- 1Department of Anesthesiology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 3Department of Anesthesiology, Liaoning Cancer Hospital and Institute, Shenyang, China
- 4Department of Anesthesiology, The First Affiliated Hospital of China Medical University, Shenyang, China
- 5Department of Anesthesiology, The Affiliated Hospital of Qingdao University, Qingdao, China
Emergence delirium is a common postoperative complication in patients undergoing general anesthesia, especially in children. In severe cases, it can cause unnecessary self-harm, affect postoperative recovery, lead to parental dissatisfaction, and increase medical costs. With the widespread use of inhalation anesthetic drugs (such as sevoflurane and desflurane), the incidence of emergence delirium in children is gradually increasing; however, its pathogenesis in children is complex and unclear. Several studies have shown that age, pain, and anesthetic drugs are strongly associated with the occurrence of emergence delirium. Alterations in central neurophysiology are essential intermediate processes in the development of emergence delirium. Compared to adults, the pediatric nervous system is not fully developed; therefore, the pediatric electroencephalogram may vary slightly by age. Moreover, pain and anesthetic drugs can cause changes in the excitability of the central nervous system, resulting in electroencephalographic changes. In this paper, we review the pathogenesis of and prevention strategies for emergence delirium in children from the perspective of brain electrophysiology—especially for commonly used pharmacological treatments—to provide the basis for understanding the development of emergence delirium as well as its prevention and treatment, and to suggest future research direction.
1 Introduction
Emergence delirium (ED) in children is defined as the impairment of a child’s attention and awareness of the environment with disorientation and altered perception, including hypersensitivity to stimuli and hyperactive motor behavior, which occurs immediately after surgery. Children with ED exhibit irritability, uncooperativeness, sadness, crying, moaning, writhing, and kicking (Eckenhoff et al., 1961). Although most ED cases are self-limiting or present as a benign process (Jalili et al., 2019), it may cause unnecessary violence and self-harm, prolong the stay in the post-anesthesia care unit (PACU) affect the child’s postoperative recovery, cause parental dissatisfaction, and increase medical costs (Urits et al., 2020; Simonini et al., 2021). Additionally, children, 1 week after surgery, are more likely to experience behavioral changes such as maladjustment, insomnia, bedwetting, and lack of attention (Urits et al., 2020). One pilot study found no significant long-term effects of pediatric delirium on overall cognition, executive function, or behavior; however, baseline levels of cognition and behavior were not assessed (Meyburg et al., 2018). More prospective trials are needed to confirm and analyze the recovery process of brain function and consciousness after pediatric delirium, and its long-term adverse effects. Therefore, research on the mechanisms and prevention of ED is crucial.
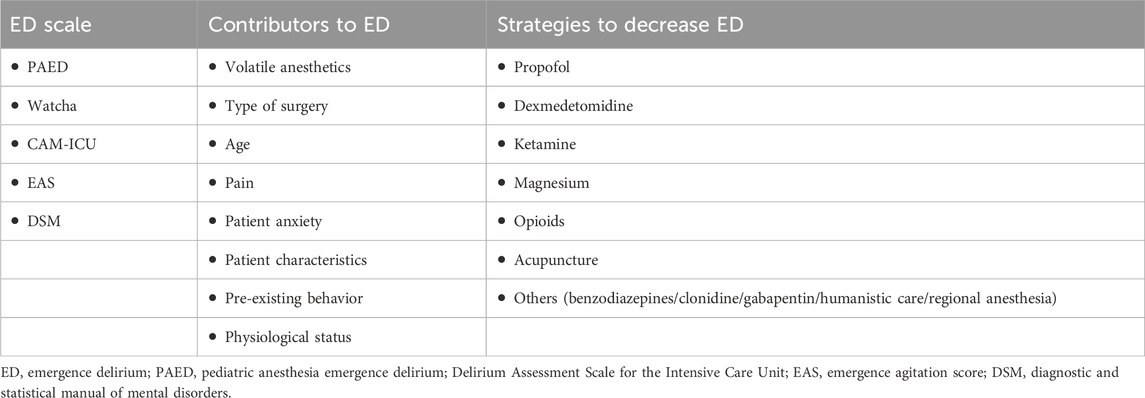
Several studies have explored the mechanisms of ED, finding that it is associated with inhaled anesthetics (such as sevoflurane), preschool age, type of surgery (including eye, ear, nose, and throat surgery), pain, preoperative anxiety, psychological immaturity, personality/temperament, and physiological conditions (Moore and Anghelescu, 2017) (Table 1). The incidence of ED in children after sevoflurane anesthesia ranges from 10% to 80%, which is 3–8 times higher than that in adults (Urits et al., 2020). Among them, in preschool and school-age children, the incidence is 40% and 11.5%, respectively (Dahmani et al., 2014). Currently, the most prevailing hypothesis for the occurrence of ED in patients under general anesthesia is that the clearance rate for these agents varies between different sites in the central nervous system, resulting in different rates of functional recovery at these sites. Compared to other brain functions (auditory and motor), cognitive function recovers late, and variations in brain function recovery contribute to the occurrence of ED (Dahmani et al., 2014).
Brain function is signalized in the form of brain electricity, the generation and conduction of neuronal action potentials and inter-synaptic transmission are the basis of neurophysiological activity, and brain waves are the overall reflection of the electrophysiological activity of the central nerve cells in the cerebral cortex or on the surface of the scalp. Changes in brain electricity are the basis of brain function. Many drugs can reduce the incidence of ED, such as dexmedetomidine, fentanyl, ketamine, and propofol supplementation at the end of sevoflurane anesthesia (Ng et al., 2019; Jiao et al., 2020; Haile et al., 2021; Zhang et al., 2022)). The most recognized effective adjuvant drug is dexmedetomidine, which has intrinsic sedative and analgesic effects. These drugs cause patient-specific electroencephalographic (EEG) changes to achieve ED prevention and control.
EEG is a direct and real-time measurement of neural activity in the brain, responding to brain changes in real time. There is a lack of evidence on the use of EEG to predict ED in children. In this paper, we will provide an overview of the mechanisms and preventive measures of ED from the perspective of changes in brain wave power during awakening from anesthesia, alterations in functional connectivity of the brain, abnormal discharges during the induction period of anesthesia, physiological development of the brain, and pain (Figure 1). The adverse effects of general anesthesia on children are manifested by a series of different behavioral abnormalities experienced during the awakening period, including ED, emergence excitation, and emergence agitation. These terms are not synonymous but are used interchangeably to describe behavioral disorders following anesthesia, especially in pediatric patients, because it is difficult to clearly define the mental state and behavior of children during the awakening period (Mason, 2017; Larsen et al., 2022). Herein, we uniformly use ED to describe abnormal behaviors during the waking period.
2 Physiological EEG and anesthesia EEG
2.1 EEG in the physiological state
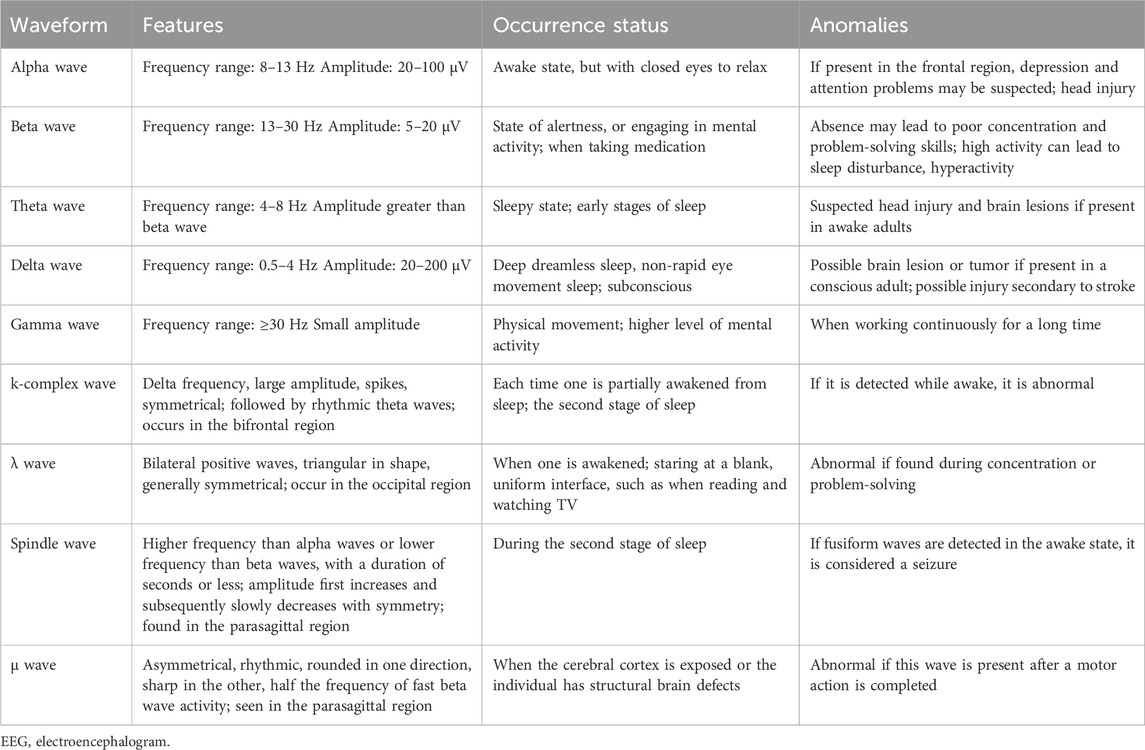
The basic EEG waveforms are alpha, beta, theta, gamma, and delta waves. Some normal EEG waves with special waveforms—such as hump, σ wave, λ wave, k-complex wave, and μ wave—can also appear during sleep. During sleep, the EEG changes are divided into two states: non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. The first stage of NREM sleep is dominated by theta and beta waves, which is the transition stage between full wakefulness and sleep. Spindle and k-complex waves dominate the second stage of NREM sleep. The spindle wave is a typical marker of sleep, appearing when consciousness disappears. It does not appear during wakefulness or REM sleep and is abnormally reduced in insomnia. Notably, the sleep spindle wave, one of many important measurable indicators of brain activity during sleep, provides a window into the current state of brain health and an individual’s risk for brain disease or cognitive decline. Slower delta waves begin to dominate during stage III NREM sleep; stage IV is entered when delta waves comprise more than 50% of the EEG waves. The EEG reveals a mixture of low frequency and low amplitude activity, including beta, theta, and alpha waves (Brown et al., 2012; Schwartz and Kilduff, 2015) (Table 2). In conclusion, the waveforms of brain waves are diversified and dynamically change with the changes of brain consciousness and sleep cycles, and the EEG waveforms are consistent with the conscious state of the brain, which is an important indicator for monitoring the central conscious activity and arousal state.
