
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 08 February 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1348019
This article is part of the Research Topic Drug Discovery Derived from Herbal Medicine/Polypeptide for Neurological Diseases View all 14 articles
Depression is a prevalent mental disorder. However, clinical treatment options primarily based on chemical drugs have demonstrated varying degrees of adverse reactions and drug resistance, including somnolence, nausea, and cognitive impairment. Therefore, the development of novel antidepressant medications that effectively reduce suffering and side effects has become a prominent area of research. Polysaccharides are bioactive compounds extracted from natural plants that possess diverse pharmacological activities and medicinal values. It has been discovered that polysaccharides can effectively mitigate depression symptoms. This paper provides an overview of the pharmacological action and mechanisms, intervention approaches, and experimental models regarding the antidepressant effects of polysaccharides derived from various natural sources. Additionally, we summarize the roles and potential mechanisms through which these polysaccharides prevent depression by regulating neurotransmitters, HPA axis, neurotrophic factors, neuroinflammation, oxidative stress, tryptophan metabolism, and gut microbiota. Natural plant polysaccharides hold promise as adjunctive antidepressants for prevention, reduction, and treatment of depression by exerting their therapeutic effects through multiple pathways and targets. Therefore, this review aims to provide scientific evidence for developing polysaccharide resources as effective antidepressant drugs.
Depression, as a common mental disorder, seriously endangers human physical and mental health (Paykel, 2008). People with depression usually show symptoms such as lack of interest, slow thinking, low mood, loss of appetite, insomnia and sleeplessness, and decreased willpower (Thapar et al., 2022). With socio-economic development and incremental pressure on people, the number of people suffering from depression has increased dramatically, bringing serious costs to society and families. Because the severe illness is often accompanied by self-harm, suicidal tendencies and violent tendencies, making depression become the disease with the highest suicide rate in the world. At the same time, the disease can seriously affect or even damage the patient’s digestive system, immune system, and nervous system (Fries et al., 2023). The pathogenesis of depression is complex (Guo et al., 2023), and its pathogenic mechanism is not yet clear. Existing studies have shown that the main features of depression are abnormal neurotransmitter levels in patients, abnormal function of the hypothalamic-pituitary-adrenal (HPA) axis, hormonal imbalance in the organism with inflammation and oxidative stress, neurotrophic factors, tryptophan metabolism, and the occurrence of gut microbiota-related diseases, as shown in Figure 1.
At present, depression is mostly treated with chemical drugs, antipsychotic drugs, which can be divided into first- and second-generation drugs (Rybakowski, 2023). The first-generation drugs are mainly dopamine receptor blockers, which have antipsychotic effects by blocking dopamine receptors in the dopamine pathway of the central nervous system. And their long-term use can cause side effects such as drowsiness and cardiac rhythm disturbances. In response to the problems caused by the first-generation drugs, the second generation of drugs represented by fluoxetine and paroxetine combines therapeutic efficacy and low side effects, which improves depression and relieve anxiety though inhibiting the reuptake of 5-serotonin (5-HT) (Grinchii and Dremencov, 2020). 5-HT, as one of the indole derivatives, is a neurotransmitter with the effects on mood regulation, memory improvement and cognitive enhancement that exerts inhibitory effects in the neuroendocrine system, which is considered to be a messenger of pleasure (Moncrieff et al., 2022). The reduction of its levels is closely correlated with the occurrence of depression and anxiety. However, second-generation drugs tend to have slow onset of action, weak anxiolytic effects and are associated with prolonged cognitive impairment (Barbuti et al., 2023). While these drugs can relieve the symptoms of depression, there is currently no cure for depression. At the same time, antipsychotic drugs are limited by the fact that they can only act at one site or one type of target site. As a result, depression is characterized by low remission rate, high recurrence rate and significant side effects for patients, and only 12.7% of patients received minimal adequate treatment (Grinchii and Dremencov, 2020). Despite the clear target and rapid efficacy of Western clinical antidepressant drugs, they also have the disadvantages of low efficiency, single pathway, and serious adverse effects. Due to the complexity of the pathogenesis of depression, single-target drugs have limited amelioration of depression and serious side effects, the exploitation of new multi-target drugs for the treatment of depression is particularly important.
Therefore, the development of an antidepressant drug, with high efficiency, long-term use, and low side effects, has become a recent research hotspot. Polysaccharides from natural sources have received widespread attention due to their wide range of pharmacological activities and fewer side effects (Song et al., 2021). Polysaccharides are the biologically active macromolecules formed by monosaccharides linked by glycosidic bonds, which are formed by polyhydroxy polymers and their derivatives. They have widespread in natural products of higher plants, animals, microorganisms and algae. Numerous studies have shown that polysaccharides have anti-inflammatory, antioxidant, antiviral, immune regulation, hypoglycemic and hypolipidemic effects, and regulation of gut microbiota (Mu et al., 2021; Ji et al., 2023). Meanwhile, existing studies have demonstrated that the polysaccharide molecules in natural products can effectively alleviate depression, and their mechanism of action has attracted extensive attention from researchers (Yi et al., 2009; Song et al., 2022; Guo et al., 2023). The progress has been reported in the study of the multi-targeted therapeutic effects of polysaccharides in depression. Some researchers have pointed out that the mechanism is achieved through the modulation of brain function, biological and immune barriers. The polysaccharide molecules in natural products can usually be used in multiple targets with significant therapeutic effects and fewer side effects (Wang et al., 2022b). However, there are fewer reports on the relationship between polysaccharides and depression. This review summarizes the recent progress of research on the antidepressant effects of polysaccharides by intervening in different mechanisms in recent years, providing references for further research on the prevention, alleviation and treatment of depression and the development of therapeutic drugs.
Polysaccharides in natural plant sources alleviate depression by regulating the expression of neurotransmitters and their receptors, HPA axis, neurotrophic factors, neuroinflammatory, oxidative stress, tryptophan metabolism and gut microbiota. The various plant polysaccharides from different sources can induce antidepressant effects through diverse mechanisms of action, as summarized in Table 1.
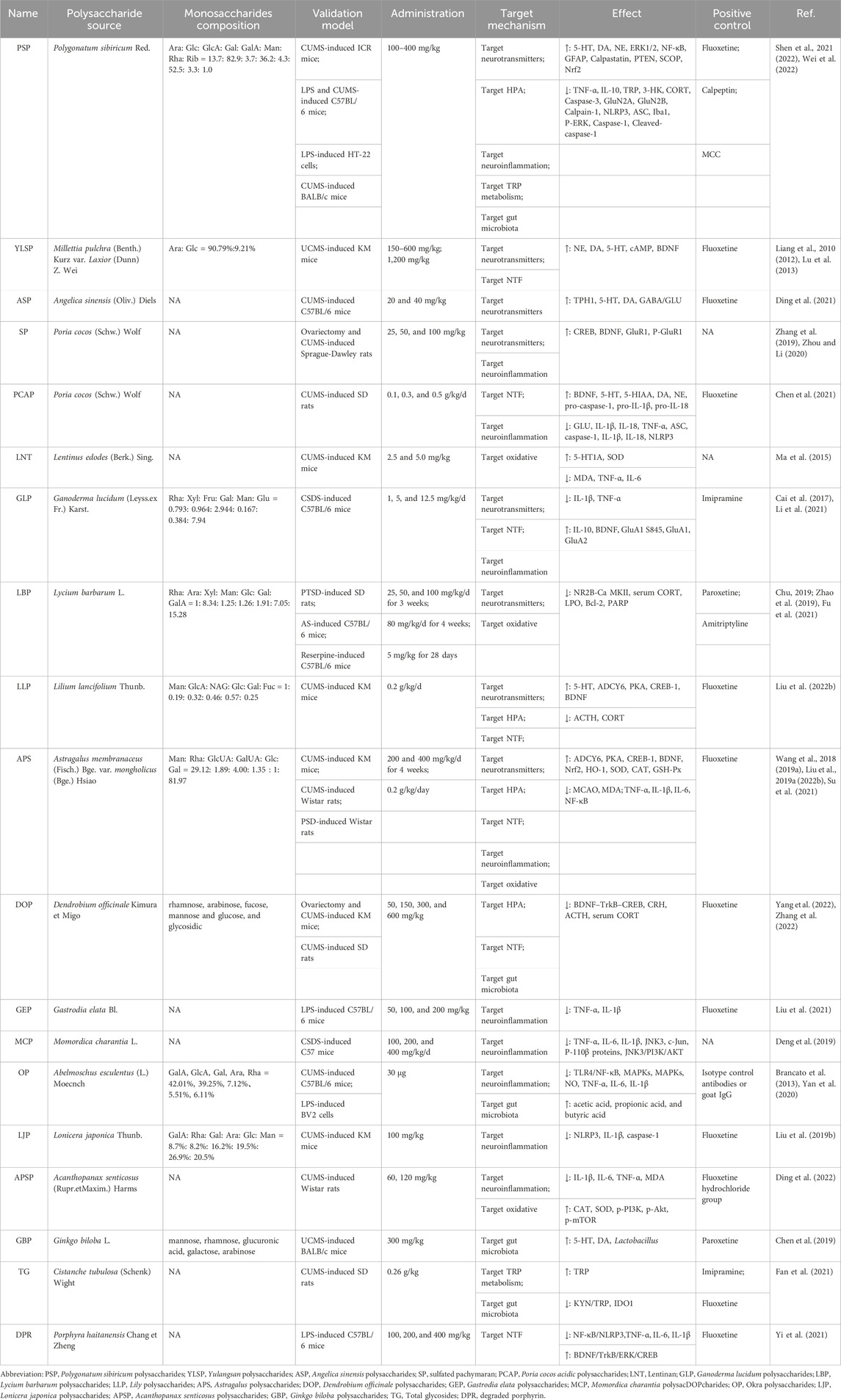
TABLE 1. Validation models, targeting mechanisms and intervention results of natural plant polysaccharides.
The mechanism of action underlying the majority of current antidepressants is predicated upon the hypothesis pertaining to the monoaminergic system. According to the hypothesis of the monoaminergic system, depression is characterized by a reduction in levels of 5-hydroxytryptamine, dopamine (DA), and norepinephrine (NE) within the central nervous system. The pathogenesis of depression is rooted in the dysregulation of 5-HT, DA, and NE neurotransmitter systems. The upregulation of type A monoamine oxidase (MAOA) and the downregulation of serotonin (5-hydroxytryptamine, 5-HT) and NE levels in the brain are considered to be the primary etiological factors underlying depression. The MAOA enzyme is responsible for the breakdown of monoamine neurotransmitters, including 5-HT, NE, and DA. It significantly contributes to the pathogenesis, progression, and treatment of various neuropsychiatric conditions. Studies have demonstrated that DA, 5-HT, and other neurotransmitters are crucial in maintaining chemical homeostasis within the brain. An imbalance of these substances, whether it be an excess or deficiency of neurotransmitters, can result in abnormalities within the signaling system of the brain, ultimately leading to the onset and development of depression (Kim et al., 2019).
Serotonin, also known as 5-HT, is a monoamine neurotransmitter that accumulates in nerve endings through the action of the 5-hydroxytryptamine transporter (SERT). This transporter facilitates the uptake of 5-HT into the cytoplasm to replenish synaptic vesicles and terminate its extracellular effects. In addition to regulating behavioral, emotional, and memory processes in the human body, 5-HT also plays a crucial role in the treatment of various psychiatric and neurological disorders and is pivotal in understanding depression’s pathogenesis and treatment. In addition, 5-HT exhibits a distinctive form of neuroplasticity that encompasses synaptic plasticity. It has been demonstrated that addressing the deficit in synaptic plasticity caused by neuronal atrophy and cell death can be beneficial in the treatment of depression (Popa et al., 2010). The substance NE is derived from adrenaline by removing the N-methyl group, and it plays a crucial role in regulating the functions of various internal organs, glands, and the immune system. Additionally, NE serves as a vital neurotransmitter within the central nervous system (CNS). NE neurons originate in the locus coeruleus (LC) of the brain and extend their axons through different regions such as the CNS cortex, hypothalamus, amygdala, cerebellum, and spinal cord to reach primary nerve centers or other neurons (Jovanovic et al., 2022). The serotonin and noradrenaline reuptake inhibitor (SNRI) functions as a modulator of NE neurotransmission, making it an effective first-line medication for depression treatment. Its antidepressant effect is attributed to its regulation of NE levels in the body. DA serves as the predominant catecholamine neurotransmitter in the brain and plays a crucial role in transmitting feelings of excitement and happiness, as well as being involved in learning and rewarding activities. It is closely associated with human eroticism and sensation, transmitting pleasure and excitement while participating in learning, motor function, and reward-related processes. Dopaminergic activity is regulated by the ventral inferior colliculus of the hippocampus and basolateral amygdala regions. Studies have demonstrated deficits within the dopaminergic system among patients with depression, suggesting that these deficiencies may originate from dysfunctions within their afferent circuits (Belujon and Grace, 2017). The existing body of evidence strongly indicates an association between dysfunction in the dopaminergic system and depression. The excitatory neurotransmitter glutamate (Glu), which is found in the central nervous system (CNS), functions as an amino acid neurotransmitter and interacts with both ionotropic and metabotropic receptors. The production of Glu in neurons occurs through glucose-derived intermediates of the tricarboxylic acid cycle and branched-chain amino acids. It is subsequently released at synapses in the brain, exerting short-term effects on postsynaptic excitability and longer-term effects on synaptic strength and neural plasticity. This process involves modulation of the second-messenger system, downstream effects on the activity of various membrane-bound receptors, nuclear gene expression, and translation. Abnormalities in the transmission of excitatory or inhibitory neurotransmitters and neuronal plasticity can result in dysfunctions in brain function, while altered levels of Glu and γ-aminobutyric acid (GABA) have been associated with dysfunction of neural networks. Clinical studies conducted on depression have also identified changes in the concentration and activity of Glu and GABA (Duman et al., 2019), suggesting that dysfunctions in excitatory and inhibitory neurotransmitter signaling mechanisms may play a significant role in depression. The findings of other studies have demonstrated that Glu neurotransmission plays a crucial role in the pathophysiology and therapeutic response to depression, leading to a reduction in depressive symptoms (Fond et al., 2014; Newport et al., 2015). The inhibitory neurotransmitter GABA is naturally present in the human nervous system, acting as a non-protein amino acid responsible for precise control and regulation of excitatory transmission. The neurotransmitter GABA is a significant target for 5-HT afferent fibers (Möhler, 2012), and its physiological effects encompass the modulation of synaptic transmission, facilitation of neuronal development, as well as prevention of insomnia and depression.
