
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 15 April 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1370444
This article is part of the Research TopicInnovative approaches to drug discovery in musculoskeletal diseaseView all 6 articles
 Yanling Wang1
Yanling Wang1 Shijie Yin1,2
Shijie Yin1,2 Shixiong Wang1,2
Shixiong Wang1,2 Kuan Rong3
Kuan Rong3 Xiang-He Meng1
Xiang-He Meng1 Huashan Zhou1
Huashan Zhou1 Luo Jiao1
Luo Jiao1 Da Hou1
Da Hou1 Zhongjing Jiang4*
Zhongjing Jiang4* Jun He1*
Jun He1* Zenghui Mao1
Zenghui Mao1Introduction: The escalating global surge in Rifampicin-resistant strains poses a formidable challenge to the worldwide campaign against tuberculosis (TB), particularly in developing countries. The frequent reports of suboptimal treatment outcomes, complications, and the absence of definitive treatment guidelines for Rifampicin-resistant spinal TB (DSTB) contribute significantly to the obstacles in its effective management. Consequently, there is an urgent need for innovative and efficacious drugs to address Rifampicin-resistant spinal tuberculosis, minimizing the duration of therapy sessions. This study aims to investigate potential targets for DSTB through comprehensive proteomic and pharmaco-transcriptomic analyses.
Methods: Mass spectrometry-based proteomics analysis was employed to validate potential DSTB-related targets. PPI analysis confirmed by Immunohistochemistry (IHC) and Western blot analysis.
Results: The proteomics analysis revealed 373 differentially expressed proteins (DEPs), with 137 upregulated and 236 downregulated proteins. Subsequent Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses delved into the DSTB-related pathways associated with these DEPs. In the context of network pharmacology analysis, five key targets—human leukocyte antigen A chain (HLAA), human leukocyte antigen C chain (HLA-C), HLA Class II Histocompatibility Antigen, DRB1 Beta Chain (HLA-DRB1), metalloproteinase 9 (MMP9), and Phospholipase C-like 1 (PLCL1)—were identified as pivotal players in pathways such as “Antigen processing and presentation” and “Phagosome,” which are crucially enriched in DSTB. Moreover, pharmaco-transcriptomic analysis can confirm that 58 drug compounds can regulate the expression of the key targets.
Discussion: This research confirms the presence of protein alterations during the Rifampicin-resistant process in DSTB patients, offering novel insights into the molecular mechanisms underpinning DSTB. The findings suggest a promising avenue for the development of targeted drugs to enhance the management of Rifampicin-resistant spinal tuberculosis.
Tuberculosis (TB) is a communicable disease resulting from Mycobacterium tuberculosis (M. tuberculosis) infection (Nahid et al., 2016; Sable et al., 2019). The global impact of TB in 2021 was staggering, with an estimated 10.6 million people falling ill, equating to 134 cases per 100,000 population. Geographically, South-East Asia accounted for 45% of TB cases, underscoring its pervasive presence (Bagcchi, 2023). Within the spectrum of TB cases, 10% manifest as extrapulmonary TB, with half affecting the musculoskeletal system. The spine, comprising 1%–2% of cases, emerges as the most prevalent site in extrapulmonary TB (Dunn and Ben Husien, 2018).
However, Rifampicin resistance in M. tuberculosis, the causative agent of tuberculosis (TB), is a growing global concern that has significant implications for public health. Studies have indicated that over 90% of tuberculosis cases resistant to Rifampicin also exhibit resistance to isoniazid, establishing Rifampicin resistance as a reliable surrogate indicator for multidrug-resistant TB (MDR-TB) (Morgan et al., 2005; Cavusoglu et al., 2002).The emergence of drug-resistant strains of M. tuberculosis, particularly MDR-TB and extensively drug-resistant TB (XDR-TB), presents a formidable challenge to TB control efforts (Bagcchi, 2023). The proliferation of drug-resistant TB strains not only complicates treatment but also heightens mortality rates, amplifies healthcare costs, and intensifies the risk of further transmission (Zumla et al., 2015). Thus, this sparked interest in exploring the mechanism of Rifampicin-resistant for new multidrug-resistant therapy.
The crux of drug resistance in clinical M. tuberculosis (MTB) strains predominantly resides in chromosomal mutations, precipitating several pivotal mechanisms. These mechanisms include alterations in drug targets through genetic changes, reducing susceptibility to anti-TB drugs. Overexpression of drug targets further diminishes drug effectiveness, while disruptions in prodrug activation impede the conversion of prodrugs into active forms within bacterial cells (Lata et al., 2015; de Keijzer et al., 2016; Banaei-Esfahani et al., 2017). Additionally, the activation of efflux pumps, specialized transport proteins expelling drugs from bacterial cells, contributes to resistance against anti-TB drugs (Miotto et al., 2018). However, the challenge of diminishing drug resistance or discovering novel and effective treatments for Rifampicin-resistant spinal tuberculosis (TB) or extensively drug-resistant TB (XDR-TB) persists without a clear solution.
In this study, our primary focus was to identify potential targets based on proteomics and explore the mechanism of Rifampicin-resistance using pharmaco-transcriptomic analyses. The graphical abstract of our study was presented in Figure 1.
