
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 08 July 2024
Sec. Inflammation Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1376708
This article is part of the Research TopicCancer and inflammatory diseases research: from the basics to the precision medicineView all 11 articles
Aims: Persistent uncertainties exist surrounding the therapeutic drug monitoring (TDM) of adalimumab in clinical settings. To address these issues, we conducted a systematic review to assess the current evidence regarding the benefits of TDM for adalimumab.
Methods: PubMed, EMBASE, and Cochrane Databases were searched from inception to October 2022. The trials regarding to the list three key questions were considered: 1) Could routine proactive TDM assist in improving outcomes in patients receiving adalimumab? 2) Could reactive TDM assist in guiding subsequent treatment strategies for patients with treatment failure to adalimumab? 3) Could TDM assist in informing dose reduction or discontinuation in patients with low disease activity or in remission treated with adalimumab? Two reviewers independently selected the studies and extracted the data. Meta-analysis was performed to calculate the relative risk (RR) and 95% confidence interval (CI).
Results: A total of 9 studies was included in this review. For proactive TDM, meta-analysis indicated that proactive TDM (n = 163/257, 63.42%) showed no significant superiority over reactive TDM and/or conventional management (n = 336/606, 55.44%) in achieving and/or maintaining clinical remission by random effects model (RR: 1.24, 95% CI 0.98–1.58, I2 = 73%). There were three studies that supporting the reactive TDM, low drug levels in the absence of anti-drug antibodies (ADA) strongly indicate the need for dose intensification, and infliximab is a feasible choice for patients with low drug levels and ADA positivity. While swapping to another class should be considered in patients with adequate drug levels. In addition, TDM can help clinicians optimize dosing schedules and prevent overtreatment in patients who have achieved low disease activity and sufficient drug concentrations, with no predictive value for successful adalimumab discontinuation.
Conclusion: Current evidence suggests that proactive TDM is numerically but not statistically significant superiority over reactive TDM and/or conventional management. Reactive TDM can aid in understanding treatment failure and developing subsequent therapy. For patients reaching low disease activity and remission, TDM can help successful dose reduction, while it cannot inform the successful drug discontinuation. However, existing trials are limited, and more well-designed trials are necessary to clarify the role of TDM in adalimumab treatment.
Adalimumab (ADM), a fully human monoclonal antibody that neutralizes tumor necrosis factor-α (TNF-α), was initially approved for the treatment of moderate to severe rheumatoid arthritis (RA) in 2002 (Rau, 2002). Since then, it has been found to be effective in treating a variety of other conditions, such as ankylosing spondylitis, psoriasis, Crohn’s disease (CD), ulcerative colitis (UC), uveitis, and juvenile idiopathic arthritis, making it the most widely used agent.
Therapeutic drug monitoring (TDM) is a practical method used to monitor the drug concentration and their metabolites in the blood, which can help guide clinical medication decisions, enhance drug effectiveness, prevent drug toxicity, and establish personalized treatment schedules. Recently, TDM has become essential in biological therapy due to the impact of drug concentrations of TNF-α inhibitors on clinical outcomes (Pouw et al., 2015; Rinawi et al., 2021). Anti-drug antibodies (ADA) play a significant role in the inter-individual variability of drug clearance, leading to insufficient drug exposure and treatment failure, such as primary non-response (PNR) and loss-of-response (LOR) (Bartelds et al., 2011; Baert et al., 2016; Ding et al., 2020). Reactive TDM refers to measure biological concentration and ADA in patients experiencing treatment failure. This approach is endorsed by the American Gastroenterological Association and expert consensus statements to understand treatment failure (Feuerstein et al., 2017; Mitrev et al., 2017; Cheifetz et al., 2021; Krieckaert et al., 2023), despite the limited quality of evidence. The supported evidence comes primary from studies involving infliximab therapy. It is not yet clear how many benefits of TDM can bring to the clinical application of ADM. However, the use of proactive TDM, which involves scheduled testing and adjusting dosages to achieve predefined target concentrations, lacks consistent recommendations (Feuerstein et al., 2017; Cheifetz et al., 2021). There are persistent uncertainties surrounding the most effective use of TDM in clinical settings. Specifically, the evidence supporting the use of TDM to guide dose reduction or discontinuation in patients achieving deep remission has not been reviewed.
To systematically review the value of TDM in optimizing ADM therapy, three key questions throughout the entire drug treatment process were considered: 1) Could routine proactive TDM assist in improving outcomes in patients receiving ADM? 2) Could reactive TDM assist in guiding subsequent treatment strategies for patients PNR or LOR to ADM? 3) Could TDM assist in informing dose reduction or discontinuation in patients with low disease activity or in remission treated with ADM?
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021). We systematically searched PubMed, EMBASE and Cochrane Database from inception to October 2022 to identify applicable studies. A search strategy was created based on the PICO (Population, Intervention, Comparison, Outcomes) questions. The search terms used were combinations of text-free terms and Medical Subject Headings (MeSH) terms as follows: ADM, therapeutic drug monitoring, therapeutic monitoring, serum concentration monitoring. There were no language or publication date restrictions. The full search terminology was included in the Supplementary Table S1. We also hand-searched trial registries such as ClinicalTrials.gov (https://clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch) and reference lists of included trials for completeness.
Studies published as full manuscripts related to the PICO questions were included. These involved studies assessing: 1) Could routine proactive TDM assist in improving outcomes in patients receiving ADM? 2) Could reactive TDM assist in guiding subsequent treatment strategies for patients PNR or LOR to ADM? 3) Could TDM assist in informing dose reduction or discontinuation in patients with low disease activity or in remission treated with ADM? There were no restrictions on disease types or TDM measurements. Reviews, editorials, guidelines, case reports, and studies that focused only on pharmacokinetics and pharmacodynamics were excluded.
