
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 March 2024
Sec. Drugs Outcomes Research and Policies
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1340255
This article is part of the Research Topic Recent Advances in Attempts to Improve Medication Adherence – from basic research to clinical practice, volume II View all 19 articles
 Catalina Lizano-Barrantes1,2,3
Catalina Lizano-Barrantes1,2,3 Olatz Garin1,2,4*
Olatz Garin1,2,4* Karina Mayoral4,5
Karina Mayoral4,5 Alexandra L. Dima4,6
Alexandra L. Dima4,6 Angels Pont1,4
Angels Pont1,4 María Araceli Caballero-Rabasco2,7
María Araceli Caballero-Rabasco2,7 Manuel Praena-Crespo8,9
Manuel Praena-Crespo8,9 Laura Valdesoiro-Navarrete10,11
Laura Valdesoiro-Navarrete10,11 María Teresa Guerra9,12
María Teresa Guerra9,12 Alberto Bercedo-Sanz9,13
Alberto Bercedo-Sanz9,13 Montse Ferrer1,2,4*on behalf of the ARCA Group
Montse Ferrer1,2,4*on behalf of the ARCA GroupIntroduction: We aimed to evaluate the longitudinal relationships, both at between- and within-person levels, that adherence to inhaled corticosteroid-based maintenance treatment and inhalation technique present with symptom control, exacerbations, and health-related quality of life (HRQoL) in children and adolescents with asthma.
Methods: Participants (6–14 years old) from the ARCA (Asthma Research in Children and Adolescents) cohort—a prospective, multicenter, observational study (NCT04480242)—were followed for a period from 6 months to 5 years via computer-assisted telephone interviews and a smartphone application. The Medication Intake Survey–Asthma (MIS-A) was administered to assess the implementation stage of adherence, and the Inhalation Technique Questionnaire (InTeQ) was used to assess the five key steps when using an inhaler. Symptom control was measured with the Asthma Control Questionnaire (ACQ), and HRQL was measured with the EQ-5D and the Patient-Reported Outcomes Measurement Information System–Pediatric Asthma Impact Scale (PROMIS-PAIS). Multilevel longitudinal mixed models were constructed separately with symptom control, exacerbation occurrence, EQ-5D, and PROMIS-PAIS as the dependent variables.
Results: Of the 360 participants enrolled, 303 (1,203 interviews) were included in the symptom control and exacerbation analyses, 265 (732) in the EQ-5D, and 215 (617) in the PROMIS-PAIS. Around 60% of participants were male subjects, and most of them underwent maintenance treatment with inhaled corticosteroids plus long-acting β-agonists in a fixed dose (73.3%). Within-person variability was 83.6% for asthma control, 98.6% for exacerbations, 36.4% for EQ-5D, and 49.1% for PROMIS-PAIS. At the within-person level, patients with higher adherence had better symptom control (p = 0.002) and HRQoL over time (p = 0.016). Patients with a better inhalation technique reported worse HRQoL simultaneously (p = 0.012), but they showed better HRQoL in future assessments (p = 0.012). The frequency of reliever use was associated with symptom control (p < 0.001), exacerbation occurrence (p < 0.001), and HRQoL (p = 0.042); and boys were more likely to present better symptom control and HRQoL than girls.
Conclusion: Our results confirm longitudinal associations at the within-person level of the two indicators of quality use of inhalers: for adherence to maintenance treatment with symptom control and HRQoL, and for the inhalation technique with HRQoL. Although treatment adherence was shown to be excellent, a third of the participants reported a suboptimal inhalation technique, highlighting the need for actions for improving asthma management of the pediatric population.
Asthma is the most common non-communicable disease in school-aged children (Bercedo Sanz et al., 2022; The Global Asthma Report, 2022) and a major public health problem worldwide (Asher et al., 2021; Bercedo Sanz et al., 2022; Song et al., 2022). In 2019, an estimated 12,900 deaths occurred and 5.1 million disability-adjusted life years were lost due to childhood asthma (Zhang and Zheng, 2022). According to the latest global report, only 44.1% of children and 55.4% of adolescents with asthma achieved a well-controlled disease stage (García-Marcos et al., 2023).
Childhood asthma is a heterogeneous and fluctuating disease, with symptoms that vary in time and intensity (Global Initiative for Asthma; von Mutius and Smits, 2020). Therefore, management is mainly based on a continuous personalized cycle of assessment of asthma control (symptom control and risk factors for future exacerbations), any comorbidities that could contribute to symptom burden and poor health-related quality of life (HRQoL), and treatment (Global Initiative for Asthma, 2023). Intake of daily inhaled corticosteroids (ICS) is the currently recommended pharmacologic maintenance therapy in individuals of all ages (Montuschi and Barnes, 2011; National Heart Lung and Blood Institute (NHLBI), 2020; Global Initiative for Asthma, 2023). Research has shown that adherence to ICS and inhalation technique are dynamic and complex (Vrijens et al., 2016; Azzi et al., 2017; Almomani et al., 2021), with studies indicating generally low adherence to maintenance medication (20%–70%) (Herndon et al., 2012; Boutopoulou et al., 2018) and suboptimal inhalation technique (8%–22%) (Gillette et al., 2016) in children and/or adolescents.
Most evidence from systematic reviews suggests that whether it is children (Everhart and Fiese, 2009; Engelkes et al., 2015; Silva et al., 2015; Kocks et al., 2018; Usmani et al., 2018), adolescents (Engelkes et al., 2015; Silva et al., 2015; Kocks et al., 2018; Usmani et al., 2018; Vazquez-Ortiz et al., 2020; Roche et al., 2022), or adults (Bårnes and Ulrik, 2015; Engelkes et al., 2015; Kocks et al., 2018; Usmani et al., 2018; Vazquez-Ortiz et al., 2020; Roche et al., 2022), higher levels of adherence (Bårnes and Ulrik, 2015; Engelkes et al., 2015; Vazquez-Ortiz et al., 2020) and better inhalation technique (Kocks et al., 2018; Usmani et al., 2018; Roche et al., 2022), analyzed separately, are associated with better outcomes (symptom control, exacerbations, and/or HRQoL), although an inverse or null association has also been found (Bårnes and Ulrik, 2015; Engelkes et al., 2015; Kocks et al., 2018; Usmani et al., 2018; Vazquez-Ortiz et al., 2020; Roche et al., 2022). Furthermore, impaired HRQoL has also been linked with asthma-associated factors, such as severity (Everhart and Fiese, 2009), disease control, and exacerbations (Silva et al., 2015; Vazquez-Ortiz et al., 2020) in children, adolescents, and young adults.
These systematic reviews (Bårnes and Ulrik, 2015; Engelkes et al., 2015; Silva et al., 2015; Kocks et al., 2018; Usmani et al., 2018; Vazquez-Ortiz et al., 2020; Roche et al., 2022) show that more than 160 studies have been conducted involving patients with asthma that evaluated the relationships between adherence, inhalation technique, asthma control, asthma exacerbation, and/or HRQoL. However, only 22 of these studies included longitudinal analyses, with nine focusing exclusively on children and/or adolescents (Bukstein et al., 2007; Camargo et al., 2007; Delea et al., 2008; Hagmolen of ten Have et al., 2008; Lasmar et al., 2009; Elkout et al., 2012; Herndon et al., 2012; Krishnan et al., 2012; Tiggelman et al., 2015) and 13 encompassing adults as well (Osman et al., 1999; Balkrishnan and Christensen, 2000; McMahon et al., 2000; Stern et al., 2006; Santos et al., 2008; McNally et al., 2009; Smith et al., 2009; Mattke et al., 2010; Rohan et al., 2010; Sundell et al., 2011; Williams et al., 2011; Hyland et al., 2012; Yildiz et al., 2014). Notably, none of them considered the temporal stages of adherence (initiation, implementation, and discontinuation) described in 2012 (Vrijens et al., 2012). Although the systematic reviews were published between 2015 and 2022, none of the studies including longitudinal analyses, were conducted after 2013. Therefore, they probably do not reflect the Food and Drug Administration (FDA) requirement changes, contraindicating the use of long-acting beta-agonists (LABAs) without concurrent ICS (Chowdhury and Dal Pan, 2010).
The Global Asthma Initiative Guideline (GINA) (Global Initiative for Asthma, 2023) has continued to incorporate changes due to the collection of new evidence related to the efficacy and safety of ICS, LABA, and short-acting beta-agonists (SABAs). More recent longitudinal studies (Azzi et al., 2017; Johnson et al., 2017; Souverein et al., 2017; Papi et al., 2018; Dima et al., 2019; Vervloet et al., 2020; Vervloet et al., 2022; Hale et al., 2023; Sousa-Pinto et al., 2023) have presented further evidence of the long-term role of ICS adherence in asthma. However, none were conducted specifically on a pediatric population, only one included HRQoL (Hale et al., 2023), few specified the adherence stage considered (Souverein et al., 2017; Dima et al., 2019; Vervloet et al., 2020; Vervloet et al., 2022), and only one included medication adherence alongside the inhalation technique (Hale et al., 2023). These last two concepts are closely related, with the poor inhalation technique even being considered an unintentional form of adherence (van Boven et al., 2015), but they are usually identified as independent concepts (Monteiro et al., 2022). Two of the aforementioned systematic reviews (Engelkes et al., 2015; Kocks et al., 2018) have highlighted the scarcity of studies evaluating the impact of adherence and inhalation technique, assessed together, on asthma outcomes, despite the association that has been observed between them (Giraud et al., 2011; Maricoto et al., 2020).
A deeper insight into how adherence and inhaler technique evolve over time and affect the clinical outcomes and HRQoL in children could foster a ‘quality use of medications’ strategy (Braido et al., 2016), aligning with the current guidelines. Therefore, we aimed to evaluate the longitudinal relationships, both at between- and within-person levels, that adherence to ICS (alone or in combination with LABA) and inhalation technique present with symptom control, exacerbations, and HRQoL in children and adolescents with asthma.
