
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 09 February 2024
Sec. Neuropharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1361651
Insulin resistance in brain and amyloidogenesis are principal pathological features of diabetes-related cognitive decline and development of Alzheimer’s disease (AD). A growing body of evidence suggests that maintaining glucose under control in diabetic patients is beneficial for preventing AD development. Dipeptidyl peptidase 4 inhibitors (DDP4is) are a class of novel glucose-lowering medications through increasing insulin excretion and decreasing glucagon levels that have shown neuroprotective potential in recent studies. This review consolidates extant evidence from earlier and new studies investigating the association between DPP4i use, AD, and other cognitive outcomes. Beyond DPP4i’s benefits in alleviating insulin resistance and glucose-lowering, underlying mechanisms for the potential neuroprotection with DPP4i medications were categorized into the following sections: (Ferrari et al., Physiol Rev, 2021, 101, 1,047–1,081): the benefits of DPP4is on directly ameliorating the burden of β-amyloid plaques and reducing the formation of neurofibrillary tangles; DPP4i increasing the bioactivity of neuroprotective DPP4 substrates including glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), and stromal-derived factor-1α (SDF-1α) etc.; pleiotropic effects of DPP4is on neuronal cells and intracerebral structure including anti-inflammation, anti-oxidation, and anti-apoptosis. We further revisited recently published epidemiological studies that provided supportive data to compliment preclinical evidence. Given that there remains a lack of completed randomized trials that aim at assessing the effect of DPP4is in preventing AD development and progression, this review is expected to provide a useful insight into DPP4 inhibition as a potential therapeutic target for AD prevention and treatment. The evidence is helpful for informing the rationales of future clinical research and guiding evidence-based clinical practice.
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder with insidious onset. The underlying pathologic process of AD involves the accumulation of extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (Ferrari and Sorbi, 2021). These neuropathological changes contribute to the loss of neurons and synapses, triggering progressive cognitive impairment and further leading to the development of AD. AD disproportionately affects the elderly population. Effective treatments for preventing and curing AD are still lacking. Current treatments including cholinesterase inhibitors (e.g., Donepezil, Galantamine) and N-methyl-D-aspartate receptors (e.g., Memantine) focus primarily on managing clinical symptoms and have shown no clear benefits in preventing disease progression. As per data provided by the World Health Organization, the global population of individuals aged 60 and older is estimated to increase twofold by the year 2050, reaching 2.1 billion; and the number of individuals aged 80 or older is expected to reach 426 million (World Health Organization, 2023). Demographic aging will undoubtedly lead to an exponential rise in the new cases of AD patients and its prevalence, exacerbating AD-related societal and public health burden. In 2018, Alzheimer’s Disease International estimated that there are around 50 million people worldwide living with dementia, and this number is expected to triple by 2050 (Alzheimers Disease International, 2023). The epidemiology of AD highlights the urgency of exploring an effective therapeutic approach for preventing AD from happening and slowing its progression.
Ample evidence has suggested an association between type 2 diabetes (T2DM), cognitive decline, and AD (Talbot et al., 2012). Maintaining glucose under control in T2DM patients may serve as an effective way for preventing AD development. This hypothesis is supported by existing evidence. First, hyperglycemia was found that can substantially increase levels of Aβ protein (Yang et al., 2013; Chao et al., 2016). Second, the increased formation and accumulation of methylglyoxal through glycolytic pathways in individuals with diabetes has been linked to an increased risk of AD (Angeloni et al., 2014). Methylglyoxal, a highly reactive dicarbonyl metabolite, serves as a potent precursor for advanced glycation end products (AGEs) (Waqas et al., 2022). Both methylglyoxal and its derived AGEs are implicated in etiopathogenesis and progression of AD, inducing extensive protein cross-linking, mitochondrial dysfunction, oxidative stress, and neuronal cell death (Angeloni et al., 2014; Brings et al., 2017; Akhter et al., 2021). Studies have also found that AGEs can accumulate in neurons and astroglia, contributing the formation of neuritic amyloid plaques and neurofibrillary tangles (Srikanth et al., 2011; Fawver et al., 2012; Angeloni et al., 2014; Twarda-Clapa et al., 2022). Additionally, methylglyoxal was found that can impair the integrity of blood brain barrier (BBB), further elevating the risks of various neurodegenerative disorders, including AD and cerebrovascular diseases (Daneman and Prat, 2015; Profaci et al., 2020; Chojdak-Lukasiewicz et al., 2021; Berends et al., 2023). Third, AD is called ‘type 3’ diabetes due to the involvement of insulin resistance in its pathology (Michailidis et al., 2022). Dysfunction in brain insulin signaling is likely a pivotal factor initiating pathological changes in AD (De Felice et al., 2022). The classical insulin signaling pathway in the brain involves the activation of the PI3-K/Akt pathway by insulin receptor substrate (IRS) (Kothari et al., 2017; Salas et al., 2018; Gabbouj et al., 2019). Inhibition of this pathway can result in the blockade of downstream GSK-3, which is implicated in tau protein hyperphosphorylation, augmentation of Aβ production, neuroinflammation, and memory impairment (Chami et al., 2016; Huang et al., 2018). An alternative insulin signaling pathway, the MAPK pathway comprising ERK, JNK, and p38 kinases, was identified to be associated with cell apoptosis, neuroinflammation, and oxidative stress in the brain, contributing to the development of AD (Figure 1) (Morrison, 2012; Asih et al., 2020; Tian et al., 2023; Zhang et al., 2023).
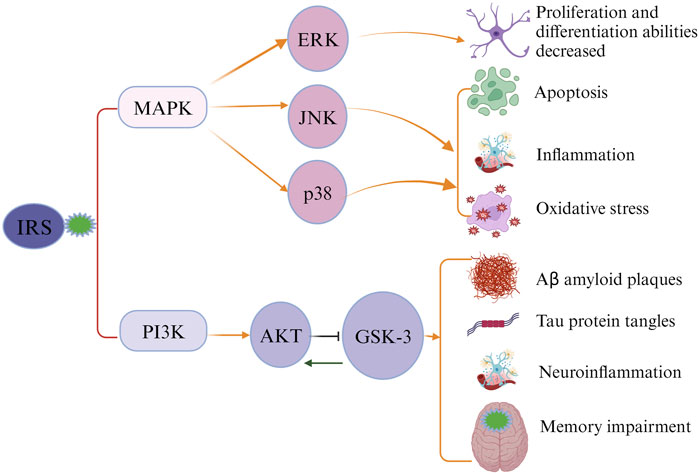
FIGURE 1. Impaired insulin signaling leads to the development of Alzheimer’s disease (AD). Insulin receptor substrate (IRS) activates the classical insulin signaling pathway. When the signaling pathway is compromised, IRS cannot activate PI3K and MAPK, causing alterations in the levels of downstream proteins and subsequently triggering the pathological characteristics of AD.
Except for insulin resistance, common pathological features shared by T2DM and AD also include amyloid accumulation, and accumulated amylin in T2DM has a similar structural morphology as abnormal Aβ peptides in AD (Stanciu et al., 2020). Diabetes and AD have also been found to share many risk factors, which include but not limit to hyperlipidemia, metabolic syndromes, oxidative stress and inflammation, mitochondrial dysfunction, as well as genetic (e.g., amyloid precursor protein [APP] gene) and lifestyle factors (e.g., sedentary lifestyle and poor dietary patterns) (Stanciu et al., 2020; Michailidis et al., 2022). Diabetes can also cause intracerebral micro- and macro-vascular lesions to disrupt brain blood flow, contributing to an increased AD risk. An experimental study of mice models exposed to hyperglycemia condition revealed that high blood glucose levels can augment the vulnerability of endothelial cells in brain’s blood vessels to the toxicity of abnormal Aβ protein. This increased susceptibility consequently contributes to the impairment of BBB, reduced blood flow and slow clearance of Aβ protein (Carvalho et al., 2014).
