
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 May 2024
Sec. Pharmacoepidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1372401
 Dan Li1
Dan Li1 Yuan Zhang1
Yuan Zhang1 Jia Qi Ni2,3,4
Jia Qi Ni2,3,4 Juan Zhu1
Juan Zhu1 Wen Ting Lu1
Wen Ting Lu1 Ya Lin Chen1
Ya Lin Chen1 Lei Cheng1
Lei Cheng1 Yu Qi Wang1
Yu Qi Wang1 Qian Jiang Li1
Qian Jiang Li1 Jie Wang1
Jie Wang1 Yan Bing Lu1
Yan Bing Lu1 Jia Chen5*
Jia Chen5* Li Chen2,3,6*
Li Chen2,3,6*Objective: Bendamustine was approved for treating chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma. Despite its therapeutic benefits, the long-term safety of bendamustine in a large population remains inadequately understood. This study evaluates the adverse events (AEs) associated with bendamustine, using a real-world pharmacovigilance database to support its clinical application.
Methods: We conducted a post-marketing risk analysis to assess the association between bendamustine and its AEs. Data were extracted from the US FDA’s Adverse Event Reporting System (FAERS), covering the period from January 2017 to September 2023. The characteristics of bendamustine-associated AEs and the onset time were further analyzed. Statistical analysis was performed using MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2016, and Minitab 21.0.
Results: 9,461,874 reports were collected from the FAERS database, 9,131 identified bendamustine as the “primary suspected” drug. We identified 331 significant disproportionality preferred terms (PTs). Common AEs included pyrexia, neutropenia, infusion site reaction, progressive multifocal leukoencephalopathy (PML), injection site vasculitis, and pneumonia—all documented on bendamustine’s label. Notably, 16 unexpected and significant AEs were discovered, including hypogammaglobulinemia, which is concerning due to its potential to increase infection susceptibility following bendamustine treatment. Other significant findings were anaphylactic reactions, PML, and cutaneous malignancies, suggesting updates to the drug’s label may be necessary. Physicians should monitor for neurological and skin changes in patients and discontinue treatment if PML is suspected. Moreover, the median onset time for bendamustine-associated AEs was 13 days, with an interquartile range [IQR] of 0–59 days, predominantly occurring on the first day post-initiation. The β of bendamustine-related AEs suggested risk reduction over time.
Conclusion: Our study uncovered some potential pharmacovigilance signals for bendamustine, providing important insights for its safe and effective clinical use.
Bendamustine is a bifunctional molecule combining alkylating and antimetabolic properties, which has demonstrated enhanced efficacy across various lymphoma pathologies. Numerous studies have documented the effectiveness of bendamustine, either alone or in combination with other agents, in treating conditions such as indolent non-Hodgkin lymphoma (NHL), chronic lymphocytic leukemia, and mantle cell lymphoma (Flinn, 2019; Wang et al., 2022). These treatments have been shown to improve progression-free survival, reduce residual tumor cells, and minimize tumor burden (Merryman et al., 2020).
In 2008, the U.S. Food and Drug Administration approved bendamustine for patients with rituximab-resistant indolent NHL and chronic lymphocytic leukemia (Lalic et al., 2022). Given its beneficial effects in treating relapsed or refractory hematologic malignancies and its synergistic potential with other antineoplastic agents, bendamustine has attracted significant recent interest due to its immunomodulatory effects (Palumbo et al., 2015; Arulogun et al., 2023; Gao et al., 2023). However, adverse effects such as fever, memory loss, anxiety, and rash have been noted during or after discontinuation of treatment (Yi et al., 2022). Furthermore, serious complications like progressive multifocal leukoencephalopathy (PML) have been reported, including a case in a patient with non-Hodgkin follicular lymphoma post hematopoietic stem cell transplantation and rituximab-bendamustine therapy, where JC Virus DNA was detected in both peripheral blood and cerebrospinal fluid (Uchida Trociukas et al., 2020). A retrospective study of 95 patients also highlighted non-prior chemotherapy as a significant risk factor for skin toxicities among NHL patients treated with bendamustine alone or in combination with rituximab (BR therapy).
Subsequently, on 24 March 2021, the UK Medicines and Health Products Regulatory Agency issued an advisory highlighting the increased risks of non-melanoma skin cancer and PML associated with bendamustine (GOV.UK, 2021).
Despite these insights, most safety data on bendamustine derive from clinical trials and literature reviews (Flinn et al., 2014; Shotton et al., 2024; Wang et al., 2023), with a lack of systematic research into AE signals based on extensive international and real-world databases. This study aims to fill that gap by analyzing AEs associated with bendamustine using the US FDA Adverse Event Reporting System (FAERS) database, providing valuable insights for its clinical application and future research.
We conducted an observational retrospective disproportionality analysis using a case/non-case study design to assess potential associations between bendamustine and various AEs (Almenoff et al., 2007; Bate and Evans, 2009). In this study, AEs associated with bendamustine were identified as signals when they were reported more frequently than other drug events in the background information within the database. We included all reports submitted to the FDA Adverse Event Reporting System (FAERS) from January 2017 to September 2023, reflecting the most recent update of the FAERS database at the time of our analysis.
