- 1Affiliated Hospital of Hubei University of Chinese Medicine, Wuhan, China
- 2Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3School of Clinical Traditional Chinese Medicine, Hubei University of Chinese Medicine, Wuhan, China
- 4Hubei Key Laboratory of Theory and Application Research of Liver and Kidney in Traditional Chinese Medicine, Affiliated Hospital of Hubei University of Chinese Medicine, Hubei Provincial Hospital of Traditional Chinese Medicine, Wuhan, China
- 5Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 6Department of Nephrology, Renmin Hospital of Wuhan University, Wuhan University, Wuhan, China
- 7Traditional Chinese Medicine Research Institute, Guangdong Pharmaceutical University, Guangzhou, China
- 8Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Aims: This study aimed to synthesize the evidence of the comparative effectiveness and safety of Ophiocordyceps sinensis (OS) preparations combined with renin–angiotensin system inhibitors (RASi) for diabetic kidney disease (DKD).
Methods: Eight databases were searched from their inception to May 2023. Systematic reviews (SRs) of OS preparations combined with RASi for DKD were identified. Randomized controlled trials (RCTs) from the included SRs and additional searching were performed for data pooling. Cochrane risk-of-bias 2 (RoB 2) tool and AMSTAR 2 were used to evaluate the methodological quality of RCTs and SRs, respectively. A Bayesian network meta-analysis was performed to compare the add-on effect and safety of OS preparations for DKD. The certainty of evidence was graded using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.
Results: Fourteen SRs were included, whose methodological quality was assessed as high (1/14) or critically low (13/14). After combining additional searching, 157 RCTs were included, involving 13,143 participants. The quality of the RCTs showed some concerns (155/157) or high risk (2/157). Jinshuibao capsules and tablets, Bailing capsules and tablets, and Zhiling capsules were evaluated. Compared to RASi, adding either of the OS capsular preparations resulted in a decreased 24-h urinary total protein levels. OS preparations ranked differently in each outcome. Jinshuibao capsules plus RASi were beneficial in reducing urinary protein, serum creatinine, serum urea nitrogen, and blood glucose levels, with moderate-certainty evidence. No serious adverse events were observed after adding OS to RASi.
Conclusion: Combining OS capsular preparations with RASi appeared to be associated with decreased urinary total protein levels in DKD patients. Further high-quality studies are needed to confirm.
Systematic Review Registration: INPASY202350066.
1 Introduction
The prevalence of diabetes mellitus (DM) has increased rapidly over the past decades worldwide (Lovic et al., 2020). As one of the common and serious microvascular complications of diabetes, the prevalence of diabetic kidney disease (DKD) has also increased significantly (Tuttle et al., 2022). Although the spectrum for the etiology of chronic kidney disease (CKD) in China differs from that in Western countries (Wang and He, 2021), such a trend has also been found in recent studies. The results of the Sixth China Chronic Disease and Risk Factor Surveillance showed that the prevalence of CKD associated with DM increased proportionally, despite the observed decreasing trend in the overall prevalence of CKD in China (Zhang et al., 2016; Wang et al., 2023). DKD patients have a poor prognosis compared to non-DKD patients. DKD substantially increases the risk of kidney failure and cardiovascular events (Tuttle et al., 2022). Therefore, DKD has become a global public health problem with a significant disease burden.
At present, the basic prevention and treatment therapies for DKD are lifestyle change and risk factor control, including exercise, nutrition, smoking cessation, glycemic control, blood pressure control, and lipid management (KDIGO, 2022). In addition, renin–angiotensin system inhibitors (RASi) have a renal protective effect independent of decreasing blood pressure (Leoncini et al., 2020). Previously, only RASi with multidisciplinary therapy were effective for DKD. Recent studies on some newly developed hypoglycemic drugs suggested that they may have a potential renal protective effect, such as sodium–glucose cotransporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP−1) receptor agonists (Kristensen et al., 2019; Zelniker et al., 2019). Despite the remarkable progress, there is still a substantial residual risk of disease progression with existing therapies (Tuttle et al., 2022). In addition, side effects and the requirement for the estimated glomerular filtration rate (eGFR) in the indications of drug use still limit the clinical applications of these drugs. Overall, DKD is undoubtedly a medical challenge all over the world.
Ophiocordyceps sinensis (OS), popularly known as caterpillar mushroom, is a non-toxic, medicinal fungus growing in the Himalayan hills in Nepal, India, and Tibet, China (Singh et al., 2018). OS contains cordycepin, carbohydrate d-mannitol, vitamin B12, six essential amino acids, and unsaturated fatty acids (Singh et al., 2018). A number of scientific research studies have indicated that OS has anti-inflammatory, anticancer, antidiabetic, analgesic, antioxidant, anti-allergic, and anti-obesity effects (Olatunji et al., 2018). According to the traditional Chinese medicine (TCM) theory, OS has the function of securing the essence and strengthening Qi, reinforcing the lung and kidney. In particular, OS has a good therapeutic effect on lung and renal diseases (Zhang et al., 2015; Wang et al., 2021). Because wild OS is rare and expensive, it can no longer meet the market demand. As the fermentation technology of medicinal strains isolated from OS is becoming increasingly mature, preparations composed of artificially fermented OS powder have become more and more common in clinical applications. At present, the main OS preparations of Chinese patent medicine products include Jinshuibao capsule/tablet (species name: Paecilomyces hepiali chen), Bailing capsule/tablet/granule (Hirsutella sinensis Liu, Guo, Yu-et Zeng), Zhiling capsule (Mortierella sp.), Xinganbao capsule (Gliocladium roseum (link) Thom), Yong Chong Cao capsule (Cordyceps militaris L. Link), and Ningxinbao capsule (Cephalosporium sinensis Chen. sp. nov) (Zheng et al., 2011; Wang et al., 2016). Even if derived from the same species name, the preparations of different dosage forms are not exactly the same. A study comparing the amino acid fingerprint and common peak map of Jinshuibao capsules and tablets clarified the differences between the two, which may be used as the basis for distinguishing different preparations (Zhao, 2019).
Accumulating research on OS preparations combined with RASi for DKD treatment exists, but there is a lack of summary of the overall evidence and comparison between different OS preparations. The objective of this study is to evaluate the methodological quality of existing systematic reviews (SRs) and compare the effectiveness and safety of different OS preparations (including different dosage forms) when used in combination with RASi in DKD patients. The umbrella review and network meta-analysis (NMA) approaches were used to provide comprehensive evidence and reference for clinical rational drug use.
2 Methods
2.1 Protocol and registration
This study followed the methodological process of the Joanna Briggs Institute for an “umbrella review” (Aromataris et al., 2015; Aromataris et al., 2020). The protocol of this review was specified in advance and registered on the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY.COM) (Registration number: 202350066). The study was reported according to the Preferred Reporting Items for Overviews of Reviews (PRIOR) statement (Gates et al., 2022).
2.2 Eligibility criteria
2.2.1 Types of participants
All participants met the diagnostic criteria for DKD (Kopel et al., 2019; Expert Group of Chinese Society of Nephrology, 2021). None of the participants entered renal replacement therapies, including peritoneal dialysis, hemodialysis, and kidney transplant. There were no restrictions on age, sex, race, stage of disease, or source of cases.
2.2.2 Types of interventions
In addition to basic treatment (BT), any OS preparation added was included in the experimental group. BT referred to exercise, nutrition, smoking cessation, glycemic control, blood pressure control, lipid management, and RASi use.
2.2.3 Types of comparisons
The control group involved “BT alone” or “BT plus placebo” or “BT plus different OS preparation.” There is no restriction on the form or dosage of OS preparation.
2.2.4 Types of outcomes
We specified the relative importance of the outcomes according to the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach (Guyatt et al., 2011) by referring to the clinical practice guideline of KDIGO (2022) and consulting clinical doctors. SRs that reported at least one of the following outcomes were included in this study. Critical outcomes included (Lovic et al., 2020) end-point events including all-cause mortality, doubling of the serum creatinine (Scr) level, decrease in the eGFR by more than 50% from the baseline level, or entry into renal replacement therapies (Tuttle et al., 2022); albuminuria progression including the onset of albuminuria, moderately increased (formerly known as microalbuminuria) to severely increased albuminuria (formerly known as macroalbuminuria) (Wang and He, 2021); and major adverse cardiovascular and cerebrovascular events (MACCEs) including cardiovascular mortality, acute myocardial infarction, hospitalization for congestive heart failure, intraparenchymal hemorrhage, subarachnoid hemorrhage, or cerebral infarction. Important but not critical outcomes involved urine protein testing, renal function testing, and adverse events (AEs). The specific outcomes on urine protein testing included 24-h urinary total protein (24-h UTP), urinary albumin excretion rate (UAER), and urinary albumin/creatinine ratio (UACR). The specific outcomes on renal function included Scr, serum urea nitrogen (SUN), and eGFR. Other outcomes were fasting plasma glucose (FPG), glycated hemoglobin A1c (HbA1c), and patient-reported symptoms.
2.2.5 Types of studies
All the SRs and meta-analysis of randomized controlled trials (RCTs) were included in this umbrella review. All RCTs included in SRs and RCTs obtained after additional retrieval were used for network meta-analysis after overlapping was excluded.
2.3 Search strategy
We searched the following Chinese and English databases from their inception to May 2023. Chinese databases included the China National Knowledge Infrastructure (CNKI), Wanfang, Chinese Science and Technology Journal Database (VIP), and SINOMED database. English databases included PubMed, Embase, the Cochrane Library, and the Web of Science. Two international platforms of registered SR and meta-analysis protocols including INPLASY and PROSPERO were also searched. No language or publication type is imposed. Additional retrieval for RCTs was conducted from the date of the most recently searched data on published SRs to 15 May 2023. The retrieval strategies for SRs and RCTs are specified in Supplementary Table S1.
2.4 Study selection and data extraction
Two authors (X Xue and KY Li) independently screened the eligible SRs and then screened the RCTs from the SRs for inclusion in the network meta-analysis. Disagreements of selection and data extraction were resolved through discussions with the corresponding author.
Two authors (KY Li and XL Ye) extracted the data, and two authors (X Xue and JX Li) checked those. The following information was extracted: first author, publication year, design and the number of included studies, participants, intervention/comparison measures, outcomes, and methodological quality evaluation information.
For the network meta-analysis, the following items were collected from the RCTs: study identification, characteristics of participants, trade names of OS preparations, daily dosage, treatment duration, outcomes, and adverse events.
2.5 Methodological quality assessment
The AMSTAR 2 tool (Shea et al., 2017) was applied to appraise the methodological quality of the included SRs by two authors (X Xue and XY Jin) independently. Two authors (X Xue and XY Jin) assessed the quality of RCTs using the risk-of-bias 2 (RoB 2) tool from the Cochrane Library (Sterne et al., 2019). Any disagreements were resolved by discussion with the corresponding author.
2.6 Data synthesis and statistical methods
R software version 4.2.1 was used for analysis. The pooled results were presented as the mean difference (MD) or risk ratio (RR) with 95% confidence intervals (CIs). A p-value of <0.05 was considered statistically significant. The Bayesian network meta-analysis was performed using Markov chain Monte Carlo (MCMC) simulation (Phillippo et al., 2020). A potential scale reduction factor (PSRF) value, the median, and 97.5% of the PSRF value close to or equal to 1 indicate good convergence of the MCMC algorithm (1 < PSRF ≤ 1.05) (Tunaru, 2002; Valkenhoef et al., 2012). If the PSRF value is not in this range, the iteration continues manually until the PSRF value reaches the range standard. An evidence network is performed according to each outcome of our interest. The data are analyzed by adjusting the indirect comparison approach if closed loops are unavailable, while the mixed treatment comparison approach is performed when one or more closed loops are available. For the local inconsistency estimate based on the existence of one or more closed loops, the consistency of direct and indirect comparisons is assessed by the node-split model. The consistency model is adopted if the p-value is > 0.05, whereas the inconsistent model is used for analysis (Dias et al., 2010; Van Valkenhoef et al., 2016). The results of the NMA showed that the ranking probability plot and the surface under the cumulative ranking (SUCRA) plot are generated and sorted by dominance. The value of SUCRA is between 0 and 1. When the SUCRA value is 1, it indicates that the intervention is absolutely effective. The effect of interventions can be ranked based on the value of SUCRA.
If closed loops are available, both the consistency model and unrelated mean effects model are adopted as sensitivity analysis to explore the robustness of the results. Regarding the choice of random-effects model or fixed-effects model, we refer to values of DIC and Dbar. If the difference in the DIC value between models is greater than 5, the model with the smaller DIC is selected. When the difference is between 3 and 5, the model with the lower Dbar value is adopted; if the difference is less than 3, the fixed-effects model is used. If more than 10 trials are included, counter-enhanced funnel plots combined with the trim and fill method are applied to evaluate publication bias.
2.7 Assessment of the certainty of evidence
The certainty of the evidence was assessed by RCTs after excluding overlapping. Two authors (X Xue and XY Jin) independently assessed direct evidence, indirect evidence, and combined evidence for outcomes using the GRADE approach (Atkins et al., 2004) if closed loops were available in the network. When closed loops were unavailable, the direct and indirect evidence for significant outcomes was assessed.