Two reviewers (Yun Li and Cheng Xie) independently assessed studies for possible inclusion by reading titles and/or abstracts, then viewed the full texts of the remaining publications to pick up the ultimately available studies. Data extraction was done by one reviewer (Yun Li), and subsequently cross-checked by the other reviewer (Cheng Xie). Any divergences were discussed or determined by a third investigator (Xiaoliang Ding). Following information was abstracted: the first author and publication year, country, study type, sample size, baseline, patients feature, treatment feature, follow-up time, the clinical outcomes and their definitions.
Two reviewers (Yun Li and Cheng Xie) independently evaluated the quality of the studies. Disagreements were resolved through discussion and consultation with the third investigator (Xiaoliang Ding). The risk of bias in the randomized controlled trial (RCT) was evaluated according to the standards developed by the Cochrane Bias Risk Tool (Sterne et al., 2019). The quality of the observational studies was assessed using the Newcastle–Ottawa scale (NOS) (Stang, 2010).
In this systematic review, we conducted a narrative review and utilized meta-analysis when dichotomous outcomes were sufficiently similar across studies, considering the diversity of these focused questions. Both fixed-effect and random-effects model were employed to calculate the relative risk (RR) and 95% confidence interval (CI). Heterogeneity of effect size across the studies was tested using Q statistics at the p < 0.10 level of significance. We also calculated the I2 statistic with a quantitative measure of inconsistency across the studies. The data were pooled by random-effects model in case significant heterogeneity (Cochran test with p < 0.10 or I2 > 50%) was found. Otherwise, the fixed-effects model was used. Subgroup analysis, sensitivity analysis, and publication bias analysis were not conducted due to the limited number of included studies. The analysis was carried out using the “meta” package in R (version 4.3.2).
Figure 1 shows the research selection process for inclusion in the systematic review. The initial search generated 4764 references. After deleting 1325 duplicate articles titles and abstracts of all the articles were reviewed. A total of 109 studies were reviewed in full, while 100 studies were excluded because of not meet the inclusion criteria. The main reasons for excluding full articles were the inability to extract data related to ADM alone, noncompliance with research objectives, review articles and editorials/letters to editors. The final 9 studies were included (Papamichael et al., 2019; Assa et al., 2019; D’Haens et al., 2022; Panes et al., 2022; Roblin et al., 2014; Roblin et al., 2022; Ulijn et al., 2020; Chen et al., 2016; Lamers-Karnebeek et al., 2019) and the details are shown in Table 1.
A summary of the bias risk data is shown in Figure 2 and Table 2. The quality evaluation of the RCTs revealed that three trials were at high risk of bias across one domain (randomization domain, PAILOT; Other bias, SERENE UC and SERENE CD). PAILOT study is an Open-label study, and most outcomes likely to be influenced. There was no sample size calculation for the maintenance study in SERENE UC and SERENE CD studies. All six observational studies received 8–9 stars out of 9 on the NOS, indicating low risk of bias. Four studies did not fully meet the scoring criteria in terms of inter group comparability and population representativeness. In Roblin’s study, they combined CD and UC together, which may affect the comparability of the results (Roblin et al., 2014; Roblin et al., 2022). In addition, therapeutic groups were not fully comparable at baseline, especially in terms of disease (Roblin et al., 2022). In Lamers Karnebeek’s study, the included population had a longer duration of disease (average of 9 years), which may not fully represent the population of patients with RA (Lamers-Karnebeek et al., 2019). In Papamichael’s research the control group received standard of care which was defined as empirical dose escalation and/or reactive TDM. Therefore, it is not possible to draw clear conclusions between proactive TDM and reactive TDM, as well as between proactive TDM and empirical dose escalation (Papamichael et al., 2019).