Bendamustine, an antineoplastic drug approved by the FDA, was the primary suspect in the reports analyzed. We utilized fuzzy matching in MySQL on the “drug name” field to filter out reports specifically mentioning bendamustine and to remove duplicates. Key search terms included “Bendamustine,” “BELRAPZO,” “BENDEKA,” “TREANDA,” and “VIVIMUSTA.” AEs data within FAERS were coded according to the preferred terminology from the International Medical Dictionary for Regulatory Activities (MedDRA), version 26.1 (Zhang et al., 2023). MedDRA organizes terms into five hierarchical levels: System Organ Class (SOC), High-Level Group Terms (HLGT), High-Level Terms, Preferred Term (PT), and Lowe Level Terms (LLT), which facilitated the categorization and searchability of data at various levels (Shu et al., 2023).
Given the limitations of the FAERS database, specifically the absence of denominator data, we cannot directly calculate the incidence of AEs. However, disproportionality analysis serves as a robust method in pharmacovigilance to detect signals of disproportionate reporting related to bendamustine (Ahdi et al., 2023). In our study, we applied both Bayesian and Frequentist approaches to assess the association between bendamustine and AEs. We utilized several statistical measures, including the Reporting Odds Ratio (ROR), the Proportional Reporting Ratio (PRR), the Information Component (IC), and the Empirical Bayes Geometric Mean (EBGM) (Sakaeda et al., 2013; Moore et al., 2020; Yao et al., 2020).
A positive signal of disproportionality was defined according to several criteria: a PRR of at least two, a chi-squared value (χ2) of at least four, three or more reported cases, an IC05 > 0, EBGM>2 (Kinoshita et al., 2020). All statistical analyses were performed using Microsoft Excel 2016.
Following the initial disproportionality analysis, detailed data concerning the AEs were collected, including patient characteristics (such as gender and age), reporting areas, indications for drug use, outcomes of the events, and the identity of the reporters. The methodology for data extraction, processing, and analysis is illustrated in Figure 1.
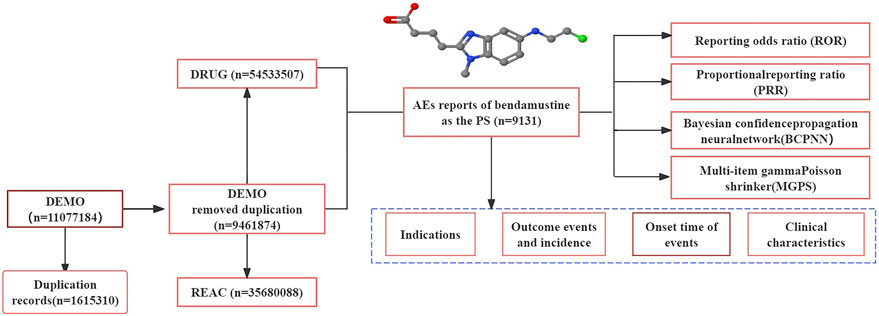
Figure 1. Process of retrieving bendamustine-associated AEs from the FAERS database. PS: primary suspected, AEs: adverse events.
To analyze the time-to-onset data, we employed median and quartile calculations along with Weibull shape parameter (WSP) tests (Chen et al., 2021). These analyses were conducted from the initial date of drug administration to the occurrence of specific AEs. We ensured the accuracy of our calculations by using only data that was fully reported with complete dates in the YYYYMMDD format, excluding cases with partial or missing dates (Zhao et al., 2023). Additionally, we removed instances with input errors, such as those where the AE occurrence was reported to precede the start of drug administration.
The tendency for AEs to occur was modeled using the two or three-parameter WSP. The tendency of AE occurrence can be predicted with the two or three-parameter WSP (Brucato et al., 2015; Mazhar Chiappini et al., 2021). The WSP is defined by two parameters: scale (α) and shape (β). A constant hazard over time corresponds to a β value of 1. Conversely, a β value less than one indicates a decreasing hazard over time, while a value greater than one suggests an increasing hazard. All analyses were performed using Minitab software (version 21.0; Minitab LLC, State College, PA, USA).
Our study analyzed 27 quarters of adverse drug event (ADE) data from the FAERS, spanning from the first quarter of January 2017 to September 2023. We extracted a total of 9,461,874 AEs reports from the FAERS database, and after removing duplicates, 9,131 reports were initially identified with bendamustine as the suspected drug. Further refinement by eliminating duplicate and misleading records reduced the sample to 5,195 reports for final analysis.
Demographic analysis revealed that patients over the age of 65 comprised 48.61% of the study sample, with a median age of 67 and an average age of 62.8 years. The data showed a higher incidence of AEs in male patients compared to females. Severe outcomes, including hospitalization and death, were reported in 2,406 cases, accounting for 30.38% and 20.41% of the severe reports, respectively.
The majority of AE reports were submitted by physicians, who contributed 45.44% of the data. This was followed by consumers (24.77%), other health professionals (13.85%), healthcare professionals (11.33%), and pharmacists (4.62%). The top five countries reporting the most bendamustine-associated AEs were the United States, Japan, France, Germany, and Britain, with Japan alone accounting for 33.49% of the cases. The summarized data and further details are presented in Table 1.
