- 1The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, Henan, China
Objectives: The purpose of the study was to comprehensively evaluate efficacy and safety of CDDP in patients with AMI undergoing PCI.
Methods: A computerised search was conducted on the CNKI, WF, VIP, CBM, PubMed, Embase, Web of Science, and Cochrane Library databases for RCTs of CDDP adjuvant therapy for AMI up to May 2023. STATA 17.0 was used to perform meta-analyses, sensitivity analyses, subgroup analyses, meta-regression, and publication bias assessments. TSA 0.9.5.10 Beta was used for trial sequential analysis (TSA). Evidence confidence of meta results was evaluated by GRADE (Grading of Recommendations Assessment, Development and Evaluation) according to the instructions.
Results: The results of the meta-analysis showed that CDDP combined with conventional western treatment (CWT) was superior to CWT in increasing LVEF and TCER and decreasing LVEDD, hs-CRP, IL-6 and TNF-α. The quality of evidence for TCER was moderate, LVEF, LVEDD, IL-6, and TNF-α were low. The TSA results showed that the total number of samples collected in this study met the requirements for meta-analysis and excluded the possibility of false positives, further confirming the efficacy of CDDP for the treatment of AMI undergoing PCI.
Conclusion: Adjuvant treatment of AMI with CDDP has shown exciting and safe benefits in improving cardiac function and reducing inflammatory response in patients with AMI undergoing PCI, but the quality of some of the included studies was poor, and the results should be interpreted with caution until further confirmation by well-designed RCTs.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/#recordDetails], identifier [CRD42023453293].
Highlights
1. This study is the first trial sequential analysis and meta-analysis of the treatment of AMI undergoing PCI with CDDP.
2. CDDP has been shown to have a positive effect on improving cardiac function and suppressing inflammation.
3. The complementary or alternative therapies combined with conventional medicine are beneficial to the AMI undergoing PCI.
1 Introduction
AMI is myocardial necrosis caused by acute and persistent ischemia and hypoxia of the coronary arteries, with clinical manifestations of severe and persistent retrosternal pain, increased myocardial enzyme activity, and progressive changes in the electrocardiogram, which can involve respiratory, digestive, and cardiovascular systems and be accompanied by cardiac failure, arrhythmia, and cardiogenic shock, which can seriously endanger the lives of patients (Bhatt et al., 2022). Annually, about 7.2 million people in the United States are affected by AMI, with patients over the age of 75 accounting for approximately 30%–40% of hospital admissions (Damluji et al., 2023). Since 2005, the mortality rate for AMI patients has increased rapidly, with female patients showing a tendency to have a higher mortality rate than male patients (Shengshou et al., 2019). AMI has a high morbidity and mortality rate, posing a serious threat to human health and life safety, and placing a heavy burden on patients and their families (Roohafza et al., 2023).
AMI can be categorized into ST segment elevation myocardial infarction (STEMI) and non-ST segment elevation myocardial infarction (NSTEMI). This classification is based on the presence or absence of ST segment elevation on electrocardiogram (ECG). STEMI is caused by the complete occlusion of coronary arteries, and ischemia-reperfusion therapy should be implemented as early as possible to open the infarcted vessel, reducing the infarcted area or the extent of myocardial ischemia, decreasing the rate of death, and improving prognosis. For patients with NSTEMI, the GRACE and TIMI risk scores are commonly used in clinical practice to identify their ischemic risk. It is also important to consider early implementation of ischemia-reperfusion therapy for high-risk NSTEMI patients (Ibanez et al., 2018). PCI is currently considered the most effective treatment for STEMI. However, it is important to note that sudden reperfusion of ischemic myocardium can cause ischemia-reperfusion injury (IRI) (Yellon and Hausenloy, 2007; Hausenloy and Yellon, 2008), IRI-induced cardiac dysfunction comprises systolic dysfunction, reperfusion arrhythmias, endothelial dysfunction, and lethal reperfusion injury (Qin et al., 2009). The mortality rate at 90 days after ischemia-reperfusion can be as high as 5% (Stebbins et al., 2010). Therefore, it is urgent to find effective targeted drugs to inhibit IRI (Rout et al., 2020).
CDDP is a Chinese medicine that combines modern medical technology with traditional Chinese medicine (TCM) theories. It has successfully passed Phase III clinical trials by the U.S. FDA, making it the first proprietary Chinese medicine to do so (Liang et al., 2020). CDDP is mainly concocted from three herbs, Salviae Miltiorrhizae Radix et Rhizoma, Notoginseng Radix, and Borneolum Syntheticum. Pharmacological studies have shown that CDDP has the ability to inhibit oxidative stress, platelet aggregation, and calcium channels, as well as dilate coronary blood vessels (Li, 2018). Currently, there is a growing number of clinical studies on the treatment of AMI. CWT has been found to be effective in treating AMI, but it is also associated with more adverse reactions. The dosage and types of drugs used are also increasing, leading to a corresponding increase in side effects. TCM has distinct advantages in treating cardiovascular diseases due to its lower incidence of side effects and superior long-term effectiveness (Wang et al., 2018). This study aims to comprehensively and systematically evaluate the efficacy and safety of CDDP in treating AMI undergoing PCI, following PRISMA guidelines. The goal is to provide a more reliable evidence-based basis for the clinical use of CDDP.
2 Information and methods
2.1 Protocol and registration
This systematic review and meta-analysis of RCTs were conducted by PRISMA guidelines (Moher et al., 2009). The protocol studied has been registered with PROSPERO under the registration number CRD42023453293.
2.2 Literature sources
A computerised search was conducted on the CNKI, WF, VIP, CBM, PubMed, Embase, Web of Science, and Cochrane Library databases for RCTs of CDDP adjuvant therapy for AMI up to May 2023. There is no restriction on the language of the search using subject terms. The search terms are Compound Danshen Dropping Pill, Danshen Dropping Pill, Acute Myocardial Infarction, Percutaneous Coronary Intervention, PCI, and AMI. Search strategies were provided in Supplementary Material S1.
2.3 Inclusion criteria for screening studies
2.3.1 Type of participants (P)
Compliance with the Guidelines for Integrating Chinese and Western Medicine in the Diagnosis and Treatment of Acute Myocardial Infarction or (Zhang, 2018) the American College of Cardiology/American Heart Association (ACC/AHA) diagnostic criteria for AMI (Levine et al., 2016), without restriction on age, gender, or history of smoking or alcohol use.
2.3.2 Type of interventions (I and C)
Control group: Conventional western treatment (CWT), including PCI, antiplatelet, anticoagulation, β-receptor blocker, lipid modulation, angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB), CWT were consistent between groups in each study. Treatment group: CDDP plus CWT.
2.3.3 Type of outcome measures (O)
Primary indicator included left ventricular ejection fraction (LVEF) and total clinical effective rate (TCER) = (obvious + effective) cases/total cases × 100%, obvious: no shift or less than 0.05 mV shift of the ST segment on the ECG, effective: ECG ST segment returns more than 0.05 mV to baseline, ineffective: ECG not improved (Xiaoyu, 2002). Secondary indicator included left ventricular end-diastolic internal diameter (LVEDD), N-terminal pro-B-type natriuretic peptide (NT-proBNP), troponin T (cTnT), creatine kinase isoenzyme (CK-MB), high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and adverse reactions/adverse events.
2.3.4 Types of studies (S)
RCTs of CDDP in combination with CWT for AMI.
Exclusion criteria
Mechanistic studies, reviews, lessons learned, and case reports.
Duplicate publications.
Incomplete documentation
2.4 Literature screening and extraction
Two researchers independently read the full text and extracted relevant information. The extracted information included basic information, intervention methods, risk of bias assessment, relevant outcome indicators and adverse reactions, etc. When the two parties disagreed on the inclusion of the literature, it was referred to a third party for discussion and judgment.
2.5 Literature quality assessment
The Cochrane risk-of-bias tool for randomized trials was used to assess each study’s risk of bias (Sterne et al., 2019). This tool comprises a five-item checklist: 1) randomization process; 2) deviations from the intended interventions; 3) missing outcome data; 4) measurement of the outcome; and 5) selection of the reported result. The risk of each domain was examined as “low”, “high” and “some concerns”.
2.6 Statistical processing
The included data were statistically analyzed using STATA 17.0. Continuous data was pooled with Std mean difference (SMD) and 95% confidence interval (CI), dichotomous data was pooled with relative risk (RR) and 95% CI. Heterogeneity was judged based on the results of the I2 test; when I2<50%, a fixed-effects model was used, and when I2 ≥ 50% indicated that inter-study heterogeneity was significant, so the reasons for heterogeneity were analyzed. First, the raw data were checked for correctness, and second, if heterogeneity was attributed to treatment duration, sample size, publication time, etc., meta-regression and subgroup analyses could be used to investigate the sources of heterogeneity. Sensitivity analysis evaluates the robustness and reliability of the results. If an outcome had more than 10 articles, a funnel plot was analysed for publication bias and publication bias was evaluated using Egger’s test with STATA 12.0.
2.7 Trial sequential analysis (TSA) and evidence confidence
Trial sequential analysis (TSA) was performed using TSA 0.9.5.10 Beta software (Greco and Capodanno, 2024). Evidence confidence of meta results was evaluated by GRADE (Grading of Recommendations Assessment, Development and Evaluation) according to the instructions (Brozek et al., 2009).
