accepted, individualized treatment can be selected based on age (see Figure 1).
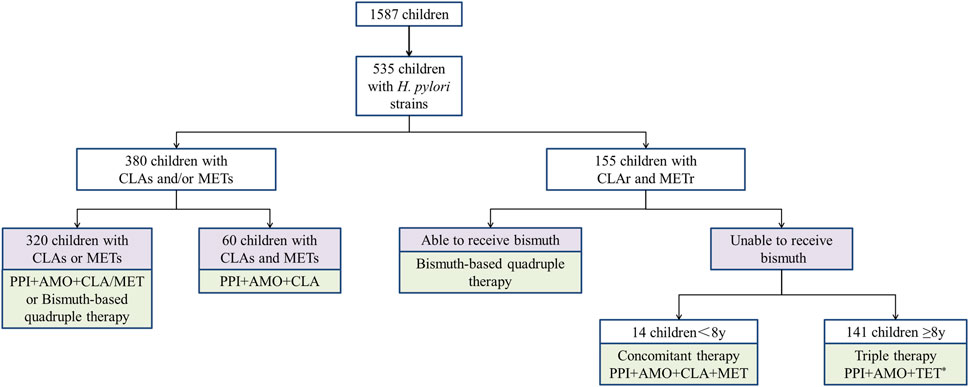
Figure 1. Individualized treatment strategies for Helicobacter pylori in Chinese children based on susceptibility testing. * 43 children above 14 years old could use TET or FZD. PPI proton pump inhibitors, AMO amoxicillin, CLA clarithromycin, MET metronidazole, TET tetracycline.
4.3 Avoid these factors associated with treatment failure
We collected epidemiology and current resistance data on H. pylori in children in a specific area. Individualized treatment based on culture tests is a universally acknowledged strategy. However, recent research showed that the eradication rate by therapies based only on susceptibility testing does not reach 90% in children (Le et al., 2022). Insufficient acid-suppressive and poor therapy compliance may be the reasons for failure.
H. pylori eradication depends on a combination of acid suppressants and antibiotics. Gastric acid suppressants reduce the minimal inhibitory concentration of antibiotics by increasing the gastric pH and increase the antibacterial drug concentration by diminishing the gastric juice volume. PPIs are commonly used for acid suppression in pediatric eradication treatments. The insufficient acid-suppressive capacity of PPIs is related to under-dosing, ingestion of drugs at inappropriate times (not prior to meals), and genetic polymorphisms of hepatic CYP enzyme activity (Litalien et al., 2005). First-generation PPIs, including lansoprazole, pantoprazole, and omeprazole, and second-generation PPIs, such as dexlansoprazole, are mainly metabolized by CYP2C19 and less metabolized by CYP3A4. More than 90% of omeprazole is metabolized by CYP2C19. Other second-generation PPIs including esomeprazole and rabeprazole are less dependent on CYP2C19, and rabeprazole is mainly eliminated through non-enzymatic mechanisms (El Rouby et al., 2018). The CYP2C19 genotype is associated with PPI exposure and affects drug efficacy and causes adverse reactions. Individuals are divided into extensive metabolizers (EM), intermediate metabolizers (IM), and poor metabolizers (PM), and metabolic phenotypes vary among different regions. Research on Chinese children showed that the proportions of EM, IM, and PM are 38.04%–49.1%, 40.2%–53%, and 10%–10.7%, respectively (Zhang et al., 2020; Zhou et al., 2020; Luo et al., 2023). Extensive metabolism may cause the failure of omeprazole triple therapy to eradicate H. pylori, but does not substantially impact rabeprazole triple therapy (Kuo et al., 2014; Lin et al., 2018). Similar conclusions were reported in another study, where the eradication rates of the EM, IM, and PM groups were 60.7%, 84.2%, and 100%, respectively, when the same antibacterial drug was used in combination with omeprazole, whereas this difference did not occur in combination treatment with rabeprazole (Lin et al., 2017). In Europe, esomeprazole (60%) was the most used PPI for tailored triple therapy in children, followed by omeprazole (32%), pantoprazole (6%), and lansoprazole (2%) (Le et al., 2023). In addition, CYP2C19 activity is higher during puberty, and higher doses may be required to achieve ideal acid inhibition (El Rouby et al., 2018). Hence, children should receive higher PPIs doses than the dose administered to adults based on body weight. The updated pediatric guidelines recommend higher PPIs doses for all regimens, with omeprazole doses ranging from 1 to 2 mg/kg/day (Jones et al., 2017; Manfredi et al., 2023). However, omeprazole doses below 1 mg/kg/day have been used for many years in China, and recently updated guidelines for children recommend 1 mg/kg/day for IM. Therefore, it is necessary to consider the CYP2C19 genotype in Chinese children or amend the PPI types used to increase the acid inhibition ability to treat H. pylori infection in Chinese children.
