- Department of Orthopaedics, The Second Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
Background: In recent years, with the continuous expansion of the application scope of Tranexamic acid (TXA), its usage has surged. Despite numerous studies demonstrating its powerful efficacy, concerns regarding its adverse reactions persist, necessitating comprehensive safety assessment. This study analyzed real-world data from the U.S. Food and Drug Administration to investigate TXA-related adverse events, aiming to elucidate its safety and optimize patient treatment.
Methods: The adverse drug event data concerning TXA from 2004 Q1 to 2023 Q3 were collected. Following data standardization, a variety of signal quantification techniques, including the reporting odds ratios, proportional reporting ratios, Bayesian confidence propagation neural network, and empirical Bayes geometric mean were used for analysis.
Results: After analyzing 16,692,026 adverse event reports, a total of 1,574 cases of adverse events related to TXA were identified, spanning 23 system organ classes and 307 preferred terms. In addition to the common thrombosis-related Vascular disorders (n = 386) and Cardiac disorders (n = 377), adverse reactions in the Nervous system disorders category were also observed (n = 785), including Myoclonus (n = 70), Status epilepticus (n = 43), and Myoclonic epilepsy (n = 17). Furthermore, this study uncovered adverse effects such as Renal cortical necrosis, Hepatic cyst rupture, and Vascular stent stenosis, which were not previously mentioned in the instructions. Although these occurred infrequently, they exhibited high signal strength. Both Retinal artery occlusion and Vascular stent thrombosis disorder were frequent and exhibited high signal strength as well. It is worth noting that 78 cases of adverse reactions were caused by confusion between incorrect product administration.
Conclusion: Our research suggests that TXA has some adverse reactions that are being overlooked. As a cornerstone medication in hemorrhage treatment, it’s crucial to monitor, identify, and address these adverse reactions effectively.
1 Introduction
1. Background
Since its development and release in the early 1960s, tranexamic acid (TXA), an indirect fibrinolytic inhibitor, has been utilized extensively. Initially, it was prescribed for heavy menstrual bleeding in females and individuals with hereditary bleeding disorders. Over time, its usage expanded to include elective surgery due to its effectiveness in reducing blood loss (Tengborn et al., 2015).
In recent years, there has been a steady uptick in the usage of TXA, with its applications continually broadening. TXA gained global recognition following the 2010 Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage (CRASH-2) trial, with the result notably decreasing the risk of bleeding-related mortality by approximately one-sixth and overall mortality by about a 10th (Shakur et al., 2010). In 2011, the World Health Organization recommended TXA as an essential medicine for managing acute bleeding in patients with trauma, cardiopulmonary bypass, or postpartum hemorrhage (Sixty-third World Health Assembly, 2010). Subsequently, the 2013 European guidelines advocated for TXA’s use in prophylactic treatment during major surgeries to mitigate perioperative blood loss and allogeneic blood transfusion (Kozek-Langenecker et al., 2013). Additionally, the 2015 American Society of Anesthesiologists practice guidelines recommended considering TXA for surgical patients experiencing excessive bleeding (American Society of Anesthesiologists Task Force on Perioperative Blood Management, 2015). TXA has consistently demonstrated its significance in blood conservation and reducing perioperative blood loss across various medical domains, including trauma, cardiac, orthopedic, neurological, craniofacial, obstetrical, and gynecological surgeries (Ortmann et al., 2013).
Nevertheless, with the ongoing rise in utilization and expanding indications, numerous inquiries persist regarding the additional clinical effects of TXA. These include its potential anti-inflammatory response during cardiopulmonary bypass, the risk of thromboembolic events, adverse neurological effects such as seizures, as well as its incidence and mortality rates (Ortmann et al., 2013). Hence, there is an urgent necessity to thoroughly assess the safety profile of TXA across various medical disciplines. In this data analysis, we utilize real-world pharmacovigilance data, predominantly from the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS), to scrutinize adverse events (AEs) linked with TXA administration. Through meticulous examination of these reports, our objective is to illuminate the safety landscape of TXA, pinpoint potential risk factors, and offer valuable insights to clinicians for optimizing patient care strategies.
2 Materials and methods
2.1 Data source
Given TXA’s market release date, this study retrieved the American Standard Code for Information Interchange (ASCII) report files from the FAERS database spanning from the first quarter of 2004 to the third quarter of 2023. The data underwent processing using R_4.3.2 software.
2.2 Data extraction and analysis
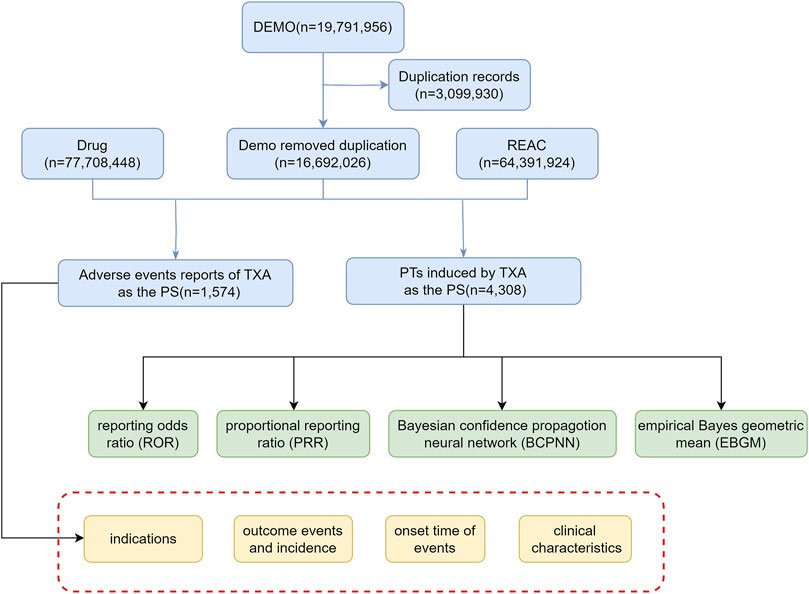
To ensure data integrity, duplicate reports were removed. For reports sharing the same CASE number, the latest FDA_DT is chosen. In cases where both the CASE number and FDA_DT are identical, the report with the higher ISR number is selected. Screening is conducted based on the drug name: tranexamic acid, with the suspicion level of reports limited to “Primary Suspect (PS)”. Finally, we obtain adverse event reports (AERs) of TXA and preferred terms (PTs) induced by TXA (Figure 1). The relationships among datasets were established using the primaryid field. Standardization of drug names was achieved through the Medex_UIMA_1.8.3 system.
