
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 10 May 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1374680
 Hao Cheng1†
Hao Cheng1† Chenglong Yang2†
Chenglong Yang2† Pengliang Ge3†
Pengliang Ge3† Yi Liu1
Yi Liu1 Muhammad Mubashar Zafar1
Muhammad Mubashar Zafar1 Beibei Hu2
Beibei Hu2 Tong Zhang4
Tong Zhang4 Zengchun Luo4
Zengchun Luo4 Siyu Lu4
Siyu Lu4 Qin Zhou4
Qin Zhou4 Abdul Jaleel5*
Abdul Jaleel5* Maozhi Ren1,2*
Maozhi Ren1,2*Safflower (Carthamus tinctorius L.), a member of the Asteraceae family, is widely used in traditional herbal medicine. This review summarized agronomic conditions, genetic diversity, clinical application, and phytochemicals and pharmacological properties of safflower. The genetic diversity of the plant is rich. Abundant in secondary metabolites like flavonoids, phenols, alkaloids, polysaccharides, fatty acids, polyacetylene, and other bioactive components, the medicinal plant is effective for treating cardiovascular diseases, neurodegenerative diseases, and respiratory diseases. Especially, Hydroxysafflor yellow A (HYSA) has a variety of pharmacological effects. In terms of treatment and prevention of some space sickness in space travel, safflower could be a potential therapeutic agent. Further studies are still required to support the development of safflower in medicine. Our review indicates that safflower is an important medicinal plant and research prospects regarding safflower are very broad and worthy of further investigation.
Metabolic Syndrome (MetS), Coronary Heart Disease (CHD), hypertension, dysmenorrhoea, and amenorrhoea are typical public’s health problems. Specifically, MetS is a cluster of metabolic risk factors that lead to 84 Cardiovascular Diseases (CVD) and diabetes (Ruyvaran et al., 2022). As a common heart disease, CHD is the leading cause of morbidity and mortality world-wide and has been characterized as a chronic immunoinflammatory, fibroproliferative disease fueled by lipids (Dong et al., 2019; Shaya et al., 2022). Hypertension is a fatal yet preventable risk factor for cardiovascular disease and is responsible for the majority of cardiovascular mortality (Alpsoy, 2020). Dysmenorrhoea is the term for painful menstruation. It is a common gynecological complaint among female adolescents, which reduces the quality of women’s life and is still an important public health problem (Barcikowska et al., 2020). As for Amenorrhoea, it is regarded as a kind of menstrual disorder in a woman of reproductive age, due to an abnormality in the hypothalamic-pituitary-ovarian axis, anatomical abnormalities of the genital tract or functional causes (Ghadirkhomi et al., 2022). Nowadays, many medicinal plants and their derivatives have been traditionally used for the prevention and treatment of different types of diseases and disorders, with high healing but minor toxic side effects (Abuova et al., 2022). Among those plants, safflower is effective for some typical public health problems.
Safflower, which originates from Ethiopia, Afghanistan, and Arab countries, has a long history of cultivation and is widely distributed worldwide (Ruyvaran et al., 2022). This crop can provide commercial oil and natural pigments for the food area, which is of great industrial importance. Flavonoids, quinochalcones, alkaloids, polyacetylenes, fatty acids, proteins, lignans, steroids, and polysaccharides are compound families for safflower. Among these chemical components, flavonoids and quinochalcones are the main active components with various pharmacological effects (Ren et al., 2020; Adamska and Biernacka, 2021; Rodríguez-Félix et al., 2021). Besides, the flowers and seeds of safflower are rich in oils, which makes it a dryland oilseed crop yielding high quality edible oil (Mani et al., 2020). In some Asian countries, the plant’s petals and oil are used as medicine to treat diseases, including coronary heart disease, hypertension, dysmenorrhoea, and amenorrhoea (Zhang et al., 2016). Additionally, the leaf and stem of safflower (>80% of the total plant) are considered by-products of the oil industry in Mexico and other countries (Del-Toro-Sánchez et al., 2021).
In order to fully develop safflower in the future, we examined the current advancements in genetic diversity, clinical application, phytochemicals and pharmacological properties of safflower. The potential application in space disease was also emphasized in this paper.
The life cycle of safflower includes seed germination, seedling growth, flower blooming, and harvest. Information about the medicinal plant’s genome, botanical characteristics, growth conditions, and application is depicted in Figure 1. Safflower is considered a long-day plant, and most “spring” cultivars can be sown in late winter or early spring and flower rapidly in the absence of overwintering, dominating global safflower production. Some winter-hardy accessions that can be sown in autumn and survive winter conditions were also reported in Australia (Cullerne et al., 2021). As a promising oilseed crop with appreciable seed and oil yields, it has shown great suitability in arid regions mainly due to its high tolerance to cold, drought and soil salinity, which allows its cultivation in regions that experience dry spells like central Southern Italy, Brazilian Cerrado, and Xinjiang of China (de Oliveira Neto et al., 2022; Licata et al., 2023). The wide adaptation ability of safflower is associated with its deep root system, which may take up moisture and nutrients, especially nitrogen that has been leached below the rooting zone of most other crops, especially in sandy soils which are already deprived of essential plant nutrients (Hussain and Al-Dakheel, 2018). However, safflower is significantly sensitive to many soil and plant pathogens associated with wet conditions, with a particular aversion to wet soil during the period of germination (Joshi et al., 2021). In terms of irrigation, compared with normal irrigation, water shortage during the reproductive stage of safflower severely influenced production. Similarly, water stress could decline photosynthesis and crop nutrient uptake, decreasing safflower seed yield (Bijanzadeh et al., 2022). Temperature is a major environmental factor for safflower. During the rosette stage, seedlings of safflower exhibited good adaptability to temperature change and can tolerate even −5°C. In Sichuan, China, the optimum growth temperature is 15–18°C during the seedling stage (Li et al., 2022). Dormancy of safflower seeds is pronounced soon after removal from the parent plant. It was indicated that recently harvested seeds could efficiently germinate at 10°C in the dark, while seeds dry-stored at 20°C had low germination percentages (Silva et al., 2023). As for fertilizers, the application of plant growth promoting rhizobacteria (Azospirillum and Azotobacter) not only reduced the use of nitrogen and phosphate (NP) fertilizers up to 50%–75%, but also improved the seed protein and oil quality (Nosheen et al., 2016; Nosheen et al., 2018). Leaf miners, like Liriomyza sativae and L. huidobrensis, could cause yield loss and poor quality seriously for safflower. 2% emulsifiable concentrate, or the mixture of three insecticides (bifenthrin 20% water emulsions, thiamethoxam 25% water dispersible granule, abamectin 2% emulsifiable concentrate = 1:1:1) are suggested to spray on the plant’s leaves at squaring stage. Along with the safflower’s color turning from yellow to red, the flower could be harvested for different purposes. In the early stage of blooming, yellow pigment accumulates much, which can be used to prepare medicines and for soft drink dyeing; As the content of red pigments gradually increased in the middle stage, flowers are suggested to be harvested quickly and dried in the shade for the application of herbal medicine; While yellow parts are barely visible, safflower could be processed for dyeing fabrics chocolate, and cosmetics (Pu et al., 2019).
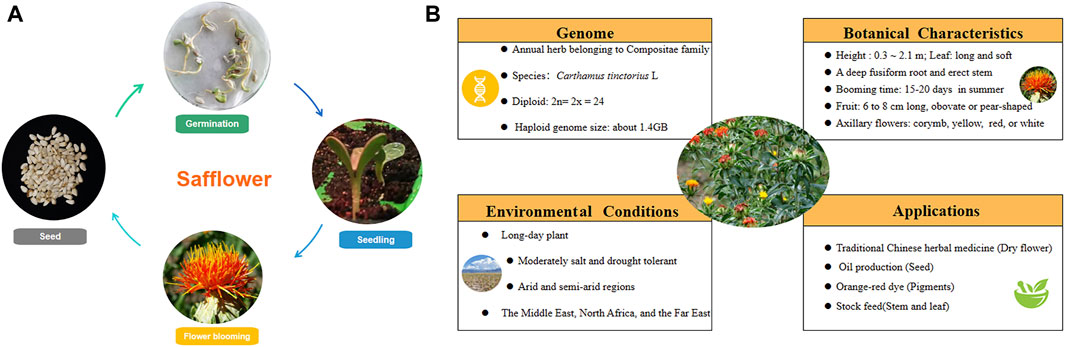
Figure 1. The biological characteristics and application of safflower. (A) the life cycle of safflower; (B) the characters of safflower.
Safflower is best known as “kusum” (in India and Pakistan), a term derived from the Sanskrit word “kusumbha.” Meanwhile, this crop is known as “Golrang” in Iran and “honghua” (red flower) in China (Abuova et al., 2022). The use of safflower by humans dates back to ancient times; remains have been found at an archaeological site in Syria, believed to be from 7500 B.C. The wild ancestor of safflower is believed to be Carthamus palestinus Eig, and its domestication led to its cultivation in the Middle East, North Africa, India, and the Far East (Pearl et al., 2014; Ali et al., 2019; Ali et al., 2020). Phylogenetic analysis showed that safflower likely diverged from artichokes (Cynara cardunculus) and sunflowers (Helianthus annuus) approximately 30.7 and 60.5 million years ago, respectively (Wu et al., 2021).
Safflower was cultivated in Italy, France, and Spain as early as the Middle Ages (fifth to fifteenth centuries). The crop was first introduced to North America in the late 1890s and commercial cultivation began in the 1950s (Turgumbayeva et al., 2018). It was also introduced in northern Mexico and is becoming an increasingly attractive crop for farmers, which is cultivated for oil extraction purposes from seed (Del-Toro-Sánchez et al., 2021). According to the “Compendium of Materia Medica,” safflower was introduced to China via the Silk Road during the Han Dynasty (119 B.C.) and has been cultivated in China for more than 2,000 years. At present, the main production areas in China are Henan, Sichuan, Zhejiang, and Xinjiang Uyghur Autonomous Region. Among them, the safflower variety ‘Chuanhonghua’ is used as a medicinal herb in Sichuan, China (Liang and Wang, 2022). Good light conditions in Xinjiang make it the largest producer of safflower in China, accounting for more than 80% of the national production (Lin et al., 2022).
