
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 05 April 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1362739
 Rehab Ahmed1,2*
Rehab Ahmed1,2* Sawsan A. Zaitone3,4*
Sawsan A. Zaitone3,4* Asmaa K. K. Abdelmaogood5
Asmaa K. K. Abdelmaogood5 Huda M. Atef6
Huda M. Atef6 Mona F. M. Soliman7,8
Mona F. M. Soliman7,8 Alaa M. Badawy9
Alaa M. Badawy9 Howaida S. Ali10,11
Howaida S. Ali10,11 AbdelNaser Zaid12,13
AbdelNaser Zaid12,13 Hatem I. Mokhtar14
Hatem I. Mokhtar14 Lamiaa M. Elabbasy15,16
Lamiaa M. Elabbasy15,16 Emad Kandil17
Emad Kandil17 Asmaa Mokhtar Yosef18
Asmaa Mokhtar Yosef18 Rama I. Mahran19
Rama I. Mahran19Introduction: Betanin (C₂₄H₂₆N₂O₁₃) is safe to use as food additives approved by the FDA with anti-inflammatory and anticancer effects in many types of cancer cell lines. The current experiment was designed to test the chemotherapeutic effect of the combination of betanin with the standard chemotherapeutic agent, capecitabine, against chemically induced colon cancer in mice.
Methods: Bioinformatic approach was designed to get information about the possible mechanisms through which the drugs may control cancer development. Five groups of mice were assigned as, (i) saline, (ii) colon cancer, (iii) betanin, (iv) capecitabine and (v) betanin/capecitabine. Drugs were given orally for a period of six weeks. Colon tissues were separated and used for biological assays and histopathology.
Results: In addition, the mRNA expression of TNF-α (4.58-fold), NFκB (5.33-fold), IL-1β (4.99-fold), cyclin D1 (4.07-fold), and IL-6 (3.55-fold) and protein levels showed several folds increases versus the saline group. Tumor histopathology scores in the colon cancer group (including cryptic distortion and hyperplasia) and immunostaining for NFκB (2.94-fold) were high while periodic-acid Schiff staining demonstrated poor mucin content (33% of the saline group). These pathologic manifestations were reduced remarkably in betanin/capecitabine group.
Conclusion: Collectively, our findings demonstrated the usefulness of betanin/capecitabine combination in targeting colon cancer and highlighted that betanin is a promising adjuvant therapy to capecitabine in treating colon cancer patients.
Colon cancer typically begins as a growth called a polyp on the inner lining of the colon that becomes cancerous overtime (Fabregas et al., 2022). Its characteristics include uncontrolled cellular growth and proliferation involving the colonic crypt epithelial lining, emerging in the form of hyperplasia and gradually developing into an invasive carcinoma (Almet et al., 2020). Many experimental animal models have been established for studying the molecular pathogenesis of colon cancer and testing preventive nutritional and pharmacologic agents (Alshaman et al., 2022). A 1,2-dimethylhydrazine (DMHZ) model is a common chemically induced animal model of dysplastic colon and aberrant cryptic foci (ACF) that are considered prerequisites for colon cancer, which is widely used by researchers for studying colon cancer pathogenesis (Attia et al., 2021).
Commonly used chemotherapies for treating colon cancer comprise capecitabine, 5-fluorouracil, trifluridine, tipiracil, and oxaliplatin (Gill et al., 2003). Capecitabine is a prodrug that is often used alone or in combinations to treat different types of malignancies (Feng et al., 2020), including colon cancer (Knikman et al., 2020). Capecitabine is a fluoropyrimidine antimetabolite agent. Its mechanism of action as an antimetabolite leads to cell cycle arrest and blockage of DNA polymerase. Its pharmacokinetic properties show inter-individual variability; this may be attributed to the variation in enzyme activity (Deng et al., 2015). Capecitabine has estrogenic, cytotoxic, and teratogenic properties (Huo et al., 2020). Toxic adverse effects of capecitabine also include hair loss, myelosuppression, and gastrointestinal problems (Fernández et al., 2021). Hence, toxicity may raise concerns about the utilized doses. It is well known that capecitabine is frequently combined with other drugs (Pouya et al., 2021; Kibudde and Begg, 2022) to treat cancer. Treating cancer with a combination of nutraceuticals and anticancer drugs is a good strategy (Singh et al., 2018; Milella et al., 2023). Nowadays, capecitabine in combination with other non-chemotherapeutic agents has also been proven effective (Ruiz-Rabelo et al., 2011; Lin et al., 2020).
Betalains are water-soluble, orally bioavailable pigments that are extracted from beetroots (Vieira Teixeira da Silva et al., 2019). Red beet pigments have been recognized for their multiorgan anticancer effects in vitro and in vivo (Kapadia and Rao, 2012) and reduced chemically induced esophageal tumor incidence (Lechner et al., 2010).
Betanin (C₂₄H₂₆N₂O₁₃, betanidin-5-O-β-glucoside) is the most common betacyanin pigment in the plant kingdom. Betanin is a safe component for use as a food additive and food coloring and is approved by the FDA and the European Union (Vieira Teixeira da Silva et al., 2019). Studies have shown potential health benefits of betanin, primarily as an anti-inflammatory agent (ElSayed et al., 2023a). Betanin has anticancer effects and inhibits inflammatory cytokines in microglial cells (Ahmadi et al., 2020). It shows significant anticancer effects in human hepatic cell lines (Krajka-Kuźniak et al., 2013), U87MG human glioma cells (Salimi et al., 2021), growth of the breast cancer cell line MCf7 (Reddy et al., 2005), and Caco-2 colon cancer cells (Zielińska-Przyjemska et al., 2016). In addition, betanin inhibits the proliferation of the human colon cancer cell line HCT116 (Reddy et al., 2005). Betanin significantly reduced tumor multiplicity and tumor load after oral administration in female A/J mice (Zhang et al., 2013) but was not tested previously in vivo in combination with capecitabine.
To date, the effect of combining betanin with capecitabine has not been pursued. In the present study, our aim was to test the chemopreventive activity of the betanin/capecitabine combination in a model of chemically induced colon cancer and explore the possible inhibition of NFκB signaling and cyclin D1 protein using bioinformatic tools.
The KEGG database-refined tool, KEGG MEDICUS (www.kegg.jp/kegg/medicus), was used to explore the genes and proteins interacting in colon cancer. This health-related information tool integrates the KEGG network, disease, and drug with drug categories (ElSayed et al., 2023b; Kanehisa et al., 2023). Colon cancer was used as the entry category in the search box. Chemical carcinogenesis—receptor activation pathway (Pathway ID: hsa05207)—best described and reflected the postulation of the current experiment.
Two other freely available databases were also used to incorporate more targets, namely, Online Mendelian Inheritance in Man (OMIM), available at https://omim.org/ (Amberger et al., 2015), and DisGeNET (v7.0), available at http://www.disgenet.org/, with the gda index set at ≥ 0.1 for the simplification and summarization of the results (Piñero et al., 2019). Gene/protein lists from previous entries were exported from all the datasets and combined with for the removal of duplicates. This was followed by an investigation via FunRich software version 3.1.3 (www.funrich.org) (Pathan et al., 2015; Mohammad et al., 2023a) to annotate and visualize the overlapping genes.
The PubChem database (https://pubchem.ncbi.nlm.nih.gov) was accessed on the 10th of November 2023 and was used to obtain the isomeric Simplified Molecular-Input Line-Entry System (SMILES) for betanin (phytolaccanin) (Kim et al., 2023). The SMILES served as the input in the search box for the SwissTargetPrediction tool of the Swiss Institute of Bioinformatics database (http://www.swisstargetprediction.ch/) (Daina et al., 2019), SuperPred database (http://prediction.charite.de) (Dunkel et al., 2008), and STITCH (v 5.0) database (http://stitch.embl.de) (Kuhn et al., 2008). These tools were used to create a list of proteins/genes that are most likely targeted by this novel drug, all of which were combined and the duplicates removed.
FunRich software version 3.1.3 (www.funrich.org) was used again to reveal the shared putative targets between those of colon cancer and betanin and compare, identify, and visualize the overlapping genes.
The overlapping genes from the two lists were pasted into the gene enrichment graphical tool ShinyGO 0.77 (bioinformatics.sdstate.edu/go) for the exploration of the enriched pathways and the incriminated targets that require investigations (Ge et al., 2020).
A total of 60 male Swiss albino mice (8–10 weeks old with a body weight of 21–29 g) were purchased from Mustafa Rashed Company (Cairo, Egypt). The mice were housed in plastic cages in a normal diurnal cycle and with food and water available ad libitum. Acclimatization to the housing conditions was performed for 1 week, and then, the mice were randomly assigned to five groups. The experimental procedures were performed following the National Research Council’s Guide for the Care and Use of Laboratory Animals, and all procedures were performed in compliance with relevant laws and institutional guidelines. The experimental procedures were approved by the Research Ethics Committee of the Faculty of Pharmacy (202211RA9) and the Faculty of Medicine (Research 4664#) at the Suez Canal University. All measures were undertaken to minimize animal suffering. The experimental reports were performed following the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
In this study, 1,2,-dimethylhydrazine (DMHZ; Sigma-Aldrich, United States) was used to induce colon cancer in mice, as reported previously (Hamiza et al., 2012; Bahr et al., 2021). In brief, DMHZ was dissolved according to the manufacturer’s instructions and diluted in phosphate-buffered saline (PBS) before use. The mice were randomly assigned to negative control and test groups (n = 12). The mice in the saline group received intraperitoneal injections of PBS once weekly for 12 weeks. The mice in the test group received the DMHZ solution (I.P.) once weekly for 12 weeks at 25 mg/kg. The other groups were divided into “colon cancer” control (DMHZ-treated for 12 weeks) and drug-treated groups (Figure 1). Betanin (901266-1G, molecular weight = 550.5 g/mol) was obtained from Sigma-Aldrich and dissolved in distilled water, while capecitabine was a product of Roche (California, United States).
