
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 July 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1378140
This article is part of the Research Topic Real-World Evidence of Natural Products, Herbal Medicines, and Traditional Medicine Treatments Volume II View all 15 articles
Background: Within-day glycemic variability (GV), characterized by frequent and significant fluctuations in blood glucose levels, is a growing concern in hospitalized patients with type 2 diabetes mellitus (T2DM). It is associated with an increased risk of hypoglycemia and potentially higher long-term mortality rates. Robust clinical evidence is needed to determine whether traditional Chinese medicine (TCM) decoctions can be a beneficial addition to the management of within-day GV in this patient population.
Methods: This retrospective cohort study utilized data from adult inpatients diagnosed with T2DM admitted to the Traditional Chinese Medicine Hospital of Kaifeng. The primary outcome investigated was the association between the use of TCM decoctions and improved stability of within-day GV. Blood glucose variability was assessed using the standard deviation of blood glucose values (SDBG). For each patient, the total number of hospitalization days with SDBG below 2 mmol/L was calculated to represent within-day GV stability. Hospitalization duration served as the secondary outcome, compared between patients receiving TCM decoctions and those who did not. The primary analysis employed a multivariable logistic regression model, with propensity score matching to account for potential confounding variables.
Results: A total of 1,360 patients were included in the final analysis. The use of TCM decoctions was significantly associated with enhanced stability of within-day GV (OR = 1.77, 95% CI: 1.34–2.33, P < 0.01). This association was most prominent in patients with a diagnosis of deficiency syndrome (predominantly qi-yin deficiency, accounting for 74.8% of cases) and a disease duration of less than 5 years (OR = 2.28, 95% CI: 1.21–4.29, P = 0.03). However, TCM decoctions did not exert a statistically significant effect on hospitalization duration among patients with T2DM (OR = 0.96, 95% CI: 0.91–1.01, P = 0.22).
Conclusion: This study suggests that TCM decoctions may be effective in improving within-day GV stability in hospitalized patients with T2DM. This effect appears to be most pronounced in patients diagnosed with deficiency syndrome, particularly those with qi-yin deficiency and a shorter disease course. Further investigation is warranted to confirm these findings and elucidate the underlying mechanisms.
While glycated hemoglobin (HbA1c) has served as the gold standard for assessing glycemic control in patients with type 2 diabetes, its sole reliance for this purpose may be inadequate (Hirsch, 2015). This limitation stems from the inherent nature of HbA1c, which reflects average blood glucose levels over the preceding 2–3 months, failing to capture daily acute fluctuations or hypoglycemic episodes. Consequently, patients with T2D can exhibit significant glycemic variability (GV) even when achieving target HbA1c levels (Kovatchev and Cobelli, 2016; Dandona, 2017). GV, characterized by short-term oscillations in plasma glucose, typically refers to fluctuations within a 24-h window, known as within-day GV (Julla et al., 2021). Frequent and extensive within-day GV independently increases the risk of T2D inpatients experiencing hypoglycemia (Kauffmann et al., 2011; Monnier et al., 2011; Bajaj et al., 2017) and cardiovascular complications (Monnier et al., 2006; Saisho, 2014; Nusca et al., 2018; Scott et al., 2020). This association has further been linked to prolonged hospital stays and increased long-term mortality rates (Mendez et al., 2013; Akirov et al., 2017; Timmons et al., 2017; Akirov et al., 2019; Jordán-Domingo et al., 2021). The growing recognition of stable within-day GV’s importance in T2D management highlights its potential as a novel target for glycemic control therapy.
Traditional Chinese medicinal (TCM) decoction therapy is widely used in diabetes management due to its perceived gentle effects and reported glucose-lowering efficacy (Hu and Jia, 2019). However, robust clinical evidence regarding the effectiveness of TCM decoctions in specifically reducing within-day glycemic variability (GV) and promoting glycemic stability remains limited. This study aimed to investigate the association between the administration of oral TCM decoctions and the maintenance of stable within-day GV in hospitalized patients diagnosed with type 2 diabetes mellitus (T2DM). The primary objective was to compare the impact of TCM decoctions on within-day GV stability with that of conventional antidiabetic Western medications in this patient population. Besides, we sought to evaluate the potential benefits of TCM decoctions in reducing the duration of hospitalization.
This retrospective cohort study utilized the electronic medical record (EMR) database of the Department of Endocrinology at Kaifeng Traditional Chinese Medicine Hospital in Henan Province, China. The database encompassed demographic information, vital signs, past and current diagnoses, clinical symptoms, laboratory results, medication administration details (including both traditional Chinese medicine and Western medications), and self-monitoring of blood glucose (SMBG) data for 12,664 patients admitted between 1 January, 2017, and 16 June 2021. The datasets were linked using unique patient identifiers.
