
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 22 February 2024
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1344828
This article is part of the Research Topic Pharmacological Therapy in Patients with Arrhythmias View all 6 articles
 Yang-Qi Pan†
Yang-Qi Pan† Lu-Shen Jin†
Lu-Shen Jin† Sang Qian†
Sang Qian† Ting Jiang
Ting Jiang Zhe-Ning Wang
Zhe-Ning Wang Yi-Lian Chen
Yi-Lian Chen Yi-Xuan Qiu
Yi-Xuan Qiu Yi-Hao Wu
Yi-Hao Wu Jia-Yang Fu
Jia-Yang Fu Ling Li
Ling Li Yuan-Nan Lin
Yuan-Nan Lin Yue-Chun Li*
Yue-Chun Li*Background and aim: Rivaroxaban is an emerging oral anticoagulant for postoperative anticoagulation after percutaneous left atrial appendage closure (LAAC). Because a once-daily dosing regimen of rivaroxaban causes fluctuations in the drug plasma concentration, we studied the feasibility and safety of twice-daily rivaroxaban as a postoperative anticoagulation regimen for patients with atrial fibrillation (AF) undergoing LAAC.
Methods: This study involved patients with AF who underwent LAAC and took rivaroxaban postoperatively. A total of 326 patients who received a standard total dose (15 or 20 mg) of rivaroxaban based on their creatinine clearance rate were divided into the twice-daily (BID) rivaroxaban group (n = 208) and once-daily (QD) rivaroxaban group (n = 118) according to their anticoagulation strategy. Transesophageal echocardiography was recommended at 3–6 months postoperatively to check for device-related thrombosis (DRT). Clinical outcomes were evaluated during postoperative anticoagulation.
Results: The median CHA2DS2-VASc score (4 [3, 5] vs. 4 [3, 5], p = 0.28) and HAS-BLED score (2 [2, 3] vs. 2 [2, 3], p = 0.48) were not significantly different between the groups. During the anticoagulation period (4.1 ± 0.7 vs. 4.1 ± 0.9 months, p = 0.58), 148 (71.2%) patients in the BID group and 75 (63.6%) in the QD group underwent follow-up transesophageal echocardiography. There were no statistically significant differences between the two groups in terms of DRT (1.4% vs. 2.7%, p = 0.60), minor bleeding (8.2% vs. 11.0%, p = 0.39), thromboembolic events (1.0% vs. 0.8%, p = 1.00), major bleeding (0.5% vs. 0.8%, p = 1.00), or death.
Conclusion: A short course of twice-daily rivaroxaban following LAAC is a feasible alternative regimen with a low rate of major bleeding events, DRT, and thromboembolic events for patients with AF.
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia and is associated with high risks of ischemic stroke and other thromboembolic events. Because the left atrial appendage (LAA) is the origin of most atrial thrombi, percutaneous LAA closure (LAAC) has been used for thromboprophylaxis in patients with AF (Blackshear and Odell, 1996). Previous studies have evaluated once-daily dosing of rivaroxaban as a postoperative anticoagulation regimen in patients undergoing LAAC (Bösche et al., 2015; Bergmann et al., 2017; Enomoto et al., 2017; Gu et al., 2020; Zhu and Xu, 2021) and have demonstrated a close correlation between pharmacodynamic effects and pharmacokinetic profiles (Kubitza et al., 2005a; Kubitza et al., 2005b). However, the plasma concentration peaked as soon as 2–4 h after rivaroxaban administration, and Factor Xa activity and prolongation of prothrombin time returned to within 10% of baseline values at 24 h after rivaroxaban intake. In contrast, twice-daily administrations of rivaroxaban results in a more stable plasma concentration. A study revealed that patients who received a once-daily regimen exhibited a 60% decrease in trough plasma concentration and a 20% increase in maximum concentration, compared to those who received a twice-daily regimen, while maintaining an equivalent total dosage (Mueck et al., 2011). Therefore, twice-daily rivaroxaban has a smoother efficacy, and may be a better option after LAAC. This study was performed to assess the safety and efficacy of twice-daily rivaroxaban as an alternative regimen for patients undergoing LAAC.