The different groups can be summarized as follows:
Group I: Mice treated with PBS injections weekly at the same time as DMHZ injections.
Group II: Mice treated with DMHZ (I.P.) once weekly for 12 weeks (Hamiza et al., 2012; Bahr et al., 2021).
Group III: Mice treated with DMHZ (I.P.) weekly for 12 weeks and treated with oral doses of capecitabine (60 mg/kg; Roche) every 48 h for 6 weeks (Manu et al., 2014).
Group IV: Mice treated with DMHZ (I.P.) weekly for 12 weeks and treated with oral doses of betanin (50 mg/kg; Sigma-Aldrich, MO, United States) (ElSayed et al., 2023a) every day for 6 weeks.
Group V: Mice treated with DMHZ (I.P.) weekly for 12 weeks and treated with capecitabine and betanin—in the same aforementioned schedule—for 6 weeks.
In general, therapeutic regimens were administered by oral gavage starting from week 7 until the end of week 12. The animals were inspected daily to investigate any signs of distress. At the end of the experiment, the mice were anesthetized using ketamine and euthanized by cervical dislocation.
We rapidly dissected the descending colon, washed it with PBS, and divided it into two portions, as described previously (Attia et al., 2021). One portion was fixed in 10% paraformaldehyde for immunohistochemical analysis. Three other portions were quickly frozen at −80°C for further analysis.
Tumoral homogenates were obtained by homogenizing a frozen colon specimen in RIPA buffer and used to estimate TNF-α (SEA133Mu) and NFκB (SEB824Mu) levels using kits provided by Cloud-Clone Corp. (Katy, TX, United States). IL-1β (Cat. No. 432601, BioLegend, San Diego, CA, United States) and IL6 (201-02-0050, SunRedBio, China) kits were utilized to determine these parameters.
From the colon tissue (50 mg) homogenate, the total RNA was extracted utilizing TRIzol (Invitrogen, United States). The quantity (RNA yield) and purity (A260/230 and A260/280 ratios) of the total RNA were measured using the NanoDrop ND-1000 spectrophotometer (Thermo Scientific, United States).
Complementary DNA (cDNA) was synthetized from 1 μg RNA utilizing an Applied Biosystems High-Capacity cDNA Reverse Transcription Kit (United States). Real-time PCR was conducted using the SYBR Green Master Kit (Fermentas, United States) and Applied Biosystems software version 3.1 (StepOneTM, United States). Twenty microliters were utilized for the quantitative real-time PCR (qRT-PCR). A housekeeping control gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), was utilized in order to normalize the expression of the genes and selected based on a previous study (Attia et al., 2021). The comparative Ct method (2−ΔΔCT) was employed for the calculation of the fold changes in gene expression (Livak and Schmittgen, 2001). These Ct values were calculated using StepOne Real-Time PCR detection software. Primer3 software (version 4.1.0) was used to allocate the primer sets, and the specificity of these sets was determined using the Primer-BLAST program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Table 1 shows the list of primer sets.
The formalin-fixed tissues were dehydrated in ascending ethanol, followed by clearing tissues in xylene and embedding them in liquid paraffin wax. Cut sections (5 µm) were prepared and stained with hematoxylin and eosin (H&E) staining, Feulgen staining, and periodic acid Schiff (PAS) stain following standard protocols. PAS staining is usually used to stain neutral mucins. Mucins are mucopolysaccharides, which are long chains of sugar molecules found throughout the body, essential for life, and significant in maintaining the structural integrity of bone, cartilage, skin, elastic tissue, and membranes. They are important in cell growth as they help regulate the flow of nutrients between capillaries and cells and are known as the “glue of life.”
Additionally, the cut sections were subjected to antigen retrieval in the Tris-EDTA solution (pH 9) and blocked in 5% normal goat serum, as detailed previously (Mohammad et al., 2023b). Next, an anti-NFκB antibody (diluted as 1:100; Cat. #RB-1638-R7, Thermo Scientific, Fremont, CA 94538, United States) was added to colon sections, followed by the appropriate biotin-conjugated secondary antibodies (Catalog #BSB 0205, Mouse–Rabbit PolyDetector, DAB HRP Brown Detection System). Lastly, the tissue sections were stained with the DAB reagent and counterstained using Mayer’s hematoxylin (Ali et al., 2019). The slides were examined under a light microscope [Leica, Model: DM 1000, Wetzlar, Germany] equipped with a PC-driven digital camera [Leica, Wetzlar, Germany]. H&E-stained sections were blindly scored according to the dysplastic and histopathological changes, as detailed previously (Alshaman et al., 2022), whereas the Feulgen-stained area, PAS-stained goblet cell area, and immunohistochemically stained sections were examined and identified using ImageJ software (NIH, Bethesda, United States) following previously described procedures (Ali et al., 2015).
The general architecture of colon specimens stained with H&E was inspected, and imaging was done for A) colon dysplasia, B) hyperplasia, C) cryptic distortion, D) stromal cell infiltration (the degree of cell invasion into the connective tissue surrounding the tumor), and E) goblet cell depletion. ACF are defined as “clusters of abnormal tube-like glands in the lining of the colon and rectum.” ACF form before colorectal polyps and are one of the earliest changes that can be observed in the colon that may lead to cancer.
Blind scoring was performed according to 0–3 grading based on the severity of the findings. Next, the grand score was calculated for each group and presented as medians. In addition, we measured the area % of inflammatory filtrates and the thickness of mucosal layers in two sections per mouse and used six mice from each group. The images were analyzed on an Intel® Core I7®-based computer using VideoTest Morphology® software (Russia).
After data collection, they were presented as the mean ± SD. We set a p-value < 0.05 as the acknowledged significance level. The variances between the experimental groups were estimated using the ANOVA test, followed by Bonferroni’s test to show the pair-wise group comparisons.
For colon cancer, the list generated from the three datasets was investigated using FunRich software version 3.1.3, which revealed shared putative targets, and we compared the lists (Figure 2A). Four genes were consistent in the three databases (AKT1, CCND1, PIK3CA, and SRC).
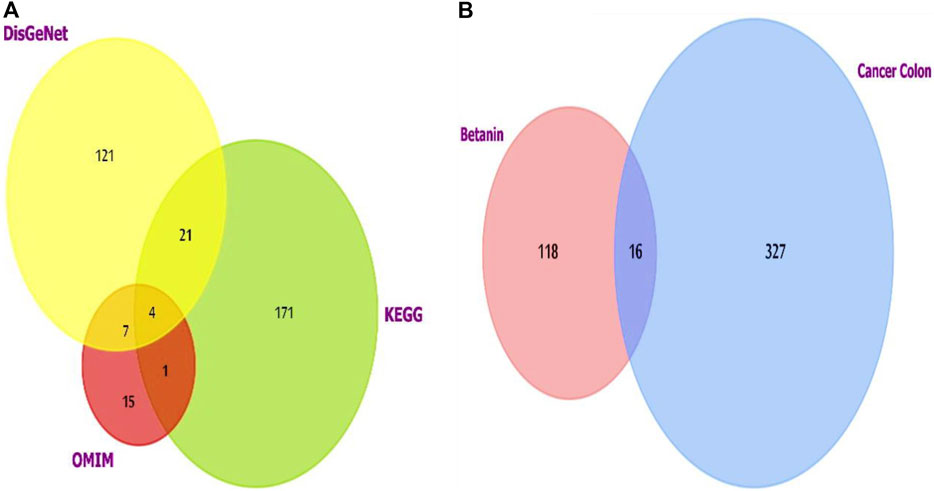
Figure 2. (A) Venn diagram showing the overlapping targets of the three databases used to investigate colon cancer-targeted proteins/genes. CCND1 was one of the four overlapped targets. (B) Venn diagram showing the shared targets of both colon cancer and betanin. Analysis and figure construction were done using FunRich software version 3.1.3.
G1/S-specific cyclin D1 (CCND1) was chosen for the downstream experiment due to its pivotal role in the cell cycle and carcinogenesis as it is a part of various cellular complexes that represent major integrators of many mitogenic and antimitogenic signals.
Colon cancer and betanin targets also showed intercepting shared targets, as explored using FunRich (Figure 2B). The 16 targets included many genes from which NFκB1 was chosen for estimation. It is a pleiotropic transcription factor existing in nearly all types of cells. Moreover, it is a cross-point in various signal transduction events that are triggered by chronic inflammation, apoptosis, and oncogenesis. TNF-α, IL-1β, and IL-6 are pivotal inducers of the NFκB complex and also one of its target genes; therefore, they will be included in future measurements. The addition of cyclin D will help understand the effect of the novel drug on cell cycle progression in normal and transformed scenarios through its relation to the growth-promoting effects of NFκB.
The in-depth gene characteristic analysis and exploration of the enrichment using ShinyGO 0.77 of those inter-shared genes revealed intercalating and overlapping networks that all seemed to be strongly involved in the pathway proposed by our protocol as chemical carcinogenesis receptor activation pathways and microRNAs in cancer (Figures 3A, B).