According to the Food and Agricultural Organization, the yield of safflower has increased over the past 60 years (Figure 2A). Almost 20 countries have cultivated safflower on a large scale, with a total planting area of 1,140,002 ha and an annual production of 948,516 tons in 2019 (Ali et al., 2019). In 2021, the global harvested safflower area was 850,431 ha, with safflower seed production of 631,051.6 tons. Kazakhstan accounted for the highest production of safflower seed (223,895.45 tons; area harvested, 43.27% of the global harvested area), followed by the Russian Federation, the United States of America, Mexico, India, China, and Turkey (Figure 2B).
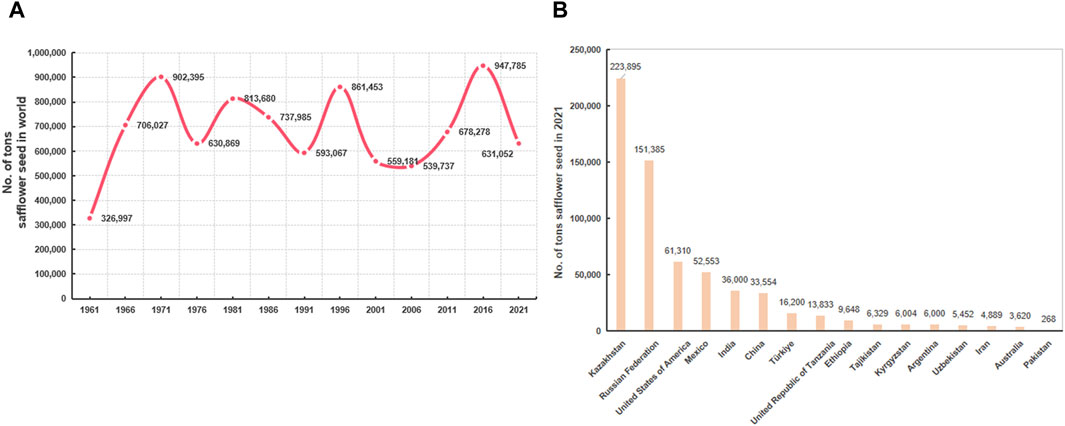
Figure 2. The distribution and yield of safflower around the world. (A) The changes in safflower yield in recent 60 years; (B) list of world’s top safflower-producing countries in 2021.
Germplasm resources, which serve as repositories for diverse traits, are essential for crop improvement and industry development. Various DNA molecular markers, including simple sequence repeat (SSR), amplified fragment length polymorphism (AFLP), inter-simple sequence repeat (ISSR), expressed sequence tag SSR (EST-SSR), random amplified polymorphic DNA (RAPD), inter-primary binding site (iPBS)-retrotransposon, sequence-related amplified polymorphism (SRAP), and peroxidase gene polymorphism (POGP) have been used to assess the genetic diversity of safflower. The analysis has received considerable attention from the scientific community in the last decade (Figure 3).
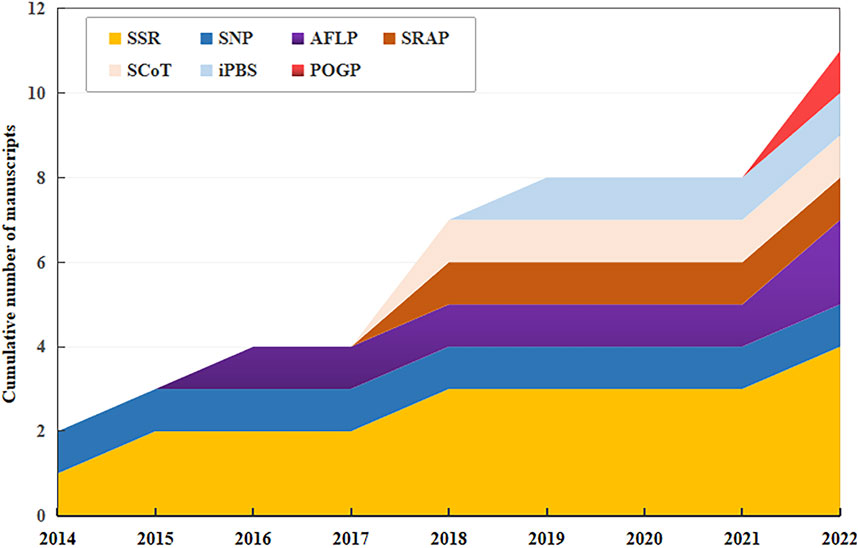
Figure 3. Cumulative number of manuscripts published between 2014 and 2022 reporting in the use of DNA molecular markers for safflower (Carthamus tinctorius) genotyping. SSR, simple sequence repeat; SNP, single-nucleotide polymorphism; AFLP, amplified fragment length polymorphism; SRAP, sequence-related amplified polymorphism; ScoT, start codon target; iPBS, inter-primary binding site; POGP, peroxidase gene polymorphism. The same as below.
Research led by scientists has revealed the rich genetic diversity of safflower (Table 1). 190 safflower individuals were genotyped using 133 single-nucleotide polymorphism (SNP) markers and identified previously undocumented genetic diversity. The results showed a modest reduction in gene diversity was observed in the commercial breeding lines (Pearl et al., 2014). Interestingly, most safflower breeding lines come from the Old World safflower germplasm (Lee et al., 2014).
Microsatellite markers (SSR) confirmed that 93 markers were polymorphic, and wild safflower species showed significant cross-species transfer (Ambreen et al., 2015). Using AFLP markers, the phenotypes of 531 representative safflower resources worldwide were evaluated for genetic diversity significant variation in agronomic traits was observed, with the highest variability and unique traits found in materials from the Indian subcontinent and from the America (Kumar et al., 2016). Genetic diversity analysis of 100 safflower genotypes from different geographical regions worldwide was performed using 12 polymorphic SRAPs and 11 polymorphic start codon target (SCoT) markers, showing that the genotypes of cultivated safflower were classified into the following five taxa: the Middle East (Iran, Iraq, Turkey, and Tajikistan), the Far East (India, Pakistan, and Korea), Europe, the American continent, and Africa (Egypt, Sudan, and Libya) (Golkar and Mokhtari, 2018). A safflower panel (CtAP) of 124 accessions from two core collections was analyzed using SSR markers. The Shannon diversity index (H = 0.7537) and Nei’s expected heterozygosity (I = 0.4432) revealed significant genetic diversity of CtAP (Ambreen et al., 2018). Additionally, 131 safflower germplasm resources from 28 countries were analyzed using 13 iPBS-retrotransposon markers, revealing the genetic diversity and population structure of seven inferred similarity centers (Ali et al., 2019). POGP markers were also used to analyse the genetic diversity of 131 safflower samples, which demonstrated that safflower germplasm resources collected from the Fertile Crescent region were clustered (Yildiz et al., 2022). Moreover, structural analysis of safflowers based on 155 AFLP and 144 SSR markers identified three main subpopulations (K = 3) with approximately 35% admixtures in the panel (Singh et al., 2022). In China, 13 local safflower varieties, including Chuanhonghua No. 2, Yunhonghua No. 2, and Xinhonghua No. 7, were breeded using safflower resources.
There are some limits on the molecular marker information for evaluation of genetic diversity of safflower. Recently, With next-generation sequencing methods developing, omics and biotechnology have been used for investigating the genetic diversity of safflower (Table 2). 509 putative genomic SSR markers for sufficient genome coverage were acquired (Lee et al., 2014). Genetic mapping of millions of SNPs in safflower via whole-genome resequencing showed there were a total of 57,270 scaffolds, each containing five or more mapped SNPs (Bowers et al., 2016). A microarray based Diversity Array Technology (DArT) marker has been developed and used as an effective tool for genome diversity and population structure, and mapping construction in safflower. In-depth genome diversity analysis of worldwide diverse safflower accessions using NGS data generated by DArTseq technology was conducted, which confirmed the hypothesis that safflower was domesticated in the western Fertile Crescent and then spread to Africa and Europe (Hassani et al., 2020). Various mean genetic diversity parameters like expected heterozygosity (0.32) for 94 safflower accessions originating from 26 countries exhibited sufficient genetic diversity using 12232 silicoDArT markers. Interestingly, two DArTseq markers (DArT-45483051 and DArT-15672391) significantly associated with (p < 0.01) for 100-seed weight were identified, which may help develop high-yielding cultivars of safflower through marker-assisted breeding (Ali et al., 2020). cDNA-derived SSR markers are suitable for evaluation of genetic diversity. 35 SSR primer pairs were detected a high rate of polymorphism (>57%) among safflower accessions, physically mapped on safflower genome and could clearly discriminate the cultivated accessions from wild relatives (Ahmadi and Ahmadikhah, 2022). Additionally, a 1.17-Gb assembly with a contig N50 of 1.08 Mb was obtained for ‘Chuanhonghua 1’ using an integrated strategy combining Illumina, Oxford Nanopore, and Hi-C sequencing, 220 safflower lines were re-sequenced, resulting in the acquisition of a total of 7 402 693 high-quality SNPs (Chen et al., 2023). These studies showed that safflower germplasms had high genetic diversity, providing raw plant materials for alternative safflower breeding program.
