- Department of Chinese Medicine, The First Affiliated Hospital of Bengbu Medical University, Bengbu, Anhui, China
Introduction: Xiaoyaosan (XYS), a traditional Chinese formula, not only has good antitumor effects but also attenuates distress, anorexia, and quality of life (QoL) by regulating neurology, the microbiota, immunology, and oxidative stress. This study aimed to assess the effect of XYS on QoL, psychological pressure, and spiritual well-being in breast cancer patients undergoing adjuvant chemotherapy.
Methods: This prospective cohort study enrolled 176 postoperative breast cancer patients who received adjuvant chemotherapy combined with (n = 81) or without (n = 95) XYS for comparison. The Quality-of-Life Questionnaire Core-30 (QLQ-C30), Hospital Anxiety and Depression Scale (HADS), University of California Los Angeles (UCLA-LS), and Functional Assessment of Chronic Illness Therapy–Spiritual Well-being (FACIT–Sp) scores were evaluated before adjuvant chemotherapy (T0) and after the first (T1), second (T2), third (T3), and fourth cycles (T4) of adjuvant chemotherapy.
Results: XYS improved the QLQ-C30 score at T2 (p = 0.043), T3 (p = 0.021), and T4 (p = 0.040) and the QLQ-C30 score at T4 (p = 0.027); moreover, XYS attenuated the QLQ-C30 score at T2 (p = 0.040), T3 (p = 0.023), and T4 (p = 0.027). Regarding distress, XYS reduced the HADS-anxiety score at T2 (p = 0.010), T3 (p = 0.025), and T4 (p = 0.019) and the HADS-defined anxiety score at T3 (p = 0.038). XYS also decreased the HADS-depression score at T2 (p = 0.016), T3 (p = 0.018), and T4 (p = 0.017) and the HADS-defined depression rate at T2 (p = 0.047), T3 (p = 0.012), and T4 (p = 0.013). In addition, XYS decreased the UCLA-LS at T2 (p = 0.023) but enhanced the FACIT-Sp at T2 (p = 0.029) and T4 (p = 0.026). Furthermore, after adjustment via propensity score matching, most of the significant findings remained.
Discussion: The addition of XYS to adjuvant chemotherapy improved QoL, psychological health, and spiritual well-being in breast cancer patients.
1 Introduction
According to a recent global cancer statistical report, breast cancer ranks high among all cancer cases in terms of both incidence and mortality (Sung et al., 2021). Profiting from screening technology and national health literacy education, an increasing number of breast cancer patients are being diagnosed at an early stage and are able to receive mastectomy, with a good prognosis estimation (Yang S. et al., 2022a; Ren et al., 2022; Alkabban and Ferguson, 2023). However, a large proportion of patients are indicated to receive adjuvant chemotherapy with or without anti-HER2 agents based on the type of agent used after surgery, which has a considerable impact on patients’ quality of life (QoL), vasomotor symptoms, distress, social functions, and so on (Grimison and Stockler, 2007; Liu et al., 2022; Pereira et al., 2022). These factors make improving QoL, mental health, and spiritual well-being essential for postoperative breast cancer patients undergoing adjuvant chemotherapy (Krzyzanowska et al., 2021; He et al., 2022).
Xiaoyaosan (XYS) is a traditional Chinese formula commonly consisting of eight main components, Radix Bupleuri, Radix Angelicae sinensis, Radix Paeoniae alba, Rhizoma Atractylodis macrocephala, Poria, Rhizoma Zingiberis Recens, Radix Glycyrrhizae, and Herba Menthae, which can also be modified by the addition of other individual components (Hu et al., 2021). Multiple types of XYS have been shown to have good antitumor effects on several cancers, such as ovarian cancer (Li et al., 2021), colorectal cancer (Zhang et al., 2020), and especially breast cancer (Chen et al., 2012). Moreover, numerous studies have reported the excellent effects of XYS on depression, anxiety, anorexia, and QoL, which may be attributed to its regulation of the hypothalamic‒pituitary‒adrenal axis, neural and synaptic plasticity, neuronal loss, microbiota components, immunology, and oxidative stress, as well as its ability to modify several key biological pathways, including the PI3K/AKT, TLR4/NLRP3, RAGE, and JAK/STAT pathways (Li et al., 2017; Zhao et al., 2020; Zhou et al., 2021; Zhu et al., 2021; Chen et al., 2022; Yan et al., 2022; Zeng et al., 2022; Jiao et al., 2023). Recently, XYS has been applied along with adjuvant chemotherapy in breast cancer patients, which attenuates the postoperative complications and toxic reactions of chemotherapy and prolongs disease-free survival and overall survival to some degree (Wang et al., 2006; Du et al., 2015; Suo et al., 2018; Lu, 2019). However, its impact on QoL, psychological pressure, and spiritual well-being regarding adjuvant therapy in breast cancer patients has been less reported.
The current study compared adjuvant chemotherapy plus XYS versus adjuvant chemotherapy alone in postoperative breast cancer patients, aiming to investigate the effect of XYS on QoL, anxiety, depression, loneliness, and spiritual well-being in postoperative patients with breast cancer.
2 Methods
2.1 Subjects
This prospective cohort study enrolled 176 breast cancer patients who planned to receive adjuvant chemotherapy or adjuvant chemotherapy plus XYS between August 2020 and October 2022. The inclusion criteria were as follows: 1) histologically confirmed breast cancer; 2) aged older than 18 years; 3) scheduled for adjuvant chemotherapy or adjuvant chemotherapy plus XYS; 4) adequate hepatic, kidney, and bone marrow function according to the investigator; and 5) willing to cooperate with this study. The exclusion criteria were as follows: 1) had other primary cancers; 2) had malignant hematological diseases; 3) had distant metastases; 4) had allergies or physical intolerance to the study drugs; and 5) were pregnant women or nursing mothers. Approval for this study was granted via the Ethics Committee of The First Affiliated Hospital of Bengbu Medical University with the approval number 2020159. After explaining the details of the study to the patients, the investigator asked the patients whether they would participate in the study. The participants signed the consent form if they agreed to participate in this study.
2.2 Data collection and sample detection
Clinical characteristics, which included the age, marital status, employment status, education level, residence status, lesion site, histologic grade, and tumor–node–metastasis (TNM) stage, were collected for all subjects. In addition, estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), and Ki67 expression were detected in the tumor tissue by immunohistochemistry.
2.3 Treatment
Patients received adjuvant chemotherapy or adjuvant chemotherapy plus XYS. This study did not intervene in patients’ treatments, which were based on their disease status, their own wishes, or doctors’ suggestions. XYS was administered once a day, 30 min prior to a meal, for four cycles (3 weeks per cycle). The contents of XYS included bupleurum (10 g), white peony (15 g), Tuckahoe (15 g), Atractylodis Macrocephalae Rhizoma (10 g), Angelica sinensis (10 g), Radix glycyrrhizae preparata (10 g), Zingiber officinale (5 g), jujube (10 g), and Mentha (4 g) (Hu et al., 2021). Furthermore, the XYS regimen was adjusted according to the condition of the patients. In detail, patients with diarrhea received Dioscorea opposita Thunb. or Lablab purpureus (L.) Sweet; patients with poor sleep quality (such as insomnia) received Semen Ziziphi Spinosae. In addition, adjuvant chemotherapy included the following regimens: AC-T, AC-TH, AC, AC-THP, and AC + H. ‘A’ represents anthracyclines, ‘C’ represents cyclophosphamide, ‘T’ represents taxane, ‘H’ represents trastuzumab, and ‘P’ represents pertuzumab. Conventionally, four cycles of adjuvant chemotherapy (‘A,’ ‘C,’ and ‘T’) were administered (3 weeks per cycle). Patients with a HER2-positive status received 1 year of targeted therapy (‘H’ and ‘P’) in addition to adjuvant chemotherapy. The detailed regimen used was described in the guidelines of the Chinese Society of Clinical Oncology (CSCO) for breast cancer 2020 and is available at http://www.csco.org.cn/cn/index.aspx.