We have secured the proper approval from the Ethics Committee of Hunan Academy of Traditional Chinese Medicine Affiliated Hospital for our study. The investigation involved the examination of forty cases of spinal tuberculosis lesion tissues, collected from the Hunan Academy of Traditional Chinese Medicine Affiliated Hospital. This dataset encompassed 20 patients with non Rifampicin-resistant spinal tuberculosis (NDSTB) forming the control group, and an additional 20 patients with Rifampicin-resistant spinal tuberculosis (DSTB) constituting the experimental group. Surgical removal of spinal tuberculosis lesions was performed on all patients between January 2020 and June 2023, adhering to the inclusion and exclusion criteria outlined in Table 1. Clinical information, including sex, age, history of tuberculosis, regular use of anti-tuberculosis drugs, and T-SPOT.TB test results (Table 2), and the site of spinal infection were meticulously extracted from medical records. After the surgeries, postoperative pathological examinations and culture results unequivocally confirmed the presence of MTB infection in the collected tissue samples. This robust confirmation served as the cornerstone for the study’s investigation into Rifampicin-resistance in spinal tuberculosis.
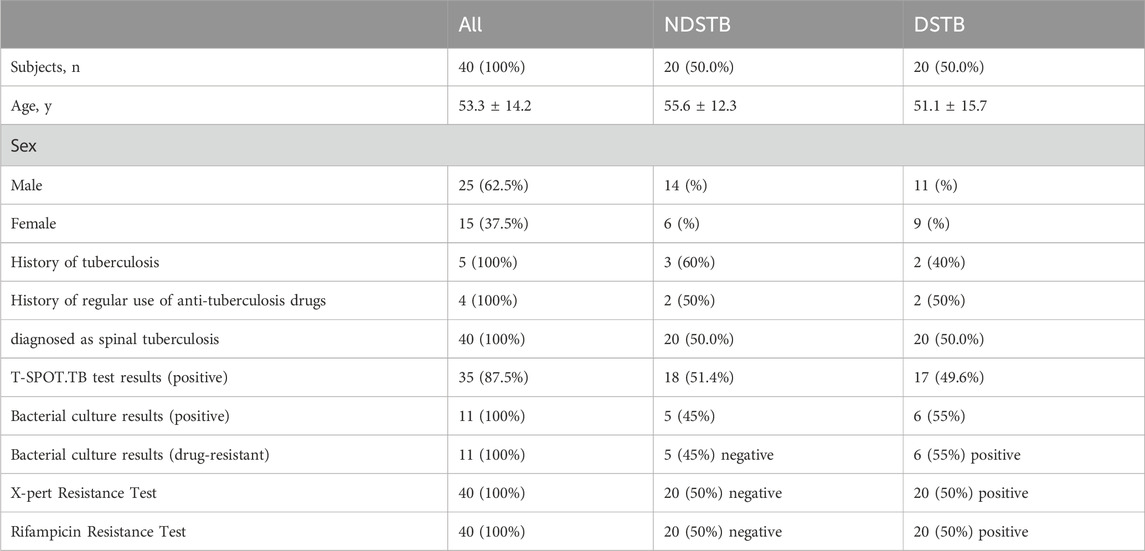
Table 2. Baseline characteristics of participants and comparison between NDSTB groups and DSTB group. Participants without a tuberculosis history or a regular use of anti-tuberculosis drugs were not documented. Additionally, any test results that went undetected were not recorded.
First, we obtained tissues we obtained within 30 min of surgical removal and the samples were rapidly frozen using liquid nitrogen. Then, the frozen tissue samples were preserved in a −80°C refrigerator for subsequent analysis. The tissues of spinal tuberculosis lesions were thawed on ice and then lysed with a lysis buffer (Beyotime, P0013J, Shanghai, China). Subsequently, we added 1% PMSF (Beyotime, ST507-10 mL) and 1% inhibitor cocktail (Roche, 4693116001, Basle, Switzerland) and lysed the mixture for 40 min. Further, the lysis samples were centrifuged for 25 min at 4°C, 12,000 g in a 1.5 mL EP tube. The supernatant was isolated and quantified protein concentration in a new tube.
The process of ultrafiltration (Microcon units, 30 kD) was repeated several times using 200 µL of UA buffer (8 M Urea, 150 mM Tris-HCl pH 8.0) in combination with centrifugation to remove the detergent and other low-molecular-weight components from the mixture. To block reduced cysteine residues, we added 100 µL of 0.05 M iodoacetamide in the UA buffer. Subsequently, the lucifugous precipitate was incubated for 20 min. To proceed, we had to properly wash the sample, so 25 mM NH4HCO3 was added twice for 100 µL. Next, Trypsin (Promega) was used to digest the protein suspension overnight at 37°C in 40 µL of 25 mM NH4HCO3. The filtrate peptides were concentrated with OD280 using a Nanodrop device.
The peptide mixtures underwent labeling with TMT reagents (Thermo Scientific, CA, United States) following the manufacturer’s instructions. Each aliquot (100 µg of peptide equivalent) was treated with one tube of TMT reagent. Subsequently, 100 µL of 0.05 M TEAB (pH 8.5) was added to the mixtures. The TMT reagents were dissolved in 41 µL of 100% acetonitrile (ACN) before being applied for labeling at room temperature. Following the addition of TMT reagents to the peptide mixtures, the combined solutions were incubated for 1 hour. To halt the reaction, 8 µL of 5% hydroxylamine was introduced and allowed to incubate for 15 min. The Multiplex labeled samples were then pooled together and subjected to lyophilization.
Sample labeled TMT fractionation was completed using Waters XBridge BEH130 column (C18, 3.5 μm, 2.1 × 150 mm) on an Agilent 1290 HPLC operating at 0.3 mL/min. We finished the elution of peptides using buffers (Buffer A: 10 mM ammonium formate; Buffer B: 10 mM ammonium formate with 90% ACN, PH 10) at a flow rate of 100 μL/min. For each peptide mixture, 30 fractions were collected, and they were concatenated into 15 final ones (pooling equal intervals of RPLC fractions). Elution materials were freeze-dried using vacuum centrifugation for LC–MS analysis (−20°C stored).
