- 1Department of Rehabilitation Medicine, People’s Hospital of Longhua, Shenzhen, China
- 2Institute of Biomedicine and Biotechnology, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 3Department of Pharmacy, Shenzhen Bao’an Traditional Chinese Medicine Hospital, Shenzhen, China
- 4The First School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Department of Urology Surgery, Guangzhou Baiyun District Maternal and Child Health Hospital, Guangzhou, China
The increasing prevalence of depression is a major societal burden. The etiology of depression involves multiple mechanisms. Thus, the outcomes of the currently used treatment for depression are suboptimal. The anti-depression effects of traditional Chinese medicine (TCM) formulations have piqued the interest of the scientific community owing to their multi-ingredient, multi-target, and multi-link characteristics. According to the TCM theory, the functioning of the kidney is intricately linked to that of the brain. Clinical observations have indicated the therapeutic potential of the kidney-tonifying formula Erxian Decoction (EXD) in depression. This review aimed to comprehensively search various databases to summarize the anti-depression effects of EXD, explore the underlying material basis and mechanisms, and offer new suggestions and methods for the clinical treatment of depression. The clinical and preclinical studies published before 31 August 2023, were searched in PubMed, Google Scholar, China National Knowledge Infrastructure, and Wanfang Database. This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Clinical studies have demonstrated that EXD exhibits therapeutic properties in patients with menopausal depression, postpartum depression, and maintenance hemodialysis-associated depression. Meanwhile, preclinical studies have reported that EXD and its special chemical markers exert anti-depression effects by modulating monoamine neurotransmitter levels, inhibiting neuroinflammation, augmenting synaptic plasticity, exerting neuroprotective effects, regulating the hypothalamic-pituitary-adrenal axis, promoting neurogenesis, and altering cerebrospinal fluid composition. Thus, the anti-depression effects of EXD are mediated through multiple ingredients, targets, and links. However, further clinical and animal studies are needed to investigate the anti-depression effects of EXD and the underlying mechanisms and offer additional evidence and recommendations for its clinical application. Moreover, strategies must be developed to improve the quality control of EXD. This review provides an overview of EXD and guidance for future research direction.
1 Introduction
The World Health Organization has raised concerns about the increasing incidence of depression, a psychiatric condition characterized by enduring feelings of sadness, diminished drive, despair, and the inability to experience pleasure. Depression is the third most onerous ailment worldwide and is expected to majorly contribute to the disease burden by 2030 (Malhi and Mann, 2018). The global incidence of depression was further exacerbated by the COVID-19 pandemic. Approximately 40% of Chinese adults were reported to exhibit manifestations of depressive symptoms during the COVID-19 pandemic (Qin et al., 2018; Collaborators, 2021).
The etiology of depression has not been elucidated. Early studies suggested that the downregulation of monoamine neurotransmitters contributes to the onset of depression. Thus, patients with depression are currently treated with antidepressants to augment these neurotransmitter levels (Haase and Brown, 2015). However, recent studies suggest that the etiology of depression cannot be attributed to a single mechanism. Various hypotheses have been proposed to explain the pathogenesis of depression (Blier and El Mansari, 2013; Huang et al., 2020; Tartt et al., 2022), including the neuroplasticity hypothesis, the neuroinflammation hypothesis, and the hippocampal damage hypothesis. Currently, the consensus for the treatment of depression is psychotherapy in combination with antidepressants. However, the currently used antidepressants are associated with unsatisfactory outcomes, adverse reactions, limited clinical efficacy, and poor tolerability. Hence, there is an urgent need to elucidate the pathogenesis of depression and develop efficacious antidepressant drugs.
Traditional Chinese medicine (TCM) has considerable expertise in the treatment of depression. The attributes of TCM formulations include multi-target and multi-link regulation, as well as a high safety profile (Xu et al., 2020; Zhang et al., 2022). TCM offers several advantages for the treatment of depression, such as comprehensive therapeutic approaches that address both symptomatic manifestations and underlying causes, yielding stable curative effects and preventing recurrence. According to TCM theory, the functions of the kidney and brain are correlated. Thus, the administration of kidney-tonifying formulations is beneficial for maintaining brain health, repairing brain damage, and alleviating depression.
Erxian Decoction (EXD), which was initially introduced by Zhang Bo-Ne in the early 1950s, is a kidney-tonifying formulation. The composition of EXD is as follows: Epimedium brevicornu Maxim (Berberidaceae; Epimedii folium; Yin Yang Huo in Chinese; 10–15 g), Curculigo orchioides Gaertn (Hypoxidaceae; Curculiginis rhizoma; Xian Mao in Chinese; 3–15 g), Morinda officinalis F.C.How (Rubiaceae; Morindae officinalis radix; Ba Ji Tian in Chinese; 10–15 g), Angelica sinensis (Oliv.) Diels (Apiaceae; Angelicae sinensis radix; Dang Gui in Chinese; 4.5–9 g), Phellodendron chinense C.K.Schneid. (Rutaceae; Phellodendri chinensis cortex; Huang Bo in Chinese; 5–15 g), and Anemarrhena asphodeloides Bunge (Asparagaceae; Anemarrhenae rhizome; Zhi Mu in Chinese; 6–15 g). EXD was developed to treat the syndromes of kidney-yang and kidney-yin deficiency, as well as to harmonize the yin-yang balance (Li et al., 2007; Zhang et al., 2020). Epimedii folium and Curculiginis rhizoma, which are the monarch drugs in EXD, can invigorate the kidney-yang and replenish the kidney-essence (Li et al., 2007; Wang Y. et al., 2019). Morindae officinalis radix, a minister drug in EXD, exerts therapeutic effects by warming and tonifying the kidney-yang, complementing the warming and nourishing properties of the monarch drugs (Wang Y. et al., 2019). Anemarrhenae rhizome and Phellodendri chinensis cortex, which are the assistant drugs in EXD, can nourish the kidney-yin and mitigate the strong and intense properties of Curculiginis rhizoma and Epimedii folium (Wang Y. et al., 2019). Angelicae sinensis radix, which is the envoy drug of EXD, nourishes the blood and softens the liver, facilitating blood circulation and supporting the regulatory and nourishing activities of the monarch drugs on the Chong and Ren meridians (Wang Y. et al., 2019). These six herbal medicines interact synergistically and are interconnected in their actions (Zhang et al., 2021a).
Previous phytochemical studies have demonstrated that EXD water extract contains several active ingredients, such as icariin, curculigoside, ferulic acid, berberine, timosaponin B-Ⅱ, mangiferin, quercetin, kaempferol, and luteolin (Wang N. et al., 2019; Zhang et al., 2020). According to the Chinese Pharmacopoeia (2020 edition), the threshold concentration of different bioactive components is as follows: icariin (Epimedii folium marker), should not be <5.0% in Epimedii folium decoction pieces. curculigoside (Curculiginis rhizome marker), should not be <0.10% in Curculiginis rhizoma decoction pieces; ferulic acid (A. sinensis radix marker), should not be <0.050% in A. sinensis radix decoction pieces; berberine (Phellodendri chinensis cortex marker), should not be <3.0% in Phellodendri chinensis cortex decoction pieces; timosaponin B-Ⅱ and mangiferin (Anemarrhenae rhizome markers), should not be <3.0% and 0.5%, respectively. Icariin, curculigoside, ferulic acid, berberine, timosaponin B-Ⅱ, and mangiferin are the special chemical markers of EXD. The concentrations of these compounds in EXD water extract serve as major parameters for assessing the quality of EXD.
The effects of EXD, which was initially developed to treat perimenopausal syndrome in women, have been examined in clinical practice and experimental studies, which have demonstrated its anti-depression potential with favorable outcomes in diverse forms of depression (Zhang et al., 2020). Moreover, preclinical studies have demonstrated that EXD exhibits distinctive advantages, such as multi-link, multi-target, and multi-pathway characteristics, as well as minimal adverse reactions. Consequently, the elucidation of the anti-depression mechanisms of EXD has clinical significance. This review provides a comprehensive summary of the clinical and preclinical studies on the anti-depression effects of EXD and its special chemical markers. Additionally, this review provides a theoretical foundation for future studies on the anti-depression effects of EXD.
2 Methodology
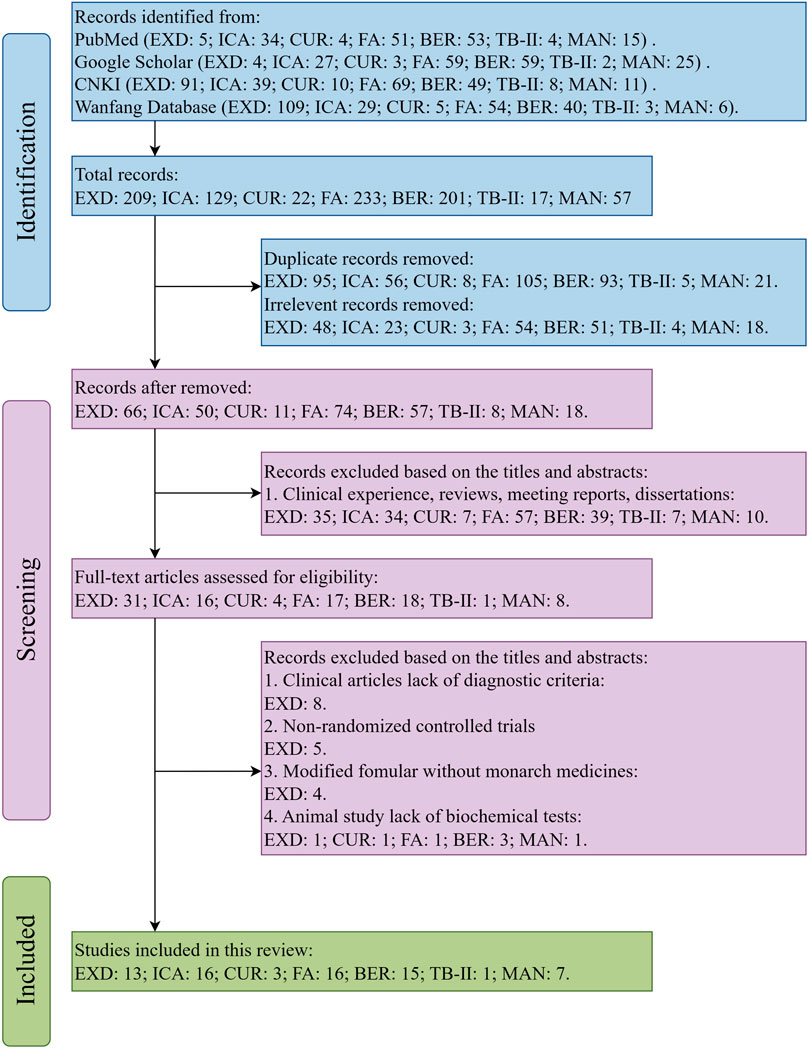
This review comprehensively searched the electronic databases based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The flow diagram of the search strategy is shown in Figure 1.
The terms “Erxian Decoction,” “Erxian Tang,” “Erxian,” “depressive disorder,” “depression,” “depressive symptom,” and “anti-depressant” and the special chemical markers of EXD were searched in the PubMed, Google Scholar, Chinese National Knowledge Infrastructure (CNKI), and Wanfang Database to retrieve studies examining the effects of EXD and its special chemical markers on depression.
The inclusion criteria employed in this study were as follows: 1) clinical and animal (in vivo) studies assessing the effectiveness of EXD (or its modified formula) in treating depression; 2) clinical studies that employed the Classification And Diagnostic Criteria Of Mental Disorders In China-Third-Edition (CCMD-3) or International Classification of diseases 10th Revision criteria for diagnosing patients with depression; 3) clinical studies that included a treatment group using EXD (or its modified formula) either as a standalone therapy or in conjunction with TCM formulations or antidepressants; (4) animal (in vivo) studies assessing the effectiveness of EXD markers in treating depression; 5) studies performed before 31 August 2023.
Meanwhile, the exclusion criteria employed in this study were as follows: 1) clinical studies that did not provide diagnostic criteria; 2) non-randomized controlled trials; 3) studies on modified formula that excluded the monarch drugs Epimedii folium and (or) Curculiginis rhizoma; 4) animal studies that lack behavioral experiments or biochemical tests; 5) studies written in languages other than Chinese and English; 6) clinical experience, reviews, meeting reports, dissertations, or meta-analysis.
