- 1School of Medicine and Health Management, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2School of Psychology and Public Health, La Trobe University, Melbourne, VIC, Australia
- 3Major Disciplinary Platform Under Double First-Class Initiative for Liberal Arts at Huazhong University of Science and Technology (Research Center for High-Quality Development of Hospitals), Wuhan, Hubei, China
- 4Key Research Institute of Humanities and Social Sciences of Hubei Provincial Department of Education, Wuhan, Hubei, China
Objectives: To assess the effects of the transparent online open procurement arrangement on the prices, volumes, and costs of medicines in Ningxia, China.
Methods: Data were extracted from the Ningxia pharmaceutical procurement platform, covering 16 months of purchase orders (December 2019 to March 2021) prior to the implementation of the transparent online open procurement policy and 20 months of purchase orders after the implementation of the policy (April 2021 to November 2022). Interrupted time series (ITS) analysis was performed to evaluate the effects of the transparent online open procurement policy on the prices, volumes, and total costs of the purchase orders.
Results: After implementation of the transparent online open procurement policy, the average price of purchased medicines showed a declining trend by 0.012 Yuan per month, while the total volume of purchase orders declined at a rate by 1.741 million per month measured by the smallest formulation units and the total costs of the purchase orders decreased at a rate by 5.525 million Yuan per month.
Conclusion: The transparent online open procurement policy resulted in reduced prices, lowered volumes, and lowered total costs of purchased orders of medicines.
1 Introduction
According to the most recent statistics provided by the global pharmaceutical information company IQVIA, global expenditure on medicatioxns - defined as the total amount allocated for the procurement of pharmaceuticals from manufacturers, exclusive of off-invoice discounts and rebates -is projected to reach $1.9 trillion by the year 2027 and this expenditure is anticipated to grow steadily at a rate ranging between 3% and 6% annually (IQVIA, 2023). Notably, medications constitute a significant portion of healthcare expenditures in low- and middle-income countries, accounting for 20%–60% of their healthcare spending, in contrast to the 18% observed in countries affiliated with the Organization for Economic Co-operation and Development (OECD) (World Health Organization, 2015). It is crucial to acknowledge that in developing nations, up to 90% of the population rely on out-of-pocket payments for the purchase of medications, resulting in pharmaceuticals being the most substantial household expense, second only to food (World Health Organization, 2015). In China, out-of-pocket payments accounted for 27.0% of its total health expenditure in 2022 (National Health Commission of the People’s Republic of China, 2023b).
China held the position of the world’s second largest consumer in the pharmaceutical sector in 2018, with an impressive expenditure of $137 billion (IQVIA, 2023). The share of China’s consumption of medicines in the world increased from 17.6% in 2015 to 20.3% in 2022 (Fei et al., 2023). Projections suggest that the pharmaceutical market in China is poised for substantial growth, with a volume increase of 8% over the upcoming 5 years (IQVIA, 2023). This surge in demand will be mirrored by a noteworthy 19% increase in pharmaceutical spending, ultimately reaching an estimated range of $140 to $170 billion by the year 2023 (IQVIA, 2023). Notably, from 2010 to 2020, a substantial portion of the total healthcare expenditure, ranging from 30% to 40%, were spent in pharmaceuticals (National Health Commission of the People’s Republic of China, 2023a; Yan et al., 2022). This figure surpassed not only the corresponding percentages observed in the United States (11.0%), Japan (20.7%), and Korea (18.6%) but also exceeded the average of OECD countries (15.9%) (OECD, 2023).
Extensive research has been conducted to investigate the factors contributing to the escalating pharmaceutical expenditure (Main et al., 2022). Pharmaceutical pricing remains a pivotal and predominant factor that significantly contributes to the escalating pharmaceutical expenditure (de Wolf et al., 2005; Kadkhodamanesh et al., 2021; Mirzoev et al., 2021; Akbarpour et al., 2023), gaining increased prominence with each passing year (Ling-hua and Jie-yuan, 2022). Governments worldwide have introduced a range of interventions and policy measures aimed at fostering the rationalisation of drug prices, thereby curtailing pharmaceutical expenses (Lee et al., 2015; Murthy and Okunade, 2016; Hassali and Wong, 2018). These multifaceted strategies encompass diverse pricing mechanisms, as well as practices such as pooled procurement, tendering, and negotiation. These efforts collectively seek to address the challenge of burgeoning pharmaceutical costs (World Health Organization, 2020; Mirzoev et al., 2021). At present, the evidence regarding the effectiveness of these policies in reducing medication prices and enhancing access to pharmaceuticals is notably mixed. These multifaceted considerations underscore the complexity of achieving a balanced and effective pharmaceutical pricing policy (World Health Organization, 2020; Kakkar, 2021).
The fundamental principle of advancing market transparency for pharmaceuticals lies in the public dissemination of information regarding the net prices of healthcare products (World Health Organization, 2019). This concept was initially championed by the 72nd World Health Assembly (WHA), which underscored the critical need for reliable data concerning medicine prices. In this context, the guidelines set forth by the World Health Organization (WHO) on country pharmaceutical pricing policies recommend that nations should actively “Share the net transaction prices of pharmaceutical products with relevant stakeholders, within and external to the country” (World Health Organization, 2020; Riccaboni et al., 2022). The significance of promoting price transparency has been underscored by various initiatives and regulations aimed at enhancing clarity in pricing practices. One notable example is the Medicines Transparency Alliance (MeTA), an initiative led by WHO. This initiative aspired to establish national-level, multi-stakeholder platforms for the sharing of comprehensive data related to the selection, procurement, quality, availability, pricing, promotion, and utilisation of medicines (Paschke et al., 2018). Another pertinent example is the European Union (EU) Transparency Directive, which mandates the public disclosure of list prices for all reimbursable medicines across Europe (European Union, 1988).
