
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 26 July 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1377690
Introduction: Inhibitors of programmed cell death 1 (PD1) and its ligand (PDL1) have exhibited favorable long-term survival in many types of advanced-stage cancer and current approvals have to date been granted in certain tumour types irrespective of PD-L1 status.
Methods: We extracted the following information: study sample size, trial period, cancer types, intervention of treatment, type of PD-L1 antibody, immunohistochemistry (IHC) scoring method, number and percentage of PD-L1 < 1% population, and median follow- up time. PD-L1 expression was defined as percentage of number of PD-L1-stained tumor cells (TPS), area of tumor infiltrated by PD-L1-stained immune cells (IPS), number of PD-L1-stained cells (tumor cells, lymphocytes and macrophages; CPS). Different trials used distinct method to define low PD-L1 expression. The risk of bias of the included trials was assessed by using the Cochrane risk of bias tool for RCTs.
Results: Here, a total of 34 trials were included to extract individual patient data (IPD) to evaluate the survival benefit of first line PD1/PDL1 inhibitors vs. standard-of-care (SOC) in patients with PDL1 < 1%. In term of anti-PD-1/PD-L1 monotherapy, OS (HR = 0.90, 0.81−1.01) and PFS (HR = 1.11, 0.97−1.27) between PD-1/PD-L1 inhibitor group and SOC group were comparable. In term of anti-PD-1/PD-L1 combination therapy, PD-1/PD-L1 inhibitor group exhibited longer OS (median 19.5 months vs. 16.3 months; HR = 0.83, 0.79−0.88, p < 0.001) and PFS than those of SOC group (median 8.11 months vs. 6.96 months; HR = 0.82, 0.77−0.87, p < 0.001).Subgroup analysis showed that survival benefit was mainly observed in non-small cell lung cancer (NSCLC) (HROS = 0.74; HRPFS = 0.69; p < 0.001), small-cell lung cancer (SCLC) (HROS = 0.58, p < 0.001; HRPFS = 0.55, p = 0.030), esophageal squamous cell carcinoma (ESCC) (HROS = 0.62, p = 0.005; HRPFS = 0.79, p < 0.001), melanoma (HROS = 0.53, p < 0.001) and nasopharyngeal carcinoma (NPC) (HRPFS = 0.35, p = 0.013).
Conclusion: Anti-PD-1/PD-L1 combinational therapy rather than monotherapy exhibit survival benefit in the low PD-L1 population in the first-line setting, and the survival benefit was mainly observed in specific tumor types.
Therapeutic blockade targeting programmed cell death 1 (PD-1) and its ligand (PD-L1) is one of the most important advances in the history of cancer treatment (Ribas and Wolchok, 2018). PD-1/PD-L1 inhibitors in the first-line setting, alone or in combination with other antitumor therapies, are increasingly being demonstrated to exhibit favorable long-term survival in many types of advanced-stage cancer, including melanoma, lung cancer, esophageal squamous cell carcinoma (ESCC), gastric carcinoma (GC) and many others (Doroshow et al., 2021).
Notably, recent randomized controlled trials (RCTs) of PD-1/PD-L1 inhibitors preferred to set the primary endpoints of survival in PD-L1-positive and intention-to-treat (ITT) populations (Rini et al., 2019; Emens et al., 2021; Miles et al., 2021). Most of these RCTs always published the data of ITT and PD-L1-positive populations, with a lack of presentation of the low PD-L1-expression subgroup.
CheckMate 648 showed that overall survival (OS) and progression-free survival (PFS) were significantly longer with nivolumab plus chemotherapy or ipilimumab than chemotherapy alone in all randomly assigned patients with ESCC, without reporting the Kaplan‒Meier (KM) curves for patients with absent or low PD-L1 expression (Doki et al., 2022). Similar observations were found in CheckMate 649 of GC (Janjigian et al., 2021) and CheckMate 743 of malignant pleural mesothelioma (MPM) (Peters et al., 2022). However, while two recent meta-analyses showed that PD1-/PD-L1 inhibitors failed to exhibit a survival benefit in the GC or ESCC patients with absent or low PD-L1 expression (Zhao et al., 2022a; Yap et al., 2023), the post hoc analysis of JUPITER-06 and meta-analysis showed superiority of PD-1 inhibitor with chemotherapy in advanced ESCC patients with absent or low PD-L1 expression. (Wu et al., 2022). Therefore, there are several critical and debatable issues: whether survival benefit in the randomized assigned population is largely derived from those in the PD-L1-positive population and whether PD-1/PD-L1 inhibitors can exhibit a survival benefit in patients with absent or low PD-L1 expression remain uncertain.
Here, we reconstructed individual patient data (IPD) of absent or low PD-L1 expression (PD-L1 < 1%) populations from the reported KM curves of high-quality RCTs, using a novel workflow, KMSubtraction (Zhao et al., 2022a; Zhao et al., 2022b; Yu et al., 2022). Given that the hazards in the trials of anti-PD-1/PD-L1 therapy are always not proportional during the entire study period, we used the approaches of log-rank test, Bayesian hierarchical model, and restricted mean survival time (RMST) to comprehensively evaluate the survival benefit of first line PD-1/PD-L1 inhibitor vs. standard-of-care (SOC) in patients with PD-L1 < 1%.
This study was conducted following the Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data (PRISMA-IPD) protocol (Stewart et al., 2015).
Two investigators (RCN and YBC) conducted independent literature searches of PubMed, Web of Science and Embase for eligible publications between 1 January 2015, and 8 February 2023, using the key words PD-1, PD-L1, checkpoint inhibitor, and phase 3 clinical trial (eBox 1).
Phase 3 RCTs were included if first line PD-1/PD-L1 inhibitors, alone or combined with other antitumor therapies (e.g., chemotherapy, targeted therapy or immunotherapy), were compared with SOC in patients with advanced tumors. The other criterion is that trials must report the hazard ratio (HR) of OS and/or PFS between PD-1/PD-L1 inhibitors and SOC in patients with low PD-L1 expression. We excluded reviews, conference abstracts and non-English-language articles. In the case of repeated studies reporting the same population, the most recent and most informative study was eligible.
We extracted the following information: study sample size, trial period, cancer types, intervention of treatment, type of PD-L1 antibody, immunohistochemistry (IHC) scoring method, number and percentage of PD-L1 < 1% population, and median follow-up time. PD-L1 expression was defined as percentage of number of PD-L1-stained tumor cells (TPS), area of tumor infiltrated by PD-L1-stained immune cells (IPS), number of PD-L1-stained cells (tumor cells, lymphocytes and macrophages; CPS). Different trials used distinct method to define low PD-L1 expression. In this study, low PD-L1 expression was defined as TPS <1%, IPS <1%, TPS&IPS <1% or CPS <1. CPS = 10 can be equal to TPS = 1% (Wu et al., 2022; Yap et al., 2023). The risk of bias of the included trials was assessed by RCN and YBC using the Cochrane risk of bias tool for RCTs (Higgins, 2008).
For trials reporting KM curves of the PD-L1 < 1% population, IPD was extracted and decoded from the reported KM curves using the “IPDfromKM” package (Liu et al., 2021). The quality of reconstruction was evaluated by checking the at-risk tables, HRs, and shape of the KM curves.
For trials reporting KM curves of overall and PD-L1 ≥ 1% population, IPD was extracted using the “IPDfromKM” and “KMSubtraction” packages (Zhao et al., 2022b), which can derive unreported subgroup survival data from known subgroups. For KMSubtraction process, minimal-cost bipartite matching was adopted as the primary algorithm. Monte Carlo simulations with 1,000 iterations were used to evaluate the limits of error (Zhao et al., 2022b).