2.4 Questionnaires
The Quality-of-Life Questionnaire Core 30 (QLQ-C30) is a scale that contains 30 items. The first 28 items of the questionnaire used a four-point response scale ranging from 1 to 4. Items 29 and 30 were designed to evaluate global health status and quality of life (QoL), respectively, and used a response scale ranging from 1 to 7. All the raw data were transformed to a 0- to 100-point scale. Higher scores for the functional scales and global health status indicated better functioning and overall QoL, while a high score for the symptom scale represented a high level of symptom distress. The Cronbach’s α coefficient for this QLQ-C30 exceeded 0.70 (Tung et al., 2016). The Hospital Anxiety and Depression Scale (HADS) for anxiety (HADS-A) subscale comprises seven items with scores ranging from 0 to 3 for each item, and the total scores range from 0 to 21; the higher the score is, the more severe the anxiety. The HADS for depression (HADS-D) subscale comprises seven items with scores ranging from 0 to 3 for each item, and the total score ranges from 0 to 21; the higher the score is, the more severe the depression. A previous study reported good internal consistency for both the anxiety (0.71) and depression (0.67) subscales (Su et al., 2012). The University of California Los Angeles Loneliness Scale (UCLA-LS) scale contains 20 items (each item has a four-point frequency score ranging from 1 to 4), of which nine items are scored in a reverse order. The higher the total score is, the greater the degree of loneliness. The internal consistency of the UCLA-LS was good (Cronbach’s α coefficient was 0.94) (Liu and Guo, 2008). The Functional Assessment of Chronic Illness Therapy–Spiritual Wellbeing (FACIT-Sp) scale contains 12 items, which involves three dimensions. Each item was scored from 0 to 4 by the Likert 5 grading method. The Cronbach’s α coefficient for the FACIT-Sp subscales ranged from 0.711 to 0.920 (Liu et al., 2016). These questionnaires were administered in written form in Chinese.
2.5 Outcome measures
The QLQ-C30, HADS-A, HADS-D, UCLA-LS, and FACIT-Sp scores were used for evaluating quality of life, anxiety, depression, loneliness, and spiritual well-being, respectively (Aaronson et al., 1993; Russell, 1996; Peterman et al., 2002; Snaith, 2003). The above parameters were assessed before adjuvant chemotherapy (T0) and after the first (T1), second (T2), third (T3), and fourth cycles (T4) of adjuvant chemotherapy.
2.6 Statistics
SPSS (IBM, United States) version 26.0 was used for data processing. Student’s t-test, the Mann‒Whitney U test, the χ2 test, and Fisher’s exact test were utilized for comparison analyses. To adjust the imbalance of the characteristics between the two groups, propensity score analysis with the nearest neighbor matching method was used. The matched analysis included a comprehensive assessment of various factors, including the age, marital status, employment status, level of education, place of residence, lesion site, histologic grading, TNM stage, ER status, PR status, HER-2 status, and Ki67. Thus, the two study groups were matched at a 1:1 ratio. p < 0.05 indicated statistical significance.
3 Results
3.1 Patient characteristics
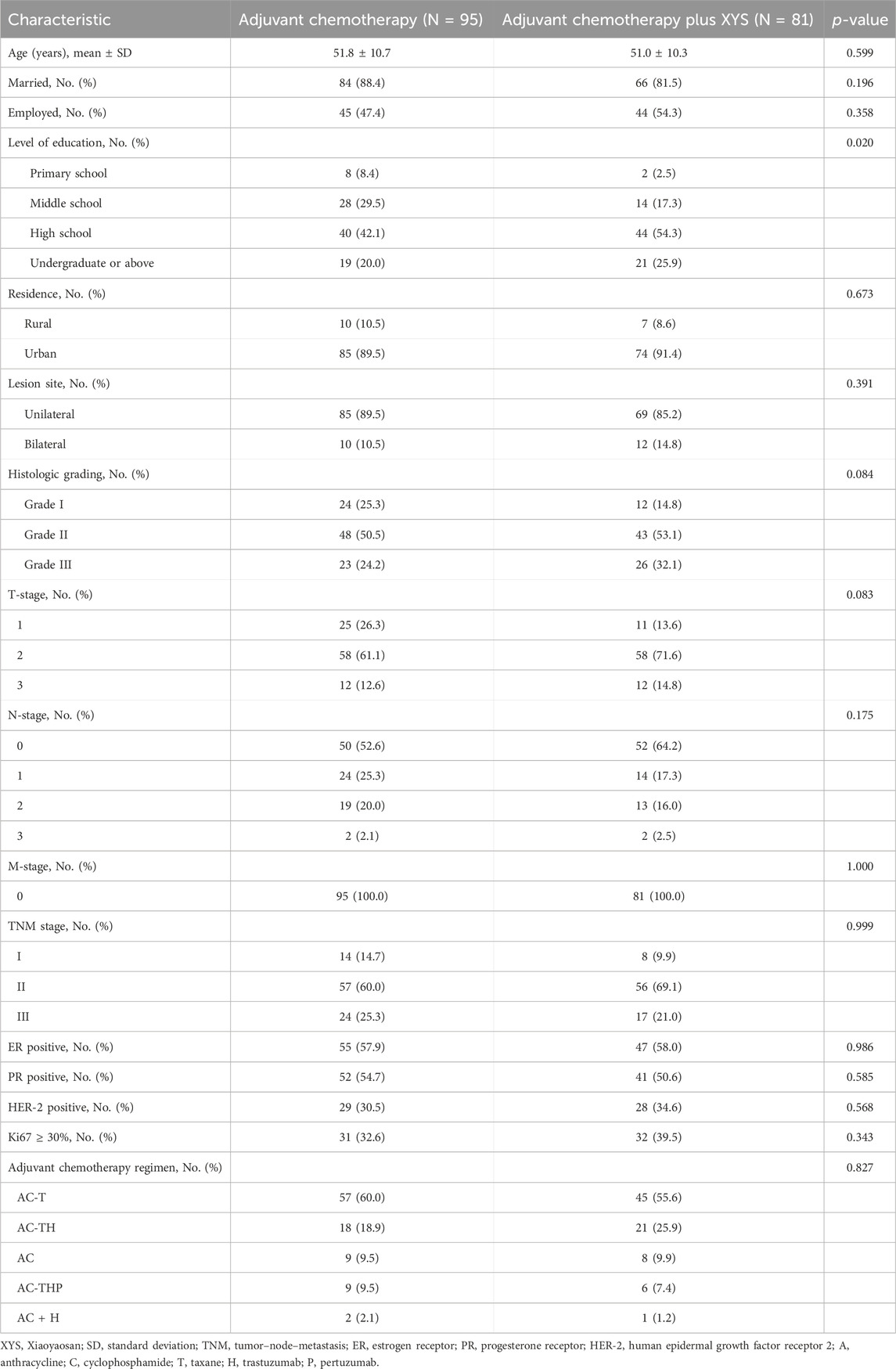
A total of 176 eligible patients were analyzed in the study, consisting of 81 patients in the adjuvant chemotherapy plus XYS group and 95 patients in the adjuvant chemotherapy group (Table 1). In the adjuvant chemotherapy plus XYS group, the mean age was 51.0 ± 10.3 years; 9.9%, 69.1%, and 21.0% of patients were at TNM stage I, II, and III, respectively; and 55.6%, 25.9%, 9.9%, 7.4%, and 1.2% of patients received AC-T, AC-TH, AC, AC-THP, and AC + H as adjuvant chemotherapy regimens, respectively. In the adjuvant chemotherapy group, the mean age was 51.8 ± 10.7 years; 14.7%, 60.0%, and 25.3% of patients were at TNM stage I, II, and III, respectively; and 60.0%, 18.9%, 9.5%, 9.5%, and 2.1% of patients received AC-T, AC-TH, AC, AC-THP, and AC + H as adjuvant chemotherapy regimens, respectively. In comparison, most of the patients’ characteristics did not differ between the two groups, but the level of education was greater in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (p = 0.020).