Asthma Research in Children and Adolescents (ARCA) is a longitudinal, prospective, multicenter, observational study (NCT04480242) designed to provide evidence about the evolution of young patients with persistent asthma through regular follow-ups.
Patients were consecutively recruited from five outpatient pediatric pulmonology hospital units and nine primary care pediatric centers in Spain from January 2018 to March 2023 and were thus followed for a period from 6 months to 5 years. The inclusion criteria were as follows: aged 6–14, with a clinical diagnosis of asthma (history of characteristic symptoms and objective signs of variable airflow limitation) (Global Initiative for Asthma, 2017), undergoing treatment with ICS (alone or combined with LABA) for more than 6 months in the previous year, no concomitant respiratory diseases, and with access to a smartphone (their own or their parents’). Written informed consent was requested from the parents or legally authorized representatives of all participants, and additionally, oral consent was obtained from the children.
The participants were followed via the ARCA smartphone application (Mayoral et al., 2021) monthly and via computer-assisted telephone interviews (CATIs) performed by trained interviewers at enrollment, every 6 months (regular CATIs), and after each exacerbation (post-exacerbation CATIs). The ARCA application is available in three age versions: proxy response for children aged 6–7 years and self-response for participants aged 8–11 and ≥12 years. Through the application, participants reported any new exacerbations and completed the HRQoL instruments. Two versions of the CATIs were administered, one for parents or guardians of children under 8 years old (proxy response) and one for participants aged 8 and older (self-response). CATIs collected information on asthma symptom control, exacerbations, asthma treatments (maintenance and reliever), adherence to maintenance medication, inhalation technique, reliever use, and exacerbation occurrence for the period immediately before the interview. Demographic and clinical information was collected from medical records at enrollment.
For this analysis, we selected participants who had valid registries of at least two CATIs during a period with an ICS-based treatment prescribed for regular use (maintenance).
The ESPACOMP Medication Adherence Reporting Guideline (EMERGE) was followed (Adams N. P. et al., 2005).
Medication information was collected at every CATI, including the active drug component, dose, type of inhaler device (pressurized metered-dose inhaler—pMDI and dry-powder inhaler—DPI) for the maintenance treatment, and the frequency of reliever medication use. Maintenance treatment was grouped into two categories: ICS in a fixed-dose combination with LABA (ICS plus LABA) and single ICS inhaler. Both categories were classified following the GINA preferred track steps, according to the ICS dose (low/medium/high) (Global Initiative for Asthma, 2023). The frequency of reliever medication use was measured with the following question: How often have you usually taken your “reliever medication” (brand name) in the past 4 weeks: every day, almost every day, once or twice every week, or less than once a week? This variable was grouped into the following: almost never (participants with no SABA prescribed and those reporting used less than once a week) and usually (participants reporting the first three response options).
Medication adherence was measured with the Medication Intake Survey–Asthma (MIS-A) (Dima et al., 2017), a validated instrument for telephone interviews, which assesses the implementation stage of adherence separately for each maintenance inhaler based on the self-reported prescription start date, daily dosage recommendations, and questions on maintenance use over increasing periods. Percentages of used versus prescribed medication are calculated first for each question and, subsequently, as composite scores. We used 1-month composite scores based on inhalations used the day before (Q1), days on which no inhalations were taken in the past 7 days (Q2), days on which all prescribed inhalations were used in the past 7 days (Q3), and days on which all prescribed inhalations were used in the past 28 days (Q4). MIS-A was administered at enrollment and at every 6 months in the regular CATIs and in the post-exacerbation CATIs. When patients used more than one inhaler containing ICS, we computed scores for each inhaler and averaged across them. MIS-A has been validated (Dima et al., 2017) using self-response in adult patients and teenagers and a proxy version for the caregivers of children in English and French. The MIS-A was linguistically adapted into Spanish for the pediatric population within the ARCA study, according to the recommended methodology (double direct translation, translation synthesis, back-translation, and cognitive debriefing) (Wild et al., 2005).
The inhalation technique was measured with the Inhaler Technique Questionnaire (InTeQ) (Lizano-Barrantes et al., 2022; Lizano-Barrantes et al., 2023a), an instrument that assesses the frequency of performing five key steps when using the inhaler in the previous 6 months with a five-level Likert scale (from “always” to “never”). The InTeQ was administered in the CATIs at enrollment and yearly. A global score was calculated as a sum of the InTeQ items answered “always,” among the four, which demonstrated unidimensionality in children and adolescents (Lizano-Barrantes et al., 2023a), and was categorized into the following: 4–3 (good inhaler technique), 2 (fair), and 1–0 (poor). The InTeQ has been validated for telephone interviews (Lizano-Barrantes et al., 2023a) using self-response in children aged 8 and older and proxy response for parents or guardians of children under 8 years old. As the InTeQ was only administered yearly, the missing values were replaced by data from the previous interview.
Symptom control was measured with the asthma control questionnaire (ACQ)– symptoms only (Juniper et al., 2005a), which was administered in the regular and post-exacerbation CATIs. It assesses the presence and intensity of night-time waking, symptoms on waking, activity limitation, shortness of breath, and wheezing during the previous week on a 7-level Likert scale from 0 (no impairment) to 6 (maximum impairment). The overall score, calculated as the mean item responses, ranges from 0 to 6. Cut-off points of 1.5 and 0.75 were established to define not well- and well-controlled asthma, respectively (Juniper et al., 2006). The ACQ has been validated (Juniper et al., 2005b) using self-administration in adolescents and interviewer administration in children.
Asthma exacerbations were identified in the regular CATIs administered every 6 months or by reporting them through the application, which prompted an alert to the research team that was followed by a post-exacerbation CATI to confirm its occurrence. In both cases, exacerbations were defined through three questions that were constructed applying the definitions by the American Thoracic Society and the European Respiratory Society (Reddel et al., 2009): Did you visit or phone your family doctor or outpatient emergency department because your asthma got worse? Did you call an ambulance or go to the hospital because of your asthma? Did you take steroid tablets or syrup (such as prednisolone or Deltacortril) for at least 2 days because of your asthma? If the participant answers “yes” to at least one of the three questions, an asthma exacerbation is confirmed.
Health-related quality of life (HRQoL) was measured using two complementary instruments, the EuroQol generic questionnaire (EQ-5D) (Ravens-Sieberer et al., 2010; Wille et al., 2010; Gusi et al., 2014) and the disease-specific questionnaire Patient-Reported Outcomes Measurement Information System–Pediatric Asthma Impact Scale (PROMIS-PAIS) (Yeatts et al., 2010), which were administered through the ARCA application. The EQ-5D was administered at enrollment and every 6 months. It consists of five dimensions, namely, “mobility,” “looking after myself,” “doing usual activities,” “having pain/discomfort,” and “feeling worried/sad/unhappy, with a time frame of “today.” According to the age, we used the EQ-5D-Y-3L proxy-version (6–7 years), the self-administered EQ-5D-Y-3L (8–11 years), and the self-administered EQ-5D-5L (≥12 years). A single preference-based utility index was calculated ranging from 1 (the best health state) to negative values (health states valued by society as worse than death), where 0 is equal to death. Preference value sets applied to generate this utility index were those obtained from Spanish adults for the EQ-5D-5L (Ramos-Goñi et al., 2018) and those obtained from Spanish adults thinking as a hypothetical 10-year-old child for the EQ-5D-Y (Ramos-Goñi et al., 2020; Ramos-Goñi et al., 2024). The short form 8a version of the PROMIS-PAIS (v2.0) was administered at 4 months from enrollment and at every 6 months thereafter. Its items ask about the past 7 days in a 5-level Likert response scale (1–5) with the following options: never, almost never, sometimes, often, and almost always. It is available for self-response for ages 8–17 and for proxy response for children starting at age 5. The total raw score is calculated by adding the values of the response to each question, ranging from 8 to 40 (a lower score indicates better HRQoL) (A brief guide to the PROMIS, 2023).
To specifically examine the impact of the implementation stage of adherence to an ICS-based maintenance treatment (i.e., the degree to which patients follow their prescribed doses during treatment), we censored from the dataset reports under certain conditions: no prescribed daily ICS at all, ICS prescribed on an as-needed basis, or prescribed other asthma maintenance treatment (such as tiotropium). Descriptive analyses were performed of patients’ follow-up, reports, patient characteristics, treatment, and outcomes by calculating the percentages or means and standard deviations. Differences between the patients included and not included in the analysis of each outcome (asthma symptom control, exacerbation, EQ-5D, and PROMIS-PAIS) were assessed with a chi-squared or t-test, according to the type of variable.
Continuous time-varying predictors (adherence and the inhalation technique) were decomposed into three variables to distinguish the between-person effects and the simultaneous and sequential within-person effects. Average adherence was calculated as the mean score for each patient across all reports (one score per patient) and used for examining whether differences in adherence between patients predict the outcomes. Current fluctuation was computed as the difference between a patient’s average adherence and their score in a given report (multiple scores per patient) to examine whether changes in adherence within patients are associated with concomitant changes in the outcome (i.e., measured in the same report). Prior fluctuation was computed as a lagged variable, i.e., the difference between a patient’s average and the score in their previous report, usually 6 months earlier (multiple scores per patient), to examine whether changes in adherence predict the outcomes measured in the subsequent report.