The quality of the reconstructed IPD was evaluated before the pooled analysis. Reconstruction KM curves of overall, subgroups with PD-L1 ≥ 1% and PD-L1 < 1% were compared with the original published KM curves, regarding the HRs, at-risk tables, and shape of the KM curves. In addition, we estimated the correlation between the reconstructed and reported outcomes using the Pearson correlation test.
An important aspect of validity is the representativeness of reconstructed IPD. To evaluate the representativeness of trials with available IPD, we performed standard meta-analysis models to combine aggregate data (from trials of non-IPD) with the available IPD. A random-effects model was used for this meta-analysis. Egger’s test and funnel plot analyses were assessed the presence of publication bias (Egger et al., 1997), with a two-tailed p < 0.05 considered statistically significant. Then, the HRs of trials with IPD and trials with total data (IPD and aggregate data) were compared.
The clinical benefit was graded by a simplified ESMO-Magnitude of Clinical Benefit Scale version 1.1 (Cherny et al., 2017; Korn et al., 2022) (Supplementary Table S1). In this study, grade 3/4 clinical benefit was considered meaningful. If the median OS for standard treatment was ≤12 months, the experimental arm median OS better by ≥ 2 months was considered clinically meaningful; if the median OS for standard treatment was >12 months and ≤24 months, the experimental arm median OS better by ≥ 3 months was considered meaningful. If median OS was not reached, a 10% increase in 2-year OS was considered meaningful. The upper limit of the 95% confidence interval (CI) for the HR should be less than 1.
The primary outcomes were OS and PFS. OS was defined from the date of randomization to death from any cause. PFS was defined from the date of randomization to progressive disease as per RECIST guidelines (version 1.1) or death from any cause, whichever occurred first.
In this study, 1-stage approach was used to evaluate the survival benefit in subgroup of PD-L1 < 1%, through three different approaches. The primary analysis applied the log-rank test and marginal Cox model. To account for the between-study heterogeneity, the shared-frailty model was adopted to incorporate a random-effects terms, and the HRs were adjusted by the effect of cancer types, anti-PD-1/PD-L1 drugs, treatment of control arm. The subgroup analysis of different cancer types, anti-PD-1/PD-L1 drugs, PD-1/PD-L1 inhibitors (PD-1 or PD-L1), treatment regimens (single PD-1/PD-L1 regimen or combination PD-1/PD-L1 regimen), treatment of control arm, PD-L1 clone, and PD-L1 IHC scoring method was performed. In this study, we modified the predictive value of PD-L1 expression describe by Yoon et al. (Yoon et al., 2022), defined as log transformation of the ratio of HR of PDL1 < 1% versus ≥1% population.
We also applied a Bayesian hierarchical model with a time-varying hazard ratio (HR) (Berry, 2006). We modeled the time-varying HR effect by assuming that the hazards were constant within each 3-month follow-up and truncated the results at 60 months. Each 3-month segment had its own hazard rate and HR. The average HR adjusting the effect of cancer types, PD-1/PD-L1 agents, treatment of control arm was calculated. Markov chain Monte Carlo (MCMC) methods (1,000 iterations) (Gelman et al., 2013) were used to calculate the posterior mean of OS and PFS distributions and their corresponding 95% CI. The priors were set as default using the stan_surv function by rstanarm package. Rhat statistic was used to assess the convergence of the MCMC chains, with Rhat statistic less than 1.1 indicating the good evidence in favor of convergence (Carpenter et al., 2017).
The RMST was the nonparametric alternative strategy of the HR that does not rely on proportional hazards (Royston and Parmar, 2013). The RMST difference, the area bounded by 2 KM plots, represents the absolute gain or loss in survival. In this study, the truncation times were 2 years and 1 year for OS and PFS, respectively. If the minimum of the largest observed time in each of the two groups was shorter than 2 years for OS or 1 year for PFS, the truncation time was equal to this minimum of the largest observed time.
All statistical analyses were performed using R software, version 4.2.0 (http://www.r-project.org). p < 0.05 was considered statistically significant.
Of 7,592 reports identified by the search strategy, 287 full-text articles met the eligibility criteria for detailed review. Of these, 49 phase 3 RCTs (Motzer et al., 2018; Paz-Ares et al., 2018; Socinski et al., 2018; Rini et al., 2019; West et al., 2019; Choueiri et al., 2020; Galsky et al., 2020; Jotte et al., 2020; Powles et al., 2020; Rudin et al., 2020; Motzer et al., 2021; Powles et al., 2021; Sun et al., 2021; Cheng et al., 2022; Wang et al., 2022; Cheng et al., 2022; Dummer et al., 2022; Gogishvili et al., 2022; Kang et al., 2022; Lu et al., 2022; Motzer et al., 2022; Shitara et al., 2022; Wu et al., 2022; Ascierto et al., 2023; de Castro et al., 2023; Johnson et al., 2023) involving 14,677 patients with PD-L1 < 1% met the inclusion criteria and were included (Supplementary Figure S1). The percentage of the PD-L1 < 1% population in each trial varied from 14.3% to 85.7% (Table 1).
These studies covered 28 trials with anti-PD-1 (including 12 with nivolumab, 9 with pembrolizumab, 2 with camrelizumab, 2 with toripalimab, 1 with sintilimab, 1 with serplulimab, and 1 with spartalizumab) and 21 trials with anti-PD-L1 (including 12 with atezolizumab, 3 with avelumab, 3 with durvalumab, 1 with adebrelimab, 1 with cemiplimab, and 1 with sugemalimab) agents. Fifteen trials were conducted in patients with non-small cell lung cancer (NSCLC), six trials in patients with renal cell carcinoma (RCC), five trials in patients with small-cell lung cancer (SCLC), five trials in patients with ESCC, three trials in patients with triple-negative breast cancer (TNBC), three trials in patients with GC, three trial in patients with melanoma, two trials in patients with ovarian cancer (OC), two trial in patients with hepatocellular carcinoma (HCC), two trials in patients with urothelial cancer (UC),1 trial in patients with head and neck squamous cell carcinoma (HNSCC), one trial in patients with MPM, and one trial in patients with nasopharyngeal carcinoma (NPC). Of the 49 trials, fourteen trials assessed PD-L1 expression with the use of IHC antibody 28–8, 14 trials with 22C3, 11 trials with SP142, eight trials with SP263, and two trial with JS311. In terms of the PD-L1 IHC scoring method, 24 trials defined PD-L1 expression with the use of the TPS, eight trials with the IPS, eight trials with TPS&IPS, and nine trials with the CPS.
Among the 49 trials, 34 trials were available for the IPD and 15 trials were available for the aggregate data, with a total of 52 comparisons of OS and 49 comparisons of PFS. A total of 13 comparisons of single anti-PD-1/PD-L1 agents (OS: 8; PFS: 5), 88 comparisons of combination anti-PD-1/PD-L1 agents (OS: 44; PFS: 44) were included (Table 1). Table 1 provides further information on the study characteristics.
The quality of most included trials was generally high (Supplementary Table S2), and no publication bias was observed (Supplementary Figure S2) in the IPD and aggregate data. The random-effect model was used to evaluate the pooled effect of OS and PFS. We obtained a pooled HR of 0.82 (0.77–0.87) for OS. Of note, we found that patients with PD-L1 < 1% can benefit from combination PD-1/PD-L1 regimens (HR 0.80, 0.75–0.86) rather than single PD-1/PD-L1 regimens (HR 0.93, 0.81–1.07). The subgroup difference was significant (p = 0.049) (Supplementary Figure S3). Similar results were found regarding PFS (Supplementary Figure S4).
