
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 23 February 2024
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1364924
This article is part of the Research Topic Methods in Gastrointestinal and Hepatic Pharmacology 2023 View all 9 articles
 Yue Chen1,2†
Yue Chen1,2† Yutao Wang2,3†
Yutao Wang2,3† Jin Lei2
Jin Lei2 Bowen Chen2,4
Bowen Chen2,4 Xinfeng Zhang2,5
Xinfeng Zhang2,5 Liangzheng Chang2
Liangzheng Chang2 Zhangli Hu6
Zhangli Hu6 Yun Wang6*
Yun Wang6* Yinying Lu1,2,6*
Yinying Lu1,2,6*Background and aims: Tyrosine kinase inhibitors (TKIs) combined with programmed cell death protein-1 (PD-1) have significantly improved survival in patients with unresectable hepatocellular carcinoma (uHCC), but effective biomarkers to predict treatment efficacy are lacking. Peripheral blood bile acids (BAs) are associated with tumor response to therapy, but their roles in HCC remain unclear.
Methods: This retrospective study included HCC patients who received first-line TKIs combined with PD-1 inhibitors treatment (combination therapy) in our clinical center from November 2020 to June 2022. The aim of this study was to analyze the changes in plasma BA profiles before and after treatment in both the responding group (Res group) and the non-responding group (Non-Res group). We aimed to explore the potential role of BAs in predicting the response to combination therapy in HCC patients.
Results: Fifty-six patients with HCC who underwent combination therapy were included in this study, with 28 designated as responders (Res group) and 28 as non-responders (Non-Res group). There were differences in plasma BA concentrations between the two groups before systemic therapy. Plasma taurohyocholic acid (THCA) levels in the Res group were significantly lower than those in the Non-Res group. Patients with low levels of THCA exhibited superior median progression-free survival (7.6 vs. 4.9 months, p = 0.027) and median overall survival (23.7 vs. 11.6 months, p = 0.006) compared to those of patients with high levels of THCA.
Conclusion: Peripheral blood BA metabolism is significantly correlated with combination therapy response and survival in patients with HCC. Our findings emphasize the potential of plasma BAs as biomarkers for predicting combination therapy outcomes and offering novel therapeutic targets for modulating responses to systemic cancer therapy.
Hepatocellular carcinoma (HCC) is currently the third most common cause of cancer-related deaths globally, and its incidence is on the rise (Devarbhavi et al., 2023). Systemic therapy is the first-line treatment option for advanced HCC, encompassing targeted therapy, such as tyrosine kinase inhibitors (TKIs), immune-checkpoint inhibitors (ICIs), including programmed cell death-1 (PD-1) inhibitors, and combined therapies (Xu et al., 2018). Study 117 found that lenvatinib plus the anti-PD-1 monoclonal antibody nivolumab resulted in an objective response rate of 66.7% and a clinical benefit rate of 70.8% in patients with advanced HCC (Kudo et al., 2020). LEAP-002 study showed that the lenvatinib combined with the pembrolizumab regimen had a trend of better benefit compared with lenvatinib monotherapy in the Asian subgroup (approximately 50% of Chinese patients), with a median PFS increase of 1.8 months, an ORR increase of 9.2%, and a median OS increase of approximately 4 months (Llovet et al., 2023). Another multicenter real-world study conducted by our clinical center and Peking Union Medical College Hospital showed that the median OS was 17.8 months, the median PFS was 6.9 months, and the ORR was 19.6% (Yang et al., 2023). Despite the effectiveness of these therapies for the treatment of HCC in terms of tumor response and patient survival, only a proportion of patients fully benefit from immunotherapy. Therefore, it is imperative to identify biomarkers that can predict outcomes of combination therapies to screen for patients with high potential long-term benefits and promote the development of personalized and precision medicine.
Dysregulation of bile acids (BAs) has been noted in various liver diseases, such as liver cirrhosis, nonalcoholic fatty liver disease, and HCC (Puri et al., 2018; Xie et al., 2021; Shen et al., 2022). In our previous study, we found significant changes in BA levels in patients with primary liver cancer compared with those with cirrhosis and healthy individuals, especially a significant increase in tauroursodeoxycholic acid (TUDCA) levels in plasma, which was negatively correlated with survival (Jia et al., 2020). Chen et al. (2011) found that the levels of glycocholic acid (GCA) and chenodeoxycholic acid glycine conjugate (GCDCA) were negatively correlated with the stage of HCC after analyzing the plasma BA components of patients with HCC at different stages, suggesting that the levels of these two components had the potential to predict the progression of HCC. In addition, several studies have suggested a role for BAs in treatment response. Patients who reacted to ICI had significantly greater amounts of ursodeoxycholic acid (UDCA) and ursocholic acid present in their feces compared to those who did not react (Lee et al., 2022). Secondary BAs have been demonstrated to regulate the build-up of CXCR6+ natural killer T cells in preclinical tumor models, inhibiting the progression of liver cancer, which is a potential mechanism underlying the role of BAs in responding to immunotherapy (Ma et al., 2018). Nevertheless, the relationship between plasma BAs and target-immune combination therapy in HCC remains unclear.
Furthermore, a similar phenomenon has been observed in patients with breast cancer, with a robust connection between plasma BA metabolite composition, host immune activation, and therapeutic response (Wu et al., 2022).
