
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 27 May 2024
Sec. Pharmacology of Infectious Diseases
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1367686
Background: The therapeutic effects of vitamin D supplementation on Coronavirus disease 2019 (COVID-19) aggravation remain controversial and inconclusive. To probe into this contentious issue, we performed the present meta-analysis of randomized controlled trials (RCTs).
Methods: Literature published up to June 2023 was retrieved from Cochrane Library, PubMed, Web of Science and Embase. RCTs assessing mortality, intensive care unit (ICU) admission, mechanical ventilation (MV), length of hospitalization (LOH), and inflammatory markers containing C-reactive protein (CRP), D-dimer, interleukin-6 (IL-6), lactate dehydrogenase (LDH) were included. 19 RCTs were involved in the analysis and were conducted subgroup analyses on the baseline COVID-19 severity and vitamin D administration.
Results: In the severity subgroup, statistically significant effects in moderate to severe group were observed in ICU admission (OR 0.43, 95% CI 0.23, 0.80; p = 0.008), MV (OR 0.44, 95% CI 0.27, 0.72; p = 0.001) and LOH (SMD –0.49, 95% CI –0.92, −0.06; p = 0.027). In the administration subgroup, effects of ICU admission (OR 0.39, 95% CI 0.16, 0.97; p = 0.044), MV (OR 0.18, 95% CI 0.07, 0.46; p = 0.000) and LOH (SMD –0.50, 95% CI –0.96, −0.04; p = 0.034) were more pronounced in patients supplied with multiple-dose vitamin D than single-dose. Although the result of mortality showed no statistically significant effect, it indicated a reduced trend (OR 0.87, 95% CI 0.63, 1.12; p > 0.05). The results of inflammatory markers reached no statistical differences.
Conclusion: This meta-analysis revealed that moderate to severe COVID-19 patients supplied with multiple doses of vitamin D were less apt to need ICU admission, mechanical ventilation and have shorter hospital stays.
A downward trend of the Coronavirus disease 2019 (COVID-19) outbreak can be witnessed throughout the world in 2023, but the COVID-19 pandemic has not gone away, with an estimated 767 million confirmed cases and 6.9 million fatalities up to June 2023, according to epidemiological data on the Coronavirus Dashboard of World Health Organization (World Health Organization, 2023). COVID-19, due to the highly infectious SARS-CoV-2 virus, is a respiratory disease of which symptoms range from mild, moderate, and even severe and critical (COVID-19 Treatment Guidelines Panel, 2023). Despite the perception that COVID-19 is primarily a respiratory illness, certain research suggested that the nutritional status of infected individuals may influence the progression of COVID-19 (Li et al., 2021; Silverio et al., 2021). Reportedly, vitamin D insufficiency has come forth as a potential but modifiable risk factor with important implications, and vitamin D’s significance in lowering the severity and incidence of COVID-19 is increasingly established (Im et al., 2020). Observational studies that underwent meta-analyses (Ben-Eltriki et al., 2022; Wang et al., 2022) revealed that COVID-19 patients had noticeably lower vitamin D concentrations in serum and greater odds of SARS-CoV-2 infection, and worse prognosis than healthy controls. Meanwhile, low vitamin D levels are closely tied to rising inflammatory marker levels (Hopefl et al., 2022). In the period of COVID-19, inadequate intake of vitamin D and the status of hypovitaminosis D has developed into public health concern that requires addressing.
During COVID-19, numerous randomized controlled trials (RCTs) have been stimulated to elucidate whether additional intake of vitamin D could prevent COVID-19 aggravation. However, the results were mixed, with some studies claiming statistically significant protective benefits and others reporting null results. Up to this point, published meta-analyses of non-RCTs on this topic account for a larger portion, whereas the number of RCTs in meta-analyses of vitamin D supplementation and COVID-19 is fairly limited. In the meta-analysis with 6 RCTs by Varikasuvu et al. (Varikasuvu et al., 2022), COVID-19 patients supplemented with vitamin D showed fewer rates but no statistically significant differences of ICU admission and mortality, which was consistent with another meta-analysis with 8 RCTs by Kümmel et al. (Kümmel et al., 2022). Intriguingly, the pooled analyses of ICU admission reached statistical significance in the meta-analysis with 9 RCTs by Zaazouee et al. (Zaazouee et al., 2023) and the meta-analysis with 9 RCTs and 14 non-RCTs by Hosseini et al. (Hosseini et al., 2022). Hence, it is urgent to conduct a new meta-analysis of RCTs with a larger sample size to collect emerging evidence and to provide more convincing and valuable information. In addition, evidence supporting the therapeutic effects of vitamin D supplementation with different doses (single dose/multiple doses) on COVID-19 patients with different severity (particularly in mild to moderate/moderate to severe COVID-19 patients) is still not entirely inconclusive. Based on these factors, we aim to collect updated published RCTs and conduct a meta-analysis with larger sample sizes to better illustrate the connection between COVID-19 and vitamin D supplementation. A high focus will be placed on the following investigative questions: 1) in COVID-19 patients with different severity, is vitamin D supplementation a new approach to mitigate the risk of mortality, ICU admission, and mechanical ventilation, and to reduce length of hospitalization and levels of inflammatory markers? 2) in terms of administration, could single-high-dose vitamin D improve the curative effect of COVID-19 in comparison with multiple-dose vitamin D?
This meta-analysis was performed and reported in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Page et al., 2021).
The research problem was put forward by the principal investigator (YWX). With the database including PubMed, EMBASE, Web of Science, and Cochrane Library, a thorough retrieval of relevant and available literature published up to 19 June 2023, was performed independently by two investigators (YYY and WLS). The search strategy is shown detailedly in Additional Supplementary Table S1. After deduplication, the title and abstract of each retrieved literature were evaluated independently by two co-authors (FY and SPS) to exclude articles that were not related to our study. Any differences were reconciled by consensus or by another two reviewers (GXZ and XYL).
To better establish the framework of the research questions and seek evidence, we used the PICOS strategy (patient, intervention, comparison, outcome, study). Finally, we adopted the inclusion as follows: 1) inpatients or outpatients diagnosed with COVID-19, severity at baseline ranged from asymptomatic, mild, moderate, and severe; no limitations on age, gender, or ethnicity; 2) comparing administration of single-dose or multiple-dose vitamin D to placebo or standardized therapy for COVID-19; no limitations on the route of administration, duration of medication, and type of vitamin D; 3) reporting baseline COVID-19 severity, endpoints including ICU admission, mortality, mechanical ventilation, length of hospitalization, and inflammatory markers before and after the intervention; 4) randomized controlled trials published with no restriction in language; exclusion criteria as follows: 1) pregnant or lactating women; 2) taking vitamin D supplementation before/at the recruiting time; 3) types of clinical trials other than RCT such as retrospective studies, observational studies, and pilot protocols.