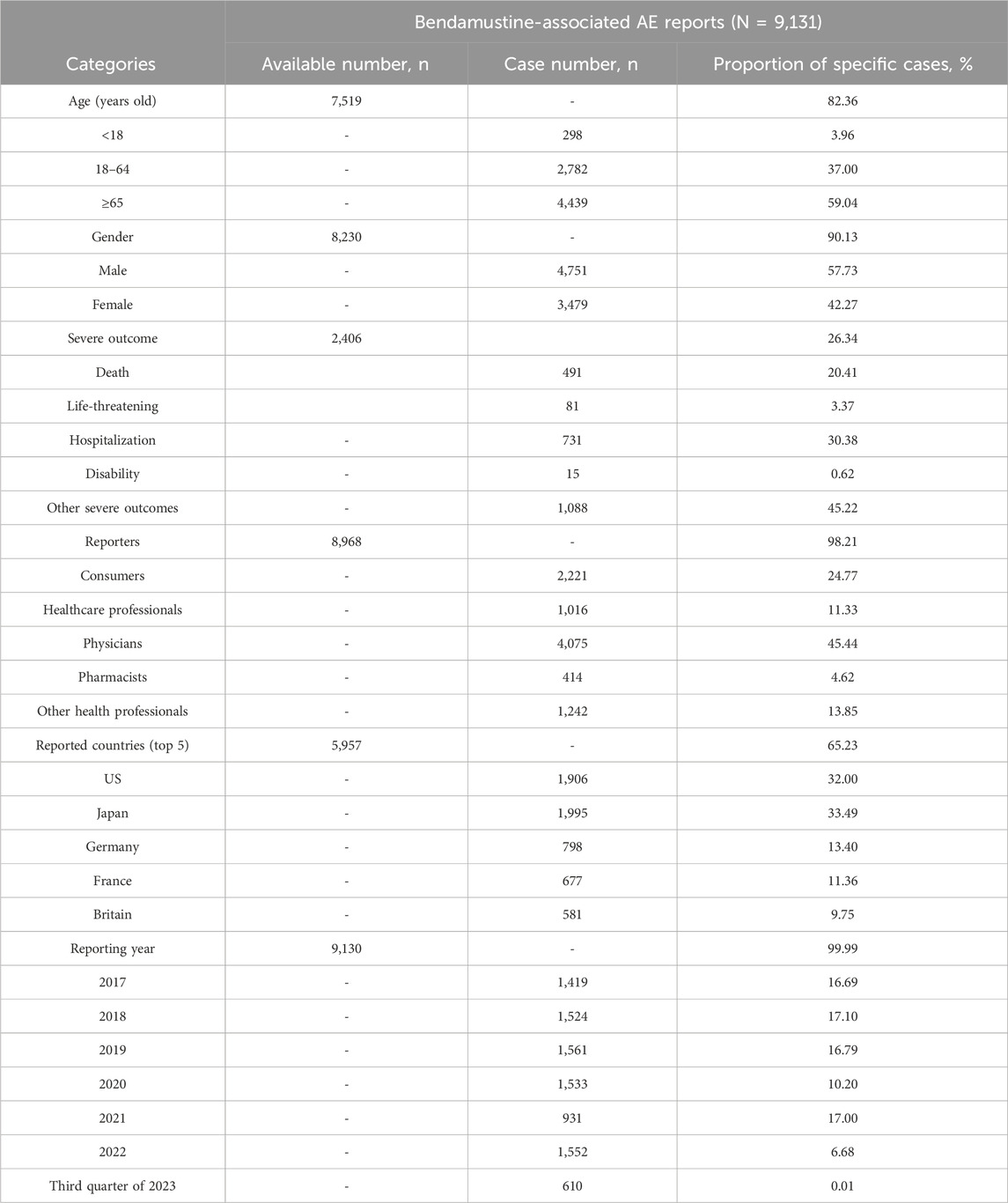
Table 1. Information of reports with bendamustine as the primary suspected drug from the FAERS database (January 2017 to September 2023).
In our examination of the FAERS database, signal strengths and bendamustine-related reports at the SOC level are detailed in Figure 2. We analysis identified statistically significant signals across 320 PTs within 17 SOCs, after excluding non-adverse drug reaction signals such as product issues, social environment factors, injuries, poisonings, operational complications, and effects from various surgical and medical procedures.
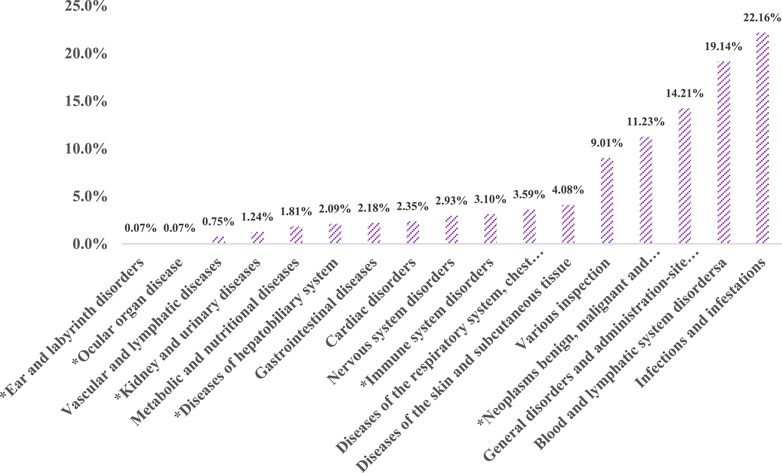
Figure 2. Proportion of bendamustine-associated AEs in different organ systems. Note: *New findings of bendamustine-associated AEs compared to the package insert of the drug.
The SOC category “Infections and Infestations” reported the highest cumulative number of AE cases, accounting for 22.16% of all cases (945 cases). This was followed by disorders of the blood and lymphatic system (816 cases, 19.14%), and systemic diseases and reactions at the site of drug administration (606 cases, 14.21%).
A comparison with the AEs listed on the package insert for Cunda (bendamustine hydrochloride for injection, manufactured by Pharmachemie B.V.) revealed unexpected AEs affecting six organ systems. Notable among these were diseases of the blood and lymphatic system, which included six PTs such as embolism, hematoma, vasculitis, and vascular pain, accounting for a composition ratio of 0.75%. Ear and labyrinth disorders (PT: neurosensory deafness) and ocular diseases each accounted for 0.07% of reports.