The study cohort comprised hospitalized adult patients (aged ≥ 18 years) diagnosed with type 2 diabetes mellitus. Patients were categorized into two groups: those receiving TCM decoctions (TCM treatment group) and those receiving antidiabetic Western medications (non-TCM treatment group). Medical records with admission diagnoses other than T2DM or its complications were excluded. Additionally, patients whose TCM diagnosis did not correspond to the term “XiaoKe” (representing T2DM in TCM theory) were excluded. To minimize the influence of repeated hospitalizations, patients with multiple admissions within the study timeframe were excluded if the interval between admissions was less than 1 year. Admissions exceeding 1 year from the previous admission were considered new admissions. Patients receiving both TCM decoctions and antidiabetic Western medications concurrently were also excluded. To ensure adequate exposure to TCM decoction treatment, patients with a hospitalization duration of less than 7 days and receiving TCM decoctions fewer than seven times were excluded. The study baseline was defined as 24 h after patient admission. All laboratory tests were performed on fasting blood samples collected within 24 h of admission. The second-day SMBG data served as the entry point for patients into the cohort. The final analysis included 1,361 patients (Figure 1).
This study was conducted in accordance with ethical principles governing real-world research and non-interventional studies. All data pertaining to research participants received approval from the Ethics Committee of Kaifeng Traditional Chinese Medicine Hospital approval number: 2022-ky-006.
In our analysis, we adjusted for potential confounding variables including age, gender, duration of diabetes, body mass index (BMI), baseline HbA1c, hypertension, LDL cholesterol, fasting insulin levels, C-peptide, average fasting plasma glucose (FPG) during hospitalization, comorbidities, and TCM syndrome. Diabetes duration was categorized into four groups: 0 to less than 3 years, 3 to less than 5 years, 5 to less than 10 years, and 10 or more years. Following the consensus of Chinese experts in medical nutrition therapy for overweight/obesity (CecCooOMN, 2016), BMI was divided into four categories: less than or equal to 18 kg/m2, 18–23.9 kg/m2, 24–27.9 kg/m2, and greater than or equal to 28 kg/m2. LDL cholesterol, fasting insulin level, and C-peptide were also categorized. LDL was classified as either less than 2.6 mmol/L or greater than or equal to 2.6 mmol/L. Fasting insulin levels were grouped as less than 10 μU/mL, 10–15 μU/mL, and greater than 15 μU/mL. C-peptide levels were categorized as less than 1.71 ng/mL, 1.71–2.51 ng/mL, and greater than or equal to 2.51 ng/mL. We considered 13 common comorbidities associated with diabetes, including liver diseases, hypertension, hyperlipidemia, osteoarthropathy, coronary atherosclerosis, chronic kidney disease, cerebral infarction, ischemic cerebrovascular disease, ischemic heart disease, diabetic retinopathy, diabetic macrovascular disease, diabetic polyneuropathy, and diabetic peripheral vascular disease. Finally, TCM syndromes were categorized into four groups: deficiency syndromes, phlegm syndromes, liver stagnation and spleen deficiency syndrome, and dampness-heat syndrome.
All patients in the TCM treatment group received decoctions according to the TCM treatment modalities for Type 2 diabetes as practiced at Kaifeng Traditional Chinese Medicine Hospital (Pang et al., 2019). The primary decoctions administered included: 1) Qingre Yangyin Tiaotang decoction; 2) Yiqi Yangyin Tiaotang Decoction; 3) Shugan Jianpi Tiaotang decoction; 4) Hezhong Jiangzhuo tiaotang decoction; 5) Qingre Huashi Tiaotang decoction; and 6) Jianpi Yishen Tiaotang decoction (details of the composition of each decoction are provided in Supplementary Table S1). Additional classical TCM decoctions, such as Shenqi Dihuang Tang, were also considered (complete description of classical TCM decoctions summarized in Supplementary Table S1).
The selection and adjustment of these decoctions were carried out by experienced TCM practitioners, adhering to the principles of TCM syndrome differentiation, tailored to the individual symptoms of each patient. Each participant received 400 mL of the decoction daily, divided into two doses of 200 mL each, taken in the morning and evening. The duration of treatment was no less than seven consecutive days. Patients assigned to the TCM group were permitted to receive usual care medications; however, the concurrent use of any Western medications or Commercial Chinese Polyherbal Preparation with known glucose-lowering effects was strictly prohibited.
The non-TCM treatment group received standard Western medical treatment for hyperglycemia, as outlined in the Chinese guidelines for the prevention and treatment of type 2 diabetes (Jia et al., 2019). These therapies included oral anti-hyperglycemic agents (Thiazolidinediones, Glucagon-like peptide1 agonist, Dipeptidyl peptidase-4 inhibitors, Glinide, SGLT2 inhibitors, Sulfonylurea, α-glucosidase inhibitors, and Metformin) and insulin, with the specific medication regimen selected by attending physicians based on each patient’s individual clinical presentation and adherence to the routine diabetes management protocol for inpatients at Kaifeng Traditional Chinese Medicine Hospital. Treatment selection for the control group was not prescriptive, allowing for flexibility based on individual needs.