Since long time, safflower has been used as a traditional herbal medicine in Asian countries (Table 3). In Iranian folklore medicine, safflower is an indispensable element for treating melancholy humor, vitiligo and black spots, rheumatism and paralysis, mouth ulcers, phlegm humor, numb limbs, diabetes, melancholia, dropsy, and the like. In Ayurveda, the herb is typically used for arthritis, scabies, and mastalgia. In Thailand, aqueous extract of safflower flowers is regarded as the hair color promoter, which has been largely used clinically (Delshad et al., 2018). Safflower is included as a traditional Chinese medicine in the Pharmacopoeia of the People’s Republic of China with the application of drugs for the treatment of amenorrhea, gastric tumors, as well as wounds. Especially, about 80 herbal medical products in Chinese Pharmacopoeia (2015 Ed.) are connected with safflower (Tong et al., 2021). Safflower has effects on relieving pain, dispersing blood stasis, and activating blood circulation, and a natural pigment named safflower yellow (SY) from safflower petals has been extensively applied in the medical field (Chen et al., 2022).
Safflower preparations, including safflower injection, safflower yellow injection, and safflower soothing and revitalizing compresses, have been used clinically in modern medicine (Wu et al., 2021). For example, the water extract of safflower has been developed as an intravenous injection in China, which is extensively applied to treat cardiovascular diseases clinically (Turgumbayeva et al., 2018). In addition to treating physical diseases, a recent survey of 752 Saudis who had previously tried safflower for depression and anxiety showed that 279 (37.1%) reported that safflower was effective, whereas 389 (51.73%) reported some improvement (Albaiz, 2022). Consistent with the survey, a systematic review of scientific articles published between 2010 and 2020 showed that safflower flower extracts have an anxiolytic effect as effective as diazepam (Meneses et al., 2023). Due to its nutritional and healthy function, many safflower products, such as painkillers, health drinks, skin lotions, tablets, and other nutritional supplements, are currently on the market. The combination of safflower and other ingredients is effective in the treatment of some diseases. It was reported that GuHong injection, composed the safflower and chemical drug N-acetyl-L-glutamine, has great value in clinical for cerebrovascular diseases, such as ischemic stroke and related diseases (Wang et al., 2023a). Safflower and peach kernel herb-pair are widely used in traditional Chinese medicine for the treatment of liver fibrosis (Huang et al., 2023). The safflower-astragalus herbal pair has synergistic effects in the treatment of CHD (Yuan et al., 2023).
Some bioactive compounds of safflower is with great potential in clinical research. For example, Hydroxysafflor yellow A (HYSA), a natural compound from safflower, has a good effect of alleviating atherosclerosis, and clinical trials is needed (Xue et al., 2021). A meta-analysis with 31 groups containing a total of 2487 participants confirmed that HSYA can effectively treat diabetic kidney disease (DKD), which may provide therapies for DKD with new insights and promote its application in clinical practice (Fu et al., 2022). Besides, as unique organelles with a natural resilience to environmental stresses, safflower oleosomes may serve as a novel therapeutic agent in the field of dermatology, and human clinical research is required to determine their efficacy and safety (Patel et al., 2023).
The obvious medical effects are associated with the chemical composition and pharmacological properties of safflower. In recent years, large amounts of bioactive components have been determined using various analytical methods, including liquid chromatography-mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), high-throughput metabolic fingerprinting, and nuclear magnetic resonance (NMR) (Mani et al., 2020). Safflower contains several bioactive components, such as vitamins A and E, polyunsaturated fatty acids (with linoleic acid being predominant at 70%), monounsaturated oleic acid (10%), small amounts of stearic acid, flavonoids, alkaloids, and polyalkenes (Liang and Wang, 2022). The main bioactive compounds and chemical structures of safflower were depicted in Figure 4.
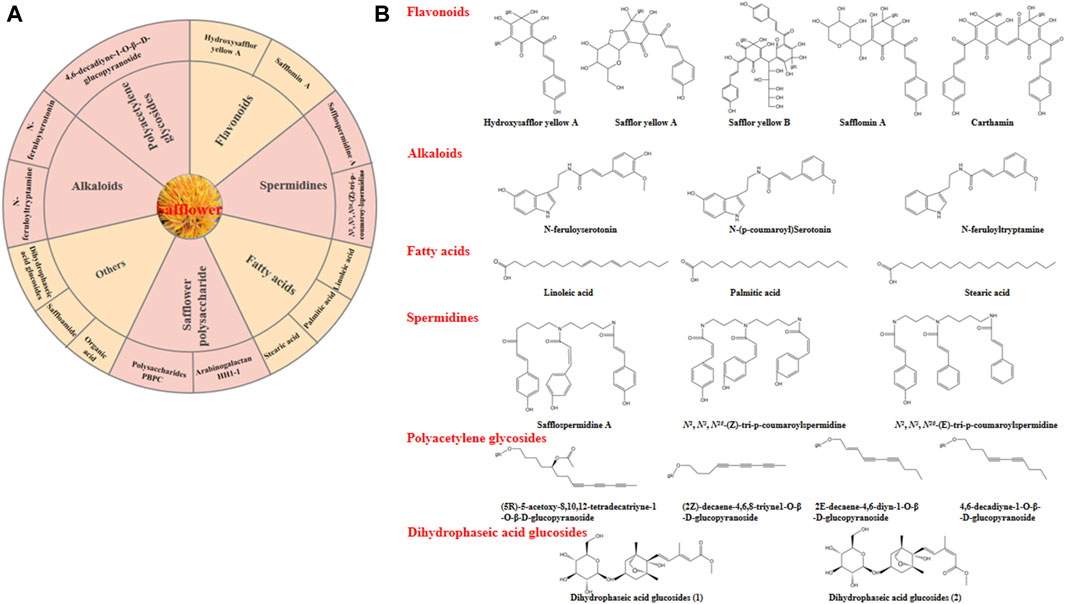
Figure 4. The main bioactive compounds of safflower. (A) some important bioactive compounds identified in safflower; (B) The chemical structures of the main bioactive compounds in safflower.
Flavonoids and their glycosides (O- and C-glycosides) are an important group of phytochemicals in safflower. It is reported that the flavonoid content of safflower polyphenol extracts from flowers is 330 ± 23 catechin equivalent/100 g (Bacchetti et al., 2020). Previous studies have identified more than 104 compounds, among which six characteristic quinochalcone C-glycosides (QCGs), including HYSA and its two isomers, anhydrosafflor yellow B (AHSYB), safflomin C, and isosafflomin C, serve as biomarkers of safflower, suggesting that quinone chalcone and flavonoid compounds are active components (Si et al., 2016). Ultra-performance liquid chromatography coupled with triple-quadrupole linear ion-trap tandem mass spectrometry (UPLC-QTRAP®/MS2) analysis revealed that 16 components, including the signature components HYSA, AHSYB, safflomin C, and 13 other flavonoid glycosides, were related to the chromaticity characteristic of safflower (Pu et al., 2019). Similarly, ultra-high-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry (UHPLC/Q-TOF-MS) analysis revealed that the flowers of safflower are rich in natural red and yellow pigments, which are flavonoid components such as safflomin A, safflomin C, and safflomin yellow B (Wang et al., 2021). A hybrid HDMSE-HDDDA method for non-targeted characterization identified 41 chalcones, 66 flavanols/flavones, 11 flavanones, six organic acids, one polyacetylene, and 16 others in safflower (Qian et al., 2022). UHPLC ESI-MS/MS analysis uncovered 212 flavonoid metabolites in safflower, including 64 flavones, 41 flavonols, 40 flavone-C-glycosides, 22 flavonones, 10 isoflavones, 10 catechin derivatives, 19 anthocyanins, 2 quinonechalcones, 2 flavonolignans, 1 alkaloid, and 1 proanthocyanidin (Ren et al., 2022). Recent studies have isolated a new flavonoid-saffloflavone, and six known compounds: kaempferol-3-O-rutinoside, kaempferol-3-O-sophoroside, quercetin-3-O-β-d-glucoside, quercetin-7-O-β-d-glucoside, luteolin-7-O-β-d-glucoside, and kaempferol-3-O-β-d-glucoside, from flowers of safflowers. All of these flavonoids can protect against H2O2-induced injury in H9c2 cells (Wang et al., 2022).
Alkaloids such as N-feruloyltryptamine and serotonin derivatives are widely distributed in the flowers of C. tinctorius and exhibit significant anticoagulant, hepatoprotective, neuroprotective, and antioxidant effects. Among them, 5-hydroxytryptamine derivatives, particularly N-(p-coumaroyl) serotonin and N-feruloylserotonin, are the main active alkaloids (Zhang et al., 2016). The main phenolic compounds of safflower seeds are N-feruloylserotonin-5-O-β-D-glucoside, 8-hydroxyarctigenin-4-O-β-D-glucoside, luteolin-7-O-β-D-glucoside, and N-feruloylserotonin (Mani et al., 2020).
Polysaccharides are the main bioactive components in safflower. A large number of soluble polysaccharides (SPS) from C. tinctorius, which have been proved to have antitumor, immunomodulatory, anti-cancer, and anti-diabetic effects (Zhou et al., 2018; Lin et al., 2022). HH1-1, an arabinogalactan with a relative molecular weight of 70.9 kDa, has been isolated from safflower flowers. The HH1-1 structure comprises a backbone of 1,6-linked Galp which is branched at C-3 position by a side chain of 1,3-linked Galp with sub-branches attached to the C-3 position (Yao et al., 2018). PBPC, a polysaccharide extracted from safflower bee pollen, has attracted the attention of the scientific community owing to its unique origin and biological activity (Wu et al., 2021). Four purified safflower polysaccharide fractions (named SSP1, SSP2, SSP3, and SSP4, respectively) were extracted from safflower by ultrasonic assisted extraction, among which SSP3 exhibited relatively higher antiproliferative activity, Fe+3-reduction activity, and ABTS+ scavenging activity (Wang et al., 2023b).
Oleic, linoleic, palmitic, and stearic acids are the most common fatty acids extracted from safflower. Safflower oil contains both saturated (palmitic, C16:0, and stearic C18:0) and unsaturated (oleic-C18:1, linoleic-C18:2, and linolenic-C18:3) fatty acids (Conte et al., 2016). The volatile oils from safflower variety ‘Ak-Mai’ of Kazakhstan were rich in undecanoic acid, heneicosanoic acid, octane, octadecanoic acid, 2-nonen-1-ol, 1.3-cyclohexadiene, myrtenoic acid, 1-eicosanol, hexcosane, and heptocosane (Turgumbayeva et al., 2018). The primary component of safflower seed oil is linoleic acid (84.48%), followed by palmitic acid (6.54%) and stearic acid (3.77%) (Alimi et al., 2022).