The lyophilized eluates underwent analysis using Easy nLC (Ultimate RSLC Nano, Thermo Scientific), equipped with a C18-reversed phase column (12 cm long, 75 μm ID, 3 μm). The sample was absorbed by buffer A (2% ACN and 0.1% Formic acid) and subsequently separated by buffer B (90% ACN and 0.1% Formic acid) at a flow rate of 300 nL/min for a duration of 90 min. The gradient used was as follows: 0–2 min, 2%–5% buffer B; 2–62 min, 5%–20% buffer B; 62–80 min, 20%–35% buffer B; 80–83 min, 35%–90% buffer B; 83–90 min, buffer B 90%. Data of MS was collected by dynamically selected the most abundant precursor ions from the survey scan (300–1800 m/z). This was followed by a full Orbitrap MS scan (AGC target: 1e6, maximum injection time: 50 ms), and data-dependent Orbitrap MS/MS scans (isolation window = 1.5, injection time: 100 ms, activation type: HCD, AGC target: 1e5, max.) with a dynamic exclusion duration of 30 s. The resolution for survey scans was set at 70,000 at m/z 200, and for HCD spectra, it was set at 35,000 at m/z 200. The collision energy normalized to 30 was measured, and the instrument was configured with the peptide recognition mode enabled.
Our data analysis was demonstrated with Proteome Discoverer Version 1.6.0.16 and an initial mass windows of 10 ppm were used as precursor. We properly followed the cleavage rules of Trypsin/P, so a maximum of two missed cleavage sites were allowed, as well as a mass tolerance of 20 ppm for fragment ions. Fixed modification: Carbamidomethyl (C), TMT10plex(K), TMT10plex (N-term), Variable modification: Oxidation(M), and Acetyl (Protein N-term).
Differentially significant expressed proteins (DEP) were screened using log2FC > 1 or log2FC ≤ −1; p < 0.05 as the threshold and protein-level hierarchical clustering was used to group expression data. We annotated the sequences using UniProtKB/Swiss-Prot, KEGG, and Gene Ontology (GO) and also conducted Fisher’s exact test to analyze GO and KEGG enrichment. All genes were ranked based on their degree of differential expression in the two groups of samples, followed by applying a statistical method to assess whether the predetermined set of genes was enriched at the top or bottom of the ranked list. Gene Set Enrichment Analysis (GSEA) involves three primary steps: calculating the Enrichment Score, estimating the significance level of the Enrichment Score, and conducting multiple hypotheses testing. GSEA utilizes both Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases. In the results of GO analysis, gene sets represent GO terms, while in KEGG analysis, gene sets represent individual pathways.
Key targets in Rifampicin-resistant spinal tuberculosis were identified by intersecting two disease-related pathways in WGCNA with DEPs (version 7.5.1). The obtained results were utilized to construct a protein-protein interaction network using the STRING database and visualized through Cytoscape (version 3.9.0).
To propose novel treatment strategies for Rifampicin-resistant spinal tuberculosis (TB), we performed a pharmaco-transcriptomic analysis using the Integration of the Drug–Gene Interaction Database (DGIdb 4.0). The DGIdb database (version 4.0) integrates information on drug-gene interactions and druggable genes sourced from publications, databases, and other web-based resources (Freshour et al., 2021). It serves as a comprehensive, publicly accessible web database. Finally, we utilized Cytoscape to assess and visualize the impact of drug molecule metabolism on the regulation of genes.
Focal tissues from all patients in each group were immersed in 4% paraformaldehyde and subsequently embedded in paraffin. The paraffin-embedded slices underwent a 30-min incubation at 100°C. Following this, the samples were sequentially treated with 100% xylene, 95% xylene, 95% ethanol, 95% ethanol, and 100% ethanol, each for 5 min. Antigen repair of the slices was performed using citrate buffer. The tissues were then exposed to blocking buffer (20% heat-inactivated sheep serum, SP KIT-B2) at room temperature for 1 hour. Subsequently, they were incubated with primary antibodies (HLA-DRB1 (proteintech, 15862-1-AP), HLA-A (proteintech, 55383-1-AP), HLA-C (proteintech, 15777-1-AP), MMP9 (proteintech, 10375-2-AP), PLCL1 (Abcam, ab190225)) at a 1:50 dilution. Following a PBS wash for 5 min four times, the secondary antibody was applied for 1 hour. Once developed with DAB chromogen and counterstained with hematoxylin, the samples were observed under a microscope.
The protein samples were loaded into wells containing an SDS-PAGE gel, and electrophoresis was carried out. A solution of TBS was used to prepare a 5% skimmed milk powder and 5% BSA protein blocking solution. After successful membrane transfer, the PVDF membrane was labeled and then incubated with the blocking solution. The blocking process was performed under gentle shaking at room temperature for 1–2 h. Subsequently, the PVDF membrane was incubated overnight at 4°C with the primary antibody solution (diluted with the appropriate diluent from Biyuntian Company, PLCL1 (Abcam, ab157200) at 1:1000, other primary antibodies at 1:1000). Following incubation, the PVDF membrane was washed with TBST solution at room temperature and then incubated with the secondary antibody. An appropriate amount of ECL luminous solution was added, and the target bands were detected using a chemiluminescence imaging system (CIIC). Images were captured, and the gray value of the protein bands was calculated using ImageJ software.