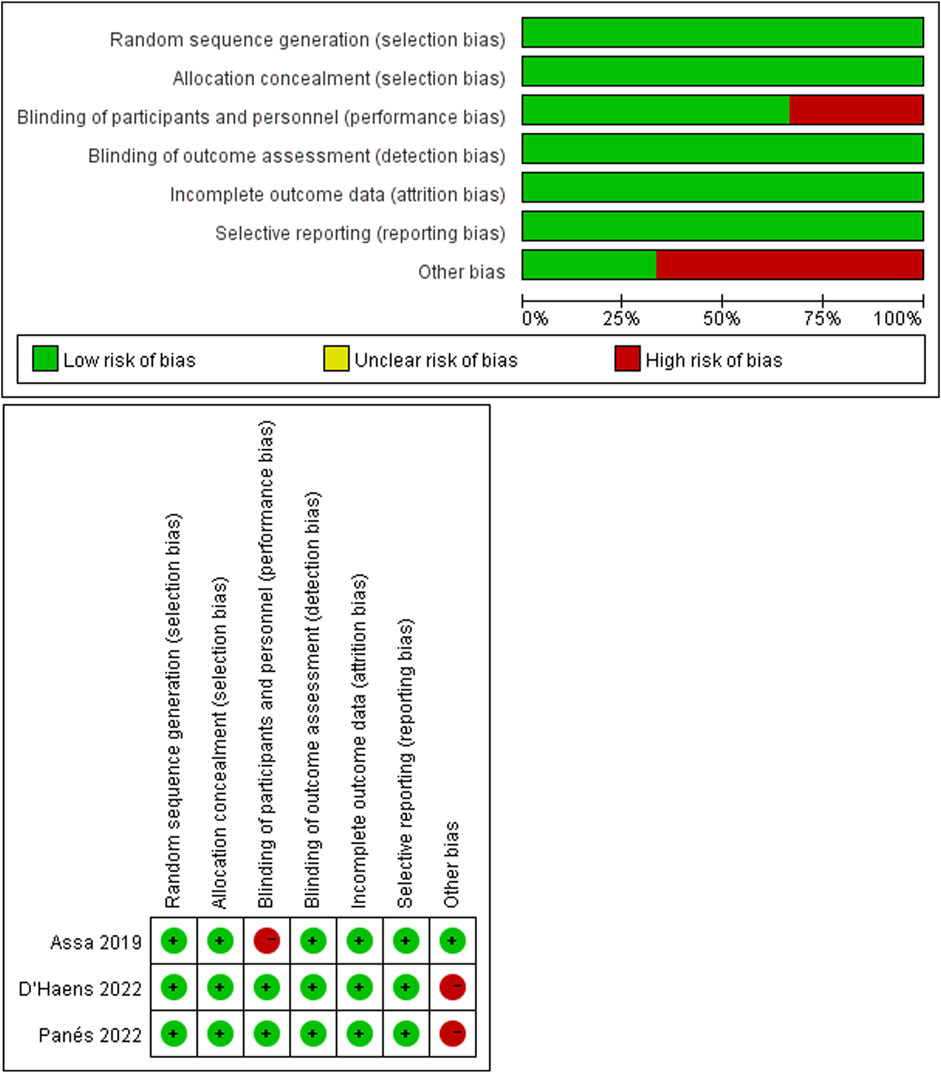
Figure 2. The risk of bias in randomized controlled trials was assessed using the Cochrane risk of bias tool.
Dosage adjustment to target and maintain a predefined drug concentration was the primary format of TDM, specifically referred to routine proactive TDM. This scenario included three RCTs (Assa et al., 2019; D’Haens et al., 2022; Panes et al., 2022) and one observational study (Papamichael et al., 2019), with detailed characteristics outlined in Table 3. Results from the meta-analysis indicated that proactive TDM (n = 163/257, 63.42%) showed no significant superiority over reactive TDM and/or conventional management (n = 336/606, 55.44%) in achieving and/or maintaining clinical remission by random effects model (RR: 1.24, 95% CI 0.98–1.58, I2 = 73%; Figure 3).
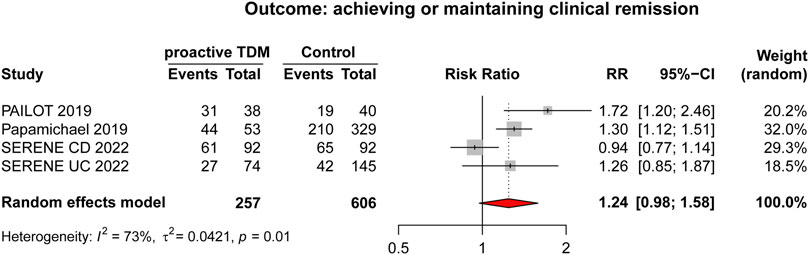
Figure 3. Forest plot comparing proactive TDM vs. control (reactive TDM and/or conventional management which is defined as empirical dose escalation), outcome (achieving or maintaining clinical remission).
Reactive TDM plays a crucial role in understanding and addressing treatment failure with ADM treatment. A total of three studies were included in this scenario and the detailed characteristics were shown in Table 3. Two retrospective cohorts (Roblin et al., 2014; Ulijn et al., 2020) were conducted to evaluate the predictive value of TDM in guiding subsequent strategies. Roblin et al. (2014) studied 82 patients with inflammatory bowel disease (IBD) who experienced disease relapse and were treated with ADM at a weekly dose of 40 mg. Results showed that after 6 months, patients with drug level <4.9 μg/mL and negative ADA tested at time of relapse had a higher clinical remission rate (67%, n = 16/24) compared to those with drug level >4.9 μg/mL (29.2%, n = 12/41) or drug level <4.9 μg/mL and ADA positive (12%, n = 2/17). Subsequently, the remaining fifty-two patients who did not respond to ADM were switched to infliximab treatment. Among these patients, those with drug level <4.9 μg/mL and ADA positive exhibited higher clinical response rate (80%, n = 12/15) than those with drug level >4.9 μg/mL (6.9%, n = 2/29) or drug level <4.9 μg/mL and ADA negative (25%, n = 2/8). Ulijn et al. (Ulijn et al., 2020) conducted a retrospectively study involving 137 RA patients who failed treatment with ADM. The study analyzed the predictive value of TDM results for the use of subsequent biological agents and did not find clear predictive value of ADM concentrations or ADA status in either the TNF-α inhibitors or non-TNF-α inhibitors groups. A nonrandomized controlled trial conducted by Roblin et al. (Roblin et al., 2022) compared dose intensification (n = 61) with swapping to different class (ustekinumab or vedolizumab, n = 70) in patients under ADM maintenance therapy who experienced LOR and had ADM concentration >4.9 μg/mL. The median time without discontinuation in the swapping group was significantly longer than that in the intensification group (24 months vs. 13.3 months, p < 0.001). In summary, reactive TDM may assist in understanding the mechanisms of treatment failure and making subsequent treatment strategies. Low drug levels in the absence of ADA strongly indicate the need for dose intensification, with infliximab being a viable option for patients with low drug levels and ADA positive. While swapping to another class should be considered in patients with adequate drug levels.