This study simultaneously employed the reporting odds ratios (ROR) (Rothman et al., 2004), proportional reporting ratios (PRR) (Evans et al., 2001), Bayesian confidence propagation neural network (BCPNN) (Bate et al., 1998), and empirical Bayes geometric mean (EBGM) (Dumouchel, 1999) techniques from the disproportionality analysis to detect ADEs. Through their joint application and cross-validation to reduce potential false positives, thresholds and variances were adjusted to enhance the detection of potentially rare adverse reactions. All algorithms were based on 2 × 2 contingency tables, as shown in Supplementary Table S1. Specific formulas and thresholds are outlined in Supplementary Table S2. Statistical analysis was conducted using R_4.3.2 software. Higher values indicate stronger signal intensity, suggesting a stronger association between the target drug and AEs.
2.3 Signal filtering and categorization
PTs with reporting counts of ≥3 were selected. The Medical Dictionary for Regulatory Activities (MedDRA) PTs and System Organ Classes (SOCs) were utilized to encode, classify, and localize signals, facilitating the analysis of specific SOCs related to AE signals.
3 Results
3.1 Basic characteristics of TXA-related ADEs
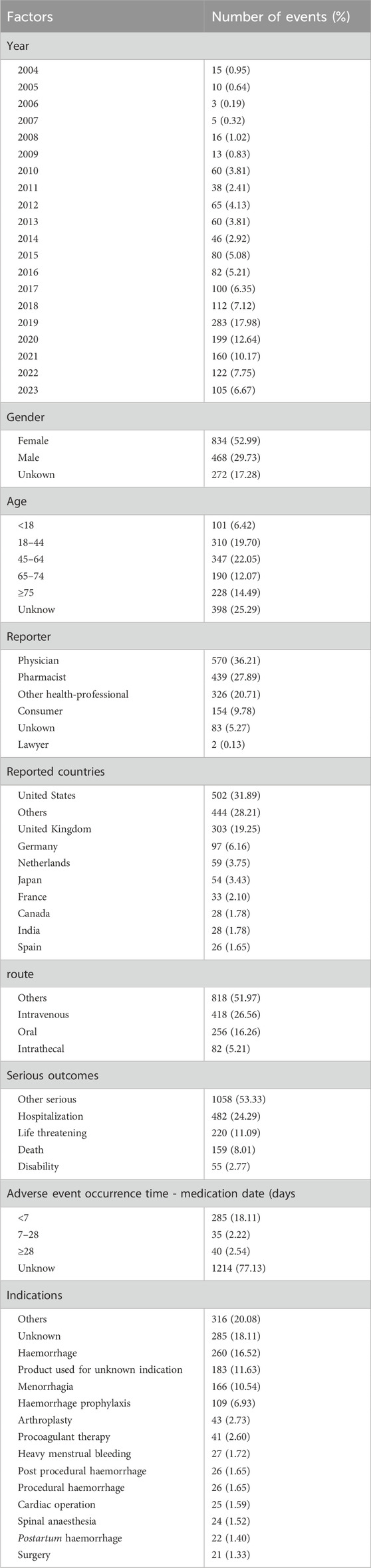
From the first quarter of 2004 to the third quarter of 2023, this study obtained a total of 16,692,026 AERs from the FAERS database. Among these reports, TXA was identified as the primary suspected drug for ADEs in 1,574 cases. Interestingly, fluctuations in the annual distribution of these reports were observed, with a notable surge in recent years, peaking in 2019 with 283 reports, representing 17.98% of the total. Gender analysis revealed a significant skew towards female patients, constituting 52.99% of the reports, compared to 29.73% for male patients. Across different age groups, the distribution of reports was relatively uniform, without any discernible variations. Notably, the majority of reports were submitted by healthcare professionals, predominantly physicians (36.21%) and pharmacists (27.89%), with consumer reports making up 9.78% of the total submissions. Geographically, the United States contributed the highest proportion of reports at 31.89%, followed by a diverse international representation. Various administration routes were documented, with intravenous (26.56%) and oral (16.26%) routes being the most prevalent. Notably, serious outcomes associated with ADEs, such as hospitalization (24.29%), life-threatening conditions (11.09%), and death (8.01%), underscore the importance of vigilant pharmacovigilance. Additionally, the majority of ADEs occurred within 7 days of medication usage, as revealed by our investigation. TXA, known for its diverse indications, was primarily associated with hemorrhage (16.52%) and menorrhagia (10.54%). Details can be found in Table 1 and Figure 2.