Currently, the primary focus of clinical depression treatment lies in targeting monoamine neurotransmitters. This is achieved by inhibiting monoamine oxidation and blocking the reuptake of 5-HT and NE, thereby increasing extracellular levels of 5-HT, DA, and NE throughout the brain. Consequently, there is an elevation in synaptic concentrations of these neurotransmitters which enhances excitatory potential transmission at neural synaptic endings to ameliorate depressive symptoms (Yang et al., 2021).
The Polygonatum sibiricum is a traditional medicinal and food plant, wherein the Polygonatum sibiricum polysaccharide (PSP) serves as one of its primary bioactive constituents. In the acute behavioral despair mouse depression model, PSP (100, 200, and 400 mg/kg) significantly reduced immobility time in tail-hanging and forced-swimming experiments. Moreover, the levels of 5-HT, DA, and NE in the cortex of mice were significantly elevated compared to those in the model group (Wei et al., 2022). In the lipopolysaccharide (LPS) depression mouse model and the chronic unpredictable stress (CUS)-induced depression mouse model, PSP were found to enhance depressive-like behavior in mice and significantly elevate hippocampal 5-HT levels (Shen et al., 2021; Shen et al., 2022). The aforementioned studies suggest that the antidepressant effects of PSP are associated with the modulation of monoamine neurotransmitters in the brain.
The Yulangsan polysaccharide (YLSP, 300 and 600 mg/kg) can also exert antidepressant effects by elevating the levels of 5-HT, DA, and NE in brain tissue (Liang et al., 2010). The Angelica sinensis polysaccharide (ASP), one of the primary active compounds derived from Angelica sinensis (Hou et al., 2021), has been found to significantly reduce the duration of forced swimming and immobility in mice with hanging tails at a dosage of 40 mg/kg (Ding et al., 2021). Moreover, it enhances sugar-water preference, increases the levels of DA and 5-HT in the hippocampus, upregulates tryptophan hydroxylase mRNA expression, and elevates the GABA/Glu ratio-an essential rate-limiting enzyme involved in 5-HT synthesis, thereby regulating monoamine neurotransmitter transmission and rectifying excitatory-inhibitory imbalances in mice. The rat depression model was established by (Zhang et al., 2019) through ovarian removal combined with CUS, aiming to explore the correlation between the antidepressant effect of sulfated pachymaran (SP) and AMPA receptors (Zhang et al., 2019). The AMPA receptor, a type of Glu receptor primarily responsible for excitatory synaptic transmission in the brain, is closely associated with the pathogenesis of depression (Shirayama et al., 2022). The administration of SP (50 and 100 mg/kg) for 21 days was found to reduce hippocampal neuronal damage and increase the expression levels of both AMPA receptor Glu R1 and p-Glu R1. However, these anti-inflammatory effects and upregulation of AMPA receptors were completely inhibited by the AMPA receptor inhibitor GYKI52466. These findings suggest that the antidepressant effect of SP may be achieved through the regulation of AMPA receptor Glu R1 expression. Ganoderma lucidum polysaccharide (GLP, at doses of 1, 5, and 12.5 mg/kg) also increased the expression levels of p-Glu A1, Glu A1, and Glu A2 in the hippocampus of mice subjected to chronic social frustration stress (Li et al., 2021).
The N-methyl-D-aspartate receptor (NMDAR) is a specific receptor for glutamate, with NR2B being the most important functional subunit. Excessive stress can lead to increased glutamate release and overactivation of NMDAR, resulting in calcium influx and subsequent activation of intracellular signaling pathways that cause neuronal atrophy and death, ultimately leading to mood disorders and depression-like symptoms (Abdoulaye et al., 2021). The administration of Lycium barbarum polysaccharide (LBP, 25, 50, and 100 mg/kg) to rats for three consecutive weeks intragastrically was found to effectively inhibit the excessive activation of NR2B in the prefrontal cortex of rats, reduce the expression of calmodulin kinase II (CaMKII), a crucial downstream signaling molecule of NMDAR, and alleviate depressive-like behaviors in rats with PTSD (Chu, 2019).
The natural plant polysaccharides have the ability to modulate neurotransmitters in the brain. They can significantly enhance the levels of 5-HT, DA, and NE; mitigate hippocampal neuronal damage and regulate excitatory synaptic transmission in the brain; as well as exert antidepressant effects. Additionally, natural plant polysaccharides can inhibit excessive activation of NR2B in the prefrontal cortex of rats, reduce expression of the pivotal signaling molecule CaMKII downstream of NMDAR, and ameliorate depressive behaviors induced by post-traumatic stress disorder. The potential mechanism of polysaccharide-targeted intervention in depression through neurotransmitters and their receptors is illustrated in Figure 2.
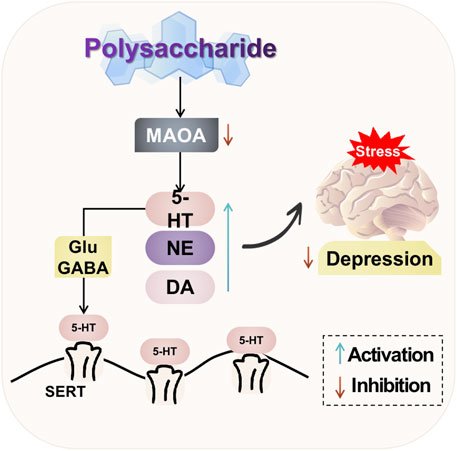
FIGURE 2. Possible antidepressant mechanism of polysaccharide targeted intervention in neurotransmitters and their receptors.
The hypothalamic-pituitary-adrenal (HPA) axis plays a crucial role in stress regulation and serves as a vital component of the neuroendocrine system, which is intricately associated with stress and stimulation (Van Den Eede and Claes, 2004; Mikulska et al., 2021). During prolonged stress and the stress response, corticosterone (CORT) levels remain elevated due to the loss of negative feedback regulation on corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) (Song et al., 2021). This leads to hyperactivity of the HPA axis, increased release of CRH, stimulation of the pituitary gland to release ACTH, and disruption in the normal synthesis, release, and degradation of corticosterone by the adrenal cortex. Consequently, this imbalance results in damage to hippocampal neurons which can trigger or exacerbate depression. In a negative feedback loop, the HPA axis suppresses the feedback signals transmitted by cortisol to the hypothalamus and pituitary gland in order to decrease CRH and ACTH production, thereby regulating its own secretion level for stress response management (Chai et al., 2022). Therefore, implementing strategies to attenuate the release of CORT, CRH, and ACTH while promoting negative feedback regulation of the HPA axis represents efficacious approaches for decelerating the progression of depression.
The administration of Polygonatum sibiricum polysaccharide (PSP) significantly decreased CORT levels in LPS and chronic unpredictable mild stress (CUMS)-induced mice, thereby demonstrating the inhibition of hyperactivity in the HPA axis (Shen et al., 2021). The Lycium barbarum polysaccharide (LBP) significantly downregulated the expression of N-methyl-D-aspartate receptor 2B subunit (NR2B) and calmodulin kinase II (CaMKII) proteins, reduced serum CORT levels, and enhanced the negative feedback regulation of the HPA axis, thereby ameliorating depressive behaviors in rats with post-traumatic stress disorder (PTSD) (Chu, 2019). Liu et al. (2022) discovered that the administration of Lily polysaccharides (LLP), Astragalus polysaccharides (APS), and their combination to CUMS-induced mice for consecutive 28 d significantly ameliorated the depression-like behavior of CUMS mice. The polysaccharides mitigated the pathological damage of neuronal cells in the hippocampal CA1 region to varying extents, and markedly reduced plasma levels of ACTH and CORT. The combined administration of LLP and APS exhibited a superior antidepressant effect compared to the individual administration of polysaccharides. The study conducted by Zhang et al. demonstrated that Dendrobium officinale polysaccharide (DOP) effectively reduced the elevated serum levels of CRH, ACTH, and CORT in depression model mice (Zhang et al., 2022). This suggests that DOP restores the HPA axis and mitigates the depressive effects induced by de-virginized and chronic mild stress-induced peri-menopausal syndrome model mice.
In summary, polysaccharides can ameliorate depressive-like behavior by inhibiting hyperactivity of the HPA axis. They have the ability to decrease levels of CRH, ACTH, and CORT, thereby facilitating negative feedback regulation of the HPA axis. Additionally, polysaccharides can mitigate hippocampal neuronal damage, suppress excessive activation of the HPA axis, exerting antidepressant effects. The potential mechanism of polysaccharide-targeted intervention in depression through HPA axis is illustrated in Figure 3.
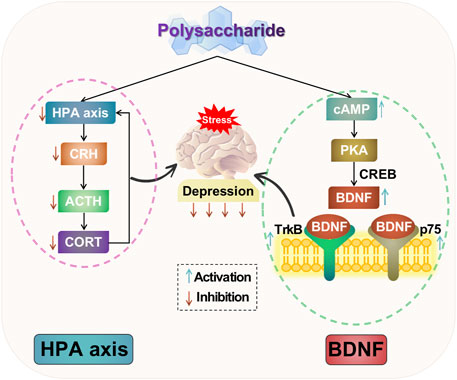
FIGURE 3. Possible antidepressant mechanism of polysaccharide targeted intervention in HPA axis and neurotrophic factors.
Neurotrophic factors (NTF), also referred to as neurotrophic agents, are a class of substances that exert a supportive influence on neuronal survival and facilitate neuronal regeneration and functional recovery. They are widely employed in the clinical management of Alzheimer’s disease, cerebral atrophy, Parkinson’s disease, and other neurological disorders (Castrén et al., 2007; PehliVan Karakas et al., 2020). Brain-derived neurotrophic factors (BDNF), the most widely distributed neurotrophic factors in the CNS, plays important roles by activating both the prokinetic myosin-associated kinase (Trk) and p75 receptor. BDNF has become a representative factor in depression research. The blood of patients with persistent and recurrent depression, as well as animal models of depression, have been consistently found to exhibit reduced levels of NTF in numerous studies (Zhao et al., 2021; Zhuang et al., 2022; Joo et al., 2023). The conventional and rapid antidepressants not only rely on the expression of BDNF and its downstream signaling for their efficacy, but they also directly interact with the transmembrane structural domain of the TrkB dimer, leading to the stabilization of a multiprotein complex conformation that facilitates TrkB binding to BDNF. NTF can activate cell signaling pathways through Trk receptors, which in turn regulate various aspects of neuronal function such as cell fate determination, axon growth, dendritic growth and pruning (Dwivedi, 2009).
Neuroplasticity is modulated in individuals with depression, where the ratio of BDNF to pro-BDNF plays a essential role in synaptic plasticity (Vigna et al., 2019). The cyclic AMP (cAMP)/cAMP-response element binding protein (CREB)/BDNF pathway serves as an important antidepressant pathway, as well as a significant route and target for pharmacotherapy in depression. The cAMP/CREB/BDNF is a crucial signaling pathway in regulating hippocampal neuronal regeneration in depression (Yamada et al., 2003). The main mechanism of action of antidepressants is to enhance the concentration of cAMP, thereby inducing alterations in the cAMP signaling cascade and its downstream target, pCREB BDNF (Gao et al., 2022).
The antidepressant effects of Yulangsan polysaccharide (YLSP) have been demonstrated in animal models of “behavioral despair” (Liang et al., 2010), exerting a suppressive effect on depression through the upregulation of monoamine neurotransmitters, enhancement of prefrontal cortical adenylate cyclase activity, and increased hippocampal expression of BDNF (Liang et al., 2012). Lu et al. (2013) proposed that the neuroprotective effect of the YLSP group in a chronic stress depression model mice may be attributed to its ability to stimulate the expression of BDNF and its receptor TrkB, thereby activating neuronal protective signaling pathways and CREB activation, which inhibits neuronal death while promoting differentiation and regeneration. The acidic polysaccharides derived from Poria cocos were found to enhance the levels of BNDF, 5-HT, 5-HIAA, DA, and NE in the hippocampus while significantly reducing Glu levels. This suggests that these polysaccharides may exert antidepressant effects by modulating relevant trophic factors and neurotransmitters in depressed rats (Chen et al., 2021). Additionally, Ganoderma lucidum polysaccharides (GLP) were observed to elevate BDNF expression in the hippocampus of mice subjected to chronic social frustration stress (Li et al., 2021).