TDM can help reduce overtreatment in patients with low disease activity or in remission by identifying higher drug concentrations. This approach allows for dose reduction or tapering while still maintaining efficacy. Two studies (Chen et al., 2016; Lamers-Karnebeek et al., 2019) were included in this scenario, and their characteristics were shown in Table 3. Chen et al. (2016) evaluated the predictive value of ADM concentrations for dose reduction. 64 RA patients who had already achieved low disease activity (LDA) or remission after receiving ADM full-dose therapy at least 2 years were included, and then received ADM dose-halving at a dose of 40 mg monthly. After 24-week follow-up, they found that ADM concentration above a cutoff of 6.4 μg/mL predicted a persistent remission (AUC: 0.998, 95% CI: 0.936-1.000, sensitivity: 100%, specificity: 93.4%), and a persistent LDA (AUC: 0.995, 95% CI: 0.931-1.000, sensitivity: 93.9%, specificity: 100%) after dose halving. ADM dose halving is feasible for patients who have achieved remission and adequate drug levels. Lamers-Karnebeek et al. (2019) investigated whether the ADM concentration and ADA status predict disease flares after ADM cessation in RA patients who received ADM therapy for more than 1 year and achieved LDA for at least 6 months. 210 RA patients with 1 year follow-up after ADM discontinuation were included and analyzed. 62 (53%) of 117 patients with ADM concentrations ≥5 μg/mL experienced a flare versus 44 (47%) of 93 patients with concentrations <5 μg/mL, with no cut-off of ADM concentration at stopping ADM clearly predicted disease flare. TDM can help clinicians optimize dosing schedules and prevent overtreatment in patients who have achieved LDA and sufficient drug concentrations, with no predictive value for successful ADM discontinuation.
In clinical setting, TDM typically involves adjusting the dosage based on blood concentrations and using pharmacometrics model to ensure that the concentration falls within the desired range to achieve optimal efficacy and avoid adverse reaction. The clinical implementation of TDM of ADM is intricate, mainly due to the need to adjust treatment plans based on different clinical scenarios and TDM results. Our study outlines the benefits of TDM in the entire clinical process of ADM treatment for various diseases. The comprehensive clinical scenarios and evidence are demonstrated in Figure 4.
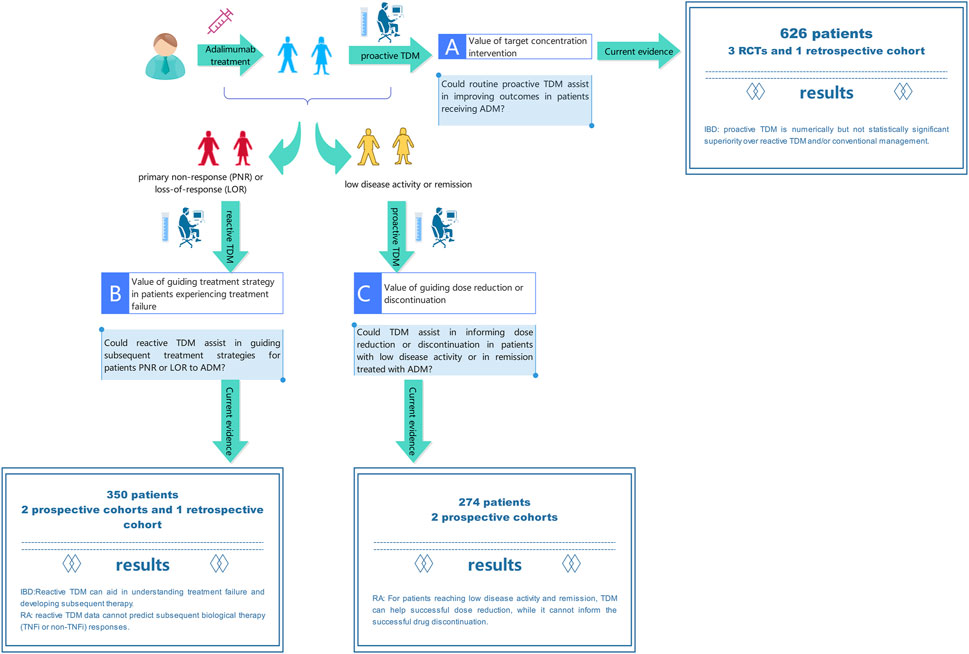
Figure 4. Current evidence on benefits of therapeutic drug monitoring throughout the entire process of adalimumab treatment.
In scenario A, people hope to obtain the drug concentration and antibody level of ADM to actively intervene and achieve better therapeutic effects. In a meta-analysis of 3 RCTs and 1 retrospective cohort involving 626 IBD patients treated with ADM. Numerically but not statistically significant superiority of proactive TDM over reactive TDM and/or conventional management in achieving and/or maintaining clinical remission was observed. Our results are in line with previous studies on TNF-α inhibitors (Nguyen et al., 2022) which included 9 RCTs (6 for infliximab and 3 for ADM) in patients with IBD. There was no significant difference in the risk of failing to maintain clinical remission in patients who underwent proactive TDM vs. conventional management. Disease duration, concomitant immunomodulators, disease activity at baseline, and optimization of therapy before randomization did not modify this association. Exposure response relationship studies in IBD patients clearly demonstrate that higher anti-TNF drug concentrations are associated with clinical, biochemical, endoscopic, and histological remission (Zittan et al., 2016; Ward et al., 2017; Papamichael et al., 2018). According to reports, proactive TDM is important not only during maintenance therapy but also during induction therapy. Research has shown that ADA can develop as early as the second week in CD patients, leading to unresponsiveness. Proactive TDM can detect low concentrations at the fourth week to avoid immunogenicity and impact patient prognosis (Ungar et al., 2016). However, it seems that we have not obtained the expected evidence of benefits of ADM proactive TDM, but it is worth noting that the included literatures varied in study design, with moderate heterogeneity. The results may be influenced by factors such as patient population, sample size, study time, and detection method, etc. Therefore, more high-quality research is needed to provide additional evidence to clarify benefits of proactive TDM. Proactive TDM may be more important in more severely active patients and those with higher drug clearance rates, such as during induction therapy and in patients with acute severe UC and severe CD. These patients have a high burden of inflammation, increased drug clearance rates, and therefore a higher risk of insufficient drug exposure, immunogenicity, and treatment failure (Brandse et al., 2015; Brandse et al., 2016; Ungar et al., 2016; Battat et al., 2021). Another population with high drug clearance rates is the pediatric population (Jongsma et al., 2020; Winter et al., 2020). Assa et al. conducted relevant studies on pediatric IBD patients and demonstrated that proactive TDM can guide higher frequency treatment strategy adjustments, resulting in higher sustained response rates in the absence of corticosteroids and biological responses (Assa et al., 2019).
