
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 23 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1389768
 Liang Li1†
Liang Li1† Xiaorong Wu2,3†
Xiaorong Wu2,3† Jili Gong2,3†
Jili Gong2,3† Zhuqiang Wang1†
Zhuqiang Wang1† Weibo Dai1†
Weibo Dai1† Li Qiu3,4
Li Qiu3,4 Hongyuan Zuo3
Hongyuan Zuo3 Mengqin Yi3
Mengqin Yi3 Hui Yuan3,4
Hui Yuan3,4 Mei Hu1,3
Mei Hu1,3 Zhaobing Gao2,3,4*
Zhaobing Gao2,3,4* Fuyun Tian3*
Fuyun Tian3*Huanglian Wendan Decoction (HWD) is a traditional Chinese medicine (TCM) prescribed to patients diagnosed with insomnia, which can achieve excellent therapeutic outcomes. As positively modulating the γ-aminobutyric acid (GABA) type A receptors (GABAARs) is the most effective strategy to manage insomnia, this study aimed to investigate whether the activation of GABAARs is involved in the anti-insomnia effect of HWD. We assessed the metabolites of HWD using LC/MS and the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and tested the pharmacological activity in vitro and in vivo using whole-cell patch clamp and insomnia zebrafish model. In HEK293 cells expressing α1β3γ2L GABAARs, HWD effectively increased the GABA-induced currents and could induce GABAAR-mediated currents independent of the application of GABA. In the LC-MS (QToF) assay, 31 metabolites were discovered in negative ion modes and 37 metabolites were found in positive ion modes, but neither three selected active metabolites, Danshensu, Coptisine, or Dihydromyricetin, showed potentiating effects on GABA currents. 62 active metabolites of the seven botanical drugs were collected based on the TCMSP database and 19 of them were selected for patch-clamp verification according to the virtual docking simulations and other parameters. At a concentration of 100 μM, GABA-induced currents were increased by (+)-Cuparene (278.80% ± 19.13%), Ethyl glucoside (225.40% ± 21.77%), and β-Caryophyllene (290.11% ± 17.71%). In addition, (+)-Cuparene, Ethyl glucoside, and β-Caryophyllene could also serve as positive allosteric modulators (PAMs) and shifted the GABA dose-response curve (DRC) leftward significantly. In the PCPA-induced zebrafish model, Ethyl glucoside showed anti-insomnia effects at concentrations of 100 μM. In this research, we demonstrated that the activation of GABAARs was involved in the anti-insomnia effect of HWD, and Ethyl glucoside might be a key metabolite in treating insomnia.
Insomnia is a prevalent sleep disorder characterized by difficulty falling or staying asleep or enduring non-restorative sleep. With a prevalence of 30%, insomnia is now a significant health and economic burden worldwide (Buysse, 2013; Morin et al., 2015; Vargas et al., 2020). This disorder is usually associated with medical and mental disorders, including fatigue, reduced energy, impaired attention, and mood disturbances, and is one of the most common complaints in medical practice. In addition, insomnia is related to a variety of other disorders, including depressive disorder (Baglioni et al., 2011), anxiety disorder (Johnson et al., 2006), hypertension (Vgontzas et al., 2009), and suicidality (Pigeon et al., 2012), and recurrent insomnia increases the risk of mortality (Parthasarathy et al., 2015).
In clinical practice, insomnia is most often managed with medications, and positively modulating the γ-aminobutyric acid (GABA) type A receptors (GABAARs) is the most effective strategy (Buysse, 2013). GABA binding can activate the GABAARs, which mediate the influx of chloride, controlling the resting membrane potential. GABAARs are pentameric ligand-gated chloride channels, which are mostly heteropentamers assembled from numerous subunits, including α1–6, β1–3, γ1–3, δ, ε, π, θ and ρ1–3. The constitute of GABAARs is variable, and the function usually depends on the alpha subunit it contains (Ghit et al., 2021). The α1 subunit encoded by the GABRA1 gene is most ubiquitously expressed in most brain regions and is closely associated with circadian rhythm and sleep regulation (Jacob et al., 2008). In sleep-awake regulation, GABAARs activation promotes sleep by inhibiting the arousal systems (Saper et al., 2010; Buysse, 2013). GABAARs modulators used for treating insomnia can be divided into Benzodiazepines (BDZ) and non-BDZ, both enhance GABA-mediated inhibition through allosteric modulation of the GABAARs (Downing et al., 2005; Solomon et al., 2019). These agents are most effective for treating sleep problems and many of them are approved by the FDA for the treatment of insomnia, such as diazepam (Morin et al., 2015). However, these agents might be problematic for drug dependence and drug abuse (Krystal, 2009), and alternative treatments are needed.
Huanglian Wendan Decoction (HWD) is a traditional Chinese medicine (TCM) derived from “six-factor syndrome differentiation” and is mainly used for clearing heat and removing annoyance, removing dampness, and resolving phlegm. HWD formula consists of seven main botanical drugs, including Coptis chinensis Franch. [Ranunculaceae; Coptis Rhizoma] (Huanglian, HL), Pinellia ternata (Thunb.) Breit. [Araceae; Pinelliae Rhizoma] (Banxia, BX), Citrus aurantium L. [Rutaceae; Aurantii Fructus Immaturus] (Zhishi, ZS), Citrus reticulata Blanco [Rutaceae; Citri Reticulatae Pericarpium] (Chenpi, CP), Faria cocos (Schw.) Wolf [Polyporaceae; Poria] (Fuling, FL), Bambusa tuldoides Munro [Poaceae; Bambusae Caulis in Taenias] (Zhuru, ZR), and Glycyrrhiza uralensis Fisch. [Fabaceae; Glycyrrhizae Radix et Rhizoma] (Gancao, GC) (Figure 1A). Studies have confirmed that HWD could be mainly used to treat vascular and metabolic disorders, including atherosclerosis and type 2 diabetes, suggesting its regulation in glucose and lipid metabolism (Li et al., 2021; Shi et al., 2022). In our clinical practice, we prescribe HWD to patients diagnosed with insomnia, which can achieve excellent therapeutic outcomes (Li et al., 2022), suggesting that HWD can be used as an alternative treatment option for insomnia. However, the potential mechanism of HWD for insomnia is not well defined. Based on the close correlation between GABAARs and insomnia, we propose a hypothesis that the effects of HWD on GABAARs were involved in the possible mechanisms of the anti-insomnia effect of HWD. This study accumulates the theoretical basis for the further rational application of HWD and lays the foundation for future clinical research.