3 Results
3.1 Characteristics of included reviews
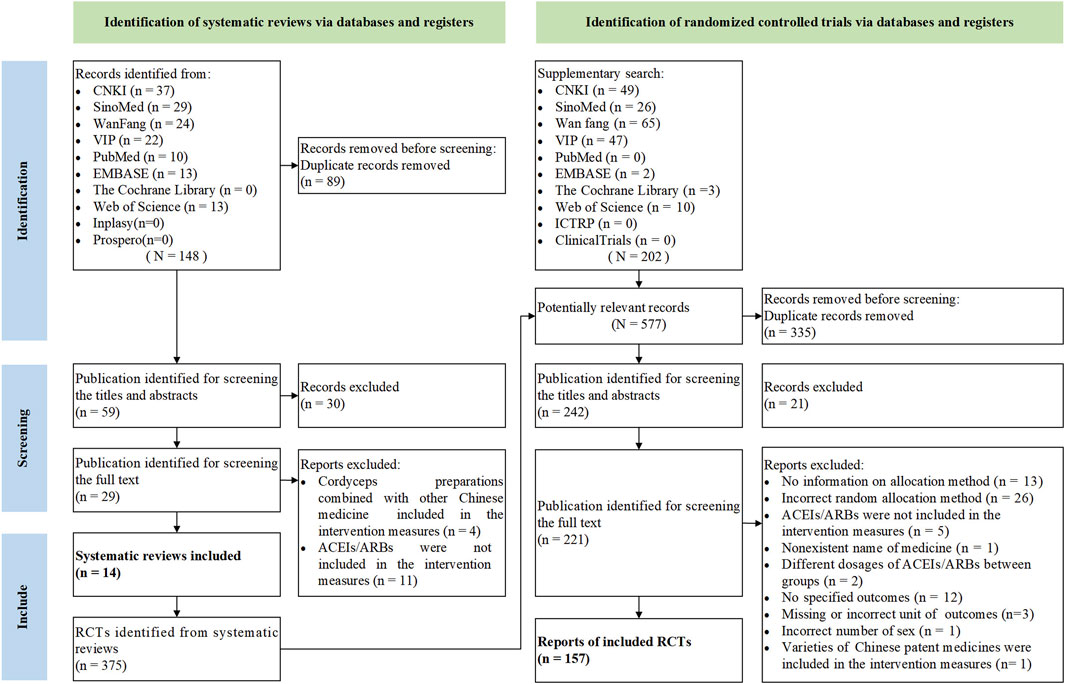
A total of 148 citations were retrieved from the initial searches, and 30 citations were screened out by reading the titles and abstracts. After scanning the full texts, 15 SRs were excluded (Zhang et al., 2012; He and Ge, 2012; Huang et al., 2012; Mao et al., 2012; Tang et al., 2013; Wang and Duan, 2013; Ji et al., 2014; Chen et al., 2017; Jing et al., 2017; Wen et al., 2018; Liu et al., 2020; Sheng et al., 2020; Gao et al., 2021; Su et al., 2021; Yu et al., 2022), and 14 SRs were included finally (Tang et al., 2013; Duan and Li, 2015; Luo et al., 2015; Cai, 2016; Li, 2016; Lu et al., 2018; Zhang, 2018; Huang et al., 2019; Li and Xu, 2019; Lian et al., 2020; Gong et al., 2021; Zhou et al., 2021; Li et al., 2022; Yan et al., 2023). The screening and selection process of the literature is shown in Figure 1. The list of exclusions during the full-text screening process is presented in Supplementary Table S2. A total of five varieties of OS preparations were involved in the 14 SRs, namely, Bailing capsule (BLC), Bailing tablet (BLT), Jinshuibao capsule (JSBC), Jinshuibao tablet (JSBT), and Zhiling capsule (ZLC).
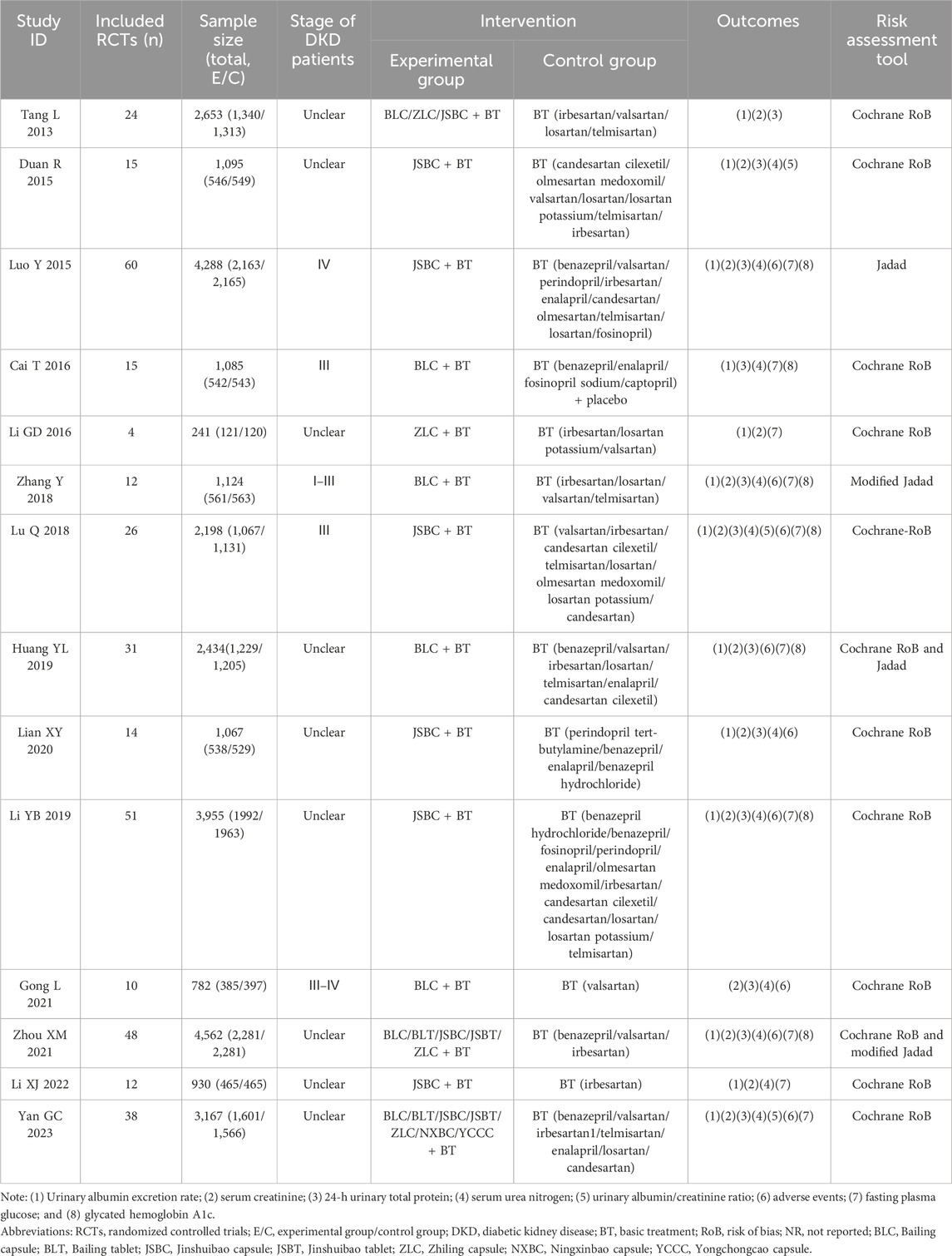
Fourteen SRs were published between 2013 and 2023, and all have been published in full text. Ten SRs were published in Chinese and four in English. Fourteen SRs were performed in China. The number of RCTs in the included SRs ranged from 4 to 60, and the total sample size ranged from 241 to 4,562 participants. Types of intervention measures were BT combined with OS preparations. Types of control measures included BT alone. The total course of treatment in 14 SRs ranged from 2 to 48 weeks. Furthermore, we did not find the critical outcomes in all the included SRs. Among the important outcomes, we found 6 relevant measures, namely, UAER (13/14, 92.86%), 24-h UTP (12/14, 85.71%), UACR (3/14, 21.43%), Scr (13/14, 92.86%), SUN (11/14, 78.57%), and adverse events (8/14, 57.14%). Regarding other outcomes, FPG was reported in 10 SRs (10/14, 71.43%), and HbA1c was reported in 7 SRs (7/14, 50.00%). No review mentioned patient-reported symptoms. The general characteristics of each review are presented in Table 1.
3.2 Methodological quality evaluation of included reviews
We appraised the methodological quality of the included SRs using AMSTAR 2. The results indicated that 1 SR was “high,” and 13 SRs were “critically low” in methodological quality, as shown in Supplementary Table S3.
3.3 Characteristics of included RCTs
A total of 375 primary RCTs were included in the SRs. We conducted an additional search of 264 RCTs. After 397 overlapping RCTs were excluded and further screened according to the eligibility criteria, a total of 157 eligible RCTs were included in the final analysis (Jia, 1999; Cao et al., 2007; Chen, 2007; Chen, 2009; Cui et al., 2009; Lei et al., 2009; Li and Liu, 2009; Chen et al., 2010; Guan et al., 2010; Hong, 2010; Huang et al., 2010; Chen, 2011; Cao, 2012; He and Lu, 2012; Hu and Wei, 2012; Gao and Wei, 2013; Ding, 2014; Cao, 2015; Diao, 2015; Chen et al., 2016; Dai, 2016; Hu, 2016; Huang and Zhou, 2016; Jin, 2016; Chen, 2017; Feng, 2017; He, 2017; Hou et al., 2017; Lei et al., 2017; Gao, 2018; Hu et al., 2018; Dai, 2019; Hu, 2019; Cheng, 2021; Du, 2021; Guan et al., 2021; Han, 2021; Jiang, 2021; Ding et al., 2022; Dong, 2022; Huang et al., 2022; Li and Lv, 2011; Li et al., 2019; Li, 2012; Li, 2010; Li, 2012; Li, 2017; Li, 2019; Li, 2023; Lin, 2009; Lin, 2016; Liu and Jian, 2020; Liu and Li, 2011a; Liu and Li, 2011b; Liu and Zhang, 2017; Liu et al., 2016a; Liu et al., 2016b; Liu, 2010; Liu, 2011; Liu, 2011; Liu, 2017; Liu, 2019; Liu, 2019; Long, 2014; Lou et al., 2010; Lu and Shi, 2020; Lu, 2010; Luo et al., 2011; Luo et al., 2018; Lv et al., 2006; Lv, 2012; Lv, 2012; Lv, 2014; Lv, 2022; Ma et al., 2011; Ma, 2017; Ma, 2018; Pan and Shang, 2016; Qiu et al., 2019; Ren and Hu, 2020; Ren et al., 2020; Shan, 2012; Shen and Chen, 2011; Shen et al., 2018; Shen, 2012; Shen et al., 2021; Shi, 2010; Song and Ning, 2017; Song et al., 2009; Sun et al., 2012; Tang, 2011; Tang, 2016; Tao and Zhou, 2009; Tian, 2019; Wang and He, 2015; Wang and Qi, 2018; Wang and Yuan, 2008; Wang and Zheng, 2010; Wang et al., 2009; Wang et al., 2020; Wang et al., 2020; Wang et al., 2020; Wang, 2007; Wang, 2011; Wang, 2011; Wang, 2012; Wei, 2010; Wu and Pan, 2016; Wu et al., 2014; Wu et al., 2019; Wu, 2008; Wu, 2012; Xiao, 2021; Xie and Mo, 2021; Xie, 2013; Yan and Wang, 2005; Xue, 2009; Yi et al., 2009; Zhang and Lu, 2009; Zeng and Zhang, 2010; Yang and Yang, 2011; Zhang, 2011; Zheng, 2011; Zhang et al., 2012; Ye et al., 2012; Yu, 2012; Zhou, 2012; Yang, 2013; Yu et al., 2013; Zhou et al., 2013; Zeng, 2014; Zhang et al., 2014; Zhang and Zuo, 2014; Zong and Ding, 2014; Xu, 2015; Zeng, 2015; Zhou et al., 2015; Zhu and Qiu, 2015; Yang et al., 2016; Zhan et al., 2016; Zhang, 2016; Yang et al., 2017; Yuan, 2017; Zhang et al., 2017; Xu, 2018; Yang, 2019; Zhang and Zhang, 2019; Xu, 2021; Yu et al., 2021; Zhang, 2021; Yu, 2022), involving 13,143 participants. Although we tried our best to contact the corresponding authors by email or phone in order to acquire the questionable information about the characteristics of RCTs, only three authors responded. Details are given in Supplementary Table S4. The recruitment timeline ranged from 1999 to 2023. All 157 trials were designed as two-armed RCTs (157/157, 100%). In addition, 151 trials were performed at a single center (151/157, 96.18%), while 1 trial was performed at a multi-center (1/157, 0.64%). The sample size of the included trials ranged from 36 to 252 participants, with an average of 84. Sex information was reported in 152 trials (152/157, 96.82%), in which 6,971 people were men and 5,682 were women. The participants’ age ranged from 32 to 85 years, while 9 trials did not report any age information (9/157, 5.73%). The comparisons of included trials were all BT plus OS preparations versus OS preparations, involving BLC, BLT, JSBC, JSBT, and ZLC. The intervention duration was arranged from 2 weeks to 12 months, with 12 weeks as common. None of the RCTs reported the critical outcomes. All 157 trials reported important but not critical outcomes (157/157, 100%), among which 127 trials involved urine protein testing (127/157, 80.89%), 118 trials involved renal function testing (118/157, 75.16%), and only 48 trials involved adverse events (48/157, 30.57%). Only one trial reported a follow-up (1/157, 0.64%). Funding sources were reported in 15 trials (15/157, 9.55%), with no funding from pharmaceutical companies. The main characteristics of the included primary trials are given in Supplementary Table S5.
3.4 Methodological quality evaluation of included randomized controlled trials
The risk of bias in the RCTs was assessed by the randomization of processes, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result using the RoB 2 tool. The results showed that all trials were assessed as having “some concerns” in the randomization process domain, which resulted in the overall methodological quality of the RCTs being “some concern” (155/157, 98.73%). As for missing outcome data and measurement of the outcome domains, all trials were assessed as “low risk.” However, two trials showed “high risk” in deviation from the intended intervention domain, and the overall methodological quality was assessed as “high risk” (2/157, 1.27%). The risk of bias in each included trial is given in Supplementary Figure S1.
3.5 Effects of interventions
The Bayesian network model was constructed using gemtc, BUGSnet, and multinma packages in R software. The trace and density plots and Brooks–Gelman–Rubin diagnostic plots show that the model convergence degree of each outcome was satisfactory (Supplementary Figure S2). Therefore, the Bayesian model established in this study can effectively predict the results.
3.5.1 Important but not critical outcome: 24-h urinary total protein
Sixty-five RCTs compared the effectiveness of reducing 24-h UTP between the experimental and control groups, including 5,733 participants. Network evidence of 24-h UTP results showed that a total of four interventions were included (Figure 2A): BT and BT plus three OS preparations (BLC, JSBC, and ZLC). As the league table shows, the effectiveness of BT combined with any of the above three OS preparations on reducing urinary total protein was better than that of BT alone (Figure 3A). We drew the ranking probability plot and the SUCRA plot, from which we obtained the order of probability ranking results. The sequence from good to bad was as follows: BT + ZLC > BT + JSBC > BT + BLC > BT (Figure 4A).