The primary outcome of interest was the stability of within-day GV. This was assessed through the standard deviation of blood glucose values (SDBG) measured by SMBG seven times a day (fasting, pre-breakfast, pre-lunch, post-lunch, pre-dinner, post-dinner, and bedtime). Following the expert consensus on diabetes mellitus glycemic variability management (CSo, 2017), a threshold of SDBG measured by SMBG less than 2 mmol/L was considered indicative of normal within-day GV for Chinese patients with diabetes. To quantify the stability of within-day GV, we calculated a score for each patient. This score reflected the proportion of hospitalization days with SDBG below 2 mmol/L. We achieved this by dividing the total number of days with SDBG below 2 mmol/L by the total number of hospitalization days and multiplying by 100. For example, consider a patient with a sequence of SDBG values: 6, 5, 0.8, 2.3, 2.6, 1.5, 1.6, 0.9, 3.6, 2.3, 2.2, and 2.7 mmol/L. If the total length of stay was 12 days and the number of days with SDBG below 2 mmol/L was four, the score would be 33 [i.e., (100 × 4)/12]. For analysis, scores were categorized into four groups: 0–20, 21–40, 41–60, and ≥ 61. For sensitivity analyses, we considered the postprandial glucose excursion (PPGE) and largest amplitude of glycemic excursions (LAGE) as additional assessment indicators for within-day GV stability (CSo, 2017). Similarly, we calculated the total number of days within the expected range for PPGE and LAGE as a measure of within-day GV stability. The secondary outcome was length of hospital stay.
Associations between treatment modality and patient demographic, clinical, and other baseline characteristics were assessed using a t-test for continuous variables and chi-square tests for categorical variables.
To investigate the association between the use of TCM decoctions and the stability of within-day GV, we employed a multivariable logistic regression model. Similarly, a Poisson regression model was used to assess the relationship between hospital stay duration and TCM decoction use. Our initial multivariable logistic regression and Poisson regression models included all potential covariates identified for the study. However, to account for potential confounding variables due to the non-randomized nature of TCM decoction administration, we implemented propensity score matching techniques. Prior to propensity score matching, we conducted a univariate analysis of all potentially explanatory factors for both the primary (within-day GV stability) and secondary (hospital stay duration) outcomes. This analysis identified 15 factors significantly associated with the primary outcome and 7 factors associated with the secondary outcome (all p-values < 0.05, detailed in Supplementary Table S2). We then utilized a logistic regression model to estimate a propensity score for each patient. This score represented the predicted probability of receiving TCM decoctions, and it was based on the 15 significant factors identified for the primary outcome (7 factors for the secondary outcome). Finally, we employed three propensity score matching methods within separate multivariable logistic regression and Poisson regression models to estimate the association between TCM decoction use and within-day GV stability and hospital stay duration, respectively.
The primary analysis employed propensity score matching to address potential confounding due to the non-randomized treatment assignment. Patients receiving TCM decoctions were matched in a 1:1 ratio to those receiving antidiabetic drugs based on their predicted probabilities of receiving TCM decoctions derived from the propensity score model. A greedy nearest neighbor matching algorithm was used with a maximum caliper width of ±1%, ensuring a close match between patients in the TCM and non-TCM groups on all measured covariates. Logistic regression and Poisson regression models were then performed using the propensity score-matched cohort data to estimate the association between TCM decoction use and the primary and secondary outcomes, respectively.
To further explore the robustness of our findings, we performed two additional prespecified sensitivity analyses for the primary outcome, investigating within-day glycemic variability stability. The first analysis employed inverse probability of treatment weighting (IPTW) on the propensity score. The second analysis incorporated the propensity score itself as an additional covariate in the regression model. Furthermore, we conducted sensitivity analyses to assess the potential influence of missing data. Here, we defined within-day GV stability using the standard deviation of %CVw values during hospitalization and utilized a complete data set for analysis.
We conducted prespecified subgroup analyses to explore the impact of TCM decoctions on within-day glycemic variability stability within specific patient populations. Patients in the TCM and non-TCM treatment groups were matched based on three criteria: TCM syndrome (deficiency syndrome vs. phlegm syndromes/liver stagnation and spleen deficiency syndrome), diabetes duration (≤5 years vs. >5 years), and the logit of the propensity score. Within each of the four resulting subgroups, a multivariable logistic regression model was used to compare the risk reduction for the primary outcome (reduced within-day GV instability) between the TCM and non-TCM treatment groups.
To address missing covariate data and minimize selection bias, we employed multivariate imputation by chained equations. This approach estimates missing values under the assumption that data are missing at random. Prior to imputation, a missing pattern analysis confirmed the presence of only monotonic missing patterns (i.e., missing data only occurred for subsequent variables in a sequence). Five covariates (BMI, LDL cholesterol, fasting insulin, C-peptide, and duration of diabetes) contained missing data, ranging from 0% to 14% across variables. Notably, 65% of patients had complete data for all covariates. To account for imputation uncertainty, five separate imputed datasets were generated for analysis. The results from each imputed dataset were subsequently pooled to estimate regression parameters.