Given their weak signals and relatively low report numbers, it is possible that false positives detected in these cases are due to the high sensitivity of the Reporting Odds Ratio (ROR) method (Gravel et al., 2023). Conversely, diseases of the liver and biliary system reported 89 cases (2.09%), with PTs such as acute hepatic failure, hepatic cirrhosis, abnormal hepatic function, hyperbilirubinemia, and veno-occlusive liver disease. The highest correlation and number of cases were seen in abnormal hepatic function (27 cases, χ2 = 117.32).
Furthermore, immune system diseases were identified in 132 cases (3.10%), encompassing 13 PTs including amyloidosis, anaphylactic reactions, cytokine release syndrome, cytokine storm, food allergy, graft versus host disease, hemophagocytic lymphohistiocytosis, and hypogammaglobulinemia. Diseases affecting the kidney and urinary system were reported in 53 cases (1.24%), including PTs like Waldenstrom’s macroglobulinemia and blastic plasmacytoid dendritic cell neoplasia.
AE signals associated with bendamustine were thoroughly analyzed. To identify the top 30 AEs with high reporting rates and robust signals, effective signals were ranked in descending order based on the number of AE reports and the lower limit of the 95% Confidence Interval (CI) for the ROR, as shown in Table 2. Since the PRR values were equivalent to the ROR values, our signal strength analysis focused solely on comparing the ROR 95%CI lower limits (Chiappini et al., 2023).
Comparison with the AEs listed in the current package insert for bendamustine revealed 16 new AEs. Among these newly identified AEs, the most significant were 169 cases of malignant neoplasm progression, 88 cases of disease progression, 58 cases of peripheral neuropathy, and 58 cases of sepsis. These findings are instrumental in providing a basis for potential updates to the AE listings in the bendamustine package insert.
From the first quarter of 2017 to the third quarter of 2023, 4,825 cases reported at the SOC level included onset time data. The median onset time was identified as 13 days with an interquartile range (IQR) of 0–59 days, predominantly occurring on the first day post-initiation. The distribution of time-to-onset (TTO) for bendamustine-associated AEs was as follows: most occurred within the first month of treatment (n = 2,836, 58.78%), with diminishing frequencies in the second (n = 418, 8.66%) and third months (n = 258, 5.35%). Notably, approximately 11.09% of AEs (n = 535) were reported 1 year after initiating treatment with bendamustine (Figure 3). The cumulative proportion of these TTOs is depicted in Figure 4. Besides, as outlined in Table 3, the results revealed variable onset times for bendamustine-associated AEs across different SOCs. In the WSP analysis, all shape parameters β were less than 1, demonstrating that all AE signals in the SOC level had early failure types.
Previous studies on bendamustine have predominantly focused on its mechanism of action, clinical trials, and literature reviews, with limited real-world research. Our study leverages the largest sample of real-world data to date, assessing post-marketing pharmacovigilance to identify new and significant AEs and evaluate the post-marketing safety of bendamustine for rational drug use.
The AEs associated with bendamustine were more commonly reported in males (57.73%) than in females (42.27%). A higher proportion of AEs occurred in elderly patients (n = 4,439, 59.04% of patients over 65 years old), likely due to the prevalence of B-cell lymphoma in middle-aged and older adults (Cesaretti et al., 2018). The clinical trial SEQUOIA (Tam et al., 2022) reported common hematological AEs such as decreased lymphocytes (87% incidence), neutrophils (83%), leukocytes (83%), and CD4 lymphocytes (77%), with Grade 3/4 decreases occurring in 77%–87% of patients. Nonhematologic AEs included nausea (73%), infusion-related reactions (63%), and constipation (50%), with severe AEs like febrile neutropenia and cytomegalovirus enterocolitis also reported, as described in the drug description of the package insert, and our study confirmed it.
Our analysis revealed that bendamustine induced AEs in 17 SOCs, including six systems not listed in the drug’s package insert. The most impacted was the immune system, followed by the kidney and urinary system. Notably, hypogammaglobulinemia presented the strongest signal (n = 44, χ2 = 2,274.55), with reports of persistent hypogammaglobulinemia in indolent NHL patients treated with bendamustine and rituximab (Sepulcri et al., 2021; Suzuki et al., 2022). Additionally, though nephrogenic diabetes insipidus had fewer reports (n = 4, χ2 = 117.48), it showed significant signal strength, with cases presenting symptoms like polyuria and low urinary osmolality shortly after treatment (Uwumugambi et al., 2016; Derman BA et al., 2017; Desjardins et al., 2022).
This comprehensive real-world analysis underscores the importance of monitoring for both expected and novel AEs in patients receiving bendamustine, enhancing our understanding of its safety profile and informing clinical practice.
It's worth noting that bendamustine most significantly prompted signals of infections, including bacterial and viral infections and viral activation. Due to the compromised immune function commonly observed in tumor patients, who often undergo radiation therapy, chemotherapy, and immunotherapy, it is challenging to distinctly attribute the high incidence of infections to therapy-induced lymphopenia or hypogammaglobulinemia. These infections may also stem from the underlying disease itself, suggesting a multifactorial etiology (Perriguey Sanchez-Gonzalez et al., 2021). Consequently, whether it's a bendamustine-induced ADR or the patient’s own underlying condition, physicians are advised to vigilantly monitor patients’ vital signs and immunoglobulin levels to preempt potential infections during bendamustine treatment is of the utmost importance.