Against this background, glucose-lowering medications may hold promise for repurposing as a preventive and therapeutic treatment for AD (Wang et al., 2023). A recent meta-analysis in 2022 including 229,110 participants found no evidence of protective effect of metformin on AD prevention, with of odds ratios (ORs) of 1.17 (Luo et al., 2022). Despite the unfavorable outcome of older glucose-lowering medications yielded in earlier studies, recent research has revealed the potential neuroprotective benefit associated with novel antidiabetic drugs, including sodium-glucose cotransporter-2 inhibitors (SGLT2i), dipeptidyl peptidase-4 inhibitors (DPP4is), and glucagon-like peptide-1 receptor agonists (GLP-1RAs) (Sim et al., 2021; Kopp et al., 2022; Yin et al., 2022; Sim et al., 2023). These findings are of particular interest to researchers and clinicians, and a timely review to consolidate existing evidence will be beneficial for informing current clinical practice and providing guidance for future research in more profound investigations. Given the limited number of clinical studies investigating the relationship between SGLT2i and GLP-1RA with AD, this review specifically focused on the relationship between DPP4i/gliptins and AD (Tang et al., 2023).
DPP4 is a type Ⅱ transmembrane protein, belonging to the serine peptidase subfamily S9B, with a typical α/β hydrolase fold (Rohrborn et al., 2015). DPP4 exists in two forms: either as a membrane-anchored protein or as a soluble form (sDPP4) comprising the majority of extracellular DPP4 protein, produced through the cleavage of membrane-bound DPP4 by matrix metalloproteinase (MMP) (Rohrborn et al., 2014). sDPP4 lacks intracellular tail and transmembrane regions but retains catalytic activity (Mulvihill and Drucker, 2014). Membrane-bound DPP4 is widely expressed on the cell surface of various tissues, including intestine, liver, pancreas, kidney, spleen, lung and bone marrow, while sDPP4 is widely distributed in serum and body fluids such as saliva, cerebrospinal fluid, seminal fluid and bile (Mulvihill and Drucker, 2014; Baggio et al., 2020). Active sDPP4 in the circulation ensures that DPP4 can play a role (DPP-4-mediated proteolysis) in the extracellular environment. sDPP4 has been identified as a new adipokine contributing to most para- and endocrine effects (Rohrborn et al., 2015). In human’s brain, DPP4 was found that is expressed in thalamus, cerebral cortex, white matter, and pons (DPP4, 2024). The structure of DPP4 includes three main regions which are catalytic region, cysteine-rich region, and highly glycosylated region (Figure 2). Glucagon-like peptide-1 (GLP-1) is a peptide hormone known to maintain glucose homeostasis by enhancing glucose-dependent insulin secretion from pancreatic beta cells and suppressing the release of glucagon. DPP4 can rapidly cleave GLP-1 into inactive fragments GLP-1 (9–36/37) to prevent it from binding to GLP-1 receptors (GLP-1R) to exert an action (Figure 2). Beyond GLP-1, DPP4 can also degrade other incretin hormones, such as glucose-dependent insulinotropic polypeptide (GIP) that plays a similar role as GLP-1 on regulation of glucose and insulin (Seino et al., 2010).
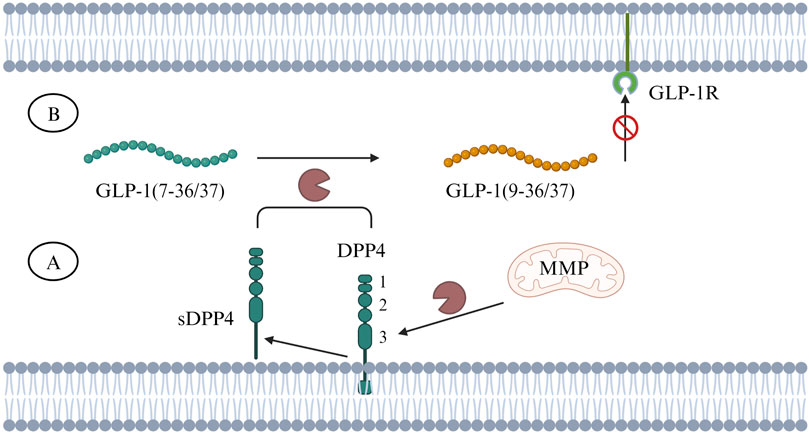
FIGURE 2. (A) DPP4 is a transmembrane protein and can be cleaved by MMP into sDPP4. The number 1, 2, 3 represent different regions of DPP4’s domain structure, 1. catalytic region, 2. cysteine-rich region, 3. highly glycosylated region. (B) Both DPP4 and sDPP4 can cleave GLP-1 (7–36/37) to inactive fragments - GLP-1 (9–36/37), making it unable to bind to GLP-1R. Abbreviations: DPP4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; GLP-1R, glucagon-like peptide-1 receptor; MMP, matrix metalloproteinase; sDDP4, soluble DDP4.
DPP4i manages diabetes by stimulating insulin secretion and inhibiting glucagon secretion through elevating endogenous GLP-1 levels. Substrate-based DPP4is are drugs that bind to the active site of the enzyme, inhibiting DPP4’s activity and leading to increased levels of GLP-1. Since DPP4i agents generally do not increase the risk of hypoglycemia and is well tolerated, they have now been widely used. At least 11 different DPP4i medications have been approved for use worldwide (Deacon, 2020). Sitagliptin was the firstly approved DPP4i used on market in the United States in 2006. Vildagliptin, saxagliptin, linagliptin and alogliptin are also commonly used DPP4is as of now. DPP4 has five binding subsites including S1, S2, S1′, S2′, and S2 extensive (Arulmozhiraja et al., 2016). DPP4is interacting with S1 and S2 subsites is mandatory for them to exert their inhibitory activity, and additional interaction with S1′, S2′, or S2 extensive will substantially increase the drug’s potency (Mathur et al., 2023). Accordingly, DPP4is were grouped into different classes according to the enzyme subsites where they bind to. For example, vildagliptin and saxagliptin binding with S1 and S2 only were categorized into Class 1, alogliptin and linagliptin binding with S1′, S2′, S1 and S2 belong to Class 2, and sitagliptin, anagliptin, gemigliptin, and teneligliptin binding with S1, S2 and S2 extensive were classified as Class 3 (Arulmozhiraja et al., 2016; Gallwitz, 2019; Mathur et al., 2023).
Accumulative evidence from human and experimental studies has suggested that inhibition of DPP4 may be protective against AD. A study of 1,229 Chinese adults aged 60 years old or older found that increased plasma DPP4 activity was associated with accelerated cognitive impairment and reduced MoCA score (all p < 0.001) (Chen et al., 2017). A significant increase in DPP4 activity was found in the brains of sporadic AD patients (Oumata et al., 2022), suggesting that increased DPP4 activity is implicated in cognitive dysfunction caused by AD. Mechanisms supporting the potential neuroprotective effects of DPP4i have been increasingly investigated and summarized as below:
The direct impact of DPP4 on the pathological process of AD is likely explained by the DPP4’s capacity to cleave two key Aβ fragments. As observed in in vitro experiments, DPP4 can cleave Aβ1-42 and Aβ1-40, the crucial components of amyloid deposits in AD patients, into Aβ3-42 and Aβ3-40. Subsequently, glutamyl cyclase (GC) catalyzes the cyclization of the N-terminal glutamate of Aβ3-42 and Aβ3-40 and transforms them into non-degradable pE-Aβ3-40/42. pE-Aβ3-40/42 aggregates to form amyloid plaques and lead to the progression of AD (Antonyan et al., 2018). DPP4i can improve AD by inhibiting Aβ plaque deposition independent of GLP-1 signaling pathways (Figure 3). An experimental study found that administering DPP4i to mice can decrease the abnormal phosphorylation of Tau and neurofilaments in mice’s brain, and attenuate intracellular Aβ deposition (Chen et al., 2019). Another study in mice of AD reported similar findings that oral DPP4i administration (Linagliptin) can significantly improve incretin levels and reduce Aβ deposition, tau phosphorylation and neuroinflammation in the brain (Kosaraju et al., 2017). In agreement with these findings, an in vitro study of human neuronal cells found that linagliptin can restore the impaired insulin signaling caused by Aβ-induced cytotoxicity and inhibit the activation of GSK3β and hyperphosphorylation of tau by restoring insulin downstream signaling (Kornelius et al., 2015). Xue’s study on elderly T2DM patients with mild cognitive impairment revealed a significant increase in the plasma Aβ1-42/Aβ1-40 ratio in the DPP4i treatment group compared to the control group (sulfonylurea) (Xue et al., 2020). The mean values before and after DPP4i treatment were 0.39 and 0.47, respectively, while in the control group, they were 0.40 and 0.43 (p < 0.001). These findings suggest an improvement in Aβ burden associated with DPP4i use. In addition, a study found that inhibition of DPP4 significantly reduced the activity of β-secretase, the most important enzyme to hydrolyze APP to produce abnormal Aβ peptides (p < 0.01) (Huang et al., 2020).
