
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 05 February 2024
Sec. Pharmacology of Anti-Cancer Drugs
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1356708
Colorectal cancer is the third most common type of cancer worldwide and has become one of the major human disease burdens. In clinical practice, the treatment of colorectal cancer has been closely related to the use of irinotecan. Irinotecan combines with many other anticancer drugs and has a broader range of drug combinations. Combination therapy is one of the most important means of improving anti-tumor efficacy and overcoming drug resistance. Reasonable combination therapy can lead to better patient treatment options, and inappropriate combination therapy will increase patient risk. For the colorectal therapeutic field, the significance of combination therapy is to improve the efficacy, reduce the adverse effects, and improve the ease of treatment. Therefore, we explored the clinical advantages of its combination therapy based on mechanism or metabolism and reviewed the rationale basis and its limitations in conducting exploratory clinical trials on irinotecan combination therapy, including the results of clinical trials on the combination potentiation of cytotoxic drugs, targeted agents, and herbal medicine. We hope that these can evoke more efforts to conduct irinotecan in the laboratory for further studies and evaluations, as well as the possibility of more in-depth development in future clinical trials.
In terms of incidence, colorectal cancer (CRC) is the second most frequent cause of cancer-related fatalities (Sung et al., 2021). Because of its high incidence and mortality worldwide, CRC has emerged as a global public health issue (Kumar et al., 2021). With the prevalence of CRC getting younger, it is expected that the economic burden will further increase, bringing great challenges to global public health (Siegel et al., 2023).
Currently, the treatment of CRC mainly includes chemotherapy, targeted therapy, radiation therapy, immunotherapy and palliative care. Chemotherapy is one of the vital means for the treatment of CRC. Chemotherapy can cooperate with other treatment methods and improve the effect of comprehensive treatment. Irinotecan is a critical component of the therapy of CRC and is typically used with other drugs to ease cancer-related symptoms and increase patients’ survival times. Irinotecan is a comprehensive anticancer therapy when combined with other drugs, such as oxaliplatin, capecitabine, 5-fluorouracil (5-FU) and leucovorin and other drugs composed of traditional chemotherapy. Molecularly targeted agents are included in the standard treatment of traditional chemotherapy, usually choosing between anti-epidermal growth factor receptor (EGFR) (cetuximab, panitumumab) and anti-vascular endothelial growth factor (VEGF) (bevacizumab) monoclonal antibodies. Although irinotecan-based combination chemotherapy improves the treatment and the survival in CRC patients, adverse events such as delayed diarrhea and neutropenia caused by irinotecan greatly limit clinical application. Therefore, it is particularly important to find treatment options with better specificity for CRC (Islam et al., 2022). At present, many studies have demonstrated that herbs can serve as an adjunctive in chemotherapy regimens related to irinotecan (Li et al., 2020; Sun et al., 2021). Herbs have the advantages of low toxicity, safety, effectiveness, and multi-targets (Qiu et al., 2018). Most importantly, herbs may achieve favorable therapeutic outcomes by alleviating the serious side effects caused by irinotecan, improving the quality of life (QoL) of patients and increasing the effectiveness of chemotherapy (Yue et al., 2021; Wang et al., 2021; Chen et al., 2022). However, their possible toxicity and side effects need to be evaluated over time. It is without a doubt that irinotecan is the main anticancer drug, no matter what type of drug it is combined with.
Irinotecan, like other camptothecin derivatives, presents dynamic equilibrium in aqueous solution in two forms: one in lactone form and the other in carboxyl form, and the equilibrium constant of this reaction is pH dependent (Figure 1). In an acidic environment, the preference is for the lactone form. It is generally believed that lactone has anti-tumor activity, while carboxylate has no inhibitory effect on tumors (Thomas and Pommier, 2019). The distribution of irinotecan in vivo is thought to be mediated by various enzyme systems. In phase Ⅰ metabolism, irinotecan passes through the peripheral bloodstream to the liver, where it is metabolized in vivo to the active metabolite 7-ethyl-10-hydroxy-camptothecin (SN-38) by the catalytic action of carboxylesterase2 (CES2) (Mathijssen et al., 2001). Irinotecan is oxidized by the cytochrome P450 isoenzyme 3A4 (CYP3A4), resulting in the production of relatively inactive metabolites 7-ethyl-10-[4-N-(5-aminopentanoic acid)-1-piperidino] carbonyloxy camptothecin (APC) and a smaller amount of metabolites 7-ethyl-10-(4-amino-1-piperidino) carbonyloxy camptothecin (NPC) (Alimonti et al., 2004). NPC and APC can be further converted to SN-38 by CES2. The binding reaction of phase Ⅱ is mainly the glucuronidation process of active metabolite SN-38. SN-38 can be rapidly metabolized to inactive SN-38 glucuronide (SN-38G) by liver uridine diphosphate-glucuronosyltransferase 1A1 (UGT1A1) (Yue et al., 2021). The elimination of irinotecan is mainly liver metabolism and bile secretion. After SN-38G is secreted into the intestinal tract through bile, it is transformed into SN-38 under the action of β-glucuronidase (BGUS) produced by the intestinal tract (Figure 2), which is reabsorbed into the blood, causing dose-limited diarrhea (de Jong et al., 2006).
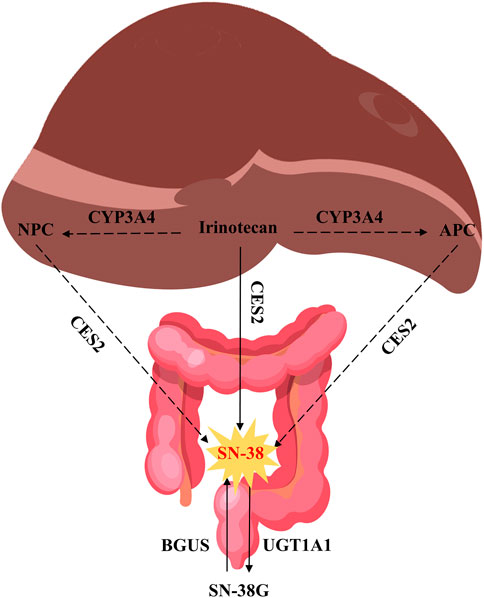
FIGURE 2. Metabolism of irinotecan. Source of liver and intestine illustrations: https://scidraw.io/.
CRC is a disease that occurs in the colon or rectum and is caused by the abnormal proliferation of colonic glandular epithelial cells. The progression of CRC is a dynamic process based on the depth of tumor infiltration, the degree of lymph node invasion, and the presence of metastases in other organs (Hossain et al., 2022). It can be divided into five disease stages: benign polyp (stage 0), invades the muscularis propria (stage Ⅰ), invades tissue in the serosa (stage Ⅱ), invades visceral peritoneum (stage Ⅲ), metastasis to other organs (stage Ⅳ) (Mahmod et al., 2022). The liver is the most common site of metastasis, followed by the lung and bone. A key factor in the treatment of patients with mCRC is to maximize the likelihood of resection. Irinotecan-based chemotherapy can shrink tumors to the point of complete resection. Triple therapy with irinotecan (FOLFOXIRI) is more toxic compared to doublet therapy (FOLFIRI), but has advantages in terms of resectability of liver metastases, and XELIRI can be used as an alternative to FOLFIRI (Sobrero and Bennicelli, 2010).
Irinotecan is the most widely studied first- and second-line anti-CRC drug. It is both a derivative of camptothecin and a prodrug of SN-38. Irinotecan is a Topoisomerase Ⅰ (Topo Ⅰ) inhibitor and does not interact directly with DNA. Irinotecan and its active metabolite SN-38 combine with the Topo Ⅰ-DNA complex to form the Topo Ⅰ-Irinotecan/SN-38-DNA ternary complex by reversibly breaking DNA single strands (Xu and Villalona-Calero, 2002). When the ternary complex collides with the progressive replication fork, it will form double-stranded DNA unwinding (Figure 3), resulting in irreversible stagnation of the replication fork and cell death, and play a highly effective anti-tumor effect by interfering with the process of DNA replication in tumor cells (Bailly, 2019; Kciuk et al., 2020).
FOLFIRI is one of the most common standard chemotherapy regimens for the therapy of CRC and consists of three drugs: irinotecan, 5-FU, and leucovorin. 5-FU is a pyrimidine antagonist. The mechanism of 5-FU activation begins with the conversion of 5-FU by the orotate phosphoribosyltransferase (OPRT) and uridine phosphorylase (UP) to fluorouridine monophosphate (FUMP) and fluorouridine (FUR), where FUR is converted indirectly via uridine kinase (UK) to FUMP, which is phosphorylated to fluorouridine diphosphate (FUDP), and then undergoes another phosphorylation process to the active metabolite fluorouridine triphosphate (FUTP) or to fluorodeoxyuridine diphosphate (FdUDP) via ribonucleotide reductase (RNR) (Sethy and Kundu, 2021). FUTP is a fluorinated analog of RNA nucleotides that can be incorrectly doped into the RNA of tumor cells resulting in RNA damage. FdUDP can be phosphorylated or dephosphorylated to generate the active metabolites fluorodeoxyuridine triphosphate (FdUTP) and fluorodeoxyuridine monophosphate (FdUMP). FdUTP is incorrectly doped into the DNA of tumor cells leading to DNA damage (Azwar et al., 2021). Another mechanism of activation is the conversion of 5-FU to fluorodeoxyuridine (FUDR) by thymidine phosphorylase (TP) and then phosphorylation to FdUMP by thymidine kinase (TK) (Figure 4). In conclusion the antitumor activity of 5-FU is the incorporation of its active metabolites into RNA and DNA to interfere with nucleoside metabolism (Vodenkova et al., 2020), and the active metabolite FdUMP irreversibly inhibits thymidylate synthase (TS), leading to DNA damage and tumor cell death. It is often used extensively in the therapy of CRC (Saltz et al., 1996).
Typically, the FOLFIRI regimen is usually administered every 2 weeks for multiple consecutive cycles. In clinical practice, it has been found that patients are prone to congenital or acquired resistance to 5-FU, which means that the efficacy of 5-FU monotherapy is limited, so the effectiveness of clinical treatment is hindered (Longley et al., 2003). 5-FU is typically administered with leucovorin, which increases the affinity of 5-FU for TS and further enhancing the efficacy of 5-FU (Benson and Goldberg, 2003). A meta-analysis also revealed the same results, with the administration of 5-FU and leucovorin increasing response rate (RR) and overall survival (OS) in comparison with 5-FU alone (Thirion et al., 2004). When irinotecan was added to the regimen, it was preferred over 5-FU and leucovorin alone in respect of progression-free survival (PFS), OS and RR, and effectively delayed the progression of cancer (Saltz et al., 2000). Irinotecan has no cross-resistance to 5-FU/leucovorin therapy, which is an essential for its use in combination therapy for CRC.