3 Results
3.1 Literature search
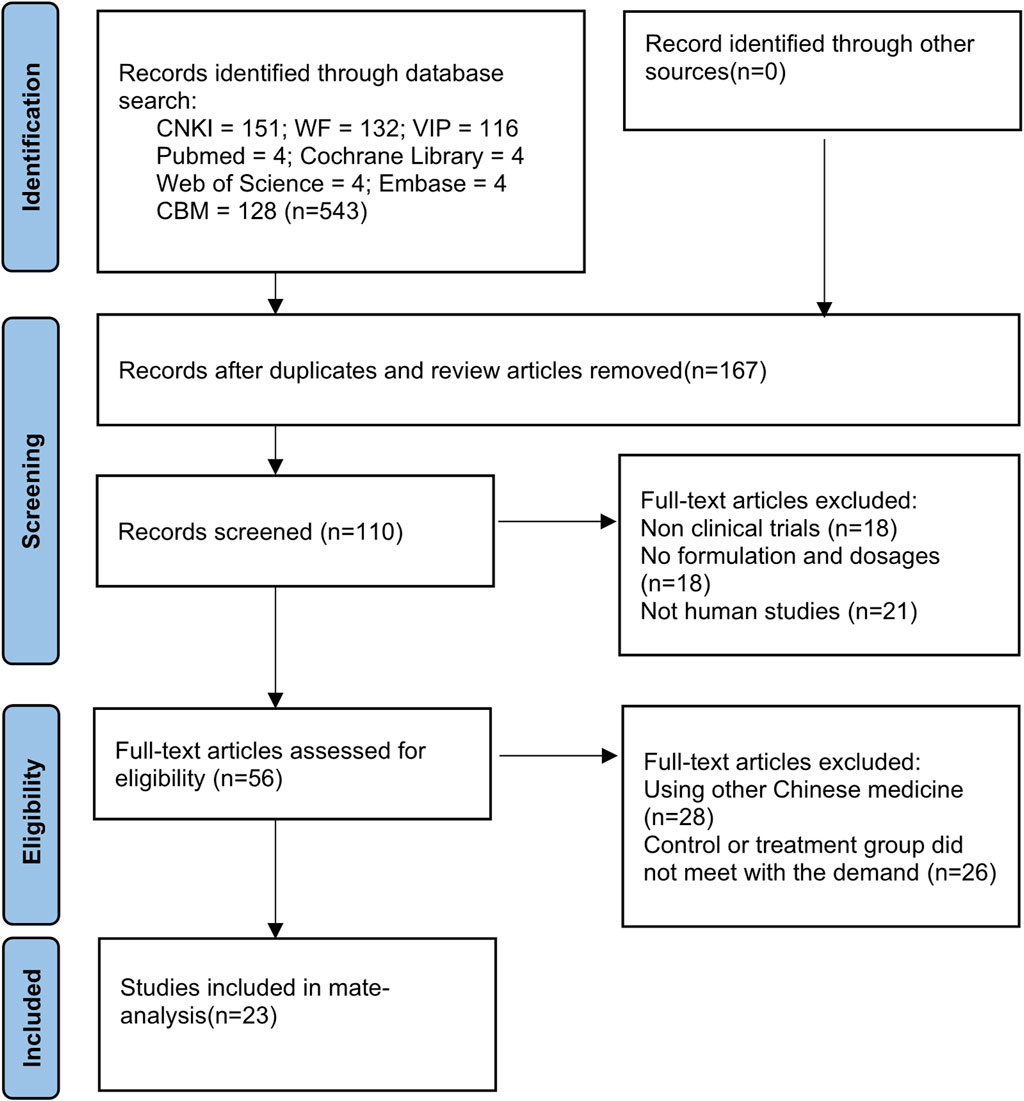
A total of 543 studies were searched. The retrieved titles were imported into EndNote X9, and 23 studies (Li et al., 2010; Li et al., 2011; Lin, 2011; Fang et al., 2017; Pan et al., 2017; Zhang, 2017; Li, 2018; Guihong et al., 2018; Hong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Shi, 2019; Su, 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Jian, 2020; Li, 2020; Niu, 2020; Shi, 2020; Chen, 2023) were finally included after checking and screening (Figure 1).
3.2 Study characteristics
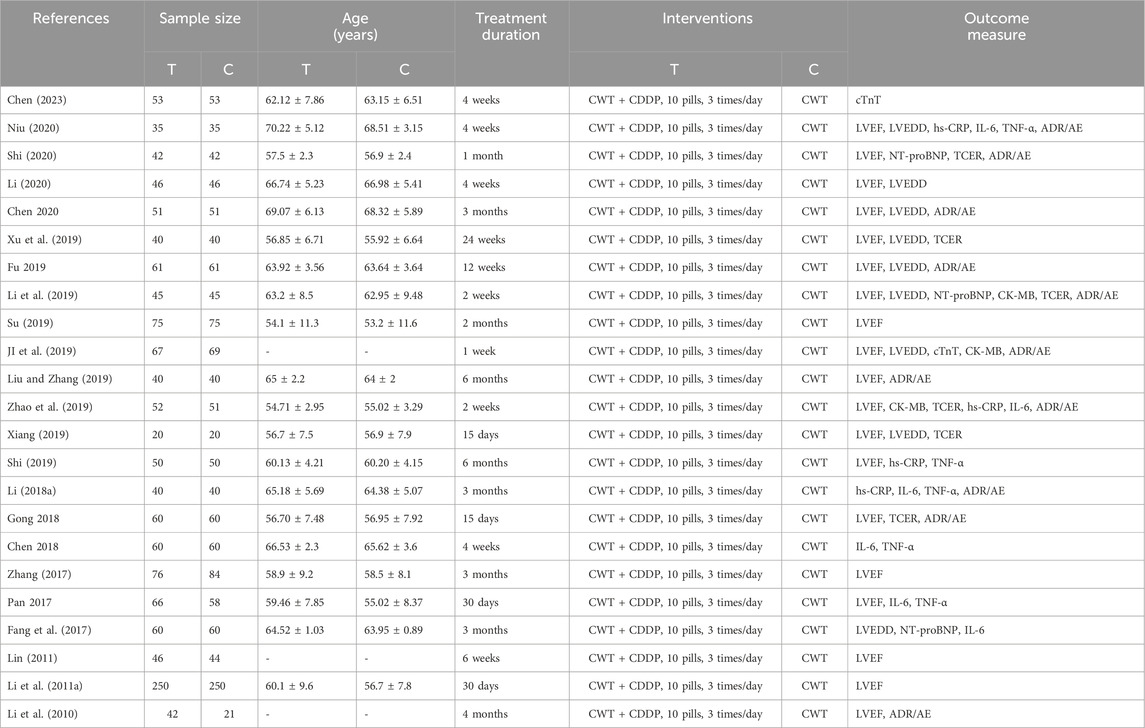
A total of 23 studies (Li et al., 2010; Li et al., 2011; Lin, 2011; Fang et al., 2017; Pan et al., 2017; Zhang, 2017; Li, 2018; Guihong et al., 2018; Hong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Shi, 2019; Su, 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Jian, 2020; Li, 2020; Niu, 2020; Shi, 2020; Chen, 2023) were included in this meta-analysis, all of which were conducted in China and involved 2,732 AMI patients, including 1,377 in the treatment group and 1,355 in the control group. In all studies, there were no statistical differences between the experimental and control groups in terms of age and sample size. LVEF was reported in 19 studies (Li et al., 2010; Li et al., 2011; Lin, 2011; Pan et al., 2017; Zhang, 2017; Guihong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Shi, 2019; Su, 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Jian, 2020; Li, 2020; Niu, 2020; Shi, 2020), TCER in 6 studies (Guihong et al., 2018; Li et al., 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Shi, 2020), LVEDD in 9 studies (Fang et al., 2017; Ji et al., 2019; Li et al., 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Jian, 2020; Li, 2020; Niu, 2020), NT-proBNP in 3 studies (Fang et al., 2017; Li et al., 2019; Shi, 2020), cTnT in 2 studies (Ji et al., 2019; Chen, 2023), CK-MB in 3 studies (Ji et al., 2019; Li et al., 2019; Zhao et al., 2019), hs-CRP in 4 studies (Li, 2018; Shi, 2019; Zhao et al., 2019; Niu, 2020), IL-6 in 6 studies (Fang et al., 2017; Pan et al., 2017; Li, 2018; Hong et al., 2018; Zhao et al., 2019; Niu, 2020), TNF-α in 5 studies (Pan et al., 2017; Li, 2018; Hong et al., 2018; Shi, 2019; Niu, 2020), and adverse reactions/adverse events in 11 studies (Li et al., 2010; Li, 2018; Guihong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Wenhui, 2019; Zhao et al., 2019; Jian, 2020; Niu, 2020; Shi, 2020) (Table 1).
Note: T: Treatment group; C: Control group; ADR/AE: Adverse Reactions/Adverse Events.