The statistical analysis of our dataset was performed in SPSS 26 and GraphPad Prism 7. All the numerical data were expressed as mean ± SD. Comparisons between groups were conducted using ANOVA with Newman–Keuls post hoc tests and t-tests. Statistical significance was defined as p < 0.05.
In the NDSTB group, there were a total of 20 participants, including 6 females and 14 males, with an average age of 55.6 ± 12.3 years. The DSTB group consisted of 9 females and 11 males, with a mean age of 51.1 ± 15.7 years (Table 2). The results showed no significant difference in age and sex between the two groups (p = 0.117, p = 0.408). All participants underwent questioning and testing for radiographic manifestations, T-SPOT.TB, Bacterial culture, X-pert Resistance Test, and Rifampicin Resistance Test in hospitals, with statistics recorded accordingly to the actual situation. Additionally, radiographic manifestations of spinal tuberculosis were documented and compared with those of a normal spine (Figure 2).
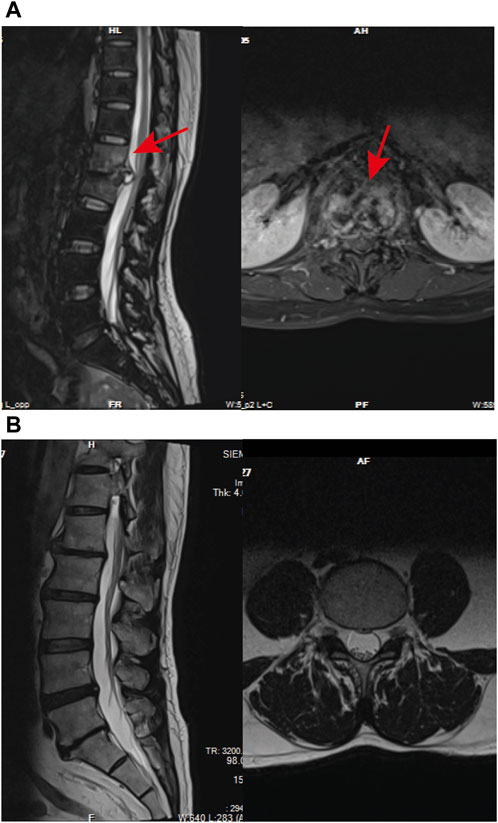
Figure 2. Radiographic manifestations of spinal tuberculosis. (A) Radiographic manifestations of spinal tuberculosis (indicated by arrows). (B) Imaging features of a normal spine.
The protein expression profile in lesion locations from study participants with NDSTB and DSTB was investigated using TMT-based proteomics. This analysis aimed to assess the underlying mechanisms associated with Rifampicin-resistant spinal tuberculosis.
We identified the sum of 24,160 peptides after conducting a TMT-based proteomic (Supplementary Table S1). According to the filter conditions, 373 differentially expressed proteins (DEPs) in NDSTB and DSTB samples were present in Figure 3, extracted from 5033 identified proteins (Supplementary Table S2, S3). Furthermore, we also demonstrated 137 upregulated proteins (log2FC ≤ −1) and 236 downregulated proteins (log2FC > 1, p < 0.05) between the NDSTB and DSTB groups.
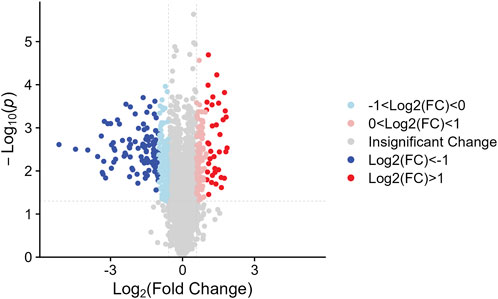
Figure 3. Two groups gomparison: Differential analysis showed that 373 were differentially expressed between the two groups with 236 downregulated proteins (log2FC ≤ −1) and 137 upregulated proteins (log2FC > 1, p < 0.05).
As per the results demonstrated in this study, NDSTB and DSTB samples exhibited various differentially expressed proteins (DEPs). Analysis of the subcellular localization showed that DEPs were diffusely distributed throughout the cytoplasm, extracellular region, lysosomes, Golgi apparatus, centrosome, membrane, mitochondrion, nuclear membrane, nucleolus, ribosome, and endoplasmic reticulum (Figure 4).
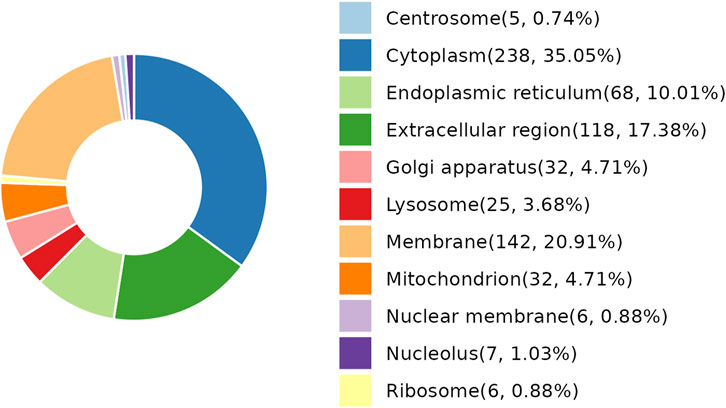
Figure 4. Subcellular localization analysis of DEPs. 25 DEPs were distributed in lysosomes, accounting for 3.68%. 238 DEPs were distributed in Cytoplasm, accounting for 35.05%.
