- Institute of Basic Theory of Traditional Chinese Medicine, China Academy of Chinese Medical Sciences, Beijing, China
Background: IgA nephropathy (IgAN), a condition posing a significant threat to public health, currently lacks a specific treatment protocol. Research has underscored the potential benefits of traditional Chinese medicine (TCM) for treating IgAN. Nevertheless, the effectiveness of various intervention strategies, such as combining TCM with angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs), lacks a comprehensive systematic comparison. Therefore, this study aimed to conduct a network meta-analysis to assess the clinical efficacy of ACEIs, ARBs, TCM, and their combinations in treating IgAN to offer novel insights and approaches for the clinical management of IgAN.
Methods: A systematic review conducted until November 2023 included relevant literature from databases such as PubMed, Embase, Cochrane, Web of Science, Scopus, CNKI, and Wanfang. Two independent researchers screened and assessed the data for quality. Network and traditional meta-analyses were performed using Stata 18.0 and RevMan 5.3 software, respectively. Outcome measures included 24-h urinary protein quantification (24 hpro), estimated glomerular filtration rate (eGFR), serum creatinine (Scr), blood urea nitrogen (BUN), and adverse event incidence rates (ADRs). Forest plots, cumulative ranking probability curves (SUCRA), and funnel plots generated using Stata 18.0 facilitated a comprehensive analysis of intervention strategies’ efficacy and safety.
Results: This study included 72 randomized controlled trials, seven interventions, and 7,030 patients. Comparative analysis revealed that ACEI + TCM, ARB + TCM combination therapy, and TCM monotherapy significantly reduced the levels of 24 hpro, eGFR, Scr, and BUN compared to other treatment modalities (p < 0.05). TCM monotherapy demonstrated the most favorable efficacy in reducing eGFR levels (SUCRAs: 78%), whereas the combination of ARB + TCM reduced Scr, 24 hpro, and BUN levels (SUCRAs: 85.7%, 95.2%, and 87.6%, respectively), suggesting that ARB + TCM may represent the optimal intervention strategy. No statistically significant differences were observed among the various treatment strategies in terms of ADR (p > 0.05).
Conclusion: The combination of ACEI or ARB with TCM demonstrated superior efficacy compared to ACEI/ARB monotherapy in the treatment of IgAN without any significant ADRs. Therefore, combination therapies can be used to enhance therapeutic outcomes based on individual patient circumstances, highlighting the use of TCM as a widely applicable approach in clinical practice.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023476674.
1 Introduction
Immunoglobulin A nephropathy (IgAN) involves the deposition of IgA- or IgA-dominant immunoglobulins in the glomerular mesangial area, leading to a range of clinical manifestations such as hematuria, proteinuria, edema, and hypertension (Matsumoto et al., 2022). The prevalence of IgAN is high in Asia, with most patients experiencing an insidious onset (Wyatt and Julian, 2013). Proteinuria is a common clinical manifestation of IgAN (Xia et al., 2022). Notably, proteinuria is the primary risk factor for renal function deterioration in individuals diagnosed with IgAN (Gadola et al., 2023). Approximately 30%–40% of IgAN cases progress to end-stage renal disease within 10–20 years after diagnosis, necessitating dialysis or kidney transplantation for life-sustaining treatment. This imposes a significant burden on patients, their families, and society (Jarrick et al., 2019; Floege et al., 2022).
The detailed pathogenesis of IgAN remains poorly understood, posing challenges to effective treatment. Some scholars have posited that dysregulation of the mucosal immune system contributes to a deficiency in the galactosylation of IgA, subsequently leading to the development of autoantibodies (IgG) targeting non-galactosylated IgA. Ultimately, the deposition of these IgG–IgA immune complexes within the mesangium culminates in glomerular inflammation, which is the primary underlying mechanism driving the disease (Rajasekaran et al., 2021; Schimpf et al., 2023).
Despite advancements in our understanding of the IgAN pathophysiology, no specific treatment is currently available. Currently, the management of patients with IgAN relies primarily on a uniform therapeutic strategy for all chronic glomerular diseases. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines for glomerular disease management emphasize that reducing proteinuria, employing renin-angiotensin receptor blockers, and controlling hypertension are the preferred treatment modalities for IgAN (Inker et al., 2014). This entails utilizing angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin II receptor blockers (ARBs) to suppress the renin-angiotensin system (RAS) for blood pressure regulation and minimize proteinuria to decelerate albuminuric IgAN progression.
After receiving supportive treatment, hormonal and immunosuppressive therapies are commonly administered to patients with IgAN if proteinuria above 1 g/day persists. However, it is important to note that these treatments carry potential risks of adverse reactions, which may impact the overall therapeutic efficacy (Lv et al., 2017). In addition to contemporary drugs, traditional Chinese medicine (TCM) has a long history. Several studies have confirmed the efficacy of Chinese herbal medicines for reducing liver and kidney injury (Samarghandian et al., 2017; Eftekhari et al., 2020). Numerous Chinese studies have confirmed the safety and efficacy of TCM in the treatment of IgAN (Li et al., 2020; Wang et al., 2021b). Several meta-analyses have validated the effects of each treatment modality on IgAN (Zhao et al., 2021b; Huo et al., 2021; Wang et al., 2022). It is imperative to investigate an optimal treatment protocol and leverage the complementary and alternative attributes of TCM to ameliorate symptoms, mitigate toxicity and adverse effects associated with contemporary medical interventions, enhance therapeutic efficacy, and diminish the likelihood of recurrence. However, the effectiveness of different intervention strategies such as combining TCM with ACEI or ARB remains unclear. Therefore, we conducted a network meta-analysis of randomized controlled trials (RCTs) to systematically evaluate the effects of various intervention strategies on key renal outcome indicators in patients with IgAN and provide insights and support for future clinical treatments.
2 Materials and methods
2.1 Study design
This study was conducted in accordance with the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines while referencing relevant sources. The study protocol was registered in the International Registry of Prospective Systematic Reviews (PROSPERO) under registration number CRD42023476674.
2.2 Search strategy
Electronic databases including PubMed, Cochrane Library, Scopus, Embase, Web of Science, China National Knowledge Infrastructure, and Wan Fang were searched. The deadline for the search was November 2023 with no language restrictions. Supplementary Table S1 provides further information on the search strategy. The retrieved articles were imported into the Endnote X9 software to eliminate duplicate entries. Two reviewers (SJ Ma and YH Jiang) independently reviewed the titles, keywords, and abstracts of the literature selected in these formats; when they had different opinions, they were discussed with a third reviewer (Linlin Qian) and then an optimal solution was found. Data extracted included the following: title, authorship, diagnostic criteria for the disease, disease stage classification, patient count, average age of the participants, sex distribution, study design type, intervention details, treatment duration, outcome measures employed, and adverse events recorded. The study methodology is illustrated in Figure 1.
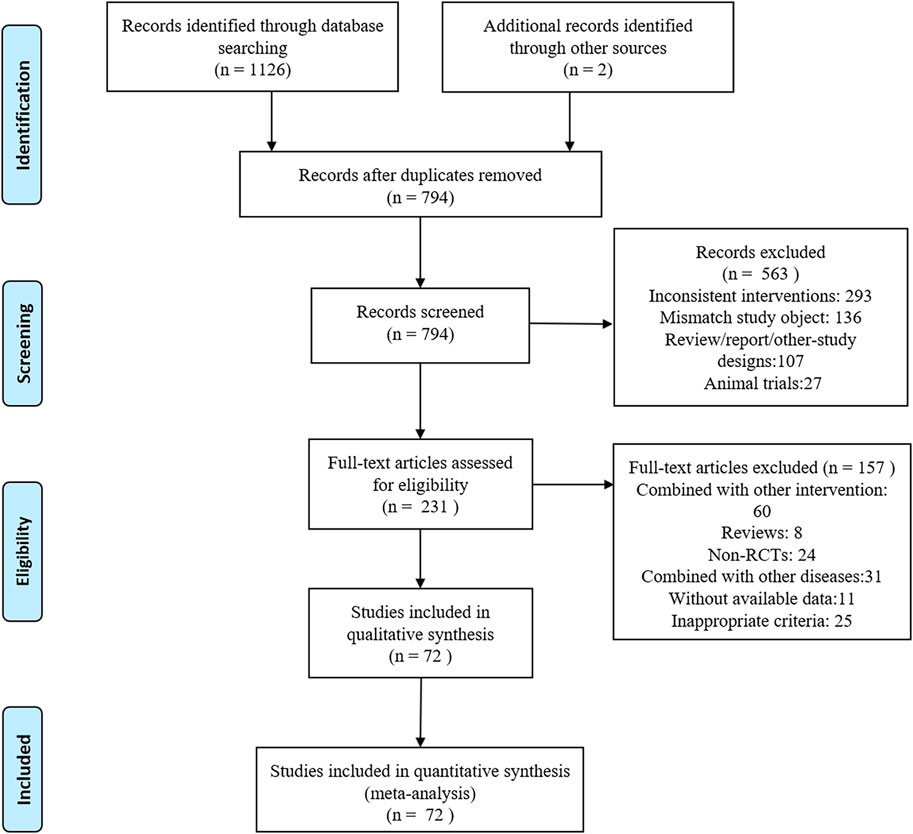
Figure 1. PRISMA flowchart of the screening process for study inclusion. RCT, randomized controlled trial.