The bioactive polysaccharide degraded porphyrin (DPR), extracted from Porphyra haitanensis, was utilized by Yi et al. to reverse depressive-like behaviors in LPS-treated mice. This treatment activated the BDNF/TrkB/ERK/CREB signaling pathway in the hippocampus of CUMS mice, offering a potential therapeutic approach for depression (Yi et al., 2021). Yang et al. (2022) discovered that the alcohol-soluble polysaccharides present in Dendrobium officinale flowers exhibit additional protective effects against neuronal apoptosis and contribute to the maintenance of the 5-HT system by activating the BDNF/TrkB/CREB pathway. Liu et al. investigated the combined effects of Lily polysaccharide (LLP) and Astragalus polysaccharide (APS) in a specific ratio on depressive-like behaviors and their modulation of the adenylyl cyclase/cyclic adenosine monophosphate/protein kinase A (AC/cAMP/PKA) signaling pathway in mice subjected to chronic stress (Liu et al., 2022). The findings suggest a significant enhancement in the antidepressant effect of LLP and APS following their combination, potentially through the modulation of brain 5-HT levels and suppression of HPA axis-induced stress, leading to activation of the AC/cAMP/PKA signaling pathway and upregulation of BDNF levels.
The studies suggest that depression induces neuronal atrophy and loss in limbic regions of the brain, such as the hippocampus, prefrontal lobe, and amygdala, along with a decrease in BDNF expression. Conversely, the administration of antidepressant drugs promotes adult hippocampal neurogenesis and an upregulation of BDNF expression. BDNF and relevant signaling pathways may exert antidepressant effects by modulating neuronal growth, differentiation, injury response, apoptosis, and regulating neuroendocrine networks. Therefore, investigating BDNF and relevant signaling pathway is crucial for studying the pathogenesis of depression. The potential mechanism of polysaccharide-targeted intervention in depression through neurotrophic factors is illustrated in Figure 3.
Neuroinflammation is an innate immune response of the nervous system that plays a pivotal role in the pathogenesis of numerous neuropsychiatric disorders (Troubat et al., 2021). Through extensive research on depression, it has been discovered that dysregulated secretion of inflammatory factors can contribute to its onset. The neuroinflammatory response assumes a critical role in mediating depression, as evidenced by elevated levels of pro-inflammatory cytokines within the central nervous system and aberrant activation of astrocytes and microglia (Benedetti et al., 2020). These findings suggest that neuroinflammation can either induce intracerebral lesions or be modulated for repairing intracerebral injuries.
Microglia are specialized cells of the central nervous system that exhibit macrophage-like properties, playing a crucial role in neuroinflammation. In response to stress, infection, trauma, or injury, microglia undergo phenotypic changes known as “microglial activation” and “microglial polarization,” which involve their conversion into pro-inflammatory (M1) and anti-inflammatory (M2) states (Orihuela et al., 2016; Feng et al., 2020; Zhou et al., 2020). The M1 phenotype of microglia is responsible for synthesizing and releasing various cytokines, including prostaglandin E2 (PGE2), CRP, and TNF-alpha, into the bloodstream (Attwells et al., 2020). Maintaining a balanced and stable state of M1/M2 microglia is crucial for normal immune function in the central nervous system. Evidence suggests that an increase in M1 microglia is associated with depression (Guo et al., 2020). The CUS activates microglia and induces depressive behaviors (Xiao et al., 2021), leading Yirmiya et al. to propose that depression can be characterized as “microgliosis” (Yirmiya et al., 2015). These studies indicate that the activation of microglia plays a significant role in the pathogenesis of depression.
Astrocytes and microglia play a crucial role in regulating inflammation within the nervous system through the secretion of various cytokines and inflammatory mediators, such as IL-1, TNF-α, and complement component 1q. The transcriptional responses of astrocytes are induced by the secretion of these molecules from microglia. Additionally, this release of inflammatory mediators leads to a decrease in phagocytosis activity and expression of neurotrophic factors (Linnerbauer et al., 2020). Animal experiments have demonstrated that inhibiting astrocyte activation can improve depressive symptoms (Wang et al., 2019b). However, there is currently limited research investigating the mechanisms underlying astrocyte action in neuroinflammation.
The nucleotide-binding oligomerization structural domain-like receptor protein 3 (NLRP3) inflammatory pathway plays a crucial role in the initiation and progression of depression (Bian et al., 2022). Activated NLRP3 inflammasomes cleave pro-IL-1β into active IL-1β, thereby triggering inflammation and concurrently promoting microglial activation while inhibiting hippocampal neurogenesis (Tastan et al., 2021).
Pro-inflammatory factors such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) are regarded as depression biomarkers (Carniel and da Rocha, 2021). The excessive production of pro-inflammatory factors leads to neuronal damage, apoptosis, disruption in neurotransmitter transmission, activation of the HPA axis, alteration in related signaling pathways, and exacerbation of various subtypes of depression. These processes primarily involve the phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt), nuclear factor-kappa B (NF-kB), NLRP3/ASC/caspase-1, and mitogen-activated protein kinases (MAPKs) signaling pathways.
The Acanthopanax senticosus polysaccharides (APSP) demonstrated a reduction in the levels of pro-inflammatory factors IL-1β, IL-6, and TNF-α, while simultaneously increasing the expression of p-PI3K, p-Akt, and p-mTOR proteins induced by chronic mild agnostic stress stimuli in rats (Ding et al., 2022). These findings suggest that the antidepressant effects of spikenard polysaccharides are associated with their modulation of the PI3K/Akt/mTOR pathway and anti-inflammatory properties.
Ganoderma lucidum polysaccharides (GLP) significantly mitigated the downregulation of pro-inflammatory cytokines in BV-2 microglia induced by LPS or aβ, while promoting the expression of anti-inflammatory cytokines in both BV-2 and primary microglia (Cai et al., 2017). Furthermore, GLP attenuated inflammation-associated microglial migration, morphological alterations, and phagocytosis potential. Studies have demonstrated that GLP possesses a modulatory effect on LPS and aβ-induced neuroinflammation, suggesting its potential antidepressant effects through modulation of microglial inflammatory and behavioral responses to achieve neuroprotective functions. Other studies have confirmed that GLP possess the ability to alleviate depressive symptoms in mouse models, inhibit microglial activation, and promote astrocyte proliferation (Li et al., 2021). Additionally, it downregulates the expression of pro-inflammatory cytokines IL-1β and TNF-α in the hippocampus of mice while up-regulating the expression of anti-infective cytokines IL-10 and BDNF.
The expression levels of AMPA receptor GluR1 and p-GluR1 were significantly elevated by sulfated pachymaran (SP), whereas the anti-inflammatory and up-regulatory effects of SP on AMPA receptors were completely inhibited by the AMPA receptor inhibitor GYKI 52466 (Zhou and Li, 2020). These findings suggest that the antidepressant effect of SP may be achieved through its anti-inflammatory properties and regulation of AMPA receptor GluR1 expression. Poria cocos acidic polysaccharides (PCAP) significantly reduced the mRNA and protein expression levels of NLRP3, ASC, caspase-1, IL-1β, and IL-18 in the prefrontal cortex of rats in a chronic unpredictable stress model (Chen et al., 2021). Conversely, they significantly increased the mRNA and protein expression levels of pro-caspase-1, pro-IL-1β, and pro-IL-18. These results indicate that PCAP may inhibit the NLRP3 inflammasome pathway and reverse serum levels of inflammatory factors by regulating associated mRNA and protein expression levels related to NLRP3.
Gastrodia elata polysaccharides (GEP) exhibited the ability to attenuate the relative mRNA expression of pro-inflammatory cytokines TNF-α and IL-1β in hippocampal tissues of depressed mice, thereby exerting a neuroprotective effect and ameliorating LPS-induced depressive-like behaviors in mice (Liu et al., 2021). Momordica charantia polysaccharides (MCP, 200 and 400 mg/kg) also decreased hippocampal levels of IL-1β, IL-6, and TNF-α, alleviating depressive behaviors in mice subjected to chronic social frustration stress (Deng et al., 2019). Additionally, MCP significantly upregulated PI3K activity and Akt phosphorylation in the hippocampus of depressed mice with chronic social frustration stress. Notably, partial inhibition of the antidepressant effects of MCP was observed upon treatment with LY294002, a PI3K inhibitor.
The intervention of Astragalus polysaccharide (APS) significantly reduced the levels of hippocampal TNF-α, IL-1β, and IL-6 in rats with CUS-induced depression (Wang et al., 2018; Wang et al., 2019a; Liu et al., 2019). Additionally, it also significantly decreased the levels of NF-κB p65, p-NF-κB p65, p-IκBα, and the DNA-binding activity of NF-κB p65. Notably, p65 is a subunit of NF-κB that plays a crucial role in this process. These findings suggest that the antidepressant effect of APS may be attributed to their ability to inhibit overactivation of NF-κB signaling pathway as well as regulate downstream inflammatory factors. APS intervention also effectively reduced the expression levels of hippocampal ERK1/2, JNK, and p38 along with their phosphorylated forms in LPS-induced depressed rats (Wang et al., 2018). Moreover, it significantly decreased the levels of downstream phosphorylated c-Fos and c-Jun compared to the model group. This indicates that APS can further suppress Activator Protein 1 (AP-1) by inhibiting MAPK signaling pathway to modulate inflammatory responses and thereby ameliorate depressive-like behaviors.
Polygonatum sibiricum polysaccharide (PSP) may exert antidepressant effects by inhibiting the activation of NF-κB expression and nuclear translocation in mouse models of depression induced by LPS and CUS (Shen et al., 2021; Shen et al., 2022). Additionally, PSP decreases the expression levels of pro-inflammatory factors IL-1β and TNF-α in hippocampal tissues. Endogenous ligands produced during brain injury activate Toll-like receptor 4 (TLR4), which in turn activates NF-κB through the MyD88-dependent signaling pathway, leading to the transcription of numerous pro-inflammatory factors (Brancato et al., 2013). PSP demonstrated inhibitory effects on the upregulation of NLRP3, ASC, caspase-1, cleaved-caspase-1, and IL-1β in LPS-induced depression model in mice (Shen et al., 2022). Additionally, they downregulated the expression of Iba-1 as a microglial activation marker and GFAP as an astrocyte activation marker, thereby suppressing the activation of microglia and astrocytes. Simultaneously, PSP exhibited potential to ameliorate depressive-like behavior by inhibiting ERK phosphorylation-mediated NF-κB activation and modulating inflammatory responses (Shen et al., 2021).
Yan et al. demonstrated that intervention with Okra polysaccharides (OP) from Abelmoschus esculentus (L.) Moecnch suppressed the expression of TLR4, MyD88, and nuclear translocation of NF-κB, suggesting that the TLR4/NF-κB pathway may be involved in the mechanism of action of OP in the brain (Yan et al., 2020). Furthermore, it has been shown that OP can significantly reduce inflammatory factor levels in mice with chronic unpredictable stress-induced depression and concurrently downregulate phosphorylated expression of ERK1/2, JNK, and p38. This suggests that OP may exert their antidepressant effects by modulating inflammatory responses through the MAPKs pathway.
Liu et al. demonstrated that Lonicera japonica polysaccharides (LJP) significantly ameliorated depression-like behavior in CUMS model mice (Liu et al., 2019). Furthermore, LJP upregulated the number of hippocampal metameres and protected their structure and arrangement from disruption. There was a significant reduction in the expression of proteins such as NLRP3, Caspase-1, and IL-1β upon treatment with LJP. These findings suggest that LJP may exert an antidepressant effect by inhibiting the NLRP3 inflammatory vesicle-mediated immune-inflammatory response.
In summary, natural plant polysaccharides can modulate the PI3K/AKT and NF-κB signaling pathways, thereby reducing the release of inflammatory factors and decreasing the downstream expression levels of inflammation-related proteins. Additionally, polysaccharides can attenuate the phosphorylated expression of ERK1/2, JNK, and p38 in the MAPKs family, thus influencing neuroinflammatory responses. Moreover, they can inhibit protein expression related to the NLRP3/ASC/caspase-1 signaling pathway, consequently impacting immune inflammation and exerting antidepressant effects. Therefore, polysaccharides play a crucial role in regulating inflammation and their ability to suppress inflammatory responses is an integral part of depression treatment. The potential mechanism of polysaccharide-targeted intervention in depression through neuroinflammatory is illustrated in Figure 4.