In this study, we examined the disparities in BA profiles between patients with HCC who exhibited clinical efficacy from combination therapy and those who did not. The aim of this study was to discover plasma biomarkers indicating target-immune combination therapy response and outcomes in patients with HCC, aiming to pinpoint subgroups that might benefit from combination therapies and maximize patient benefits.
This retrospective study was approved by the Ethics Committee of the Fifth Medical Center of the PLA General Hospital (KY-2021-12-35-1). The research was carried out in compliance with the ethical standards outlined in the Declaration of Helsinki. Patients with hepatitis B virus (HBV)-related HCC who received TKIs combined with PD-1 inhibitors therapy (combination therapy) as a first-line treatment between November 2020 and June 2022 were considered for inclusion. The inclusion criteria were as follows: 1) histologically or radiologically diagnosed HCC; 2) unresectable HCC, i.e., not eligible for curative treatment; 3) age 18 years or older; 4) Child–Pugh score class A–B; 5) Eastern Cooperative Oncology Group (ECOG) performance status (PS) scores of 0–2; 6) availability of plasma samples obtained at the beginning of systemic therapy; and 7) availability of complete clinical and follow-up data. The exclusion criteria were as follows: 1) presence of other primary malignant tumors; 2) prior treatment with systemic drug therapy; and 3) irregular medication. Plasma samples were obtained from the Biobank of the Fifth Medical Center of PLA General Hospital. All informed consent was obtained from the subjects, and all samples were promptly frozen at −80°C until analysis.
Treatment response was assessed using dynamic computed tomography or magnetic resonance imaging at the baseline and every 6–8 weeks post-treatment initiation, according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Patients achieving a complete response (CR), partial response (PR), or stable disease (SD) for a minimum of 6 months were classified as responders (Res), whereas individuals demonstrating stable disease for less than 6 months or progressing disease (PD) were categorized as non-responders (Non-Res). Overall survival (OS) was determined from the commencement of the combination therapy until death for any reason. Survivors were censored at the last follow-up. Progression-free survival (PFS) was defined as the time from the initial dose to the first radiologically confirmed tumor progression or all-cause mortality, whichever occurred first.
Plasma samples (50 μL) were added to 400 μL acetonitrile/methanol solvent and mixed by vibration for 20 min. Aliquots (250 μL) of the supernatant were then lyophilized. The samples were reconstituted prior to analysis with 40 μL acetonitrile/methanol. BA analysis was performed using ultra-high-performance liquid chromatography-tandem mass spectrometry (ACQUITY UPLC-Xevo TQ-S, Waters Corp, Milford, MA, United States) as previously described (Toledo et al., 2017), and MassLynx software version 4.1 (Waters Corp). Internal standards were added to the test samples to monitor analytical bias throughout sample processing and analysis. Quality control samples were inserted into the entire injection sequence at intervals to objectively evaluate the intra-batch repeatability of large-scale samples and to correct the inter-batch error. BAs were quantified in plasma samples using the quantification curves and mass spectra obtained from standard samples analyzed under the same conditions.
Continuous variables are presented as median (interquartile range), whilst categorical variables are represented as frequencies and percentages. Chi-squared and Fisher’s exact tests were utilized for the comparison of categorical variables, and the paired t-test, Mann–Whitney U test, and Wilcoxon signed-rank test were adopted for continuous variables. The area under the receiver operating characteristic curve (AUROC) was calculated to determine the optimal cut-off values for BA levels. The optimal cut-off was considered to be the value with the highest Youden’s index (sensitivity + specificity − 1). The PFS and OS were determined using the Kaplan–Meier methodology and analyzed using the log-rank test. Univariate Cox regression analysis was performed on clinical and BA data, and multivariate Cox regression analysis was performed on significant factors (p < 0.05) identified by the univariate analysis. All statistical analyses were conducted using R software version 4.3.1 (R Project for Statistical Computing, Vienna, Austria) and SPSS software version 26 (IBM Corp, Armonk, NY, United States). All statistical tests were conducted using a two-sided approach, and statistical significance was determined as p < 0.05.
Between November 2020 and June 2022, the study included 56 patients who met the specified criteria. According to RECIST version 1.1, 17, 20, and 19 patients exhibited PR, SD, and PD, respectively; there were 28 patients each in the Res and Non-Res groups. Most patients were male (n = 49, 87.5%) and under 60 years old (n = 37, 66.1%). At the time of enrollment, 43 (76.8%) patients exhibited portal vein invasion by the tumor, whereas 24 (42.9%) exhibited extrahepatic metastasis. The majority of the patients (n = 52, 92.9%) were classified as stage C according to the Barcelona Clinic Liver Cancer staging system, and Child–Pugh score class A (n = 42, 75%). The group was dominated by Lenvatinib plus anti-PD-1 therapy (n = 40, 71%). No significant differences were observed in tumor number, tumor size, alpha-fetoprotein levels, portal hypertension, and albumin levels between the Res and Non-Res groups, suggesting that there were no established confounding variables causing discrimination between the groups before sample gathering. The detailed baseline characteristics of the patients are outlined in Table 1.