3.2 TXA signal mining
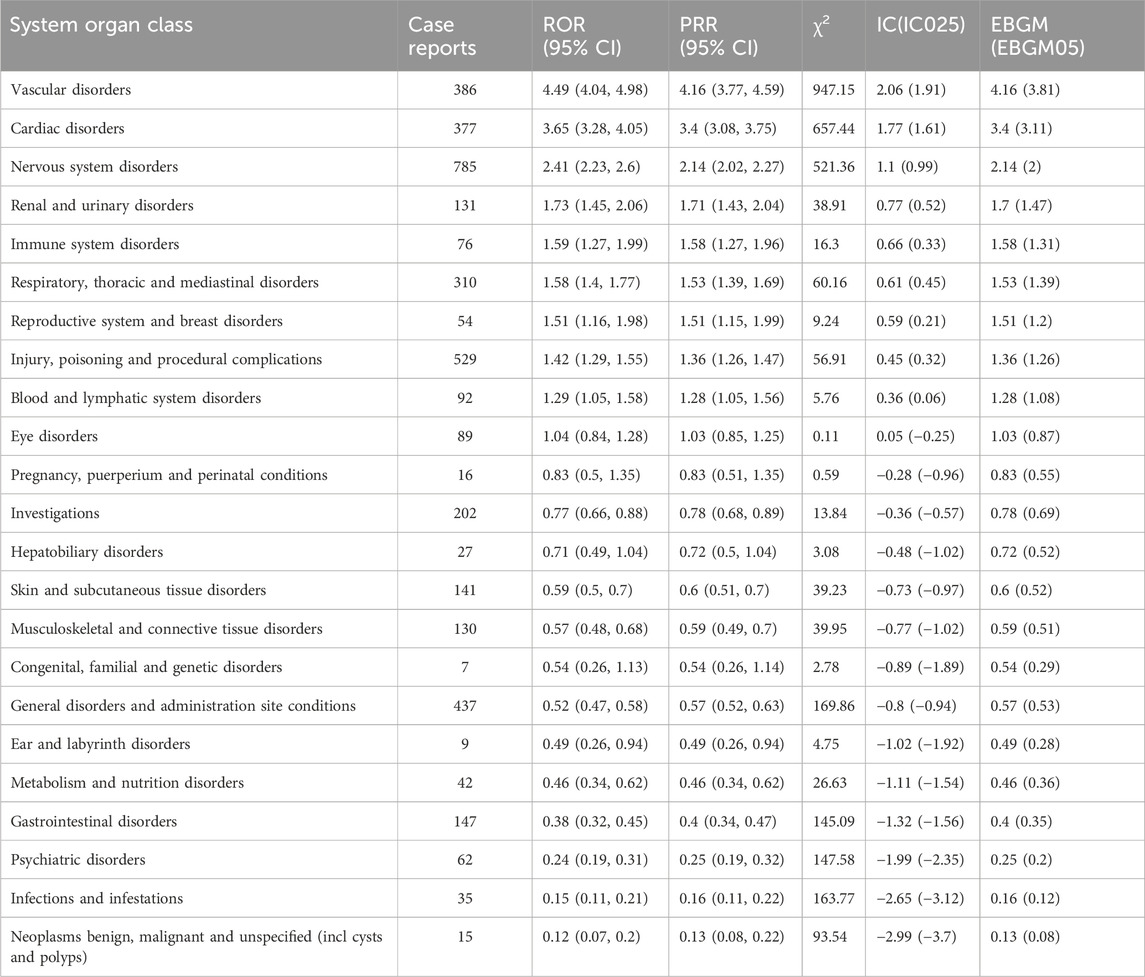
This study found that AEs related to TXA involved 23 System Organ Classes (SOCs). The three most common systems were Nervous system disorders (n = 785, ROR 2.41, PRR 2.14, IC 1.1, EBGM 2.14), Injury, poisoning and procedural complications (n = 529, ROR 1.42, PRR 1.36, IC 0.45, EBGM 1.36), and General disorders and administration site conditions (n = 437, ROR 0.52, PRR 0.57, IC −0.8, EBGM 0.57). Furthermore, Vascular disorders (n = 386, ROR 4.49, PRR 4.16, IC 2.06, EBGM 4.16) and Cardiac disorders (n = 377, ROR 3.65, PRR 3.4, IC 1.77, EBGM 3.4) exhibited the strongest signal intensities. Details can be found in Table 2.