2.3 Inclusion criteria
Studies were selected for inclusion if they met the following criteria: (1) study design: RCT methodology; (2) participants: adult patients with primary IgAN; (3) intervention: the treatment group received ACEIs, ARBs, TCM, or any appropriate combination thereof, in addition to conventional treatment if necessary, whereas the control group received a single class of the above-mentioned drugs or a placebo along with usual care; (4) outcome measures: estimated glomerular filtration rate (eGFR), 24-h urinary protein (24 hpro), serum creatinine (Scr) levels, blood urea nitrogen (BUN) levels, and adverse drug reactions (ADRs). Only articles published in peer-reviewed journals were included in this review, and both English and Chinese language publications were eligible for inclusion. The studies selected for inclusion had at least one of the aforementioned outcome measures evaluated and a minimum treatment duration of 3 months.
2.4 Exclusion criteria
We excluded duplicate publications, studies lacking sufficient data, studies without full texts, studies with unclear observational processes, studies with inconsistent baseline characteristics between the experimental and control groups, and trials involving patients under 18 years of age, secondary IgAN, pregnancy, and treatment duration of less than 3 months.
2.5 Quality assessment
Revman 5.3 software provided by Cochrane was used to analyze data quality and potential bias. The results were independently assessed by two researchers using the RCT bias risk assessment tool recommended by the Cochrane Handbook and were cross-checked for accuracy. Evaluations included random sequence generation, random concealment, blinding, data integrity, withdrawal, and other biases. The included literature was evaluated for bias risk from seven aspects using decision terms such as “high risk,” “low risk,” and “unclear risk.”
2.6 Statistical analysis
Eligible studies were used as sources for data extraction and entered into a standardized spreadsheet. Five outcome measures (eGFR, 24 hpro, Scr, BUN, and ADR) were analyzed. The standardized mean difference was used for continuous variables, whereas relative risk was used for dichotomous variables. Each effect size is expressed as a 95% confidence interval. A traditional meta-analysis using Stata18.0 software was conducted in conjunction with the I2 value to quantitatively assess the degree of heterogeneity among the studies. If the I2 value exceeded 50%, substantial heterogeneity between the groups was indicated, necessitating the use of a random-effects model. Conversely, if the I2 value was less than or equal to 50%, minimal heterogeneity between groups was indicated, which allowed for the selection of a fixed-effects model. In cases with significant heterogeneity, sensitivity, and subgroup analyses were performed to identify potential sources of heterogeneity before deciding whether inclusion should be based on these findings alone. Descriptive analyses were used when the source of heterogeneity could not be determined.
A frequency framework was adopted and a random-effects model was employed for the network meta-analysis. Data preprocessing was performed using the network group command to visualize the network relationships between the intervention measures for each outcome index comparison. Inconsistency tests were conducted when closed loops formed among the interventions to assess the level of agreement between direct and indirect comparisons. Consistency with the study was determined at p > 0.05; otherwise, statistical differences were evaluated using the node-splitting method. The surface under the cumulative ranking curve (SUCRA) was used to rank the efficacy indicators of the intervention measures and identify the most effective intervention. Higher SUCRA values indicated better intervention effects. Stata 18.0 software was used to generate a publication bias funnel plot (comparison-correction plot).
3 Results
3.1 Study characteristics
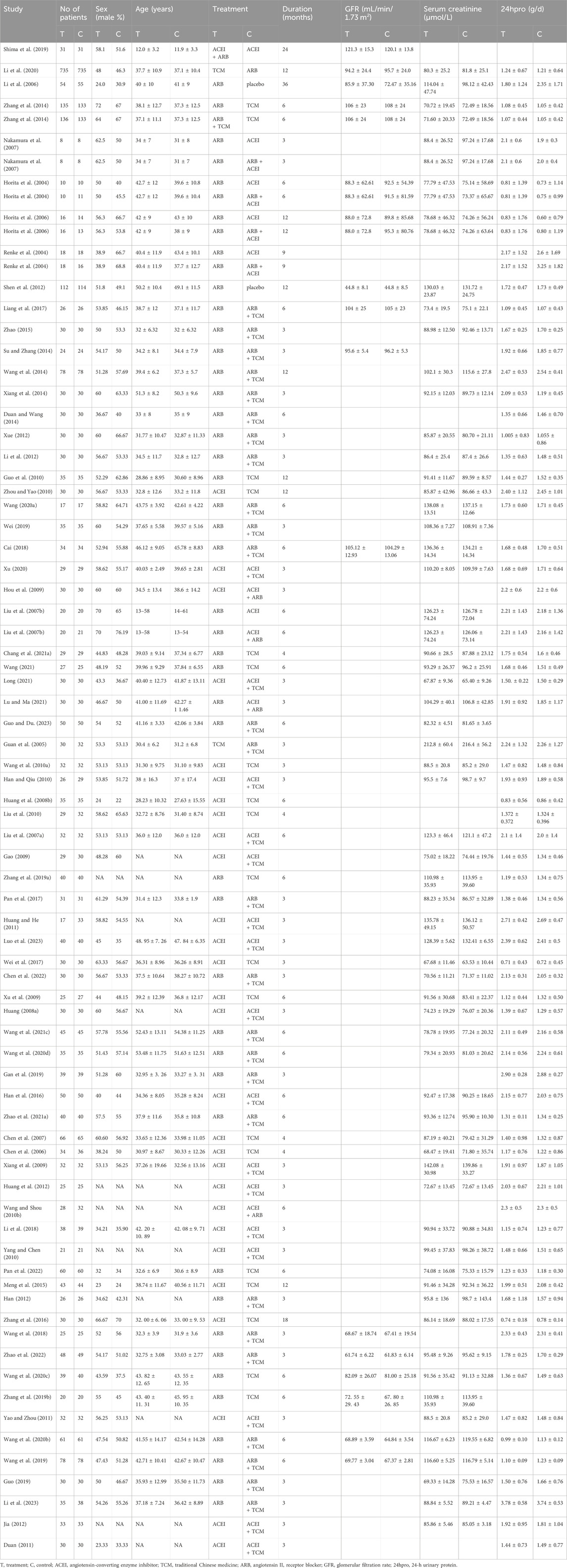
A PRISMA flowchart of the screening process used in this study is shown in Figure 1. In total, 1,128 articles were retrieved, of which 794 were excluded because of duplications in the Import Endnote software. Additionally, 563 articles did not meet the inclusion criteria and were excluded based on their titles and abstracts. We conducted a thorough review of the full text of the remaining 231 articles with potentially eligible records. Ultimately, this study included 72 articles involving 7,030 patients. The following seven therapeutic interventions were evaluated: placebo, TCM, ACEI, ARB, TCM + ACEI, TCM + ARB, and ACEI + ARB. No statistically significant differences in baseline parameters were observed between the study groups. Table 1 summarizes the clinical and methodological characteristics of each trial along with their main results.
3.2 Assessment of risk of bias
The 72 studies were assessed for risk of bias. Explicit random sequence generation was reported in 43 studies, whereas randomization was mentioned without describing the method in 26. The randomization procedures in three additional studies were inadequately implemented. Specific implementation measures for allocation concealment are mentioned in the literature. Eighteen studies mentioned specific blinding practices; however, only seven of these studies achieved the ideal implementation of blinding. Complete outcome data were available for all included studies. The results of the risk-assessment bias analysis are shown in Figure 2.
3.3 Inconsistency test
The selected treatments were subjected to a node-split analysis, which revealed no statistically significant differences (all p > 0.05). This analysis demonstrated the absence of disparities between direct and indirect evidence (Supplementary Table S2). The results of the heterogeneity tests among the multiple interventions are presented in Supplementary Table S3.