Even if a doctor formulates strict and accurate treatment, the treatment is not effective if it is insufficiently implemented in children. Compliance among patients with chronic diseases is often lower than that among those with acute diseases (Osterberg and Blaschke, 2005). Although H. pylori infection is not a chronic disease and the treatment duration does not exceed 2 weeks, poor compliance strongly impacts the eradication rate (Shoiab et al., 2018). High compliance is defined as >90% intake of the prescribed dose. Kotilea et al. demonstrated that eradication was achieved in 89.9% of children with high compliance, but in only 36.8% of children with low adherence (Kotilea et al., 2017). In another study, counseling by pharmacists increased medication adherence from 27.5% to 45%, with the eradication rate of H. pylori increasing from 28.5% to 42.5% (Shoiab et al., 2023).
Moreover, there is a lack of child-specific preparations of H. pylori treatment drugs in China. Tablets or capsules are often needed to distribute the dosage, which leads to an unpleasant taste. In addition to education on diseases and medication counseling from pharmacists, other measures may be needed. A study of non-adherence in H. pylori treatment found that 80% of patients missed doses because of forgetfulness (Shakya et al., 2016). Hence, electronic monitoring of medication records and reminders can be used to reduce the number of missed medications.
Therapy compliance includes sufficient prescription dosage and proper medication use to achieve an optimal therapeutic role. Medication for H. pylori eradication in children based on body weight is summarized in Table 4. Ingesting antibiotics after meals and PPIs before meals provides a suitable environment for antibiotics to exert their effects. Another therapeutic drug, bismuth, dissolves in the stomach at concentrations of pH four to seven, which can reduce its efficacy. Therefore, bismuth should be used before administering PPIs.
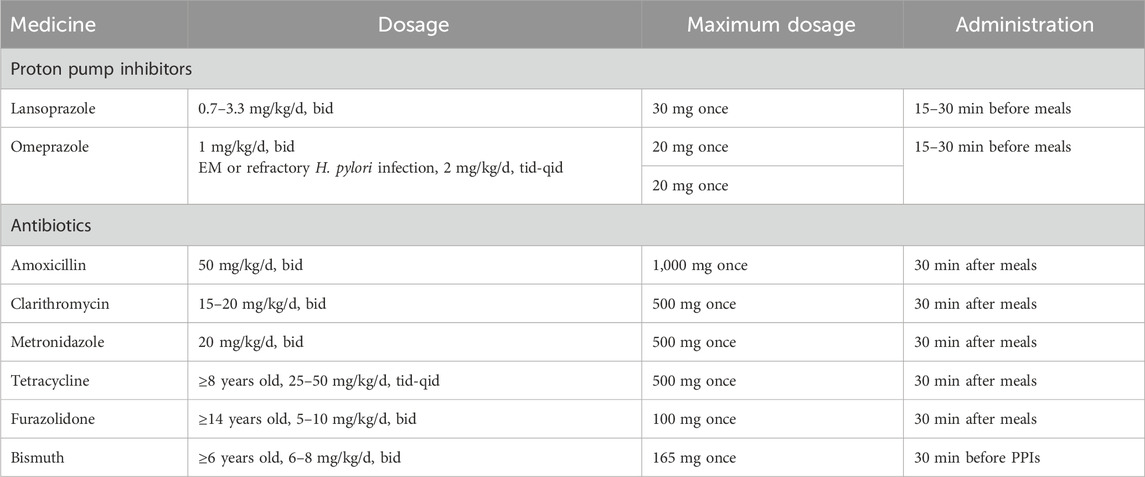
Table 4. Dosage and usage of oral therapeutic drugs available for Helicobacter pylori eradication treatment in children (recommended in China).
4.4 Other developing personalized strategies of Helicobacter pylori
Eradication of H. pylori infection in children using a combination of antimicrobials and PPIs rarely exceeds 90%; therefore, researchers have investigated alternative strategies, including probiotics, vaccines, and phytomedicines, for H. pylori eradication.