Prespecified primary outcomes were the following events encompassing need for ICU admission and MV, mortality in COVID-19 patients. The secondary outcomes were length of hospitalization and changes in the levels of inflammatory markers encompassing CRP, D-dimer, IL-6, and LDH.
Two reviewers (YYY and WLS) independently collected the eligible data from the included RCTs using a pre-designed table. Data comprised the source of study, publication year, location, study design, number of participants, baseline characteristics of participants (mean age, sex, vitamin D status), details between the intervention group and control group, and duration of follow-up. Discrepancies were settled by clear consensus. When the mean values and standard deviations (SD) of the provided outcomes were presented indirectly, we manage to derive the desired value by using an estimation formula based on the given numerical values, such as median, range, sample size, and quartile (Wan et al., 2014; Luo et al., 2016).
Two reviewers (FY and GXZ) independently conducted the quality assessment of the included studies, by the use of Cochrane Collaboration’s bias risk tool. The risk of bias for each domain was categorized as low, high, or unclear by the criteria of the Cochrane Handbook of Systematic Reviews (Higgins et al., 2011). To evaluate potential publication bias, we combined the visual perception of the funnel plot and the values of Egger’s test when the number of included studies was at least 10 (Mavridis and Salanti, 2014). We defined significant publication bias as asymmetric funnel plots or the p-value of Egger’s test <0.05. When the funnel plot asymmetry was caused by significant publication bias, we applied the trim and filling method to make the adjustment.
In this meta-analysis of RCTs, we performed all our analyses by applying Stata 17.0 (StataCorp, College Station, TX, USA). Treatment effects were summarized as odd ratios (OR) with 95% confidence intervals (CI) for dichotomous outcomes and standard mean differences (SMD) with 95% CI for continuous outcomes. Besides, we used the I2 statistic to identify the heterogeneity across studies, and we viewed I2 > 50% as statistically significant heterogeneity. When significant statistical heterogeneity was noted, we reported OR/SMD using the random effects model. When I2 < 50%, we used the fixed effects model. In conducting all the analyses, we considered the result reaching statistical significance if the p-value <0.05. Regarding sensitivity analysis, we undertook a one-study leave-out method for each outcome by eliminating one RCT at a time and by analyzing repeatedly.
According to the search strategy, we initially identified 780 articles. After removing duplicates and non-RCTs (reviews, meta-analyses, protocols, case reports, Mendelian randomization studies, etc.), 203 articles remained eventually. Of them, 153 articles were filtered as irrelevant articles after viewing the titles and abstracts; 50 articles were assessed for eligibility; 31 articles were excluded for the below reasons: articles retracted (n = 2), lack of relevant outcomes (n = 17), no vitamin D intervention (n = 7), unexpected study design (n = 3) and baseline COVID-19 severity is severe to critical (n = 2). After undergoing the above screening, 19 RCTs were included in the final meta-analysis. The flowchart diagram of this study selection is displayed in Figure 1.
The main characteristics of the 19 RCTs (Castillo et al., 2020; Maghbooli et al., 2021; Murai et al., 2021; Sabico et al., 2021; Sánchez-Zuno et al., 2021; Annweiler et al., 2022; Cannata-Andía et al., 2022; Cervero et al., 2022; De Niet et al., 2022; Elamir et al., 2022; Fernandes et al., 2022; Karonova et al., 2022; Mariani et al., 2022; Rastogi et al., 2022; Said et al., 2022; Sarhan et al., 2022; Soliman et al., 2022; Torres et al., 2022; Zurita-Cruz et al., 2022) are summarized in Table 1, with a total of 2,435 participants incorporated. Of these 19 RCTs, there are 7 RCTs making comparisons between the effects of vitamin D and placebo, 7 RCTs between the effects of vitamin D and standard of care, and 5 RCTs between the effects of different dosages of vitamin D supplementation. The majority of studies took Cholecalciferol as an intervention, and the majority of participants are vitamin D-deficient and even vitamin D-insufficient at baseline. Vitamin D was dispensed with a single-high dose or multiple doses. The baseline severity of COVID-19 patients varied across the included studies. According to the National Institutes of Health classification (COVID-19 Treatment Guidelines Panel, 2023), COVID-19 infection was categorized into mild disease (defined as non-pneumonia and pneumonia cases, such as mild respiratory symptoms and fever), moderate disease (defined as a lower respiratory disease in clinical evaluation or medical imaging manifestations and the pulse oxygen saturation (SpO2) ≥ 94% on indoor air at sea level), severe disease (defined as SpO2 < 94% on indoor air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mmHg, breathing rate >30 times/min, or pulmonary infiltration >50%), and critical disease (defined as respiratory failure, infectious shock, and/or multiple organ dysfunction). We conducted two subgroup analyses based on the baseline severity of COVID-19 (mild to moderate group, moderate to severe group) and administration of vitamin D (single-dose group, multiple-dose group).
The funnel plots and the results of Egger’s test, generated from the data of the included RCTs in the meta-analysis, are listed in Additional Figures 1–3. It was important to note that Egger’s test of ICU admission in the subgroup of severity and administration (p = 0.026), mortality in subgroup of administration (p = 0.046), showed significant publication bias. However, after further analyses with the trim-and-fill method, the publication bias did not impact the estimates (no trimming performed and no data changed), indicating that publication bias had little effect and verifying the robustness of our results (Duval and Tweedie, 2004). Cochrane Collaboration’s bias risk tool was used to assess the quality of the methodology of included RCTs. The quality assessment of included studies is summarized in Figure 2. Among the 19 RCTs, 47.4% were assessed as high risk of bias, which could be largely attributed to ambiguity blinding setting and possible selective reporting from multiple outcomes. Likewise, 26.3% were assessed as unclear of risk bias, mainly due to problems in the implementation of blinding. 26.3% were assessed as low risk of bias.
Pooled results calculated by Stata display as shown in Tables 2, 3. Analyses of primary outcomes (ICU admission, mechanical ventilation, mortality) and secondary outcomes (length of hospitalization, inflammatory markers) were assessed, disclosing results as described below.
Thirteen studies including 305 individuals in the vitamin D and the control group reported the events of ICU admission with a total rate of 17.2%. Patients’ need for ICU admission occurred at a rate of 13.6% in the intervention group and 20.9% in the control group, respectively. Patients in the vitamin D group had a decreased probability of COVID-19 progression and a lower frequency of requiring intensive care (OR: 0.49; 95% CI: 0.30, 0.79; p = 0.003; I2 = 55.2%, p = 0.008) (Figure 3A). Notably, in terms of illness severity of COVID-19 patients, comparing moderate to severe with mild to moderate in subgroup analysis, the former showed a lower frequency of ICU admission (OR: 0.43; 95% CI: 0.23, 0.80; p = 0.008; I2 = 66.3%, p = 0.002) (Figure 3A), while the latter showed higher frequency (OR: 0.64; 95% CI: 0.32, 1.28; p = 0.202; I2 = 0.0%, p = 0.934) (Figure 3A). Additionally, subgroup analysis was conducted with eleven studies according to the administration of vitamin D. With the result reaching a statistically significant effect, ICU admission in multiple-dose group (OR: 0.39; 95% CI: 0.16, 0.97; p = 0.044; I2 = 70.0%, p = 0.002) (Figure 3B) is much less frequent than single-dose group (OR: 0.67; 95% CI: 0.40, 1.14; p = 0.140; I2 = 0.0%, p = 0.546) (Figure 3B).