Statistical analyses were performed using R software version 4.3.1. A two-sided p-value less than 0.05 was considered statistically significant. For the propensity score matched cohort, a standardized difference of less than 0.1 between the TCM decoction and control groups indicated a good balance on all types of distributional characteristics between the two groups.
A total of 1,360 patients were included in the final analysis (Figure 1). The distribution of patients across the within-day glycemic variability (GV) stability score categories was as follows: 0–20 (n = 474), 21–40 (n = 398), 41–60 (n = 267), and ≥ 61 (n = 221).
Table 1 summarizes the baseline characteristics of the study participants before propensity score matching, categorized by exposure to TCM decoctions. Among the 1,360 patients, 388 (28.5%) received TCM decoctions, while the remaining 972 (71.5%) did not. In the unmatched sample, statistically significant differences in exposure to TCM decoctions were observed based on age, sex, BMI, duration of diabetes, HbA1c, FPG, fasting insulin levels, C-peptide levels, TCM syndrome diagnosis, and the presence of several comorbidities. Notably, 53.1% of patients treated with TCM decoctions had a diabetes duration of less than 3 years, while over 60% of patients who did not receive TCM decoctions had a duration of 5–10 years or longer. Additionally, patients receiving TCM decoctions exhibited lower baseline HbA1c levels compared to those who did not [median: 7.5% (9.4 mmol/L) vs. 9.5% (12.6 mmol/L)].
Following propensity score matching, a total of 282 matched patient pairs were obtained (Table 2). Importantly, there were no significant differences (p > 0.30 for all comparisons) between the TCM decoction and non-TCM decoction groups across any of the 17 baseline characteristics. Additionally, the standardized mean differences between the two groups were all less than 0.1, indicating successful covariate balancing through propensity score matching.
Overall, patients who received TCM decoctions exhibited greater stability of within-day glycemic variability compared to those who did not. This association was statistically significant in both unadjusted and adjusted analyses. In the unadjusted analysis, the odds ratio (OR) for improved within-day GV stability with TCM decoction use was 3.15 [95% confidence interval (CI): 2.62 to 3.78; p < 0.01; Table 3]. This finding remained significant after adjusting for potential confounding variables using propensity score matching (OR: 1.77; 95% CI: 1.34 to 2.33; p < 0.01; Table 3). Similar statistically significant results were obtained in both the adjusted analysis (OR: 1.77; 95% CI: 1.58 to 1.98; p < 0.001; Table 3) and the inverse probability weighted analysis (OR: 1.63; 95% CI: 1.32 to 2.04; p < 0.001; Table 3) based on propensity score. The observed association between TCM decoctions and improved within-day GV stability remained consistent when using postprandial glucose excursion (PPGE) and largest amplitude of glycemic excursions (LAGE) as alternative assessment indicators. The results of the sensitivity analysis are in Supplementary Table S3. Additionally, the analysis of complete cases excluding missing data yielded comparable results (Supplementary Table S3). Subgroup analyses by TCM syndrome and diabetes duration categories revealed a significant benefit for patients diagnosed with deficiency syndromes (predominantly deficiency of qi-yin, accounting for 74.8%) and a diabetes duration of less than 5 years (OR: 2.28; 95% CI: 1.21 to 4.29; p = 0.03; Table 4). However, no statistically significant benefits were observed in the other three subgroups (Table 4).
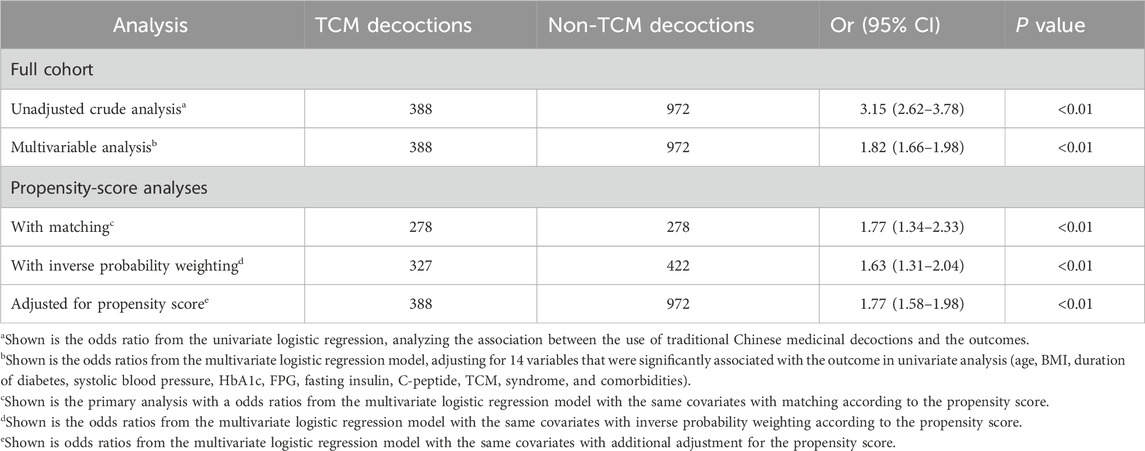
Table 3. Associations between TCM decoction use and the stability of within-day GV in the crude analysis, multivariable analysis, and propensity-score analyses.