3.2 QoL comparison
Generally, in both the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group, the QLQ-C30 global health status and functional scale showed a decreasing trend from T0 to T1 and then gradually increased thereafter to T4 (Figures 1A, B); the QLQ-C30 symptom scale revealed the opposite trend (Figure 1C).
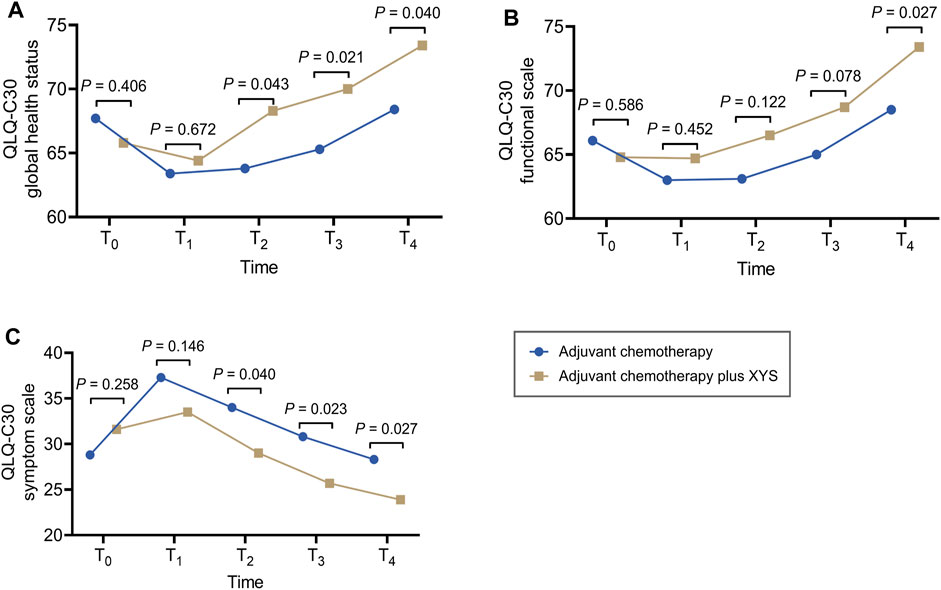
Figure 1. XYS improved QoL. Comparison of the QLQ-C30 global health status (A), functional scale (B), and symptom scale (C) at T0, T1, T2, T3, and T4 between the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group.
In comparison, the QLQ-C30 global health status did not differ between T0 (p = 0.406) and T1 (p = 0.672) but was greater at T2 (p = 0.043), T3 (p = 0.021), and T4 (p = 0.040) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Figure 1A). The QLQ-C30 functional scale did not vary at T0 (p = 0.586), T1 (p = 0.452), T2 (p = 0.122), or T3 (p = 0.078) and was greater only at T4 (p = 0.027) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Figure 1B). In addition, the QLQ-C30 symptom scale did not differ at T0 (p = 0.258) or T1 (p = 0.146) but was lower at T2 (p = 0.040), T3 (p = 0.023), and T4 (p = 0.027) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Figure 1C).
3.3 Anxiety comparison
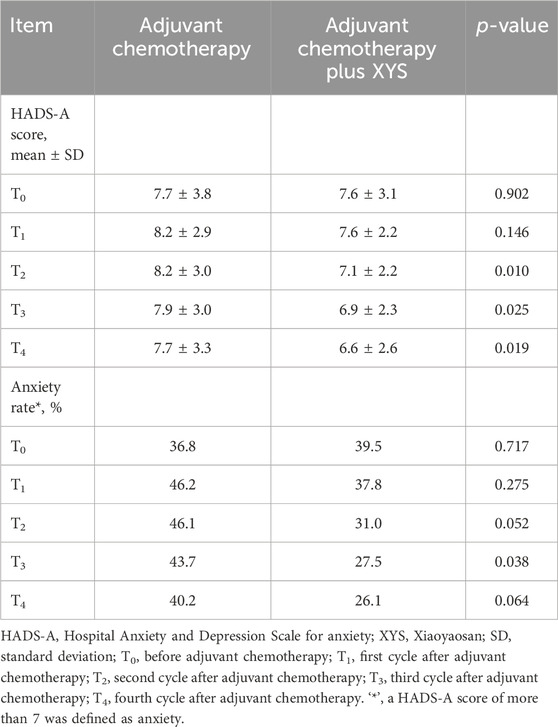
The HADS-A score did not differ at T0 (p = 0.902) or T1 (p = 0.146) but was lower at T2 (p = 0.010), T3 (p = 0.025), or T4 (p = 0.019) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Table 2). Moreover, the HADS-defined anxiety rate did not vary at T0 (p = 0.717), T1 (p = 0.275), T2 (p = 0.052), or T4 (p = 0.064) but was lower at T3 (p = 0.038) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group.
3.4 Depression comparison
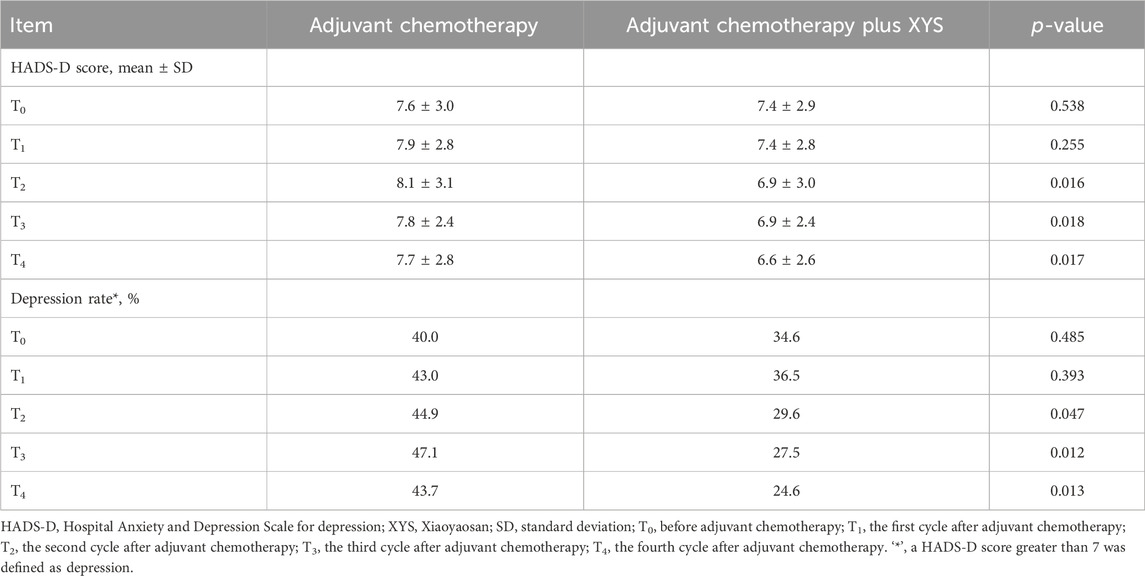
The HADS-D score did not differ at T0 (p = 0.538) or T1 (p = 0.255) but was lower at T2 (p = 0.016), T3 (p = 0.018), or T4 (p = 0.017) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Table 3). Moreover, the HADS-defined depression rate did not differ at T0 (p = 0.485) or T1 (p = 0.393) but was lower at T2 (p = 0.047), T3 (p = 0.012), or T4 (p = 0.013) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group.