4.4.1 Probiotics
The use of antibiotics and PPIs for H. pylori eradication alters the gastrointestinal mucosal immune response and intestinal inflammatory microenvironment, resulting in a microbiota imbalance (Manfredi et al., 2023 ). Moreover, initiating H. pylori eradication can alter gut microbiota diversity, which may have a long-lasting effect (Sitkin et al., 2022). Hence, supplementation with probiotics and microbial metabolites should be considered to reduce the negative effects of eradication. However, whether probiotics improve the eradication rate of H. pylori is unclear. Published studies are inconsistent with respect to the types, dose, timing, and duration of probiotic use. Furthermore, the studies used different eradication therapies. Therefore, the use of probiotics as and adjuvant treatment for H. pylori eradication varies by country (Jones et al., 2017; Kato et al., 2019; Kato et al., 2020; Leung et al., 2023). The Maastricht VI/Florence guidelines state that Lactobacillus, Bifidobacterium, and S. boulardii can improve eradication rates in adults (Malfertheiner et al., 2022). However, there is a lack of agreement regarding the effect on eradication of adding single or combination probiotics, especially in children (Daelemans et al., 2022).
4.4.2 Vaccines
The annual recurrence rate of H. pylori worldwide is 4.3% (Hu et al., 2017), and is considerably higher in developing countries (13%) than in developed countries (2.7%) (Sun and Zhang, 2019). Although the infection rate of H. pylori is decreasing, the recurrence rate has not decreased (Hu et al., 2017). Recurrence is generally associated with severe resistance, which increases the difficulty to treatment. Vaccines have the potential to achieve eradication and prevention (Ding et al., 2018; Wang et al., 2021). Most vaccines are in the early stage of development (Phase I or preclinical studies), and studies have had variable results (Stubljar et al., 2018; Dos Santos et al., 2021). However, the results of a phase III clinical trial of a H. pylori subunit vaccine, conducted in children in China aged 6–15 years, showed a 1-year efficacy rate of 71.8% and a 3-year efficacy rate of 65% (Zeng et al., 2015). Vaccines are a promising strategy for use in children because children are infected at a younger age and have a high recurrence rate. However, effective, safe, and immunogenic vaccines are not currently available.
4.4.3 Phytomedicines
Many plant products or their bioactive compounds have antibacterial properties. Several in vitro studies have found that certain plant extracts, such as blueberry and grape seed, cinnamon extract, and flavonoids, have anti-H. pylori activity (Liu et al., 2018; Sousa et al., 2022). Currently, all in vivo studies have been conducted in animals, and no human safety or efficacy studies, or clinical studies on children, are available.
5 Conclusion
We observed antibiotic resistance to CLA, MET, AMO, TET, FZD, and LEV in clinical strains isolated from Chinese children between 2019 and 2022. Individualized treatment was designed based on the results of antimicrobial susceptibility testing. The antibiotic resistance rate of H. pylori in Chinese children is high; thus, to achieve a 90% eradication rate, it is also necessary to avoid inappropriate use of PPIs and poor therapy compliance. Measures are needed to strengthen the management of antibiotics, reduce unnecessary exposure to antibiotics, and curb the deterioration of drug resistance. Additionally, eradication plans must be standardized to improve the eradication rate.