Facing the problem of high drug prices and opacity of the drug procurement process in hospitals, in 2000, the Chinese government issued the first policy document on public disclosure of drug price transparency. This retailing price ceilings of drugs set by the government has enabled further regional exploration of drug procurement procedures. In 2005, Sichuan, a western province of China, innovatively piloted centralised drug procurement via the internet and purchasing at the provincial level, with reference to the price ceilings (Jun-feng, 2006). In 2006, Guangdong implemented transparent online open procurement of medicines through price-limit bidding (Jun-he, 2007). In 2009, to further decrease the inflated drug price, the zero-markup for drugs (ZMD) policy was introduced (Ni et al., 2021). In 2015, the milestone national policy document on transparent drug procurement was announced: All localities were encouraged to actively explore and pilot market-oriented drug pricing mechanism instead of strict and static drug retail price cap restriction by government alone. In 2017, the Chinese government issued several policy documents concerning reforming drug procurement and delivery. Standardizations of the Comprehensive National Information Management Platform as well as the Provincial Centralised Drug Procurement Platforms were put forward as core measures to monitor drug prices and supplies. Drug procurement related data, including drug prices were encouraged to be shared with the public. In 2019, the Chinese government issued the “Pilot Plan for National Centralised Drug Procurement”.
The fundamental rationale for promoting price transparency is that it may improves economic efficiency; assists policymakers and researchers with reliable price information; empowers buyers to negotiate more strategically; increases the accountability of manufacturers and governments for prices; and facilitates cost-effective decision-making by prescribers and patients (World Health Organization, 2018; Ahmad et al., 2020). Disclosure and control of drug prices in this study was one of the four aspects of transparency that can occur (World Health Organization, 2018). There was inconsistency in the impact of price transparency on drug prices in current literature. Some studies demonstrated that transparency have brought down the price of medicines, such as a pricing system in the private market conducted in South Africa (Moodley and Suleman, 2019a; Moodley and Suleman 2019b). However, some studies have concluded that transparency has no obvious expenditure saving effect. A previous study in the United Kingdom, for example, found that expenditure on inhaled glucocorticosteroids was unchanged after cost information was provided (Langley et al., 2018). Transparency has even been found to drive prices up, such as a South African study of transparent pricing systems (Bangalee and Suleman, 2016). A systematic review in 2023 concluded that the impact of drug price transparency was still of controversial although important, calling for stronger evidence on aspects as prices, quantites and expenditure (Joosse et al., 2023).
This study was conducted in Ningxia, an underdeveloped province located in northwestern China. In December 2020, Ningxia Public Resources Trading Administration, an agency for administration of governmental related products procurement including pharmaceuticals, announced the notice and rules for transparent online open procurement of medicines (Ningxia Public Resources Trading Administration, 2020a; Ningxia Public Resources Trading Administration, 2020b), with an intention to address the issues of supply shortage of essential medicines by engaging more qualified suppliers and hospitals in free bargaining. It was also expected to bring down transaction costs for both suppliers and hospitals. This study aimed to evaluate the effects of the transparent online open procurement arrangement on the prices, volumes, and total costs of the purchase orders in Ningxia, China. This study was expected to provide evidence of the effectiveness of drug price transparency. Although a previous study conducted in Shanghai, China explored these themes, the evidences were qualitative in nature (Cheng and Bo, 2021). This study used a institutional data with a quasi-experimental design. The findings of this current study were expected to not only address the gap in the literature, but also provide practical reference regarding drug price transparency practice for low and middle income countries similar to China.
2 Methods
2.1 Study setting
This study was conducted in Ningxia Hui Autonomous Region in northwest China, which covers an area of 66,400 square kilometres with 7.25 million residents (in 2021). It has the largest group of Hui ethnicity. Ningxia is deemed an underdeveloped province in China in terms of both GDP and per capita GDP. In 2021, Ningxia ranked bottom third among the 31 provinces/regions in mainland China in total GDP (452.23 billion Yuan, or $US 63 billion) and 20th in per capita GDP (62,549 Yuan, or $US 8701). Its per capita disposable income reached 38,291 Yuan ($US 5326) for urban and 15,337 Yuan ($US 2133) for rural residents, respectively. In 2021, Ningxia had 4,571 healthcare institutions (including both hospitals and primary care centres), 5.68 inpatient beds per 1,000 population (compared with 6.7 national average), and 8.36 skilled health workers per 1,000 population (compared with 7.97 national average) (Ningxia Hui Autonomous Region Statistics Bureau, 2023; National Health Commission of the People’s Republic of China, 2023a).
2.2 Intervention measures
The transparent online open procurement arrangements in Ningxia involve several components.
First, the “Ningxia Pharmaceutical Procurement Platform” (the Platform hereafter) was established by integrating and upgrading the “Ningxia Drug Tendering and Purchasing Platform” and the “Ningxia Centralized Drug Purchasing Network”. The two platforms used to realize the bidding function and the procurement function, respectively. In January 2021, the “Ningxia Pharmaceutical Procurement Platform” overseen by the Ningxia Public Resources Trading Administration was officially put into use and in April 2021, the first batch of drug purchases officially started.
Second, it mandates all qualified suppliers publicly disclosing necessary product information, including the generic names, specifications, dosage forms, and prices of all drugs, to be listed on the official public website on a timely manner. Hospitals are required to procure all of their medicines in line with the product information disclosed on the Platform. The Platform is deemed the only legal channel for public hospitals to make purchase orders.
Third, the Public Resources Trading Administration also coordinates reviews of the product prices negotiated conducted by a group of medical and pharmaceutical experts drawn from the expert database. Those experts will step in for further negotiations for overpriced products. Public hospitals negotiate with the listed suppliers under the supervision of the Public Resources Trading Administration. The price ceiling and average price relating to each of the procured medicines in Ningxia guided the negotiation. After that, medical institutions, on the basis of the listed price of drugs on the platform, combined with their own purchasing volume, bargain with manufacturers through the bargaining function of the Ningxia Pharmaceutical Procurement Platform. Hospitals were allowed to negotiate with listed enterprises on pharmaceutical prices.