Douillard et al. (2000) included 387 patients in the study of advanced CRC and randomly divided them into two groups, one of which was applied irinotecan in combination with 5-FU and leucovorin, and the other group applied only 5-FU and leucovorin. According to the findings, the irinotecan had a higher RR (49% vs. 31%, p < 0.001), a longer PFS (6.7 vs. 4.4 months, p < 0.001), and a longer OS (17.4 vs. 14.1 months, p = 0.031) than the non-irinotecan group. While diarrhea and neutropenia in the irinotecan group were more common and severe, several toxic events in grade 3 and 4 were also noticeably more prevalent. Comparison with irinotecan alone, FOLFIRI can reduce the rate of adverse events such as alopecia and diarrhea, and did not affect the effect of clinical treatment. There were no significant differences in terms of change in overall QoL, RR, PFS, or OS between the two groups (Clarke et al., 2011). The key clinical study V308 proved that irinotecan was the first option for first-line treatment of advanced metastatic colorectal cancer (mCRC), which compared the effectiveness of sequential usage of FOLFIRI and FOLFOX6 (oxaliplatin, 5-FU/leucovorin) for the treatment of advanced mCRC. The results showed that FOLFIRI regimen as the first-line treatment option for advanced mCRC had lower overall adverse reactions and was more tolerable (Tournigand et al., 2004).
Subgroup analysis indicated that elderly patients treated with FOLFOXIRI (irinotecan, oxaliplatin, 5-FU/leucovorin) had a higher incidence of severe diarrhea, which did not appear to have a significant benefit in elderly mCRC patients compared with FOLFIRI (Vamvakas et al., 2010). Therefore, FOLFIRI two-drug regimen for first-line chemotherapy is a rational choice in elderly mCRC patients. It is obvious that irinotecan in combination with 5-FU/leucovorin is an advantageous treatment, especially in elderly patients.
In Japan, the cost of chemotherapy with FOLFIRI was lower than that of oxaliplatin-based chemotherapy regimens (FOLFOX, oxaliplatin plus 5-FU/leucovorin), and the use of lower-cost chemotherapy regimens as first-line chemotherapy could reduce the overall cost of the entire chemotherapy course (Yajima et al., 2015). Due to the differences in toxicity between the two regimens, the FOLFIRI regimen is superior to the FOLFOX regimen from the standpoint of long-term health outcomes (Qingwei et al., 2020).
The FOLFOXIRI regimen, which combines irinotecan, oxaliplatin, and 5-FU/leucovorin, is a high-intensity chemotherapy regimen. The mechanism of action of oxaliplatin, a cytotoxic drug in this regimen, is not yet fully understood, but according to existing studies, it exerts its cytotoxic effects mainly through DNA damage (O’Dowd et al., 2023). The main target of oxaliplatin is DNA. When oxaliplatin enters the cell, the platinum atoms can combine with the DNA of the tumor cell to form a Pt-DNA adduct, which affects the transcription and replication functions of DNA and ultimately leads to the death of the tumor cell (Szefler and Czeleń, 2023) (Figure 5). The oxaliplatin antitumor process can be divided into four phases, including cellular uptake of the drug, hydration or activation of the drug, DNA platinization, and intracellular processing. The FOLFOXIRI regimen is mainly characterized by good efficacy in terms of objective response rate, PFS, and OS, with manageable and well-tolerated side effects.
At present, FOLFOXIRI regimen has emerged as one of the chemotherapeutic regimens recommended by the guidelines of the Chinese Society of Clinical Oncology (CSCO), European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) for the treatment of advanced CRC. Initially, there was clinical evidence that concomitant therapy with irinotecan and oxaliplatin for CRC was feasible and effective (Scheithauer et al., 1999). The safety and efficacy of FOLFOXIRI as a first-line therapeutic treatment for mCRC was initially reported in 2002 (Falcone et al., 2002). In an attempt to compare the advantages of the three-agent chemotherapeutic regimen FOLFOXIRI versus the two-agent chemotherapeutic regimen FOLFIRI in the first-line treatment of mCRC, the Gruppo Oncologico Nord Ovest (GONO) carried out a phase III study. The FOLFOXIRI group had a significantly beneficial effect in RR (66% vs. 41%, p = 0.0002), OS (22.6 vs. 16.7 months, p = 0.032) and median PFS (9.8 vs. 6.9 months, p = 0.0006), and adverse events like diarrhea, peripheral nerve reactions and neutropenia were also increased significantly in the FOLFOXIRI group. The occurrence of diarrhea was also significantly increased, and the risk of toxicity was increased with the use of FOLFOXIRI in elderly patients, so the FOLFOXIRI regimen should be used with caution in elderly patients with poor physical condition (Falcone et al., 2007). The HORG study had a total of 283 patients were enrolled, which indicated a significantly more frequent incidence of alopecia, diarrhea, and neurotoxicity in the FOLFOXIRI group in comparison with the FOLFIRI group. The treatment endpoint OS (21.5 vs. 19.5 months, p = 0.337) improved in the FOLFOXIRI group as compared with the FOLFIRI group, but the difference was not statistically significant (Souglakos et al., 2006).
Due to poorer tolerability in Asian individuals compared with European populations, the clinical application of the conventional FOLFOIRI regimen in CRC is greatly restricted in China and even throughout the entire Asian region. In China, the dosage of irinotecan in the FOLFOXIRI regimen was adjusted downward from 180 mg/m2 to 150–165 mg/m2, which is more suitable for the dosage intensity of the Chinese population (Cai et al., 2018). However, the assessment of the efficacy and adverse effects of the modified regimen needs to be confirmed by further clinical studies, due to the limited number of reported cases in this study.
The FOLFOXIRI regimen showed promising outcomes with a PFS of 13.37 ± 9 months, an overall response rate of 79.4%, a significant decrease in the risk of early disease progression, and side effects within the acceptable range for mCRC first-line therapy (Huy et al., 2019). More importantly, the FOLFOXIRI regimen increased the rate of radical surgery for initially unresectable mCRC, and the long-term survival of radically resected patients was particularly notable, with a benefit of 42% and 33% in 5- and 8-year survival rates, respectively (Masi et al., 2009). Compared to the FOLFIRI regimen, FOLFOXIRI provided clinically significant improvements in long-term outcomes, with an absolute benefit in 5-year survival of 7% and improvements in both PFS and OS at long-term follow-up (Masi et al., 2011). Compared to FOLFIRI, the FOLFOXIRI regimen increased drug costs due to the addition of oxaliplatin.
The regimen of irinotecan in combination with capecitabine (XELIRI) requires only 2–3 h of infusion every 3 weeks. A phase II single-arm study with mCRC patients showed favorable efficacy and safety (Garcia-Alfonso et al., 2009). Capecitabine is an orally available fluorouracil prodrug with an oral bioavailability of nearly 100%. It has superior safety and convenience and has significant anti-tumor activity (García-Alfonso et al., 2021). Capecitabine is relatively non-cytotoxic in vitro and, after oral administration, is readily absorbed through the intestinal mucosa, where it is first catalytically metabolized to 5′-deoxy-5-fluorocytidine (5′-DFCR) in the liver by CES (Walko and Lindley, 2005). Cytidine deaminase (CD) is an enzyme that is found widely and in high concentrations in most tissues, including the liver and tumors (Alzahrani et al., 2023). 5′-DFCR is catalytically converted to 5′-deoxy-fluorouracil (5′-DFUR) by CD in the liver and tumor cells, and finally converted to 5-FU by TP to exert antitumor effects (de With et al., 2023) (Figure 6). The last metabolic step is thought to occur preferentially in tumor tissues because TP activity is very low in normal tissues, reducing the exposure of 5-FU in normal tissues, whereas the concentration of this enzyme is markedly elevated in tumor tissues, and thus capecitabine is effective in enhancing antitumor effects and reducing systemic toxicity (Pentheroudakis and Twelves, 2002).
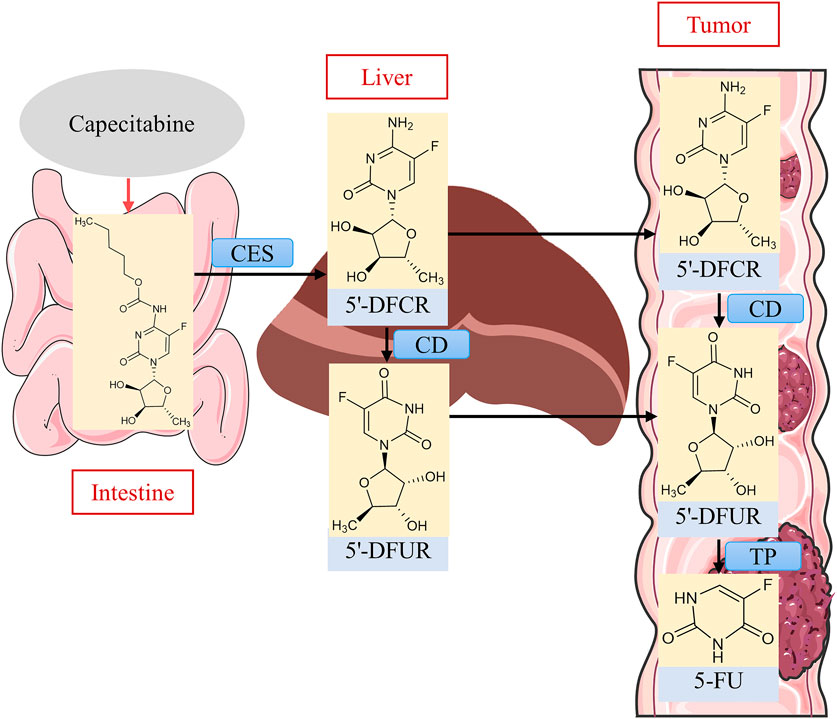
FIGURE 6. Metabolic activation process of capecitabine. Source of liver illustration: https://scidraw.io/.
When neoadjuvant preoperative radiotherapy of irinotecan in combination with capecitabine was guided by the UGT1A1 gene, increasing the dose of irinotecan can enhance the pathologic complete response rate from 15% to 30%, which may become an improved strategy for locally advanced CRC patients to achieve better tumor regression, significantly improve the clinical effective rate, and the toxicity and side effects are within the acceptable range (Zhu et al., 2020). A meta-analysis that compared the efficacy of the XELIRI and FOLFIRI regimens for the first-line therapy of mCRC indicated no apparent distinction in terms of OS, overall response rate or PFS, and the safety profiles of the two regimens were comparable (Guo et al., 2014). The combined treatment of irinotecan and capecitabine is logical, which can not only maintain the efficacy of the combination regimen, but also take advantage of the convenience of capecitabine in the treatment process. From a long-term safety perspective, the XELIRI regimen increased gastrointestinal toxicity compared to FOLFIRI (Montagnani et al., 2010).