2.2 EEG under anesthesia
To elucidate the relationship between anesthesia and EEG, Chander et al. used standardized terms to define specific EEG changes during anesthesia maintenance, similar to those used to describe natural sleep: slow-wave anesthesia (SWA), predominantly delta and spindle wave activity, non–slow-wave anesthesia (NSWA), very low spindle wave and delta power, delta-dominant slow-wave anesthesia (ddSWA), and spindle-dominant slow-wave anesthesia (sdSWA)) (Chander et al., 2014). The spectral power corresponding to the four fundamental EEG frequency ranges was integrated to obtain the absolute power of each wave. The relative power (RP) of each wave is the ratio of the absolute power of each wave to the sum of the powers in all EEG frequency ranges. From the beginning of the awakening period (cessation of drug administration) to the end, approximately half of the patients showed typical EEG phase changes: the absence of delta waves followed by the absence of spindle waves followed by a period of NSWA (Chander et al., 2014). The change in consciousness level from anesthesia sleep to wakefulness is customarily accompanied by a gradual transition between low- and high-frequency EEG oscillations at multiple frequencies and closely resembles awakening from natural sleep.
Sevoflurane is a commonly used pediatric anesthetic, and like propofol, enhanced GABAergic inhibition may be its primary mechanism of action. At sub-MAC concentrations, sevoflurane exhibited EEG manifestations similar to those of propofol, enhanced GABAergic inhibition of the thalamic reticular nucleus (TRN)to reduce excitatory inputs from the thalamus to the cortex, resulting in alpha oscillations and beta oscillations; enhanced inhibition of GABAergic projections to brainstem arousal centers, and enhanced hyperpolarization of cortical pyramidal neurons, which facilitated the emergence of slow oscillations and delta oscillations. In addition, sevoflurane also blocks K+ channels and hyperpolarization-activated cyclic nucleotide-gated cation channels, binds to NMDA receptors, and blocks the release of glutamate (Cohen et al., 2003; Hemmings et al., 2005; Moore and Anghelescu, 2017). When the concentration of sevoflurane is increased to the level of MAC and above, theta oscillations will also occur, forming a unique pattern of uniform distribution from the slow oscillation range to the alpha range (Purdon et al., 2015).
3 ED and EEG
3.1 ED and delta and alpha waves
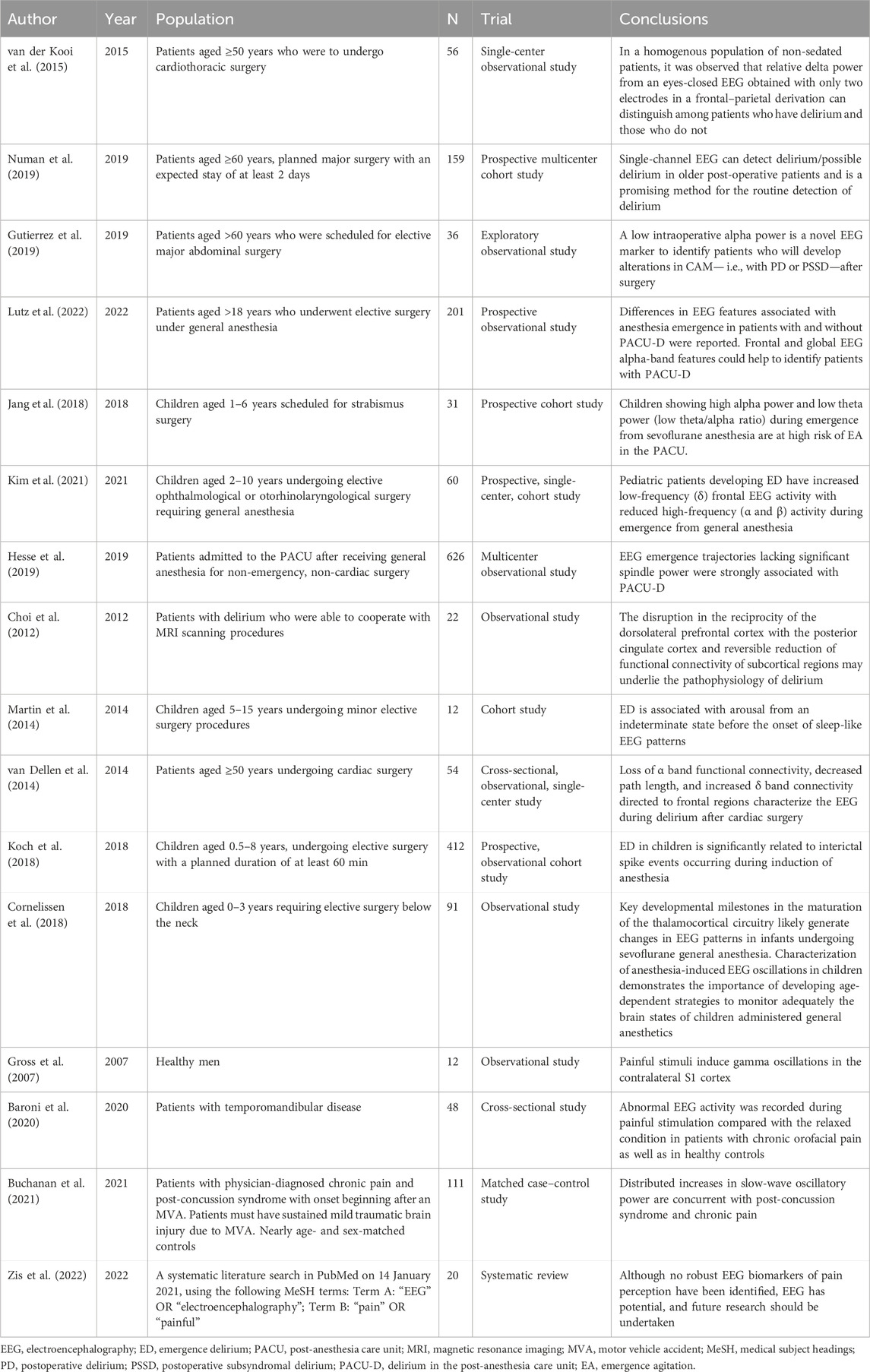
Recently, several studies have suggested that the occurrence of postoperative delirium (POD) in the elderly is closely related to high delta RP and low alpha RP (van der Kooi et al., 2015; Gutierrez et al., 2019; Numan et al., 2019; Lutz et al., 2022). Lutz et al. (Lutz et al., 2022) looked at the EEG performance of the frontal lobe during the awakening period under static-absorbent combined anesthesia in patients (age >18 years) undergoing elective surgery, and found that patients who developed delirium in the PACU had higher delta RP and lower alpha RP.
Due to incomplete brain development, the EEG characteristics of children differ greatly from those of adults (Cornelissen et al., 2018). More clinical and preclinical evidence is needed to clarify whether the adult findings can be directly extrapolated to children. Bruni et al. found that children exhibited crying, screaming, and night terrors when continuous delta activity was interrupted during NREM sleep. This is similar to the manifestations of irritability, uncooperativeness, sadness, crying, moaning, writhing, and kicking in children with delirium. We speculate that the occurrence of ED in children may be related to continuous delta frequency activity.
Kim et al. observed the two-channel frontal EEG of 60 children aged 2–10 years undergoing ophthalmological or otorhinolaryngological surgery under sevoflurane maintenance anesthesia. Although the emergence trajectories did not differ significantly between the ED and non-ED groups, the frontal delta RP during emergence was greater, and the alpha and beta RPs were lower, in the ED group. This is similar to the EEG trajectory during emergence found in adults with ED as mentioned earlier. Additionally, they found that the ratio of low- (delta and theta) to high-frequency (alpha and beta) waves, the delta/alpha ratio, was positively correlated with PAED scores (Kim et al., 2021). The results of this study further confirm that the occurrence of ED in children somewhat resembles that in adults, and that the increased delta/alpha ratio during emergence is an important factor in the occurrence of ED in pediatric patients.
However, Jang et al. (Jang et al., 2018) concluded that children with a decreased theta/alpha ratio during the awakening period had a high risk of postoperative ED, which may be caused by the small sample size of his study, different assessments of ED (Sikich and Lerman, 2004), and different definitions of the awakening period. We believe that the high delta/alpha ratio during emergence is suggestive of ED occurrence.
3.2 ED and spindle waves
The spindle wave is a characteristic sleep EEG wave of NREM stage II and occurs after loss of consciousness. The mechanism of spindle wave generation based on the recruitment of T-type calcium channels in the thalamocortex (TC) and TRN has been widely accepted. Activation of T-type calcium channels on GABAergic neurons in the TRN generates a low-threshold calcium potential and the release of GABA from the GABAergic neurons acts on the glutamatergic neurons in the TC to generate a GABAAipsp, which is followed by the Restoration of the membrane potential triggers the opening of T-type calcium channels on glutamatergic neurons, releasing glutamatergic neurotransmitters, and glutamate in turn causes neurons in the NRT to produce EPSP, which helps trigger electrical activity at the spindle frequency (Crunelli et al., 2014). In addition, Astori found that CaV3.3 channels have a central role in generating sleep spindle wave rhythms (Astori et al., 2011). By performing frontal EEG in 626 patients undergoing general anesthesia for elective non-cardiac surgery, Hesse et al. found that those with no sdSWA emergence trajectories had an increased probability of ED in the PACU, whereas those with sdSWA emergence trajectories had a decreased probability (Hesse et al., 2019). This suggests that the occurrence of ED is not only associated with an increase in delta RP and a decrease in alpha RP but is also inextricably linked to a decrease in spindle wave power. We hypothesize that the decrease in spindle wave power during emergence from general anesthesia also contributes to the occurrence of ED. However, there is a lack of studies on the correlation between spindle wave changes during pediatric emergence from general anesthesia and the incidence of ED after surgery, which is one of our future research directions.