In recent years, bioactive components, such as spermidine and polyacetylene, have been identified in safflower. Two new spermidine compounds (safflospermidine A and safflospermidine B) and two previously identified compounds (N1,N5,N10-(Z)-tri-p-coumaroylspermidine and N1,N5,N10-€-tri-p-coumaroylspermidine) were isolated from safflower (Zhang et al., 2016). In addition, Polyacetylene glucosides including (5R)-5-acetoxy-8,10,12-tetradecatriyne-1-O-β-D-glucopyranoside, (2Z)-decaene-4,6,8-triyne-1-O-β-D-glucopyranoside, (8Z)-1-[(3-O-β-D-glucosyl)-isovaleroyloxy]-8-decaene-4,6-diyne, (8Z)-decaene-1-isovaleroyloxy −4,6-diyne-10-O-β-D-glucopyranoside, and (2E,8E)-decadiene-4,6-diyne-1-O-β -D-glucopyranoside, were also identified from safflower buds (Li et al., 2021). A new flavonoid (saffloflavanside (1)), sesquiterpene (safflomegastigside (2)), and amide (saffloamide (3)), in addition to 22 known compounds from safflower were also isolated (Liu et al., 2022). Two dihydrophasic acid glycosides, dihydrophasic acid glucoside, which has anti-obesity effects, were also been isolated from C. tinctorius florets (Baek et al., 2020). Five new sesquiterpenoids (1–5) were isolated from the florets of safflower, which was named as (−)-(1R,4S,9S,11R)-caryophyll-8 (13)-en-14-ol-5-one (1), (+)-(1R,4R,9S,11R)-caryophyll-8 (13)-en-14-ol-5-one (2), (−)-(3Z,1R,5S,8S,9S,11R)-5,8-epoxycaryophyll-3-en-14-O-β-D-glucopyranoside (3), (+)-(1S,7R,10S)-guai-4-en-3-one-11-O-β-D-fucopyranoside (4), and (−)-(2R,5R,10R)-vetispir-6-en-8-one-11-O-β-D-fucopyranoside (5). These compounds have therapeutic effects for the treatment of atherosclerosis (Li et al., 2022).
Safflower is widely used in traditional Chinese medicine owing to its health benefits. It has a wide range of biological effects, including coronary artery dilation, alleviation of myocardial ischaemia, modulation of the immune system, and anticoagulant, antithrombotic, antioxidant, anti-aging, anti-hypoxic, anti-fatigue, anti-inflammatory, anti-liver fibrosis, anti-tumour, and analgesic activities (Figure 5).
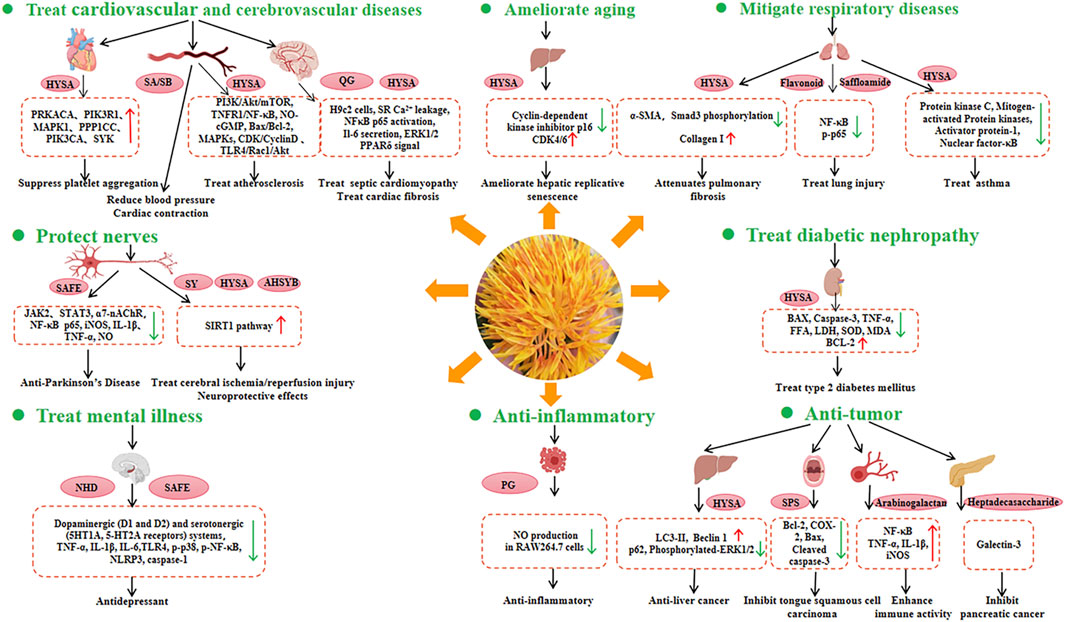
Figure 5. Health benefits and related molecular mechanisms of safflower. The red arrow represents increasing expression of genes, while the green arrow represents decreasing expression of genes. HYSA, Hydroxysafflor yellow A; SA, Safflomin A; SB, Safflomin B; QC, Quinochalcone C-Glycosides; SAFE, Safflower flavonoid extract; SY, Safflower yellow; AHSYB, Anhydrosafflor yellow B; NHD, N-Hexadecanoic acid; PG, Polyacetylene glucosides; SPS, Safflower polysaccharide.
The incidence of CCVDs is increasing globally and constitutes a significant public health burden. Modern pharmacological studies have reported that safflower can be used to treat CCVDs. The therapeutic effects of hydroxysafflor yellow A (HYSA) on CCVD are due to its antioxidant, anti-inflammatory, and neuroprotective properties, which are mediated through complex signaling pathways (Wu et al., 2018; Bai et al., 2020). Safflower may regulate core genes (PRKACA, PIK3R1, MAPK1, PPP1CC, PIK3CA, and SYK) involved in the platelet activation pathway, potentially inhibiting platelet aggregation and having therapeutic effects in cardiovascular diseases (Yu et al., 2019).
In traditional Chinese medicine, safflower injection is used to improve blood circulation, because it can activate and promote blood circulation, as well as exert potent regulatory effects on the intrinsic coagulation system (Wang et al., 2018). HYSA has protective effects against atherosclerosis by regulating processes such as reverse cholesterol transport, fatty acid synthesis, oxidative stress, PI3K/Akt/mTOR, NLRP3 inflammasome, TNFR1/NF-κB, NO-cGMP, Bax/Bcl-2, mitogen-activated protein kinases (MAPKs), CDK/CyclinD, and TLR4/Rac1/Akt signaling pathways (Xue et al., 2021).
In terms of cardiomyopathy, hydroxysafflor yellow B and hydroxysafflor yellow C isolated from safflower florets have been shown to protect cultured H9c2 cardiomyocytes against H2O2-induced cytotoxicity by exerting antioxidant effects (Yue et al., 2014). Additionally, quercetin-7-O-β-d-glucoside, luteolin-7-O-β-d-glucoside, and kaempferol 3-O-β-d-glucoside have been found to protect H9c2 cells against H2O2-induced injury (Wang et al., 2022). C. tinctorius ethanolic extract was shown to alleviate lipopolysaccharide (LPS)-induced cardiac fibrosis through the ERK1/2 pathway, suggesting that safflower may be a potential cardioprotective agent against LPS-induced cardiac fibrosis (Han et al., 2017). One of the main functional components of safflower is HYSA. On the one hand, HYSA is a potential antioxidant with protective effects against myocardial injury, which can be attributed to its antioxidant effects (Yao et al., 2021). Moreover, it can enhance endurance performance by activating PPARδ signaling and promoting the utilisation of substrates in myocytes from glucose to fat (Sun et al., 2021).
Parkinson’s disease (PD) and Alzheimer’s disease (AD) are neurodegenerative diseases. Safflower petal extracts have been shown free radical scavenging and neuroprotective effects (Abuova et al., 2022). The safflower flavonoid extract (SAFE) showed significant anti-PD effects, which might be due to the anti-inflammatory activity of flavonoids (Lei et al., 2020). Molecular docking analysis revealed that key components of SAFE, such as kaempferol-3-O-rutinoside or AHSYB, can bind to proteins such as TH, JAK2, STAT3, and α7-nAChR (Ablat et al., 2022). Thus, SAFE is a potential drug candidate for PD prevention.
SY and HYSA can protect nerves by alleviating amyloid β1-42-induced glutamate cycle disorder in an AD rat model and by improving synaptic structural plasticity, leading to enhanced learning and memory (Hou et al., 2020). In particular, HYSA can partially inhibit the expression of NF-κB p65 and iNOS, and downregulate the levels of IL-1β, TNF-α, and NO, leading to the suppression of inflammatory responses, attenuation of LPS-induced midbrain neurotoxicity and neuroinflammation, and alleviation of LPS-induced dopaminergic neuronal damage (Tiwari et al., 2018). Furthermore, HYSA and AHSYB may improve cell viability, decrease neuronal apoptosis, reduce infarct volume, improve neurological function, inhibite apoptosis, and reduce oxidative stress, which suggest that HYSA and AHSYB are potential drugs for the treatment of brain ischaemia/reperfusion (I/R) injury via the SIRT1 pathway (Fangma et al., 2021).