3 Results
3.1 Clinical studies on the anti-depression effects of EXD
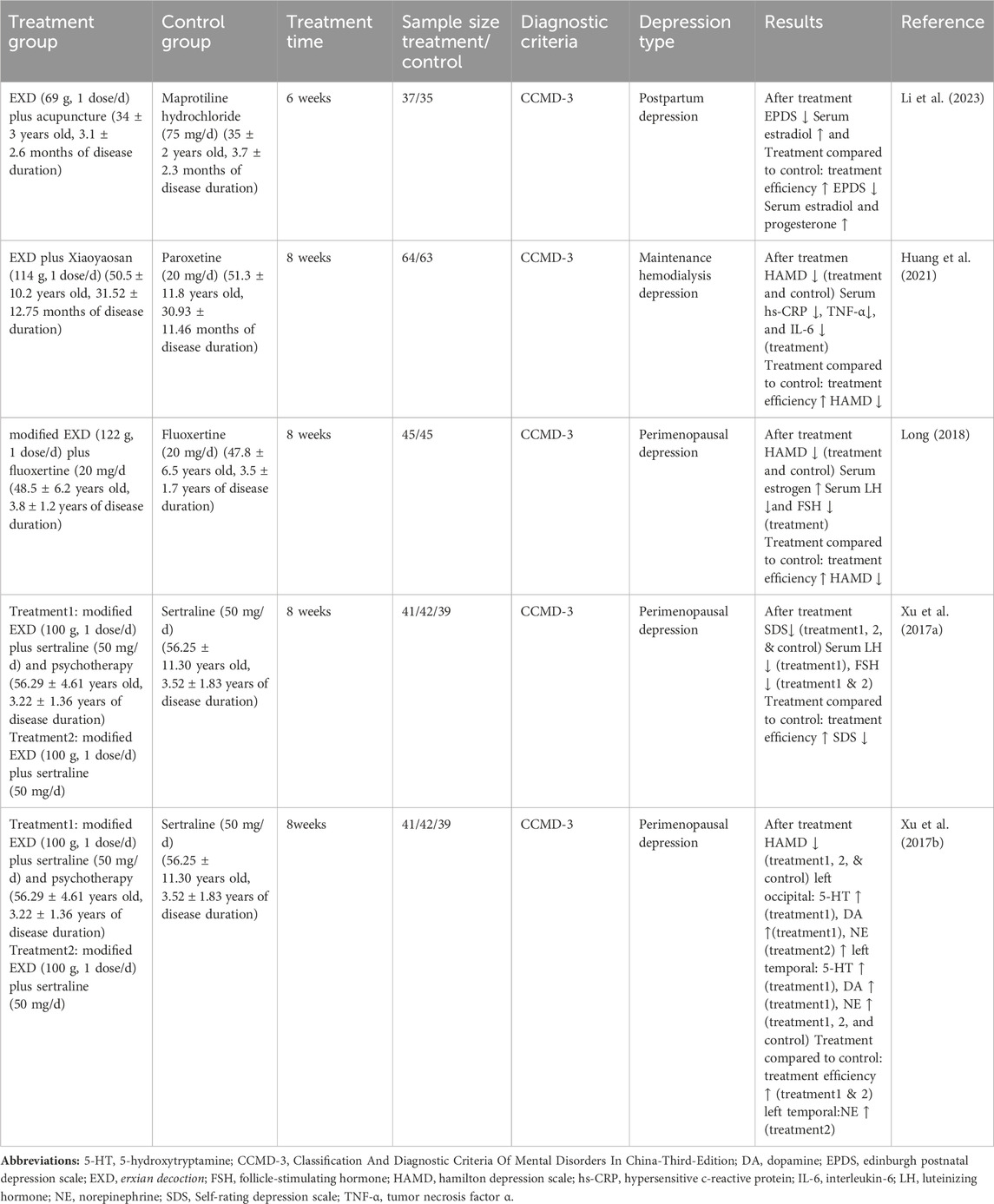
The etiology of depression has not been elucidated. Previous studies have demonstrated that the pathological process of depression is associated with various factors, including neurotransmitter dysregulation, inflammation, and neuronal impairment. EXD has been used in clinical practice for the treatment of depression in its native or modified forms in combination with other TCM formulations and conventional antidepressants. This treatment approach is effective in ameliorating depressive symptoms, yielding a favorable therapeutic effect. In this section, the studies examining the clinical anti-depression effects of EXD have been reviewed (Table 1).
3.1.1 Therapeutic effects of EXD on perimenopausal depression
Perimenopausal depression, also known as menopausal depression, is a depressive disorder that emerges during the menopausal period. In a clinical study by Xu et al. (2017b), female patients with perimenopausal depression were randomly assigned to the western medicine, integrative medicine, and integrative physical and mental treatment groups. Patients in the western medicine group were orally administered with sertraline hydrochloride (50 mg/d), while those in the integrative medicine group were orally administered with sertraline hydrochloride and modified EXD (100 g, 1 dose/d). Meanwhile, patients in the integrative physical and mental treatment group were provided psychological counseling and orally administered modified EXD and sertraline hydrochloride. After 8 weeks of treatment, the Hamilton Depression Scale (HAMD) scores in the integrative medicine group and integrative physical and mental treatment groups were lower than those in the western medicine group. Based on the HAMD score, the total effective rates were 48.89% (22/45), 78.78% (35/45), and 80.00% (36/45) in the western medicine, integrative medicine, and integrative physical and mental treatment groups, respectively. Monoamine neurotransmitter deficiency in the brain is a major etiological factor of perimenopausal depression. The administration of sertraline hydrochloride alone did not upregulate the brain levels of 5-hydroxytryptamine (5-HT), norepinephrine (NE), and dopamine (DA) in patients with perimenopausal depression. In contrast, the combination of modified EXD and sertraline hydrochloride significantly upregulated the NE levels in the left occipital region, while the combination of modified EXD, sertraline hydrochloride, and psychological counseling upregulated the levels of 5-HT and DA in both the left occipital and left anterior temporal regions. Additionally, analysis of the blood pressure, heart rate, liver and kidney functions, and electrocardiogram findings did not reveal any marked alterations (Xu et al., 2017b).
Another clinical study by Xu et al. (2017a) revealed that based on the Self-rating Depression Scale (SDS) scores, the total effective rates in the western medicine, integrative medicine, and integrative physical and mental treatment groups were 51.28%, 69.05%, and 80.49%, respectively. The ovarian function declines in perimenopausal women, leading to the dysregulation of the secretion of hormones, such as estrogen, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). This interferes with the negative feedback regulation of the hypothalamic-pituitary-gonadal axis, resulting in decreased secretion of neurotransmitters by the hypothalamus. The administration of sertraline hydrochloride alone did not significantly affect the serum levels of estrogen, FSH, and LH in patients with perimenopausal depression. However, the combination of modified EXD and sertraline hydrochloride, as well as the combination of modified EXD, sertraline hydrochloride, and psychological counseling, downregulated the FSH levels. Furthermore, the combination of modified EXD, sertraline hydrochloride, and psychological counseling downregulated the LH levels.
In a study by Long, (2018), female patients with perimenopausal depression were randomly divided into the observation and control groups (45 cases/group). Patients in the control group were orally administered with fluoxetine (20 mg/d, 8 weeks), while those in the observation group were orally administered with modified EXD (122 g, 1 dose/d, 8 weeks) and fluoxetine. The HAMD scores in the observation group were lower than those in the control group. Based on the HAMD score, the total effective rates in the control and observation groups were 53.33% and 91.11%, respectively. The serum estrogen, FSH, and LH levels were not affected in the control group. In contrast, the serum levels of estrogen were upregulated and the serum levels of FSH and LH were downregulated in the observation group.
3.1.2 Therapeutic effects of EXD on postpartum depression
Postpartum depression, which is characterized by the enduring presentation of depressive symptoms in women after childbirth, usually manifests within 6 weeks of childbirth. The incidence rate of postpartum depression is in the range of 2.1%–31.6% (Gressier et al., 2015). Furthermore, 20%–30% of patients with postpartum depression experience a relapse during subsequent pregnancies (Fisher et al., 2016). In a study by Li et al. (2023), 72 female patients with postpartum depression were randomly divided into the observation (37 cases) and western medicine groups (35 cases). Patients in the observation group were subjected to acupuncture and orally administered with EXD (69 g, 1 dose/d), while those in the western medicine group were orally administered with maprotiline hydrochloride (75 mg/d). After 6 weeks of treatment, compared with those in the western medicine group, the Edinburgh Postnatal Depression Scale scores were lower, the estrogen levels were higher, and the progesterone levels were downregulated in the observation group. The total effective rates in the observation and western medicine groups were 94.6% (35/37) and 62.9% (22/35), respectively. Furthermore, analysis of bodyweight, blood pressure, heart rate, or liver and kidney functions did not reveal marked alterations throughout the treatment period.
3.1.3 Therapeutic effects of EXD on maintenance hemodialysis-associated depression
Patients undergoing maintenance hemodialysis exhibit psychological distress owing to the protracted nature of their illness, exorbitant treatment costs, and the prevalence of various severe complications. These patients are susceptible to develop negative affective states, such as anxiety and depression. In particular, depression is a prevailing mental disorder among patients undergoing maintenance hemodialysis with incidence rates in the range of 22.8%–62.0% (Griva et al., 2018; Zamanian et al., 2018). Prolonged depressive episodes adversely affect the quality of life of these patients and may increase the risk of sudden mortality. In a study by Huang et al., 2021, 130 patients with maintenance hemodialysis-associated depression were administered conventional treatment for the underlying disease and were randomly divided into control (32 male cases and 33 female cases) and observation groups (34 male cases and 31 female cases). Patients in the control group were orally administered with paroxetine (20 mg/d), while those in the observation group were orally administered with EXD and XiaoYaoSan (114 g, 1 dose/d) for 8 weeks. The total effective rates in the observation and control groups were 79.69% (51/64) and 69.84% (44/63), respectively. The HAMD scores in the observation group were lower than those in the control group. The serum levels of hypersensitive C-reactive protein (hs-CRP), interleukin (IL)-6, and tumor necrosis factor-alpha (TNF-α) were not affected in the control group but were significantly upregulated in the observation group.
3.2 Animal studies on the anti-depression effects of EXD
Previous studies have examined the effectiveness of EXD in ameliorating depressive symptoms and cognitive impairment in different depression models, including the maternal separation + chronic restraint stress (MS-RS)-induced, chronic unpredictable mild stress (CUMS)-induced, and reserpine-induced depression models, using a series of behavioral experiments. The findings of these experiments indicate that EXD exerts anti-depression effects by inhibiting neuroinflammation, enhancing synaptic plasticity, upregulating monoamine neurotransmitter levels, and alleviating neuronal damage. This section reviews the mechanisms through which EXD exerts anti-depression effects in the animal depression models (Table 2).
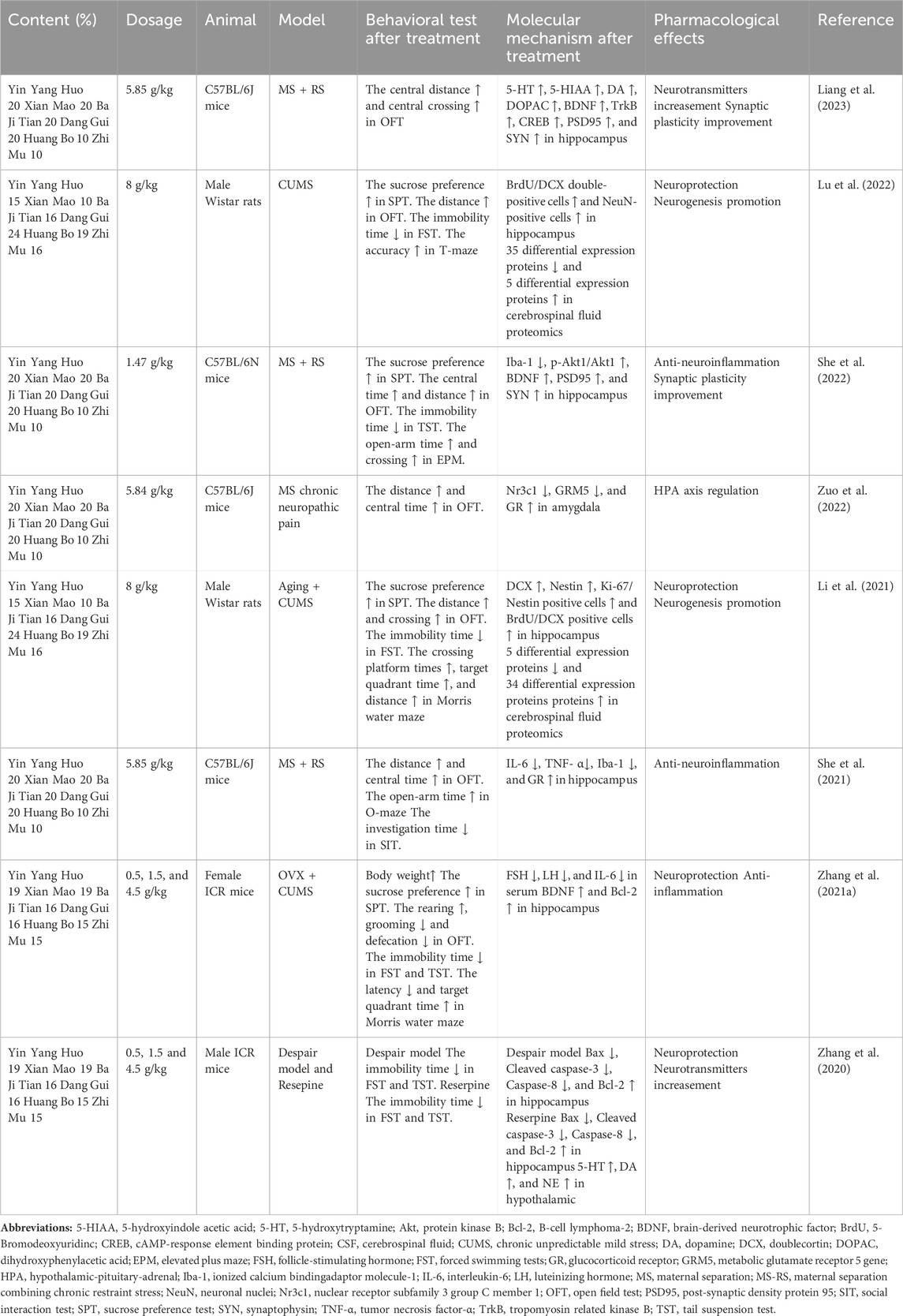
Table 2. Summary of animal studies on the anti-depression effects of EXD and the underlying molecular mechanisms.
