
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 27 March 2024
Sec. Respiratory Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1354806
 Fei-Fei Gao1†
Fei-Fei Gao1† Dian-Qing Chen2†
Dian-Qing Chen2† Yue-Tong Jiang3†
Yue-Tong Jiang3† Cui-Fei Han3
Cui-Fei Han3 Bi-Yun Lin4
Bi-Yun Lin4 Zhan Yang4
Zhan Yang4 Juan-Hua Quan5
Juan-Hua Quan5 Ying-Huan Xiong4*
Ying-Huan Xiong4* Xin-Tian Chen5*
Xin-Tian Chen5*Lung injury leads to respiratory dysfunction, low quality of life, and even life-threatening conditions. Circular RNAs (circRNAs) are endogenous RNAs produced by selective RNA splicing. Studies have reported their involvement in the progression of lung injury. Understanding the roles of circRNAs in lung injury may aid in elucidating the underlying mechanisms and provide new therapeutic targets. Thus, in this review, we aimed to summarize and discuss the characteristics and biological functions of circRNAs, and their roles in lung injury from existing research, to provide a theoretical basis for the use of circRNAs as a diagnostic and therapeutic target for lung injury.
Lung injury, which can be caused by sepsis, pneumonia, trauma, aspiration pneumonia, and even some treatments, leads to respiratory dysfunction, and seriously affects the quality of life (Matthay et al., 2019). Acute lung injury (ALI) has a high morbidity and mortality of approximately 30%. When the lung tissue fails to fully repair, the lung inflammatory responses may ultimately lead to chronic obstructive pulmonary disease, which is the fourth leading reason of death globally (Tsushima et al., 2009; Pelgrim et al., 2019). Even there are some studies focused on human embryonic stem cells (Wu et al., 2020), utilizing lung spheroid cell-secretome (LSC-Sec) and exosomes (LSC-Exo) for lung injury and fibrosis treatments (Dinh et al., 2020), the challenges of consistency, safety, and clinical applicability of those therapies are not be ignored. Therefore, fully elucidating the underlying development mechanism of lung injury is expected to fundamentally improve the treatment.
Circular RNAs (circRNAs) exist widely. Several specialized computational tools and databases based on different identification strategies have been combined with next-generation sequencing and bioinformatic analysis to identify and analyze circRNAs(Chen et al., 2021). circRNAs are reported to be not only involved in cardiovascular biology (Aufiero et al., 2019), brain injury (Zhu et al., 2020), kidney-related diseases (Chen et al., 2021), but also tumor progression (Shang et al., 2019). However, the knowledge regarding why circRNAs exist in various diseases remains limited, and potential roles in lung injury progression are unclear.
To this end, we aimed to review the characteristics of circRNAs and discuss their potential roles in lung injury caused due to multiple factors. The finding of this review may aid in underscoring the potential of circRNAs to be used as a target for the diagnosis and treatment of lung injuries (Figure 1).
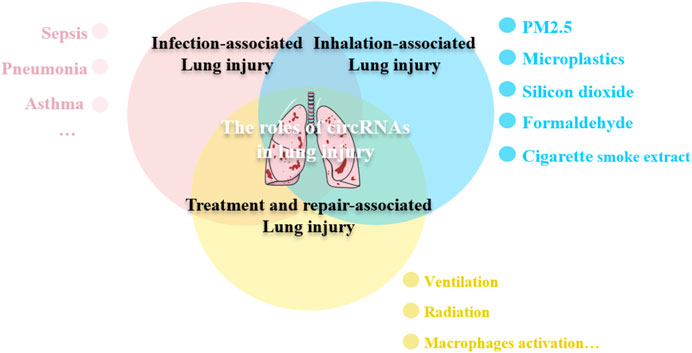
Figure 1. circRNAs participate in the lung injury. Lung injury can be caused by various etiologies, including infection, toxic substances inhalation, even some treatments maybe the contributing injury factors. circRNAs are involved in the pathological process of lung injury through different mechanisms.
CircRNAs range from <100 nucleotides to multiple kilobases (Lasda and Parker, 2014; Aufiero et al., 2019). CircRNAs including exonic circRNAs (ecircRNAs), circular intron circRNAs (ciRNAs), and exon- and intron-derived or retained intron circRNAs (EIciRNA) (Han et al., 2017), are formed by intron pairing, RNA-binding protein, and lariat-driven circularization mechanisms (Aufiero et al., 2019).
These different splicing mechanisms confer consistent characteristics on circRNAs. They are highly conserved in different tissues and conditions; the covalently closed loop structures endow them with RNase resistance, thereby providing them with the properties of a biomarker (Li et al., 2015). Moreover, the specific location and expression of circRNAs lead to different biological functions. The majority of circRNAs are ecircRNAs, which are located in the cytoplasm and can interact with target miRNAs, thereby acting as miRNA sponges or reservoirs. The miRNA sponges cause an increase in the expression of target mRNAs, whereas the miRNA reservoir decreases the target mRNA expression (Memczak et al., 2013; Qu et al., 2015).
circRNAs have been reported to be involved in the development of the human brain, kidney, and liver (Xu et al., 2017). By analyzing the database analysis available on the circBase database, 9,698 circRNA candidates have been detected in fetal lung tissues, which is eight times more than those found in adult lung tissues. RNA sequencing analysis in humans has further verified that the expression of 1,701 circRNAs in fetal lung samples is higher than that in the corresponding adult lung. 452 unique circRNAs are enriched in the lung than in the other organs, suggesting that circRNAs may play crucial roles in human lung development (Xu et al., 2017; Tong et al., 2023).
Bronchopulmonary dysplasia (BPD) is the most common complication associated with extremely preterm infants and its prevalence has been increasing worldwide (Thébaud et al., 2019). circABCC4 promotes BPD progression by facilitating PLA2G6 expression by sequestering miR-663a (Chen et al., 2020). PLA2G6, which belongs to the phospholipase A2 family that is involved in signal transduction and phospholipid homeostasis (Deng et al., 2023), further aggravates lung inflammation by promoting the production of arachidonic acid metabolites (Bellido-Reyes et al., 2006). circABPD1 was also found highly expressed in preterm colostrum milk exosomes, it can alleviate lung injury by targeting the miR-330–3p/HIF1α axis (Li et al., 2023). Three upregulated circRNAs (hsa_circ_0005389, hsa_circ_0000367, and hsa_circ_0059571) and two downregulated circRNAs (hsa_circ_0058495, hsa_circ_0006608) were found in neonatal acute respiratory distress syndrome (NARDS) through high-throughput sequencing in ten clinical blood samples of newborns (Zhou et al., 2021). These findings provide a new therapeutic direction to use circRNAs as molecular markers for early diagnosis of lung injury; nevertheless, existing studies focus only on the changes in circRNA expression levels, based on human or animal models, dynamic observation of circRNA with neonates at different stages as research objects may be solid evidence in clarifying the production and function of circRNAs in lung development.
Sepsis is a systemic inflammatory response syndrome that is triggered by infection with pathogenic bacteria, viruses, or fungi; it is also the major cause of ALI (Wang et al., 2018). Lipopolysaccharide (LPS) is a vital medium for sepsis. The role of circRNAs in infection-associated lung injury has been validated primarily by using clinical sample combined with multiple models of LPS-induced lung injury in vivo and in vitro (As shown in Figure 2).

Figure 2. circRNAs participate in infection-associated lung injury. The schematic diagram depicts the known role of circRNAs in infection-associated lung injury progression.