3.4 Outcome measures
3.4.1 eGFR
Seventeen studies involving 2,974 patients with changes in eGFR from baseline were included in the analysis (Figure 3A). The network graph revealed that most comparisons were made between the ARB and TCM + ARB groups. Subsequently, we conducted 15 pairwise comparisons using network meta-analyses, two of which showed statistically significant results (Figure 4A). Forest plots generated using Stata software, which indicate the prediction intervals, are presented in Figure 5A. In our analysis, placebo was used as the control group. The eGFRs of the patients were improved by TCM, ACEI, ARB, TCM + ARB, and ACEI + ARB compared with those of the control group. According to the SUCRA ranking analysis for improving eGFR levels (Figure 6A), TCM intervention was found to be the most effective, followed by TCM + ARB, ACEI, ACEI + ARB, ARB, and placebo (Supplementary Table S4). The publication bias assessment is presented in Figure 7A, where each dot represents an included study and different colors indicate different interventions. The comparatively corrected funnel plot suggested acceptable symmetry but also indicated the possibility of publication bias.
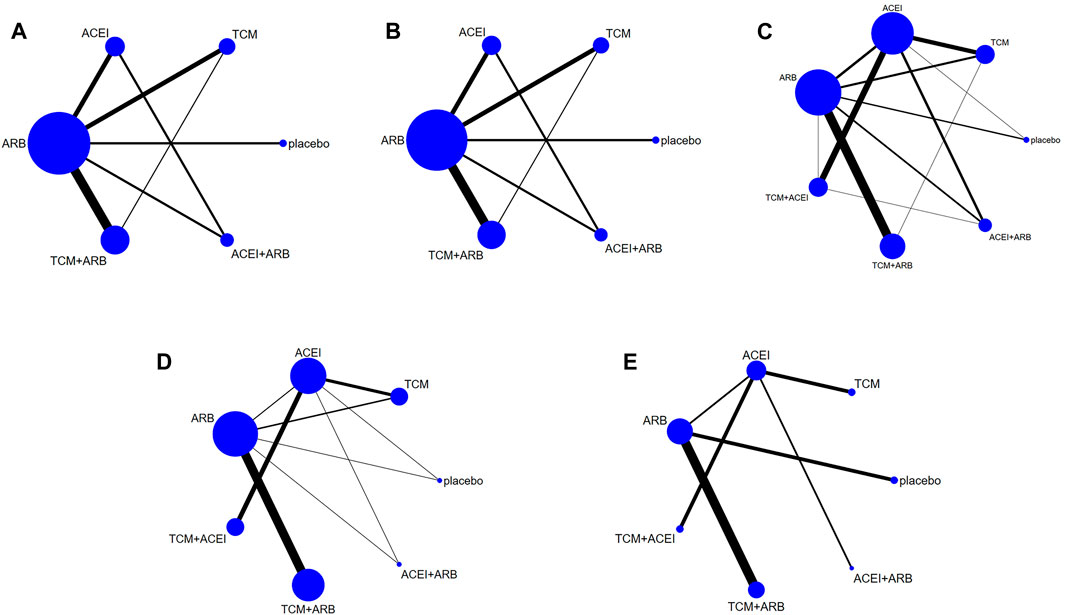
Figure 3. Observation index network for the outcomes (A) estimated glomerular filtration rate, (B) serum creatinine, (C) 24-h urinary protein, (D) blood urea nitrogen, and (E) adverse drug reactions. TCM, traditional Chinese medicine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
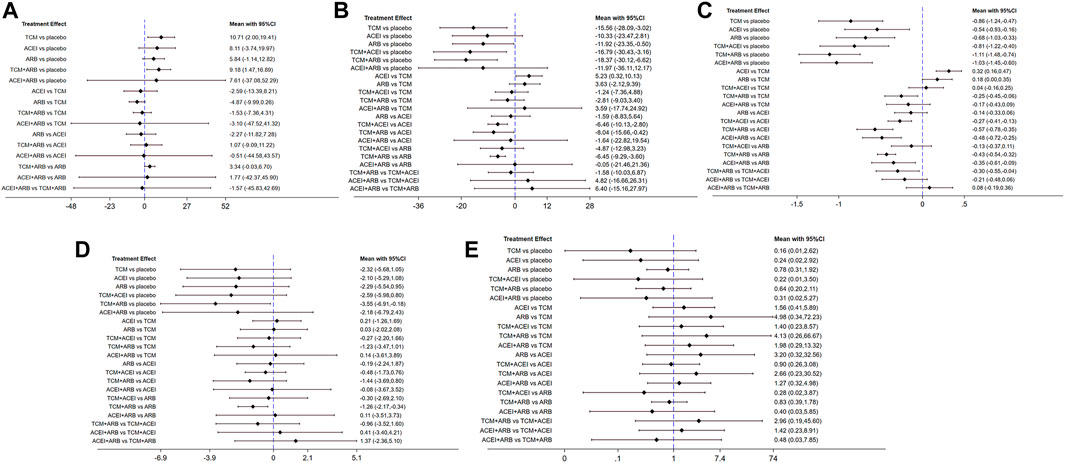
Figure 4. Forest plots for prediction through network meta-analysis using observational indices for the outcomes (A) estimated glomerular filtration rate, (B) serum creatinine, (C) 24-h urinary protein, (D) blood urea nitrogen, and (E) adverse drug reactions. TCM, traditional Chinese medicine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
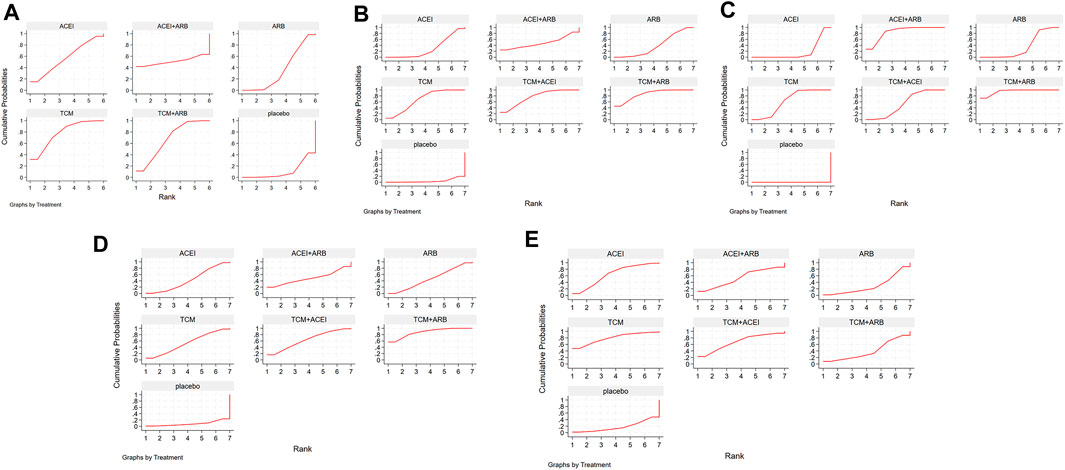
Figure 5. Surface under the cumulative ranking curves depicting the impact of diverse clinical interventions on various indicators: (A) estimated glomerular filtration rate, (B) serum creatinine, (C) 24-h urinary protein, (D) blood urea nitrogen, and (E) adverse drug reactions. TCM, traditional Chinese medicine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
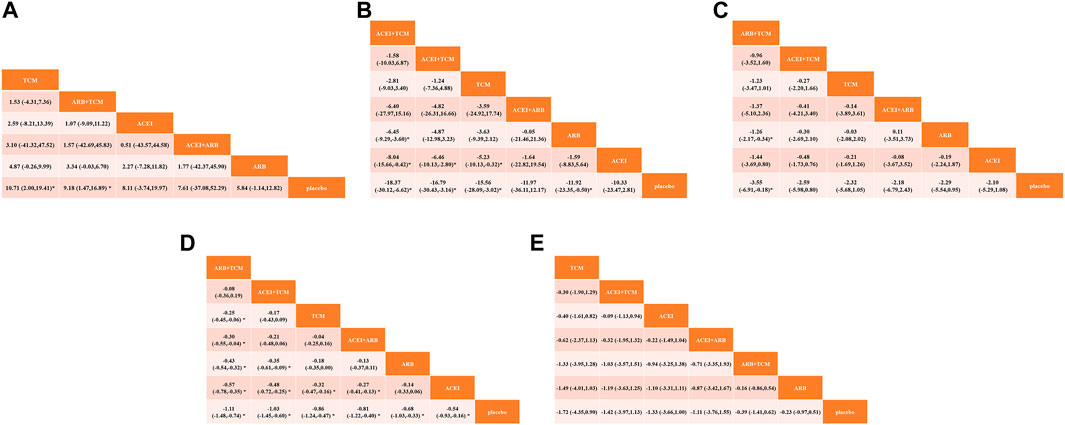
Figure 6. Comparative analysis of therapeutic interventions on diverse clinical indicators: (A) estimated glomerular filtration rate, (B) serum creatinine, (C) 24-h urinary protein, (D) blood urea nitrogen, and (E) adverse drug reactions. TCM, traditional Chinese medicine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
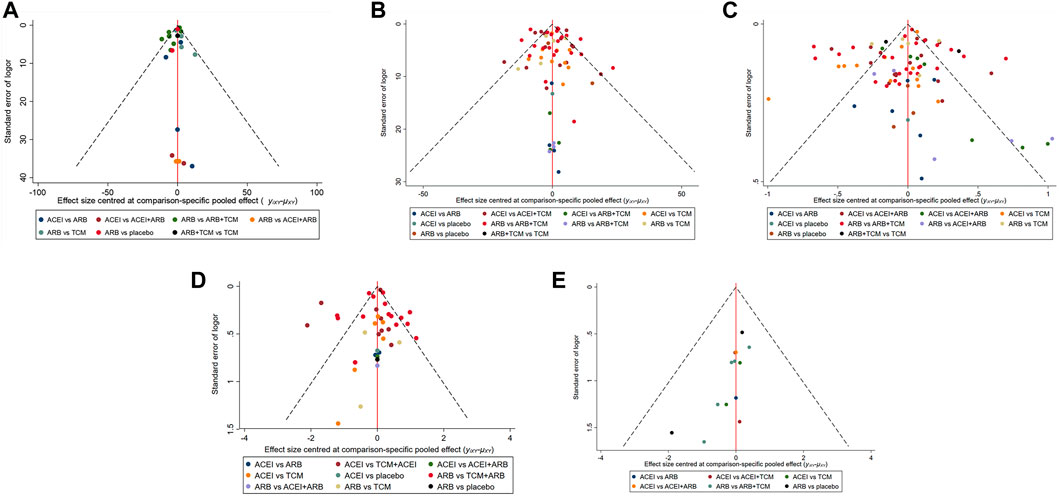
Figure 7. Funnel plot illustrating the treatment effects of diverse clinical interventions on various indicators: (A) estimated glomerular filtration rate, (B) serum creatinine, (C) 24-h urinary protein, (D) blood urea nitrogen, and (E) adverse drug reactions. TCM, traditional Chinese medicine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
3.4.2 Scr
A total of 57 studies involving 4,365 patients with changes in Scr levels from baseline were included (Figure 3B). The network graph revealed that most comparisons were between the ARB and TCM + ARB groups. We conducted a network meta-analysis of Scr reduction based on the included studies, resulting in 21 paired comparisons, of which eight showed statistical significance (Figure 4B). Forest plots displaying the prediction intervals are presented in Figure 5B, with the placebo intervention serving as a control. Compared with the control group, the TCM, ACEI, ARB, TCM + ARB, and ACEI + ARB interventions demonstrated a reduction in the patients’ Scr levels. According to the SUCRA ranking analysis (Figure 6B), TCM + ARB intervention was the most effective approach for reducing Scr levels. The order of probability size was as follows: TCM + ARB > TCM + ACEI > ACEI + ARB > ARB > ACEI > placebo (Supplementary Table S4). The results of the publication bias assessment are shown in Figure 7B. Each dot represents an included study, with different colors indicating different interventions. The comparatively corrected funnel plot suggested acceptable symmetry but also indicated the possibility of publication bias.
3.4.3 24hpro
A total of 68 studies involving 6,520 patients with changes at 24 hpro from baseline were included (Figure 3C). The network graph revealed that the majority of comparisons were made between the ARB and TCM + ARB groups. We conducted a network meta-analysis of the included studies to assess their efficacy in reducing 24 hpro, resulting in 21 paired comparisons, of which eight showed statistical significance (Figure 4C). The generated forest plots are presented in Figure 5C, where the placebo intervention was used as the control group. Compared to the control group, the TCM, ACEI, ARB, TCM + ARB, and ACEI + ARB groups demonstrated a significant reduction in 24 hpro levels. According to the SUCRA ranking analysis, TCM + ARB intervention was the most effective approach for reducing 24 hpro levels (Figure 6C). The order of probability size was as follows: TCM + ARB > ACEI + ARB > TCM > ACEI + TCM > ARB > ACEI > placebo (Supplementary Table S4). The results of the publication bias assessment are shown in Figure 7C. Each dot represents an included study, and different colors indicate different interventions. The comparative corrected funnel plot indicated acceptable symmetry but also suggested the possibility of publication bias.