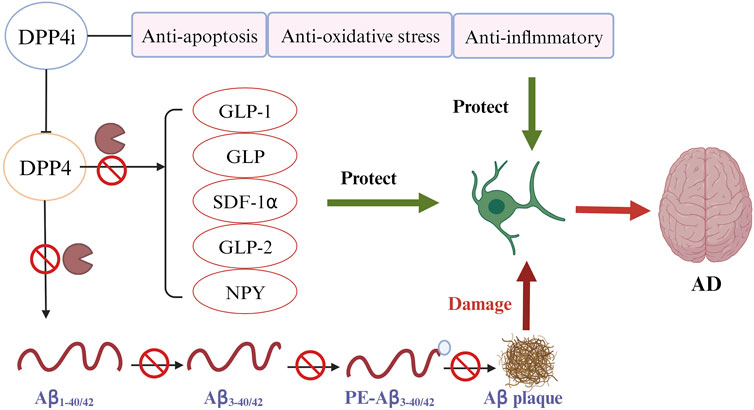
FIGURE 3. DPP4 inhibitors (DPP4i) hold potential of neuroprotective effects by inhibiting DPP4 via different mechanisms (Ferrari and Sorbi, 2021): DPP4i may be directly implicated in the prevention of Aβ plaque deposition (World Health Organization, 2023); DPP4i can increase the bioavailability of various DPP4i substrates to reduce the Aβ accumulation, tau phosphorylation and neuroinflammation (Alzheimer’s Disease International, 2023); DDP4i exhibits other beneficial properties for preventing AD, including anti-inflammation, anti-oxidation, and anti-apoptosis.
Beyond a direct effect on preventing Aβ accumulation, DPP4i may also offer benefits by increasing the expression of neuroprotective DPP4 substrates (Figure 3) (Angelopoulou and Piperi, 2018; Yin et al., 2022). GLP-1 and GIP, the best characterized DPP4 substrates, have shown their potential of neuroprotection in many studies (Holscher, 2014; Angelopoulou and Piperi, 2018; Reich and Holscher, 2022). GLP-1 and GIP can penetrate the BBB and bind to their receptors in brain tissues to exert an effect (Athauda and Foltynie, 2016; Reich and Holscher, 2022). Use of DPP4i can significantly increase the bioavailability of these two incretin hormones (Yin et al., 2022).
The neuroprotective effects of GLP-1 are mostly studied. Both human studies and studies of animal models found significantly reduced expression of GLP-1 and GLP-1R in the AD brain compared with controls without AD (Chen et al., 2019). Previous studies found extra-pancreatic effects of GLP-1 analogues which are independent of their role in glucose homeostasis. GLP-1 crosses BBB to exert neuroprotective benefits through decreasing the levels of APP and glycogen synthase kinase- 3β (GSK-3β), reducing Aβ deposition and tau phosphorylation, which are hallmarks of AD, as well as increasing insulin secretion and insulin receptor sensitivity and restoring insulin signaling pathway (Siddiqui et al., 2021). GLP-1 can also protect against neuronal degeneration by improving mitochondrial function and cellular proliferation, alleviating neuroinflammation and apoptosis (Athauda and Foltynie, 2016). Similarly, studies found that GLP-1R exists in the pyramidal neurons of hippocampus and Purkinje cells in the cerebellum. GLP-1R exerts classical type growth effects by influencing the expression of genes that are involved in cell growth and repairment. Mice overexpressing GLP-1R in hippocampus showed improved synaptic growth and cognitive function, while mice with GLP-1R knockout showed reduced synaptic plasticity and deficits in learning and memory (Du et al., 2022). The endogenous GLP-1 is quickly deactivated by the endogenous DPP4, transforming it into a metabolite that is incapable of binding to GLP-1R. While studies suggested that most DPP4is have limited ability to penetrate BBB, its inhibition of peripheral DPP4 raises the serum level of GLP-1 (Lin et al., 2023). Increasing circulated GLP-1 can penetrate BBB to exert neurocognitive benefits (Shannon, 2013). In another word, DPP4is may prevent AD development and progression by prolonging the circulating half-life of endogenous GLP-1 (Chen et al., 2019).
GIP can inhibit the apoptosis of cerebellar granule cells, and the activation of GIP receptor can promote the proliferation of neuronal progenitor cells. GIP analogues D-ala2-GIP and N-glyc-GIP have been shown to promote hippocampal synaptic plasticity and memory, while the antagonist of GIP (Pro 3-GIP) reduces hippocampal synaptic plasticity and memory (Ji et al., 2016). A novel long-acting GIP analogue DAla2GIP-Glu-PAL was shown to improve cognitive behavior, synaptic plasticity and alleviate central pathological progression in AD mice, with the underlying mechanism pertaining to the inhibition of neuroinflammation and the upregulation of cAMP-/PKA/CREB signaling pathway (Yuan et al., 2021).
Stromal-derived factor-1α (SDF-1α), also named CXCL12, is another physiological DPP4 substrate associated with neuroprotection and neurogenesis in experimental studies of AD (Chalichem et al., 2017). The expression level of SDF-1α is significantly reduced in AD patients and negatively correlated with markers of synaptic loss and microglia activation (Sanfilippo et al., 2020). A study of AD mice found that SDF-1α can facilitate bone marrow-derived microglia to migrate into the brain, leading to a reduction in Aβ accumulation by enhancing the Aβ phagocytosis (Wang et al., 2012). A decrease in SDF-1α expression is related to the excessive production of APP in transgenic mice, contributing to cognitive defects, while SDF-1α pretreatment in AD mice model was found to reduce neuronal dendritic degeneration and neuronal apoptosis (Raman et al., 2011).
Other DPP4 substrates have also been shown to be neuroprotective, and use of DPP4i can increase their expression levels. Neuropeptide-Y (NPY) is the best characterized DPP4 substrate in blood circulation. NPY and its receptors are also widely expressed in the central nerve system (CNS) showing to attenuate neuroinflammation, promote neuro-proliferation and the production of sufficient trophic support for the growth of new neurons (Duarte-Neves et al., 2016). Studies in both humans and animal models reported decreased NPY levels in hippocampus and cerebral cortex regions in AD patients (Ye et al., 2018). Overexpression of NPY via DPP4i may be protective against AD.
In addition to the mechanisms illustrated previously, DPP4i may offer additional neurocognitive benefits through anti-inflammation, anti-oxidation, and anti-apoptosis (Figure 3) (Yin et al., 2022).
The anti-inflammatory feature of DPP4i has been documented in various neurodegenerative disorders. Studies of rat models showed that pro-inflammatory cytokines in the hippocampus, the prone factors of AD (Calsolaro and Edison, 2016), such as TNF-α, IL-6, and NF-κB, were significantly reduced after the administration of sitagliptin (El-Sahar et al., 2015; Siddiqui et al., 2021). In mice with moderate traumatic brain injury, sitagliptin was observed to exert a neuroprotective effect by increasing the expression of anti-inflammatory factor IL-10 in the cerebral cortex and striatum (Hung et al., 2020). Reducing the expression of these cytokines via DPP4i can also inhibit the expression of NF-κB and further reduce the level of BACE-1 enzyme in neurons, a key component involved in the amyloidogenic pathway to produce Aβ oligomers (Chen et al., 2012). In addition, DPP4i was shown to reduce the differentiation of macrophages into M1 phenotype, with the latter associated with neuroinflammation, and to induce differentiation of macrophages into M2 phenotype to exert neuroprotective effects (Wiciński et al., 2018).