Moreover, our study also found about severe signals of anaphylactic reaction induced by bendamustine(n = 22, χ2 = 36.10). Several reports have also highlighted bendamustine-induced anaphylactic reactions, presenting symptoms such as throat discomfort, pruritus, hives, general erythema, and facial swelling within 8 hours of administration (Sanchez-Gonzalez Bilò and Barbarroja-Escudero, 2014). Anaphylaxis represents a severe emergency medical condition that can escalate rapidly, potentially leading to death within minutes (Bilò et al., 2021). These reactions are not currently listed in the bendamustine’s package insert, posing a risk of being overlooked in clinical practice.
Conversely, while the package insert for bendamustine mentions psychiatric disorders and skeletal muscle-related AEs, our study did not detect active signals for these conditions. Only a few reports have linked bendamustine with AEs affecting these systems. Zimmer P et al. (Zimmer et al., 2015) observed that patients treated with bendamustine combined with rituximab reported a decline in cognitive perception, fatigue, and emotional functioning compared to those on conventional chemotherapy regimens (cyclophosphamide, doxorubicin, vincristine, prednisone). Additionally, research by Esposito et al. (Esposito et al., 2023) indicated a potential reduction in bone mineral density in patients treated with a chemotherapy regimen that includes bendamustine. However, these AEs could also be attributable to the concomitant use of rituximab, complicating the isolation of bendamustine’s specific effects. Given the inherent biases in self-reported FAERS data, clinicians must exercise caution when interpreting these findings and applying them to patient care. The identification of new and significant AEs not listed in the drug’s insert underscores the necessity of continual vigilance in the clinical monitoring of bendamustine use.
Recent findings have heightened concerns regarding bendamustine, particularly its association with non-melanoma skin cancers and progressive multifocal leukoencephalopathy (PML). In March 2021, the Medicines and Healthcare Products Regulatory Agency (MHRA) issued a risk warning following an increase in reported cases, highlighting the need for added precautions in the drug’s usage (MHRA, 2021). Our study validated these concerns, ranking PML 17th among the top 30 AE signals for bendamustine (showed as Table 2), with a significant number of reports (n = 6, χ2 = 1988.96, 95% CI lower limit = 34.71%). This supports the European review’s recommendation to include these risks in the Summary of Product Characteristics (SmPC), advising periodically monitor patients for skin changes in patients using a bendamustine-containing regimen (Rosas Cancio-Suarez et al., 2020).
A clinical retrospective study (Warsch et al., 2012) showed that during the period analyzed (7 January 2018, to 6 January 2020), 42 cases of PML were reported globally, including 11 deaths, compared to 9 cases in the previous period (7 January 2017 to 6 January 2018). Notably, 31 of these cases reported bendamustine as the most recent treatment prior to PML onset, underscoring a probable link to the drug, despite concurrent administration of other medications like rituximab and obituzumab. Given these findings, it is crucial for clinicians to remain vigilant for new or worsening neurological, cognitive, or behavioral signs that may suggest PML. If PML is suspected, bendamustine should be withheld until PML is ruled out. Diagnostic evaluations for PML should include Magnetic Resonance Imaging (MRI), lumbar puncture (cerebrospinal fluid John H. K. Cunningham Virus DNA test), and other tests. (https://www.medicines.org.uk/emc/product/9844/smpc).
Additionally, our analysis identified 95 cases related to skin system diseases from the standard MedDRA analysis query (SMQ), which included cutaneous malignant tumors like basal cell carcinoma (n = 17, χ2 = 101.90), squamous cell carcinoma (n = 6, χ2 = 33.73), and other serious skin reactions (shown as Table 4). Nonetheless, data from two clinical trials (the BRIGHT and GALLIUM trials) (Hiddemann et al., 2018; Flinn, 2019) have shown that the number of cases of non-melanoma skin cancers was higher in patients treated with a bendamustine-containing regimen compared to patients treated with other lymphoma regimens. Bendamustine causes prolonged lymphopenia and depletion of CD4-positive T cells. This effect was more significant when it was combined with rituximab. Our findings are consistent with clinical trial data suggesting an elevated risk of non-melanoma skin cancers in patients treated with bendamustine-containing regimens.
These results suggest a need for enhanced patient education and regular skin monitoring for those on bendamustine, with specific attention to suspicious skin changes. This approach should be integrated into the summary of product characteristics and patient information leaflets to mitigate the risk of severe AEs associated with bendamustine treatment.
Our study corroborated earlier findings (Czuczman et al., 2015) showing that most AEs associated with bendamustine occur early in the treatment course, typically within the first 3 months, with a median onset time of 13 days. The WSP test indicated an early failure type profile for all bendamustine-associated AEs at the SOC level, suggesting an increased risk of AEs shortly after treatment initiation, which then diminishes over time. Nonetheless, some AEs manifested after prolonged treatment, highlighting the need for extended follow-up in future clinical studies.
AE signal detection addresses the limitations of drug package inserts, such as delays in reporting, uncertainty, and incompleteness, by leveraging extensive data from spontaneous AE report databases to better reflect the drug’s safety profile (Feng et al., 2023). However, the reliance on the FAERS database, which primarily includes U.S. reports, introduces potential biases (Mai et al., 2015). Notably, the spontaneous nature of the reports often results in missing data, such as patient demographics, and the results may not be universally applicable due to population differences (Yin et al., 2022). Additionally, while methods like the ROR and PRR are straightforward, they are susceptible to false positives under certain conditions. Our analysis could not adjust for multiple confounding factors, such as concurrent drug use and patient comorbidities, which could influence AE outcomes.