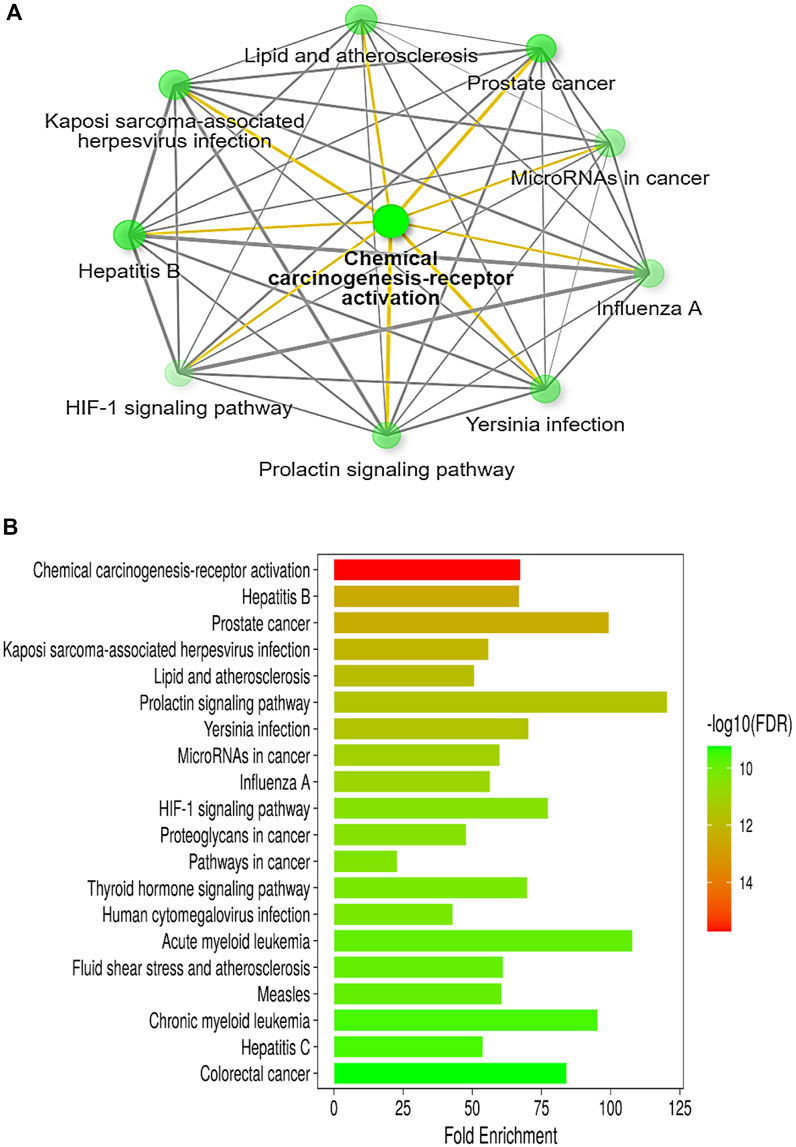
Figure 3. (A) Illustrative network of the top pathways (sorted by fold enrichment) targeted by colon cancer and betanin according to the KEGG pathway database. (B) A bar-plot chart of the top 20 pathways, sorted by FDR, enriched in both colon cancer and betanin-predicted target pathways. Nearly all the intercalating pathways are related to carcinogenesis and chronic insults. Both figures were constructed using the network tool, ShinyGO 0.77 software (bioinformatics.sdstate.edu/go).
The current results demonstrated elevated levels for TNF-α (376.2 ± 20.9 vs. 94.8 ± 18.3) and NFκB (3,287.7 ± 264.7 vs. 724.5 ± 79.1) (Figures 4A, B) and for the downstream products IL-1β (446.9 ± 23.3 vs. 85.25 ± 13.5) and IL-6 (258.0 ± 34.7 vs. 20.7 ± 9.8) (Figures 4C, D) in the colon cancer group versus the saline group. Per se treatment with betanin or capecitabine reduced the level of these inflammatory parameters compared to the colon cancer group. Interestingly, the betanin/capecitabine combination suppressed these inflammatory parameters to a greater extent than the betanin per se treatment (p < 0.05; Figures 4A–D).
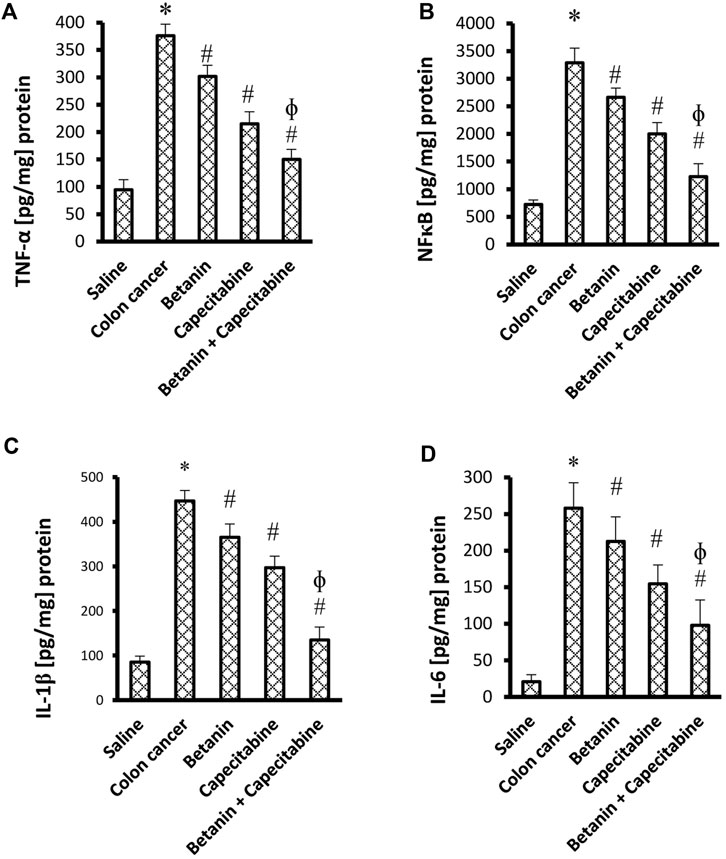
Figure 4. Impact of the betanin/capecitabine combination on tissue inflammatory parameters: (A) NFκB, (B) TNF-α, (C) IL-1β, and (D) IL-6. The data are presented as the mean ± SD and compared at p < 0.05. [*] versus saline control, [#] versus colon cancer control, and [Ф] versus the betanin group, n = 6.
Figures 5A–E show significant increases in the mRNA expression of TNF-α (4.58-fold), NFκB (5.33-fold), IL-1β (4.99-fold), cyclin D1 (4.07-fold), and IL-6 (3.55-fold) in the colon cancer group compared to the saline group (p < 0.05; Figures 5D–G). Therapeutic doses of the betanin/capecitabine combination significantly downregulated the mRNA expression of the parameters TNF-α (Figures 5A), NFκB (Figures 5B), IL-1β (Figures 5C), IL-6 (Figures 5D), and cyclin D1 (Figures 5E) compared to the colon cancer control.
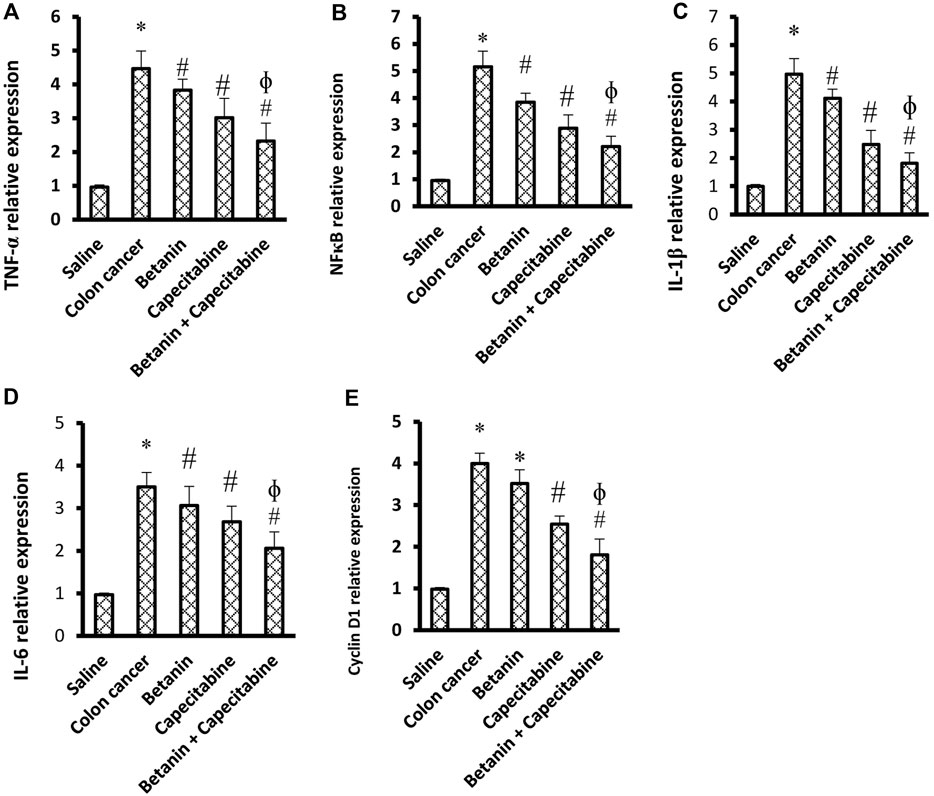
Figure 5. Impact of the betanin/capecitabine combination on tissue inflammation parameters and cyclin D1 in colon tissue specimens: (A) TNF-α, (B) NFκB, (C) IL-1β, (D) IL-6, and (E) cyclin D1. Data are presented as the mean ± SD and compared at p < 0.05. [*] versus saline control, [#] versus colon cancer control, and [Ф] versus the betanin group.
Hematoxylin and eosin staining shown in Figure 6 demonstrate colon specimens from the saline group. Panels A–A2 show H&E staining for sections from the saline control group. Colon sections of the saline group showed the mucosal layer containing many tubular intestinal glands cut transversely or longitudinally; the glands extend as deep as the muscularis mucosa. The submucosal layer consists of well-vascularized connective tissue, followed by the muscularis externa of the inner circular and outer longitudinal layers (ME). Panels B–B2 show sections from the colon cancer group showing hyperplasia and irregular-shaped mucosa lined by a dysplastic epithelium of hyperchromatic nuclei and columnar cells with pyknotic nuclei. Our results showed histological changes in the crypt glands, defined as hyperplastic and dysplastic changes. This is obviously shown in the H&E-stained sections (B1–B2) and in Supplementary Figure S1.