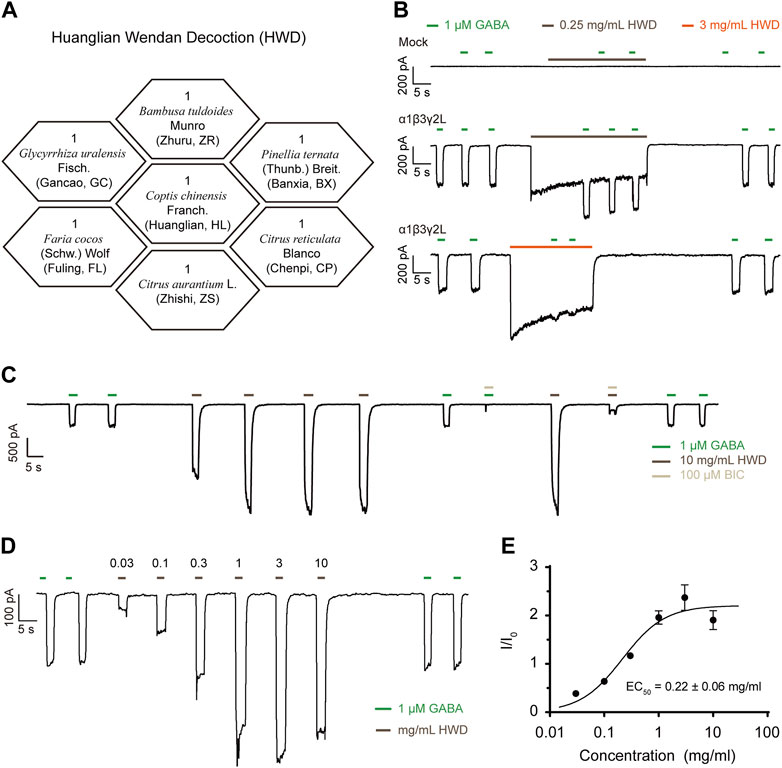
Figure 1. HWD served as a positive modulator of α1β3γ2L GABAARs. (A) The botanical drugs composition of HWD. (B) The effects of HWD on HEK293 cells expressing α1β3γ2L GABAARs. (C) The effects of BIC on HWD- or GABA-induced currents. (D) Representative trace induced by HWD of different concentrations. (E) DRC of the agonist effect of HWD.
Traditional Chinese medicine (TCM) decoction pieces used in the experiment were purchased from the Chinese herbal pharmacy of Zhongshan Traditional Chinese Medicine Hospital and certified as genuine by Chief Chinese Pharmacist Weibo Dai. The seven TCM pieces of Huanglian Wendan Decoction are Coptis chinensis Franch. [Ranunculaceae; Coptis Rhizoma], Pinellia ternata (Thunb.) Breit. [Araceae; Pinelliae Rhizoma], Citrus aurantium L. [Rutaceae; Aurantii Fructus Immaturus], Citrus reticulata Blanco [Rutaceae; Citri Reticulatae Pericarpium], Faria cocos (Schw.) Wolf [Polyporaceae; Poria], Bambusa tuldoides Munro [Poaceae; Bambusae Caulis in Taenias], and Glycyrrhiza uralensis Fisch. [Fabaceae; Glycyrrhizae Radix et Rhizoma].
According to the decocting process of traditional Chinese medicine, 200 g of each of the above seven TCM decoction pieces was mixed, and 14 L of water was added to soak the TCM decoction pieces for 30 min. After that, the TCM decoction pieces were boiled over the fire and simmered with the gentle fire for 1 h. After pouring out the medicinal liquid of the 1st decoction, the dregs of the TCM decoction pieces were added to 14 L of water to decoct for the second time, and the time of the 2nd decoction was the same as that of the 1st decoction. After the 2nd decoction was combined and filtered through gauze, it was concentrated to 1 g/mL by rotary evaporator, quickly added liquid nitrogen, and placed in a vacuum freeze-drying machine (freeze-drying temperature −45°C, vacuum degree of 1,000 Pa) to dry for 72 h. The lyophilized powder of HWD solution obtained from the preparation was quickly transferred to a self-sealing bag, and placed in a low-temperature, dry, and light-avoiding place for storage.
Four- Chloro-DL-phenylalanine (PCPA) was purchased from the Harveybio (Beijing, China). Melatonin was purchased from Aladdin (Shanghai, China). Stiripentol was purchased from Macklin (Shanghai, China). All active metabolites of the botanical drugs were purchased from Topscience (Shanghai, China).
The lyophilized powder of HWD was dissolved in 20% methanol-water by ultrasonication, and the solution with a concentration of 78.3 mg/mL was filtered by 0.22 μm microporous membrane, then put into a liquid injection vial and stored at 4°C for testing. Metabolites were identified based on accurate molecular weight combined with secondary spectrum fragment analysis and an Agilent TCM database. Chromatographic conditions: LC 1290-UPLC (Agilent, USA) with a Zorbax eclipse Plus C18 column (150 mm × 3.0 mm, 1.8 µm) and mobile phase consisting of ultrapure water containing 0.1% formic acid (A) and acetonitrile(B); sample volume: 0.5 μL; flow rate: 0.5 mL/min; column temperature: 30 °C; elution conditions: gradient elution program (0–2 min, 5% B; 2–20 min, 5%–35% B; 20–24 min, 35%–40% B; 24–25 min, 40%–50% B; 25–30 min, 50%–95%B; 30–35 min, 95% B; 35–38 min, 5% B). Mass spectrometry conditions: Q-TOF 6545 (Agilent, USA) high-resolution mass spectrometry system with ESI electrospray ion source; instrument settings: both negative and positive ion mode for detection; drying gas temperature: 300°C; drying gas flow rate: 8 L/min; Sheath gas temperature: 350 °C; Sheath gas flow rate: 11 L/min; atomizing gas pressure: 45 psi; cracking voltage: 130 V; capillary voltage: 4000 V (+)/3500 V (−); nozzle voltage: 0 V (+)/1000 V (−); primary mass scanning range: 100–1700 m/z; the scanning range of child ion: 20–1,200 m/z; the collision energy (CE): 10/20/40 V.