3.3 Quality assessment
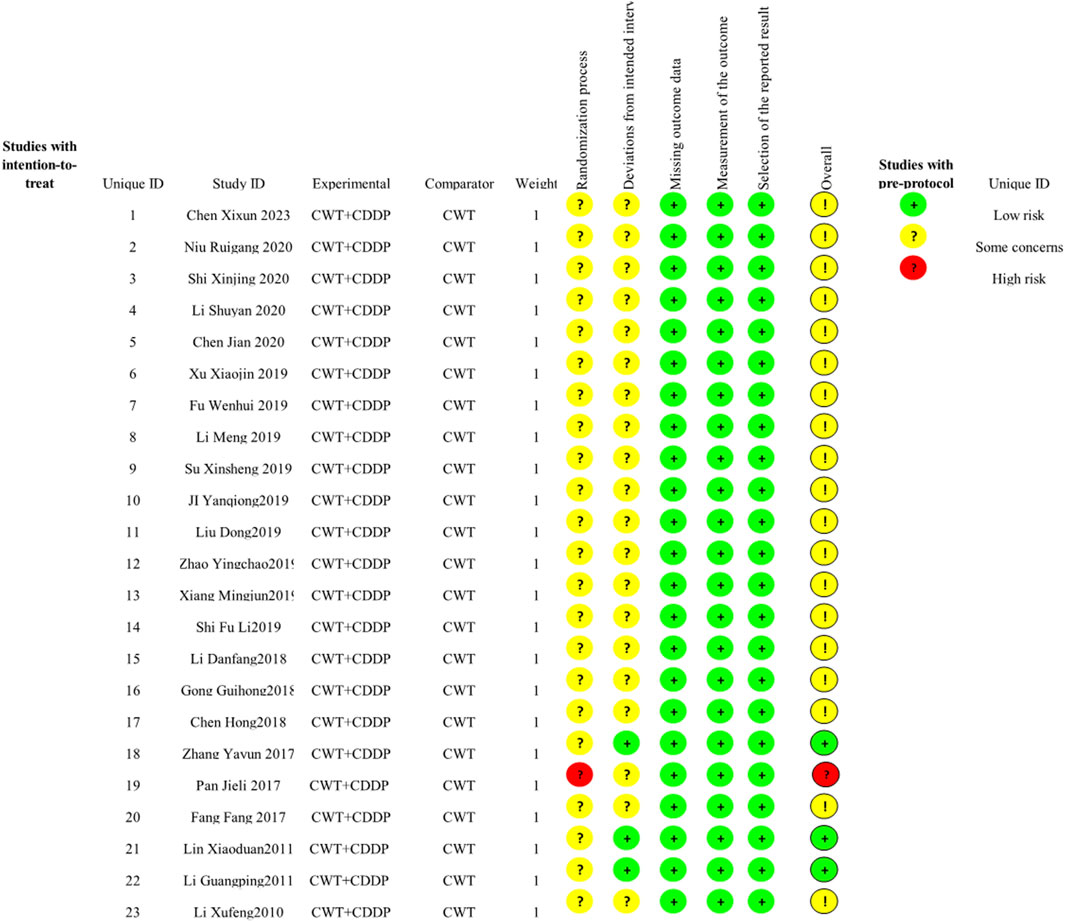
In the 23 included studies, 13 used a random-number table, and the aspect of whether the order of allocation was randomized was described as “low risk”. One study was randomized using order of presentation, which may have been at risk of bias, described as “high risk”. All studies did not mention allocation hiding, so the randomization process section was listed as “some concern”. Three studies were blinded to subjects and implementers, and no information was described for the other 19 studies. There was no missing data and no selective results in any of the studies, so these sections were described as “low risk” (Figure 2).
4 Meta-analysis results
4.1 Cardiac function
4.1.1 LVEF
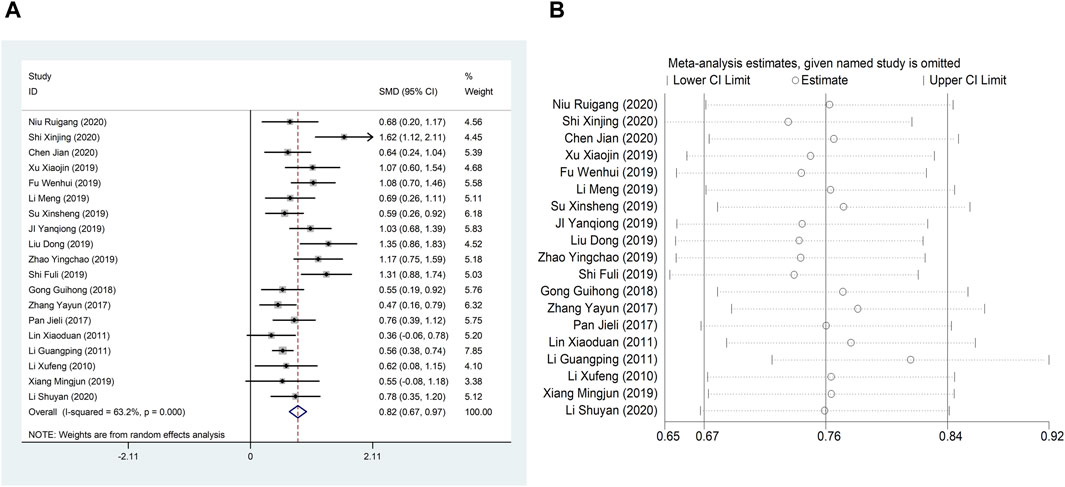
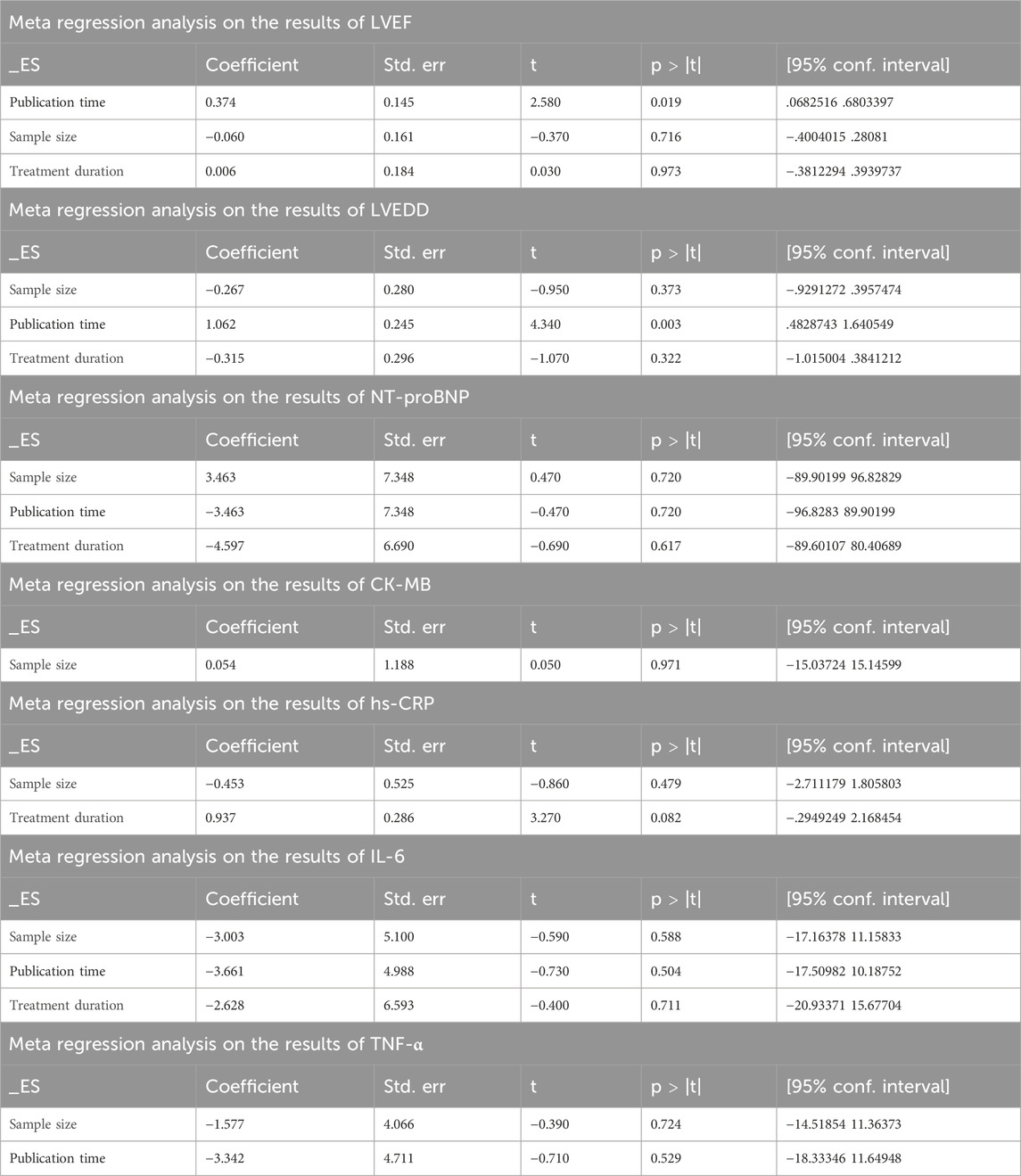
Nineteen studies (Li et al., 2010; Li et al., 2011; Lin, 2011; Pan et al., 2017; Zhang, 2017; Guihong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Shi, 2019; Su, 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Jian, 2020; Li, 2020; Niu, 2020; Shi, 2020) reported LVEF, and due to the large heterogeneity (I2 = 63.2%), so meta-analysis using a random effects model showed statistically significant differences (SMD = 0.82, 95%CI (0.67, 0.97), p < 0.0001), and this result indicated that CDDP added to CWT was superior to CWT in improving LVEF (Figure 3A). Because of inter-study heterogeneity, we performed a sensitivity analysis to exclude any study that did not affect the overall estimate of effect (Figure 3B). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the publication time (Coed. = 0.374, p = 0.019), sample size (Coed. = −0.014, p = 0.991), and treatment duration (Coed. = −0.219, p = 0.856), the results suggest that publication time may be a source of high heterogeneity (Table 2). Subgroup analyses confirmed less heterogeneity for publication dates before 2018. Subgroup analyses also found significantly less heterogeneity in subgroups with a treatment duration of less than 4 weeks (Table 3, Supplementary Material S2).