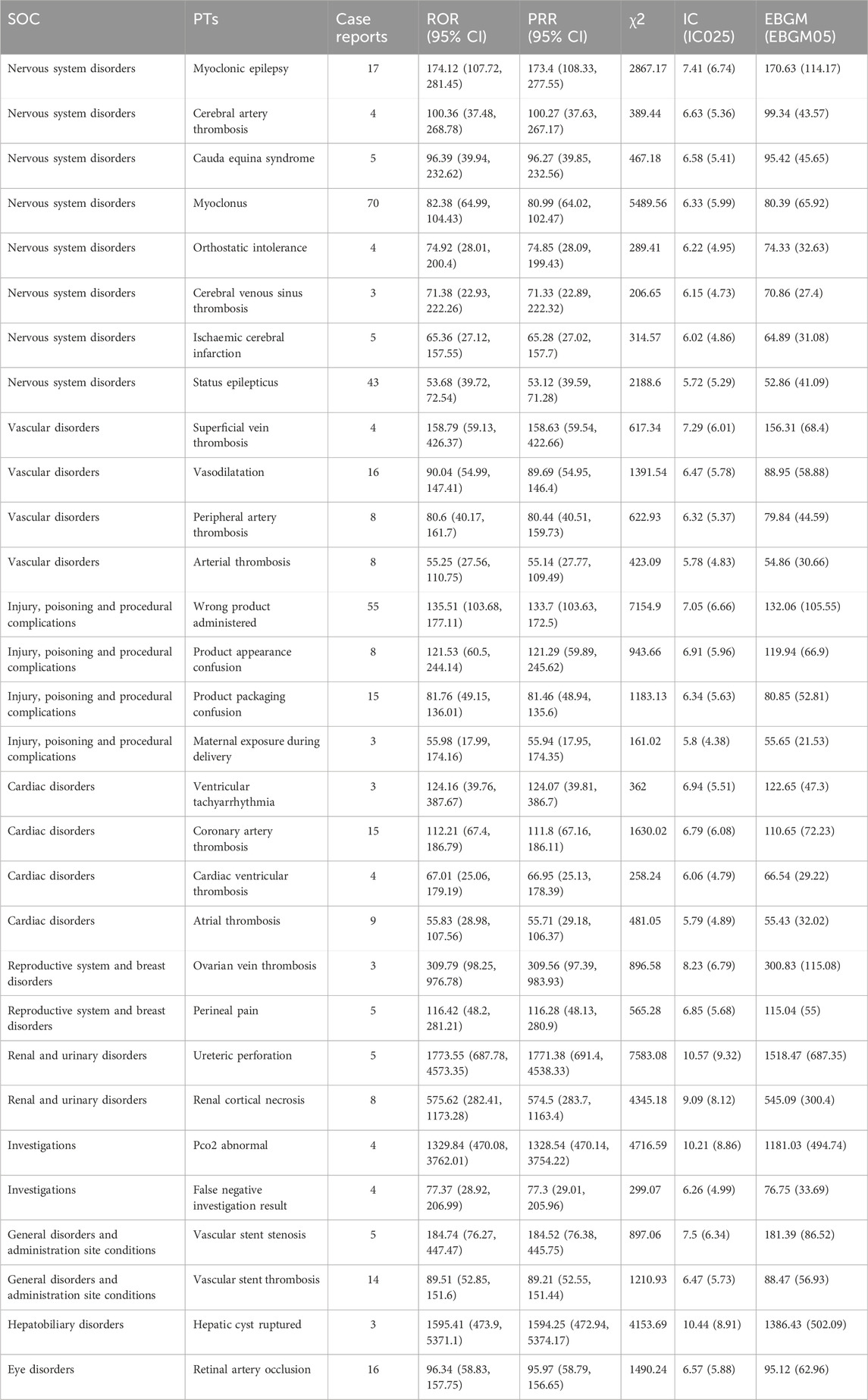
A total of 307 PTs, with the top 30 PTs ranked by the ROR algorithm displayed in Table 3. The results indicated that Ureteric perforation (n = 5, ROR 1773.55, PRR 1771.38, IC 10.57, EBGM 1518.47), Myoclonic epilepsy (n = 17, ROR 174.12, PRR 172.4, IC 7.41, EBGM 170.63), and Superficial vein thrombosis (n = 4, ROR 158.79, PRR 158.63, IC 7.29, EBGM 156.31) were high signal focal points. The most common AEs were Myoclonus (n = 70, ROR 82.38, PRR 80.99, IC 6.33, EBGM 80.39) and Status epilepticus (n = 43, ROR 53.68, PRR 53.12, IC 5.72, EBGM 52.86). In addition to adverse effects mentioned in the product label, this study also discovered rare but high signal intensity adverse events such as Renal cortical necrosis, Hepatic cyst ruptured, and Vascular stent stenosis. Both Retinal artery occlusion and Vascular stent thrombosis disorder were observed with high frequencies and signal intensities. Notably, there were 78 instances of injury, poisoning, and procedural complications attributed to Tranexamic acid, stemming from incidents of wrong product administration, confusion regarding product appearance, and ambiguity in product packaging.
4 Discussion
TXA, among antifibrinolytic agents, has demonstrated efficacy in preventing bleeding complications across various hemostatic challenges while minimizing adverse effects (Hunt, 2015; Chornenki et al., 2019). The landmark WOMAN study illustrated the survival benefits of TXA in postpartum hemorrhage patients (WOMAN Trial Collaborators, 2017). Additionally, a systematic review revealed TXA’s ability to reduce blood loss in surgical patients by nearly one-third compared to placebo (Ker et al., 2013). The CRASH-2 trial further confirmed TXA’s effectiveness in acute traumatic hemorrhage, showing a one-third reduction in mortality when administered within 3 hours of the inciting event (Roberts et al., 2013).
In addition to these extensively researched indications, TXA has shown promising clinical benefits in various other areas. Some emerging fields currently under investigation include gastrointestinal bleeding, subdural and subarachnoid hemorrhage, spontaneous chronic urticaria, chemotherapy-induced thrombocytopenia, and ruptured abdominal aortic aneurysm (Cai et al., 2020). In clinical practice, TXA is widely applied across a spectrum of specialties, including obstetrics (WOMAN Trial Collaborators, 2017), acute trauma (Perel et al., 2013), orthopedic surgery (Elwatidy et al., 2008), cardiothoracic surgery (Myles et al., 2017), dental procedures (Myles et al., 2017), hemoptysis (Márquez-Martín et al., 2010), epistaxis (Zahed et al., 2018), as well as primary and secondary hemostatic disorders (Estcourt et al., 2016).