3.2.1 Modulation of monoamine neurotransmitter levels
The pathogenesis of depression involves multiple mechanisms, including the downregulation of monoamine neurotransmitters in the brain. NE, 5-HT, and DA are critical for the regulation of human emotions and cognitive processes. The downregulation of these neurotransmitters in the brain tissue can induce neuronal hypoactivity, leading to depression and cognitive dysfunction (Malhi and Mann, 2018). Various antidepressant medications aim to modulate the levels of these neurotransmitters. For example, selective serotonin reuptake inhibitors can augment serotonin release and concentrations in patients with depression, eliciting an antidepressant response.
Liang et al. (2023) reported that EXD upregulated the 5-HT, DA, 5-hydroxyindoleacetic acid (5-HTAA), and dihydroxyphenylacetic acid (DOPAC) levels in the hippocampus of the MS-RS-induced depression mouse model. Zhang et al. (2020) focused on the hypothalamus and established a depression mouse model by intraperitoneally injecting reserpine. The authors demonstrated that EXD upregulates the levels of 5-HT, DA, and NA in the hypothalamus of the depression mouse model. These findings suggest a correlation between the anti-depression effects of EXD and the upregulation of monoamine neurotransmitters in the brain.
3.2.2 Alleviation of neuroinflammation
Recent studies have reported the upregulation of inflammatory markers in patients diagnosed with depression, suggesting a correlation between depression and neuroinflammation (Xie et al., 2023). Microglia, which are immune cells residing in the brain, maintain cerebral homeostasis and facilitate nerve restoration. Activated microglia differentiate into M1 and M2 subtypes. M1 microglia promote neuroinflammation, whereas M2 microglia exhibit anti-inflammatory properties (Wang H. et al., 2022). Ionized calcium-binding adapter molecule 1 (Iba-1) serves as a surface marker for M1 microglia. After activation, M1 microglia promote the secretion of inflammatory cytokines, including IL-1β, IL-6, and TNF-α, eliciting protective responses in the nervous system against detrimental stressors (Wang Y. L. et al., 2018). Prolonged and excessive microglial activation promotes the generation of various inflammatory mediators, resulting in neuroinflammation, exacerbation of neurotoxicity, and neuronal damage (Beurel et al., 2020; Guo et al., 2020).
She et al., 2021 demonstrated that EXD significantly decreased the mRNA and protein expression levels of Iba-1, as well as the levels of IL-6 and TNF-α in the hippocampus, in the MS-RS-induced depression mouse model. Additionally, Zhang et al. (2021b) reported that EXD downregulated the serum IL-6 contents in a perimenopausal depression mouse model.
The mitogen-activated protein kinase (MAPK) signaling pathway facilitates the proinflammatory process of microglia and is strongly correlated with impaired synaptic plasticity and neuroinflammation during the pathogenesis of depression. She et al., 2022 performed a network pharmacology analysis and reported that the MAPK signaling pathway is the principal pathway mediating the anti-depression effects of EXD. However, this finding was not experimentally verified.
3.2.3 Augmentation of synaptic plasticity
Impaired synaptic plasticity has been a focus area of studies on the pathogenesis of depression. Synaptic plasticity, which encompasses alterations in the structure and function of synapses, serves as the foundation of neural plasticity. The release of mature brain-derived neurotrophic factor (BDNF) from dendrites is critical for the diverse manifestations of synaptic plasticity. BDNF binds and stimulates tyrosine receptor kinase B (TrkB) receptors, initiating the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt) pathway, facilitating cAMP-response element binding protein (CREB) activation, and subsequently exerting regulatory effects on synaptic plasticity (Zhang and Liao, 2020; Rana et al., 2021; Wang et al., 2021). Chronic stress is reported to downregulate the expression of BDNF and impair signal transduction. This leads to neuronal atrophy and synaptic dysfunction, diminishing stress resilience, enhancing stress susceptibility, and promoting the development of depression (Castrén and Rantamäki, 2010).
The PI3K/Akt signaling pathway enhances the viability and proliferation of neuronal cells. Additionally, the PI3K/Akt signaling pathway mediates the mechanism of action of various antidepressant medications (Zeng et al., 2022). Activated PI3K promotes the phosphorylation of Akt, which subsequently upregulates BDNF and downregulates apoptotic genes. These molecular events further contribute to the enhancement of synaptic plasticity and the maintenance of neuronal homeostasis by supporting essential processes, such as neuronutrition, neuronal survival, and the inhibition of apoptosis. Synaptophysin (SYN), a calcium-binding protein predominantly found in presynaptic terminals, serves as an indirect indicator of synaptic count, distribution, and density. Postsynaptic density protein 95 (PSD95) plays a crucial role in postsynaptic remodeling and signal transmission on the postsynaptic membrane (Li et al., 2022). Network pharmacology studies by Luo et al., 2020 and She et al., 2022 have predicted that the PI3K/Akt pathway is the major pathway mediating the anti-depression effect of EXD with Akt1 serving as the central target. In a subsequent validation experiment, She et al., 2022 demonstrated that EXD significantly upregulates the phosphorylation of Akt1 and the protein and mRNA expression levels of BDNF, PSD95, and SYN in the hippocampus of the MS-RS-induced depression mouse model.
CREB regulates various neuronal processes, including growth, development, synaptic plasticity, and the formation of long-term memory. The phosphorylation of CREB exerts beneficial effects, such as the upregulation of BDNF, the inhibition of cell apoptosis, and the facilitation of cell differentiation and repair after injury. Liang et al., 2023 demonstrated that EXD significantly enhanced the protein and mRNA levels of BDNF, TrkB, and CREB in the hippocampus of the MS-RS-induced depression mouse model. Additionally, Zhang et al. (2021a) utilized ovariectomy combined with CUMS to establish a mouse model of menopausal depression and demonstrated that EXD upregulated the BDNF levels in the hippocampus of the perimenopausal depression mouse model.
3.2.4 Protection of neurons and induction of neurogenesis
Hippocampal damage is reported to be involved in the etiology of depression. Dysfunctional neurogenesis and neural loss in the hippocampus are therapeutic targets for depression (Huang et al., 2020). Cognitive impairments, including deficits in memory and learning, frequently manifest in patients with depression, indicating a strong correlation between hippocampal dysfunction and the initiation and progression of depressive symptoms. Clinical autopsies have consistently demonstrated decreased hippocampal volume along with neuronal atrophy and loss in patients diagnosed with depression. Preclinical studies have yielded empirical evidence for the reduction in the quantity and length of dendritic branches in the hippocampal neurons along with dysfunctional neurogenesis in depression animal models. Thus, the disruption of the structure and function of the hippocampus is associated with the onset and progression of depressive disorders.
Bax and B-cell lymphoma-2 (Bcl-2) proteins, which are members of the Bcl-2 family, exert pro-apoptotic and anti-apoptotic effects, respectively. Additionally, caspase-3 and caspase-8 serve as crucial initiators of apoptosis. Zhang et al., 2020 demonstrated that EXD dose-dependently upregulated Bcl-2 expression and downregulated Bax, caspase-8, and cleaved caspase-3 expression in the hippocampus of the despair mouse models and reserpine-treated mice. Additionally, another study by Zhang et al. (2021a) demonstrated that EXD dose-dependently upregulated Bcl-2 expression in the hippocampus of a menopausal depression mouse model.
Hippocampal neurogenesis encompasses the intricate mechanisms of neural stem cell proliferation and differentiation and the survival of newly formed neural cells. Previous studies have revealed a strong correlation between the dysregulation of hippocampal neurogenesis and the initiation and progression of depressive disorders. Prolonged stress impedes the proliferation of hippocampal neural stem cells, resulting in the downregulation of cell proliferation in the dentate gyrus (DG) region, which disrupts the process of neuronal differentiation and generation. Lu et al., 2022 demonstrated that EXD augmented the number of newborn precursor neurons and mature neurons in the hippocampal DG region of the CUMS-induced depression rat model. Li et al., 2021 used a combination of natural aging and CUMS to establish a rat model of late-onset depression (LOD) and demonstrated that EXD effectively increased the population of newborn neural stem cells, precursor neurons, and mature neurons in the hippocampal DG region of rats with LOD.
3.2.5 Alteration of the composition of cerebrospinal fluid (CSF)
The CSF can be used for investigating central nervous system diseases as it is in direct contact with the central nervous system. Thus, CSF offers valuable insights into the physiological and pathological conditions of the brain, encompassing metabolic and biochemical reactions. The dysregulation of CSF composition promotes both physiological and pathological alterations in the brain. Positioning close to the lateral ventricle, the hippocampus is associated with the CSF. The components of the CSF can directly affect the structure and function of the hippocampus. The CSF proteome is markedly altered in patients diagnosed with depression. These differentially expressed proteins are closely linked to central nervous system damage and dysfunction in patients with depression.
Lu et al., 2022 performed proteomics analysis to identify changes in the CSF proteome of the CUMS-induced depression rat model treated with EXD. EXD mitigated the CUMS-induced dysregulation of 40 proteins in the rat CSF. These differentially expressed proteins were primarily associated with the ribosome and ubiquitin-mediated proteolysis pathway. In particular, ribosomal protein S19, ribosomal protein S12, ribosomal protein S14, vimentin, and ubiquitin-like modifier activating enzyme 1 mediated the therapeutic effects of EXD on depression and hippocampal damage. Another proteomics study by Li et al., 2021 demonstrated that EXD mitigated the CUMS-induced dysregulation of 39 proteins in the CSF of naturally aging rats. Additionally, some proteins involved in promoting neurogenesis, such as growth differentiation factor 11, neuronal cell adhesion molecule, and Ghrelin were downregulated in the CSF after CUMS modeling but were upregulated after EXD treatment.
3.2.6 Regulation of the hypothalamic-pituitary-adrenal (HPA) axis
The hyperactivity of the HPA axis, a prevalent neurobiological manifestation of depression, can impair neuronal function and activity, resulting in the development of clinical symptoms of depression. Zuo et al., 2022 demonstrated that EXD upregulated glucocorticoid receptor (GR) and downregulated GR (Nr3c1) and glutamate metabotropic receptor 5 (GRM5) in the amygdala of the depression mouse model. She et al., 2021 demonstrated that EXD upregulated the expression of GR in the hippocampus of the MS-RS-induced depression mouse model.
3.3 Studies on the anti-depression effects of EXD special chemical markers
Icariin, curculigoside, ferulic acid, berberine, timosaponin B-Ⅱ, and mangiferin are the special chemical markers of EXD. The quantification of these components in EXD serves as a criterion for assessing EXD quality. This section primarily focuses on icariin, curculigoside, ferulic acid, berberine, timosaponin B-Ⅱ, and mangiferin as they are considered the special chemical markers that mediate the anti-depression effects of EXD (Supplementary Table S1).
3.3.1 Anti-depression effects of icariin
Icariin is the most important and principal bioactive constituent in Epimedii folium. Previous studies have examined the effects of icariin, especially the anti-depression effects. The findings of these studies indicate that icariin is a potential candidate for the development of antidepressant medications.
Icariin is reported to exhibit anti-inflammatory properties. Previous studies have demonstrated that icariin can ameliorate depressive-like behavior in the CUMS-induced depression rat model by inhibiting the nuclear factor kappa-B (NF-κB) signaling pathway and the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome/Caspase-1/IL-1β axis, suppressing the release of TNF-α (Liu et al., 2015). Furthermore, icariin alleviates neuroinflammation in the hippocampus of mice with depression by inhibiting the high mobility group box-1 (HMGB1)/receptor for advanced glycation end-products (RAGE) signaling pathway and activating the X-box binding protein 1 spliced (XBP1s)/NF-κB signaling pathway (Liu et al., 2019). Additionally, icariin downregulates the serum levels of IL-6 and TNF-α in rats with depression (Pan et al., 2006).
The hyperactivity of the HPA axis, which is a prevalent neurobiological manifestation of depression, can impair hippocampal function and neuronal activity, resulting in the development of depression-related clinical symptoms. Icariin exerts anti-depression effects by regulating the HPA axis (Wu et al., 2011; Wei et al., 2016). Icariin can effectively downregulate the expression of corticotropin-releasing factor (CRF) in the hypothalamus and adrenocorticotropic hormone (ACTH) and corticosterone (CORT) in the pituitary gland, as well as upregulate the expression of GR in the liver, contributing to the amelioration of depressive behavior in mice (Liu et al., 2022). Furthermore, icariin downregulates the expression of GR in the hippocampus and prefrontal cortex and CRF in the serum, cortex, hippocampus, corpus striatum, and medulla oblongata in rats with depression (Pan et al., 2010; Pan et al., 2007).