3.5 Loneliness and spiritual well-being comparison
UCLA-LS increased from T0 to T1 and then continuously decreased from T1 to T4 in both the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group. In comparison, UCLA-LS was not different at T0 (p = 0.473), T1 (p = 0.219), T3 (p = 0.093), or T4 (p = 0.159) but was lower at T2 (p = 0.023) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Figure 2A).
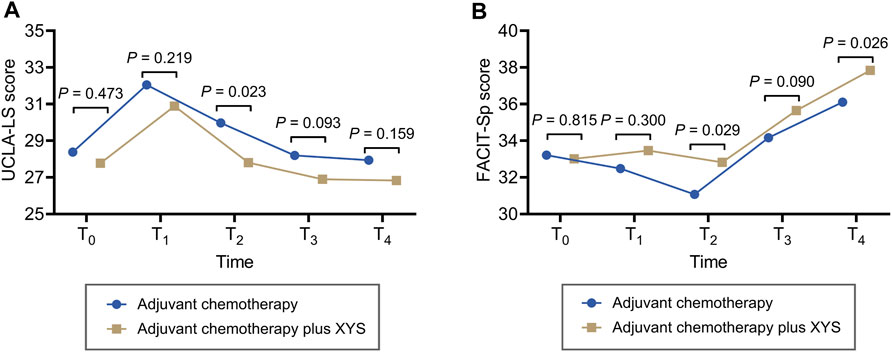
Figure 2. XYS attenuated loneliness and improved spiritual well-being. Comparisons of the UCLA-LS score (A) and FACIT-Sp score (B) at T0, T1, T2, T3, and T4 between the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group.
FACIT-Sp did not fluctuate obviously from T0 to T2 and then greatly increased from T2 to T4 in the adjuvant chemotherapy plus XYS group; however, it decreased from T0 to T2 first and then gradually increased from T2 to T4 in the adjuvant chemotherapy group. In comparison, FACIT-Sp did not vary at T0 (p = 0.815), T1 (p = 0.300), or T3 (p = 0.090) but was greater at T2 (p = 0.029) and T4 (p = 0.026) in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Figure 2B).
3.6 Findings after the adjustment by propensity score matching
Considering that some of the characteristics differed between the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group, propensity score matching was performed for adjustment. All of the characteristics did not differ between the two groups after adjustment by propensity score matching (Table 4).
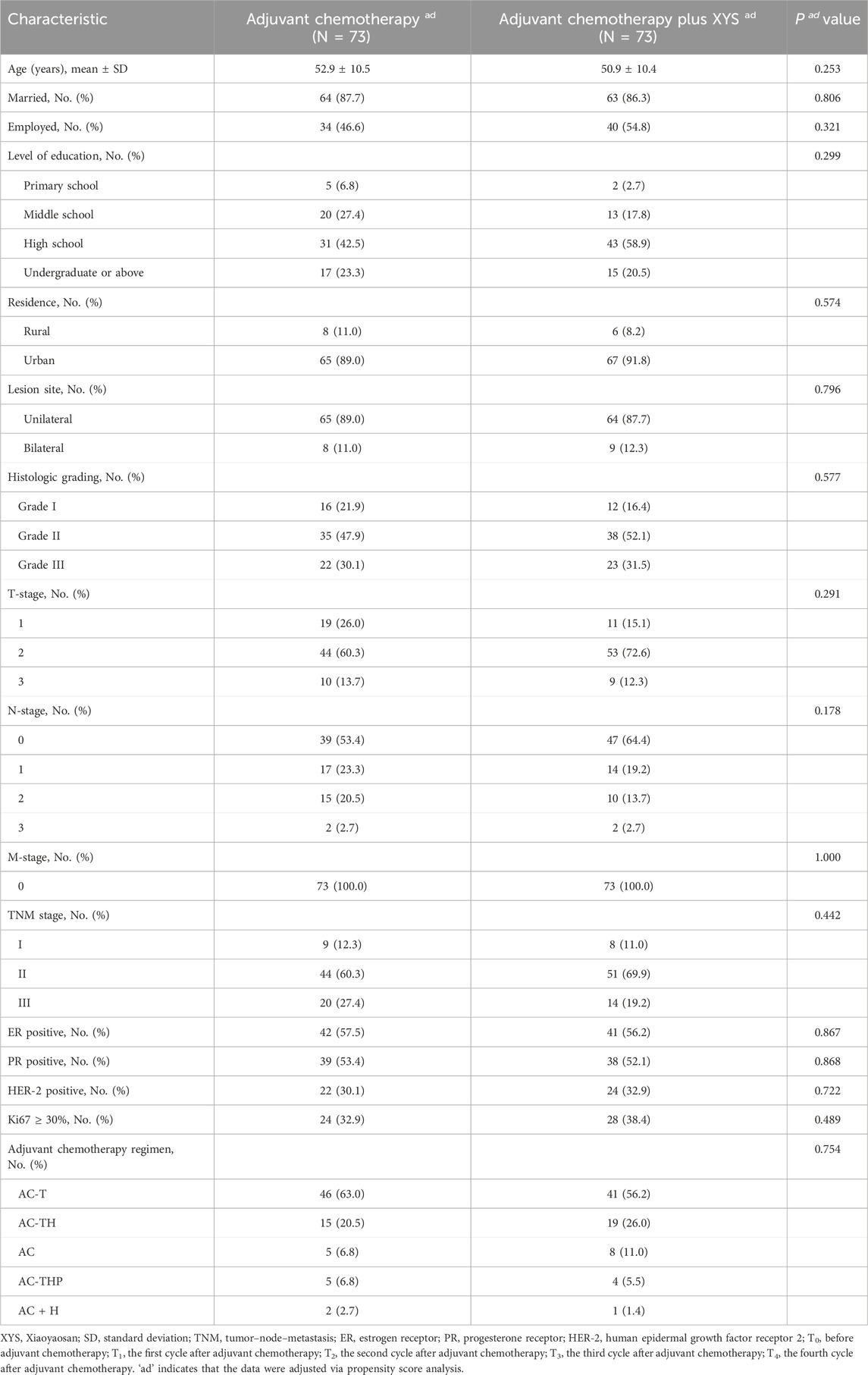
Table 4. Clinical characteristics of breast cancer patients after adjustment via propensity score analysis.
After adjustment, the QLQ-C30 global health status (p = 0.991), functional scale score (p = 0.909), symptom scale score (p = 0.645), HADS-A score (p = 0.700), HADS-defined anxiety rate (p = 0.389), HADS-D score (p = 0.749), HADS-defined depression rate (p = 1.000), UCLA-LS score (p = 0.570), and FACIT-Sp score (p = 0.558) were not different at T0 between the adjuvant chemotherapy plus XYS group and the adjuvant chemotherapy group. Importantly, the QLQ-C30 global health status (p = 0.013), QLQ-C30 functional scale score (p = 0.010), and FACIT-Sp score (p = 0.035) were greater at T4 in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group. Moreover, the HADS-D (p = 0.002) and HADS-defined depression rates (p = 0.010) were lower at T4 in the adjuvant chemotherapy plus XYS group than in the adjuvant chemotherapy group (Table 5). Furthermore, no liver- or kidney-related adverse events occurred in this study.