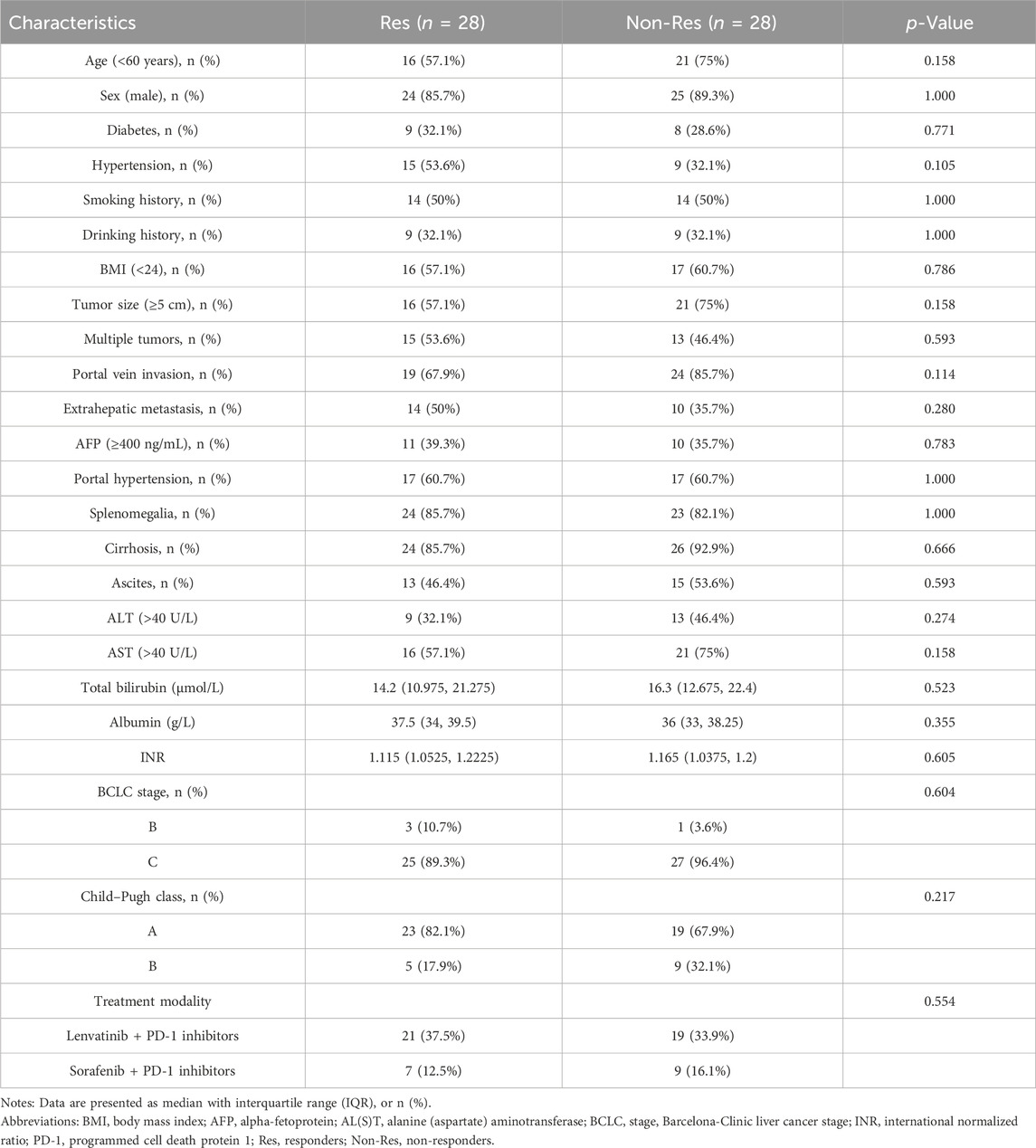
TABLE 1. Clinical characteristics of 56 patients with unresectable hepatocellular carcinoma receiving tyrosine kinase inhibitors combined with programmed cell death-1 inhibitors therapy.
The baseline plasma BA profiles in patients were examined using mass spectrometry to evaluate the levels and compositional features of BAs. The results of the BA measurements are shown in Figure 1. There were variations in BA composition between the two groups, and trends were observed in the heat map (Figure 1A). Although no significant differences were observed in the absolute concentrations of primary BAs between the two groups, the levels of primary BAs were generally higher in the Non-Res group than in the Res group (Figure 1B). Notably, among the secondary BAs, only taurohyocholic acid (THCA) levels were significantly reduced in the Res group compared with the Non-Res group (p < 0.05, Figure 1C).To investigate the correlation between BA metabolism and treatment response further, we categorized the 56 patients with HCC into high and low THCA groups. The optimal cut-off value for baseline serum THCA levels was determined to be 7.48 nmol/L by plotting the receiver operating characteristics (ROC) curve with the maximum Youden index. Our findings demonstrated that the response rate in the low THCA group was significantly better than that in the high THCA group. (73.7% vs. 37.8%, p < 0.05, Figure 1D). Taken together, these results suggest that baseline plasma BAs, especially THCA, correlate with the response to combination therapy in patients diagnosed with HCC, indicating that plasma BAs might be effective biomarkers for predicting the response to systematic drug treatment.