Fourth, dynamic price monitoring and adjustment mechanisms were established. Inner reference pricing was used for price adjustment. For enterprises, they are required to actively report the new products prices to Ningxia Public Resources Trading Administration if the prices of the same product listed on other provincial procurement platform was lower. Ningxia Public Resources Trading Administration were then responsible for adjusting the listed price on the platform with reference to the new prices that the enterprises declared. For enterprises that failed to report such information or those with fraud practices, they may be disqualified from the platform. The thorough monitoring and reviewing of pharmaceutical prices are conducted every 6 months.
2.3 Study design
This study employed a pre-post design with segmented time series analysis. An interrupted time series is a strong quasi-experimental design in which data are collected at multiple time points before and after the intervention. The advantage of this design is that it can detect a possible underlying secular trend which occurs after the intervention (Koskinen et al., 2015).
The procurement information from December 2019 to November 2022 recorded in Ningxia Pharmaceutical Procurement Platform were extracted for analysis. We aggregated the original data into monthly indicators for the purposed of the study. The basic idea behind the A.M. index system analysis method (Addis A. & Magrini N.’ method) of drug expenditure was that changes in price, volume, and structure were the three main drivers of changes in drug expenditure (Addis and Magrini, 2002). Based on this idea, we chose these variables to explore changes in expenditure, and we did not include structure changes in our study because there was no systematic difference in structure before and after the intervention (Ningxia Public Resources Trading Administration, 2017; Ningxia Public Resources Trading Administration, 2020a). Three indicators were synthesized for analysis: monthly procurement price, volume and cost of medicines. These variables were largely used in current drug cost related studies (Wouters et al., 2017; Dos Santos et al., 2022). Both monthly procurement price and volume were standardized by smallest pack unit (bottle, bag, box, etc.), a commonly used standardization for pharmaceutical price and volume (Wouters et al., 2017). The monthly procurement volume of pharmaceuticals was calculated as sums-up of procurement volumes of each specific product in each month. The monthly procurement price of pharmaceuticals was calculated by dividing total monthly pharmaceutical cost by total monthly procurement volume.
One wave of segmented analysis was conducted in this study. The purchasing information over a 36-month period (from December 2019 to November 2022) were collected. April 2021 (i.e., the 17th month) was chosen as the intervention point for the implementation of pharmaceutical transparent online open procurement policy. This analysis enable us to explore the impact of Ningxia pharmaceutical transparent online open procurement policy on pharmaceutical procurement.
2.4 Statistical analysis
The interrupted time series (ITS) was used to analyze the procurement price (CNY), total volume of purchase orders measured by the smallest formulation units (millions of units) and total costs of the purchase orders (CNY in millions), they acted as dependent variables in this study. The linear regression equation is constructed as follows:
In this model, Yt is the outcome indicator in month t; Tt is a continuous variable indicating the months passed at month t since the start of the observation period; It represents the two periods before (value = 0) and after (value = 1) the intervention; T is a continuous variable indicating months passed since the intervention (time prior to the intervention is coded 0). β0 is the initial level estimate, i.e. the study variable at t = 0; β1 is the trend estimate of the change in time variable t in the pre-intervention study variable, i.e., the baseline slope estimate; β2 was the level of change in the study variables before and after the intervention; β3 is the trend change of the study variable after the intervention, that is, the difference between the post-intervention slope and the pre-intervention slope; β1+β3 is the slope of the trend of study variables after intervention; εt is random error.
The level of autocorrelation in the model was estimated using the Durbin-Watson test (Savin and White, 1977). If autocorrelation exists, the Prais-Winsten method was used to deal with autocorrelation (Turner et al., 2021). All of the analyses were performed using STATA17.0 software (Stata Corp LP, College Station, TX, United States).
3 Results
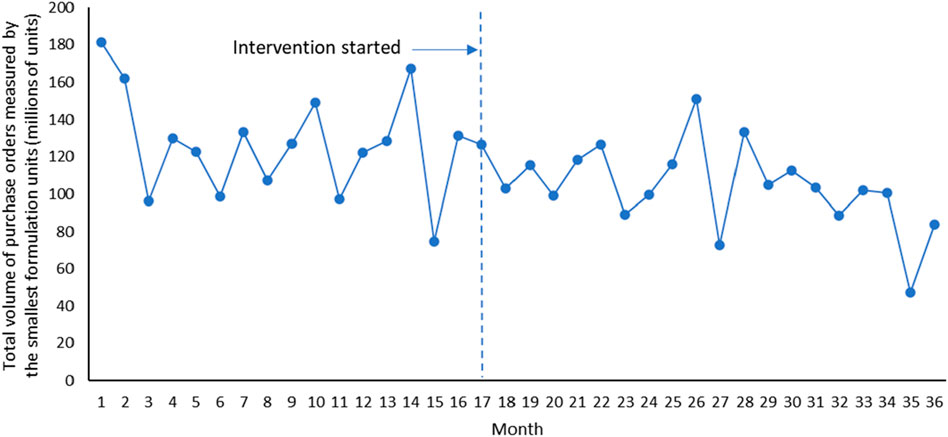
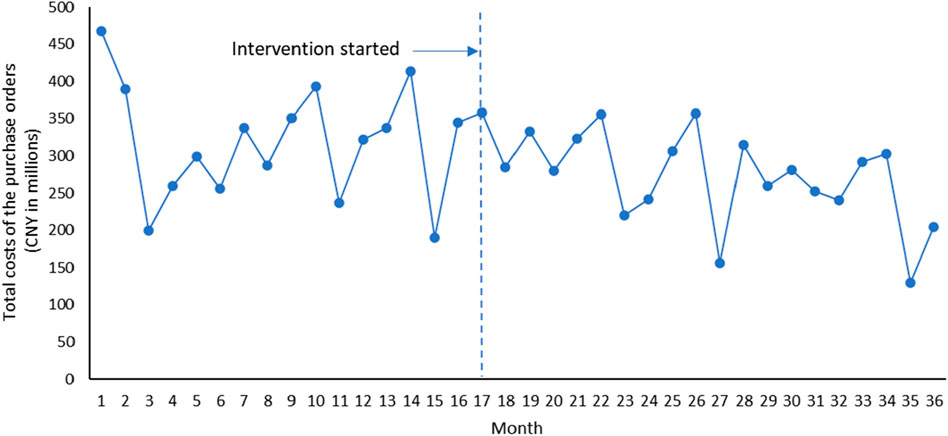
Overall, it can be seen that after the implementation of Ningxia pharmaceutical transparent online open procurement, the average price of purchased medicines has changed from an upward trend to a downward trend (Figure 1). The downward trend of total volume of purchase orders measured by the smallest formulation units and total costs of the purchase orders has accelerated significantly after the implementation of Ningxia pharmaceutical transparent online open procurement (Figures 2, 3).