Icariin exerts anti-depression effects by augmenting the monoamine neurotransmitter levels. Previous studies have demonstrated that icariin upregulates the concentrations of 5-HT, DA, and NA in the cortex, hippocampus, and striatum of the CUMS-induced depression rat model (Zhang et al., 2018). Furthermore, icariin upregulates the serum levels of 5-HT, DA, and NA in rats with perimenopausal depression (Cao et al., 2019). Additionally, the anti-depression effects of icariin include the regulation of glutamate reuptake (Zhang et al., 2017).
Icariin exerts inhibitory effects on hippocampal apoptosis in the CUMS-induced depression rat model by downregulating the expression levels of Bax, caspase-3, cleaved caspase-3, and cytochrome C and upregulating the expression of Bcl-2 (Wu et al., 2023). Moreover, icariin promotes hippocampal neurogenesis in the CUMS-induced depression rat model as evidenced by the upregulation of the number of precursor neurons and mature neurons. The icariin-mediated neurogenesis in the CUMS-induced depression rat model is closely related to alterations in CSF proteomics, especially differentially expressed proteins associated with the ribosome, PI3K/Akt, and IL-17 signaling pathways (Zeng et al., 2022). Furthermore, the icariin-mediated upregulation of BNDF and synapse activity in rats with depression involved the modulation of various proteins, including Akt, CREB, TrkB, and MAPK (Gong et al., 2016; Di et al., 2024). Additionally, the neuroprotective properties of icariin are associated with the inhibition of oxidative stress (Xue et al., 2021).
3.3.2 Anti-depression effects of curculigoside
Curculiginis rhizoma comprises various compounds, including polysaccharides, saponins, phenols, glycosides, and terpenes. Of these, curculigoside is the sole constituent included in the Chinese Pharmacopoeia as a quality control marker for Curculiginis rhizoma decoction pieces.
Recent studies have provided evidence for the anti-depression properties of curculigoside. In particular, curculigoside effectively mitigates depression-like behavior in mice subjected to the learned helplessness paradigm through the upregulation of protein kinase A (PKA) pathway, the downregulation of granule cell apoptosis in the hippocampal DG region, and the inhibition of astrocyte activation (Shen et al., 2019). Alternatively, curculigoside may exert antidepressant effects by promoting the expression of hippocampal BDNF and activating the hippocampal Akt/mammalian target of rapamycin (mTOR) signaling pathway (Yang et al., 2019). Furthermore, curculigoside alleviates depression-like behaviors in the perimenopausal depression mouse model by upregulating the 5-HT and DA levels (Miao et al., 2017).
3.3.3 Anti-depression effects of ferulic acid
Ferulic acid is a reliable marker for A. sinensis radix. Previous studies have reported that ferulic acid mitigates atypical depressive behavior in different animal models of depression, indicating that it is a potential anti-depression agent.
Studies examining the anti-depression properties of ferulic acid revealed that it exerts anti-neuroinflammatory effects (Singh et al., 2017). Ferulic acid inhibited the activation of microglia and significantly decreased the contents of IL-1β, IL-6, and TNF-α in the prefrontal cortex of the CUMS-induced depression mouse model by inhibiting the NLRP3/caspase-1/NF-κB pathway (Liu et al., 2017c). Furthermore, ferulic acid downregulates the levels of proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6) and upregulates the levels of anti-inflammatory cytokines (such as IL-10) in the hippocampus of the rat depression model by inhibiting the phosphorylation of the NF-κB pathway-related proteins (Zheng et al., 2019).
Ferulic acid exerts regulatory effects on monoamine neurotransmitters. In particular, ferulic acid inhibits the reuptake of 5-HT, NE, and DA, enhancing their concentrations in different brain regions, including the hippocampus and frontal cortex (Zhang et al., 2011; Xu et al., 2013; Zhang et al., 2013; Chen et al., 2015; Sasaki et al., 2019). Ferulic acid is also reported to facilitate synaptic plasticity. Ferulic acid can upregulate the expression of BDNF and PSD95 in the prefrontal cortex and hippocampus of the depressed mouse (Yabe et al., 2010; Liu et al., 2017a), and active the CREB/BDNE/TrkB signaling pathway in the hippocampus of depressive Goto-Kakizaki rats induced by CUMS (Wang et al., 2020). Ferulic acid exerts anti-depression effects through the regulation of the HPA axis. Previous studies have reported that ferulic acid downregulates the serum ACTH and CORT levels and upregulates hippocampal GR expression in the rat depression model (Zheng et al., 2019). Additionally, the neuroprotective properties of ferulic acid are associated with the inhibition of oxidative stress, mitochondrial dysfunction, and apoptosis (Zeni et al., 2012; Lenzi et al., 2015; Zeni et al., 2017; Sasaki et al., 2019; Li et al., 2020). Furthermore, some studies have suggested a correlation between the anti-depression effect of ferulic acid and the modulation of gut microbiota (Deng et al., 2022).
3.3.4 Anti-depression effects of berberine
Berberine, a prominent constituent of Phellodendri chinensis cortex, is a potential therapeutic for depression. Recently, berberine was reported to exert anti-depression effects in diverse animal models of depression.
Berberine effectively mitigates depression-like symptoms by exerting anti-neuroinflammation (Liu et al., 2017b and Xu et al., 2018) effects. Additionally, berberine significantly downregulated the contents of IL-1β, IL-18, pro-IL-1β, pro-IL-18, and TNF-α in the hippocampus of the CUMS-induced depression mouse model by inhibiting the activity of microglia and downregulating the expression of tripartite motif 65 (Trim65), NLRP3, caspase-1, apoptosis-associated speckle-like protein (ASC), and gasdermin D (GSDMD) (Yang et al., 2023).
Berberine significantly enhances synaptic plasticity. Previous studies have demonstrated that berberine effectively upregulates the expression of hippocampal PSD95 and SYN in mice with depression, reversing the decreased density of dendritic spines, mushroom spines, and thin spines, as well as promoting the length and depth of postsynaptic dendrites (Qin et al., 2023).
Additionally, berberine promotes the expression of monoamine neurotransmitters, upregulating the levels of 5-HT, DA, NE, and gamma-aminobutyric acid in various brain regions, including the hippocampus, cortex, striatum, and amygdala (Wang Q. et al., 2022; Ge et al., 2023).
Previous studies have reported that berberine upregulates BDNF levels, promotes neuronal survival, and stimulates neurogenesis in animals exhibiting depressive symptoms (Lee et al., 2012; Shen et al., 2016; Fan et al., 2017; Gong et al., 2019; Lu et al., 2021; Yi et al., 2021; Zhan et al., 2021; Ge et al., 2023; Qin et al., 2023). The alteration of gut microbiota has also been implicated in the anti-depression effects of berberine (Huang et al., 2023). Berberine can reverse the physical damage of gastrointestinal tract in CUMS rats (Zhu et al., 2017).
3.3.5 Anti-depression effects of timosaponin B-Ⅱ and mangiferin
Saponins serve as the primary bioactive constituents in Anemarrhenae rhizome. Among these saponins, timosaponin B-Ⅱ constitutes 50% of the total saponin content in Anemarrhenae rhizome. Thus, the timosaponin B-II content is a crucial parameter for ensuring the quality control of Anemarrhenae rhizome decoction pieces. Timosaponin B-Ⅱ is a major bioactive compound in Anemarrhenae rhizome. Previous studies have demonstrated that timosaponin B-Ⅱ inhibits the reuptake of brain neurotransmitters (5-HT, NE, and DA), exerting an anti-depression effect (Lu et al., 2010).
Mangiferin can also be used to ensure the quality control of Anemarrhenae rhizome decoction pieces. The anti-depression properties of mangiferin are primarily attributed to its anti-inflammatory activity (Tao et al., 2023). Mangiferin treatment suppresses microglial activity and downregulates the levels of TNF-α, IL-6, and IL-1β in the hippocampus of mice with postpartum depression (Yan et al., 2022). Furthermore, mangiferin downregulates the contents of IL-18, IL-1β, IL-6, and TNF-α in the hippocampus of the CUMS-induced depression mouse model by inhibiting the NLRP3/ASC/caspase-1 pathway (Cao et al., 2017). Additionally, mangiferin exerts neuroprotective effects by alleviating oxidative stress levels and upregulating BDNF in the hippocampus and prefrontal cortex of mice with depression (Fu et al., 2013; Jangra et al., 2014; Luo et al., 2021).
4 Discussion
The prevalence of depression has recently increased. However, the currently used treatments are associated with limited efficacy and side effects and are ineffective in preventing recurrence. The pathogenesis of depression is characterized by multifactorial and intricate mechanisms. Consequently, an effective approach to treat depression should be based on multiple targets, pathways, and mechanisms. The efficacy and safety profiles of TCM formulations are higher than those of conventional prescription antidepressants owing to their multiple components and mechanisms and the ability to modulate multiple targets and pathways.
According to the basic theory of TCM, “the brain is the place where the primordial spirit resides” and “the kidney stores willpower.” “Huangdi Neijing,” which is the most influential classic of TCM, emphasizes that “the brain is the sea of marrow” and that “the kidney stores essence and mainly induces bones to produce marrow.” Additionally, “Huangdi Neijing” revealed that “if the kidney does not grow, the marrow cannot be full.” The kidney-essence has a critical role in maintaining diverse mental processes and emotional fluctuations. Thus, a deficiency in kidney-essence results in a depletion of the marrow sea, while a lack of kidney-yang leads to inadequate warmth nourishment and transpiration, rendering the brain susceptible to emotional and cognitive dysfunctions, including depression, anxiety, and dementia. Conversely, when the kidney is abundant in essence and qi, the marrow is adequately replenished, enhancing the resistance of the brain to illnesses. The “kidney-brain axis” theory suggests that a deficiency of kidney function plays a major role in the pathogenesis of depression and that kidney-tonifying therapy is a potential therapeutic strategy for depression.
EXD, a popular kidney-toning prescription, was originally developed for the treatment of menopausal syndrome in women. Most of the previous review articles on EXD have focused on its anti-menopausal effect. This review summarized the clinical and preclinical studies on the anti-depression effect of EXD, as well as the therapeutic effects of its special chemical markers on depression, based on current research on the pathogenesis of depression (Figure 2). To the best of our knowledge, this is the first study to review the effect of EXD on depression.
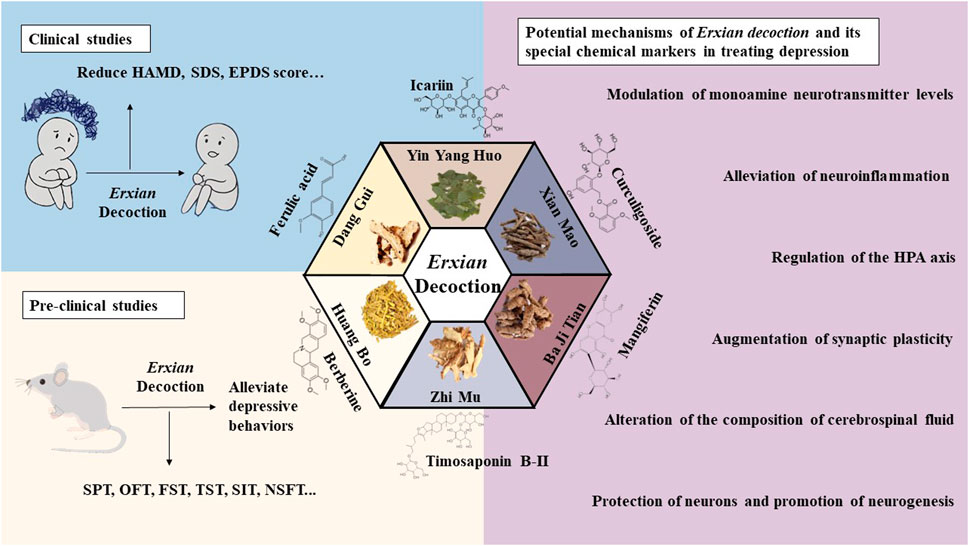
Figure 2. Anti-depression effects of EXD. EXD significantly decreased depression-related scores, improved the quality of life of patients, and exerted therapeutic effects on depressive disorder with limited side effects. EXD and its special chemical markers exert anti-depression effects by modulating monoamine neurotransmitter levels, inhibiting neuroinflammation, augmenting synaptic plasticity, protecting neurons, promoting neurogenesis, regulating the HPA axis, and altering the composition of CSF.
Various clinical investigations have reported the efficacy of EXD in mitigating depressive symptoms among patients with depression stemming from diverse etiologies, encompassing general depression, menopausal depression, postpartum depression, LOD, and other depressive disorders secondary to diseases. Moreover, EXD alone or in combination with antidepressants, TCM formulations, or therapeutic modalities was efficacious in the management of depressive symptoms.
This review screened clinical studies examining the anti-depression effects of EXD. Studies lacking clear diagnostic criteria, control groups, or sufficient sample sizes were excluded from this review. These exclusion criteria were set as they indicate a lack of rigorous experimental design and limited support for the translational medicine applications of EXD to treat depression. Additionally, in clinical practice, TCM practitioners may modify the prescription based on the condition of the patient. As Epimedii folium and Curculiginis rhizoma serve as the monarch drugs in EXD, studies on modified EXD without Epimedii folium and Curculiginis rhizoma as its constituents were excluded from this review.