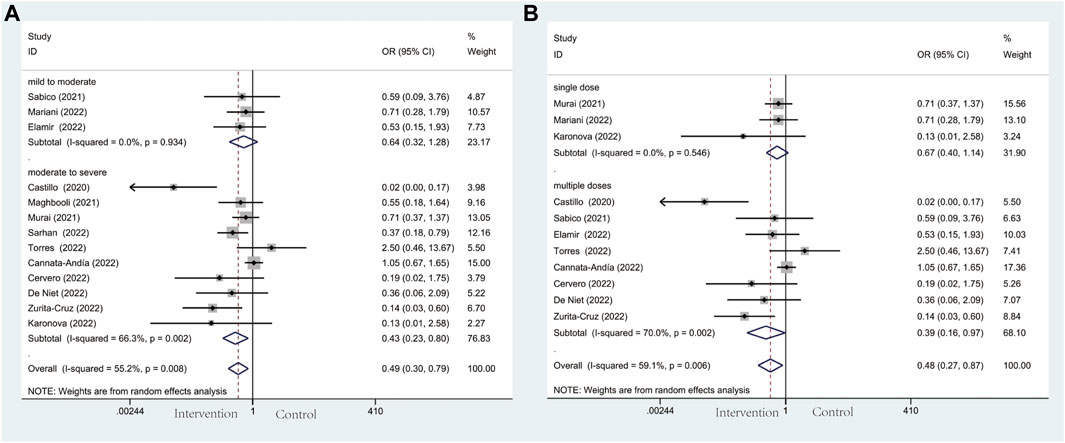
Figure 3. Forest plots of RCT for the association of vitamin D supplementation and ICU admission in the severity subgroup (A) and administration subgroup (B).
Modalities of mechanical ventilation can be both invasive and non-invasive. The proportion of need for MV in the vitamin D group and the control groups was 21.1% versus 27.6%. Compared with the control group, vitamin D supplementation with lower odds of undergoing mechanical ventilation could be observed (OR: 0.46; 95% CI: 0.29, 0.72; p = 0.001; I2 = 6.0%, p = 0.385) (Figure 4A). Results of subgroup analysis, same as ICU admission, represented more beneficial effects in patients with moderate to severe COVID-19 (OR: 0.44; 95% CI: 0.27, 0.72; p = 0.001; I2 = 20.1%, p = 0.276) (Figure 4A) than in mild to moderate group (OR: 0.58; 95% CI: 0.19, 1.73; p = 0.327; I2 = 0.0%, p = 0.411) (Figure 4A). What’s more, patients who accepted multiple doses of vitamin D (OR: 0.18; 95% CI: 0.07, 0.46; p = 0.000; I2 = 0.0%, p = 0.948) (Figure 4B) are less likely to need MV than those supplied with a single high dose of vitamin D (OR: 0.59; 95% CI: 0.32, 1.07; p = 0.080; I2 = 0.0%, p = 0.821) (Figure 4B).
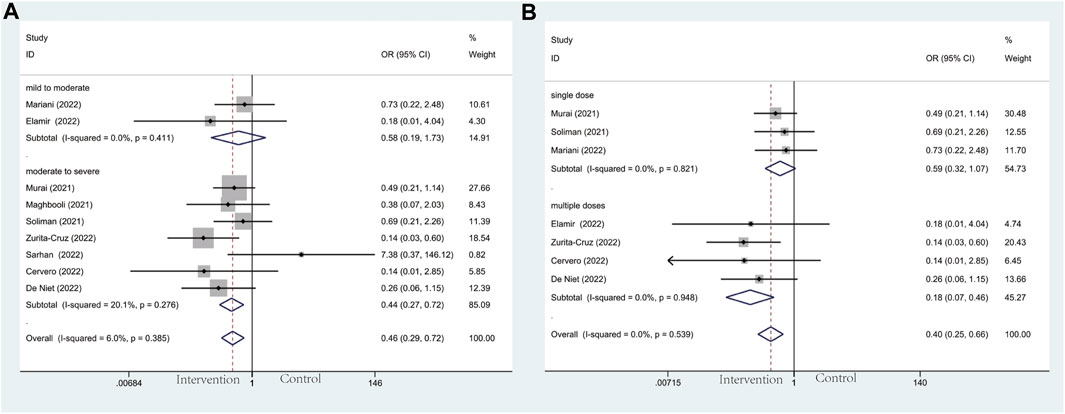
Figure 4. Forest plots of RCT for the association of vitamin D supplementation and mechanical ventilation in the severity subgroup (A) and administration subgroup (B).
For mortality, seventeen trials including 2,175 patients reported this clinical outcome. No deaths occurred at three of these seventeen trials. The death rate in the vitamin D and the control group was 7.6% versus 8.4%. Out of line with our hypothesis, the results we performed were not statistically significant in the subgroup analysis of baseline COVID-19 severity (OR: 0.87; 95% CI: 0.63, 1.22; p = 0.425; I2 = 0.0%, p = 0.532) (Figure 5A). However, the results of severity subgroup demonstrated a trend of declining mortality in the intervention group intriguingly. Among these results in detail, no effects were observed in mild to moderate group (OR: 1.09; 95% CI: 0.36, 3.32; p = 0.881; I2 = 34.9.%, p = 0.215) (Figure 5A) and moderate to severe group (OR: 0.86; 95% CI: 0.60, 1.21; p = 0.377; I2 = 0.0%, p = 0.573) (Figure 5A). Similarly, in another subgroup of vitamin D administration, no significant effects were observed in the single-dose group (OR: 1.47; 95% CI: 0.68, 3.17; p = 0.322; I2 = 0.0%, p = 0.719) (Figure 5B) and multiple-dose group (OR: 0.91; 95% CI: 0.54, 1.53; p = 0.718; I2 = 12.1%, p = 0.335) (Figure 5B).