3.4.4 BUN
A total of 36 studies involving 2,358 patients with a change in BUN from baseline (Figure 3D) were included. The network graph revealed that most comparisons were made between the ARB and TCM + ARB groups. We conducted a network meta-analysis of the included studies to assess the reduction in BUN levels, which resulted in 21 paired comparisons, two of which showed statistical significance (Figure 4D). Forest plots are presented in Figure 5D, with the placebo intervention serving as the control group. Compared to the control, TCM, ACEI, ARB, TCM + ARB, and ACEI + ARB demonstrated efficacy in reducing BUN levels. According to the SUCRA ranking analysis (Figure 6D), the TCM + ARB intervention was the most effective approach for BUN reduction among all the interventions considered. The order of probability size was as follows: TCM + ARB > TCM + ACEI > TCM > ACEI + ARB > ARB > ACEI > placebo (Supplementary Table S4). Publication bias assessment results are displayed in Figure 7D, where each dot represents an included study, and different colors indicate different interventions. The comparatively corrected funnel plot suggested acceptable symmetry but also indicated the possibility of publication bias.
3.4.5 ADR
Thirteen studies were included, with 88 adverse events reported in 1,158 patients (Figure 3E). The network graph illustrates the predominant comparisons between the ARB and TCM + ARB groups. We conducted a network meta-analysis of the included studies on ADRs, comprising 21 paired comparisons; however, none of these comparisons yielded statistically significant results (Figure 4E), suggesting that there may be no difference in terms of ADR causation among the interventions. Forest plots displaying the prediction intervals are shown in Figure 5E, with the placebo intervention serving as a control. Compared with the control, TCM, ACEI, ARB, TCM + ARB, and ACEI + ARB interventions demonstrated reductions in patient ADRs. According to the SUCRA rankings for ADR reduction (Figure 6E), the TCM intervention appeared to be potentially superior among all interventions evaluated. The order of probability size was as follows: TCM > TCM + ACEI > ACEI > ACEI + ARB > ARB + TCM > ARB > placebo (Supplementary Table S4). Publication bias analysis results are presented in Figure 7A, where each dot represents an included study and different colors indicate different interventions. The comparatively corrected funnel plot indicated acceptable symmetry but also suggested potential publication bias.
4 Discussion
In this network meta-analysis, we conducted a comprehensive evaluation of the effects of ACEIs, ARBs, TCM, and combination treatment strategies on key renal outcomes in patients with IgAN. Our findings demonstrate that the addition of TCM significantly enhances the efficacy of ACEI or ARB therapy compared with ACEI or ARB alone, as well as with placebo. Specifically, the combined use of TCM with ACEI or ARB effectively reduced 24 hpro, Scr, and BUN levels. Moreover, our results suggest that combining an ACEI with TCM is more effective than using an ACEI alone for preserving renal function and that combining an ARB with TCM is more effective than using an ARB alone. No statistically significant differences in ADRs were observed among the studies.
ACEIs and ARBs are recommended by the KDIGO for the initial treatment of IgAN, as they exhibit a favorable renal protective effect, which has been substantiated in some preliminary studies (Coppo, 2019; Rovin et al., 2021; Floege et al., 2022). However, robust evidence comparing the efficacies of ACEIs and ARBs is lacking. The efficacy of RAS-blockers is often suboptimal in patients with hyperkalemia and severe renal dysfunction. Moreover, these drugs are often associated with adverse effects including dry cough, edema, and fatigue (Whitlock et al., 2023; Yao et al., 2023).
TCM and its decoctions have been extensively used in China to treat IgAN and its associated complications, resulting in a vast accumulation of clinical experience. In recent years, TCM has been extensively used to manage IgAN and its associated complications. Moreover, an increasing number of reports have highlighted the efficacy of TCM combined with ACEIs/ARBs for the treatment of IgAN. Numerous studies have demonstrated that this combination therapy exerts a significant therapeutic effect on IgAN by controlling disease progression and improving clinical symptoms, with minimal side effects and high safety (Li et al., 2020; Wang et al., 2021b; Wang et al., 2022; Pan et al., 2023). The findings of our study also demonstrated that the combination of TCM with ACEI/ARB exhibited superior efficacy compared to ACEI/ARB monotherapy in terms of reducing 24 hpro, eGFR, Scr, and BUN levels and ultimately delayed kidney disease progression.