FOLFIRI regimen requires 46 h of infusion every 2 weeks, and patients need to undergo central venous intubation before treatment, which is inconvenient to use. Based on the limitations of the FOLFIRI regimen, the toxicity caused by the standard dose of XELIRI and the difference in tolerance among different populations (Haller et al., 2006; Fuchs et al., 2007). The AXEPT study, the first large multicenter randomized controlled phase III study of the modified XELIRI (mXELIRI, capecitabine plus irinotecan) regimen compared to the FOLFIRI regimen, supported the use of the mXELIRI regimen as an alternative second-line treatment option for patients with mCRC (Xu et al., 2018). A cost-benefit analysis showed that compared with FOLFIRI, mXELIRI regimen is a cost-effective second-line treatment for mCRC in China (Wu et al., 2020). According to the AXEPT study, OS with mXELIRI in combination with or without bevacizumab (16.8 vs. 15.4 months, p < 0.0001) was no less than with the standard FOLFIRI regimen. Regarding safety, the incidence of neutropenia, the most prevalent grade 3–4 adverse event, was considerably lower in the mXELIRI group in 17% (52 of 310 patients) than in the FOLFIRI group in 43% (133 of 310 patients). Grade 3–4 diarrhea in the mXELIRI arm than the incidence in most previous full-dose XELIRI trials (Koopman et al., 2007; Köhne et al., 2008; Souglakos et al., 2012). The incidence of serious adverse incidents was 15% in the mXELIRI group, whereas it was 20% in the FOLFIRI group. There was no discernible difference in PFS between the two groups. According to the overall findings, modified XELIRI may be an efficacious, tolerable, and more convenient therapeutic option replace FOLFIRI as a standard second-line backbone regimen for Asian mCRC patients. Considering the results of this study, the modified XELIRI regimen will be expected to replace the FOLFIRI regimen as the new standard chemotherapy regimen for advanced CRC patients worldwide, especially in Asia, changing the current clinical practice.
The US Food and Drug (FDA) authorized bevacizumab as the first targeted drug in 2004 for CRC treatment, signaling the start of a new series of anti-cancer therapies (Heinemann and Hoff, 2010). Bevacizumab is still the most widely used and characterized anti-vascular endothelial growth factor monoclonal antibody. VEGF is the most important angiogenic player, and VEGF stimulates endothelial cell proliferation and survival and increases vascular permeability, thereby supporting the metabolic demands of tumor growth (Apte et al., 2019). Among them, Vascular endothelial growth factor A (VEGF-A) is a major mediator of tumor angiogenesis and induces angiogenesis through direct action on endothelial cells. VEGF-A activates VEGF signaling in endothelial cells by binding to VEGF rceptor-1 (VEGFR-1) and VEGF receptor-2 (VEGFR-2) (Méndez-Valdés et al., 2023). VEGFR-2 is mainly involved in tumor pathological processes such as tumor angiogenesis and is the most important inducer, and VEGFR-1 plays an important role mainly in tumor growth and progressive inflammatory processes (Melincovici et al., 2018). Bevacizumab exerts anti-tumor effects by binding to VEGF-A, preventing VEGF-A from interacting with VEGFR-1 and VEGFR-2, blocking the signaling pathway of angiogenesis, and inhibiting the formation of tumor neovasculature, thus inhibiting the growth of tumor cells (Gerber and Ferrara, 2005) (Figure 7).
Bevacizumab is commonly administered as an addition to standard chemotherapy regimens to provide an effective therapeutic option for a spectrum of CRC patients with a poor prognosis. For induction and maintenance therapy, the combination of bevacizumab with chemotherapeutic drugs is advised. The inclusion of bevacizumab to irinotecan-based backbone chemotherapy regimens is a standard choice for first-line treatment for mCRC patients (Garcia et al., 2020). However, adverse events of proteinuria and thromboembolism occurred in mCRC patients chronically treated with bevacizumab when the dose of the drug exceeded the threshold dose (Fukuda et al., 2023). Adding bevacizumab to first- and second-line therapy increased costs by $60,000 and $40,000, respectively, and prolonged median OS by 6 weeks for both, but bevacizumab was more cost-effective in second-line therapy due to the shorter duration of therapy (Goldstein et al., 2015, 2017).
With the addition of bevacizumab to FOLFIRI significantly improved RR (44.8% vs. 34.8%, p = 0.004), as well as prolonged median PFS (10.6 vs. 6.2 months, p < 0.001) and OS (20.3 vs. 15.6 months, p = 0.00003) in AVF2107g, the first phase 3 study to evaluate the effect of bevacizumab in the treatment of first-line mCRC patients (Hurwitz et al., 2004). Bevacizumab and two irinotecan-based backbone chemotherapy regimens FOLIRI and XELIRI were useful first-line therapies for the treatment of mCRC patients, with similar safety profiles and expected endpoints, according to a randomized, non-controlled study (Ducreux et al., 2013). A meta-analysis published in 2020 reviewed 11 studies including 5632 patients to compare the effectiveness and safety of bevacizumab with irinotecan-based or oxaliplatin-based dual-backbone chemotherapy as first-line treatment options for mCRC. The findings showed that bevacizumab and irinotecan-based chemotherapy had more advantages in improving PFS. The study recommended that bevacizumab plus irinotecan-based backbone chemotherapy is the first-line therapeutic choice for prolonging PFS (Ren et al., 2021).
The TRIBE-2 study demonstrated that FOLFOXIRI plus bevacizumab improved OS (29.8 vs. 25.8 months, p = 0.03) in patients with mCRC in comparison with FOLFIRI plus bevacizumab (Cremolini et al., 2015). A randomized controlled trials found that FOLFOXIRI combined with bevacizumab led to an improved prognosis in mCRC patients, but the frequency of adverse events was also relatively increased (Loupakis et al., 2014). These provide compelling support for the first-line treatment of irinotecan-based three-drug regimen FOLFOXIRI combined with bevacizumab. For patients with mCRC who are generally in good condition, the strategy of FOLFOXIRI palliative care, sequential maintenance therapy, and treatment progression followed by FOLFOXIRI plus bevacizumab can be used (Cremolini et al., 2020). At present, the TRIBE-C (NCT04230187) study is in progress to assess whether the Chinese modified version of FOLFOXIRI combined with bevacizumab for advanced CRC can further improve the efficacy, safety and feasibility compared with the traditional regimen combined with bevacizumab.
Panitumumab, an epidermal growth factor receptor (EGFR) antagonist, is indicated for the treatment of EGFR-expressing mCRC (Hoy and Wagstaff, 2006). EGFR is highly or aberrantly expressed in a variety of cancers, stimulates proliferation, angiogenesis, and metastasis, and protects tumor cells from apoptosis (Uribe et al., 2021). Panitumumab is the first fully human monoclonal antibody approved for the treatment of CRC, so it is less likely to induce an immunogenic response (Ohishi et al., 2023). Panitumumab is an IgG2 monoclonal antibody that has a stronger affinity for EGFR than cetuximab, binds more readily to EGFR, and effectively blocks the binding of epidermal growth factor (EGF) or transforming growth factor-alpha (TGF-α) ligands to EGFR (Rastin et al., 2024) (Figure 8). Panitumumab acts as a functional antagonist of EGF and TGF-α ligands, leading to the internalization and degradation of antibody-receptor complexes, thereby inhibiting the EGFR-mediated signaling pathway, and the signaling blockage leads to the inhibition of tumor cell division, which inhibits tumor growth, metastasis, and angiogenesis and promotes apoptosis of tumor cells (Adebayo et al., 2023).
Panitumumab may also play a direct or indirect antitumor role by enhancing the cytotoxic effects of other drugs and may be used as monotherapy for irinotecan-resistant tumors or in combination with irinotecan for the treatment of mCRC. Panitumumab plus irinotecan appeared to be no less effective than cetuximab combined with irinotecan in the therapy of KRAS wild-type exon 2 mCRC patients (Daisuke et al., 2020). Clinical studies have indicated that cetuximab decreased efficacy in patients previously treated with bevacizumab (Sato et al., 2015). In contrast, panitumumab was more effective than cetuximab individuals previously treated with bevacizumab (Price et al., 2016). Panitumumab is used in combination regimens and has been studied for potential interactions with chemotherapeutic agents. The pharmacokinetic profile of irinotecan co-administered with or without panitumumab is almost identical. Panitumumab does not effect on the pharmacokinetics of irinotecan or there are synergistic effects (Yang et al., 2013).
The PICCOLO trial included 460 advanced KRAS wild-type CRC patients that had not been treated previously with EGFR-targeted agents; the PFS in the panitumumab plus irinotecan group was markedly superior to that of the irinotecan group alone, and there was no differentiation in the OS between the two groups, with the combination of irinotecan plus panitumumab not improving the OS in patients with KRAS wild-type tumors (Seymour et al., 2013). In treatment-refractory mCRC, treatment is palliative rather than curative, and the main goal is to maximize patient survival and maintain QoL. In KRAS wild-type patients, the irinotecan-based chemotherapy regimen FOLFIRI combined with panitumumab achieved a median PFS of 5.9 months, which was a significant increase versus 3.9 months in the FOLFIRI group (p = 0.004). With the administration of panitumumab, the RR increased from 10% to 35% although the OS did not dramatically increase (Peeters et al., 2010). The combination of FOLFIR with panitumumab was seen to be effective. In the presence of irinotecan dose reduction, panitumumab in combination with FOLFOXIRI reduced irinotecan-induced diarrhea (Fornaro et al., 2013). In addition, the JACCRO CC-14 study supported that the FOLFOXIRI plus panitumumab regimen was well tolerated in RAS wild-type mCRC patients with irinotecan dose reduction (Satake et al., 2018). With an objective response rate of 87.3% (87.3% vs. 60.06%, p = 0.004) and a secondary resection rate of metastases with panitumumab of33.3% (33.3% vs. 12.1%, p = 0.02), the combination of panitumumab with the modified FOLFOXIRI regimen improved the objective response rate and secondary resection rate of metastases in RAS wild-type mCRC patients (Modest et al., 2019). It is clear that the efficacy of panitumumab in a highly active chemotherapy backbone is not reduced.
A case of a patient with metastatic chemotherapy-refractory CRC who was treated with panitumumab monotherapy for more than 65 months was reported in 2010, with sustained efficacy over a prolonged period of time, significant prolongation of PFS, and persistent and generally stable skin toxicity (Seront et al., 2010). In the RAS wild-type subgroup of mCRC, the use of anti-EGFR (panitumumab or cetuximab) in first-line therapy was more favorable cost-effective compared to anti-VEGF (bevacizumab) (Koilakou and Petrou, 2021).
The chemotherapeutic regimen of panitumumab combined with the cytotoxic drug irinotecan prolonged the PFS or the toxicity of the drug was tolerable in RAS wild-type mCRC patients, but there are no remarkable results in OS. Overall, irinotecan combined with panitumumab is positive, safe and feasible as a salvage treatment.
With solid preclinical evidence, cetuximab was considered as the first mouse-human monoclonal antibody targeting EGFR in 1995, and the FDA authorized cetuximab for the therapeutic use of mCRC in 2004 (Mendelsohn et al., 2015). Cetuximab, similar to panitumumab, is an EGFR inhibitor that suppresses tumor cell growth and metastasis by inhibiting the EGFR signaling pathway. However, unlike panitumumab, cetuximab has antibody-dependent cell mediated cytotoxicity (ADCC) effect that triggers immune anti-tumor effects (García-Foncillas et al., 2019). Natural killer (NK) cells are activated by binding to cetuximab uploaded onto the EGFR, and released interferon-gamma (IFN-γ) activates dendritic cells, which further activates NK cells (Xiong et al., 2023) (Figure 9). Cetuximab-induced ADCC releases antigens, which are captured by activated dendritic cells and presented to T cells, and in turn, mature dendritic cells are able to activate a variety of additional immunogenic processes, including antigen presentation to cytotoxic T cells and further activation of NK cells (Kasi et al., 2023). IFN-γ mediated crosstalk between macrophages and other immune cells is critical for bringing additional active cytotoxic T cells into the intra-tumor space, and these neurotoxic T cells can subsequently undergo lysogenic activity against tumor cells, resulting in the production of additional tumor antigens and further stimulation of long-term immune responses (Ferris et al., 2018).