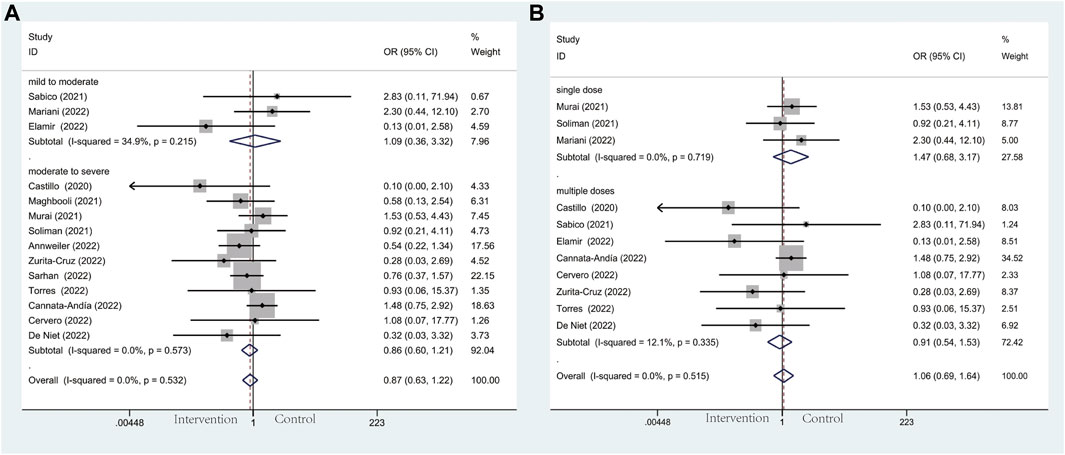
Figure 5. Forest plots of RCT for the association of vitamin D supplementation and mortality in the severity subgroup (A) and administration subgroup (B).
Regarding the length of hospitalization, it was the secondary outcome of this meta-analysis. Based on the observation of the severity subgroup, patients in the moderate to severe group tend to have shorter hospital stays than those in controls (SMD: −0.49; 95% CI: −0.92, −0.06; p = 0.027; I2 = 78.7%, p = 0.003) (Figure 6A), whereas mild to moderate group did not reach statistically significant effect (SMD: −0.12; 95% CI: −0.62, 0.39; p = 0.652; I2 = 76.5%, p = 0.014) (Figure 6A). As for the administration subgroup, a significant difference was observed in the multiple-dose group (SMD: −0.50; 95% CI: −0.96, −0.04; p = 0.034; I2 = 68.2%, p = 0.024) (Figure 6B). However, no noticeable effect was observed in the single-dose group (SMD: 0.07; 95% CI: −0.35, 0.49; p = 0.749; I2 = 80.9%, p = 0.022) (Figure 6B).
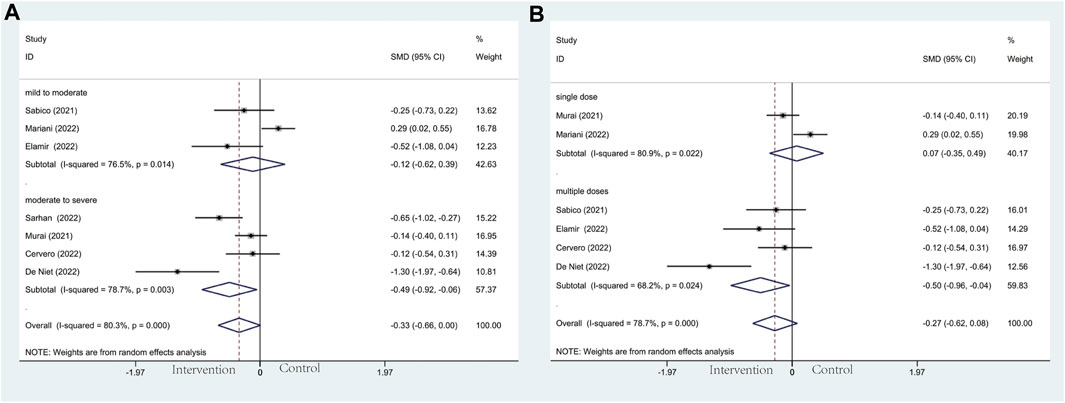
Figure 6. Forest plots of RCT for the association of vitamin D supplementation and length of hospitalization in the severity subgroup (A) and administration subgroup (B).
In the subgroup of severity, the results did not reach statistically significant differences between the intervention group and control group in CRP (SMD: 0.04; 95% CI: −0.37, 0.46; p = 0.836; I2 = 91.8%, p = 0.000) (Figure 7A), D-dimer (SMD: 0.08; 95% CI: −0.21, 0.37; p = 0.606; I2 = 59.1%, p = 0.032) (Figure 8A), IL-6 (SMD: −0.09; 95% CI: −0.26, 0.09; p = 0.337; I2 = 0.0%, p = 0.886) (Figure 9A), LDH (SMD: 0.12; 95% CI: −0.06, 0.30; p = 0.176; I2 = 0.0%, p = 0.538) (Figure 10). Likewise in the administration subgroup, no statistical difference was observed in CRP (SMD: 0.11; 95% CI: −0.34, 0.55; p = 0.645; I2 = 91.9%, p = 0.000) (Figure 7B), D-dimer (SMD: −0.01; 95% CI: −0.20, 0.17; p = 0.903; I2 = 42.0%, p = 0.142) (Figure 8B), IL-6 (SMD: −0.10; 95% CI: −0.27, 0.08; p = 0.292; I2 = 0.0%, p = 0.907) (Figure 9B). Administration subgroup of LDH failed to meet the condition to conduct further analysis due to inadequate studies.
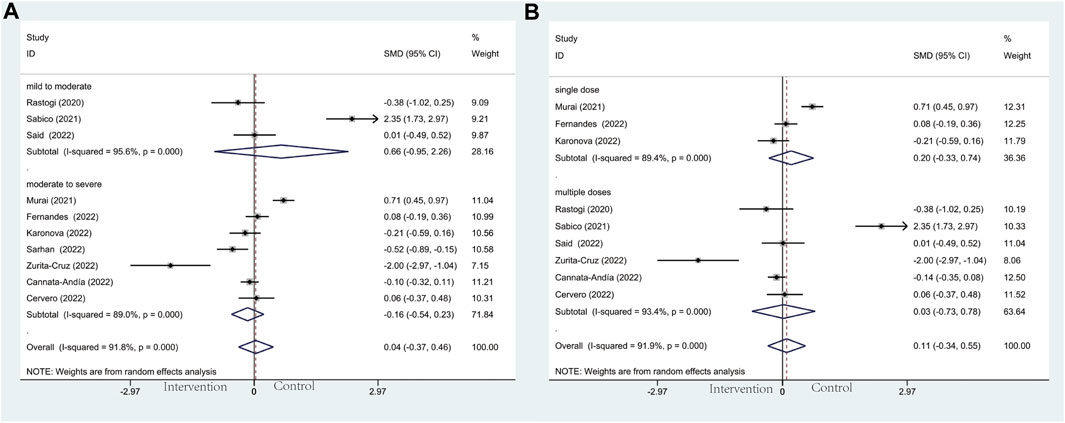
Figure 7. Forest plots of RCT for the association of vitamin D supplementation and CRP levels in the severity subgroup (A) and administration subgroup (B).