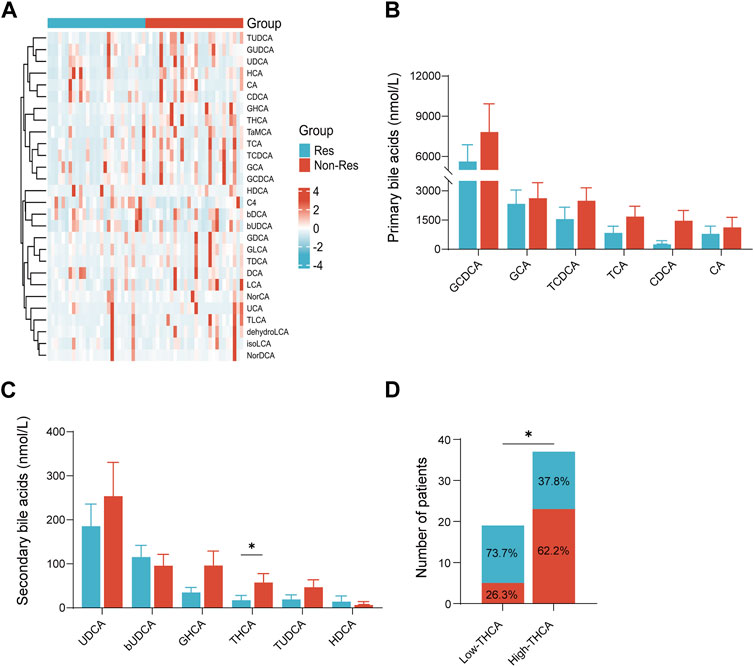
FIGURE 1. Plasma BA concentration in patients with hepatocellular carcinoma treated with tyrosine kinase inhibitors combined with programmed cell death-1 inhibitors therapy. (A) Heatmap of serum BA profiles in the Res and Non-Res groups; (B) primary BA levels in the Res and Non-Res groups; (C) secondary BA levels in the Res and Non-Res groups; and (D) The number of Res and Non-Res patients in the low and high THCA groups. *p < 0.05. BA, bile acid; Res, responders; Non-Res, non-responders; THCA, taurohyocholic acid.
To understand the changes in BA metabolism during treatment, plasma THCA levels were detected at baseline and 3 months after treatment (three patients in the Res group were excluded from this analysis owing to a lack of 3-month post-treatment plasma samples). Compared to baseline plasma BA profiles, the Res group showed a significant decrease in THCA levels after treatment (p < 0.05, Figure 2A), whereas no significant change was observed in the Non-Res group. Furthermore, the Res group exhibited lower post-treatment THCA levels than the Non-Res group (p < 0.05, Figure 2A).
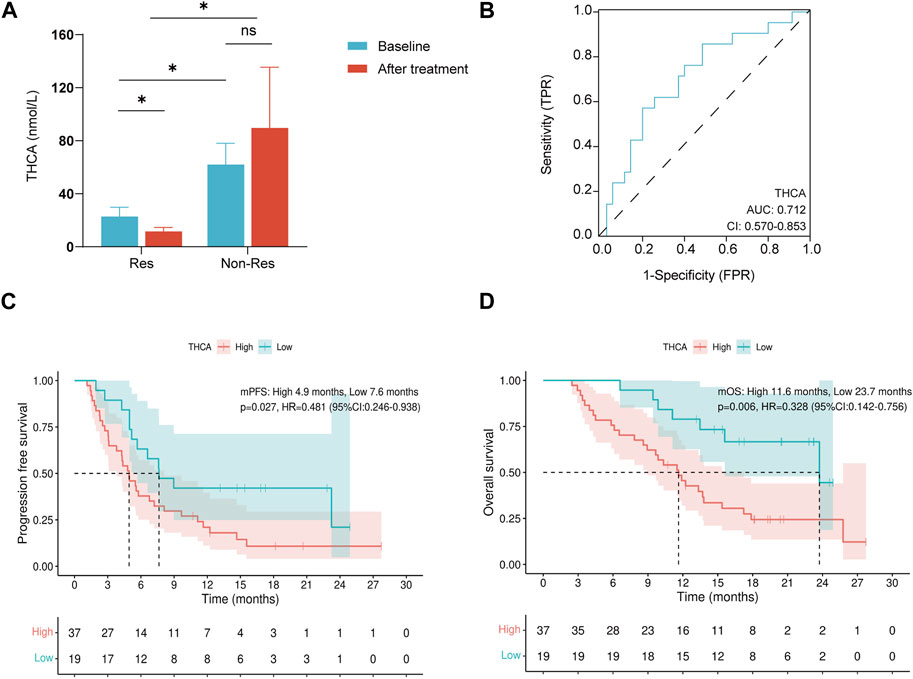
FIGURE 2. THCA is associated with tyrosine kinase inhibitors combined with programmed cell death-1 inhibitors therapy outcomes in patients with hepatocellular carcinoma. (A) Changes in plasma THCA levels at baseline and after treatment; (B) Receiver operating characteristic curve of THCA for overall survival in HCC; (C) Kaplan–Meyer curves for progression-free survival in patients with high and low baseline THCA levels; (D) Kaplan–Meyer curves for overall survival in patients with high and low baseline THCA levels. ns, not significant; *p < 0.05. THCA, taurohyocholic acid.