Some circRNAs are found in samples of patients with ALI. Compared to those in healthy controls, 35 circRNAs were upregulated and 9 were downregulated in patients with sepsis, hsa_circ_0003091 (mmu_circ_0015268) were found to be significantly elevated both in ALI patient and mice. Mechanically, hsa_circ_0003091 sponged miR-149 to upregulate the expression of Smad2, thereby contributing to pulmonary injury, cell apoptosis, and inflammatory responses (Shen et al., 2022). Rho-associated coiled-coil-containing protein kinase I (ROCK1), a member of the serine/threonine protein kinase family, primarily exists in the lung tissues and exhibits facilitating effect on inflammation in ALI (Ishizaki, 2003; Meng et al., 2019). circANKRD36 expression was significantly elevated in the serum of patients with sepsis-induced ALI. circANKRD36 serves as a sponge for miR-330, leading to the increase of ROCK1 expression and, aggravating inflammation of LPS-stimulated RAW264.7 cells (Lin et al., 2021). Programmed cell death 4 (PDCD4), a well-known tumor suppressive protein has been demonstrated as a novel modulator in inflammation response by activating several inflammatory signaling, including the NF-κB pathway (Su et al., 2015). circ-UQCRC2 is upregulated in the serum of patients with pneumonia and LPS-treated MRC-5 cells. circ-UQCRC2 directly target miR-326 to upregulate PDCD4 expression for the activation of NF-κB pathway (Zhou et al., 2021).
Mitogen-activated protein kinase 14 (MAPK14) is ubiquitously expressed in various cell types and, exhibits a vital role in response to inflammation (Zhang et al., 2021). hsa_circ_0026579 (circESPL1) expression is significantly upregulated in patients with pneumonia and acts as a sponge of miR-326 for MAPK14 activation during LPS-induced lung cell injury (Liang et al., 2022).
The roles of circRNA in lung injury have been validated both in vivo and in vitro. 20 circRNAs were found to be upregulated and 18 were downregulated in ALI mice induced by cecal ligation and puncture. These circRNAs were found to be closely associated with the inflammatory response (e.g., the TGF-β, MAPK, Fc gamma R-mediated phagocytic, TNF, and chemokine signaling pathways) using bioinformatics analyses (Yuan et al., 2020; Teng et al., 2021). circPTK2 was upregulated in cecal ligation and puncture-based mouse and LPS-based alveolar type II cell (RLE-6TN), and reasonable for the ATP efflux, pyroptosis, and inflammation through upregulating eIF5A expression by competitively adsorbing miR-766 (Ding et al., 2023). Similarly, upregulated circTDRD9 acted as miR-223-3p sponge to increase RAB10 expression, also promoting LPS-induced lung injuries (Zhang et al., 2023).
CircRNAs could play a protective role against lung injury. Reportedly, 21 upregulated and 55 downregulated circRNAs are involved in the progression of LPS-induced autophagy in human bronchial epithelial cell 16HBE(Liu et al., 2021). Furthermore adipose-derived stem cell exosomes have high levels of the circular RNA (circ)-Fryl, which plays a protective role against sepsis-induced mouse lung injury by decreasing apoptosis and inflammatory factor expression. Mechanistically, miR-490-3p and SIRT3 are downstream targets of circ-Fryl. circ-Fryl overexpression promotes autophagy by inducing SIRT3/AMPK signaling and sponging miR-490-3p (Shen et al., 2022).
Moreover, circC3P1 is downregulated in ALI mice induced by sepsis; it attenuates pro-inflammatory cytokine production and cell apoptosis through the modulation of miR-21 (Jiang et al., 2020). Elevated circVMA21 levels suppress oxidative stress, apoptosis, and inflammation via mediating the miR-497-5p/CD2AP axis to mitigate ALI in sepsis rats (Ke et al., 2022). circ_0038467 knockdown alleviates LPS-induced inflammatory injury in 16HBE cells by sponging miR-338-3p and inhibiting the activation of JAK/STAT3 pathway (Liu et al., 2020). Similarly, circHECTD1 is downregulated in LPS-induced human and mouse AECs [HBE and murine lung epithelial-12 (MLE-12)]; it inhibits the apoptosis of AECs through the miR-320a/PIK3CA and miR-136/Sirt1 pathways (Li et al., 2022).
Phospholamban (Pln), cadherin-2 (Cdh2) and Nprl3 are found to participate in the pathogenesis of sepsis and promote inflammation (Black et al., 2018; Weng et al., 2019; Zhuang et al., 2020). mmu_circ_0001679 is reported to regulate the expression of Nprl3, and mmu_circ_0001212 similarly regulates Pln, Cdh2 and Nprl3 expression, which were all increased in the sepsis mice (Zou et al., 2020). ROCK2 aggravates sepsis-caused ALI through association with miR-424 and transendothelial migration of polymorphonuclear leukocytes (Li et al., 2010; Chen et al., 2020). CircPALM2 is increased, and involved in LPS-caused MLE-12 cell damage by targeting miR-330-5p, thereby leading to ROCK2 activation (Ren et al., 2022).
circRNAs can aggravate lung injury by maintaining the activation of the NF-κB, MAPK, and WNT pathways. Alveolar epithelial cell-produced thymic stromal lymphopoietin (TSLP) has been shown to worsen ALI by triggering airway inflammation. miR-291a-3p can directly bind to the 3′-UTR of TSLP and suppress TSLP expression. circNCLN has been identified to act as a sponge to antagonize miR-291a-3p and thereby maintain the expression of TSLP (Cao et al., 2022). circ_0054633 is over-expressed in LPS-induced rats and murine pulmonary microvascular endothelial cells, through activating the NF-κB pathways (Yang et al., 2021). Similarly, circANKRD36 is upregulated in LPS-induced MRC-5 cells, and associated with cell injury through regulating miR-31/MyD88-mediated activation of the NF-κB pathway (Guo et al., 2020).
Activation of the MAPK and Wnt pathways are responsible for neutrophil infiltration and pro-inflammatory cytokine production (Cheng et al., 2018). Circ_0001679 is upregulated in LPS-induced MLE-12 cells, and maintains a high expression of MAPK1 by suppressing miR-338-3p, leading to the increased apoptosis (Lu et al., 2022). It is found that mmu_circRNA_42341, mmu_circRNA_44122, and mmu_circRNA_44123 were substantially upregulated, whereas mmu_circRNA_010498, mmu_circRNA_25030, and mmu_circRNA_010498 were significantly downregulated through microchip analysis. These differentially expressed circRNAs were chiefly involved in the MAPK and Wnt signaling pathways (Li et al., 2019).
C–X–C motif chemokine receptor 1 (CXCR1) is necessary for the activation of inflammatory mediators, CXCR1 antagonism has been proposed as a protective strategy against bacterial pneumonia (Wei et al., 2013; Ha et al., 2017). LPS upregulates circTMOD3 expression in normal lung fibroblast (WI-38) cells, and circTMOD3 functions as a competing endogenous RNA for miR-146b-3p to induce CXCR1 expression (Ma et al., 2021). Similarly, Kruppel-like transcription factor 4 (KLF4) is an inflammatory palliative in sepsis (Li et al., 2018). circ_VMA21 was downregulated in pneumonia samples and LPS-treated WI-38 cells, and circ_VMA21 could sponge miR-409-3p to induce the expression of KLF4 (Wang et al., 2021).
In addition to bacterial inflammation, lung injury can be caused by other pathogens. circRNAs Slco3a1 and Wdr33 were aberrantly expressed in the plasma of influenza A virus-induced ALI patients. Biological process analysis revealed that both circRNAs might be involved in the mitochondrial function and superoxide metabolic process (Wang et al., 2021). Moreover, house dust mite is the major allergen contributor to asthma, circRNAs vacuolar protein sorting 33A (circVPS33A, circ_0000455) was highly expressed in a murine asthma model and Dermatophagoides pteronyssinus peptidase 1-treated BEAS-2B cells. circVPS33A targeted miR-192-5p to upregulate the expression of high-mobility group box 1 (HMGB1), a strong pro-inflammatory mediator in the pathogenesis of asthma, leading to lung injury (Imbalzano et al., 2017; Su et al., 2021).