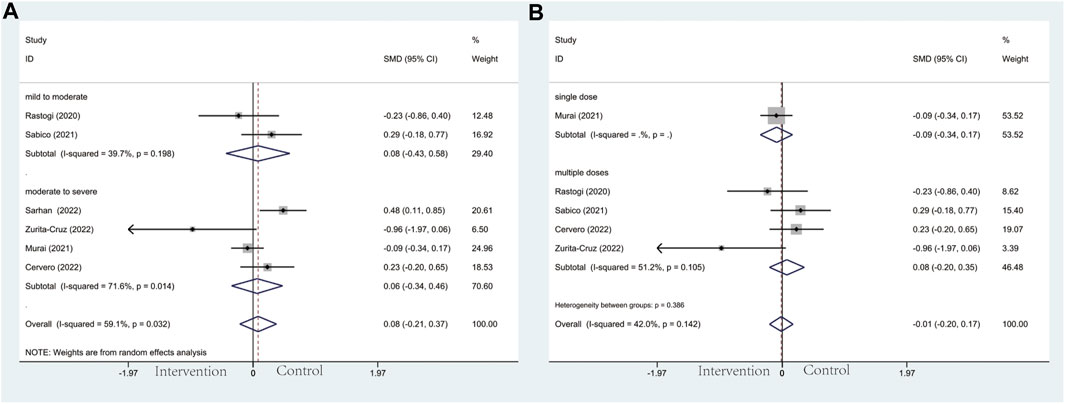
Figure 8. Forest plot of RCT for the association of vitamin D supplementation and D-dimer levels in the severity subgroup (A) and administration subgroup (B).
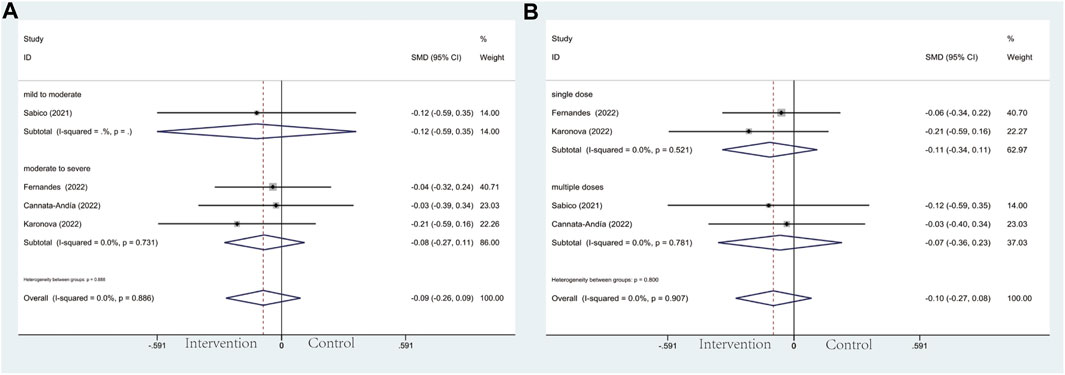
Figure 9. Forest plot of RCT for the association of vitamin D supplementation and IL-6 levels in the severity subgroup (A) and administration subgroup (B).
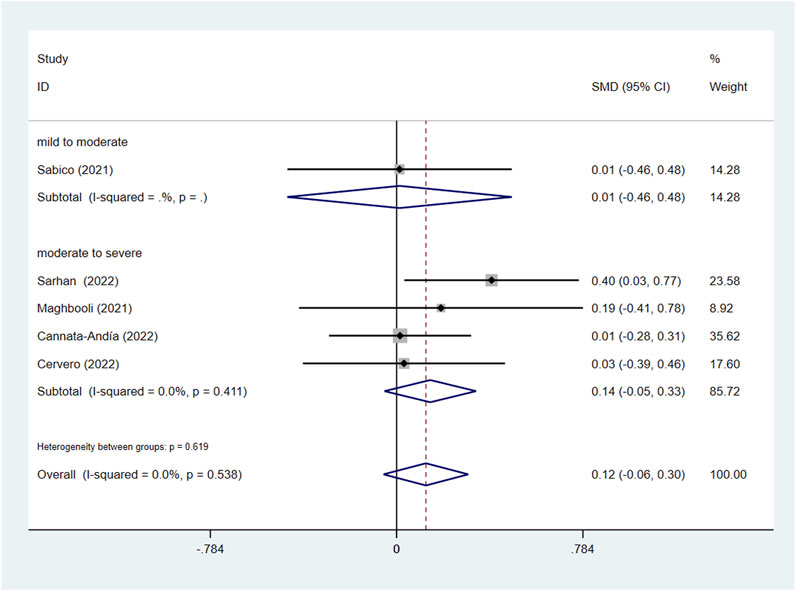
Figure 10. Forest plot of RCT for the association of vitamin D supplementation and LDH levels in the severity subgroup.
We conducted a sensitivity analysis to identify the impact of each trial on the effective index, and ultimately the significant effects of any individual study were unobserved (Additional Supplementary Figures S4–S11).
Centering on the question of whether vitamin D supplementation could diminish the progression of COVID-19, this meta-analysis of 19 RCTs including a distinctly larger sample size than ever before is the first to explore the efficacy of vitamin D in COVID-19 patients with different baseline severity. More convincing than previously published results, the pooled analyses disclosed newfound and statistically significant results that vitamin D supplementation reduced the likelihood of admission to ICU, the need for mechanical ventilation, and the length of hospitalization, especially in moderate to severe COVID-19 patients and those administrated with multiple doses of vitamin D. Nevertheless, the intervention did not significantly transform into decline in mortality or decreased level of inflammatory markers.