To assess longitudinal relationships of adherence to ICS-based maintenance treatment and the inhalation technique with outcomes, we followed established procedures for hierarchical longitudinal modeling (Singer and Willett, 2003). Four multilevel longitudinal mixed models were constructed separately for asthma symptom control, exacerbation occurrence, EQ-5D, and PROMIS-PAIS (as dependent variables). In all cases, models were constructed to assess the role of the two time-varying variables, adherence and inhalation technique (which are the main explanatory variables), including them together with the type of ICS-based maintenance treatment and sociodemographic variables that can be potential confounders (model A); then, other factors that are part of the implicit standard for asthma management were added (Dima et al., 2016; Global Initiative for Asthma, 2023), namely, the use of a reliever, asthma symptom control, and the occurrence of exacerbations, except in models where they were the dependent variables (model B). Time was modeled as years since the first interview per patient (random and fixed), and interactions between the independent variables and time were tested. In addition to the p-values of each coefficient or odds ratio (OR) provided by the models, ANOVA was applied to test the significance corresponding to each independent variable. Sensitivity analyses were performed with 1-week adherence scores.
R (version 4.2.2) and RStudio (2022.07.2 Build 576) were used to construct all the models, except for the exacerbation occurrence, which was constructed with SAS 9.4.
Out of the 360 participants enrolled from January 2018 to March 2023 (Figure 1), we excluded the following from the analysis: 10 who did not respond to any CATI, 42 with only one valid CATI, and 5 without ICS-based maintenance treatment. Then, 303 valid participants (who responded to a total of 1,203 CATIs) were included in the analysis of asthma symptom control and exacerbation, 265 participants (with 732 questionnaires completed) in the EQ-5D analysis, and 215 (with 617 questionnaires completed) in the analysis of PROMIS-PAIS. Globally, patients provided 2–9 reports (Q2 (median) = 4, Q1-Q3 = 2–5), with a mean (SD) follow-up of 692 (419) days (range 116–1,759 days) in the analyses of symptom control and exacerbation occurrence. For the EQ-5D and PROMIS-PAIS analyses, reports per patient ranged from 1 to 8, with medians of 2 (Q1–Q3 = 2–4) and 3 (Q1–Q3 = 2–4), respectively.
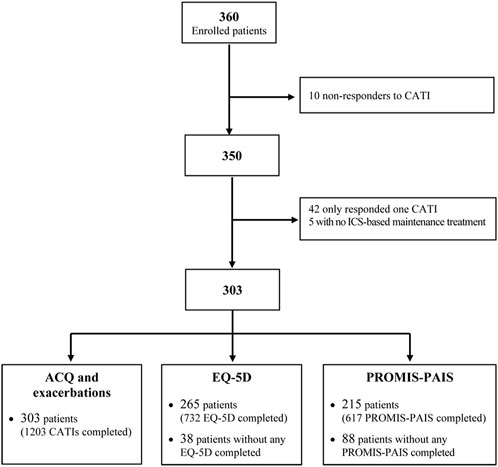
FIGURE 1. Flowchart of the selection of patients meeting the analysis criteria. CATI: computer-assisted telephone interview; ICS: inhaled corticosteroids; ACQ: Asthma Control Questionnaire; PROMIS-PAIS: Patient-Reported Outcomes Measurement Information System–Pediatric Asthma Impact Scale.
Figure 2A shows the number of patients who started follow-up in each year and were valid for analysis. For example, of the patients followed since 2018 in the ARCA cohort, 43 were included in the analysis of symptom control and exacerbation occurrence (white bar), 34 in the EQ-5D one (light gray bar), and 28 in the PROMIS-PAIS (dark gray bar) analysis. Enrollment for the study peaked in 2019 and then faced challenges in 2020 due to the SARS-CoV-2 pandemic, but it was sustained through effective mitigation efforts. The mean number of reports completed per patient is shown in Figure 2B. For instance, the 43 patients who started follow-up in 2018 provided a mean of 5.7 reports, while the 66 patients who started follow-up in 2022 provided 2.3 reports on average. These differences in the number of valid reports per participant are due to the duration of the follow-up, according to the year of enrollment, which was 1,318 vs. 299 days of median (Q2) for patients followed since 2018 and 2022, respectively, in the analyses of symptom control and exacerbation occurrence.
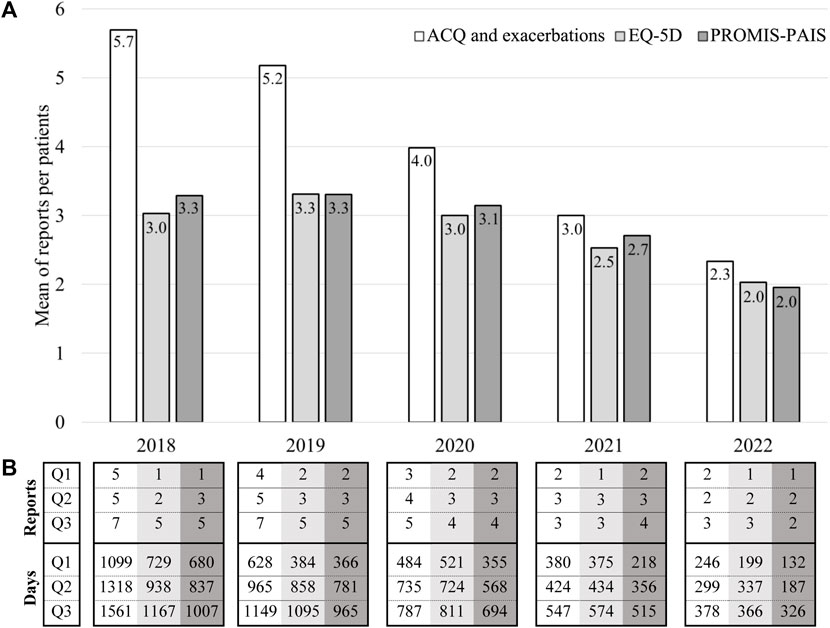
FIGURE 2. Description of patients, reports, and days analyzed by the year of follow-up initiation. ((A)-bar chart) Number of patients by the year of follow-up initiation. ((B)-table) Number of reports and days analyzed per patient by the year of follow-up initiation. n: number of patients; %: percentage of patients included in the EQ-5D and PROMIS-PAIS analyses relative to those included in the ACQ and exacerbation analyses; Q1: percentile 25; Q2: percentile 50 or median; Q3: percentile 75.
The characteristics of the participants are presented in Table 1. The majority were male subjects (60.7%), reported using relievers less than once a week (55.4%), undergoing maintenance treatment with ICS combined with LABA in a fixed dose (73.3%), administered by pMDI (74.6%), and similarly distributed among steps 2–3 (33.0%), step 4 (37.4%), and step 5 (29.5%) of the GINA preferred track, according to the dose of ICS. The mean 1-month adherence score was 87.8%; 45.2% of participants reported a good inhalation technique, and 64.2% had well-controlled symptoms. Experiencing exacerbations were reported by 37.8% of the participants. The HRQoL score measured with the EQ-5D was 0.93 (1, best health state to negative values, worse than death), and when measured with the PROMIS-PAIS, it was 13.0 (8, best health state to 40, worst).
Table 2 shows the results for the longitudinal associations that maintenance treatment adherence and the inhalation technique present with asthma symptom control (left column) and exacerbations (right column). The proportion of between-person variation was 16.4% for asthma control and 1.4% for exacerbations. Model A with asthma symptom control shows that, at the within-person level, patients reporting higher adherence to maintenance medication also reported better control in the next interview (prior fluctuation; p = 0.002). On the contrary, both models A and B show that girls (p = 0.006 and p = 0.012) were more likely to report worse control of asthma symptoms. Furthermore, model B shows that patients who reported using reliever medication ≥1–2 times per week (p < 0.001) and having an exacerbation (p < 0.001) were also more likely to present uncontrolled asthma symptoms. Age, the inhalation technique, and the type of maintenance treatment (ICS alone or in combination with LABA) did not present any statistically significant association with asthma symptom control.
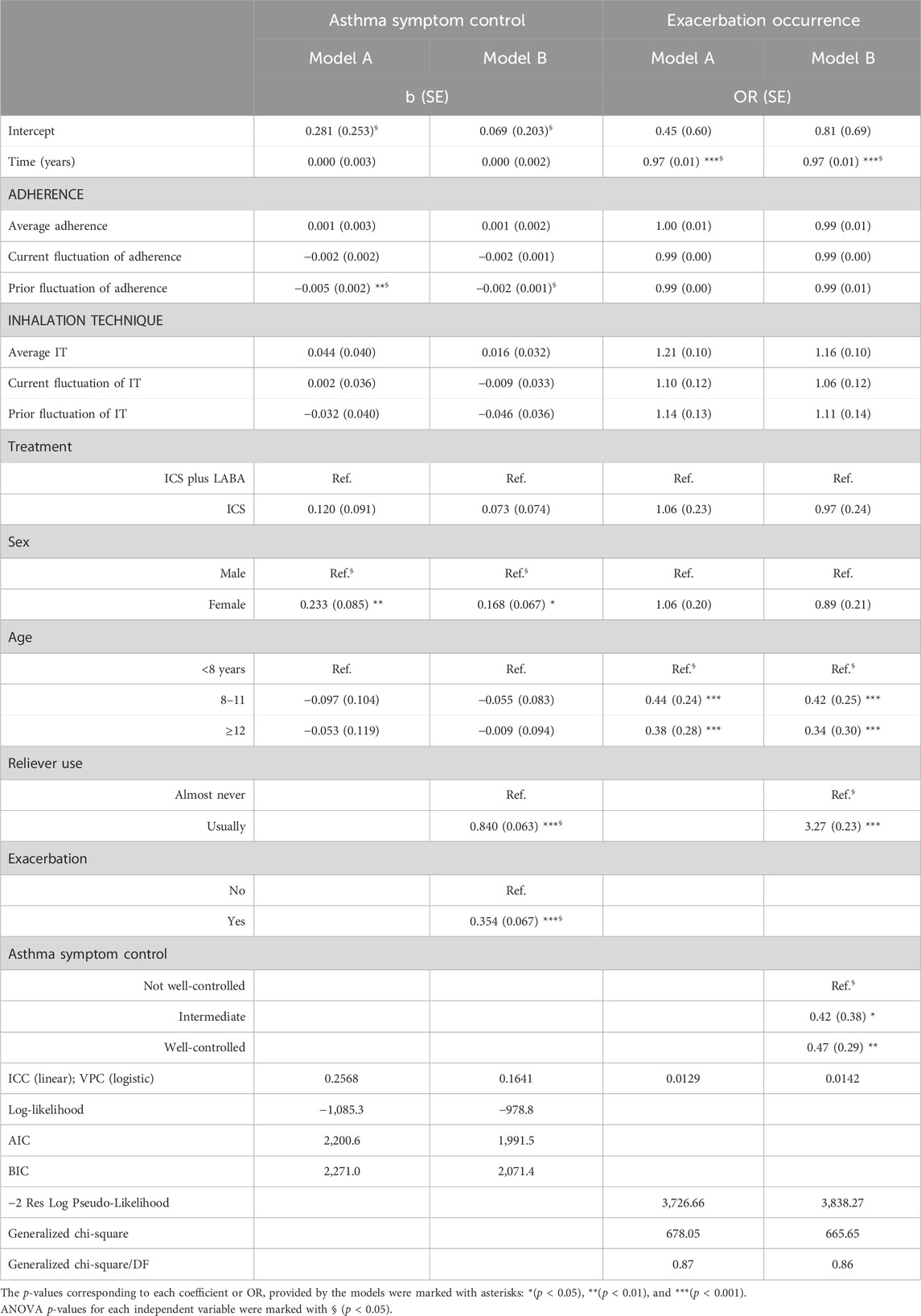
TABLE 2. Multilevel models of asthma symptom control (linear) and exacerbation occurrence (logistic).