In scenario B, reactive TDM is performed when the patient experiencing treatment failure (Krieckaert et al., 2015; Irving and Gecse, 2022; Papamichael et al., 2022). For example, approximately one-third of IBD patients do not respond to TNF-α inhibitors treatment, and among those who initially respond, the LOR is an important clinical issue. In the first year of treatment, up to 40% of patients experience this condition (Colombel et al., 2007). For unresponsive patients, empirical dose escalation therapy may incur significant additional costs, leading to potential ineffective treatment and delaying more effective treatment. In addition, in patients with immune-mediated pharmacokinetic failure (for which ADA was established), additional drug exposure may lead to hypersensitivity reactions. Similarly, excessive drug exposure can lead to a higher risk of drug-related adverse events (such as severe infections). Roblin’s two studies confirmed that different levels of drug and ADA in the IBD population are associated with corresponding treatment adjustment strategies (Roblin et al., 2014; Roblin et al., 2022). Although there was no RCTs to demonstrate superior clinical outcomes of reactive TDM compared to empirical care, the use of TDM can elucidate the mechanism of LOR, whether the lack of response is caused by pharmacokinetic issues, insufficient drug levels, or pharmacological issues of ineffective ADA. TDM provides information for clinical decision-making in unresponsive patients and has intuitive benefits, such as preventing ineffective and potentially dangerous dose escalation in high-titer ADA patients. These results lay the foundation for the guiding the role of TDM in clinical practice and have been introduced in clinical guidelines and expert consensuses to support reactive TDM in ADM treatment (Feuerstein et al., 2017; Khan et al., 2019; Cheifetz et al., 2021; Krieckaert et al., 2023). In addition, Ulijn et al. conducted a study on RA and reported that reactive TDM data cannot predict subsequent biological therapy (TNF-α inhibitors or a non TNF-α inhibitors) responses in patients who failed treatment with ADM (Ulijn et al., 2020). On this issue, current researches have not reached a consistent convincing conclusion. In previous studies, it has been suggested that the measurement of ADM serum levels and/or ADA might be helpful for channeling the right patients to a TNF-α inhibitors or a non TNF-α inhibitors, thus increasing overall response chances (Bartelds et al., 2010; Jamnitski et al., 2011; Plasencia et al., 2013). There may be several reasons for these different results. In Ulijn’s study, samples were not collected at the trough level but were randomly collected after injection of ADM. This might have reduced the association between ADA and response. Second, as this was a retrospective study, serum samples and clinical results were not always available, which may have led to selection bias. In summary, further prospective studies with larger sample sizes are needed to confirm whether drug and ADA levels indeed cannot predict disease activity.
In scenario C, due to the considerable interindividual variability in ADM concentrations and the existing exposure-response relationship, a considerable number of patients may experience overtreatment, leading to a higher risk of infection and increased costs. It is crucial in clinical practice to taper the dose to the lowest effective level, considering cost-effectiveness and potential adverse reactions. For patients who have achieved remission, sufficient ADM concentrations (≥6.4 μg/mL) can support successful ADM dose reduction (halving the dose to 40 mg monthly) (Chen et al., 2016). This approach has been validated by a RCT (l’Ami et al., 2018), RA patients with ADM concentrations >8 μg/mL can potentially prolong dosing interval to once every 3 weeks without loss of disease control, leading to reduced drug costs. While other biomarkers, involving patient, treatment, disease activity, and laboratory and imaging measurements, have not shown predictive value for successful dose reduction (Tweehuysen et al., 2017). It is hypothesized that patients who have achieved LDA and have undetectable drug concentrations may be considered for discontinuation of ADM, as the maintenance of LDA may be independent of the drug. However, data from the POET study (Chen et al., 2016) revealed that a significant proportion of patients (48%) experienced disease flare even with low or undetectable ADM concentrations, indicating that drug concentrations alone may not be sufficient to guide discontinuation decisions. Alternative strategies, such as disease activity-guided dose reduction and withdrawal or step-down approaches, may also be worth considering (van Herwaarden et al., 2015; Fautrel et al., 2016).