Wild-type AB strain zebrafish were purchased from China Zebrafish Resource Center, CZRC (Wuhan, China). Adult zebrafish were reared at 28°C on a 14/10 h light/dark cycle. Following natural spawning, eggs were collected and maintained in an embryo buffer under continuous light conditions at 28°C. The embryo buffer contained (in mM): 5 NaCl, 0.17 KCl, 0.33 MgSO4, and 0.33 CaCl2. At 5 days post fertilization (dpf), zebrafish larvae were pre-treated with fish water containing active metabolites, melatonin, or 1% DMSO for 2 hours and then were exposed to 10 mM PCPA for 8 h; the moving of the zebrafish larvae was recorded in the observation box and analyzed with Noldus EthoVision XT (Noldus XT, Wageningen, Netherlands).
Human GABRA1 and GABRB3 were cloned into pEG BacMam plasmids, respectively; a plasmid expressing human GABRG2 from the CMV promoter and EGFP from the EF1a promoter was constructed to ensure the presence of γ2 subunits based on the green fluorescence.
The Human embryonic kidney 293T (HEK293T) cell lines were grown in DMEM basal medium (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and maintained kept at 37°C in a 5% CO2 incubator. The cells were passaged the day before transfection at a ratio of 1:4. The transfections were performed using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions, and the fluid was changed 6 h after transfection. To express GABAARs, pcDNA3.1 plasmids containing wild-type GABRA1 were co-transfected with GABRB3 and GABRG2 at a ratio of 1:1:1.
Whole-cell patch-clamp recordings were performed at room temperature (22°C–25°C) using an Axon 700B amplifier and a Digidata 1550B digitizer (Axon Instruments, Molecular Devices). Pipettes were pulled with resistances of 3–7 MΩ when filled with the intracellular solution. The pipette solution contained (in mM): 153 KCl, 1 MgCl2, 10 HEPES, and 5 EGTA (280–290 mOsm), with pH adjusted to 7.4 using KOH. The extracellular solution contained (in mM): 142 NaCl, 8 KCl, 1 CaCl2, 6 MgCl2, and 10 HEPES (290–300 mOsm), with pH adjusted to 7.4 using NaOH. Metabolite or botanical drug powder solutions were applied through an RSC-200 Rapid Solution Changer (BioLogic, France). Recordings were performed at a holding potential of −50 mV. Voltage clamp mode was used and the signal was sampled at 10 kHz and low pass filtered at 2 kHz.
The information on the active metabolites that interact with GABRA1 was downloaded from the TCMSP database, and medicine, metabolites, and target information were visualized using Cytoscape. This network showed that the drugs, metabolites, and GABRA1 were expressed as nodes, whereas their interactions were as edges.
PubChem was used to obtain 3D structures of metabolites. Chem 3D was used to optimize small molecules and export them to the MOL2 format. The RCSB Protein Data Bank (PDB) was used to obtain the 3D structure of GABRA1 in PDB format (7QNE), and PyMOL was used to analyze protein dehydration and hydrogenation. AutoDock software was used to convert the metabolite and target protein format to the PDBQT format. Finally, AutoDock Vina was run for virtual docking. It is generally accepted that the lower the energy is, the higher the binding possibility.
Patch-clamp data were analyzed using Clampfit 10.6 (Molecular Device, United States) and GraphPad Prism 8 (GraphPad Software, United States). Concentration-response curves were fitted using the following four-parameter Hill equation: Y=Bottom + (Top-Bottom)/(1 + 10^((LogEC50-X)*HillSlope)), where Bottom and Top represent the channel’s maximum and minimum responses to the metabolites, respectively; X is the value of the logarithm of the concentration, Y is the Idrug/Icontrol value, EC50 is the concentration producing a half-maximum response, and Hillslope is the factor to describe the steepness of the curve. All results were presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using Student’s t-test, *p < 0.05, **p < 0.01 and ***p < 0.001.
The GABAAR α1 subunit encoded by GABRA1 is mainly associated with sleep regulation; thus, we co-transfected the GABRA1 gene in HEK293 cells with the GABRB3 and GABRG2 gene that is widely expressed in the brain and tested the effect of HWD freeze-dried powder on the α1β3γ2L GABAARs in vitro. We used GABA of 1 μM as ligands to induce current and pre-treated transfected cells with HWD for 15 s before co-treatment with GABA to ensure an adequate reaction between the active metabolites and the membrane receptors.
Interestingly, the application of HWD (freeze-dried powder) of 0.25 mg/mL or 3 mg/mL enhanced the GABA-induced currents significantly and induced an inward current itself (Figure 1B). Neither HWD- nor GABA-induced inward current was present in the mock control, suggesting that the current was mediated by α1β3γ2L GABAARs and HWD could serve as an agonist as GABA. In addition, HWD induced inward currents in cells expressed α2β3γ2L, α3β3γ2L, or α6β3γ2L (Supplementary Figure S1). We further analyzed the direct activation of HWD and found that HWD of 10 mg/mL could effectively induce currents independent of GABA (Figure 1C). The HWD-induced current was sensitive to bicuculline (BIC), an antagonist of GABAARs, which further demonstrated that the current induced by HWD was mediated by GABAARs rather than off-target effects on the background current of HEK293 (Figure 1C). In addition, the HWD concentration-response curve of agonist effect revealed a bell shape, the EC50 was 0.22 ± 0.06 mg/mL, with a maximum response at 3 mg/mL (Figures 1D,E), suggesting that the active metabolites in the HWD mixture may include both agonists and inhibitors.