This study included consecutive patients who underwent successful implantation of either the Watchman (Boston Scientific, Marlborough, MA, USA) or the LAmbre (LifeTech Scientific, Shenzhen, China) at our institution between August 2018 and December 2020 and received rivaroxaban after the procedure. The indication for LAAC was nonvalvular AF with a CHA2DS2-VASc score of ≥2 in a patient deemed to be a poor candidate for long-term oral anticoagulation. The exclusion criteria were an intracardiac thrombus, a mechanical prosthetic heart valve, LAA obliteration, a left ventricular ejection fraction of <30%, heart transplantation, significant mitral valve stenosis, patent foramen ovale, and end-stage renal disease (creatinine clearance rate of <15 mL/min). Informed consent was obtained from all patients. Data collection and all study procedures were conducted according to the protocol approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University.
The patients underwent either a one-stop procedure (combined CA and LAAC) or LAAC. Catheter ablation was performed as previously described (Ke et al., 2022). Antral ring electrical isolation of the left and right pulmonary veins was performed in all patients, and additional linear ablation was individualized according to requirements for persistent forms of AF or for redo ablation procedures. LAAC with the Watchman or LAmbre device was undertaken either before or after AF ablation according to the operator’s experience. The implantation procedure has been described in detail elsewhere (Sick et al., 2007; Lam, 2013). Briefly, the device was implanted under transesophageal echocardiography (TEE), LAA angiography, and fluoroscopic guidance via right femoral venous and transseptal access into the LAA. Ultrasonography examination was performed before discharge.
After LAAC, the patients received rivaroxaban for anticoagulation twice daily or once daily (total dose of 15 mg or 20 mg) depending on the physicians. Rivaroxaban was routinely administered with discontinuation depending on the results of TEE, which was recommended at 3–6 months. Rivaroxaban was replaced by clopidogrel if TEE confirmed satisfactory device positioning and residual flow of <3 mm without device-related thrombosis (DRT) (defined as detection of thrombosis on the lumen side of the device). In patients without TEE imaging, the decision to discontinue anticoagulation was at the discretion of the physicians. During anticoagulation, we evaluated the incidence of thromboembolic and bleeding events according to the Munich consensus document (Tzikas et al., 2017). Thromboembolic events included transient ischemic attack, stroke, and systemic embolism. Bleeding events were classified as major bleeding or minor bleeding. Major bleeding was defined as pericardial bleeding, a ≥3 g/dL drop in hemoglobin or transfusion of two or more units of whole blood or red blood cells, hospitalization because of bleeding, requirement for additional surgery, life-threatening bleeding, or disabling bleeding (Tzikas et al., 2017). A bleeding event that did not meet the above criteria was defined as minor bleeding.
Continuous variables are reported as mean ± standard deviation or median [interquartile range], and categorical variables are expressed as count and percentage (%). Continuous variables were compared between groups using the t-test or Mann–Whitney U test as appropriate, and categorical variables were compared using the chi-square test or Fisher’s exact test. Analyses were performed with SPSS Version 26.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
In total, 326 patients were included in our study and divided into two groups (Figure 1). After LAAC, 208 patients received rivaroxaban twice daily (BID group) and 118 received rivaroxaban once daily (QD group). The number of patients receiving a total dose of 20 mg was 118 in the BID group and 56 in the QD group (56.7% vs. 47.5%, p = 0.11). The patients’ baseline characteristics are shown in Table 1. The median CHA2DS2-VASc score (4 [3, 5] vs. 4 [3, 5], p = 0.28) and HAS-BLED score (2 [2, 3] vs. 2 [2, 3], p = 0.48) were similar between the two groups. There were no statistically significant differences in the proportion of persistent AF (65.4% vs. 63.6%, p = 0.74) or any other baseline characteristics between the BID and QD groups.
The procedural characteristics and periprocedural adverse events are shown in Table 2. The one-stop procedure was performed in 151 (72.6%) and 85 (72.0%) patients in the BID and QD groups, respectively, while the others underwent LAAC. One major bleeding event occurred in each group (0.5% vs. 0.8%, p = 1.0). One patient in the BID group underwent pericardial tamponade 6 h after the combined procedure, and the patient’s hemodynamics stabilized after pericardiocentesis. One patient in the QD group developed a cerebral hemorrhage on the second postoperative day. Eight and six minor bleeding events occurred in the BID and QD groups, respectively, but did not lead to discontinuation of rivaroxaban (3.8% vs. 5.1%, p = 0.6). No other complications occurred during the perioperative period.