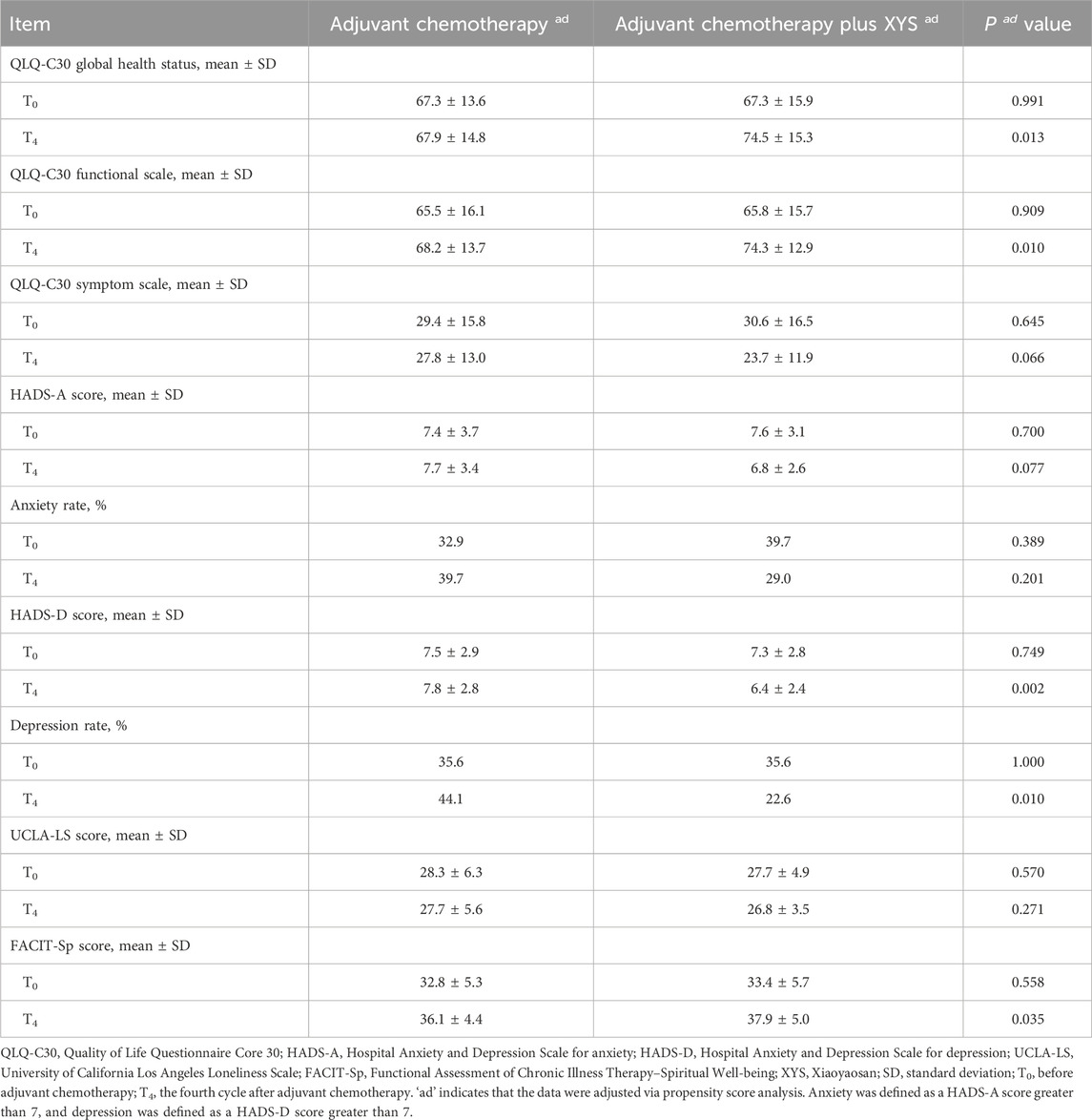
Table 5. Comparisons of the QLQ-C30 scores, HADS-A scores, anxiety rates, HADS-D scores, depression rates, UCLA-LS scores, and FACIT-Sp scores between the two groups after adjustment.
3.7 Subgroup analysis
The subgroup analysis was carried out based on the TNM stage. These findings indicated that most outcomes were not statistically significant in the subgroup analysis, even though a tendency still existed. Specifically, the QLQ-C30 global health status score, QLQ-C30 functional scale score, and FACIT-Sp score were numerically greater, while the QLQ-C30 symptom scale score, HADS-A score, anxiety rate, HADS-D score, depression rate, and UCLA-LS score were numerically lower at T4 in the adjuvant chemotherapy plus XYS subgroup than in the adjuvant chemotherapy subgroup in the TNM stage I, II, and III subgroups (Supplementary Table S1).
4 Discussion
Previous studies have focused mainly on the ability of XYS to alleviate anxiety or depression, and some studies have explored its potential mechanism (Wang et al., 2024; Wu et al., 2024). For instance, one study indicated that XYS might inhibit neuroinflammation to treat depression by regulating the TRIM31/NLRP3 inflammasome (Wang et al., 2024). However, the effect of XYS on QoL in breast cancer patients has seldom been reported. In this study, the addition of XYS to chemotherapy might improve the QLQ-C30 global health and symptom scores. These findings were similar to those of the previous studies, which also reported that XYS might alleviate depression symptoms in patients with depression (Hu et al., 2021; Liu et al., 2021). Even though this study did not carry out any mechanistic experiment, the mechanism of XYS on improving the QLQ-C30 global health and symptom scores might be explained by referring to the previous study, which reported that XYS could relieve depression and anxiety through several mechanisms, such as participating in the autophagy of hypothalamic neurons (Peterman et al., 2002; Zeng et al., 2022; Wang et al., 2024), regulating synapses or synaptic-associated signals (Wu et al., 2024), regulating neurotransmitters (Hagiwara et al., 2023; Javan Biparva et al., 2023; Gudhoor et al., 2024), and regulating the brain–gut axis (Chen et al., 2022), thereby alleviating mental disorders to further improve the global health status and reduce the symptoms of breast cancer patients. However, these hypotheses about the mechanism still need further exploration. However, the functional score of the QLQ-C30 improved relatively little, which was mainly because the QLQ-C30 functional score mainly consisted of the physical function, role function, emotional function, cognitive function, and social function ((Hagiwara et al., 2023; Biparva et al., 2023)); XYS could only have a specific effect on emotional function and social function but had little impact on physical function, role function, or cognitive function. Therefore, chemotherapy plus XYS improved the QLQ-C30 functional score only to a certain degree (the significance of the difference between the two groups was observed only at T4).