Moreover, while the AE signals identified suggest an association between bendamustine use and potential AEs, they do not establish causality. This underscores the necessity for further post-marketing clinical trials to confirm these associations and to enhance the understanding of bendamustine’s safety profile.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
DL: Writing–review and editing, Writing–original draft, Data curation. YZ: Funding acquisition, Writing–review and editing, Methodology. JQN: Writing–review and editing, Data curation. JZ: Writing–review and editing. WTL: Writing–review and editing, Methodology. YLC: Writing–review and editing, Software. LeiC: Writing–review and editing, Methodology. YQW: Writing–review and editing, Data curation. QJL: Writing–review and editing, Data curation. JW: Writing–review and editing, Formal Analysis, Data curation. YBL: Writing–review and editing, Formal Analysis. JC: Writing–review and editing, Writing–original draft, Methodology, Formal Analysis, Data curation. LiC: Writing–review and editing, Writing–original draft, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Bijie Science and Technology Bureau Joint Major Project(2023)2-1, Joint project of Bijie Science and Technology Bureau (2023) NO.66.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahdi, H. S., Wichelmann, T. A., Pandravada, S., and Ehrenpreis, E. D. (2023). Medication-induced osteonecrosis of the jaw: a review of cases from the food and drug administration adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 24 (1), 15. doi:10.1186/s40360-023-00657-y
Almenoff, J. S., Pattishall, E. N., Gibbs, T. G., DuMouchel, W., Evans, S. J., and Yuen, N. (2007). Novel statistical tools for monitoring the safety of marketed drugs. Clin. Pharmacol. Ther. 82 (2), 157–166. doi:10.1038/sj.clpt.6100258
Arulogun, S. O., Brian, D., Goradia, H., Cooney, A., Menne, T., Koo, R., et al. (2023). Bendamustine plus rituximab for the treatment of Waldenström Macroglobulinemia: patient outcomes and impact of bendamustine dosing. Am. J. Hematol. 98 (5), 750–759. doi:10.1002/ajh.26895
Bate, A., and Evans, S. J. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 18 (6), 427–436. doi:10.1002/pds.1742
Bilò, M. B., Martini, M., Tontini, C., Corsi, A., and Antonicelli, L. (2021). Anaphylaxis. Eur. Ann. Allergy Clin. Immunol. 53 (1), 4–17. doi:10.23822/EurAnnACI.1764-1489.158
Brucato, A., Maestroni, S., Masciocco, G., Ammirati, E., Bonacina, E., and Pedrotti, P. (2015). Cardiac involvement in Churg-Strauss syndrome. G. Ital. Cardiol. (Rome) 16 (9), 493–500. doi:10.1714/1988.21524
Cesaretti, M., Loustau, M., Robba, C., Senescende, L., and Zarzavadjian Le Bian, A. (2018). Reappraisal of primary hepatic lymphoma: is surgical resection underestimated? Crit. Rev. Oncol. Hematol. 123, 1–6. doi:10.1016/j.critrevonc.2018.01.004
Chen, C., Wu, B., Zhang, C., and Xu, T. (2021). Immune-related adverse events associated with immune checkpoint inhibitors: an updated comprehensive disproportionality analysis of the FDA adverse event reporting system. Int. Immunopharmacol. 95, 107498. doi:10.1016/j.intimp.2021.107498
Chiappini, S., Vickers-Smith, R., Harris, D., Papanti Pelletier, G. D., Corkery, J. M., Guirguis, A., et al. (2023). Is there a risk for semaglutide misuse? Focus on the food and drug administration's FDA adverse events reporting system (FAERS) pharmacovigilance dataset. Pharm. (Basel). 16 (7), 994. doi:10.3390/ph16070994
Czuczman, M. S., Goy, A., Lamonica, D., Graf, D. A., Munteanu, M. C., and van der Jagt, R. H. (2015). Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: efficacy, tolerability, and safety findings. Ann. Hematol. 94 (12), 2025–2032. doi:10.1007/s00277-015-2478-9
Derman, B. A., Jain, M., McAninch, E. A., and Gashti, C. (2017). Bendamustine-induced nephrogenic diabetes insipidus. Clin. Nephrol. 87 (1), 47–50. doi:10.5414/CN108908
Desjardins, A., Le-Nguyen, V., Turgeon-Mallette, L., Vo, C., Boudreault, J. S., Rioux, J. P., et al. (2022). Bendamustine-induced nephrogenic diabetes insipidus - a case report. J. Oncol. Pharm. Pract. 28 (1), 205–210. doi:10.1177/10781552211013878
Du, J., Yu, D., Han, X., Zhu, L., and Huang, Z. (2021). Comparison of allogeneic stem cell transplant and autologous stem cell transplant in refractory or relapsed peripheral T-cell lymphoma: a systematic review and meta-analysis. JAMA Netw. Open 4 (5), e219807. doi:10.1001/jamanetworkopen.2021.9807
Esposito, F., Pascale, M. R., Tesei, C., Gigliotti, P. E., Luciano, A., Angeloni, C., et al. (2023). Body composition in patients with follicular lymphoma: asso-ciations between changes in radiomic parameters in patients treated with R-CHOP-like and R-B regimens: LyRa 01F. Cancers (Basel) 15 (4), 999. doi:10.3390/cancers15040999
Feng, G., Zhou, X., Chen, J., Li, D., and Chen, L. (2023). Platinum drugs-related safety profile: the latest five-year analysis from FDA adverse event reporting system data. Front. Oncol. 12, 1012093. doi:10.3389/fonc.2022.1012093
Flinn, I. W., van der Jagt, R., Kahl, B., Wood, P., Hawkins, T., MacDonald, D., et al. (2019). First-Line Treatment of patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma with bendamustine plus rituximab versus R-CHOP or R-CVP: results of the BRIGHT 5-year follow-up study. J. Clin. Oncol. 37, 984–991. doi:10.1200/JCO.18.00605
Flinn, I. W., van der Jagt, R., Kahl, B. S., Wood, P., Hawkins, T. E., Macdonald, D., et al. (2014). Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 123 (19), 2944–2952. doi:10.1182/blood-2013-11-531327
Gao, Y., Liu, Y., Wang, Y., Zhang, Q., Wu, D., Ye, X., et al. (2023). Bendamustine in the treatment of patients with indolent non-Hodgkin lymphoma refractory or relapse to rituximab treatment: an open-label, single-agent, multicenter study in China. Cancer 129 (4), 551–559. doi:10.1002/cncr.34544
GOV.UK (2021). Bendamustine (Levact): increased risk of non-melanoma skin cancer and progressive multifocal encephalopathy (PML). Available at: https://www.gov.uk/drug-safety-update/bendamustine-levact-increased-risk-of-non-melanoma-skin-cancer-and-progressive-multifocal-encephalopathy-pml.