Worldwide, a very high prevalence of hypovitaminosis D status has been reported in many countries (van Schoor and Lips, 2017). As an essential nutrient for the human body, vitamin D, of which active metabolite is 1,25(OH)2D3, plays a participating role in regulating immunoreaction and inflammatory responses to microorganism infections, such as Epstein-Barr Virus, Human Immunodeficiency Virus, Hepatitis B Virus, Human Papilloma Virus, Influenza (Teymoori-Rad et al., 2019), and SARS-CoV-2 (Bilezikian et al., 2020). Malnutrition such as hypocalcemia, hypovitaminosis D in patients has been consistently linked to COVID-19 progression and a worsened prognosis. In a retrospective study conducted by Minasi et al. (Minasi et al., 2023), the relationship between hypocalcemia and adverse clinical outcomes in COVID-19 patients was investigated. The study revealed a significant correlation between serum calcium levels and circulating 25(OH)D. However, the researchers hypothesized that the observed association with the severity of COVID-19 was not directly related to the role of vitamin D in regulating calcium homeostasis. Instead, they suggested that vitamin D might influence the immune response and the production of proinflammatory cytokines. With a direct antiviral effect, vitamin D induces antimicrobial peptides (part of the innate immune system) against enveloped/non-enveloped viruses (Hansdottir et al., 2008; Youssef et al., 2011), and reinforces the barriers made up of cells to help fight off invasive viruses via E-cadherin (Oh et al., 2019). Additionally, vitamin D can suppress cytokine storms which account for acute respiratory distress syndrome (ARDS), by decreasing the production of proinflammatory T-Helper-1 cells (Th-1) and T-Helper-17 cells (Th-17) (Tomaszewska et al., 2022) and increasing the expression of anti-inflammatory cytokines under the regulation of inflammation-related genes (Wöbke et al., 2014). Under most circumstances, the immunopathogenesis of COVID-19 infection involves disruptions in inflammatory mediators. While these disturbances may not directly cause the disease, they contribute to its progression. Some studies claimed that level of Th-17 cells increased in critical COVID-19 patients on account of their body releasing excessive amounts of IL-6 (Xu et al., 2020), indicating the interaction of IL-6 and Th-17 cells in the pathogenesis of COVID-19 with poor prognosis. Most importantly, SARS-CoV-2 infects host cells by utilizing angiotensin-converting enzyme 2 (ACE2) as its receptor (Hoffmann et al., 2020) and leads to the downregulation of ACE2 expression (Lu et al., 2022). Meanwhile, vitamin D can reduce the pulmonary permeability of ARDS by means of mediating the renin-angiotensin system (Malek Mahdavi, 2020). Hence, in COVID-19 patients, downregulation of ACE2 tends to trigger an inflammatory chain reaction and the cytokine storm complicated by ARDS. Vitamin D could be a promising therapeutic approach in patients during COVID-19.
It is noteworthy that vitamin D supplementation shows no significant linkage with mortality and inflammatory markers according to our results, which is consistent with some previous meta-analyses (Hosseini et al., 2022; Kümmel et al., 2022; Varikasuvu et al., 2022; Zaazouee et al., 2023). In accordance with previous evidence, pre-existing vitamin D deficiency predisposes COVID-19 patients to suffer from a worse prognosis. Reportedly, decreased synthesis of vitamin D-binding protein tends to be more common in critical illness, potentially on account of inflammation, injury, disrupted metabolism, and hepatic dysfunction (Jeng et al., 2009; Madden et al., 2015), suggesting that long-term supplemental vitamin D rather than single high-dose vitamin D is preferable. What’s more, a recent study revealed that immune responses of the first line of the human body against viral replication or spread turned out to be slight and delayed, especially in critical COVID-19 patients (Liu et al., 2021). This may help to explain why in the administration subgroup multiple-dose group had more effective results in ICU admission, mechanical ventilation, and length of hospitalization than single-dose group. It is regular long-term vitamin D supplementation that takes a protective effect and offers the human body a relatively suitable circumstance allowing the various beneficial effects to be manifested and reinforced in preventing COVID-19.
Owing to pre-designed study protocols and rigorous screening methods, participants in the majority of previous research were restricted to take vitamin D supplementation before/at the recruiting time, and most of them were at the status of chronic hypovitaminosis D. This may thus be considered as a reason for unimproved mortality and levels of inflammatory markers in COVID-19 patients. At the genetic level, Mendelian randomization studies (Butler-Laporte et al., 2021; Daniel et al., 2022) did not find strong evidence supporting that increasing levels of 25(OH)D protect against COVID-19 severity, but the difference between the finding may be attributed to socioeconomic status and other medical comorbidities.
We acknowledge the limitations of our study. First, participants from single- or multi-centric, open-label, or double-blinded RCTs had several different coexisting diseases and interventions (different dosages, frequencies of administration, and medication duration), leading to the formation of heterogeneity. Second, the absence of reported serum 25(OH)D level after intervention in most studies, limits us to making further evaluations on the efficacy of various administrations and maintenance of optimum dose. Further investigations on vitamin D supplementation maintaining an optimum range of 25(OH)D serum concentration to prevent and alleviate the aggravation of COVID-19 are needed. In the meantime, the most effective and safe method of vitamin D supplementation concerning dosage, route, and duration of administration are all unneglected considerations.
In conclusion, multiple-dose vitamin D supplementation is closely linked with significantly lower odds of ICU admission, mechanical ventilation, and shorter hospital stays in patients with moderate to severe COVID-19. In the unending era of COVID-19, long-term adherence to a daily intake of vitamin D is recommended to stimulate the immune system and promote anti-inflammatory effects for the purpose of preventing aggravation and poor prognosis after infection with the SARS-CoV-2 virus.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YY: Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Writing–original draft. WS: Data curation, Investigation, Methodology, Supervision, Writing–review and editing. FY: Investigation, Software, Supervision, Validation, Writing–review and editing. GZ: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing. XL: Conceptualization, Supervision, Validation, Writing–review and editing. SS: Supervision, Validation, Writing–review and editing. YX: Funding acquisition, Investigation, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the CACMS Innovation Fund (Grant No. CI 2021A00919), the National Natural Science Foundation of China (Grant Nos. 81725024 and 81430098), the National Key R&D Program of China (Grant Nos. 2018YFC1704901 and 2018YFC1704900) and the High Level Chinese Medical Hospital Promotion Project (No. HLCMHPP2023090).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1367686/full#supplementary-material
COVID-19, Coronavirus disease 2019; RCTs, Randomized controlled trials; ICU, Intensive care unit; MV, Mechanical ventilation; LOH, Length of hospitalization; CRP, C-reactive protein; IL-6, Interleukin-6; LDH, Lactate dehydrogenase; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis; OR, Odds ratio; SMD, Standardized mean difference; CI, Confidence interval; Th-1 cell, T-Helper-1 cell; Th-17 cell, T-Helper-17 cell.