Our systematic review and meta-analysis summarized the benefits of TDM in the entire clinical process of ADM treatment for various diseases. However, there are some limitations to consider. In terms of data sources, limitations in data collection methods or sources may affect the reliability and universality of research results. Grey literature, as an important source of information, plays an indispensable role in literature search. Unlike traditional commercial publications, gray literature is usually published by institutions, enterprises, government agencies, professional conferences, and individuals. Its uniqueness makes it important, such as providing comprehensive information, reflecting practical experience and policy advocacy, timely grasping the latest research results, and eliminating publication bias. However, in our study, we only manually searched the trial registry and the list of references included in the trial, which made our search for grey literature incomplete and needed improvement in future research. Secondly, the included literatures varied in study design and quality. The results may be influenced by factors such as patient population, sample size, study time, and experimental environment, etc. as we excluded studies for which we were unable to extract individual ADM data; consequently, studies related to certain diseases, such as psoriasis and ankylosing spondylitis, were not included. Although evidence of benefits, including CD, UC, and RA, was ultimately included, the patient population, research perspectives, and outcome indicators of these studies were not the same, making it difficult to quantitatively summarize and perform meta-analyses for all literature results. Thirdly, it should be noted that assays used in TDM are varied and not yet standardized and may explain the deviation in results from different studies. Finally, our results are mainly based on the Western population, which means that it is difficult to generalize globally. However, within the scope of the currently published research, this article provides the latest results on the benefits of TDM in the entire process of clinical use and management of ADM.
The systematic review highlights the current evidence of TDM in ADM treatment. We addressed three clinical concerns regarding the benefits of TDM throughout the ADM treatment process. Current evidence suggests that proactive TDM is numerically but not statistically significant superiority over reactive TDM and/or conventional management in achieving and/or maintaining clinical remission. For patients experiencing treatment failure, reactive TDM can aid in understanding the reasons for treatment failure and developing subsequent treatment schedule. For patients reaching LDA or remission, monitoring drug concentrations can help identify and reduce overtreatment, while it cannot inform the successful drug discontinuation. Evidence was observed across various populations, including those with CD, UC, and RA. They encompass optimizing treatment strategies, enhancing clinical outcomes, improving drug utilization, and reducing treatment costs. However, existing clinical trials are limited and of varying quality. More well-designed, high-quality clinical studies are needed to clarify the role of TDM in different clinical settings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YL: Writing–original draft, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration. CX: Writing–original draft, Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration. XD: Writing–original draft, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software. ZW: Writing–review and editing, Conceptualization, Formal Analysis, Investigation, Methodology. JJZ: Writing–review and editing, Data curation, Formal Analysis, Investigation, Methodology. JGZ: Writing–review and editing, Conceptualization, Project administration, Supervision, Validation. LM: Writing–review and editing, Conceptualization, Formal Analysis, Funding acquisition, Project administration, Supervision, Validation.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82003857), Key R&D Program of Jiangsu Province (BE2021644), Suzhou Health Leading Talent (GSWS2019001), the National Clinical Research Center for Hematologic Diseases, the First Affiliated Hospital of Soochow University (2020WSC07), the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD) and the Project of Suzhou Science and Technology Development Plan (SKJY2023182).
We thank all the members of our study team for their whole-hearted cooperation and the original authors of the included studies for their excellent work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1376708/full#supplementary-material
Assa, A., Matar, M., Turner, D., Broide, E., Weiss, B., Ledder, O., et al. (2019). Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn's disease compared with reactive monitoring. Gastroenterology 157 (4), 985–996. doi:10.1053/j.gastro.2019.06.003
Baert, F., Kondragunta, V., Lockton, S., Vande Casteele, N., Hauenstein, S., Singh, S., et al. (2016). Antibodies to adalimumab are associated with future inflammation in Crohn's patients receiving maintenance adalimumab therapy: a post hoc analysis of the Karmiris trial. Gut 65 (7), 1126–1131. doi:10.1136/gutjnl-2014-307882
Bartelds, G. M., Krieckaert, C. L., Nurmohamed, M. T., van Schouwenburg, P. A., Lems, W. F., Twisk, J. W., et al. (2011). Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA 305 (14), 1460–1468. doi:10.1001/jama.2011.406