The follow-up results are shown in Table 3. During follow-up, 148 patients in the rivaroxaban BID group and 75 in the rivaroxaban QD group underwent postoperative TEE (71.2% vs. 63.6%, p = 0.16) and the mean time from the procedure to TEE was 4.2 ± 0.6 vs. 4.1 ± 1.0 months (p = 0.26). All patients showed residual peri-device flow of <3 mm and complete sealing of the LAA. TEE DRT was detected in two (0.7%) patients in each of the two groups by TEE (1.4% vs. 2.7%, p = 0.60). These four patients with a history of persistent AF were free of thromboembolic events during the follow-up period. After intensive anticoagulation, the DRT dissolved in all patients, and long-term clopidogrel was then initiated.
During the postoperative anticoagulation period (4.1 ± 0.7 vs. 4.1 ± 0.9 months, p = 0.58), no patients died and no major bleeding events occurred (Table 3). Sixteen patients developed minor bleeding during rivaroxaban administration. Symptoms of minor bleeding events in the BID group were oral mucosal bleeding (n = 3), conjunctival congestion (n = 1), bloody stool (n = 2), hematuria (n = 1), and skin ecchymoses (n = 2). Symptoms of minor bleeding events in the QD group included oral mucosal bleeding (n = 2), hematuria (n = 1), bloody stool (n = 1), and skin ecchymoses (n = 3). Two patients receiving 10 mg rivaroxaban twice daily and one receiving 20 mg rivaroxaban once daily developed a stroke (1.0% vs. 0.8%, p = 1.00). During follow-up, there was no significant difference in various adverse events between the two groups (Figure 2).
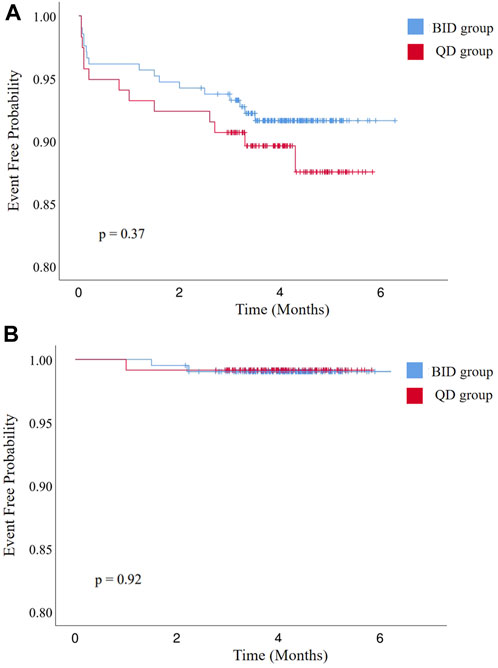
FIGURE 2. Kaplan–Meier curves of (A) freedom from bleeding events and (B) all-cause stroke, transient ischemic attack, and systemic embolism. BID, twice daily; QD, once daily.
This study is the first to evaluate the feasibility and safety of taking rivaroxaban twice daily after LAAC. This medication regimen had rates of DRT, bleeding events, and thromboembolic events comparable to those of once-daily rivaroxaban, suggesting that twice-daily administration is a safe and effective postoperative antithrombotic regimen.
Rivaroxaban at 20 mg once daily has been used to prevent thrombosis and stroke after LAAC (Gu et al., 2020). The standard dose of rivaroxaban was determined by data from studies of deep vein thrombosis and exposure–response regression analysis, and subsequent studies of rivaroxaban for postoperative anticoagulation in patients undergoing LAAC followed this regimen (Mueck et al., 2011). However, the data could not reveal slight but relevant differences in major bleeding and thrombotic events between once-daily and twice-daily dosing regimens (Mainbourg et al., 2021). On the one hand, the pharmacodynamic effects of rivaroxaban are closely correlated with pharmacokinetic profiles (Kubitza et al., 2005a; Kubitza et al., 2005b). Research has shown that once-daily dosing regimens cause greater fluctuation in the rivaroxaban plasma concentration than twice-daily dosing (the maximum plasma concentration is 20% higher and the minimum is 60% lower (Mueck et al., 2011)). On the other hand, the Factor Xa activity and prolongation of prothrombin time returned to within 10% of baseline values 24 h after taking the rivaroxaban tablet (Kubitza et al., 2013). Therefore, twice-daily dosing of rivaroxaban provides better continuity of the drug plasma level than does once-daily dosing (Vrijens and Heidbuchel, 2015). In summary, compared with once-daily dosing, twice-daily dosing of rivaroxaban may be a better choice for preventing bleeding events and thrombosis after LAAC.