Based on drug resistance issues and previous studies, we summarized personalized treatment strategies for regional resistance to achieve an initial eradication rate of over 90%. However, there is no practical support for individualized treatment strategy analyses. Therefore, we plan to conduct prospective single or multicenter studies based on these treatment strategies to explore real-world data.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Approval Number: WXCH2023-04-070. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
DZ: Conceptualization, Writing–original draft. WW: Conceptualization, Formal Analysis, Writing–review and editing. LG: Data curation, Methodology, Writing–review and editing. MH: Formal Analysis, Investigation, Writing–review and editing. WH: Data curation, Methodology, Writing–review and editing. JH: Data curation, Methodology, Software, Visualization, Writing–review and editing. QL: Conceptualization, Investigation, Methodology, Resources, Validation, Writing–review and editing. YW: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Technology Development Fund Project of Wuxi Key Medical Disciplines (Grant number: 2024SZD-YXB), Wuxi Science and Technology Development Fund (Grant number: Y20232008), and Pharmaceutical Fund of the Jiangsu Pharmaceutical Association (Grant number: Q202224 and A202135).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abadi, A. T. B. (2017). Resistance to clarithromycin and gastroenterologist’s persistence roles in nomination for Helicobacter pylori as high priority pathogen by World Health Organization. World. J. Gastroenterol. 23 (35), 6379–6384. doi:10.3748/wjg.v23.i35.6379
Agudo, S., Alarcón, T., Urruzuno, P., Martínez, M. J., and López-Brea, M. (2010). Detection of Helicobacter pylori and clarithromycin resistance in gastric biopsies of pediatric patients by using a commercially available real-time polymerase chain reaction after NucliSens semiautomated DNA extraction. Diagn. Microbiol. Infect. Dis. 67 (3), 213–219. doi:10.1016/j.diagmicrobio.2010.02.021
Alfaro, E., Sostres, C., and Lanas, A. (2023). Diagnosis and treatment of Helicobacter pylori infection in real practice-new role of primary care services in antibiotic resistance era. Diagn. (Basel) 13 (11), 1918. doi:10.3390/diagnostics13111918
Alkim, H., Koksal, A. R., Boga, S., Sen, I., and Alkim, C. (2017). Role of bsmuth in the eradication of Helicobacter pylori. Am. J. Ther. 24 (6), e751–e757. doi:10.1097/MJT.0000000000000389
Borka, B. R., Meliț, L. E., and Mărginean, C. O. (2022). Worldwide prevalence and risk factors of Helicobacter pylori infection in children. Child. 9 (9), 1359. doi:10.3390/children9091359
Borka, B. R., Meliț, L. E., and Mărginean, C. O. (2023). Current worldwide trends in pediatric Helicobacter pylori antimicrobial resistance. Child. (Basel). 10 (2), 403. doi:10.3390/children10020403
Chey, W. D., Leontiadis, G. I., Howden, C. W., and Moss, S. F. (2017). ACG clinical guideline: treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 112 (2), 212–239. doi:10.1038/ajg.2016.563
Daelemans, S., Deseck, V., Levy, E. I., and Vandenplas, Y. (2022). Are pro- and/or synbiotics beneficial in Helicobacter pylori eradication therapy in children? A narrative review. Eur. J. Pediatr. 181 (9), 3225–3234. doi:10.1007/s00431-022-04523-7
Ding, C., Ma, J., Dong, Q., and Liu, Q. (2018). Live bacterial vaccine vector and delivery strategies of heterologous antigen: a review. Immunol. Lett. 197, 70–77. doi:10.1016/j.imlet.2018.03.006
Ding, S. Z., Du, Y. Q., Lu, H., Wang, W. H., Cheng, H., Chen, S. Y., et al. (2022). Chinese consensus report on family-based Helicobacter pylori infection control and management (2021 Edition). Gut 71 (2), 238–253. doi:10.1136/gutjnl-2021-325630