One major cause of oxidative stress in the human body is hyperglycemia (Fiorentino et al., 2013). DPP4i has been shown to alleviate oxidative stress. An early study of rat models with lipopolysaccharide (LPS)-induced sepsis found that linagliptin can reduce LPS-induced endothelial dysfunction, reactive oxygen species (ROS) generation, the NADPH oxidase subunits expression and aortic infiltration with inflammatory cells in the vascular and cardiac tissues and blood (Kroller-Schon et al., 2012). After the treatment of sitagliptin, the levels of glutamate and nitric oxide decreased significantly in the hippocampus of ischemic rats, while the concentration of glutathione increased significantly (El-Sahar et al., 2015). Li et al. assessing the effect of sitagliptin combined with quercetin for the treatment of AD found that the combined administration not only significantly reduced the level of Aβ, but also enhanced the Nrf2/HO-1 pathway and improved the antioxidant activity in the brain of rat models (Li et al., 2019).
Moreover, DPP4i was found to reduce neuronal cell apoptosis and promote neurogenesis (Kornelius et al., 2015). In an experimental study of human neuronal cells, linagliptin was observed to mitigate Aβ-induced cytotoxicity by activating the AMPK-Sirt1 signaling pathways. The proportion of apoptotic cells in the total cells decreased significantly from 35% to 20% (p < 0.01) (Kornelius et al., 2015). Vildagliptin use was found to prevent neuronal apoptosis in hippocampus, reduce the expression of apoptosis-related proteins and increased neurotrophic factors in rat models of T2DM (Zhang et al., 2018).
There are other mechanisms underlying the potential neuroprotective effects of DPP4i. For example, Sakr et al. studied the working memory and reference memory of T2DM rats with and without sitagliptin treatment by using the hole-board memory test and isolated rat’s hypothalamus to measure levels of acetylcholine and adiponectin receptor 1 (Adipo R1) mRNA expression (Sakr, 2013). The results showed that sitagliptin treatment significantly improved working memory from 50.67% to 63.5% and reference memory from 43.33% to 69.76% in T2DM rats (both p < 0.0001). In addition, sitagliptin significantly increased the content of acetylcholine and the expression of Adipo R1 in the hypothalamus, providing an insight into the mechanisms underlying the neuroprotective effects of sitagliptin (Sakr, 2013). Dong et al. reported that sitagliptin can improve learning and memory function by enhancing synaptic plasticity through stimulating BDNF-TrkB signal transduction pathway (Dong et al., 2019). Recent studies have shown that sitagliptin improved L-methionine-induced vascular dementia and cognitive deficits through its antioxidant, anti-inflammatory, anti-apoptosis and neurotrophic effects (Khodir et al., 2022). In addition, DPP4i was found to increase serum Sirtuin 1 level in T2DM patients with AD (Kornelius et al., 2015). Sirtuin 1 is a class III histone deacetylase known for its benefit in cognitive function by enhancing synaptic plasticity, improving memory through regulation of CREB and BDNF expression, and reducing Aβ accumulation, oxidative stress, and neuronal loss (Kumar et al., 2013). Substance P, a neuropeptide widely presented in the CNS and a substrate of DPP4, was found to be protective against AD by ameliorating Aβ-induced neuronal apoptosis in the brain secondary to the stimulation of non-amyloidogenic APP processing (Severini et al., 2016). The increased bioactivity of GLP-2 by DPP4i may improve spatial working memory in juvenile diabetic rats through MEK/ERK pathway (Sasaki-Hamada et al., 2021).
In addition to the experimental evidence presented both in vitro and in vivo, accumulative evidence from clinical studies have supported the neuroprotective effects of DPP4i in AD (Table 1). In a small-scale study of older patients with T2DM and mild cognitive impairment (n = 250), DPP4i-based glucose-lowering therapy was associated with a slower deterioration of cognitive function, mainly attentional and executive functions, compared to the sulfonylurea-based glucose-lowering therapy over a 2-year follow-up (Odds ratio: 0.88, 95% CI 0.45–0.99, p = 0.03) (Rizzo et al., 2014). This association appeared to be independent of its sustained hyperglycemia and glucose excursion. Another small-scale study of 253 older T2DM patients with and without AD yielded a similar conclusion that DPP4i use was associated with better cognitive performance compared with metformin (mean [SD] change in MMSE score in sitagliptin group versus metformin group over 6 months in patients without AD: 0.95 ± 2.17 versus −2.50 ± 3.03) (Isik et al., 2017). Nasir et al. found that, when used in combination with metformin, DPP4i was associated with better cognition, when compared with sulphonylureas, alpha glucosidase inhibitors, and thiazolidinediones (p < 0.05), with mean and SD in MMSE score of 29.11 ± 0.19, 24.64 ± 0.38, 25.33 ± 0.73, and 21.36 ± 1.77, respectively (Nair et al., 2019). Akimoto et al. conducting a regression analysis for the risk of AD on different antidiabetic drug therapies found that the risk of AD was significantly reduced by sitagliptin treatment compared with metformin monotherapy (adjusted odds ratio: 0.75; 95% confidence interval [CI]: 0.60–0.93; p = 0.011) (Akimoto et al., 2020).
There are more clinical studies being conducted in recent years. A Korean study using health insurance claim database found that DPP4i use versus sulfonylurea use was associated with a 34% reduced risk of incident all-cause dementia (95% CI: 0.56–0.78; p < 0.001) and 36% reduced risk of AD (95% CI: 0.52–0.79; p < 0.001) in older adults with T2DM (Kim et al., 2018). This finding in Asian populations was agreed by another study using data of European cohort, in which DPP4i use was found to be associated with a slower cognitive decline in patients with T2DM who were recently diagnosed with dementia, when compared with no treatment use, insulin, and sulfonylureas (Secnik et al., 2021). A recent meta-analysis of observational studies published in 2021 reported that DPP4i use was associated with a significantly reduced risk of all-cause dementia (Hazard ratio [HR]: 0.65, 95% CI, 0.55–0.76) and AD (HR: 0.48, 95% CI, 0.25–0.92), when compared with no glucose-lowering treatment (Zhou et al., 2020). Another newer meta-analysis yielded a similar conclusion that DPP4i use was associated with a significantly reduced risk of all-cause dementia (Risk Ratio [RR], 0.84; 95% CI, 0.74–0.94) and vascular dementia (RR, 0.59; 95% CI, 0.47–0.75) compared with no DPP4i use (Tang et al., 2023). However, when compared with other glucose-lowering medications, the association between DPP4i and AD (RR, 0.82; 95% CI, 0.63–1.08) is not statistically significant but there is still a trend towards positive despite a high between-study heterogeneity (Tang et al., 2023).
As of now, no randomized trial has been completed yet to investigate the effect of DPP4i on prevention and treatment of AD. Clinical studies in GLP-1RAs and SGLT2is for treating AD are lesser. GLP-1RAs work by directly activating GLP-1 receptors on pancreatic islet β-cells, δ-cells, and α-cells to increase insulin release and decrease glucagon secretion (Ussher and Drucker, 2023). SGLT2i lowers blood glucose and increase glycosuria levels by preventing glucose reabsorption in kidney through inhibiting SGLT2, the primary sodium-coupled glucose transporter in renal proximal tubules (Cassis et al., 2018). Like DPP4is, both SGLT2is and GLP1-RAs have been shown in many pre-clinical studies that can confer neuroprotective benefits beyond their effects on glucose-lowering. These include ameliorating the accumulation of Aβ plaques, oxidative stress and neuroinflammation, inhibiting acetylcholinesterase activity, and reducing cerebrovascular damage and neuronal cell death (Hayden et al., 2019; Hierro-Bujalance et al., 2020; Kaneto et al., 2021; Du et al., 2022; Chen et al., 2023; Mancinetti et al., 2023; Pelle et al., 2023; Piatkowska-Chmiel et al., 2023). Given that these novel anti-diabetic medications hold great promise for reducing AD in T2DM patients, well-performed randomized trials with sufficient sample size and follow-up are urgently needed to inform evidence-based clinical practice and new therapeutic approaches for dementia and AD.