Twice-daily dosing of rivaroxaban has been studied in other cardiovascular diseases. A phase II trial of rivaroxaban in proximal deep vein thrombosis showed that more patients receiving rivaroxaban at 20 mg twice daily improved thrombus burden compared to patients receiving rivaroxaban once daily at the same total dose (Agnelli et al., 2007). In a randomized study of acute coronary artery disease, participants in separate groups received different total doses of rivaroxaban (5, 10, or 20 mg) by a once-daily or twice-daily regimen. The rate of clinically significant bleeding was higher in the once-daily dosing groups than in the twice-daily dosing groups, although no statistically significant difference was observed. Nevertheless, because of the lower troughs and higher peaks of plasma concentration associated with once-daily dosing, the twice-daily regimen (2.5 mg twice daily) was chosen for a phase III clinical trial and subsequently as a long-term antithrombotic treatment for secondary prevention in patients with coronary artery disease (Mega et al., 2009; Collet et al., 2020). In a noninferiority, open-label, event-driven phase II trial, 2420 patients with acute symptomatic pulmonary embolism received rivaroxaban at 15 mg twice daily for 21 days followed by 20 mg once daily. Compared with the vitamin K antagonist group, the rivaroxaban group showed noninferior primary efficacy and a reduced major bleeding rate (Kuuskne and Dankoff, 2014). However, twice-daily rivaroxaban has not been studied in patients after LAAC. Our study investigated the effectiveness and safety of a twice-daily rivaroxaban regimen in patients with AF after LAAC.
DRT is considered to develop secondary to exposure of the occluder to the circulation and is one of the main factors adversely affecting the benefit of LAAC. The PROTECT-AF study, which initially used postoperative antithrombotic therapy with warfarin, showed a DRT rate of 4.2% (20/478) (Holmes et al., 2009). In a subsequent study of DRT that analyzed 1739 patients from the PROTECT, PREVAIL, CAP, or CAP2 studies, the overall incidence of DRT was 3.7% (Dukkipati et al., 2018). The incidence of DRT after LAAC followed by non-vitamin K oral anticoagulant (NOAC) therapy in the EWOLUTION study (Bergmann et al., 2017) and in a study by Enomoto et al. (Enomoto et al., 2017) was 1.3% (1/77) and 0.9% (2/214), respectively, which was comparable to the incidence in the BID group of the present study (2/148, 1.4%) with a similar duration of follow-up. Notably, these data were obtained only for the 3-month postoperative follow-up period in the EWOLUTION study (Bergmann et al., 2017), and 60% of the patients in the study by Enomoto et al. (Enomoto et al., 2017) were followed up for only 45 days postoperatively; these factors may have resulted in a lower rate of DRT. The prospective ASAP study reported a DRT incidence of 4.0% with dual-antiplatelet therapy (DAPT) in patients contraindicated to oral anticoagulants (Reddy et al., 2013). The 3-month data from the EWOLUTION registry showed that 60.2% patients treated with DAPT had a DRT rate of 3.1% and 7.0% patients treated with single-antiplatelet therapy had a DRT rate of 3.8% (Bergmann et al., 2017), which was higher than the BID group in the present study. In our study, DRT was detected in two patients receiving the twice-daily rivaroxaban regimen and in two patients receiving the once-daily rivaroxaban regimen. Although not statistically different, the incidence of DRT was lower in the BID group than in the QD group.
The etiology of DRT remains incompletely understood. In our study, the reasons for the occurrence of DRT are unclear because most patients were highly compliant and achieved complete sealing of the LAA with a well-positioned device. Nevertheless, a study of DRT identified that DRT is closely related to a history of stroke/transient ischemic attack, a larger LAA diameter, a lower left ventricular ejection fraction, vascular disease, and permanent AF (Dukkipati et al., 2018). In addition, in two studies examining DRT, the researchers found that a dead-end structure may increase thrombogenicity and serve as a nidus for thrombus formation (Sedaghat et al., 2017; Pracon et al., 2018). Intraprocedural three-dimensional echocardiography or cardiac computed tomography may help to achieve complete coverage of the LAA ostium (Wunderlich et al., 2015; Wang et al., 2016). Moreover, close clinical follow-up and echocardiography should be performed for patients with a high risk of DRT.