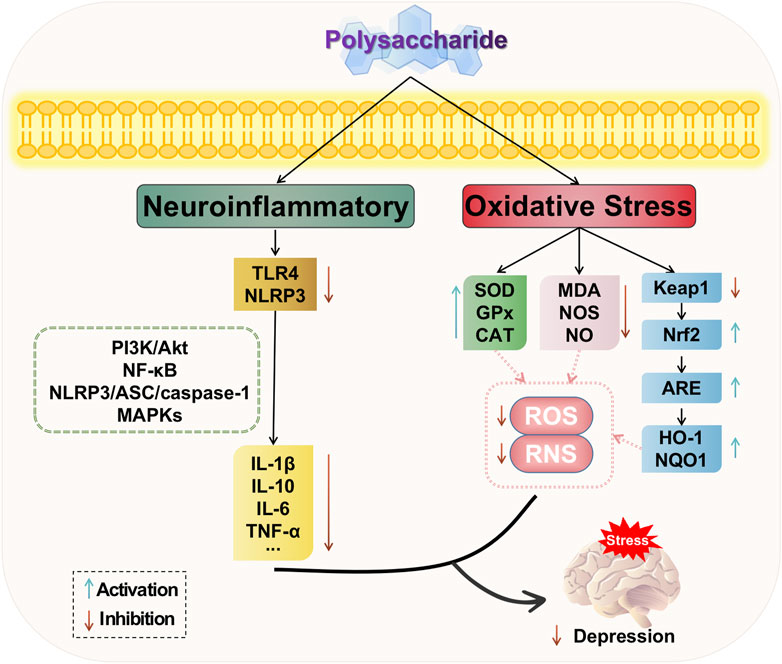
FIGURE 4. Possible antidepressant mechanism of polysaccharide targeted intervention in neuroinflammatory and oxidative stress.
The “oxidative stress hypothesis of depression” posits that oxidative stress is accountable for the structural alterations in the brains of individuals with depression. Oxidative stress refers to an imbalance between the oxidizing and antioxidant effects within the body, leading to tissue damage. Elevated levels of oxidative stress result in an excess production of reactive oxygen radicals, known as reactive oxygen species (ROS), and reactive nitrogen radicals, referred to as reactive nitrogen species (RNS), which have potential neurotoxicity (Bhatt et al., 2020). The role of oxidative stress is pivotal in the pathological changes associated with stress-related diseases, and it constitutes a significant component in the pathogenesis of depression (Kim et al., 2016). Clinical studies investigating oxidative stress in depression have demonstrated that a key characteristic of oxidative stress in depression is the diminished antioxidant capacity and inadequate blood levels of antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT). Conversely, serum levels of lipid markers associated with oxidative stress are elevated, including reactive oxygen species (ROS) and nitrogen species (NS), such as malondialdehyde (MDA), Malondialdehyde (MDA), nitric oxide (NO) and nitric oxide synthase (NOS) (Moylan et al., 2014; Nobis et al., 2020; Manosso et al., 2022). Additionally, there is evidence of protein, DNA, and mitochondrial damage; along with secondary autoimmune responses targeting redox-modified nitrosylated proteins and oxidation-specific epitopes. Therefore, the reduction of oxidative stress damage through elevation of antioxidant levels such as SOD and GSH, along with inhibition of MDA production, contributes to alleviating depressive symptoms in PSD. Moreover, nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor, emerges as a pivotal regulator of antioxidant signaling pathways and holds promise as a therapeutic target for depression (Bouvier et al., 2017; Zuo et al., 2022; Luo et al., 2023).
It has been observed that conventional pharmaceutical drugs exhibit limited efficacy in alleviating symptoms and may even induce severe adverse reactions in 30%–60% of patients (Liang et al., 2021). Medicinal plants and their bioactive constituents offer substantial health-promoting advantages, thereby warranting their utilization for the management of various ailments such as cancer, gastrointestinal disorders, and hepatic injury (Cheema and Singh, 2021; Khosravifarsani et al., 2022; Parganiha et al., 2022). Plant-derived polysaccharides represent crucial active components possessing anti-inflammatory, antioxidant, and immunomodulatory properties (Farahani et al., 2015; Wang et al., 2022a; Liaqat et al., 2022b), with notable evidence supporting their potential antidepressant effects.
In recent years, Polygonatum sibiricum has been utilized in various formulations for the treatment of depression with promising outcomes (Liu et al., 2022). It encompasses multiple bioactive constituents such as polysaccharides, steroidal saponins, and flavonoids. Polygonatum sibiricum polysaccharide (PSP) is a key constituent of PS and exhibits diverse biological activities including antitumor, antioxidant, anti-inflammatory, immunomodulatory effects, as well as regulation of blood glucose and lipids. Shen et al. discovered that the administration of PSP effectively reversed the alterations in reduced SOD levels and increased MDA levels induced by LPS-induced depression model in rats (Shen et al., 2021). PSP may exert its preventive effects on depressive-like behavior through the inhibition of reactive oxygen species (ROS), hyperfunctioning HPA axis, and ERK/NF-κB-mediated inflammatory response. Additionally, Shen et al. employed the LPS and CUMS-induced model to investigate the antidepressant effects of PSP and elucidate its underlying mechanism of action. Their findings revealed that PSP exerts its antidepressant properties by modulating the oxidative stress-calpain-1-NLRP3 signaling axis (Shen et al., 2022). These studies provide experimental foundation for the development of efficacious antidepressant medications.
The Lycium barbarum polysaccharide (LBP) is a bioactive compound derived from the Lycium barbarum L., which is widely used in traditional Chinese medicine. It possesses various pharmacological properties, including immunomodulatory and anti-aging effects (Xiao et al., 2022). Additionally, LBP exhibits neuroprotective properties that can be attributed to its antioxidant and anti-inflammatory activities (Zhu et al., 2015; Zhao et al., 2017). For instance, studies have demonstrated the protective effects of LBP in models of partial optic dissection injury and focal cerebral ischemic injury (Chu et al., 2013). Recent studies have indicated that LBP may possess therapeutic potential in the treatment of depression (Fu et al., 2021; Li et al., 2022). Zhao et al. conducted an assessment on the antidepressant activity of LBP in rifampicin-induced depressed mice, and proposed a possible mechanism of action whereby LBP attenuates the reduction in apoptosis inhibitors Bcl-2 and PARP by suppressing lipid peroxidation (LPO) production, subsequently leading to a decrease in apoptosis within striatal neurons (Zhao et al., 2019).
The authors hypothesized that Astragalus polysaccharide (APS) may exert protective and antidepressant effects on the hippocampus by inhibiting CMUS-induced oxidative stress, which is achieved through APS activation of the Nrf2-ARE pathway in depressed rats (Su et al., 2021). After the study, it was observed that APS intervention significantly upregulated hippocampal Nrf2 gene expression, total Nrf2 protein levels, and nuclear translocation in depressed rats. This finding suggests that APS enhances Nrf2 activation and its subsequent translocation to the nucleus, thereby promoting the antioxidative stress mechanism in rats. Furthermore, APS demonstrated regulatory effects on SOD, GSH-Px, CAT, HO-1 enzymes, indicating its ability to activate the Nrf2-ARE pathway in the hippocampus of depressed rats. Additionally, intervention with Acanathopanax senticosus polysaccharides significantly increased CAT and SOD activities while effectively reducing malondialdehyde (MDA) levels in rat hippocampal tissues [69]. The intervention of Lentinan (LNT, 2.5 and 5 mg/kg) also significantly enhanced the activity of superoxide dismutase (SOD) and reduced the levels of malondialdehyde (MDA) in the hippocampus of mice with chronic unpredictable stress-induced depression (Ma et al., 2015).
Based on the aforementioned studies, natural plant polysaccharides exert anti-oxidative stress effects by upregulating the levels of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), while downregulating the levels of malondialdehyde (MDA) as an oxidizing agent. Polysaccharides possess the ability to modulate the oxidative stress response in the depressed hippocampus through multiple pathways, with potential targets including Nrf2-related pathways for polysaccharide-based treatment of depression. The potential mechanism of polysaccharide-targeted intervention in depression through oxidative stress is illustrated in Figure 4.
Tryptophan (TRP) is one of the essential amino acids in the human body and serves as a precursor to 5-HT. Being the sole “raw material” for 5-HT synthesis, it also plays a significant role in the development of depression. Tryptophan undergoes metabolism at three primary sites: the brain, intestine, and liver; among these, the colon stands out as the most crucial site for TRP absorption (O’Mahony et al., 2015; Comai et al., 2020). There are two primary metabolic pathways for tryptophan (Breda et al., 2016; Agus et al., 2018), namely the serotonin pathway and the kynurenine (KNY) pathway (KP). The former involves conversion of tryptophan to 5-HT by tryptophan hydroxylase (TPH), followed by metabolism to 5-hydroxyindoleacetic acid (5-HIAA) via monoamine oxidase in blood. The latter pathway entails generation of kynurenine from tryptophan through the action of indoleamine-2,3-dioxygenase (IDO) or tryptophan-2,3-dioxygenase (TDO). The conversion of tryptophan to kynurenine is catalyzed by the enzyme kynurenine-3-monooxygenase, resulting in the production of 3-hydroxykynurenine (3-HK), which has been implicated in oxidative stress and neurotoxicity. Only a small fraction, less than 5%, of tryptophan is converted to 5-HT, with the majority being metabolized through the KP—the primary route for tryptophan metabolism (Höglund et al., 2019). Studies have demonstrated that acute TRP depletion is associated with the emergence of depressive symptoms, potentially attributed to a significant reduction in 5-HT production (Chen et al., 2022). Simultaneously, an elevation in KYN, another metabolite of TRP, contributes to the development of depressive symptoms through immune dysregulation and induction of neuroinflammation (Schwarcz et al., 2012; Gong et al., 2023). It is evident that there exists a strong correlation between TRP metabolism and depression.
The regulation of key enzyme activities by natural plant polysaccharides can contribute to the modulation and equilibrium of metabolites with specific neuroactive properties, thereby promoting TRP metabolism to 5-HT while inhibiting TRP metabolism to 3-HK (Liaqat et al., 2022a).
Intervention with Polygonatum sibiricum polysaccharides (PSP) was found to downregulate TRP and 3-HK levels in the hippocampus of mice in a behavioral despair model (Wei et al., 2022). Furthermore, considering the concurrent increase in 5-HT levels, it is postulated that these polysaccharides may modulate TRP metabolism towards the TRP/5-HT pathway by enhancing TPH enzyme activity and inhibiting IDO enzyme activity. PSP may attenuate kynurenine metabolism and reduce downstream 3-HK levels, thereby exerting their antidepressant effects. The total glycosides (TG) of Cistanche tubulosa significantly elevate serum TRP levels in chronically unpredictable stressed rats (p < 0.05), while reducing the levels of serum KYN and KYN/TRP ratio (p < 0.05) (Fan et al., 2021). Simultaneously, they downregulate the expression of IDO protein in the colon and hippocampus of rats (p < 0.05). These findings suggest that these glycosides possess an inhibitory effect on TRP metabolism into KNY, thereby promoting increased conversion of TRP to serotonin and exerting an antidepressant effect. Chen et al. demonstrated the specific protective effect of Tongxieyaofang polysaccharide in mitigating TRP metabolism disorders induced by CUS (Chen et al., 2023). They observed that the administration of polysaccharide solution effectively suppressed the transcriptional activity of IDO1 in the colon, thereby preventing biased TRP metabolism towards the KP. This led to a reduction in serum KYN concentration and KYN/TRP ratio, ultimately resulting in decreased colonic 5-HT levels, increased hippocampal 5-HT levels, and alleviation of depressive symptoms.
Cheng et al. obtained Tiansi Liquid, formulated with Morinda officinalis How polysaccharides and Cuscuta chinensis polysaccharides in a 1:1 ratio. After conducting a preliminary investigation, Tiansi liquid exhibits certain effects on monoamine neurotransmission and neuronal morphology in brain tissue by inhibiting the activity of IDO and reducing the expression of its mRNA (Zhou et al., 2015). The activity of IDO is reduced, leading to a decrease in the expression of its mRNA. This results in IDO inhibition, which regulates the TRP/KYN pathway and subsequently impacts monoamine neurotransmitter transmission and neuron morphology in brain tissue, thereby exerting a certain antidepressant effect. Subsequently, the groups investigated the antidepressant mechanism of Tiansi liquid on a hydrocortisone-induced depression rat model (Cheng et al., 2018). The metabolomics results demonstrated that Tiansi liquid effectively downregulated TDO, IDO, and quinoline (QUIN) levels while simultaneously reducing the KYN/TRP ratio. It significantly increased kynurenic acid and 5-HT levels. These findings suggest that Tiansi liquid alleviates depressive symptoms in rats by modulating gut microbiota composition and metabolites within the TRP/KYN pathway.
Tryptophan metabolism is implicated in several hypotheses regarding the pathogenesis of depression. Firstly, under stress conditions, TRP conversion to 5-HT and KYN is affected, leading to decreased expression of TPH and insufficient production of 5-HT, resulting in a deficiency of monoamine transmitters. Secondly, excessive TRP to KYN conversion is closely associated with the body’s inflammatory response and overexpression of IDO. Lastly, downstream products of KYN exert excitotoxic effects that can trigger apoptosis through activation of ionotropic glutamate receptors impacting neuroplasticity. The two key enzymes, TPH and IDO, exert control over the direction of TRP metabolism, which is a dynamic factor in depression and various other neuropsychiatric disorders. Consequently, these enzymes may emerge as potential targets for future research on natural plant polysaccharides intervention in depression. The potential mechanism of polysaccharide-targeted intervention in depression through tryptophan metabolism is illustrated in Figure 5.