Before the reconstruction of IPD, the HRs of trials with IPD and trials with total data (IPD and aggregate data) were compared. Of note, the HRs of trials with IPD and trials with total data were comparable (Supplementary Figure S5), indicating that treatment effect estimated by trials with IPD can effectively represent those by total data.
We summarized the extraction process of IPD of PD-L1 < 1% in Supplementary Tables S3-S4. The reconstructed KM curves of overall, subgroups with PD-L1 ≥ 1% and PD-L1 < 1% were similar to those of original curves (Supplementary Table S4), and the limits of error of KMSubtraction of extraction of unreported subgroup were small and negligible (Supplementary Figure S6). Then, we calculated the correlation between the reconstructed outcomes and reported outcomes from the original articles. As expected, we observed extremely strong associations in terms of HR, median survival time, OS rate and PFS rate (all Pearson correlation coefficients >0.99 and all p < 0.001, Supplementary Figure S7), indicating that the reconstructed IPD could effectively represent the original data.
No publication bias was observed in the IPD analysis (Supplementary Figure S8). Next, we conducted survival analysis using the log-rank test, stratified by single/combination PD-1/PD-L1 inhibitors. The IPD of OS from 33 trials were available for 9,686 patients. In the analysis for OS of single PD-1/PD-L1 inhibitors, the median OS was 14.1 months (12.5–16.2) in the PD-1/PD-L1 inhibitor group and 13.6 months (12.5–15.0) in the SOC group (adjusted HR 0.90, 0.81–1.01, p = 0.063) (Figure 1A), with no clinical benefit. Subgroup analysis stratified by cancer types further showed no statistically significant difference for single PD-1/PD-L1 inhibitors compared with SOC (Supplementary Figure S9). In the analysis for OS of combination PD-1/PD-L1 inhibitors, the median OS was 19.5 months (18.5–20.1) in the PD-1/PD-L1 inhibitor group and 16.3 months (15.5–17.2) in the SOC group (adjusted HR 0.83, 0.79–0.88, p < 0.001) (Figure 1B), with grade 3 clinical benefit. Then, we explored the subgroup of OS in patients treated with combination PD-1/PD-L1 inhibitors, and found that the PD-1/PD-L1 inhibitor group only showed OS benefit in patients with NSCLC, SCLC, ESCC and melanoma (Figure 2; Supplementary Figure S10). Interestingly, we found that PD-1 inhibitors rather than PD-L1 inhibitors showed OS benefit (p = 0.001 for subgroup difference; Supplementary Figure S10; Supplementary Figure S11A, B). Of note, if TPS or TPS&IPS was used to assess PD-L1 expression, PD-1/PD-L1 inhibitors exhibited OS benefit and clinical benefit in patients with PD-L1 < 1% (Supplementary Figure S10).
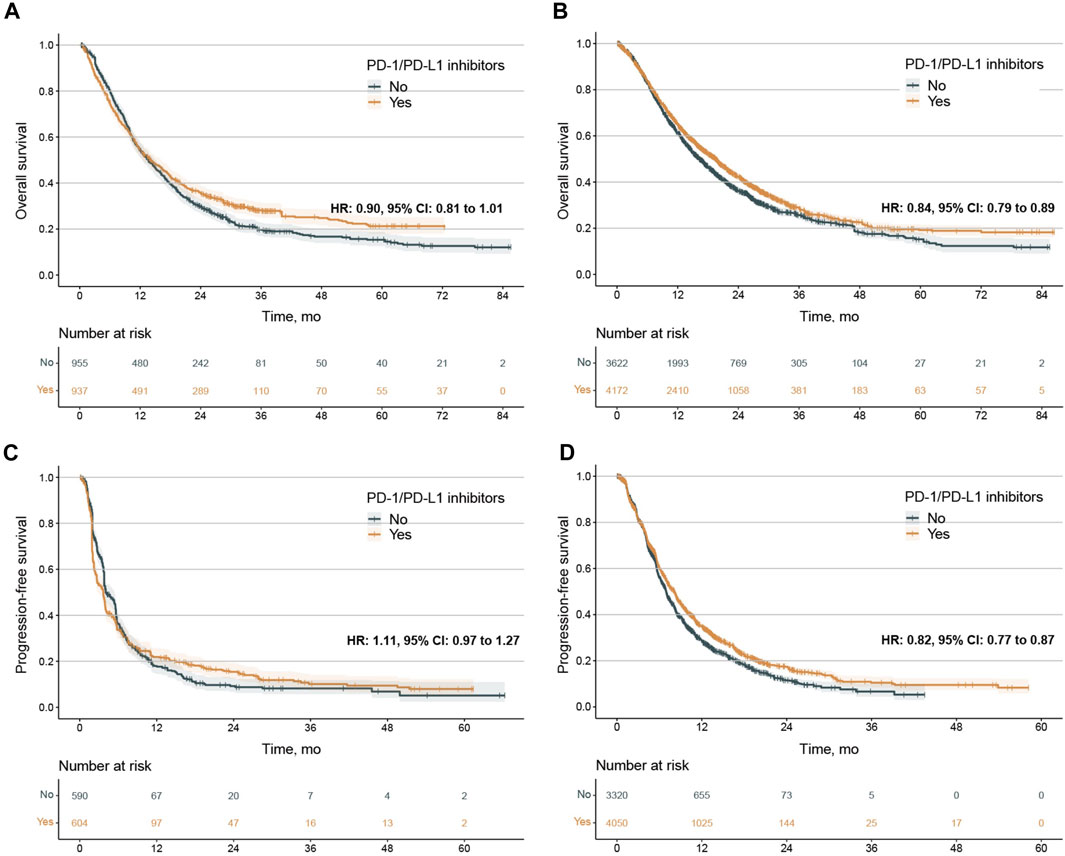
Figure 1. Kaplan‒Meier estimates of overall survival and progression-free survival. (A, B), overall survival (OS) in the PD-L1 < 1% population treated with PD-1/PD-L1 single agents (A) and combination agents (B). C-D, progression-free survival (PFS) In the PD-L1 < 1% population treated with PD-1/PD-L1 single agents (C) and combination agents (D). The 2-year OS was 35.6% (32.6%–38.9%) vs. 29.6% (26.7%–32.8%) for patients treated PD-1/PD-L1 single agents or not, 43.2% (41.5%–44.9%) vs. 37.2% (35.4%–39.0%) or patients treated PD-1/PD-L1 combination agents or not. The 1-year PFS was 21.9% (18.6%–25.8%) vs. 18.0% (14.7%–21.9%) for patients treated PD-1/PD-L1 single agents or not, 35.5% (33.8%-37.2) vs. 29.4% (27.7%–31.3%) or patients treated PD-1/PD-L1 combination agents or not. PD-1, programmed death-1; PD-L1, programmed death-ligand 1; HR, hazard ratio; CI, confidence interval.