This study observed a significant increase in AERs related to TXA in recent years, with a notable surge in adverse reactions occurring in 2019. This trend aligns with the rising utilization rate and expanding indications of TXA during this period. Several studies suggest that 2019 may have been a period when the clinical application of TXA became more widely recognized (Ahmadzia et al., 2020; Sterling et al., 2023). TXA was more extensively used in 2019 to treat various types of bleeding symptoms, such as those resulting from surgeries, injuries, or postpartum haemorrhage (Bolliger and Tanaka, 2020; Bharath et al., 2019; CRASH-3 trial collaborators, 2019; Brenner et al., 2019). With the widening scope of medication usage, more patients received treatment, potentially accompanied by less cautious application by healthcare practitioners, thereby resulting in the notable increase in adverse reaction reports.
Additionally, AERs associated with TXA were more commonly reported among female patients compared to male patients. This could be attributed to females being more inclined to report AEs frequently or to the more prevalent use of TXA in treating conditions such as menorrhagia and postpartum hemorrhage among females. Regarding age, the distribution of reports across different age groups was relatively uniform, indicating no significant age bias in TXA adverse reactions. It is noteworthy that the majority of reports were submitted by healthcare professionals, ensuring the professionalism and authenticity of AERs. Since most reports originated from the United States, this may also reflect reporting trends specific to certain regions or cultures, necessitating further investigation to confirm potential regional or cultural biases. Finally, the majority of ADEs occurred within 7 days of medication use, with indications primarily associated with bleeding and menorrhagia, aligning with the pharmacological characteristics of TXA as an indirect fibrinolysis inhibitor and its primary indications.
AEs associated with TXA primarily involve Vascular disorders, Cardiac disorders, Nervous system disorders, Injury, poisoning, and procedural complications, as well as General disorders and administration site conditions. Of particular note, Nervous system disorders are specific to this medication and are not mentioned in the drug’s labeling, warranting further investigation and attention. At the PT level, higher rates of myoclonus, thrombosis, and epilepsy were observed. Additionally, this study identified AEs not documented in the drug’s labeling, such as ureter perforation, renal cortical necrosis, hepatic cyst rupture, and retinal artery occlusion. These AEs all exhibited high signals in quantitative signal detection, indicating a certain level of associated risk with the use of TXA.
TXA is available in both intravenous and oral formulations, acting as an antifibrinolytic agent by blocking lysine binding sites on plasminogen molecules. This action inhibits the interaction between plasminogen and formed plasmin and fibrin, thereby stabilizing the preformed fibrin meshwork generated during secondary hemostasis (McCormack, 2012; Stansfield et al., 2020; Wu et al., 2020). However, this mechanism also presents risks of thrombosis and subsequent vascular and cardiovascular complications, which may be inevitable. Nonetheless, studies suggest that early administration of TXA to trauma patients with significant bleeding or at risk of bleeding can reduce the risk of bleeding-related mortality without a notable increase in fatal or non-fatal thrombotic events. Additionally, tranexamic acid can significantly lower overall mortality (Shakur et al., 2010). Nevertheless, caution should be exercised when using TXA in patients with active intravascular coagulation, active thromboembolic disease, or an imbalance in the hemostatic system favoring thrombus formation, to prevent thrombus formation.
Compared to common AEs within the cardiovascular system, the strong association between TXA and AEs in the nervous system, particularly seizures, is unexpected and has not received widespread attention in drug labeling. There have been reports linking TXA to adverse neurological reactions, primarily occurring in the early postoperative period following cardiac surgery (de Leede-van der Maarl et al., 1999; Keyl et al., 2011; Koster et al., 2013). Several animal studies suggest that TXA’s proconvulsant properties may stem from its direct impact on the central nervous system. Administration of TXA to the cortex or injection into the brain ventricles of experimental animals can induce generalized seizures (Yamaura et al., 1980; Pellegrini et al., 1982; Schlag et al., 2002). Clinical research also supports this notion, indicating that TXA acts as a competitive antagonist of the receptors for glycine and gamma-aminobutyric acid (Furtmüller et al., 2002). Consequently, higher concentrations of TXA in the brain may heighten the risk of seizures. Studies further suggest that isoflurane or propofol could potentially prevent or treat early postoperative TXA-induced seizures (Mohseni et al., 2009; Kaabachi et al., 2011; Lecker et al., 2012), although additional research is warranted to mitigate the occurrence of severe neurological side effects.
Hemorrhagic rupture is a rare but life-threatening complication of simple hepatic cysts, often presenting with acute abdominal pain (Marion et al., 2013; Simon et al., 2015). Reports suggest that the acute administration of antithrombotic drugs in patients with hepatic cysts can exacerbate intraperitoneal hemorrhage and lead to hemodynamic instability (Tong et al., 2019). However, there are currently no other reported studies investigating the use of antifibrinolytic agents such as TXA in causing hepatic cyst rupture. For patients suspected of having hepatic cysts, abnormalities in liver function may accelerate intravascular coagulation and fibrinolysis (O'Leary et al., 2019). Therefore, during episodes of acute bleeding, extra caution should be exercised, with careful consideration given to the risk of hemorrhagic rupture.
TXA has been linked to ureter perforation and renal cortical necrosis, in line with relevant reports (Lee et al., 2022; Maresca et al., 2022). TXA treatment may induce the formation of urinary clots in hematuria patients, leading to secondary ureter perforation and other related adverse reactions, potentially culminating in renal failure (Lee et al., 2022). However, existing evidence has not conclusively demonstrated an increased risk of renal function failure in hematuria patients exposed to TXA. Further research is needed to explore this relationship.