The results of studies that satisfied the inclusion criteria demonstrated that EXD decreases the HAMD and SDS scores, upregulates the monoamine neurotransmitter levels in the brain, and alleviates sex hormone imbalance in patients with menopausal depression. Additionally, EXD ameliorates depressive symptoms in patients with postpartum depression and maintenance hemodialysis-associated depression. However, these findings do not indicate that EXD is solely effective for menopausal depression, postpartum depression, and maintenance hemodialysis-associated depression. Several studies have reported the therapeutic effects of EXD on general depression and LOD. These studies were excluded from this review due to unclear diagnostic criteria, absence of control groups, and small sample sizes. Additionally, the optimal therapeutic regimen involving EXD must include psychotherapy. Limited numbers of clinical trials have investigated the efficacy of the combination of EXD and psychotherapy in treating depression. Thus, the existing clinical studies have provided some evidence for the therapeutic potential of EXD in depression. However, the quality of these clinical studies is not satisfactory. Hence, a large number of standardized and rigorous clinical trials must be performed to enable the application of EXD as an alternative therapy or a complementary therapy in the future.
This review also screened the preclinical studies evaluating the anti-depression mechanisms of EXD. Studies lacking behavioral experiments or performing only cellular experiments were excluded as the effect of EXD on depressive symptoms cannot be determined based on these experiments. Preclinical studies have demonstrated that EXD and its special chemical markers ameliorate depressive symptoms, such as anhedonia and suppress autonomic activity and hopelessness in diverse depression models. The anti-depression effect of EXD and its special chemical markers may be attributed to the following mechanisms: 1) the modulation of monoamine neurotransmitter levels (Figure 3): EXD and its special chemical markers can promote the synthesis of 5-HT, NE, and DA, inhibit the reuptake of 5-HT, NE, and DA, and regulate the expression of postsynaptic neurotransmitter receptors; 2) the inhibition of neuroinflammation (Figure 4): EXD and its special chemical markers can inhibit the NLRP3/caspase-1/IL-1β, HMGB1/RAGE, and XBP1/NF-κB pathways to suppress the release of cytokines, such as TNF-α, IL-1β, IL-6, and IL-18; 3) the augmentation of synaptic plasticity (Figure 5): EXD and its special chemical markers can activate the PI3K/Akt, HMGB1/RAGE, CREB/BDNF, and BDNF/TrkB pathways, promote the release of BDNF, and upregulate the expression of PSD95 and SYN; 4) the regulation of the HPA axis (Figure 6): EXD and its special chemical markers can inhibit the secretion of CRF, ACTH, and CORT, downregulate the contents of CRF, ACTH, and CORT in the blood, and regulate the expression of HPA-related hormone receptors in the brain and liver; 5) exerting neuroprotective effects (Figure 7): EXD and its special chemical markers exert neuroprotective effects by inhibiting apoptosis, suppressing autophagy, alleviating oxidative stress, upregulating energy metabolism, promoting neurogenesis, regulating gut microbiota, and altering CSF composition. Several high-quality preclinical studies have revealed that EXD exerts anti-depression effects through multi-ingredient, multi-target, and multi-mechanism properties, providing strong evidence for the therapeutic application of EXD in depression.
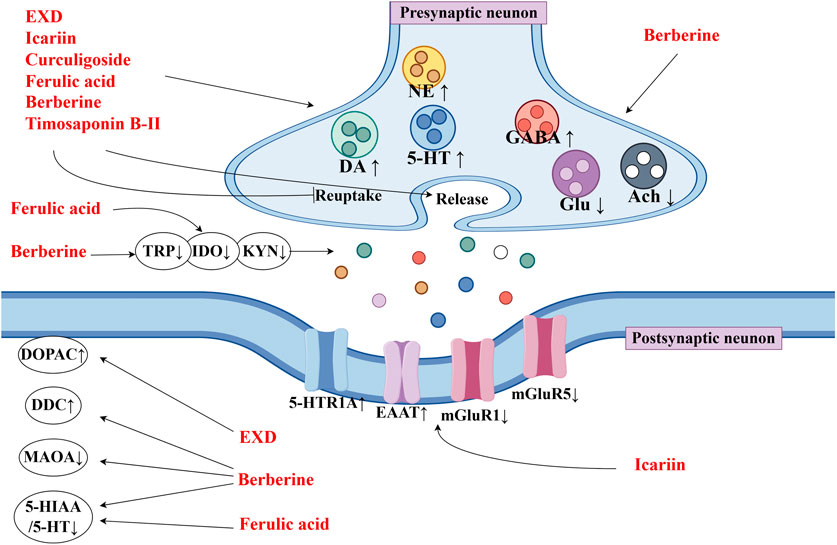
Figure 3. EXD modulates the monoamine neurotransmitter levels. EXD and its special chemical markers promote the synthesis of 5-HT, NE, and DA, inhibit the reuptake of 5-HT, NE, and DA, and regulate the expression of postsynaptic neurotransmitter receptors.
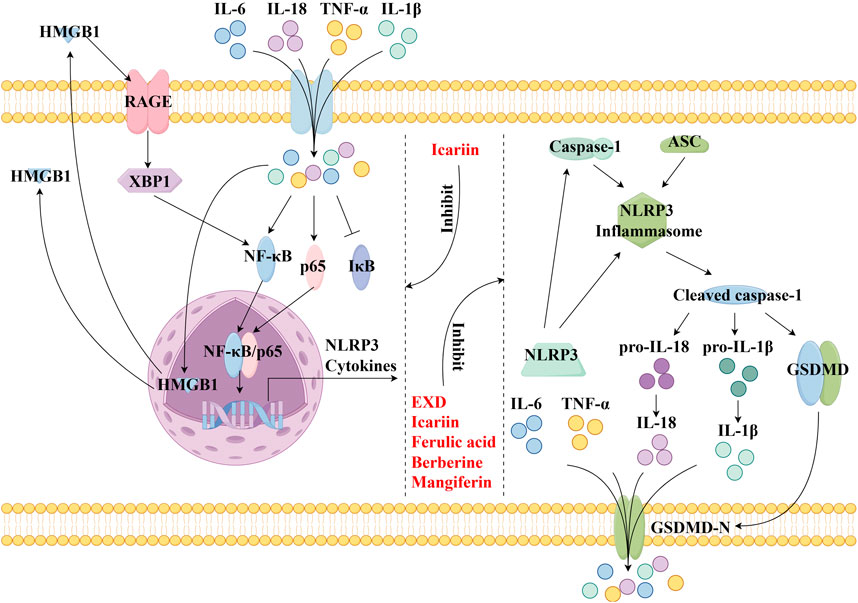
Figure 4. EXD inhibits neuroinflammation. EXD and its special chemical markers inhibit the NLRP3/caspase-1/IL-1β, HMGB1/RAGE, and XBP1/NF-κB pathways, suppressing the release of cytokines, such as TNF-α, IL-1β, IL-6, and IL-18.
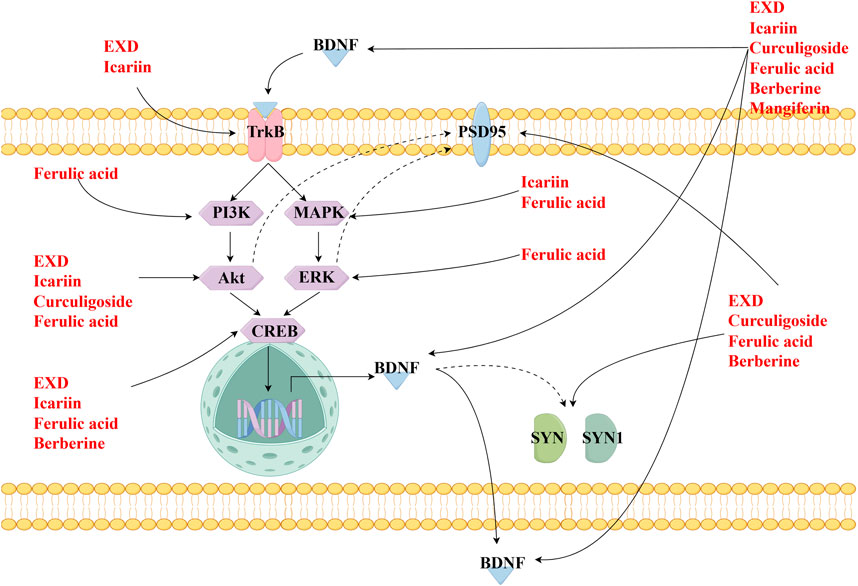
Figure 5. EXD augments synaptic plasticity. EXD and its special chemical markers activate the PI3K/Akt, HMGB1/RAGE, CREB/BDNF, and BDNF/TrkB pathways, promote the release of BDNF, and upregulate the expression of PSD95 and SYN.
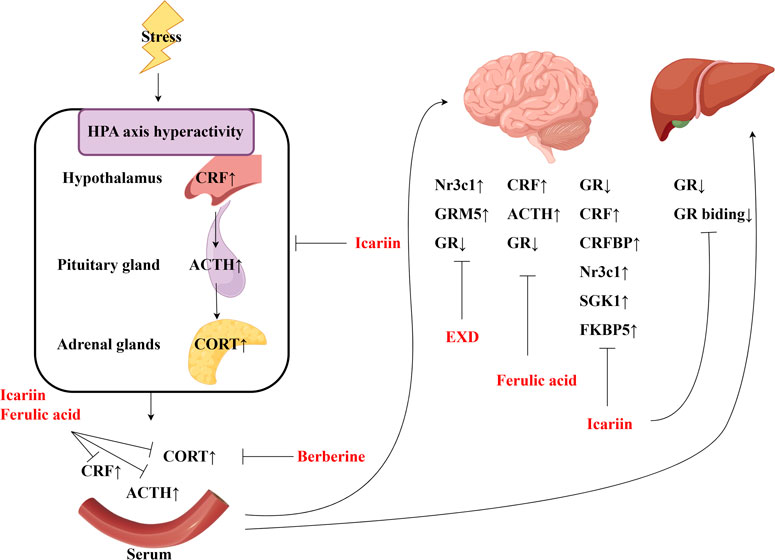
Figure 6. EXD regulates the HPA axis. EXD and its special chemical markers can inhibit the secretion of CRF, ACTH, and CORT, downregulate the contents of CRF, ACTH, and CORT in the blood, and regulate the expression of HPA-related hormone receptors in the brain and liver.
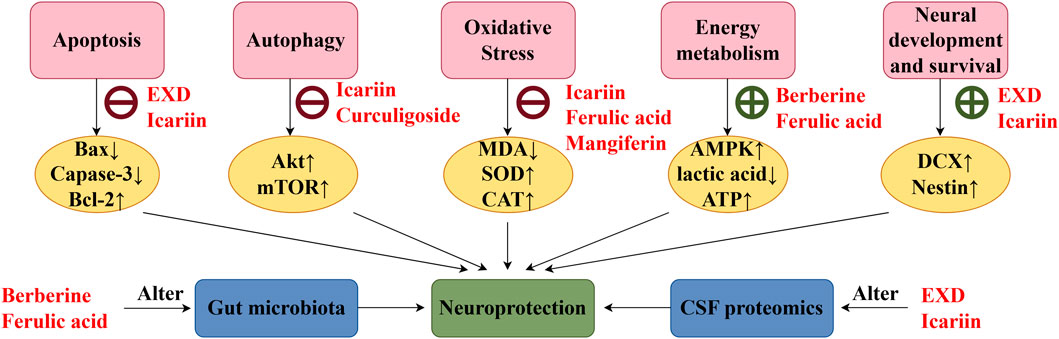
Figure 7. EXD exerts neuroprotective effects. EXD and its special chemical markers exert neuroprotective effects by inhibiting apoptosis, suppressing autophagy, alleviating oxidative stress, enhancing energy metabolism, promoting neurogenesis, regulating gut microbiota, and altering cerebrospinal fluid composition.
However, further preclinical studies are needed to address some limitations. The current preclinical studies of EXD have not utilized a standardized herbal preparation, resulting in heterogeneous concentrations of active components in the water extract. The yields of icariin, curculigoside, berberine, and ferulic acid in the water extract of EXD prepared with Epimedii folium, Curculiginis rhizoma, Morindae officinalis radix, A. sinensis radix, Phellodendri chinensis cortex, and Anemarrhenae rhizome at a ratio of 9:9:9:9:6:6 were 1.605, 0.002, 1.814, and 0.007 mg/g, respectively (Wong et al., 2021). Meanwhile, the yields of icariin, curculigoside, berberine, ferulic acid, and mangiferin in the EXD water extract prepared with Epimedii folium, Curculiginis rhizoma, Morindae officinalis radix, A. sinensis radix, Phellodendri chinensis cortex, and Anemarrhenae rhizome at a ratio of 12:12:10:9:10:9 were 1.490, 0.002, 1.001, 0.1999, and 0.6591 mg/g, respectively (Cheung et al., 2017). Although all the herbs in EXD exert anti-depression effects, further studies are needed to develop a standardized herbal preparation that can enhance the effectiveness of depression treatment.