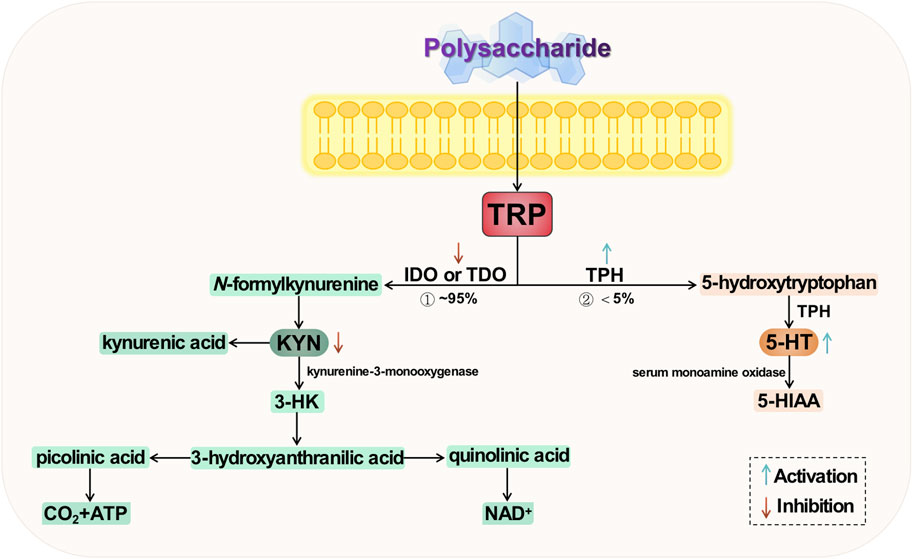
FIGURE 5. Possible antidepressant mechanism of polysaccharide targeted intervention in tryptophan metabolism.
The gut serves as a habitat for parasitic or commensal bacteria and plays a crucial role in maintaining the homeostasis of the internal milieu (Ji et al., 2020). The gut establishes a bidirectional regulatory mechanism with the brain through the mediation of gut microbiota, known as the microbiota-gut-brain axis (MGBA). This axis encompasses the central nervous system, enteric nervous system, autonomic nervous system, as well as neuroendocrine, enteroendocrine, and neuroimmune systems (Appleton, 2018). The gut microbiota is influenced by stress, leading to changes in its composition and diversity. Disruption of the gastrointestinal microbiota can activate the gut-brain axis, resulting in alterations in mental mood and depressive behaviors (Pferschy-Wenzig et al., 2022). The modulation of the gut-brain axis (MGBA) involves the integration of neural, hormonal, immune signals, and other factors between the gut and brain. This provides a potential pathway for the entry of gut microbiota and its metabolites into the brain. Consequently, regulating MGBA has emerged as a promising approach for treating psychiatric disorders such as depression (Rathour et al., 2023).
The gut microbiota communicates with the brain by activating the HPA axis, altering neurotransmitters, producing microbial metabolites, influencing neurotrophic factors, modulating immune and inflammatory responses, and engaging vagus nerve pathways to regulate brain function and activity (Han et al., 2022). These interactions subsequently impact the pathogenesis of depression. Dysbiosis of the gut microbiota can lead to hyperactivity of the HPA axis, resulting in elevated levels of ACTH and CORT, as well as reduced BDNF. Conversely, nutritional therapy involving the administration of beneficial bacteria such as Bifidobacteria, Lactobacillus, and probiotics can attenuate HPA axis activity, restore normal ACTH, CORT, and BDNF levels, and exert an antidepressant effect (Sun et al., 2022). Dysbiosis of the gut microbiota also leads to dysfunction of specific cellular microbiota (such as enterochromaffin cells and citrobacter), thereby impacting the involvement of gut microbiota in the synthesis and secretion of key neurotransmitters and factors, including GABA, Glu, NE, 5-HT, and DA (Rajanala et al., 2021). These dysfunctions contribute to the development of depression. The metabolites produced by the gut microbiota, such as short-chain fatty acids (SCFAs), play a crucial role as signaling molecules that regulate the production of intestinal peptides through enteroendocrine cells (EECs) (Averina et al., 2020). These peptides are responsible for governing the gut-brain axis and stimulating the synthesis of gut-derived 5-HT by enterochromaffin cells (ECs), subsequently influencing hormone communication between the gut and brain (Eicher and Mohajeri, 2022). Thus, a healthy gut microbiota promotes brain development and regulation. Disrupted gut microbiota can impact the expression levels of BNDF, potentially leading to depression (Zhao et al., 2022). Therefore, the treatment of depression may involve regulating the balance of intestinal microbiota, modulating BDNF-related pathways, and promoting both BDNF secretion levels and gene expression. The communication between gut microbiota and the central nervous system (CNS) is facilitated by neuroactive substances produced by the former through the vagus nerve (Tan et al., 2022). Neurotoxic metabolites produced by gut microbiota can be transmitted to the central nervous system (CNS) through the vagus nerve, thereby influencing brain function and inducing depression. Disturbances in gut microbiota may result in the release of bacteria into the bloodstream, leading to excessive production of LPS, which causes oxidative stress and inflammatory responses. These processes can disrupt the blood-brain barrier, allowing inflammatory factors to enter the CNS and subsequently activate neuroglia through various signaling pathways such as NF-κB and cholinergic mechanisms, ultimately promoting the development of depression (Han et al., 2022).
A variety of herbal compounds, monotherapies, and their active ingredients have demonstrated remarkable efficacy in reducing depression symptoms with minimal side effects when utilizing traditional herbal treatments (Bi et al., 2022; Shao et al., 2022). Natural plant polysaccharides can effectively regulate gut microbiota disorders and intervene in the development of depression through MGBA. The intervention of natural plant polysaccharides to improve imbalances in gut microbiota provides a novel target for the prevention and treatment of depression (Sun et al., 2021).
Yan et al. discovered that Okra polysaccharides (OP) from Abelmoschus esculentus (L.) Moecnch exhibited significant improvements in depression-like behavior and alterations in the structure and abundance of gut microbiota within a depressed mouse model of CUMS (Yan et al., 2020). Specifically, at the phylum level, there was a notable reduction in the relative abundance of Bacteroidetes and Actinobacteria, accompanied by an increase in the relative abundance of Firmicutes. At the genus level, there was a significant decrease in the relative abundance of Barnesiella and Bacteroides, while Lactobacillus showed a substantial increase. The relative abundance of Lactobacillus was increased, while the levels of Barnesiella and Bacteroides were decreased by OP. The antidepressant effects mediated by gut microbiota were further elucidated through fecal microtransplantation technique (FMT). Furthermore, OP significantly reversed the reduction in acetic acid, propionic acid, and butyric acid levels, as well as the elevation in isovaleric acid level within the intestines of CUMS mice. These findings suggest that OP may exert their antidepressant effects by modulating both the composition of gut microbiota and the levels of various metabolites such as SCFAs. Chen et al. discovered that the administration of Ginkgo biloba polysaccharides (GBP, 300 mg/kg) for consecutive 28 days effectively mitigated the antidepressant effects induced by chronic stress in mice, while also exerting antidepressant effects through rectifying dysregulated gut homeostasis, elevating brain levels of 5-HT and DA, and enhancing the abundance of Lactobacillus in the intestine (Chen et al., 2019). The administration of Total Cistanche polysaccharides effectively mitigated the inflammatory response in the colon and ameliorated gut barrier disruption by modulating the dysbiosis of gut microbiota in rats subjected to chronic unpredictable stress, indicating that the antidepressant effect of Cistanche polysaccharides is also associated with their regulation on gut microbiota (Fan et al., 2021). The polysaccharides derived from Polygonum sibiricum exhibited the ability to suppress depression-like behavior in perimenopausal mice induced by ovariectomy plus chronic mild stress (Zhang et al., 2022). Additionally, they demonstrated the capacity to mitigate the inflammatory response via modulation of the MGBA and inhibit excessive activation of the HPA axis. Zhang and others discovered that Polygonum sibiricum polysaccharides (PSP) were capable of elevating serum levels of 5-HT and NE, reducing pro-inflammatory cytokine levels in the hippocampus, as well as inhibiting the PI3K/AKT/TLR4/NF-κB and ERK/CREB/BDNF pathways in mice with depression induced by CUMS (Zhang et al., 2023). The PSP compound exerts antidepressant-like behavior by modulating the MGBA axis.
Based on the aforementioned studies, it can be concluded that natural plant polysaccharides have a significant impact on regulating gut microbiota disorders, improving depressive symptoms through the MGBA axis, and playing a crucial role in modulating host-microbe crosstalk. Current research exploring the relationship between depression and various gut microbiota is still in its preliminary stage. The specific key microbiota responsible for depression has not been identified, leaving ample room for further investigation into utilizing gut microbiota regulation as a broader therapeutic approach to treating depression. Polysaccharide intervention aimed at ameliorating imbalances in gut microbiota offers a novel therapeutic strategy for both preventing and treating depression. The potential mechanism of polysaccharide-targeted intervention in depression through gut microbiota is illustrated in Figure 6.
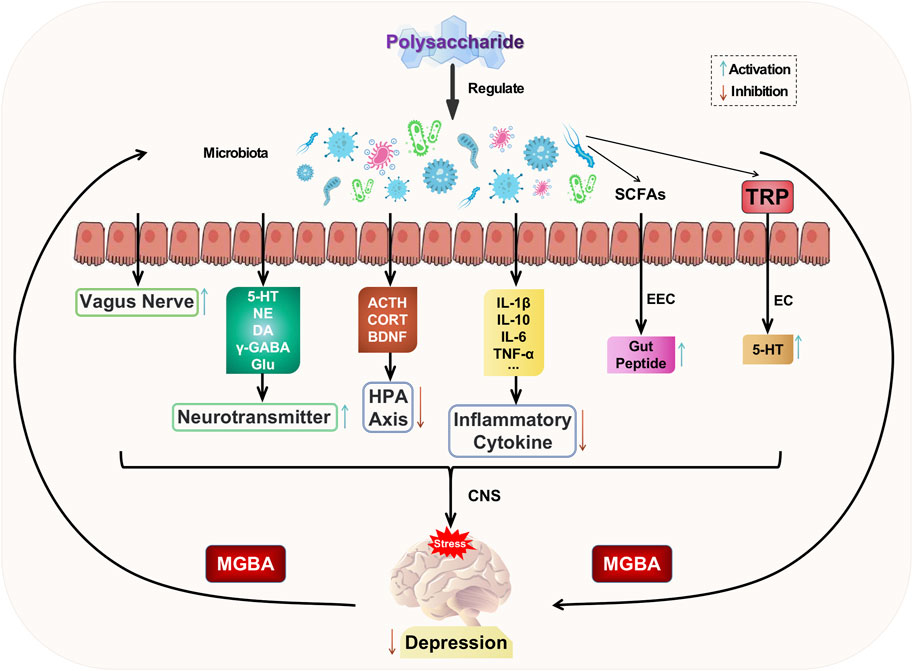
FIGURE 6. Possible antidepressant mechanism of polysaccharide targeted intervention in gut microbiota.
Due to the intricate pathogenesis of depression, the current antidepressants exhibit limited efficacy and are associated with potential toxic side effects. Hence, there is an urgent need to develop drugs that can effectively prevent or treat depression while minimizing adverse reactions. Natural plants derived from diverse sources offer minimal harm to the human body and possess the advantage of being multi-targeted in their actions. Several traditional Chinese medicine polysaccharide drugs, such as Astragalus polysaccharide injection, Ginseng polysaccharide injection, and Poria cocos acidic polysaccharide oral solution, have been developed for clinical use due to their immunomodulatory effects in improving chemotherapy-induced immune deficiency among tumor patients. The discovery has revealed that natural plant polysaccharides possess the ability to exert antidepressant effects through the regulation of neurotransmitters and their receptors, modulation of the HPA axis, control of inflammatory responses, management of oxidative stress, modulation of neurotrophic factors, regulation of gut microbiota and tryptophan metabolism, as well as other pathways.
In the experimental investigation of natural plant polysaccharide antidepressants, researchers conducted studies using various stress models including chronic unpredictable mild stress, social isolation-induced stress, social defeat stress, post-traumatic stress disorder, and acute behavioral despair depression. It is important to note that many depressed patients also suffer from comorbid psychiatric disorders such as obsessive-compulsive disorder and anxiety disorder. Therefore, future studies should examine the efficacy of polysaccharides in comorbidity models with these factors and explore combination therapy to enhance or optimize depression treatment.
Currently, mice and rats are the most commonly utilized models for depression research and testing of potential depression protective agents. Rodent models offer significant advantages over other mammals due to their metabolic and disease characteristics that closely resemble those of humans. Additionally, their smaller size, docile temperament, and ease of husbandry make them highly suitable for experimental purposes. However, it is important to acknowledge that rodents differ from mammals in terms of brain and neural structural organization; therefore, future studies should pay more attention to the specificities associated with rodent models. The higher complexity of human thinking and emotions compared to other species, as well as the similarity between non-human primate brains and those of humans in comparison to rodents, necessitate further preclinical studies involving higher animals to validate the alleviating and therapeutic effects of polysaccharides on depression. Ultimately, this will lead to real clinical trials involving human subjects.