In the unadjusted analysis, use of TCM decoctions was associated with a statistically significant reduction in hospital length of stay [odds ratio (OR), 0.93; 95% confidence interval (CI), 0.90 to 0.96; p < 0.001; Table 5]. However, this association was not robust, as it was no longer statistically significant after adjusting for confounding variables using propensity score matching (OR, 0.96; 95% CI, 0.91 to 1.01; p = 0.22; Table 5) or other methods.
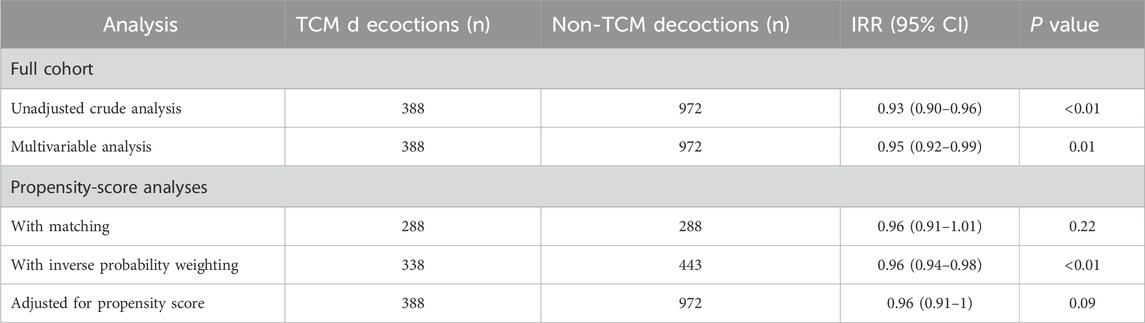
Table 5. Associations between TCM decoctions use and the length of stay in hospital in the crude analysis, multivariable analysis, and propensity-score analyses.
This propensity score-matched cohort study provides valuable insights for clinicians in considering the potential effectiveness of TCM decoctions for maintaining within-day glycemic variability (GV) stability. Our findings suggest an association between TCM decoction use and improved within-day GV stability in hospitalized patients, which persisted after adjusting for potential confounding variables. This association was further corroborated through comprehensive sensitivity analyses. Furthermore, the study revealed that TCM decoction treatment may be most beneficial for patients diagnosed with deficiency syndromes, particularly those with a predominant deficiency of Qi-yin (comprising 74.8% of this subgroup) and a diabetes duration of less than 5 years. Notably, while a small reduction in hospital length of stay was observed among patients receiving TCM decoctions, this effect became statistically non-significant after propensity score matching.
Our study facilitated a more robust investigation into the potential association between TCM decoctions and the stability of within-day GV. The findings hold promise for future clinical practice considerations in T2D management. Strict glycemic control in hospitalized settings, while necessary to minimize hyperglycemia’s detrimental effects in uncontrolled T2D patients (Nusca et al., 2018), can lead to rapid reductions in overall blood glucose levels, potentially increasing within-day GV (Braithwaite, 2013; Akirov et al., 2018). Frequent short-term fluctuations in blood glucose can have a significant impact on various physiological functions. Short-term glucose fluctuations, through the excessive production of reactive oxygen species (ROS), reactive nitrogen species (RNS), inflammatory cytokines, and oxidative stress, can ultimately lead to β-cell apoptosis and a decline in β-cell function (Kohnert et al., 2012), accelerating the deterioration of glycemic control. Additionally, evidence suggests that short-term acute blood glucose fluctuations may induce a greater degree of oxidative stress compared to chronic sustained hyperglycemia, potentially contributing to the development of diabetes complications (Monnier et al., 2006; Ceriello et al., 2008). Individuals with T2D experiencing excessive within-day GV are also at an increased risk of hypoglycemia (Murata et al., 2004; Monnier et al., 2011), lower quality of life and negative moods (Penckofer et al., 2012). Given the significant impact of these adverse effects on T2D management, GV emerges as a crucial target for glycemic control interventions. However, a growing body of research suggests that traditional antidiabetic pharmacotherapies, including basal insulin and intensive treatment strategies, may not necessarily improve GV stability despite achieving glycemic targets (Gerstein et al., 2008; Duckworth et al., 2009; Zenari and Marangoni, 2013). In contrast, our research findings suggest that TCM decoction treatment might offer a unique opportunity to maintain more stable GV, providing a potential approach for future clinical consideration.