Exacerbations models A and B show less risk of occurrence in children aged 8 years or older (p ≤ 0.001 in both models) and participants reporting better asthma symptom control (p = 0.023 and p = 0.008). Conversely, the risk of exacerbation occurrence is higher in participants reporting using reliever medication ≥1–2 times per week (p < 0.001). Neither average adherence and the inhalation technique nor their prior or simultaneous fluctuations were associated with exacerbation occurrence.
The proportion of between-person variation was 63.6% and 50.9% for HRQoL (Table 3), EQ-5D, and PROMIS-PAIS, respectively. The EQ-5D models reveal that when participants reported a better inhalation technique, they reported worse HRQoL simultaneously (current fluctuation; p = 0.012 and p = 0.012), but they also reported better HRQoL in the next interview (prior fluctuation; p = 0.005 and p = 0.012). Furthermore, worse HRQoL was more likely in girls (p = 0.037 and p = 0.036). Age, adherence, type of treatment, the use of reliever medication, and the occurrence of exacerbations were not statistically significantly associated with EQ-5D.
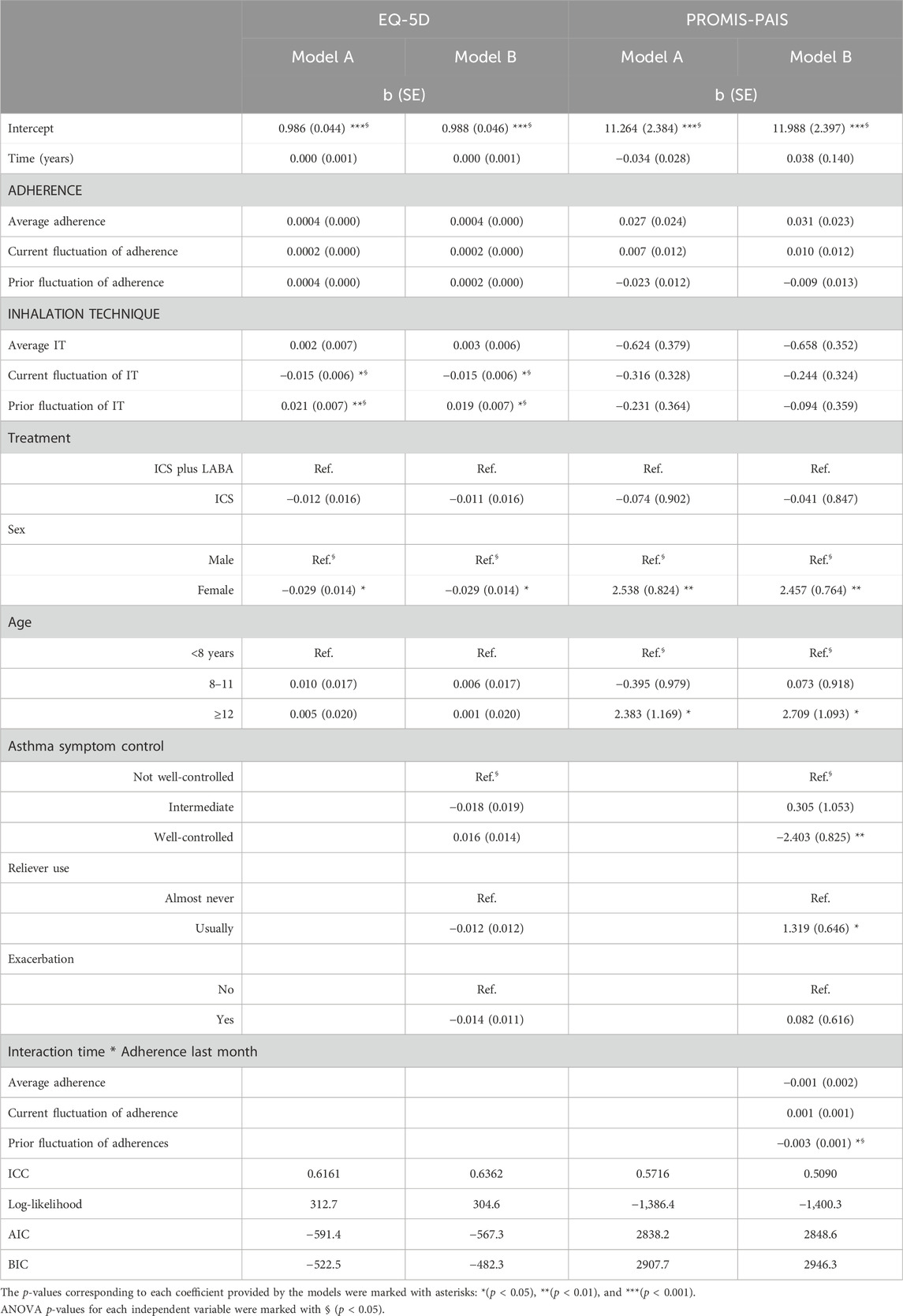
TABLE 3. Multilevel models of health-related quality of life measured with the EQ-5D and PROMIS-PAIS (linear).
In PROMIS-PAIS models, the interaction between time and adherence reveals an increase in HRQoL over time, correlating with higher levels of patient-reported adherence in subsequent interviews (prior fluctuation; p = 0.016). Furthermore, better asthma symptom control was also associated with better HRQoL (p = 0.004). Conversely, worse HRQoL was more likely for adolescents compared to children under 12 years of age (p = 0.043 and p = 0.014), girls (p = 0.002 and p = 0.002), and the use of reliever medication ≥1–2 times a week (p = 0.042). The type of maintenance treatment regimen, the inhalation technique, and exacerbation did not present a statistically significant association with PROMIS-PAIS.
Sensitivity analysis with 1-week adherence scores showed similar results (Supplementary Material).
This study provides evidence regarding the longitudinal relationships that maintenance treatment adherence and the inhaler technique present with asthma symptom control, exacerbations occurrence, and HRQoL in pediatric asthma patients. We gathered comprehensive patient-reported data using a combination of the ARCA application and CATIs. We found that better asthma symptom control over time (future assessments) was more likely in patients with higher adherence to treatment, while boys and those participants who reported almost never using reliever medication or no exacerbations generally had better symptom control. In the same direction, a lower risk of exacerbations was found in older children, those reporting well-controlled symptoms, and in those who almost never used reliever medication. Better HRQoL over time was observed in patients who reported better adherence and inhalation technique. Additionally, boys and participants with better symptom control generally had better HRQoL.
The level of adherence to maintenance treatment in ARCA participants is high on average; they reported having administered 88% of the prescribed dose during the previous month, which is above the range of 20%–70% identified by a systematic review (Herndon et al., 2012; Boutopoulou et al., 2018) in children and/or adolescents.
Consistent with our hypothesis, we found that higher adherence was associated with better asthma symptom control in future assessments, despite the inconsistent results reported both by systematic reviews (Gillette et al., 2016; Vazquez-Ortiz et al., 2020), which mainly included cross-sectional studies, and by more recent longitudinal studies (Dima et al., 2019; Vervloet et al., 2020; Vervloet et al., 2022; Sousa-Pinto et al., 2023). Consistently with our finding, a longitudinal study in French and English adults and children with asthma (Dima et al., 2019) showed that patients maintaining high ICS adherence over time have better asthma control. In the same line, a study of the large Nivel Primary Care Database in the Netherlands shows an association between poor ICS adherence and uncontrolled asthma (Papi et al., 2018). Conversely, a United Kingdom study (Vervloet et al., 2020) using the Optimum Patient Care Research Database (OPCRD) found that patients might adjust their ICS based on the current needs without this necessarily impacting later in hospitalizations, emergency visits, outpatient visits, or the need for oral corticosteroids or antibiotics. Additionally, a longitudinal study in patients from 27 countries with ICS plus LABA maintenance treatment pointed out that most patients only use medication when they are not well (Sousa-Pinto et al., 2023). Overall, these findings lead us to incorporate nuances into our hypothesis: the association between adherence and asthma control might be driven by an increased adherence as a reactive response to uncontrolled symptoms, which could eventually lead to increased symptom control over time.