3.3 ED and functional brain connectivity
The human brain has a high density of short connections within brain regions and sparse long connections between brain regions. This property allows the real-time transmission of messages between multiple systems, an efficient organization of internal and external information, and an efficient exchange of information between different functional brain regions, i.e., the network property reflects the information exchange property of brain functional differentiation and integration. There are four types of “connectivity” frequently measured in the brain (Mashour and Avidan, 2014). In 1993, Friston et al. defined functional connectivity as the temporal correlations between spatially remote neurophysiological events (Friston et al., 1993). Functional brain connectivity is a measure of temporal correlations and functional activity dependencies between spatially separated brain regions and is one of the effective means of describing synergistic working patterns between brain regions. Additionally, there exists structural, directional, and efficiency connectivity (Mashour and Avidan, 2014). What is the relationship between the occurrence of ED during anesthesia awakening and the functional connectivity of the brain?
In 2012, Choi et al. used a functional magnetic resonance imaging (MRI) seed-based correlation and found increased functional connectivity between the prefrontal cortex and posterior cingulate cortex during the onset of delirium in 22 patients (Choi et al., 2012). In 2014, Martin et al. used multichannel EEG to monitor the EEG activity of children during the emergence period and used global efficiency (GE) and global coherence of the whole brain, frontal lobe, and parietal lobe as the response form of brain functional connectivity. The whole brain and parietal functional connectivity did not significantly differ between the ED and control group, although the mean GE values for the frontal network were significantly higher in the ED group. This study suggests that increased connectivity after discontinuation of sevoflurane anesthesia may be closely related to the development of ED (Martin et al., 2014). However, given the small sample size of this study, individual differences, condition restrictions, and preoperative medications may have significantly affected the experimental results. Thus, more rigorous studies with larger sample sizes are needed to further explore the relationship between functional brain connectivity and ED during anesthesia awakening and the related molecular mechanisms in the future. Additionally, Dellen et al. analyzed the functional connectivity of EEG time series using the phase lag index, directed phase lag index, and functional brain network topology with graph analysis, and found that patients with delirium after cardiac surgery had a loss of functional connectivity in the alpha band, reduced pathway length, and increased connectivity in the delta band (van Dellen et al., 2014). This is consistent with our previous findings regarding the relationship between alpha and delta waves and ED, suggesting a potential association between ED and altered functional connectivity of the brain, in addition to specific cortical sites, and specific EEG frequency fractions.
Therefore, increased functional connectivity may reflect a state of cortical hyperexcitability, and greater network connectivity implies increased responsiveness to internal and external stimuli. This increased responsiveness may be an important cause of ED.
3.4 ED and epileptiform discharges
Recent studies have found that epileptiform discharges during general anesthesia induction are also involved in the mechanism of ED. Epileptiform discharges are abnormal waveforms on the EEG that indicate the possible presence of epilepsy and manifest as sharp waves, spike waves, sharp-and-slow-wave complexes, or spike-and-slow-wave complexes. A sharp wave is a triangle wave with an 80–300 ms time limit; a wave amplitude of 200 µV or more; a fast, straight rise; and a slow fall. A spike wave is an abnormal waveform with a 20–70 ms time limit, an amplitude exceeding 20 μV, a steep rise, and falling branches, and an overall thorn shape. Sharp and spike waves can be combined with slow waves. Some studies have reported that epileptiform discharges are frequently observed during general anesthesia induction with sevoflurane (Vakkuri et al., 2001; Schultz et al., 2012). According to Vakkuri et al., the following types of epileptiform discharges were described during sevoflurane induction: delta with spikes (DSP), rhythmic polyspikes (PSR), periodic epileptiform discharges (PED), and suppression with spikes (Vakkuri et al., 2001). Moreover, Kreuzer et al. found a reduction in EEG epileptiform activity in children anesthetized with 6% sevoflurane compared with 8% sevoflurane (Kreuzer et al., 2014). Hesdorffer et al. found that most children with attention deficit disorder and hyperactivity develop epileptiform activity on EEG (Hesdorffer et al., 2004). This suggests that epileptiform activity may be associated with behavioral hyperactivity. ED is characterized by hypersensitivity to stimuli and hyperactive motor behavior, leading us to hypothesize that epileptiform EEG is most likely associated with ED.
Koch et al. analyzed dual-channel EEG signals during anesthesia induction in 62 children aged 0.5–8 years and found that epileptiform discharges were more common in children with ED. They concluded that the occurrence of ED in children was significantly associated with epileptiform discharges during general anesthesia induction, especially PSR, PED, and DSP. They also confirmed a higher incidence of epileptiform discharges during sevoflurane than propofol anesthesia-induced loss of consciousness (Koch et al., 2018). Interestingly, the incidence of epileptiform discharges induced by propofol and sevoflurane was consistent with the clinical occurrence of ED, indirectly confirming the correlation between epileptiform discharges during induction and ED in pediatric patients. There is insufficient evidence on the correlation between EEG performance during induction and the occurrence of ED when comparing general intravenous anesthesia with sevoflurane inhalation anesthesia—this is a direction for our future research.
3.5 ED and physiological brain development
Voepel-Lewis et al. assessed 521 children who underwent outpatient surgery and found that age was an independent risk factor for ED—the younger the child, the higher the risk (Voepel-Lewis et al., 2003). However, during infancy and early childhood, EEG performance under sevoflurane anesthesia differs from that of older children and adults because the nervous system is rapidly developing. Cornelissen et al. found slow oscillations (0.1–1 Hz) and delta oscillations (1–4 Hz) on the EEG of neonates, infants, and toddlers during sevoflurane general anesthesia, and the consistency of slow and delta oscillations was the highest at 3 months; thereafter, it decreased and became discordant at 9 months. Alpha oscillations appear in the frontal lobe at approximately 3–4 months and increase steadily until they reach a plateau and become coherent at 10 months. That is, infants have coordinated local frontal slow and delta oscillations during the first 8 months of life, while from 10 months, fine-tuning of coordinated alpha oscillations begins to appear, resembling an adult EEG (Cornelissen et al., 2018). Therefore, the EEG mechanisms underlying the occurrence of ED in infants and children need to be analyzed considering the EEG characteristics caused by age itself, which may be one of the mechanisms behind the large differences in ED incidence between adults and children.
3.6 Pain and EEG
Recently, several studies have shown that pain is an independent risk factor for the development of ED (Moore and Anghelescu, 2017; Wei et al., 2021). Gross et al. found that painful stimuli induced gamma oscillations in the primary somatosensory cortex by laser stimulation of the right dorsum in healthy volunteers, and that the amplitude of these oscillations increased with increasing objective stimulus intensity and subjective pain intensity (Gross et al., 2007). Similarly, Baroni et al. observed increased gamma activity in the central and frontal regions of adult patients with chronic orofacial pain compared with controls (Baroni et al., 2020), while Buchanan et al. found significantly higher delta and theta power in patients with post-concussive syndrome and chronic pain following a motor vehicle collision compared with controls (Buchanan et al., 2021). Hence, pain may cause changes in various EEG activities. Zis et al. reviewed 20 relevant articles and found that EEG performance was consistent with an increase in delta and gamma wave power, despite inconsistent conclusions on the relationship between delta, theta, alpha, beta, and gamma activities and painful stimuli due to differences in individuals, stimulus intensity, stimulus location, and state at the time of recording (eyes open/closed). The authors concluded that these increases are characteristic EEG manifestations of nociception (Zis et al., 2022). This led us to further confirm that pain causes changes in brain waves, with enhanced changes in delta and gamma wave activity in particular. We hypothesize that once anesthesia is discontinued after surgery, pain caused by surgical stimulation and intolerance caused by artificial ventilation devices (e.g., tracheal tubes) would cause EEG changes during the emergence period. This could lead to an increase in delta and gamma wave power, which could lead to the development of ED.
Hence, EEG delta and alpha wave power imbalance during emergence from general anesthesia, decreased or absent spindle waves, increased functional brain connectivity, epileptiform discharges during anesthesia induction, brain underdevelopment, and pain-induced increases in EEG delta and gamma wave power are all involved in the pathogenesis of pediatric ED. The electrophysiological mechanism of pediatric ED in the brain is based on multiple factors. A single factor cannot clarify the pathogenesis of ED (Table 3)—various aspects have not yet been investigated, and extensive basic and clinical neurophysiological studies are still required.
4 Prevention and treatment
Currently, there are no definitive clinical methods to effectively prevent or treat ED given its unknown pathogenesis. Nonetheless, many recent clinical and preclinical studies have shown interest in the prevention and treatment of ED, and an increasing number of measures have been suggested. These include pharmacologic and nonpharmacologic treatments (Cui et al., 2020). Below, we discuss several ED prevention measures commonly used in clinical practice based on the electrophysiologic aspects of the brain mentioned above (Table 4).
4.1 Decrease in delta/alpha ratio
4.1.1 Propofol
In recent years, studies have found that general anesthetics are closely associated with the development of ED. Propofol and sevoflurane, the most widely used anesthetics in clinical practice, are widely used in the induction and maintenance of general anesthesia by activating the functional Cl-channels formed by the β-subunit of GABA A receptor, and the increase in the inward Cl-current causes the post-synaptic neuron to hyperpolarize and agonize the GABA A receptor, exerting central inhibitory effects (Sanna et al., 1995).