The precise mechanisms by which TCM decoctions improve within-day GV stability remain largely unclear. Some studies suggest that TCM may exert regulatory effects that correct internal environment imbalances caused by various pathogenic factors, enabling the body to restore homeostasis more promptly and potentially contribute to reduced glycemic fluctuations (Liu et al., 2015). Our study observed that patients with a shorter disease duration (less than 5 years) and a diagnosis of deficiency syndrome, particularly Qi-Yin deficiency (comprising 74.8% of this subgroup), exhibited greater stability in within-day GV. From the perspective of traditional Chinese medicine (TCM), blood glucose is considered a nutritional substance produced by the transport and transformation function of spleen-stomach (Ke et al., 2022). Therefore, spleen and stomach Qi deficiency is seen as a basic TCM pathogenesis for GV in diabetic patients (Ke et al., 2022). Li, (2023) research on the correlation between symptom scores of Qi deficiency syndrome and blood glucose fluctuations in type 2 diabetes patients revealed a positive correlation between Qi deficiency and within-day GV. Similarly, Zhang et al. (2024) found that patients with severe Yin deficiency exhibited greater glycemic fluctuations. Zheng, (2023) study further corroborated these findings, showing that patients with deficiency syndromes, particularly those with both Qi and Yin deficiencies, experienced significant within-day GV. Additionally, a clinical study by Pang et al. indicated that patients with Qi and Yin deficiency who received TCM treatment aimed at replenishing Qi and nourishing Yin exhibited the least within-day GV (Pang and Sun, 2017). These findings collectively suggest a significant positive correlation between the severity of Qi and Yin deficiencies and the stability of glycemic levels. Correcting the states of Qi and Yin deficiencies through TCM treatment could therefore be effective in maintaining stable glycemic levels in type 2 diabetes patients. Furthermore, patients with a shorter disease duration, whose islet function is not yet severely compromised, tend to have relatively stable glycemic levels, and may experience more pronounced benefits from TCM treatments (Zhang et al., 2015). However, limited clinical research currently exists regarding the efficacy of TCM in specifically reducing glycemic fluctuations, and the underlying mechanisms are yet to be fully elucidated. Additionally, no prior studies have definitively identified superior glycemic stability with TCM interventions in specific patient populations.
Our analysis revealed a small reduction in the length of hospital stay for patients receiving TCM decoctions. However, this finding did not reach statistical significance. Furthermore, propensity score matching, which aimed to account for potential confounding variables, resulted in the loss of this observed association. Therefore, our results should be interpreted with caution and further research is warranted to validate this potential benefit of TCM decoctions on hospital stay duration.
This study possesses several key strengths. First, we leveraged a population-based cohort exclusively receiving TCM decoctions. This unique design allowed for a direct comparison of outcomes between patients receiving TCM decoctions and those receiving concurrent antidiabetic Western medications (controls) using a rigorous propensity score matching approach. Furthermore, we employed a series of analyses utilizing various propensity score methods to enhance the robustness and reliability of our findings. The consistency observed across these multiple sensitivity analyses strengthens our confidence in the results.
Our study has several limitations that should be acknowledged. First, the lack of access to a larger population dataset restricted our ability to subdivide the control group into categories based on specific Western medications. This limited our capacity to directly compare the outcomes of traditional Chinese medicine decoctions with a particular anti-diabetic medication. Second, the absence of continuous glucose monitoring (CGM) data is a noteworthy limitation. CGM data provides minute-by-minute glycemic fluctuations, offering a more precise metric for assessing glycemic variability compared to the SMBG employed in this study. Additionally, limitations include missing data for certain variables and the potential for inaccuracies in electronic health records. We have addressed missing data to minimize bias, but this remains a potential source of error. Finally, the single-center observational design may limit the generalizability of our results. Future studies with larger cohorts, extended follow-up periods, and the incorporation of CGM data are necessary to further elucidate the relationship between TCM decoction therapy and enhanced GV stability. These studies would strengthen the evidence for the potential of TCM interventions to achieve better glycemic maintenance and stability in hospitalized type 2 diabetes patients. Additionally, further research is needed to understand the mechanisms underlying these potential associations.