4.1.2 LVEDD
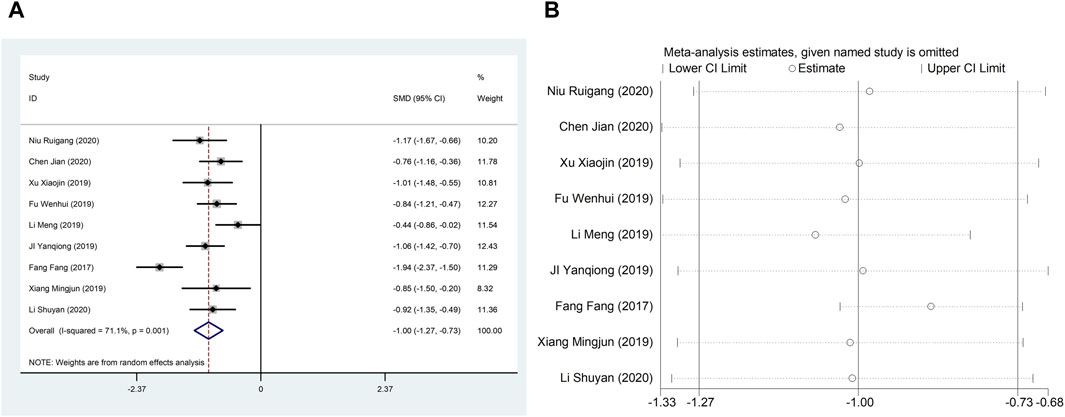
Nine studies (Fang et al., 2017; Ji et al., 2019; Li et al., 2019; Wenhui, 2019; Xiang, 2019; Xu et al., 2019; Jian, 2020; Li, 2020; Niu, 2020) reported LVEDD with large heterogeneity (I2 = 71.1%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −1.00, 95%CI (−1.27, −0.73), p < 0.0001), which indicated that CDDP added to CWT was superior to CWT in improving LVEDD (Figure 4A). Because of inter-study heterogeneity, we performed a sensitivity analysis to exclude any study that did not affect the overall estimate of effect (Figure 4B). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the publication time (Coed. = 1.062, p = 0.003), sample size (Coed. = −0.267, p = 0.373), and treatment duration (Coed. = −0.315, p = 0.322), the results suggest that publication time may be a source of high heterogeneity (Table 2). Subgroup analyses confirmed less heterogeneity in studies published after 2018. This may have been influenced by the rapid development of pharmaceutical technology in recent years and the further optimization of therapeutic options. Subgroup analyses also confirmed better homogeneity with sample sizes less than 50 cases (Table 3, Supplementary Material S2).
4.1.3 NT-proBNP
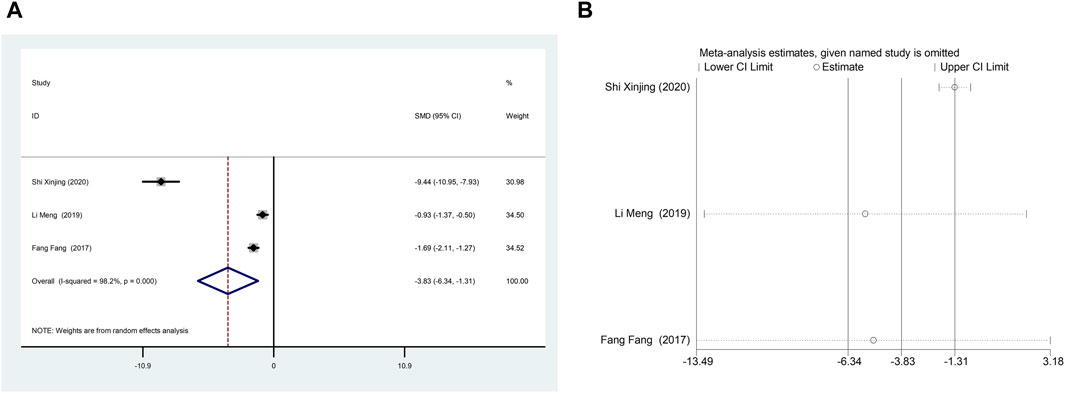
Three studies (Fang et al., 2017; Li et al., 2019; Shi, 2020) reported NT-proBNP with large heterogeneity (I2 = 98.2%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −3.83, 95%CI (−6.34, −1.31), p = 0.003), which indicated that CDDP added to CWT was superior to CWT in improving NT-proBNP (Figure 5A). Because of inter-study heterogeneity, we performed a sensitivity analysis to exclude any study that did not affect the overall estimate of effect (Figure 5B). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the publication time (Coed. = −3.463, p = 0.720), sample size (Coed. = 3.463, p = 0.720), and treatment duration (Coed. = −4.597, p = 0.617), the results did not reveal the source of heterogeneity among studies (Table 2). Subgroup analyses showed that subgroups with a sample size greater than 50 cases and a treatment duration less than 4 weeks were better at improving NT-proBNP (Table 3, Supplementary Material S2).
4.1.4 cTnT
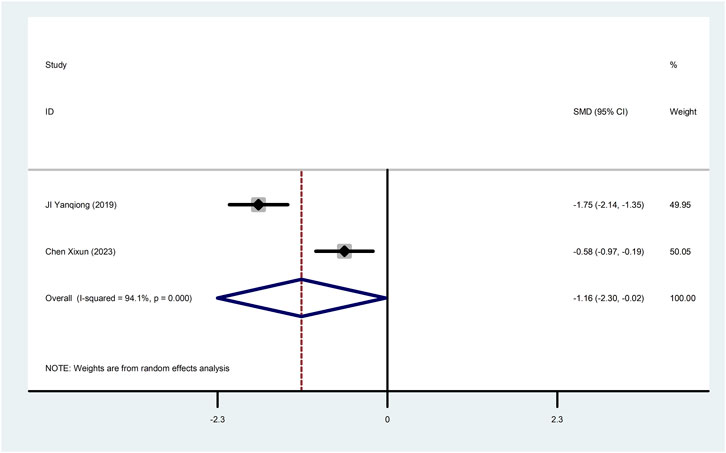
Two studies (Ji et al., 2019; Chen, 2023) reported cTnT with greater heterogeneity (I2 = 94.1%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −1.16, 95%CI (−2.30, −0.02), p = 0.045), which indicated that CDDP added to CWT was superior to CWT in improving cTnT (Figure 6).
4.1.5 CK-MB
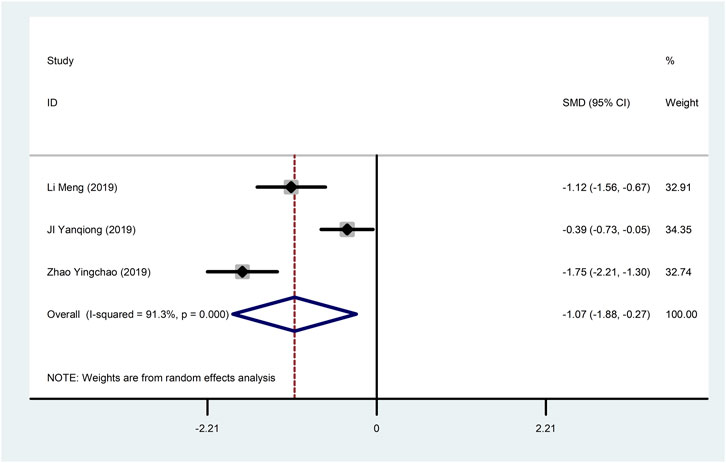
Three studies (Ji et al., 2019; Li et al., 2019; Zhao et al., 2019) reported CK-MB with large heterogeneity (I2 = 91.3%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −1.07, 95%CI (−1.88, −0.27), p = 0.009), which indicated that CDDP added to CWT was superior to CWT in improving CK-MB (Figure 7). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the sample size (Coed. = 0.054, p = 0.971), the results did not reveal the source of heterogeneity among studies (Table 2). Subgroup analyses showed that subgroups with sample sizes greater than 50 cases were better at improving CK-MB (Table 3, Supplementary Material S2).
4.2 TCER
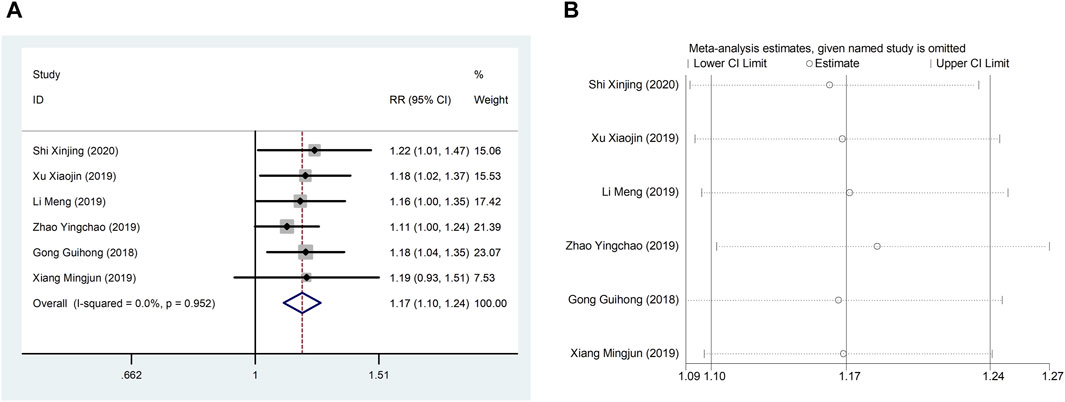
Six studies (Guihong et al., 2018; Li et al., 2019; Xiang, 2019; Xu et al., 2019; Zhao et al., 2019; Shi, 2020) reported TCER with little heterogeneity (I2 = 71.1%), therefore, meta-analysis using fixed effect model showed statistically significant between the two groups (RR = 1.17, 95% CI (1.10, 1.24), p < 0.0001), which result suggests that CDDP added to CWT is superior to CWT in improving TCER (Figure 8A). Sensitivity analysis showed that excluding any study did not affect the overall estimate of effect (Figure 8B).