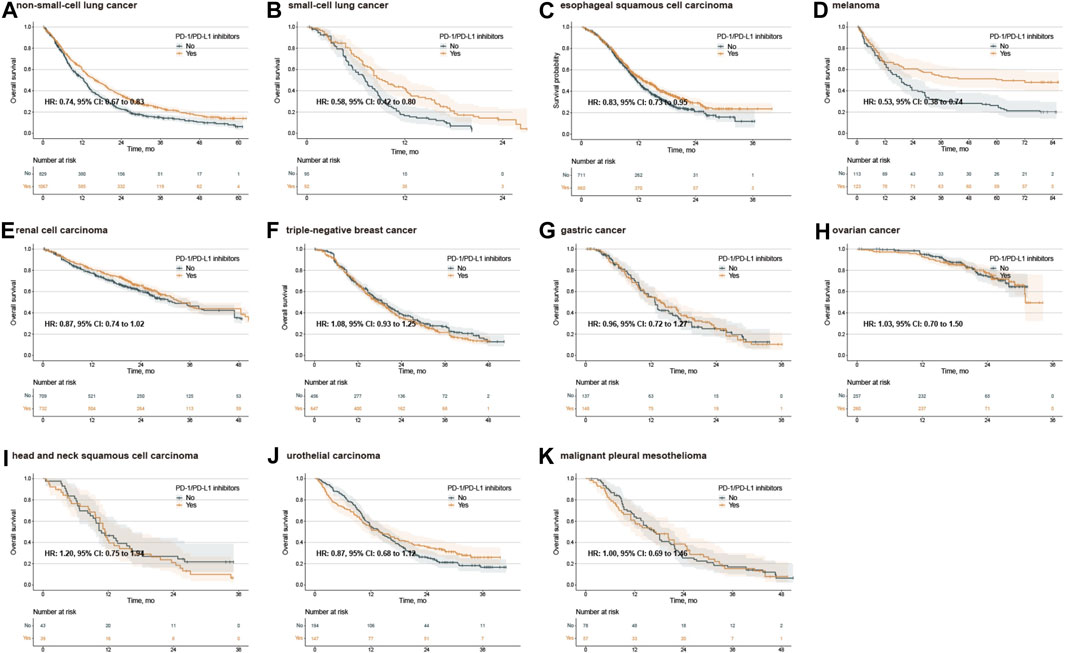
Figure 2. Kaplan‒Meier estimates of overall survival treated with combination agents, stratified by cancer type. Overall survival in the PD-L1 < 1% population with non-small-cell lung cancer (A), small-cell lung cancer (B), esophageal squamous cell carcinoma (C), melanoma (D), renal cell carcinoma (E), triple-negative breast cancer (F), gastric cancer (G), ovarian cancer (H), head and neck squamous cell carcinoma (I), urothelial carcinoma (J), and malignant pleural mesothelioma (K). PD-1, programmed death-1; PD-L1, programmed death-ligand 1; HR, hazard ratio; CI, confidence interval.
IPD of PFS from 33 trials were available for 8,217 patients. In the PFS analysis of single PD-1/PD-L1 inhibitors, the PFS between PD-1/PD-L1 inhibitor group and SOC group were comparable (median 3.68 months, 2.95 to 3.91 vs. 4.05 months, 3.81 to 5.40; adjusted HR 1.11, 0.97 to 1.27, p = 0.122) (Figure 1C). Subgroup analysis stratified by cancer types further showed no PFS difference for single PD-1/PD-L1 inhibitors compared with SOC (Supplementary Figure S12). In the analysis for PFS of combination PD-1/PD-L1 inhibitors, PD-1/PD-L1 inhibitor group exhibited longer PFS than those of SOC group (median 8.11 months, 7.58 to 8.33 vs.6.96 months, 6.78 to 7.11; adjusted HR 0.82, 0.77 to 0.87, p < 0.001) (Figure 1D). Then, we explored the subgroup of PFS in patients treated with combination PD-1/PD-L1 inhibitors. The PD-1/PD-L1 inhibitor group showed a PFS benefit in patients with NSCLC, SCLC, ESCC and NPC (Figure 3; Supplementary Figure S13). Similarly, treatment effect of PD-1 inhibitors was higher than those of PD-L1 inhibitors (p = 0.111 for subgroup difference; Supplementary Figure S11C, D; Supplementary Figure S13), and if TPS or TPS&IPS was used to assess PD-L1 expression, PD-1/PD-L1 inhibitors exhibited a PFS benefit in patients with PD-L1 < 1% (Supplementary Figure S13B).
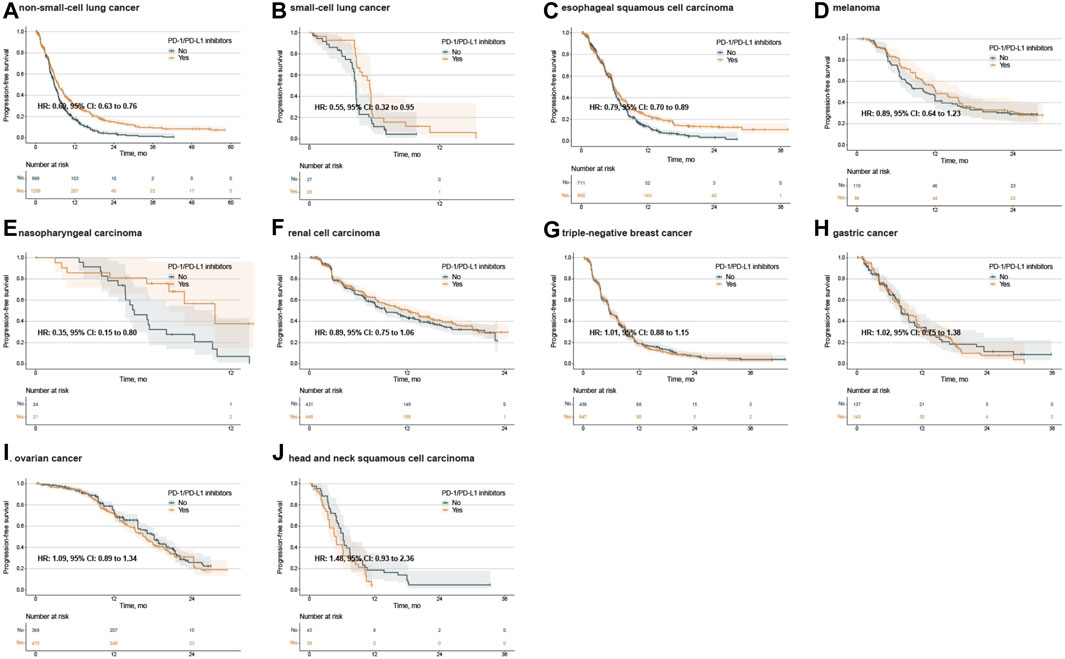
Figure 3. Kaplan‒Meier estimates of progression-free survival treated with combination agents, stratified by cancer type. Progression-free survival in the PD-L1 < 1% population with non-small-cell lung cancer (A), small-cell lung cancer (B), esophageal squamous cell carcinoma (C), melanoma (D), nasopharyngeal carcinoma (E), renal cell carcinoma (F), triple-negative breast cancer (G), gastric cancer (H), ovarian cancer (I), and head and neck squamous cell carcinoma (J). PD-1, programmed death-1; PD-L1, programmed death-ligand 1; HR, hazard ratio; CI, confidence interval.
We next conducted survival analysis using a Bayesian hierarchical model. The survival curves for the PD-1/PD-L1 inhibitor and SOC groups are shown in Supplementary Figure S14. In term of single PD-1/PD-L1 inhibitors, both OS and PFS were similar between patients treated with PD-1/PD-L1 inhibitors and those treated with SOC, regardless of cancer types (Supplementary Figure S14A, C; Supplementary Figure S15, 16). In term of combination PD-1/PD-L1 inhibitors, PD-1/PD-L1 inhibitor group exhibited OS and PFS benefit. At 2 years, the estimated OS was 44.4% (43.0%–45.9%) for the combination PD-1/PD-L1 inhibitor group and 39.2% (37.8%–40.8%) for the SOC group. The average adjusted HR for OS was 0.83 (0.78–0.88) (Supplementary Figure S14B). At 1 year, the estimated PFS was 40.0% (38.7%–41.3%) for the combination PD-1/PD-L1 inhibitor group and 33.4% (31.8%–34.9%) for the SOC group. The average adjusted HR for PFS was 0.79 (0.74–0.82) (Supplementary Figure S14D). Subgroup analysis stratified by cancer types also demonstrated similar results to those of the log-rank test (Supplementary Figure SS17, 18).