DPP4i, a class of novel glucose-lowering medications that has been used in recent years, holds a great promise in preventing AD development and progression. Beyond its efficacy in glucose control and improvement of neuronal insulin resistance, DPP4i may provide neurocognitive benefits by directly reducing Aβ deposition and tau hyperphosphorylation. DPP4i can also increase the expression and bioavailability of neuroprotective DPP4 substrates such as GLP-1, GIP, SDF-1α, and NPY. Furthermore, a growing body of evidence substantiates the diverse biological functions of DPP4i in the brain, including anti-inflammatory, anti-oxidative, and anti-apoptotic effects, along with the promotion of neurogenesis. These properties collectively contribute to the amelioration of neurodegeneration and provide direct protection against Aβ-induced neurotoxicity. Randomized trials are needed to provide a definitive conclusion on the effect of DPP4i on AD prevention and treatment in T2DM patients and individuals at high risk of AD.
XJ: Conceptualization, Writing–original draft. JL: Conceptualization, Writing–original draft. XY: Writing–review and editing. HD: Writing–review and editing. AG: Writing–review and editing. ZZ: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Projects Fund of Baoying People’s Hospital (202004), the Open Project of Jiangsu Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Senile Diseases (202119), the General Program of Jiangsu Provincial Health Commission (M2022034), and the Medical Research project of Yangzhou Municipal Health Commission (2023-2-23).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akhter, F., Chen, D., Akhter, A., Yan, S. F., and Yan, S. S. (2021). Age-dependent accumulation of dicarbonyls and advanced glycation endproducts (AGEs) associates with mitochondrial stress. Free Radic. Biol. Med. 164, 429–438. doi:10.1016/j.freeradbiomed.2020.12.021
Akimoto, H., Negishi, A., Oshima, S., Wakiyama, H., Okita, M., Horii, N., et al. (2020). Antidiabetic drugs for the risk of alzheimer disease in patients with type 2 DM using FAERS. Am. J. Alzheimers Dis. Other Demen 35, 1533317519899546. doi:10.1177/1533317519899546
Alzheimers Disease International (2023). World alzheimer report 2018. Available at: https://www.alzint.org/u/WorldAlzheimerReport2018.pdf (Accessed on December 21, 2023).
Angeloni, C., Zambonin, L., and Hrelia, S. (2014). Role of methylglyoxal in Alzheimer's disease. Biomed. Res. Int. 2014, 238485. doi:10.1155/2014/238485
Angelopoulou, E., and Piperi, C. (2018). DPP-4 inhibitors: a promising therapeutic approach against Alzheimer's disease. Ann. Transl. Med. 6, 255. doi:10.21037/atm.2018.04.41
Antonyan, A., Schlenzig, D., Schilling, S., Naumann, M., Sharoyan, S., Mardanyan, S., et al. (2018). Concerted action of dipeptidyl peptidase IV and glutaminyl cyclase results in formation of pyroglutamate-modified amyloid peptides in vitro. Neurochem. Int. 113, 112–119. doi:10.1016/j.neuint.2017.12.001
Arulmozhiraja, S., Matsuo, N., Ishitsubo, E., Okazaki, S., Shimano, H., and Tokiwa, H. (2016). Comparative binding analysis of dipeptidyl peptidase IV (DPP-4) with antidiabetic drugs - an ab initio fragment molecular orbital study. PLoS One 11, e0166275. doi:10.1371/journal.pone.0166275
Asih, P. R., Prikas, E., Stefanoska, K., Tan, A. R. P., Ahel, H. I., and Ittner, A. (2020). Functions of p38 MAP kinases in the central nervous system. Front. Mol. Neurosci. 13, 570586. doi:10.3389/fnmol.2020.570586
Ates Bulut, E., Sahin Alak, Z. Y., Dokuzlar, O., Kocyigit, S. E., Soysal, P., Smith, L., et al. (2020). Cognitive and metabolic outcomes of vildagliptin addition to the therapy in patients with type 2 diabetes mellitus: 26 week follow-up study. Arch. Gerontol. Geriatr. 88, 104013. doi:10.1016/j.archger.2020.104013
Athauda, D., and Foltynie, T. (2016). The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson's disease: mechanisms of action. Drug Discov. Today 21, 802–818. doi:10.1016/j.drudis.2016.01.013
Baggio, L. L., Varin, E. M., Koehler, J. A., Cao, X., Lokhnygina, Y., Stevens, S. R., et al. (2020). Plasma levels of DPP4 activity and sDPP4 are dissociated from inflammation in mice and humans. Nat. Commun. 11, 3766. doi:10.1038/s41467-020-17556-z
Berends, E., van Oostenbrugge, R. J., Foulquier, S., and Schalkwijk, C. G. (2023). Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 20, 75. doi:10.1186/s12987-023-00477-6
Biessels, G. J., Verhagen, C., Janssen, J., van den Berg, E., Zinman, B., Rosenstock, J., et al. (2019). Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care 42, 1930–1938. doi:10.2337/dc19-0783
Bohlken, J., Jacob, L., and Kostev, K. (2018). Association between the use of antihyperglycemic drugs and dementia risk: a case-control study. J. Alzheimers Dis. 66, 725–732. doi:10.3233/JAD-180808
Borzi, A. M., Condorelli, G., Biondi, A., Basile, F., Vicari, E. S. D., Buscemi, C., et al. (2019). Effects of vildagliptin, a DPP-4 inhibitor, in elderly diabetic patients with mild cognitive impairment. Arch. Gerontol. Geriatr. 84, 103896. doi:10.1016/j.archger.2019.06.001
Brings, S., Fleming, T., Freichel, M., Muckenthaler, M. U., Herzig, S., and Nawroth, P. P. (2017). Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 18, 984. doi:10.3390/ijms18050984
Calsolaro, V., and Edison, P. (2016). Neuroinflammation in Alzheimer's disease: current evidence and future directions. Alzheimers Dement. 12, 719–732. doi:10.1016/j.jalz.2016.02.010
Carvalho, C., Katz, P. S., Dutta, S., Katakam, P. V., Moreira, P. I., and Busija, D. W. (2014). Increased susceptibility to amyloid-β toxicity in rat brain microvascular endothelial cells under hyperglycemic conditions. J. Alzheimers Dis. 38, 75–83. doi:10.3233/JAD-130464
Cassis, P., Locatelli, M., Cerullo, D., Corna, D., Buelli, S., Zanchi, C., et al. (2018). SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight 3, e98720. doi:10.1172/jci.insight.98720
Chalichem, N. S. S., Gonugunta, C., Krishnamurthy, P. T., and Duraiswamy, B. (2017). DPP4 inhibitors can Be a drug of choice for type 3 diabetes: a Mini review. Am. J. Alzheimers Dis. Other Demen 32, 444–451. doi:10.1177/1533317517722005