To research the therapeutic effectiveness of cetuximab plus irinotecan and cetuximab monotherapy in the treatment of patients with refractory CRC, the researchers recruited 329 patients. The results indicated that the combined treatment group had a significantly greater RR (22.9% vs. 10.8%, p = 0.007) than the monotherapy group and a significantly prolonged median time to progression (4.1 vs. 1.5 months, p < 0.001) (Cunningham et al., 2004). It has been suggested that cetuximab can restore the sensitivity of irinotecan. In the EPIC trial, it was showed that the additional administration of cetuximab to irinotecan cloud improve PFS and RR in mCRC patients, with a QoL superior to that of irinotecan alone and without causing a significant increase in toxicity (Sobrero et al., 2008). The AGITG ICECREAM trial confirmed that irinotecan combined with cetuximab had significant benefits in RAS wild-type mCRC patients compared with cetuximab alone. Cetuximab in combination with irinotecan improved the RR and delay the disease progression of RAS wild-type CRC resistant to irinotecan (Shapiro et al., 2018). This retrospective analysis showed that irinotecan combined with cetuximab improved objective response rate, PFS, and QoL in RAS wild-type mCRC patients (Sobrero et al., 2021). The combination of irinotecan and cetuximab appears to be more advantageous for the treatment of RAS wild-type mCRC than irinotecan or cetuximab monotherapy.
The FIRE-3 research found that in KRAS exon 2 wild-type mCRC patients, cetuximab in addition to with the standard irinotecan-based chemotherapy regimen FOLFIRI was superior to bevacizumab combined with FOLFIRI, and patients achieved higher objective response rate and prolonged OS (Heinemann et al., 2014). The AIO KRK-0306 trial further supported that FOLFIRI plus cetuximab was more beneficial than FOFLIRI plus bevacizumab in the treating RAS wild-type mCRC (Stintzing et al., 2016). The POCHER study indicated that for RAS/BRAF wild-type patients, FOLFOXIRI in combination with cetuximab treated patients with initially unresectable CRC liver metastases with a resection rate of up to 60% (Garufi et al., 2010). The MACBETH study demonstrated that overall response rate of 71.6% for FOLFOXIRI in combination with cetuximab in RAS/BARF wild-type patients (Cremolini et al., 2018). A phase Ib study in Japan also revealed that combination of cetuximab and FOLFOXIRI had controllable toxicity and good efficacy in RAS wild-type mCRC (Kadowaki et al., 2021).
A retrospective subgroup analysis showed that cetuximab in combination with irinotecan-based FOLFIRI is an ideal regimen to promote an increase in objective response rate, with higher objective response rate promoting resectability, which in turn contributes to improved long-term survival (Köhne et al., 2016). For unselected patients with advanced CRC, the incremental cost of cetuximab is high, and when limited to KRAS wild-type patients, the incremental cost is low (Mittmann et al., 2009). In RAS wild-type mCRC patients, the use of cetuximab added to irinotecan-based backbone chemotherapy regimens is feasible, demonstrating positive antitumor activity. However, further controlled trials are required to ascertain whether it prolongs patient survival.
PHY-906 is derived from a traditional formula used for thousands of years, Huang Qin Tang (HQT), a traditional formula used for thousands of years in Zhang Zhongjing’s The Treatise on Typhoid Fever of the Eastern Han Dynasty, which is primarily used for treating gastrointestinal disorders including diarrhea, abdominal cramps, nausea and vomiting (Qin et al., 2022). It is composed of Paeonia lactiflora Pall, Glycyrrhiza uralensis Fisch, Scutellaria baicalensis Georgi and Ziziphus jujuba Mill (Tilton et al., 2010). PHY-906, a natural mixture extracted from these four herbs, is a unique novel anti-tumor drug candidate developed based on an integrated systems biology approach. However, it differs from HQT in terms of drug source, biological activity, and pharmacodynamic composition (Liu and Cheng, 2012). PHY-906 is a kind of strictly in accordance with the Current Good Manufacture Practices (cGMP) specifications, each production step has strict standard operating procedures, which fundamentally ensures the consistency of drug quality (Lam et al., 2018).
Diarrhea is one of the most important dose-limiting toxic reactions of irinotecan in the treatment of CRC, and the opioid receptor agonist loperamide is commonly used clinically to alleviate the diarrhea induced by irinotecan without reaping satisfactory results (Abigerges et al., 1994). According to preclinical studies, PHY-906 enhances the anticancer activity of irinotecan while decreasing irinotecan-induced weight loss and mortality. PHY-906 reduces irinotecan-induced inflammation by decreasing neutrophil or macrophage infiltration, decreasing the expression of tumor necrosis factor-alpha (TNF-α) in the intestines, and decreasing plasma concentrations of proinflammatory cytokines. A variety of PHY-906's chemical constituents or metabolites can effectively inhibit the NF-κB pathway and directly inhibit cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) activity to mediate this mechanism. PHY-906 also promotes the growth of intestinal progenitor and stem cells through activation of the Wnt signaling, thereby accelerating the regeneration and recovery of damaged gastrointestinal tissues (Lam et al., 2010). When PHY-906 is administered with irinotecan, PHY-906 causes activation of the IRF-5/Myd88 pathway and reverses the inhibition of the STAT-1/IRF-1 pathway, with significant immune effects (Wang et al., 2011) (Figure 10). Researchers screened baicalin, baicalein, glycyrrhizic acid and wogonin in PHY-906 through in vivo and in vitro assays and identified them as key bioactive constituents with the ability to enhance the anticancer effects of irinotecan while reducing the intestinal toxicity induced by irinotecan (Dou-Dou et al., 2021).
The combination of PHY-906 with irinotecan does not change the pharmacokinetic parameters of irinotecan or influence the transformation of irinotecan to its active metabolite SN-38. A double-blind, randomized, dose-escalation, placebo-controlled phase I trial included 17 advanced CRC patients (Kummar et al., 2011). Investigators found that during irinotecan in combination with PHY-906 chemotherapy, patients had no treatment-related, life-threatening grade IV adverse incidents, and the general incidence of grade 3/4 diarrhea, as well as the frequency and severity of patient vomiting, was low. In contrast, there were two life-threatening grade IV adverse cases in the placebo group, including neutropenia and gastrointestinal bleeding. The Phase I/IIA clinical study used a multicenter, double-blind, randomized, placebo-controlled, dose-escalation cross-over clinical trial methodology (Farrell and Kummar, 2003), in which patients with advanced CRC were randomized into 2 groups, both of which were treated with FOLFIRI or irinotecan, with group 1 receiving PHY-906 in the first cycle and placebo in the second cycle, and group 2 receiving the opposite. The results of the study showed that PHY-906 could improve the anti-tumor efficacy of chemotherapeutic drugs, significantly reduce the intestinal adverse effects caused by irinotecan, especially diarrhea, and PHY-906 did not adversely impact the anti-tumor activity of irinotecan. It is suggested that PHY-906 can reduce the adverse reactions caused by irinotecan without affecting the anti-tumor activity of irinotecan. Based on the interaction of irinotecan combined with PHY-906 in the inflammatory process of the tumor microenvironment, it was found that PHY-906 could enhance the anti-tumor activity of irinotecan by promoting apoptosis of tumor cells and polarization of macrophages into M1-type macrophages. Most of the herbal remedies used in traditional Chinese medicine have been developed for long-term use, and they have certain long-term survival benefits (Rieder, 2015). Although clinical studies have shown promising results with PHY-906, there is insufficient evidence to conclude that PHY-906 is cost-effective in providing symptomatic relief for CRC survivors.
Silymarin is a natural flavonoid found in the perennial herb artichoke, and the main active ingredient of silymarin is silybin (Koushki et al., 2023). Silymarin is non-toxic even at very high doses and can disrupt with cycle regulation, apoptosis, angiogenesis, and expression of proteins associated with multidrug resistance (Koltai and Fliegel, 2022). On the contrary, the good antioxidant activity of silymarin makes it a useful agent for the prevention of cancer and has a certain place in cancer therapy (Wang et al., 2023).
According to preclinical studies, silymarin leads to inhibition of Wnt signaling in human CRC cells through downregulation of β-catenin and TCF4 (Eo et al., 2016). In vitro experiments confirmed the strong anti-angiogenic effect and anti-proliferative activity of silymarin on LoVo CRC cell lines (Yang et al., 2003; Colombo et al., 2011). The anti-CRC activity of the silymarin-oxidized azoxymethane (AOM)-induced colon cancer model was also validated in that silymarin reduced the number of aberrant crypt foci (ACF) in the colon, and dietary intake of silymarin reduced colonic BGUS activity (Kohno et al., 2002). Also in the AOM colitis-associated cancer model, the researchers found that silymarin, the active ingredient of silymarin, significantly downregulated interleukin-6 (IL-6), that interleukin-1beta (IL-1β) and TNF-α could act as prophylactic agents for colitis-associated cancers, and that silymarin prevented colitis-associated tumorigenesis in mice by inhibiting IL-6/STAT3 signaling pathway (Zheng et al., 2018). Silymarin also inhibits 1,2-dimethylhydrazine (DMH)-induced colon carcinogenesis by modulating the activity of intestinal microbial enzymes, the level of colonic oxidative stress, and the Wnt/β-catenin signaling to exert its antiproliferative effects (Sangeetha et al., 2009; 2010b; 2010a). Silymarin significantly reduces survival and induces apoptosis and autophagy in mouse CT26 CRC cells by up-regulating Bax and Caspase-3 and down-regulating Bcl-2 expression (Sameri et al., 2021) (Figure 11).
Silymarin does not work as a stand-alone anticancer agent and may be an important factor in multidrug combination anticancer regimens. For patients with mCRC, the irinotecan-based FOLFIRI chemotherapy regimen has demonstrated its benefit in first-line treatment; however, this regimen causes significant intestinal toxicity, which directly affects the clinical treatment effect of patients with CRC. The main reason for the intestinal reaction caused by irinotecan and metabolites excreted into the intestines via the biliary are converted in the action of BGUS to active SN-38, which leads to delayed diarrhea (Cao, 2019; Huang and Zhu, 2021). Animal experiments inhibited chemically evoked colon cancer in rats by dietary intake of silymarin (Kohno et al., 2002). Silymarin and its components may decrease risk factors for colon cancer by blocking hydrolysis of glucuronides in metabolites (Kim et al., 1994). Silymarin may serve as a good adjuvant to irinotecan in combination, which opens up the possibility of irinotecan in combination with silymarin for treating CRC. A prospective open-label pilot clinical trial evaluated silymarin as a supplement in irinotecan-based therapy in mCRC patients (Chang et al., 2021). FOLFIRI plus the targeted agent bevacizumab was administered to 35 patients with mCRC as the group of control, and 35 patients in the study group received oral silymarin capsules on the basis of control group chemotherapy. With the administration of silymarin capsules, the occurrence of toxic incidents was lower in the study group, with lower rates of nausea (27.0% vs. 40.2%, p = 0.005) and diarrhea (5.4% vs. 14.6%, p = 0.002). The addition of silymarin slowed the incidence of nausea and diarrhea in patients. Neither short-term (4 days) nor longer-term (12 days) ingestion of silymarin had a significant effect on the clearance of irinotecan (van Erp et al., 2005).