The difference in RMST between the PD-1/PD-L1 inhibitor and SOC groups was further estimated. In term of single PD-1/PD-L1 inhibitors, the RMST difference between the two groups failed to exhibit statistical significance (Supplementary Figure S19, 20). In term of combination PD-1/PD-L1 inhibitors, the RMST difference between the two groups started to exhibit statistical significance at truncation time points of13 months for OS and 8 months for PFS (Supplementary Figure S19). The 2-year RMST difference between the two groups was 0.79 months (0.41–1.16) for OS, and the 1-year RMST difference between the two groups was 0.40 months (0.21–0.59) for PFS. Notably, we observed that only seven trials showed a significant 2-year RMST difference for OS, and nine trials showed a significant 1-year RMST difference for PFS (Supplementary Figure S21).
Finally, we estimated the predictive value of PD-L1 expression. PD-L1 expression ranged from −0.41 to 0.67 (Supplementary Table S5; Supplementary Figure S22), and −0.52 to 0.94 for each subgroup (Supplementary Figure S23).
To our knowledge, this is the largest IPD meta-analysis that investigates the survival benefit of first-line anti-PD-1/PD-L1 therapy in patients with PD-L1 < 1%. The results suggest that anti-PD-1/PD-L1 monotherapy failed to exhibit survival benefit in the low PD-L1 population. The magnitude of the survival benefit associated with anti-PD-1/PD-L1 combinational therapy in the low PD-L1 population was moderate (grade 3 clinical benefit). In addition, a survival benefit of anti-PD-1/PD-L1 combinational therapy was mainly observed in specific tumor types, including NSCLC, SCLC, ESCC, melanoma and NPC.
Recently, there have been an increasing number of RCTs demonstrating the survival benefit of PD-1/PD-L1 inhibitors for the treatment of patients with late-stage tumors in the first-line setting, accelerating regulatory approval by the FDA. These approvals have promoted the exploration of the efficacy of PD-1/PD-L1 inhibitors in earlier-stage settings (Ascierto et al., 2020; Forde et al., 2022; Schmid et al., 2022). A previous meta-analysis reported that PD-1/PD-L1 inhibitors prolonged the survival in patients with PD-L1 negative in the second and later line setting (Shen and Zhao, 2018). However, this issue in the first line setting is datable. In terms of mechanism, PD-L1 expressed on tumor cells promotes immune evasion (Topalian et al., 2015; Sanmamed and Chen, 2018), and therapeutic blockade of the PD-1 pathway theoretically requires the expression of PD-L1 on antigen-presenting cells and tumor cells (Yamaguchi et al., 2022).
In the present study, we noted that the proportion of the PD-L1 < 1% population was high (39.6%, 14,677/37,036; range: 14.3%–85.7%), which warrants a deeper analysis to identify whether the absent or low PD-L1 population can truly benefit from PD-1/PD-L1 inhibitors. We utilized a novel approach (KMSubtraction) to extract the unreported subgroups of IPD of the PD-L1 < 1% population from 34 high-quality phase 3 RCTs. The reconstructed IPD were representative. Overall, our findings suggested that the use of PD-1/PD-L1 inhibitors alone in the first line setting failed to provide OS or PFS benefit in patients with absent or low PD-L1 expression compared with SOC, which suggested the importance of PD-L1 expression in PD-1/PD-L1 blockade therapy. Anti-PD-1/PD-L1 combinational therapy exhibited OS and PFS benefit in the low PD-L1 population, which can be explained that chemotherapy and targeted therapy can induce PD-L1 expression (Akbay et al., 2013; Parra et al., 2018). Nonetheless, we also found that the timepoint at which PD-1/PD-L1 inhibitors initially exhibited a survival benefit was lagging (13 months for OS and 8 months for PFS). In addition, most of the eligible trials and subgroups appeared to have a positive predictive value for PD-L1 expression, consistent with a previous study (Yoon et al., 2022). Together, these results suggested that most patients with absent or low PD-L1 expression should not be indicated for PD-1/PD-L1 inhibitors.
The large IPD of this study allowed for relevant subgroup analyses. The efficacy of PD-1/PD-L1 inhibitors may differ across cancer types (Morad et al., 2021). Therefore, we first assessed the efficacy of PD-1/PD-L1 inhibitors in different cancer types. A total of 11 cancer types were included. The efficacy of PD-1/PD-L1 inhibitors in advanced ESCC with low PD-L1 expression was debatable (Wu et al., 2022; Yap et al., 2023). In this study, we extracted IPD from five trials (CheckMate 648 6, ESCORT-first (Luo et al., 2021), JUPITER-06 11, KEYNOTE-590 49, ORIENT-15 29), and found that advanced ESCC patients with low PD-L1 expression can still benefit from anti-PD-1/PD-L1 combinational therapy. The efficacy of PD-1/PD-L1 inhibitors in other cancer types were investigated. Overall, PD-1/PD-L1 inhibitors did not show a survival benefit in most cancer types but were associated with a modestly improved survival benefit in patients with NSCLC, SCLC, melanoma and NPC. The treatment effect of anti-PD-1 and anti-PD-L1 therapy may be different. Our findings indicated that the treatment effect of anti-PD-1 therapy in the first-line setting may be stronger than those of anti-PD-L1 therapy, consistent with previous study (Duan et al., 2020).
In clinical practice, IHC is the most common technology to quantify PD-L1 expression on tumor cells and tumor-infiltrating immune cells (Doroshow et al., 2021). RCTs in which patients receive different PD-1/PD-L1 inhibitors often used different PD-L1 IHC assays. Of note, when the 28–8 assay was used to identify the status of PD-L1 < 1%, PD-1/PD-L1 inhibitors still showed OS and PFS benefits. Interestingly, the predictive values of PD-L1 expression diagnosed by the 28–8 assay were 0.12 for OS and 0.10 for PFS, which indicated that PD-L1 expression diagnosed by the 28–8 assay is a biomarker to predict the intensity of efficacy of PD-1/PD-L1 inhibitors but not a biomarker to select patients who should receive anti-PD-1/PD-L1 therapy.
The IHC scoring algorithm involved the evaluation of TPS, IPS, or CPS (Doroshow et al., 2021). Notably, we found that if CPS was used to assess PD-L1 expression, PD-1/PD-L1 inhibitors exhibited OS (HR 0.75, 0.68–0.83) and PFS (HR 0.77, 0.60–0.99) benefits in patients with PD-L1 ≥ 1% but no OS (HR 0.91, 0.76–1.09) and PFS (HR 1.35, 0.76–2.39) benefits in patients with PD-L1 < 1%. Furthermore, the predictive value of CPS-based PD-L1 expression was the highest (0.19 for OS and 0.56 for PFS) among the IHC scoring algorithms, suggesting that CPS at a cutoff point of 1 may be powerful for selecting patients for anti-PD-1/PD-L1 immunotherapy. Nevertheless, it should be noted that the intraclass correlation coefficients for a CPS of ≥1 were relatively low (0.39 and 0.26 using the 22C3 and SP263 assays, respectively) (Park et al., 2020). Therefore, further research is warranted.