The association found between increased treatment adherence and increased HRQoL over time is also consistent with our hypotheses as it could reflect an individual’s overall investment in maintaining their health and well-being through effective asthma management practices. This association was particularly identified with the asthma-specific questionnaire PROMIS-PAIS, likely due to its focused content, which is potentially more responsive to asthma symptoms (Wiebe et al., 2003). Although the specific association of adherence with HRQoL has been less frequently examined, our results are consistent with findings of a systematic review in adolescents (Usmani et al., 2018) and a multicenter, observational, prospective study in Greek adults with variable asthma severity (Exarchos et al., 2022). Additionally, a longitudinal study in Dutch adolescents (Tiggelman et al., 2015) indicated that higher HRQoL at baseline predicted increased medication adherence at follow-up, although good medication adherence did not predict an increase in HRQoL over time. These results line up with our enhanced hypothesis, distinguishing patients with regular adherence who actively integrate treatment into their daily routines, recognizing its importance, from those with “reactive adherence” who strictly follow treatments only when they feel that their asthma is out of control.
Although there is a substantial body of evidence from RCTs (Adams N. et al., 2005; Adams N. P. et al., 2005; Pauwels et al., 2003; O’byrne et al., 2001) showing that ICS-based maintenance treatment reduces exacerbation risks, there is less consistency in its association with adherence to this type of treatment. Our findings indicate a lack of association between adherence and exacerbation occurrence, which were consistent with observations from the abovementioned longitudinal studies in France, the United Kingdom, and the Netherlands (Dima et al., 2019; Vervloet et al., 2020; Vervloet et al., 2022), and a meta-analysis centered on the effect of interventions to improve adherence to ICS-based maintenance treatments, indicating that they may not always correlate with enhanced clinical outcomes (Normansell et al., 2017). Nevertheless, they contrast with a meta-analysis showing the association between treatment adherence and severe asthma exacerbations (Chongmelaxme et al., 2020). On one hand, it is important to highlight that response bias cannot be discarded in our study since interviews were performed immediately after experiencing an exacerbation, which could have made patients feel accountable, i.e., the patient’s behavior may be influenced by the expectation of social interactions with healthcare providers (Oussedik et al., 2017). On the other hand, taking into account that almost 70% of the participants in our study received a medium or high ICS dose, some of them may be candidates for a step-up in treatment, as suggested by a United Kingdom large cohort of adult patients in GINA step 3 or 4 of asthma management (Papi et al., 2018).
In our study, 32% of participants reported poor inhalation technique, which is above the proportion of the suboptimal inhalation technique reported by the studies of children and/or adolescents with asthma (8%–22%) identified in a systematic review (Gillette et al., 2016).
Given the recognized importance of both inhalation technique and adherence in impacting actual drug exposure (Global Initiative for Asthma, 2023; Ramos-Goñi et al., 2024), we hypothesized finding a similar association when both factors were analyzed together. Our results focusing on within-person fluctuations of the inhalation technique revealed that when participants temporarily improved their technique, their HRQoL decreased during that same period, but it improved afterward. This is also consistent with our hypothesis distinguishing between regular and reactive behaviors, suggesting similar patterns for inhalation technique and adherence, where a proactive approach to asthma management, even if initially challenging, ultimately contributes to enhanced HRQoL. These fluctuations are likely due to factors changing within patients with asthma over time rather than stable differences between patients, as highlighted in the longitudinal study involving French and English adults and children (Dima et al., 2019).
Three systematic reviews (Kocks et al., 2018; Usmani et al., 2018; Roche et al., 2022) supported that better inhalation technique, analyzed without considering adherence, are consistently associated with exacerbations (Usmani et al., 2018; Roche et al., 2022) and HRQoL (Kocks et al., 2018; Usmani et al., 2018; Roche et al., 2022), but there are less consistent results with asthma symptoms control (Kocks et al., 2018; Usmani et al., 2018; Roche et al., 2022). However, evidence on the relationship between inhalation technique and HRQoL remains limited. For instance, one of the reviews (Engelkes et al., 2015) included one single prospective longitudinal clinical study with a small sample size. Another review (Kocks et al., 2018) referenced only two intervention-focused studies to enhance inhalation technique. The third review (Vazquez-Ortiz et al., 2020) exclusively referenced a cross-sectional study assessing HRQoL, which found no significant outcome differences between patients based on the inhalation technique. This highlights the need for further comprehensive research to fully understand the impact of inhalation technique on various asthma-related outcomes. The lack of a statistically significant association between inhalation technique and the other outcomes of our study deserves further research.
Our findings about the association of the frequent use of reliever medications with uncontrolled asthma symptoms and exacerbation occurrence align with those from the Nivel Primary Care Database from the Netherlands (Vervloet et al., 2022), which also observed them. Two studies conducted across European countries (Quint et al., 2022) and Canada (Noorduyn et al., 2022) also reported the association between the use of SABA and exacerbations occurrence. Furthermore, our study revealed an association between frequent reliever use and worse HRQoL, a relationship that has been explored less. A cross-sectional analysis of the study in France and the United Kingdom measuring the impact of asthma (Hernandez et al., 2018) showed statistically significant differences of HRQoL, according to the frequency of reliever medication use; among women, those using reliever medication almost or every day presented the biggest deviation from the reference norms.
Our findings suggest that the frequent use of reliever medication, which potentially reflects a reactive approach to asthma management, negatively impacts HRQoL. This observation ties in with our earlier hypothesis regarding adherence and inhalation technique, where proactive self-management practices are contrasted with reactive behaviors. Such patterns underline the complex dynamics of asthma self-management and emphasize the need for future research to conduct a more in-depth exploration of the within-person fluctuations in reliever use and its impact on HRQoL.
The positive long-term association between asthma symptom control and HRQoL found in our study was consistent with a longitudinal study in dyads of asthmatic children and their parents in USA (Li et al., 2017), showing that poorly controlled asthma status was associated with poor HRQoL. Additionally, a systematic review on adolescents (Vazquez-Ortiz et al., 2020) identified poor disease control, exacerbations, and asthma severity as the main factors associated with impaired HRQoL. In contrast, the longitudinal Dutch study in adolescents (Tiggelman et al., 2015) found that higher HRQoL at baseline did not predict changes in asthma control over time. On the other hand, the lower risk of exacerbations among patients with better asthma symptom control observed in our study aligns with the Asthma Care logic process model (Dima et al., 2016) and the GINA guideline (Global Initiative for Asthma, 2023), which position asthma control as directly related to exacerbations.
Our research identified gender differences in asthma outcomes, with girls experiencing worse asthma symptom control and HRQoL compared to boys. This finding is supported by literature reviews (Vazquez-Ortiz et al., 2020; Chowdhury et al., 2021; Jenkins et al., 2022) that also show an association of HRQoL and asthma control impairment with the female gender. Additionally, we observed that individuals aged 12 years and older showed a decreased HRQoL. These associations could be attributed to hormonal changes impacting airway inflammation, potential variances in immune responses, and the distinctive psychosocial challenges faced by female subjects and adolescents, as previously explained (de Benedictis and Bush, 2017; Chowdhury et al., 2021; Jenkins et al., 2022). These factors might collectively contribute to worsened asthma symptoms and treatment outcomes, subsequently affecting HRQoL. Nevertheless, the Dutch study in adolescents (Tiggelman et al., 2015) observed an increase in adolescents’ HRQoL over time, attributing this to the possibility that they may perceive their illness as less of a concern. Furthermore, we found that a lower risk of exacerbations was associated with a higher age, which could be related to fewer virus-induced exacerbations, since they are more common in younger children (Ramsahai et al., 2019).
Interpreting our findings requires taking into account various limitations. First, we did not consider the interplay of other important factors, such as comorbidities (rhinitis, obesity, and anxiety among others) and environmental triggers. Second, our results do not preclude the potential benefits of a deliberate effort to improve the overall adherence and inhalation technique due to the participation in a study, which could potentially impact their relationship with outcomes and the outcomes themselves. Third, the InTeQ’s reliance on a long recall period (previous 6 months) introduces a potential recall bias. Fourth, the measurement of adherence and the inhalation technique is based on the patient or proxy reporting. Thus, future research could benefit from pharmacy claims, performance tests, and smart inhalers for studying these complex relationships.
Finally, our analysis did not differentiate among specific LABA drugs in the ICS fixed-dose combination treatments (Global Initiative for Asthma, 2023) nor between the types of inhaler devices. Unfortunately, our sample size misbalance among the treatments used (712 reports of ICS-salmeterol, 124 ICS-vilanterol, and 69 ICS-formoterol; 226 patients used pMDI vs. 59 using DPI) prevented carrying out stratified analysis to explore the differences. However, the associations of LABA drugs and the type of inhaler device with adherence were not statistically significant (data not shown). Differences were only found between both inhaler devices among the patients presenting good inhalation technique (41.6% with pMDI vs. 60.7% with DPI, p = 0.002), as expected, since children are most likely to use pMDI with a spacer. Therefore, the impact of different inhaler devices on the association between inhaler technique and clinical outcomes merits further research.
To the best of our knowledge, this is the first longitudinal study specifically conducted in pediatric patients to assess both HRQoL and clinical outcomes (asthma symptom control and exacerbation occurrence), allowing for the evaluation of their longitudinal relationships with two of the main indicators of the quality use of inhalers (i.e., adherence and the inhalation technique). Methodologically, the hierarchical mixed model approach adopted has the advantage of describing how each person changes over time (within-person) and how these changes differ across people (between-person). In addition, conceptually, the timelines–events–objectives–sources (TEOS) framework (Li et al., 2017) has been applied to operationalize adherence.