Inhalation exposure to toxic substances, such as PM2.5, polystyrene microplastics (PS-MPs), phagocytosis of silicon dioxide (SiO2), formaldehyde (FA), and cigarette smoke extract (CSE) could compromise respiratory epithelial barrier integrity and induce inflammation and lung injury. Multiple studies have defined circRNAs as potential disease modifier in lung injury caused by multiple environmental factors (Table.1).
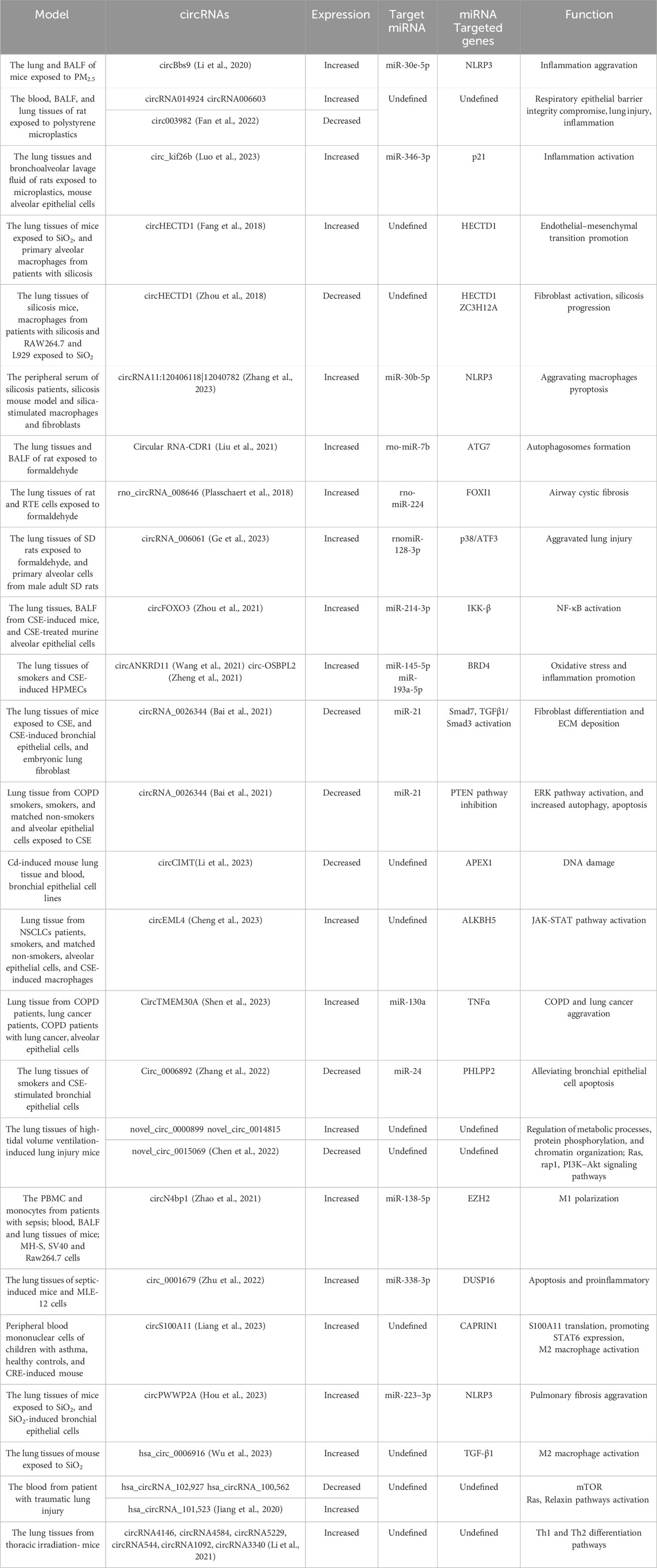
Table 1. circRNAs are involved in inhalation-induced lung injury and lung treatment and repair. Multiple circRNAs are involved in lung injury and repair.
PM2.5 inhalation upregulates the expression of circBbs9, which binds to miR-30e-5p for the activation of NLRP3, aggravating lung inflammation (Li et al., 2020). circRNA 014924 and circRNA 006603 were upregulated and circ003982 was downregulated in the rat lung tissues on PS-MP exposure (Fan et al., 2022). What’s more, PS-MPs inhalation increased circ_kif26b levels in alveolar epithelial cells, which upregulated the expression p21 by binding to miR-346-3p. The increased p21 expression activated SASP and increased the secretion of inflammatory factors IL-6 and IL-8, promoting alveolar epithelial cell senescence and participating in inflammatory lung injury (Luo et al., 2023)
Similarly, SiO2 exposure is the biggest promoter of silicosis. SiO2 exposure downregulates circHECTD1 levels and increased HECTD1 protein expression. The increased HECTD1 protein expression is associated with macrophage activation and contributing to the progression of silicosis (Fang et al., 2018; Zhou et al., 2018). It is found that circRNA11:120406118|12040782 was increased in the peripheral serum of silicosis patients, which facilitated the progress of silicosis by aggravating NLRP3-mediated macrophages pyroptosis through sponging miR-30b-5p (Zhang et al., 2023).
Formaldehyde, a prevailing air pollutant, has seriously threatened public health in recent years (Zhao et al., 2021). Long-term formaldehyde inhalation upregulates the expression of circRNA-CDR1 in rat lung tissues in a dose-dependent manner. Mechanistically, circRNA CDR1 suppresses rno-miR-7b to elevate ATG7 expression, which is necessary for the formation of autophagosomes, consequently resulting lung injury (Tanida et al., 2012; Liu et al., 2021). Similarly, rno_circRNA_008646 and circRNA_006061 were also significantly high in rat lung tissues when exposed to formaldehyde (Yang et al., 2022). rno_circRNA_008646 sponges rno-miR-224 to upregulate the expression of forkhead box I1 (FOXI1) (Plasschaert et al., 2018), and circRNA_006061 activated p38/ATF3 pathway expression via sponging the rnomiR-128-3p (Ge et al., 2023), contributing to airway cystic fibrosis.
To date, evidence indicates that smoking, including e-cigarettes, also can induce lung inflammation and injury. The roles of circRNAs in CSE-induced lung injury cannot be overlooked. circFOXO3 is significantly upregulated in cigarette smoke-exposed mice lungs and CSE-treated murine alveolar epithelial cells. circFOXO3 sponge miR-214-3p to the upregulate IKK-β mRNA, thereby resulting in NF-κB signaling activation (Zhou et al., 2021). Bromo-domain-containing 4(BRD4) participates in promoting inflammation and oxidative stress (Song et al., 2020). circANKRD11 and circ-OSBPL2 are highly expressed in the lung tissues of smokers and CSE-induced human pulmonary microvascular endothelial and bronchial epithelioid cells (Wang et al., 2021). circANKRD11 can sponge miR-145-5p to upregulate the expression of BRD4, and circ-OSBPL2 serve as a sponge for miR-193a-5p, which also upregulates BRD4 in HBECs(Zheng et al., 2021). Meanwhile, circRNA_0026344 is downregulated in CSE-induced bronchial epithelial cells, with increasing levels of miR-21. The elevated miR-21 can be transported to bronchial fibroblasts through exosomes, leading to the inhibition of Smad7 expression and activation of the TGFβ1/Smad3 pathway, thereby contributing to bronchial fibroblast differentiation and ECM deposition (Bai et al., 2021). In alveolar epithelial cells, CS decreases circRNA_0026344 levels, which sponges miR-21 to inhibit the PTEN, leading to the activation of ERK pathway and increased autophagy and apoptosis, contributing to emphysema (Bai et al., 2021).