According to the ROC analysis, THCA was an acceptable predictor of survival (AUROC: 0.712, Figure 2B). Therefore, THCA levels were investigated in survival analyses. Patients with lower THCA levels experienced a longer median PFS than patients with higher THCA levels (7.6 months vs. 4.9 months, p = 0.027, Figure 2C). An OS benefit was also observed in patients with lower THCA levels (median OS: 23.7 months vs. 11.6 months, p = 0.006, Figure 2D). Child–Pugh class (hazard ratio [HR]: 2.079, 95% confidence interval [CI]: 1.077–4.014, p = 0.029) and THCA (HR: 2.084, 95% CI: 1.065–4.078, p = 0.032) were identified as independent prognostic factors for PFS in multivariate Cox regression analysis (Table 2). In the univariate analysis of OS, only a high THCA level (HR, 3.049; 95% CI, 1.322–7.032; p = 0.009) was independently associated with poor OS (Table 3). Therefore, BAs are linked to survival in patients receiving combination therapy. Taken together, our findings indicate that the low THCA level in plasma might enhance the clinical response and prolong the survival of combination therapy, which can predict the survival benefit for patients with HCC receiving TKIs combined with PD-1 inhibitors therapy.
To validate the above results, we repeated the survival analyses in the patient subgroup that received lenvatinib combined with sintilimab, the largest subgroup in our cohort. Of the 28 patients in the subgroup cohort, those with low THCA levels experienced a significantly prolonged PFS than patients with high THCA levels (median PFS: 9.0 months vs. 4.6 months, p = 0.028, Figure 3A). Importantly, patients with low THCA levels also experienced a significantly prolonged OS compared to those with high THCA levels (median OS: not reached [NR] vs. 9.8 months, p = 0.011, Figure 3B).
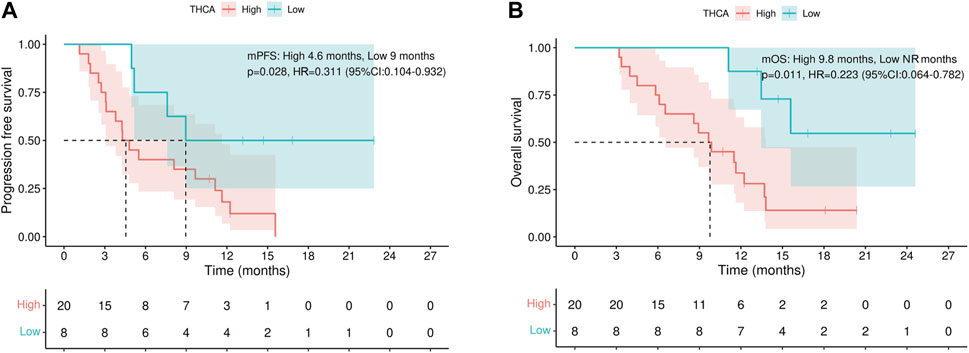
FIGURE 3. Progression-free and overall survival subgroup analysis. (A) THCA levels are associated with progression-free survival and (B) overall survival. NR, not reached; THCA, taurohyocholic acid.
To the best of our knowledge, this is the first study to link plasma BA metabolism with the treatment response to TKIs combined with PD-1 inhibitors treatment and survival in patients with HCC. Using plasma BA metabolic profile analysis, we demonstrated that plasma BA concentrations differed between the combination therapy Res and Non-Res groups of patients with HCC. Further analysis identified THCA as an independent predictor of survival. THCA levels were negatively associated with combination therapy response and survival in patients with HCC.
HCC is a solid tumor characterized by high heterogeneity, with varying immune microenvironments depending on the underlying liver disease. Immunotherapy combined with targeted therapy has proven more effective for virus-associated HCC (Qin et al., 2023). Immune checkpoint inhibitors contribute to antiviral therapy while exerting their anti-tumor mechanism of action. This may explain why the Chinese subgroups of the LEAP-002 and CARES-310 studies achieved a longer median overall survival. Patients with HBV-associated HCC in China bear a significant disease burden. Our study exclusively included patients with HBV-associated HCC, effectively mitigating disease background as a confounding factor. Further clinical studies are warranted to ascertain whether our current findings are applicable to hepatocellular carcinoma caused by other factors, such as HCV-associated, alcoholic, and non-alcoholic steatosis.