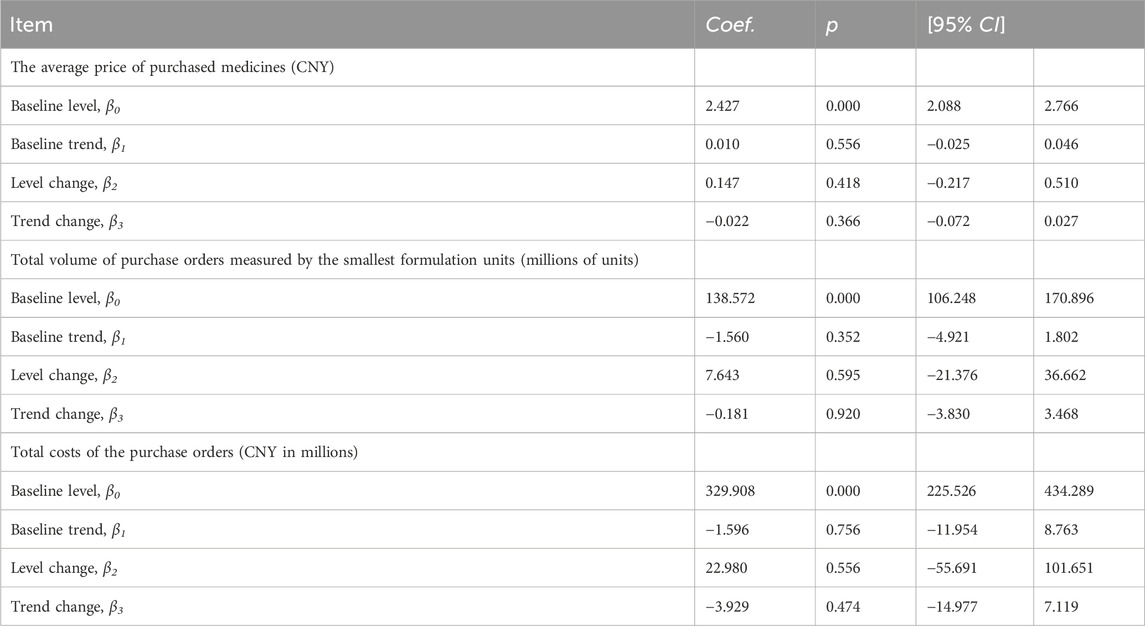
ITS analysis shows that before the implementation of the pharmaceutical transparent online open procurement policy, the average price of purchased medicines is increasing at a trend of 0.010 yuan per month [p = 0.556, CI = (−0.025, 0.046)]. In the first month of policy intervention, the average price of purchased medicines increased by 0.147 yuan, and the change was not statistically significant [p = 0.418, CI = (−0.217, 0.510)]. Compared with the upward trend of 0.010 yuan before policy intervention, the upward trend of the average price of purchased medicines decreased by 0.022 yuan, and the change was not statistically significant [p = 0.366, CI = (−0.072, 0.027)], that is, the average price of purchased medicines decreased at 0.012 yuan per month after policy intervention (β1+β3) (Table 1).
The total volume of purchase orders was decreasing at a trend of 1.560 million smallest formulation units per month, and the change was not statistically significant [p = 0.352, CI = (−4.921, 1.802)]. In the first month of policy intervention, total volume of purchase orders increased by 7.643 million smallest formulation units, with no statistically significant change [p = 0.595, CI = (−21.376, 36.662)]. Compared with the downward trend of 1.560 million before the policy intervention, the upward trend of total volume of purchase orders increased by 0.181 million smallest formulation units, and the change was not statistically significant [p = 0.920, CI = (−3.830, 3.468)], that is, the total volume of purchase orders after the policy intervention decreased at a trend of 1.741 million smallest formulation units per month (β1+β3) (Table 1).
The total costs of the purchase orders was decreasing at a trend of 1.596 million yuan per month, and the change was not statistically significant [p = 0.756, CI = (−11.954, 8.763)]. In the first month of policy intervention, the total costs of the purchase orders increased by 22.980 million yuan, and the change was not statistically significant [p = 0.556, CI = (−55.691, 101.651)]. Compared with the downward trend of −1.596 million before the policy intervention, the upward trend of the total costs of the purchase orders decreased by 3.929 million yuan, and the change was not statistically significant [p = 0.474, CI = (−14.977, 7.119)], that is, the total costs of the purchase orders after the policy intervention decreased at a monthly trend of 5.525 million yuan (β1+β3) (Table 1).