Mechanistic studies have suggested that TCM exerts its therapeutic effects on IgAN via multiple targets and pathways (Ma et al., 2023). Importantly, extensive and in-depth data mining regarding the application of TCM in treating IgAN facilitates the acquisition of ancient clinical experiences, enhances the understanding of the theoretical and practical aspects of TCM for IgAN treatment, and enriches the therapeutic options for managing this condition. Our findings provide evidence supporting the efficacy and safety of current clinical treatments for IgAN to a certain extent, thereby offering a new and comprehensive framework for promoting clinical practice guidelines. This contributes to expanding the range of therapeutic options available for the management of IgAN.
Common ADRs associated with ACEIs include cough, dizziness, and headache. However, no instances of liver damage have been reported. Common adverse effects of ARBs include hyperkalemia, abdominal distension, and dizziness. The adverse effects observed with ACEIs and ARBs were similar to those observed with ACEIs or ARBs alone. TCM commonly elicits abdominal distension and diarrhea as its main ADR. Mild liver damage and menstrual disturbances have been predominantly observed in studies involving Tripterygium wilfordii and are linked to gonadal impairment (Fu et al., 2012; Chang et al., 2021b). It is worth mentioning that the differences in the incidence of ADRs among the various interventions were not statistically significant. Nonetheless, it is advisable to regularly monitor hepatic function, sperm motility, and the menstrual cycle in patients undergoing treatment with TCM Tripterygium wilfordii. Furthermore, prudent consideration should be given to the appropriate administration of hepatoprotective agents.
The strengths of this study lie primarily in the implementation of a systematic search method to minimize the potential impact of publication bias, the rationality of the analysis, and the reliability of the obtained results. Network meta-analysis was employed to construct a comprehensive network that incorporated both direct and indirect comparisons, enabling a more thorough comparative analysis of various interventions for IgAN treatment. Sensitivity analyses demonstrated minimal changes in primary outcome measures, thus enhancing the overall credibility of these assessments.
Unfortunately, the limited use of TCM outside China has led us and other researchers to include studies from the same population, which introduces the possibility of publication bias. In our study, there were also insufficient data on patients using different treatment regimens, owing to the inclusion of patients at various stages of IgAN. Therefore, we assessed the efficacy of the major drug classes in the treatment of IgAN. Subgroup analyses involving drugs, different dosages, and varying stages of IgAN failed to clarify the effectiveness of diverse treatment regimens for different stages of IgAN. Among the RCTs included in our analysis, patients exhibited varying degrees of severity at 24 hpro, eGFR, Scr, and BUN levels, along with other indicators, resulting in inconsistent treatment outcomes that may contribute to heterogeneity and impact the study results. Additionally, some studies were deemed to be of low quality as they did not explicitly describe whether allocation concealment or blinding occurred; this lack of information could potentially influence the analysis outcomes.
In the heterogeneity correlation test, heterogeneity among several interventions was not statistically significant and was often below 50%, which is considered within an acceptable range. Only a few studies on TCM interventions have shown a heterogeneity of >50%. We attribute this to the substantial differences in the various interventions included in TCM, variations in the composition of the TCM decoction, and discrepancies in patient indications, such as qi and yin deficiency, qi stagnation, and blood stasis. In summary, it is crucial to conduct high-quality, multicenter, large-sample, double-blind RCTs with long-term follow-up to enhance the robustness of our findings.
5 Conclusion
This network meta-analysis integrated multiple clinical datasets and connected multiple RCTs to achieve an indirect comparison of seven different interventions for the treatment of IgAN, effectively addressing the issue of disparate efficacy and priority resulting from a lack of direct comparisons. The results demonstrated that the combination of ACEI + TCM and ARB + TCM, along with TCM treatment alone, significantly decreased the 24 hpro, eGFR, Scr, and BUN levels in patients with IgAN. Among these, the combination of ARBs and TCM resulted in the most favorable outcomes. Combining TCM with conventional therapy for patients with IgAN enhances its protective effects and superiority without increasing the occurrence of ADRs. The pathogenesis of IgAN, which is one of the most prevalent primary glomerular diseases in clinical practice, is complex. Thus, the treatment challenge lies in the heterogeneity of its clinical manifestations and prognosis, necessitating individualized treatment plans (El Karoui et al., 2024). Therefore, combination TCM therapies can be used to enhance therapeutic outcomes based on individual patient circumstances. The severity of symptoms and signs must be considered when devising individualized treatment plans while simultaneously emphasizing the advantages of comprehensive TCM treatments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
SM: Writing–original draft, Visualization, Software, Formal Analysis, Data curation, Conceptualization. YJ: Writing–original draft, Visualization, Software, Methodology, Formal Analysis, Data curation. LQ: Writing–original draft, Methodology, Formal Analysis, Data curation. MW: Writing–original draft, Visualization, Software, Methodology, Formal Analysis. SX: Writing–review and editing, Validation, Supervision, Software, Conceptualization. GW: Writing–review and editing, Visualization, Supervision, Software.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Science (grant number: CI 2021B001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1374377/full#supplementary-material
Abbreviations
24 hpro, 24-h urinary protein; ACEI, angiotensin-converting enzyme inhibitor; ADR, adverse drug reaction; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; IgAN, immunoglobulin A nephropathy; KDIGO, Kidney Disease: Improving Global Outcomes; RAS, renin-angiotensin system; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International Registry of Prospective Systematic Reviews; RCT, randomized controlled trial; Scr, serum creatinine; SUCRA, surface under the cumulative ranking curve; TCM, traditional Chinese medicine.
References
Cai, Y. P. (2018). Effect of Tripterygium wilfordii polyglycosides combined with telmisartan in the treatment of patients with primary IgA nephropathy with moderate proteinuria. Henan Med. Res. 27, 3726–3728.
Chang, M. Y., Zhao, M. M., Yu, Y., Wang, R. M., Ma, S. J., and Zhang, Y. (2021a). Effect of modified Huangqi Chifeng decoction on proteinuria and urinary podocyte-related protein expression in patients with IgA nephropathy of qi deficiency, blood stasis and pathogenic wind and heat toxin syndrome. J. Tradit. Chin. Med. 62, 971–976. doi:10.13288/j.11-2166/r.2021.11.011
Chang, Z., Qin, W., Zheng, H., Schegg, K., Han, L., Liu, X., et al. (2021b). Triptonide is a reversible non-hormonal male contraceptive agent in mice and non-human primates. Nat. Commun. 12, 1253. doi:10.1038/s41467-021-21517-5
Chen, W. D., Lv, Y., Shang, L. W., Luan, M., and Ren, K. J. (2022). Clinical effect of Oiteng Xiaozhuo granule in treatment of lgA nephropathy withSpleen-kidney deficiency and blood stasis. J. Anhui Univ. China Med. 41, 18–23. doi:10.3969/j.issn.2095-7246.2022.04.006
Chen, X. M., Chen, J., Chen, Y. P., Zhou, Z. L., He, Y. N., Li, P., et al. (2007). Multicentered randomized controlled clinical trial on patients with IgA nephropathy of Qi yin deficiency syndrome type. Chin. J. Integr. Tradit. West. Med. 27, 101–105.
Chen, X. M., Chen, Y. P., Zhou, Z. L., Chen, J., Wei, R. B., Deng, Y. Y., et al. (2006). Prospective multi-centered randomized and controlled trial on effect of Shenle capsule in treating patients with IgA nephropathy of Fei Pi Qi deficiency syndrome. Chin. J. Integr. Tradit. West. Med. 26, 1061–1065.
Coppo, R. (2019). Towards a personalized treatment for IgA nephropathy considering pathology and pathogenesis. Nephrol. Dial. Transpl. 34, 1832–1838. doi:10.1093/ndt/gfy338
Duan, X. F. (2011). “Clinical efficacy on treating IgA nephropathywith Zini-Shenyan decoction,”. Master’s thesis (Fujian: Fujian University of Traditional Chinese Medicine).