In addition to participating in the normal cholesterol metabolism pathway, BAs have been demonstrated to have a crucial role in the development of tumors and HCC (Sun et al., 2016; Wang et al., 2016). For instance, elevated levels of glycine- and taurine-conjugated primary bile acids have been linked to a heightened likelihood of chronic hepatitis B/C-related hepatocellular carcinoma (Petrick et al., 2020). Among patients undergoing anti-PD-1 immunotherapy for hepatobiliary tumors, those with elevated BA levels exhibited poorer treatment responses and prognosis (Mao et al., 2021). In the current study, the Res group exhibited lower levels of total bile acid (TBA) than the Non-Res group. In addition, a lower treatment response rate was observed in patients with high TBA levels (36.4% vs. 63.3%, Supplementary Figure S1A). However, these differences were not statistically significant, presumably owing to the relatively small cohort sample size. Further analysis of TBA levels before and after treatment revealed that TBA levels in the Res group were significantly decreased (p < 0.05, Supplementary Figure S1B). These observations suggest a close relationship between BAs and the response to immunotherapy. There were no significant differences between the two groups in the absolute concentrations of BAs, including chenodeoxycholic acid (CDCA), cholic acid (CA), and UDCA, which have been the focus of previous research (Figures 1A, B) (Ding et al., 2015; Liu et al., 2018; Režen et al., 2022). Another study reported that fecal UDCA concentrations were notably elevated in ICI responders compared to non-responders, whereas fecal lithocholic acid (LCA) concentrations were higher in patients with PD (Lee et al., 2022). This seems to disagree with our findings, in which an upward trend in plasma UDCA levels was observed in the Non-Res group. These conflicting results may be related to the different metabolic environments of plasma and feces; for example, the effects of intestinal flora and permeability (Mao et al., 2021; Shi et al., 2023). Previous studies have also indicated that the proportion of BAs in plasma and feces differ (Jia et al., 2020; Kasai et al., 2022). Recent studies have highlighted the crucial roles played by BAs in the development and function of T cells (Ding et al., 2022). The transformation of 3-oxoLCA into isoalloLCA by gut Bacteroidetes enhances naïve T cell differentiation into regulatory T cells, thus maintaining immune homeostasis (Hang et al., 2019; Song et al., 2020; Li et al., 2021).
Similar observations have been noted amongst patients with melanoma wherein elevated levels of circulating myeloid-derived suppressor cells (MDSCs) have been reported in non-responders to immunotherapy (Meyer et al., 2014; Tobin et al., 2017). This increase in the number of MDSCs may be due to taurodeoxycholate, which may also affect the immune-regulatory functions of these cells (Chang et al., 2018). These findings imply that patients with tumors experience diverse changes in BA concentration and/or composition, which could potentially regulate their response to antitumor therapies through mechanisms related to immune cells and intestinal flora.
Our study found that, among the BAs examined, THCA showed the most distinct differentiation between patients with HCC who responded to combination treatment and those who did not. Taurocholic acid (TCA) levels were also higher in the Non-Res group compared to the Res group. However, this difference did not reach statistical significance (Figure 1B), which could be due to the limited sample size of our study. In addition, THCA can be transformed into TCA. High levels of plasma TCA promote HCC development by inducing M2-like macrophage polarization and generating an immunosuppressive tumor microenvironment (Sun et al., 2022). Therefore, the interconversion of primary and secondary BAs has the potential to induce changes in immune cells within the tumor microenvironment, which may subsequently affect the treatment response. Further research is required to fully comprehend the mechanisms behind this association and explore potential therapeutic strategies targeting BA metabolism to improve treatment responses.
Multivariate Cox regression analysis demonstrated that the Child–Pugh class was an autonomous predictor of PFS, which may be related to changes in BAs. Liver dysfunction can impact BA metabolism in numerous ways, including hampered hepatic BA clearance, heightened BA synthesis, and alterations in the gut microbiome. These changes may cause modifications to the production of secondary and tertiary BAs (Fuchs and Trauner, 2022). Conversely, the dysregulation of BA metabolism may lead to impaired liver function (Ahmad and Haeusler, 2019). Alterations in liver function can affect the response to systemic therapies. Therefore, it is crucial to consider liver function and BA metabolism when evaluating the efficacy and outcomes of anti-tumor therapies in patients with HCC.
Furthermore, we assessed whether variations in the impact of distinct bile acids on patients’ overall and progression-free survival existed. Subsequent analysis revealed that the concentration of certain bile acids, including C4, TLCA, and TUDCA, significantly affected patients’ PFS and/or OS despite no differences in baseline levels (Supplementary Tables S1, S2). The small sample size may account for this, and the findings in this study segment require validation in a larger patient cohort.
Our study has some limitations that must be considered. First, this was a retrospective, single-center study with a limited sample size. This may have resulted in a degree of information and selection bias. Therefore, prospective studies with larger clinical cohorts are required to validate our findings and hypotheses. Second, the treatment regimen for this study cohort was relatively complex. Therefore, to strengthen the hypothesis of our study, we conducted a subgroup analysis specifically focusing on the treatment regimen of lenvatinib plus sintilimab, and we will explore subgroup analyses of other combination regimens in the future with an expanded sample size. Our study lacked fecal metagenomic and metabolomic information, which would aid the understanding of the relationship between the gut microbiota and host metabolism. Finally, we investigated the potential reasons for the lack of response to systemic therapy by examining plasma BA metabolism. However, the specific mechanisms underlying this lack of treatment response require further investigation, both in vivo and in vitro.