4 Discussion
Our research found that the implementation of Ningxia pharmaceutical transparent online open procurement policy resulted in reduced prices, lowered volumes, and lowered total costs of purchased orders of medicines, and the implementation of this policy can be considered to be quite effective. The average price of purchased medicines changed from an upward trend to a downward trend after the policy intervention, and the downward trend of total volume of purchase orders and total costs of the purchase orders accelerated significantly.
The transparent online open procurement of drugs in Ningxia encompassed some major aspects: First, information on products prices were publicly disclosure under public scrutiny. On the one hand hospitals may benefit with the possibility of expenditure rationalization, and suppliers on the other hand with sales on a larger scale (Sigulem and Zucchi, 2009). Second, a unified platform integration of two separated systems enables unified and simplified government supervision, especially the transaction process by hospitals, manufacturers and deliver enterprises. Comprehensive information may also promote rational procurement decisions by hospitals regarding drug alternatives, etc. Thirdly, public disclosures of prices benefits government regulators for a clearer picture on products prices all over China, leaving more information supporting bargaining with enterprises about their listed prices, largely simplified local price supervision process. Fourth, all qualified drug manufacturers were encouraged to participate in open bidding, competition among suppliers were intensified. Fifth, the openness and transparency of the whole transaction process may also effectively prevent corruptions. From this research, such comprehensive intervention had the potential to bring down drug prices. Cost-savings can also be achieved. The procurement volumes of drugs by hospitals were decreased.
The results of this study are due to a combination of factors, in which transparency may have played a key role in lowering drug prices, but there may have been other elements in the overall policy that played a role, such as upgrading the consolidation of drug purchasing platforms and encouraging companies to bid for medicines, etc., and it is worthwhile to explore further the role played by the specific individual elements. Increasing transparency is one of the solutions proposed by both researcher and practitioners to reduce prices (Franzen et al., 2020). This is especially important for underdeveloped countries where drug corruptions were common. Peru, Zambia, and Jamaica, for example, have promoted the smooth procurement of medicines mainly by strengthening transparent legislation, open procurement process and related information transparency (World Health Organization. Regional Office for the Eastern Mediterranean, 2009). Simply public disclosure of pharmaceutical prices on the internet would make a great positive impact (Paschke et al., 2018). It was also believed that increased manufacturer transparency would improve the overall fairness and efficiency of price negotiation system (Moon, 2018; Colbert et al., 2020), leading to price reduction on both originator and generic medicines. Previous studies in China have shown that transparency of information on the procurement of essential medicines reduces the number of high-priced medicines procured, which is conducive to promoting the accessibility of essential medicines and the rational use of medicines (Xin et al., 2013).
Information technology in public contracting helps creating a more competitive, transparent, and accountable procurement system. Ukraine, for example, introduced a centralized e-procurement system that is known as ProZorro (Law of Ukraine, 2015). Public dissemination about the procurement process combined with an electronic bidding system is considered an efficient strategy for price competition and also an anti-corruption tool (Cohen and Montoya, 2001). Example of how transparency improved procurement processes is also found in Chile. It’s pharmaceutical procurement system CENABAST had historically been inefficient and non-transparent. Empirical analysis showed that savings from use of the reformed system were estimated to be between 5% and 7%, with demonstrated price reductions (Cohen and Montoya, 2001; Singer et al., 2009). In 2013 their efforts reportedly increased transparency in 180 hospitals (Navarrete, 2014). Merely publishing purchase prices may be insufficient to reduce prices. Full bidding and fulfillment via electronic means are key enabling factors for medication price reduction. This has been explored by several Brazilian studies. A 2009 study of an e-procurement system introduced to nine Brazilian hospitals revealed a decrease in the price over ten percent (Sigulem and Zucchi, 2009). While such decrease can’t be attributable to transparency alone, volume purchasing arrangement may also play a crucial part (Sigulem and Zucchi, 2009). Price reductions may be attributable to greater purchasing leverage, in addition to improved transparency. Later on, another study in 2015 verified such hypothesis that an Internet-based strategy to improve pricing transparency did not lead to statistically significant reductions in actual purchase price, suggesting that merely publishing purchase prices for medications may be insufficient to reduce prices (Kohler et al., 2015).
In addition to pharmaceutical transparency, many benefits have been found in other areas of transparent online, some studies have emphasized the economic and social significance of adopting online pharmacies (Al Halbusi, 2024), finding that social media use and technological turbulence in SMEs can have a positive impact on business performance (Al Halbusi, 2022a; Hassani et al., 2022), as well as on e-entrepreneurial intentions (Al Halbusi, 2022a; Al Halbusi, 2022b). In addition, the adoption of knowledge management systems and artificial intelligence can improve business sustainability (Al Halbusi, 2023).
The results of the study can be better understood when considering the unique context of Ningxia. Ningxia, was an autonomous region in China, with a distinctive economic and medical background. Additionally, the technological upgrades of the Ningxia pharmaceutical procurement platform facilitated government supervision and drug transactions, which also played a crucial role in observing the results. As stated in the study setting in the Methods section, Ningxia as an economically and medically underdeveloped province needs efficient spending, which aligns with the goals of the transparent online open procurement policy. The observed reduction in medicine prices and costs is reflective of the region’s need to optimize limited resources while maintaining access to essential medicines.
This study suffered from major limitations. First, we only had access to institutional data of Ningxia, the generality of the results may be limited since nationwide data would be more applicable. The data we got was the average price of purchased medicines, the total volume of purchase orders, and the total costs of the purchase orders for each month of transparent online open procurement, and we got synthetic data rather than original data. Second, when dealing with drug prices and volumes, Defined Daily Dose (DDD) standardization would have be more concise, but since our access to the detailed data was restricted, the smallest package unit were used as an alternative. In addition, this study lacked control variables, data to adjust for drug type were not available, and administrative costs associated with the procurement process were not considered. Ningxia, as a less developed provincial level autonomous region, faced with similar problems as those undeveloped countries, including insufficient medical resources and government revenue. The demand for pharmaceuticals was also low with limited population. Thus, even with these flaws, this study makes significant contribution to the international readers especially for developing countries facing with souring pharmaceutical expenditure issues and medical corruption issues. This study evaluated the policy effects, provided new evidence that expands public understanding of the impact of transparent online open procurement, may provide valuable lessons for health policy decision makers elsewhere.