The persistent lung inflammation induced by the inhalation of environmental pollutants causes chronic morbidity, as well as leads to sudden and fatal lung dysfunction. It is also reported that CSE-induced circRNAs are associated with tumor progression. Exposure of human lung tissue to Cadmium (Cd) is mainly through the inhalation of cigarette smoke and airborne particulate. Lower circCIMT expression was associated with DNA damage in the mouse lung tissue and blood after Cd exposure, which contributing to the acquisition of tumor characteristics of lung epithelial cells (Li et al., 2023). What’s more, smoking-induced M2 macrophages via circEML4 in extracellular vesicles promote the non small cell lung cancer progression through ALKBH5-regulated m6A modification of SOCS2, which leading to the activation of JAK-STAT pathway (Cheng et al., 2023). Similarly, CircTMEM30A was highly expressed in COPD patients with lung cancer, and it regulated the expression of TNFα through miR-130a, thereby promoting the progression of COPD and lung cancer (Shen et al., 2023). Whereas, circRNAs have also been reported to play protective roles in CSE-induced lung injury. PH domain and leucinerich repeat protein phosphatase 2 (PHLPP2) inhibit inflammation in the progression of lung cancer and injury (Gu et al., 2018; Yan et al., 2018). circ_0006892 is downregulated in lung tissues of smokers and CSE-stimulated bronchial epithelial cells. It can promote PHLPP2 expression via regulating miR-24 and alleviating CSE-induced apoptosis and inflammatory response (Zhang et al., 2022).
Pulmonary dysfunction caused by lung injury triggers a self-repair process and may partly require the auxiliary treatment of mechanical ventilation. Improper use of a ventilator can worsen lung injury. Numerous significant circRNAs likely participate in the pathological process (Table.1).
Compared to those in the control group, 171 circRNAs were significantly upregulated and 114 were significantly downregulated in the lung tissues of high-tidal volume ventilation-induced mice. novel_circ_0000899 and novel_circ_0014815 were identified to be the most upregulated circRNAs, whereas novel_circ_0015069 was the most downregulated circRNA. These circRNAs were found to be involved in metabolic processes, and in the pathway of Ras, Rap1, and PI3K/Akt (Chen et al., 2022).
Macrophages can be activated and polarized in response to lung injury. The classically activated pro-inflammatory macrophage (M1) and alternatively activated anti-inflammatory macrophage (M2) have been extensively investigated in lung injury, repair, and fibrosis (Sica and Mantovani, 2012; Cheng et al., 2021). The circRNA expression patterns in macrophage activation in lung injury were analyzed by many studies.
11 and 126 circRNAs were found to be significantly upregulated and downregulated, respectively, in pulmonary macrophage polarization. Further biological analysis revealed that the upregulated circRNAs were involved in mitochondrion distribution regulation and Notch binding, whereas the downregulated ones were primarily mainly involved in histone H3K27 methylation (Bao et al., 2019).
EZH2, a histone methyltransferase, is involved in sepsis-induced inflammation and lung injury through modulating macrophage M1 polarization (Zhang et al., 2019). circN4bp1was overexpressed in PBMC and monocytes, and was correlated with a poor prognosis in sepsis induced ALI patients. circN4bp1 can sponge miR-138-5p for the expression of EZH2 (Zhao et al., 2021). Similarly, dual-specificity phosphatases 16 (DUSP16) could be inducible in macrophages, and negatively regulate the JNK pathway to attenuate metabolic stress-triggered hepatic steatosis (Zhang et al., 2015; Wu et al., 2020). circ_0001679 was overexpressed in sepsis-induced ALI mice and MLE-12 cells. It bound to mmu-miR-338-3p and miR-338-3p targeted DUSP16 3′-UTR to reduce DUSP16 expression and aggravate injury (Zhu et al., 2022). circS100A11 was dominantly expressed in monocytes and significantly upregulated in children with asthma, reasonably for the M2 macrophage activation. Mechanistically, circS100A11 promoted S100A11 translation, which liberated SP3 from nucleolin and increased STAT6 expression (Liang et al., 2023). Similarily, circPWWP2A could adsorb miR-223–3p to regulate NLRP3 after silica stimulation in pulmonary fibrosis (Hou et al., 2023), and hsa_circ_0006916 was upregulated in pulmonary fibrosis, associated with the high expression level of M2 molecule TGF-β1, playing an important role in the activation of M1-M2 polarization (Wu et al., 2023).
In this review, we summarized the origin and functions of circRNAs, and discussed their roles in lung development and injury caused by different etiologies. However, their role in lung injury remains mostly unelucidated, and the functions of most circRNAs are still not fully analyzed and require further exploration.
First, various factors can cause lung injury. Hemorrhagic shock and thoracic trauma can lead to lethal lung injuries. Reportedly, 13 circRNAs were significantly upregulated and 16 were downregulated in hemorrhagic shock-induced ALI rat lung tissues; these circRNAs might participate in DNA damage recognition and repair (Wang et al., 2021). Furthermore, downregulated hsa_circRNA_102,927 and hsa_circRNA_100,562, and upregulated hsa_circRNA_101,523 were identified in the plasma samples of patients with traumatic lung injury (Jiang et al., 2020). Radiation-induced lung injury (RILI) is a key threat to patients who undergo thoracic radiotherapy, in the thoracic irradiation-induced RILI mice, 10 circRNAs were downregulated and 17 were upregulated (including circRNA4146, circRNA4584, circRNA5229, circRNA544, circRNA1092, and circRNA3340), which are reported related to the Th1 and Th2 differentiation pathways (Li et al., 2021). Those results suggest that circRNAs are involved in the process of lung injury caused by various etiologies but are limited to undefined specific pathology. Current studies are mainly focused on the relationship between circRNA and a certain signaling pathway in a lung injury-related model, and changes in signaling pathways further regulate inflammatory. The crosstalk of various pathways of inflammatory, possible role of circRNA in the regulation of those pathways, and whether some specific circRNAs are involved in all the processes of lung injury caused by multiple etiologies still need to be explored.
Second, circRNAs are universal and stable, and may serve as novel biomarkers. The downregulated hsa_circRNA_042882 and upregulated hsa_circRNA_104034 in bronchoalveolar lavage fluid were regarded as promising diagnostic biomarkers for patients with ARDS caused by severe pneumonia (Sun et al., 2023). Blood samples are mainly collected from patients for the current study of lung injury. Whether circRNAs exist in patients’ sputum, urine, and other body fluids remains unclear. Although animal studies have reproduced the expression of some circRNAs in humans, the screening process for these circRNAs involves a small size of patients; thus, larger patient cohort studies are warranted. Moreover, even most circRNAs are reported as biomarkers for qualitative diagnosis, the correlation between the circRNA levels and the degree of disease severity is not well analyzed, and whether circRNA expression is associated with lung function requires further exploration.
Third, interpreting the role of macrophages in lung injury repair and fibrosis is complicated. In the lung injury stage, macrophages display a pro-inflammatory phenotype aggravating injury chief by M1. However, during the lung repair process, the function of M2 is dominant; it contributes to lung fibroblast cell proliferation and differentiation, leading to the risk of pulmonary fibrosis. The critical division in the circRNA balance of macrophage polarization is still unknown. Moreover, three different populations of macrophages are existed, namely, airway, alveolar, and interstitial macrophages (Jiang and Zhu, 2016; Hesketh et al., 2017; Joshi et al., 2018). The effect of circRNAs on different populations of macrophages requires further research. In addition to macrophages, other inflammatory cells, such as neutrophils, are involved in the inflammatory process. Whether circRNAs are involved in regulating neutrophils in the process of lung injury needs to be further explored.
What’s more, antisense oligonucleotides are artificially synthesized specific nucleic acid sequences that specifically target ncRNAs, such as lncRNAs and circRNAs. They have been approved by the FDA for clinical application, making it possible for selectively targeted circRNAs to be used in diagnostic and therapeutic approaches (Chan et al., 2006; Das et al., 2021). Whether the antisense oligonucleotides can be used for lung injury treatment are still a long way off.
In summary, circRNA has great potential as a diagnostic, therapeutic and prognostic target in lung injury diseases, fully elucidating the underlying mechanism of circRNAs in lung injury may radically improve the treatment. Continuous development of biotechnology and further exploration of circRNAs would greatly benefit patients with ALI.