4.3 Inflammation indicators
4.3.1 hs-CRP
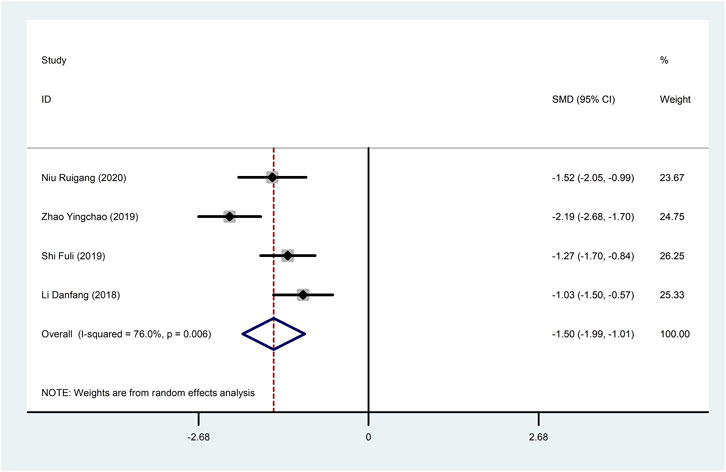
Four studies (Li, 2018; Shi, 2019; Zhao et al., 2019; Niu, 2020) reported hs-CRP with large heterogeneity (I2 = 76.0%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −1.50, 95%CI (−1.99, −1.01), p < 0.0001), which indicated that CDDP added to CWT was superior to CWT in reducing hs-CRP (Figure 9). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the sample size (Coed. = −0.453, p = 0.479) and treatment duration (Coed. = 0.937, p = 0.082), the results did not reveal the source of heterogeneity among studies (Table 2). Subgroup analyses showed better homogeneity in subgroups with sample size of less than 50 cases and treatment duration greater than or equal to 4 weeks (Table 3, Supplementary Material S2).
4.3.2 IL-6
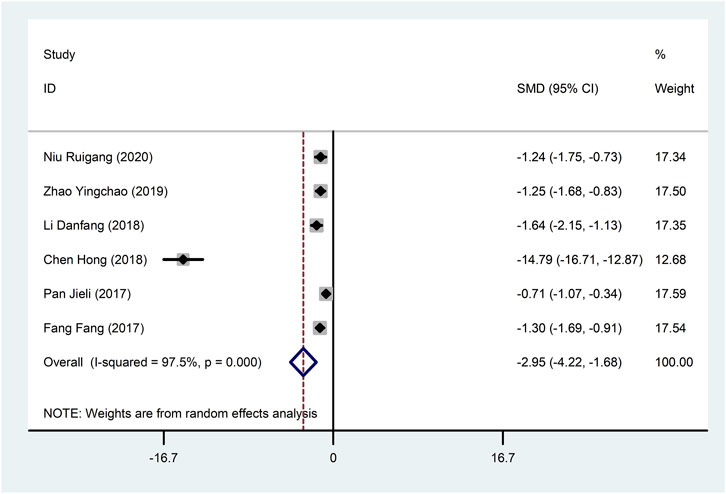
Six studies (Fang et al., 2017; Pan et al., 2017; Li, 2018; Hong et al., 2018; Zhao et al., 2019; Niu, 2020) reported IL-6 with large heterogeneity (I2 = 97.5%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −2.95, 95%CI (−4.22, −1.68), p < 0.0001), which indicated that CDDP added to CWT was superior to CWT in reducing IL-6 (Figure 10). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the publication time (Coed. = −3.463, p = 0.720), sample size (Coed. = 3.463, p = 0.720), and treatment duration (Coed. = −4.597, p = 0.617), the results did not reveal the source of heterogeneity among studies (Table 2). Subgroup analyses showed better homogeneity in subgroups with sample sizes less than 50 cases (Table 3, Supplementary Material S2).
4.3.3 TNF-α
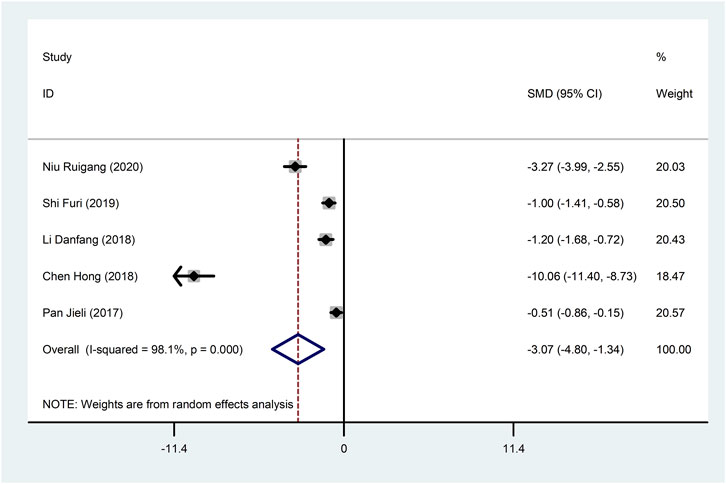
Five studies (Pan et al., 2017; Li, 2018; Hong et al., 2018; Shi, 2019; Niu, 2020) reported TNF-α with large heterogeneity (I2 = 98.1%), so meta-analysis using a random effects model showed statistically significant differences (SMD = −3.07, 95%CI (−4.80, −1.34), p = 0.001), which indicated that CDDP added to CWT was superior to CWT in reducing TNF-α (Figure 11). To further clarify the sources of heterogeneity, we also conducted meta-regression to evaluate the effects of the publication time (Coed. = −3.342, p = 0.529) and sample size (Coed. = −1.577, p = 0.724), the results did not reveal the source of heterogeneity among studies (Table 2). The results of the subgroup analysis failed to identify sources of heterogeneity (Table 3).
4.4 Adverse reactions/adverse events
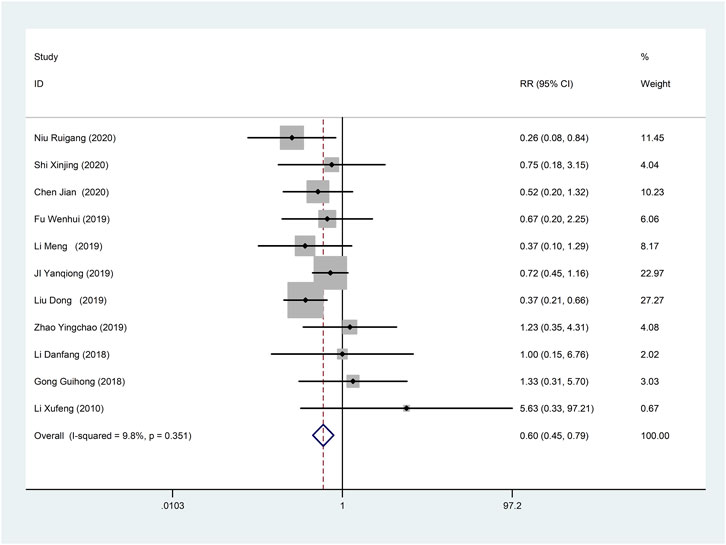
Eleven studies (Li et al., 2010; Li, 2018; Guihong et al., 2018; Ji et al., 2019; Li et al., 2019; Liu and Zhang, 2019; Wenhui, 2019; Zhao et al., 2019; Jian, 2020; Niu, 2020; Shi, 2020) reported adverse reactions/adverse events with fine homogeneity, so meta-analysis using fixed-effects model showed statistically significant between the two groups (RR = 0.60, 95% CI (0.45, 0.79), p < 0.0001), suggesting that CDDP added to CWT was superior to CWT (Figure 12). The incidence of adverse reactions/adverse events was 11.6% (62/535) in the treatment group and 18.6% (96/515) in the control group.
4.5 Results of publication bias assess
We assessed publication bias for metrics that included more than 10 studies. The LVEF funnel plot exhibited left-right asymmetric distribution characteristics, indicating potential publication bias. The quantitative results of Egger’s test are consistent with the qualitative results of the funnel plot (t = 2.19, 95%CI, 0.096 to 4.895, p = 0.042) (Figures 13A,B). It may be related to the quality and sample size of the included studies, and selective reporting cannot be ruled out due to lack of information on clinical trial registration or study protocols. The adverse reaction/adverse event funnel plot exhibited left-right symmetry, and the quantitative results of the egger’s test were consistent with the qualitative results of the funnel plot (t = −2.13, 95%CI, −397.27 to−2.28, p = 0.048) (Figures 13C,D).