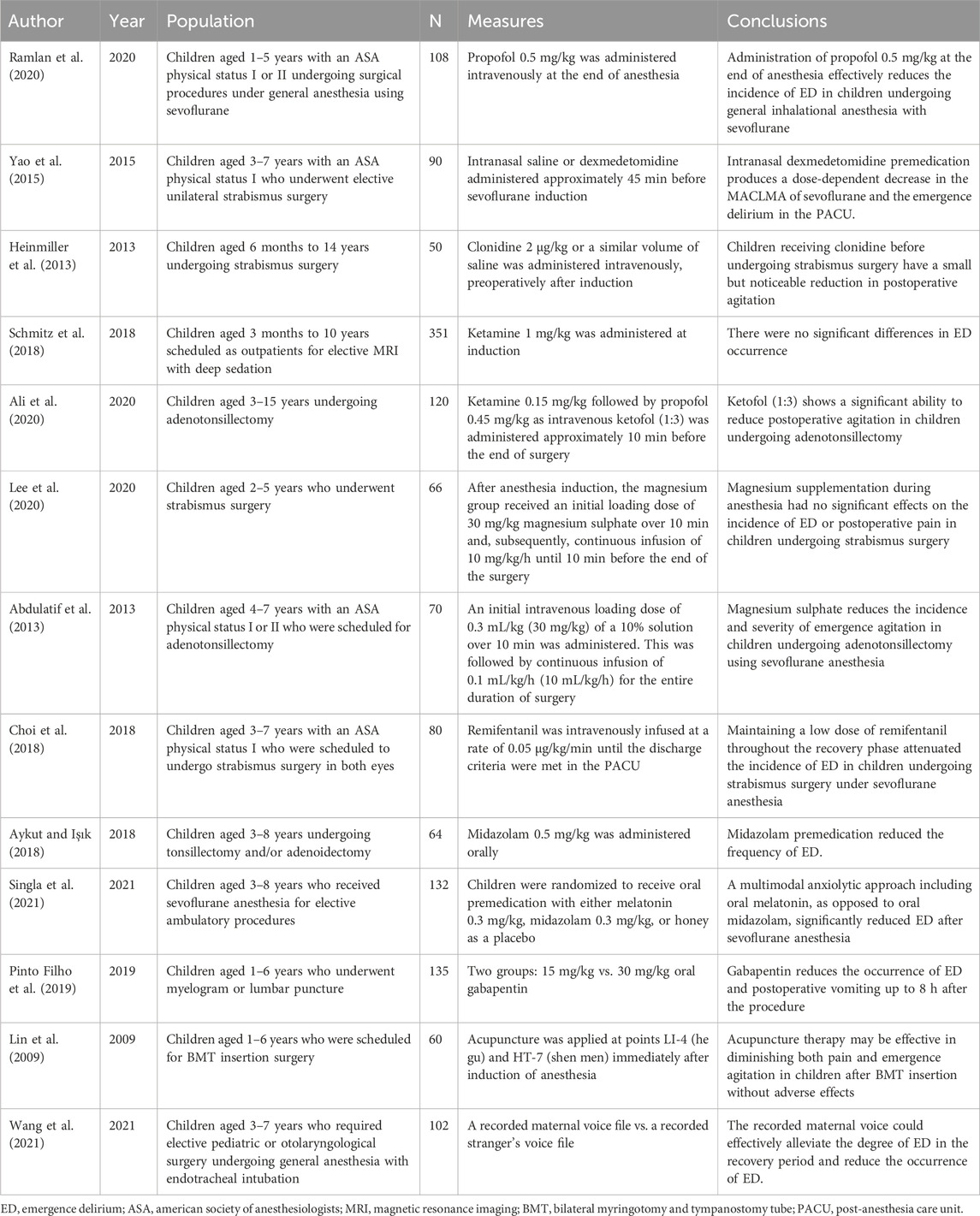
Clinical studies have shown a higher ED incidence in children induced with inhalational than with intravenous anesthetics (Peker and Polat, 2020). Furthermore, the incidence of ED after sevoflurane administration is higher than that after desflurane (Wu et al., 2019b); however, propofol can reduce the incidence of ED after sevoflurane anesthesia (Wu et al., 2019a; Ramlan et al., 2020). Wu et al. retrospectively analyzed the data of 200 children undergoing inguinal hernia, where the experimental group received 2 mg/kg propofol intravenously, 5 min after discontinuation of sevoflurane, and the control group received sodium chloride 0.9%. Using the PAED scale, they concluded that propofol administered after discontinuation of sevoflurane inhalation anesthesia could prevent the development of pediatric ED (Wu et al., 2019a). This corresponds with the results of a double-blind RCT conducted by Ramlan and colleagues. The Aono and PAED scales were used to assess the incidence and severity of ED in children aged 1–5 years in the PACU within 30 min. They found that propofol 0.5 mg/kg administration immediately after resumption of spontaneous breathing following cessation of inhaled sevoflurane was effective in reducing the incidence of ED (Ramlan et al., 2020).
Previously, Ching et al. demonstrated that propofol can stimulate increased alpha wave power in the frontal cortex as well as hypersynchronous neurobiological manifestations. They hypothesized that propofol-induced increases in thalamocortical hypersynchrony and alpha wave power may interfere with the flexible cortical communication required for brain consciousness, causing selective inhibition of frontoparietal feedback connectivity and ultimately loss of consciousness (Ching et al., 2010; Dehaene and Changeux, 2011). Therefore, combined with previous findings on the relationship between alpha waves and neurological connectivity of the brain regions and ED occurrence, we believe that the propofol-induced increased alpha wave power and reduced brain connectivity may have a protective effect on ED occurrence.
4.1.2 Magnesium
Magnesium is a noncompetitive blocker of NMDA receptors (Mayer et al., 1984), reduces the incidence of postoperative shivering, and has good analgesic properties (Lysakowski et al., 2007). Clinically, the most commonly used magnesium-containing drug is magnesium sulfate (MgSO4). Recently, MgSO4 has been used as an adjunct to general anesthetic agents for sedation and analgesia, and may potentially prevent ED.
There is no consensus on whether MgSO4 can reduce the incidence of postoperative ED in children (Abdulatif et al., 2013; Lee et al., 2020; Koo et al., 2022; Shen et al., 2022). An RCT by Lee et al. included 66 children aged 2–5 years undergoing daytime strabismus surgery who received MgSO4 30 mg/kg as an initial loading dose after anesthesia induction, followed by continuous infusion of 10 mg/kg/h. They found no significant effect of magnesium supplementation during anesthesia on the incidence of ED (Lee et al., 2020). A meta-analysis by Shen et al. including 635 patients aged <18 years from eight RCTs also concluded that MgSO4 did not reduce the incidence of ED in children (Shen et al., 2022). However, Abdulatif et al. observed 65 children undergoing adenotonsillectomy with sevoflurane induction followed by intravenous administration of MgSO4 and found the incidence of ED in the experimental group and the control group was 36% and 72%, respectively. They concluded that MgSO4 reduced the incidence and severity of ED in these children (Abdulatif et al., 2013). A meta-analysis by Koo et al. on the effect of intraoperative MgSO4 administration on postoperative agitation and delirium in pediatric patients also reported that MgSO4 has a positive effect on reducing ED incidence (Koo et al., 2022). The discrepancy in the results of these RCTs may be owing to the different types of surgery—the high ED incidence following ENT surgery is more likely to deliver positive results—and the different induction methods and doses of MgSO4 administered. Overall, we believe that MgSO4 has a role in preventing the occurrence of ED in children.
Magnesium is involved in energy metabolism, preserving brain glucose or pyruvate in tissues to meet energy demands during cerebral ischemia and reperfusion, and suppressing brain lactate and elevated glutamate levels (Lin et al., 2002; Bariskaner et al., 2003). Brain tissue energy metabolism is closely related to EEG grading. An increase in EEG grading is manifested by a decrease in EEG wave amplitude, a decrease in alpha band activity, and a gradual increase in theta or delta band activity. As the grading continues to increase, the EEG reveals an absence of brain wave activity (Oğün et al., 2002). Bariskaner et al. found that brain lactate and malondialdehyde concentrations positively correlate with EEG grading. Thus, when the brain lactate and malondialdehyde concentrations were suppressed after intravenous MgSO4 injection, the EEG grading decreased, EEG amplitude increased, alpha band activity increased, and delta band activity decreased (Bariskaner et al., 2003). As mentioned above, the decrease in alpha RP and increase in delta RP during the emergence period is one of the main EEG manifestations of ED in children. Therefore, we speculate that MgSO4 changes the delta/alpha ratio on the EEG during the emergence period by inhibiting the production of lactate and malondialdehyde in the brain, thus reducing ED occurrence. Further research is required to elucidate how lactate and malondialdehyde affect ion channels or protein receptors in neuronal cells, affecting EEG conduction and thus cortical EEG waveforms.
4.2 Increase in spindle waves
4.2.1 Dexmedetomidine
Dexmedetomidine is a selective α2-adrenoceptor agonist with sedative, analgesic, anxiolytic, and sympatholytic effects. It may produce sedative effects by agonizing endogenous pro-sleep pathways through presynaptic mechanisms (Weerink et al., 2017). Under physiological conditions, the LC acts as an inhibitor on the hypothalamus-preoptic area (HPOA) via adrenergic neurons, inhibiting the brainstem and thalamus via GABAergic neurons. The activity of the brainstem reticular formation is associated with arousal. The LC also transmits excitation to the cerebral cortex via adrenergic neurons. The binding of dexmedetomidine to the α2 receptors hyperpolarizes LC neurons, decreasing norepinephrine release. This weakens HPOA inhibition by the LC, and neuron activation in the HPOA inhibits the brainstem arousal center, while cortical excitability decreases, resulting in a sedated state in patients (Brown et al., 2010; Brown et al., 2011). Recently, an increasing number of research related to dexmedetomidine and ED have demonstrated its diverse positive effects on ED (Yao et al., 2015; Lin et al., 2017; Sun et al., 2017; Sadeghi et al., 2022).