HYSA can exert various pharmacological effects, including preventive and therapeutic effects on some respiratory diseases such as acute lung injury and chronic obstructive pulmonary disease. Specifically, HYSA can mitigate the effects of bleomycin (BLM)-induced pulmonary fibrosis in mice (Jin et al., 2016). It also has the potential to be a novel drug for asthma owing to its ability to inhibit the upregulation of inflammatory factor expression, disruption of cellular barrier function, and suppression of the expression of protein kinase C, MAPK, activator protein-1, and nuclear factor-κB activation (Guo et al., 2018). Concerning lung injury, one new flavonoid (1), one new sesquiterpene (2), one new amide (3), and 22 known compounds (4–25) have been isolated from safflower flowers. Compounds 2–3, 8–11, and 15–19 demonstrated protective effects against LPS-induced BEAS-2B cell injury, suggesting that safflower could be developed as a drug for the treatment of lung injury (Liu et al., 2022).
Safflower contains various active ingredients that exhibit antitumor and anti-inflammatory effects (Zhou et al., 2018). HYSA induced autophagy in hepatocellular carcinoma cells by promoting Beclin 1 expression and inhibiting ERK phosphorylation, indicating HYSA may be a potential therapeutic agent for hepatocellular carcinoma (Chen et al., 2020). SPS is one of the most important active components of safflower and has been found to exert therapeutic effects on tongue squamous cell carcinoma by regulating the expression of Bcl-2, COX-2, Bax, and cleaved CASP3 (Zhou et al., 2018). Safflower arabinogalactan HH1-1 polysaccharide enhances immune activity by activating the NF-κB signaling pathway (Yao et al., 2018). Additionally, a highly branched heptadecasaccharide can target galectin-3 and inhibit pancreatic cancer cell growth (Hu et al., 2022). The polyacetylene glycosides (5R)-5-acetoxy-8,10,12-tetradecatriyne-1-O-β-D-glucopyranoside exhibited anti-inflammatory activity by inhibiting LPS-induced NO production in RAW264.7 cells (Li et al., 2021).
Anxiety and depression are some of the causes of human suffering. However, the diagnosis and treatment remain challenging. Safflower can control monoamine transporters, resulting in the alleviation of neuropsychological damage. Moreover, safflower components (especially n-hexadecanoic acid) exhibit antidepressant-like effects by interacting with dopaminergic (D1 and D2) and serotonergic (5HT1A and 5-HT2A receptors) systems (Abbasi-Maleki and Mousavi, 2017). In addition, it can regulate the TLR4-NF-κB-NLRP3 signaling pathway, which contributes to its antidepressant effects (Chen et al., 2021). Clinically, Safflower has been applied to the Chinese herb DANSHEN-honghua (DSHH) to treat cognitive impairment after hippocampal ischaemic injury (Gu et al., 2018).
Safflower are found to have antiaging effects. HYSA is likely to regulate downstream genes such as CCNE1, CCNA2, P107, and MCM4, resulting in the amelioration of D-gal-induced hepatic replicative senescence (Min et al., 2020). The incidence of diabetic nephropathy (DN), a serious complication of diabetes, is the main cause of end-stage renal failure. HYSA was reported to show renal protective effects in high-fat diet-fed rats and streptozotocin-induced DN by inhibiting oxidative stress, attenuating the inflammatory response, and downregulating renal apoptosis. The results indicated the potential application of HYSA for the treatment of type 2 diabetes (Lee et al., 2020). Safflower by-products like leaf and stem are sources of obtaining phenolic compounds. Compounds from the by-products have been confirmed to have a protective effect against human erythrocytes, due to their ability to inhibit oxidative damage to erythrocyte membrane lipids as a result of free radicals (Del-Toro-Sánchez et al., 2021).
The space environment alters human physiology, including fluid shifts, cardiovascular deconditioning, bone and muscle density loss, immune system dysregulation, and changes in the gastrointestinal tract as well as metabolic enzymes (Eyal and Derendorf, 2019). Cardiovascular system may be especially vulnerable due to the combined impacts of space radiation exposure, lack of gravity, and other spaceflight hazards (Huff et al., 2022). Long-term exposure to space microgravity also alters the time-frequency of heart rate in astronauts (Otsuka et al., 2016). Space motion sickness, which affects around 70% of astronauts, has symptoms of fatigue, vertigo, nausea, dizziness, headaches, vomiting, and cold sweating. It might cause potential problems for mission-critical tasks and astronauts’ wellbeing (Khalid et al., 2023). Additionally, astronauts also exhibit immune system dysregulation during space flight (Baba et al., 2020). Some possible hazards of space travel could be related to physical and psychological consequences on astronauts (Marazziti et al., 2022). Thus, medications are commonly administered during missions to treat space sickness.
Safflower contains various active ingredients that exert pharmacological effects on the cardiovascular system, including ischemic myocardium-alleviating, anti-myocardial fibrosis, vascular protection, anti-thrombosis, lipid-lowering, antihypertensive, and anti-myocardial hypertrophic effects (Yu et al., 2019). Thus, the herb and its components could be used to treat some space sickness. Moreover, owing to anti-anxiety and antidepressant effects, the herb could be a potential therapeutic agent to alleviate psychological and psychopathological issues associated with space exploration. It was reported that an efficient space farming system is essential for human survival in space, and a whole-body edible and elite plant (WBEEP) strategy for space crop improvement is suggested (Liu et al., 2021). As safflower has advantages such as various bioactive compounds, medicinal values, and excellent adaptability, it could be WBEEP for providing medicine and food for human in space travel, with the application of biotechnology, plant factory, and cultivating strategy in space (Figure 6).
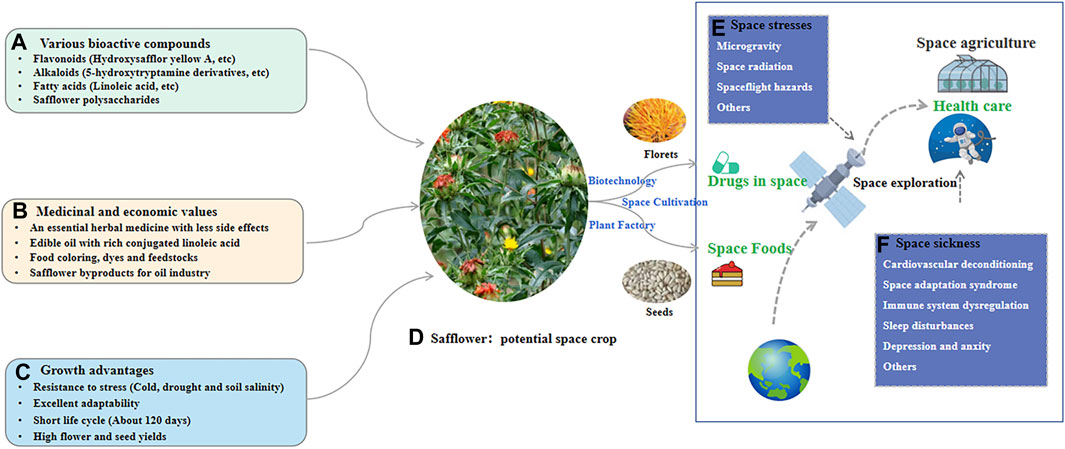
Figure 6. The potential application of safflower in space to supply space drugs and foods. (A) The bioactive compounds for safflower; (B) The medicinal and economic values for safflower; (C) The growth advantages for safflower; (D) The potential of safflower as space crop for providing both drugs and foods. (E) The main stresses for human in space; (F) Some space sickness during space exploration.
As a high-value medicinal plant with a wide range of genetic diversity, safflower is cultivated on a large scale in many countries, such as Kazakhstan, China, Russia, and the United States, where it is used to produce medicines, edible oils, food colouring agents, dyes, and fodders. Especially, it is rich in pharmacologically significant secondary metabolites, such as flavonoids, phenols, alkaloids, polysaccharides, and polyacetylene, with therapeutic effects on a variety of diseases, including cardiovascular diseases, neurodegenerative diseases, respiratory diseases, tumors, dysregulated inflammation, psychological diseases, aging, and diabetes. The phytochemistry and pharmacological activities of safflower have been investigated systematically in recent years. Considering its efficacy for the treatment of many physical and mental diseases, safflower could be herbal medicine for potential health challenges associated with space travel.
At present, the utilization of safflower relies on traditional planting and picking, which is low yield, labor cost, and easily affected by weather. As synthetic biology is a kind of biotechnology for efficient production of active ingredients, it is suggested to express key enzymes related to medicinal components in other organism like potato, yeast, and microalgae. Moreover, nanotechnology has shown promising advancements in the field of drug development and its delivery, with an opportunity to achieve better-targeted delivery, effective treatment, and an improved safety profile. It is suggested to apply nanotechnology for the controlled release of safflower components clinically. Although safflower has been recognized for its medicinal value, there have been limited studies on breeding, pharmacology, synthetic biology, and drug development. Hence, further research may focus on the following aspects: (1) utilizing the safflower germplasm resources to breed new safflower varieties with biotechnology; (2) understanding the phytochemistry and pharmacology of safflower, and exploring its new therapeutic potential; (3) uncovering the molecular mechanisms of safflower’s bioactive components synthesis, and conducting biosynthesis of pharmacologically active secondary metabolites of safflower by microorganisms like yeast and microalgae; (4) developing nanotechnology-based therapeutic agents with safflower and evaluating their efficacy and safety; (5) advancing medical research and clinical trial of safflower in treating and preventing space sickness.