To filter out the active metabolites in HWD, we tested the agonist effect of the seven botanical drugs, respectively. Surprisingly, all seven botanical drugs (freeze-dried powder) could induce a visible inward current in the HEK293 cells expressing α1β3γ2L GABAARs at a concentration of 10 mg/mL, and GC and ZR showed the most potent effects. (Supplementary Figure S1). Considering the general effectiveness of the seven botanical drugs, we performed an LC-MS (QToF) assay in positive and negative ion modes to identify the main metabolites in HWD rather than in a particular botanical drug. As shown in Supplementary Table S1, 2 31 metabolites were discovered in negative ion modes and 37 metabolites were found in positive ion modes.
We selected Coptisine, Danshensu, and Dihydromyricetin as representative metabolites to validate the effect of the main metabolites on α1β3γ2L GABAARs using patch-clamp electrophysiology. None of the three metabolites showed an agonist effect that induce a chloride current itself at concentrations of 100 μM or 300 μM. Interestingly, all of them displayed inhibitory effects. At a concentration of 100 μM, Coptisine and Dihydromyricetin showed significant inhibitory effects (I/I0 = 0.59 ± 0.05 and I/I0 = 0.63 ± 0.04, respectively); at a higher concentration of 300 μM, Danshensu slightly decrease GABA-induced current (I/I0 = 0.89 ± 0.02) (Figure 2), which might contribute to bell-shape concentration-response curve of HWD.
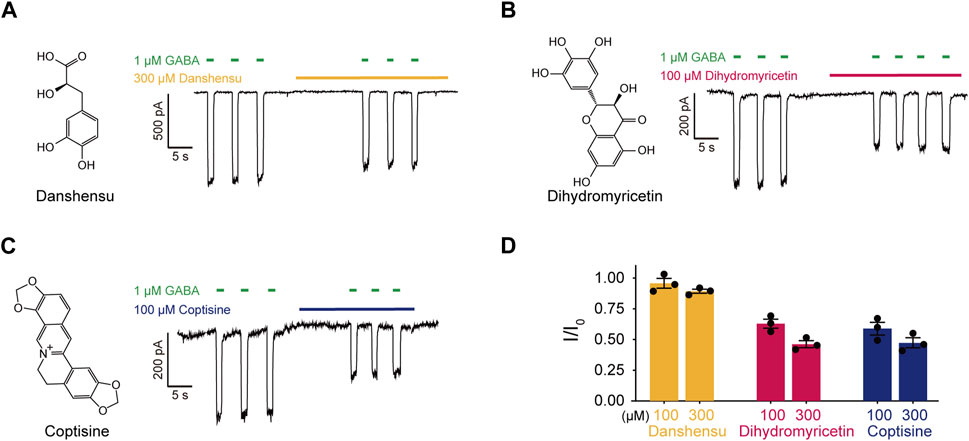
Figure 2. The effects of main metabolites in HWD on α1β3γ2L GABAARs. (A–C) Molecular structures and effects on α1β3γ2L GABAARs of (A) Danshensu, (B) Dihydromyricetin, and (C) Coptisine. (D) Statistic graph of the effects of Coptisine, Danshensu, and Dihydromyricetin on GABA-induced current.
Network pharmacology has emerged as a valuable tool for analyzing complex systems in pharmacology studies; here, we tried to screen the active metabolites targeting GABRA1 in HWD using the TCMSP (Traditional Chinese Medicine Systems Pharmacology) database. Due to the general agonist effect of the seven botanical drugs (Supplementary Figure S1), we used all seven botanical drugs as keywords in the TCMSP database to obtain the active metabolites list and filtered out 62 active metabolites interacting with GABRA1 (Figure 3). As shown in Table 1, the 62 metabolites were sorted according to the binding affinity. The active metabolites list included 16 in BX, four in HL, 18 in CP, 15 in ZS, and 14 in RG, and several active metabolites were found in multiple botanical drugs, including quercetin (HL and GC), (−)-α-Terpineol (CP and GC), EIC (BX and ZS), and so on. However, no metabolites co-existed in all the seven botanical drugs, suggesting the existence of several potential modulators of GABAARs in HWD.
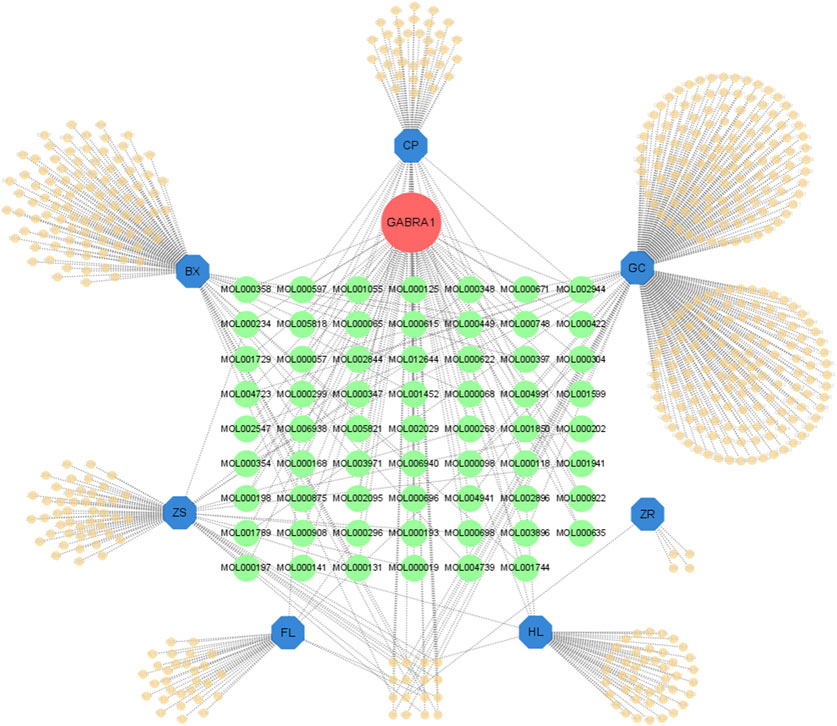
Figure 3. The botanical drugs-metabolites-GABRA1 network. Green circles represent metabolites that may act on GABRA1.