ENT surgery is a known risk factor for pediatric ED. In 2020, a meta-analysis by Jiao et al. showed that dexmedetomidine reduced the incidence of ED in children undergoing adenotonsillectomy under sevoflurane anesthesia and was superior to other drugs (Jiao et al., 2020). However, large, high-quality RCTs are required to confirm its superiority, because the included studies did not standardize the administration dose, route, or timing, and did not limit the age of inclusion. A subsequent RCT conducted by Lin et al. demonstrated that intravenous dexmedetomidine 1 μg/kg for maintenance of anesthesia in children undergoing odontotherapy significantly reduced the incidence of ED (Lin et al., 2017). Dexmedetomidine can be administered intranasally and intravenously. In an RCT conducted among 90 children aged 3–7 years who underwent unilateral strabismus surgery, Yao and colleagues found that intranasal administration of dexmedetomidine 1 μg/kg and 2 μg/kg 45 min before induction reduced the incidence of pediatric ED (Yao et al., 2015). Sadeghi et al. reported that, for ED prevention, intravenous dexmedetomidine 1 μg/kg 10 min before the end of adenotonsillectomy or cleft palate repair surgery was superior to the equivalent dose 10 min after the start of surgery (Sadeghi et al., 2022). Besides the administration route and timing, the dose is also a factor influencing the effect of dexmedetomidine. Intravenous administration of dexmedetomidine 0.25 μg/kg, 0.5 μg/kg, and 1.0 μg/kg 10 min before the end of laparoscopic hernia repair in children prevented ED occurrence—the fewest cases occurred in the 1.0 ug/kg group (Sun et al., 2017).
In 2016, Akeju et al. assessed EEG changes throughout the cortex during intravenous infusion of dexmedetomidine 1 μg/kg (within 10 min) followed by continuous infusion at 0.7 μg/kg/h for 50 min in eight healthy adult volunteers aged 18–36 years by 64-channel EEG. They found an increase in delta oscillations, a decrease in beta oscillations, an increase in occipital theta oscillations, and an increase in frontal spindle waves. These changes are similar to those in the second phase of NREM. They further found that intravenous dexmedetomidine 0.5 μg/kg or 1 μg/kg alone promoted NREM stage III in a dose-dependent manner (Akeju et al., 2018). The NREM stage III EEG waves were dominated by delta waves. Dexmedetomidine prolongs NREM and reduces wakefulness time, and this effect is dose-dependent (Feng et al., 2018). In 2020, Chamadia et al. found that oral dexmedetomidine increased the duration of NREM stage II and decreased the duration of REM sleep in an RCT conducted among 15 adults. They also suggested that dexmedetomidine promotion of NREM stage II or III may be related to the route and time of administration (Chamadia et al., 2020).
Overall, EEG characteristics after dexmedetomidine administration are unclear in children. Therefore, we hypothesize that dexmedetomidine may increase EEG spindle waves and prolong N2 sleep duration. Although delta oscillations may also increase, low CNS excitability persists, thereby preventing ED occurrence.
4.3 Decreased brain connectivity
4.3.1 Ketamine
Ketamine is a non-competitive NMDA receptor antagonist with dose-dependent sedative and analgesic effects (Mihaljević et al., 2020). The analgesic action of ketamine is multimodal and mainly produced by blocking NMDA receptors in the spinal cord (Oye et al., 1992); secondly, it acts on opioid receptors to produce analgesic effects (Pacheco Dda et al., 2014). More studies on ketamine are emerging, aimed at preventing pediatric ED (Dalens et al., 2006; Ali et al., 2020).
Ali et al. analyzed the data of 120 children aged 3–15 years who underwent adenotonsillectomy and found that the incidence of ED in children who were given 0.15 mg/kg ketamine intravenous 10min before the end of the operation was 13.33% and that in the control group was 48.33% (Ali et al., 2020). Similarly, a randomized controlled study involving 90 children undergoing MRI found that 0.25 mg of ketamine at the end of surgery reduced the incidence of ED by about 13% compared with a control group (Dalens et al., 2006). However, a study by Schmitz et al., involving 351 children aged 3 months to 10 years undergoing MRI, found no difference in the incidence of ED between ketamine given 1 mg/kg at induction of anesthesia and the control group (Schmitz et al., 2018). In the former study, sevoflurane was used for induction and maintenance of anesthesia, while propofol was used for maintenance of anesthesia in the latter, and the incidence of ED after propofol was lower than that after sevoflurane. Secondly, different doses of ketamine, different time points of administration, and different ages of included children may cause inconsistent conclusions.
Lee et al. observed changes in frontal and parietal EEG among 30 surgery patients after intravenous induction with ketamine 2 mg/kg, and found an increase in delta, theta, and gamma RP (25–35 Hz) and a decrease in alpha and beta RP, with diminished brain connectivity (Lee et al., 2013). In 2015, by observing changes in the EEG of rats under ketamine anesthesia, Pal et al. found that gamma activity in the mid-high range (65–175 Hz) was significantly decreased after ketamine-induced loss of consciousness compared to the awake state; activity in other frequency bands did not markedly change. Delta activity was decreased and gamma wave activity was increased during the emergence period. They also found that cortical connectivity was reduced under ketamine anesthesia (Pal et al., 2015). An EEG after ketamine sedation is characterized by beta and gamma oscillations (Purdon et al., 2015). From the abovementioned study, we found that low-frequency gamma activity is a characteristic EEG manifestation of ketamine sedation. Although the EEG pattern under ketamine action is relatively active and different from that of propofol, Lee et al. suggested that both ketamine and propofol result in reduced frontoparietal connectivity and thus a sedated, unconscious state.
We hypothesize that cortical connectivity is reduced after intravenous ketamine administration, which reduces CNS excitability even though EEG activity appears relatively active. Ketamine may prevent ED occurrence by reducing the cortical connectivity of the child and its analgesic effect.
4.4 Pain
4.4.1 Opioids
Opioids can be divided into three categories: natural (morphine, codeine, papaverine), synthetic (methadone, meperidine, fentanyl, alfentanil, sufentanil, remifentanil), and semisynthetic (hydromorphone). They produce analgesic effects by acting on μ, κ, and δ receptors. Opioids are widely used in clinical practice and are one of the essential drug classes used for general anesthesia. Recently, several opioids have been used to prevent ED in children (An et al., 2017; Kim et al., 2017; Choi et al., 2018; Chu and Pan, 2018).
Fentanyl is commonly used in pediatric anesthesia. In 2017, Kim et al. analyzed 10 RCTs and found that intravenous fentanyl before and after the end of surgery reduced the incidence of pediatric ED with sevoflurane general anesthesia (Kim et al., 2017). In addition to fentanyl, remifentanil, and sufentanil are commonly used in pediatric anesthesia and positively affect ED incidence. An RCT by Choi et al. revealed that, in children aged 3–7 years, remifentanil 0.05 μg/kg/min infusion at the end of strabismus surgery after discontinuation of sevoflurane, the incidence of ED was reduced by 35% (Choi et al., 2018). It has also been reported that administration of 0.1 mg/kg dezocine at the end of surgery has reduced the incidence of ED in children undergoing laparoscopic inguinal hernia repair (An et al., 2017) and that intravenous infusion of 0.01 mg/kg hydromorphone 10 min before surgery prevented ED in children undergoing strabismus surgery (Chu and Pan, 2018).
We believe that the mechanism of opioid prevention of ED is inextricably linked to its analgesic effect, which reduces the incidence of ED by suppressing pain, reduces sensitivity to external stimuli, and more closely resembles the natural arousal of NREM sleep.
4.4.2 Acupuncture
Acupuncture is a type of acupuncture therapy that involves the insertion of fine needles into acupuncture points on the skin to a certain depth and for a sustained period of time in order to alleviate symptoms and cure diseases (Nakajima et al., 2022). In recent years, some studies have reported that acupuncture therapy can prevent the occurrence of postoperative ED in pediatric patients. Yuanchi Lin et al. observed 80 children aged 1–6 years who underwent tympanotomy with tympanic ventricular tubing, and acupuncture treatment was performed for 10 min at bilateral Hegu and Senmen points after induction of sevoflurane anesthesia, and it was found that the acupuncture therapy significantly reduced the severity of postoperative pain and agitation in children (Lin et al., 2009). However, contrastingly, a study by Martin et al. employed acupuncture at bilateral HT-7 and ear shen men points of children after anesthesia induction until the end of surgery and showed no significant difference in ED incidence between the two groups (Martin et al., 2020). The discrepancy in results may be due to the difference in acupuncture points and duration. Some studies have shown that acupuncture therapy may activate the release of opioid peptides in the adult CNS (Chernyak and Sessler, 2005; Zhao, 2008). Others have suggested that it can affect the release of visceral pain–modulating substances, such as increasing the secretion of β-endorphin and decreasing epinephrine, cortisol, and prostaglandin E2 levels (Lee et al., 2019). We believe that acupuncture therapy may prevent ED occurrence by reducing pain. However, it is unclear how it affects EEG changes, and the optimal acupuncture site, timing of initiation, and duration of treatment for the prevention of pediatric ED need to be further explored.
In summary, these interventions alter the EEG during wakefulness through their respective sedative and analgesic effects, increase the power of spindle or alpha waves, reduce the connectivity of t the brain, so that the EEG during the emergence period will be closer to the natural awakening EEG changes, and render the childless perceptive to external stimuli and reduce CNS excitability, thereby preventing the occurrence of pediatric ED. Furthermore, other interventions such as colistin, benzodiazepines, melatonin, gabapentin (Pinto Filho et al., 2019; Archana et al., 2022; Yang et al., 2022) and recorded maternal voice during emergence, and regional block can reduce the incidence of ED (Byun et al., 2018; Zhong et al., 2018; Yang et al., 2020). Interestingly, after intravenous administration of clonidine to achieve sedation in healthy volunteers, their EEG showed a decrease in dominant alpha activity and an increase in delta oscillations (Bischoff et al., 2004; Miyazaki et al., 2004). However, this EEG change is inconsistent with our previous findings that the increase in delta RP and decrease in alpha RP are associated with a high ED incidence. Nevertheless, sufficient clinical evidence supports the beneficial effect of clonidine on ED outcomes; more relevant studies are required to elaborate on the EEG changes after clonidine administration in children.