Silymarin has shown superiority as an effective and well-tolerated supplement in irinotecan-based chemotherapy regimens, and silymarin may be a safe and effective option as a complementary therapy to irinotecan-containing chemotherapy regimens. Coupled with the superior cost-effectiveness of silymarin as a natural product, the use of silymarin to enhance the effectiveness of existing anticancer drugs is a very promising approach (Dheeraj et al., 2018).
The chemotherapy strategy for CRC is determined based on the patient’s condition, and survival outcome will be the primary determinant of the choice of any one strategy. Irinotecan has promising antitumor activity in CRC, and irinotecan-based regimens have a survival advantage and can enhance the effect of irinotecan-based chemotherapy. Various combinations of irinotecan-based combinations are recommended by NCCN guidelines and CSCO guidelines for chemotherapy of different types of colorectal cancer. The current irinotecan-based combination chemotherapy regimens, FOLFIRI, FOLFOXIRI and XELIRI, are also the backbone of clinical chemotherapy for CRC. The role that the combinations play in the treatment process is in part dependent on the irinotecan backbone-based chemotherapy regimen used. With the development and marketing of a variety of molecular targeted drugs that include bevacizumab, cetuximab, and panitumumab, targeted therapies in combination with chemotherapy provide a more efficient and targeted treatment option for CRC. Irinotecan in combination with different targeted agents has its own specific adverse events, and the choice of the optimal combination and sequencing depends on the pre-molecular characteristics of CRC.
Herbal medicines such as silymarin capsules and PHY-906 have had a significant impact on CRC as complementary and alternative therapies. Irinotecan-based regimens combining herbs have shown superior performance in improving patients’ QoL. The chemical composition in herbal medicines is diverse and mechanistically complex, and further research should concentrate on characterizing bioactive compounds with therapeutic effects and delving into their mechanisms of action. Many of the clinical trials were poorly designed, included small patient samples, had poor methodological control, short treatment and follow-up periods, lacked a more rigorous and robust clinical rationale, and the results obtained need to be treated with caution. In the future, larger and more methodologically justified randomized controlled trials should be conducted, otherwise it will be difficult for clinical studies to demonstrate the benefits exerted by herbal medicines.
Toxic reactions to irinotecan are reversible, non-cumulative and controllable. Irinotecan-based chemotherapy regimens can be used at various times during CRC treatment, whether during neoadjuvant, transformational or palliative care and play an irreplaceable and important role. We expect that more personalized irinotecan-based therapies will be optimized or developed, enabling patients to achieve longer survival with fewer adverse effects.
In order to explore the prospects for further use of irinotecan, several studies of irinotecan and its chemotherapy combinations in the treatment of CRC are still underway. The TRIPLETE study was designed to explore whether the first-line treatment of patients with RAS/BRAF wild-type mCRC with the three-agent combination of panitumumab in combination with irinotecan, mFOLFOXIRI, would result in increased efficacy compared to the mFOLFOX6 regimen. The primary study endpoint was not met, and the combination of mFOLFOXIRI with panitumumab did not provide a therapeutic benefit and increased the incidence of gastrointestinal toxicity (Cremolini et al., 2022). The triple combination of anti-EGFR monoclonal antibody combined with irinotecan may provide tumor remission and survival benefit for RAS wild-type mCRC. In contrast, the TRIPLETE study showed that irinotecan-based triple-agent combination regimens did not show added value versus mFOLFOX6 chemotherapy when compared to mFOLFOX6 chemotherapy in clinically selected molecularly based populations, which is different from previous knowledge. This suggests that the three-agent chemotherapy regimen of irinotecan is not inapplicable to the population, and that further refinement of the population is needed if better efficacy is to be achieved by increasing the intensity of chemotherapy. The three-agent chemotherapy regimen of irinotecan can be tried in young patients with significant symptomatic disease, patients with insensitivity to chemotherapeutic agents, patients with large tumor loads, and patients with advanced colorectal cancer with peritoneal or multiple metastases. In clinical practice, investigators still need to choose the appropriate regimen from patient characteristics, tumor characteristics and other multifactorial considerations to obtain better survival outcomes.
YC: Data curation, Resources, Writing–original draft. J-LL: Data curation, Visualization, Writing–original draft. SZ: Formal Analysis, Visualization, Writing–original draft. NL: Conceptualization, Project administration, Supervision, Writing–review and editing. D-QX: Formal Analysis, Investigation, Visualization, Writing–original draft. W-JL: Writing–review and editing. R-JF: Writing–review and editing. Y-PT: Conceptualization, Funding acquisition, Project administration, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by National Natural Science Foundation of China (82274084), The Entrusted service project of Shaanxi Administration of Traditional Chinese Medicine (ZYJXG-L23001), Subject Innovation Team of Shaanxi University of Chinese Medicine (2019-YL10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abigerges, D., Armand, J. P., Chabot, G. G., Da Costa, L., Fadel, E., Cote, C., et al. (1994). Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J. Natl. Cancer Inst. 86 (6), 446–449. doi:10.1093/jnci/86.6.446
Adebayo, A. S., Agbaje, K., Adesina, S. K., and Olajubutu, O. (2023). Colorectal cancer: disease process, current treatment options, and future perspectives. Pharmaceutics 15 (11), 2620. doi:10.3390/pharmaceutics15112620
Alimonti, A., Gelibter, A., Pavese, I., Satta, F., Cognetti, F., Ferretti, G., et al. (2004). New approaches to prevent intestinal toxicity of irinotecan-based regimens. Cancer Treat. Rev. 30 (6), 555–562. doi:10.1016/j.ctrv.2004.05.002
Alzahrani, S. M., Al Doghaither, H. A., Al-Ghafari, A. B., and Pushparaj, P. N. (2023). 5-Fluorouracil and capecitabine therapies for the treatment of colorectal cancer (Review). Oncol. Rep. 50 (4), 175–216. doi:10.3892/or.2023.8612
Apte, R. S., Chen, D. S., and Ferrara, N. (2019). VEGF in signaling and disease: beyond discovery and development. Cell 176 (6), 1248–1264. doi:10.1016/j.cell.2019.01.021
Azwar, S., Seow, H. F., Abdullah, M., Faisal Jabar, M., and Mohtarrudin, N. (2021). Recent updates on mechanisms of resistance to 5-fluorouracil and reversal strategies in colon cancer treatment. Biol. (Basel) 10 (9), 854. doi:10.3390/biology10090854
Bailly, C. (2019). Irinotecan: 25 years of cancer treatment. Pharmacol. Res. 148, 104398. doi:10.1016/j.phrs.2019.104398
Benson, A. B., and Goldberg, R. M. (2003). Optimal use of the combination of irinotecan and 5-fluorouracil. Semin. Oncol. 30 (3 Suppl. 6), 68–77. doi:10.1016/S0093-7754(03)00127-1
Cai, Y., Deng, R., Hu, H., Zhang, J., Ling, J., Wu, Z., et al. (2018). Analysis on safety and preliminary efficacy of dose-modified regimen of 5-fluorouracil plus oxaliplatin and irinotecan (FOLFOXIRI) in advanced colorectal cancer. Zhonghua Wei Chang. Wai Ke Za Zhi Chin. J. Gastrointest. Surg. 21 (9), 1045–1050. doi:10.3760/cma.j.issn.1671-0274.2018.09.013
Cao, J. (2019). UGT1A1∼* 28 Association of gene polymorphisms with irinotecan-related toxicity and chemotherapy efficacy. J. Mod. Oncol. 27 (6), 1087–1089. doi:10.3969/j.issn.1672-4992.2019.06.045
Chang, T.-K., Yin, T.-C., Su, W.-C., Tsai, H.-L., Huang, C.-W., Chen, Y.-C., et al. (2021). A pilot study of silymarin as supplementation to reduce toxicities in metastatic colorectal cancer patients treated with first-line FOLFIRI plus bevacizumab. Oncol. Res. 28 (7), 801–809. doi:10.3727/096504021X16218531628569
Chen, J., Lin, D., Yang, J., Cai, X.-T., Wei, G.-L., and Cao, P. (2022). Advances in the synergistic and toxic attenuating effect of traditional Chinese medicine in cancer treatment. Sci. CHINA LIFE Sci. 52 (6), 920–934. doi:10.1360/ssv-2021-0389
Clarke, S. J., Yip, S., Brown, C., van Hazel, G. A., Ransom, D. T., Goldstein, D., et al. (2011). Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis [corrected]. Eur. J. Cancer Oxf. Engl. 47 (12), 1826–1836. doi:10.1016/j.ejca.2011.04.024
Colombo, V., Lupi, M., Falcetta, F., Forestieri, D., D’Incalci, M., and Ubezio, P. (2011). Chemotherapeutic activity of silymarin combined with doxorubicin or paclitaxel in sensitive and multidrug-resistant colon cancer cells. Cancer Chemother. Pharmacol. 67 (2), 369–379. doi:10.1007/s00280-010-1335-8
Cremolini, C., Antoniotti, C., Lonardi, S., Aprile, G., Bergamo, F., Masi, G., et al. (2018). Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 4 (4), 529–536. doi:10.1001/jamaoncol.2017.5314
Cremolini, C., Antoniotti, C., Rossini, D., Lonardi, S., Loupakis, F., Pietrantonio, F., et al. (2020). Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 21 (4), 497–507. doi:10.1016/S1470-2045(19)30862-9
Cremolini, C., Loupakis, F., Antoniotti, C., Lupi, C., Sensi, E., Lonardi, S., et al. (2015). FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 16 (13), 1306–1315. doi:10.1016/S1470-2045(15)00122-9
Cremolini, C., Rossini, D., Lonardi, S., Antoniotti, C., Pietrantonio, F., Marmorino, F., et al. (2022). Modified FOLFOXIRI plus panitumumab (mFOLFOXIRI/PAN) versus mFOLFOX6/PAN as initial treatment of patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer (mCRC): results of the phase III randomized TRIPLETE study by GONO. J. Clin. Oncol. 40 (17_Suppl. l), LBA3505. doi:10.1200/JCO.2022.40.17_suppl.LBA3505
Cunningham, D., Humblet, Y., Siena, S., Khayat, D., Bleiberg, H., Santoro, A., et al. (2004). Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351 (4), 337–345. doi:10.1056/NEJMoa033025
Daisuke, S., Hiroya, T., Naotoshi, S., Takao, T., Tomohiro, N., Hiroki, H., et al. (2020). Randomised phase II study of panitumumab plus irinotecan versus cetuximab plus irinotecan in patients with KRAS wild-type metastatic colorectal cancer refractory to fluoropyrimidine, irinotecan and oxaliplatin (WJOG 6510G). Eur. J. Cancer Oxf. Engl. 1990 (135), 11–21. doi:10.1016/j.ejca.2020.04.014
de Jong, F. A., de Jonge, M. J. A., Verweij, J., and Mathijssen, R. H. J. (2006). Role of pharmacogenetics in irinotecan therapy. Cancer Lett. 234 (1), 90–106. doi:10.1016/j.canlet.2005.04.040
de With, M., van Doorn, L., Maasland, D. C., Mulder, T. A. M., Oomen-de Hoop, E., Mostert, B., et al. (2023). Capecitabine-induced hand-foot syndrome: a pharmacogenetic study beyond DPYD. Biomed. Pharmacother. 159, 114232. doi:10.1016/j.biopha.2023.114232
Dheeraj, A., Tailor, D., Singh, S. P., and Singh, R. P. (2018). Anticancer Attributes of silibinin: chemo- and radiosensitization of cancer. Role Nutraceuticals Cancer Chemosensitization 2, 199–220. doi:10.1016/B978-0-12-812373-7.00010-3
Dou-Dou, X., Xiao-Ying, H., Ou, W., Di, W., Dan-Ting, L., Si-Yuan, Q., et al. (2021). A four-component combination derived from Huang-Qin Decoction significantly enhances anticancer activity of irinotecan. Chin. J. Nat. Med. 19 (5), 364–375. doi:10.1016/S1875-5364(21)60034-1