Functional enrichment analysis demonstrated that the DEPs were primarily involved in the immune system process and response to stimulus, which might be associated with the Rifampicin-resistant mechanism of spinal tuberculosis induced by the immune system process (Figure 5A). Notably, five proteins annotated with the term human leukocyte antigen, A Chain ((HLA-A), human leukocyte antigen, C Chain ((HLA-C), HLA Class II Histocompatibility Antigen, DRB1 Beta Chain (HLA-DRB1), metalloproteinase 9 (MMP9) and Phospholipase C-like 1 (PLCL1) were significantly changed compared with NDSTB (Supplementary Table S4). KEGG pathway analysis demonstrated that DEPs were mainly associated with “Antigen processing and presentation” and “Phagosome” (Figure 5B). These findings contribute novel perspectives to our understanding of the etiology and comorbidities associated with drug-resistant spinal tuberculosis.
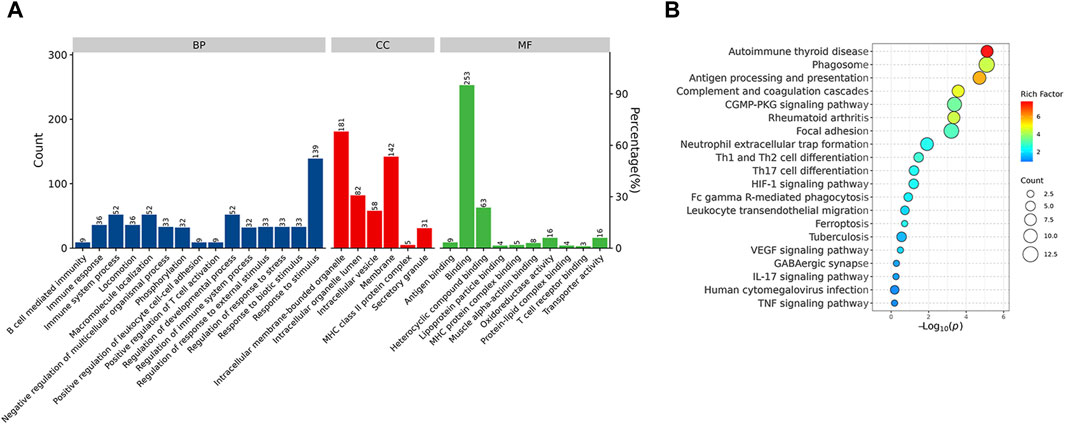
Figure 5. (A) BP, the cellular component category (CC), the molecular function category (MF) phantom enrich mentanalysis of DEPs. The left vertical coordinate is count (number of differential proteins annotated to the term), and the right vertical coordinate is percentage (number of differential proteins annotated to the term/total number of differential proteins with GO annotations). (B) KEGG enrichment pathway analysis of DEPs. The abscissa represents the negative logarithmic transformation of the Enrichment factor, and the ordinate represented the specific path. The column color indicates -log10 (p-value). Specific counts and p-values are presented on the right side of the column. KEGG, Kyoto Protocol Encyclopedia of Genes and Genomes; DEPs, differentially expressed proteins.
Through KEGG signaling pathways analysis, it was revealed that HLA-DRB1, HLA-A, HLA-C, MMP9, and PLCL1 among the 25 DEPs were linked to “Antigen processing and presentation” and “Phagosome” pathways (as depicted in Figure 6A). To further exprole, results revealed that the expression of MMP9 and PLCL1 were remarkably decreased in the focal tissues of DSTB, compared with NDSTB. Contrarily, the HLA-A, HLA-C, and HLA-DRB1 were significantly increased in the focal tissues of DSTB, compared with NDSTB (Figures 6B,C), suggesting that five proteins (HLA-DRB1, HLA-A, HLA-C, MMP9 and PLCL1) may be involved in Rifampicin-resistant STB.