In conclusion, our study demonstrates that, among hospitalized patients diagnosed with type 2 diabetes, TCM decoctions are more efficacious in maintaining stable within-day GV compared to antidiabetic medications derived from Western medicine. This beneficial effect is particularly pronounced in patients presenting with deficiency syndrome and a disease course of less than 5 years. However, it is important to note that our findings do not support a reduction in hospitalization duration associated with TCM decoction therapy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Kaifeng Traditional Chinese Medicine Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YX: Data curation, Investigation, Methodology, Software, Writing–original draft, Writing–review and editing. PL: Data curation, Resources, Writing–review and editing. GP: Data curation, Methodology, Resources, Supervision, Writing–review and editing. HZ: Funding acquisition, Resources, Writing–review and editing. TW: Formal Analysis, Funding acquisition, Methodology, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82374624), Beijing Natural Science Foundation (7232306), Key Research and Development Program of Ministry of Science and Technology (2017YFC1703501), Major Special Projects of Ministry of Science and Technology (2012ZX10005009), and Science and Technology Innovation Project of China Academy of Chinese Medical Sciences (CI2021A05501).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1378140/full#supplementary-material
Akirov, A., Amitai, O., Masri-Iraqi, H., Diker-Cohen, T., Shochat, T., Eizenberg, Y., et al. (2018). Predictors of hypoglycemia in hospitalized patients with diabetes mellitus. Intern Emerg. Med. 13 (3), 343–350. doi:10.1007/s11739-018-1787-0
Akirov, A., Diker-Cohen, T., Masri-Iraqi, H., and Shimon, I. (2017). High glucose variability increases mortality risk in hospitalized patients. J. Clin. Endocrinol. Metab. 102 (7), 2230–2241. doi:10.1210/jc.2017-00450
Akirov, A., Shochat, T., Dotan, I., Diker-Cohen, T., Gorshtein, A., and Shimon, I. (2019). Glycemic variability and mortality in patients hospitalized in general surgery wards. Surgery 166 (2), 184–192. doi:10.1016/j.surg.2019.02.022
Bajaj, H. S., Venn, K., Ye, C., Patrick, A., Kalra, S., Khandwala, H., et al. (2017). Lowest glucose variability and hypoglycemia are observed with the combination of a GLP-1 receptor agonist and basal insulin (VARIATION study). Diabetes Care 40 (2), 194–200. doi:10.2337/dc16-1582
Braithwaite, S. S. (2013). Glycemic variability in hospitalized patients: choosing metrics while awaiting the evidence. Curr. Diab Rep. 13 (1), 138–154. doi:10.1007/s11892-012-0345-9
CecCooOMN (2016). The consensus of Chinese experts in medical nutrition therapy for overweight/obesity. Chin. J. Diabetes Mellit. 10 (10), 451–455. doi:10.3760/cma.j.issn.1674-5809.2016.09.004
Ceriello, A., Esposito, K., Piconi, L., Ihnat, M. A., Thorpe, J. E., Testa, R., et al. (2008). Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57 (5), 1349–1354. doi:10.2337/db08-0063
Cso, E. (2017). Experts consensus on management of glycemic variability of diabetes mellitus. Chin. J. Endocr. Metabolism (08), 633–636. doi:10.3760/cma.j.issn.1000-6699.2017.08.002
Dandona, P. (2017). Minimizing glycemic fluctuations in patients with type 2 diabetes: approaches and importance. Diabetes Technol. Ther. 19 (9), 498–506. doi:10.1089/dia.2016.0372
Duckworth, W., Abraira, C., Moritz, T., Reda, D., Emanuele, N., Reaven, P. D., et al. (2009). Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360 (2), 129–139. doi:10.1056/NEJMoa0808431
Gerstein, H. C., Miller, M. E., Byington, R. P., Goff, D. C., Bigger, J. T., Buse, J. B., et al. (2008). Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358 (24), 2545–2559. doi:10.1056/NEJMoa0802743
Hirsch, I. B. (2015). Glycemic variability and diabetes complications: does it matter? Of course it does. Diabetes Care 38 (8), 1610–1614. doi:10.2337/dc14-2898
Hu, C., and Jia, W. (2019). Therapeutic medications against diabetes: what we have and what we expect. Adv. Drug Deliv. Rev. 139, 3–15. doi:10.1016/j.addr.2018.11.008
Jia, W., Weng, J., Zhu, D., Ji, L., Lu, J., Zhou, Z., et al. (2019). Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 35 (6), e3158. doi:10.1002/dmrr.3158
Jordán-Domingo, M., Gimeno-Orna, J. A., Lahoza-Pérez, M. C., Ilundain-González, A. I., Agudo-Tabuenca, A., and Sáenz-Abad, D. (2021). Effect of in-hospital glycemic variability on mortality in patients with diabetes. Rev. Clin. Esp. 221 (6), 323–330. doi:10.1016/j.rceng.2019.12.014
Julla, J. B., Jacquemier, P., Fagherazzi, G., Vidal-Trecan, T., Juddoo, V., Jaziri, A., et al. (2021). Is the consensual threshold for defining high glucose variability implementable in clinical practice? Diabetes Care 44 (7), 1722–1725. doi:10.2337/dc20-1847
Kauffmann, R. M., Hayes, R. M., Buske, B. D., Norris, P. R., Campion, T. R., Dortch, M., et al. (2011). Increasing blood glucose variability heralds hypoglycemia in the critically ill. J. Surg. Res. 170 (2), 257–264. doi:10.1016/j.jss.2011.03.008
Ke, R., Liu, Y., Zhu, J., Zhu, Q., Kang, J., and Zhang, H. (2022). Treatment of glucose fluctuation of type 2 diabetes mellitus based on transfer function of spleen and stomach qi. Liaoning J. traditional Chin. Med. 49 (06), 67–69. doi:10.13192/j.issn.1000-1719.2022.06.018
Kohnert, K. D., Freyse, E. J., and Salzsieder, E. (2012). Glycaemic variability and pancreatic β-cell dysfunction. Curr. Diabetes Rev. 8 (5), 345–354. doi:10.2174/157339912802083513
Kovatchev, B., and Cobelli, C. (2016). Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care 39 (4), 502–510. doi:10.2337/dc15-2035
Li, G. (2023). Study on TCM syndrome characteristics of blood glucose fluctuation in type 2 diabetes mellitus. master. Tianjin, China: Tianjin University of Traditional Chinese Medicin.