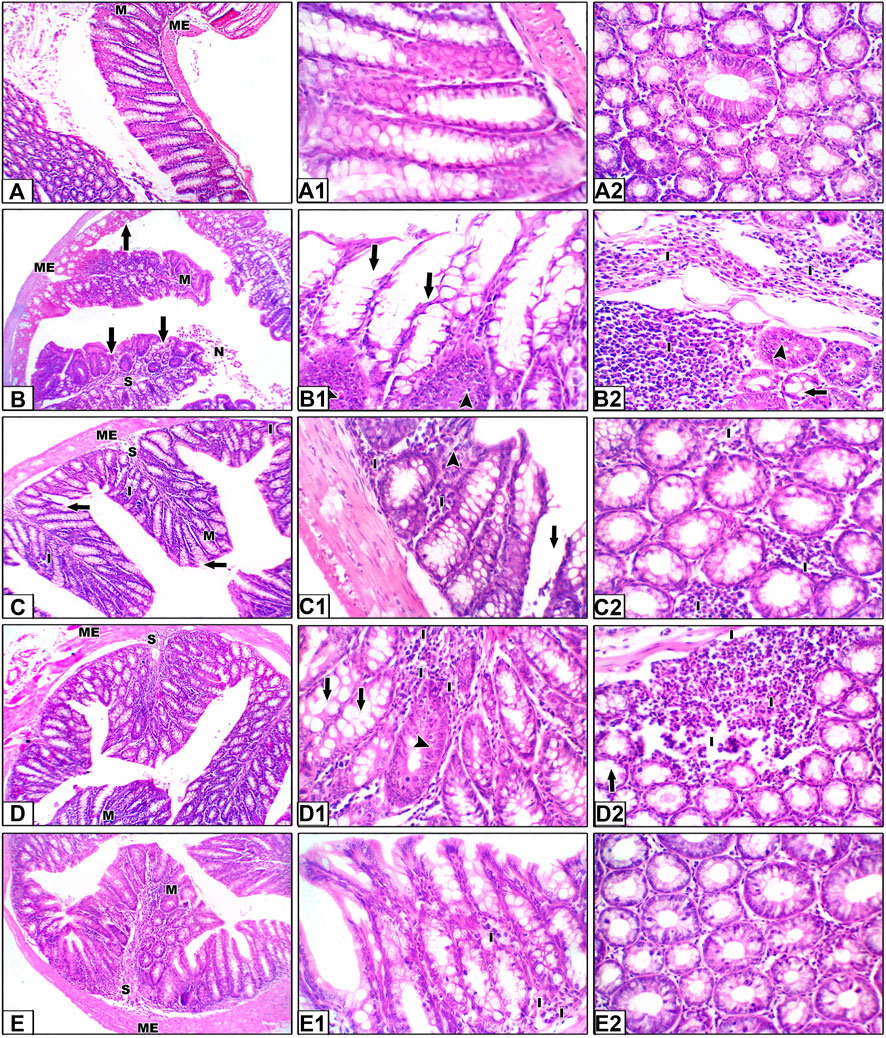
Figure 6. Hematoxylin and eosin staining for colon specimens. Panels (A–A2) Saline control group showing the mucosal layer (M) with normal-shaped crypts (C) lined by simple columnar epithelia with goblet cells, with minimal free lymphocytes in between. The submucosal connective tissue layer (S) is followed by the muscularis externa (ME). Panels (B–B2) Sections from the colon cancer group, distorted crypts lined by a dysplastic epithelium and goblet cells (arrows). Some crypts show hyperplastic changes (head arrows). Areas of dirty necrosis are observed in the lumen. A severe inflammatory infiltrate is noted in the connective tissue of the lamina propria and submucosa, even infiltrating the muscularis layer (I). Panels (C–C2) Sections from the capecitabine group show some disrupted crypts and a mild inflammatory infiltrate (I). Panels (D–D2) Sections from the betanin group showing few disrupted crypts and a moderate inflammatory infiltrate (I). Panels (E–E2) Sections from the betanin/capecitabine group showing normal crypts with minimal inflammatory infiltrate (I). Power × 100 (A, B, C, D, E) and power × 400 (A1, A2, B1, B2, C1, C2, D1, D2, E1, and E2).
In panels C–C2, H&E staining for sections from the capecitabine group show moderate dysplasia of the epithelial lining, with some disrupted crypts and severe inflammatory infiltrates, whereas sections from the betanin group show partial dysplasia of the epithelial lining, with few disrupted crypts and moderate inflammatory infiltrates (Panels D–D2). Finally, H&E staining for sections from the betanin/capecitabine group show minimal dysplasia of the epithelial lining and normal crypts. Few inflammatory infiltrates are observed (Panels E–E2).
Furthermore, histopathologic scoring indicated greater scores for colon dysplasia (Figure 7A), hyperplasia (Figure 7B), cryptic distortion (Figure 7C), stromal cell infiltration (Figure 7D), goblet cell depletion (Figure 7E), and total histopathology (Figure 7F) in the colon cancer group than those in the saline group. Treatment with the betanin/capecitabine combination produced significant reductions in the individual scores and the total score compared to the colon cancer group (p < 0.05; Figure 7). In addition, the colon cancer group showed high mucosal thickness and leukocyte infiltrated compared to the saline group.
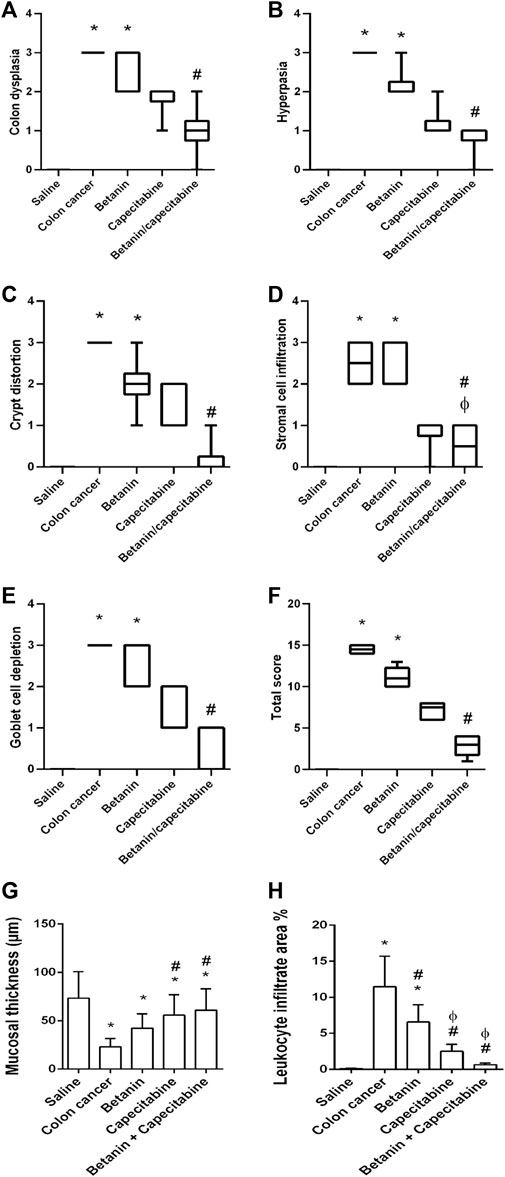
Figure 7. Scores of histopathology findings in colon sections in the experimental groups. (A) Colon dysplasia, (B) hyperplasia, (C) cryptic distortion, (D) stromal cell infiltration, (E) goblet cell depletion, (F) total histopathology, (G) mucosal thickness, and (H) leukocyte infiltrate area%. Data from six mice are graphically presented as boxplots, demonstrating the median value and the quartiles. [*] versus saline control, [#] versus colon cancer control, and [Ф] versus the betanin group at p < 0.05.
Figure 8 shows microscopic images of the colon sections stained with Feulgen stain, which represents nuclear staining for DNA. Feulgen-stained colon sections in the saline group show dark-stained nuclei of cell lining crypts (Figures 8A,A1) in a regular arrangement. The colon cancer control group (Figures 8B,B1) shows faint staining with nuclei showing pyknosis or fragmentation. The capecitabine group (Figures 8C, C1) shows some cells with dark nuclei and others with faint nuclei. The betanin group (Figures 8D, D1) shows more faint-stained nuclei than the saline group, whereas the betanin/capecitabine group (Figures 8E, E1) shows a marked increase in the number of dark-stained nuclei. Panel 8F represents the mean area of staining in the groups and shows that the betanin/capecitabine group produced a significant increase in Feulgen staining in nuclei compared (p < 0.05) to both the colon cancer group and betanin monotherapy group.
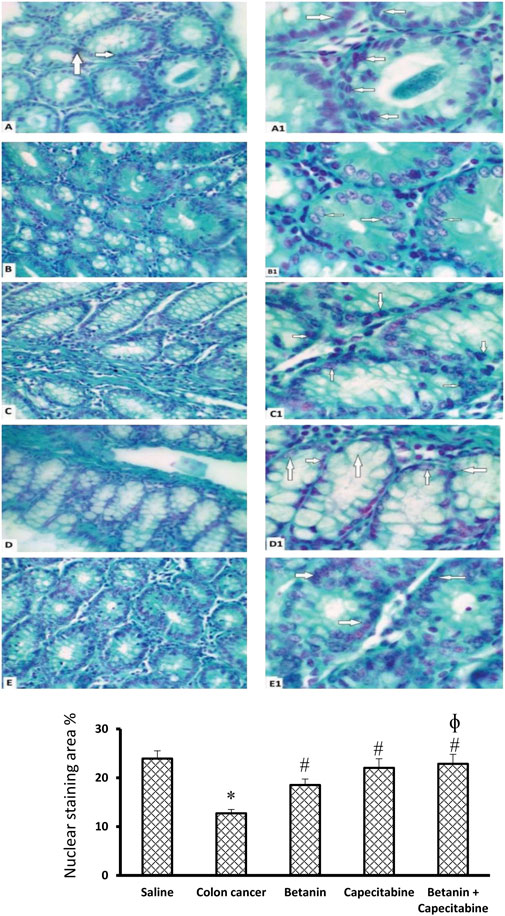
Figure 8. Microscopic images of colon sections stained with Feulgen stain. Photomicrograph of Feulgen-stained colon sections showing nuclei (white arrows) of cell lining crypts of the saline group (A, A1) dark-stained nuclei with regular shape and arrangement. The colon cancer control group (B, B1) showing faint-stained nuclei with some pyknotic nuclei, and others are fragmented. The capecitabine group (C, C1) shows some cells with dark-stained nuclei (white arrows) and others with faint-stained nuclei. The betanin group (D, D1) shows more faint-stained nuclei (white arrows) than the normal group, whereas the betanin/capecitabine group (E, E1) shows a marked increase in the number of dark-stained nuclei (white arrows). Power × 400 (A, B, C, D, E) and ×1,000 (A1, B1, C1, D1, E1). (F) Column charts for the PAS-positive stained area. Data from six mice are graphically presented as boxplots, demonstrating the median value and the quartiles. [*] versus saline control and [#] versus colon cancer control at p < 0.05.