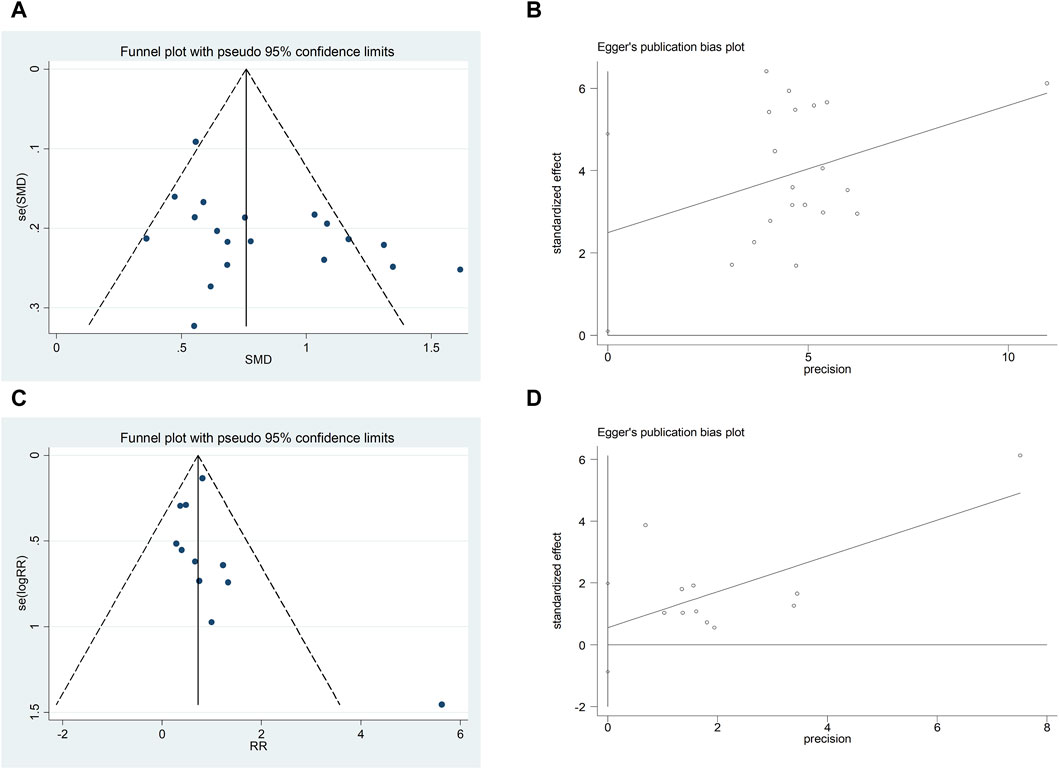
Figure 13. A-B Funnel plot and Egger’s test for LVEF; C-D Funnel plot and Egger’s test for adverse reaction/adverse event.
4.6 GRADE evidence evaluation
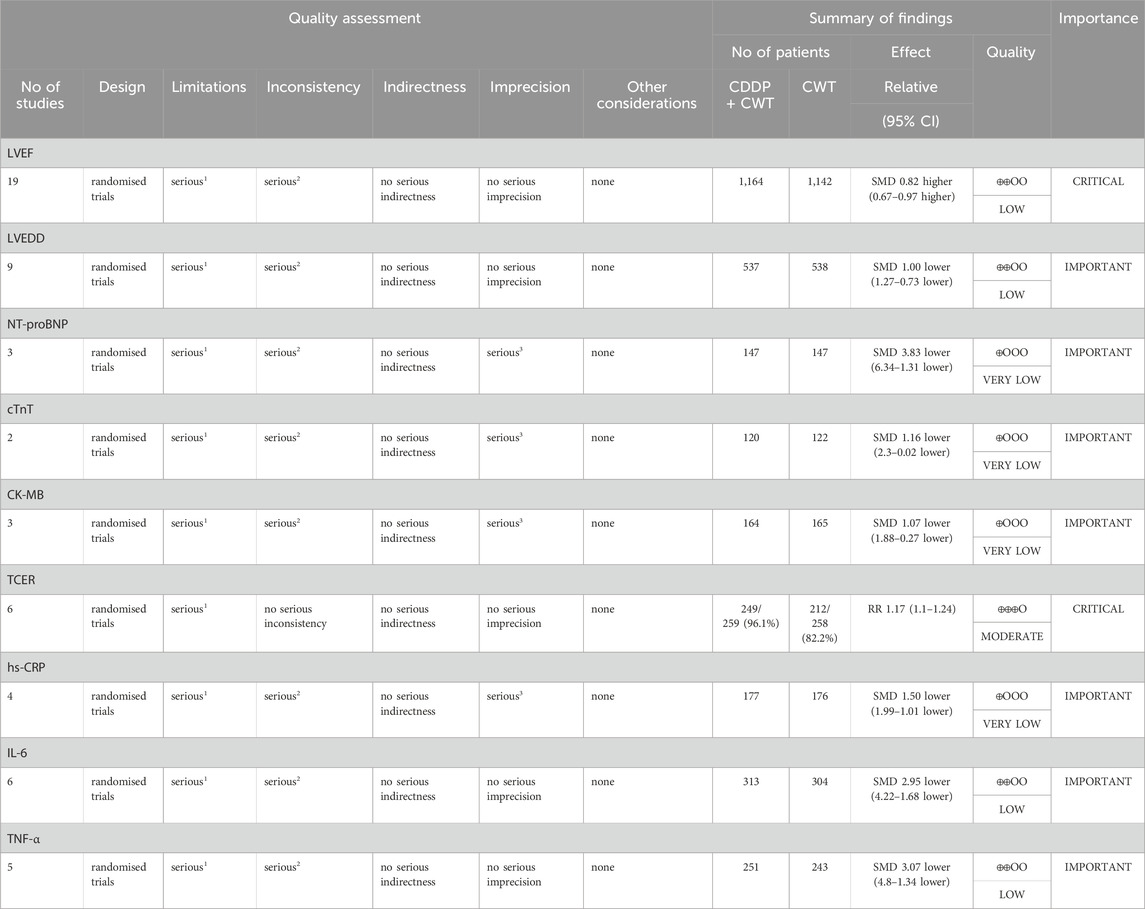
The GRADE evaluation tool was used to grade the evidence for each study’s outcome indicators, taking into account the risk of bias, inconsistency of results, directness of evidence, precision of evidence, and publication bias. Downgrading the quality of the evidence when one or more factors are present in the results of each study. TCER was evaluated as moderate. LVEF, LVEDD, IL-6, TNF-α were evaluated as low. hs-CRP, CK-MB, cTnT, and NT-proBNP were evaluated as very low because of the risk of bias associated with small sample sizes for these indicators (Table 4).
Note: 1the included studies have certain defects in randomization, allocation concealment, and blinding; 2 the included studies are highly heterogeneous; 3 Relatively few patients were included.
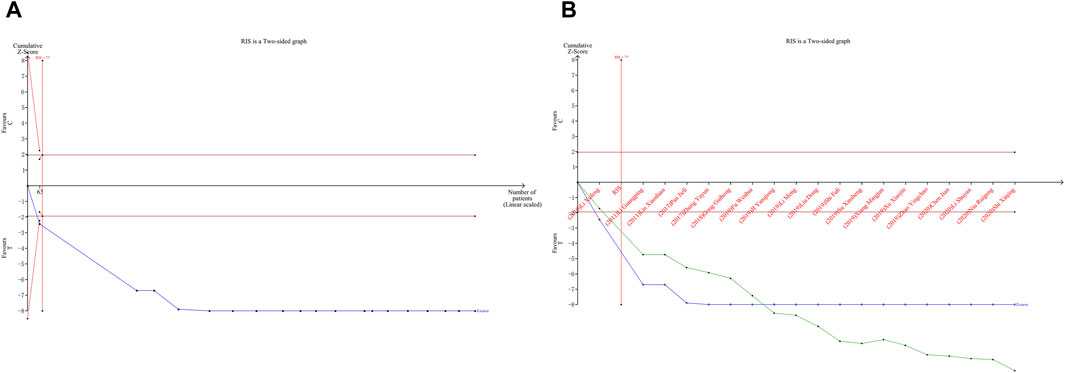
4.7 Results of TSA
TSA was performed on LVEF. Type I error was defined as 5%, the information axis was set to a cumulative sample size, statistical efficacy was 80%, and the cumulative Z-value crossed the traditional and TSA bounds after item 2 to obtain a positive conclusion in advance, using the sample size as the required information size (RIS). The penalized curve exceeded the traditional bound of Z = 1.96, further confirming the clinical efficacy of CDDP in improving LVEF (Figures 14A,B).
5 Discussion
Early reperfusion is a typical therapy for AMI that can effectively restore blood flow to ischemic myocardial tissue. However, reperfusion itself will increase irreversible damage to coronary artery circulation and accelerate and expand the IRI (Qi et al., 2021). One of the ways in which TCM has made an outstanding contribution to the development of medicine in the world is through the availability of effective natural compounds. TCM is characterised by the adjustment of multiple components and multiple targets to transform the organism from an abnormal to a normal state (Yin-Yang balance) (Wang et al., 2018).
5.1 From bench to bedside
CDDP is a highly dispersed state of Chinese patent medicine made of Salviae Miltiorrhizae Radix et Rhizoma, Notoginseng Radix, and Borneolum Syntheticum as the main ingredients and processed by modern technology, which has the effect of activating blood and activating stasis, regulating qi and relieving pain. The inflammatory response induced after PCI promotes an excessive increase in oxygen free radicals, which triggers ventricular remodeling and myocardial injury and is an initiating factor for thrombus re-formation (Li et al., 2023). Pharmacological studies have found CDDP to have coronary artery dilation, anti-inflammatory, anti-thrombotic, and vascular endothelial and cardiomyocyte protection effects, thereby further reversing IRI (Bei et al., 2017). Panax notoginseng (PNS), an active ingredient in Notoginseng Radix, can effectively inhibit oxygen sugar deprivation-induced apoptosis, probably by inhibiting oxidative stress and inducing Akt phosphorylation (Chen et al., 2011). PNS also reduces the expression of pro-inflammatory factors by inhibiting the RAGE, MAPK signaling pathway in apoE (−/−) mouse lesions (Dou et al., 2012). Ginsenosides Rb1 and Rg1 activate nitric oxide (NO) and promote endothelium-dependent vasodilatation by regulating endothelial cell PI3K/Akt/eNOS pathway and L-arginine transport (Pan et al., 2012). Ginsenoside Rd prevents the development of atherosclerosis by inhibiting Ca2+ inward flow through voltage-independent Ca2+ channels (Li et al., 2011). CDDP has a wide range of applications in cardiovascular disease, and most RCTs have demonstrated significant clinical efficacy of CDDP-assisted therapy in AMI undergoing PCI. However, trials with large sample sizes are lacking. This meta-analysis provides a valid evidence-based rationale for the clinical use of CDDP in the treatment of AMI undergoing PCI.