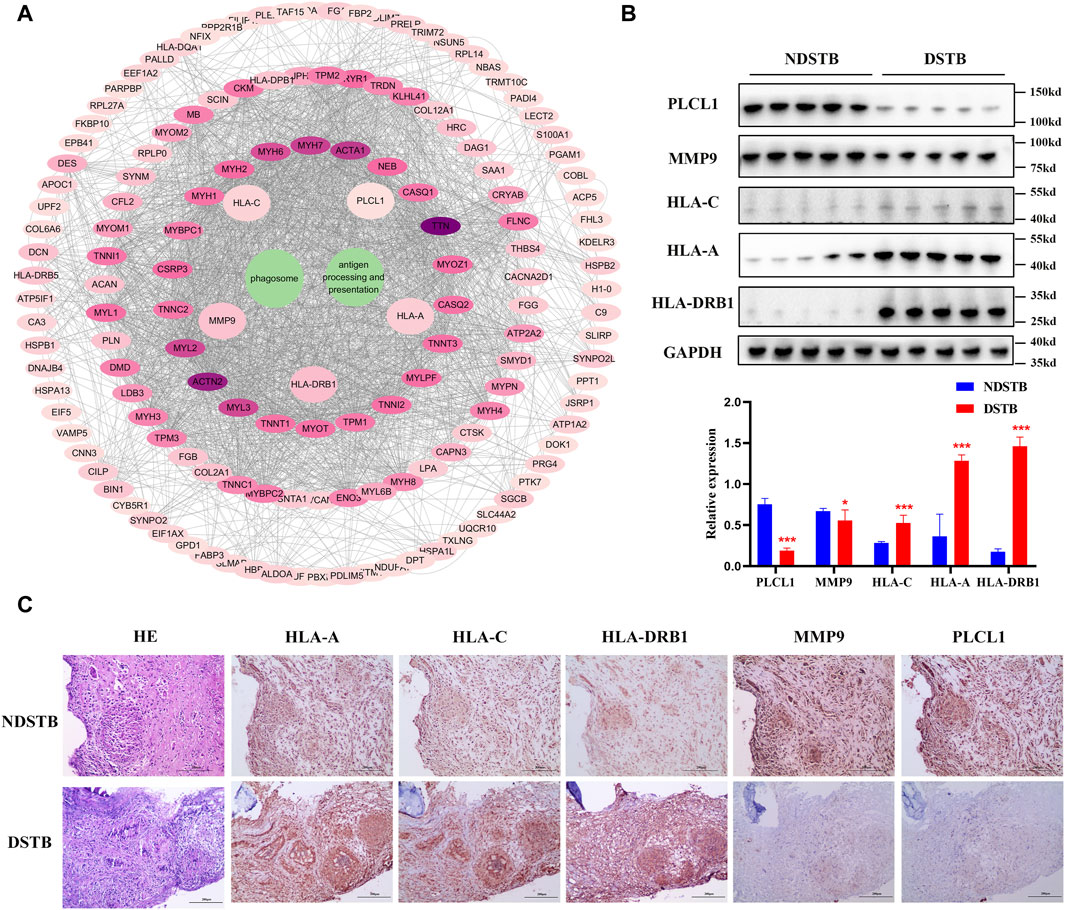
Figure 6. (A) “DEPs-drug-resistant spinal TB- “Antigen processing and presentation “pathway -“Phagosome” pathway -DEPs-Targets” network. Protein-protein interaction network using STRING. A PPI of all detected DEPs. The significantly enriched pathways (p < 0.05) and protein/protein coding gene and protein-protein interaction network associated with the pathways. The innermost circle is 5 of the key proteins of DEPs. The darker the color, the greater the degree value. The criteria for sub-setting was the more and denser the edges, the more central the protein was. The innermost five key proteins in the circle are most relevant to these two pathways. These proteins are particularly relevant to the mentioned pathways, with green highlighting their involvement in “Antigen processing and presentation” and “Phagosome.” (B) Relative expression of MMP9, PLCL1, HLA-A, HLA-C and HLA-DRB1 measured using the tissue protein of DSTB and NDSTB were tested by Western blot (n = 5). Statistically significant differences between groups were determined using a Student’s t-test or two-tailed one-way analysis of variance, followed by a Student-Newman-Keuls test; *p < 0.05. (C) IHC staining of tissue in the focal tissues of DSTB (n = 20) and NDSTB (n = 20) for MMP9, PLCL1, HLA-A, HLA-C and HLA-DRB1. scale bars = 200 µm.
Based on the above results, HLA-A, HLA-C, HLA-DRB1, MMP9 and PLCL1 may be potential therapeutic targets for DSTB. Therefore, the results of our pharmaco-transcriptomic analysis can confirm that 13 drug compounds (e.g., Floxacillin, Clavulanic, and Amoxicillin) can regulate the expression of HLA-C. Similarly, there were 12 kinds of drug compounds found regulating MMP9, such as Celecoxib, Marimastat, and Andecaliximab. Moreover, 15 kinds of drug compounds (Terbinafine, Zonisamide, Ticlopidine, etc.) regulated the expression of HLA-A. In addition, Flupirtine, Fluvastatin, Methimazole, and other 18 drug compounds were confirmed to regulate the HLA-DRB1 (Figure 7). These experimental results will help us provide new insights into the treatment of DSTB.
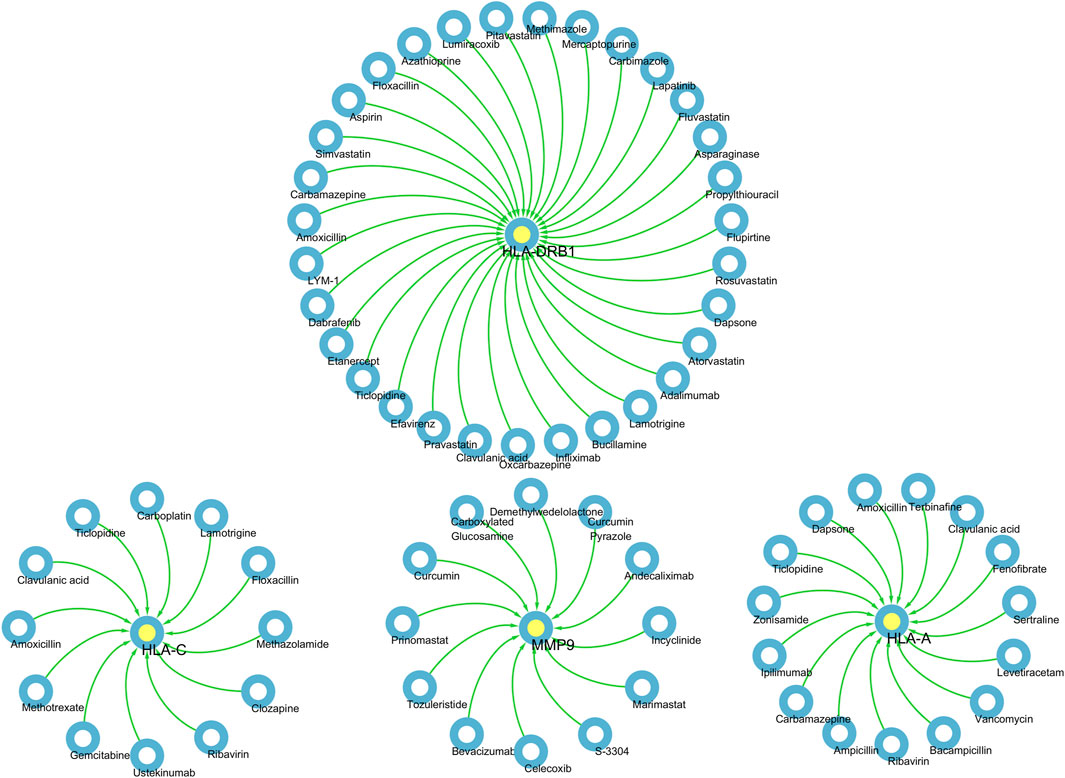
Figure 7. We performed pharmaco-transcriptomics analysis by the results of key proteins for the PPI network.pharmaco-transcriptomic analysis used the Integration of the Drug–Gene Interaction Database (DGIdb 4.0).