Retinal artery occlusion is also a secondary adverse reaction caused by TXA-induced thrombosis, with limited relevant reports and the underlying mechanism remaining unclear (Parsons et al., 1988; Roumeliotis et al., 2020; Kiser et al., 2021). Healthcare professionals, both medical and non-medical, should be aware of this rare but potential AEs.
Finally, it’s important to note that many AEs result from errors in product administration, confusion in product appearance, and unclear product packaging. Therefore, there should be strengthened pharmaceutical regulation of TXA to prevent adverse reactions from occurring.
While this study offers robust scientific evidence for assessing the safety of TXA from various angles, it does have certain limitations. Primarily, the reliance on spontaneous reporting for data collection introduces potential biases and incomplete information. Consumer-reported data, for example, may not be as reliable or thorough as that provided by medical professionals. Moreover, there could be sampling biases in regions or countries with higher report numbers. Additionally, the data did not account for comorbidities associated with reported events, which could potentially confound any inference of causality between TXA and adverse events. Therefore, despite observing signals, establishing a causal relationship with TXA may prove challenging. Furthermore, limitations of the database, the retrospective nature of the study, and possible reporting biases could impact these findings. To achieve a more comprehensive and precise assessment, future research endeavors could explore employing stricter prospective methodologies, integrating clinical trials with epidemiological studies, thereby enhancing the accuracy of safety risk evaluations associated with TXA.
5 Conclusion
TXA stands as a cornerstone therapeutic agent in the management of hemorrhage across diverse medical specialties. While its efficacy in mitigating bleeding complications is well-established, comprehensive pharmacovigilance is imperative to discern and address potential adverse reactions associated with its administration. This study found that, apart from common adverse reactions, particular attention should be paid to adverse neurological effects, especially seizures. Additionally, caution is advised when administering TXA to patients with impaired liver or kidney function. Healthcare practitioners should be aware of the potential occurrence of rare thrombotic secondary adverse reactions. Lastly, there should be strengthened pharmaceutical regulation of TXA to prevent AEs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
NT: Data curation, Investigation, Methodology, Writing–original draft, Writing–review and editing, Conceptualization, Software, Validation. YS: Investigation, Writing–original draft, Methodology, Conceptualization, Data curation, Software. YL: Conceptualization, Data curation, Investigation, Writing–original draft, Methodology, Software. JJ: Investigation, Methodology, Writing–original draft, Conceptualization. SC: Investigation, Methodology, Writing–original draft. HH: Writing–original draft, Investigation, Methodology. YZ: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing. ZL: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foundation of Jiangsu Provincial Science and Technology Plan Special Fund Key R&D Plan Social Development Project (BE2023787), the Elderly Health Research Project of Jiangsu Commission of Health (LKZ2022008), the Natural Science Foundation of Nanjing University Of Chinese Medicine (XZR2021060), and the Foundation of The Second Affiliated Hospital of Nanjing University of Chinese Medicine (SEZYB2023004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1388138/full#supplementary-material
Abbreviations
ADEs, adverse drug events; AEs, adverse events; AERs, adverse event reports; ASCII, the American Standard Code for Information Interchange; BCPNN, Bayesian confidence propagation neural network; CRASH-2, Clinical Randomization of an Antifibrinolytic in Significant Hemorrhage; EBGM, empirical Bayes geometric mean; FAERS, FDA Adverse Event Reporting System; MedDRA, Medical Dictionary for Regulatory Activities; PRR, proportional reporting ratios; PS, primary suspect; PTs, preferred terms; ROR, reporting odds ratios; SOCs, System Organ Classes; TXA, Tranexamic acid.
References
Ahmadzia, H. K., Hynds, E. B., Amdur, R. L., Gimovsky, A. C., James, A. H., and Luban, N. L. C. (2020). National trends in tranexamic acid use in the peripartum period, 2015-2019. J. Thromb. Thrombolysis 50, 746–752. doi:10.1007/s11239-020-02141-4
American Society of Anesthesiologists Task Force on Perioperative Blood Management (2015). Practice guidelines for perioperative blood management: an updated report by the American society of Anesthesiologists Task Force on perioperative blood management*. Anesthesiology 122, 241–275. doi:10.1097/aln.0000000000000463
Bate, A., Lindquist, M., Edwards, I. R., Olsson, S., Orre, R., Lansner, A., et al. (1998). A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 54, 315–321. doi:10.1007/s002280050466