In conclusion, we have demonstrated the significant role of BAs in the therapeutic outcomes of HCC. In addition, we suggest, for the first time, that peripheral blood THCA might be a valid biomarker, the levels of which independently predict TKIs combined with PD-1 inhibitors therapy response in patients with HCC. These findings highlight a promising therapeutic approach to enhance the treatment efficacy of systemic anti-tumor agents through the modification of BA metabolites. More research is needed to clarify the role of BAs in the treatment of HCC completely. However, by considering the role of BAs in the treatment response, we may be able to develop personalized treatment approaches that consider individual variations in BA metabolism, ultimately leading to improved outcomes in patients with HCC.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the Fifth Medical Center of the PLA General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YC: Conceptualization, Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. YW: Formal Analysis, Methodology, Writing–original draft, Writing–review and editing. JL: Data curation, Validation, Writing–review and editing. BC: Data curation, Validation, Writing–review and editing. XZ: Data curation, Investigation, Writing–review and editing. LC: Data curation, Investigation, Writing–review and editing. ZH: Supervision, Writing–review and editing, Funding acquisition. YW: Conceptualization, Funding acquisition, Project administration, Writing–review and editing. YL: Conceptualization, Funding acquisition, Project administration, Resources, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science, Technology and Innovation Commission of Shenzhen Municipality (KCXFZ202002011006448, KCXFZ20211020164013021 and XMHT20220104019), the National Natural Science Foundation of China (No. 82272956), and the Guangdong Key R & D Project (2022B1111070005).
The authors would like to thank all patients and their families.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1364924/full#supplementary-material
Ahmad, T. R., and Haeusler, R. A. (2019). Bile acids in glucose metabolism and insulin signalling—mechanisms and research needs. Nat. Rev. Endocrinol. 15, 701–712. doi:10.1038/s41574-019-0266-7
Chang, S., Kim, Y. H., Kim, Y. J., Kim, Y. W., Moon, S., Lee, Y. Y., et al. (2018). Taurodeoxycholate increases the number of myeloid-derived suppressor cells that ameliorate sepsis in mice. Front. Immunol. 9, 1984. doi:10.3389/fimmu.2018.01984
Chen, T., Xie, G., Wang, X., Fan, J., Qiu, Y., Zheng, X., et al. (2011). Serum and urine metabolite profiling reveals potential biomarkers of human hepatocellular carcinoma. Mol. Cell. Proteomics. 10. M110.004945, doi:10.1074/mcp.M110.004945
Devarbhavi, H., Asrani, S. K., Arab, J. P., Nartey, Y. A., Pose, E., and Kamath, P. S. (2023). Global burden of liver disease: 2023 update. J. Hepatol. 79, 516–537. doi:10.1016/j.jhep.2023.03.017
Ding, C., Hong, Y., Che, Y., He, T., Wang, Y., Zhang, S., et al. (2022). Bile acid restrained T cell activation explains cholestasis aggravated hepatitis B virus infection. FASEB J. 36, e22468. doi:10.1096/fj.202200332R
Ding, L., Yang, L., Wang, Z., and Huang, W. (2015). Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm. Sin. B 5, 135–144. doi:10.1016/j.apsb.2015.01.004
Fuchs, C. D., and Trauner, M. (2022). Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 19, 432–450. doi:10.1038/s41575-021-00566-7
Hang, S., Paik, D., Yao, L., Kim, E., Trinath, J., Lu, J., et al. (2019). Bile acid metabolites control TH17 and Treg cell differentiation. Nature 576, 143–148. doi:10.1038/s41586-019-1785-z
Jia, X., Lu, S., Zeng, Z., Liu, Q., Dong, Z., Chen, Y., et al. (2020). Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatology 71, 893–906. doi:10.1002/hep.30852
Kasai, Y., Kessoku, T., Tanaka, K., Yamamoto, A., Takahashi, K., Kobayashi, T., et al. (2022). Association of serum and fecal bile acid patterns with liver fibrosis in biopsy-proven nonalcoholic fatty liver disease: an observational study. Clin. Transl. Gastroenterol. 13, e00503. doi:10.14309/ctg.0000000000000503
Kudo, M., Ikeda, M., Motomura, K., Okusaka, T., Kato, N., Dutcus, C. E., et al. (2020). A phase Ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J. Clin. Oncol. 38, 513. doi:10.1200/JCO.2020.38.4_suppl.513
Lee, P. C., Wu, C. J., Hung, Y. W., Lee, C. J., Chi, C. T., Lee, I. C., et al. (2022). Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor–treated unresectable hepatocellular carcinoma. J. Immunother. Cancer. 10, e004779. doi:10.1136/jitc-2022-004779
Li, W., Hang, S., Fang, Y., Bae, S., Zhang, Y., Zhang, M., et al. (2021). A bacterial bile acid metabolite modulates Treg activity through the nuclear hormone receptor NR4A1. Cell. Host Microbe 29, 1366–1377.e9. doi:10.1016/j.chom.2021.07.013
Liu, T., Yang, H., Fan, W., Tu, J., Li, T. W. H., Wang, J., et al. (2018). Mechanisms of MAFG dysregulation in cholestatic liver injury and development of liver cancer. Gastroenterology 155, 557–571. doi:10.1053/j.gastro.2018.04.032