Currently, numerous studies have substantiated the antidepressant effects of natural plant polysaccharides and elucidated their mechanisms of action from various perspectives. Furthermore, ongoing research continues to unveil additional insights into the antidepressant mechanisms of natural plant polysaccharides. As one of the primary bioactive constituents found in plants, natural plant polysaccharides exhibit a broad spectrum of biological activities. Therefore, conducting research on the antidepressant effects of natural plant polysaccharides holds immense significance for the development and utilization of natural plant-based polysaccharide antidepressant products.
Recently, there have been advancements in both preclinical and clinical research on the utilization of natural plant polysaccharides for depression treatment. A randomized, double-blind, placebo-controlled trial was conducted by experts to assess the effectiveness of LBP (Lycium barbarum polysaccharides, 300 mg/d for 6 weeks) in adolescents with subthreshold depression. The findings demonstrated that LBP effectively alleviated depressive symptoms such as cognitive impairments, retardation, and hopelessness in adolescents with subthreshold depression without any reported adverse events (Li et al., 2022). This experiment serves as a valuable point of reference for the application of natural plant polysaccharides at a human level. We eagerly anticipate further preclinical and clinical trials to validate the efficacy of natural plant polysaccharides in treating depression.
However, there are still numerous issues that require further exploration in the study of the antidepressant effects of natural plant polysaccharides. Firstly, the pathogenesis of depression is diverse and intricate, and natural plant polysaccharides predominantly exert their influence through multiple pathways, thereby rendering the mechanism behind their antidepressant properties even more complex. Although research on the antidepressant effect of polysaccharides has progressed from general observations to molecular-level investigations, our understanding of their signaling pathways and mechanisms remains insufficiently clear and comprehensive. Secondly, while cellular and animal studies on polysaccharides have increased significantly, clinical research in this area is relatively scarce. As research continues to advance, finding ways to extend the study of polysaccharide antidepressant effects to human subjects will remain a crucial point awaiting breakthroughs. Lastly, the physical properties, primary structure, advanced structure, and chemical modification of polysaccharides are closely linked to their biological activity; thus, investigating the conformational relationship between different natural plant polysaccharides with similar efficacy holds great significance for a thorough examination of their biological activity. However, research on the correlation between structure and antidepressant effects of natural plant polysaccharides is still in its early stages. With advancements in current technology for isolating and purifying polysaccharides as well as deepening studies into structural analysis over time, this may become a new avenue for future research on the antidepressant effects of natural plant polysaccharides.
Many active polysaccharides also play a significant role in the treatment of cardiovascular diseases in traditional Chinese medicine. However, most studies on the therapeutic potential of traditional Chinese medicine polysaccharides for depression are limited to animal and cellular models, which may not fully reflect their actual effects in humans. Currently, research on natural plant polysaccharides for depression treatment lacks depth and there is a scarcity of clinical trial studies, hindering widespread implementation in clinical settings and limiting their application within the field of medicine. The mechanism of action and signaling pathways of many natural plant polysaccharides for antidepressants remain unclear due to limitations in research methods, necessitating further investigation. Future studies should delve deeper into the mechanisms underlying natural plant polysaccharides’ ability to improve depression, clarifying previously studied but ambiguous mechanisms as well as exploring unreported ones. Additionally, future research should focus on clinical applications of polysaccharides, addressing gaps in knowledge such as tissue distribution and target specificity while also gathering more clinical evidence.
In conclusion, due to their unique advantages, polysaccharides derived from natural resources offer potential for investigating the mechanism of depression and serve as a valuable reference for further research on natural plant polysaccharides. Moreover, this exploration may lay the foundation for clinical applications. Furthermore, the development of antidepressant polysaccharides with minimal adverse reactions and high efficacy holds promise in expanding treatment options for depression.
Y-HY: Conceptualization, Writing–original draft. C-XL: Investigation, Visualization, Writing–original draft. R-BZ: Project administration, Writing–original draft. YS: Project administration, Writing–review and editing. X-JX: Writing–review and editing. Q-MY: Funding acquisition, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Education Department of Heilongjiang Provincial Basic research expenses of undergraduate universities (No. 2023-KYYWF-1030), Traditional Chinese medicine research project of Heilongjiang Provincial Administration of Traditional Chinese medicine (No. ZHY2023-198), and Harbin University of Commerce doctoral research support program (No. 22BQ43).
The authors would like to thank the reviewers and authors of all references.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdoulaye, I. A., Wu, S., Chibaatar, E., Yu, D., Le, K., Cao, X., et al. (2021). Ketamine induces lasting antidepressant effects by modulating the NMDAR/CaMKII-mediated synaptic plasticity of the hippocampal dentate gyrus in depressive stroke model. Neural Plast. 2021, e6635084. doi:10.1155/2021/6635084
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi:10.1016/j.chom.2018.05.003
Appleton, J. (2018). The gut-brain axis: influence of microbiota on mood and mental health. Integr. Med. Clin. J. 17, 28–32.
Attwells, S., Setiawan, E., Wilson, A. A., Rusjan, P. M., Miler, L., Xu, C., et al. (2020). Replicating predictive serum correlates of greater translocator protein distribution volume in brain. Neuropsychopharmacology 45, 925–931. doi:10.1038/s41386-019-0561-y
Averina, O. V., Zorkina, Y. A., Yunes, R. A., Kovtun, A. S., Ushakova, V. M., Morozova, A. Y., et al. (2020). Bacterial metabolites of human gut microbiota correlating with depression. Int. J. Mol. Sci. 21, 9234. doi:10.3390/ijms21239234
Barbuti, M., Menculini, G., Verdolini, N., Pacchiarotti, I., Kotzalidis, G. D., Tortorella, A., et al. (2023). A systematic review of manic/hypomanic and depressive switches in patients with bipolar disorder in naturalistic settings: the role of antidepressant and antipsychotic drugs. Eur. Neuropsychopharmacol. 73, 1–15. doi:10.1016/j.euroneuro.2023.04.013
Belujon, P., and Grace, A. A. (2017). Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 20, 1036–1046. doi:10.1093/ijnp/pyx056
Benedetti, F., Aggio, V., Pratesi, M. L., Greco, G., and Furlan, R. (2020). Neuroinflammation in bipolar depression. Front. Psychiatry 11, 71. doi:10.3389/fpsyt.2020.00071
Bhatt, S., Nagappa, A. N., and Patil, C. R. (2020). Role of oxidative stress in depression. Drug Discov. Today 25, 1270–1276. doi:10.1016/j.drudis.2020.05.001
Bi, C., Guo, S., Hu, S., Chen, J., Ye, M., and Liu, Z. (2022). The microbiota–gut–brain axis and its modulation in the therapy of depression: comparison of efficacy of conventional drugs and traditional Chinese medicine approaches. Pharmacol. Res. 183, 106372. doi:10.1016/j.phrs.2022.106372
Bian, H., Yan, F., Li, W., Tu, W., and Ji, X. (2022). Tert-butylhydroquinone prevents neuroinflammation and relieves depression via regulation of NLRP3 signaling in mice. Int. Immunopharmacol. 107, 108723. doi:10.1016/j.intimp.2022.108723
Bouvier, E., Brouillard, F., Molet, J., Claverie, D., Cabungcal, J.-H., Cresto, N., et al. (2017). Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatry 22, 1701–1713. doi:10.1038/mp.2016.144
Brancato, S. K., Thomay, A. A., Daley, J. M., Crane, M. J., Reichner, J. S., Sabo, E., et al. (2013). Toll-like receptor 4 signaling regulates the acute local inflammatory response to injury and the fibrosis/neovascularization of sterile wounds. Wound Repair Regen. 21, 624–633. doi:10.1111/wrr.12061
Breda, C., Sathyasaikumar, K. V., Sograte Idrissi, S., Notarangelo, F. M., Estranero, J. G., Moore, G. G. L., et al. (2016). Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc. Natl. Acad. Sci. 113, 5435–5440. doi:10.1073/pnas.1604453113
Cai, Q., Li, Y., and Pei, G. (2017). Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflammation 14, 63. doi:10.1186/s12974-017-0839-0
Carniel, B. P., and da Rocha, N. S. (2021). Brain-derived neurotrophic factor (BDNF) and inflammatory markers: perspectives for the management of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 108, 110151. doi:10.1016/j.pnpbp.2020.110151
Castrén, E., Võikar, V., and Rantamäki, T. (2007). Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 7, 18–21. doi:10.1016/j.coph.2006.08.009
Chai, Y., Li, Q., Wang, Y., Tao, E., and Asakawa, T. (2022). The value of HPA axis hormones as biomarkers for screening and early diagnosis of postpartum depression: updated information about methodology. Front. Endocrinol. 13, 916611. doi:10.3389/fendo.2022.916611
Cheema, H. S., and Singh, M. P. (2021). The use of medicinal plants in digestive system related disorders: a systematic review. J. Ayurvedic Herb. Med. 7, 182–187. doi:10.31254/jahm.2021.7303
Chen, G., Zhou, S., Chen, Q., Liu, M., Dong, M., Hou, J., et al. (2022). Tryptophan-5-HT pathway disorder was uncovered in the olfactory bulb of a depression mice model by metabolomic analysis. Front. Mol. Neurosci. 15, 965697. doi:10.3389/fnmol.2022.965697
Chen, H., Kan, Q., Zhao, L., Ye, G., He, X., Tang, H., et al. (2023). Prophylactic effect of Tongxieyaofang polysaccharide on depressive behavior in adolescent male mice with chronic unpredictable stress through the microbiome-gut-brain axis. Biomed. Pharmacother. 161, 114525. doi:10.1016/j.biopha.2023.114525
Chen, K., Chen, S., Ren, J., Lin, S., Xiao, M., Cheng, L., et al. (2021). Antidepressant effect of acidic polysaccharides from Poria and their regulation of neurotransmitters and NLRP3 pathway. China J. Chin. Mat. Medica 46, 5088–5095. doi:10.19540/j.cnki.cjcmm.20210610.705
Chen, P., Hei, M., Kong, L., Liu, Y., Yang, Y., Mu, H., et al. (2019). One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 10, 8161–8171. doi:10.1039/C9FO01178A
Cheng, D., Chang, H., Ma, S., Guo, J., She, G., Zhang, F., et al. (2018). Tiansi Liquid modulates gut microbiota composition and tryptophan–kynurenine metabolism in rats with hydrocortisone-induced depression. Molecules 23, 2832. doi:10.3390/molecules23112832
Chu, P. H. W., Li, H.-Y., Chin, M.-P., So, K., and Chan, H. H. L. (2013). Effect of Lycium barbarum (Wolfberry) polysaccharides on preserving retinal function after partial optic nerve transection. PLOS ONE 8, e81339. doi:10.1371/journal.pone.0081339
Chu, S. (2019). Protective effects of Lycium barbarum polysaccharide on depression rats with post-traumatic stress disorder. Mod. Prev. Med. 46, 2622–2637.