Gravel, C. A., Krewski, D., Mattison, D. R., Momoli, F., and Douros, A. (2023). Concomitant use of statins and sodium-glucose co-transporter 2 inhibitors and the risk of myotoxicity reporting: a disproportionality analysis. Br. J. Clin. Pharmacol. 89 (8), 2430–2445. doi:10.1111/bcp.15711
Hiddemann, W., Barbui, A. M., Canales, M. A., Cannell, P. K., Collins, G. P., Dürig, J., et al. (2018). Immunochemotherapy with obinutuzumab or rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. J. Clin. Oncol. 36, 2395–2404. doi:10.1200/JCO.2017.76.8960
Kinoshita, S., Hosomi, K., Yokoyama, S., and Takada, M. (2020). Time-to-onset analysis of amiodarone-associated thyroid dysfunction. J. Clin. Pharm. Ther. 45 (1), 65–71. doi:10.1111/jcpt.13024
Lalic, H., Aurer, I., Batinic, D., Visnjic, D., Smoljo, T., and Babic, A. (2022). Bendamustine: a review of pharmacology, clinical use and immunological effects (Review). Oncol. Rep. 47 (6), 114. doi:10.3892/or.2022.8325
Mai, F., Tomoya, H., Kouichi, H., and Takada, M. (2015). Association between statin use and cancer: data mining of a spontaneous reporting database and a claims database. Int. J. Med. Sci. 12 (3), 223–233. doi:10.7150/ijms.10656
Mazhar Chiappini, F., Battini, V., Gringeri, M., Pozzi, M., Mosini, G., Marran, A. M. N., et al. (2021). The impact of anti-TNFα agents on weight-related changes: new insights from a real-world pharmacovigilance study using the FDA adverse event reporting system (FAERS) database. Expert Opin. Biol. Ther. 21 (9), 1281–1290. doi:10.1080/14712598.2021.1948529
Merryman, R. W., Edwin, N., Redd, R., Bsat, J., Chase, M., LaCasce, A., et al. (2020). Rituximab/bendamustine and rituximab/cytarabine induction therapy for transplant-eligible mantle cell lymphoma. Blood Adv. 4 (5), 858–867. doi:10.1182/bloodadvances.2019001355
Moore, T. J., Morrow, R. L., Dormuth, C. R., and Mintzes, B. (2020). US food and drug administration safety advisories and reporting to the adverse event reporting system (FAERS). Pharm. Med. 34 (2), 135–140. doi:10.1007/s40290-020-00329-w
Palumbo, A., Offidani, M., Patriarca, F., Petrucci, M. T., and Cavo, M. (2015). Bendamustine for the treatment of multiple myeloma in first-line and relapsed-refractory settings: a review of clinical trial data. Leuk. Lymphoma 56 (3), 559–567. doi:10.3109/10428194.2014.915545
Perriguey Sanchez-Gonzalez, M., Maarouf, A., Stellmann, J. P., Rico, A., Boutiere, C., Demortiere, S., et al. (2021). Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol. Neuroimmunol. Neuroinflamm 9 (1), e1115. doi:10.1212/nxi.0000000000001115
Rosas Cancio-Suárez, M., Barbolla Díaz, I., and González-García, A. (2020). Progressive multifocal leukoencephalopathy in the course of combined therapy with bendamustine and rituximab. Med. Clin. Barc. 155 (11), 513–514. doi:10.1016/j.medcli.2019.07.010
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Sanchez-Gonzalez Bilò, M. J., Barbarroja-Escudero, J., Antolin-Amerigo, D., Marco-Martin, G., Alvarez-De-Mon, M., and Rodriguez-Rodriguez, M. (2014). Immunological reactions induced by bendamustine. Clinical&Translational Allergy 4 (3 Suppl. l), P56. doi:10.1186/2045-7022-4-s3-p56
Sepulcri, C., Dentone, C., Mikulska, M., Bruzzone, B., Lai, A., Fenoglio, D., et al. (2021). The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient-A case study. Open Forum Infect. Dis. 8 (11), ofab217. doi:10.1093/ofid/ofab217
Shotton, R., Broadbent, R., Alchawaf, A., Mohamed, M. B., Gibb, A., Martinez-Calle, N., et al. (2024). Safety of bendamustine for the treatment of indolent non-Hodgkin lymphoma: a UK real-world experience. Blood Adv. 8 (4), 878–888. doi:10.1182/bloodadvances.202301130
Shu, Y., Wang, L., Ding, Y., and Zhang, Q. (2023). Disproportionality analysis of abemaciclib in the FDA adverse event reporting system: a real-world post-marketing pharmacovigilance assessment. Drug Saf. 46 (9), 881–895. doi:10.1007/s40264-023-01334-z