Our findings highlight the multifaceted nature of asthma in children and adolescents, getting closer to a comprehensive understanding of the dynamic process of asthma treatment and outcomes over time. It is remarkable how although treatment adherence showed to be excellent, a third of the participants reported a suboptimal inhalation technique, supporting the need of actions for improvement in the asthma management of pediatric population. We found longitudinal associations at the within-person level of the two indicators of quality use of inhalers: for adherence to ICS-based maintenance treatment with symptom control and HRQoL, as well as for the inhalation technique with HRQoL. This reinforces the importance of further examining changes over time alongside the changes across people. Notably, the frequency of reliever use was associated with symptom control, exacerbation occurrence, and HRQoL; this pointed out the need for examining within-person changes in reliever use, which is further than the usually assessed between-person differences. Finally, due to the differences observed between boys and girls, it is especially important to apply a gender perspective in clinical practice and future studies on children and adolescents with asthma.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of clinical research of the Parc de Salut Mar (nº 2015/62/12l) and of the participant centers, following national and international guidelines (code of ethics, Helsinki Declaration), as well as legislation on data confidentiality (Spanish Organic Law 3/2018 of December 5 on the Protection of Personal Data and the Guarantee of Digital Rights). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
CL-B: conceptualization, investigation, methodology, project administration, visualization, writing–original draft, and writing–review and editing. OG: conceptualization, methodology, visualization, and writing–review and editing. KM: conceptualization, methodology, visualization, and writing–review and editing. AD: methodology and writing–review and editing. AP: data curation, formal analysis, and writing–review and editing. MC-R: investigation and writing–review and editing. MP-C: investigation and writing–review and editing. LV-N: investigation and writing–review and editing. MG: investigation and writing–review and editing. AB-S: investigation and writing–review and editing. MF: conceptualization, funding acquisition, methodology, project administration, supervision, visualization, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support for this study was provided through grants by the Instituto de Salud Carlos III FEDER: Fondo Europeo de Desarrollo Regional (PI15/00449) and Generalitat de Catalunya (AGAUR2021 SGR 00624, 2017 SGR 452). The following researchers have worked on this manuscript while funded by grants: CL-B (University of Costa Rica OAICE-85-2019) and KM (ISCIII FI16/00071).
The authors would like to thank the participants of the study. They also thank Áurea Martín for her support in English editing and proofreading. ARCA Group: MF, KM, CL-B, OG, YP, and ÀP (Hospital del Mar Research Institute); MC-R (Hospital del Mar); MP-C (Centro de Salud la Candelaria); LV-N (Hospital Universitario Parc Taulí); Ines de Mir (Hospital Vall d'Hebron); Gimena Hernandez, Camila Maroni (CAP La Sagrera); AB-S (Centro de Salud Los Castros); Jose Antonio Castillo (Hospital Infantil Universitario Miguel Servet); MG (Centro de Salud Jerez Sur), Olga Cortés (Centro de Salud Canillejas); Eva Tato (Hospital Universitario Araba); Pilar Ortiz, Marta Ortega, Alberto Servan (Centro de Salud Dos de Mayo), María Ángela Carrasco (Consultorio Sevilla la Nueva); AD (Institut de Recerca Sant Joan de Déu); Eric van Ganse (University Claude Bernard Lyon); and Marijn de Bruin (Radboud UniversityMedical Center). The preprint version of this manuscript is available in the medRxiv repository (Lizano-Barrantes et al., 2023b).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1340255/full#supplementary-material
ICS, inhaled corticosteroids; ICS plus LABA, inhaled corticosteroids plus long-acting beta-agonist; ICC, intra-class correlation coefficient; VPC, variance partition coefficient; AIC, Akaike information criterion; BIC, Bayesian information criterion.
A brief guide to the PROMIS (2023). A brief guide to the PROMIS© asthma impact instruments. Available from: http://www.healthmeasures.net/images/PROMIS/manuals/PROMIS_Asthma_Impact_Scoring_Manual.pdf (Accessed July 20, 2023).
Adams, N., , Bestall, J., Lasserson, T., Jones, P., and Cates, C. (2005). “Fluticasone versus placebo for chronic asthma in adults and children,” in Cochrane Database of systematic reviews. Editor N. Adams (Chichester, UK: John Wiley & Sons, Ltd).
Adams, N. P., Bestall, J. C., Malouf, R., Lasserson, T. J., and Jones, P. (2005). Beclomethasone versus placebo for chronic asthma. Cochrane Database Syst. Rev. 2008 (4), 2008. doi:10.1002/14651858.cd002738.pub2
Almomani, B. A., Al-Qawasmeh, B. S., Al-Shatnawi, S. F., Awad, S., and Alzoubi, S. A. (2021). Predictors of proper inhaler technique and asthma control in pediatric patients with asthma. Pediatr. Pulmonol. 56 (5), 866–874. doi:10.1002/ppul.25263
Asher, M. I., Rutter, C. E., Bissell, K., Chiang, C. Y., El Sony, A., Ellwood, E., et al. (2021). Worldwide trends in the burden of asthma symptoms in school-aged children: global Asthma Network Phase I cross-sectional study. Lancet 398 (10311), 1569–1580. doi:10.1016/S0140-6736(21)01450-1
Azzi, E., Srour, P., Armour, C. L., Rand, C., and Bosnic-Anticevich, S. (2017). Practice makes perfect: self-reported adherence a positive marker of inhaler technique maintenance. Prim. Care Respir. J. 27 (1), 29. doi:10.1038/s41533-017-0031-0
Balkrishnan, R., and Christensen, D. B. (2000). Inhaled corticosteroid nonadherence and immediate avoidable medical events in older adults with chronic pulmonary ailments. J. Asthma 37 (6), 511–517. doi:10.3109/02770900009055478
Bårnes, C. B., and Ulrik, C. S. (2015). Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir. Care 60 (3), 455–468. doi:10.4187/respcare.03200
Bercedo Sanz, A., Martínez-Torres, A., González Díaz, C., López-Silvarrey Varela, Á., Pellegrini Belinchón, F. J., Aguinaga-Ontoso, I., et al. (2022). Prevalence and temporal evolution of asthma symptoms in Spain. Global Asthma Network (GAN) study. An Pediatr (Engl Ed). 97 (3), 161–171. doi:10.1016/j.anpede.2021.10.005
Boutopoulou, B., Koumpagioti, D., Matziou, V., Priftis, K. N., and Douros, K. (2018). Interventions on adherence to treatment in children with severe asthma: a systematic review. Front. Pediatr. 6, 232. doi:10.3389/fped.2018.00232
Braido, F., Chrystyn, H., Baiardini, I., Bosnic-Anticevich, S., van der Molen, T., Dandurand, R. J., et al. (2016). “Trying, but failing” — the role of inhaler technique and mode of delivery in respiratory medication adherence. J. Allergy Clin. Immunol. Prac. 4 (5), 823–832. doi:10.1016/j.jaip.2016.03.002
Bukstein, D. A., Murphy, K. R., Katz, L. M., Ramachandran, S., Doyle, J. J., and Stern, L. S. (2007). Outcomes among a young population of pediatric asthma patients using controller therapies: results from a retrospective database analysis. Pediatr. Asthma Allergy Immunol. 20 (4), 211–222. doi:10.1089/pai.2006.024
Camargo, C. A., Ramachandran, S., Ryskina, K. L., Lewis, B. E., and Legorreta, A. P. (2007). Association between common asthma therapies and recurrent asthma exacerbations in children enrolled in a state Medicaid plan. Am. J. Health Syst. Pharm. 64 (10), 1054–1061. doi:10.2146/ajhp060256
Chongmelaxme, B., Chaiyakunapruk, N., and Dilokthornsakul, P. (2020). Association between adherence and severe asthma exacerbation: a systematic review and meta-analysis. J. Am. Pharm. Assoc. 60 (5), 669–685. doi:10.1016/j.japh.2020.02.010
Chowdhury, B. A., and Dal Pan, G. (2010). The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N. Engl. J. Med. 362 (13), 1169–1171. doi:10.1056/NEJMp1002074
Chowdhury, N. U., Guntur, V. P., Newcomb, D. C., and Wechsler, M. E. (2021). Sex and gender in asthma. Eur. Respir. Rev. 30 (162), 210067. doi:10.1183/16000617.0067-2021
de Benedictis, D., and Bush, A. (2017). Asthma in adolescence: is there any news? Pediatr. Pulmonol. 52 (1), 129–138. doi:10.1002/ppul.23498
Delea, T. E., Stanford, R. H., Hagiwara, M., and Stempel, D. A. (2008). Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs. Curr. Med. Res. Opin. 24 (12), 3435–3442. doi:10.1185/03007990802557344
Dima, A. L., de Bruin, M., and Van Ganse, E.ASTRO-LAB group (2016). Mapping the asthma care process: implications for research and practice. J. Allergy Clin. Immunol. Prac. 4 (5), 868–876. doi:10.1016/j.jaip.2016.04.020
Dima, A. L., van Ganse, E., Laforest, L., Texier, N., and de Bruin, M.The Astro-Lab Group (2017). Measuring medication adherence in asthma: development of a novel self-report tool. Psychol. Health 32 (10), 1288–1307. doi:10.1080/08870446.2017.1290248
Dima, A. L., van Ganse, E., Stadler, G., and de Bruin, M.ASTRO-LAB group, Members of the ASTRO-LAB group were: (2019). Does adherence to inhaled corticosteroids predict asthma-related outcomes over time? A cohort study. Eur. Respir. J. 54 (6), 1900901. doi:10.1183/13993003.00901-2019
Elkout, H., Helms, P. J., Simpson, C. R., and McLay, J. S. (2012). Adequate levels of adherence with controller medication is associated with increased use of rescue medication in asthmatic children. PLoS One 7 (6), e39130. doi:10.1371/journal.pone.0039130
Engelkes, M., Janssens, H. M., De Jongste, J. C., Sturkenboom, MCJM, and Verhamme, K. M. C. (2015). Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur. Respir. J. 45 (2), 396–407. doi:10.1183/09031936.00075614
Everhart, R. S., and Fiese, B. H. (2009). Asthma severity and child quality of life in pediatric asthma: a systematic review. Patient Educ. Couns. 75 (2), 162–168. doi:10.1016/j.pec.2008.10.001
Exarchos, K. P., Rovina, N., Krommidas, G., Latsios, D., Gogali, A., and Kostikas, K. (2022). Adherence and quality of life assessment in patients with asthma treatment with budesonide/formoterol via the Elpenhaler device: the COMPLETE study. BMC Pulm. Med. 22 (1), 254. doi:10.1186/s12890-022-02049-0
García-Marcos, L., Chiang, C. Y., Asher, M. I., Marks, G. B., El Sony, A., Masekela, R., et al. (2023). Asthma management and control in children, adolescents, and adults in 25 countries: a Global Asthma Network Phase I cross-sectional study. Lancet Glob. Health 11 (2), e218–e228. doi:10.1016/S2214-109X(22)00506-X
Gillette, C., Rockich-Winston, N., Kuhn, J. A., Flesher, S., and Shepherd, M. (2016). Inhaler technique in children with asthma: a systematic review. Acad. Pediatr. 16, 605–615. doi:10.1016/j.acap.2016.04.006
Giraud, V., Allaert, F. A., and Roche, N. (2011). Inhaler technique and asthma: feasability and acceptability of training by pharmacists. Respir. Med. 105 (12), 1815–1822. doi:10.1016/j.rmed.2011.07.004
Global Initiative for Asthma. Global strategy for asthma management and prevention. Updated 2017. Available from: http://www.ginasthma.org.2017.