Bleeding risk is inevitable with postoperative anticoagulation. A rational regimen for LAAC requires a balance between prevention of thromboembolic events and reduction of bleeding events. Several clinical studies have explored the safety of NOAC therapy after LAAC. The EWOLUTION study showed that the rate of major bleeding was lower in patients receiving NOACs (1.9%) than in those receiving warfarin (2.0%) (Bergmann et al., 2017). In a retrospective study by Zhu and Xu (Zhu and Xu, 2021), although the rates of major bleeding and thromboembolic events were similar between the NOAC and warfarin groups, the patients in the NOAC group had a significantly lower rate of minor bleeding events (5% vs. 30%, p < 0.01). In addition, Gu et al. (Gu et al., 2020) explored the safety and efficacy of rivaroxaban after LAAC, and Bösche et al. (Bösche et al., 2015) compared the effectiveness of NOACs with DAPT. However, the event rates in both studies were low because of the small number of cases and the short follow-up period (Bösche et al., 2015; Gu et al., 2020). Regarding antiplatelet regimens, DAPT was found to have a 2.3-fold increase in bleeding events compared to NOAC in the real-world study, while single-antiplatelet therapy had a 4.4-fold, although this was influenced by patient selection (Bergmann et al., 2017). In the study by Reddy et al., DAPT had an estimated annual bleeding rate of 6.6% (Reddy et al., 2013). In our study, the rate of all bleeding events was lower in the BID than QD group (8.6% vs. 11.9%, p = 0.37), but not significantly. The rate of major bleeding events in the rivaroxaban BID group was lower than in previous studies but was not significantly different from that in the QD group (0.5% vs. 0.8%, p = 1.00). Notably, the patient with major bleeding in the BID group of our study had undergone the one-stop procedure, and the major bleeding event was mainly attributed to operative injury caused by catheter ablation. By contrast, the patient in the QD group developed cerebral hemorrhage. Compared with serious adverse events such as stroke and major bleeding, minor bleeding events are not critical to the benefit of procedure. A few studies have documented them and there is a wide variation in incidence rates. Nonetheless, the rate of minor bleeding event in the BID group in this study was lower than the QD group without a significant difference (8.2% vs. 11.0%, p = 0.39). These findings suggest potentially better effects of twice-daily rivaroxaban in terms of reducing the incidence of bleeding events.
Except for percutaneous LAAC, other devices such as Lariat (SentreHEART, Inc., Redwood City, CA) and AtriClip (AtriCure, Inc., West Chester, OH) are used for epicardial exclusion of LAA. Both procedures do not require postoperative anticoagulant medications due to the absence of intracardiac devices and can be performed with acceptable rates of adverse events and success. However, both procedures are considered more complicated than LAAC and require general anesthesia. LAAC is more appealing to patients, especially those without concurrent cardiac surgery (Di Biase et al., 2024).
The main limitations of this study are its single-center retrospective nature, limited sample size, and relatively low event rates. Another limitation is that our study involved patients who underwent the one-stop procedure and LAAC only to investigate the effect of taking rivaroxaban twice daily, which may have affected the rates of DRT and thrombotic events. Furthermore, the incidence of DRT may have been underestimated because of the possibility of undetected small DRT and the low frequency of TEE monitoring. Finally, the Watchman and LAmbre devices were used for LAAC in this study, and the results should not be generalized to other LAAC systems. In summary, the superiority of this anticoagulation regimen needs to be confirmed by further large-sample real-world studies and randomized trials.