Annweiler, C., Beaudenon, M., Gautier, J., Gonsard, J., Boucher, S., Chapelet, G., et al. (2022). High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): a multicenter, open-label, randomized controlled superiority trial. PLoS Med. 19 (5), e1003999. doi:10.1371/journal.pmed.1003999
Ben-Eltriki, M., Hopefl, R., Wright, J. M., and Deb, S. (2022). Association between vitamin D status and risk of developing severe COVID-19 infection: a meta-analysis of observational studies. J. Am. Nutr. Assoc. 41 (7), 679–689. doi:10.1080/07315724.2021.1951891
Bilezikian, J. P., Bikle, D., Hewison, M., Lazaretti-Castro, M., Formenti, A. M., Gupta, A., et al. (2020). MECHANISMS in endocrinology: vitamin D and COVID-19. Eur. J. Endocrinol. 183 (5), R133–R147. doi:10.1530/EJE-20-0665
Butler-Laporte, G., Nakanishi, T., Mooser, V., Morrison, D. R., Abdullah, T., Adeleye, O., et al. (2021). Vitamin D and COVID-19 susceptibility and severity in the COVID-19 host genetics initiative: a mendelian randomization study. PLoS Med. 18 (6), e1003605. doi:10.1371/journal.pmed.1003605
Cannata-Andía, J. B., Díaz-Sottolano, A., Fernández, P., Palomo-Antequera, C., Herrero-Puente, P., Mouzo, R., et al. (2022). A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: the COVID-VIT-D—a randomised multicentre international clinical trial. BMC Med. 20 (1), 83. doi:10.1186/s12916-022-02290-8
Castillo, M., Entrenas Costa, L. M., Vaquero Barrios, J. M., Alcalá Díaz, J. F., López Miranda, J., Bouillon, R., et al. (2020). Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 203, 105751. doi:10.1016/j.jsbmb.2020.105751
Cervero, M., López-Wolf, D., Casado, G., Novella-Mena, M., Ryan-Murua, P., Taboada-Martínez, M. L., et al. (2022). Beneficial effect of short-term supplementation of high dose of vitamin D3 in hospitalized patients with COVID-19: a multicenter, single-blinded, prospective randomized pilot clinical trial. Front. Pharmacol. 13, 863587. doi:10.3389/fphar.2022.863587
COVID-19 Treatment Guidelines Panel (2023). Coronavirus disease 2019 (COVID-19) treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed June 20, 2023).
Daniel, N., Bouras, E., Tsilidis, K. K., and Hughes, D. J. (2022). Genetically predicted circulating concentrations of micronutrients and COVID-19 susceptibility and severity: a mendelian randomization study. Front. Nutr. 9, 842315. doi:10.3389/fnut.2022.842315
De Niet, S., Trémège, M., Coffiner, M., Rousseau, A.-F., Calmes, D., Frix, A.-N., et al. (2022). Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: a randomized, double-blind, placebo-controlled trial. Nutrients 14 (15), 3048. doi:10.3390/nu14153048
Duval, S., and Tweedie, R. (2004). Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Elamir, Y. M., Amir, H., Lim, S., Rana, Y. P., Lopez, C. G., Feliciano, N. V., et al. (2022). A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 154, 116175. doi:10.1016/j.bone.2021.116175
Fernandes, A. L., Murai, I. H., Reis, B. Z., Sales, L. P., Santos, M. D., Pinto, A. J., et al. (2022). Effect of a single high dose of vitamin D3 on cytokines, chemokines, and growth factor in patients with moderate to severe COVID-19. Am. J. Clin. Nutr. 115 (3), 790–798. doi:10.1093/ajcn/nqab426
Hansdottir, S., Monick, M. M., Hinde, S. L., Lovan, N., Look, D. C., and Hunninghake, G. W. (2008). Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 181 (10), 7090–7099. doi:10.4049/jimmunol.181.10.7090
Higgins, J. P. T., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2), 271–280. doi:10.1016/j.cell.2020.02.052
Hopefl, R., Ben-Eltriki, M., and Deb, S. (2022). Association between vitamin D levels and inflammatory markers in COVID-19 patients: a meta-analysis of observational studies. J. Pharm. Pharm. Sci. 25, 124–136. doi:10.18433/jpps32518
Hosseini, B., El Abd, A., and Ducharme, F. M. (2022). Effects of vitamin D supplementation on COVID-19 related outcomes: a systematic review and meta-analysis. Nutrients 14 (10), 2134. doi:10.3390/nu14102134
Im, J. H., Je, Y. S., Baek, J., Chung, M.-H., Kwon, H. Y., and Lee, J.-S. (2020). Nutritional status of patients with COVID-19. Int. J. Infect. Dis. 100, 390–393. doi:10.1016/j.ijid.2020.08.018
Jeng, L., Yamshchikov, A. V., Judd, S. E., Blumberg, H. M., Martin, G. S., Ziegler, T. R., et al. (2009). Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 7 (1), 28. doi:10.1186/1479-5876-7-28
Karonova, T. L., Golovatyuk, K. A., Kudryavtsev, I. V., Chernikova, A. T., Mikhaylova, A. A., Aquino, A. D., et al. (2022). Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID-19 patients: a randomized, open-label, single-center study. Nutrients 14 (13), 2602. doi:10.3390/nu14132602
Kümmel, L. S., Krumbein, H., Fragkou, P. C., Hünerbein, B. L., Reiter, R., Papathanasiou, K. A., et al. (2022). Vitamin D supplementation for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 13, 1023903. doi:10.3389/fimmu.2022.1023903
Li, Y., Tong, S., Hu, X., Wang, Y., Lv, R., Ai, S., et al. (2021). The relationship between nutritional status and the prognosis of COVID-19: a retrospective analysis of 63 patients. Medicine 100 (14), e25287. doi:10.1097/MD.0000000000025287
Liu, C., Martins, A. J., Lau, W. W., Rachmaninoff, N., Chen, J., Imberti, L., et al. (2021). Time-resolved systems immunology reveals a late juncture linked to fatal COVID-19. Cell 184 (7), 1836–1857.e22. doi:10.1016/j.cell.2021.02.018
Lu, Y., Zhu, Q., Fox, D. M., Gao, C., Stanley, S. A., and Luo, K. (2022). SARS-CoV-2 down-regulates ACE2 through lysosomal degradation. Mol. Biol. Cell 33 (14), ar147. doi:10.1091/mbc.E22-02-0045
Luo, D., Wan, X., Liu, J., and Tong, T. (2016). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Madden, K., Feldman, H. A., Chun, R. F., Smith, E. M., Sullivan, R. M., Agan, A. A., et al. (2015). Critically ill children have low vitamin D-binding protein, influencing bioavailability of vitamin D. Ann. Am. Thorac. Soc. 12 (11), 1654–1661. doi:10.1513/AnnalsATS.201503-160OC
Maghbooli, Z., Sahraian, M. A., Jamalimoghadamsiahkali, S., Asadi, A., Zarei, A., Zendehdel, A., et al. (2021). Treatment with 25-hydroxyvitamin D3 (calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: a pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 27 (12), 1242–1251. doi:10.1016/j.eprac.2021.09.016
Malek Mahdavi, A. (2020). A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: implications for a potential treatment for COVID-19. Rev. Med. Virol. 30 (5), e2119. doi:10.1002/rmv.2119