Chami, B., Steel, A. J., De La Monte, S. M., and Sutherland, G. T. (2016). The rise and fall of insulin signaling in Alzheimer's disease. Metab. Brain Dis. 31, 497–515. doi:10.1007/s11011-016-9806-1
Chao, A. C., Lee, T. C., Juo, S. H., and Yang, D. I. (2016). Hyperglycemia increases the production of amyloid beta-peptide leading to decreased endothelial tight junction. CNS Neurosci. Ther. 22, 291–297. doi:10.1111/cns.12503
Chen, B., Zheng, T., Qin, L., Hu, X., Zhang, X., Liu, Y., et al. (2017). Strong association between plasma dipeptidyl peptidase-4 activity and impaired cognitive function in elderly population with normal glucose tolerance. Front. Aging Neurosci. 9, 247. doi:10.3389/fnagi.2017.00247
Chen, K. C., Chung, C. H., Lu, C. H., Tzeng, N. S., Lee, C. H., Su, S. C., et al. (2020). Association between the use of dipeptidyl peptidase 4 inhibitors and the risk of dementia among patients with type 2 diabetes in taiwan. J. Clin. Med. 9, 660. doi:10.3390/jcm9030660
Chen, S., Liu, A. R., An, F. M., Yao, W. B., and Gao, X. D. (2012). Amelioration of neurodegenerative changes in cellular and rat models of diabetes-related Alzheimer's disease by exendin-4. Age (Dordr) 34, 1211–1224. doi:10.1007/s11357-011-9303-8
Chen, S., Zhou, M., Sun, J., Guo, A., Fernando, R. L., Chen, Y., et al. (2019). DPP-4 inhibitor improves learning and memory deficits and AD-like neurodegeneration by modulating the GLP-1 signaling. Neuropharmacology 157, 107668. doi:10.1016/j.neuropharm.2019.107668
Chen, S. D., Chuang, Y. C., Lin, T. K., and Yang, J. L. (2023). Alternative role of glucagon-like Peptide-1 receptor agonists in neurodegenerative diseases. Eur. J. Pharmacol. 938, 175439. doi:10.1016/j.ejphar.2022.175439
Chojdak-Lukasiewicz, J., Dziadkowiak, E., Zimny, A., and Paradowski, B. (2021). Cerebral small vessel disease: a review. Adv. Clin. Exp. Med. 30, 349–356. doi:10.17219/acem/131216
Daneman, R., and Prat, A. (2015). The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7, a020412. doi:10.1101/cshperspect.a020412
Deacon, C. F. (2020). Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 16, 642–653. doi:10.1038/s41574-020-0399-8
De Felice, F. G., Gonçalves, R. A., and Ferreira, S. T. (2022). Impaired insulin signalling and allostatic load in Alzheimer disease. Nat. Rev. Neurosci. 23, 215–230. doi:10.1038/s41583-022-00558-9
Dong, Q., Teng, S. W., Wang, Y., Qin, F., Li, Y., Ai, L. L., et al. (2019). Sitagliptin protects the cognition function of the Alzheimer's disease mice through activating glucagon-like peptide-1 and BDNF-TrkB signalings. Neurosci. Lett. 696, 184–190. doi:10.1016/j.neulet.2018.12.041
DPP4 (2024). The human protein atlas. Available at: https://www.proteinatlas.org/ENSG00000197635-DPP4/brain; Accessed on 25 January 2024.
Du, H., Meng, X., Yao, Y., and Xu, J. (2022). The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer's disease. Front. Endocrinol. (Lausanne) 13, 1033479. doi:10.3389/fendo.2022.1033479
Duarte-Neves, J., Pereira de Almeida, L., and Neuropeptide, C. Y. (2016). Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol. Dis. 95, 210–224. doi:10.1016/j.nbd.2016.07.022
El-Sahar, A. E., Safar, M. M., Zaki, H. F., Attia, A. S., and Ain-Shoka, A. A. (2015). Sitagliptin attenuates transient cerebral ischemia/reperfusion injury in diabetic rats: implication of the oxidative-inflammatory-apoptotic pathway. Life Sci. 126, 81–86. doi:10.1016/j.lfs.2015.01.030
Fawver, J. N., Schall, H. E., Petrofes Chapa, R. D., Zhu, X., and Murray, I. V. (2012). Amyloid-β metabolite sensing: biochemical linking of glycation modification and misfolding. J. Alzheimers Dis. 30, 63–73. doi:10.3233/JAD-2012-112114
Ferrari, C., and Sorbi, S. (2021). The complexity of Alzheimer's disease: an evolving puzzle. Physiol. Rev. 101, 1047–1081. doi:10.1152/physrev.00015.2020
Fiorentino, T. V., Prioletta, A., Zuo, P., and Folli, F. (2013). Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 19, 5695–5703. doi:10.2174/1381612811319320005
Gabbouj, S., Ryhanen, S., Marttinen, M., Wittrahm, R., Takalo, M., Kemppainen, S., et al. (2019). Altered insulin signaling in Alzheimer's disease brain - special emphasis on PI3K-akt pathway. Front. Neurosci. 13, 629. doi:10.3389/fnins.2019.00629
Gallwitz, B. (2019). Clinical use of DPP-4 inhibitors. Front. Endocrinol. (Lausanne) 10, 389. doi:10.3389/fendo.2019.00389
Hayden, M. R., Grant, D. G., Aroor, A. R., and DeMarco, V. G. (2019). Empagliflozin ameliorates type 2 diabetes-induced ultrastructural remodeling of the neurovascular unit and neuroglia in the female db/db mouse. Brain Sci. 9, 57. doi:10.3390/brainsci9030057
Hierro-Bujalance, C., Infante-Garcia, C., Del Marco, A., Herrera, M., Carranza-Naval, M. J., Suarez, J., et al. (2020). Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer's disease and type 2 diabetes. Alzheimers Res. Ther. 12, 40. doi:10.1186/s13195-020-00607-4
Holscher, C. (2014). Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J. Endocrinol. 221, T31–T41. doi:10.1530/JOE-13-0221
Huang, C. N., Wang, C. J., Lin, C. L., Li, H. H., Yen, A. T., and Peng, C. H. (2020). Abelmoschus esculentus subfractions attenuate Aβ and tau by regulating DPP-4 and insulin resistance signals. BMC Complement. Med. Ther. 20, 370. doi:10.1186/s12906-020-03163-4
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14, 1483–1496. doi:10.7150/ijbs.27173
Hung, Y. W., Wang, Y., and Lee, S. L. (2020). DPP-4 inhibitor reduces striatal microglial deramification after sensorimotor cortex injury induced by external force impact. Faseb J. 34, 6950–6964. doi:10.1096/fj.201902818R
Isik, A. T., Soysal, P., Yay, A., and Usarel, C. (2017). The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res. Clin. Pract. 123, 192–198. doi:10.1016/j.diabres.2016.12.010
Ji, C., Xue, G. F., Li, G., Li, D., and Hölscher, C. (2016). Neuroprotective effects of glucose-dependent insulinotropic polypeptide in Alzheimer's disease. Rev. Neurosci. 27, 61–70. doi:10.1515/revneuro-2015-0021
Kaneto, H., Obata, A., Kimura, T., Shimoda, M., Kinoshita, T., Matsuoka, T. A., et al. (2021). Unexpected pleiotropic effects of SGLT2 inhibitors: pearls and pitfalls of this novel antidiabetic class. Int. J. Mol. Sci. 22, 3062. doi:10.3390/ijms22063062