In this study, TMT labeled Quantitative Proteomicswas employed to scrutinize the protein expression profiles of focal tissues in DSTB patients as compared to NDSTB. The identified signaling pathways, specifically “Antigen processing and presentation” and “Phagosome,” linked to DSTB, shed light on potential mechanisms underlying Rifampicin-resistance. Furthermore, five proteins (HLA-DRB1, HLA-A, HLA-C, MMP9 and PLCL1) were identified to be potential targets for DSTB.
It is worth noting that drug resistance of patients with spinal TB underlines at a higher rate under clinical use of four first-line drug combinations than theoretically predicted (Gao et al., 2016). Nahid et al. highlighted the choice and number of drugs significantly impact the speed of drug resistance spread, and exproled the mechanism of drug-resistant STB was extremely urgent (Nahid et al., 2019). Notably, our screening process identified five key proteins, HLA-DRB1, HLA-A, HLA-C, MMP9, and PLCL1, with distinct upregulation or downregulation in Rifampicin-resistant STB patients. Recent research has demonstrated that the HLA class II presentation of Mycobacterium bacterial antigens to CD4+ cells is one of the most crucial biological steps regarding the outcome of the infection (Magira et al., 2012), CD8 T lymphocytes recognize bacteria exposed at the membrane of infected cells. In addition, our study revealed the involvement of HLA-DRB1, HLA-A, HLA-C in the signaling pathways of antigen processing and presentation, suggesting their potential as targets by CD4+ for STB Rifampicin-resistance. MMP9 was reported closely involved in immune inflammation. Several cell types, including epithelial cells, macrophages, fibroblasts, and neutrophils, produce pro-MMP9 (Van den Steen et al., 2002). Therefore, MMP9-inhibiting monocytes can suppress the invasion of T lymphocytes into the walls of blood vessels, causing decreasing the density of an immune response that leads to vasculitis (Watanabe et al., 2018). These results indicate that HLA-DRB1, HLA-A, HLA-C, MMP9, and PLCL1 may play pivotal roles in the mechanism of Rifampicin-resistant spinal TB, potentially serving as diagnostic markers and promising therapeutic targets for DSTB.
Interestingly, HLA-DRB1, HLA-A and HLA-C were significantly increased in DSTB group, but MMP9 and PLCL1 were substantially decreased in DSTB group. The pharmaco-transcriptomic analysis identified various drugs (Floxacillin, Clavulanic, Ustekinumab and so on) capable of regulating the expression of these proteins. Ustekinumab, a human monoclonal antibody, was kown as a target inhibition of interleukin-12 and −23. Moreover, Morelli et al., 2022 demonstrated that interleukin-12/23 was related to HLA-C, since they preferentially present (auto) antigens, for example the metalloprotease (MMP) domain, to CD8 T cells. Our paper demonstrated that Ustekinumab could regulate the protein of HLA-C, which might be one of the mechanisms of Ustekinumab-induced upregulated risk of Rifampicin-resistant of spinal TB disease.
Moreover, our study faced certain limitations. Initially, the sample size was insufficient. Instead of conducting an analysis on a larger scale, we utilized only twenty pairs of 40 samples for the protein park test, which proved to be inadequate. Additionally, further validation is required through cell and animal experiments to bolster the findings of this study.
To sum up, the involvement of HLA-DRB1, HLA-A, HLA-C, MMP9, and PLCL1 in the mechanism of Rifampicin-resistant spinal tuberculosis is evident. These factors may serve as crucial components in both diagnosis and potential therapeutic targeting for Rifampicin-resistant spinal TB.
The datasets presented in this study can be found in online repositories (https://www.scidb.cn/en/detail?dataSetId=64acfda7ef7546e694e45476c4766720&version=V1)/Supplementary Material.