Douillard, J. Y., Cunningham, D., Roth, A. D., Navarro, M., James, R. D., Karasek, P., et al. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet lond. Engl. 355 (9209), 1041–1047. doi:10.1016/s0140-6736(00)02034-1
Ducreux, M., Adenis, A., Pignon, J.-P., François, E., Chauffert, B., Ichanté, J. L., et al. (2013). Efficacy and safety of bevacizumab-based combination regimens in patients with previously untreated metastatic colorectal cancer: final results from a randomised phase II study of bevacizumab plus 5-fluorouracil, leucovorin plus irinotecan versus bevacizumab plus capecitabine plus irinotecan (FNCLCC ACCORD 13/0503 study). Eur. J. Cancer 49 (6), 1236–1245. doi:10.1016/j.ejca.2012.12.011
Eo, H. J., Park, G. H., and Jeong, J. B. (2016). Inhibition of Wnt signaling by silymarin in human colorectal cancer cells. Biomol. Ther. Seoul. 24 (4), 380–386. doi:10.4062/biomolther.2015.154
Falcone, A., Masi, G., Allegrini, G., Danesi, R., Pfanner, E., Brunetti, I. M., et al. (2002). Biweekly chemotherapy with oxaliplatin, irinotecan, infusional fluorouracil, and leucovorin: a pilot study in patients with metastatic colorectal cancer. J. Clin. Oncol. 20 (19), 4006–4014. doi:10.1200/JCO.2002.12.075
Falcone, A., Ricci, S., Brunetti, I., Pfanner, E., Allegrini, G., Barbara, C., et al. (2007). Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord ovest. J. Clin. Oncol. 25 (13), 1670–1676. doi:10.1200/JCO.2006.09.0928
Farrell, M. P., and Kummar, S. (2003). Phase I/IIA randomized study of PHY906, a novel herbal agent, as a modulator of chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer 2 (4), 253–256. doi:10.3816/CCC.2003.n.007
Ferris, R. L., Lenz, H.-J., Trotta, A. M., García-Foncillas, J., Schulten, J., Audhuy, F., et al. (2018). Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat. Rev. 63, 48–60. doi:10.1016/j.ctrv.2017.11.008
Fornaro, L., Lonardi, S., Masi, G., Loupakis, F., Bergamo, F., Salvatore, L., et al. (2013). FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann. Oncol. 24 (8), 2062–2067. doi:10.1093/annonc/mdt165
Fuchs, C. S., Marshall, J., Mitchell, E., Wierzbicki, R., Ganju, V., Jeffery, M., et al. (2007). Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C study. J. Clin. Oncol. 25 (30), 4779–4786. doi:10.1200/JCO.2007.11.3357
Fukuda, S., Niisato, Y., Tsuji, M., Fukuda, S., Hagiwara, Y., Onoda, T., et al. (2023). Relationship between safety and cumulative bevacizumab dose in patients with metastatic colorectal cancer who received long-term bevacizumab treatment. Anticancer Res. 43 (5), 2085–2090. doi:10.21873/anticanres.16369
Garcia, J., Hurwitz, H. I., Sandler, A. B., Miles, D., Coleman, R. L., Deurloo, R., et al. (2020). Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 86, 102017. doi:10.1016/j.ctrv.2020.102017
Garcia-Alfonso, P., Muñoz-Martin, A., Mendez-Ureña, M., Quiben-Pereira, R., Gonzalez-Flores, E., and Perez-Manga, G. (2009). Capecitabine in combination with irinotecan (XELIRI), administered as a 2-weekly schedule, as first-line chemotherapy for patients with metastatic colorectal cancer: a phase II study of the Spanish GOTI group. Br. J. Cancer 101 (7), 1039–1043. doi:10.1038/sj.bjc.6605261
García-Alfonso, P., Muñoz Martín, A. J., Ortega Morán, L., Soto Alsar, J., Torres Pérez-Solero, G., Blanco Codesido, M., et al. (2021). Oral drugs in the treatment of metastatic colorectal cancer. Ther. Adv. Med. Oncol. 13, 17588359211009001. doi:10.1177/17588359211009001
García-Foncillas, J., Sunakawa, Y., Aderka, D., Wainberg, Z., Ronga, P., Witzler, P., et al. (2019). Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front. Oncol. 9, 849. doi:10.3389/fonc.2019.00849
Garufi, C., Torsello, A., Tumolo, S., Ettorre, G. M., Zeuli, M., Campanella, C., et al. (2010). Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br. J. Cancer 103 (10), 1542–1547. doi:10.1038/sj.bjc.6605940
Gerber, H.-P., and Ferrara, N. (2005). Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 65 (3), 671–680. doi:10.1158/0008-5472.671.65.3
Goldstein, D. A., Chen, Q., Ayer, T., Chan, K. K. W., Virik, K., Hammerman, A., et al. (2017). Bevacizumab for metastatic colorectal cancer: a global cost-effectiveness analysis. Oncol. 22 (6), 694–699. doi:10.1634/theoncologist.2016-0455
Goldstein, D. A., Chen, Q., Ayer, T., Howard, D. H., Lipscomb, J., El-Rayes, B. F., et al. (2015). First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States–based cost-effectiveness analysis. J. Clin. Oncol. 33 (10), 1112–1118. doi:10.1200/JCO.2014.58.4904
Guo, Y., Shi, M., Shen, X., Yang, C., Yang, L., and Zhang, J. (2014). Capecitabine plus irinotecan versus 5-FU/leucovorin plus irinotecan in the treatment of colorectal cancer: a meta-analysis. Clin. Colorectal Cancer 13 (2), 110–118. doi:10.1016/j.clcc.2013.12.004
Haller, D. G., Cassidy, J., Clarke, S., Cunningham, D., Van Cutsem, E., Hoff, P., et al. (2006). Tolerability of fluoropyrimidines appears to differ by region. J. Clin. Oncol. 24 (18_Suppl. l), 3514. doi:10.1200/jco.2006.24.18_suppl.3514
Heinemann, V., and Hoff, P. M. (2010). Bevacizumab plus irinotecan-based regimens in the treatment of metastatic colorectal cancer. Oncology 79 (1-2), 118–128. doi:10.1159/000314993
Heinemann, V., von Weikersthal, L. F., Decker, T., Kiani, A., Vehling-Kaiser, U., Al-Batran, S.-E., et al. (2014). FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 15 (10), 1065–1075. doi:10.1016/S1470-2045(14)70330-4
Hossain, M. S., Karuniawati, H., Jairoun, A. A., Urbi, Z., Ooi, D. J., John, A., et al. (2022). Colorectal cancer: a review of carcinogenesis, global Epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers 14 (7), 1732. doi:10.3390/cancers14071732
Hoy, S. M., and Wagstaff, A. J. (2006). Panitumumab: in the treatment of metastatic colorectal cancer. Drugs 66 (15), 2005–2014. discussion 2015-2016. doi:10.2165/00003495-200666150-00011
Huang, C.-Y., and Zhu, J. (2021). Irinotecan in neoadjuvant radiotherapy for rectal cancer. J. Chin. Oncol. 27 (10), 791–797. doi:10.11735/j.issn.1671-170X.2021.10.B001
Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., et al. (2004). Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350 (23), 2335–2342. doi:10.1056/NEJMoa032691
Huy, T. L., Bui, M. H., Dinh, T. C., and Xuyen, H. T. H. (2019). Efficacy and toxicity of folfoxiri for patients with metastatic colorectal cancer. Open Access Maced. J. Med. Sci. 7 (24), 4244–4249. doi:10.3889/oamjms.2019.368
Islam, M. R., Akash, S., Rahman, M. M., Nowrin, F. T., Akter, T., Shohag, S., et al. (2022). Colon cancer and colorectal cancer: prevention and treatment by potential natural products. Chem. Biol. Interact. 368, 110170. doi:10.1016/j.cbi.2022.110170
Kadowaki, S., Masuishi, T., Ura, T., Sugiyama, K., Mitani, S., Narita, Y., et al. (2021). A triplet combination of FOLFOXIRI plus cetuximab as first-line treatment in RAS wild-type, metastatic colorectal cancer: a dose-escalation phase Ib study. Int. J. Clin. Oncol. 26 (4), 701–707. doi:10.1007/s10147-020-01842-3
Kasi, P. M., Afable, M. G., Herting, C., Lukanowski, M., and Jin, Z. (2023). Anti-EGFR antibodies in the management of advanced colorectal cancer. Oncologist 28 (12), 1034–1048. doi:10.1093/oncolo/oyad262
Kciuk, M., Marciniak, B., and Kontek, R. (2020). Irinotecan-still an important player in cancer chemotherapy: a comprehensive overview. Int. J. Mol. Sci. 21 (14), E4919. doi:10.3390/ijms21144919
Kim, D. H., Jin, Y. H., Park, J. B., and Kobashi, K. (1994). Silymarin and its components are inhibitors of beta-glucuronidase. Biol. Pharm. Bull. 17 (3), 443–445. doi:10.1248/bpb.17.443
Köhne, C.-H., De Greve, J., Hartmann, J. T., Lang, I., Vergauwe, P., Becker, K., et al. (2008). Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann. Oncol. 19 (5), 920–926. doi:10.1093/annonc/mdm544
Köhne, C.-H., Poston, G., Folprecht, G., Ciardiello, F., Ronga, P., Beier, F., et al. (2016). FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic colorectal cancer: a retrospective subgroup analysis of the CRYSTAL study. Eur. J. Surg. Oncol. EJSO 42 (10), 1540–1547. doi:10.1016/j.ejso.2016.05.038
Kohno, H., Tanaka, T., Kawabata, K., Hirose, Y., Sugie, S., Tsuda, H., et al. (2002). Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Int. J. Cancer 101 (5), 461–468. doi:10.1002/ijc.10625
Koilakou, S., and Petrou, P. (2021). Economic evaluation of monoclonal antibodies in metastatic colorectal cancer: a systematic review. Mol. Diagn. Ther. 25 (6), 715–734. doi:10.1007/s40291-021-00560-4
Koltai, T., and Fliegel, L. (2022). Role of silymarin in cancer treatment: facts, hypotheses, and questions. J. Evid.-Based Integr. Med. 27, 2515690X211068826. doi:10.1177/2515690X211068826
Koopman, M., Antonini, N. F., Douma, J., Wals, J., Honkoop, A. H., Erdkamp, F. L., et al. (2007). Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 370 (9582), 135–142. doi:10.1016/S0140-6736(07)61086-1
Koushki, M., Farrokhi Yekta, R., and Amiri-Dashatan, N. (2023). Critical review of therapeutic potential of silymarin in cancer: a bioactive polyphenolic flavonoid. J. Funct. Foods 104, 105502. doi:10.1016/j.jff.2023.105502
Kumar, R., Harilal, S., Carradori, S., and Mathew, B. (2021). A comprehensive overview of colon cancer- A grim reaper of the 21st century. Curr. Med. Chem. 28 (14), 2657–2696. doi:10.2174/0929867327666201026143757
Kummar, S., Copur, M. S., Rose, M., Wadler, S., Stephenson, J., O’Rourke, M., et al. (2011). A phase I study of the Chinese herbal medicine PHY906 as a modulator of irinotecan-based chemotherapy in patients with advanced colorectal cancer. Clin. Colorectal Cancer 10 (2), 85–96. doi:10.1016/j.clcc.2011.03.003
Lam, W., Bussom, S., Guan, F., Jiang, Z., Zhang, W., Gullen, E. A., et al. (2010). The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci. Transl. Med. 2 (45), 45ra59. doi:10.1126/scitranslmed.3001270
Lam, W., Ren, Y., Guan, F., Jiang, Z., Cheng, W., Xu, C.-H., et al. (2018). Mechanism based quality control (mbqc) of herbal products: a case study YIV-906 (PHY906). Front. Pharmacol. 9, 1324. doi:10.3389/fphar.2018.01324
Li, B.-L., Chen, H.-G., Zhao, Z., and Zhou, X. (2020). Progress of research on the mechanism of action of traditional Chinese medicine against colorectal cancer. Nat. Prod. Res. Dev. 32 (12), 2132–2141. doi:10.16333/j.1001-6880.2020.12.019
Liu, S.-H., and Cheng, Y.-C. (2012). Old formula, new Rx: the journey of PHY906 as cancer adjuvant therapy. J. Ethnopharmacol. 140 (13), 614–623. doi:10.1016/j.jep.2012.01.047
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3 (5), 330–338. doi:10.1038/nrc1074
Loupakis, F., Cremolini, C., Masi, G., Lonardi, S., Zagonel, V., Salvatore, L., et al. (2014). Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl. J. Med. 371 (17), 1609–1618. doi:10.1056/NEJMoa1403108
Mahmod, A. I., Haif, S. K., Kamal, A., Al-ataby, I. A., and Talib, W. H. (2022). Chemoprevention effect of the Mediterranean diet on colorectal cancer: current studies and future prospects. Front. Nutr. 9, 924192. doi:10.3389/fnut.2022.924192
Masi, G., Loupakis, F., Pollina, L., Vasile, E., Cupini, S., Ricci, S., et al. (2009). Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann. Surg. 249 (3), 420–425. doi:10.1097/SLA.0b013e31819a0486
Masi, G., Vasile, E., Loupakis, F., Cupini, S., Fornaro, L., Baldi, G., et al. (2011). Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. JNCI J. Natl. Cancer Inst. 103 (1), 21–30. doi:10.1093/jnci/djq456
Mathijssen, R. H. J., van Alphen, R. J., Verweij, J., Loos, W. J., Nooter, K., Stoter, G., et al. (2001). Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 7 (8), 2182–2194.