We applied oral bioavailability (OB), drug-likeness (DL), blood-brain barrier penetration, structural characterization, and easy availability as screening criteria to select active metabolites for the measurement of their activation on α1β3γ2L GABAARs using patch clamp (Table 1). Similar to the HWD test protocol, we used GABA of 1 μM as ligand and pre-treated transfected cells with metabolites for 15 s before co-treatment with GABA. After filtering, 19 molecules were chosen for patch-clamp evaluation. Among these metabolites, kaempferol and corydaldine suppressed the GABA-induced current by more than half at a concentration of 100 μM, which may explain the bell-shape DRC of the agonist effect of HWD (Figure 4A). Seven molecules effectively enhanced the GABA-induced current and three of them could increase the GABA current more than two-fold at a concentration of 100 μM, including β-Caryophyllene, (+)-Cuparene, and Ethyl glucoside (Figure 4). In addition, all the three molecules increased the GABA-induced current in a dose-dependent manner, and the EC50 was 15.11 ± 5.94 μM ((+)-Cuparene), 37.03 ± 8.56 μM (Ethyl glucoside), and 46.88 ± 6.33 μM (β-Caryophyllene), respectively (Figure 4B). Interestingly, (+)-Cuparene itself could also induce an inward current independent on the application of GABA, which may be responsible for the agonist effects of HWD (Figure 4C).
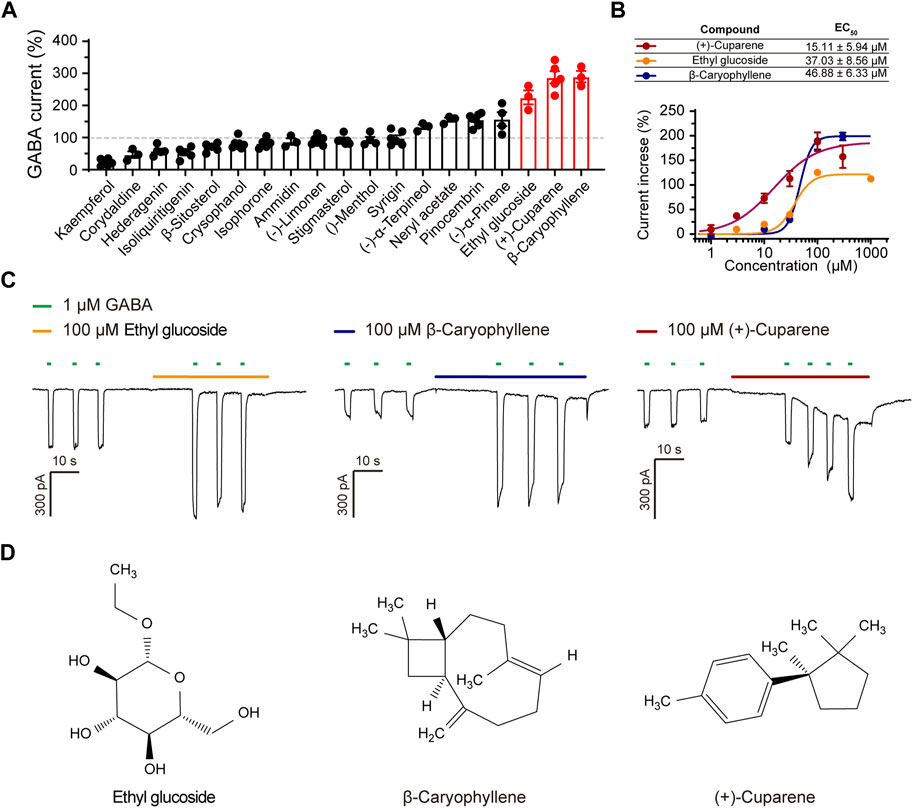
Figure 4. The effects of 19 active metabolites on α1β3γ2L GABAARs. (A) Statistic graph of the effects of 19 active metabolites on α1β3γ2L GABAARs; the test concentration was 100 μM. (B) DRC, (C) representative current trace, and (D) molecular structure of (+)-Cuparene, Ethyl glucoside, and β-Caryophyllene.
We then tested whether the three metabolites could serve as PAMs of α1β3γ2L GABAARs. We pre-treated the HEK293 cells expressing α1β3γ2L with the test metabolites at a concentration of around EC50, and then co-treated with GABA of different concentrations to obtain a GABA dose-response curve (DRC). All three molecules shifted the GABA DRC leftward; the GABA EC50 was 9.35 ± 1.38 μM (control), 2.47 ± 0.88 μM ((+)-Cuparene), 2.99 ± 0.61 μM (Ethyl glucoside), and 3.06 ± 1.11 μM (β-Caryophyllene), respectively, demonstrating a PAM effect (Figure 5). In addition, β-Caryophyllene and (+)-Cuparene could increase the GABA-induced current in cells expressed α2β3γ2L, α3β3γ2L, or α6β3γ2L at a concentration of 30 μM; Ethyl glucoside showed weak effects on α2β3γ2L or α6β3γ2L and no effect on α3β3γ2L (Supplementary Figure S3).