Figure 9 shows the PAS reaction in the mucosal goblet cells. The saline group revealed abundant PAS-positive staining in goblet cells (Panels 9A, 9A1, 9A2). The colon cancer group showed a marked reduction in the number of stained PAS-positive goblet cells (Panels 9B, 9B1, 9B2). The capecitabine group displayed a reduced number of positive goblet cells (Panels 9C, 9C1, 9C2). The betanin-treated group showed a moderate number of stained positive cells (Panels 9D, 9D1, 9D2), and the betanin/capecitabine group showed marked increases in positive-stained goblet cells (Panel 9E, 9E1, 9E2). The mean area of PAS staining was enhanced in the betanin/capecitabine group compared (p < 0.05) to both the colon cancer group and betanin monotherapy group (Panel 9F).
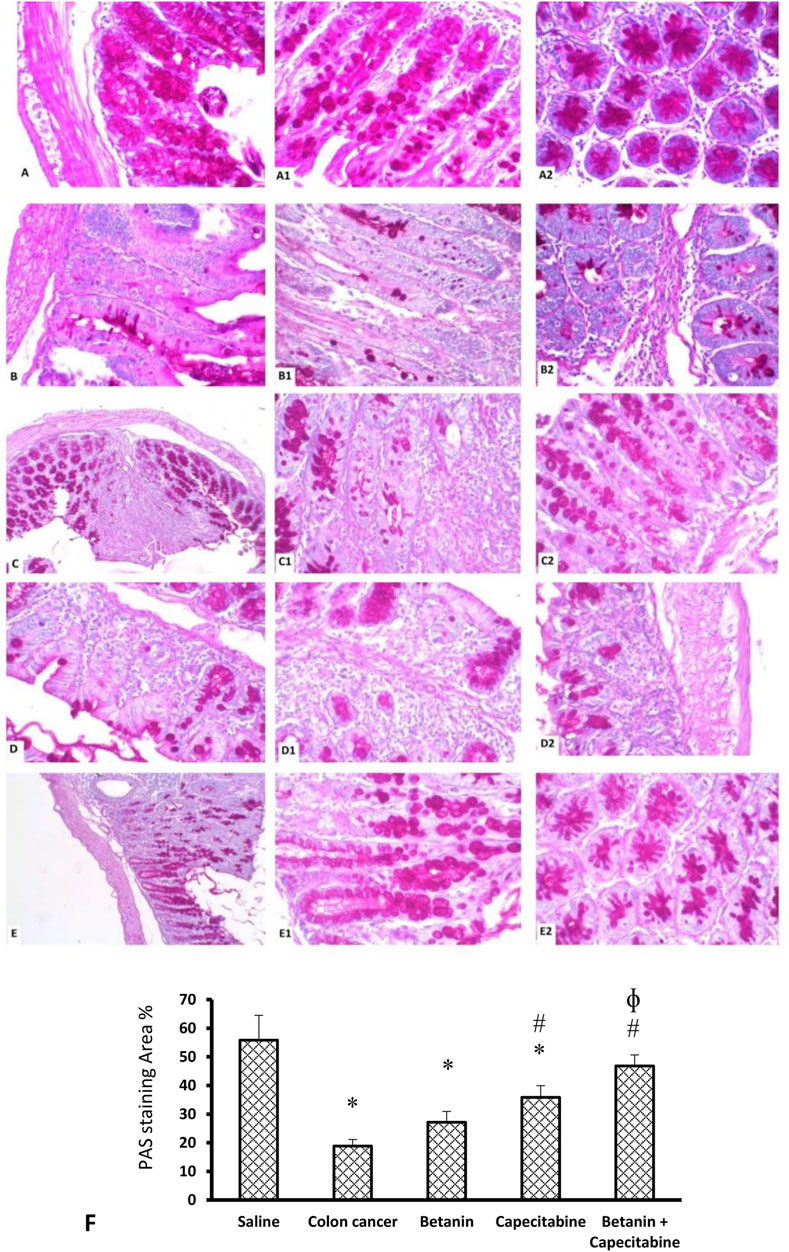
Figure 9. Microscopic images for colon sections stained with periodic acid Schiff stain. Panels (A, A1, A2) Saline group revealed abundant staining of PAS-positive goblet cells. Panels (B, B1, B2) Colon hyperplasia group shows a marked reduction in the number of stained PAS-positive goblet cells. Panels (C, C1, C2) The capecitabine group displays an increased number of positive goblet cells compared to the colon cancer control. Panels (D, D1, D2) The betanin-treated group shows a mild number of stained-positive cells. Panels (E, E1, E2) show a marked increase in positive-stained goblet cells in the betanin/capecitabine group. (A–E) Power × 100 (A1, B1, C1, D1, E1) and × 400 (A2, B2, C2, D2, E2). (F) Column charts for the PAS-positive stained area. Data from six mice are graphically presented as boxplots, demonstrating the median value and the quartiles. [*] versus saline control and [#] versus colon cancer control at p < 0.05.
Figure 10 shows immunostaining for NFκB. Immunostained colon sections from the saline group showed negative staining (19.2 ± 3.8; Panels 10A and 10A1), whereas sections from the colon cancer control group showed a strong reaction within a hyperplastic colon (56.4 ± 8.1; Panels 10B and 10B1). Immunostained colon sections from the capecitabine group showed a mild–moderate positive reaction (43.8 ± 3.4; Panels 10C and 10C1), whereas the betanin group showed a moderate reaction (54.38 ± 5.5; Panels 10D and 10D1). The photomicrograph of immunostained colon sections from the betanin/capecitabine group showed minimal reaction compared to other groups (36.33; Panels 10E and 10E1). Panel 10F represents the mean area of staining in the groups and shows that the betanin/capecitabine group produced a significant decrease in immunostaining compared (p < 0.05) to both the colon cancer group and betanin per se group.
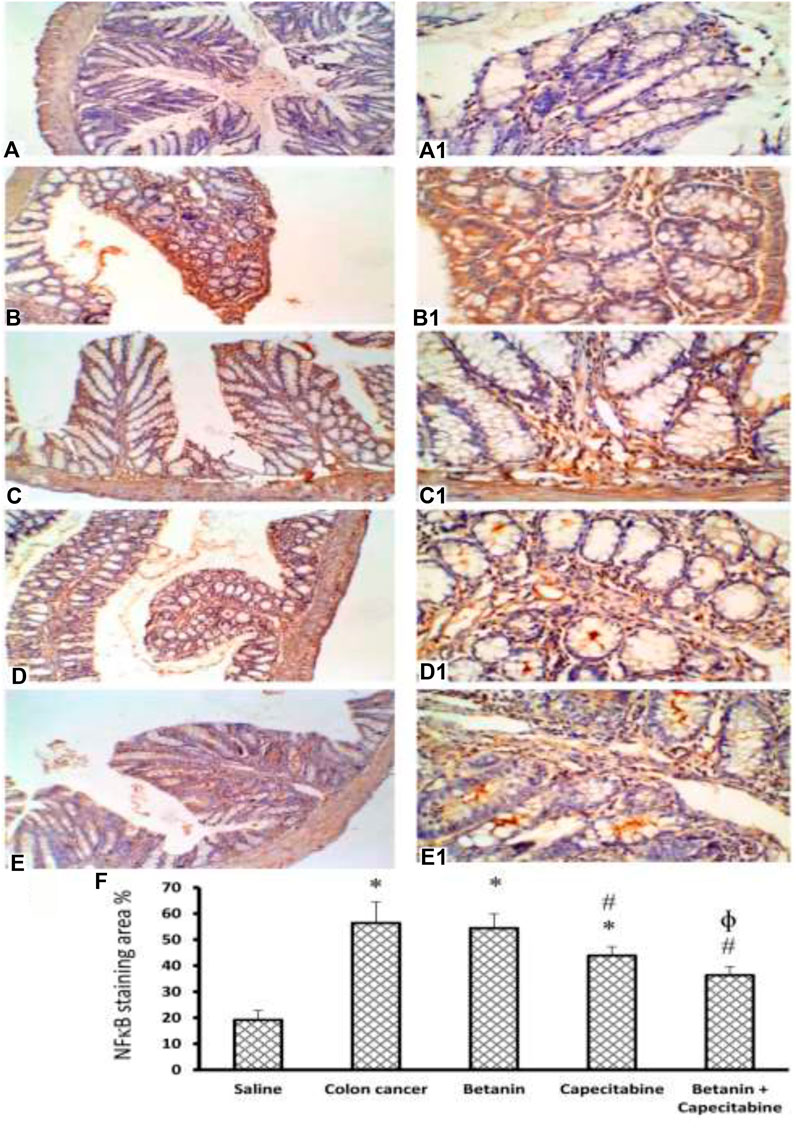
Figure 10. Microscopic images for colon sections stained for NFκB. Panels (A, A1) Photomicrograph from the saline group shows negative staining. Panels (B, B1) Photomicrograph of immunostained colon sections from the colon cancer control group shows a strong reaction within the hyperplastic colon. Panels (C, C1) Photomicrograph of the capecitabine group shows a mild positive reaction compared to other groups (D, D1). Panels (E, E1) Photomicrograph of immunostained colon sections from the betanin/capecitabine group shows minimal reaction compared to other groups. Power × 100 (A, B, C, D, E) and power × 400 (A1, B1, C1, D1, E1). Panel (F) Column charts for the caspase 3-positive stained area. Data from six mice are graphically presented as boxplots, demonstrating the median value and the quartiles. [*] versus saline control and [#] versus colon cancer control at p < 0.05.