F-FG: Writing–original draft. D-QC: Writing–original draft. Y-TJ: Writing–original draft. C-FH: Investigation, Visualization, Writing–review and editing. B-YL: Investigation, Writing–review and editing. ZY: Visualization, Writing–review and editing. J-HQ: Investigation, Visualization, Writing–review and editing. Y-HX: Writing–review and editing. X-TC: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Youth Cultivation Fund of Guangdong Medical University (GDMUQ2022002), and Start-up Fund of Scientific Research for High-level talents (2081z20220003), and the Competitive Allocation Project of Zhanjiang Municipal Science and Technology Development Special Fund (2022A01156), and Guangdong Medical Science and Technology Research Foundation Project (A2023338).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aufiero, S., Reckman, Y. J., Pinto, Y. M., and Creemers, E. E. (2019). Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. 16, 503–514. doi:10.1038/s41569-019-0185-2
Bai, J., Deng, J., Han, Z., Cui, Y., He, R., Gu, Y., et al. (2021). CircRNA_0026344 via exosomal miR-21 regulation of Smad7 is involved in aberrant cross-talk of epithelium-fibroblasts during cigarette smoke-induced pulmonary fibrosis. Toxicol. Lett. 347, 58–66. doi:10.1016/j.toxlet.2021.04.017
Bao, X., Zhang, Q., Liu, N., Zhuang, S., Li, Z., Meng, Q., et al. (2019). Characteristics of circular RNA expression of pulmonary macrophages in mice with sepsis-induced acute lung injury. J. Cell. Mol. Med. 23, 7111–7115. doi:10.1111/jcmm.14577
Bellido-Reyes, Y. A., Akamatsu, H., Kojima, K., Arai, H., Tanaka, H., and Sunamori, M. (2006). Cytosolic phospholipase A2 inhibition attenuates ischemia-reperfusion injury in an isolated rat lung model. Transplantation 81, 1700–1707. doi:10.1097/01.tp.0000226065.82066.21
Black, M., Milewski, D., Le, T., Ren, X., Xu, Y., Kalinichenko, V. V., et al. (2018). FOXF1 inhibits pulmonary fibrosis by preventing CDH2-CDH11 cadherin switch in myofibroblasts. Cell Rep. 23, 442–458. doi:10.1016/j.celrep.2018.03.067
Cao, J., Kuang, D., Luo, M., Wang, S., and Fu, C. (2022). Targeting circNCLN/miR-291a-3p/TSLP signaling axis alleviates lipopolysaccharide-induced acute lung injury. Biochem. Biophys. Res. Commun. 617, 60–67. doi:10.1016/j.bbrc.2022.05.095
Chan, J. H., Lim, S., and Wong, W. S. (2006). Antisense oligonucleotides: from design to therapeutic application. Clin. Exp. Pharmacol. Physiol. 33, 533–540. doi:10.1111/j.1440-1681.2006.04403.x
Chen, H., Hu, X., Li, R., Liu, B., Zheng, X., Fang, Z., et al. (2020a). LncRNA THRIL aggravates sepsis-induced acute lung injury by regulating miR-424/ROCK2 axis. Mol. Immunol. 126, 111–119. doi:10.1016/j.molimm.2020.07.021
Chen, S., Xia, J., Zhan, Q., and Zhang, Y. (2022). Microarray analysis reveals the changes in circular RNA expression and molecular mechanisms in mice with ventilator-induced lung injury. Front. Physiol. 13, 838196. doi:10.3389/fphys.2022.838196
Chen, X. T., Li, Z. W., Zhao, X., Li, M. L., Hou, P. F., Chu, S. F., et al. (2021). Role of circular RNA in kidney-related diseases. Front. Pharmacol. 12, 615882. doi:10.3389/fphar.2021.615882
Chen, Y. F., Feng, D. D., Wu, S. H., Lu, H. Y., Banu Pasha, A., Permall, D. L., et al. (2020b). Promotion of bronchopulmonary dysplasia progression using circular RNA circabcc4 via facilitating PLA2G6 expression by sequestering miR-663a. Front. Cell Dev. Biol. 8, 585541. doi:10.3389/fcell.2020.585541
Cheng, C., Wang, P., Yang, Y., Du, X., Xia, H., Liu, J., et al. (2023). Smoking-induced M2-TAMs, via circEML4 in EVs, promote the progression of NSCLC through ALKBH5-regulated m6A modification of SOCS2 in NSCLC cells. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 10, doi:10.1002/advs.202300953
Cheng, L., Zhao, Y., Qi, D., Li, W., and Wang, D. (2018). Wnt/β-catenin pathway promotes acute lung injury induced by LPS through driving the Th17 response in mice. Biochem. Biophys. Res. Commun. 495, 1890–1895. doi:10.1016/j.bbrc.2017.12.058
Cheng, P., Li, S., and Chen, H. (2021). Macrophages in lung injury, repair, and fibrosis. Cells 10 . doi:10.3390/cells10020436
Das, D., Das, A., and Panda, A. C. (2021). Antisense oligo pulldown of circular RNA for downstream analysis. Bio Protoc. 11, e4088. doi:10.21769/BioProtoc.4088
Deng, X., Yuan, L., Jankovic, J., and Deng, H. (2023). The role of the PLA2G6 gene in neurodegenerative diseases. Ageing Res. Rev. 89, 101957. doi:10.1016/j.arr.2023.101957
Ding, F., Zhu, J., and Hu, Y. (2023). Circular RNA protein tyrosine kinase 2 aggravates pyroptosis and inflammation in septic lung tissue by promoting microRNA-766/eukaryotic initiation factor 5A axis-mediated ATP efflux. Acta Cir. Bras. 38, e380323. doi:10.1590/acb380323
Dinh, P. C., Paudel, D., Brochu, H., Popowski, K. D., Gracieux, M. C., Cores, J., et al. (2020). Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 11, 1064. doi:10.1038/s41467-020-14344-7
Fan, Z., Xiao, T., Luo, H., Chen, D., Lu, K., Shi, W., et al. (2022). A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics. Environ. Int. 163, 107223. doi:10.1016/j.envint.2022.107223
Fang, S., Guo, H., Cheng, Y., Zhou, Z., Zhang, W., Han, B., et al. (2018). circHECTD1 promotes the silica-induced pulmonary endothelial-mesenchymal transition via HECTD1. Cell Death Dis. 9, 396. doi:10.1038/s41419-018-0432-1
Ge, P., Yuan, X. W., Zhang, X., Liu, Z. H., Wang, S. Y., Yang, Y. Q., et al. (2023). Rno_circRNA_006061 participates in apoptosis induced by formaldehyde via activating p38/ATF3 pathway. Chem. Biol. Interact. 381, 110584. doi:10.1016/j.cbi.2023.110584
Gu, W., Yuan, Y., Yang, H., Wu, H., Wang, L., Tang, Z., et al. (2018). Role of miR-195 in cigarette smoke-induced chronic obstructive pulmonary disease. Int. Immunopharmacol. 55, 49–54. doi:10.1016/j.intimp.2017.11.030
Guo, R., Zhang, L., and Meng, J. (2020). Circular RNA ANKRD36 attends to lipopolysaccharide-aroused MRC-5 cell injury via regulating microRNA-31-3p. Biofactors 46, 391–401. doi:10.1002/biof.1592
Ha, H., Debnath, B., and Neamati, N. (2017). Role of the CXCL8-CXCR1/2 Axis in cancer and inflammatory diseases. Theranostics 7, 1543–1588. doi:10.7150/thno.15625
Han, Y. N., Xia, S. Q., Zhang, Y. Y., Zheng, J. H., and Li, W. (2017). Circular RNAs: a novel type of biomarker and genetic tools in cancer. Oncotarget 8, 64551–64563. doi:10.18632/oncotarget.18350