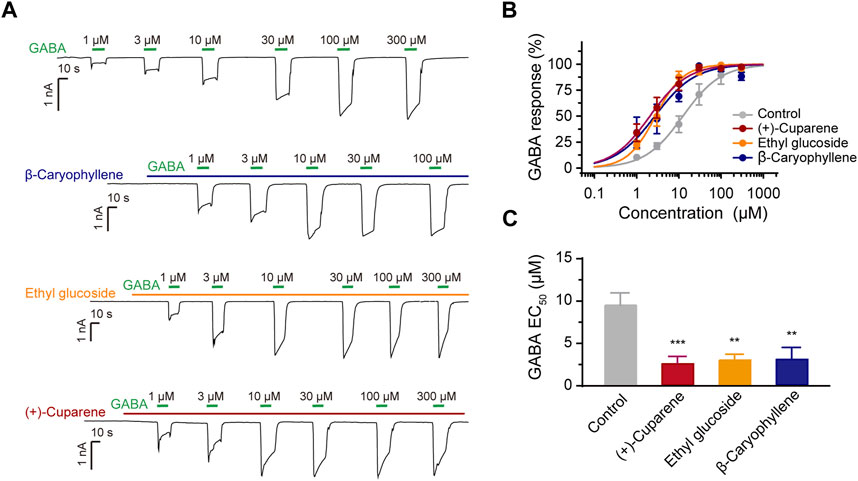
Figure 5. The PAM effects of three active metabolites on α1β3γ2L GABAARs. (A) Representative traces of GABA-induced currents after the application of the three active metabolites. (B) GABA DRC and (C) EC50 after the application of the three active metabolites.
Zebrafish have been widely used to construct neurological disorder models for high-throughput drug screenings in vivo, which reduces the costs substantially compared to mammalian models. To verify the involvement of GABAARs in the anti-insomnia effects of HWD, we used para-chlorophenylacetic acid (PCPA)-induced insomnia zebrafish models to evaluate the hypnotic effects of the three most potent molecules. PCPA is a serotonin (5-HT) synthesis inhibitor that induces insomnia by disturbing the serotonergic system, which is necessary for sleep onset and maintenance. As shown in Figure 6B, the application of PCPA could increase the moving duration in a concentration-dependent manner, suggesting an insomnia-like behavior. Based on the dose-response relationship and the limited solubility, PCPA of 10 mM was used to establish the model, and melatonin was used as a positive control.
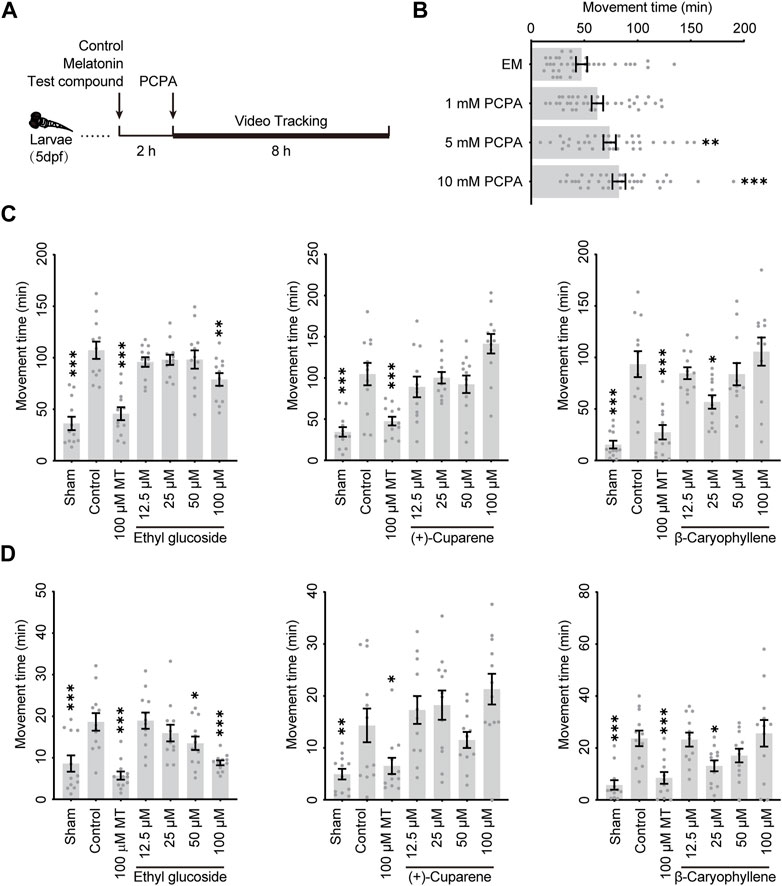
Figure 6. The anti-insomnia effect of three active metabolites in zebrafish model (A) Schema of the experimental regimen. (B) The dose-response relationship of PCPA The anti-insomnia effect of three active metabolites in the zebrafish model during (C) an 8-h recording or (D) the last 2 hours.
To evaluate the anti-insomnia effect of three metabolites, larvae of five dpf were treated with the test metabolite, control, or melatonin for 2 h. The larvae were exposed to PCPA to induce an insomnia-like phenotype, and their movements would be recorded by a camera and analyzed with tracking software (Figure 6A). As shown in Figure 6C, PCPA increased the movement duration significantly during an 8-h recording, and melatonin could effectively decrease insomnia. During an 8-h recording, Ethyl glucoside could relieve the hyperactive movement at a concentration of 100 μM, while β-Caryophyllene could relieve the hyperactive movement at a concentration of 25 μM. In addition, the decrements of movement duration were more significant during the last 2 hours (Figure 6D).
HWD is a TCM that could be used in insomnia treatment. This study reveals that HWD can serve as a ligand of α1β3γ2L GABAARs, which may be responsible for their anti-insomnia effects. Based on the prediction of TCMSP and the confirmation using patch clamp, we find several monomeric metabolites as PAMs of α1β3γ2L GABAARs, which may be involved in the active metabolites of HWD in treating insomnia.
HWD can be an effective TCM recipe for treating insomnia, but the underlying mechanisms responsible for the anti-insomnia effects remain poorly defined. In a previous work, the mechanism of HWD against insomnia was investigated using a network pharmacology approach, demonstrating that this effect involves multiple signaling pathways, such as neurotransmitter signaling and NF-κB pathway (Dong et al., 2021). In our previous work on an insomnia rat model, several serum biochemical parameters were significantly changed, such as BDNF and GFAP; the concentration of several neurotransmitters in the brain was also different after the application of HWD, including GABA, 5-HT, and glutamate (Li et al., 2022). Here in this work, we report that HWD could activate GABAARs directly for the first time, which is consistent with the underlying mechanism of BZD, the most effective treatment against insomnia in clinical.