Increased consumption of fruits and vegetables is a very effective strategy for enhancing antioxidant intake and decreasing oxidative stress and may result in a reduction in the risk of developing chronic diseases, such as cancer (Song et al., 2010).
In the current study, capecitabine per se controlled tumor development pathologic parameters. Cancer chemotherapies are non-invasive agents that lead to delaying, inhibition, or reversing carcinogenesis. Despite that, various chemotherapies have been established, and there are still high numbers of recorded mortalities and morbidities among cancer patients (Kundu et al., 2006). Hence, natural agents with potential antitumor and anti-inflammatory activities are very promising as adjuvant therapies with standard chemotherapies. Hence, in this research article, we focused on exploring the chemotherapeutic potential of the betanin/capecitabine combination versus each per se regimen.
Our study revealed histopathological and immunohistochemical changes in the current chemically induced colon cancer mouse model. ACF were originally recognized on the colonic mucosa of the rodents subjected to chemical colorectal carcinogens and have been long considered preneoplastic lesions (Mori et al., 2005). In the present study, DMHZ was employed to induce colon cancer in mice. DMHZ induced various pathologic manifestations such as crowded and proliferating tubular glands, which were characterized by irregular sizes and shapes in addition to distortion in crypts. The mucosal lining demonstrated hyperchromatic nuclei accompanied by severe levels of inflammatory infiltration, as shown in the lamina propria, submucosa, and muscularis layer. Importantly, these pathologic manifestations were partly alleviated in groups receiving the betanin/capecitabine combination or each per se therapeutic intervention. These results are supported by those of similar studies using chemical carcinogens in mouse in vivo experiments (Abd El-Fadeal et al., 2021; Mohamed et al., 2022).
On the other hand, Feulgen staining is widely used to demonstrate the DNA concentration in tissue specimens. Our results showed a moderate degree of magenta of the Feulgen reaction, reflecting the DNA concentration in the colon specimens of the saline group accompanied by normally distributed columnar epithelia in the colon crypts. Moreover, the significant decrease in magenta in the colon cancer control group reflects DNA damage that was partly restored in groups receiving various treatment strategies. It is known that chemical carcinogens modify the molecular structure of DNA, produce genetic errors, and lead to mutations throughout DNA synthesis. The formation of DNA adducts results in the activation of proto-oncogenes or inactivation of tumor suppressor genes, which is assumed as a tumor initiator step (Hursting et al., 1999).
Quantitative analysis of data on the current colon specimens stained with PAS showed different extents of PAS staining among the study groups, and the greatest difference was recorded between the colon cancer control group and the saline group. Similar results were obtained previously (Mondal et al., 2014; Bahr et al., 2021) as the authors documented a decrease in PAS staining in the hyperplastic colons affected by a chemical carcinogen compared to normal colon samples. These results correspond with the results obtained by other authors (Danquah et al., 2017; Kasprzak et al., 2019). The PAS technique is a very versatile and commonly utilized technique for demonstrating mucin, carbohydrates, and glycoproteins. PAS staining is sensitive particularly for detecting neutral mucins and acid mucins, which incorporate substantial concentrations of sialic acid (Ullah, 2012).
Mucins are complex carbohydrates excreted by the epithelia and connective tissues. Mucin glycoproteins play a crucial role in intestinal protection from injuries; however, the protective mechanism and probable relationship between the structure and function of mucins are only partly known. From a structural point of view, purified intestinal mucins are large-molecular weight glycoproteins of different compositions according to the region and developmental stage. Mucin glycoproteins are implicated in the pathology of epithelial malignancy (Niv, 1994). Modifications in mucin expression patterns have been described in carcinomas and their precursor lesions (Hakomori, 1989).
In the current study, NFκB expression in the colon was elevated in the DMHZ-induced colon cancer control group; this was evident from the mRNA expression and strong immunohistochemical reaction. The mechanisms of NFκB activation in colon cancer are yet to be completely described. Many factors can trigger this activation, such as inflammation mediators, bacterial products, and reactive oxygen species (Baeuerle, 1991; Barnes and Karin, 1997). Indeed, the activation of NFκB in tumors is thought to be associated with malignancy (Raziuddin et al., 1997).
The current results demonstrated the upregulated expression and protein levels of inflammatory mediators (NFκB, TNF-α, IL-1β, and IL-6). Inflammation is mediated through accumulating different immune and inflammatory cells and inflammatory molecules. The interaction between these cells and cytokines results in generating signals that encourage the growth and progression of tumor cells. There is a clearly identified relationship between inflammation and colon cancer, and factors that initiate inflammation can establish colon cancer (Janakiram and Rao, 2014).
Betanin can, at least partly, through anti-inflammatory activity, abate these inflammatory mediators. A recent research paper documented that using gamma-tocopherol and aspirin synergistically defeats colitis-associated colon carcinogenesis and inhibits the growth of human colon cancer cells (Liu et al., 2023). Similarly, it was reported that decreased colon cancer mortality was observed with the regular use of NSAIDs (Zell et al., 2009). In observational studies, aspirin and non-steroidal anti-inflammatory drugs decrease the colon cancer incidence (Nan et al., 2015). Therefore, agents with anti-inflammatory activities are very promising as adjuvant therapies with standard chemotherapies.
The current results demonstrated upregulated expression levels of cyclin D1. Cyclins are chief regulating factors for the progression of the cell cycle and tumor survival. In particular, cyclins D (cyclin D1, cyclin D2, and cyclin D3) are vital intermediaries between proliferation pathways and the cell cycle machinery in the nucleus. Dysregulated expression of cyclins leads to impairing cancer development and carcinogenesis (Pawlonka et al., 2021).
Substantial attention has been focused on the use of beetroot extract or its ingredients for dietary supplementation for preventing carcinogenesis (Gescher et al., 1998; Lee et al., 2004; Stintzing and Carle, 2004; Pan and Ho, 2008; Song et al., 2010). This evidence depends on its apparent capacity to control overoxidative stress that initiates and aggravates cancer and the predominant agreement that long-term exposure to fine amounts of diets rich in antioxidants has cancer chemopreventive potential (Stanner et al., 2004; Azeredo, 2009; Boivin et al., 2009). Betanin has anti-inflammatory activity and suppresses NFκB in rats subjected to acute renal injury (Tan et al., 2015). Similarly, Reddy et al. showed that betanin suppressed 97% of cyclooxygenase-2 (COX-2) enzyme activity (Reddy et al., 2005).
Betanin was confirmed for its anticancer effects and showed significant anticancer effects in human hepatic cell lines (Krajka-Kuźniak et al., 2013), inhibited inflammatory cytokines in microglial cells (Ahmadi et al., 2020), and induced cytotoxicity in U87MG human glioma cells (Salimi et al., 2021). Betanin also inhibits the growth of the breast cancer cell line MCf7 (Reddy et al., 2005) and initiates apoptosis in Caco-2 colon cancer cells (Zielińska-Przyjemska et al., 2016). In addition, betanin inhibits the proliferation of the human colon cancer cell line HCT116 (Reddy et al., 2005). Betanin significantly reduced tumor multiplicity and tumor load after oral administration in female A/J mice (Zhang et al., 2013) but was not tested previously in combination with capecitabine. Hence, the identification of natural compounds that are effective and safe combined with conventional chemotherapeutic drugs can be an effective strategy to combat colon cancer and improve the therapeutic outcome.
The present study highlighted for the first time that betanin augments the anticancer effect of capecitabine and provides evidence that the mechanism was, at least partly, mediated via its strong anti-inflammatory activity besides the downregulation of cyclin D1 expression.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the Research Ethics Committee of the Faculty of Pharmacy, Suez Canal University, Ismailia, Egypt. The study was conducted in accordance with the local legislation and institutional requirements.