Duan, X. F., and Wang, S. Z. (2014). Clinical observation of modified Erzhi pill in the treatment of IgA nephropathy. New J. Tradit. Chin. Med. 46, 68–70. doi:10.13457/j.cnki.jncm.2014.05.027
Eftekhari, A., Hasanzadeh, A., Khalilov, R., Hosainzadegan, H., Ahmadian, E., and Eghbal, M. A. (2020). Hepatoprotective role of berberine against paraquat-induced liver toxicity in rat. Environ. Sci. Pollut. Res. Int. 27, 4969–4975. doi:10.1007/s11356-019-07232-1
El Karoui, K., Fervenza, F. C., and De Vriese, A. S. (2024). Treatment of IgA nephropathy: a rapidly evolving field. J. Am. Soc. Nephrol. 35, 103–116. doi:10.1681/ASN.0000000000000242
Floege, J., Wied, S., and Rauen, T. (2022). Assessing prognosis in IgA nephropathy. Kidney Int. 102, 22–24. doi:10.1016/j.kint.2022.04.018
Fu, Y., Zhao, Z., Wu, Y., Wu, K., Xu, X., Liu, Y., et al. (2012). Therapeutic mechanisms of Tongmai Dasheng Tablet on Tripterygium glycosides induced rat model for premature ovarian failure. J. Ethnopharmacol. 139, 26–33. doi:10.1016/j.jep.2011.08.077
Gadola, L., Cabrera, M. J., Garau, M., Coitiño, R., Aunchayna, M. H., Noboa, O., et al. (2023). Long-term follow-up of an IgA nephropathy cohort: outcomes and risk factors. Ren. Fail. 45, 2152694. doi:10.1080/0886022X.2022.2152694
Gan, W. Y., Wang, Y., and Xiao, W. (2019). Clinical efficacy of Qiangshen Quyu Tongluo prescription and olmesartan Medoxomi I in treatment of kidney deficiency and blood stasis in Collatarels type IgA nephropathy. Chin. Arch. Tradit. Chin. Med. 37, 1966–1970. doi:10.13193/j.issn.1673-7717.2019.08.042
Gao, S. (2009). “Clinical efficacy on treating IgA nephropathywith Shenmao-Shiwei decoction,”. Master’s thesis (Fujian: Fujian University of Traditional Chinese Medicine).
Guan, X. D., Wu, Y. F., and Zhao, W. (2005). Clinical observation on treatment of lgA nephropathy with Huobahuagen Tablets and irbesartan. Chin. Integr. Med. 3, 36–39.
Guo, D. Z., Bian, D., Wang, Y. H., Chen, Z. Q., Han, P. Y., and Shang, Y. R. (2010). Effect of treatment of non-nephrotic syndrome IgA nephropathy with Shenyanning. Chin. J. Integr. Tradit. West. Med. 30, 841–844.
Guo, M., and Du, Y. L. (2023). Effect and mechanism of Huozhuohuagen tablets combined with losartan potassium tablets in the treatment of IgA nephropathy. Pract. Clin. J. Integr. Tradit. Chin. West. Med. 23, 21–23. doi:10.13638/j.issn.1671-4040.2023.07.006
Guo, S. F. (2019). “Clinical study of Zishen Huoxue decoction in the treatment ofIgA nephropathy with liver-kidney yin deficiency and blood stasis,”. Master’s thesis (Yunnan: Yunnan University of Traditional Chinese Medicine).
Han, C. E. (2012). “Observation on the curative effect of Qi-yin nourishing method on IgA nephropathy with deficiency of Qi and Yin,”. Master’s thesis (Chengdu: Chengdu University of Traditional Chinese Medicine).
Han, L. X., Guo, R. R., Zhang, R., Xing, G. Y., and Han, X. (2016). Clinical observation of ginseng Guipi pill combined with benazepril in the treatment of 50 cases of IgA nephropathy. Hebei J. Tradit. Chin. Med. 38, 46–48. doi:10.3969/j.issn.1002-2619.2016.01.013
Han, Y. R., and Qiu, Z. Y. (2010). Clinical study of Huangkui capsule combined with benazepril in the treatment of primary IgA nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 11, 998–999.
Horita, Y., Tadokoro, M., Taura, K., Suyama, N., Taguchi, T., Miyazaki, M., et al. (2004). Low-dose combination therapy with temocapril and losartan reduces proteinuria in normotensive patients with immunoglobulin A nephropathy. Hypertens. Res. 27, 963–970. doi:10.1291/hypres.27.963
Horita, Y., Taura, K., Taguchi, T., Furusu, A., and Kohno, S. (2006). Aldosterone breakthrough during therapy with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in proteinuric patients with immunoglobulin A nephropathy. Nephrol. Carlt. 11, 462–466. doi:10.1111/j.1440-1797.2006.00665.x
Hou, J. R., An, Y. H., and Li, X. F. (2009). Clinical study of candesartan combined with benazepril hydrochloride in the treatment of IgA nephropathy proteinuria. J. Clin. Exp. Med. 8, 89.
Huang, G. D., He, X. P., Xiang, S. W., Ma, X. L., Yu, C. J., and Wen, H. T. (2008b). Clinical observation of compound Xiancao capsule in the treatment of latent IgA nephropathy. J. Guangxi Univ. China Med. 11, 11–13.
Huang, J., Wang, L. X., Hou, H. J., and Hu, Q. Q. (2012). Gastritis Kangfu tablet combined with fosinopril in the treatment of 25 cases of IgA nephropathy. Henan. J. Tradit. Chin. Med. 32, 1646–1647. doi:10.16367/j.issn.1003-5028.2012.12.093
Huang, L. L. (2008a). “Clinical efficacy on treating IgA nephropathywith oilian maogen decoction,”. Master’s thesis (Fujian: Fujian University of Traditional Chinese Medicine).
Huang, Q., and He, J. Q. (2011). Clinical observation of Bailing capsule combined with benazepril in the treatment of 33 cases of IgA nephropathy. J. Guizhou Univ. J. Tradit. Chin. Med. 33, 29–31. doi:10.3969/j.issn.1002-1108.2011.01.15
Huo, Z., Ye, H., Ye, P., Xiao, G., Zhang, Z., and Kong, Y. (2021). Comparative efficacy of different renin angiotensin system blockade therapies in patients with IgA nephropathy: a Bayesian network meta-analysis of 17 RCTs. PeerJ 9, e11661. doi:10.7717/peerj.11661
Inker, L. A., Astor, B. C., Fox, C. H., Isakova, T., Lash, J. P., Peralta, C. A., et al. (2014). KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 63, 713–735. doi:10.1053/j.ajkd.2014.01.416
Jarrick, S., Lundberg, S., Welander, A., Carrero, J. J., Höijer, J., Bottai, M., et al. (2019). Mortality in IgA nephropathy: a nationwide population-based cohort study. J. Am. Soc. Nephrol. 30, 866–876. doi:10.1681/ASN.2018101017
Jia, X. C. (2012). Clinical study on self-design ZiCao DiHuang DiYuTang in treating IgA nephropathy of YinD efficiency type and dual defficiency type of Qi and Yin: a report of 33 cases. West. J. Tradit. Chin. Med. 25, 8–10.
Li, G. H., Zhang, D., Zhang, L., Guan, Y. M., Gao, Y. X., Zhou, Z., et al. (2018). Study of urinary IL-6 and serum secretory IgA in IgA nephropathy treated with purging kidney and cooling blood. Chin. J. Integr. Tradit. West. Nephrol. 19, 311–314.
Li, J., He, A. D., and Tao, B. J. (2023). Clinical study on Ziyin Yishen prescription combined with telmisartan for primary IgA nephropathy. New J. Tradit. Chin. Med. 55, 69–72. doi:10.13457/j.cnki.jncm.2023.06.015
Li, L. S., Yan, C. P., and Zhou, Z. H. (2012). Clinical study of Huangkui Capsule combined with olmesartan in the treatment of IgA nephropathy with mild to moderate proteinuria. Chin. Her. 9, 74–75.
Li, P., Lin, H., Ni, Z., Zhan, Y., He, Y., Yang, H., et al. (2020). Efficacy and safety of Abelmoschus manihot for IgA nephropathy: a multicenter randomized clinical trial. Phytomedicine 76, 153231. doi:10.1016/j.phymed.2020.153231
Li, P. K., Leung, C. B., Chow, K. M., Cheng, Y. L., Fung, S. K., Mak, S. K., et al. (2006). Hong Kong study using valsartan in IgA nephropathy (HKVIN): a double-blind, randomized, placebo-controlled study. Am. J. Kidney Dis. 47, 751–760. doi:10.1053/j.ajkd.2006.01.017
Liang, Y., Zhang, L. P., Lu, Y. B., and Guo, W. (2017). Clinical observation of Huangkui capsule combined with losartan potassium in the treatment of IgA nephropathy. Shaanxi J. Tradit. Chin. Med. 38, 1192–1193. doi:10.3969/j.issn.1000-7369.2017.09.018
Liu, C. Y., Ren, Y. Y., Leng, W., and Chen, L. X. (2010). Compound Shenfu tablets in the treatment of 32 cases of IgA nephropathy. Mod. Tradit. Chin. Med. 30, 50–52. doi:10.13424/j.cnki.mtcm.2010.06.023
Liu, X. W., Chen, W., Xu, G. S., Sun, S. R., Liu, H. B., Zhang, P., et al. (2007a). Therapeutic efficacy of fleabane combined with fosinopril for IgA nephropathy. J. Air Force Med. Univ., 1805–1807.