Dos Santos, V. I., Cordeiro Santos, M. L., Santos Marques, H., Lima de Souza Gonçalves, V., Bittencourt de Brito, B., França da Silva, F. A., et al. (2021). Vaccine development against Helicobacter pylori: from ideal antigens to the current landscape. Expert Rev. Vaccines 20 (8), 989–999. doi:10.1080/14760584.2021.1945450
El Rouby, N., Lima, J. J., and Johnson, J. A. (2018). Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert. Opin. Drug. Metab. Toxicol. 14 (4), 447–460. doi:10.1080/17425255.2018.1461835
Garcés-Duran, R., Kindt, S., Kotilea, K., François, S., Rasschaert, G., Smet, A., et al. (2023). Belgian consensus for Helicobacter pylori management 2023. Acta. Gastroenterol. belg. 86 (1), 74–91. doi:10.51821/86.1.11327
Graham, D. Y., and Fischbach, L. (2010). Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 59 (8), 1143–1153. doi:10.1136/gut.2009.192757
Hu, F., Zhu, D., Wang, F., and Wang, M. (2018). Current status and trends of antibacterial resistance in China. Clin. Infect. Dis. 67 (-2), S128–S134. doi:10.1093/cid/ciy657
Hu, Y., Wan, J. H., Li, X. Y., Zhu, Y., Graham, D. Y., and Lu, N. H. (2017). Systematic review with meta-analysis: the global recurrence rate of Helicobacter pylori. Aliment. Pharmacol. Ther. 46 (9), 773–779. doi:10.1111/apt.14319
Jones, N. L., Koletzko, S., Goodman, K., Bontems, P., Cadranel, S., Casswall, T., et al. (2017). Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J. Pediatr. Gastroenterol. Nutr. 64 (6), 991–1003. doi:10.1097/MPG.0000000000001594
Katelaris, P., Hunt, R., Bazzoli, F., Cohen, H., Fock, K. M., Gemilyan, M., et al. (2023). Helicobacter pylori world gastroenterology organization global guideline. J. Clin. Gastroenterol. 57 (2), 111–126. doi:10.1097/MCG.0000000000001719
Kato, M., Ota, H., Okuda, M., Kikuchi, S., Satoh, K., Shimoyama, T., et al. (2019). Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter 24 (4), e12597. doi:10.1111/hel.12597
Kato, S., Shimizu, T., Toyoda, S., Gold, B. D., Ida, S., Ishige, T., et al. (2020). The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr. Int. 62 (12), 1315–1331. doi:10.1111/ped.14388
Ko, S. W., Kim, Y. J., Chung, W. C., and Lee, S. J. (2019). Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: systemic review and meta-analysis. Helicobacter 24, e12565. doi:10.1111/hel.12565
Kotilea, K., Mekhael, J., Salame, A., Mahler, T., Miendje-Deyi, V. Y., Cadranel, S., et al. (2017). Eradication rate of Helicobacter Pylori infection is directly influenced by adherence to therapy in children. Helicobacter 22 (4), e12383. doi:10.1111/hel.12383
Kuo, C. H., Lu, C. Y., Shih, H. Y., Liu, C. J., Wu, M. C., Hu, H. M., et al. (2014). CYP2C19 polymorphism influences Helicobacter pylori eradication. World. J. Gastroenterol. 20 (43), 16029–16036. doi:10.3748/wjg.v20.i43.16029
Le, L. T. T., Nguyen, T. A., Nguyen, N. A., Nguyen, Y. T. H., Nguyen, H. T. B., Nguyen, L. T., et al. (2022). Helicobacter pylori eradication efficacy of therapy based on the antimicrobial susceptibility in children with gastritis and peptic ulcer in Mekong Delta, Vietnam. Child. (Basel) 9 (7), 1019. doi:10.3390/children9071019
Le, T. T. G., Werkstetter, K., Kotilea, K., Bontems, P., Cabral, J., Cilleruelo, P. M. L., et al. (2023). Management of Helicobacter pylori infection in paediatric patients in Europe: results from the EuroPedHp Registry. Infection 51 (4), 921–934. doi:10.1007/s15010-022-01948-y
Leung, W. K., Cheung, K. S., Sham, P. C. O., Tang, R. S. Y., Loo, C. K., Hsu, A. S. J., et al. (2023). Consensus recommendations for the screening, diagnosis, and management of Helicobacter pylori infection in Hong Kong. Hong. Kong. Med. J. 29 (6), 532–541. doi:10.12809/hkmj2210321
Li, L., Ke, Y., Yu, C., Li, G., Yang, N., Zhang, J., et al. (2017). Antibiotic resistance of Helicobacter pylori in Chinese children: a multicenter retrospective study over 7 years. Helicobacter 22 (3), e12373. doi:10.1111/hel.12373