RA: conceptualization, funding acquisition, validation, visualization, and writing–original draft. SZ: conceptualization, formal analysis, investigation, methodology, project administration, visualization, and writing–review and editing. AA: investigation, software, visualization, and writing–original draft. HA: methodology, software, visualization, and writing–original draft. MS: methodology, software, visualization, and writing–original draft. AB: resources, validation, visualization, and writing–review and editing. HA: conceptualization, validation, visualization, and writing–original draft. AZ: resources, validation, visualization, and writing–original draft. HM: formal analysis, software, visualization, and writing–review and editing. LE: investigation, methodology, visualization, validation, writing–original draft. EK: conceptualization, validation, visualization, and writing–review and editing. AY: formal analysis, visualization, writing–original draft, and writing–review and editing. RM: conceptualization, resources, visualization, and writing–original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deanship of Scientific Research at the University of Tabuk for funding this work through research no. S-1443-0193.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1362739/full#supplementary-material
Abd El-Fadeal, N. M., Nafie, M. S., El-kherbetawy, K., El-mistekawy, A., Mohammad, H. M. F., Elbahaie, A. M., et al. (2021). Antitumor activity of nitazoxanide against colon cancers: molecular docking and experimental studies based on wnt/β-catenin signaling inhibition. IJMS 22, 5213. doi:10.3390/ijms22105213
Ahmadi, H., Nayeri, Z., Minuchehr, Z., Sabouni, F., and Mohammadi, M. (2020). Betanin purification from red beetroots and evaluation of its anti-oxidant and anti-inflammatory activity on LPS-activated microglial cells. PLoS One 15, e0233088. doi:10.1371/journal.pone.0233088
Ali, S. A., Zaitone, S. A., Dessouki, A. A., and Ali, A. A. (2019). Pregabalin affords retinal neuroprotection in diabetic rats: suppression of retinal glutamate, microglia cell expression and apoptotic cell death. Exp. Eye Res. 184, 78–90. doi:10.1016/j.exer.2019.04.014
Ali, S. A., Zaitone, S. A., and Moustafa, Y. M. (2015). Boswellic acids synergize antitumor activity and protect against the cardiotoxicity of doxorubicin in mice bearing Ehrlich’s carcinoma. Can. J. physiology Pharmacol. 93(8), 695–708. doi:10.1139/cjpp-2014-0524
Almet, A. A., Maini, P. K., Moulton, D. E., and Byrne, H. M. (2020). Modeling perspectives on the intestinal crypt, a canonical system for growth, mechanics, and remodeling. Curr. Opin. Biomed. Eng. 15, 32–39. doi:10.1016/j.cobme.2019.12.012
Alshaman, R., Alattar, A., El-Sayed, R. M., Gardouh, A. R., Elshaer, R. E., Elkazaz, A. Y., et al. (2022). Formulation and characterization of doxycycline-loaded polymeric nanoparticles for testing antitumor/antiangiogenic action in experimental colon cancer in mice. Nanomaterials 12, 857. doi:10.3390/nano12050857
Amberger, J. S., Bocchini, C. A., Schiettecatte, F., Scott, A. F., and Hamosh, A. (2015). OMIM.org: online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic acids Res. 43, D789–D798. doi:10.1093/nar/gku1205
Attia, M. A., Enan, E. T., Hashish, A. A., El-kannishy, S., Gardouh, A. R., Tawfik, K., et al. (2021). Chemopreventive effect of 5-flurouracil polymeric hybrid PLGA-lecithin nanoparticles against colon dysplasia model in mice and impact on p53 apoptosis. Biomolecules 11, 109. doi:10.3390/biom11010109
Azeredo, H. M. C. (2009). Betalains: properties, sources, applications, and stability – a review. Int. J. Food Sci. Technol. 44, 2365–2376. doi:10.1111/j.1365-2621.2007.01668.x
Baeuerle, P. A. (1991). The inducible transcription activator NF-κB: regulation by distinct protein subunits. Biochimica Biophysica Acta (BBA) - Rev. Cancer 1072, 63–80. doi:10.1016/0304-419X(91)90007-8
Bahr, H. I., Ibrahiem, A. T., Gabr, A. M., Elbahaie, A. M., Elmahdi, H. S., Soliman, N., et al. (2021). Chemopreventive effect of α-hederin/carboplatin combination against experimental colon hyperplasia and impact on JNK signaling. Toxicol. Mech. Methods 31, 138–149. doi:10.1080/15376516.2020.1849483
Barnes, P. J., and Karin, M. (1997). Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071. doi:10.1056/NEJM199704103361506
Boivin, D., Lamy, S., Lord-Dufour, S., Jackson, J., Beaulieu, E., Côté, M., et al. (2009). Antiproliferative and antioxidant activities of common vegetables: a comparative study. Food Chem. 112, 374–380. doi:10.1016/j.foodchem.2008.05.084
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic acids Res. 47, W357–W364. doi:10.1093/nar/gkz382
Danquah, K. O., Adjei, E., Quayson, S., Adankwah, E., Gyamfi, D., Sampene, P. P., et al. (2017). Mucin expression patterns in histological grades of colonic cancers in Ghanaian population. Pan Afr. Med. J. 27, 267. doi:10.11604/pamj.2017.27.267.9793
Deng, P., Ji, C., Dai, X., Zhong, D., Ding, L., and Chen, X. (2015). Simultaneous determination of capecitabine and its three nucleoside metabolites in human plasma by high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 989, 71–79. doi:10.1016/j.jchromb.2015.03.002
Dunkel, M., Günther, S., Ahmed, J., Wittig, B., and Preissner, R. (2008). SuperPred: drug classification and target prediction. Nucleic acids Res. 36, W55–W59. doi:10.1093/nar/gkn307
ElSayed, M. H., Atif, H. M., Eladl, M. A., Elaidy, S. M., Helaly, A. M. N., Hisham, F. A., et al. (2023a). Betanin improves motor function and alleviates experimental Parkinsonism via downregulation of TLR4/MyD88/NF-κB pathway: molecular docking and biological investigations. Biomed. Pharmacother. 164, 114917. doi:10.1016/j.biopha.2023.114917
ElSayed, M. H., Elbayoumi, K. S., Eladl, M. A., Mohamed, A. A., Hegazy, A., El-Sherbeeny, N. A., et al. (2023b). Memantine mitigates ROS/TXNIP/NLRP3 signaling and protects against mouse diabetic retinopathy: histopathologic, ultrastructural and bioinformatic studies. Biomed. Pharmacother. 163, 114772. doi:10.1016/j.biopha.2023.114772
Fabregas, J. C., Ramnaraign, B., and George, T. J. (2022). Clinical updates for colon cancer Care in 2022. Clin. Colorectal Cancer 21, 198–203. doi:10.1016/j.clcc.2022.05.006
Feng, Z., Yan, P., Hou, X., Feng, J., He, X., and Yang, K. (2020). The efficacy and safety of capecitabine-based versus S-1-based chemotherapy for metastatic or recurrent gastric cancer: a systematic review and meta-analysis of clinical randomized trials. Ann. Palliat. Med. 9, 883–894. doi:10.21037/apm.2020.04.26
Fernández, J., Silván, B., Entrialgo-Cadierno, R., Villar, C. J., Capasso, R., Uranga, J. A., et al. (2021). Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 143, 112241. doi:10.1016/j.biopha.2021.112241
Ge, S. X., Jung, D., and Yao, R. (2020). ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinforma. Oxf. Engl. 36, 2628–2629. doi:10.1093/bioinformatics/btz931
Gescher, A., Pastorino, U., Plummer, S. M., and Manson, M. M. (1998). Suppression of tumour development by substances derived from the diet—mechanisms and clinical implications. Brit J. Clin. Pharma 45, 1–12. doi:10.1046/j.1365-2125.1998.00640.x
Gill, S., Thomas, R. R., and Goldberg, R. M. (2003). Review article: colorectal cancer chemotherapy. Aliment. Pharmacol. Ther. 18, 683–692. doi:10.1046/j.1365-2036.2003.01735.x
Hakomori, S. (1989). Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 52, 257–331. doi:10.1016/S0065-230X(08)60215-8
Hamiza, O. O., Rehman, M. U., Tahir, M., Khan, R., Khan, A. Q., Lateef, A., et al. (2012). Amelioration of 1,2 dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in wistar rats. Asian Pac. J. Cancer Prev. 13, 4393–4402. doi:10.7314/APJCP.2012.13.9.4393
Huo, Z., Wang, S., Shao, H., Wang, H., and Xu, G. (2020). Radiolytic degradation of anticancer drug capecitabine in aqueous solution: kinetics, reaction mechanism, and toxicity evaluation. Environ. Sci. Pollut. Res. 27, 20807–20816. doi:10.1007/s11356-020-08500-1
Hursting, S. D., Slaga, T. J., Fischer, S. M., DiGiovanni, J., and Phang, J. M. (1999). Mechanism-based cancer prevention approaches: targets, examples, and the use of transgenic mice. JNCI J. Natl. Cancer Inst. 91, 215–225. doi:10.1093/jnci/91.3.215
Janakiram, N. B., and Rao, C. V. (2014). “The role of inflammation in colon cancer,” in Inflammation and cancer advances in experimental medicine and biology. Editors B. B. Aggarwal, B. Sung, and S. C. Gupta (Basel: Springer Basel), 25–52. doi:10.1007/978-3-0348-0837-8_2
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M., and Ishiguro-Watanabe, M. (2023). KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592. doi:10.1093/nar/gkac963
Kapadia, G. J., and Rao, G. S. (2012). “Anticancer effects of red beet pigments,” in Red beet biotechnology: food and pharmaceutical applications. Editor B. Neelwarne (Boston, MA: Springer US), 125–154. doi:10.1007/978-1-4614-3458-0_7
Kasprzak, A., Seraszek-Jaros, A., Jagielska, J., Helak-Łapaj, C., Siodła, E., Szmeja, J., et al. (2019). The histochemical alterations of mucin in colorectal carcinoma quantified by two efficient algorithms of digital image analysis. Int. J. Mol. Sci. 20, 4580. doi:10.3390/ijms20184580
Kibudde, S., and Begg, W. (2022). Capecitabine plus oxaliplatin in the treatment of metastatic colorectal cancer at Tygerberg hospital: a retrospective study. Pan Afr. Med. J. 42, 141. doi:10.11604/pamj.2022.42.141.31234