Comai, S., Bertazzo, A., Brughera, M., and Crotti, S. (2020). Tryptophan in health and disease. Adv. Clin. Chem. 95, 165–218. doi:10.1016/bs.acc.2019.08.005
Deng, Z., Yuan, C., Yang, J., Peng, Y., Wang, W., Wang, Y., et al. (2019). Behavioral defects induced by chronic social defeat stress are protected by Momordica charantia polysaccharides via attenuation of JNK3/PI3K/AKT neuroinflammatory pathway. Ann. Transl. Med. 7, 6. doi:10.21037/atm.2018.12.08
Ding, C., Xu, Y., and Ge, Y. (2021). Research on the mechanism and the effects of Angelica polysaccharide on the behavior of chronic stress depression mice. West. J. Tradit. Chin. Med. 34, 21–27. doi:10.12174/j.issn.2096-9600.2021.06.06
Ding, J., Jiang, C., Yang, L., and Wang, X. (2022). Ameliorative effect of Acanathopanax senticosus polysaccharides on depressive behavior in rats by regulating PI3K/Akt/mTOR pathway. Sci. Technol. Food Ind. 43, 369–375. doi:10.13386/j.issn1002-0306.2021090141
Duman, R. S., Sanacora, G., and Krystal, J. H. (2019). Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102, 75–90. doi:10.1016/j.neuron.2019.03.013
Dwivedi, Y. (2009). Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr. Dis. Treat. 5, 433–449. doi:10.2147/ndt.s5700
Eicher, T. P., and Mohajeri, M. H. (2022). Overlapping mechanisms of action of brain-active bacteria and bacterial metabolites in the pathogenesis of common brain diseases. Nutrients 14, 2661. doi:10.3390/nu14132661
Fan, L., Peng, Y., Wang, J., Ma, P., Zhao, L., and Li, X. (2021). Total glycosides from stems of Cistanche tubulosa alleviate depression-like behaviors: bidirectional interaction of the phytochemicals and gut microbiota. Phytomedicine 83, 153471. doi:10.1016/j.phymed.2021.153471
Farahani, M. S., Bahramsoltani, R., Farzaei, M. H., Abdollahi, M., and Roja, R. (2015). Plant-derived natural medicines for the management of depression: an overview of mechanisms of action. Rev. Neurosci. 26, 305–321. doi:10.1515/revneuro-2014-0058
Feng, X., Fan, Y., and Chung, C. Y. (2020). Mefenamic acid can attenuate depressive symptoms by suppressing microglia activation induced upon chronic stress. Brain Res. 1740, 146846. doi:10.1016/j.brainres.2020.146846
Fond, G., Loundou, A., Rabu, C., Macgregor, A., Lançon, C., Brittner, M., et al. (2014). Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacol. (Berl.) 231, 3663–3676. doi:10.1007/s00213-014-3664-5
Fries, G. R., Saldana, V. A., Finnstein, J., and Rein, T. (2023). Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 28, 284–297. doi:10.1038/s41380-022-01806-1
Fu, Y., Peng, Y., Huang, X., Yang, Y., Huang, L., Xi, Y., et al. (2021). Lycium barbarum polysaccharide-glycoprotein preventative treatment ameliorates aversive. Neural Regen. Res. 16, 543–549. doi:10.4103/1673-5374.293156
Gao, F., Yang, S., Wang, J., and Zhu, G. (2022). cAMP-PKA cascade: an outdated topic for depression? Biomed. Pharmacother. 150, 113030. doi:10.1016/j.biopha.2022.113030
Gong, X., Chang, R., Zou, J., Tan, S., and Huang, Z. (2023). The role and mechanism of tryptophan–kynurenine metabolic pathway in depression. Rev. Neurosci. 34, 313–324. doi:10.1515/revneuro-2022-0047
Grinchii, D., and Dremencov, E. (2020). Mechanism of action of atypical antipsychotic drugs in mood disorders. Int. J. Mol. Sci. 21, 9532. doi:10.3390/ijms21249532
Guo, X., Rao, Y., Mao, R., Cui, L., and Fang, Y. (2020). Common cellular and molecular mechanisms and interactions between microglial activation and aberrant neuroplasticity in depression. Neuropharmacology 181, 108336. doi:10.1016/j.neuropharm.2020.108336
Guo, Y., Chen, X., Gong, P., Li, Z., Wu, Y., Zhang, J., et al. (2023). Advances in the mechanisms of polysaccharides in alleviating depression and its complications. Phytomedicine 109, 154566. doi:10.1016/j.phymed.2022.154566
Han, W., Wang, N., Han, M., Ban, M., Sun, T., and Xu, J. (2022). Reviewing the role of gut microbiota in the pathogenesis of depression and exploring new therapeutic options. Front. Neurosci. 16, 1029495. doi:10.3389/fnins.2022.1029495
Höglund, E., Øverli, Ø., and Winberg, S. (2019). Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front. Endocrinol. 10, 158. doi:10.3389/fendo.2019.00158
Hou, C., Yin, M., Lan, P., Wang, H., Nie, H., and Ji, X. (2021). Recent progress in the research of Angelica sinensis (Oliv.) Diels polysaccharides: extraction, purification, structure and bioactivities. Chem. Biol. Technol. Agric. 8, 13. doi:10.1186/s40538-021-00214-x
Ji, X., Guo, J., Cao, T., Zhang, T., Liu, Y., and Yan, Y. (2023). Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci. Hum. Wellness 12, 1969–1980. doi:10.1016/j.fshw.2023.03.017
Ji, X., Hou, C., Gao, Y., Xue, Y., Yan, Y., and Guo, X. (2020). Metagenomic analysis of gut microbiota modulatory effects of Jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 11, 163–173. doi:10.1039/C9FO02171J
Joo, M.-K., Ma, X., Yoo, J.-W., Shin, Y.-J., Kim, H.-J., and Kim, D.-H. (2023). Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 25, 105116. doi:10.1016/j.micinf.2023.105116
Jovanovic, P., Wang, Y., Vit, J.-P., Novinbakht, E., Morones, N., Hogg, E., et al. (2022). Sustained chemogenetic activation of locus coeruleus norepinephrine neurons promotes dopaminergic neuron survival in synucleinopathy. PLOS ONE 17, e0263074. doi:10.1371/journal.pone.0263074
Khosravifarsani, M., Tolouian, R., Yadollahifarsani, S., Soleimani, P., Sarazen, M., Mostafizi, P., et al. (2022). Medical plants for lung cancer: an overview of current knowledge. Immunopathol. Persa 9, e38455. doi:10.34172/ipp.2022.38455
Kim, Y.-K., Na, K.-S., Myint, A.-M., and Leonard, B. E. (2016). The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 277–284. doi:10.1016/j.pnpbp.2015.06.008
Kim, Y.-S., O’Sullivan, D. M., and Shin, S.-K. (2019). Can 24 weeks strength training reduce feelings of depression and increase neurotransmitter in elderly females? Exp. Gerontol. 115, 62–68. doi:10.1016/j.exger.2018.11.009
Li, H., Xiao, Y., Han, L., Jia, Y., Luo, S., Zhang, D., et al. (2021). Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system. Brain Res. Bull. 171, 16–24. doi:10.1016/j.brainresbull.2021.03.002
Li, X., Mo, X., Liu, T., Shao, R., Teopiz, K., McIntyre, R., et al. (2022). Efficacy of Lycium barbarum polysaccharide in adolescents with subthreshold depression: interim analysis of a randomized controlled study. Neural Regen. Res. 17, 1582–1587. doi:10.4103/1673-5374.330618
Liang, S., Huang, R., Lin, X., Huang, J., Huang, Z., and Liu, H. (2012). Effects of Yulangsan polysaccharide on monoamine neurotransmitters, adenylate cyclase activity and brain-derived neurotrophic factor expression in a mouse model of depression induced by unpredictable chronic mild stress. Neural Regen. Res. 7, 191–196. doi:10.3969/j.issn.1673-5374.2012.03.006
Liang, S., Wang, N., Huang, R., Tan, H., and Fu, S. (2010). Study on anti-depression effect of Yulangsan polysaccharides. Lishizhen Med. Mat. Medica Res. 21, 241–242.
Liang, X., Wang, X., Zhao, G., Huang, X., Xu, X., and Dong, W. (2021). Research progress of essential oil as a new complementary therapy in the treatment of depression. Mini Rev. Med. Chem. 21, 2276–2289. doi:10.2174/1389557521666210219161747
Liaqat, H., Parveen, A., and Kim, S. Y. (2022a). Neuroprotective natural products’ regulatory effects on depression via gut–brain axis targeting tryptophan. Nutrients 14, 3270. doi:10.3390/nu14163270
Liaqat, H., Parveen, A., and Kim, S.-Y. (2022b). Antidepressive effect of natural products and their derivatives targeting BDNF-TrkB in gut–brain axis. Int. J. Mol. Sci. 23, 14968. doi:10.3390/ijms232314968
Linnerbauer, M., Wheeler, M. A., and Quintana, F. J. (2020). Astrocyte crosstalk in CNS inflammation. Neuron 108, 608–622. doi:10.1016/j.neuron.2020.08.012
Liu, D., Tang, W., Han, C., and Nie, S. (2022a). Advances in Polygonatum sibiricum polysaccharides: extraction, purification, structure, biosynthesis, and bioactivity. Front. Nutr. 9, 1074671. doi:10.3389/fnut.2022.1074671
Liu, J., Wang, Y., Zhao, H., Shen, Y., Wang, C., Li, S., et al. (2022b). Effect and mechanism of Lily polysaccharide combined with Astragalus polysaccharide on depressive behavior in chronic stress mice. Chin. J. Exp. Tradit. Med. Formulae 24, 62–70. doi:10.13422/j.cnki.syfjx.20220539
Liu, L., Liu, W., Fan, L., and Guo, L. (2019a). Effects of Astragalus polysaccharide on post-stroke depression in rats. China Med. Her. 16, 11–14.
Liu, M., Zhang, Y., Zhang, Y., Wang, G., and Zhou, B. (2021). Effects and potential mechanisms of Gastrodia Elata polysaccharides in LPS-induced depression model mice. China Pharm. 24, 2018–2023. doi:10.19962/j.cnki.issn1008-049X.2021.11.011
Liu, P., Bai, X., Zhang, T., Zhou, L., Li, J., and Zhang, L. (2019b). The protective effect of Lonicera japonica polysaccharide on mice with depression by inhibiting NLRP3 inflammasome. Ann. Transl. Med. 7, 811. doi:10.21037/atm.2019.12.64
Lu, Y., Huang, R., and He, P. (2013). Effects of Yulangsan polysaccharides on behavior, structure of hippocampus in mice with chronic stress-induced depression. Chin. J. Exp. Tradit. Med. Formulae 19, 220–223. doi:10.13422/j.cnki.syfjx.2013.01.068
Luo, J., Li, J., Shen, Z., Lin, X., Chen, A., Wang, Y., et al. (2023). Advances in health-promoting effects of natural polysaccharides: regulation on Nrf2 antioxidant pathway. Front. Nutr. 10, 1102146. doi:10.3389/fnut.2023.1102146
Ma, Q., Pu, Y., Yuan, W., Lv, L., Du, Z., and Li, W. (2015). Antidepressant effect of lentinan in chronically stressed mice and its possible mechanism. Chin. J. Immunol. 31, 329–333. doi:10.3969/j.issn.1000-484X.2015.03.009
Manosso, L. M., Camargo, A., Dafre, A. L., and Rodrigues, A. L. S. (2022). Vitamin E for the management of major depressive disorder: possible role of the anti-inflammatory and antioxidant systems. Nutr. Neurosci. 25, 1310–1324. doi:10.1080/1028415X.2020.1853417
Mikulska, J., Juszczyk, G., Gawrońska-Grzywacz, M., and Herbet, M. (2021). HPA axis in the pathomechanism of depression and schizophrenia: new therapeutic strategies based on its participation. Brain Sci. 11, 1298. doi:10.3390/brainsci11101298
Möhler, H. (2012). The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology 62, 42–53. doi:10.1016/j.neuropharm.2011.08.040
Moncrieff, J., Cooper, R. E., Stockmann, T., Amendola, S., Hengartner, M. P., and Horowitz, M. A. (2022). The serotonin theory of depression: a systematic umbrella review of the evidence. Mol. Psychiatry. 28, 3243–3256. doi:10.1038/s41380-022-01661-0
Moylan, S., Berk, M., Dean, O. M., Samuni, Y., Williams, L. J., O’Neil, A., et al. (2014). Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev. 45, 46–62. doi:10.1016/j.neubiorev.2014.05.007
Mu, S., Yang, W., and Huang, G. (2021). Antioxidant activities and mechanisms of polysaccharides. Chem. Biol. Drug Des. 97, 628–632. doi:10.1111/cbdd.13798
Newport, D. J., Carpenter, L. L., McDonald, W. M., Potash, J. B., Tohen, M., Nemeroff, C. B., et al. (2015). Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 172, 950–966. doi:10.1176/appi.ajp.2015.15040465
Nobis, A., Zalewski, D., and Waszkiewicz, N. (2020). Peripheral markers of depression. J. Clin. Med. 9, 3793. doi:10.3390/jcm9123793
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi:10.1016/j.bbr.2014.07.027
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi:10.1111/bph.13139
Parganiha, R., Tripathi, A., Prathyusha, S., Baghel, P., Lanjhiyana, S., Lanjhiyana, S., et al. (2022). A review of plants for hepatic disorders. J. Complement. Med. Res. 13, 46. doi:10.5455/jcmr.2022.13.04.10
Paykel, E. S. (2008). Basic concepts of depression. Dialogues Clin. Neurosci. 10, 279–289. doi:10.31887/DCNS.2008.10.3/espaykel
PehliVan Karakas, F., Coşkun, H., Soytürk, H., and Bozat, B. G. (2020). Anxiolytic, antioxidant, and neuroprotective effects of goji berry polysaccharides in ovariectomized rats: experimental evidence from behavioral, biochemical, and immunohistochemical analyses. Turk. J. Biol. 44, 238–251. doi:10.3906/biy-2003-8
Pferschy-Wenzig, E.-M., Pausan, M. R., Ardjomand-Woelkart, K., Röck, S., Ammar, R. M., Kelber, O., et al. (2022). Medicinal plants and their impact on the gut microbiome in mental health: a systematic review. Nutrients 14, 2111. doi:10.3390/nu14102111