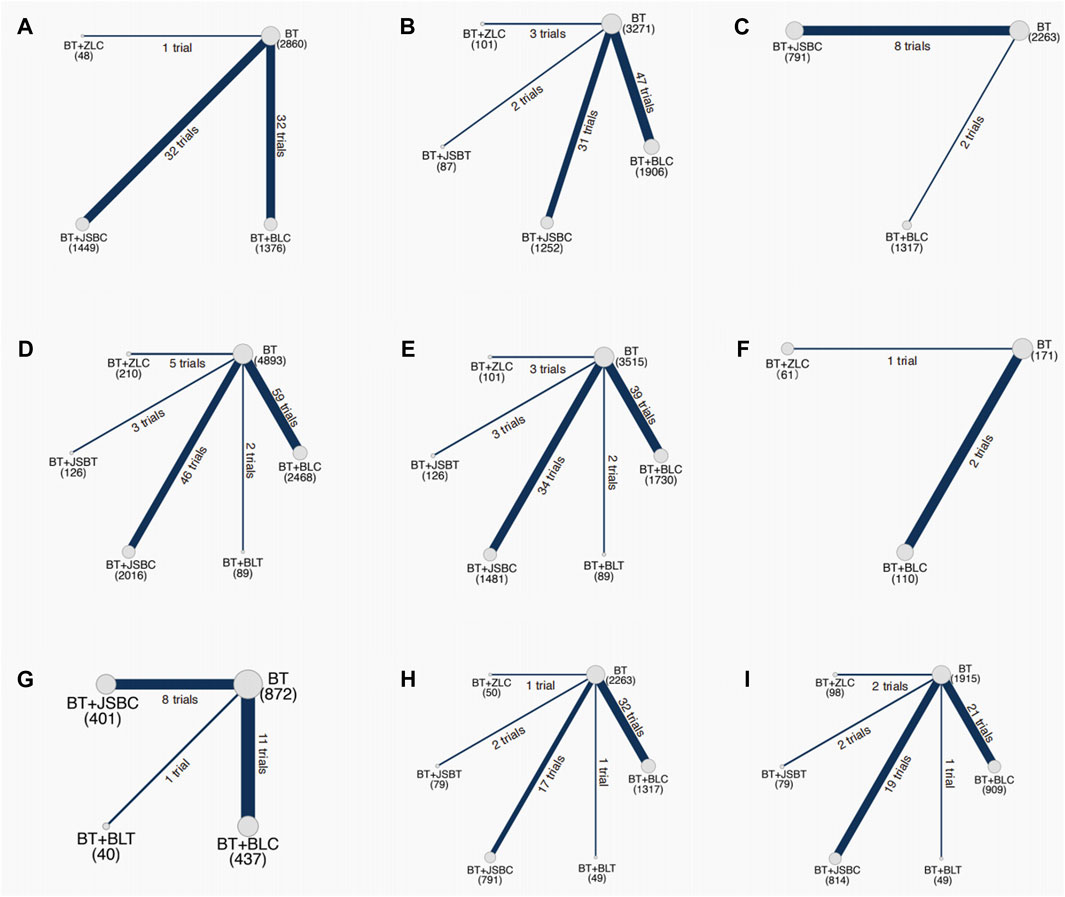
Figure 2. Network evidence on outcomes. Note: (A) 24-h urinary total protein; (B) urinary albumin excretion rate; (C) urinary albumin/creatinine ratio; (D) serum creatinine; (E) serum urea nitrogen; (F) estimated glomerular filtration rate; (G) adverse events; (H) fasting plasma glucose; and (I) glycated hemoglobin A1c. BT, basic treatment; BLC, Bailing capsule; BLT, Bailing tablet; JSBC, Jinshuibao capsule; JSBT, Jinshuibao tablet; and ZLC, Zhiling capsule.
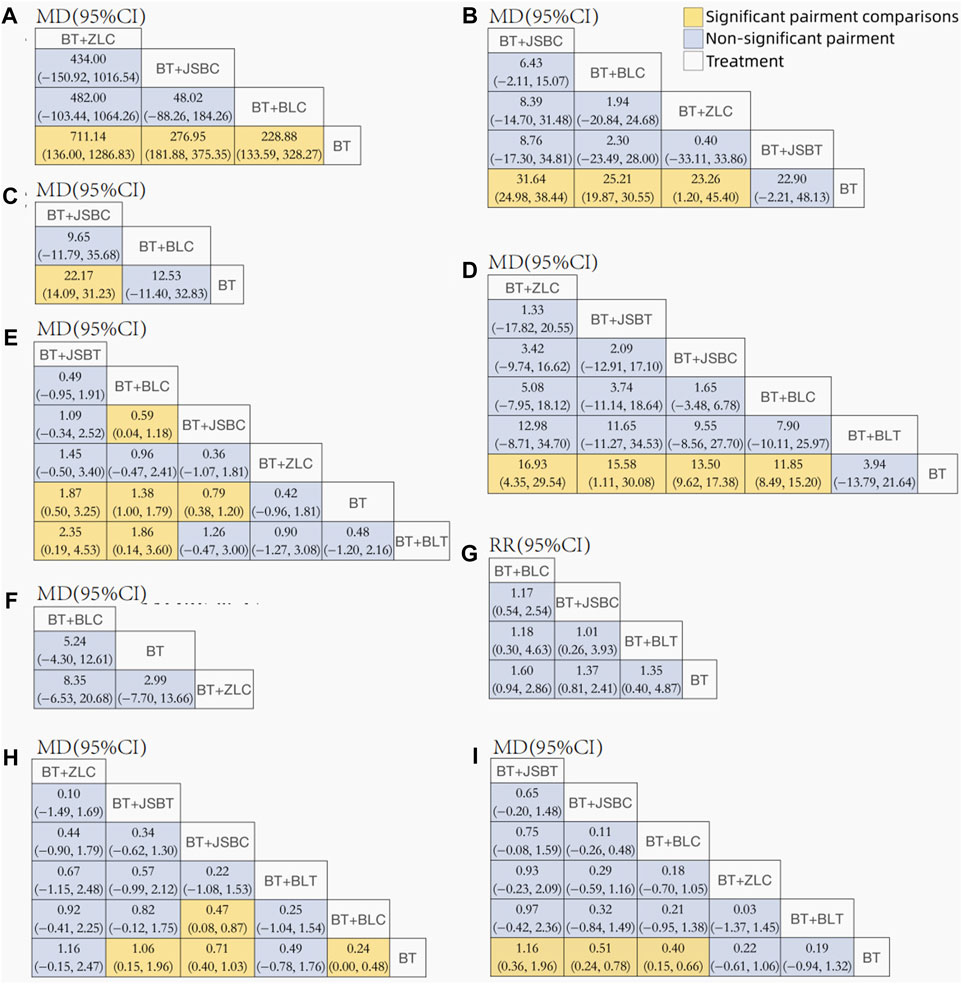
Figure 3. League tables for comparison between each intervention. Note: (A) 24-h urinary total protein; (B) urinary albumin excretion rate; (C) urinary albumin/creatinine ratio; (D) serum creatinine; (E) serum urea nitrogen; (F) estimated glomerular filtration rate; (G) adverse events; (H) fasting plasma glucose; and (I) glycated hemoglobin A1c. BT, basic treatment; BLC, Bailing capsule; BLT, Bailing tablet; JSBC, Jinshuibao capsule; JSBT, Jinshuibao tablet; and ZLC, Zhiling capsule.
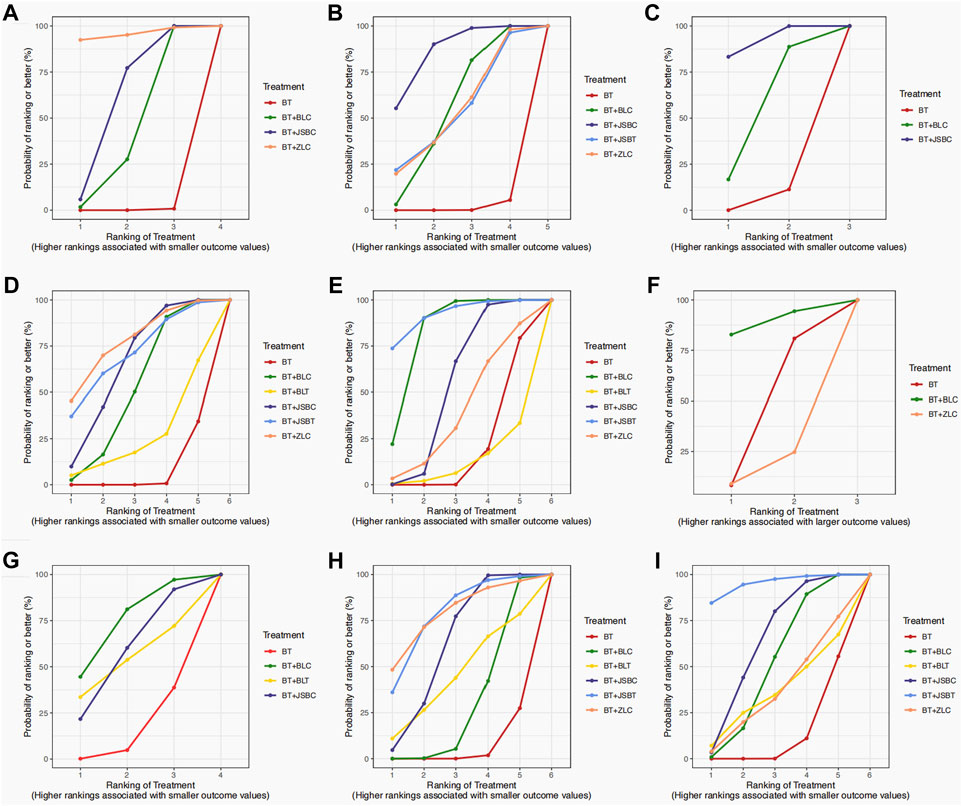
Figure 4. Surface under the cumulative ranking (SUCRA) plots on outcomes. Note: (A) 24-h urinary total protein; (B) urinary albumin excretion rate; (C) urinary albumin/creatinine ratio; (D) serum creatinine; (E) serum urea nitrogen; (F) estimated glomerular filtration rate; (G) adverse events; (H) fasting plasma glucose; and (I) glycated hemoglobin A1c. BT, basic treatment; BLC, Bailing capsule; BLT, Bailing tablet; JSBC, Jinshuibao capsule; JSBT, Jinshuibao tablet; and ZLC, Zhiling capsule.
3.5.2 Important but not critical outcome: urinary albumin excretion rate
The UAER was reported in 83 trials including 5 interventions (BT and BT plus 4 OS preparations) and 6,617 patients. The four OS preparations were BLC, JSBC, JSBT, and ZLC (Figure 2B). The league table shows that the effectiveness of BT combined with any of the three OS preparations (BLC, JSBC, and ZLC) was superior to BT alone on reducing the UAER (Figure 3B). However, the combination of JSBT on reducing the UAER in DKD patients was not more significant than that of BT alone, and the difference was not statistically significant (MD 22.90, 95% CI −2.21 to 48.13, two trials). The SUCRA plot is sequenced as BT + JSBC, BT + BLC, BT + ZLC, BT + JSBT, and BT (Figure 4B).
3.5.3 Important but not critical outcome: urinary albumin/creatinine ratio
A total of 10 RCTs, including 626 participants, were reported in the UACR. Network evidence of UACR results indicated that a total of three interventions were included (Figure 2C): BT and BT plus two OS preparations (BLC and JSBC). The league table showed that, when compared with BT, the MD [95% CI] for each intervention was as follows: BT + JSBC (−22.17 −[−31.23 to −14.09], eight trials) and BT + BLC (−12.53 [−32.83 to 11.40], two trials) (Figure 3C). According to the SUCRA values, “BT + JSBC” showed the highest effectiveness (SUCRA value of 91.58%), followed by “BT + BLC” (52.8%), while BT showed the lowest effectiveness (5.61%) (Figure 4C).
3.5.4 Important but not critical outcome: serum creatinine
A total of 115 trials with 6 interventions and 9,802 individuals were included in the NMA of Scr. Network evidence of UACR results manifested that a total of six interventions were included: BT and BT plus all five OS preparations (Figure 2D). As the league table shows, the effectiveness of BT combined with any of the four OS preparations (BLC, JSBT, JSBC, or ZLC) was better than that of BT alone in reducing Scr levels (Figure 3D). However, the effectiveness of BT plus BLT in reducing Scr levels in DKD patients was not more significant than that of BT, and the difference was not statistically significant (MD 3.94, 95% CI −13.79 to 21.64, two trials). As the SUCRA plot shows, the sequence from good to bad was as follows: BT + ZLC > BT + JSBT > BT + JSBC > BT + BLC > BT + BLT > BT alone (Figure 4D).
3.5.5 Important but not critical outcome: serum urea nitrogen
Eighty-one RCTs reported the effectiveness in reducing SUN levels, including 7,042 participants. Network evidence of SUN results indicated that a total of six interventions were included: BT and BT plus all five OS preparations (Figure 2E). The results of the NMA showed that the effectiveness of BT combined with BLC, JSBT, or JSBC was better than that of BT alone in reducing SUN levels. Nevertheless, the combination of BLT or ZLC did not have a superior effect in reducing SUN levels in DKD patients compared to BT alone (Figure 3E). The indirect comparison results suggested that “BT plus BLC” had a better effect than “BT plus JSBC” in decreasing SUN levels (MD −0.59, 95% CI −1.18 to −0.04); “BT combined with JSBT” was superior to “BT combined with BLT” (MD −2.35, 95% CI −4.53 to −0.19); and “BT plus BLC” was better than “BT plus BLT,” with an MD of −1.86 and a 95% CI (−3.60 to −0.14). The SUCRA plot is sequenced as BT + JSBT, BT + BLC, BT + JSBC, BT + ZLC, BT + BLT, and BT alone (Figure 4E).
3.5.6 Important but not critical outcome: estimated glomerular filtration rate
A total of three trials with three interventions (BT, BT plus ZLC, and BLC) and 342 individuals were included in the NMA of the eGFR (Figure 2F). The league table showed no significant differences among the three interventions in improving the eGFR (Figure 3F). As the SUCRA plot shows, the sequence from good to bad was as follows: BT + BLC > BT alone > BT + ZLC (Figure 4F).
3.5.7 Important but not critical outcome: adverse events
Twenty RCTs compared the AEs between groups. Network evidence of AE results showed that a total of four interventions were included: BT and BT plus JSBC, BLC, and BLT (Figure 2G). No serious AEs occurred in all included trials. The detailed adverse events are given in Supplementary Table S6. We analyzed the incidence of overall AEs between groups. As revealed in the league table, the results indicated that there was no significant statistical difference between the experimental group (co-intervention of BT and any of the three OS preparations) and the control group (BT alone) in the incidence of AEs (Figure 3G). On the basis of SUCRA values, the “BT + BLC” ranked the lowest in the incidence of an AE (SUCRA value of 74.23%), followed by “BT + JSBC” (57.63%) and “BT + BLT” (53.42%). BT was the highest in the incidence of an AE (14.72%) (Figure 4G).