Llovet, J. M., Kudo, M., Merle, P., Meyer, T., Qin, S., Ikeda, M., et al. (2023). Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial. Lancet Oncol. 24 (12), 1399–1410. doi:10.1016/S1470-2045(23)00469-2
Ma, C., Han, M., Heinrich, B., Fu, Q., Zhang, Q., Sandhu, M., et al. (2018). Gut microbiome–mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931. doi:10.1126/science.aan5931
Mao, J., Wang, D., Long, J., Yang, X., Lin, J., Song, Y., et al. (2021). Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer. 9, e003334. doi:10.1136/jitc-2021-003334
Meyer, C., Cagnon, L., Costa-Nunes, C. M., Baumgaertner, P., Montandon, N., Leyvraz, L., et al. (2014). Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257. doi:10.1007/s00262-013-1508-5
Petrick, J. L., Florio, A. A., Koshiol, J., Pfeiffer, R. M., Yang, B., Yu, K., et al. (2020). Prediagnostic concentrations of circulating bile acids and hepatocellular carcinoma risk: REVEAL-HBV and HCV studies. Int. J. Cancer. 147, 2743–2753. doi:10.1002/ijc.33051
Puri, P., Daita, K., Joyce, A., Mirshahi, F., Santhekadur, P. K., Cazanave, S., et al. (2018). The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 67, 534–548. doi:10.1002/hep.29359
Qin, S., Chan, S. L., Gu, S., Bai, Y., Ren, Z., Lin, X., et al. (2023). Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet 402 (10408), 1133–1146. doi:10.1016/S0140-6736(23)00961-3
Režen, T., Rozman, D., Kovács, T., Kovács, P., Sipos, A., Bai, P., et al. (2022). The role of bile acids in carcinogenesis. Cell. Mol. Life Sci. 79, 243. doi:10.1007/s00018-022-04278-2
Shen, R., Ke, L., Li, Q., Dang, X., Shen, S., Shen, J., et al. (2022). Abnormal bile acid-microbiota crosstalk promotes the development of hepatocellular carcinoma. Hepatol. Int. 16, 396–411. doi:10.1007/s12072-022-10299-7
Shi, L., Jin, L., and Huang, W. (2023). Bile acids, intestinal barrier dysfunction, and related diseases. Cells 12, 1888. doi:10.3390/cells12141888
Song, X., Sun, X., Oh, S. F., Wu, M., Zhang, Y., Zheng, W., et al. (2020). Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415. doi:10.1038/s41586-019-1865-0
Sun, L., Beggs, K., Borude, P., Edwards, G., Bhushan, B., Walesky, C., et al. (2016). Bile acids promote diethylnitrosamine-induced hepatocellular carcinoma via increased inflammatory signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G91–G104. doi:10.1152/ajpgi.00027.2015
Sun, R., Zhang, Z., Bao, R., Guo, X., Gu, Y., Yang, W., et al. (2022). Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J. Hepatol. 77, 453–466. doi:10.1016/j.jhep.2022.02.030
Tobin, R. P., Davis, D., Jordan, K. R., and McCarter, M. D. (2017). The clinical evidence for targeting human myeloid-derived suppressor cells in cancer patients. J. Leukoc. Biol. 102, 381–391. doi:10.1189/jlb.5VMR1016-449R
Toledo, J. B., Arnold, M., Kastenmüller, G., Chang, R., Baillie, R. A., Han, X., et al. (2017). Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement. 13, 965–984. doi:10.1016/j.jalz.2017.01.020
Wang, H., Shang, X., Wan, X., Xiang, X., Mao, Q., Deng, G., et al. (2016). Increased hepatocellular carcinoma risk in chronic hepatitis B patients with persistently elevated serum total bile acid: a retrospective cohort study. Sci. Rep. 6, 38180. doi:10.1038/srep38180
Wu, R., Yu, I., Tokumaru, Y., Asaoka, M., Oshi, M., Ishikawa, T., et al. (2022). Decreased bile acid metabolism and association with prognosis reflecting microbiome in tumor microenvironment involved in cancer cell proliferation in breast cancer. J. Clin. Oncol. 40, e12539. doi:10.1200/JCO.2022.40.16_suppl.e12539
Xie, G., Jiang, R., Wang, X., Liu, P., Zhao, A., Wu, Y., et al. (2021). Conjugated secondary 12α-hydroxylated bile acids promote liver fibrogenesis. EBiomedicine 66, 103290. doi:10.1016/j.ebiom.2021.103290
Xu, F., Jin, T., Zhu, Y., and Dai, C. (2018). Immune checkpoint therapy in liver cancer. J. Exp. Clin. Cancer Res. 37, 110. doi:10.1186/s13046-018-0777-4
Yang, X., Chen, B., Wang, Y., Wang, Y., Long, J., Zhang, N., et al. (2023). Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol. Int. 17 (3), 709–719. doi:10.1007/s12072-022-10480-y
Keywords: hepatocellular carcinoma, tyrosine kinase inhibitor, programmed death-1 inhibitor, biomarkers, bile acid, treatment response
Citation: Chen Y, Wang Y, Lei J, Chen B, Zhang X, Chang L, Hu Z, Wang Y and Lu Y (2024) Taurohyocholic acid acts as a potential predictor of the efficacy of tyrosine kinase inhibitors combined with programmed cell death-1 inhibitors in hepatocellular carcinoma. Front. Pharmacol. 15:1364924. doi: 10.3389/fphar.2024.1364924
Received: 03 January 2024; Accepted: 06 February 2024;
Published: 23 February 2024.
Edited by:
Norsham Juliana, Universiti Sains Islam Malaysia, MalaysiaReviewed by:
Khairun Nain Bin Nor Aripin, Islamic Science University of Malaysia, MalaysiaCopyright © 2024 Chen, Wang, Lei, Chen, Zhang, Chang, Hu, Wang and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Wang, eXVud0BzenUuZWR1LmNu; Yinying Lu, bHV5aW55aW5nMTk3M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.