Mariani, J., Antonietti, L., Tajer, C., Ferder, L., Inserra, F., Sanchez Cunto, M., et al. (2022). High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: multicentre randomized controlled clinical trial. PLoS One 17 (5), e0267918. doi:10.1371/journal.pone.0267918
Mavridis, D., and Salanti, G. (2014). How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid. Based Ment. Health 17 (1), 30. doi:10.1136/eb-2013-101699
Minasi, A., Andreadi, A., Maiorino, A., Giudice, L., De Taddeo, S., D'Ippolito, I., et al. (2023). Hypocalcemia is associated with adverse outcomes in patients hospitalized with COVID-19. Endocrine 79 (3), 577–586. doi:10.1007/s12020-022-03239-w
Murai, I. H., Fernandes, A. L., Sales, L. P., Pinto, A. J., Goessler, K. F., Duran, C. S. C., et al. (2021). Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA 325 (11), 1053–1060. doi:10.1001/jama.2020.26848
Oh, C., Kim, H. J., and Kim, H.-M. (2019). Vitamin D maintains E-cadherin intercellular junctions by downregulating MMP-9 production in human gingival keratinocytes treated by TNF-α. J. Periodontal Implant Sci. 49 (5), 270–286. doi:10.5051/jpis.2019.49.5.270
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Rastogi, A., Bhansali, A., Khare, N., Suri, V., Yaddanapudi, N., Sachdeva, N., et al. (2022). Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 98 (1156), 87–90. doi:10.1136/postgradmedj-2020-139065
Sabico, S., Enani, M. A., Sheshah, E., Aljohani, N. J., Aldisi, D. A., Alotaibi, N. H., et al. (2021). Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate covid-19: a randomized clinical trial. Nutrients 13 (7), 2170. doi:10.3390/nu13072170
Said, S. A., Abdulbaset, A., El-Kholy, A. A., Besckales, O., and Sabri, N. A. (2022). The effect of Nigella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: a randomized controlled clinical trial. Front. Pharmacol. 13, 1011522. doi:10.3389/fphar.2022.1011522
Sánchez-Zuno, G. A., González-Estevez, G., Matuz-Flores, M. G., Macedo-Ojeda, G., Hernández-Bello, J., Mora-Mora, J. C., et al. (2021). Vitamin D levels in COVID-19 outpatients from western Mexico: clinical correlation and effect of its supplementation. J. Clin. Med. 10 (11), 2378. doi:10.3390/jcm10112378
Sarhan, N., Abou Warda, A. E., Sarhan, R. M., Boshra, M. S., Mostafa-Hedeab, G., Alruwaili, B. F., et al. (2022). Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: a randomized clinical trial. Medicina 58 (10), 1358. doi:10.3390/medicina58101358
Silverio, R., Gonçalves, D. C., Andrade, M. F., and Seelaender, M. (2021). Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? Adv. Nutr. Bethesda, Md 12 (3), 682–692. doi:10.1093/advances/nmaa125
Soliman, A. R., Abdelaziz, T. S., and Fathy, A. (2022). Impact of vitamin D therapy on the progress COVID-19: six weeks follow-up study of vitamin D deficient elderly diabetes patients. Proc. Singap. Healthc. 31, 201010582110414. doi:10.1177/20101058211041405
Teymoori-Rad, M., Shokri, F., Salimi, V., and Marashi, S. M. (2019). The interplay between vitamin D and viral infections. Rev. Med. Virol. 29 (2), e2032. doi:10.1002/rmv.2032
Tomaszewska, A., Rustecka, A., Lipińska-Opałka, A., Piprek, R. P., Kloc, M., Kalicki, B., et al. (2022). The role of vitamin D in COVID-19 and the impact of pandemic restrictions on vitamin D blood content. Front. Pharmacol. 13, 836738. doi:10.3389/fphar.2022.836738
Torres, M., Casado, G., Vigón, L., Rodríguez-Mora, S., Mateos, E., Ramos-Martín, F., et al. (2022). Changes in the immune response against SARS-CoV-2 in individuals with severe COVID-19 treated with high dose of vitamin D. Biomed. Pharmacother. 150, 112965. doi:10.1016/j.biopha.2022.112965
van Schoor, N., and Lips, P. (2017). Global overview of vitamin D status. Endocrinol. Metab. Clin. North Am. 46 (4), 845–870. doi:10.1016/j.ecl.2017.07.002
Varikasuvu, S. R., Thangappazham, B., Vykunta, A., Duggina, P., Manne, M., Raj, H., et al. (2022). COVID-19 and vitamin D (Co-VIVID study): a systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-infective Ther. 20 (6), 907–913. doi:10.1080/14787210.2022.2035217
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14 (1), 135. doi:10.1186/1471-2288-14-135
Wang, Z., Joshi, A., Leopold, K., Jackson, S., Christensen, S., Nayfeh, T., et al. (2022). Association of vitamin D deficiency with COVID-19 infection severity: systematic review and meta-analysis. Clin. Endocrinol. 96 (3), 281–287. doi:10.1111/cen.14540
Wöbke, T. K., Sorg, B. L., and Steinhilber, D. (2014). Vitamin D in inflammatory diseases. Front. Physiol. 5, 244. doi:10.3389/fphys.2014.00244
World Health Organization (2023). WHO COVID-19 dashboard data. Available at: https://data.who.int/dashboards/covid19/data.
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 8 (4), 420–422. doi:10.1016/S2213-2600(20)30076-X
Youssef, D. A., Miller, C. W. T., El-Abbassi, A. M., Cutchins, D. C., Cutchins, C., Grant, W. B., et al. (2011). Antimicrobial implications of vitamin D. Dermato-Endocrinology 3 (4), 220–229. doi:10.4161/derm.3.4.15027
Zaazouee, M. S., Eleisawy, M., Abdalalaziz, A. M., Elhady, M. M., Ali, O. A., Abdelbari, T. M., et al. (2023). Hospital and laboratory outcomes of patients with COVID-19 who received vitamin D supplementation: a systematic review and meta-analysis of randomized controlled trials. Naunyn-Schmiedeberg's Archives Pharmacol. 396 (4), 607–620. doi:10.1007/s00210-022-02360-x
Zurita-Cruz, J., Fonseca-Tenorio, J., Villasís-Keever, M., López-Alarcón, M., Parra-Ortega, I., López-Martínez, B., et al. (2022). Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: a randomized controlled trial. Front. Pediatr. 10, 943529. doi:10.3389/fped.2022.943529
Keywords: vitamin D, COVID-19, mortality, ICU admission, mechanical ventilation, meta-analysis
Citation: Yang Y, Sun W, Yang F, Zhang G, Li X, Sun S and Xing Y (2024) Therapeutic effects of vitamin D supplementation on COVID-19 aggravation: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 15:1367686. doi: 10.3389/fphar.2024.1367686
Received: 09 January 2024; Accepted: 07 May 2024;
Published: 27 May 2024.
Edited by:
Karunakaran Kalesh, Teesside University, United KingdomReviewed by:
Aikaterini Andreadi, University of Rome Tor Vergata, ItalyCopyright © 2024 Yang, Sun, Yang, Zhang, Li, Sun and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanwei Xing, eGluZ3lhbndlaTEyMzQ1QDE2My5jb20=; Shipeng Sun, c2hpcGVuZ3N1bkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.