3.5.8 Other outcomes: fasting plasma glucose
FPG was reported in 53 studies including 6 interventions (BT and BT plus 5 OS preparations) and 4,549 patients (Figure 2H). BT combined with JSBC, JSBT, or BLC had better FPG reduction effectiveness than BT alone. However, the combination of ZLC or BLT has not been found. The indirect comparison results indicated that the effectiveness of BT combined with JSBC was higher than that of BT combined with BLC (MD −0.47, 95% CI −0.87 to −0.08) (Figure 3H). According to the SUCRA values, the “BT + ZLC” had the highest effectiveness (SUCRA value of 78.78%), followed by “BT + JSBT” (78.58%), “BT + JSBC” (62.36%), “BT + BLT” (45.13%), and “BT + BLC” (29.27%), while the BT had the lowest effectiveness (5.89%) (Figure 4H).
3.5.9 Other outcomes: HbA1c
HbA1c was reported in 45 studies including 6 interventions (BT and BT plus 5 OS preparations) and 3,864 participants (Figure 2I). BT combined with JSBC, JSBT, or BLC had better HbA1c reduction effectiveness than BT alone. However, the combination of ZLC or BLT has not been found (Figure 3I). The SUCRA plot is sequenced as BT + JSBT, BT + JSBC, BT + BLC, BT + ZLC, BT + BLT, and BT alone (Figure 4I).
3.6 Sensitivity analyses
Seven trials did not report the course of OS preparations. We removed them and performed sensitivity analyses. We found that the addition of any of the OS capsular preparations was superior to RASi alone in reducing UAER levels. The remaining results of the direct comparisons were consistent with our main findings (Supplementary Figure S3). In addition, given that chronic diseases require long-term medication, we removed trials with treatment courses of 1 month or less, as well as trials without reported courses (18 trials). After removal, only the outcomes of Scr, SUN, and AEs were reported in the remaining trials that included the BLT intervention. The remaining results of the direct comparisons were consistent with the above sensitivity analysis results.
3.7 Publication bias
For the outcome measures of eGFR and UACR, since there were not enough (no more than 10 studies) included trials, no publication bias test was performed. The results of the counter-enhanced funnel plots combined with the trim and fill method showed that no significant publication bias appears to be found for the remaining outcome indicators (Supplementary Figure S2).
3.8 Grading of the evidence certainty
Combined evidence was not available because there was no closed loop. Overall, the evidence for nine outcomes was assessed. On the basis of GRADE assessment results, the overall certainty of direct evidence for main findings ranged from “very low” to “moderate,” and the certainty of indirect evidence ranged from “very low” to “low” (Supplementary Table S7). Among them, compared to RASi alone, the combination of JSBC and RASi showed beneficial effects in reducing 24-h UTP, UAER, UACR, Scr, SUN, FPG, and HbA1c levels, with moderate-certainty direct evidence. In addition, BLC plus RASi suggested significant effects in decreasing 24-h UTP, UAER, Scr, and SUN levels, with moderate-certainty direct evidence.
4 Discussion
4.1 Implications for healthcare and clinical practice
As a commonly used adjuvant medicine, OS preparations are used in the clinical practice of DKD treatment. Although the main medicinal ingredients of OS preparations on the market are isolated from wild OS, there may be differences in the therapeutic effects due to the differences in species name, fermentation techniques, and dosage forms. This is the first umbrella review and network meta-analysis generating the effectiveness and safety rankings of different OS preparations for DKD treatment, with a critical assessment of existing SRs. These results have brought beneficial implications for healthcare and clinical practice.
In this umbrella review, we specifically and comprehensively analyzed the effectiveness and safety of the combination of RASi and OS in treating DKD in the included 157 RCTs involving 13,130 patients. All RCTs included did not report end-point events, albuminuria progression, MACCEs, or patient-reported outcomes (PROs). For DKD, the prognosis is different in different stages of the disease. The cause–glomerular filtration rate–albuminuria (CGA) stage was proposed in the KDIGO Guidelines for the evaluation and management of CKD in 2012. Among them, G represents the eGFR degree, A represents the UACR degree, and the diagnostic stages are expressed by G1–5 and A1–3 (KDIGO, 2012). We found that Mogensen staging was used in most of the included RCTs, and some trials did not report disease staging. In addition, we know that Mogensen staging seems more appropriate for DKD caused by type 1 diabetes. Since the role of agents may not be the same in different stages of the disease, we need to select the most effective drugs at the specific stage of the intervention. This is something that needs to be improved in clinical practice and research in the future, with careful reporting of disease staging. Regarding the dose and duration of the intervention, this should be clearly reported in the RCT as the specifications vary from drug to drug. Unfortunately, the RCTs we included did not report well in this area. Therefore, we cannot analyze and explore the dose–response relationship between agents and diseases.
As is well known, urinary protein is a vital independent risk factor that affects the progression of DKD (Neuen et al., 2021). A Bayesian network meta-analysis revealed that the addition of any of the OS capsular preparations was superior to RASi alone in reducing 24-h UTP levels without additional adverse reactions. The “JSB capsule + RASi” therapy appeared to be beneficial in reducing urinary protein levels (including 24-h UTP, UAER, and UACR levels), protecting renal function (including Scr and SUN levels), and lowering blood glucose levels (including FPG and HbA1c levels) in patients with DKD. Although a few OS preparations did not have positive results in the outcomes of UAER, UACR, Scr, SUN, eGFR, FPG, and HbA1c levels, we found that the number of trials related to these outcomes was very rare, with almost only two trials. Therefore, the results need to be explained with caution as negative results may be related to a small number and insufficient statistical efficiency.
A recently published SR indicated that OS preparation combined with ACEIs/ARBs has a beneficial influence on renal function, proteinuria, and inflammation in DKD patients. However, no significant change in FPG and HbA1c levels was detected (Yan et al., 2023). Our results showed that RASi combined with JSBC, JSBT, or BLC had better FPG and HbA1c reduction effectiveness than BT alone. The reason may be that we used the Bayesian network meta-analysis method to analyze the effects of five different OS preparations on FPG and HbA1c levels. In contrast, only three OS preparations were included in the recent SR, and the number of studies and sample size corresponding to each OS preparation were lower than those included in our study. We found in some previous studies that OS also reduces insulin resistance and hypoglycemia in vitro and animal experiments (Kim et al., 2017; Liu et al., 2019). More clinical practice and studies are needed to confirm the effect of OS preparations on blood glucose levels in the future.
Based on the current results, OS preparations appear generally safe, and no serious adverse events were observed. Liver function impairment in individual patients in the experimental group was reported in three RCTs, one of which showed a doubling of aminotransferase levels, followed by a return to normal levels without stopping the intervention. The other two trials only reported improvement in liver function after symptomatic treatment, but no specific situation was reported. Renal function impairment in the experimental group was reported in four RCTs, one of which showed increased Scr levels but less than 30% of pre-medication levels. The research studies did not end the intervention and did not report subsequent prognosis. The other three trials only reported an improvement in kidney function after symptomatic treatment, but no detailed data on therapies and review results were reported. Because the subjects in the experimental group were taking OS and RASi at the same time, it is difficult to determine a causal relationship between the drugs. In addition, no follow-up information was recorded in the RCTs included, so long-term safety remains to be observed and determined in future healthcare and clinical practice.
Various factors are involved in the pathogenesis of DKD, including metabolic disorders, hemodynamic abnormalities, oxidative stress, inflammatory responses, immune abnormality, and epigenetics (Reddy et al., 2013; Ilyas et al., 2017; Tang and Yiu, 2020). RASi treat DKD mainly by improving hemodynamics (Reddy et al., 2013), and their inhibition of epigenetic changes and anti-inflammatory and antioxidant effects seems to be still being proven. OS can inhibit inflammation and improve antioxidation and immune regulation, thereby protecting the kidneys (Liu et al., 2022; Liu et al., 2022). Among the RCTs we included, there were trials that focused on the immune cells and inflammatory factors such as CD4/CD8 (5 trials), interleukin-6 (7 trials), and tumor necrosis factor-α (10 trials). The results all found that loading OS could significantly elevate the ratio of CD4/CD8 and reduce the levels of inflammation compared with basic treatment alone. We speculate that the combination of OS and RASi not only improves hemodynamics but also modulates immunity, anti-inflammatory, and antioxidant effects, which may be why the effect after adding OS based on the basic treatment is more significant. Of course, this is just the simplest hypothesis. Further in-depth studies are needed in future to elucidate the herb–drug interaction.
4.2 Future research recommendations to improve transparency and readability
In this study, the AMSTAR 2 tool was applied to appraise the methodological quality of the included SRs. Considering that the AMSTAR 2 scale was published in September 2017 (Shea et al., 2017), SRs published before 2017 may affect the quality inevitably due to the uncertainty of the evaluation criteria. Five of the SRs we included were published before 2017. We evaluated SRs published from 2013 to 2016 with “critically low” quality and SRs published from 2018 to 2023 with “high” and “critically low” quality. Regarding the critical items, the main problems focused on the non-reporting of research protocols, as well as literature exclusion lists. About the non-critical items, the problems centered on the unreported sources of funding and conflicts of interest. We used the RoB 2 tool to assess the methodological quality of the included RCTs. Because none of the trials had placebos, blinding of subjects and researchers could not be performed. The outcomes were almost all laboratory test indicators, which may be less affected by blinding. The current problem mainly focuses on the unclear reporting of randomized methods and selective reporting of outcomes. In addition, when it comes to patient-reported outcomes in future studies, the implementation of blinding is critical to the presentation of actual effects. Since the results may be affected by the subjective factors of participants and researchers, it may elevate the risk of detection bias and performance bias.
All of these problems and recommendations are intended to ensure scientific rigor and are essential to conducting high-quality research. In future studies, attention should be paid not only to the recommendations mentioned above to increase research transparency and methodological quality but also to present findings in accordance with international reporting standards and evaluate the preparations according to the core outcome set. This will make the results more readable and can be disseminated more widely for readers to obtain high-level research evidence.
4.3 Limitations
This review has several limitations. First, there were significant differences in the number and sample size of RCTs related to the five OS preparations. For example, the Zhiling capsule included only five trials and 421 participants, which may have an impact on the results. Second, the included RCTs did not report about allocation concealment, which could easily cause selective bias and potentially affect the accuracy and reliability of the analysis results. Third, all the subjects in this study were from China, and the generalizability of the results was limited. Fourth, there were no follow-up assessments conducted at the end of the intervention, which limits the ability to observe long-term effectiveness and safety. At last, only five trials of OS preparations for DKD treatment were retrieved. No evidence of other OS preparations for DKD was available, such as the Xinganbao capsule, Yong Chong Cao capsule, and Ningxinbao capsule. The evidence will need to be updated in the future.
5 Conclusion
In conclusion, there are some favorable effects in decreasing 24-h UTP levels in DKD patients using OS capsular preparations plus RASi. This study presents the effectiveness and safety ranking of OS preparations for DKD treatment. Our results need to be supplemented and improved by more high-quality and larger sample RCTs with a longer follow-up in the future. Future studies should focus more on the benefits of kidney disease progression and cardiovascular events. The herb–drug interaction should be explored in depth.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
XX: conceptualization, investigation, analysis, methodology, writing–original draft, funding acquisition. X-YJ: investigation, analysis, methodology, writing–original draft. X-LY: investigation, analysis, visualization, writing–original draft. K-YL: investigation, analysis, writing–original draft. J-XL: investigation, analysis, writing–original draft. X-HL: analysis, writing–review and editing. JB: investigation, writing–original draft. QL: investigation, writing–review and editing. B-RZ: analysis, writing–original draft. X-RZ: writing–review and editing. JY: writing–review and editing. C-LL: analysis, writing–review and editing, funding acquisition. F-FZ: writing–review and editing. J-PL: supervision, writing–review and editing. X-QW: supervision, writing–review and editing, funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors acknowledge the financial support from the National Natural Science Foundation of China [No. 82204989 (Youth Project) and No. 82374384], the Natural Science Foundation of Hubei Province [No. 2022CFC056], and Guangzhou Basic and Applied Basic Research Topics (SL 2024A04J02040).
Acknowledgments
The authors acknowledge the National Administration of TCM for the construction project of the Hubei Province Academy of TCM and TCM evidence-based ability improvement project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1360633/full#supplementary-material
References
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2015). Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Healthc. 13 (3), 132–140. doi:10.1097/XEB.0000000000000055
Aromataris, E., Fernandez, R., Godfrey, C. M., Holly, C., Khalil, H., and Tungpunkom, P. (2020). “Chapter 10: umbrella reviews,” in Synth. Editors E. Aromataris, Z. Munn, and E. JBI, 13, 132–140. doi:10.46658/JBIMES-20-11
Atkins, D., Best, D., Briss, P. A., Eccles, M., Falck-Ytter, Y., Flottorp, S., et al. (2004). Grading quality of evidence and strength of recommendations. Br. Med. J. Clin. Res. Ed. . 328 (7454), 1490. doi:10.1136/bmj.328.7454.1490
Cai, T. (2016). Effect of bailing capsule combined with angiotensin-converting enzyme inhibitors in the treatment of diabetic kidney disease: a meta-analysis. Shandong Univ. Tradit. Chin. Med.
Cao, L. M. (2012). Clinical effects of Bailing capsule combined with valsartan in treatment of patients with diabetic nephropathy. Chin. J. Med. Guide. 14 (09), 1571–1572.
Cao, X. C. (2015). Effect analysis of valsartan combined with Bailing capsule in the treatment of early-stage diabetic nephropathy. CHN Mod. Med. 22 (04), 97–98+101.
Cao, X. X., Zhang, P. R., and Yang, J. K. (2007). Clinical observation on treatment of diabetic nephropathy with Jinshuibao capsules and valsartan. Chin. J. New Drugs. 16, 1303–1306. doi:10.3321/j.issn:1003-3734.2007.16.021
Chen, F., Chen, B. P., and Shi, J. (2010). Clinical observation on treatment of early diabetic nephropathy with Bailing capsules and valsartan. CHN Pharm. 13 (04), 551–552. doi:10.3969/j.issn.1008-049X.2010.04.046
Chen, J. (2009). The clinical studies on the prophylactic and therapeutic effects of Bailing capsule in early diabetic nephropathy. Chin. J. Health Care Med. 11 (05), 371–373.