5 Conclusion
Based on the EEG level analysis, we concluded that the mechanism affecting ED occurrence is not unique but a combination of several factors. We hypothesize that the phenomena of increased EEG delta and alpha RP ratio, decreased spindle wave power, increased cerebral connectivity, increased neuronal excitability during anesthesia emergence and epileptiform discharges during anesthesia induction can be used as indicators for identifying ED, which allows us to target the administration of drugs related to the prevention of ED and to change the EEG manifestations of the affected children to prevent the occurrence of ED. In addition, the neurophysiological development of the child himself and factors such as pain are also associated with the development of ED. However, at present, our understanding of the bottom-up process from neural activity to behavior is very incomplete; and at the mechanism level, the causal relationship between EEG and ED performance has not yet been clearly established; the detailed mechanism of preventive medications and therapeutic EEGs in children, as well as the detailed physiological mechanisms behind the mechanism of EEGs in children with ED, have yet to be investigated, and more basic and clinical studies are needed in the future.
Author contributions
XG: Methodology, Writing–review and editing, Writing–original draft. ZL: Methodology, Writing–review and editing. JC: Writing–review and editing, Conceptualization. SL: Writing–review and editing, Software. XP: Writing–review and editing, Visualization, Software. JL: Writing–review and editing, Software. LL: Writing–review and editing, Software. SQ: Writing–review and editing, Visualization. YK: Writing–review and editing, Visualization. YZ: Writing–review and editing, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdulatif, M., Ahmed, A., Mukhtar, A., and Badawy, S. (2013). The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia 68 (10), 1045–1052. doi:10.1111/anae.12380
Akeju, O., Hobbs, L. E., Gao, L., Burns, S. M., Pavone, K. J., Plummer, G. S., et al. (2018). Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: a pilot study. Clin. Neurophysiol. 129 (1), 69–78. doi:10.1016/j.clinph.2017.10.005
Ali, I., Alahdal, M., XiaShiqian, H. H., and Yao, S. (2020). Ketofol performance to reduce postoperative emergence agitation in children undergoing adenotonsillectomy. Libyan J. Med. 15 (1), 1688450. doi:10.1080/19932820.2019.1688450
An, L. J., Zhang, Y., Su, Z., Zhang, X. L., Liu, H. L., Zhang, Z. J., et al. (2017). A single dose of dezocine suppresses emergence agitation in preschool children anesthetized with sevoflurane-remifentanil. BMC Anesthesiol. 17 (1), 154. doi:10.1186/s12871-017-0446-8
Archana, K. N., Vyshnavi, S., and Ganesh, V. (2022). Effect of caudally administered clonidine on sevoflurane induced emergence agitation-A randomized trial. J. Anaesthesiol. Clin. Pharmacol. 38 (2), 196–200. doi:10.4103/joacp.JOACP_248_20
Astori, S., Wimmer, R. D., Prosser, H. M., Corti, C., Corsi, M., Liaudet, N., et al. (2011). The Ca(V)3.3 calcium channel is the major sleep spindle pacemaker in thalamus. Proc. Natl. Acad. Sci. U. S. A. 108 (33), 13823–13828. doi:10.1073/pnas.1105115108
Aykut, A., and Işık, B. (2018). Emotion regulation and premedication success relationship in children who underwent general anesthesia. Turk J. Med. Sci. 48 (2), 217–222. doi:10.3906/sag-1702-117
Bariskaner, H., Ustun, M. E., Ak, A., Yosunkaya, A., Ulusoy, H. B., and Gurbilek, M. (2003). Effects of magnesium sulfate on tissue lactate and malondialdehyde levels after cerebral ischemia. Pharmacology 68 (3), 162–168. doi:10.1159/000070174
Baroni, A., Severini, G., Straudi, S., Buja, S., Borsato, S., and Basaglia, N. (2020). Hyperalgesia and central sensitization in subjects with chronic orofacial pain: analysis of pain thresholds and EEG biomarkers. Front. Neurosci. 14, 552650. doi:10.3389/fnins.2020.552650
Bischoff, P., Schmidt, G. N., Scharein, E., Bromm, B., and Schulte am Esch, J. (2004). Clonidine induced sedation and analgesia--an EEG study. J. Neurol. 251 (2), 219–221. doi:10.1007/s00415-004-0283-9
Brown, E. N., Lydic, R., and Schiff, N. D. (2010). General anesthesia, sleep, and coma. N. Engl. J. Med. 363 (27), 2638–2650. doi:10.1056/NEJMra0808281
Brown, E. N., Purdon, P. L., and Van Dort, C. J. (2011). General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu. Rev. Neurosci. 34, 601–628. doi:10.1146/annurev-neuro-060909-153200
Brown, R. E., Basheer, R., McKenna, J. T., Strecker, R. E., and McCarley, R. W. (2012). Control of sleep and wakefulness. Physiol. Rev. 92 (3), 1087–1187. doi:10.1152/physrev.00032.2011
Buchanan, D. M., Ros, T., and Nahas, R. (2021). Elevated and slowed EEG oscillations in patients with post-concussive syndrome and chronic pain following a motor vehicle collision. Brain Sci. 11 (5), 537. doi:10.3390/brainsci11050537
Byun, S., Song, S., Kim, J. H., Ryu, T., Jeong, M. Y., and Kim, E. (2018). Mother's recorded voice on emergence can decrease postoperative emergence delirium from general anaesthesia in paediatric patients: a prospective randomised controlled trial. Br. J. Anaesth. 121 (2), 483–489. doi:10.1016/j.bja.2018.01.042
Chamadia, S., Hobbs, L., Marota, S., Ibala, R., Hahm, E., Gitlin, J., et al. (2020). Oral dexmedetomidine promotes non-rapid eye movement stage 2 sleep in humans. Anesthesiology 133 (6), 1234–1243. doi:10.1097/aln.0000000000003567
Chander, D., García, P. S., MacColl, J. N., Illing, S., and Sleigh, J. W. (2014). Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS One 9 (9), e106291. doi:10.1371/journal.pone.0106291
Chernyak, G. V., and Sessler, D. I. (2005). Perioperative acupuncture and related techniques. Anesthesiology 102 (5), 1031–1049. quiz 1077-1038. doi:10.1097/00000542-200505000-00024
Ching, S., Cimenser, A., Purdon, P. L., Brown, E. N., and Kopell, N. J. (2010). Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc. Natl. Acad. Sci. U. S. A. 107 (52), 22665–22670. doi:10.1073/pnas.1017069108
Choi, E. K., Lee, S., Kim, W. J., and Park, S. J. (2018). Effects of remifentanil maintenance during recovery on emergence delirium in children with sevoflurane anesthesia. Paediatr. Anaesth. 28 (8), 739–744. doi:10.1111/pan.13446
Choi, S. H., Lee, H., Chung, T. S., Park, K. M., Jung, Y. C., Kim, S. I., et al. (2012). Neural network functional connectivity during and after an episode of delirium. Am. J. Psychiatry 169 (5), 498–507. doi:10.1176/appi.ajp.2012.11060976
Chu, L. Y., and Pan, C. X. (2018). Study of the effects of hydromorphone on emergence agitation of children anesthetized by sevoflurane. Zhonghua Yi Xue Za Zhi 98 (28), 2250–2253. doi:10.3760/cma.j.issn.0376-2491.2018.28.008
Cohen, I. T., Finkel, J. C., Hannallah, R. S., Hummer, K. A., and Patel, K. M. (2003). Rapid emergence does not explain agitation following sevoflurane anaesthesia in infants and children: a comparison with propofol. Paediatr. Anaesth. 13 (1), 63–67. doi:10.1046/j.1460-9592.2003.00948.x
Cornelissen, L., Kim, S. E., Lee, J. M., Brown, E. N., Purdon, P. L., and Berde, C. B. (2018). Electroencephalographic markers of brain development during sevoflurane anaesthesia in children up to 3 years old. Br. J. Anaesth. 120 (6), 1274–1286. doi:10.1016/j.bja.2018.01.037
Crunelli, V., David, F., Leresche, N., and Lambert, R. C. (2014). Role for T-type Ca2+ channels in sleep waves. Pflugers Arch. 466 (4), 735–745. doi:10.1007/s00424-014-1477-3
Cui, Y., Li, G., Cao, R., Luan, L., and Kla, K. M. (2020). The effect of perioperative anesthetics for prevention of postoperative delirium on general anesthesia: a network meta-analysis. J. Clin. Anesth. 59, 89–98. doi:10.1016/j.jclinane.2019.06.028
Dahmani, S., Delivet, H., and Hilly, J. (2014). Emergence delirium in children: an update. Curr. Opin. Anaesthesiol. 27 (3), 309–315. doi:10.1097/aco.0000000000000076
Dalens, B. J., Pinard, A. M., Létourneau, D. R., Albert, N. T., and Truchon, R. J. (2006). Prevention of emergence agitation after sevoflurane anesthesia for pediatric cerebral magnetic resonance imaging by small doses of ketamine or nalbuphine administered just before discontinuing anesthesia. Anesth. Analg. 102 (4), 1056–1061. doi:10.1213/01.ane.0000200282.38041.1f
Dehaene, S., and Changeux, J. P. (2011). Experimental and theoretical approaches to conscious processing. Neuron 70 (2), 200–227. doi:10.1016/j.neuron.2011.03.018
Eckenhoff, J. E., Kneale, D. H., and Dripps, R. D. (1961). The incidence and etiology of postanesthetic excitment. A clinical survey. A Clin. Surv. Anesthesiol. 22, 667–673. doi:10.1097/00000542-196109000-00002
Feng, Z. X., Dong, H., Qu, W. M., and Zhang, W. (2018). Oral delivered dexmedetomidine promotes and consolidates non-rapid eye movement sleep via sleep-wake regulation systems in mice. Front. Pharmacol. 9, 1196. doi:10.3389/fphar.2018.01196
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow. Metab. 13 (1), 5–14. doi:10.1038/jcbfm.1993.4
Gross, J., Schnitzler, A., Timmermann, L., and Ploner, M. (2007). Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 5 (5), e133. doi:10.1371/journal.pbio.0050133