GABAARs are ligand-gated chloride channels that mediate fast neuronal inhibition in the brain. Based on their physiological roles, they are important drug targets for treating epilepsy, anxiety, and insomnia, and most of these drugs act through positive modulation of GABAARs. However, drugs targeting GABAARs often lead to side effects, including fatigue, dizziness, memory loss, or even mental symptoms such as anxiety, and some of these drugs can lead to tolerance and dependence. The effects of HWD in treating insomnia have been proven, demonstrating it can be used as replacement therapy in treating insomnia.
None of the three most potent metabolites, β-Caryophyllene, (+)-Cuparene, or Ethyl glucoside, have been reported as GABAARs positive modulators. The effects of β-caryophyllene on GABAARs have been tested before, but no increased effect of GABA-induced current was detected, possibly due to the different subunit composition (Janzen et al., 2021). β-caryophyllene has been reported as a neuropharmacological protector in seizure, anxiety, and depression, which may involve its effect on GABAARs (da Silva Oliveira et al., 2021). In addition, (−)-α-pinene was found to bind to aromatic residues of α1 and γ2 subunits of GABAA-BZD receptors in the molecular model (Yang et al., 2016), which was functionally verified in this work. Menthol and Stigmasterol have been reported as positive modulators of GABAARs (Watt et al., 2008; Karim et al., 2021), but they only showed slight effects at a concentration of 100 μM, which may be due to the different test systems and concentrations; both Stigmasterol and Menthol showed positive modulation at a concentration of 300 μM, and Stigmasterol also showed an agonist effect at this concentration (Supplementary Figure S2).
Zebrafish were widely used to establish neural disease models such as insomnia. Here, we first used PCPA to establish a novel insomnia model in zebrafish. PCPA can inhibit the synthesis of 5-HT by antagonizing tryptophan hydrogenase, which is usually used to establish the insomnia rat model. In our results, PCPA can induce a dose-dependent increase in moving duration, which could be effectively relieved by applying melatonin. Using this novel insomnia model, we successfully certificated the anti-insomnia effects of the PAMs of GABAARs screened from HWD. This novel model may help to establish a drug screen system to identify novel sleeping aids.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was approved by the Zhongshan institute for drug discovery IACUC. The study was conducted in accordance with the local legislation and institutional requirements.
LL: Conceptualization, Funding acquisition, Validation, Writing–original draft. XW: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. JG: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–original draft. ZW: Formal Analysis, Methodology, Project administration, Writing–original draft. WD: Funding acquisition, Investigation, Methodology, Writing–original draft. LQ: Data curation, Formal Analysis, Methodology, Writing–original draft. HZ: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. MY: Data curation, Formal Analysis, Methodology, Writing–original draft. HY: Methodology, Writing–original draft. MH: Formal Analysis, Validation, Writing–original draft. ZG: Conceptualization, Funding acquisition, Writing–review and editing. FT: Conceptualization, Funding acquisition, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ZG received funding from the High-level New R&D Institute (2019B090904008) and the High-level Innovative Research Institute (2021B0909050003) of the Department of Science and Technology of Guangdong Province, National Science and Technology Innovation 2030 Major Program (2021ZD0200900), Zhongshan Municipal Bureau of Science and Technology (CXTD2022013), Zhongshan Municipal Natural Science Foundation (221018194369472), and the National Science Fund for Distinguished Young Scholars (81825021). LL received funding from Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (20201379); Science and Technology Major Project of Zhongshan City, Guangdong Province (2019B1016). WD received funding from Project of Guangdong Provincial Bureau of Traditional Chinese Medicine (20201379); Zhongshan City Social Public Welfare and Basic Research Project (2019B1016, and 2021B3009). FT received funding from Zhongshan Municipal Bureau of Science and Technology (210724194041939).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1389768/full#supplementary-material
GABAAR, γ-aminobutyric acid type A receptor; HWD, Huanglian Wendan Decoction; TCM, traditional Chinese medicine; LC-MS, liquid chromatography-mass spectrometry; TCMSP, Traditional Chinese Medicine Systems Pharmacology; DRC, dose-response curve; PAM, positive allosteric modulator; BDZ, Benzodiazepine; PCPA, 4-Chloro-DL-phenylalanine; HL, Huanglian, Coptis chinensis Franch; BX, Banxia, Pinellia ternata (Thunb.); ZS, Zhishi, Citrus × aurantium L; CP, Chenpi, Citrus reticulata Blanco; FL, Fuling, Smilax glabra Roxb; ZR, Zhuru, Bambusa tuldoides Munro; GC, Gancao, Glycyrrhiza glabra L; PDB, Protein Data Bank.