HC: Investigation, Writing–original draft, Writing–review and editing. CY: Investigation, Writing–original draft. PG: Writing–original draft, Data curation. YL: Investigation, Writing–review and editing. MZ: Visualization, Writing–review and editing. BH: Data curation, Writing–review and editing. TZ: Resources, Writing–review and editing. ZL: Resources, Writing–review and editing. SL: Resources, Writing–review and editing. QZ: Resources, Writing–review and editing. AJ: Supervision, Writing–review and editing. MR: Funding acquisition, Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chinese Academy of Agricultural Sciences, grant number (34-IUA-02), Sichuan Science and Technology Program, grant number (2022112), Chengdu Technology Program, grant number (N5101012023000213), Central Public-interest Scientific Institution Basal Research Fund (S2023011), and the Local Financial Funds of Chengdu National Agricultural Science and Technology Center (NASC2021ST08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbasi-Maleki, S., and Mousavi, Z. (2017). Hydroethanolic extract of Carthamus tinctorius induces antidepressant-like effects: modulation by dopaminergic and serotonergic systems in tail suspension test in mice. Iran. J. Basic Med. Sci. 20 (9), 1063–1073. doi:10.22038/IJBMS.2017.9277
Ablat, N., Liu, R., Ablimit, M., Sun, Y., Xu, F., Zhao, X., et al. (2022). Preventive effects of a standardized flavonoid extract of safflower in rotenone-induced Parkinson's disease rat model. Neuropharmacology 217, 109209. doi:10.1016/j.neuropharm.2022.109209
Abuova, Z., Turgumbayeva, A., Jumagaziyeva, A., Rakhimov, K., and Jussupkaliyeva, A. (2022). Study of component composition and antimicrobial activity of the ophthalmic emulsion based on the safflower flowers (Carthamus tinctorius L.). Int. J. Microbiol. 2022, 3181270–3181278. doi:10.1155/2022/3181270
Adamska, I., and Biernacka, P. (2021). Bioactive substances in safflower flowers and their applicability in medicine and health-promoting foods. Int. J. Food Sci. 2021, 6657639–6657723. doi:10.1155/2021/6657639
Ahmadi, A. J., and Ahmadikhah, A. (2022). Occurrence of simple sequence repeats in cDNA sequences of safflower (Carthamus tinctorius) reveals the importance of SSR-containing genes for cell biology and dynamic response to environmental cues. Front. Plant Sci. 13, 991107. doi:10.3389/fpls.2022.991107
Albaiz, A. S. (2022). The Use of Safflower (Carthamus tinctorius) in treating depression and anxiety. Cureus J. Med. Sci. 2 (14), e22278. doi:10.7759/cureus.22278
Ali, F., Nadeem, M. A., Barut, M., Habyarimana, E., Chaudhary, H. J., Khalil, I. H., et al. (2020). Genetic diversity, population structure and marker-trait association for 100-seed weight in international safflower panel using silicoDArT marker information. Plants 9 (5), 652. doi:10.3390/plants9050652
Ali, F., Yılmaz, A., Nadeem, M. A., Habyarimana, E., Subaşı, I., Nawaz, M. A., et al. (2019). Mobile genomic element diversity in world collection of safflower (Carthamus tinctorius L.) panel using iPBS-retrotransposon markers. PLoS One 14 (2), e0211985. doi:10.1371/journal.pone.0211985
Alimi, D., Hajri, A., Jallouli, S., and Sebai, H. (2022). Efficacy of synergistic activity of seed oils from Carthamus tinctorius (Safflower) and Nasturtium officinale (Watercress) on lethality of the cattle tick Hyalomma scupense (Acari: ixodidae). Open Vet. J. 12 (1), 80–90. doi:10.5455/OVJ.2022.v12.i1.10
Alpsoy, S. (2020). Exercise and hypertension. Adv. Exp. Med. Biol. 1228, 153–167. doi:10.1007/978-981-15-1792-1_10
Ambreen, H., Kumar, S., Kumar, A., Agarwal, M., Jagannath, A., and Goel, S. (2018). Association mapping for important agronomic traits in safflower (Carthamus tinctorius L.) core collection using microsatellite markers. Front. Plant Sci. 9, 402. doi:10.3389/fpls.2018.00402
Ambreen, H., Kumar, S., Variath, M. T., Joshi, G., Bali, S., Agarwal, M., et al. (2015). Development of genomic microsatellite markers in Carthamus tinctorius L. (safflower) using next generation sequencing and assessment of their cross-species transferability and utility for diversity analysis. PLoS One 10 (8), e0135443. doi:10.1371/journal.pone.0135443
Baba, S., Smith, T., Hellmann, J., Bhatnagar, A., Carter, K., Vanhoover, A., et al. (2020). Space flight diet-induced deficiency and response to gravity-free resistive exercise. Nutrients 12 (8), 2400. doi:10.3390/nu12082400
Bacchetti, T., Morresi, C., Bellachioma, L., and Ferretti, G. (2020). Antioxidant and pro-oxidant properties of Carthamus tinctorius, hydroxy safflor yellow a, and safflor yellow A. Antioxidants 9 (2), 119. doi:10.3390/antiox9020119
Baek, S. C., Lee, B. S., Yi, S. A., Yu, J. S., Lee, J., Ko, Y., et al. (2020). Discovery of dihydrophaseic acid glucosides from the florets of Carthamus tinctorius. Plants 9 (7), 858. doi:10.3390/plants9070858
Bai, X., Wang, W., Fu, R., Yue, S., Gao, H., Chen, Y., et al. (2020). Therapeutic potential of hydroxysafflor yellow A on cardio-cerebrovascular diseases. Front. Pharmacol. 11, 01265. doi:10.3389/fphar.2020.01265
Barcikowska, Z., Rajkowska-Labon, E., Grzybowska, M. E., Hansdorfer-Korzon, R., and Zorena, K. (2020). Inflammatory markers in dysmenorrhea and therapeutic options. Int. J. Environ. Res. Public Health. 17 (4), 1191. doi:10.3390/ijerph17041191
Bijanzadeh, E., Moosavi, S., and Bahadori, F. (2022). Quantifying water stress of safflower (Carthamus tinctorius L.) cultivars by crop water stress index under different irrigation regimes. Heliyon 8 (3), e09010. doi:10.1016/j.heliyon.2022.e09010
Bowers, J. E., Pearl, S. A., and Burke, J. M. (2016). Genetic mapping of millions of SNPs in safflower (Carthamus tinctorius L.) via whole-genome resequencing. G3 (Bethesda) 6 (7), 2203–2211. doi:10.1534/g3.115.026690
Chen, H., Ma, Y., Chen, M., Chen, J., and Chen, J. (2021). Safflower extract improves depression in mice by inhibiting the TLR4-NLRP3 inflammation signaling pathway. Ann. Pallliat. Med. 10 (7), 8015–8023. doi:10.21037/apm-21-1728
Chen, J., Guo, S., Hu, X., Wang, R., Jia, D., Li, Q., et al. (2023). Whole-genome and genome-wide association studies improve key agricultural traits of safflower for industrial and medicinal use. Hortic. Res. 10 (11), uhad197. doi:10.1093/hr/uhad197
Chen, Y., Li, M., Wen, J., Pan, X., Deng, Z., Chen, J., et al. (2022). Pharmacological activities of safflower yellow and its clinical applications. Evid. Based Complement. Altern. Med. 2022, 2108557. doi:10.1155/2022/2108557
Chen, Z., Liu, L., Liu, Y., Wang, S., Zhang, S., Dong, R., et al. (2020). Hydroxysafflor yellow A induces autophagy in human liver cancer cells by regulating Beclin 1 and ERK expression. Exp. Ther. Med. 19 (4), 2989–2996. doi:10.3892/etm.2020.8552
Conte, R., Gullich, L. M. D., Bilibio, D., Zanella, O., Bender, J. P., Carniel, N., et al. (2016). Pressurized liquid extraction and chemical characterization of safflower oil: a comparison between methods. Food Chem. 213, 425–430. doi:10.1016/j.foodchem.2016.06.111
Cullerne, D., Fjellheim, S., Spriggs, A., Eamens, A., Trevaskis, B., and Wood, C. (2021). A vernalization response in a winter safflower (Carthamus tinctorius) involves the upregulation of homologs of FT, FUL, and MAF. Front. Plant Sci. 12, 639014. doi:10.3389/fpls.2021.639014
de Oliveira Neto, S. S., Zeffa, D. M., Freiria, G. H., Zoz, T., da Silva, C. J., Zanotto, M. D., et al. (2022). Adaptability and Stability of Safflower Genotypes for Oil Production. Plants (Basel) 11 (5), 708. doi:10.3390/plants11050708
Delshad, E., Yousefi, M., Sasannezhad, P., Rakhshandeh, H., and Ayati, Z. (2018). Medical uses of Carthamus tinctorius L. (Safflower): a comprehensive review from traditional medicine to modern medicine. Electron Physician. 10 (4), 6672–6681. doi:10.19082/6672
Del-Toro-Sánchez, C. L., Rodríguez-Félix, F., Cinco-Moroyoqui, F. J., Juárez, J., Ruiz-Cruz, S., Wong-Corral, F. J., et al. (2021). Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. FOOD PROCESS Pres. 45 (9), e15765. doi:10.1111/jfpp.15765
Dong, Y., Chen, H., Gao, J., Liu, Y., Li, J., and Wang, J. (2019). Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 136, 27–41. doi:10.1016/j.yjmcc.2019.09.001
Eyal, S., and Derendorf, H. (2019). Medications in space: in search of a pharmacologist's guide to the galaxy. Pharm. Res. 36 (10), 148. doi:10.1007/s11095-019-2679-3
Fangma, Y., Zhou, H., Shao, C., Yu, L., Yang, J., Wan, H., et al. (2021). Hydroxysafflor yellow A and anhydrosafflor yellow B protect against cerebral ischemia/reperfusion injury by attenuating oxidative stress and apoptosis via the silent information regulator 1 signaling pathway. Front. Pharmacol. 12, 739864. doi:10.3389/fphar.2021.739864