Some of the constituents of EXD are reported to exert toxic effects. The maximum oral dose of Epimedii folium water and alcohol extracts for mice is 80 g/kg bodyweight, which is 560 times the maximum clinical dose for humans. In long-term toxicity experiments, continuous gavage of Epimedii folium water and alcohol extracts at a dose of 80 g/kg bodyweight for 8 weeks significantly decreased the bodyweight of mice and dysregulated the liver and kidney function indicators in the serum. The effect of Epimedii folium water and alcohol extracts on the liver and kidney function indicators was mitigated at a dose of 20 g/kg bodyweight, which was 140 times the maximum dose for humans (Wang Q. et al., 2018). The maximum oral dose of Curculiginis rhizoma water extract for mice is 206 g/kg bodyweight, which is 1384 times the maximum clinical daily dose for humans. The half-maximal lethal dose (LD50) of Curculiginis rhizoma alcohol extract is 215.9 g/kg bodyweight, which is 1439 times the maximum clinical daily dose for humans. In acute toxicity experiments, treatment with Curculiginis rhizoma water extract at a dose of 206 g/kg bodyweight did not cause death in mice, while treatment with Curculiginis rhizoma water extract at a dose of 90 g/kg bodyweight did not exert toxic effects (Chen et al., 2021). Additionally, treatment with Phellodendri chinensis cortex water extract at a dose of 80 g/kg bodyweight results in acute toxicity and can cause gastrointestinal reactions (Qiu et al., 2004). The toxicity of A. sinensis radix, Anemarrhenae rhizome, and Morindae officinalis radix is not substantial. The conventional equivalent dose of A. sinensis radix is 2 g/kg bodyweight, and no acute toxic effects were observed upon treatment with A. sinensis radix water extract at a dose of 80 g/kg bodyweight (Min et al., 2012). The maximum oral tolerance of Anemarrhenae rhizome water extract for mice is 35.0 g/kg bodyweight (equivalent to 145–291 times the human dose), while that of Anemarrhenae rhizome alcohol extract for mice is 37.5 g/kg bodyweight (equivalent to 156–312 times the human dose) (Liu et al., 2014). Currently, the dose of EXD used in clinics is lower than that used in toxicity experiments. Epimedii folium can mitigate the toxicity of Curculiginis rhizoma (Zhu et al., 2015), while Morindae officinalis radix can alleviate the toxicity of Epimedii folium (Ling et al., 2018). Most current publications do not indicate the toxic effects of EXD. Previous studies have suggested that EXD is safe and reliable. However, long-term and high-dose oral administration of these herbal extracts may result in potential toxicity. Future studies must focus on decreasing the toxic effects of EXD. Additionally, the liver and kidney functions must be regularly monitored after the oral administration of high doses of EXD for a prolonged period.
In summary, clinical and experimental studies have reported the therapeutic potential of EXD in alleviating depression. However, several issues must be addressed. The availability of contemporary studies on the clinical utilization of EXD for depression treatment is limited with small sample sizes and diminished quality of evidence. Additionally, the optimal treatment regimen involving EXD must include psychotherapy. However, the concurrent application of EXD and psychological therapy is uncommon. Furthermore, the inadequate standardization of EXD, encompassing factors such as herbal proportion, ingredient concentration, and dose control, is a major limitation for a comprehensive analysis of the dose-response relationship. Although EXD has several targets for the treatment of depression, limited studies have identified these targets and their interconnections. All constituent herbs of EXD exhibit anti-depression properties. However, the specific component primarily mediating the anti-depression effect and the interactions among these constituents have not been elucidated. Finally, depression is a central nervous system disease. The impact of EXD on blood-brain barrier permeability has not been established.
Future studies should focus on the application of EXD, especially the combination of EXD and psychotherapy, as a therapeutic for depression by implementing multi-center, large-scale, and rigorous randomized controlled trials. Additionally, the technical barriers associated with developing standardized quality control of EXD and minimizing toxicity must be addressed to ensure the safety, efficacy, and stability of the treatment. Furthermore, contemporary methodologies, such as metabolomics, genomics, proteomics, high-performance liquid chromatography, and network pharmacology must be integrated to establish a “herbal medicine-ingredient-target-pathway” network of EXD, facilitate the elucidation of the anti-depression mechanism, and the identification of the active ingredients of EXD. These approaches will provide a theoretical foundation for the translational medicine application of EXD to treat depression.
5 Limitations
This study has several limitations. 1) The strict exclusion criteria of this study resulted in the non-inclusion of several studies with unsatisfactory quality. This may affect the elucidation of the therapeutic potential of EXD in depression. 2) Although nystose serves as a chemical marker of Morindae officinalis radix, limited studies have examined its anti-depression properties. 3) Various active ingredients of EXD, such as quercetin, luteolin, and kaempferol exhibit anti-depression properties attributed to EXD. However, these compounds are also prevalent in other herbs and are not special chemical markers of EXD. This review did not focus on these compounds.
6 Conclusion
Depression is a psychiatric disorder with adverse effects on the physical and mental health of patients. The etiology of depression cannot be attributed to a single mechanism. Clinical studies have demonstrated that EXD exhibits therapeutic properties in patients with menopausal depression, postpartum depression, and maintenance hemodialysis-associated depression, suggesting the therapeutic potential of EXD in depression. Meanwhile, experimental studies have confirmed the therapeutic effects of EXD on depression-like behavior and demonstrated its multi-ingredient, multi-target, and multi-mechanism characteristics. These studies have provided evidence for the anti-depression effects of EXD. The development of EXD as an alternative or complementary therapy for depression has a promising future. However, large-scale studies on the efficacy and side effects of EXD are lacking. Additionally, strategies to ensure the quality control of EXD are inadequate. Moreover, the anti-depression mechanisms of EXD must be further elucidated. Thus, extensive clinical and animal studies must be performed to thoroughly investigate the anti-depression effects and mechanisms, ingredients, and quality control of EXD. These studies will provide high-quality evidence and recommendations for the clinical application of EXD.
Author contributions
N-XZ: Conceptualization, Funding acquisition, Investigation, Writing–original draft, Writing–review and editing. HL: Investigation, Writing–original draft, Writing–review and editing. M-YS: Investigation, Writing–review and editing. XC: Investigation, Writing–review and editing. X-YY: Investigation, Writing–review and editing. MS: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Construction Funds of Key Medical Disciplines in Longhua District, Shenzhen (MKD202007090206, to MS); the Guangdong Basic and Applied Basic Research Foundation (2022A1515110668, to N-XZ); the China Postdoctoral Science Foundation (2022M723310, to N-XZ); the Inflammation and Immune Mediated Diseases Laboratory of Anhui Province Open Project (IMMDL20220005, to N-XZ).
Acknowledgments
Figures were illustrated by Figdraw.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1377079/full#supplementary-material
References
Beurel, E., Toups, M., and Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron 107 (2), 234–256. doi:10.1016/j.neuron.2020.06.002
Blier, P., and El Mansari, M. (2013). Serotonin and beyond: therapeutics for major depression. Philosophical Trans. R. Soc. Lond. Ser. B, Biol. Sci. 368 (1615), 20120536–20120541-20120536-7. doi:10.1098/rstb.2012.0536
Cao, C., Su, M., and Zhou, F. (2017). Mangiferin inhibits hippocampal NLRP3 inflammasome and exerts antidepressant effects in a chronic mild stress mice model. Behav. Pharmacol. 28 (5), 356–364. doi:10.1097/FBP.0000000000000305
Cao, L. H., Qiao, J. Y., Huang, H. Y., Fang, X. Y., Zhang, R., Miao, M. S., et al. (2019). PI3K-AKT signaling activation and icariin: the potential effects on the perimenopausal depression-like rat model. Molecules 24 (20), 3700. doi:10.3390/molecules24203700
Castrén, E., and Rantamäki, T. (2010). The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev. Neurobiol. 70 (5), 289–297. doi:10.1002/dneu.20758
Chen, J., Guo, X. H., Zhang, X. Q., and Liu, X. (2021). Research progress on chemical constituents, pharmacological and toxicology effects of Curculigo orchioides Gaertn. China J. Traditional Chin. Med. Pharm. 36 (07), 4151–4158.
Chen, J., Lin, D., Zhang, C., Li, G., Zhang, N., Ruan, L., et al. (2015). Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 30 (1), 129–136. doi:10.1007/s11011-014-9635-z
Cheung, H. P., Wang, S. W., Ng, T. B., Zhang, Y. B., Lao, L. X., Zhang, Z. J., et al. (2017). Comparison of chemical profiles and effectiveness between erxian decoction and mixtures of decoctions of its individual herbs: a novel approach for identification of the standard chemicals. Chin. Med. 12, 1. doi:10.1186/s13020-016-0123-8
Collaborators, C.-M. D. (2021). Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 398 (10312), 1700–1712. doi:10.1016/S0140-6736(21)02143-7
Deng, L., Zhou, X., Tao, G., Hao, W., Wang, L., Lan, Z., et al. (2022). Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 162 (Pt A), 111887. doi:10.1016/j.foodres.2022.111887
Di, X., Wan, M., Bai, Y. N., Lu, F., Zhao, M., Zhang, Z., et al. (2024). Exploring the mechanism of Icariin in the treatment of depression through BDNF-TrkB pathway based on network pharmacology. Naunyn-Schmiedeberg's Archives Pharmacol. 397 (1), 463–478. doi:10.1007/s00210-023-02615-1
Fan, J., Li, B., Ge, T., Zhang, Z., Lv, J., Zhao, J., et al. (2017). Berberine produces antidepressant-like effects in ovariectomized mice. Sci. Rep. 7 (1), 1310. doi:10.1038/s41598-017-01035-5
Fisher, S. D., Wisner, K. L., Clark, C. T., Sit, D. K., Luther, J. F., and Wisniewski, S. (2016). Factors associated with onset timing, symptoms, and severity of depression identified in the postpartum period. J. Affect. Disord. 203, 111–120. doi:10.1016/j.jad.2016.05.063
Fu, Y. Y., Song, Y. J., Yang, Y. H., and Duan, J. Y. (2013). The efects of mangiterin on the improvement of behavior and expression of brain-derived neurotrophic factor in the hippocampus of chronic stress-induced depression model mice. Chin. J. Behav. Med. Brain Sci. 22 (10), 883–885. doi:10.3760/cma.j.issn.1674-6554.2013.10.006
Ge, P. Y., Qu, S. Y., Ni, S. J., Yao, Z. Y., Qi, Y. Y., Zhao, X., et al. (2023). Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting Ido1-associated with tryptophan metabolism. Phytotherapy Res. 37 (1), 342–357. doi:10.1002/ptr.7616
Gong, M. J., Han, B., Wang, S. M., Liang, S. W., and Zou, Z. J. (2016). Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats. J. Pharm. Biomed. Analysis 123, 63–73. doi:10.1016/j.jpba.2016.02.001