Most importantly, this study is the largest IPD meta-analysis of this topic. In addition, rather than extracting and pooling the study-level HR estimates, we applied an advanced method to reconstruct the IPD from published KM curves, which enables more elaborate survival analysis. The reconstructed IPD were validated through elaborate analysis, and can reflect the original data and represent the non-IPD trials. In addition to the log-rank test, we applied a Bayesian hierarchical model and RMST analysis to integrate survival data, which can overcome the potential limitations of proportional hazards modeling.
This study also has several notable limitations. These in any such meta-analysis include the potential for publication bias that not all the RCTs in the first-line setting report the KM plots of PD-L1 < 1% population or total and PD-L1 ≥ 1% population. Second, the PD-L1 expression of different tissue (primary versus metastatic samples) and intratumoral position (different spatiotemporal part of the same samples) may be different. Third, although we adopted random-effects model and calculated HRs adjusting other covariates, some between-study heterogeneities were still inevitable, such as different criterion to define PD-L1 < 1% (TPS, IPS or CPS; different PD-L1 clones). Fourth, PD-L1 expression might change after receiving another therapy, one-timepoint assessment rather than dynamic records of PD-L1 is inadequate. Fifth, although we performed methodological precautions to ensure the reconstructed KM curves and HRs for low PD-L1 expression subgroups are close to the reported data, we acknowledged some minute differences. Finally, the safety data were unavailable in this study.
Compared with SOC, anti-PD-1/PD-L1 monotherapy failed to exhibit survival benefit in the low PD-L1 population in the first-line setting. The magnitude of the survival benefit associated with anti-PD-1/PD-L1 combinational therapy in the low PD-L1 population was moderate, and the survival benefit was mainly observed in specific tumor types, including NSCLC, SCLC, ESCC, melanoma and NPC.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
FZ: Writing–original draft, Validation, Methodology, Investigation, Formal Analysis, Data curation. GC: Writing–original draft, Project administration, Methodology. YY: Writing–original draft, Data curation. XC: Writing–original draft, Methodology, Formal Analysis. RN: Writing–review and editing, Writing–original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Funding acquisition, Formal Analysis, Conceptualization. YC: Writing–review and editing, Supervision.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the grants of the National Key R&D Program of China (2017YFC1309001), Guangzhou Science and Technology Plan Projects (Health Medical Collaborative Innovation Program of Guangzhou; 201803040019 and 202201010885), National Natural Science Foundation of China (82103586, 81730072, 81672407, 81872001, 81902411, and 81772589), and Beijing Xisike Clinical Oncology Research Foundation (Y-tongshu2021/qn-0227).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1377690/full#supplementary-material
Akbay, E. A., Koyama, S., Carretero, J., Altabef, A., Tchaicha, J. H., Christensen, C. L., et al. (2013). Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 3 (12), 1355–1363. doi:10.1158/2159-8290.CD-13-0310
Ascierto, P. A., Del Vecchio, M., Mandala, M., Gogas, H., Arance, A. M., Dalle, S., et al. (2020). Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 21 (11), 1465–1477. doi:10.1016/S1470-2045(20)30494-0
Ascierto, P. A., Stroyakovskiy, D., Gogas, H., Robert, C., Lewis, K., Protsenko, S., et al. (2023). Overall survival with first-line atezolizumab in combination with vemurafenib and cobimetinib in BRAF(V600) mutation-positive advanced melanoma (IMspire150): second interim analysis of a multicentre, randomised, phase 3 study. Lancet Oncol. 24 (1), 33–44. doi:10.1016/S1470-2045(22)00687-8
Baas, P., Scherpereel, A., Nowak, A. K., Fujimoto, N., Peters, S., Tsao, A. S., et al. (2021). First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 397 (10272), 375–386. doi:10.1016/S0140-6736(20)32714-8
Berry, D. A. (2006). Bayesian clinical trials. Nat. Rev. Drug Discov. 5 (1), 27–36. doi:10.1038/nrd1927
Burtness, B., Rischin, D., Greil, R., Soulières, D., Tahara, M., de Castro, G., et al. (2022). Pembrolizumab alone or with chemotherapy for recurrent/metastatic head and neck squamous cell carcinoma in KEYNOTE-048: subgroup analysis by programmed death ligand-1 combined positive score. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 40 (21), 2321–2332. doi:10.1200/JCO.21.02198
Carpenter, B., Gelman, A., Hoffman, M. D., Lee, D., Goodrich, B., Betancourt, M., et al. (2017). Stan: a probabilistic programming language. J. Stat. Softw. 76, 1. doi:10.18637/jss.v076.i01
Cheng, A. L., Qin, S., Ikeda, M., Galle, P. R., Ducreux, M., Kim, T. Y., et al. (2022a). Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 76 (4), 862–873. doi:10.1016/j.jhep.2021.11.030
Cheng, Y., Han, L., Wu, L., Chen, J., Sun, H., Wen, G., et al. (2022b). Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA 328 (12), 1223–1232. doi:10.1001/jama.2022.16464
Cherny, N. I., Dafni, U., Bogaerts, J., Latino, N. J., Pentheroudakis, G., Douillard, J. Y., et al. (2017). ESMO-magnitude of clinical benefit Scale version 1.1. Ann. Oncol. 28 (10), 2340–2366. doi:10.1093/annonc/mdx310
Choueiri, T. K., Motzer, R. J., Rini, B. I., Haanen, J., Campbell, M. T., Venugopal, B., et al. (2020). Updated efficacy results from the JAVELIN Renal 101 trial: first-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 31 (8), 1030–1039. doi:10.1016/j.annonc.2020.04.010
Cortes, J., Rugo, H. S., Cescon, D. W., Im, S. A., Yusof, M. M., Gallardo, C., et al. (2022). Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387 (3), 217–226. doi:10.1056/NEJMoa2202809
de Castro, G., Rizvi, N. A., Schmid, P., Syrigos, K., Martin, C., Yamamoto, N., et al. (2023). NEPTUNE: phase 3 study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J. Thorac. Oncol. 18 (1), 106–119. doi:10.1016/j.jtho.2022.09.223
Doki, Y., Ajani, J. A., Kato, K., Xu, J., Wyrwicz, L., Motoyama, S., et al. (2022). Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 386 (5), 449–462. doi:10.1056/NEJMoa2111380
Doroshow, D. B., Bhalla, S., Beasley, M. B., Sholl, L. M., Kerr, K. M., Gnjatic, S., et al. (2021). PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 18 (6), 345–362. doi:10.1038/s41571-021-00473-5
Duan, J., Cui, L., Zhao, X., Bai, H., Cai, S., Wang, G., et al. (2020). Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 6 (3), 375–384. doi:10.1001/jamaoncol.2019.5367
Dummer, R., Long, G. V., Robert, C., Tawbi, H. A., Flaherty, K. T., Ascierto, P. A., et al. (2022). Randomized phase III trial evaluating spartalizumab plus dabrafenib and trametinib for BRAF V600-mutant unresectable or metastatic melanoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 40 (13), 1428–1438. doi:10.1200/JCO.21.01601
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Emens, L. A., Adams, S., Barrios, C. H., Diéras, V., Iwata, H., Loi, S., et al. (2021). First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 32 (8), 983–993. doi:10.1016/j.annonc.2021.05.355
Forde, P. M., Spicer, J., Lu, S., Provencio, M., Mitsudomi, T., Awad, M. M., et al. (2022). Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386 (21), 1973–1985. doi:10.1056/NEJMoa2202170
Galsky, M. D., Arija, J. A. A., Bamias, A., Davis, I. D., De Santis, M., Kikuchi, E., et al. (2020). Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395 (10236), 1547–1557. doi:10.1016/S0140-6736(20)30230-0
Gelman, A., Carlin, J. B., Stern, H. S., Dunson, D. B., Vehtari, A., and Rubin, D. B. (2013). Bayesian data analysis. 3rd ed. Chapman and Hall/CRC.