Bartelds, G. M., Wijbrandts, C. A., Nurmohamed, M. T., Stapel, S., Lems, W. F., Aarden, L., et al. (2010). Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann. Rheum. Dis. 69 (5), 817–821. doi:10.1136/ard.2009.112847
Battat, R., Hemperly, A., Truong, S., Whitmire, N., Boland, B. S., Dulai, P. S., et al. (2021). Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin. Gastroenterol. Hepatol. 19 (3), 511–518.e6. doi:10.1016/j.cgh.2020.03.072
Brandse, J. F., Mathot, R. A., van der Kleij, D., Rispens, T., Ashruf, Y., Jansen, J. M., et al. (2016). Pharmacokinetic features and presence of antidrug antibodies associate with response to infliximab induction therapy in patients with moderate to severe ulcerative colitis. Clin. Gastroenterol. Hepatol. 14 (2), 251–258. doi:10.1016/j.cgh.2015.10.029
Brandse, J. F., van den Brink, G. R., Wildenberg, M. E., van der Kleij, D., Rispens, T., Jansen, J. M., et al. (2015). Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology 149 (2), 350–355. doi:10.1053/j.gastro.2015.04.016
Cheifetz, A. S., Abreu, M. T., Afif, W., Cross, R. K., Dubinsky, M. C., Loftus, E. V., et al. (2021). A comprehensive literature review and expert consensus statement on therapeutic drug monitoring of biologics in inflammatory bowel disease. Am. J. Gastroenterol. 116 (10), 2014–2025. doi:10.14309/ajg.0000000000001396
Chen, D. Y., Chen, Y. M., Hsieh, T. Y., Hung, W. T., Hsieh, C. W., Chen, H. H., et al. (2016). Drug trough levels predict therapeutic responses to dose reduction of adalimumab for rheumatoid arthritis patients during 24 weeks of follow-up. Rheumatol. Oxf. 55 (1), 143–148. doi:10.1093/rheumatology/kev298
Colombel, J. F., Sandborn, W. J., Rutgeerts, P., Enns, R., Hanauer, S. B., Panaccione, R., et al. (2007). Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 132 (1), 52–65. doi:10.1053/j.gastro.2006.11.041
D’Haens, G. R., Sandborn, W. J., Loftus, E. V., Hanauer, S. B., Schreiber, S., Peyrin-Biroulet, L., et al. (2022). Higher vs standard adalimumab induction dosing regimens and two maintenance strategies: randomized SERENE CD trial results. Gastroenterology 162 (7), 1876–1890. doi:10.1053/j.gastro.2022.01.044
Ding, X., Zhu, R., Wu, J., Xue, L., Gu, M., and Miao, L. (2020). Early adalimumab and anti-adalimumab antibody levels for prediction of primary nonresponse in ankylosing spondylitis patients. Clin. Transl. Sci. 13 (3), 547–554. doi:10.1111/cts.12738
Fautrel, B., Pham, T., Alfaiate, T., Gandjbakhch, F., Foltz, V., Morel, J., et al. (2016). Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: spacing of TNF-blocker injections in Rheumatoid ArthritiS Study). Ann. Rheum. Dis. 75 (1), 59–67. doi:10.1136/annrheumdis-2014-206696
Feuerstein, J. D., Nguyen, G. C., Kupfer, S. S., Falck-Ytter, Y., and Singh, S.American Gastroenterological Association Institute Clinical Guidelines C (2017). American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 153 (3), 827–834. doi:10.1053/j.gastro.2017.07.032
Irving, P. M., and Gecse, K. B. (2022). Optimizing therapies using therapeutic drug monitoring: current strategies and future perspectives. Gastroenterology 162 (5), 1512–1524. doi:10.1053/j.gastro.2022.02.014
Jamnitski, A., Bartelds, G. M., Nurmohamed, M. T., van Schouwenburg, P. A., van Schaardenburg, D., Stapel, S. O., et al. (2011). The presence or absence of antibodies to infliximab or adalimumab determines the outcome of switching to etanercept. Ann. Rheum. Dis. 70 (2), 284–288. doi:10.1136/ard.2010.135111
Jongsma, M. M. E., Winter, D. A., Huynh, H. Q., Norsa, L., Hussey, S., Kolho, K. L., et al. (2020). Infliximab in young paediatric IBD patients: it is all about the dosing. Eur. J. Pediatr. 179 (12), 1935–1944. doi:10.1007/s00431-020-03750-0
Khan, A., Berahmana, A. B., Day, A. S., Barclay, M. L., and Schultz, M. (2019). New Zealand society of gastroenterology guidelines on therapeutic drug monitoring in inflammatory bowel disease. N. Z. Med. J. 132 (1491), 46–62.
Krieckaert, C. L., Nair, S. C., Nurmohamed, M. T., van Dongen, C. J., Lems, W. F., Lafeber, F. P., et al. (2015). Personalised treatment using serum drug levels of adalimumab in patients with rheumatoid arthritis: an evaluation of costs and effects. Ann. Rheum. Dis. 74 (2), 361–368. doi:10.1136/annrheumdis-2013-204101
Krieckaert, C. L., van Tubergen, A., Gehin, J. E., Hernandez-Breijo, B., Le Meledo, G., Balsa, A., et al. (2023). EULAR points to consider for therapeutic drug monitoring of biopharmaceuticals in inflammatory rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 82 (1), 65–73. doi:10.1136/annrheumdis-2022-222155
Lamers-Karnebeek, F. B. G., Jacobs, J. W. G., Radstake, T., van Riel, P., and Jansen, T. L. (2019). Adalimumab drug and antidrug antibody levels do not predict flare risk after stopping adalimumab in RA patients with low disease activity. Rheumatol. Oxf. 58 (3), 427–431. doi:10.1093/rheumatology/key292
l’Ami, M. J., Krieckaert, C. L., Nurmohamed, M. T., van Vollenhoven, R. F., Rispens, T., Boers, M., et al. (2018). Successful reduction of overexposure in patients with rheumatoid arthritis with high serum adalimumab concentrations: an open-label, non-inferiority, randomised clinical trial. Ann. Rheum. Dis. 77 (4), 484–487. doi:10.1136/annrheumdis-2017-211781
Mitrev, N., Vande Casteele, N., Seow, C. H., Andrews, J. M., Connor, S. J., Moore, G. T., et al. (2017). Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 46 (11-12), 1037–1053. doi:10.1111/apt.14368