5 Conclusion
Ningxia pharmaceutical transparent online open procurement policy was characterized by a high transparency on pharmaceutical prices. The whole transaction process between hospitals, manufacturers and delivery enterprises were under strict government scrutiny. Public disclosure of pharmaceutical prices enabled an inner reference price adjustment. From our empirical assessment, this comprehensive procurement strategy resulted in reduced prices, lowered volumes, and lowered total costs of purchased orders of medicines. While the effect of such comprehensive reform on patient outcome or drug access needs further investigation.
Data availability statement
The data that support the findings of this study are available from Ningxia Public Resources Trading Administration but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Ningxia Public Resources Trading Administration.
Author contributions
JG: Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. CL: Conceptualization, Writing–original draft, Writing–review and editing. YL: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft. XC: Data curation, Formal Analysis, Investigation, Writing–original draft. TX: Investigation, Writing–original draft. YT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Fundamental Research Funds for the Central Universities (Grant Number: 2019kfyXJJS171).
Acknowledgments
The authors are also grateful to the staff in Ningxia Public Resources Trading Administration for their kind help in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Addis, A., and Magrini, N. (2002). New approaches to analysing prescription data and to transfer pharmacoepidemiological and evidence-based reports to prescribers. Pharmacoepidemiol Drug Saf. 11 (8), 721–726. doi:10.1002/pds.785
Ahmad, N. S., Makmor-Bakry, M., and Hatah, E. (2020). Drug price transparency initiative: a scoping review. Res. Soc. Adm. Pharm. 16 (10), 1359–1369. doi:10.1016/j.sapharm.2020.01.002
Akbarpour, Z., Zarei, L., Varahrami, V., Peiravian, F., and Yousefi, N. (2023). Main drivers of diabetes pharmaceuticals expenditures: evidence from OECD countries and Iran. J. Diabetes & Metabolic Disord. 22 (1), 431–442. doi:10.1007/s40200-022-01161-6
Al Halbusi, H., Abdeslam, H., Mosconi, E., and Ahmad, A. (2023). Knowledge management systems and artificial intelligence adoption for increasing business sustainability. AMCIS.
Al Halbusi, H., Alhaidan, H., Abdelfattah, F., T, R., and Cheah, J.-H. (2022a). Exploring social media adoption in small and medium enterprises in Iraq: pivotal role of social media network capability and customer involvement. Technol. Analysis Strategic Manag. 36, 2052–2069. doi:10.1080/09537325.2022.2125374
Al Halbusi, H., Al-Sulaiti, K., Abdelfattah, F., Ahmad, A. B., and Hassan, S. (2024). Understanding consumers’ adoption of e-pharmacy in Qatar: applying the unified theory of acceptance and use of technology. J. Sci. Technol. Policy Manag. ahead-of-print(ahead-of-print). doi:10.1108/JSTPM-03-2023-0042
Al Halbusi, H., Soto-Acosta, P., and Popa, S. (2022b). Entrepreneurial passion, role models and self-perceived creativity as antecedents of e-entrepreneurial intention in an emerging Asian economy: the moderating effect of social media. Asia Pac. J. Manag. doi:10.1007/s10490-022-09857-2
Bangalee, V., and Suleman, F. (2016). Towards a transparent pricing system in South Africa: trends in pharmaceutical logistics fees. South Afr. Health Rev.
Cheng, Z., and Bo, G. (2021). Practice and effect of optimizing the supervision mechanism of online bargaining procurement of drugs in Shanghai. China Health Insur. (08), 46–48. doi:10.19546/j.issn.1674-3830.2021.8.006
Cohen, J. C., and Montoya, J. C. (2001). Using technology to fight corruption in pharmaceutical purchasing: lessons learned from the Chilean experience. World Bank Publications.
Colbert, A., Rintoul, A., Simão, M., Hill, S., and Swaminathan, S. (2020). Can affordability and innovation coexist for medicines? Bmj 368, l7058. doi:10.1136/bmj.l7058
de Wolf, P., Brouwer, W. B., and Rutten, F. F. (2005). Regulating the Dutch pharmaceutical market: improving efficiency or controlling costs? Int. J. Health Plann Manage 20 (4), 351–374. doi:10.1002/hpm.819
Dos Santos, R. L. B., Pepe, V. L. E., and Osorio-de-Castro, C. G. S. (2022). Public procurement of antineoplastic agents used for treating breast cancer in Brazil between 2013 and 2019. BMC Cancer 22 (1), 769. doi:10.1186/s12885-022-09851-3
European Union (1988). Council Directive 89/105/EEC of 21 December 1988 relating to the transparency of measures regulating the prices of medicinal products for human use and their inclusion in the scope of national health insurance systems. Official J. Eur. Communities. Available at: http://data.europa.eu/eli/dir/1989/105/oj (Accessed June 15, 2023).
Fei, K., Yuan, C., Ming, X., Haiyan, L., and Jie, Q. (2023). Analysis and prospect of the pharmaceutical industry development trend in China. Strategic Study CAE 25 (05), 1–10. doi:10.15302/j-sscae-2023.05.003
Franzen, N., Retèl, V. P., Schats, W., and van Harten, W. H. (2020). Evidence underlying policy proposals for sustainable anticancer drug prices: a systematic review. JAMA Oncol. 6 (6), 909–916. doi:10.1001/jamaoncol.2019.6846
Hassali, M. A., and Wong, Z. Y. (2018). “Chapter 12 - overcoming challenges of generic medicines utilization in low- and middle-income countries: lessons learned from international experiences,” in Social and administrative aspects of pharmacy in low- and middle-income countries. Editors M. I. M. Ibrahim, A. I. Wertheimer, and Z.-U.-D. Babar (Academic Press), 197–210. doi:10.1016/B978-0-12-811228-1.00012-1
Hassani, A., Halbusi, H. A., and Gharbaoui, O. E. (2022). “Technological turbulence and innovation performance: mediating role of absorptive capacity in Industrial SMEs,” in Paper presented at the 2022 IEEE international conference on technology management, operations and decisions (ICTMOD). doi:10.1109/ICTMOD55867.2022.10041884
IQVIA (2023). The global use of medicines 2023 and outlook to 2027. Available at: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/the-global-use-of-medicines-2023 (Accessed June 13, 2023).