Popa, D., Cerdan, J., Repérant, C., Guiard, B. P., Guilloux, J.-P., David, D. J., et al. (2010). A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur. J. Pharmacol. 628, 83–90. doi:10.1016/j.ejphar.2009.11.037
Rajanala, K., Kumar, N., and Chamallamudi, M. R. (2021). Modulation of gut-brain axis by probiotics: a promising anti-depressant approach. Curr. Neuropharmacol. 19, 990–1006. doi:10.2174/1570159X19666201215142520
Rathour, D., Shah, S., Khan, S., Singh, P. K., Srivastava, S., Singh, S. B., et al. (2023). Role of gut microbiota in depression: understanding molecular pathways, recent research, and future direction. Behav. Brain Res. 436, 114081. doi:10.1016/j.bbr.2022.114081
Rybakowski, J. K. (2023). Application of antipsychotic drugs in mood disorders. Brain Sci. 13, 414. doi:10.3390/brainsci13030414
Schwarcz, R., Bruno, J. P., Muchowski, P. J., and Wu, H.-Q. (2012). Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477. doi:10.1038/nrn3257
Shao, J., Wei, Y., and Wei, X. (2022). A comprehensive review on bioavailability, safety and antidepressant potential of natural bioactive components from tea. Food Res. Int. 158, 111540. doi:10.1016/j.foodres.2022.111540
Shen, F., Song, Z., Xie, P., Li, L., Wang, B., Peng, D., et al. (2021). Polygonatum sibiricum polysaccharide prevents depression-like behaviors by reducing oxidative stress, inflammation, and cellular and synaptic damage. J. Ethnopharmacol. 275, 114164. doi:10.1016/j.jep.2021.114164
Shen, F., Xie, P., Li, C., Bian, Z., Wang, X., Peng, D., et al. (2022). Polysaccharides from Polygonatum cyrtonema Hua reduce depression-like behavior in mice by inhibiting oxidative stress-calpain-1-NLRP3 signaling axis. Oxid. Med. Cell. Longev. 2022, 2566917–17. doi:10.1155/2022/2566917
Shirayama, Y., Iwata, M., Fujita, Y., Oda, Y., and Hashimoto, K. (2022). The Toll-like receptor 4 antagonist TAK-242 induces antidepressant-like effects in a rat learned helplessness model of depression through BDNF-TrkB signaling and AMPA receptor activation. Behav. Brain Res. 423, 113769. doi:10.1016/j.bbr.2022.113769
Song, L., Wu, X., Wang, J., Guan, Y., Zhang, Y., Gong, M., et al. (2021a). Antidepressant effect of catalpol on corticosterone-induced depressive-like behavior involves the inhibition of HPA axis hyperactivity, central inflammation and oxidative damage probably via dual regulation of NF-κB and Nrf2. Brain Res. Bull. 177, 81–91. doi:10.1016/j.brainresbull.2021.09.002
Song, Q., Wang, Y., Huang, L., Shen, M., Yu, Y., Yu, Q., et al. (2021b). Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 140, 109858. doi:10.1016/j.foodres.2020.109858
Song, Z., Cheng, L., Liu, Y., Zhan, S., Wu, Z., and Zhang, X. (2022). Plant-derived bioactive components regulate gut microbiota to prevent depression and depressive-related neurodegenerative diseases: focus on neurotransmitters. Trends Food Sci. Technol. 129, 581–590. doi:10.1016/j.tifs.2022.10.019
Su, H., Wang, D., Zhang, T., Zhang, X., Mao, S., and Li, C. (2021). Effects of Astragalus polysaccharide on depressive behaviors and hippocampal Nrf2-ARE signaling pathway in rats. Chin. Pharmacol. Bull. 37, 839–843. doi:10.3969/j.issn.1001-1978.2021.06.018
Sun, Q., Ho, C.-T., Zhang, X., Liu, Y., Zhang, R., and Wu, Z. (2022). Strategies for circadian rhythm disturbances and related psychiatric disorders: a new cue based on plant polysaccharides and intestinal microbiota. Food Funct. 13, 1048–1061. doi:10.1039/D1FO02716F
Sun, Y., Cheng, L., Zeng, X., Zhang, X., Liu, Y., Wu, Z., et al. (2021). The intervention of unique plant polysaccharides-Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 170, 336–342. doi:10.1016/j.ijbiomac.2020.12.164
Tan, C., Yan, Q., Ma, Y., Fang, J., and Yang, Y. (2022). Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front. Neurol. 13, 1015175. doi:10.3389/fneur.2022.1015175
Tastan, B., Arioz, B. I., Tufekci, K. U., Tarakcioglu, E., Gonul, C. P., Genc, K., et al. (2021). Dimethyl fumarate alleviates NLRP3 inflammasome activation in microglia and sickness behavior in LPS-challenged mice. Front. Immunol. 12, 737065. doi:10.3389/fimmu.2021.737065
Thapar, A., Eyre, O., Patel, V., and Brent, D. (2022). Depression in young people. Lancet 400, 617–631. doi:10.1016/S0140-6736(22)01012-1
Troubat, R., Barone, P., Leman, S., Desmidt, T., Cressant, A., Atanasova, B., et al. (2021). Neuroinflammation and depression: a review. Eur. J. Neurosci. 53, 151–171. doi:10.1111/ejn.14720
Van Den Eede, F., and Claes, S. J. (2004). Mechanisms of depression: role of the HPA axis. Drug Discov. Today Dis. Mech. 1, 413–418. doi:10.1016/j.ddmec.2004.11.021
Vigna, L., Morelli, F., Agnelli, G. M., Napolitano, F., Ratto, D., Occhinegro, A., et al. (2019). Hericium erinaceus improves mood and sleep disorders in patients affected by overweight or obesity: could circulating pro-BDNF and BDNF be potential biomarkers? Evid. Based Complement. Altern. Med. 2019, 7861297–7861312. doi:10.1155/2019/7861297
Wang, X., Chen, X., Tang, Y., Wu, J., Qin, D., Yu, L., et al. (2022a). The therapeutic potential of plant polysaccharides in metabolic diseases. Pharmaceuticals 15, 1329. doi:10.3390/ph15111329
Wang, X., Cheng, L., Liu, Y., Zhang, R., Wu, Z., Weng, P., et al. (2022b). Polysaccharide regulation of intestinal flora: a viable approach to maintaining normal cognitive performance and treating depression. Front. Microbiol. 13, 807076. doi:10.3389/fmicb.2022.807076
Wang, Y., Deng, W., Li, X., and Peng, J. (2019a). Experimental observation on effect of Astragalus polysaccharides on learning ability in rats with post-stroke depression. Lab. Anim. Comp. Med. 39, 443–448. doi:10.3969/j.issn.1674-5817.2019.06.004
Wang, Y., Li, C., Qu, J., Li, H., Chen, B., Nie, K., et al. (2018). Effects of Astragalus polysaccharide on hippocampal NF-κB signaling in rats with depressive behaviors. Chin. Pharmacol. Bull. 34, 836–840. doi:10.3969/j.issn.1001-1978.2018.06.019
Wang, Y., Ni, J., Zhai, L., Gao, C., Xie, L., Zhao, L., et al. (2019b). Inhibition of activated astrocyte ameliorates lipopolysaccharide-induced depressive-like behaviors. J. Affect. Disord. 242, 52–59. doi:10.1016/j.jad.2018.08.015
Wei, Z., Song, H., An, F., Sun, J., Li, S., Jiang, N., et al. (2022). Protective effects and mechanism of polysaccharide from Polygonatum sibiricum on behavioral despair mice. Sci. Technol. Food Ind. 43, 351–357. doi:10.13386/j.issn1002-0306.2021060137
Xiao, K., Luo, Y., Liang, X., Tang, J., Wang, J., Xiao, Q., et al. (2021). Beneficial effects of running exercise on hippocampal microglia and neuroinflammation in chronic unpredictable stress-induced depression model rats. Transl. Psychiatry 11, 461–512. doi:10.1038/s41398-021-01571-9
Xiao, Z., Deng, Q., Zhou, W., and Zhang, Y. (2022). Immune activities of polysaccharides isolated from Lycium barbarum L. What do we know so far? Pharmacol. Ther. 229, 107921. doi:10.1016/j.pharmthera.2021.107921
Yamada, S., Yamamoto, M., Ozawa, H., Riederer, P., and Saito, T. (2003). Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J. Neural Transm. 110, 671–680. doi:10.1007/s00702-002-0810-8
Yan, T., Nian, T., Liao, Z., Xiao, F., Wu, B., Bi, K., et al. (2020). Antidepressant effects of a polysaccharide from okra (Abelmoschus esculentus (L) Moench) by anti-inflammation and rebalancing the gut microbiota. Int. J. Biol. Macromol. 144, 427–440. doi:10.1016/j.ijbiomac.2019.12.138
Yang, H., Li, M., Zhou, M., Xu, H., Huan, F., Liu, N., et al. (2021). Links between gut dysbiosis and neurotransmitter disturbance in chronic restraint stress-induced depressive behaviours: the role of inflammation. Inflammation 44, 2448–2462. doi:10.1007/s10753-021-01514-y
Yang, Y., Fan, L., Peng, Y., Peng, C., and Li, X. (2022). Alcohol–soluble polysaccharides from Dendrobium officinale flowers as an antidepressant by regulating the gut–brain axis. Int. J. Biol. Macromol. 216, 836–849. doi:10.1016/j.ijbiomac.2022.07.220
Yi, L., Zhang, L., Ding, A., Xu, Q., Zhu, Q., and Kong, L. (2009). Orthogonal array design for antidepressant compatibility of polysaccharides from Banxia-Houpu decoction, a traditional Chinese herb prescription in the mouse models of depression. Arch. Pharm. Res. 32, 1417–1423. doi:10.1007/s12272-009-2011-6
Yi, L., Zhang, M., Cheng, J., Wan, H., Li, C., Zhu, J., et al. (2021). Antidepressant-like effects of degraded porphyran isolated from Porphyra haitanensis. Mol. Nutr. Food Res. 65, 2000869. doi:10.1002/mnfr.202000869
Yirmiya, R., Rimmerman, N., and Reshef, R. (2015). Depression as a microglial disease. Trends Neurosci. 38, 637–658. doi:10.1016/j.tins.2015.08.001
Zhang, J., Tang, J., Zhang, Q., Wang, X., and Li, X. (2019). Effects of sulfated pachymaran on AMPA receptor expression in hippocampi of depression model rats. Chin. J. Clin. Psychol. 27, 1086–1091. doi:10.16128/j.cnki.1005-3611.2019.06.003
Zhang, Q., Cheng, J., Liu, Q., Xu, G., Li, C., and Yi, L. (2022). Dendrobium officinale polysaccharides alleviate depression-like symptoms via regulating gut microbiota-neuroinflammation in perimenopausal mice. J. Funct. Foods 88, 104912. doi:10.1016/j.jff.2021.104912
Zhang, Y., Sun, Y., Liu, Y., Liu, J., Sun, J., Liu, X., et al. (2023). Polygonum sibiricum polysaccharides exert the antidepressant-like effects in chronic unpredictable mild stress-induced depressive mice by modulating microbiota-gut-brain axis. Phytother. Res. 37, 3408–3423. doi:10.1002/ptr.7813
Zhao, K., Yao, M., Zhang, X., Xu, F., Shao, X., Wei, Y., et al. (2022). Flavonoids and intestinal microbes interact to alleviate depression. J. Sci. Food Agric. 102, 1311–1318. doi:10.1002/jsfa.11578
Zhao, P., Zhou, R., Zhu, X.-Y., Liu, G., Zhao, Y.-P., Ma, P.-S., et al. (2017). Neuroprotective effects of Lycium barbarum polysaccharide on focal cerebral ischemic injury in mice. Neurochem. Res. 42, 2798–2813. doi:10.1007/s11064-017-2293-x
Zhao, R., Master, B. Q., Master, B. M., and Cai, Y. (2019). Improving activity of Lycium Barbarum polysaccharide on depressive mice induced by reserpine. Iran. J. Pharm. Res. IJPR 18, 1556–1565. doi:10.22037/ijpr.2019.1100763
Zhao, S., Rong, C., Gao, Y., Wu, L., Luo, X., Song, S., et al. (2021). Antidepressant-like effect of Ganoderma lucidum spore polysaccharide-peptide mediated by upregulation of prefrontal cortex brain-derived neurotrophic factor. Appl. Microbiol. Biotechnol. 105, 8675–8688. doi:10.1007/s00253-021-11634-y
Zhou, H., and Li, T. (2020). Antidepressant mechanism of sulfated pachymaran. J. Wannan Med. Coll. 39, 209–213. doi:10.3969/j.issn.1002-0217.2020.03.002
Zhou, J., Lu, Y., Xu, X., Zhang, J., Li, H., and Chang, H. (2015). Effect of antidepression and mechanism of regulation on Ido of Tiansi Liquid. J. Beijing Univ. Tradit. Chin. Med. 38, 182–185. doi:10.3969/j.issn.1006-2157.2015.03.008
Zhou, S., Chen, S., Xie, W., Guo, X., and Zhao, J. (2020). Microglia polarization of hippocampus is involved in the mechanism of Apelin-13 ameliorating chronic water immersion restraint stress-induced depression-like behavior in rats. Neuropeptides 81, 102006. doi:10.1016/j.npep.2020.102006
Zhu, X., Hu, S., Zhu, L., Ding, J., Zhou, Y., and Li, G. (2015). Effects of Lycium barbarum polysaccharides on oxidative stress in hyperlipidemic mice following chronic composite psychological stress intervention. Mol. Med. Rep. 11, 3445–3450. doi:10.3892/mmr.2014.3128
Zhuang, Y., Zeng, R., Liu, X., Yang, L., and Chan, Z. (2022). Neoagaro-oligosaccharides ameliorate chronic restraint stress-induced depression by increasing 5-HT and BDNF in the brain and remodeling the gut microbiota of mice. Mar. Drugs 20, 725. doi:10.3390/md20110725
Keywords: polysaccharides, natural plant, depression, pharmacological action, mechanism
Citation: Yang Y-H, Li C-X, Zhang R-B, Shen Y, Xu X-J and Yu Q-M (2024) A review of the pharmacological action and mechanism of natural plant polysaccharides in depression. Front. Pharmacol. 15:1348019. doi: 10.3389/fphar.2024.1348019
Received: 01 December 2023; Accepted: 29 January 2024;
Published: 08 February 2024.
Edited by:
Peng Sang, Zhengzhou University, ChinaCopyright © 2024 Yang, Li, Zhang, Shen, Xu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin-Ming Yu, eXVxaW5taW5nMjAyM0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.