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., et al. (2023). PubChem 2023 update. Nucleic Acids Res. 51, D1373–D1380. doi:10.1093/nar/gkac956
Knikman, J. E., Rosing, H., Guchelaar, H., Cats, A., and Beijnen, J. H. (2020). A review of the bioanalytical methods for the quantitative determination of capecitabine and its metabolites in biological matrices. Biomed. Chromatogr. 34, e4732. doi:10.1002/bmc.4732
Krajka-Kuźniak, V., Paluszczak, J., Szaefer, H., and Baer-Dubowska, W. (2013). Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 110, 2138–2149. doi:10.1017/S0007114513001645
Kuhn, M., von Mering, C., Campillos, M., Jensen, L. J., and Bork, P. (2008). STITCH: interaction networks of chemicals and proteins. Nucleic acids Res. 36, D684–D688. doi:10.1093/nar/gkm795
Kundu, J. K., Choi, K.-Y., and Surh, Y.-J. (2006). beta-Catenin-mediated signaling: a novel molecular target for chemoprevention with anti-inflammatory substances. Biochimica Biophysica Acta (BBA) - Rev. Cancer 1765, 14–24. doi:10.1016/j.bbcan.2005.08.006
Lechner, J. F., Wang, L.-S., Rocha, C. M., Larue, B., Henry, C., McIntyre, C. M., et al. (2010). Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N-Nitrosomethylbenzylamine-Treated rats. J. Med. Food 13, 733–739. doi:10.1089/jmf.2008.0280
Lee, J., Koo, N., and Min, D. B. (2004). Reactive oxygen species, aging, and antioxidative nutraceuticals. Comp. Rev. Food Sci. Food Safe 3, 21–33. doi:10.1111/j.1541-4337.2004.tb00058.x
Lin, S., Chang, C., Hsu, C., Tsai, M., Cheng, H., Leong, M. K., et al. (2020). Natural compounds as potential adjuvants to cancer therapy: preclinical evidence. Br. J Pharmacol. 177, 1409–1423. doi:10.1111/bph.14816
Liu, K. Y., Wang, Q., Nakatsu, C. H., Jones-Hall, Y., and Jiang, Q. (2023). Combining gamma-tocopherol and aspirin synergistically suppresses colitis-associated colon tumorigenesis and modulates the gut microbiota in mice, and inhibits the growth of human colon cancer cells. Eur. J. Pharmacol. 946, 175656. doi:10.1016/j.ejphar.2023.175656
Manu, K. A., Shanmugam, M. K., Li, F., Chen, L., Siveen, K. S., Ahn, K. S., et al. (2014). Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappa B-regulated gene products. J. Mol. Med. 92, 267–276. doi:10.1007/s00109-013-1095-0
Milella, R. A., De Rosso, M., Gasparro, M., Gigante, I., Debiase, G., Forleo, L. R., et al. (2023). Correlation between antioxidant and anticancer activity and phenolic profile of new Apulian table grape genotypes (V. Vinifera L.). Front. Plant Sci. 13, 1064023. doi:10.3389/fpls.2022.1064023
Mohamed, S. M. S., Amin, A. M., Skander, S. W., Hewala, T. I. M., Saleh, E. O. M., Salih, A. M. O., et al. (2022). Chemoprevention of 1,2 dimethyl hydrazine-induced colon tumor in Albino rat by meloxicam and its correlation with immunoassay of serum CEA. Am. J. Biomed. Sci., 154–166. doi:10.5099/aj220400154
Mohammad, H. M., Eladl, M. A., Abdelmaogood, A. K., Elshaer, R. E., Ghanam, W., Elaskary, A., et al. (2023a). Protective effect of topiramate against diabetic retinopathy and computational approach recognizing the role of NLRP3/IL-1β/TNF-α signaling. Biomedicines 11 (12), 3202. doi:10.3390/biomedicines11123202
Mohammad, H. M., Gouda, S. G., Eladl, M. A., Elkazaz, A. Y., Elbayoumi, K. S., Farag, N. E., et al. (2023b). Metformin suppresses LRG1 and TGFβ1/ALK1-induced angiogenesis and protects against ultrastructural changes in rat diabetic nephropathy. Biomed. Pharmacother. 158, 114128. doi:10.1016/j.biopha.2022.114128
Mondal, S., Sinha, S., Mukhopadhyay, M., Chakraborty, I., and Jain, P. (2014). Diagnostic and prognostic significance of different mucin expression, preoperative CEA, and CA-125 in colorectal carcinoma: a clinicopathological study. J. Nat. S. C. Biol. Med. 5, 404–408. doi:10.4103/0976-9668.136207
Mori, H., Hata, K., Yamada, Y., Kuno, T., and Hara, A. (2005). Significance and role of early-lesions in experimental colorectal carcinogenesis. Chemico-Biological Interact. 155, 1–9. doi:10.1016/j.cbi.2005.04.005
Nan, H., Hutter, C. M., Lin, Y., Jacobs, E. J., Ulrich, C. M., White, E., et al. (2015). Association of aspirin and NSAID use with risk of colorectal cancer according to genetic variants. JAMA 313, 1133–1142. doi:10.1001/jama.2015.1815
Niv, Y. (1994). Mucin and colorectal cancer metastasis. Am. J. Gastroenterol. 89, 665–669. doi:10.1097/MCG.0000000000001050
Pan, M.-H., and Ho, C.-T. (2008). Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 37, 2558–2574. doi:10.1039/b801558a
Pathan, M., Keerthikumar, S., Ang, C.-S., Gangoda, L., Quek, C. Y. J., Williamson, N. A., et al. (2015). FunRich: an open access standalone functional enrichment and interaction network analysis tool. Proteomics 15, 2597–2601. doi:10.1002/pmic.201400515
Pawlonka, J., Rak, B., and Ambroziak, U. (2021). The regulation of cyclin D promoters – review. Cancer Treat. Res. Commun. 27, 100338. doi:10.1016/j.ctarc.2021.100338
Piñero, J., Ramírez-Anguita, J. M., Saüch-Pitarch, J., Ronzano, F., Centeno, E., Sanz, F., et al. (2019). The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 48, D845-D855. doi:10.1093/nar/gkz1021
Pouya, F. D., Rasmi, Y., Camci, I. Y., Tutar, Y., and Nemati, M. (2021). Performance of capecitabine in novel combination therapies in colorectal cancer. J. Chemother. 33, 375–389. doi:10.1080/1120009X.2021.1920247
Raziuddin, A., Court, D., Sarkar, F. H., Liu, Y.-L., Kung, H., and Raziuddin, R. (1997). A c-erbB-2 promoter-specific nuclear matrix protein from human breast tumor tissues mediates NF-kappaB DNA binding activity. J. Biol. Chem. 272, 15715–15720. doi:10.1074/jbc.272.25.15715
Reddy, M. K., Alexander-Lindo, R. L., and Nair, M. G. (2005). Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 53, 9268–9273. doi:10.1021/jf051399j
Ruiz-Rabelo, J., Vázquez, R., Arjona, Á., Perea, D., Montilla, P., Túnez, I., et al. (2011). Improvement of capecitabine antitumoral activity by melatonin in pancreatic cancer. Pancreas 40, 410–414. doi:10.1097/MPA.0b013e318201ca4f
Salimi, A., Bahiraei, T., Ahdeno, S., Vatanpour, S., and Pourahmad, J. (2021). Evaluation of cytotoxic activity of betanin against U87MG human glioma cells and normal human lymphocytes and its anticancer potential through mitochondrial pathway. Nutr. Cancer 73, 450–459. doi:10.1080/01635581.2020.1764068
Singh, K., Bhori, M., Kasu, Y. A., Bhat, G., and Marar, T. (2018). Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity – exploring the armoury of obscurity. Saudi Pharm. J. 26, 177–190. doi:10.1016/j.jsps.2017.12.013
Song, W., Derito, C. M., Liu, M. K., He, X., Dong, M., and Liu, R. H. (2010). Cellular antioxidant activity of common vegetables. J. Agric. Food Chem. 58, 6621–6629. doi:10.1021/jf9035832
Stanner, S., Hughes, J., Kelly, C., and Buttriss, J. (2004). A review of the epidemiological evidence for the ‘antioxidant hypothesis. Public Health Nutr. 7, 407–422. doi:10.1079/PHN2003543
Stintzing, F. C., and Carle, R. (2004). Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 15, 19–38. doi:10.1016/j.tifs.2003.07.004
Tan, D., Wang, Y., Bai, B., Yang, X., and Han, J. (2015). Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 78, 141–146. doi:10.1016/j.fct.2015.01.018
Ullah, E. (2012). Mucin histochemistry in tumours of colon, ovaries and lung. J. Cytol. Histol. 3. doi:10.4172/2157-7099.1000163
Vieira Teixeira da Silva, D., dos Santos Baião, D., de Oliveira Silva, F., Alves, G., Perrone, D., Mere Del Aguila, E., et al. (2019). Betanin, a natural food additive: stability, bioavailability, antioxidant and preservative ability assessments. Molecules 24, 458. doi:10.3390/molecules24030458
Zell, J. A., Ziogas, A., Bernstein, L., Clarke, C. A., Deapen, D., Largent, J. A., et al. (2009). Nonsteroidal anti-inflammatory drugs: effects on mortality after colorectal cancer diagnosis. Cancer 115, 5662–5671. doi:10.1002/cncr.24705
Zhang, Q., Pan, J., Wang, Y., Lubet, R., and You, M. (2013). Beetroot red (betanin) inhibits vinyl carbamate- and benzo(a)pyrene-induced lung tumorigenesis through apoptosis. Mol. Carcinog. 52, 686–691. doi:10.1002/mc.21907
Zielińska-Przyjemska, M., Olejnik, A., Dobrowolska-Zachwieja, A., Łuczak, M., and Baer-Dubowska, W. (2016). DNA damage and apoptosis in blood neutrophils of inflammatory bowel disease patients and in Caco-2 cells in vitro exposed to betanin. Postepy Hig. Med. Dosw (Online) 70, 265–271. doi:10.5604/17322693.1198989
Keywords: betanin, bioinformatic study, capecitabine, cyclin D1, experimental colon cancer, mouse, NFκB
Citation: Ahmed R, Zaitone SA, Abdelmaogood AKK, Atef HM, Soliman MFM, Badawy AM, Ali HS, Zaid A, Mokhtar HI, Elabbasy LM, Kandil E, Yosef AM and Mahran RI (2024) Chemotherapeutic potential of betanin/capecitabine combination targeting colon cancer: experimental and bioinformatic studies exploring NFκB and cyclin D1 interplay. Front. Pharmacol. 15:1362739. doi: 10.3389/fphar.2024.1362739
Received: 28 December 2023; Accepted: 13 March 2024;
Published: 05 April 2024.
Edited by:
Yasmina Mohammed Abd-Elhakim, Zagazig University, EgyptReviewed by:
Venkateshwar Madka, University of Oklahoma Health Sciences Center, United StatesCopyright © 2024 Ahmed, Zaitone, Abdelmaogood, Atef, Soliman, Badawy, Ali, Zaid, Mokhtar, Elabbasy, Kandil, Yosef and Mahran. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rehab Ahmed, cmFobWVkQHV0LmVkdS5zYQ==; Sawsan A. Zaitone, c3phaXRvbmVAdXQuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.