Chen, Q. J. (2017). Curative effect observation of losartan potassium combined with Jinshuibao capsule on early diabetic nephropathy. Chin. J. Urban Rural. Enterp. Hyg. 32 (11), 101–102. doi:10.16286/j.1003-5052.2017.11.044
Chen, Q. S., Zhang, P., and Liang, H. (2016). Application of benazepril combined with Bai Ling capsule in the treatment of early diabetic nephropathy. CHN Pract. Med. 11 (31), 102–103. doi:10.14163/j.cnki.11-5547/r.2016.31.063
Chen, R. C., Xiang, J. Q., and Chen, H. Y. (2017). Systematic evaluation of the efficacy of Cordyceps sinensis in the treatment of diabetic nephropathy. Chin. J. Integr. Tradit. West Med. 18, 141–146. doi:10.3969/j.issn.1009-587X.2017.02.015
Chen, W. J. (2011). Observation of the effect of Zhiling capsule combined with irbesartan treating early diabetic nephropathy. Mod. Hosp. 11 (08), 57–58. doi:10.3969/j.issn.1671-332X.2011.08.026
Chen, Y. (2007). Clinical observation of the effect of Bailing capsule combined with fosinopril treating early diabetic nephropathy. Guangxi Univ. Chin. Med.
Cheng, J. (2021). Analysis of clinical efficacy of Bailing capsule combined with irbesartan in the treatment of diabetic nephropathy. Diabetes New World 24 (13), 190–193. doi:10.16658/j.cnki.1672-4062.2021.13.190
Cui, T. X., Jin, S. K., Qian, B. Y., and Zhu, W. P. (2009). Effect of telmisartan combined with Bailing capsule on pulse pressure and proteinuria in diabetic nephropathy patients with hypertension. Chin. J. New Drugs Clin. Rem. 28 (09), 716–718.
Dai, G. J. (2019). Effects of Jinshuibao capsule combined with irbesartan on renal function and proteinuria in patients with diabetic nephropathy. Drug Eval. 16, 19–20. doi:10.3969/j.issn.1672-2809.2019.21.008
Dai, H. H. (2016). Clinical observation of valsartan combined with Jinshuibao capsule treating diabetic nephropathy. J. Henan Med. Coll. 28 (01), 12–14.
Diao, L. (2015). Curative effects of valsartan combined with Bailing capsule for the treatment of diabetic nephropathy. Chin. J. Prim. Med. Pharm. 6, 886–888. doi:10.3760/cma.j.issn.1008-61706.2015.06.030
Dias, S., Welton, N. J., Caldwell, D. M., and Ades, A. E. (2010). Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 29 (7–8), 932–944. doi:10.1002/sim.3767
Ding, L., Guan, C. R., and Zhu, X. Y. (2022). Clinical effect of Bailing capsules combined with western medicine on diabetic nephropathy and its influence on kidney function. New Chin. Med. 54 (07), 93–96. doi:10.13457/j.cnki.jncm.2022.07.021
Ding, T. (2014). Clinical observation of the effect of irbesartan combined with Jinshuibao treating diabetic nephropathy. J. Math. Med. 27 (02), 207–208. doi:10.3969/j.issn.1004-4337.2014.02.030
Dong, Y. Q. (2022). Effects of Bailing capsules combined with Irbesartan in treatment of patients with diabetic nephropathy. Med. J. Chin. Peoples Health. 34 (3), 87–89. doi:10.3969/j.issn.1672-0369.2022.03.028
Du, N. (2021). Clinical observation of Bailing capsule combined with irbesartan treating diabetic nephropathy. J. Pract. Trad. Chin. Med. 37 (10), 1680–1681.
Duan, R., and Li, Z. X. (2015). Combined Jinshuibao and angiotensin receptor blocker in treatment of diabetic nephropathy: a Meta-analysis. Drug Eval. Res. 38 (01), 78–84. doi:10.7501/j.issn.1674-6376.2015.01.016
Expert Group of Chinese Society of Nephrology (2021). Chinese guidelines for diagnosis and treatment of diabetic kidney disease. Chin. J. Nephrol. 37 (3), 255–304. doi:10.3760/cma.j.cn441217-20201125-00041
Feng, Z. L. (2017). Clinical evaluation of Jinshuibao capsules combined with valsartan for treating early diabetic nephropathy with micro-inflammation in 43 cases. CHN Pharm. 26 (23), 62–64. doi:10.3969/j.issn.1006-4931.2017.23.021
Gao, T. W., and Wei, j S. (2013). Analysis of the efficacy of Jinshuibao in treating 100 cases of diabetic nephropathy. Health. Must Read. 12 (3), 291.
Gao, X. (2018). Clinical observation of the effect of losartan potassium combined with Jinshuibao capsule treating early type 2 diabetic nephropathy in 76 cases. Guide CHN Med. 16 (06), 102–103. doi:10.15912/j.cnki.gocm.2018.06.088
Gao, Y., Song, Z. L., Duan, Y. H., and Zhang, X. K. (2021). Meta-analysis of Bailing Capsule in treatment of diabetic kidney disease. Drug Eval. Res. 44 (01), 161–169. doi:10.7501/j.issn.1674-6376.2021.01.024
Gates, M., Gates, A., Pieper, D., Fernandes, R. M., Tricco, A. C., Moher, D., et al. (2022). Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ 378, e070849. doi:10.1136/bmj-2022-070849
Gong, L., Huang, Y. L., Xie, S. Y., Chen, Y. T., Wang, G. J., and Xie, J. C. (2021). Meta-analysis of clinical efficacy and safety of bailing capsules combined with valsartan in the treatment of diabetic kidney disease. J. Guangzhou Univ. Tradit. Chin. Med. 38 (05), 1061–1066. doi:10.13359/j.cnki.gzxbtcm.2021.05.036
Guan, C. A., Chen, D. J., Duan, X. F., Jiang, Z. H., and Chen, L. (2021). Effects of Bailing capsule and candesartan medoxomil on renal function, inflammatory factors and hemorheology in patients with early diabetic nephropathy. Chin. Arch. Tradit. Chin. Med. 39 (05), 247–250. doi:10.13193/j.issn.1673-7717.2021.05.060
Guan, H. B., He, K. P., Heng, W. M., Lv, L., Huang, H., and Wang, B. (2010). Effect of irbesartan with Bailing capsules on microalbuminuria-to-creatinine ratio and high-sensitive C-reactive protein in patients with early diabetic nephropathy. Chin. Gen. Pract. 13 (26), 2934–2936. doi:10.3969/j.issn.1007-9572.2010.26.013
Guyatt, G. H., Oxman, A. D., Kunz, R., Atkins, D., Brozek, J., Vist, G., et al. (2011). GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 64 (4), 395–400. doi:10.1016/j.jclinepi.2010.09.012
Han, P. P. (2021). Clinical effect of Jinshuibao capsule combined with irbesartan in the treatment of patients with diabetic nephropathy. Inn. Mong. J. Tradit. Chin. Med. 40 (09), 64–66. doi:10.16040/j.cnki.cn15-1101.2021.09.038
He, P., and Lu, B. D. (2012). Observation of the effect of valsartan combined with Jinshuibao capsule treating type 2 diabetic nephropathy. Med. J. Chin. Peoples Health. 24 (12), 1452–1453. doi:10.3969/j.issn.1672-0369.2012.12.026
He, X. H., and Ge, Z. Y. (2012). Meta-analysis of the efficacy of Bailing capsule in the prevention and treatment of diabetic nephropathy. Clin. focus (Lond). 27 (13), 1164–1166.
He, X. J. (2017). Clinical analysis of valsartan combined with Bailing capsule in early diabetic nephropathy. J. Front. Med. 7 (19), 151–152. doi:10.3969/j.issn.2095-1752.2017.19.126
Hong, Y. Q. (2010). Observation of the effect of Bailing capsule combined with irbesartan treating type 2 diabetic nephropathy in the elderly. Chin. J. Misdiagnostics. 10 (24), 5857–5858.
Hou, K., Bao, M., and Ren, H. L. (2017). Clinical observation of valsartan combined with Bailing capsule treating early diabetic nephropathy. J. CHN Prescr. Drug. 15 (07), 71–72. doi:10.3969/j.issn.1671-945X.2017.07.047
Hu, W. F., and Wei, Y. (2012). Clinical observation of the effect of Bailing capsule combined with irbesartan treating early diabetic nephropathy. Chin. J. Tradit. Med. Sci. Technol. 19 (02), 173–174. doi:10.3969/j.issn.1005-7072.2012.02.059
Hu, X. J. (2019). Evaluation of the efficacy of Jinshuibao capsule combined with benazepril in the treatment of early diabetic nephropathy. Diabetes New World 22 (07), 174–175. doi:10.16658/j.cnki.1672-4062.2019.07.174
Hu, Y. (2016). Clinical efficacy of Bailing capsule combined with irbesartan in the treatment of diabetic nephropathy. J. Baotou Med. Coll. 32 (10), 71–72. doi:10.16833/j.cnki.jbmc.2016.10.042
Hu, Y., Zhang, Q., Zou, Y., Zhu, Y., and Hu, L. (2018). Systematic evaluation of DaiWen combined with Jinshuibao capsule in the treatment of diabetic nephropathy. Jiangxi Med. J. 43 (6), 720–724. doi:10.3969/j.issn.1006-2238.2018.12.024
Huang, D., Chen, S. J., and Chen, Q. (2022). Bailing capsule combined with candesartan cilexetil in the treatment of diabetic nephropathy in older patients. Intern. J. Geriatr. 43 (6), 720–724. doi:10.3969/j.issn.1674-7593.2022.06.017
Huang, J. Y., and Zhou, C. X. (2016). Analysis of Bailing capsule combined losartan for UMA and lipid metabolism in patients with early diabetic nephropathy. Chin. J. Biochem. Pharm. 36 (12), 144–146+150. doi:10.3969/j.issn.1005-1678.2016.12.041
Huang, T., Sun, H., and Wu, T. Y. (2010). Effects of perindopril combined with Jinshuibao capsules on renal function in elderly patients with early diabetic nephropathy. Her. Med. 29 (07), 890–892. doi:10.3870/yydb.2010.07.022
Huang, Y. L., Huang, G. D., Cai, L. K., Lin, J. Y., Huang, Q. H., Gan, J. L., et al. (2019). Effect and safety of bailing capsule combined with RAAS inhibitors in treatment of early diabetic nephropathy:A systematic review. Chin. Arch. Tradit. Chin. Med. 37 (06), 1290–1297. doi:10.13193/j.issn.1673-7717.2019.06.002
Huang, Y. L., Yang, H., and Wang, S. M. (2012). Systematic evaluation on Bailing Capsule for treating early diabetic nephropathy. Chin. Pharm. J. 21 (20), 18–21. doi:10.3969/j.issn.1006-4931.2012.20.009
Ilyas, Z., Chaiban, J. T., and Krikorian, A. (2017). Novel insights into the pathophysiology and clinical aspects of diabetic nephropathy. Rev. Endocr. Metab. Disord. 18, 21–28. doi:10.1007/s11154-017-9422-3
Ji, X. X., Xu, J. J., and Tang, X. (2014). Systematic review of effect of Bailing Capsule on renal functional level of diabetic nephropathy. Hubei J. Tradit. Chin. Med. 36 (12), 3–5.
Jia, Z. R. (1999). Influnces of treatment with captopril combined with cordyceps preparation on microalbuminuria of early diabetic nephropathy in Ⅱ type diabetes. Integr. Tradit. Chin. West Med. Pract. Crit. Care. Med. 35 (02), 39–42. doi:10.3321/j.issn:1008-9691.1999.12.015
Jiang, T. (2021). Clinical observation of ramipril combined with Jinshuibao capsule in the treatment of early diabetic nephropathy. J. Pract. Tradit. Chin. Intern. Med. 35 (02), 39–42. doi:10.13729/j.issn.1671-7813.z20201413
Jin, X. B. (2016). Clinical analysis of 50 cases of early diabetic nephropathy treated with Bailing capsule combined with valsartan capsule. J. New Chin. Med. 48 (08), 99–101. doi:10.13457/j.cnki.jncm.2016.08.042
Jing, Y. F., Hu, J. L., and Tang, S. Y. (2017). Systematic evaluation of Cordyceps sinensis in the treatment of diabetic nephropathy stages III-IV. Tianjin Pharm. 29 (02), 32–37. doi:10.3969/j.issn.1006-5687.2017.02.011
KDIGO (2012). Clinical practice guideline for the evaluation and management of chronic kidney disease. doi:10.7326/0003-4819-158-11-201306040-00007
KDIGO (2022). KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102 (5), S1–S127. doi:10.1016/j.kint.2022.06.008
Kim, D. J., Kang, Y. H., Kim, K. K., Kim, T. W., Park, J. B., and Choe, M. (2017). Increased glucose metabolism and alpha-glucosidase inhibition in Cordyceps militaris water extract-treated HepG2 cells. Nutr. Res. Pract. 11 (3), 180–189. doi:10.4162/nrp.2017.11.3.180
Kopel, J., Pena-Hernandez, C., and Nugent, K. (2019). Evolving spectrum of diabetic nephropathy. World J. Diabetes 10 (5), 269–279. doi:10.4239/wjd.v10.i5.269
Kristensen, S. L., Rørth, R., Jhund, P. S., Docherty, K. F., Sattar, N., Preisset, D., et al. (2019). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7 (10), 776–785. doi:10.1016/S2213-8587(19)30249-9
Lei, G. Y., Zheng, L., and Yan, X. Y. (2017). Application value of telmisartan combined with Bai Ling capsule in the treatment of diabetic nephropathy. Chin. Community Dr. 33 (11), 89+91.
Lei, S. H., Li, J., Lai, X. Y., Zhang, M. Y., Zhang, L., and Xiong, Y. (2009). Observation of the effect of Jinshuibao combined with ambevi treating non-hypertensive diabetic nephropathy. Shandong Med. J. 49 (01), 98–99. doi:10.3969/j.issn.1002-266X.2009.01.041
Leoncini, G., Viazzi, F., De Cosmo, S., Russo, G., Fioretto, P., and Pontremoli, R. (2020). Blood pressure reduction and RAAS inhibition in diabetic kidney disease: therapeutic potentials and limitations. J. Nephrol. 33, 949–963. doi:10.1007/s40620-020-00803-3
Li, G. D. (2016). Zhiling capsule combined conventional treatment for early disbetic nephropathy:A systematic review. Med. Inf. 29 (22), 39–41. doi:10.3969/j.issn.1006-1959.2016.22.026
Li, H., and Lv, J. N. (2011). Nephroprotective effect of irbesartan combined with Bailing capsules in diabetic nephropathy combined with hypertension. CHN Mod. Med. 18 (16), 56–57. doi:10.3969/j.issn.1674-4721.2011.16.031
Li, H. S. (2023). The clinical value of Jinshuibao tablets combined with irbesartan in the treatment of early diabetic nephropathy. Chin. J. Mod. Drug Appl. 17 (04), 136–139. doi:10.14164/j.cnki.cn11-5581/r.2023.04.042
Li, J. E., and Liu, H. F. (2009). Nephroprotective effect of valsartan combined with Bailing capsules in elderly patients with type 2 diabetics. Clin. Nephrol. 9 (4), 172–173.