The outcomes of this study suggest that taking rivaroxaban twice daily after LAAC can prevent bleeding complications without increasing the risk of DRT and thromboembolic complications and might be a viable anticoagulation alternative for patients with AF after LAAC.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Y-QP: Writing–original draft, Writing–review and editing, Conceptualization, Data curation, Supervision. L-SJ: Writing–original draft, Writing–review and editing, Conceptualization, Methodology. SQ: Writing–original draft, Writing–review and editing, Conceptualization, Methodology. TJ: Investigation, Writing–review and editing. Z-NW: Investigation, Supervision, Writing–review and editing. Y-LC: Investigation, Writing–review and editing. Y-XQ: Data curation, Writing–review and editing. Y-HW: Visualization, Writing–review and editing. J-YF: Visualization, Writing–review and editing. LL: Software, Writing–review and editing. Y-NL: Writing–review and editing. Y-CL: Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank all the volunteers who participated in this study. We also thank Angela Morben, DVM, ELS, from Liwen Bianji (Edanz) (www.liwenbianji.cn), for editing the English text of a draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agnelli, G., Gallus, A., Goldhaber, S. Z., Haas, S., Huisman, M. V., Hull, R. D., et al. (2007). Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (oral direct factor Xa inhibitor BAY 59-7939 in patients with acute symptomatic deep-vein thrombosis) study. Circulation 116, 180–187. doi:10.1161/CIRCULATIONAHA.106.668020
Bergmann, M. W., Betts, T. R., Sievert, H., Schmidt, B., Pokushalov, E., Kische, S., et al. (2017). Safety and efficacy of early anticoagulation drug regimens after WATCHMAN left atrial appendage closure: three-month data from the EWOLUTION prospective, multicentre, monitored international WATCHMAN LAA closure registry. EuroIntervention 13, 877–884. doi:10.4244/EIJ-D-17-00042
Blackshear, J. L., and Odell, J. A. (1996). Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 61, 755–759. doi:10.1016/0003-4975(95)00887-X
Bösche, L. I., Afshari, F., Schöne, D., Ewers, A., Mügge, A., and Gotzmann, M. (2015). Initial experience with novel oral anticoagulants during the first 45 Days after left atrial appendage closure with the watchman device. Clin. Cardiol. 38, 720–724. doi:10.1002/clc.22478
Collet, J.-P., Thiele, H., Barbato, E., Barthélémy, O., Bauersachs, J., Bhatt, D. L., et al. (2020). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 42, 1289–1367. doi:10.1093/eurheartj/ehaa575(2021)
Di Biase, L., Lakkireddy, D. J., Marazzato, J., Velasco, A., Diaz, J. C., Navara, R., et al. (2024). Antithrombotic therapy for Patients Undergoing cardiac electrophysiological and Interventional Procedures: JACC state-of-the-art review. J. Am. Coll. Cardiol. 83, 82–108. doi:10.1016/j.jacc.2023.09.831
Dukkipati, S. R., Kar, S., Holmes, D. R., Doshi, S. K., Swarup, V., Gibson, D. N., et al. (2018). Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation 138, 874–885. doi:10.1161/CIRCULATIONAHA.118.035090
Enomoto, Y., Gadiyaram, V. K., Gianni, C., Horton, R. P., Trivedi, C., Mohanty, S., et al. (2017). Use of non-warfarin oral anticoagulants instead of warfarin during left atrial appendage closure with the Watchman device. Heart rhythm. 14, 19–24. doi:10.1016/j.hrthm.2016.10.020
Gu, Z.-C., Qiao, Z. Q., Hao, Z. Y., Li, Z., Jiang, L. S., Ge, H., et al. (2020). Initial anticoagulation experience with standard-dose rivaroxaban after Watchman left atrial appendage occlusion. Ann. Transl. Med. 8, 105. doi:10.21037/atm.2019.12.116
Holmes, D. R., Reddy, V. Y., Turi, Z. G., Doshi, S. K., Sievert, H., Buchbinder, M., et al. (2009). Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374, 534–542. doi:10.1016/S0140-6736(09)61343-X
Ke, J.-Y., Jin, L. S., Lin, Y. N., Xu, J., Liu, W. K., Fu, J. Y., et al. (2022). Combined atrial fibrillation ablation and left atrial appendage closure: Watchman vs LAmbre devices. Front. Cardiovasc Med. 9, 1011037. doi:10.3389/fcvm.2022.1011037
Kubitza, D., Becka, M., Roth, A., and Mueck, W. (2013). The influence of age and gender on the pharmacokinetics and pharmacodynamics of rivaroxaban--an oral, direct Factor Xa inhibitor. J. Clin. Pharmacol. 53, 249–255. doi:10.1002/jcph.5