Baglioni, C., Battagliese, G., Feige, B., Spiegelhalder, K., Nissen, C., Voderholzer, U., et al. (2011). Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J. Affect Disord. 135, 10–19. doi:10.1016/j.jad.2011.01.011
da Silva Oliveira, G. L., da Silva, J., Dos Santos, C. L. d.S. A. P., Feitosa, C. M., and de Castro Almeida, F. R. (2021). Anticonvulsant, anxiolytic and antidepressant properties of the β-caryophyllene in Swiss mice: involvement of benzodiazepine-GABAAergic, serotonergic and nitrergic systems. Curr. Mol. Pharmacol. 14, 36–51. doi:10.2174/1874467213666200510004622
Dong, W. R., Li, H., Li, Y. F., Wang, N., Ma, B. Y., Lu, G. L., et al. (2021). Mechanism of Huanglian Wendan Decoction in improving impaired glucose tolerance based on skeletal muscle NLRP3/caspase-1/IL-1beta, IL-18 pathway. Zhongguo Zhong Yao Za Zhi 46, 4480–4487. doi:10.19540/j.cnki.cjcmm.20210621.401
Downing, S. S., Lee, Y. T., Farb, D. H., and Gibbs, T. T. (2005). Benzodiazepine modulation of partial agonist efficacy and spontaneously active GABA(A) receptors supports an allosteric model of modulation. Br. J. Pharmacol. 145, 894–906. doi:10.1038/sj.bjp.0706251
Ghit, A., Assal, D., Al-Shami, A. S., and Hussein, D. E. E. (2021). GABA(A) receptors: structure, function, pharmacology, and related disorders. J. Genet. Eng. Biotechnol. 19, 123. doi:10.1186/s43141-021-00224-0
Jacob, T. C., Moss, S. J., and Jurd, R. (2008). GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343. doi:10.1038/nrn2370
Janzen, D., Slavik, B., Zehe, M., Sotriffer, C., Loos, H. M., Buettner, A., et al. (2021). Sesquiterpenes and sesquiterpenoids harbor modulatory allosteric potential and affect inhibitory GABA(A) receptor function in vitro. J. Neurochem. 159, 101–115. doi:10.1111/jnc.15469
Johnson, E. O., Roth, T., and Breslau, N. (2006). The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J. Psychiatr. Res. 40, 700–708. doi:10.1016/j.jpsychires.2006.07.008
Karim, N., Khan, I., Abdelhalim, A., Halim, S. A., Khan, A., and Al-Harrasi, A. (2021). Stigmasterol can be new steroidal drug for neurological disorders: evidence of the GABAergic mechanism via receptor modulation. Phytomedicine 90, 153646. doi:10.1016/j.phymed.2021.153646
Krystal, A. D. (2009). A compendium of placebo-controlled trials of the risks/benefits of pharmacological treatments for insomnia: the empirical basis for U.S. clinical practice. Sleep. Med. Rev. 13, 265–274. doi:10.1016/j.smrv.2008.08.001
Li, H., Wang, N., and Ma, B. Y. (2021). Mechanism of Huanglian Wendan Decoction in intervening IGT IR based on liver cell pyroptosis. Zhongguo Zhong Yao Za Zhi 46, 3394–3401. doi:10.19540/j.cnki.cjcmm.20210319.401
Li, L., Wang, Z., Hu, Y., Dai, W., Guo, W., Chen, Q., et al. (2022). Exploring the therapeutic effect and mechanism of huanglian wendan decoction on insomnia rats based on neurotransmitter and 5-HT1A/Gαi/o/cAMP signaling pathway. Tradit. Chin. Drug Research&Clinical Pharmacol. 34, 591–598. doi:10.19378/j.issn.1003-9783.2023.05.003
Morin, C. M., Drake, C. L., Harvey, A. G., Krystal, A. D., Manber, R., Riemann, D., et al. (2015). Insomnia disorder. Nat. Rev. Dis. Prim. 1, 15026. doi:10.1038/nrdp.2015.26
Parthasarathy, S., Vasquez, M. M., Halonen, M., Bootzin, R., Quan, S. F., Martinez, F. D., et al. (2015). Persistent insomnia is associated with mortality risk. Am. J. Med. 128, 268–275. doi:10.1016/j.amjmed.2014.10.015
Pigeon, W. R., Pinquart, M., and Conner, K. (2012). Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J. Clin. Psychiatry 73, e1160–e1167. doi:10.4088/JCP.11r07586
Saper, C. B., Fuller, P. M., Pedersen, N. P., Lu, J., and Scammell, T. E. (2010). Sleep state switching. Neuron 68, 1023–1042. doi:10.1016/j.neuron.2010.11.032
Shi, N., Zhou, Y., and Ma, H. (2022). A network pharmacology study of mechanism and efficacy of Jiawei Huanglian-Wendan decoction in polycystic ovary syndrome with insulin resistance. Med. Baltim. 101, e32057. doi:10.1097/MD.0000000000032057
Solomon, V. R., Tallapragada, V. J., Chebib, M., Johnston, G. A. R., and Hanrahan, J. R. (2019). GABA allosteric modulators: an overview of recent developments in non-benzodiazepine modulators. Eur. J. Med. Chem. 171, 434–461. doi:10.1016/j.ejmech.2019.03.043
Vargas, I., Nguyen, A. M., Muench, A., Bastien, C. H., Ellis, J. G., and Perlis, M. L. (2020). Acute and chronic insomnia: what has time and/or hyperarousal got to do with it? Brain Sci. 10, 71. doi:10.3390/brainsci10020071
Vgontzas, A. N., Liao, D., Bixler, E. O., Chrousos, G. P., and Vela-Bueno, A. (2009). Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 32, 491–497. doi:10.1093/sleep/32.4.491
Watt, E. E., Betts, B. A., Kotey, F. O., Humbert, D. J., Griffith, T. N., Kelly, E. W., et al. (2008). Menthol shares general anesthetic activity and sites of action on the GABA(A) receptor with the intravenous agent, propofol. Eur. J. Pharmacol. 590, 120–126. doi:10.1016/j.ejphar.2008.06.003
Keywords: Huanglian Wendan Decoction (HWD), GABA A type receptor (GABAAR), insomnia, zebrafish, traditional Chinese medicine systems pharmacology (TCMSP)
Citation: Li L, Wu X, Gong J, Wang Z, Dai W, Qiu L, Zuo H, Yi M, Yuan H, Hu M, Gao Z and Tian F (2024) Activation of GABA type A receptor is involved in the anti-insomnia effect of Huanglian Wendan Decoction. Front. Pharmacol. 15:1389768. doi: 10.3389/fphar.2024.1389768
Received: 22 February 2024; Accepted: 01 May 2024;
Published: 23 May 2024.
Edited by:
Zhengyu Cao, China Pharmaceutical University, ChinaReviewed by:
Wuguang Lu, Nanjing University of Chinese Medicine, ChinaCopyright © 2024 Li, Wu, Gong, Wang, Dai, Qiu, Zuo, Yi, Yuan, Hu, Gao and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaobing Gao, emJnYW9Ac2ltbS5hYy5jbg==; Fuyun Tian, dGlhbmZ1eXVuQHppZGQuYWMuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.