Li, N. (2019). Clinical effect of Bailing capsules on early diabetic nephropathy. Chin. J. New Clin. Med. 12 (03), 308–310. doi:10.3969/j.issn.1674-3806.2019.03.18
Li, Q. Y. (2012a). Clinical observation of the effect of irbesartan combined with Ophiocordyceps sinensis treating type 2 diabetic nephropathy. Contemp. Med. 18 (22), 82–83. doi:10.3969/j.issn.1009-4393.2012.22.059
Li, W. (2017). Clinical observation of the effect of Jinshuibao capsule combined with benazepril treating early diabetic nephropathy. World J. Clin. Med. 11 (1), 90–91.
Li, X. (2012b). Observation of the effect of Jinshuibao combined with benazepril treating diabetic nephropathy. CHN Foreign Med. Treat. 31 (28), 112–114. doi:10.16662/j.cnki.1674-0742.2012.28.070
Li, X. J., Qie, S. H., and Liu, Y. (2022). Systematic review on Jinshuibao capsules combined with irbesartan for the treatment of diabetic kidney disease. CHN J. Pharm. Econ. 17 (9), 80–85. doi:10.12010/j.issn.1673-5846.2022.09.014
Li, X. L. (2010). Observation of the effect of Cosuya combined with Bailing capsule treating early diabetic nephropathy. J. CHN Tradit. Chin. Med. Inf. 02 (30), 184–185.
Li, Y. B., and Xu, G. S. (2019). Clinical efficacy and safety of Jinshuibao combined with ACEI/ARB in the treatment of diabetic kidney disease: a meta-analysis of randomized controlled trials. J. Ren. Nutr. 30 (02), 92–100. doi:10.1053/j.jrn.2019.03.083
Li, Z., Zhang, G. M., Zheng, S., Zheng, J. J., Zhang, Y., and Yuan, P. (2019). Effects of Bailing capsule combined with irbesartan on oxidative stress, inflammatory response and immune function in patients with diabetic nephropathy. J. Hainan Med. Univ. 25 (09), 670–673. doi:10.13210/j.cnki.jhmu.20190322.004
Lian, X. Y., Liu, H. F., Wang, X. M., and Zhang, Y. L. (2020). Meta-analysis on Jinshuibao capsules combined with angiotensin converting enzyme inhibitor for the treatment of diabetic kidney disease. Chin. J. Inf. Tradit. Chin. Med. 27 (09), 103–107. doi:10.3969/j.issn.1005-5304.201911310
Lin, J. (2009). Protective effects of valsartan and Jinshuibao in diabetic nephropathy. Tianjin Pharm. 21 (06), 37–39. doi:10.3969/j.issn.1006-5687.2009.06.020
Lin, Z. N. (2016). Clinical observation of Bailing capsule combined with benazepril treating early diabetic nephropathy. Contemp. Med. 22 (24), 150–151. doi:10.3969/j.issn.1009-4393.2016.24.104
Liu, C. P., and Li, M. J. (2011a). Effect of combined therapy with Bailing capsule and irbesartan on urinary albumin excretion rate and C-reactive protein in patients with early diabetic nephropathy. Hebei Med. J. 33 (13), 1990–1991. doi:10.3969/j.issn.1002-7386.2011.13.036
Liu, C. P., and Li, M. J. (2011b). Observation of the effect of Bailing capsule combined with irbesartan treating early diabetic nephropathy. Hebei Med. J. 33 (11), 1661–1662. doi:10.3969/j.issn.1002-7386.2011.11.031
Liu, C. Y., Chen, Y. X., Su, J. P., Liu, X. R., and Zhu, C. J. (2016a). Effects of Bailing capsule on blood glucose, blood pressure and blood lipid in patients with diabetic nephropathy. Hebei Med. J. 38 (02), 209–211. doi:10.3969/j.issn.1002-7386.2016.02.014
Liu, C. Y., Chen, Y. X., Su, J. P., Liu, X. R., and Zhu, C. J. (2016b). The effect of Bailing capsule on renal function in patients with diabetic nephropathy. Hebei Med. J. 38 (1), 52–54. doi:10.3969/j.issn.1002-7386.2016.01.015
Liu, D. D., Wang, X. S., Wang, J. M., and Du, J. M. (2020). Cordyceps preparation combined with angiotensin receptor blocker in treating diabetic nephropathy: meta analysis. World J. Integr. Tradit. West Med. 5 (05), 835–840. doi:10.13935/j.cnki.sjzx.200511
Liu, H. Y. (2019a). Systematic evaluation of benazepril combined with Jinshuibao in the treatment of diabetic nephropathy. CHN Rural. Health 11 (08), 55. doi:10.3969/j.issn.1674-361X.2019.08.059
Liu, J., and Jian, H. (2020). Clinical effect of Jinshuibao capsule combined with western medicine on early diabetic nephropathy. Contemp. Med. 26 (30), 91–93. doi:10.3969/j.issn.1009-4393.2020.30.037
Liu, M. J. (2019b). Effects of Bailing capsule combined with high-dose ramipril on renal function and glycemic control in patients with early diabetic nephropathy. J. North Pharm. 16 (05), 91–93. doi:10.3969/j.issn.1672-8351.2019.05.073
Liu, M. W. (2011a). Evaluation of the efficacy of losartan combined with Bailing capsule in the treatment of diabetic nephropathy. Chin. J. Misdiagnostics. 11 (26), 6336.
Liu, R. M., Dai, R., Luo, Y., and Xiao, J. H. (2019). Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice. BMC Complement. Altern. Med. 19, 230. doi:10.1186/s12906-019-2646-x
Liu, W., Gao, Y., Zhou, Y., Yu, F., Li, X., and Zhang, N. (2022b). Mechanism of cordyceps sinensis and its extracts in the treatment of diabetic kidney disease: a review. Front. Pharmacol. 13, 881835. doi:10.3389/fphar.2022.881835
Liu, W. Y., and Zhang, H. J. (2017). Curative effect observation of Jinshuibao capsule combined with valsartan on early diabetic nephropathy. Health Guide 30, 267. doi:10.3969/j.issn.1006-6845.2017.30.248
Liu, X. D. (2011b). Clinical research of Ophiocordyceps sinensis in combination with benazepril in the treatment of early diabetic nephropath. Asia. Pac. Tradit. Med. 7 (12), 135–136.
Liu, Y. (2010). Effections of telmisartan and Jinshuibao capsule on patients with type 2 diabetic kidney disease. Chin. J. Hosp. Pharm. 30 (18), 1573–1575.
Liu, Y., Guo, Z. J., and Zhou, X. W. (2022a). Chinese cordyceps: bioactive components, antitumor effects and underlying mechanism-A review. Mol. Basel, Switz. 27 (19), 6576. doi:10.3390/molecules27196576
Liu, Y. X. (2017). The Influence of Zhiling capsule combined valsartan dispersible tablets in early diabetic nephropathy. Nei Mong. J. Tradit. Chin. Med. 36 (10), 7–8. doi:10.16040/j.cnki.cn15-1101.2017.10.007
Long, Y. T. (2014). Clinical observation of Bailing capsule combined with enalapril on the C-reactive protein and Homa IR of patients with early type 2 diabetic nephropathy. Chin. J. Ethnobiol. Ethnomed. 23 (21), 37–38. doi:10.3969/j.issn.1007-8517.2014.21.zgmzmjyyzz201421021
Lou, P. H., Long, X. S., and Li, L. B. (2010). Clinical observation on threatment of early diabetic nephropathy with valsartan and Bailing capsule. J. Pract. Med. 26 (20), 3780–3782. doi:10.3969/j.issn.1006-5725.2010.20.054
Lovic, D., Piperidou, A., Zografou, I., Grassos, H., Pittaras, A., and Manolis, A. (2020). The growing epidemic of diabetes mellitus. Curr. Vasc. Pharmacol. 18 (2), 104–109. doi:10.2174/1570161117666190405165911
Lu, J. Y., and Shi, D. Y. (2020). Observations on the effect of Bailing capsule of and perindopril on patients with diabetic nephropathy. Renowned Dr. 10, 299–300.
Lu, Q., Li, C., Chen, W., Shi, Z., Zhan, R., and He, R. (2018). Clinical efficacy of Jinshuibao capsules combined with angiotensin receptor blockers in patients with early diabetic nephropathy: a meta-analysis of randomized controlled trials. Evid. Based Complement. Altern. Med. 2018, 6806943. doi:10.1155/2018/6806943
Lu, Y. L. (2010). Observation of the effect of Bailing capsule combined with telmisartan treating diabetic nephropathy. Chin. J. Clin. Ration. Drug Use. 3 (21), 43–44. doi:10.15887/j.cnki.13-1389/r.2010.21.093
Luo, F., Cao, S., and Sun, X. Y. (2011). Clinical research on early diabetic nephropathy treated by Bailing capsule combined with irbesartan. Chin. Med. 26 (04), 466–467. doi:10.16368/j.issn.1674-8999.2011.04.051
Luo, J. G., Su, X. H., Dai, S. Z., and Cai, J. S. (2018). Observation on the efficacy of Bailing capsule combined with irbesartan in the treatment of early diabetic nephropathy. Chin. Foreign Med. Res. 16 (18), 1–3. doi:10.14033/j.cnki.cfmr.2018.18.001
Luo, Y., Yang, S. K., Zhou, X., Wang, M., Tang, D., Liu, F. Y., et al. (2015). Use of Ophiocordyceps sinensis (syn. Cordyceps sinensis) combined with angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) versus ACEI/ARB alone in the treatment of diabetic kidney disease: a meta-analysis. Ren. Fail. 37, 614–634. doi:10.3109/0886022X.2015.1009820
Lv, F. (2012a). Efficacy observation of treating early DN with Jin Shui Bao capsule. Clin. J. Chin. Med. 4 (13), 23–24. doi:10.3969/j.issn.1674-7860.2012.13.010
Lv, H., Liu, J. Q., Jia, Z. X., and He, H. M. (2006). Observation of the effect of Jinshuibao combined with valsartan treating early diabetic nephropathy. J. Chin. Physician. S1, 291–292.
Lv, W. Q. (2022). Effects of Jinshuibao tablet combined with irbesartan for treatment on oxidative stress, inflammatory response and immune function in patients with diabetic nephropathy. Med. Diet. Health. 20 (02), 80–83.
Lv, X. Q. (2014). Observation of the effect of 62 patients with early diabetic nephropathy treated with Jinshuibao capsule. CHN Pract. Med. 9 (05), 180–181. doi:10.14163/j.cnki.11-5547/r.2014.05.010
Lv, Z. M. (2012b). Clinical study of Zhiling capsule combined with losartan potassium in treatment of early diabetic nephropathy. Strait Pharm. J. 24 (02), 160–161. doi:10.3969/j.issn.1006-3765.2012.02.083
Ma, J. (2017). Clinical efficacy study of irbesartan combined with Jinshuibao capsule in elderly diabetic nephropathy. Nei Mong. J. Tradit. Chin. Med. 36 (02), 60. doi:10.16040/j.cnki.cn15-1101.2017.02.061
Ma, J. L. (2018). Observation on the effect of benazepril combined with Jinshuibao in the treatment of diabetic nephropathy with proteinuria. Contin. Med. Educ. 32 (05), 155–157. doi:10.3969/j.issn.1004-6763.2018.05.086
Ma, Y. L., Chen, F., and Chen, B. P. (2011). Effect of Bailing capsule combined with irbesartan on blood IL-18 levels in early diabetic nephropathy. CHN Pharm. 14 (07), 1027–1028. doi:10.3969/j.issn.1008-049X.2011.07.048
Mao, J. X., Cheng, M., and Tang, X. J. (2012). Systematic evaluation on Jinshuibao for treating diabetic nephropathy. Chin. J. Integr. Tradit. West Med. 13 (06), 526–530. doi:10.3969/j.issn.1009-587X.2012.06.017
Neuen, B. L., Weldegiorgis, M., Herrington, W. G., Ohkuma, T., Smith, M., and Woodward, M. (2021). Changes in GFR and albuminuria in routine clinical practice and the risk of kidney disease progression. Am. J. Kidney Dis. 78 (03), 350–360.e1. doi:10.1053/j.ajkd.2021.02.335
Olatunji, O. J., Tang, J., Tola, A., Auberon, F., Oluwaniyi, O., and Ouyang, Z. (2018). The genus Cordyceps: an extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 129, 293–316. doi:10.1016/j.fitote.2018.05.010
Pan, J., and Shang, S. Y. (2016). Observation of the effect of telmisartan combined with Jinshuibao capsule treating diabetic nephropathy. Chin. J. Geriatr. Care. 14 (01), 40–41. doi:10.3969/j.issn.1672-4860.2016.01.018
Pan, X., Sun, J. M., and Liao, W. J. (2021). Clinical observation of Jinshuibao tablet combined with irbesartan tablet treating diabetic nephropathy. J. Pract. Tradit. Chin. Med. 37 (11), 1855–1856.