Liu, Y., Kang, J., and Gao, H. (2015). Discussion based on theory of homogeny of pi and yi of decreasing glucose fluctuation of diabetes by TCM syndrome differentiation and treatment. Liaoning J. traditional Chin. Med. 42 (07), 1246–1247. doi:10.13192/j.issn.1000-1719.2015.07.037
Mendez, C. E., Mok, K. T., Ata, A., Tanenberg, R. J., Calles-Escandon, J., and Umpierrez, G. E. (2013). Increased glycemic variability is independently associated with length of stay and mortality in noncritically ill hospitalized patients. Diabetes Care 36 (12), 4091–4097. doi:10.2337/dc12-2430
Monnier, L., Mas, E., Ginet, C., Michel, F., Villon, L., Cristol, J. P., et al. (2006). Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Jama 295 (14), 1681–1687. doi:10.1001/jama.295.14.1681
Monnier, L., Wojtusciszyn, A., Colette, C., and Owens, D. (2011). The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol. Ther. 13 (8), 813–818. doi:10.1089/dia.2011.0049
Murata, G. H., Hoffman, R. M., Shah, J. H., Wendel, C. S., and Duckworth, W. C. (2004). A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: the Diabetes Outcomes in Veterans Study (DOVES). Arch. Intern Med. 164 (13), 1445–1450. doi:10.1001/archinte.164.13.1445
Nusca, A., Tuccinardi, D., Albano, M., Cavallaro, C., Ricottini, E., Manfrini, S., et al. (2018). Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab. Res. Rev. 34 (8), e3047. doi:10.1002/dmrr.3047
Pang, G., and Sun, F. (2017). Clinical study on the relationship between TCM syndrome types and dynamic blood glucose in type 2 diabetes mellitus. China J. Traditional Chin. Med. Pharm. 32 (03), 1384–1386.
Pang, G., Wang, K., Zhu, P., Lou, J., Gao, Y., and Sun, F. (2019). Sequential three methods of Chinese medicine for the treatment of type 2 diabetes mellitus. J. Traditional Chin. Med. 60 (14), 1243–1246. doi:10.13288/j.11-2166/r.2019.14.016
Penckofer, S., Quinn, L., Byrn, M., Ferrans, C., Miller, M., and Strange, P. (2012). Does glycemic variability impact mood and quality of life? Diabetes Technol. Ther. 14 (4), 303–310. doi:10.1089/dia.2011.0191
Saisho, Y. (2014). Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int. J. Mol. Sci. 15 (10), 18381–18406. doi:10.3390/ijms151018381
Scott, E. S., Januszewski, A. S., O’Connell, R., Fulcher, G., Scott, R., Kesaniemi, A., et al. (2020). Long-term glycemic variability and vascular complications in type 2 diabetes: post hoc analysis of the FIELD study. J. Clin. Endocrinol. Metab. 105 (10), dgaa361. doi:10.1210/clinem/dgaa361
Timmons, J. G., Cunningham, S. G., Sainsbury, C. A., and Jones, G. C. (2017). Inpatient glycemic variability and long-term mortality in hospitalized patients with type 2 diabetes. J. Diabetes Complicat. 31 (2), 479–482. doi:10.1016/j.jdiacomp.2016.06.013
Zenari, L., and Marangoni, A. (2013). What are the preferred strategies for control of glycaemic variability in patients with type 2 diabetes mellitus? Diabetes Obes. Metab. 15 (Suppl. 2), 17–25. doi:10.1111/dom.12143
Zhang, H., Zhou, J., Zhang, L., Ma, J., Sun, Y., and Zhao, Y. (2015). Characteristics of blood glucose excursions in type 2 diabetes mellitus patients with three different Traditional Chinese Medicine syndromes. J. Tradit. Chin. Med. 35 (5), 537–545. doi:10.1016/s0254-6272(15)30136-9
Zhang, Q., Qu, L., Shi, X., Yang, D., Zhao, F., Wang, Q., et al. (2024). Study on the association between glucose variability of patients with diabetes mellitus and traditional Chinese medicine syndrome. J. Traditional Chinese Med. Pharm. 39 (02), 956–961.
Keywords: within-day glycemic variability, glycemic fluctuations, glycemic stability, type 2 diabetes, traditional Chinese medicine decoction therapy
Citation: Xing Y, Li P, Pang G, Zhao H and Wen T (2024) Observational study on stability of within-day glycemic variability of type 2 diabetes inpatients treated with decoctions of traditional Chinese medicine. Front. Pharmacol. 15:1378140. doi: 10.3389/fphar.2024.1378140
Received: 29 January 2024; Accepted: 04 July 2024;
Published: 19 July 2024.
Edited by:
Jiajia Song, Southwest University, ChinaReviewed by:
Aikaterini Andreadi, University of Rome Tor Vergata, ItalyCopyright © 2024 Xing, Li, Pang, Zhao and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiancai Wen, d2VudGlhbmNhaUBuZGN0Y20uY24=; Guoming Pang, a2Zzenl5cGdtQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.