Kubitza, D., Becka, M., Voith, B., Zuehlsdorf, M., and Wensing, G. (2005a). Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin. Pharmacol. Ther. 78, 412–421. doi:10.1016/j.clpt.2005.06.011
Kubitza, D., Becka, M., Wensing, G., Voith, B., and Zuehlsdorf, M. (2005b). Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur. J. Clin. Pharmacol. 61, 873–880. doi:10.1007/s00228-005-0043-5
Kuuskne, M., and Dankoff, J. (2014). Oral rivaroxaban for the treatment of symptomatic pulmonary embolism: are we ready? CJEM 16, 155–157. doi:10.2310/8000.2013.131073
Lam, Y.-Y. (2013). A new left atrial appendage occluder (Lifetech LAmbre Device) for stroke prevention in atrial fibrillation. Cardiovasc. Revascularization Med. 14, 134–136. doi:10.1016/j.carrev.2013.04.003
Mainbourg, S., Cucherat, M., Provencher, S., Bertoletti, L., Nony, P., Gueyffier, F., et al. (2021). Twice- or once-daily dosing of direct oral anticoagulants, a systematic review and meta-analysis. Thromb. Res. 197, 24–32. doi:10.1016/j.thromres.2020.10.011
Mega, J. L., Braunwald, E., Mohanavelu, S., Burton, P., Poulter, R., Misselwitz, F., et al. (2009). Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet 374, 29–38. doi:10.1016/S0140-6736(09)60738-8
Mueck, W., Lensing, A. W. A., Agnelli, G., Decousus, H., Prandoni, P., and Misselwitz, F. (2011). Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 50, 675–686. doi:10.2165/11595320-000000000-00000
Pracon, R., Bangalore, S., Dzielinska, Z., Konka, M., Kepka, C., Kruk, M., et al. (2018). Device thrombosis after percutaneous left atrial appendage occlusion is related to patient and procedural characteristics but not to duration of postimplantation dual antiplatelet therapy. Circ. Cardiovasc Interv. 11, e005997. doi:10.1161/CIRCINTERVENTIONS.117.005997
Reddy, V. Y., Möbius-Winkler, S., Miller, M. A., Neuzil, P., Schuler, G., Wiebe, J., et al. (2013). Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J. Am. Coll. Cardiol. 61, 2551–2556. doi:10.1016/j.jacc.2013.03.035
Sedaghat, A., Schrickel, J. W., Andrié, R., Schueler, R., Nickenig, G., and Hammerstingl, C. (2017). Thrombus Formation after left atrial appendage occlusion with the amplatzer amulet device. JACC Clin. Electrophysiol. 3, 71–75. doi:10.1016/j.jacep.2016.05.006
Sick, P. B., Schuler, G., Hauptmann, K. E., Grube, E., Yakubov, S., Turi, Z. G., et al. (2007). Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J. Am. Coll. Cardiol. 49, 1490–1495. doi:10.1016/j.jacc.2007.02.035
Tzikas, A., Holmes, D. R., Gafoor, S., Ruiz, C. E., Blomström-Lundqvist, C., Diener, H. C., et al. (2017). Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 19, 4–15. doi:10.1093/europace/euw141
Vrijens, B., and Heidbuchel, H. (2015). Non-vitamin K antagonist oral anticoagulants: considerations on once-vs. twice-daily regimens and their potential impact on medication adherence. Europace 17, 514–523. doi:10.1093/europace/euu311
Wang, D. D., Eng, M., Kupsky, D., Myers, E., Forbes, M., Rahman, M., et al. (2016). Application of 3-dimensional computed tomographic image guidance to WATCHMAN implantation and impact on Early operator learning curve: single-center experience. JACC Cardiovasc. Interv. 9, 2329–2340. doi:10.1016/j.jcin.2016.07.038
Wunderlich, N. C., Beigel, R., Swaans, M. J., Ho, S. Y., and Siegel, R. J. (2015). Percutaneous interventions for left atrial appendage exclusion: options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc. Imaging 8, 472–488. doi:10.1016/j.jcmg.2015.02.002
Keywords: rivaroxaban, left atrial appendage occlusion, atrial fibrillation, bleeding events, device-related thrombosis, stroke
Citation: Pan Y-Q, Jin L-S, Qian S, Jiang T, Wang Z-N, Chen Y-L, Qiu Y-X, Wu Y-H, Fu J-Y, Li L, Lin Y-N and Li Y-C (2024) Twice-daily rivaroxaban after percutaneous left atrial appendage closure for atrial fibrillation. Front. Pharmacol. 15:1344828. doi: 10.3389/fphar.2024.1344828
Received: 26 November 2023; Accepted: 02 February 2024;
Published: 22 February 2024.
Edited by:
Antonio Lax, University of Murcia, SpainReviewed by:
Zefferino Palamà, Università degli Studi dell'Aquila, ItalyCopyright © 2024 Pan, Jin, Qian, Jiang, Wang, Chen, Qiu, Wu, Fu, Li, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue-Chun Li, bGl5dWVjaHVuMTk4MEBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.