Phillippo, D. M., Dias, S., Ades, A. E., Belger, M., Brnabic, A., Schacht, A., et al. (2020). Multilevel network meta-regression for population-adjusted treatment comparisons. J. R. Stat. Soc. 183 (3), 1189–1210. doi:10.1111/rssa.12579
Qian, G. F., and Qin, J. H. (2012). Clinical observation of the effect of Jinshuibao capsule combined with enalapril treating diabetic nephropathy. Chin. J. Mod. Drug Appl. 6 (10), 96–97. doi:10.14164/j.cnki.cn11-5581/r.2012.10.057
Qiao, A. M. (2013). Observation of the effect of 62 patients with early diabetic nephropathy treated with Bailing capsule combined with telmisartan. CHN Pract. Med. 8 (28), 177–178. doi:10.14163/j.cnki.11-5547/r.2013.28.113
Qiu, L. F., Yang, Z. W., and Zeng, X. H. (2019). Curative effect observation of candesartan combined with Bailing capsule on early type 2 diabetic nephropathy in 48 cases. Med. Forum. 23 (32), 4712–4713. doi:10.19435/j.1672-1721.2019.32.090
Reddy, M. A., Tak Park, J., and Natarajan, R. (2013). Epigenetic modifications in the pathogenesis of diabetic nephropathy. Semin. Nephrol. 33 (04), 341–353. doi:10.1016/j.semnephrol.2013.05.006
Ren, X., Yuan, Q. Z., Gan, X. F., Li, B., and Shi, M. J. (2020). Therapeutic effect of Bailing Capsule in treating early diabetic nephropathy and its influence on hemorheological and inflammatory factors. J. Clin. Med. Pract. 24 (10), 64–67. doi:10.7619/jcmp.202010016
Ren, Y. F., and Hu, R. C. (2020). Clinical study on the treatment of diabetic nephropathy with Bailing capsule in combination with simvastatin. Mod. J. Integr. Tradit. Chin. West Med. 29 (19), 2102–2105. doi:10.3969/j.issn.1008-8849.2020.19.012
Shan, G. H. (2012). Observation of the effect of adjuvant therapeutic dffects of Bailing Capsule on the treatment of early diabetic nephropathy. Chin. J. Clin. Ration. Drug Use. 5 (31), 81–82. doi:10.15887/j.cnki.13-1389/r.2012.31.094
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Shen, L. P., Ying, Z., Yu, L. L., Liu, X., and Jin, Q. Y. (2021). Clinical Effect of Bailing tablet combined with routine treatment on early diabetic nephropathy in elderly. Chin. Tradit. Pat. Med. 43 (10), 2926–2928. doi:10.3969/j.issn.1001-1528.2021.10.063
Shen, S. M. (2012). Observation of the effect of 86 patients with early diabetic nephropathy treated with Bailing Capsule combined with Valsartan. Chin. J. Prim. Med. Pharm. 19 (01), 1008–6706. doi:10.3760/cma.j.issn.1008-6706.2012.01.091
Shen, X. Y., Wu, J., Shao, X. H., Zhu, C. L., Zhou, J., Lu, K., et al. (2018). Effect of Jinshui bao capsule combined with irbesartan tablets on early diabetic nephropathy and its influence on urinary albumin and oxidative stress. Mod. J. Integr. Tradit. Chin. West Med. 27 (21), 2281–2284+2288. doi:10.3969/j.issn.1008-8849.2018.21.001
Shen, Y. L., and Chen, K. (2011). Observation of the effect of Bailing capsule combined with valsartan treating diabetic nephropathy. Chin. J. Pract. Med. 38 (2), 124–125. doi:10.3969/j.issn.1008-8849.2018.21.001
Sheng, X. H., Dong, Y., Cheng, D. S., Wang, N. S., and Guo, Y. P. (2020). Efficacy and safety of Bailing capsules in the treatment of type 2 diabetic nephropathy: a meta-analysis. Ann. Palliat. Med. 9 (6), 3885–3898. doi:10.21037/apm-20-1799
Shi, H. B. (2010). Clinical observation on threatment of early type 2 diabetic nephropathy with perindopril and Jinshuibao. Chin. J. Pract. Med. 37 (7), 46–47. doi:10.3760/cma.j.issn.1674-4756.2010.07.023
Singh, R., Negi, P. S., and Dwivedi, S. K. (2018). “Ophiocordyceps sinensis: the medicinal caterpillar mushroom,” in New age herbals: resource, quality and pharmacognosy. Editors B. Singh, and K. V. Peter (Singapore: Springer), 115–133. doi:10.1007/978-981-10-8291-76
Song, J., Li, Y. H., Yang, X. D., Guo, L., and Hu, Z. (2009). Effect of combined therapy with Bailing capsule and benazepril on urinary albumin excretion rate and C-reactive protein in patients with early diabetic nephropathy. Chin. J. Integr. Tradit. 29 (09), 791–793. doi:10.3321/j.issn:1003-5370.2009.09.006
Song, X. P., and Ning, Y. Y. (2017). Curative effect observation of benazepril combined with Jinshuibao on diabetic nephropathy. Chin. J. Clin. Ration. Drug Use. 10 (11), 65–66. doi:10.15887/j.cnki.13-1389/r.2017.11.044
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898–8. doi:10.1136/bmj.l4898
Su, F., Fan, W. X., Chen, G. M., and Li, Q. F. (2021). Meta-analysis of the clinical efficacy of Bailing capsule combined with liraglutide in the treatment of diabetic nephropathy. Health Guide 5, 23.
Sun, Q. H., Wang, J., Liu, X. L., Zhang, W. J., and Wang, Q. (2012). Clinical effect of enalapril combined with Bailing capsule in treatment of old patients with early diabetic kidney disease. Strait Pharm. J. 24 (10), 115–117. doi:10.3969/j.issn.1006-3765.2012.10.055
Tang, L., Feng, Z., and Chen, X. M. (2013b). Combined cordyceps sinensis and angiotension receptor blocker in treatment of diabetic nephropathy: a meta-analysis. Acad. J. Chin. PLA Med. Sch. 34 (07), 732–736. doi:10.3969/j.issn.2095-5227.2013.07.021
Tang, R., Chen, L. J., Huang, L., Xiang, F., and Hu, Z. B. (2013a). Systematic review on Bailing Capsule combined conventional treatment for treating early diabetic nephropathy. Chin. Pharm. J. 22 (14), 19–23.
Tang, S. C. W., and Yiu, W. H. (2020). Innate immunity in diabetic kidney disease. Nephrol 16 (4), 206–222. doi:10.1038/s41581-019-0234-4
Tang, X. D. (2016). Exploring the efficacy of irbesartan combined with Bailing capsule in the treatment of early diabetic nephropathy. World Latest Med. Inf. 16 (97), 161+169. doi:10.3969/j.issn.1671-3141.2016.97.112
Tang, Y. Z. (2011). Clinical observation of small-dose telmisartan combined with Jinshuibao in the treatment of early diabetic nephropathy with normal blood pressure. Guide CHN Med. 9 (27), 210–211. doi:10.15912/j.cnki.gocm.2011.27.182
Tao, S. C., and Zhou, Q. T. (2009). Observation of the effect of Jinshuibao treating early diabetic nephropathy. J. Clin. Res. 26 (7), 1271–1272. doi:10.3969/j.issn.1671-7171.20
Tian, X. Y. (2019). Observation on the effect of Bailing capsule combined with valsartan in the treatment of patients with early diabetic nephropathy. Pract. Clin. J. Integr. Tradit. Chin. West Med. 19 (07), 18–19. doi:10.15912/j.cnki.gocm.2011.27.182
Tunaru, R. (2002). Hierarchical bayesian models for multiple count data. Austrian J. Stat. 31 (3), 221–229. doi:10.17713/ajs.v31i2&3.484
Tuttle, K. R., Agarwal, R., Alpers, C. E., Bakris, G. L., Brosius, F. C., Kolkhof, P., et al. (2022). Molecular mechanisms and therapeutic targets for diabetic kidney disease. Kidney Intern 102 (2), 248–260. doi:10.1016/j.kint.2022.05.012
Valkenhoef, G., Lu, G., Brock, B., Hillege, H., Ades, A. E., and Welton, N. J. (2012). Automating network meta-analysis. Res. Synth. Methods. 3 (4), 285–299. doi:10.1002/jrsm.1054
Van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods. 7 (1), 80–93. doi:10.1002/jrsm.1167
Wang, A. Y., Zhang, T., Wu, W. P., Tang, W. J., Wang, Y., and Han, X. D. (2020c). Effect of Jinshuibao capsule combined with candesartan tablets in the treatment of early diabetic nephropathy. Contemp. Med. Symp. 8 (12), 58–59. doi:10.3969/j.issn.2095-7629.2020.12.034
Wang, F., and He, W. M. (2021). Progress of traditional Chinese medicine understanding and pharmacological research of Cordyceps sinensis in the treatment of Chronic Kidney Disease. J. Pract. Tradit. Chin. Intern. Med. 35 (11), 135–138. doi:10.13729/j.issn.1671-7813.Z20201196
Wang, H. P. (2007). Clinical observation on treatment of early type 2 diabetic nephropathy with Jinshuibao and fosinopril. Tianjin Pharm. 19 (3), 27–28. doi:10.3969/j.issn.1006-5687.2007.03.013
Wang, J., Lv, J., He, K., Wang, F., Gao, B., Zhao, M. H., et al. (2021). Longitudinal follow-up and outcomes for Chinese patients with stage 1-4 chronic kidney disease. Kidney Dis. 8 (1), 72–81. doi:10.1159/000519190
Wang, K. Y., Li, Y. S., and Dai, Z. Y. (2020b). The efficacy of Bailing capsule combined with valsartan in the treatment of diabetic nephropathy and its effect on UAR and UAER. Mod. Pract. Med. 32 (02), 171–173. doi:10.3969/j.issn.1671-0800.2020.02.014
Wang, L., Xu, X., Zhang, M., Hu, C., Zhang, X., Li, C., et al. (2023). Prevalence of chronic kidney disease in China: results from the Sixth China chronic disease and risk factor surveillance. JAMA Intern. Med. 183 (4), 298–310. doi:10.1001/jamainternmed.2022.6817
Wang, Q. F., and Duan, X. J. (2013). Clinical meta-analysis of artificial cordyceps preparations for intervention in early type 2 diabetic nephropathy. Chin. J. Gerontol. 33 (17), 4145–4147. doi:10.3969/j.issn.1005-9202.2013.17.019
Wang, R. F. (2011a). Clinical observation on the treatment of diabetic nephropathy by benazepril hydrochloride combined with Jinshuibao. J. Huaihai Med. 29 (05), 454–455. doi:10.14126/j.cnki.1008-7044.2011.05.023
Wang, S. Y., Wu, C. F., Zhang, H., Shi, J., and Chen, B. P. (2009). Efficacy of Bailing capsules combined with benazepril in the treatment of early diabetic nephropathy. CHN Pharm. 12 (4), 501–502. doi:10.3969/j.issn.1008-049X.2009.04.042
Wang, W., and Qi, J. W. (2018). Clinical study of Zhiling Capsule combined with telmisartan in treatment of early diabetic nephropathy. Drugs Clin. 33 (6), 1494–1497. doi:10.7501/j.issn.1674-5515.2018.06.045
Wang, W. (2012). Effect of captopril combined with Bailing capsule on microalbuminuria in 950 diabetic nephropathy. J. Huaihai Med. 30 (02), 146–147. doi:10.14126/j.cnki.1008-9517044.2012.02.03
Wang, X., Zhang, P., Wei, F., Qu, Y. Z., and Ma, S. C. (2016). Research progress on quality control methods of fermented Cordyceps powder and its preparation. Asia. Pac. Tradit. Med. 12 (10), 63–66. doi:10.11954/ytctyy.201610025
Wang, X. H., and He, X. R. (2015). Effect of Jinshuibao plus benazepril on proteinuria in patients with diabetic nephropathy. Eval. Anal. Drug-Use Hosp. China 15 (04), 450–452. doi:10.14009/j.issn.1672-2124.2015.04.006
Wang, Y. (2011b). Clinical observation on the treatment of diabetic nephropathy by Bailing capsule. Chin. Med. J. Metall. Ind. 28 (02), 214–215. doi:10.13586/j.cnki.yjyx1984.2011.02.010
Wang, Y. D., Yao, Q. S., and Lin, X. C. (2020a). Curative effect observation of Bailing capsule combined with benazepril on early diabetic nephropathy. Electron. J. Pract. Gynecol. Endocrinol. 7 (31), 170–171. doi:10.16484/j.cnki.issn2095-8803.2020.31.121
Wang, Y. H., and Yuan, G. F. (2008). Clinical observation on treatment of early diabetic nephropathy with Bailing capsules and telmisartan. J. Clin. Res. 25 (5), 927–928. doi:10.3969/j.issn.1671-7171.2008.05.067
Wang, Y. Z., and Zheng, L. Q. (2010). Observation of the effect of Bailing capsule combined with benazepril treating early diabetic nephropathy. J. Emerg. Tradit. Chin. Med. 9 (5), 754–756. doi:10.3969/j.issn.1004-745X.2010.05.018
Wei, S. J. (2010). Observation of the effect of benazepril combined with Jinshuibao capsule treating early type 2 diabetic nephropathy. Heilongjiang Med. Pharm. 33 (1), 50. doi:10.3969/j.issn.1008-0104.2010.01.044
Wen, L., Tian, X., Wang, D., Xia, R., Fei, Y., Huang, N., et al. (2018). Patent of ophiocordyceps sinensis (Jin Shui Bao) for diabetic kidney disease: a systematic review and meta-analysis. Glob. Adv. Health Med. 7, 197. doi:10.1177/2164956118773837
Wu, H. J., Wang, Y., Hu, F. Q., and Cao, J. H. (2019). Clinical effects of Jinshuibao capsules combined with irbesartan on patients with diabetic nephropathy. Chin. Tradit. Pat. Med. 41 (01), 75–78. doi:10.3969/j.issn.1001-1528.2019.01.016
Wu, L., Yan, D. J., Jing, S. J., Yu, J. M., and Geng, J. L. (2014). Observation on the influence of valsartan combined with Bailing capsules on urinary albumin excretion rate in early stage of type 2 diabetic nephropathy. CHN Pharm. 17 (09), 1532–1534. doi:10.3969/j.issn.1008-049X.2014.09.032
Wu, Q. F., and Pan, X. Q. (2016). Study on the efficacy of candesartan cilexetil combined with Jinshuibao in the treatment of patients with early type 2 diabetic nephropathy. Chin. Manipulation Rehabil. Med. 21, 42–44.
Wu, W. T. (2012). Effect analysis of valsartan and cordyceps preparation in the treatment of type 2 diabetic nephropathy. CHN Mod. Med. 19 (27), 71–72. doi:10.3969/j.issn.1674-4721.2012.27.034
Wu, Z. F. (2008). Clinical observation on treatment of diabetic nephropathy with Jinshuibao and irbesartan. J. Pract. Diabetol. 4 (06), 18–19.
Xiao, Y. (2021). Clinical study on the treatment of early diabetic nephropathy with Bailing capsule in combination with irbesartan. J. Pract. Tradit. Chin. Med. 37 (08), 1356–1358.
Xie, M., and Mo, Y. (2021). Clinical study on the treatment of diabetic nephropathy with Jinshuibao capsule in combination with irbesartan tablet. Diabetes New World (6), 96–97.