Liang, J., Li, J., Han, Y., Xia, J., Yang, Y., Li, W., et al. (2012). Helicobacter pylori eradication with ecabet sodium, omeprazole, amoxicillin, and clarithromycin versus bismuth, omeprazole, amoxicillin, and clarithromycin quadruple therapy: a randomized, open-label, phase IV trial. Helicobacter 17 (6), 458–465. doi:10.1111/j.1523-5378.2012.00971.x
Lin, T. J., Lee, H. C., Lin, C. L., Wang, C. K., Chen, K. Y., and Wu, D. C. (2018). CYP2C19 polymorphism has no influence on rabeprazole-based hybrid therapy for Helicobacter pylori eradication. World. J. Clin. cases. 6 (12), 514–520. doi:10.12998/wjcc.v6.i12.514
Lin, Y. A., Wang, H., Gu, Z. J., Wang, W. J., Zeng, X. Y., Du, Y. L., et al. (2017). Effect of CYP2C19 gene polymorphisms on proton pumpinhibitor, amoxicillin, and levofloxacin triple therapy for eradication of Helicobacter Pylori. Med. Sci. Monit. 23, 2701–2707. doi:10.12659/msm.901514
Litalien, C., Théorêt, Y., and Faure, C. (2005). Pharmacokinetics of proton pump inhibitors in children. Pharmacokinet. 44 (5), 441–466. doi:10.2165/00003088-200544050-00001
Liu, D. S., Wang, Y. H., Zhu, Z. H., Zhang, S. H., Zhu, X., Wan, J. H., et al. (2019). Characteristics of Helicobacter pylori antibiotic resistance: data from four different populations. Antimicrob. Resist. Infect. Control 8, 192. doi:10.1186/s13756-019-0632-1
Liu, Q., Meng, X., Li, Y., Zhao, C. N., Tang, G. Y., Li, S., et al. (2018). Natural products for the prevention and Management of Helicobacter pylori infection. Compr. Rev. Food Sci. Food Saf. 17 (4), 937–952. doi:10.1111/1541-4337.12355
Lu, H. H., Lai, F. P., Lo, H. Y., Sheu, B. S., and Yang, Y. J. (2019). Increasing antimicrobial resistance to clarithromycin and metronidazole in pediatric Helicobacter pylori infection in southern Taiwan: a comparison between two decades. Helicobacter 24 (5), e12633. doi:10.1111/hel.12633
Luo, L., Huang, Y., Liang, X., Ji, Y., Yu, L., and Lu, H. (2020). Susceptibility-guided therapy for Helicobacter pylori-infected penicillin-allergic patients: a prospective clinical trial of first-line and rescue therapies. Helicobacter 25 (4), e12699. doi:10.1111/hel.12699
Luo, L. L., Chen, B., Shu, X. L., Zheng, W., Long, G., and Jiang, M. Z. (2023). The relationship between genetic polymorphism of CYP2C19 and the efficacy of Helicobacter pylori eradication therapy in children. Zhonghua. Er. Ke. Za. Zhi. 61 (7), 600–605. doi:10.3760/cma.j.cn112140-20221230-01076
Malfertheiner, P., Megraud, F., O’Morain, C., Bazzoli, F., El-Omar, E., Graham, D., et al. (2007). Current concepts in the management of Helicobacter pylori infection: the maastricht III consensus report. Gut 56 (6), 772–781. doi:10.1136/gut.2006.101634
Malfertheiner, P., Megraud, F., O’Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut 66 (1), 6–30. doi:10.1136/gutjnl-2016-312288
Malfertheiner, P., Megraud, F., Rokkas, T., Gisbert, J. P., Liou, J. M., Schulz, C., et al. (2022). Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 71 (9), 1724–1762. doi:10.1136/gutjnl-2022-327745
Manfredi, M., Gargano, G., Gismondi, P., Ferrari, B., and Iuliano, S. (2023). Therapeutic eradication choices in Helicobacter pylori infection in children. Therap. Adv. Gastroenterol. 16, 1–17. doi:10.1177/17562848231170052
Martin-Nuñez, G. M., Cornejo-Pareja, I., Clemente-Postigo, M., and Tinahones, F. J. (2021). Gut microbiota: the missing link between Helicobacter pylori infection and metabolic disorders. Front. Endocrinol. (Lausanne). 12, 639856. doi:10.3389/fendo.2021.639856
Megraud, F., Bruyndonckx, R., Coenen, S., Wittkop, L., Huang, T. D., Hoebeke, M., et al. (2021). Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 70 (10), 1815–1822. doi:10.1136/gutjnl-2021-324032
Mehata, S., Parajuli, K. R., Pant, N. D., Rayamajhee, B., Yadav, U. N., Mehta, R. K., et al. (2021). Prevalence and correlates of Helicobacter pylori infection among under-five children, adolescent and non-pregnant women in Nepal: further analysis of Nepal national micronutrient status survey 2016. PLoS. Negl. Trop. Dis. 15 (6), e0009510. doi:10.1371/journal.pntd.0009510