5.2 Robustness of meta-analysis and credibility of evidence
Meta-analysis results showed that CDDP has good positive utility for AMI undergoing PCI treatment, can reduce hs-CRP, TNF-alpha, IL-6 levels, inhibit myocardial local inflammatory response, improve myocardial injury indexes such as CK-MB, NT-proBNP, cTnT, improve LVEF, reduce LVEDD, and at the same time, it shows strong advantages in reducing the incidence of adverse reactions/adverse events. Due to the low heterogeneity, we can clarify that CDDP combined with CWT is superior to CWT in improving TCER. Interestingly despite our choice of randomized models for data statistics, sensitivity analyses, meta-regression analyses, and subgroup analyses to eliminate heterogeneity between studies, a small number of outcome metrics remained highly heterogeneous, prompting us to be cautious in interpreting the results in clinical practice. GRADE results showed that the quality of evidence ratings for most outcome indicators were very low and low. Currently, a number of studies are rated as low quality due to methodological limitations, mainly due to risk of bias, inconsistent results, indirect evidence. Most studies lacked blinding and allocation concealment designs during randomization. However, the emerging view is that unblinded pragmatic trials should be recommended because they emphasize practical applicability and extrapolation to improve the external validity of real-world trials rather than treatment effects, even though this approach is a major contributor to the low quality of included studies (Sox and Lewis, 2016). TSA was performed to exclude possible false-positive results in order to improve the robustness of the results of the meta-analysis. The results of the TSA showed that the total number of samples collected in this study met the requirements of the meta-analysis, which excluded the possibility of false-positive results, and further confirmed the efficacy of the CDDP for the treatment of AMI undergoing PCI.
5.3 Limitations and perspectives
The purpose of this study is to summarize and evaluate the efficacy and safety of CDDP for the treatment of AMI undergoing PCI according to the updated and optimized methods of PRISMA, so that the results are sufficiently comparable and convincing. Nevertheless, despite the encouraging results, there are inevitable limitations to this study: 1) The overall quality of the studies was poor, with most being single-center, small-sample trials and with a lack of uniformity in reporting methods for outcome indicators. The poor quality of some of the studies resulted in high risk and heterogeneity between studies, which may reduce the robustness of the results of meta-analysis. 2) As original studies related to most of the outcome metrics were scarce, only studies on LVEF improvement were assessed for publication bias. 3) In addition to the indicators analyzed in this study, there are individual studies that reported TCM efficacy scores, triglycerides (TG), and total cholesterol (TC), etc. However, due to the small number of studies and the inconsistency in the way the outcome indicators were measured, this study did not perform a combined analysis, which is to be enriched and improved in the future. 4) The evaluation of drug efficacy in the related field requires a long period of time to be completed due to the lengthy process of further treatment after PCI. The study’s inclusion of a short course of RCT and the lack of long-term follow-up means that the drug’s long-term efficacy is not yet known. 5) There were few studies that explicitly stated that they conducted their RCTs in accordance with the Consolidated Standards of Reporting Trials (CONSORT), and most did not provide registry information. 6) The lack of relevant information on blinding and allocation concealment in a small number of studies may lead to an exaggerated effect of the results.
In the future, clinical RCTs on the adjuvant treatments of CDDP against AMI should be improved in the following aspects. First, at the design stage, protocols for RCTs should comply with the CONSORT statement. High-quality clinical data conforming to international standards such as the CONSORT statement is absolutely essential for the further development and acceptance of TCM, and the improvement of the quality of clinical data will be a key step in the future of TCM. Second, RCTs should use a rigorous blinded design with recognized and uniform outcomes to evaluate the therapeutic efficacy of CDDP. Third, RCTs should perform sample size estimation and be designed with a sufficiently long follow-up period for evaluation. Fourth, improvements in TCM symptoms should be recorded in detail to provide evidence for future TCM consultations and treatments to better improve quality of life.
6 Conclusion
In conclusion, adjuvant treatment of AMI with CDDP has shown exciting and safe benefits in improving cardiac function and reducing inflammatory response in patients with AMI undergoing PCI. Nevertheless, due to the poor quality of some of the included studies, it needs to use RCTs with rigorous designs and long follow-up periods to confirm and update the results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GF: Conceptualization, Data curation, Investigation, Software, Visualization, Writing–original draft, Writing–review and editing. ML: Conceptualization, Investigation, Methodology, Software, Supervision, Writing–review and editing. HS: Conceptualization, Investigation, Software, Writing–review and editing. YW: Conceptualization, Funding acquisition, Investigation, Project administration, Software, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project is supported by the National Key R&D Program “Modernization of Chinese Medicine” (2018YFC1707402).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1345897/full#supplementary-material
Abbreviations
AMI, acute myocardial infarction; CDDP, compound danshen drip pill; STEMI, ST-segment elevation myocardial infarction; NSTEMI, Non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; GRADE, grading of recommendations assessment, development and evaluation; TSA, trial sequential analysis; TCM, traditional Chinese medicine; CWT, conventional western treatment; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; LVEF, left ventricular ejection fraction; TCER, total clinical effective rate; NT-proBNP, N-terminal pro-B-type natriuretic peptide; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha; CK-MB, creatine kinase isoenzyme; IRI, ischemia reperfusion injury; PNS, panax notoginseng; NO, nitric oxide; CONSORT, consolidated standards of reporting trials.
References
Bei, C., Xiaoying, L., Keqiang, L., Lin, W., Weiping, W., and Hao, X. (2017). Compound danshen dropping pill clinical application Chinese Expert Advice. Chin. J. Integr. Tradit. West Med. 37, 17–22.
Bhatt, D. L., Lopes, R. D., and Harrington, R. A. (2022). Diagnosis and treatment of acute coronary Syndromes: a review. Jama 327, 662–675. doi:10.1001/jama.2022.0358
Brozek, J. L., Akl, E. A., Alonso-Coello, P., Lang, D., Jaeschke, R., Williams, J. W., et al. (2009). Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64, 669–677. doi:10.1111/j.1398-9995.2009.01973.x
Chen, S., Liu, J., Liu, X., Fu, Y., Zhang, M., Lin, Q., et al. (2011). Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J. Ethnopharmacol. 137, 263–270. doi:10.1016/j.jep.2011.05.011
Chen, X. (2023). Analysis of the effectiveness of compound danshen drip pill combined with clopidogrel after coronary intervention in patients with acute myocardial infarction. China J. Cardiovasc Dis. 13, 20–22.
Damluji, A. A., Forman, D. E., Wang, T. Y., Chikwe, J., Kunadian, V., Rich, M. W., et al. (2023). Management of acute coronary syndrome in the Older Adult Population: a Scientific statement from the American Heart association. Circulation 147, e32–e62. doi:10.1161/cir.0000000000001112
Dou, L., Lu, Y., Shen, T., Huang, X., Man, Y., Wang, S., et al. (2012). Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell Physiol. Biochem. 29, 875–882. doi:10.1159/000315061
Fang, F., Beneficiary, G., Guangrui, F., Hui, Y., and Xiaonan, Z. (2017). Effect of long-term administration of compound danshen drip pill on left ventricular remodeling and inflammatory factor levels after PCI in elderly patients with acute myocardial infarction. Prog. Mod. Biomed. 17, 544–546+566. doi:10.13241/j.cnki.pmb.2017.03.038
Greco, A., and Capodanno, D. (2024). Trial sequential analysis methodology for interpreting meta-analytical findings. Eur. J. Intern Med. 121, 1–3. doi:10.1016/j.ejim.2023.12.029
Guihong, G., Jie, J., Jiantao, Y., and Min, L. (2018). Clinical study of compound Danshen dripping pills combined with recombinant human prourokinase in the treatment of acute ST-segment elevation myocardial infarction. Drugs Clin. 33, 1376–1379.
Hausenloy, D. J., and Yellon, D. M. (2008). Time to take myocardial reperfusion injury seriously. N. Engl. J. Med. 359, 518–520. doi:10.1056/NEJMe0803746
Hong, C., Dongmei, W., Li, Y., Gang, Y., Jiangshu, L., and Xiaoning, Y. (2018). Effect of compound danshen drip pill on serum inflammatory factors and T-cell subsets after PCI in elderly patients with acute myocardial infarction. Chin. J. Gerontol. 38, 2586–2588.
Ibanez, B., James, S., Agewall, S., Antunes, M. J., Bucciarelli-Ducci, C., Bueno, H., et al. (2018). 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177. doi:10.1093/eurheartj/ehx393
Ji, Y., Li, Z., and Zuo, J. (2019). To observe the effect of compound Danshen dropping pills in the adjuvant treatment of acute myocardial infarction after percutaneous coronary intervention. Chin. J. Crit. Care Med. 25, 243–245.
Jian, C. (2020). Effect of compound danshen drip pill on the improvement of cardiac function after acute percutaneous coronary intervention. Hang K’ung Hang T’ien Ta Hsueh Hsueh Pao 31, 75–77.