Hesketh, M., Sahin, K. B., West, Z. E., and Murray, R. Z. (2017). Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 18, 1545. doi:10.3390/ijms18071545
Hou, L., Zhu, Z., Jiang, F., Zhao, J., Jia, Q., Jiang, Q., et al. (2023). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circPWWP2A/miR-223-3p/NLRP3 axis. Ecotoxicol. Environ. Saf. 251, 114537. doi:10.1016/j.ecoenv.2023.114537
Imbalzano, E., Quartuccio, S., Di Salvo, E., Crea, T., Casciaro, M., and Gangemi, S. (2017). Association between HMGB1 and asthma: a literature review. Clin. Mol. allergy CMA 15, 12. doi:10.1186/s12948-017-0068-1
Ishizaki, T. (2003). Rho-mediated signal transduction and its physiological roles. Nihon yakurigaku Zasshi. Folia Pharmacol. Jpn. 121, 153–162. doi:10.1254/fpj.121.153
Jiang, W. Y., Ren, J., Zhang, X. H., Lu, Z. L., Feng, H. J., Yao, X. L., et al. (2020a). CircC3P1 attenuated pro-inflammatory cytokine production and cell apoptosis in acute lung injury induced by sepsis through modulating miR-21. J. Cell. Mol. Med. 24, 11221–11229. doi:10.1111/jcmm.15685
Jiang, Y., Zhu, F., Wu, G. S., Wang, K. A., Wang, C., Yu, Q., et al. (2020b). Microarray and bioinformatics analysis of circular RNAs expression profile in traumatic lung injury. Exp. Ther. Med. 20, 227–234. doi:10.3892/etm.2020.8686
Jiang, Z., and Zhu, L. (2016). Update on the role of alternatively activated macrophages in asthma. J. Asthma Allergy 9, 101–107. doi:10.2147/JAA.S104508
Joshi, N., Walter, J. M., and Misharin, A. V. (2018). Alveolar macrophages. Cell. Immunol. 330, 86–90. doi:10.1016/j.cellimm.2018.01.005
Ke, J., Chen, M., Ma, S., Zhang, L., and Zhang, L. (2022). Circular RNA VMA21 ameliorates lung injury in septic rat via targeting microRNA-497-5p/CD2-associated protein axis. Bioengineered 13, 5453–5466. doi:10.1080/21655979.2022.2031406
Lasda, E., and Parker, R. (2014). Circular RNAs: diversity of form and function. RNA 20, 1829–1842. doi:10.1261/rna.047126.114
Li, H., Ma, K., Dou, H., Liu, L., Qian, Y., Li, S., et al. (2023a). CircABPD1 alleviates oxidative lung injury of bronchopulmonary dysplasia through regulating miR-330-3p/HIF1α axis. Int. J. Biochem. Cell Biol. 163, 106464. doi:10.1016/j.biocel.2023.106464
Li, H., Niu, X., Shi, H., Feng, M., Du, Y., Sun, R., et al. (2022). circHECTD1 attenuates apoptosis of alveolar epithelial cells in acute lung injury. Lab. Invest. 102, 945–956. doi:10.1038/s41374-022-00781-z
Li, M., Chen, W., Cui, J., Lin, Q., Liu, Y., Zeng, H., et al. (2023b). circCIMT silencing promotes cadmium-induced malignant transformation of lung epithelial cells through the DNA base excision repair pathway. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 10, doi:10.1002/advs.202206896
Li, M., Hua, Q., Shao, Y., Zeng, H., Liu, Y., Diao, Q., et al. (2020). Circular RNA circBbs9 promotes PM(2.5)-induced lung inflammation in mice via NLRP3 inflammasome activation. Environ. Int. 143, 105976. doi:10.1016/j.envint.2020.105976
Li, X., Yuan, Z., Chen, J., Wang, T., Shen, Y., Chen, L., et al. (2019). Microarray analysis reveals the changes of circular RNA expression and molecular mechanism in acute lung injury mouse model. J. Cell. Biochem. 120, 16658–16667. doi:10.1002/jcb.28924
Li, Y., Wu, Y., Wang, Z., Zhang, X. H., and Wu, W. K. (2010). Fasudil attenuates lipopolysaccharide-induced acute lung injury in mice through the Rho/Rho kinase pathway. Med. Sci. Monit. 16, BR112–118.
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984. doi:10.1038/cr.2015.82
Li, Y., Zou, L., Chu, L., Ye, L., Ni, J., Chu, X., et al. (2021). Identification and integrated analysis of circRNA and miRNA of radiation-induced lung injury in a mouse model. J. Inflamm. Res. 14, 4421–4431. doi:10.2147/JIR.S322736
Li, Z., Jia, Y., Han, S., Wang, X., Han, F., Zhang, J., et al. (2018). Klf4 alleviates lipopolysaccharide-induced inflammation by inducing expression of MCP-1 induced protein 1 to deubiquitinate TRAF6. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol. 47, 2278–2290. doi:10.1159/000491538
Liang, Q., Fu, J., Wang, X., Liu, L., Xiao, W., Gao, Y., et al. (2023). circS100A11 enhances M2a macrophage activation and lung inflammation in children with asthma. Allergy 78, 1459–1472. doi:10.1111/all.15515
Liang, Y., Miao, Y., and Xiang, J. (2022). Circular RNA circESPL1 knockdown alleviates lipopolysaccharide (LPS)-induced lung cell injury via sponging miR-326 to regulate MAPK14. Int. Immunopharmacol. 112, 109146. doi:10.1016/j.intimp.2022.109146
Lin, Q., Liang, Q., Qin, C., and Li, Y. (2021). CircANKRD36 knockdown suppressed cell viability and migration of LPS-stimulated RAW264.7 cells by sponging MiR-330. Inflammation 44, 2044–2053. doi:10.1007/s10753-021-01480-5
Liu, G., Wan, Q., Li, J., Hu, X., Gu, X., and Xu, S. (2020). Circ_0038467 regulates lipopolysaccharide-induced inflammatory injury in human bronchial epithelial cells through sponging miR-338-3p. Thorac. Cancer 11, 1297–1308. doi:10.1111/1759-7714.13397
Liu, J. Y., Jiang, Y. X., Zhang, M. Y., Huo, C., Yang, Y. C., Ji, X. L., et al. (2021a). Comprehensive bioinformatics analysis of lipopolysaccharide-induced altered autophagy in acute lung injury and construction of underlying competing endogenous RNA regulatory mechanism. Biomed. Res. Int. 2021, 6831770. doi:10.1155/2021/6831770
Liu, Q. P., Ge, P., Wang, Q. N., Zhang, S. Y., Yang, Y. Q., Lv, M. Q., et al. (2021b). Circular RNA-CDR1as is involved in lung injury induced by long-term formaldehyde inhalation. Inhal. Toxicol. 33, 325–333. doi:10.1080/08958378.2021.1999350
Lu, S., Wu, X., Xin, S., Zhang, J., Lin, H., Miao, Y., et al. (2022). Knockdown of circ_0001679 alleviates lipopolysaccharide-induced MLE-12 lung cell injury by regulating the miR-338-3p/mitogen-activated protein kinase 1 axis. Bioengineered 13, 5803–5817. doi:10.1080/21655979.2022.2034564
Luo, H., Xiao, T., Sun, X., Song, Y., Shi, W., Lu, K., et al. (2023). The regulation of circRNA_kif26b on alveolar epithelial cell senescence via miR-346-3p is involved in microplastics-induced lung injuries. Sci. Total Environ. 882, 163512. doi:10.1016/j.scitotenv.2023.163512
Ma, K., Wang, W., Gao, C., and He, J. (2021). The role of circTMOD3 in regulating LPS-induced acute inflammation and injury in human lung fibroblast WI-38 cells. Exp. Lung Res. 47, 311–322. doi:10.1080/01902148.2021.1940376