Liu, X. W., Wang, H. M., Chen, W., Li, H. P., Xu, G. S., Liu, H. B., et al. (2007b). Comparison of therapeutic effect of higher-dose valsatan combined with higher dose of lotensin for IgA nephropathy. J. Xi’an Jiaot. Univ. Med. Sci. 28, 437–439.
Long, P. (2021). “Clinical observation on the effect of modified Shenqi Dihuang decoction on proteinuria and hematuria of IgA nephropathy with deficiency of qi and yin,”. Master’s thesis (Guangxi: Guangxi University of Traditional Chinese Medicine).
Lu, J. W., and Ma, J. P. (2021). Shaoyang decoction combined with irbesartan tablets in the treatment of IgA nephropathy of Shaoyang syndrome Clinical study of 30 cases. Jiangsu J. Tradit. Chin. Med. 53, 38–41. doi:10.19844/j.cnki.1672-397X.2021.02.015
Luo, L., Liu, Z. Q., and Fan, Y. J. (2023). Effects of Bailing capsules assisting valsartan in the treatment of IgA nephropathy and its influence on renal function, Th1/Th2 drift, urine PCX and B7-1 levels. Guangzhou Med. J. 54, 46–49. doi:10.3969/j.issn.1000-8535.2023.02.009
Lv, J., Zhang, H., Wong, M. G., Jardine, M. J., Hladunewich, M., Jha, V., et al. (2017). Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 318, 432–442. doi:10.1001/jama.2017.9362
Ma, S., Zhao, M., Chang, M., Shi, X., Shi, Y., Zhang, Y., et al. (2023). Effects and mechanisms of Chinese herbal medicine on IgA nephropathy. Phytomedicine. 117, 154913. doi:10.1016/j.phymed.2023.154913
Matsumoto, Y., Aryal, R. P., Heimburg-Molinaro, J., Park, S. S., Wever, W. J., Lehoux, S., et al. (2022). Identification and characterization of circulating immune complexes in IgA nephropathy. Sci. Adv. 8, eabm8783. doi:10.1126/sciadv.abm8783
Meng, Y., Chang, Z., Meng, Y., Cai, Z., and Zhang, S. R. (2015). Clinical research of yiqi yangshen decoction curing qiyinliangxu of IgA nephropathy. Chin. J. Integr. Tradit. West. Nephrol. 16, 131–133.
Nakamura, T., Inoue, T., Sugaya, T., Kawagoe, Y., Suzuki, T., Ueda, Y., et al. (2007). Beneficial effects of olmesartan and temocapril on urinary liver-type fatty acid-binding protein levels in normotensive patients with immunoglobin A nephropathy. Am. J. Hypertens. 20, 1195–1201. doi:10.1016/j.amjhyper.2007.06.003
Pan, L., Wang, H. C., He, Z., Hu, H. P., Zhang, M. J., Wang, H. S., et al. (2022). Clinical observation of Yiqi Huoxue Tongluo recipe in the treatment of IgA nephropathy of qi deficiency and blood stasis type. Mod. J. Integr. Tradit. Chin. West. Med. 31, 220–223. doi:10.3969/j.issn.1008-8849.2022.02.015
Pan, L. J., Zhan, J. H., and Cai, Q. (2017). Clinical observation of bushen tiaogan huayu method in treatment of IgA nephropathy. Chin. Arch. Tradit. Chin. Med. 35, 3131–3133. doi:10.13193/j.issn.1673-7717.2017.12.038
Pan, Z., Zhao, M., Chang, M., Shi, X., Ma, S., and Zhang, Y. (2023). Clinical efficacy of supplementing qi dispelling wind and activating blood circulation method in the treatment of IgA nephropathy: a meta-analysis. Med. (Baltim.). 102, e33123. doi:10.1097/MD.0000000000033123
Rajasekaran, A., Julian, B. A., and Rizk, D. V. (2021). IgA nephropathy: an interesting autoimmune kidney disease. Am. J. Med. Sci. 361, 176–194. doi:10.1016/j.amjms.2020.10.003
Renke, M., Tylicki, L., Rutkowski, P., and Rutkowski, B. (2004). Low-dose angiotensin II receptor antagonists and angiotensin II-converting enzyme inhibitors alone or in combination for treatment of primary glomerulonephritis. Scand. J. Urol. Nephrol. 38, 427–433. doi:10.1080/00365590410015687
Rovin, B. H., Adler, S. G., Barratt, J., Bridoux, F., Burdge, K. A., Chan, T. M., et al. (2021). Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int. 100, 753–779. doi:10.1016/j.kint.2021.05.015
Samarghandian, S., Azimi-Nezhad, M., Farkhondeh, T., and Samini, F. (2017). Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed. Pharmacother. 87, 223–229. doi:10.1016/j.biopha.2016.12.105
Schimpf, J., Kronbichler, A., Windpessl, M., Zitt, E., Eller, K., Säemann, M. D., et al. (2023). Diagnosis and treatment of IgA nephropathy-2023. Wien. Klin. Wochenschr 135 (5), 621–627. doi:10.1007/s00508-023-02257-6
Shen, P. C., He, L. Q., Yang, X. J., and Cao, H. X. (2012). Renal protection of losartan 50 mg in normotensive Chinese patients with nondiabetic chronic kidney disease. J. Investig. Med. 60, 1041–1047. doi:10.2310/JIM.0b013e31826741d2
Shima, Y., Nakanishi, K., Sako, M., Saito-Oba, M., Hamasaki, Y., Hataya, H., et al. (2019). Lisinopril versus lisinopril and losartan for mild childhood IgA nephropathy: a randomized controlled trial (JSKDC01 study). Pediatr. Nephrol. 34, 837–846. doi:10.1007/s00467-018-4099-8
Su, B. F., and Zhang, H. D. (2014). Effect of Huangkui capsule combined with irbesartan in the treatment of IgA nephropathy. Chin. J. Prev. Control. Chronic Non-Commun. Dis. 22, 585–586. doi:10.16386/j.cjpccd.issn.1004-6194.2014.05.043
Wang, J., Zhang, X. X., Chen, X. N., Lu, J. R., Wang, Z. H., Hu, J., et al. (2019). Treatment of severe IgA nephropathy patients with liver and kidney yin deficiency syndrome by traditional Chinese medicine syndrome differentiation Combinated with losartan potassium: a prospective and multicentered clinical study. J. Tradit. Chin. Med. 60, 1929–1934. doi:10.13288/j.11-2166/r.2019.22.010
Wang, J., Zhang, X. X., Chen, X. N., Wang, Z. H., Gao, Y. C., Xu, H., et al. (2020b). TCM syndrome differentiation-based treatment combined with losartan potassium in treating severe IgA nephropathy with yin deficiency of liver and kidney syndrome:A multicenter randomized controlled trial. China J. Tradit. Chin. Med. Pharm. 35, 5319–5324. doi:10.13288/j.11-2166/r.2019.22.010
Wang, L. P., Zhang, Y., Chen, J., Zhuang, Y. Z., and Du, J. (2010a). Therapeutic effect of Huangkui Capsule on IgA nephropathy with damp-heat syndrome. Chin. Tradit. Pat. Med. 32, 18–21.
Wang, Q. Q., Lv, Y., Tang, Y., Deng, Y. Y., Gao, J. D., Chen, Y., et al. (2020c). Treatment outcome of TCM and WM therapy on IgA nephropathy with Chaiqin decoction. Chin. J. Integr. Tradit. West. Nephrol. 21, 309–312.
Wang, R. M. (2021). “Effects of modified Huangqi Chifeng decoction on proteinuria and urinary TGF-β1 and MCP-1 in patients with IgA nephropathy,”. Master’s thesis (China: China Academy of Chinese Medical Sciences).