Fu, S., Zhou, Q., Gao, Y., Yang, Y., Chen, H., Yuan, L., et al. (2022). Antioxidant and anti-inflammatory properties of hydroxyl safflower yellow a in diabetic nephropathy: a meta-analysis of randomized controlled trials. Front. Pharmacol. 13, 929169. doi:10.3389/fphar.2022.929169
Ghadirkhomi, E., Ghdirkhomi, A., and Angaji, S. A. (2022). Cytogenetic studies in primary amenorrhoea cases. J. Hum. Reprod. Sci. 15 (2), 187–190. doi:10.4103/jhrs.jhrs_13_22
Golkar, P., and Mokhtari, N. (2018). Molecular diversity assessment of a world collection of safflower genotypes by SRAP and SCoT molecular markers. Physiol. Mol. Biol. Plants. 24 (6), 1261–1271. doi:10.1007/s12298-018-0545-0
Gu, S., Ma, Y., Ge, K., Nie, R., Wu, E., and Li, Y. (2018). Danshen-honghua ameliorates stress-induced menopausal depression in rats. Neural. Plast. 2018, 6589608–6589615. doi:10.1155/2018/6589608
Guo, X., Zheng, M., Pan, R., Zang, B., and Jin, M. (2018). Hydroxysafflor yellow A suppresses platelet activating factor-induced activation of human small airway epithelial cells. Front. Pharmacol. 9, 859. doi:10.3389/fphar.2018.00859
Han, C., Tien, Y., Jine-Yuan Hsieh, D., Ho, T., Lai, C., Yeh, Y., et al. (2017). Attenuation of the LPS-induced, ERK-mediated upregulation of fibrosis-related factors FGF-2, uPA, MMP-2, and MMP-9 by Carthamus tinctorius L in cardiomyoblasts. Environ. Toxicol. 32 (3), 754–763. doi:10.1002/tox.22275
Hassani, S., Talebi, R., Pourdad, S., Naji, A., and Fayaz, F. (2020). In-depth genome diversity, population structure and linkage disequilibrium analysis of worldwide diverse safflower (Carthamus tinctorius L.) accessions using NGS data generated by DArTseq technology. Mol. Biol. Rep. 47 (3), 2123–2135. doi:10.1007/s11033-020-05312-x
Hou, J., Wang, C., Zhang, M., Ren, M., Yang, G., Qu, Z., et al. (2020). Safflower yellow improves the synaptic structural plasticity by ameliorating the disorder of glutamate circulation in aβ1-42-induced AD model rats. Neurochem. Res. 45 (8), 1870–1887. doi:10.1007/s11064-020-03051-w
Hu, C., Wu, S., He, F., Cai, D., Xu, Z., Ma, W., et al. (2022). Convergent synthesis and anti-pancreatic cancer cell growth activity of a highly branched heptadecasaccharide from Carthamus tinctorius. Angew. Chem. 134 (32). doi:10.1002/ange.202202554
Huang, L., Yu, Q., Peng, H., and Zhen, Z. (2023). The mechanism of peach kernel and safflower herb-pair for the treatment of liver fibrosis based on network pharmacology and molecular docking technology: a review. Med. Baltim. 102 (16), e33593. doi:10.1097/MD.0000000000033593
Huff, J. L., Plante, I., Blattnig, S. R., Norman, R. B., Little, M. P., Khera, A., et al. (2022). Cardiovascular disease risk modeling for astronauts: making the leap from earth to space. Front. Cardiovasc. Med. 9, 873597. doi:10.3389/fcvm.2022.873597
Hussain, M., and Al-Dakheel, A. (2018). Effect of salinity stress on phenotypic plasticity, yield stability, and signature of stable isotopes of carbon and nitrogen in safflower. Environ. Sci. Pollut. Res. Int. 25 (24), 23685–23694. doi:10.1007/s11356-018-2442-z
Jin, M., Wu, Y., Wang, L., Zang, B., and Tan, L. (2016). Hydroxysafflor yellow A attenuates bleomycin-induced pulmonary fibrosis in mice. Phytother. Res. 30 (4), 577–587. doi:10.1002/ptr.5560
Joshi, S., Thoday-Kennedy, E., Daetwyler, H., Hayden, M., Spangenberg, G., and Kant, S. (2021). High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS One 16 (7), e0254908. doi:10.1371/journal.pone.0254908
Khalid, A., Prusty, P., Arshad, I., Gustafson, H., Jalaly, I., Nockels, K., et al. (2023). Pharmacological and non-pharmacological countermeasures to Space Motion Sickness: a systematic review. Front. Neural Circuits 17, 1150233. doi:10.3389/fncir.2023.1150233
Kumar, S., Ambreen, H., Variath, M. T., Rao, A. R., Agarwal, M., Kumar, A., et al. (2016). Utilization of molecular, phenotypic, and geographical diversity to develop compact composite core collection in the oilseed crop, safflower (Carthamus tinctorius L.) through maximization strategy. Front. Plant Sci. 7, 1554. doi:10.3389/fpls.2016.01554
Lee, G., Sung, J., Lee, S., Chung, J., Yi, J., Kim, Y., et al. (2014). Genetic assessment of safflower (Carthamus tinctorius L.) collection with microsatellite markers acquired via pyrosequencing method. Mol. Ecol. Resour. 14 (1), 69–78. doi:10.1111/1755-0998.12146
Lee, M., Zhao, H., Liu, X., Liu, D., Chen, J., Li, Z., et al. (2020). Protective effect of hydroxysafflor yellow a on nephropathy by attenuating oxidative stress and inhibiting apoptosis in induced type 2 diabetes in rat. Oxidative Med. Cell. Longev. 2020, 7805393–7805411. doi:10.1155/2020/7805393
Lei, H., Ren, R., Sun, Y., Zhang, K., Zhao, X., Ablat, N., et al. (2020). Neuroprotective effects of safflower flavonoid extract in 6-hydroxydopamine-induced model of Parkinson's disease may be related to its anti-inflammatory action. Molecules 25 (21), 5206. doi:10.3390/molecules25215206
Li, L., Liu, J., Li, X., Guo, Y., Fan, Y., Shu, H., et al. (2022). Sesquiterpenoids from the florets of Carthamus tinctorius (safflower) and their anti-atherosclerotic activity. Nutrients 14 (24), 5348. doi:10.3390/nu14245348
Li, X., Liu, J., Peng, C., Zhou, Q., Liu, F., Guo, L., et al. (2021). Polyacetylene glucosides from the florets of Carthamus tinctorius and their anti-inflammatory activity. Phytochemistry 187, 112770. doi:10.1016/j.phytochem.2021.112770
Liang, Y., and Wang, L. (2022). Carthamus tinctorius L.: a natural neuroprotective source for anti-Alzheimer's disease drugs. J. Ethnopharmacol. 298, 115656. doi:10.1016/j.jep.2022.115656
Licata, M., Farruggia, D., Iacuzzi, N., Matteo, R., Tuttolomondo, T., and Miceli, G. (2023). Effects of genotype and climate on productive performance of high oleic Carthamus tinctorius L. under rainfed conditions in a semi-arid environment of sicily (Italy). Plants (Basel) 12 (9), 1733. doi:10.3390/plants12091733
Lin, D., Xu, C., Liu, Y., Zhou, Y., Xiong, S., Wu, H., et al. (2022). Chemical structures and antioxidant activities of polysaccharides from Carthamus tinctorius L. Polymers 14 (17), 3510. doi:10.3390/polym14173510
Liu, Y., Wang, M., Cao, Y., Zeng, M., Zhang, Q., Ren, Y., et al. (2022). Chemical constituents from the flowers of Carthamus tinctorius L. and their lung protective activity. Molecules 27 (11), 3573. doi:10.3390/molecules27113573
Liu, Y., Xie, G., Yang, Q., and Ren, M. (2021). Biotechnological development of plants for space agriculture. Nat. Commun. 12 (1), 5998. doi:10.1038/s41467-021-26238-3
Mani, V., Lee, S., Yeo, Y., and Hahn, B. (2020). A metabolic perspective and opportunities in pharmacologically important safflower. Metabolites 10 (6), 253. doi:10.3390/metabo10060253
Marazziti, D., Arone, A., Ivaldi, T., Kuts, K., and Loganovsky, K. (2022). Space missions: psychological and psychopathological issues. CNS Spectr. 27 (5), 536–540. doi:10.1017/S1092852921000535
Meneses, C., Valdes-Gonzalez, M., Garrido-Suárez, B. B., and Garrido, G. (2023). Systematic review on the anxiolytic and hypnotic effects of flower extracts in in vivo pre-clinical studies published from 2010 to 2020. Phytother. Res. 37 (5), 2144–2167. doi:10.1002/ptr.7830
Min, F., Sun, H., Wang, B., Ahmad, N., Guo, H., Gao, H., et al. (2020). Hepatoprotective effects of hydroxysafflor yellow A in D-galactose-treated aging mice. Eur. J. Pharmacol. 881, 173214. doi:10.1016/j.ejphar.2020.173214
Nosheen, A., Bano, A., Yasmin, H., Keyani, R., Habib, R., Shah, S., et al. (2016). Protein quantity and quality of safflower seed improved by NP fertilizer and rhizobacteria (Azospirillum and Azotobacter spp.). Front. Plant Sci. 7, 104. doi:10.3389/fpls.2016.00104
Nosheen, A., Naz, R., Tahir, A., Yasmin, H., Keyani, R., Mitrevski, B., et al. (2018). Improvement of safflower oil quality for biodiesel production by integrated application of PGPR under reduced amount of NP fertilizers. PLoS One 13 (8), e0201738. doi:10.1371/journal.pone.0201738
Otsuka, K., Cornelissen, G., Furukawa, S., Kubo, Y., Hayashi, M., Shibata, K., et al. (2016). Long-term exposure to space's microgravity alters the time structure of heart rate variability of astronauts. Heliyon 2 (12), e00211. doi:10.1016/j.heliyon.2016.e00211
Patel, P., Wang, J. Y., Mineroff, J., and Jagdeo, J. (2023). The potential cutaneous benefits of Carthamus tinctorius oleosomes. Arch. Dermatol Res. 316 (1), 26. doi:10.1007/s00403-023-02750-y