Suzuki, D. M., Koyama, D., Ikeda, S., Sukegawa, M., Teshirogi, M., Misawa, K., et al. (2022). Serum IgG and lymphocyte counts are useful for the early detection of infection in patients receiving bendamustine-rituximab therapy. J. Clin. Exp. Hematop 62 (2), 91–98. doi:10.3960/jslrt.21031
Tam, C. S., Brown, J. R., Kahl, B. S., Ghia, P., Giannopoulos, K., Jurczak, W., et al. (2022). Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. 23 (8), 1031–1043. doi:10.1016/S1470-2045(22)00293-5
Uchida Trociukas, M., Mori, Y., Akiba, K., Miyasaka, M., Hirano, T., Ikesue, H., et al. (2020). Risk factors for skin toxicities associated with bendamustine-based chemotherapy in patients with non-hodgkin lymphoma. Biol. Pharm. Bull. 43 (10), 1577–1582. doi:10.1248/bpb.b20-00428
Uwumugambi, N. A., Sanchorawala, V., Shelton, A. C., Stern, L., and Gordon, C. E. (2017). Bendamustine-induced nephrogenic diabetes insipidus in a patient with AL amyloidosis. Am. J. Kidney Dis. 69 (2), 317–319. doi:10.1053/j.ajkd.2016.07.037
Wang, M., Munoz, J., Goy, A., Locke, F. L., Jacobson, C. A., Hill, B. T., et al. (2023). Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J. Clin. Oncol. 41 (3), 555–567. doi:10.1200/JCO.21.02370
Wang, M. L., Jurczak, W., Jerkeman, M., Trotman, J., Zinzani, P. L., Belada, D., et al. (2022). Ibrutinib plus bendamustine and rituximab in untreated mantle-cell lymphoma. N. Engl. J. Med. 386 (26), 2482–2494. doi:10.1056/NEJMoa2201817
Warsch, S., Hosein, P. J., Maeda, L. S., Alizadeh, A. A., and Lossos, I. S. (2012). A retrospective study evaluating the efficacy and safety of bendamustine in the treatment of mantle cell lymphoma. Leuk. Lymphoma 53 (7), 1299–1305. doi:10.3109/10428194.2011.649476
Yao, X., Tsang, T., Sun, Q., Quinney, S., Zhang, P., Ning, X., et al. (2020). Mining and visualizing high-order directional drug interaction effects using the FAERS database. BMC Med. Inf. Decis. Mak. 20 (Suppl. 2), 50–65. doi:10.1186/s12911-020-1053-z
Yi, J. H., Kim, S. J., Lee, J. O., Lee, G. W., Kwak, J. Y., Eom, H. S., et al. (2022). Bendamustine plus rituximab for mantle cell lymphoma: a Korean, multicenter retrospective analysis. Anticancer Res. 42 (12), 6083–6089. doi:10.21873/anticanres.16120
Yin, Y., Shu, Y., Zhu, J., Li, F., and Li, J. (2022). A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci. Rep. 12 (1), 19555. doi:10.1038/s41598-022-23834-1
Zhang, Q., Ding, Y., Shu, Y., and Chen, J. (2023). A real-world disproportionality analysis of rucaparib: post-marketing pharmacovigilance data. BMC Cancer 23 (1), 745–758. doi:10.1186/s12885-023-11201-w
Zhao, H., Li, J. M., Li, Z. R., Zhang, Q., Zhong, M. K., Yan, M. M., et al. (2023). Major adverse cardiovascular events associated with testosterone treatment: a pharmacovigilance study of the FAERS database. Front. Pharmacol. 14, 1182113. doi:10.3389/fphar.2023.1182113
Zimmer, P., Mierau, A., Bloch, W., Strüder, H. K., Hülsdünker, T., Schenk, A., et al. (2015). Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk. Lymphoma 56 (2), 347–352. doi:10.3109/10428194.2014.915546
Keywords: adverse events, data mining, post-marketing risk analysis, FAERS, pharmacovigilance, bendamustine
Citation: Li D, Zhang Y, Ni JQ, Zhu J, Lu WT, Chen YL, Cheng L, Wang YQ, Li QJ, Wang J, Lu YB, Chen J and Chen L (2024) Post-marketing risk analysis of bendamustine: a real-world approach based on the FAERS database. Front. Pharmacol. 15:1372401. doi: 10.3389/fphar.2024.1372401
Received: 18 January 2024; Accepted: 29 April 2024;
Published: 13 May 2024.
Edited by:
Anoop Kumar, Delhi Pharmaceutical Sciences and Research University, IndiaReviewed by:
Shirley D'Sa, University College London Hospitals NHS Foundation Trust, United KingdomCopyright © 2024 Li, Zhang, Ni, Zhu, Lu, Chen, Cheng, Wang, Li, Wang, Lu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Chen, NzUxNDMzNjA0QHFxLmNvbQ==; Li Chen, Y2hlbmxfaHhleUBzY3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.