Khodir, S. A., Faried, M. A., Abd-Elhafiz, H. I., and Sweed, E. M. (2022). Sitagliptin attenuates the cognitive deficits in L-methionine-induced vascular dementia in rats. Biomed. Res. Int. 2022, 7222590. doi:10.1155/2022/7222590
Kim, Y. G., Jeon, J. Y., Kim, H. J., Kim, D. J., Lee, K. W., Moon, S. Y., et al. (2018). Risk of dementia in older patients with type 2 diabetes on dipeptidyl-peptidase IV inhibitors versus sulfonylureas: a real-world population-based cohort study. J. Clin. Med. 8, 28. doi:10.3390/jcm8010028
Kopp, K. O., Glotfelty, E. J., Li, Y., and Greig, N. H. (2022). Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: implications for neurodegenerative disease treatment. Pharmacol. Res. 186, 106550. doi:10.1016/j.phrs.2022.106550
Kornelius, E., Lin, C. L., Chang, H. H., Li, H. H., Huang, W. N., Yang, Y. S., et al. (2015). DPP-4 inhibitor linagliptin attenuates aβ-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci. Ther. 21, 549–557. doi:10.1111/cns.12404
Kosaraju, J., Holsinger, R. M. D., Guo, L., and Tam, K. Y. (2017). Linagliptin, a dipeptidyl peptidase-4 inhibitor, mitigates cognitive deficits and pathology in the 3xTg-AD mouse model of Alzheimer's disease. Mol. Neurobiol. 54, 6074–6084. doi:10.1007/s12035-016-0125-7
Kothari, V., Luo, Y., Tornabene, T., O'Neill, A. M., Greene, M. W., Geetha, T., et al. (2017). High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 499–508. doi:10.1016/j.bbadis.2016.10.006
Kroller-Schon, S., Knorr, M., Hausding, M., Oelze, M., Schuff, A., Schell, R., et al. (2012). Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 96, 140–149. doi:10.1093/cvr/cvs246
Kumar, R., Chaterjee, P., Sharma, P. K., Singh, A. K., Gupta, A., Gill, K., et al. (2013). Sirtuin1: a promising serum protein marker for early detection of Alzheimer's disease. PLoS One 8, e61560. doi:10.1371/journal.pone.0061560
Li, Y., Tian, Q., Li, Z., Dang, M., Lin, Y., and Hou, X. (2019). Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer's disease in rats. Drug Dev. Res. 80, 837–845. doi:10.1002/ddr.21567
Lin, Y. H., Hsu, C. C., Liu, J. S., Chang, K. C., and Huang, J. A. (2023). Use of dipeptidyl peptidase-4 inhibitors was associated with a lower risk of Parkinson's disease in diabetic patients. Sci. Rep. 13, 22489. doi:10.1038/s41598-023-49870-z
Luo, A., Ning, P., Lu, H., Huang, H., Shen, Q., Zhang, D., et al. (2022). Association between metformin and Alzheimer's disease: a systematic review and meta-analysis of clinical observational studies. J. Alzheimers Dis. 88, 1311–1323. doi:10.3233/JAD-220180
Mancinetti, F., Xenos, D., De Fano, M., Mazzieri, A., Porcellati, F., Boccardi, V., et al. (2023). Diabetes-Alzheimer's connection in older age: SGLT2 inhibitors as promising modulators of disease pathways. Ageing Res. Rev. 90, 102018. doi:10.1016/j.arr.2023.102018
Mathur, V., Alam, O., Siddiqui, N., Jha, M., Manaithiya, A., Bawa, S., et al. (2023). Insight into structure activity relationship of DPP-4 inhibitors for development of antidiabetic agents. Molecules 28, 5860. doi:10.3390/molecules28155860
Michailidis, M., Moraitou, D., Tata, D. A., Kalinderi, K., Papamitsou, T., and Papaliagkas, V. (2022). Alzheimer's disease as type 3 diabetes: common pathophysiological mechanisms between Alzheimer's disease and type 2 diabetes. Int. J. Mol. Sci. 23, 2687. doi:10.3390/ijms23052687
Morrison, D. K. (2012). MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 4, a011254. doi:10.1101/cshperspect.a011254
Mulvihill, E. E., and Drucker, D. J. (2014). Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 35, 992–1019. doi:10.1210/er.2014-1035
Nair, N. K., Vidhya, N., Mingate, M. D., and Vijayakumar, P. R. A. (2019). Comparison of different classes of oral antidiabetic drugs in combination with metformin on cognitive functions. Int. J. Pharm. Sci. Res. 10, 3455–3460. doi:10.13040/IJPSR.0975-8232.10(7).3455-60
Oumata, N., Lu, K., Teng, Y., Cavé, C., Peng, Y., Galons, H., et al. (2022). Molecular mechanisms in Alzheimer's disease and related potential treatments such as structural target convergence of antibodies and simple organic molecules. Eur. J. Med. Chem. 240, 114578. doi:10.1016/j.ejmech.2022.114578
Pelle, M. C., Zaffina, I., Giofre, F., Pujia, R., and Arturi, F. (2023). Potential role of glucagon-like peptide-1 receptor agonists in the treatment of cognitive decline and dementia in diabetes mellitus. Int. J. Mol. Sci. 24, 11301. doi:10.3390/ijms241411301
Piatkowska-Chmiel, I., Herbet, M., Gawronska-Grzywacz, M., Pawlowski, K., Ostrowska-Lesko, M., and Dudka, J. (2023). Molecular and neural roles of sodium-glucose cotransporter 2 inhibitors in alleviating neurocognitive impairment in diabetic mice. Psychopharmacol. Berl. 240, 983–1000. doi:10.1007/s00213-023-06341-7
Profaci, C. P., Munji, R. N., Pulido, R. S., and Daneman, R. (2020). The blood-brain barrier in health and disease: important unanswered questions. J. Exp. Med. 217, e20190062. doi:10.1084/jem.20190062
Raman, D., Milatovic, S. Z., Milatovic, D., Splittgerber, R., Fan, G. H., and Richmond, A. (2011). Chemokines, macrophage inflammatory protein-2 and stromal cell-derived factor-1α, suppress amyloid β-induced neurotoxicity. Toxicol. Appl. Pharmacol. 256, 300–313. doi:10.1016/j.taap.2011.06.006
Reich, N., and Holscher, C. (2022). The neuroprotective effects of glucagon-like peptide 1 in Alzheimer's and Parkinson's disease: an in-depth review. Front. Neurosci. 16, 970925. doi:10.3389/fnins.2022.970925
Rizzo, M. R., Barbieri, M., Boccardi, V., Angellotti, E., Marfella, R., and Paolisso, G. (2014). Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1122–1131. doi:10.1093/gerona/glu032
Rohrborn, D., Eckel, J., and Sell, H. (2014). Shedding of dipeptidyl peptidase 4 is mediated by metalloproteases and up-regulated by hypoxia in human adipocytes and smooth muscle cells. FEBS Lett. 588, 3870–3877. doi:10.1016/j.febslet.2014.08.029
Rohrborn, D., Wronkowitz, N., and Eckel, J. (2015). DPP4 in diabetes. Front. Immunol. 6, 386. doi:10.3389/fimmu.2015.00386
Sakr, H. F. (2013). Effect of sitagliptin on the working memory and reference memory in type 2 diabetic Sprague-Dawley rats: possible role of adiponectin receptors 1. J. Physiol. Pharmacol. 64, 613–623.