Melby, K. K., Carlsen, K. L., Håland, G., Samdal, H. H., and Carlsen, K. H. (2020). Helicobacter pylori in early childhood and asthma in adolescence. Bmc. Res. Notes. 13 (1), 79. doi:10.1186/s13104-020-04941-6
Miyata, E., Kudo, T., Ikuse, T., Tokita, K., Arai, N., Oka, I., et al. (2021). Eradication therapy for Helicobacter pylori infection based on the antimicrobial susceptibility test in children: a single-center study over 12 years. Helicobacter 26 (1), e12764. doi:10.1111/hel.12764
Moghadam, M. T., Chegini, Z., Norouzi, A., Dousari, A. S., and Shariati, A. (2021). Three-decade failure to the eradication of refractory Helicobacter pylori infection and recent efforts to eradicate the infection. Curr. Pharm. Biotechnol. 22 (7), 945–959. doi:10.2174/1389201021666200807110849
Nguyen, T. V., Bengtsson, C., Yin, L., Nguyen, G. K., Hoang, T. T., Phung, D. C., et al. (2012). Eradication of Helicobacter pylori in children in Vietnam in relation to antibiotic resistance. Helicobacter 17 (4), 319–325. doi:10.1111/j.1523-5378.2012.00950.x
Okuda, M., Lin, Y., and Kikuchi, S. (2019). Helicobacter pylori infection in children and adolescents. Adv. Exp. Med. Biol. 1149, 107–120. doi:10.1007/5584_2019_361
Osterberg, L., and Blaschke, T. (2005). Adherence to medication. N. Engl. J. Med. 353 (5), 487–497. doi:10.1056/NEJMra050100
Pacifico, L., Osborn, J. F., Anania, C., Vaira, D., Olivero, E., and Chiesa, C. (2012). Review article: bismuth-based therapy for Helicobacter pylori eradication in children. Aliment. Pharmacol. Ther. 35 (9), 1010–1026. doi:10.1111/j.1365-2036.2012.05055.x
Pan, J., Shi, Z., Lin, D., Yang, N., Meng, F., Lin, L., et al. (2020). Is tailored therapy based on antibiotic susceptibility effective? a multicenter, open-label, randomized trial. Front. Med. 14 (1), 43–50. doi:10.1007/s11684-019-0706-8
Peleteiro, B., Bastos, A., Ferro, A., and Lunet, N. (2014). Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig. Dis. Sci. 59 (8), 1698–1709. doi:10.1007/s10620-014-3063-0
Peretz, A., Paritsky, M., Nasser, O., Brodsky, D., Glyatman, T., Segal, S., et al. (2014). Resistance of Helicobacter pylori to tetracycline, amoxicillin, clarithromycin and metronidazole in Israeli children and adults. J. Antibiot. (Tokyo) 67 (8), 555–557. doi:10.1038/ja.2014.38
Ren, S., Cai, P., Liu, Y., Wang, T., Zhang, Y., Li, Q., et al. (2022). Prevalence of Helicobacter pylori infection in China: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 37 (3), 464–470. doi:10.1111/jgh.15751
Romano, M., Gravina, A. G., Eusebi, L. H., Pellegrino, R., Palladino, G., Frazzoni, L., et al. (2022). Management of Helicobacter pylori infection: guidelines of the Italian society of gastroenterology (SIGE) and the Italian society of digestive endoscopy (SIED). Dig. Liver. Dis. 54 (9), 1153–1161. doi:10.1016/j.dld.2022.06.019
Schwarzer, A., Bontems, P., Urruzuno, P., Kalach, N., Iwanczak, B., Roma-Giannikou, E., et al. (2016). Sequential therapy for Helicobacter pylori infection in treatment-naive children. Helicobacter 21 (2), 106–113. doi:10.1111/hel.12240
Schwarzer, A., Urruzuno, P., Iwańczak, B., Martínez-Gómez, M. Z., Kalach, N., Roma-Giannikou, E., et al. (2011). New effective treatment regimen for children infected with a double-resistant Helicobacter pylori strain. J. Pediatr. Gastroenterol. Nutr. 52 (4), 424–428. doi:10.1097/MPG.0b013e3181fc8c58
Seo, J. H., Bortolin, K., and Jones, N. L. (2020). Review: Helicobacter pylori infection in children. Helicobacter 25 (1), e12742. doi:10.1111/hel.12742
Shakya, S. S., Bhandari, M., Thapa, S. R., Shrestha, R., Poudyal, R., Purbey, B., et al. (2016). Medication adherence pattern and factors affecting adherence in Helicobacter Pylori eradication therapy. Kathmandu. Univ. Med. J. 14 (53), 58–64.