Global Initiative for Asthma Global strategy for asthma management and prevention. Available from: http://www.ginasthma.org.2023 (Cite May, 2023).
Gusi, N., Perez-Sousa, M. A., Gozalo-Delgado, M., and Olivares, P. R. (2014). Validity and reliability of the Spanish EQ-5D-Y Proxy version. An Pediatr (Engl Ed). 81 (4), 212–219. doi:10.1016/j.anpedi.2013.11.028
Hagmolen of ten Have, W., Van De Berg, N. J., Bindels, P. J. E., Van Aalderen, W. M. C., and Van Der Palen, J. (2008). Assessment of inhalation technique in children in general practice: increased risk of incorrect performance with new device. J. Asthma 45 (1), 67–71. doi:10.1080/02770900701815834
Hale, E. M., Greene, G., Mulvey, C., Mokoka, M. C., van Boven, J. F. M., Cushen, B., et al. (2023). Use of digital measurement of medication adherence and lung function to guide the management of uncontrolled asthma (INCA Sun): a multicentre, single-blinded, randomised clinical trial. Lancet Respir. Med. 11 (7), 591–601. doi:10.1016/S2213-2600(22)00534-3
Hernandez, G., Dima, A. L., Pont, À., Garin, O., Martí-Pastor, M., Alonso, J., et al. (2018). Impact of asthma on women and men: comparison with the general population using the eq-5d-5l questionnaire. PLoS One 13 (8), 02026244–e202718. doi:10.1371/journal.pone.0202624
Herndon, J. B., Mattke, S., Evans Cuellar, A., Hong, S. Y., and Shenkman, E. A. (2012). Anti-inflammatory medication adherence, healthcare utilization and expenditures among medicaid and children’s health insurance program enrollees with asthma. Pharmacoeconomics 30 (5), 397–412. doi:10.2165/11586660-000000000-00000
Hyland, M. E., Whalley, B., Halpin, D. M. G., Greaves, C. J., Seamark, C., Blake, S., et al. (2012). Frequency of non-asthma GP visits predicts asthma exacerbations: an observational study in general practice. Prim. Care Respir. J. 21 (4), 405–411. doi:10.4104/pcrj.2012.00061
Jenkins, C. R., Boulet, L. P., Lavoie, K. L., Raherison-Semjen, C., and Singh, D. (2022). Personalized treatment of asthma: the importance of sex and gender differences. J. Allergy Clin. Immunol. Pract. 10 (4), 963–971.e3. doi:10.1016/j.jaip.2022.02.002
Johnson, K. M., FitzGerald, J. M., Tavakoli, H., Chen, W., and Sadatsafavi, M. (2017). Stability of asthma symptom control in a longitudinal study of mild-moderate asthmatics. J. Allergy Clin. Immunol. Pract. 5 (6), 1663–1670. doi:10.1016/j.jaip.2017.04.006
Juniper, E. F., Bousquet, J., Abetz, L., and Bateman, E. D.GOAL Committee (2006). Identifying ‘ well-controlled ’ and ‘ not well-controlled ’ asthma using the Asthma Control Questionnaire. Respir. Med. 616–21, 616–621. doi:10.1016/j.rmed.2005.08.012
Juniper, E. F., Svensson, K., christin, Mo A., and Sta, E. (2005a). Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med. 99, 553–558. doi:10.1016/j.rmed.2004.10.008
Juniper, E. F., Svensson, K., Mörk, A. C., and Ståhl, E. (2005b). Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir. Med. 99 (5), 553–558. doi:10.1016/j.rmed.2004.10.008
Kocks, J. W. H., Chrystyn, H., van der Palen, J., Thomas, M., Yates, L., Landis, S. H., et al. (2018). Systematic review of association between critical errors in inhalation and health outcomes in asthma and COPD. NPJ Prim. Care Respir. Med. 28 (1), 43. doi:10.1038/s41533-018-0110-x
Krishnan, J. A., Bender, B. G., Wamboldt, F. S., Szefler, S. J., Adkinson, N. F., Zeiger, R. S., et al. (2012). Adherence to inhaled corticosteroids: an ancillary study of the Childhood Asthma Management Program clinical trial. J. Allergy Clin. Immunol. 129 (1), 112–118. doi:10.1016/j.jaci.2011.10.030
Lasmar, L., Camargos, P., Champs, N. S., Fonseca, M. T., Fontes, M. J., Ibiapina, C., et al. (2009). Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy 64 (5), 784–789. doi:10.1111/j.1398-9995.2008.01877.x
Li, Z., Leite, W. L., Thompson, L. A., Gross, H. E., Shenkman, E. A., Reeve, B. B., et al. (2017). Determinants of longitudinal health-related quality-of-life change in children with asthma from low-income families: a report from the PROMIS® Pediatric Asthma Study. Clin. Exp. Allergy 47 (3), 383–394. doi:10.1111/cea.12827
Lizano-Barrantes, C., Garin, O., Dima, A. L., Mayoral, K., Pont, A., Ortiz, E. M., et al. (2023a). Inhaler Technique Questionnaire (InTeQ) in pediatric patients with asthma. World J. Pediatr. 19 (8), 798–804. doi:10.1007/s12519-023-00695-w
Lizano-Barrantes, C., Garin, O., Dima, A. L., van Ganse, E., de Bruin, M., Belhassen, M., et al. (2022). The inhaler technique questionnaire (InTeQ): development and validation of a brief patient-reported measure. Int. J. Environ. Res. Public Health 19 (5), 2591. doi:10.3390/ijerph19052591
Lizano-Barrantes, C., Garin, O., Mayoral, K., Dima, A. L., Pont, A., Caballero-Rabasco, M. A., et al. (2023b). Impact of treatment adherence and inhalation technique on asthma outcomes of pediatric patients: a longitudinal study. medRxiv. doi:10.1101/2023.11.30.23299186
Maricoto, T., Santos, D., Carvalho, C., Teles, I., Correia-de-Sousa, J., and Taborda-Barata, L. (2020). Assessment of poor inhaler technique in older patients with asthma or copd: a predictive tool for clinical risk and inhaler performance. Drugs Aging 37, 605–616. doi:10.1007/s40266-020-00779-6
Mattke, S., Martorell, F., Hong, S. Y., Sharma, P., Cuellar, A., and Lurie, N. (2010). Anti-inflammatory medication adherence and cost and utilization of asthma care in a commercially insured population. J. Asthma 47 (3), 323–329. doi:10.3109/02770900903497196
Mayoral, K., Garin, O., Caballero-Rabasco, M. A., Praena-Crespo, M., Bercedo, A., Hernandez, G., et al. (2021). Smartphone App for monitoring Asthma in children and adolescents. Qual. Life Res. 30 (11), 3127–3144. doi:10.1007/s11136-020-02706-z
McMahon, A. D., Lipworth, B. J., Davey, P. G., Morris, A. D., and MacDonald, T. M. (2000). Continuity of prescribing with inhaled corticosteroids and control of asthma. Pharmacoepidemiol Drug Saf. 9 (4), 293–303. doi:10.1002/1099-1557(200007/08)9:4<293::AID-PDS502>3.0.CO;2-S
McNally, K. A., Rohan, J., Schluchter, M., Riekert, K. A., Vavrek, P., Schmidt, A., et al. (2009). Adherence to combined montelukast and fluticasone treatment in economically disadvantaged African American youth with asthma. J. Asthma 46 (9), 921–927. doi:10.3109/02770900903229651
Monteiro, C., Maricoto, T., Prazeres, F., Augusto Simões, P., and Augusto Simões, J. (2022). Determining factors associated with inhaled therapy adherence on asthma and COPD: a systematic review and meta-analysis of the global literature. Respir. Med., 191.
Montuschi, P., and Barnes, P. J. (2011). New perspectives in pharmacological treatment of mild persistent asthma. Drug Discov. Today 16 (23–24), 1084–1091. doi:10.1016/j.drudis.2011.09.005
National Heart Lung and Blood Institute (NHLBI) (2020). 2020 focused updates to the asthma management guidelines. Clinician’s Guide. Available at: https://www.nhlbi.nih.gov/resources/clinician-guide-2020-focused-updates-asthma-management-guidelines.