Mental disorders, including anxiety and depression, are frequently reported in cancer patients, especially in breast cancer patients undergoing adjuvant chemotherapy, who might suffer from adverse reactions to mastectomy and chemotherapy (Hashemi et al., 2020; Biparva et al., 2023). It has been reported that the depression and anxiety rates in breast cancer patients are as high as 30.2% and 41.9%, respectively (Hashemi et al., 2020; Biparva et al., 2023). These mental disorders further induce low compliance with subsequent therapy and even lead to suicide (Bui and Fava, 2017). Hence, it is essential to relieve mental illness in patients with breast cancer. XYS is a traditional Chinese medicine consisting of Angelicae Sinensis Radix, Bupleuri Radix, Paeoniae Radix Alba, Atractylodis Macrocephalae Rhizoma, Poria, Zingiberis Rhizoma Recens, Glycyrrhizae Radix et Rhizoma, and Menthae Haplocalycis Herba, which can alleviate depression-like behavior by regulating necroptosis-related cellular senescence in the hypothalamus in a mouse model (Jiao et al., 2023). However, its clinical application for relieving mental disorders in breast cancer patients is scarce. In this study, treatment with adjuvant chemotherapy plus XYS decreased the depression rate from 34.6% to 24.6% and the anxiety rate from 39.5% to 26.1% in breast cancer patients, which was more satisfactory than that of adjuvant chemotherapy monotherapy. These findings could be explained as follows: (Sung et al., 2021): XYS might have an antidepressant effect through various mechanisms, such as downregulating A2AR signaling, participating in the autophagy of hypothalamic neurons by regulating GLUT4 expression, and promoting hippocampal neurogenesis (Yang FR. et al., 2022; Zeng et al., 2022; Zhu et al., 2022; Ren et al., 2022). XYS can also regulate synapse- or synaptic-associated signaling pathways, including the neurotrophin signaling and PI3K/AKT signaling pathways, thereby alleviating depression (Meng et al., 2023; Yang S. et al., 2022). XYS has a specific effect on regulating neurotransmitters such as dopamine, 5-hydroxytryptophan, and thyroid hormone; the latter plays an essential role in the pathogenesis of depression and anxiety (Kong et al., 2010; Ding et al., 2014; Zhang et al., 2021; Alkabban and Ferguson, 2023). XYS can also regulate the brain–gut axis, neuroinflammation, and the neuroendocrine axis, thus further acting on its antidepressant effect (Chen et al., 2022). Hence, XYS might play an antidepressant role in breast cancer patients in this study.
Additionally, XYS improved loneliness (as reflected by the UCLA-LS score) and spiritual well-being (as reflected by the FACIT-Sp score) in this study. These findings could be explained as follows: loneliness was positively associated with depression symptoms (Friedman-Ezra et al., 2024). XYS might alleviate depression symptoms through several mechanisms, as mentioned above; therefore, feelings of loneliness are relieved accordingly. Therefore, spiritual well-being is a subjective feeling based on comprehensive assessments of the overall status of the patients themselves. When the QoL improved, anxiety and depression were relieved, and their spiritual well-being improved accordingly.
There was an imbalance in the baseline (T0) characteristics between the two groups. Hence, the adjustment of the baseline characteristics via propensity score methods was carried out to eliminate this potential confounder (D Agostino, 1998). After the adjustment, there was no difference in the baseline characteristics between the two groups, and the difference in primary outcomes, including anxiety, depression, QoL, loneliness, and spiritual well-being, did not change between the two groups. These findings further validated the effectiveness of chemotherapy plus XYS in breast cancer patients.
Although some methods, such as the propensity score method, have been used for remedying methodological deficiencies, our study has several limitations (Sung et al., 2021). The sample size was small, and this limitation was aggravated after adjustment by the propensity score method. Hence, further studies with larger sample sizes are needed (Ren et al., 2022). The follow-up period should be prolonged due to the short follow-up period in this study (Yang S. et al., 2022). The TNM stage was mainly I–II in most patients (approximately 70%) in this study, indicating a relatively low disease burden. However, treatment strategies differ between early-stage and advanced-stage breast cancer patients, which means that the outcomes and treatment-related side effects also differ. Hence, further studies should be conducted to determine the efficacy of chemotherapy plus XYS in breast cancer patients with greater disease burdens, such as those with advanced breast cancer (Alkabban and Ferguson, 2023). When we would like to apply XYS in treating breast cancer patients, some research gaps needed to be solved, such as the detailed mechanism, its efficacy and safety, how to be involved in the current treatment strategy, treatment timing, dosage, and individualized treatment. Our study only preliminarily explored its efficacy and safety. However, more studies are needed to resolve other research gaps before extensively applying XYS in treating breast cancer patients.
In conclusion, adding XYS to adjuvant chemotherapy improved QoL, psychological pressure, and spiritual well-being in postoperative patients with breast cancer, and these advancements remained even after adjustment via propensity score analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The First Affiliated Hospital of Bengbu Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CW: conceptualization, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. LY: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, supervision, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study is supported by Natural Science Research Project of Anhui Educational Committee (No. KJ 2021A0810).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1388646/full#supplementary-material
References
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., et al. (1993). The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 85 (5), 365–376. Epub 1993/03/03. doi:10.1093/jnci/85.5.365
Alkabban, F. M., and Ferguson, T. (2023). Breast cancer. StatPearls. Treasure island (FL) ineligible companies. Discl. Troy Ferguson declares no relevant financial Relat. ineligible Co.
Biparva, A. J., Raoofi, S., Rafiei, S., Masoumi, M., Doustmehraban, M., Bagheribayati, F., et al. (2023). Global depression in breast cancer patients: systematic review and meta-analysis. Meta-Analysis PLoS One 18 (7), e0287372. doi:10.1371/journal.pone.0287372
Bui, E., and Fava, M. (2017). From depression to anxiety, and back. Acta Psychiatr. Scand. 136 (4), 341–342. PubMed PMID: 28865404. doi:10.1111/acps.12801
Chen, J., Lei, C., Li, X., Wu, Q., Liu, C., Ma, Q., et al. (2022). Research progress on classical traditional Chinese medicine formula xiaoyaosan in the treatment of depression. Front. Pharmacol. 13, 925514. PubMed PMID: 35991880; PubMed Central PMCID: PMCPMC9386002. doi:10.3389/fphar.2022.925514
Chen, W. F., Xu, L., Yu, C. H., Ho, C. K., Wu, K., Leung, G. C., et al. (2012). The in vivo therapeutic effect of free wanderer powder (xiao yao san, xiaoyaosan) on mice with 4T1 cell induced breast cancer model. J. Tradit. Complement. Med. 2 (1), 67–75. PubMed PMID: 24716117; PubMed Central PMCID: PMCPMC3943014. doi:10.1016/s2225-4110(16)30073-6
D Agostino, R. B. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17 (19), 2265–2281. PubMed PMID: 9802183. doi:10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
Ding, X. F., Zhao, X. H., Tao, Y., Zhong, W. C., Fan, Q., Diao, J. X., et al. (2014). Xiao yao san improves depressive-like behaviors in rats with chronic immobilization stress through modulation of locus coeruleus-norepinephrine system. Evid. Based Complement. Altern. Med. 2014, 605914. PubMed PMID: 25610478; PubMed Central PMCID: PMCPMC4291141. doi:10.1155/2014/605914
Du, Y. L., Wang, Z. M., Wang, F., and Lu, D. Y. (2015). Observation of efficacy of modified Xiaoyaosan combined with chemotherapy on postoperative breast cancer. Mod. J. Integr. Traditional Chin. West. Med. 3, 295–297.