Joosse, I. R., Tordrup, D., Glanville, J., Kotas, E., Mantel-Teeuwisse, A. K., and van den Ham, H. A. (2023). Evidence on the effectiveness of policies promoting price transparency - a systematic review. Health Policy 134, 104681. doi:10.1016/j.healthpol.2022.11.002
Jun-feng, Y. (2006). New model of centralized tendering and procurement of medicines. Chin. J. Hosp. Pharm. (09), 1148–1150.
Jun-he, Y. (2007). Experiences of online medicine procurement sunshine Project in Guangdong province. China Pharm. 19, 1441–1443.
Kadkhodamanesh, A., Varahrami, V., Zarei, L., Peiravian, F., Hadidi, M., and Yousefi, N. (2021). Investigation the determinants of pharmaceutical expenditure share of GDP in Iran and selected OECD countries. J. Pharm. Policy Pract. 14 (1), 82. doi:10.1186/s40545-021-00371-2
Kakkar, A. K. (2021). Pharmaceutical price regulation and its impact on drug innovation: mitigating the trade-offs. Expert Opin. Ther. Pat. 31 (3), 189–192. doi:10.1080/13543776.2021.1876029
Kohler, J. C., Mitsakakis, N., Saadat, F., Byng, D., and Martinez, M. G. (2015). Does pharmaceutical pricing transparency matter? Examining Brazil's public procurement system. Glob. Health 11, 34. doi:10.1186/s12992-015-0118-8
Koskinen, H., Mikkola, H., Saastamoinen, L. K., Ahola, E., and Martikainen, J. E. (2015). Time series analysis on the impact of generic substitution and reference pricing on antipsychotic costs in Finland. Value Health 18 (8), 1105–1112. doi:10.1016/j.jval.2015.08.014
Langley, T., Lacey, J., Johnson, A., Newman, C., Subramanian, D., Khare, M., et al. (2018). An evaluation of a price transparency intervention for two commonly prescribed medications on total institutional expenditure: a prospective study. Future Healthc. J. 5 (3), 198–202. doi:10.7861/futurehosp.5-3-198
Law of Ukraine (2015). Law No. 922-VIII on public procurement. Available at: https://leap.unep.org/en/countries/ua/national-legislation/law-no-922-viii-public-procurement (Accessed October 18, 2023).
Lee, I. H., Bloor, K., Hewitt, C., and Maynard, A. (2015). International experience in controlling pharmaceutical expenditure: influencing patients and providers and regulating industry - a systematic review. J. Health Serv. Res. Policy 20 (1), 52–59. doi:10.1177/1355819614545675
Ling-hua, G., and Jie-yuan, Z. (2022). Analysis on influencing factors of hospital drug cost control. Strait Pharm. J. 34 (09), 167–171.
Main, B., Csanadi, M., and Ozieranski, P. (2022). Pricing strategies, executive committee power and negotiation leverage in New Zealand's containment of public spending on pharmaceuticals. Health Econ. Policy Law 17 (3), 348–365. doi:10.1017/s1744133122000068
Mirzoev, T., Koduah, A., Cronin de Chavez, A., Baatiema, L., Danso-Appiah, A., Ensor, T., et al. (2021). Implementation of medicines pricing policies in sub-Saharan Africa: protocol for a systematic review. BMJ Open 11 (2), e044293. doi:10.1136/bmjopen-2020-044293
Moodley, R., and Suleman, F. (2019a). Evaluating the impact of the single exit price policy on a basket of originator medicines in South Africa from 1999 to 2014 using a time series analysis. BMC Health Serv. Res. 19 (1), 576. doi:10.1186/s12913-019-4403-8
Moodley, R., and Suleman, F. (2019b). The impact of the single exit price policy on a basket of generic medicines in South Africa, using a time series analysis from 1999 to 2014. PLoS One 14 (7), e0219690. doi:10.1371/journal.pone.0219690
Murthy, V. N. R., and Okunade, A. A. (2016). Determinants of U.S. health expenditure: evidence from autoregressive distributed lag (ARDL) approach to cointegration. Econ. Model. 59, 67–73. doi:10.1016/j.econmod.2016.07.001
National Health Commission of the People’s Republic of China (2023a). China health statistical yearbook 2022. Available at: http://www.nhc.gov.cn/mohwsbwstjxxzx/tjtjnj/202305/6ef68aac6bd14c1eb9375e01a0faa1fb.shtml (Accessed September 6, 2023).
Navarrete, E. (2014). CUENTA PÚBLICA 2013. Central de Abastecimiento S.N.S.S. Ministerio de Salud Gobierno de Chile: Cenabast. Available at: https://www.cenabast.cl/wp-content/uploads/2017/09/Cuenta_publica_Cenabast_01_08_2014.pdf (Accessed October 18, 2023).