The studies involving humans were approved by the Ethics Committee of Hunan Academy of Traditional Chinese Medicine Affiliated Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YW: Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Validation, Writing–original draft, Writing–review and editing. SY: Conceptualization, Data curation, Investigation, Writing–original draft. SW: Data curation, Formal Analysis, Methodology, Writing–original draft. KR: Conceptualization, Formal Analysis, Methodology, Resources, Writing–review and editing. XM: Data curation, Formal Analysis, Methodology, Visualization, Writing–review and editing. HZ: Data curation, Formal Analysis, Methodology, Writing–review and editing. LJ: Data curation, Formal Analysis, Methodology, Writing–review and editing. DH: Data curation, Methodology, Supervision, Writing–review and editing. ZJ: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Writing–original draft, Writing–review and editing. JH: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Visualization, Writing–original draft, Writing–review and editing. ZM: Funding acquisition, Investigation, Writing–original draft, Resources, Methodology.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was provided by grants from the Changsha Chinese Medicine Research (B202314).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor ZP declared a past co-authorship with the author YW.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1370444/full#supplementary-material
Bagcchi, S. (2023). WHO's global tuberculosis report 2022. Lancet. Microbe 4, e20. doi:10.1016/S2666-5247(22)00359-7
Banaei-Esfahani, A., Nicod, C., Aebersold, R., and Collins, B. C. (2017). Systems proteomics approaches to study bacterial pathogens: application to Mycobacterium tuberculosis. Curr. Opin. Microbiol. 39, 64–72. doi:10.1016/j.mib.2017.09.013
De Keijzer, J., Mulder, A., De Haas, P. E. W., De Ru, A. H., Heerkens, E. M., Amaral, L., et al. (2016). Thioridazine alters the cell-envelope permeability of Mycobacterium tuberculosis. J. Proteome Res. 15, 1776–1786. doi:10.1021/acs.jproteome.5b01037
Dunn, R. N., and Ben Husien, M. (2018). Spinal tuberculosis: review of current management. Bone and Jt. J. 100-B, 425–431. doi:10.1302/0301-620X.100B4.BJJ-2017-1040.R1
Freshour, S. L., Kiwala, S., Cotto, K. C., Coffman, A. C., Mcmichael, J. F., Song, J. J., et al. (2021). Integration of the drug-gene interaction database (DGIdb 4.0) with open crowdsource efforts. Nucleic Acids Res. 49, D1144–D1151. doi:10.1093/nar/gkaa1084
Gao, J., Ma, Y., Du, J., Zhu, G., Tan, S., Fu, Y., et al. (2016). Later emergence of acquired drug resistance and its effect on treatment outcome in patients treated with Standard Short-Course Chemotherapy for tuberculosis. BMC Pulm. Med. 16, 26. doi:10.1186/s12890-016-0187-3
Lata, M., Sharma, D., Deo, N., Tiwari, P. K., Bisht, D., and Venkatesan, K. (2015). Proteomic analysis of ofloxacin-mono resistant Mycobacterium tuberculosis isolates. J. Proteomics 127, 114–121. doi:10.1016/j.jprot.2015.07.031
Magira, E. E., Papasteriades, C., Kanterakis, S., Toubis, M., Roussos, C., and Monos, D. S. (2012). HLA-A and HLA-DRB1 amino acid polymorphisms are associated with susceptibility and protection to pulmonary tuberculosis in a Greek population. Hum. Immunol. 73, 641–646. doi:10.1016/j.humimm.2012.03.008
Miotto, P., Zhang, Y., Cirillo, D. M., and Yam, W. C. (2018). Drug resistance mechanisms and drug susceptibility testing for tuberculosis. Respirol. Carlt. Vic. 23, 1098–1113. doi:10.1111/resp.13393
Morelli, M., Galluzzo, M., Scarponi, C., Madonna, S., Scaglione, G. L., Girolomoni, G., et al. (2022). Allelic variants of HLA-C upstream region, PSORS1C3, MICA, TNFA and genes involved in epidermal homeostasis and barrier function influence the clinical response to anti-IL-12/IL-23 treatment of patients with psoriasis. Vaccines (Basel) 10, 1977. doi:10.3390/vaccines10111977
Nahid, P., Dorman, S. E., Alipanah, N., Barry, P. M., Brozek, J. L., Cattamanchi, A., et al. (2016). Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. 63, e147–e195. doi:10.1093/cid/ciw376
Nahid, P., Mase, S. R., Migliori, G. B., Sotgiu, G., Bothamley, G. H., Brozek, J. L., et al. (2019). Treatment of drug-resistant tuberculosis. An official ATS/CDC/ERS/IDSA clinical practice guideline. Am. J. Respir. Crit. Care Med. 200, e93–e142. doi:10.1164/rccm.201909-1874ST
Sable, S. B., Posey, J. E., and Scriba, T. J. (2019). Tuberculosis vaccine development: progress in clinical evaluation. Clin. Microbiol. Rev. 33, e00100-19. doi:10.1128/CMR.00100-19
Van den Steen, P. E., Dubois, B., Nelissen, I., Rudd, P. M., Dwek, R. A., and Opdenakker, G. (2002). Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 37, 375–536. doi:10.1080/10409230290771546
Watanabe, R., Maeda, T., Zhang, H., Berry, G. J., Zeisbrich, M., Brockett, R., et al. (2018). MMP (matrix metalloprotease)-9-producing monocytes enable T cells to invade the vessel wall and cause vasculitis. Circ. Res. 123, 700–715. doi:10.1161/CIRCRESAHA.118.313206
Keywords: DSTB, NDSTB, HLA, DEPs, MMP9, PLCL1
Citation: Wang Y, Yin S, Wang S, Rong K, Meng X-H, Zhou H, Jiao L, Hou D, Jiang Z, He J and Mao Z (2024) Proteomics study the potential targets for Rifampicin-resistant spinal tuberculosis. Front. Pharmacol. 15:1370444. doi: 10.3389/fphar.2024.1370444
Received: 14 January 2024; Accepted: 11 March 2024;
Published: 15 April 2024.
Edited by:
Zhihong Peng, Hubei University, ChinaReviewed by:
Haroon Kalam, Broad Institute, United StatesCopyright © 2024 Wang, Yin, Wang, Rong, Meng, Zhou, Jiao, Hou, Jiang, He and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun He, Y25oamRvY0AxMjYuY29t; Zhongjing Jiang, emhvbmdqaW5nQGNzdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.