Pearl, S. A., Bowers, J. E., Reyes-Chin-Wo, S., Michelmore, R. W., and Burke, J. M. (2014). Genetic analysis of safflower domestication. BMC Plant Biol. 14, 43. doi:10.1186/1471-2229-14-43
Pu, Z., Yue, S., Zhou, G., Yan, H., Shi, X., Zhu, Z., et al. (2019). The comprehensive evaluation of safflowers in different producing areas by combined analysis of color, chemical compounds, and biological activity. Molecules 24 (18), 3381. doi:10.3390/molecules24183381
Qian, Y., Zhao, D., Wang, H., Sun, H., Xiong, Y., Xu, X., et al. (2022). An ion mobility-enabled and high-efficiency hybrid scan approach in combination with ultra-high performance liquid chromatography enabling the comprehensive characterization of the multicomponents from Carthamus tinctorius. J. Chromatogr. A 1667, 462904. doi:10.1016/j.chroma.2022.462904
Ren, C., Chen, C., Dong, S., Wang, R., Xian, B., Liu, T., et al. (2022). Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in flowers of safflower (Carthamus tinctorius L.) during colour-transition. PeerJ 10, e13591. doi:10.7717/peerj.13591
Ren, C., Wang, J., Xian, B., Tang, X., Liu, X., Hu, X., et al. (2020). Transcriptome analysis of flavonoid biosynthesis in safflower flowers grown under different light intensities. PeerJ 8, e8671. doi:10.7717/peerj.8671
Rodríguez-Félix, F., López-Cota, A. G., Moreno-Vásquez, M. J., Graciano-Verdugo, A. Z., Quintero-Reyes, I. E., Del-Toro-Sánchez, C. L., et al. (2021). Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon 7 (4), e06923. doi:10.1016/j.heliyon.2021.e06923
Ruyvaran, M., Zamani, A., Mohamadian, A., Zarshenas, M. M., Eftekhari, M. H., Pourahmad, S., et al. (2022). Safflower (Carthamus tinctorius L.) oil could improve abdominal obesity, blood pressure, and insulin resistance in patients with metabolic syndrome: a randomized, double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 282, 114590. doi:10.1016/j.jep.2021.114590
Shaya, G. E., Leucker, T. M., Jones, S. R., Martin, S. S., and Toth, P. P. (2022). Coronary heart disease risk: low-density lipoprotein and beyond. Trends cardiovasc. Med. 32 (4), 181–194. doi:10.1016/j.tcm.2021.04.002
Si, W., Yang, W., Guo, D., Wu, J., Zhang, J., Qiu, S., et al. (2016). Selective ion monitoring of quinochalcone C -glycoside markers for the simultaneous identification of Carthamus tinctorius L. in eleven Chinese patent medicines by UHPLC/QTOF MS. J. Pharm. Biomed. Anal. 117, 510–521. doi:10.1016/j.jpba.2015.09.025
Silva, B., Masetto, T., Zanzi, J., and Souza, L. (2023). Regulation of dormancy break and germination of safflower seeds: the role of GA3, light and cold temperatures. Braz. J. Biol. 83, e270354. doi:10.1590/1519-6984.270354
Singh, K. N., Rawat, S., Kumar, K., Agarwal, S. K., Goel, S., Jagannath, A., et al. (2022). Correction to: identification of significant marker-trait associations for Fusarium wilt resistance in a genetically diverse core collection of safflower using AFLP and SSR markers. J. Appl. Genet. 63 (4), 815. doi:10.1007/s13353-022-00709-9
Sun, Y., Wang, Z., Nie, C., Xue, L., Wang, Y., Song, C., et al. (2021). Hydroxysafflor yellow A alters fuel selection from glucose to fat by activating the PPARδ pathway in myocytes. J. Agric. Food Chem. 69 (46), 13838–13848. doi:10.1021/acs.jafc.1c06034
Tiwari, K., Gatto, C., and Wilkinson, B. (2018). Interrelationships between fatty acid composition, staphyloxanthin content, fluidity, and carbon flow in the staphylococcus aureus membrane. Molecules 23 (5), 1201. doi:10.3390/molecules23051201
Tong, X., Yang, J., Zhao, Y., Wan, H., He, Y., Zhang, L., et al. (2021). Greener extraction process and enhanced in vivo bioavailability of bioactive components from Carthamus tinctorius L. by natural deep eutectic solvents. Food Chem. 348, 129090. doi:10.1016/j.foodchem.2021.129090
Turgumbayeva, A. A., Ustenova, G. O., Yeskalieva, B. K., Ramazanova, B. A., Rahimov, K. D., Aisa, H., et al. (2018). Volatile oil composition of Carthamus tinctorius L. flowers grown in Kazakhstan. Ann. Agr. Env. Med. 25 (1), 87–89. doi:10.5604/12321966.1235170
Wang, K., Li, S., Zhao, Y., Li, H., and Zhang, L. (2018). In vitro anticoagulant activity and active components of safflower injection. Molecules 23 (1), 170. doi:10.3390/molecules23010170
Wang, M. N., Cao, Y. G., Wei, Y. X., Ren, Y. J., Liu, Y. L., Chen, X., et al. (2022). Saffloflavone, a new flavonoid from the flowers of Carthamus tinctorius L. and its cardioprotective activity. Nat. Prod. Res. 36 (13), 3317–3323. doi:10.1080/14786419.2020.1855167
Wang, Q., Liu, S., Xu, L., Du, B., and Song, L. (2023b). Purification, characterization and bioactivities of polysaccharides extracted from safflower (Carthamus tinctorius L.). Molecules 28 (2), 596. doi:10.3390/molecules28020596
Wang, Q., Yang, Z., Guo, L., Li, Z., Liu, Y., Feng, S., et al. (2023a). Chemical composition, pharmacology and pharmacokinetic studies of GuHong injection in the treatment of ischemic stroke. Front. Pharmacol. 14, 1261326. doi:10.3389/fphar.2023.1261326
Wang, R., Ren, C., Dong, S., Chen, C., Xian, B., Wu, Q., et al. (2021). Integrated metabolomics and transcriptome analysis of flavonoid biosynthesis in safflower (Carthamus tinctorius L.) with different colors. Front. Plant Sci. 12, 712038. doi:10.3389/fpls.2021.712038
Wu, L., Tang, Y., Shan, C., Chai, C., Zhou, Z., Shi, X., et al. (2018). A comprehensivein vitro andin vivo metabolism study of hydroxysafflor yellow A. J. Mass Spectrom. 53 (2), 99–108. doi:10.1002/jms.4041
Wu, Z., Liu, H., Zhan, W., Yu, Z., Qin, E., Liu, S., et al. (2021). The chromosome-scale reference genome of safflower (Carthamus tinctorius) provides insights into linoleic acid and flavonoid biosynthesis. Plant Biotechnol. J. 19 (9), 1725–1742. doi:10.1111/pbi.13586
Xue, X., Deng, Y., Wang, J., Zhou, M., Liao, L., Wang, C., et al. (2021). Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine 91, 153694. doi:10.1016/j.phymed.2021.153694
Yao, R., Cao, Y., Jiang, R., Zhang, X., Li, F., and Wang, S. (2021). Pharmacokinetic characteristics of hydroxysafflor yellow A in normal and diabetic cardiomyopathy mice. Biomed. Chromatogr. 35 (10), e5173. doi:10.1002/bmc.5173
Yao, Y., Yao, J., Du, Z., Wang, P., and Ding, K. (2018). Structural elucidation and immune-enhancing activity of an arabinogalactan from flowers of Carthamus tinctorius L. Carbohydr. Polym. 202, 134–142. doi:10.1016/j.carbpol.2018.08.098
Yildiz, M., Altaf, M. T., Baloch, F. S., Koçak, M., Sadık, G., Kuzğun, C., et al. (2022). Assessment of genetic diversity among 131 safflower (Carthamus tinctorius L.) accessions using peroxidase gene polymorphism (POGP) markers. Mol. Biol. Rep. 49 (7), 6531–6539. doi:10.1007/s11033-022-07485-z
Yu, G., Luo, Z., Zhou, Y., Zhang, L., Wu, Y., Ding, L., et al. (2019). Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach. Biomed. Pharmacother. 117, 109094. doi:10.1016/j.biopha.2019.109094
Yuan, Y., Liu, H., and Meng, Q. (2023). The cardioprotective effects and mechanisms of astragalus-safflower herb pairs on coronary heart disease identified by network pharmacology and experimental verification. Front. Biosci. (Landmark Ed). 28 (5), 94. doi:10.31083/j.fbl2805094
Yue, S., Tang, Y., Xu, C., Li, S., Zhu, Y., and Duan, J. (2014). Two new quinochalcone c-glycosides from the florets of Carthamus tinctorius. Int. J. Mol. Sci. 15 (9), 16760–16771. doi:10.3390/ijms150916760
Zhang, L., Tian, K., Tang, Z., Chen, X., Bian, Z., Wang, Y., et al. (2016). Phytochemistry and pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 44 (02), 197–226. doi:10.1142/S0192415X16500130
Zhou, H., Yang, J., Zhang, C., Zhang, Y., Wang, R., Li, X., et al. (2018). Safflower polysaccharide inhibits the development of tongue squamous cell carcinoma. World J. Surg. Oncol. 16 (1), 167. doi:10.1186/s12957-018-1441-3
Keywords: safflower, genetic diversity, medical applications, phytochemicals and pharmacological effects, space sickness
Citation: Cheng H, Yang C, Ge P, Liu Y, Zafar MM, Hu B, Zhang T, Luo Z, Lu S, Zhou Q, Jaleel A and Ren M (2024) Genetic diversity, clinical uses, and phytochemical and pharmacological properties of safflower (Carthamus tinctorius L.): an important medicinal plant. Front. Pharmacol. 15:1374680. doi: 10.3389/fphar.2024.1374680
Received: 22 January 2024; Accepted: 22 April 2024;
Published: 10 May 2024.
Edited by:
Diego Rivera, University of Murcia, SpainReviewed by:
Meili Guo, Second Military Medical University, ChinaCopyright © 2024 Cheng, Yang, Ge, Liu, Zafar, Hu, Zhang, Luo, Lu, Zhou, Jaleel and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Jaleel, YWJkdWwuamFsZWVsQHVhZXUuYWMuYWU=; Maozhi Ren, cmVubWFvemhpMDFAY2Fhcy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.