Salas, I. H., Weerasekera, A., Ahmed, T., Callaerts-Vegh, Z., Himmelreich, U., D'Hooge, R., et al. (2018). High fat diet treatment impairs hippocampal long-term potentiation without alterations of the core neuropathological features of Alzheimer disease. Neurobiol. Dis. 113, 82–96. doi:10.1016/j.nbd.2018.02.001
Sanfilippo, C., Castrogiovanni, P., Imbesi, R., Nunnari, G., and Di Rosa, M. (2020). Postsynaptic damage and microglial activation in AD patients could be linked CXCR4/CXCL12 expression levels. Brain Res. 1749, 147127. doi:10.1016/j.brainres.2020.147127
Sasaki-Hamada, S., Fujiwara, A., Satoh, S., Iwai, T., and Oka, J. I. (2021). GLP-2 restores impairments in spatial working memory and hippocampal LTD via the MEK/ERK pathway in juvenile-onset diabetes rats. Behav. Brain Res. 406, 113235. doi:10.1016/j.bbr.2021.113235
Secnik, J., Xu, H., Schwertner, E., Hammar, N., Alvarsson, M., Winblad, B., et al. (2021). The association of antidiabetic medications and Mini-Mental State Examination scores in patients with diabetes and dementia. Alzheimers Res. Ther. 13, 197. doi:10.1186/s13195-021-00934-0
Seino, Y., Fukushima, M., and Yabe, D. (2010). GIP and GLP-1, the two incretin hormones: similarities and differences. J. Diabetes Investig. 1, 8–23. doi:10.1111/j.2040-1124.2010.00022.x
Severini, C., Petrella, C., and Calissano, P. (2016). Substance P and Alzheimer's disease: emerging novel roles. Curr. Alzheimer Res. 13, 964–972. doi:10.2174/1567205013666160401114039
Shannon, R. P. (2013). DPP-4 inhibition and neuroprotection: do mechanisms matter? Diabetes 62, 1029–1031. doi:10.2337/db12-1794
Siddiqui, N., Ali, J., Parvez, S., Zameer, S., Najmi, A. K., and Akhtar, M. (2021). Linagliptin, a DPP-4 inhibitor, ameliorates Aβ (1-42) peptides induced neurodegeneration and brain insulin resistance (BIR) via insulin receptor substrate-1 (IRS-1) in rat model of Alzheimer's disease. Neuropharmacology 195, 108662. doi:10.1016/j.neuropharm.2021.108662
Sim, A. Y., Barua, S., Kim, J. Y., Lee, Y. H., and Lee, J. E. (2021). Role of DPP-4 and SGLT2 inhibitors connected to alzheimer disease in type 2 diabetes mellitus. Front. Neurosci. 15, 708547. doi:10.3389/fnins.2021.708547
Sim, A. Y., Choi, D. H., Kim, J. Y., Kim, E. R., Goh, A. R., Lee, Y. H., et al. (2023). SGLT2 and DPP4 inhibitors improve Alzheimer's disease-like pathology and cognitive function through distinct mechanisms in a T2D-AD mouse model. Biomed. Pharmacother. 168, 115755. doi:10.1016/j.biopha.2023.115755
Srikanth, V., Maczurek, A., Phan, T., Steele, M., Westcott, B., Juskiw, D., et al. (2011). Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol. Aging 32, 763–777. doi:10.1016/j.neurobiolaging.2009.04.016
Stanciu, G. D., Bild, V., Ababei, D. C., Rusu, R. N., Cobzaru, A., Paduraru, L., et al. (2020). Link between diabetes and Alzheimer's disease due to the shared amyloid aggregation and deposition involving both neurodegenerative changes and neurovascular damages. J. Clin. Med. 9, 1713. doi:10.3390/jcm9061713
Talbot, K., Wang, H. Y., Kazi, H., Han, L. Y., Bakshi, K. P., Stucky, A., et al. (2012). Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Invest. 122, 1316–1338. doi:10.1172/JCI59903
Tang, H., Shao, H., Shaaban, C. E., Yang, K., Brown, J., Anton, S., et al. (2023). Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J. Am. Geriatr. Soc. 71, 2096–2106. doi:10.1111/jgs.18306
Tian, Y., Jing, G., and Zhang, M. (2023). Insulin-degrading enzyme: roles and pathways in ameliorating cognitive impairment associated with Alzheimer's disease and diabetes. Ageing Res. Rev. 90, 101999. doi:10.1016/j.arr.2023.101999
Tseng, C. H. (2021). Vildagliptin has a neutral association with dementia risk in type 2 diabetes patients. Front. Endocrinol. (Lausanne) 12, 637392. doi:10.3389/fendo.2021.637392
Twarda-Clapa, A., Olczak, A., Bialkowska, A. M., and Koziolkiewicz, M. (2022). Advanced glycation end-products (AGEs): formation, chemistry, classification, receptors, and diseases related to AGEs. Cells, 11. doi:10.3390/cells11081312
Ussher, J. R., and Drucker, D. J. (2023). Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 20, 463–474. doi:10.1038/s41569-023-00849-3
Wang, Q., Xu, Y., Chen, J. C., Qin, Y. Y., Liu, M., Liu, Y., et al. (2012). Stromal cell-derived factor 1α decreases β-amyloid deposition in Alzheimer's disease mouse model. Brain Res. 1459, 15–26. doi:10.1016/j.brainres.2012.04.011
Wang, Y., Hu, H., Liu, X., and Guo, X. (2023). Hypoglycemic medicines in the treatment of Alzheimer's disease: pathophysiological links between AD and glucose metabolism. Front. Pharmacol. 14, 1138499. doi:10.3389/fphar.2023.1138499
Waqas, K., Muller, M., Koedam, M., El Kadi, Y., Zillikens, M. C., and van der Eerden, B. C. J. (2022). Methylglyoxal - an advanced glycation end products (AGEs) precursor - inhibits differentiation of human MSC-derived osteoblasts in vitro independently of receptor for AGEs (RAGE). Bone 164, 116526. doi:10.1016/j.bone.2022.116526
Wiciński, M., Wódkiewicz, E., Słupski, M., Walczak, M., Socha, M., Malinowski, B., et al. (2018). Neuroprotective activity of sitagliptin via reduction of neuroinflammation beyond the incretin effect: focus on Alzheimer's disease. Biomed. Res. Int. 2018, 6091014. doi:10.1155/2018/6091014
Wium-Andersen, I. K., Osler, M., Jorgensen, M. B., Rungby, J., and Wium-Andersen, M. K. (2019). Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case-control study. Eur. J. Endocrinol. 181, 499–507. doi:10.1530/EJE-19-0259
World Health Organization (2023). Ageing and health. Available at: https://www.who.int/zh/news-room/fact-sheets/detail/ageing-and-health (Accessed on December 21, 2023).
Xue, J., Wang, C., Pan, C., Xing, H., Xu, L., Chen, X., et al. (2020). Effect of DPP-4 inhibitor on elderly patients with T2DM combined with MCI. Exp. Ther. Med. 19, 1356–1362. doi:10.3892/etm.2019.8339
Yang, Y., Wu, Y., Zhang, S., and Song, W. (2013). High glucose promotes Aβ production by inhibiting APP degradation. PLoS One 8, e69824. doi:10.1371/journal.pone.0069824
Ye, H., Li, H., and Gao, Z. (2018). Copper binding induces nitration of NPY under nitrative stress: complicating the role of NPY in Alzheimer's disease. Chem. Res. Toxicol. 31, 904–913. doi:10.1021/acs.chemrestox.8b00128
Yin, R., Xu, Y., Wang, X., Yang, L., and Zhao, D. (2022). Role of dipeptidyl peptidase 4 inhibitors in antidiabetic treatment. Molecules 27, 3055. doi:10.3390/molecules27103055
Yuan, L., Zhang, J., Guo, J. H., Holscher, C., Yang, J. T., Wu, M. N., et al. (2021). DAla2-GIP-GLU-PAL protects against cognitive deficits and pathology in APP/PS1 mice by inhibiting neuroinflammation and upregulating cAMP/PKA/CREB signaling pathways. J. Alzheimers Dis. 80, 695–713. doi:10.3233/JAD-201262
Zhang, D. D., Shi, N., Fang, H., Ma, L., Wu, W. P., Zhang, Y. Z., et al. (2018). Vildagliptin, a DPP4 inhibitor, alleviates diabetes-associated cognitive deficits by decreasing the levels of apoptosis-related proteins in the rat hippocampus. Exp. Ther. Med. 15, 5100–5106. doi:10.3892/etm.2018.6016
Zhang, W., Xiao, D., Mao, Q., and Xia, H. (2023). Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 8, 267. doi:10.1038/s41392-023-01486-5
Zhou, B., Zissimopoulos, J., Nadeem, H., Crane, M. A., Goldman, D., and Romley, J. A. (2021). Association between exenatide use and incidence of Alzheimer's disease. Alzheimers Dement. (N Y) 7, e12139. doi:10.1002/trc2.12139
Zhou, J. B., Tang, X., Han, M., Yang, J., and Simo, R. (2020). Impact of antidiabetic agents on dementia risk: a Bayesian network meta-analysis. Metabolism 109, 154265. doi:10.1016/j.metabol.2020.154265
Keywords: Alzheimer’s disease, neurodegenerative diseases, type 2 diabetes mellitus, dipeptidyl peptidase 4, glucagon-like peptide-1
Citation: Jiang X, Li J, Yao X, Ding H, Gu A and Zhou Z (2024) Neuroprotective effects of dipeptidyl peptidase 4 inhibitor on Alzheimer’s disease: a narrative review. Front. Pharmacol. 15:1361651. doi: 10.3389/fphar.2024.1361651
Received: 26 December 2023; Accepted: 30 January 2024;
Published: 09 February 2024.
Edited by:
Ying Xu, The State University of New Jersey, United StatesReviewed by:
Maria Bogdan, University of Medicine and Pharmacy of Craiova, RomaniaCopyright © 2024 Jiang, Li, Yao, Ding, Gu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Ding, ZGluaGFvNzcxMDE3QDE2My5jb20=; Aihong Gu, eXpieWdhaEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.