Gogishvili, M., Melkadze, T., Makharadze, T., Giorgadze, D., Dvorkin, M., Penkov, K., et al. (2022). Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat. Med. 28 (11), 2374–2380. doi:10.1038/s41591-022-01977-y
Gutzmer, R., Stroyakovskiy, D., Gogas, H., Robert, C., Lewis, K., Protsenko, S., et al. (2020). Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395 (10240), 1835–1844. doi:10.1016/S0140-6736(20)30934-X
Higgins, J. S. G. (2008). Cochrane handbook for systematic reviews of interventions. New York, NY: Cochrane Collaboration, John Wiley and Sons.
Janjigian, Y. Y., Shitara, K., Moehler, M., Garrido, M., Salman, P., Shen, L., et al. (2021). First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398 (10294), 27–40. doi:10.1016/S0140-6736(21)00797-2
Johnson, M. L., Cho, B. C., Luft, A., Alatorre-Alexander, J., Geater, S. L., Laktionov, K., et al. (2023). Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 41 (6), 1213–1227. doi:10.1200/JCO.22.00975
Jotte, R., Cappuzzo, F., Vynnychenko, I., Stroyakovskiy, D., Rodríguez-Abreu, D., Hussein, M., et al. (2020). Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J. Thorac. Oncol. 15 (8), 1351–1360. doi:10.1016/j.jtho.2020.03.028
Kang, Y. K., Chen, L. T., Ryu, M. H., Oh, D. Y., Oh, S. C., Chung, H. C., et al. (2022). Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23 (2), 234–247. doi:10.1016/S1470-2045(21)00692-6
Korn, E. L., Allegra, C. J., and Freidlin, B. (2022). Clinical benefit scales and trial design: some statistical issues. J. Natl. Cancer Inst. 114 (9), 1222–1227. doi:10.1093/jnci/djac099
Liu, N., Zhou, Y., and Lee, J. J. (2021a). IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 21 (1), 111. doi:10.1186/s12874-021-01308-8
Liu, S. V., Reck, M., Mansfield, A. S., Mok, T., Scherpereel, A., Reinmuth, N., et al. (2021b). Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (IMpower133). J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 39 (6), 619–630. doi:10.1200/JCO.20.01055
Lu, Z., Wang, J., Shu, Y., Liu, L., Kong, L., Yang, L., et al. (2022). Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ 377, e068714. doi:10.1136/bmj-2021-068714
Luo, H., Lu, J., Bai, Y., Mao, T., Wang, J., Fan, Q., et al. (2021). Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA 326 (10), 916–925. doi:10.1001/jama.2021.12836
Mai, H. Q., Chen, Q. Y., Chen, D., Hu, C., Yang, K., Wen, J., et al. (2021). Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat. Med. 27 (9), 1536–1543. doi:10.1038/s41591-021-01444-0
Miles, D., Gligorov, J., Andre, F., Cameron, D., Schneeweiss, A., Barrios, C., et al. (2021). Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 32 (8), 994–1004. doi:10.1016/j.annonc.2021.05.801
Moehler, M., Dvorkin, M., Boku, N., Özgüroğlu, M., Ryu, M. H., Muntean, A. S., et al. (2021). Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 39 (9), 966–977. doi:10.1200/JCO.20.00892
Monk, B. J., Colombo, N., Oza, A. M., Fujiwara, K., Birrer, M. J., Randall, L., et al. (2021). Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. 22 (9), 1275–1289. doi:10.1016/S1470-2045(21)00342-9
Moore, K. N., Bookman, M., Sehouli, J., Miller, A., Anderson, C., Scambia, G., et al. (2021). Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 39 (17), 1842–1855. doi:10.1200/JCO.21.00306
Morad, G., Helmink, B. A., Sharma, P., and Wargo, J. A. (2021). Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184 (21), 5309–5337. doi:10.1016/j.cell.2021.09.020
Motzer, R., Alekseev, B., Rha, S. Y., Porta, C., Eto, M., Powles, T., et al. (2021). Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 384 (14), 1289–1300. doi:10.1056/NEJMoa2035716
Motzer, R. J., Powles, T., Burotto, M., Escudier, B., Bourlon, M. T., Shah, A. Y., et al. (2022). Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 23 (7), 888–898. doi:10.1016/S1470-2045(22)00290-X
Motzer, R. J., Tannir, N. M., McDermott, D. F., Arén Frontera, O., Melichar, B., Choueiri, T. K., et al. (2018). Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378 (14), 1277–1290. doi:10.1056/NEJMoa1712126
Nishio, M., Barlesi, F., West, H., Ball, S., Bordoni, R., Cobo, M., et al. (2021). Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J. Thorac. Oncol. 16 (4), 653–664. doi:10.1016/j.jtho.2020.11.025
Owonikoko, T. K., Park, K., Govindan, R., Ready, N., Reck, M., Peters, S., et al. (2021). Nivolumab and ipilimumab as maintenance therapy in extensive-disease small-cell lung cancer: CheckMate 451. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 39 (12), 1349–1359. doi:10.1200/JCO.20.02212
Park, Y., Koh, J., Na, H. Y., Kwak, Y., Lee, K. W., Ahn, S. H., et al. (2020). PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res. Treat. 52 (3), 661–670. doi:10.4143/crt.2019.718
Parra, E. R., Villalobos, P., Behrens, C., Jiang, M., Pataer, A., Swisher, S. G., et al. (2018). Effect of neoadjuvant chemotherapy on the immune microenvironment in non-small cell lung carcinomas as determined by multiplex immunofluorescence and image analysis approaches. J. Immunother. Cancer 6 (1), 48. doi:10.1186/s40425-018-0368-0
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gümüş, M., Mazières, J., et al. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379 (21), 2040–2051. doi:10.1056/NEJMoa1810865
Paz-Ares, L. G., Ramalingam, S. S., Ciuleanu, T. E., Lee, J. S., Urban, L., Caro, R. B., et al. (2022). First-line nivolumab plus ipilimumab in advanced NSCLC: 4-year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J. Thorac. Oncol. 17 (2), 289–308. doi:10.1016/j.jtho.2021.09.010
Peters, S., Scherpereel, A., Cornelissen, R., Oulkhouir, Y., Greillier, L., Kaplan, M. A., et al. (2022). First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann. Oncol. 33 (5), 488–499. doi:10.1016/j.annonc.2022.01.074
Powles, T., Csoszi, T., Ozguroglu, M., Matsubara, N., Géczi, L., Cheng, S. Y. S., et al. (2021). Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 22 (7), 931–945. doi:10.1016/S1470-2045(21)00152-2
Powles, T., Plimack, E. R., Soulieres, D., Waddell, T., Stus, V., Gafanov, R., et al. (2020). Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 21 (12), 1563–1573. doi:10.1016/S1470-2045(20)30436-8
Reck, M., Ciuleanu, T. E., Cobo, M., Schenker, M., Zurawski, B., Menezes, J., et al. (2021). First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 6 (5), 100273. doi:10.1016/j.esmoop.2021.100273
Ribas, A., and Wolchok, J. D. (2018). Cancer immunotherapy using checkpoint blockade. Science 359 (6382), 1350–1355. doi:10.1126/science.aar4060
Rini, B. I., Powles, T., Atkins, M. B., Escudier, B., McDermott, D. F., Suarez, C., et al. (2019). Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393 (10189), 2404–2415. doi:10.1016/S0140-6736(19)30723-8