Bharath, G., Mishra, P. R., and Aggarwal, P. (2019). Tranexamic acid: emerging therapies in hemoptysis. Chest 155, 1303–1304. doi:10.1016/j.chest.2019.02.018
Bolliger, D., and Tanaka, K. A. (2020). Tranexamic acid in vascular surgery: antifibrinolytic or clot-stabilising activity. Br. J. Anaesth. 124, 4–6. doi:10.1016/j.bja.2019.09.034
Brenner, A., Ker, K., Shakur-Still, H., and Roberts, I. (2019). Tranexamic acid for post-partum haemorrhage: what, who and when. Best. Pract. Res. Clin. Obstet. Gynaecol. 61, 66–74. doi:10.1016/j.bpobgyn.2019.04.005
Cai, J., Ribkoff, J., Olson, S., Raghunathan, V., Al-Samkari, H., DeLoughery, T. G., et al. (2020). The many roles of tranexamic acid: an overview of the clinical indications for TXA in medical and surgical patients. Eur. J. Haematol. 104, 79–87. doi:10.1111/ejh.13348
Chornenki, N. L. J., Um, K. J., Mendoza, P. A., Samienezhad, A., Swarup, V., Chai-Adisaksopha, C., et al. (2019). Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: a systematic review and meta-analysis. Thromb. Res. 179, 81–86. doi:10.1016/j.thromres.2019.05.003
CRASH-3 trial collaborators (2019). Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 394, 1713–1723. doi:10.1016/s0140-6736(19)32233-0
de Leede-van der Maarl, M. G., Hilkens, P., and Bosch, F. (1999). The epileptogenic effect of tranexamic acid. J. Neurol. 246, 843. doi:10.1007/s004150050466
Dumouchel, W. (1999). Bayesian data mining in large frequency tables, with an application to the FDA spontaneous reporting system. Am. Statistician 53, 177–190. doi:10.1080/00031305.1999.10474456
Elwatidy, S., Jamjoom, Z., Elgamal, E., Zakaria, A., Turkistani, A., and El-Dawlatly, A. (2008). Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976) 33, 2577–2580. doi:10.1097/BRS.0b013e318188b9c5
Estcourt, L. J., Desborough, M., Brunskill, S. J., Doree, C., Hopewell, S., Murphy, M. F., et al. (2016). Antifibrinolytics (lysine analogues) for the prevention of bleeding in people with haematological disorders. Cochrane Database Syst. Rev. 3, Cd009733. doi:10.1002/14651858.CD009733.pub3
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10, 483–486. doi:10.1002/pds.677
Furtmüller, R., Schlag, M. G., Berger, M., Hopf, R., Huck, S., Sieghart, W., et al. (2002). Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid(A) receptor antagonistic effect. J. Pharmacol. Exp. Ther. 301, 168–173. doi:10.1124/jpet.301.1.168
Hunt, B. J. (2015). The current place of tranexamic acid in the management of bleeding. Anaesthesia 70, 50–53. Suppl 1. doi:10.1111/anae.12910
Kaabachi, O., Eddhif, M., Rais, K., and Zaabar, M. A. (2011). Inadvertent intrathecal injection of tranexamic acid. Saudi J. Anaesth. 5, 90–92. doi:10.4103/1658-354x.76504
Ker, K., Prieto-Merino, D., and Roberts, I. (2013). Systematic review, meta-analysis and meta-regression of the effect of tranexamic acid on surgical blood loss. Br. J. Surg. 100, 1271–1279. doi:10.1002/bjs.9193
Keyl, C., Uhl, R., Beyersdorf, F., Stampf, S., Lehane, C., Wiesenack, C., et al. (2011). High-dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur. J. Cardiothorac. Surg. 39, e114–e121. doi:10.1016/j.ejcts.2010.12.030
Kiser, A. S., Cooper, G. L., Napier, J. D., and Howington, G. T. (2021). Color vision disturbances secondary to oral tranexamic acid. J. Am. Coll. Emerg. Physicians Open 2, e12456. doi:10.1002/emp2.12456
Koster, A., Börgermann, J., Zittermann, A., Lueth, J. U., Gillis-Januszewski, T., and Schirmer, U. (2013). Moderate dosage of tranexamic acid during cardiac surgery with cardiopulmonary bypass and convulsive seizures: incidence and clinical outcome. Br. J. Anaesth. 110, 34–40. doi:10.1093/bja/aes310
Kozek-Langenecker, S. A., Afshari, A., Albaladejo, P., Santullano, C. A. A., De Robertis, E., Filipescu, D. C., et al. (2013). Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. 30, 270–382. doi:10.1097/EJA.0b013e32835f4d5b
Lecker, I., Wang, D. S., Romaschin, A. D., Peterson, M., Mazer, C. D., and Orser, B. A. (2012). Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J. Clin. Invest. 122, 4654–4666. doi:10.1172/jci63375
Lee, S. G., Fralick, J., Wallis, C. J. D., Boctor, M., Sholzberg, M., and Fralick, M. (2022). Systematic review of hematuria and acute renal failure with tranexamic acid. Eur. J. Haematol. 108, 510–517. doi:10.1111/ejh.13762
Maresca, G., Royle, J., and Donaldson, J. F. (2022). Tranexamic acid-induced ureteric clot obstruction in a patient with urothelial cell carcinoma resulting in upper urinary tract perforation. BMJ Case Rep. 15, e247334. doi:10.1136/bcr-2021-247334
Marion, Y., Brevart, C., Plard, L., and Chiche, L. (2013). Hemorrhagic liver cyst rupture: an unusual life-threatening complication of hepatic cyst and literature review. Ann. Hepatology 12, 336–339. doi:10.1016/S1665-2681(19)31375-4
Márquez-Martín, E., Vergara, D. G., Martín-Juan, J., Flacón, A. R., Lopez-Campos, J. L., and Rodríguez-Panadero, F. (2010). Endobronchial administration of tranexamic Acid for controlling pulmonary bleeding: a pilot study. J. Bronchology Interv. Pulmonol. 17, 122–125. doi:10.1097/LBR.0b013e3181dc8c17
McCormack, P. L. (2012). Tranexamic acid: a review of its use in the treatment of hyperfibrinolysis. Drugs 72, 585–617. doi:10.2165/11209070-000000000-00000