Matthay, M. A., Zemans, R. L., Zimmerman, G. A., Arabi, Y. M., Beitler, J. R., Mercat, A., et al. (2019). Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 5, 18. doi:10.1038/s41572-019-0069-0
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi:10.1038/nature11928
Meng, L., Cao, H., Wan, C., and Jiang, L. (2019). MiR-539-5p alleviates sepsis-induced acute lung injury by targeting ROCK1. Folia histochem. Cytobiol. 57, 168–178. doi:10.5603/FHC.a2019.0019
Pelgrim, C. E., Peterson, J. D., Gosker, H. R., Schols, A., van Helvoort, A., Garssen, J., et al. (2019). Psychological co-morbidities in COPD: targeting systemic inflammation, a benefit for both. Eur. J. Pharmacol. 842, 99–110. doi:10.1016/j.ejphar.2018.10.001
Plasschaert, L. W., Žilionis, R., Choo-Wing, R., Savova, V., Knehr, J., Roma, G., et al. (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381. doi:10.1038/s41586-018-0394-6
Qu, S., Yang, X., Li, X., Wang, J., Gao, Y., Shang, R., et al. (2015). Circular RNA: a new star of noncoding RNAs. Cancer Lett. 365, 141–148. doi:10.1016/j.canlet.2015.06.003
Ren, Y., Li, L., Wang, M., Yang, Z., Sun, Z., Zhang, W., et al. (2022). Knockdown of circRNA paralemmin 2 ameliorates lipopolysaccharide-induced murine lung epithelial cell injury by sponging miR-330-5p to reduce ROCK2 expression. Immunol. Invest. 51, 1707–1724. doi:10.1080/08820139.2022.2027961
Shang, B. Q., Li, M. L., Quan, H. Y., Hou, P. F., Li, Z. W., Chu, S. F., et al. (2019). Functional roles of circular RNAs during epithelial-to-mesenchymal transition. Mol. Cancer 18, 138. doi:10.1186/s12943-019-1071-6
Shen, M. J., Yan, S. T., Zhang, X. Y., Li, W., Chen, X., Zheng, X. X., et al. (2022a). The circular RNA hsa_circ_0003091 regulates sepsis-induced lung injury by sponging the miR-149/Smad2 axis. Aging 14, 5059–5074. doi:10.18632/aging.204125
Shen, N., Yang, D., Hou, J., Mi, X., Xu, H., and Liu, X. (2023). CircTMEM30A/hsa-miR-130a-3p regulates TNFα and promotes the malignant progression of COPD with primary lung cancer. Minerva Med. 114, 332–344. doi:10.23736/S0026-4806.21.07121-4
Shen, W., Zhao, X., and Li, S. (2022b). Exosomes derived from ADSCs attenuate sepsis-induced lung injury by delivery of circ-fryl and regulation of the miR-490-3p/SIRT3 pathway. Inflammation 45, 331–342. doi:10.1007/s10753-021-01548-2
Sica, A., and Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. doi:10.1172/JCI59643
Song, J., Wang, Q., and Zong, L. (2020). LncRNA MIR155HG contributes to smoke-related chronic obstructive pulmonary disease by targeting miR-128-5p/BRD4 axis. Biosci. Rep. 40. doi:10.1042/BSR20192567
Su, Q., Li, L., Liu, Y., Zhou, Y., Wang, J., and Wen, W. (2015). Ultrasound-targeted microbubble destruction-mediated microRNA-21 transfection regulated PDCD4/NF-κB/TNF-α pathway to prevent coronary microembolization-induced cardiac dysfunction. Gene Ther. 22, 1000–1006. doi:10.1038/gt.2015.59
Su, Y., Geng, L., Ma, Y., Yu, X., Kang, Z., and Kang, Z. (2021). Identification of circular RNA circVPS33A as a modulator in house dust mite-induced injury in human bronchial epithelial cells. Exp. Lung Res. 47, 368–381. doi:10.1080/01902148.2021.1974125
Sun, H., Gao, W., Chen, R., Chen, S., Gu, X., Wang, F., et al. (2023). CircRNAs in BALF exosomes and plasma as diagnostic biomarkers in patients with acute respiratory distress syndrome caused by severe pneumonia. Front. Cell Infect. Microbiol. 13, 1194495. doi:10.3389/fcimb.2023.1194495
Tanida, I., Yamasaki, M., Komatsu, M., and Ueno, T. (2012). The FAP motif within human ATG7, an autophagy-related E1-like enzyme, is essential for the E2-substrate reaction of LC3 lipidation. Autophagy 8, 88–97. doi:10.4161/auto.8.1.18339
Teng, X., Liao, J., Zhao, L., Dong, W., Xue, H., Bai, L., et al. (2021). Whole transcriptome analysis of the differential RNA profiles and associated competing endogenous RNA networks in LPS-induced acute lung injury (ALI). PLoS ONE 16,. doi:10.1371/journal.pone.0251359
Thébaud, B., Goss, K. N., Laughon, M., Whitsett, J. A., Abman, S. H., Steinhorn, R. H., et al. (2019). Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 5, 78. doi:10.1038/s41572-019-0127-7
Tong, Y., Zhang, S., Riddle, S., Song, R., and Yue, D. (2023). Circular RNAs in the origin of developmental lung disease: promising diagnostic and therapeutic biomarkers. Biomolecules 13, 533. doi:10.3390/biom13030533
Tsushima, K., King, L. S., Aggarwal, N. R., De Gorordo, A., D'Alessio, F. R., and Kubo, K. (2009). Acute lung injury review. Intern. Med. Tokyo, Jpn. 48, 621–630. doi:10.2169/internalmedicine.48.1741
Wang, J., Zhang, Y., Zhu, F., Chen, L., Wei, Y., Zhu, Q., et al. (2021). CircRNA expression profiling and bioinformatics analysis indicate the potential biological role and clinical significance of circRNA in influenza A virus-induced lung injury. J. Biosci. 46, 38. doi:10.1007/s12038-021-00152-8
Wang, L., Zhao, H., and Wang, D. (2018). Inflammatory cytokine expression in patients with sepsis at an intensive care unit. Exp. Ther. Med. 16, 2126–2131. doi:10.3892/etm.2018.6376
Wang, Q., Zhang, X., and Chen, D. (2021a). circ_VMA21 protects WI-38 cells against LPS-induced apoptotic and inflammatory injury by acting on the miR-409-3p/KLF4 axis. Gen. Physiol. Biophys. 40, 275–287. doi:10.4149/gpb_2021011
Wang, Z., Chang, P., Ye, J., Ma, W., Zhou, J., Zhang, P., et al. (2021b). Genome-wide landscape of mRNAs, microRNAs, lncRNAs, and circRNAs in hemorrhagic shock-induced ALI/ARDS in rats. J. Trauma Acute Care Surg. 90, 827–837. doi:10.1097/TA.0000000000003119
Wang, Z., Zuo, Y., and Gao, Z. (2021c). CircANKRD11 knockdown protects HPMECs from cigarette smoke extract-induced injury by regulating miR-145-5p/BRD4 Axis. Int. J. Chron. Obstruct Pulmon Dis. 16, 887–899. doi:10.2147/COPD.S300332
Wei, J., Peng, J., Wang, B., Qu, H., Wang, S., Faisal, A., et al. (2013). CXCR1/CXCR2 antagonism is effective in pulmonary defense against Klebsiella pneumoniae infection. Biomed. Res. Int. 2013, 720975. doi:10.1155/2013/720975
Weng, J., Chen, M., Lin, Q., Chen, J., Wang, S., and Fang, D. (2019). Penehyclidine hydrochloride defends against LPS-induced ALI in rats by mitigating endoplasmic reticulum stress and promoting the Hes1/Notch1 pathway. Gene 721, 144095. doi:10.1016/j.gene.2019.144095
Wu, J., Song, D., Li, Z., Guo, B., Xiao, Y., Liu, W., et al. (2020a). Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 30, 794–809. doi:10.1038/s41422-020-0354-1