Rodriguez-Abreu, D., Powell, S. F., Hochmair, M. J., Gadgeel, S., Esteban, E., Felip, E., et al. (2021). Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 32 (7), 881–895. doi:10.1016/j.annonc.2021.04.008
Royston, P., and Parmar, M. K. (2013). Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 13, 152. doi:10.1186/1471-2288-13-152
Rudin, C. M., Awad, M. M., Navarro, A., Gottfried, M., Peters, S., Csőszi, T., et al. (2020). Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 38 (21), 2369–2379. doi:10.1200/JCO.20.00793
Sanmamed, M. F., and Chen, L. (2018). A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 175 (2), 313–326. doi:10.1016/j.cell.2018.09.035
Schmid, P., Cortes, J., Dent, R., Pusztai, L., McArthur, H., Kümmel, S., et al. (2022). Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386 (6), 556–567. doi:10.1056/NEJMoa2112651
Schmid, P., Rugo, H. S., Adams, S., Schneeweiss, A., Barrios, C. H., Iwata, H., et al. (2020). Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 21 (1), 44–59. doi:10.1016/S1470-2045(19)30689-8
Shen, X., and Zhao, B. (2018). Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 362, k3529. doi:10.1136/bmj.k3529
Shitara, K., Ajani, J. A., Moehler, M., Garrido, M., Gallardo, C., Shen, L., et al. (2022). Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 603 (7903), 942–948. doi:10.1038/s41586-022-04508-4
Socinski, M. A., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., et al. (2018). Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378 (24), 2288–2301. doi:10.1056/NEJMoa1716948
Socinski, M. A., Nishio, M., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., et al. (2021). IMpower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J. Thorac. Oncol. 16 (11), 1909–1924. doi:10.1016/j.jtho.2021.07.009
Spigel, D. R., Faivre-Finn, C., Gray, J. E., Vicente, D., Planchard, D., Paz-Ares, L., et al. (2022). Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 40 (12), 1301–1311. doi:10.1200/JCO.21.01308
Stewart, L. A., Clarke, M., Rovers, M., Riley, R. D., Simmonds, M., Stewart, G., et al. (2015). Preferred reporting Items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. Jama 313 (16), 1657–1665. doi:10.1001/jama.2015.3656
Sugawara, S., Lee, J. S., Kang, J. H., Kim, H. R., Inui, N., Hida, T., et al. (2021). Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 32 (9), 1137–1147. doi:10.1016/j.annonc.2021.06.004
Sun, J. M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., et al. (2021). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302), 759–771. doi:10.1016/S0140-6736(21)01234-4
Topalian, S. L., Drake, C. G., and Pardoll, D. M. (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27 (4), 450–461. doi:10.1016/j.ccell.2015.03.001
Wang, J., Zhou, C., Yao, W., Wang, Q., Min, X., Chen, G., et al. (2022a). Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 23 (6), 739–747. doi:10.1016/s1470-2045(22)00224-8
Wang, Z. X., Cui, C., Yao, J., Zhang, Y., Feng, J., et al. (2022b). Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell 40 (3), 277–288.e3. doi:10.1016/j.ccell.2022.02.007
West, H., McCleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H. J., et al. (2019). Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 20 (7), 924–937. doi:10.1016/S1470-2045(19)30167-6
Wolchok, J. D., Chiarion-Sileni, V., Gonzalez, R., Grob, J. J., Rutkowski, P., Lao, C. D., et al. (2022). Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 40 (2), 127–137. doi:10.1200/JCO.21.02229
Wu, H. X., Pan, Y. Q., He, Y., Wang, Z. X., Guan, W. L., Chen, Y. X., et al. (2022). Clinical benefit of first-line programmed death-1 antibody plus chemotherapy in low programmed cell death ligand 1-expressing esophageal squamous cell carcinoma: a post hoc analysis of JUPITER-06 and meta-analysis. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 41, 1735–1746. JCO2201490. doi:10.1200/JCO.22.01490
Yamaguchi, H., Hsu, J. M., Yang, W. H., and Hung, M. C. (2022). Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat. Rev. Clin. Oncol. 19 (5), 287–305. doi:10.1038/s41571-022-00601-9
Yap, D. W. T., Leone, A. G., Wong, N. Z. H., Zhao, J. J., Tey, J. C. S., Sundar, R., et al. (2023). Effectiveness of immune checkpoint inhibitors in patients with advanced esophageal squamous cell carcinoma: a meta-analysis including low PD-L1 subgroups. JAMA Oncol. 9 (2), 215–224. doi:10.1001/jamaoncol.2022.5816
Yau, T., Park, J. W., Finn, R. S., Cheng, A. L., Mathurin, P., Edeline, J., et al. (2022). Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 23 (1), 77–90. doi:10.1016/S1470-2045(21)00604-5
Yoon, H. H., Jin, Z., Kour, O., Kankeu Fonkoua, L. A., Shitara, K., Gibson, M. K., et al. (2022). Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol. 8 (10), 1456–1465. doi:10.1001/jamaoncol.2022.3707
Yu, Y., Zakeri, K., Sherman, E. J., and Lee, N. Y. (2022). Association of low and intermediate combined positive scores with outcomes of treatment with pembrolizumab in patients with recurrent and metastatic head and neck squamous cell carcinoma: secondary analysis of keynote 048. JAMA Oncol. 8 (8), 1216–1218. doi:10.1001/jamaoncol.2022.1846
Zhao, J. J., Syn, N. L., Tan, B. K. J., Yap, D. W. T., Teo, C. B., Chan, Y. H., et al. (2022b). KMSubtraction: reconstruction of unreported subgroup survival data utilizing published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 22 (1), 93. doi:10.1186/s12874-022-01567-z
Zhao, J. J., Yap, D. W. T., Chan, Y. H., Tan, B. K. J., Teo, C. B., Syn, N. L., et al. (2022a). Low programmed death-ligand 1-expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J. Clin. Oncol. official J. Am. Soc. Clin. Oncol. 40 (4), 392–402. doi:10.1200/JCO.21.01862
Zhou, C., Chen, G., Huang, Y., Zhou, J., Lin, L., Feng, J., et al. (2021). Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir. Med. 9 (3), 305–314. doi:10.1016/S2213-2600(20)30365-9
Zhou, C., Wang, Z., Sun, Y., Cao, L., Ma, Z., Wu, R., et al. (2022). Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 23 (2), 220–233. doi:10.1016/S1470-2045(21)00650-1
Keywords: PD-1, PD-L1, immunotherapy, survival, first-line
Citation: Zhang F, Chen G, Yin Y, Chen X, Nie R and Chen Y (2024) First-line immune checkpoint inhibitors in low programmed death-ligand 1-expressing population. Front. Pharmacol. 15:1377690. doi: 10.3389/fphar.2024.1377690
Received: 28 January 2024; Accepted: 10 July 2024;
Published: 26 July 2024.
Edited by:
Sheema Khan, The University of Texas Rio Grande Valley, United StatesReviewed by:
Rasha Cosman, St Vincent’s Hospital Sydney, AustraliaCopyright © 2024 Zhang, Chen, Yin, Chen, Nie and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingbo Chen, Y2hlbnliQHN5c3VjYy5vcmcuY24=; Runcong Nie, bmllcmNAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.