Melincovici, C. S., Boşca, A. B., Şuşman, S., Mărginean, M., Mihu, C., Istrate, M., et al. (2018). Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 59 (2), 455–467.
Mendelsohn, J., Prewett, M., Rockwell, P., and Goldstein, N. I. (2015). CCR 20th anniversary commentary: a chimeric antibody, C225, inhibits EGFR activation and tumor growth. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 21 (2), 227–229. doi:10.1158/1078-0432.CCR-14-2491
Méndez-Valdés, G., Gómez-Hevia, F., Lillo-Moya, J., González-Fernández, T., Abelli, J., Cereceda-Cornejo, A., et al. (2023). Endostatin and cancer therapy: a novel potential alternative to anti-VEGF monoclonal antibodies. Biomedicines 11 (3), 718. doi:10.3390/biomedicines11030718
Mittmann, N., Au, H.-J., Tu, D., O’Callaghan, C. J., Isogai, P. K., Karapetis, C. S., et al. (2009). Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of national cancer Institute of Canada clinical trials group CO.17 trial. JNCI J. Natl. Cancer Inst. 101 (17), 1182–1192. doi:10.1093/jnci/djp232
Modest, D. P., Martens, U. M., Riera-Knorrenschild, J., Greeve, J., Florschütz, A., Wessendorf, S., et al. (2019). FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI study (AIO KRK0109). J. Clin. Oncol. 37 (35), 3401–3411. doi:10.1200/JCO.19.01340
Montagnani, F., Chiriatti, A., Licitra, S., Aliberti, C., and Fiorentini, G. (2010). Differences in efficacy and safety between capecitabine and infusional 5-fluorouracil when combined with irinotecan for the treatment of metastatic colorectal cancer. Clin. Colorectal Cancer 9 (4), 243–247. doi:10.3816/CCC.2010.n.036
O’Dowd, P. D., Sutcliffe, D. F., and Griffith, D. M. (2023). Oxaliplatin and its derivatives – an overview. Coord. Chem. Rev. 497, 215439. doi:10.1016/j.ccr.2023.215439
Ohishi, T., Kaneko, M. K., Yoshida, Y., Takashima, A., Kato, Y., and Kawada, M. (2023). Current targeted therapy for metastatic colorectal cancer. Int. J. Mol. Sci. 24 (2), 1702. doi:10.3390/ijms24021702
Peeters, M., Price, T. J., Cervantes, A., Sobrero, A. F., Ducreux, M., Hotko, Y., et al. (2010). Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28 (31), 4706–4713. doi:10.1200/JCO.2009.27.6055
Pentheroudakis, G., and Twelves, C. (2002). Capecitabine (Xeloda): from the laboratory to the patient's home. Clin. Colorectal Cancer 2 (1), 16–23. doi:10.3816/CCC.2002.n.007
Price, T., Kim, T. W., Li, J., Cascinu, S., Ruff, P., Suresh, A. S., et al. (2016). Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur. J. Cancer 68, 51–59. doi:10.1016/j.ejca.2016.08.010
Qin, H.-R., Sun, W.-J., Zhang, H., Yan, H., Zhang, S.-W., Wang, H., et al. (2022). Overview of the mechanism and clinical application of Huang Qin Tang on the potentiation and toxicity reduction of chemotherapeutic drugs. China J. Chin. Med. 37 (1), 293–296.
Qingwei, Z., Dongsheng, H., Duo, L., Youlei, W., Songxia, Y., Ziqi, Y., et al. (2020). Fluorouracil supplemented with oxaliplatin or irinotecan for solid tumors: Indications from clinical characteristics and health outcomes of patients. Front. Oncol. 10, 1542. doi:10.3389/fonc.2020.01542
Qiu, Y.-L., Li, X.-Y., and Lin, F.-C. (2018). Progress in the study of the role of traditional Chinese medicine in the treatment of colorectal cancer and its mechanism. Elec. J. Transl. Med. 5 (9), 48–52. doi:10.12095/j.issn.2095?6894.2018.09.010
Rastin, F., Javid, H., Oryani, M. A., Rezagholinejad, N., Afshari, A.-R., and Karimi-Shahri, M. (2024). Immunotherapy for colorectal cancer: rational strategies and novel therapeutic progress. Int. Immunopharmacol. 126, 111055. doi:10.1016/j.intimp.2023.111055
Ren, T., Wang, S., Shen, Z., Xu, C., Zhang, Y., Hui, F., et al. (2021). Efficacy and safety of bevacizumab plus oxaliplatin- or irinotecan-based doublet backbone chemotherapy as the first-line treatment of metastatic colorectal cancer: a systematic review and meta-analysis. Drug Saf. 44 (1), 29–40. doi:10.1007/s40264-020-00997-2
Rieder, M. (2015). Phytotherapies from traditional Chinese medicine. Phytotherapies (John Wiley Sons, Ltd), 122–141. doi:10.1002/9781119006039.ch7
Saltz, L., Shimada, Y., and Khayat, D. (1996). CPT-11 (irinotecan) and 5-fluorouracil: a promising combination for therapy of colorectal cancer. Eur. J. Cancer Oxf. Engl. 32A (Suppl. 3), S24–S31. doi:10.1016/0959-8049(96)00294-8
Saltz, L. B., Cox, J. V., Blanke, C., Rosen, L. S., Fehrenbacher, L., Moore, M. J., et al. (2000). Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N. Engl. J. Med. 343, 905–914. doi:10.1056/NEJM200009283431302
Sameri, S., Mohammadi, C., Mehrabani, M., and Najafi, R. (2021). Targeting the hallmarks of cancer: the effects of silibinin on proliferation, cell death, angiogenesis, and migration in colorectal cancer. BMC Complement. Med. Ther. 21 (1), 160. doi:10.1186/s12906-021-03330-1
Sangeetha, N., Aranganathan, S., and Nalini, N. (2010a). Silibinin ameliorates oxidative stress induced aberrant crypt foci and lipid peroxidation in 1, 2 dimethylhydrazine induced rat colon cancer. Invest. New Drugs 28 (3), 225–233. doi:10.1007/s10637-009-9237-5
Sangeetha, N., Aranganathan, S., Panneerselvam, J., Shanthi, P., Rama, G., and Nalini, N. (2010b). Oral supplementation of silibinin prevents colon carcinogenesis in a long term preclinical model. Eur. J. Pharmacol. 643 (1), 93–100. doi:10.1016/j.ejphar.2010.05.060
Sangeetha, N., Felix, A. J. W., and Nalini, N. (2009). Silibinin modulates biotransforming microbial enzymes and prevents 1,2-dimethylhydrazine-induced preneoplastic changes in experimental colon cancer. Eur. J. Cancer Prev. 18 (5), 385–394. doi:10.1097/CEJ.0b013e32832d1b4f
Satake, H., Tsuji, A., Nakamura, M., Ogawa, M., Kotake, T., Hatachi, Y., et al. (2018). Phase I study of primary treatment with 5-FU, oxaliplatin, irinotecan, levofolinate, and panitumumab combination chemotherapy in patients with advanced/recurrent colorectal cancer involving the wild-type RAS gene: the JACCRO CC-14 study. Int. J. Clin. Oncol. 23 (3), 490–496. doi:10.1007/s10147-017-1228-5
Sato, Y., Matsusaka, S., Suenaga, M., Shinozaki, E., and Mizunuma, N. (2015). Cetuximab could be more effective without prior bevacizumab treatment in metastatic colorectal cancer patients. Onco Targets Ther. 8, 3329–3336. doi:10.2147/OTT.S89241
Scheithauer, W., Kornek, G. V., Raderer, M., Valencak, J., Weinländer, G., Hejna, M., et al. (1999). Combined irinotecan and oxaliplatin plus granulocyte colony-stimulating factor in patients with advanced fluoropyrimidine/leucovorin-pretreated colorectal cancer. J. Clin. Oncol. 17 (3), 902–906. doi:10.1200/JCO.1999.17.3.902
Seront, E., Marot, L., Coche, E., Gala, J.-L., Sempoux, C., and Humblet, Y. (2010). Successful long-term management of a patient with late-stage metastatic colorectal cancer treated with panitumumab. Cancer Treat. Rev. 36, S11–S14. doi:10.1016/S0305-7372(10)70002-5
Sethy, C., and Kundu, C. N. (2021). 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed. Pharmacother. 137, 111285. doi:10.1016/j.biopha.2021.111285
Seymour, M. T., Brown, S. R., Middleton, G., Maughan, T., Richman, S., Gwyther, S., et al. (2013). Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 14 (8), 749–759. doi:10.1016/S1470-2045(13)70163-3
Shapiro, J. D., Thavaneswaran, S., Underhill, C. R., Robledo, K. P., Karapetis, C. S., Day, F. L., et al. (2018). Cetuximab alone or with irinotecan for resistant KRAS-NRAS-BRAF- and PIK3CA-wild-type metastatic colorectal cancer: the AGITG randomized phase II ICECREAM study. Clin. Colorectal Cancer 17 (4), 313–319. doi:10.1016/j.clcc.2018.06.002
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A., and Jemal, A. (2023). Colorectal cancer statistics, 2023. Ca. Cancer J. Clin. 73 (3), 233–254. doi:10.3322/caac.21772
Sobrero, A., and Bennicelli, E. (2010). Chemotherapy: which drug and when? Ann. Oncol. 21 (Suppl. 7), vii130–133. doi:10.1093/annonc/mdq293
Sobrero, A., Lenz, H.-J., Eng, C., Scheithauer, W., Middleton, G., Chen, W., et al. (2021). Extended RAS analysis of the phase III EPIC trial: irinotecan + cetuximab versus irinotecan as second-line treatment for patients with metastatic colorectal cancer. Oncologist 26 (2), e261–e269. doi:10.1002/onco.13591