Mohseni, K., Jafari, A., Nobahar, M. R., and Arami, A. (2009). Polymyoclonus seizure resulting from accidental injection of tranexamic acid in spinal anesthesia. Anesth. Analg. 108, 1984–1986. doi:10.1213/ane.0b013e3181a04d69
Myles, P. S., Smith, J. A., Forbes, A., Silbert, B., Jayarajah, M., Painter, T., et al. (2017). Tranexamic acid in patients undergoing coronary-artery surgery. N. Engl. J. Med. 376, 136–148. doi:10.1056/NEJMoa1606424
O’Leary, J. G., Greenberg, C. S., Patton, H. M., and Caldwell, S. H. (2019). AGA clinical practice update: coagulation in cirrhosis. Gastroenterology 157, 34–43.e31. doi:10.1053/j.gastro.2019.03.070
Ortmann, E., Besser, M. W., and Klein, A. A. (2013). Antifibrinolytic agents in current anaesthetic practice. Br. J. Anaesth. 111, 549–563. doi:10.1093/bja/aet154
Parsons, M. R., Merritt, D. R., and Ramsay, R. C. (1988). Retinal artery occlusion associated with tranexamic acid therapy. Am. J. Ophthalmol. 105, 688–689. doi:10.1016/0002-9394(88)90069-4
Pellegrini, A., Giaretta, D., Chemello, R., Zanotto, L., and Testa, G. (1982). Feline generalized epilepsy induced by tranexamic acid (AMCA). Epilepsia 23, 35–45. doi:10.1111/j.1528-1157.1982.tb05051.x
Perel, P., Ker, K., Morales Uribe, C. H., and Roberts, I. (2013). Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database Syst. Rev. 2013, Cd010245. doi:10.1002/14651858.CD010245.pub2
Roberts, I., Shakur, H., Coats, T., Hunt, B., Balogun, E., Barnetson, L., et al. (2013). The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol. Assess. 17, 1–79. doi:10.3310/hta17100
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13, 519–523. doi:10.1002/pds.1001
Roumeliotis, G., Campbell, S., Das, S., Hildebrand, G. D., Charbel Issa, P., Jayamohan, J., et al. (2020). Central retinal artery occlusion following prone transcranial surgery for craniosynostosis and discussion of risk factors. J. Craniofac Surg. 31, 1597–1601. doi:10.1097/scs.0000000000006512
Schlag, M. G., Hopf, R., Zifko, U., and Redl, H. (2002). Epileptic seizures following cortical application of fibrin sealants containing tranexamic acid in rats. Acta Neurochir. (Wien) 144, 63–69. doi:10.1007/s701-002-8275-z
Shakur, H., Roberts, I., Bautista, R., Caballero, J., Coats, T., Dewan, T., et al. (2010). Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 376, 23–32. doi:10.1016/s0140-6736(10)60835-5
Simon, T., Bakker, I. S., Penninga, L., and Nellensteijn, D. R. (2015). Haemorrhagic rupture of hepatic simple cysts. BMJ Case Rep. 2015, bcr2014208676. doi:10.1136/bcr-2014-208676
Sixty-third World Health Assembly (2010). WHA63.12. Availability, safety and quality of blood products. Available at: http://apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R12-en.pdf.
Stansfield, R., Morris, D., and Jesulola, E. (2020). The use of tranexamic acid (TXA) for the management of hemorrhage in trauma patients in the prehospital environment: literature review and descriptive analysis of principal themes. Shock 53, 277–283. doi:10.1097/shk.0000000000001389
Sterling, E. K., Litman, E. A., Dazelle, W. D. H., and Ahmadzia, H. K. (2023). An update to tranexamic acid trends during the peripartum period in the United States, 2019 to 2021. Am. J. Obstet. Gynecol. MFM 5, 100933. doi:10.1016/j.ajogmf.2023.100933
Tengborn, L., Blombäck, M., and Berntorp, E. (2015). Tranexamic acid--an old drug still going strong and making a revival. Thromb. Res. 135, 231–242. doi:10.1016/j.thromres.2014.11.012
Tong, K. S., Hassan, R., Gan, J., and Warsi, A. (2019). Simple hepatic cyst rupture exacerbated by anticoagulation. BMJ Case Rep. 12, e230243. doi:10.1136/bcr-2019-230243
WOMAN Trial Collaborators (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet 389, 2105–2116. doi:10.1016/s0140-6736(17)30638-4
Wu, T. B., Orfeo, T., Moore, H. B., Sumislawski, J. J., Cohen, M. J., and Petzold, L. R. (2020). Computational model of tranexamic acid on urokinase mediated fibrinolysis. PLoS One 15, e0233640. doi:10.1371/journal.pone.0233640
Yamaura, A., Nakamura, T., Makino, H., and Hagihara, Y. (1980). Cerebral complication of antifibrinolytic therapy in the treatment of ruptured intracranial aneurysm Animal experiment and a review of literature. Eur. Neurol. 19, 77–84. doi:10.1159/000115131
Keywords: tranexamic acid, FAERS, ADE, pharmacovigilance, drug discovery
Citation: Tian N, Sun Y, Liu Y, Jin J, Chen S, Han H, Zhang Y and Li Z (2024) Safety assessment of tranexamic acid: real-world adverse event analysis from the FAERS database. Front. Pharmacol. 15:1388138. doi: 10.3389/fphar.2024.1388138
Received: 19 February 2024; Accepted: 13 May 2024;
Published: 28 May 2024.
Edited by:
Kenichi Tanaka, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Reney Henderson, University of Maryland, United StatesKenneth Stewart, University of Oklahoma, United States
Copyright © 2024 Tian, Sun, Liu, Jin, Chen, Han, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwei Li, ZnN5eTAwNzk3QG5qdWNtLmVkdS5jbg==; Ying Zhang, emhhbmd5aW5nX2xvdmV5b3VAMTYzLmNvbQ==
‡ORCID: Zhiwei Li, orcid.org/0009-0005-6044-189X
†These authors have contributed equally to this work
 Ningsheng Tian
Ningsheng Tian Yuxin Sun†
Yuxin Sun†