Friedman-Ezra, A., Keydar-Cohen, K., van Oppen, P., Eikelenboom, M., Schruers, K., and Anholt, G. E. (2024). Loneliness in OCD and its determinants. Psychiatry Res. 337, 115963. PubMed PMID: 38788555. doi:10.1016/j.psychres.2024.115963
Grimison, P. S., and Stockler, M. R. (2007). Quality of life and adjuvant systemic therapy for early-stage breast cancer. Expert Rev. Anticancer Ther. 7 (8), 1123–1134. PubMed PMID: 18028021. doi:10.1586/14737140.7.8.1123
Gudhoor, M., Mathew, A. T., Ganachari, A. M., Baiju, G., Kulkarni, S. S., and Ganachari, M. S. (2024). Utilization of European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 scale for evaluation of quality of life among cancer patients treated with chemotherapy: a hospital-based observational study. J. Oncol. Pharm. Pract. 30 (5), 844–852. PubMed PMID: 37537966. doi:10.1177/10781552231189706
Hagiwara, Y., Shiroiwa, T., Taira, N., Kawahara, T., Konomura, K., Noto, S., et al. (2023). Gradient boosted tree approaches for mapping European organization for research and treatment of cancer quality of life questionnaire Core 30 onto 5-level version of EQ-5D index for patients with cancer. Value Health 26 (2), 269–279. PubMed PMID: 36096966. doi:10.1016/j.jval.2022.07.020
Hashemi, S. M., Rafiemanesh, H., Aghamohammadi, T., Badakhsh, M., Amirshahi, M., Sari, M., et al. Prevalence of anxiety among breast cancer patients: a systematic review and meta-analysis. Breast Cancer (2020) 27(2):166–178. doi:10.1007/s12282-019-01031-9
He, X., Ng, M. S. N., Choi, K. C., and So, W. K. W. (2022). Effects of a 16-week dance intervention on the symptom cluster of fatigue-sleep disturbance-depression and quality of life among patients with breast cancer undergoing adjuvant chemotherapy: a randomized controlled trial. Int. J. Nurs. Stud. 133, 104317. PubMed PMID: 35850058. doi:10.1016/j.ijnurstu.2022.104317
Hu, J., Teng, J., Wang, W., Yang, N., Tian, H., Zhang, W., et al. (2021). Clinical efficacy and safety of traditional Chinese medicine Xiao Yao San in insomnia combined with anxiety. Med. Baltim. 100 (43), e27608. PubMed PMID: 34713840; PubMed Central PMCID: PMCPMC8556059. doi:10.1097/MD.0000000000027608
Javan Biparva, A., Raoofi, S., Rafiei, S., Masoumi, M., Doustmehraban, M., Bagheribayati, F., et al. (2023). Global depression in breast cancer patients: systematic review and meta-analysis. PLoS One 18 (7), e0287372. PubMed PMID: 37494393; PubMed Central PMCID: PMCPMC10370744. doi:10.1371/journal.pone.0287372
Jiao, H., Fan, Y., Gong, A., Li, T., Fu, X., and Yan, Z. (2023). Xiaoyaosan ameliorates CUMS-induced depressive-like and anorexia behaviors in mice via necroptosis related cellular senescence in hypothalamus. J. Ethnopharmacol. 318 (Pt A), 116938. PubMed PMID: 37495029. doi:10.1016/j.jep.2023.116938
Kong, M., Xing, C., and Shu, X. (2010). Influence of ease powder decoction on expression of Hippocampus 5-HT_ (1A) receptor and 5-HT_ (2A) receptor in rat model of sleep deprivation depression. Chin. J. Exp. Tradit. Med. Formulae, 14.
Krzyzanowska, M. K., Julian, J. A., Gu, C. S., Powis, M., Li, Q., Enright, K., et al. (2021). Remote, proactive, telephone based management of toxicity in outpatients during adjuvant or neoadjuvant chemotherapy for early stage breast cancer: pragmatic, cluster randomised trial. BMJ 375, e066588. PubMed PMID: 34880055; PubMed Central PMCID: PMCPMC8652580 at www.icmje.org/disclosure-of-interest/ and declare: support from the Ontario Institute for Cancer Research (OICR) for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; CCE and EG hold appointments at the OICR Health Services Research Programme; no other relationships or activities that could appear to have influenced the submitted work. doi:10.1136/bmj-2021-066588
Li, M., Zhang, W., Yang, L., Wang, H., Wang, Y., Huang, K., et al. (2021). The mechanism of xiaoyao san in the treatment of ovarian cancer by network pharmacology and the effect of stigmasterol on the PI3K/akt pathway. Dis. Markers 2021, 4304507. PubMed PMID: 34306252; PubMed Central PMCID: PMCPMC8263223. doi:10.1155/2021/4304507
Li, X. J., Ma, Q. Y., Jiang, Y. M., Bai, X. H., Yan, Z. Y., Liu, Q., et al. Xiaoyaosan exerts anxiolytic-like effects by down-regulating the TNF-α/JAK2-STAT3 pathway in the rat hippocampus. Sci. Rep. (2017) 7(1):353. doi:10.1038/s41598-017-00496-y
Liu, L. J., and Guo, Q. (2008). Life satisfaction in a sample of empty-nest elderly: a survey in the rural area of a mountainous county in China. Qual. Life Res. 17 (6), 823–830. PubMed PMID: 18595006. doi:10.1007/s11136-008-9370-1
Liu, Q., Shi, Z., Zhang, T., Jiang, T., Luo, X., Su, X., et al. (2021). Efficacy and safety of Chinese herbal medicine xiao yao san in functional gastrointestinal disorders: a meta-analysis and trial sequential analysis of randomized controlled trials. Front. Pharmacol. 12, 821802. PubMed PMID: 35126152; PubMed Central PMCID: PMCPMC8811448. doi:10.3389/fphar.2021.821802
Liu, W., Liu, J., Ma, L., and Chen, J. (2022). Effect of mindfulness yoga on anxiety and depression in early breast cancer patients received adjuvant chemotherapy: a randomized clinical trial. J. Cancer Res. Clin. Oncol. 148 (9), 2549–2560. PubMed PMID: 35788727; PubMed Central PMCID: PMCPMC9253261. doi:10.1007/s00432-022-04167-y
Liu, X., Wei, Di, Chen, Y., Cheng, Q., Liang, S., Xu, X., et al. (2016). Reliability and validity evaluation of the Chinese version of the therapeutic function assessment of chronic diseases - spirituality scale in cancer patients. Chin. J. Nurs. 51 (9), 1085–1090. doi:10.3761/j.issn.0254-1769.2016.09.014
Lu, H. M. (2019). Observation on the efficacy and toxicity of supplemented Xiaoyaosan combined with chemotherapy in the treatment of breast cancer. Cap. Med. 26 (18), 66.
Meng, P., Zhang, X., Liu, T. T., Liu, J., Luo, Y., Xie, M. X., et al. (2023). A whole transcriptome profiling analysis for antidepressant mechanism of Xiaoyaosan mediated synapse loss via BDNF/trkB/PI3K signal axis in CUMS rats. BMC Complement. Med. Ther. 23 (1), 198. Epub 2023/06/16. doi:10.1186/s12906-023-04000-0
Pereira, I., Pereira, M., Leite, A., and Pereira, M. G. (2022). Quality of life in women with breast cancer receiving chemotherapy and the moderating role of cortisol. Cancer Nurs. 45 (6), E856–E864. PubMed PMID: 35324503. doi:10.1097/NCC.0000000000001081
Peterman, A. H., Fitchett, G., Brady, M. J., Hernandez, L., and Cella, D. (2002). Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy--Spiritual Well-being Scale (FACIT-Sp). Ann. Behav. Med. a Publ. Soc. Behav. Med. 24 (1), 49–58. PubMed PMID: 12008794. doi:10.1207/s15324796abm2401_06
Ren, W., Chen, M., Qiao, Y., and Zhao, F. (2022). Global guidelines for breast cancer screening: a systematic review. Breast 64, 85–99. PubMed PMID: 35636342; PubMed Central PMCID: PMCPMC9142711. doi:10.1016/j.breast.2022.04.003
Russell, D. W. (1996). UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J. Pers. Assess. 66 (1), 20–40. Epub 1996/02/01. doi:10.1207/s15327752jpa6601_2
Snaith, R. P. (2003). The hospital anxiety and depression scale. Health Qual. Life Outcomes 1, 29. PubMed PMID: 12914662; PubMed Central PMCID: PMCPMC183845. doi:10.1186/1477-7525-1-29
Su, Q., Liu, Y., Cheng, Y., Lin, W., and Hong, M. (2012). The hospital anxiety depression scale in physical examination center application the reliability and validity of research. J. Sichuan Med. 33 (1), 174–176. doi:10.3969/j.issn.1004-0501.2012.01.078
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71 (3), 209–249. PubMed PMID: 33538338. doi:10.3322/caac.21660
Suo, J. J., Yang, K. Y., Han, F. M., Wang, Q., Zhang, B. J., and Wang, Y. (2018). Clinical significance of Xiaoyaosan combined with high quality nursing for the prevention of postoperative complications after radical mastectomy. Diet. Health 5 (46), 202.