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 134 (10), e123–e155. doi:10.1161/CIR.0000000000000404
Li, B., Feng, Q., Yu, C., Yang, J., Qin, X., Li, X., et al. (2023). Predictive value of serum HIF-1α and VEGF for arrhythmia in acute coronary syndrome patients. Exp. Biol. Med. (Maywood) 248, 685–690. doi:10.1177/15353702231171902
Li, D. (2018a). Clinical efficacy of compound danshen drip pill combined with atorvastatin in improving inflammatory response and myocardial perfusion after PCI for acute myocardial infarction. Cardiovasc Cerebrovasc. Prev. Treat. 18, 500–502.
Li, G., Zheng, X., and Wang, H. (2011a). Clinical effect of compound danshen drip pill on the interventional treatment of acute ST-segment elevation myocardial infarction. Chin. J. Interv. Cardiol. 19, 24–28.
Li, J., Xie, Z. Z., Tang, Y. B., Zhou, J. G., and Guan, Y. Y. (2011b). Ginsenoside-Rd, a purified component from panax notoginseng saponins, prevents atherosclerosis in apoE knockout mice. Eur. J. Pharmacol. 652, 104–110. doi:10.1016/j.ejphar.2010.11.017
Li, M., Hu, H., Mu, L., and Han, L. (2019). Effect of high loading dose of Rosuvastatin together with Compound Danshen Dropping Pill on vascular endothelial function and prognosis of patients with poor ST-segment regression on electrocardiogram after PCI for ST-segment elevation myocardial infarction. Mod. J. Tradit. West Med. 28, 2893–2898.
Li, Q. (2018b). Research progress and clinical application of compound danshen drip pill. China J. Tradit. Chin. Med. Pharm. 33, 2989–2991.
Li, S. (2020). Exploring the effect of compound danshen drip pill combined with clopidogrel in the treatment of acute myocardial infarction. Med. Adv. 10, 54–55. Available at: https://d.wanfangdata.com.cn/periodical/ChhQZXJpb2RpY2FsQ0hJTmV3MjAyMzA4MDYSDnlpeXF5MjAyMDIyMDQxGghoNXo1cnp1Yw==.
Li, X., Liu, F., and Jiang, T. (2010). Treatment of acute early myocardial infarction with compound danshen drip pill in 42 cases. Chin. J. New Drugs 19, 1699–1702.
Liang, Q., Li, W., Yang, X., Wang, W., and Tian, P. (2020). Compound danshen drip pill improves myocardial ischemia-reperfusion injury research progress. Chin. J. Mod. Appl. Pharm. 14, 236–238. doi:10.14164/j.cnki.cn11-5581/r.2020.07.108
Lin, X. (2011). Clinical study of compound danshen drip pill on early ventricular remodeling after acute infarction. World Chin. Med. 6, 111–112.
Liu, D., and Zhang, S. (2019). Observation on the effect of early intervention of clopidogrel combined with compound danshen drip pill on the long-term prognosis of acute myocardial infarction. Cardiovasc Prev. Treat. 9, 14–16.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G.PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Niu, R. (2020). Effects of compound danshen drip pill combined with resuvastatin on cardiac function and inflammatory factors in elderly patients with acute myocardial infarction after PCI surgery. Chin. J. Integr. Tradit. West Med. 18, 1110–1113.
Pan, C., Huo, Y., An, X., Singh, G., Chen, M., Yang, Z., et al. (2012). Panax notoginseng and its components decreased hypertension via stimulation of endothelial-dependent vessel dilatation. Vasc. Pharmacol. 56, 150–158. doi:10.1016/j.vph.2011.12.006
Pan, J., Jia, G., and Wang, M. (2017). Effects of the Chinese medicinal preparation, compound danshen drip pill, on nuclear factor-κB p65-mediated inflammatory response in patients with acute myocardial infarction in vivo. Pharm. Biotechnol. 24, 120–123. doi:10.19526/j.cnki.1005-8915.20170206
Qi, H., Zhang, J., Shang, Y., Yuan, S., and Meng, C. (2021). Argon inhibits reactive oxygen species oxidative stress via the miR-21-mediated PDCD4/PTEN pathway to prevent myocardial ischemia/reperfusion injury. Bioengineered 12, 5529–5539. doi:10.1080/21655979.2021.1965696
Qin, C., Yap, S., and Woodman, O. L. (2009). Antioxidants in the prevention of myocardial ischemia/reperfusion injury. Expert Rev. Clin. Pharmacol. 2, 673–695. doi:10.1586/ecp.09.41
Roohafza, H., Noohi, F., Hosseini, S. G., Alemzadeh-Ansari, M., Bagherieh, S., Marateb, H., et al. (2023). A cardiovascular risk assessment model according to Behavioral, Psychosocial and traditional factors in patients with ST-segment elevation myocardial infarction (CRAS-MI): review of literature and methodology of a Multi-center Cohort study. Curr. Probl. Cardiol. 48, 101158. doi:10.1016/j.cpcardiol.2022.101158
Rout, A., Tantry, U. S., Novakovic, M., Sukhi, A., and Gurbel, P. A. (2020). Targeted pharmacotherapy for ischemia reperfusion injury in acute myocardial infarction. Expert Opin. Pharmacother. 21, 1851–1865. doi:10.1080/14656566.2020.1787987
Shengshou, H., Runlin, G., and Lisheng, L. (2019). Summary of China cardiovascular disease Report 2018. J. Chin. Circ. 34, 209–220.
Shi, F. (2019). Effect of compound danshen drip pill on serum inflammatory factors and ventricular remodeling in patients with acute infarction. Chin. J. Clin. Healthc. 17, 74–76.
Shi, X. (2020). Danshen drip pill combined with clopidogrel in the treatment of acute myocardial infarction. Chin. J. Pract. Chin. Mod. Med. 20, 63–64. doi:10.13638/j.issn.1671-4040.2020.03.032
Sox, H. C., and Lewis, R. J. (2016). Pragmatic trials: practical Answers to "real world" Questions. Jama 316, 1205–1206. doi:10.1001/jama.2016.11409
Stebbins, A., Mehta, R. H., Armstrong, P. W., Lee, K. L., Hamm, C., Van de Werf, F., et al. (2010). A model for predicting mortality in acute ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: results from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. Circ. Cardiovasc Interv. 3, 414–422. doi:10.1161/circinterventions.109.925180
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Su, X. (2019). Effect of compound danshen drip pill on brain natriuretic peptide level and ventricular remodeling after percutaneous coronary intervention in patients with acute myocardial infarction. Chin. J. Pract. Intern Med. 46, 110–113.
Wang, J., Wong, Y. K., and Liao, F. (2018). What has traditional Chinese medicine delivered for modern medicine? Expert Rev. Mol. Med. 20, e4. doi:10.1017/erm.2018.3
Wenhui, F. (2019). Effect of compound Danshen dropping pills on BNP level and ventricular remodeling in patients with acute myocardial infarction after PCI. J. Shanxi Med. Coll. Contin. Educ. 29, 54–56. Available at: https://kns.cnki.net/kcms/detail/14.1266.R.20190930.1655.052.html.
Xiang, M. (2019). Clinical analysis of compound danshen drip pill in the treatment of acute early myocardial infarction. Psychol. Dr. 25, 180–181. Available at: https://d.wanfangdata.com.cn/periodical/ChhQZXJpb2RpY2FsQ0hJTmV3MjAyMzA4MDYSD3hseXMteDIwMTkwNDE0NxoIcTdvbDljaHk=.
Xiaoyu, Z. (2002). Guiding Principles for clinical research of New Chinese medicines (trial). Beijing: China Medical Science and Technology Press, 68–85.
Xu, X., Zhang, Y., and Zhang, S. (2019). Effect of compound danshen drip pill combined with resuvastatin on left ventricular remodeling and myocardial fibrosis after percutaneous coronary intervention for acute myocardial infarction. Chin. Med. J. 16, 61–64+77.
Yellon, D. M., and Hausenloy, D. J. (2007). Myocardial reperfusion injury. N. Engl. J. Med. 357, 1121–1135. doi:10.1056/NEJMra071667
Zhang, M. (2018). Guidelines for combined Chinese and Western medicine in the diagnosis and treatment of acute myocardial infarction. Chin. J. Integr. Tradit. West Med. 38, 272–284. Available at: https://kns.cnki.net/kcms/detail/11.2787.R.20180301.1556.004.html.
Zhang, Y. (2017). Effect of compound danshen drip pill on the efficacy of direct PCI in patients with acute ST-segment elevation myocardial infarction. Shanxi Medical University.
Keywords: Acute myocardial infarction, Compound danshen drip pill, Meta-analysis, Systematic review, Cardiac function
Citation: Fan G, Liu M, Song H and Wang Y (2024) Effect of adjuvant therapy with compound danshen drip pill on inflammatory factors and cardiac function after percutaneous coronary intervention for acute myocardial infarction: a systematic review and meta-analysis. Front. Pharmacol. 15:1345897. doi: 10.3389/fphar.2024.1345897
Received: 28 November 2023; Accepted: 19 March 2024;
Published: 16 April 2024.
Edited by:
Rong-Rong He, Jinan University, ChinaReviewed by:
Guldem Mercanoglu, University of Health Sciences, TürkiyeYong Wang, Beijing University of Chinese Medicine, China
Copyright © 2024 Fan, Liu, Song and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongxia Wang, d3l4Y2h6aHFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Genhao Fan
Genhao Fan Menglin Liu
Menglin Liu Huanhuan Song2†
Huanhuan Song2† Yongxia Wang
Yongxia Wang