Gutierrez, R., Egaña, J. I., Saez, I., Reyes, F., Briceño, C., Venegas, M., et al. (2019). Intraoperative low alpha power in the electroencephalogram is associated with postoperative subsyndromal delirium. Front. Syst. Neurosci. 13, 56. doi:10.3389/fnsys.2019.00056
Haile, S., Girma, T., and Akalu, L. (2021). Effectiveness of propofol on incidence and severity of emergence agitation on pediatric patients undergo ENT and ophthalmic surgery: prospective cohort study design. Ann. Med. Surg. (Lond) 69, 102765. doi:10.1016/j.amsu.2021.102765
Heinmiller, L. J., Nelson, L. B., Goldberg, M. B., and Thode, A. R. (2013). Clonidine premedication versus placebo: effects on postoperative agitation and recovery time in children undergoing strabismus surgery. J. Pediatr. Ophthalmol. Strabismus 50 (3), 150–154. doi:10.3928/01913913-20130205-02
Hemmings, H. C., Akabas, M. H., Goldstein, P. A., Trudell, J. R., Orser, B. A., and Harrison, N. L. (2005). Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci. 26 (10), 503–510. doi:10.1016/j.tips.2005.08.006
Hesdorffer, D. C., Ludvigsson, P., Olafsson, E., Gudmundsson, G., Kjartansson, O., and Hauser, W. A. (2004). ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch. Gen. Psychiatry 61 (7), 731–736. doi:10.1001/archpsyc.61.7.731
Hesse, S., Kreuzer, M., Hight, D., Gaskell, A., Devari, P., Singh, D., et al. (2019). Association of electroencephalogram trajectories during emergence from anaesthesia with delirium in the postanaesthesia care unit: an early sign of postoperative complications. Br. J. Anaesth. 122 (5), 622–634. doi:10.1016/j.bja.2018.09.016
Jalili, S., Esmaeeili, A., Kamali, K., and Rashtchi, V. (2019). Comparison of effects of propofol and ketofol (Ketamine-Propofol mixture) on emergence agitation in children undergoing tonsillectomy. Afr. Health Sci. 19 (1), 1736–1744. doi:10.4314/ahs.v19i1.50
Jang, Y. E., Jeong, S. A., Kim, S. Y., Song, I. K., Lee, J. H., Kim, J. T., et al. (2018). The efficacy of intraoperative EEG to predict the occurrence of emergence agitation in the postanesthetic room after sevoflurane anesthesia in children. J. Perianesth Nurs. 33 (1), 45–52. doi:10.1016/j.jopan.2015.10.001
Jiao, H., Wang, H., Jiang, Z., and Hu, J. (2020). Comparative efficacy of ancillary drugs in sevoflurane-related emergence agitation after paediatric adenotonsillectomy: a Bayesian network meta-analysis. J. Clin. Pharm. Ther. 45 (5), 1039–1049. doi:10.1111/jcpt.13133
Kim, J., Lee, H. C., Byun, S. H., Lim, H., Lee, M., Choung, Y., et al. (2021). Frontal electroencephalogram activity during emergence from general anaesthesia in children with and without emergence delirium. Br. J. Anaesth. 126 (1), 293–303. doi:10.1016/j.bja.2020.07.060
Kim, N., Park, J. H., Lee, J. S., Choi, T., and Kim, M. S. (2017). Effects of intravenous fentanyl around the end of surgery on emergence agitation in children: systematic review and meta-analysis. Paediatr. Anaesth. 27 (9), 885–892. doi:10.1111/pan.13181
Koch, S., Rupp, L., Prager, C., Wernecke, K. D., Kramer, S., Fahlenkamp, A., et al. (2018). Emergence delirium in children is related to epileptiform discharges during anaesthesia induction: an observational study. Eur. J. Anaesthesiol. 35 (12), 929–936. doi:10.1097/eja.0000000000000867
Koo, C. H., Koo, B. W., Han, J., Lee, H. T., Lim, D., and Shin, H. J. (2022). The effects of intraoperative magnesium sulfate administration on emergence agitation and delirium in pediatric patients: a systematic review and meta-analysis of randomized controlled trials. Paediatr. Anaesth. 32 (4), 522–530. doi:10.1111/pan.14352
Kreuzer, I., Osthaus, W. A., Schultz, A., and Schultz, B. (2014). Influence of the sevoflurane concentration on the occurrence of epileptiform EEG patterns. PLoS One 9 (2), e89191. doi:10.1371/journal.pone.0089191
Larsen, L. G., Wegger, M., S, L. G., Erngaard, L., and Hansen, T. G. (2022). Emergence agitation in paediatric day case surgery: a randomised, single-blinded study comparing narcotrend and heart rate variability with standard monitoring. Eur. J. Anaesthesiol. 39 (3), 261–268. doi:10.1097/eja.0000000000001649
Lee, I. S., Cheon, S., and Park, J. Y. (2019). Central and peripheral mechanism of acupuncture analgesia on visceral pain: a systematic review. Evid. Based Complement. Altern. Med. 2019, 1304152. doi:10.1155/2019/1304152
Lee, J. H., Choi, S., Lee, M., Jang, Y. E., Kim, E. H., Kim, J. T., et al. (2020). Effect of magnesium supplementation on emergence delirium and postoperative pain in children undergoing strabismus surgery: a prospective randomised controlled study. BMC Anesthesiol. 20 (1), 289. doi:10.1186/s12871-020-01192-7
Lee, U., Ku, S., Noh, G., Baek, S., Choi, B., and Mashour, G. A. (2013). Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology 118 (6), 1264–1275. doi:10.1097/ALN.0b013e31829103f5
Lin, J. Y., Chung, S. Y., Lin, M. C., and Cheng, F. C. (2002). Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 71 (7), 803–811. doi:10.1016/s0024-3205(02)01738-1
Lin, L., Yueming, Z., Meisheng, L., Jiexue, W., and Yang, J. (2017). Effect of dexmedetomidine on emergence agitation after general anesthesia in children undergoing odontotherapy in day-surgery operating room. Hua Xi Kou Qiang Yi Xue Za Zhi 35 (6), 613–617. doi:10.7518/hxkq.2017.06.010
Lin, Y. C., Tassone, R. F., Jahng, S., Rahbar, R., Holzman, R. S., Zurakowski, D., et al. (2009). Acupuncture management of pain and emergence agitation in children after bilateral myringotomy and tympanostomy tube insertion. Paediatr. Anaesth. 19 (11), 1096–1101. doi:10.1111/j.1460-9592.2009.03129.x
Lutz, R., Müller, C., Dragovic, S., Schneider, F., Ribbe, K., Anders, M., et al. (2022). The absence of dominant alpha-oscillatory EEG activity during emergence from delta-dominant anesthesia predicts neurocognitive impairment-results from a prospective observational trial. J. Clin. Anesth. 82, 110949. doi:10.1016/j.jclinane.2022.110949
Lysakowski, C., Dumont, L., Czarnetzki, C., and Tramèr, M. R. (2007). Magnesium as an adjuvant to postoperative analgesia: a systematic review of randomized trials. Anesth. Analg. 104 (6), 1532–1539. table of contents. doi:10.1213/01.ane.0000261250.59984.cd
Martin, C. S., Yanez, N. D., Treggiari, M. M., Piper, L., Cusick, J., and Lalwani, K. (2020). Randomized controlled trial of acupuncture to prevent emergence delirium in children undergoing myringotomy tube placement. Minerva Anestesiol. 86 (2), 141–149. doi:10.23736/s0375-9393.19.13591-2
Martin, J. C., Liley, D. T., Harvey, A. S., Kuhlmann, L., Sleigh, J. W., and Davidson, A. J. (2014). Alterations in the functional connectivity of frontal lobe networks preceding emergence delirium in children. Anesthesiology 121 (4), 740–752. doi:10.1097/aln.0000000000000376
Mashour, G. A., and Avidan, M. S. (2014). Postoperative delirium: disconnecting the network? Anesthesiology 121 (2), 214–216. doi:10.1097/aln.0000000000000330
Mason, K. P. (2017). Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br. J. Anaesth. 118 (3), 335–343. doi:10.1093/bja/aew477
Mayer, M. L., Westbrook, G. L., and Guthrie, P. B. (1984). Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature 309 (5965), 261–263. doi:10.1038/309261a0
Meyburg, J., Ries, M., Zielonka, M., Koch, K., Sander, A., von Haken, R., et al. (2018). Cognitive and behavioral consequences of pediatric delirium: a pilot study. Pediatr. Crit. Care Med. 19 (10), e531–e537. doi:10.1097/pcc.0000000000001686
Mihaljević, S., Pavlović, M., Reiner, K., and Ćaćić, M. (2020). Therapeutic mechanisms of ketamine. Psychiatr. Danub 32 (3-4), 325–333. doi:10.24869/psyd.2020.325
Miyazaki, S., Uchida, S., Mukai, J., and Nishihara, K. (2004). Clonidine effects on all-night human sleep: opposite action of low- and medium-dose clonidine on human NREM-REM sleep proportion. Psychiatry Clin. Neurosci. 58 (2), 138–144. doi:10.1111/j.1440-1819.2003.01207.x
Moore, A. D., and Anghelescu, D. L. (2017). Emergence delirium in pediatric anesthesia. Paediatr. Drugs 19 (1), 11–20. doi:10.1007/s40272-016-0201-5
Nakajima, D., Mihara, T., Hijikata, T., Tomita, M., and Goto, T. (2022). Effectiveness of acupuncture therapy for preventing emergence agitation in children: a protocol for systematic review and meta-analysis with trial sequential analysis. PLoS One 17 (3), e0264197. doi:10.1371/journal.pone.0264197
Ng, K. T., Sarode, D., Lai, Y. S., Teoh, W. Y., and Wang, C. Y. (2019). The effect of ketamine on emergence agitation in children: a systematic review and meta-analysis. Paediatr. Anaesth. 29 (12), 1163–1172. doi:10.1111/pan.13752
Numan, T., van den Boogaard, M., Kamper, A. M., Rood, P. J. T., Peelen, L. M., Slooter, A. J. C., et al. (2019). Delirium detection using relative delta power based on 1-minute single-channel EEG: a multicentre study. Br. J. Anaesth. 122 (1), 60–68. doi:10.1016/j.bja.2018.08.021
Oğün, C. O., Ustün, M. E., Duman, A., Gürbilek, M., and Genç, B. O. (2002). Correlation between tissue lactate levels and electroencephalogram in evaluating the severity of experimental head trauma. Crit. Care Med. 30 (9), 2123–2128. doi:10.1097/01.Ccm.0000026326.17262.F3
Oye, I., Paulsen, O., and Maurset, A. (1992). Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J. Pharmacol. Exp. Ther. 260 (3), 1209–1213.