Xie, P. (2013). Observation on the efficacy of combined Chinese and Western medicine in the treatment of early diabetic nephropathy. Yunnan J. Tradit. Chin. Med. Mater. Med. 34 (06), 82–83. doi:10.16254/j.cnki.53-1120/r.2013.06.043
Xiong, D., Hu, W., and He, Z. Y. (2010). Observation of the effect of irbesartan combined with Jinshuibao capsule treating early diabetic nephropathy. J. Med. Inf. 23 (8), 88.
Xiong, J. (2011). Effects of Bailing capsule on renal tubular marker proteins in diabetic nephropathy. ZheJiang Med. J. 33 (8), 1230–1231. doi:10.3969/j.issn.1006-2785.2011.08.052
Xiu, C. T. (2016). Observation of the effect of candesartan combined with Jinshuibao capsule treating early type 2 diabetic nephropathy. Henan Med. Res. 25 (09), 1658–1659. doi:10.3969/j.issn.1004-437X.2016.09.085
Xu, L. (2015). Clinical observation of the effect of Jinshuibao combined with irbesartan treating diabetic nephropathy. J. Henan Med. Coll. 27 (03), 355–356.
Xu, Y. Y. (2021). Effects of Jinshuibao capsule combined with losartan potassium tablet on renal function and glucose-lipid metabolism in patients with early diabetic nephropathy. Heilongjiang Med. Pharm. 44 (02), 159–160. doi:10.3969/j.issn.1008-0104.2021.02.072
Xu, Z. M. (2018). Curative effect observation of Jinshuibao capsule combined with benazepril on early diabetic nephropathy. Jilin Med. J. 39 (11), 2097–2098. doi:10.3969/j.issn.1004-0412.2018.11.041
Xue, X. H. (2009). Clinical observation on treatment of type 2 diabetic nephropathy with Bailing capsules and irbesartan. Shanxi Med. J. 38 (S1), 45–46. doi:10.3969/j.issn.0253-9926.2009.z1.029
Yan, G. C., Chang, T., Zhao, Y., Yu, M., Mi, J., Wang, G., et al. (2023). The effects of Ophiocordyceps sinensis combined with ACEI/ARB on diabetic kidney disease: a systematic review and meta-analysis. Phytomedicine 108, 154531. doi:10.1016/j.phymed.2022.154531
Yan, Y., and Wang, Z. Q. (2005). Observation of the effect of Bailing capsule treating early diabetic nephropathy. Chin. J. Integr. Tradit. West. Nephrol., 472–474. doi:10.3969/j.issn.1009-587X.2005.01.022
Yang, C. H. (2013). Observation of the effect of Jinshuibao capsule combined with losartan treating early diabetic nephropathy. Mod. J. Integr. Tradit. Chin. West Med. 22 (01), 65–66. doi:10.3969/j.issn.1008-8849.2013.01.036
Yang, G., Peng, H. X., and Liu, D. H. (2016). Observation on efficacy of bailing capsules combined with irbesartan in treatment of diabetic nephropathy. Eval. Anal. Drug-Use Hosp. CHN. 16 (10), 1320–1322. doi:10.14009/j.issn.1672-2124.2016.10.008
Yang, J. Q., and Yang, J. (2011). Observation of the effect of Jinshuibao capsule combined with benazepril hydrochloride treating early diabetic nephropathy. J. Chifeng Univ. 3 (02), 100–101.
Yang, R. (2019). Study on the clinical effect of fermented cordyceps powder in the treatment of patients with diabetic nephropathy. Chin. J. Mod. Drug Appl. 13 (03), 123–124. doi:10.14164/j.cnki.cn11-5581/r.2019.03.075
Yang, T., Zhang, T., Chen, H. Q., Zhang, R., Zhao, L., and Li, Z. D. (2017). The influence of Bailing film combined candesartan on serum Cys-C and β2-MG level with diabetic nephropathy. Prog. Mod. Biomed. 17 (19), 3722–3725. doi:10.13241/j.cnki.pmb.2017.19.031
Ye, J. B., Liu, Z. M., Li, J. J., Lin, H. Z., and Lu, J. (2012). Clinical observation of candesartan cilexetil combined with Bailing capsule for treatment of early diabetic nephropathy. Intern. Med. CHN. 7 (06), 612–613. doi:10.16121/j.cnki.cn45-1347/r.2012.06.037
Yi, J. Z., Ding, G. H., Zhu, L., and Gao, Z. (2009). The effections of irbesartan and Jinshuibao on patients with type 2 diabetic kidney disease. J. Clin. Intern. Med. 26 (11), 741–743. doi:10.3969/j.issn.1001-9057.2009.11.007
Yu, H. T., Shi, H. T., Xiao, L. L., and Dong, C. L. (2013). Observation of the effect of Jinshuibao combined with olmesartan medoxomil treating early type 2 diabetic nephropathy. Contemp. Med. 19 (23), 64–65. doi:10.3969/j.issn.1009-4393.2013.23.046
Yu, J. (2012). Efficacy and care of Ophiocordyceps sinensis mushroom powder combined with losartan potassium in the treatment of early diabetic nephropathy. Strait Pharm. J. 24 (07), 184–185. doi:10.3969/j.issn.1006-3765.2012.07.105
Yu, W. H., Duan, S., and Yu, Z. (2021). The effect of Bailing capsules combined with losartan to treat diabetic glomerulosclerosis and the combination’s effect on blood and urine biochemistry. Am. J. Transl. Res. 13 (6), 6873–6880.
Yu, X. H. (2022). Study on the efficacy of bailing capsule combined with benazepril hydrochloride in the treatment of early diabetic nephropathy. Smart Healthc. 8 (17), 107–110. doi:10.19335/j.cnki.2096-1219.2022.17.027
Yu, X. Y., Yan, D. M., Lan, Q., Fang, J. H., Ding, Z. H., Guan, Y. M., et al. (2022). Efficacy and safety of Jinshuibao capsule in diabetic nephropathy: a systematic review and meta-analysis of randomized controlled trials. Comput. Math. Method M, 9671768. doi:10.1155/2022/9671768
Yuan, Y. H. (2017). The clinical curative effect of telmisartan combined with Bailing capsule for early type ⅱ diabetic nephropathy (with 51 cases). J. Aerosp. Med. 28 (03), 270–272.
Zelniker, T. A., Stephen, D. W., Raz, I., Kyungah, I., Goodrich, E. L., Bonacaet, M. P., et al. (2019). SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 393, 31–39. doi:10.1016/S0140-6736(18)32590-X
Zeng, J. F. (2014). Clinical study of Bailing capsule combined with irbesartan in treatment of diabetic nephropathy. Guangxi Univ. Chin. Med.
Zeng, S. X., and Zhang, L. M. (2010). The effections of irbesartan and Jinshuibao on patients with type 2 diabetic kidney disease. Chin. J. Postgrad. Med. 33 (24), 35–37. doi:10.3760/cma.j.issn.1673-4904.2010.24.017
Zeng, X. M. (2015). Ninety-two cases of diabetic nephropathy treated with Jinshuibao capsule in combination with valsartan. Henan Tradit. Chin. Med. 35 (12), 3202–3204. doi:10.16367/j.issn.1003-5028.2015.12.1379
Zhan, J., Zhang, W. N., Feng, J. T., Luo, M. C., and Zeng, Q. D. (2016). Study on the effect of Bailing capsule combined with enalapril on the CRP, homa IR and prognosis of patients with early type 2 diabetic nephropathy. Clin. Med. Eng. 23 (11), 1469–1470. doi:10.3969/j.issn.1674-4659.2016.11.1469
Zhang, C., and Zuo, Z. H. (2014). Effect of Jinshuibao capsule combined with losartan potassiumon treatment of patients with early diabetic nephropathy. J. Clin. Med. Pract. 5, 84–86.
Zhang, L., Long, J., Jiang, W., Shi, Y., He, X., Zhou, Z., et al. (2016). Trends in chronic kidney disease in China. N. Engl. J. Med. 375, 905–906. doi:10.1056/NEJMc1602469
Zhang, L. H. (2016). Clinical effect of Jinshuibao combined with irbesartan in the treatment of patients with diabetic nephropathy. Med. Equip. 29 (01), 83–84. doi:10.3969/j.issn.1002-2376.2016.01.055
Zhang, L. Q., Yan, M., Xie, L. Y., and Zhou, L. F. (2015). Current research status of cordyceps sinensis in the treatment of chronic obstructive pulmonary disease. J. Emerg. Tradit. Chin. Med. 24 (12), 2157–2159+2164. doi:10.3969/j.issn.1004-745X.2015.12.029
Zhang, W., Zheng, W. L., and Wang, D. (2017). Effect of paecilomyces hepiall chen on serum inflammatory factors,oxidative stress and renal function in patients with diabetic nephropathy. J. Hainan Med. Univ. 23 (19), 2627–2630. doi:10.13210/j.cnki.jhmu.20170926.008
Zhang, Y. (2018). Effects of bailing capsual combing with angiotensin receptor blocker in the treatment of diabetic kidney disease:A meta-analysis. Hubei Univ. Chin. Med.
Zhang, Y. (2021). Clinical effect of Jinshuibao capsule combined with irbesartan tablet in the treatment of patients with early diabetic nephropathy. Med. Equip. 34 (10), 105–106. doi:10.3969/j.issn.1002-2376.2021.10.057
Zhang, Y., Shi, H. T., and Dai, H. S. (2012a). The observation on effect of urine protein and lipid metabolism to diabetic nephropathy with Jinshuibao capsules. CHN Mod. Dr. 50 (36), 64–65.
Zhang, Y. K. (2011). The effections of irbesartan and Jinshuibao on patients with type 2 diabetic kidney disease. Jilin Med. J. 32 (12), 342. doi:10.3969/j.issn.1004-0412.2011.12.040
Zhang, Y. M., Yang, L. P., and Shen, B. (2012). Jinshuibao capsule for diabetic nephropathy: a systematic review. Mod. J. Integr. Tradit. Chin. West. Med. 21 (23), 2509–2512.
Zhang, Y. Y., and Zhang, B. (2019). Clinical observation of Jinshuibao capsule combined with irbesartan tablet treating diabetic nephropathy. J. Pract. Tradit. Chin. Med. 35 (11), 1359–1360.
Zhang, Z. F., and Lu, J. (2009). Observation of the effect of Bailing capsule treating early diabetic nephropathy. Shanxi Med. J. 38 (08), 730–731.
Zhang, Z. Y., Li, Y. W., and Miao, R. (2014). Clinical observation of candesartan cilexetil combined with Jinshuibao for treatment of type 2 diabetic nephropathy. J. Guangdong Pharm. Univ. 30 (02), 241–244. doi:10.3969/j.issn.1006-8783.2014.02.028
Zhao, J. X. (2019). Study on quality control methods and batch-to-batch consistency of solid preparations of fermented Cordyceps sinensis powder. Jiangxi Univ. Tradit. Chin. Med. doi:10.27180/d.cnki.gjxzc.2019.000047
Zheng, J. X. (2011). Clinical observation of the effect of Bailing capsule combined with irbesartan treating type 2 diabetic nephropathy. CHN Mod. Med. 18 (24), 66–67. doi:10.3969/j.issn.1674-4721.2011.24.037
Zheng, Z. L., Huang, C. H., Mei, C. Y., and Han, R. C. (2011). Current advance on the research of Cordyceps militaris. J. Environ. Entomol. 33, 225–233. doi:10.3969/j.issn.1674-0858.201
Zhou, P. (2012). Clinical observation of losartan potassium with Jinshuibao capsule in patients with early type 2 diabetic nephropathy. Xinjiang Med. J. 42 (09), 21–23.
Zhou, X., Chen, G. D., and Zhao, D. Y. (2013). Observation of the effect of benazepril combined with Jinshuibao treating diabetic nephropathy. Chin. J. Integr. Tradit. West Med. Intensive Crit. Care. 3, 182–183. doi:10.3969/j.issn.1008-9691.2013.03.019
Zhou, X. M., Gu, S., Xia, Y., Shi, M. Y., and He, W. M. (2021). Fermented cordyceps powder combined with ACEI/ARB in treatment of diabetic kidney disease:A systematic review. Chin. J. Exp. Tradit. Med. Formul. 27 (18), 169–175. doi:10.13422/j.cnki.syfjx.20211012
Zhou, X. Y., Deng, E. P., and Chen, Y. J. (2015). Clinical observation of the effect of Zhiling capsule combined with valsartan dispersible tablets treating early diabetic nephropathy. J. North Pharm. 12 (08), 50–51.
Zhu, J., and Qiu, C. C. (2015). Clinical observation of Jinshuibao combined with valsartan treating early diabetic nephropathy. Res. Integr. Tradit. Chin. West Med. 7 (06), 303–304. doi:10.3969/j.issn.1674-4616.2015.06.010
Keywords: diabetic kidney disease, Ophiocordyceps sinensis preparations, renin–angiotensin system inhibitor, umbrella review, network meta-analysis
Citation: Xue X, Jin X-Y, Ye X-L, Li K-Y, Li J-X, Liu X-H, Bai J, Liu Q, Zhang B-R, Zou X-R, Yuan J, Lu C-L, Zhao F-F, Liu J-P and Wang X-Q (2024) Ophiocordyceps sinensis preparations combined with the renin–angiotensin system inhibitor for diabetic kidney disease treatment: an umbrella review of systematic reviews and network meta-analysis. Front. Pharmacol. 15:1360633. doi: 10.3389/fphar.2024.1360633
Received: 23 December 2023; Accepted: 18 March 2024;
Published: 22 April 2024.
Edited by:
Gopalakrishnan Natarajan, Madras Medical College, IndiaReviewed by:
Varadharajan Jayaprakash, SRM Medical College Hospital and Research Centre, IndiaYasser Sami Amer, King Saud University Medical City, Saudi Arabia
Copyright © 2024 Xue, Jin, Ye, Li, Li, Liu, Bai, Liu, Zhang, Zou, Yuan, Lu, Zhao, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ping Liu, TGl1anBAYnVjbS5lZHUuY24=; Xiao-Qin Wang, d2FuZ3hpYW9xaW5AaGJodGNtLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Xue Xue
Xue Xue Xin-Yan Jin2†
Xin-Yan Jin2† Jia-Xuan Li
Jia-Xuan Li Xue-Han Liu
Xue-Han Liu Bing-Rui Zhang
Bing-Rui Zhang Fang-Fang Zhao
Fang-Fang Zhao Jian-Ping Liu
Jian-Ping Liu