Gong, Q., Yan, X. J., Lei, F., Wang, M. L., He, L. L., Luo, Y. Y., et al. (2019). Proteomic profiling of the neurons in mice with depressive-like behavior induced by corticosterone and the regulation of berberine: pivotal sites of oxidative phosphorylation. Mol. Brain 12 (1), 118. doi:10.1186/s13041-019-0518-4
Gressier, F., Tabat-Bouher, M., Cazas, O., and Hardy, P. (2015). Paternal postpartum depression: a review. Presse Medicale 44 (4 Pt 1), 418–424. doi:10.1016/j.lpm.2014.09.022
Griva, K., Lam, K. F. Y., Nandakumar, M., Ng, J. H., McBain, H., and Newman, S. P. (2018). The effect of brief self-management intervention for hemodialysis patients (HED-SMART) on trajectories of depressive and anxious symptoms. J. Psychosomatic Res. 113, 37–44. doi:10.1016/j.jpsychores.2018.07.012
Guo, X., Rao, Y., Mao, R., Cui, L., and Fang, Y. (2020). Common cellular and molecular mechanisms and interactions between microglial activation and aberrant neuroplasticity in depression. Neuropharmacology 181, 108336. doi:10.1016/j.neuropharm.2020.108336
Haase, J., and Brown, E. (2015). Integrating the monoamine, neurotrophin and cytokine hypotheses of depression--a central role for the serotonin transporter? Pharmacol. Ther. 147, 1–11. doi:10.1016/j.pharmthera.2014.10.002
Huang, J. C., Mao, Y. L., Zou, W. Z., Wang, R., Liu, M. L., and Lei, L. (2021). Effects of Erxian xiaoyao powder on the hemodialysis patients complicated with depr ession belong to kidney deficiency and liver stagnation. Clin. J. Traditional Chin. Med. 33 (08), 1529–1533. doi:10.16448/j.cjtcm.2021.0830
Huang, M., He, Y., Tian, L., Yu, L., Cheng, Q., Li, Z., et al. (2023). Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect. Disord. 325, 141–150. doi:10.1016/j.jad.2022.12.166
Huang, Y. L., Zeng, N. X., Chen, J., Niu, J., Luo, W. L., Liu, P., et al. (2020). Dynamic changes of behaviors, dentate gyrus neurogenesis and hippocampal miR-124 expression in rats with depression induced by chronic unpredictable mild stress. Neural Regen. Res. 15 (6), 1150–1159. doi:10.4103/1673-5374.270414
Jangra, A., Lukhi, M. M., Sulakhiya, K., Baruah, C. C., and Lahkar, M. (2014). Protective effect of mangiferin against lipopolysaccharide-induced depressive and anxiety-like behaviour in mice. Eur. J. Pharmacol. 740, 337–345. doi:10.1016/j.ejphar.2014.07.031
Lee, B., Sur, B., Yeom, M., Shim, I., Lee, H., and Hahm, D. H. (2012). Effect of berberine on depression- and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J. Physiology Pharmacol. 16 (6), 379–386. doi:10.4196/kjpp.2012.16.6.379
Lenzi, J., Rodrigues, A. F., Rós Ade, S., de Castro, A. B., de Lima, D. D., Magro, D. D., et al. (2015). Ferulic acid chronic treatment exerts antidepressant-like effect: role of antioxidant defense system. Metab. Brain Dis. 30 (6), 1453–1463. doi:10.1007/s11011-015-9725-6
Li, H. Z., Zeng, N. X., Liu, K. G., Luo, W. L., Lu, W. J., and Wu, L. L. (2021). Preliminary study on cerebrospinal fluid proteomics of Erxian Decoction against neurogenesis impairment in late-onset depression. China J. Chin. Materia Medica 46 (23), 6231–6242. doi:10.19540/j.cnki.cjcmm.20210918.401
Li, J. H., Wang, J. Y., Li, J. L., Li, B. P., Yao, J., and Zhang, K. Z. (2023). Acupuncture combined with Erxian decoction in the treatment of postpartum depression. Chin. Med. Mod. Distance Educ. China 21 (14), 102–105. doi:10.3969/j.issn.1672⁃2779.2023.14.037
Li, J. J., Li, J. T., and Fu, J. P. (2007). Erxian tang--introduction of a Chinese herbal formula, clinical practice, and experimental studies. Chin. J. Integr. Med. 13 (1), 67–73. doi:10.1007/s11655-007-0067-z
Li, P. P., Shi, X., Wang, R., Wang, Y. W., and Wang, L. N. (2022). Effects of Yiqi-Huoxue decoction on learning, memory and hippocampal synaptic plasticity in cerebral ischemia/reperfusion rats. Chin. J. Pathophysiol. 38 (12), 2249–2257. doi:10.3969/j.issn.1000-4718.2022.12.018
Li, W., Liu, X., and Qiao, H. (2020). Downregulation of hippocampal SIRT6 activates AKT/CRMP2 signaling and ameliorates chronic stress-induced depression-like behavior in mice. Acta Pharmacol. Sin. 41 (12), 1557–1567. doi:10.1038/s41401-020-0387-5
Liang, W. Q., Cheng, K., Gong, Z. H., Yang, J. W., Meng, D. H., Meng, X. Y., et al. (2023). Study on the biological basis of syndrome in depressed nice from synaptic plasticity. J. Basic Chin. Med. 29 (3), 394–400. doi:10.19945/j.cnki.issn.1006-3250.2023.03.027
Ling, J., Wang, M., Chen, Y., Song, J., Sun, E., Shi, Z. Q., et al. (2018). Analysis of Folium Epimedium toxicity in combination with Radix Morindae Officinalis based on zebrafish toxicity/metabolism synchronization. Acta Pharm. Sin. 53 (1), 74–83. doi:10.16438/j.0513-4870.2017-0756
Liu, B., Xu, C., Wu, X., Liu, F., Du, Y., Sun, J., et al. (2015). Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 294, 193–205. doi:10.1016/j.neuroscience.2015.02.053
Liu, F., Li, L. F., Liu, G. J., Jiang, H., and Zhang, Q. Z. (2014). Study on acute toxicity of water and alcohol extract of Anemarrhenae Rhizoma. Tianjin J. Traditional Chin. Med. 31 (6), 361–364. doi:10.11656/j.issn.1672-1519.2014.06.13
Liu, L., Zhao, Z., Lu, L., Liu, J., Sun, J., and Dong, J. (2019). Icariin and icaritin ameliorated hippocampus neuroinflammation via mediating HMGB1 expression in social defeat model in mice. Int. Immunopharmacol. 75, 105799. doi:10.1016/j.intimp.2019.105799
Liu, Y. M., Hu, C. Y., Shen, J. D., Wu, S. H., Li, Y. C., and Yi, L. T. (2017a). Elevation of synaptic protein is associated with the antidepressant-like effects of ferulic acid in a chronic model of depression. Physiology Behav. 169, 184–188. doi:10.1016/j.physbeh.2016.12.003
Liu, Y. M., Niu, L., Wang, L. L., Bai, L., Fang, X. Y., Li, Y. C., et al. (2017b). Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Besearch Bull. 134, 220–227. doi:10.1016/j.brainresbull.2017.08.008
Liu, Y. M., Shen, J. D., Xu, L. P., Li, H. B., Li, Y. C., and Yi, L. T. (2017c). Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 45, 128–134. doi:10.1016/j.intimp.2017.02.007
Liu, Y. Q., Ji, H. B., Xiao, W. H., and Lin, L. (2022). Effect of icariin on neuroendocrine system function in depression animal model by regulating central CRF and HPA axis. Chongqing Med. 51 (9), 1453–1457+1462. doi:10.3969/j.issn.1671-8348.2022.09.003
Long, X. P. (2018). Efficacy analysis of modified Erxian decoction on improving symptoms and endocrine indexes for patients with perimenopausal depression. J. Sichuan Traditional Chin. Med. 36 (8), 169–172.
Lu, M. Z., Zhang, Z. Q., Yi, J., Chen, W. S., Hou, Z. H., and Li, T. J. (2010). Study on the effect and mechanisms of timosaponin B-Ⅱ on antidepressant. J. Pharm. Pract. 28 (4), 283–287. doi:10.3969/j.issn.1006-0111.2010.04.014
Lu, S. F., Wang, B. Y., Bai, M., Xu, E. P., and Li, Y. C. (2021). Effects of berberine on energy metabolism of hippocampus in depression mice. China J. Traditional Chin. Med. Pharm. 36 (6), 3580–3584.
Lu, W. J., Niu, J., Luo, W. L., Zeng, N. X., Yan, C., and Wu, L. L. (2022). Antidepressant effect of Erxian decoction based on cerebrospinal fluid proteomics. Chin. J. Integr. Traditional West. Med. 42 (10), 1231–1243. doi:10.7661/j.cjim.20210918.332
Luo, G. Q., Liu, L., Gao, Q. W., Wu, X. N., Xiang, W., and Deng, W. T. (2017). Mangiferin prevents corticosterone-induced behavioural deficits via alleviation of oxido-nitrosative stress and down-regulation of indoleamine 2,3-dioxygenase (Ido) activity. Neurological Res. 39 (8), 709–718. doi:10.1080/01616412.2017.1310705
Luo, W. L., Chen, J., Niu, J., Li, L., Zeng, N. X., Yan, C., et al. (2020). Study of treating effect of Erxian decoction on depression based on network pharmacology. Chin. Pharmacol. Bull. 36 (9), 1317–1324. doi:10.3969/j.issn.1001-1978.2020.09.024
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392 (10161), 2299–2312. doi:10.1016/S0140-6736(18)31948-2
Miao, M., Tian, S., Guo, L., Bai, M., Fang, X., and Liu, S. (2017). The effect of curculigoside on mouse model of perimenopausal depression. Saudi J. Biol. Sci. 24 (8), 1894–1902. doi:10.1016/j.sjbs.2017.11.033
Min, H., Cheng, J. L., Chen, J. W., Xu, J. Y., Deng, W., Liu, P. Q., et al. (2012). A study on the effect of the blood enrichment and acute toxicity of cell-wall broken powder of Angelica sinensis. J. Guangdong Pharm. Univ. 28 (1), 73–75. doi:10.3969/j.issn.1006-8783.2012.01.020
Pan, Y., Kong, L. D., Li, Y. C., Xia, X., Kung, H. F., and Jiang, F. X. (2007). Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats. Pharmacol. Biochem. Behav. 87 (1), 130–140. doi:10.1016/j.pbb.2007.04.009
Pan, Y., Wang, F. M., Qiang, L. Q., Zhang, D. M., and Kong, L. D. (2010). Icariin attenuates chronic mild stress-induced dysregulation of the LHPA stress circuit in rats. Psychoneuroendocrinology 35 (2), 272–283. doi:10.1016/j.psyneuen.2009.06.020
Pan, Y., Zhang, W. Y., Xia, X., and Kong, L. D. (2006). Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley rats. Biol. Pharm. Bull. 29 (12), 2399–2403. doi:10.1248/bpb.29.2399
Qin, X., Wang, S., and Hsieh, C.-R. (2018). The prevalence of depression and depressive symptoms among adults in China: estimation based on a National Household Survey. China Econ. Rev. 51, 271–282. doi:10.1016/j.chieco.2016.04.001
Qin, Z., Shi, D. D., Li, W., Cheng, D., Zhang, Y. D., Zhang, S., et al. (2023). Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflammation 20 (1), 54. doi:10.1186/s12974-023-02744-7
Qiu, S. H., Tang, H. B., Li, F. Y., Xiao, R. J., and Chen, B. Y. (2004). Experimental study of the acute toxicity on commom-used bitter and cold medicines. Cent. South Pharm. 2 (1), 37–38. doi:10.3969/j.issn.1672-2981.2004.01.014
Rana, T., Behl, T., Sehgal, A., Srivastava, P., and Bungau, S. (2021). Unfolding the role of BDNF as a biomarker for treatment of depression. J. Mol. Neurosci. 71 (10), 2008–2021. doi:10.1007/s12031-020-01754-x
Sasaki, K., Iwata, N., Ferdousi, F., and Isoda, H. (2019). Antidepressant-like effect of ferulic acid via promotion of energy metabolism activity. Mol. Nutr. Food Res. 63 (19), e1900327. doi:10.1002/mnfr.201900327
She, K. J., Gao, J. J., Gong, Z. H., Zhang, H. R., Zuo, Y., Yang, J. W., et al. (2021). Changes of microglia in hippocampus of mice induced by maternal separation with restraint stress and regulatory effect of Wenyang jieyu prescription. Chin. J. Exp. Traditional Med. Formulae 27 (18), 49–57. doi:10.13422/j.cnki.syfjx.20211837
She, K. J., Gong, Z. H., Yang, J. W., Liang, W. Q., Meng, D. H., and Yue, G. X. (2022). Feasibility of Erxian decoction and Wenshen prescription in treatment of depression based on network pharmacology and experimental verification. Chin. J. Exp. Traditional Med. Formulae 28 (16), 211–223. doi:10.13422/j.cnki.syfjx.20221542
Shen, F. M., Yang, S. J., Zhang, Z. R., and Zhu, G. Q. (2019). Effect of curculigoside on the apoptosis of hippocampal neurons in a mouse model of learned helplessness and related mechanisms. J. Anhui Univ. Chin. Med. 38 (6), 38–43. doi:10.3969/j.issn.2095-7246.2019.06.011
Shen, J. D., Ma, L. G., Hu, C. Y., Pei, Y. Y., Jin, S. L., Fang, X. Y., et al. (2016). Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 614, 77–82. doi:10.1016/j.neulet.2016.01.002
Singh, T., Kaur, T., and Goel, R. K. (2017). Ferulic acid supplementation for management of depression in epilepsy. Neurochem. Res. 42 (10), 2940–2948. doi:10.1007/s11064-017-2325-6
Tao, Y., Tian, X., Luo, J., Zhu, H., Chu, Y., and Pei, L. (2023). Mangiferin inhibits chronic stress-induced tumor growth in colorectal liver metastases via WAVE2 signaling pathway. Heliyon 9 (3), e13753. doi:10.1016/j.heliyon.2023.e13753
Tartt, A. N., Mariani, M. B., Hen, R., Mann, J. J., and Boldrini, M. (2022). Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol. Psychiatry 27 (6), 2689–2699. doi:10.1038/s41380-022-01520-y