Wang, R. X., Liao, B. Q., Chen, W., Zhang, Y. M., Tang, X. H., and Xie, F. H. (2022). A meta-analysis of effects and safety of Tripterygium wilfordii polyglycoside in the treatment of IgA nephropathy. Eur. Rev. Med. Pharmacol. Sci. 26, 8756–8770. doi:10.26355/eurrev_202212_30547
Wang, W., Gao, D., Zu, J., and He, X. (2014). Efficacy of Zhengqiang Fengtongning tablets in the treatment of IgA nephropathy with moderate amount of proteinuria. Med. J. Chin. PAPF. 25, 1021–1023. doi:10.14010/j.cnki.wjyx.2014.10.016
Wang, X. H., Lang, R., Liang, Y., Zeng, Q., Chen, N., and Yu, R. H. (2021b). Traditional Chinese medicine in treating IgA nephropathy: from basic science to clinical research. J. Transl. Int. Med. 9, 161–167. doi:10.2478/jtim-2021-0021
Wang, Y. H. (2020a). Clinical efficacy of Tripterygium Wilfordii polyglycoside combined with omesartan in the treatment of patients with primary IgA nephropathy. Guide Chin. Med. 18, 132–133. doi:10.15912/j.cnki.gocm.2020.29.063
Wang, Y. H., Shen, B. L., Qu, Q. S., Liu, B. L., and Liu, R. Y. (2018). Yishen Qufeng Chushi granule combined with losartan potassium in treatment of IgA nephropathy. China J. China Med. 33, 1333–1336. doi:10.16368/j.issn.1674-8999.2018.07.316
Wang, Y. X., Li, X., Feng, Z. F., and Xu, B. (2021c). Effect of Qiyu Qinghua decoction combined with losartan potassium tablets on TLR4 and inflammatory cytokine expression in IgA nephropathy. Mod. Tradit. Chin. Med. Mater. medica-world Sci. Tech. 23, 4165–4171. doi:10.11842/wst.20201210008
Wang, Y. X., Liu, X. X., and Xu, B. (2020d). To investigate the clinical efficacy of QishingQinghua decoction in the treatment of IgA nephropathy with spleen-kidney Yang deficiency syndrome and its effect on oxidative stress. Lishizhen Med. Mater. Med. Res. 31, 1918–1920. doi:10.3969/j.issn.1008-0805.2020.08.041
Wang, Z. J., and Shou, X. J. (2010b). Observation on the therapeutic effect of valsartan combined fosinopril in treating IgA Nephropathy. Med. Innov. China. 7, 19–20.
Wei, H. J., Duo, H. L., Liu, H. D., and Dong, S. Y. (2017). Clinical study of Tongluo-Ningxue decoction in the treatment of 30 cases of IgA nephropathy of kidney deficiency and blood stasis type. Jiangsu J. Tradit. Chin. Med. 49, 28–30.
Wei, J. W. (2019). Observation of Tripterygium wilfordii polyglycosides combined with irbesartan in the treatment of IgA nephropathy. J. Pract. Tradit. Chin. Med. 35, 187.
Whitlock, R., Leon, S. J., Manacsa, H., Askin, N., Rigatto, C., Fatoba, S. T., et al. (2023). The association between dual RAAS inhibition and risk of acute kidney injury and hyperkalemia in patients with diabetic kidney disease: a systematic review and meta-analysis. Nephrol. Dial. Transpl. 38, 2503–2516. doi:10.1093/ndt/gfad101
Wyatt, R. J., and Julian, B. A. (2013). IgA nephropathy. N. Engl. J. Med. 368, 2402–2414. doi:10.1056/NEJMra1206793
Xia, J., Wang, M., and Jiang, W. (2022). New insights into pathogenesis of IgA nephropathy. Int. Urol. Nephrol. 54, 1873–1880. doi:10.1007/s11255-021-03094-0
Xiang, Q., Song, E. F., and Liu, H. Y. (2014). Efficacy observation of IgA nephropathy treated with Tripterygium glycosides tablets and telmisartan in the middle and aged patients. Integr. Tradit. West. Med. 9, 756–758. doi:10.13935/j.cnki.sjzx.140725
Xiang, S. W., Lai, S. C., and Meng, Y. H. (2009). Clinical trial of Shenyankang decoction for the treatment of lgA nephropathy. New J. Tradit. Chin. Med. 41, 21–22. doi:10.13457/j.cnki.jncm.2009.10.014
Xu, B., Lu, Y., Liu, Y. J., Wang, N. S., and Wang, Y. (2009). Clinical research on “NianmoDecoction” in treating IgA nephropathy. Shanghai J. Tradit. Chin. Med. 43, 17–18. doi:10.16305/j.1007-1334.2009.10.001
Xu, K. (2020). Efficacy analysis of Tripterygium wilfordii polyglucoside combined with benazepril in the treatment of primary IgA nephropathy patients with moderate proteinuria. Mod. Diagn. Treat. 31, 3065–3067.
Xue, S. L. (2012). “Observation of clinical efficacy on IgA Nephropathy treated by the way of Benefiting Qi, nourishing Yin and promoting blood circulation,”. Master’s thesis (Fujian: Fujian University of Traditional Chinese Medicine).
Yang, L., and Chen, Q. K. (2010). Efficacy of benazepril hydrochloride, dipyridamole combined with Huangkui capsule in the treatment of 21 cases of IgA nephropathy. J. Nanchang Univ. Med. Sci. 50, 69–70.
Yao, C. Y., and Zhou, X. H. (2011). 32 cases of IgA nephropathy were treated with integrated traditional Chinese and western medicine. Shandong J. Tradit. Chin. Med. 30, 253–254. doi:10.16295/j.cnki.0257-358x.2011.04.035
Yao, T., Wu, Z., Wang, Z., Chen, L., Liu, B., Lu, M., et al. (2023). Association between angiotensin-converting enzyme inhibitor-induced cough and the risk of lung cancer: a Mendelian randomization study. Front. Pharmacol. 14, 1267924. doi:10.3389/fphar.2023.1267924
Zhang, C. X., Cheng, J., and Cheng, X. Y. (2016). Clinical observation of replenishing qi and nourishing Yin in the treatment of hematuria dominant IgA nephropathy. Inn. Mong. J. Tradit. Chin. Med. 35, 3–5. doi:10.16040/j.cnki.cn15-1101.2016.15.004
Zhang, L., Li, P., Xing, C. Y., Zhao, J. Y., He, Y. N., Wang, J. Q., et al. (2014). Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am. J. Kidney Dis. 64, 57–65. doi:10.1053/j.ajkd.2014.01.431
Zhang, X. X., Chen, X. N., Wang, Z. H., Lu, J. R., Hu, J., Gao, Y. C., et al. (2019a). Observing clinical effects of treatment of liver and kidney in treatment of IgA nephropathy at different time points. World Chin. Med. 14, 1074–1078. doi:10.3969/j.issn.1673-7202.2019.05.003
Zhang, X. X., Gao, Y. C., and He, L. Q. (2019b). Immune mechanism of inhibiting renal fibrosis in IgA nephropathy by integrating traditional Chinese and western medicine optimized treatment programs. World Chin. Med. 14, 1079–1083. doi:10.3969/j.issn.1673-7202.2019.05.004
Zhao, C. W., Tian, G., Li, H. G., Liu, L., and Shi, J. (2021a). Clinical study of Shenfukang Ⅱ capsules in the treatment of IgA nephropathy with renal tubulointerstitial fibrosis. J. Chang. Univ. China Med. 37, 558–562. doi:10.13463/j.cnki.cczyy.2021.03.023
Zhao, M., Wang, R., Yu, Y., Chang, M., Ma, S., Zhang, H., et al. (2021b). Efficacy and safety of angiotensin-converting enzyme inhibitor in combination with angiotensin-receptor blocker in chronic kidney disease based on dose: a systematic review and meta-analysis. Front. Pharmacol. 12, 638611. doi:10.3389/fphar.2021.638611
Zhao, Q. L., Lv, H. S., and Lv, K. (2022). Observation of therapeutic effect of Zhiwei Rehmannia decoction on IgA nephropathy with syndrome of kidney deficiency with effulgent fire. Henan. J. Tradit. Chin. Med. 42, 268–270. doi:10.16367/j.issn.1003-5028.2022.02.0058
Zhao, T. (2015). “Clinical observation of sinomenine in the treatment of IgA nephropathy of dampness and blood stasis,”. Master’s thesis (Hubei: Hubei University of Traditional Chinese Medicine).
Keywords: IgA nephropathy, traditional Chinese medicine, angiotensin receptor blockers, angiotensin-converting enzyme inhibitors, network meta-analysis
Citation: Ma S, Jiang Y, Qian L, Wang M, Xu S and Wang G (2024) Efficacy of traditional Chinese medicine versus angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and their combinations in the treatment of IgA nephropathy: a systematic review and network meta-analysis. Front. Pharmacol. 15:1374377. doi: 10.3389/fphar.2024.1374377
Received: 22 January 2024; Accepted: 07 March 2024;
Published: 21 March 2024.
Edited by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranReviewed by:
Rovshan Khalilov, Baku State University, AzerbaijanRenad Zhdanov, Kazan State Medical Academy, Russia
Copyright © 2024 Ma, Jiang, Qian, Wang, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shijie Xu, eHVzaGlqaWU2NjY2QHNvaHUuY29t; Guowei Wang, d2FuZ2d1b3dlaWRvY0AxNjMuY29t
 Sijia Ma
Sijia Ma Yuhua Jiang
Yuhua Jiang