Shoiab, A. A., Alsarhan, A., and Khashroum, A. O. (2023). Effect of pharmacist counseling on patient medication compliance and Helicobacter pylori eradication among Jordanian outpatients. Arq. Gastroenterol. 60 (1), 74–83. doi:10.1590/S0004-2803.202301000-10
Shoiab, A. A., Aziz, N. A., Hassan, Y., and Alefan, Q. (2018). The positive impact of patient’s knowledge on medication compliance among Helicobacter pylori infected outpatients. Adv. Sci. Lett. 24, 6961–6965. doi:10.1166/asl.2018.12896
Shu, X., Ye, D., Hu, C., Peng, K., Zhao, H., Li, H., et al. (2022). Alarming antibiotics resistance of Helicobacter pylori from children in Southeast China over 6 years. Sci. Rep. 12 (1), 17754. doi:10.1038/s41598-022-21661-y
Sitkin, S., Lazebnik, L., Avalueva, E., Kononova, S., and Vakhitov, T. (2022). Gastrointestinal microbiome and Helicobacter pylori: eradicate, leave it as it is, or take a personalized benefit-risk approach? World. J. Gastroenterol. 28 (7), 766–744. doi:10.3748/wjg.v28.i7.766
Sousa, C., Ferreira, R., Azevedo, N. F., Oleastro, M., Azeredo, J., Figueiredo, C., et al. (2022). Helicobacter pylori infection: from standard to alternative treatment strategies. Crit. Rev. Microbiol. 48 (3), 376–396. doi:10.1080/1040841X.2021.1975643
Stubljar, D., Jukic, T., and Ihan, A. (2018). How far are we from vaccination against Helicobacter pylori infection? Expert Rev. Vaccines 17 (10), 935–945. doi:10.1080/14760584.2018.1526680
Subspecialty Group of GastroenterologyNational Children′s Medical Center Digestive Specialist AllianceEditorial Board, Chinese Journal of Pediatrics (2023). Expert consensus on the diagnosis and management of Helicobacter pylori infection in Chinese children (2022). Zhonghua. Er. Ke. Za. Zhi 61 (7), 580–587. doi:10.3760/cma.j.cn112140-20220929-00849
Sun, Y., and Zhang, J. (2019). Helicobacter pylori recrudescence and its influencing factors. J. Cell Mol. Med. 23 (12), 7919–7925. doi:10.1111/jcmm.14682
Ueda, J., Gosho, M., Inui, Y., Matsuda, T., Sakakibara, M., Mabe, K., et al. (2014). Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 19 (2), 105–110. doi:10.1111/hel.12110
Van Thieu, H., Duc, N. M., Nghi, B. T. D., Van, B. N., Khoi, H. H., Tien, V. N. T., et al. (2021). Antimicrobial resistance and the successful eradication of Helicobacter pylori-induced gastroduodenal ulcers in Vietnamese children. Med. Arch. 75 (2), 112–115. doi:10.5455/medarh.2021.75.112-115
Wang, S., Ma, J., Ji, Q., and Liu, Q. (2021). Evaluation of an attenuated listeria monocytogenes as a vaccine vector to control Helicobacter pylori infection. Immunol. Lett. 238, 68–74. doi:10.1016/j.imlet.2021.07.010
Zeng, M., Mao, X. H., Li, J. X., Tong, W. D., Wang, B., Zhang, Y. J., et al. (2015). Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386 (10002), 1457–1464. doi:10.1016/S0140-6736(15)60310-5
Zhang, Y. D., Dong, Q. W., Zhang, S. H., Gu, F., Zhang, Y., Song, H. B., et al. (2020). Effectiveness of eradication regimen based on the bacterial susceptibility and CYP2C19 genotype in children with refractory Helicobacter pylori infection. Zhonghua. Er. Ke. Za. Zhi. 58 (1), 41–45. doi:10.3760/cma.j.issn.0578-1310.2020.01.010
Keywords: children, Helicobacter pylori, eradication, resistance, susceptibility testing
Citation: Zhou D, Wang W, Gu L, Han M, Hao W, Huang J, Lin Q and Wang Y (2024) Helicobacter pylori antibiotic resistance profile in Chinese children with upper gastrointestinal symptoms and a literature review for developing personalized eradicating strategies. Front. Pharmacol. 15:1392787. doi: 10.3389/fphar.2024.1392787
Received: 28 February 2024; Accepted: 13 May 2024;
Published: 03 June 2024.
Edited by:
Raffaele Pellegrino, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Muinah Fowora, Nigerian Institute of Medical Research (NIMR), NigeriaIrena Mladenova, Trakia University, Bulgaria
Copyright © 2024 Zhou, Wang, Gu, Han, Hao, Huang, Lin and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Lin, bGlucWlvbmc3NkAxNjMuY29t; Yan Wang, Njk5ODI1NkBxcS5jb20=
†These authors have contributed equally to this work
 Danli Zhou
Danli Zhou Wuyu Wang2†
Wuyu Wang2† Wujuan Hao
Wujuan Hao Qiong Lin
Qiong Lin