Nguyen, N. H., Solitano, V., Vuyyuru, S. K., MacDonald, J. K., Syversen, S. W., Jorgensen, K. K., et al. (2022). Proactive therapeutic drug monitoring versus conventional management for inflammatory bowel diseases: a systematic review and meta-analysis. Gastroenterology 163 (4), 937–949.e2. doi:10.1053/j.gastro.2022.06.052
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Panes, J., Colombel, J. F., D'Haens, G. R., Schreiber, S., Panaccione, R., Peyrin-Biroulet, L., et al. (2022). Higher vs standard adalimumab induction and maintenance dosing regimens for treatment of ulcerative colitis: SERENE UC trial results. Gastroenterology 162 (7), 1891–1910. doi:10.1053/j.gastro.2022.02.033
Papamichael, K., Afif, W., Drobne, D., Dubinsky, M. C., Ferrante, M., Irving, P. M., et al. (2022). Therapeutic drug monitoring of biologics in inflammatory bowel disease: unmet needs and future perspectives. Lancet Gastroenterol. Hepatol. 7 (2), 171–185. doi:10.1016/S2468-1253(21)00223-5
Papamichael, K., Juncadella, A., Wong, D., Rakowsky, S., Sattler, L. A., Campbell, J. P., et al. (2019). Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared with standard of care in patients with inflammatory bowel disease. J. Crohns Colitis 13 (8), 976–981. doi:10.1093/ecco-jcc/jjz018
Papamichael, K., Rakowsky, S., Rivera, C., Cheifetz, A. S., and Osterman, M. T. (2018). Infliximab trough concentrations during maintenance therapy are associated with endoscopic and histologic healing in ulcerative colitis. Aliment. Pharmacol. Ther. 47 (4), 478–484. doi:10.1111/apt.14458
Plasencia, C., Pascual-Salcedo, D., Garcia-Carazo, S., Lojo, L., Nuno, L., Villalba, A., et al. (2013). The immunogenicity to the first anti-TNF therapy determines the outcome of switching to a second anti-TNF therapy in spondyloarthritis patients. Arthritis Res. Ther. 15 (4), R79. doi:10.1186/ar4258
Pouw, M. F., Krieckaert, C. L., Nurmohamed, M. T., van der Kleij, D., Aarden, L., Rispens, T., et al. (2015). Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann. Rheum. Dis. 74 (3), 513–518. doi:10.1136/annrheumdis-2013-204172
Rau, R. (2002). Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials. Ann. Rheum. Dis. 61 (Suppl. 2), ii70–3. doi:10.1136/ard.61.suppl_2.ii70
Rinawi, F., Ricciuto, A., Church, P. C., Frost, K., Crowley, E., Walters, T. D., et al. (2021). Association of early postinduction adalimumab exposure with subsequent clinical and biomarker remission in children with Crohn's disease. Inflamm. Bowel Dis. 27 (7), 1079–1087. doi:10.1093/ibd/izaa247
Roblin, X., Genin, C., Nancey, S., Williet, N., Veyrard, P., Boschetti, G., et al. (2022). Swapping versus dose optimization in patients losing response to adalimumab with adequate drug levels. Inflamm. Bowel Dis. 28 (5), 720–727. doi:10.1093/ibd/izab158
Roblin, X., Rinaudo, M., Del Tedesco, E., Phelip, J. M., Genin, C., Peyrin-Biroulet, L., et al. (2014). Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am. J. Gastroenterol. 109 (8), 1250–1256. doi:10.1038/ajg.2014.146
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Sterne, J. A. C., Savovic, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Tweehuysen, L., van den Ende, C. H., Beeren, F. M., Been, E. M., van den Hoogen, F. H., and den Broeder, A. A. (2017). Little evidence for usefulness of biomarkers for predicting successful dose reduction or discontinuation of a biologic agent in rheumatoid arthritis: a systematic review. Arthritis Rheumatol. 69 (2), 301–308. doi:10.1002/art.39946
Ulijn, E., den Broeder, N., Wientjes, M., van Herwaarden, N., Meek, I., Tweehuysen, L., et al. (2020). Therapeutic drug monitoring of adalimumab in RA: no predictive value of adalimumab serum levels and anti-adalimumab antibodies for prediction of response to the next bDMARD. Ann. Rheum. Dis. 79 (7), 867–873. doi:10.1136/annrheumdis-2020-216996
Ungar, B., Mazor, Y., Weisshof, R., Yanai, H., Ron, Y., Goren, I., et al. (2016). Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment. Pharmacol. Ther. 43 (12), 1293–1299. doi:10.1111/apt.13631
van Herwaarden, N., van der Maas, A., Minten, M. J., van den Hoogen, F. H., Kievit, W., van Vollenhoven, R. F., et al. (2015). Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ 350, h1389. doi:10.1136/bmj.h1389
Ward, M. G., Warner, B., Unsworth, N., Chuah, S. W., Brownclarke, C., Shieh, S., et al. (2017). Infliximab and adalimumab drug levels in Crohn's disease: contrasting associations with disease activity and influencing factors. Aliment. Pharmacol. Ther. 46 (2), 150–161. doi:10.1111/apt.14124
Winter, D. A., Joosse, M. E., de Wildt, S. N., Taminiau, J., de Ridder, L., and Escher, J. C. (2020). Pharmacokinetics, pharmacodynamics, and immunogenicity of infliximab in pediatric inflammatory bowel disease: a systematic review and revised dosing considerations. J. Pediatr. Gastroenterol. Nutr. 70 (6), 763–776. doi:10.1097/MPG.0000000000002631
Keywords: adalimumab, biologics, therapeutic drug monitoring, benefits, optimization
Citation: Li Y, Xie C, Ding X, Wu Z, Zhang J, Zhu J and Miao L (2024) What are the benefits of therapeutic drug monitoring in the optimization of adalimumab therapy? a systematic review and meta-analysis up to 2022. Front. Pharmacol. 15:1376708. doi: 10.3389/fphar.2024.1376708
Received: 26 January 2024; Accepted: 17 June 2024;
Published: 08 July 2024.
Edited by:
Whocely Victor de Castro, Universidade Federal de São João del-Rei, BrazilReviewed by:
Vinícius Silva Belo, Universidade Federal de São João del-Rei, BrazilCopyright © 2024 Li, Xie, Ding, Wu, Zhang, Zhu and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyan Miao, bWlhb2x5c3V6aG91QDE2My5jb20=; Jianguo Zhu, MTU5NTAwMDUxOTVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.