Wu, Q., Jiao, B., Zhang, Q., Jin, C., Yu, H., and Wang, F. (2023). Identification of circRNA expression profiles and the potential role of hsa_circ_0006916 in silicosis and pulmonary fibrosis. Toxicology 483, 153384. doi:10.1016/j.tox.2022.153384
Wu, Y. K., Hu, L. F., Lou, D. S., Wang, B. C., and Tan, J. (2020b). Targeting DUSP16/TAK1 signaling alleviates hepatic dyslipidemia and inflammation in high fat diet (HFD)-challenged mice through suppressing JNK MAPK. Biochem. Biophys. Res. Commun. 524, 142–149. doi:10.1016/j.bbrc.2020.01.037
Xu, T., Wu, J., Han, P., Zhao, Z., and Song, X. (2017). Circular RNA expression profiles and features in human tissues: a study using RNA-seq data. BMC Genomics 18, 680. doi:10.1186/s12864-017-4029-3
Yan, X., Li, W., Yang, L., Dong, W., Chen, W., Mao, Y., et al. (2018). MiR-135a protects vascular endothelial cells against ventilator-induced lung injury by inhibiting PHLPP2 to activate PI3K/akt pathway. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol. 48, 1245–1258. doi:10.1159/000492010
Yang, C. L., Yang, W. K., He, Z. H., Guo, J. H., Yang, X. G., and Li, H. B. (2021). Quietness of circular RNA circ_0054633 alleviates the inflammation and proliferation in lipopolysaccharides-induced acute lung injury model through NF-κB signaling pathway. Gene 766, 145153. doi:10.1016/j.gene.2020.145153
Yang, Y. Q., Ge, P., Lv, M. Q., Yu, P. F., Liu, Z. G., Zhang, J., et al. (2022). Rno_circRNA_008646 regulates formaldehyde induced lung injury through Rno-miR-224 mediated FOXI1/CFTR axis. Ecotoxicol. Environ. Saf. 243, 113999. doi:10.1016/j.ecoenv.2022.113999
Yuan, C., Gu, J., Wu, J., Yin, J., Zhang, M., Miao, H., et al. (2020). Circular RNA expression in the lungs of a mouse model of sepsis induced by cecal ligation and puncture. Heliyon 6, e04532. doi:10.1016/j.heliyon.2020.e04532
Zhang, C., Gu, S., and Kang, X. (2022). CircRNA circ_0006892 regulates miR-24/PHLPP2 axis to mitigate cigarette smoke extract-induced bronchial epithelial cell injury. Biotechnol. Appl. Biochem. 69, 735–748. doi:10.1002/bab.2148
Zhang, H., Zheng, H., Mu, W., He, Z., Yang, B., Ji, Y., et al. (2015). DUSP16 ablation arrests the cell cycle and induces cellular senescence. FEBS J. 282, 4580–4594. doi:10.1111/febs.13518
Zhang, Q., Ban, J., Chang, S., Qu, H., Chen, J., and Liu, F. (2023a). The aggravate role of exosomal circRNA11:120406118|12040782 on macrophage pyroptosis through miR-30b-5p/NLRP3 axis in silica-induced lung fibrosis. Int. Immunopharmacol. 114, 109476. doi:10.1016/j.intimp.2022.109476
Zhang, Q., Sun, H., Zhuang, S., Liu, N., Bao, X., Liu, X., et al. (2019). Novel pharmacological inhibition of EZH2 attenuates septic shock by altering innate inflammatory responses to sepsis. Int. Immunopharmacol. 76, 105899. doi:10.1016/j.intimp.2019.105899
Zhang, R., Chen, L., Huang, F., Wang, X., and Li, C. (2021). Long non-coding RNA NEAT1 promotes lipopolysaccharide-induced acute lung injury by regulating miR-424-5p/MAPK14 axis. Genes Genomics 43, 815–827. doi:10.1007/s13258-021-01103-1
Zhang, R., Dang, X., Liu, J., Feng, H., Sun, J., and Peng, Z. (2023b). Circtdrd9 contributes to sepsis-induced acute lung injury by enhancing the expression of rab10 via directly binding to mir-223-3p. Shock 60, 206–213. doi:10.1097/SHK.0000000000002169
Zhao, D., Wang, C., Liu, X., Liu, N., Zhuang, S., Zhang, Q., et al. (2021a). CircN4bp1 facilitates sepsis-induced acute respiratory distress syndrome through mediating macrophage polarization via the miR-138-5p/EZH2 Axis. Mediat. Inflamm. 2021, 7858746. doi:10.1155/2021/7858746
Zhao, Y., Magaña, L. C., Cui, H., Huang, J., McHale, C. M., Yang, X., et al. (2021b). Formaldehyde-induced hematopoietic stem and progenitor cell toxicity in mouse lung and nose. Arch. Toxicol. 95, 693–701. doi:10.1007/s00204-020-02932-x
Zheng, C., Zhang, Y., Zhao, Y., Duan, Y., Mu, Q., and Wang, X. (2021). Circ-OSBPL2 contributes to smoke-related chronic obstructive pulmonary disease by targeting miR-193a-5p/BRD4 Axis. Int. J. Chron. Obstruct Pulmon Dis. 16, 919–931. doi:10.2147/COPD.S298465
Zhou, G., Duan, Y., Lu, C., and Wang, W. (2021a). Knockdown of circ-UQCRC2 ameliorated lipopolysaccharide-induced injury in MRC-5 cells by the miR-326/PDCD4/NF-κB pathway. Int. Immunopharmacol. 97, 107633. doi:10.1016/j.intimp.2021.107633
Zhou, H., Chanda, B., Chen, Y. F., Wang, X. J., You, M. Y., Zhang, Y. H., et al. (2021b). Microarray and bioinformatics analysis of circular RNA differential expression in newborns with acute respiratory distress syndrome. Front. Pediatr. 9, 728462. doi:10.3389/fped.2021.728462
Zhou, L., Wu, B., Yang, J., Wang, B., Pan, J., Xu, D., et al. (2021c). Knockdown of circFOXO3 ameliorates cigarette smoke-induced lung injury in mice. Respir. Res. 22, 294. doi:10.1186/s12931-021-01883-w
Zhou, Z., Jiang, R., Yang, X., Guo, H., Fang, S., Zhang, Y., et al. (2018). circRNA mediates silica-induced macrophage activation via HECTD1/ZC3H12a-dependent ubiquitination. Theranostics 8, 575–592. doi:10.7150/thno.21648
Zhu, H., Xing, Z., Zhao, Y., Hao, Z., and Li, M. (2020). The role of circular RNAs in brain injury. Neuroscience 428, 50–59. doi:10.1016/j.neuroscience.2019.12.018
Zhu, J., Zhong, F., Chen, F., Yang, Y., Liao, Y., Cao, L., et al. (2022). circRNA_0001679/miR-338-3p/DUSP16 axis aggravates acute lung injury. Open Med. Wars. Pol. 17, 403–413. doi:10.1515/med-2022-0417
Zhuang, X., Yu, Y., Jiang, Y., Zhao, S., Wang, Y., Su, L., et al. (2020). Molecular hydrogen attenuates sepsis-induced neuroinflammation through regulation of microglia polarization through an mTOR-autophagy-dependent pathway. Int. Immunopharmacol. 81, 106287. doi:10.1016/j.intimp.2020.106287
Keywords: circRNA, lung injury, inflammation, macrophage, fibrosis, smoking
Citation: Gao F-F, Chen D-Q, Jiang Y-T, Han C-F, Lin B-Y, Yang Z, Quan J-H, Xiong Y-H and Chen X-T (2024) Functional roles of circular RNAs in lung injury. Front. Pharmacol. 15:1354806. doi: 10.3389/fphar.2024.1354806
Received: 13 December 2023; Accepted: 16 January 2024;
Published: 27 March 2024.
Edited by:
Patricia Silveyra, Indiana University Bloomington, United StatesReviewed by:
Shikha Sharma, Indiana University, United StatesCopyright © 2024 Gao, Chen, Jiang, Han, Lin, Yang, Quan, Xiong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Huan Xiong, eHloODIxMjE1QDE2My5jb20=; Xin-Tian Chen, bXlzd215QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.