National Health Commission of the People's Republic of China (2023b). 2022 statistical bulletin on the development of health care in China. Available at: http://www.nhc.gov.cn/guihuaxxs/s3585u/202309/6707c48f2a2b420fbfb739c393fcca92.shtml. (Accessed November 22, 2023)
Ni, Z., Jia, J., Cui, L., Zhou, S., and Wang, X. (2021). The impact of China's zero markup drug policy on hospitalization expenses for inpatients in tertiary public hospitals: evidence based on quantile difference-in-difference models. Healthc. (Basel) 9 (7), 908. doi:10.3390/healthcare9070908
Ningxia Hui Autonomous Region Statistics Bureau (2023). Ningxia statistical yearbook 2022. Available at: https://nxdata.com.cn/files_nx_pub/html/tjnj/2022/indexfiles/indexch.htm?1=1 (Accessed June 1, 2023).
Ningxia Public Resources Trading Administration (2017). Notice on the implementation of the results of centralized purchasing of drugs for public hospitals in Ningxia Hui autonomous region (first batch). Available at: https://nxyp.nxggzyjy.org/cms/detail.html?infoId=22340&CatalogId=2 (Accessed May 10, 2023).
Ningxia Public Resources Trading Administration (2020a). Notice of the public resources trading administration of the autonomous region on the issuance of the implementation rules for the procurement of sunshine hanging drugs in Ningxia Hui autonomous region (for trial implementation). Available at: https://nxyp.nxggzyjy.org/cms/detail.html?infoId=22065&CatalogId=2 (Accessed May 10, 2023).
Ningxia Public Resources Trading Administration (2020b). Notice on carrying out the procurement of drugs in Ningxia Hui autonomous region. Available at: https://nxyp.nxggzyjy.org/cms/detail.html?infoId=21796&CatalogId=57 (Accessed May 10, 2023).
OECD (2023). Pharmaceutical spending (indicator). Available at: https://data.oecd.org/healthres/pharmaceutical-spending.htm (Accessed June 15, 2023).
Paschke, A., Dimancesco, D., Vian, T., Kohler, J. C., and Forte, G. (2018). Increasing transparency and accountability in national pharmaceutical systems. Bull. World Health Organ 96 (11), 782–791. doi:10.2471/blt.17.206516
Riccaboni, M., Swoboda, T., and Van Dyck, W. (2022). Pharmaceutical net price transparency across european markets: insights from a multi-agent simulation model. Health Policy 126 (6), 534–540. doi:10.1016/j.healthpol.2022.03.013
Savin, N. E., and White, K. J. J. E. (1977). The durbin-watson test for serial correlation with extreme sample sizes or many regressors. REGRESSORS 45, 1989–1996. doi:10.2307/1914122
Sigulem, F., and Zucchi, P. (2009). E-procurement in the Brazilian healthcare system: the impact of joint drug purchases by a hospital network. Rev. Panam. Salud Publica 26 (5), 429–434.
Singer, M., Konstantinidis, G., Roubik, E., and Beffermann, E. (2009). Does e-procurement save the state money? JOPP 9, 58–78. doi:10.1108/JOPP-09-01-2009-B002
Turner, S. L., Forbes, A. B., Karahalios, A., Taljaard, M., and McKenzie, J. E. (2021). Evaluation of statistical methods used in the analysis of interrupted time series studies: a simulation study. BMC Med. Res. Methodol. 21 (1), 181. doi:10.1186/s12874-021-01364-0
World Health Organization (2015). WHO guideline on country pharmaceutical pricing policies. Available at: https://fctc.who.int/publications/i/item/9789241549035 (Accessed June 15, 2023).
World Health Organization (2018). Technical report: pricing of cancer medicines and its impacts: a comprehensive technical report for the World Health Assembly Resolution 70.12: operative paragraph 2.9 on pricing approaches and their impacts on availability and affordability of medicines for the prevention and treatment of cancer. Available at: https://iris.who.int/handle/10665/277190 (Accessed May 30, 2024).
World Health Organization (2019). WHA72.8 - improving the transparency of markets for medicines, vaccines, and other health products. Available at: https://www.who.int/publications/m/item/wha72.8 (Accessed June 9, 2023).
World Health Organization (2020). WHO guideline on country pharmaceutical pricing policies. Available at: https://www.who.int/publications/i/item/9789240011878 (Accessed June 9, 2023).
World Health Organization. Regional Office for the Eastern Mediterranean. (2009). Measuring transparency to improve good governance in the public pharmaceutical sector: Jordan. Available at: https://iris.who.int/handle/10665/116572 (Accessed October 3, 2023).
Wouters, O. J., Kanavos, P. G., and Mc, K. M. (2017). Comparing generic drug markets in Europe and the United States: prices, volumes, and spending. Milbank Q. 95 (3), 554–601. doi:10.1111/1468-0009.12279
Xin, D., Xi, Y., Yu-qing, T., and Xin-ping, Z. (2013). Impacts of information transparency on the volume of purchased high-price medicines during essential drug purchase. Med. Soc. 26 (07), 50–52.
Keywords: interrupted time series analysis, pharmaceutical procurement policy, pharmaceutical expenditures, policy effect, price transparency
Citation: Gao J, Liu C, Li Y, Chen X, Xue T and Tang Y (2024) Effects of transparent online open procurement on prices, volumes, and costs of medicines: an interrupted time series study in Ningxia, China. Front. Pharmacol. 15:1362374. doi: 10.3389/fphar.2024.1362374
Received: 28 December 2023; Accepted: 30 July 2024;
Published: 20 August 2024.
Edited by:
Bernd Rosenkranz, Fundis African Academy of Medicines Development, South AfricaReviewed by:
Felix Khuluza, Kamuzu University of Health Sciences (Formerly College of Medicine-University of Malawi), MalawiHussam Al Halbusi, Ahmed Bin Mohammed Military College, Qatar
Copyright © 2024 Gao, Liu, Li, Chen, Xue and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqing Tang, dHlxMTk5MEBodXN0LmVkdS5jbg==
 Jingying Gao
Jingying Gao Chaojie Liu
Chaojie Liu Yinming Li1
Yinming Li1 Tianqin Xue
Tianqin Xue Yuqing Tang
Yuqing Tang