Wang, A. R., Mi, L. F., Zhang, Z. L., Hu, M. Z., Zhao, Z. Y., Liu, B., et al. (2021). Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway. Behav. Brain Res. 403, 113138. doi:10.1016/j.bbr.2021.113138
Wang, H., He, Y., Sun, Z., Ren, S., Liu, M., Wang, G., et al. (2022a). Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 19 (1), 132. doi:10.1186/s12974-022-02492-0
Wang, N., Xin, H., Xu, P., Yu, Z., and Shou, D. (2019a). Erxian decoction attenuates TNF-α induced osteoblast apoptosis by modulating the akt/nrf2/HO-1 signaling pathway. Front. Pharmacol. 10, 988. doi:10.3389/fphar.2019.00988
Wang, Q., Sun, Y. N., Zou, C. M., Zhang, T. L., Li, Z., Liu, M., et al. (2022b). Regulation of the kynurenine/serotonin pathway by berberine and the underlying effect in the hippocampus of the chronic unpredictable mild stress mice. Behav. Brain Res. 422, 113764. doi:10.1016/j.bbr.2022.113764
Wang, Q., Zhang, P. Y., Yuan, X. M., Bi, Y. N., Zhou, Q., and Zhang, Y. (2018a). Long-term toxicity of different extracts of Epimedium erevicornu Maxim in mice. Chin. J. Pharmacovigil. 15 (2), 65–69. doi:10.3969/j.issn.1672-8629.2018.02.001
Wang, W. K., Zhang, W., Sun, Y., Zhong, Q., and Xue, M. (2020). Effects of Danggui buxue decoction and its main active ingredient ferulic acid on CUMS model GK rats. Traditional Chin. Drug Res. Clin. Pharmacol. 31 (6), 649–654. doi:10.19378/j.issn.1003-9783.2020.06.005
Wang, Y., Lou, X. T., Shi, Y. H., Tong, Q., and Zheng, G. Q. (2019b). Erxian decoction, a Chinese herbal formula, for menopausal syndrome: an updated systematic review. J. Ethnopharmacol. 234, 8–20. doi:10.1016/j.jep.2019.01.010
Wang, Y. L., Han, Q. Q., Gong, W. Q., Pan, D. H., Wang, L. Z., Hu, W., et al. (2018b). Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J. Neuroinflammation 15 (1), 21. doi:10.1186/s12974-018-1054-3
Wei, K., Xu, Y., Zhao, Z., Wu, X., Du, Y., Sun, J., et al. (2016). Icariin alters the expression of glucocorticoid receptor, FKBP5 and SGK1 in rat brains following exposure to chronic mild stress. Int. J. Mol. Med. 38 (1), 337–344. doi:10.3892/ijmm.2016.2591
Wong, K. Y., Zhou, L., Yu, W., Poon, C. C., Xiao, H., Chan, C. O., et al. (2021). Water extract of Er-xian decoction selectively exerts estrogenic activities and interacts with SERMs in estrogen-sensitive tissues. J. Ethnopharmacol. 275, 114096. doi:10.1016/j.jep.2021.114096
Wu, J., Du, J., Xu, C., Le, J., Xu, Y., Liu, B., et al. (2011). Icariin attenuates social defeat-induced down-regulation of glucocorticoid receptor in mice. Pharmacol. Biochem. Behav. 98 (2), 273–278. doi:10.1016/j.pbb.2011.01.008
Wu, X., Zhang, X., Sun, L., Lu, X., and Shen, C. (2023). Icariin prevents depression-like behaviors in chronic unpredictable mild stress-induced rats through Bax/cytoplasm C/caspase-3 axis to alleviate neuronal apoptosis. Cell. Mol. Biol. (Noisy-le-Grand, France) 69 (7), 196–204. doi:10.14715/cmb/2023.69.7.32
Xie, Z., Xie, H., Peng, X., Hu, J., Chen, L., Li, X., et al. (2023). The antidepressant-like effects of Danzhi xiaoyao san and its active ingredients. Phytomedicine 119, 155015. doi:10.1016/j.phymed.2023.155015
Xu, F., Yang, J., Meng, B., Zheng, J. W., Liao, Q., Chen, J. P., et al. (2018). The effect of berberine on ameliorating chronic inflammatory pain and depression. Natl. Med. J. China 98 (14), 1103–1108. doi:10.3760/cma.j.issn.0376-2491.2018.14.011
Xu, F. Q., Zheng, Y., Xu, L. J., Shi, L., Zhang, C., Wang, C. F., et al. (2017a). Impacts of psychosomatic treatment on female hormones in the patients of climacteric pepression. World J. Integr. Traditional West. Med. 12 (7), 893–896. doi:10.13935/j.cnki.sjzx.170702
Xu, F. Q., Zheng, Y., Xu, L. J., Shi, L., Zhang, C., Wang, C. F., et al. (2017b). Effect of modified Erxian decoction combined psychological counseling on monoamine neurotransmitters of mMenopausal depression women. Chin. J. Integr. Traditional West. Med. 37 (7), 789–794. doi:10.7661/j.cjim.20170426.096
Xu, L., Zhang, L. P., Song, R. W., and Chen, Y. (2020). Research progress of anti-inflammatory treatment of depression with traditional Chinese medicine. Chin. Archives Traditional Chin. Med. 38 (3), 141–144. doi:10.13193/j.issn.1673-7717.2020.03.038
Xu, Y., Zhang, L., Shao, T., Ruan, L., Wang, L., Sun, J., et al. (2013). Ferulic acid increases pain threshold and ameliorates depression-like behaviors in reserpine-treated mice: behavioral and neurobiological analyses. Metab. Brain Dis. 28 (4), 571–583. doi:10.1007/s11011-013-9404-4
Xue, Y. L., Xu, T. J., and Qiu, J. H. (2021). Effect of icariin on depression - like behavior and neuronal damage in depression rats. J. Clin. Exp. Med. 20, 2152–2156. doi:10.3969/j.issn.1671-4695.2021.20.00
Yabe, T., Hirahara, H., Harada, N., Ito, N., Nagai, T., Sanagi, T., et al. (2010). Ferulic acid induces neural progenitor cell proliferation in vitro and in vivo. Neuroscience 165 (2), 515–524. doi:10.1016/j.neuroscience.2009.10.023
Yan, M., Bo, X., Zhang, X., Zhang, J., Liao, Y., Zhang, H., et al. (2022). Mangiferin alleviates postpartum depression-like behaviors by inhibiting MAPK signaling in microglia. Front. Pharmacol. 13, 840567. doi:10.3389/fphar.2022.840567
Yang, L., Huang, Y., Chen, F., Wang, Y., Su, K., Zhao, M., et al. (2023). Berberine attenuates depression-like behavior by modulating the hippocampal NLRP3 ubiquitination signaling pathway through Trim65. Int. Immunopharmacol. 123, 110808. doi:10.1016/j.intimp.2023.110808
Yang, S. J., Song, Z. J., Wang, X. C., Zhang, Z. R., Wu, S. B., and Zhu, G. Q. (2019). Curculigoside facilitates fear extinction and prevents depression-like behaviors in a mouse learned helplessness model through increasing hippocampal BDNF. Acta Pharmacol. Sin. 40 (10), 1269–1278. doi:10.1038/s41401-019-0238-4
Yi, L. T., Zhu, J. X., Dong, S. Q., Chen, M., and Li, C. F. (2021). Berberine exerts antidepressant-like effects via regulating miR-34a-synaptotagmin1/Bcl-2 axis. Chin. Herb. Med. 13 (1), 116–123. doi:10.1016/j.chmed.2020.11.001
Zamanian, H., Poorolajal, J., and Taheri-Kharameh, Z. (2018). Relationship between stress coping strategies, psychological distress, and quality of life among hemodialysis patients. Perspect. Psychiatric Care 54 (3), 410–415. doi:10.1111/ppc.12284
Zeng, N. X., Li, H. Z., Wang, H. Z., Liu, K. G., Gong, X. Y., Luo, W. L., et al. (2022). Exploration of the mechanism by which icariin modulates hippocampal neurogenesis in a rat model of depression. Neural Regen. Res. 17 (3), 632–642. doi:10.4103/1673-5374.320993
Zeni, A. L., Zomkowski, A. D., Maraschin, M., Rodrigues, A. L., and Tasca, C. I. (2012). Involvement of PKA, CaMKII, PKC, MAPK/ERK and PI3K in the acute antidepressant-like effect of ferulic acid in the tail suspension test. Pharmacol. Biochem. Behav. 103 (2), 181–186. doi:10.1016/j.pbb.2012.08.020
Zeni, A. L. B., Camargo, A., and Dalmagro, A. P. (2017). Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice. Steroids 125, 131–136. doi:10.1016/j.steroids.2017.07.006
Zhan, Y., Han, J., Xia, J., and Wang, X. (2021). Berberine suppresses mice depression behaviors and promotes hippocampal neurons growth through regulating the miR-34b-5p/miR-470-5p/BDNF axis. Neuropsychiatric Dis. Treat. 17, 613–626. doi:10.2147/NDT.S289444
Zhang, C., Lu, H. Q., Hu, C. X., Mei, Q. H., Zhang, X., and Huang, X. L. (2018). Effect of Icariin on behavior and monoamine neurotransmitters in rats with depression induced by chronic unpredicted mild stress. Chin. Pharm. J. 53 (15), 1280–1284. doi:10.11669/cpj.2018.15.007
Zhang, E., and Liao, P. (2020). Brain-derived neurotrophic factor and post-stroke depression. J. Neurosci. Res. 98 (3), 537–548. doi:10.1002/jnr.24510
Zhang, L., Li, J., Chen, Q., Di, L., and Li, N. (2021a). Erxian decoction, a famous Chinese medicine formula, ameliorate depression-like behavior in perimenopausal mice. Endocr. Metabolic Immune Disord. Drug Targets 21 (12), 2203–2212. doi:10.2174/1871530321666210618095856
Zhang, L., Wang, Q. D., Shi, H. M., and Pan, J. C. (2013). Influence of ferulic acid on the pain-depression dyad induced by reserpine. Acta Pharm. Sin. 48 (1), 32–37. doi:10.16438/j.0513-4870.2013.01.015
Zhang, L., Yang, Y., Di, L., Li, J. L., and Li, N. (2020). Erxian decoction, a famous Chinese medicine formula, antagonizes corticosterone-induced injury in PC12 cells, and improves depression-like behaviours in mice. Pharm. Biol. 58 (1), 498–509. doi:10.1080/13880209.2020.1765812
Zhang, L., Zhao, G. F., Li, N., Qiao, X. Q., and Jia, Y. F. (2021b). Mangiferin reduces stress induced depressive-like behavior and exerts a neuroprotective effect by inhibition of the NLRP3 inflammasome. J. Hebei Univ. Sci. Ed. 41 (3), 265–272. doi:10.3969/j.issn.1000-1565.2021.03.007
Zhang, M. Y., Xu, E. P., Shang, L. Z., and Mao, M. D. (2022). Research progress of Suanzaoren decoction and its components in treatment of depression. Chin. Archives Traditional Chin. Med. 40 (6), 48–54. doi:10.13193/j.issn.1673-7717.2022.06.010
Zhang, X., Sun, H., Su, Q., Lin, T., Zhang, H., Zhang, J., et al. (2017). Antidepressant-like activity of icariin mediated by group I mGluRs in prenatally stressed offspring. Brain & Dev. 39 (7), 593–600. doi:10.1016/j.braindev.2017.03.021
Zhang, Y. J., Huang, X., Wang, Y., Xie, Y., Qiu, X. J., Ren, P., et al. (2011). Ferulic acid-induced anti-depression and prokinetics similar to Chaihu-Shugan-San via polypharmacology. Brain Res. Bull. 86 (3-4), 222–228. doi:10.1016/j.brainresbull.2011.07.002
Zheng, X., Cheng, Y., Chen, Y., Yue, Y., Li, Y., Xia, S., et al. (2019). Ferulic acid improves depressive-like behavior in prenatally-stressed offspring rats via anti-inflammatory activity and HPA axis. Int. J. Mol. Sci. 20 (3), 493. doi:10.3390/ijms20030493
Zhu, F. F., Yang, M. H., Chen, W. J., Li, X. D., and Hu, W. (2015). Correlation between toxicity and content of Curculiginis Rhizoma and Epimedii Folium with different compatible proportion. Chin. J. Exp. Traditional Med. Formulae 21 (5), 175–177. doi:10.13422/j.cnki.syfjx.2015050175
Zhu, X., Sun, Y., Zhang, C., and Liu, H. (2017). Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays. Mol. Med. Rep. 15 (5), 3161–3171. doi:10.3892/mmr.2017.6353
Zuo, Y., Zhao, Y. L., Gong, Z. H., Meng, D. H., She, K. J., Zhang, Y. J., et al. (2022). Regulatory effect of Wenyang prescription, Jieyu prescription, and Wenyang jieyu prescription on pain sensitivity and depression-like behaviors in mice induced by maternal separation and chronic neuropathic pain. Chin. J. Exp. Traditional Med. Formulae 28 (14), 44–53. doi:10.13422/j.cnki.syfjx.20221339
Glossary
Keywords: Erxian decoction, depression, traditional Chinese medicine, antidepressant, review, active ingredient
Citation: Zeng N-X, Li H, Su M-Y, Chen X, Yang X-Y and Shen M (2024) Therapeutic potential of Erxian decoction and its special chemical markers in depression: a review of clinical and preclinical studies. Front. Pharmacol. 15:1377079. doi: 10.3389/fphar.2024.1377079
Received: 26 January 2024; Accepted: 15 May 2024;
Published: 10 June 2024.
Edited by:
Adolfo Andrade-Cetto, National Autonomous University of Mexico, MexicoReviewed by:
Juan Francisco Rodríguez-Landa, Universidad Veracruzana, MexicoRaquel Romay-Tallon, Rush University, United States
Copyright © 2024 Zeng, Li, Su, Chen, Yang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning-Xi Zeng, emVuZ25pbmd4aUAxNjMuY29t; Mei Shen, U2hlbm1laTA0MjlAMTYzLmNvbQ==
†These authors have contributed equally to this work to this study.
 Ning-Xi Zeng
Ning-Xi Zeng Han Li2,3†
Han Li2,3†