Sobrero, A. F., Maurel, J., Fehrenbacher, L., Scheithauer, W., Abubakr, Y. A., Lutz, M. P., et al. (2008). EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J. Clin. Oncol. 26 (14), 2311–2319. doi:10.1200/JCO.2007.13.1193
Souglakos, J., Androulakis, N., Syrigos, K., Polyzos, A., Ziras, N., Athanasiadis, A., et al. (2006). FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 94 (6), 798–805. doi:10.1038/sj.bjc.6603011
Souglakos, J., Ziras, N., Kakolyris, S., Boukovinas, I., Kentepozidis, N., Makrantonakis, P., et al. (2012). Randomised phase-II trial of CAPIRI (capecitabine, irinotecan) plus bevacizumab vs FOLFIRI (folinic acid, 5-fluorouracil, irinotecan) plus bevacizumab as first-line treatment of patients with unresectable/metastatic colorectal cancer (mCRC). Br. J. Cancer 106 (3), 453–459. doi:10.1038/bjc.2011.594
Stintzing, S., Modest, D. P., Rossius, L., Lerch, M. M., von Weikersthal, L. F., Decker, T., et al. (2016). FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 17 (16), 1426–1434. doi:10.1016/S1470-2045(16)30269-8
Sun, Q., He, M., Zhang, M., Zeng, S., Chen, L., Zhao, H., et al. (2021). Traditional Chinese medicine and colorectal cancer: implications for drug discovery. Front. Pharmacol. 12, 685002. doi:10.3389/fphar.2021.685002
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Szefler, B., and Czeleń, P. (2023). Will the interactions of some platinum (II)-Based drugs with B-vitamins reduce their therapeutic effect in cancer patients? Comparison of chemotherapeutic agents such as cisplatin, carboplatin and oxaliplatin—a review. Int. J. Mol. Sci. 24 (2), 1548. doi:10.3390/ijms24021548
Thirion, P., Michiels, S., Pignon, J. P., Buyse, M., Braud, A. C., Carlson, R. W., et al. (2004). Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J. Clin. Oncol. 22 (18), 3766–3775. doi:10.1200/JCO.2004.03.104
Thomas, A., and Pommier, Y. (2019). Targeting Topoisomerase I in the era of precision medicine. Clin. Cancer Res. 25 (22), 6581–6589. doi:10.1158/1078-0432.CCR-19-1089
Tilton, R., Paiva, A. A., Guan, J.-Q., Marathe, R., Jiang, Z., van Eyndhoven, W., et al. (2010). A comprehensive platform for quality control of botanical drugs (PhytomicsQC): a case study of Huangqin Tang (HQT) and PHY906. Chin. Med. 5, 30. doi:10.1186/1749-8546-5-30
Tournigand, C., André, T., Achille, E., Lledo, G., Flesh, M., Mery-Mignard, D., et al. (2004). FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 22 (2), 229–237. doi:10.1200/JCO.2004.05.113
Uribe, M. L., Marrocco, I., and Yarden, Y. (2021). EGFR in cancer: signaling mechanisms, drugs, and acquired resistance. Cancers (Basel) 13 (11), 2748. doi:10.3390/cancers13112748
Vamvakas, L., Athanasiadis, A., Karampeazis, A., Kakolyris, S., Polyzos, A., Kouroussis, Ch., et al. (2010). Clinical outcome of elderly patients with metastatic colorectal cancer treated with FOLFOXIRI versus FOLFIRI: subgroup analysis of a randomized phase III trial from the Hellenic Oncology Research Group (HORG). Crit. Rev. Oncol. Hematol. 76 (1), 61–70. doi:10.1016/j.critrevonc.2009.08.003
van Erp, N. P. H., Baker, S. D., Zhao, M., Rudek, M. A., Guchelaar, H.-J., Nortier, J. W. R., et al. (2005). Effect of Milk Thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin. Cancer Res. 11 (21), 7800–7806. doi:10.1158/1078-0432.CCR-05-1288
Vodenkova, S., Buchler, T., Cervena, K., Veskrnova, V., Vodicka, P., and Vymetalkova, V. (2020). 5-fluorouracil and other fluoropyrimidines in colorectal cancer: past, present and future. Pharmacol. Ther. 206, 107447. doi:10.1016/j.pharmthera.2019.107447
Walko, C. M., and Lindley, C. (2005). Capecitabine: a review. Clin. Ther. 27 (1), 23–44. doi:10.1016/j.clinthera.2005.01.005
Wang, E., Bussom, S., Chen, J., Quinn, C., Bedognetti, D., Lam, W., et al. (2011). Interaction of a traditional Chinese Medicine (PHY906) and CPT-11 on the inflammatory process in the tumor microenvironment. BMC Med. Genomics 4 (1), 38. doi:10.1186/1755-8794-4-38
Wang, K., Chen, Q., Shao, Y., Yin, S., Liu, C., Liu, Y., et al. (2021). Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. Biomedecine Pharmacother. 133, 111044. doi:10.1016/j.biopha.2020.111044
Wang, Y., Yuan, A.-J., Wu, Y.-J., Wu, L.-M., and Zhang, L. (2023). Silymarin in cancer therapy: mechanisms of action, protective roles in chemotherapy-induced toxicity, and nanoformulations. J. Funct. Foods 100, 105384. doi:10.1016/j.jff.2022.105384
Wu, Q., Zhang, P., Wang, X., Zhang, M., Liao, W., and Li, Q. (2020). Cost-effectiveness of Capecitabine+ irinotecan versus Leucovorin+ Fluorouracil+ irinotecan in the second-line treatment of metastatic colorectal cancer in China. Clin. Ther. 42 (11), 2148–2158. doi:10.1016/j.clinthera.2020.08.015
Xiong, F., Zhou, Y.-W., Hao, Y.-T., Wei, G.-X., Chen, X.-R., and Qiu, M. (2023). Combining anti-epidermal growth factor receptor (EGFR) therapy with immunotherapy in metastatic colorectal cancer (mCRC). Expert Rev. Gastroenterol. Hepatol. 0 (0), 1–8. doi:10.1080/17474124.2023.2232718
Xu, R.-H., Muro, K., Morita, S., Iwasa, S., Han, S. W., Wang, W., et al. (2018). Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 19 (5), 660–671. doi:10.1016/S1470-2045(18)30140-2
Xu, Y., and Villalona-Calero, M. A. (2002). Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann. Oncol. 13 (12), 1841–1851. doi:10.1093/annonc/mdf337
Yajima, S., Shimizu, H., Sakamaki, H., Ikeda, S., Ikegami, N., and Murayama, J.-I. (2015). Real-world cost analysis of chemotherapy for colorectal cancer in Japan: detailed costs of various regimens during the entire course of chemotherapy. BMC Health Serv. Res. 16 (1), 2–10. doi:10.1186/s12913-015-1253-x
Yang, B.-B., Wu, C.-Y., Chen, E., Infante, J. R., Chen, A., Gao, B., et al. (2013). Pharmacokinetics of irinotecan with and without panitumumab coadministration in patients with metastatic colorectal cancer. Clin. Pharmacol. Drug Dev. 2 (3), 205–212. doi:10.1002/cpdd.35
Yang, S.-H., Lin, J.-K., Chen, W.-S., and Chiu, J.-H. (2003). Anti-angiogenic effect of silymarin on colon cancer lovo cell line. J. Surg. Res. 113 (1), 133–138. doi:10.1016/S0022-4804(03)00229-4
Yue, B., Gao, R., Wang, Z., and Dou, W. (2021a). Microbiota-host-irinotecan Axis: a new insight toward irinotecan chemotherapy. Front. Cell. Infect. Microbiol. 11, 710945. doi:10.3389/fcimb.2021.710945
Yue, S.-J., Qin, Y.-F., Kang, A., Tao, H.-J., Zhou, G.-S., Chen, Y.-Y., et al. (2021b). Total flavonoids of Glycyrrhiza uralensis alleviates irinotecan-induced colitis via modification of gut microbiota and fecal metabolism. Front. Immunol. 12, 628358. doi:10.3389/fimmu.2021.628358
Zheng, R., Ma, J., Wang, D., Dong, W., Wang, S., Liu, T., et al. (2018). Chemopreventive effects of silibinin on colitis-associated tumorigenesis by inhibiting IL-6/STAT3 signaling pathway. Mediat. Inflamm. 2018, e1562010. doi:10.1155/2018/1562010
Zhu, J., Liu, A., Sun, X., Liu, L., Zhu, Y., Zhang, T., et al. (2020). Multicenter, randomized, phase III trial of neoadjuvant chemoradiation with capecitabine and irinotecan guided by UGT1A1 status in patients with locally advanced rectal cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 38 (36), 4231–4239. doi:10.1200/JCO.20.01932
Keywords: colorectal cancer, irinotecan, cancer therapy, drug combinations, targeted drug, herbal medicine
Citation: Chai Y, Liu J-L, Zhang S, Li N, Xu D-Q, Liu W-J, Fu R-J and Tang Y-P (2024) The effective combination therapies with irinotecan for colorectal cancer. Front. Pharmacol. 15:1356708. doi: 10.3389/fphar.2024.1356708
Received: 16 December 2023; Accepted: 19 January 2024;
Published: 05 February 2024.
Edited by:
Qingbin Cui, University of Toledo College of Medicine and Life Sciences, United StatesReviewed by:
Xiaolin Qian, Southern Research Institute, United StatesCopyright © 2024 Chai, Liu, Zhang, Li, Xu, Liu, Fu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, bmxpQG11c3QuZWR1Lm1v; Yu-Ping Tang, eXVwaW5ndGFuZ0BzbnRjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.