Pacheco Dda, F., Romero, T. R., and Duarte, I. D. (2014). Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res. 1562, 69–75. doi:10.1016/j.brainres.2014.03.026
Pal, D., Hambrecht-Wiedbusch, V. S., Silverstein, B. H., and Mashour, G. A. (2015). Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. Br. J. Anaesth. 114 (6), 979–989. doi:10.1093/bja/aev095
Peker, K., and Polat, R. (2020). Effects of intravenous and mask induction on post-operative emergence delirium in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Ir. J. Med. Sci. 189 (3), 1061–1068. doi:10.1007/s11845-020-02197-4
Pinto Filho, W. A., Silveira, L. H. J., Vale, M. L., Fernandes, C. R., and Gomes, J. A. (2019). Gabapentin in improvement of procedural sedation and analgesia in oncologic pediatric patients: a clinical trial. Anesth. Pain Med. 9 (5), e91197. doi:10.5812/aapm.91197
Purdon, P. L., Sampson, A., Pavone, K. J., and Brown, E. N. (2015). Clinical electroencephalography for Anesthesiologists: Part I: background and basic signatures. Anesthesiology 123 (4), 937–960. doi:10.1097/aln.0000000000000841
Ramlan, A. A. W., Pardede, D. K. B., Marsaban, A., Hidayat, J., and Peddyandhari, F. S. (2020). Efficacy of 0.5 mg/kg of propofol at the end of anesthesia to reduce the incidence of emergence agitation in children undergoing general anesthesia with sevoflurane. J. Anaesthesiol. Clin. Pharmacol. 36 (2), 177–181. doi:10.4103/joacp.JOACP_257_19
Sadeghi, A., Sajad Razavi, S., Eghbali, A., Alireza Mahdavi, S., Kimia, F., and Panah, A. (2022). The comparison of the efficacy of early versus late administration of dexmedetomidine on postoperative emergence agitation in children undergoing oral surgeries: a randomized clinical trial. Iran. J. Med. Sci. 47 (1), 25–32. doi:10.30476/ijms.2020.84509.1471
Sanna, E., Garau, F., and Harris, R. A. (1995). Novel properties of homomeric beta 1 gamma-aminobutyric acid type A receptors: actions of the anesthetics propofol and pentobarbital. Mol. Pharmacol. 47 (2), 213–217.
Schmitz, A., Weiss, M., Kellenberger, C., O'Gorman Tuura, R., Klaghofer, R., Scheer, I., et al. (2018). Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics-A prospective randomized double-blinded study. Paediatr. Anaesth. 28 (3), 264–274. doi:10.1111/pan.13315
Schultz, B., Otto, C., Schultz, A., Osthaus, W. A., Krauss, T., Dieck, T., et al. (2012). Incidence of epileptiform EEG activity in children during mask induction of anaesthesia with brief administration of 8% sevoflurane. PLoS One 7 (7), e40903. doi:10.1371/journal.pone.0040903
Schwartz, M. D., and Kilduff, T. S. (2015). The neurobiology of sleep and wakefulness. Psychiatr. Clin. North Am. 38 (4), 615–644. doi:10.1016/j.psc.2015.07.002
Shen, Q. H., Xu, S., Lai, L., Chen, Y. J., Liu, K., and Sun, L. J. (2022). The effect of magnesium sulfate on emergence agitation in children undergoing general anesthesia: a systematic review and meta-analysis. J. Clin. Anesth. 78, 110669. doi:10.1016/j.jclinane.2022.110669
Sikich, N., and Lerman, J. (2004). Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 100 (5), 1138–1145. doi:10.1097/00000542-200405000-00015
Simonini, A., Vittori, A., Cascella, M., Calevo, M. G., and Marinangeli, F. (2021). The impact of emergence delirium on hospital length of stay for children who underwent tonsillectomy/adenotonsillectomy: an observational retrospective study. Braz J. Anesthesiol. 73, 171–176. doi:10.1016/j.bjane.2021.10.006
Singla, L., Mathew, P. J., Jain, A., Yaddanapudi, S., and Peters, N. J. (2021). Oral melatonin as part of multimodal anxiolysis decreases emergence delirium in children whereas midazolam does not: a randomised, double-blind, placebo-controlled study. Eur. J. Anaesthesiol. 38 (11), 1130–1137. doi:10.1097/eja.0000000000001561
Sun, Y., Li, Y., Sun, Y., Wang, X., Ye, H., and Yuan, X. (2017). Dexmedetomidine effect on emergence agitation and delirium in children undergoing laparoscopic hernia repair: a preliminary study. J. Int. Med. Res. 45 (3), 973–983. doi:10.1177/0300060517699467
Urits, I., Peck, J., Giacomazzi, S., Patel, R., Wolf, J., Mathew, D., et al. (2020). Emergence delirium in perioperative pediatric care: a review of current evidence and new directions. Adv. Ther. 37 (5), 1897–1909. doi:10.1007/s12325-020-01317-x
Vakkuri, A., Yli-Hankala, A., Särkelä, M., Lindgren, L., Mennander, S., Korttila, K., et al. (2001). Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children. Acta Anaesthesiol. Scand. 45 (7), 805–811. doi:10.1034/j.1399-6576.2001.045007805.x
van Dellen, E., van der Kooi, A. W., Numan, T., Koek, H. L., Klijn, F. A., Buijsrogge, M. P., et al. (2014). Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology 121 (2), 328–335. doi:10.1097/aln.0000000000000329
van der Kooi, A. W., Zaal, I. J., Klijn, F. A., Koek, H. L., Meijer, R. C., Leijten, F. S., et al. (2015). Delirium detection using EEG: what and how to measure. Chest 147 (1), 94–101. doi:10.1378/chest.13-3050
Voepel-Lewis, T., Malviya, S., and Tait, A. R. (2003). A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth. Analg. 96 (6), 1625–1630. doi:10.1213/01.Ane.0000062522.21048.61
Wang, C., Wang, W., Wang, S., He, R., Yang, H., Jia, Y., et al. (2021). Effect of recorded maternal voice on emergence delirium in children under general anesthesia: a randomized controlled trial. J. Nerv. Ment. Dis. 209 (11), 814–819. doi:10.1097/nmd.0000000000001433
Weerink, M. A. S., Struys, M., Hannivoort, L. N., Barends, C. R. M., Absalom, A. R., and Colin, P. (2017). Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet. 56 (8), 893–913. doi:10.1007/s40262-017-0507-7
Wei, B., Feng, Y., Chen, W., Ren, D., Xiao, D., and Chen, B. (2021). Risk factors for emergence agitation in adults after general anesthesia: a systematic review and meta-analysis. Acta Anaesthesiol. Scand. 65 (6), 719–729. doi:10.1111/aas.13774
Wu, X., Cao, J., Shan, C., Peng, B., Zhang, R., Cao, J., et al. (2019a). Efficacy and safety of propofol in preventing emergence agitation after sevoflurane anesthesia for children. Exp. Ther. Med. 17 (4), 3136–3140. doi:10.3892/etm.2019.7289
Wu, X., Shan, C., Peng, B., Shi, X., Zhang, F., and Cao, J. (2019b). Comparison of desflurane and sevoflurane on postoperative recovery quality after tonsillectomy and adenoidectomy in children. Exp. Ther. Med. 17 (6), 4561–4567. doi:10.3892/etm.2019.7467
Yang, X., Lin, C., Chen, S., Huang, Y., Cheng, Q., and Yao, Y. (2022). Remimazolam for the prevention of emergence delirium in children following tonsillectomy and adenoidectomy under sevoflurane anesthesia: a randomized controlled study. Drug Des. Devel Ther. 16, 3413–3420. doi:10.2147/dddt.S381611
Yang, Y. Y., Zhang, M. Z., Sun, Y., Peng, Z. Z., Liu, P. P., Wang, Y. T., et al. (2020). Effect of recorded maternal voice on emergence agitation in children undergoing bilateral ophthalmic surgery: a randomised controlled trial. J. Paediatr. Child. Health 56 (9), 1402–1407. doi:10.1111/jpc.14948
Yao, Y., Qian, B., Lin, Y., Wu, W., Ye, H., and Chen, Y. (2015). Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr. Anaesth. 25 (5), 492–498. doi:10.1111/pan.12574
Zhang, Y. Z., Wei, X. L., Tang, B., Qin, Y. Y., Ou, M., Jiang, X. H., et al. (2022). The effects of different doses of alfentanil and dexmedetomidine on prevention of emergence agitation in pediatric tonsillectomy and adenoidectomy surgery. Front. Pharmacol. 13, 648802. doi:10.3389/fphar.2022.648802
Zhao, Z. Q. (2008). Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 85 (4), 355–375. doi:10.1016/j.pneurobio.2008.05.004
Zhong, H. Y., Deng, X. B., and Wang, Z. (2018). Effects of fascia iliaca compartment block combined with general laryngeal mask airway anesthesia in children undergoing femoral fracture surgery: a randomized trial. J. Pain Res. 11, 2821–2826. doi:10.2147/jpr.S177122
Keywords: emergence delirium, neurophysiology, electroencephalography, pediatrics, general anesthesia, treatment
Citation: Gao X, Li Z, Chai J, Li S, Pan X, Liu J, Li L, Qin S, Kang Y and Zhu Y (2024) Electroencephalographic insights into the pathophysiological mechanisms of emergence delirium in children and corresponding clinical treatment strategies. Front. Pharmacol. 15:1349105. doi: 10.3389/fphar.2024.1349105
Received: 04 December 2023; Accepted: 26 February 2024;
Published: 19 June 2024.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Yitian Yang, Henan Provincial People’s Hospital, ChinaHuanghui Wu, Shanghai Fourth People’s Hospital, China
Copyright © 2024 Gao, Li, Chai, Li, Pan, Liu, Li, Qin, Kang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chai, Y2hhaWp1bl9jbXVAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xin Gao
Xin Gao Zhichao Li
Zhichao Li Jun Chai
Jun Chai Si Li1
Si Li1