Tung, H. Y., Chao, T. B., Lin, Y. H., Wu, S. F., Lee, H. Y., Ching, C. Y., et al. (2016). Depression, fatigue, and QoL in colorectal cancer patients during and after treatment. West J. Nurs. Res. 38 (7), 893–908. Epub 2016/02/24. doi:10.1177/0193945916630256
Wang, B., Tian, L., Wu, M., Zhang, D., Yan, X., Bai, M., et al. (2024). Modified Danzhi XiaoyaoSan inhibits neuroinflammation via regulating TRIM31/NLRP3 inflammasome in the treatment of CUMS depression. Exp. Gerontol. 192, 112451. Epub 2024/05/11. doi:10.1016/j.exger.2024.112451
Wang, L. B., Yang, L. P., and Huang, H. S. (2006). Clinical observation of modified Xiaoyaosan to improve the toxic reactions of chemotherapy after breast cancer surgery. J. TCM Univ. Hunan 26 (3), 38–40.
Wu, Y., Liu, L., Zhao, Y., Li, X., Hu, J., Li, H., et al. (2024). Xiaoyaosan promotes neurotransmitter transmission and alleviates CUMS-induced depression by regulating the expression of Oct1 and Oct3 in astrocytes of the prefrontal cortex. J. Ethnopharmacol. 326, 117923. Epub 2024/02/18. doi:10.1016/j.jep.2024.117923
Yan, W., Dong, Z., Zhao, D., Li, J., Zeng, T., Mo, C., et al. (2022). Corrigendum: xiaoyaosan exerts antidepressant effect by downregulating RAGE expression in cingulate gyrus of depressive-like mice. Front. Pharmacol. 13, 982525. PubMed PMID: 36160413; PubMed Central PMCID: PMCPMC9494942. doi:10.3389/fphar.2022.982525
Yang, F. R., Zhu, X. X., Kong, M. W., Zou, X. J., Ma, Q. Y., Li, X. J., et al. (2022b). Xiaoyaosan exerts antidepressant-like effect by regulating autophagy involves the expression of GLUT4 in the mice hypothalamic neurons. Front. Pharmacol. 13, 873646. PubMed PMID: 35784760; PubMed Central PMCID: PMCPMC9243304. doi:10.3389/fphar.2022.873646
Yang, S., Li, P., Yu, L., Liu, N., Wang, J., Guo, P., et al. (2022a). Breast cancer awareness based on health information literacy and influential factors among female nursing students in China. J. Cancer Educ. 37 (3), 546–554. PubMed PMID: 32876864. doi:10.1007/s13187-020-01844-9
Zeng, J., Ji, Y., Luan, F., Hu, J., Rui, Y., Liu, Y., et al. (2022). Xiaoyaosan ethyl acetate fraction alleviates depression-like behaviors in CUMS mice by promoting hippocampal neurogenesis via modulating the IGF-1Rβ/PI3K/Akt signaling pathway. J. Ethnopharmacol. 288, 115005. PubMed PMID: 35051601. doi:10.1016/j.jep.2022.115005
Zhang, H. R., Yue, G. X., Liang, Y., Yang, J. W., Li, Y., Wu, W. N., et al. (2021). Effect of Xiaoyaosan on central dopamine and its receptors in chronic mild unpredictable stress depression rats. Chin. J. Basic Med. 27 (11), 1725–1730.
Zhang, Z., Shao, S., Zhang, Y., Jia, R., Hu, X., Liu, H., et al. (2020). Xiaoyaosan slows cancer progression and ameliorates gut dysbiosis in mice with chronic restraint stress and colorectal cancer xenografts. Biomed. Pharmacother. 132, 110916. PubMed PMID: 33113425. doi:10.1016/j.biopha.2020.110916
Zhao, H. B., Jiang, Y. M., Hou, Y. J., Yan, Z. Y., Liu, Y. Y., Li, X. J., et al. (2020). Xiaoyaosan produces antidepressant effects in rats via the JNK signaling pathway. Complement. Med. Res. 27 (1), 47–54. PubMed PMID: 31394544. doi:10.1159/000501995
Zhou, X. M., Liu, C. Y., Liu, Y. Y., Ma, Q. Y., Zhao, X., Jiang, Y. M., et al. (2021). Xiaoyaosan alleviates hippocampal glutamate-induced toxicity in the CUMS rats via NR2B and PI3K/akt signaling pathway. Front. Pharmacol. 12, 586788. PubMed PMID: 33912031; PubMed Central PMCID: PMCPMC8075411. doi:10.3389/fphar.2021.586788
Zhu, H. Z., Liang, Y. D., Hao, W. Z., Ma, Q. Y., Li, X. J., Li, Y. M., et al. (2021). Xiaoyaosan exerts therapeutic effects on the colon of chronic restraint stress model rats via the regulation of immunoinflammatory activation induced by the TLR4/NLRP3 inflammasome signaling pathway. Evid. Based Complement. Altern. Med. 2021, 6673538. PubMed PMID: 33505499; PubMed Central PMCID: PMCPMC7810549. doi:10.1155/2021/6673538
Zhu, X., Ma, Q., Yang, F., Li, X., Liu, Y., Chen, J., et al. (2022). Xiaoyaosan ameliorates chronic restraint stress-induced depression-like phenotype by suppressing A2AR signaling in the rat striatum. Front. Pharmacol. 13, 897436. PubMed PMID: 35814204; PubMed Central PMCID: PMCPMC9261476. doi:10.3389/fphar.2022.897436
Keywords: postoperative breast cancer, Xiaoyaosan, adjuvant chemotherapy, quality of life, psychological pressure
Citation: Wang C and Yin L (2024) Xiaoyaosan formula augments adjuvant therapy and enhances postoperative breast cancer care. Front. Pharmacol. 15:1388646. doi: 10.3389/fphar.2024.1388646
Received: 27 February 2024; Accepted: 12 July 2024;
Published: 09 August 2024.
Edited by:
Sheema Khan, The University of Texas Rio Grande Valley, United StatesReviewed by:
Ruo Wang, Shanghai Jiao Tong University, ChinaDalinda Isabel Sánchez-Vidaña, Hong Kong Polytechnic University, Hong Kong SAR, China
Copyright © 2024 Wang and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianfang Yin, MTgzMzY1NDczNEBxcS5jb20=
 Chao Wang
Chao Wang Lianfang Yin
Lianfang Yin