Noorduyn, S. G., Qian, C., Johnston, K. M., Soliman, M., Talukdar, M., Walker, B. L., et al. (2022). SABA use as an indicator for asthma exacerbation risk: an observational cohort study (SABINA Canada). ERJ Open Res. 8 (3), 00140-2022. doi:10.1183/23120541.00140-2022
Normansell, R., Kew, K., Stovold, E., Mathioudakis, A. G., and Dennett, E. (2017). Interventions to improve inhaler technique and adherence to inhaled corticosteroids in children with asthma. Paediatr. Respir. Rev. 23, 53–55. doi:10.1016/j.prrv.2017.03.014
O’byrne, P. M., Barnes, P. J., Rodriguez-Roisin, R., Runnerstrom, E., Sandstrom, T., Svensson, K., et al. (2001). Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am. J. Respir. Crit. Care Med. 164 (8), 1392–1397. doi:10.1164/ajrccm.164.8.2104102
Osman, L. M., Friend, J. A. R., Legge, J. S., and Douglas, J. G. (1999). Requests for repeat medication prescriptions and frequency of acute episodes in asthma patients. J. Asthma 36 (5), 449–457. doi:10.3109/02770909909087287
Oussedik, E., Foy, C. G., Masicampo, E. J., Kammrath, L. K., Anderson, R. E., and Feldman, S. R. (2017). Accountability: a missing construct in models of adherence behavior and in clinical practice. Patient Prefer Adherence 11, 1285–1294. doi:10.2147/PPA.S135895
Papi, A., Ryan, D., Soriano, J. B., Chrystyn, H., Bjermer, L., Rodríguez-Roisin, R., et al. (2018). Relationship of inhaled corticosteroid adherence to asthma exacerbations in patients with moderate-to-severe asthma. J. Allergy Clin. Immunol. Pract. 6 (6), 1989–1998. doi:10.1016/j.jaip.2018.03.008
Pauwels, R. A., Pedersen, S., Busse, W. W., Tan, W. C., Chen, Y. Z., Ohlsson, S. V., et al. (2003). Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet 361 (9363), 1071–1076. doi:10.1016/S0140-6736(03)12891-7
Quint, J. K., Arnetorp, S., Kocks, J. W. H., Kupczyk, M., Nuevo, J., Plaza, V., et al. (2022). Short-acting beta-2-agonist exposure and severe asthma exacerbations: SABINA findings from europe and north America. J. Allergy Clin. Immunol. Pract. 10 (9), 2297–2309.e10. doi:10.1016/j.jaip.2022.02.047
Ramos-Goñi, J. M., Craig, B. M., Oppe, M., Ramallo-Fariña, Y., Pinto-Prades, J. L., Luo, N., et al. (2018). Handling data quality issues to estimate the Spanish EQ-5D-5L value set using a hybrid interval regression approach. Value Health 21 (5), 596–604. doi:10.1016/j.jval.2017.10.023
Ramos-Goñi, J. M., Oppe, M., Estévez-Carrillo, A., and Rivero-Arias, O. (2024). Preference-based assessments accounting for unobservable preference heterogeneity and evaluating alternative anchoring approaches to estimate country-specific EQ-5D-Y value sets: a case study using Spanish preference data.
Ramos-Goñi, J. M., Oppe, M., Stolk, E., Shah, K., Kreimeier, S., Rivero-Arias, O., et al. (2020). International valuation protocol for the EQ-5D-Y-3L. Pharmacoeconomics 38, 653–663. doi:10.1007/s40273-020-00909-3
Ramsahai, J. M., Hansbro, P. M., and Wark, P. A. B. (2019). Mechanisms and management of asthma exacerbations. Am. J. Respir. Crit. Care Med. 199, 423–432. doi:10.1164/rccm.201810-1931CI
Ravens-Sieberer, U., Wille, N., Badia, X., Gouke, B., Burström, K., Cavrini, G., et al. (2010). Feasibility, reliability, and validity of the EQ-5D-Y: results from a multinational study. Qual. Life Res. 19, 887–897. doi:10.1007/s11136-010-9649-x
Reddel, H. K., Taylor, D. R., Bateman, E. D., Boulet, L. P., Boushey, H. A., Busse, W. W., et al. (2009). An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am. J. Respir. Crit. Care Med. 180 (1), 59–99. doi:10.1164/rccm.200801-060ST
Roche, N., Aggarwal, B., Boucot, I., Mittal, L., Martin, A., and Chrystyn, H. (2022). The impact of inhaler technique on clinical outcomes in adolescents and adults with asthma: a systematic review. Respir. Med., 202, 106949.
Rohan, J., Drotar, D., McNally, K., Schluchter, M., Riekert, K., Vavrek, P., et al. (2010). Adherence to pediatric asthma treatment in economically disadvantaged african-american children and adolescents: an application of growth curve analysis. J. Pediatr. Psychol. 35 (4), 394–404. doi:10.1093/jpepsy/jsp074
Santos, P. M., D’Oliveira, A., Noblat, L. A., Machado, A. S., Noblat, A. C., and Cruz, A. A. (2008). Predictors of adherence to treatment in patients with severe asthma treated at a referral center in Bahia, Brazil. J. Bras. Pneumol. 34 (12), 995–1002. doi:10.1590/s1806-37132008001200003
Silva, N., Carona, C., Crespo, C., and Canavarro, M. C. (2015). Quality of life in pediatric asthma patients and their parents: a meta-analysis on 20 years of research. Expert Rev. Pharmacoecon Outcomes Res. 15 (3), 499–519. doi:10.1586/14737167.2015.1008459
Singer, J., and Willett, J. (2003). Applied longitudinal data analysis: modeling change and event occurrence. New York: Oxford University Press.
Smith, K., Warholak, T., Armstrong, E., Leib, M., Rehfeld, R., and Malone, D. (2009). Evaluation of risk factors and health outcomes among persons with asthma. J. Asthma 46 (3), 234–237. doi:10.1080/02770900802627294
Song, P., Adeloye, D., Salim, H., Dos Santos, J. P., Campbell, H., Sheikh, A., et al. (2022). Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J. Glob. Health 12, 04052. doi:10.7189/jogh.12.04052
Sousa-Pinto, B., Louis, R., Anto, J. M., Amaral, R., Sá-Sousa, A., Czarlewski, W., et al. (2023). Adherence to inhaled corticosteroids and long-acting β2-agonists in asthma: a MASK-air study. Pulmonology (Aug 3), S2531–S0437. doi:10.1016/j.pulmoe.2023.07.004
Souverein, P. C., Koster, E. S., Colice, G., van Ganse, E., Chisholm, A., Price, D., et al. (2017). Inhaled corticosteroid adherence patterns in a longitudinal asthma cohort. J. Allergy Clin. Immunol. Pract. 5 (2), 448–456.e2. doi:10.1016/j.jaip.2016.09.022
Stern, L., Berman, J., Lumry, W., Katz, L., Wang, L., Rosenblatt, L., et al. (2006). Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann. Allergy Asthma Immunol. 97 (3), 402–408. doi:10.1016/S1081-1206(10)60808-3
Sundell, K., Bergström, S. E., Hedlin, G., Ygge, B. M., and Tunsäter, A. (2011). Quality of life in adolescents with asthma, during the transition period from child to adult. Clin. Respir. J. 5 (4), 195–202. doi:10.1111/j.1752-699X.2010.00218.x
Tiggelman, D., van de Ven, M. O. M., van Schayck, O. C. P., and Engels, RCME (2015). Longitudinal associations between asthma control, medication adherence, and quality of life among adolescents: results from a cross-lagged analysis. Qual. Life Res. 24 (9), 2067–2074. doi:10.1007/s11136-015-0945-3
Usmani, O. S., Lavorini, F., Marshall, J., Dunlop, W. C. N., Heron, L., Farrington, E., et al. (2018). Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir. Res. 19 (1), 10. doi:10.1186/s12931-017-0710-y
van Boven, J. F., Trappenburg, J. C., van der Molen, T., and Chavannes, N. H. (2015). Towards tailored and targeted adherence assessment to optimise asthma management. NPJ Prim. Care Respir. Med. 25, 15046. doi:10.1038/npjpcrm.2015.46
Vazquez-Ortiz, M., Angier, E., Blumchen, K., Comberiati, P., Duca, B., DunnGalvin, A., et al. (2020). Understanding the challenges faced by adolescents and young adults with allergic conditions: a systematic review. Allergy 75 (8), 1850–1880. doi:10.1111/all.14258
Vervloet, M., van Dijk, L., Spreeuwenberg, P., Price, D., Chisholm, A., Van Ganse, E., et al. (2020). The relationship between real-world inhaled corticosteroid adherence and asthma outcomes: a multilevel approach. J. Allergy Clin. Immunol. Pract. 8 (2), 626–634. doi:10.1016/j.jaip.2019.09.003
Vervloet, M., van Dijk, L., Weesie, Y. M., Kocks, J. W. H., Dima, A. L., and Korevaar, J. C. (2022). Understanding relationships between asthma medication use and outcomes in a SABINA primary care database study. NPJ Prim. Care Respir. Med. 32 (1), 43. doi:10.1038/s41533-022-00310-x
von Mutius, E., and Smits, H. H. (2020). Primary prevention of asthma: from risk and protective factors to targeted strategies for prevention. Lancet 396 (10254), 854–866. doi:10.1016/S0140-6736(20)31861-4
Vrijens, B., De Geest, S., Hughes, D. A., Przemyslaw, K., Demonceau, J., Ruppar, T., et al. (2012). A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 73 (5), 691–705. doi:10.1111/j.1365-2125.2012.04167.x
Vrijens, B., Dima, A. L., Van Ganse, E., van Boven, J. F. M., Eakin, M. N., Foster, J. M., et al. (2016). What we mean when we talk about adherence in respiratory medicine. J. Aller Cl. Imm-Pract. 4 (5), 802–812. doi:10.1016/j.jaip.2016.05.019
Wiebe, S., Guyatt, G., Weaver, B., Matijevic, S., and Sidwell, C. (2003). Comparative responsiveness of generic and specific quality-of-life instruments. J. Clin. Epidemiol. 56, 52–60. doi:10.1016/s0895-4356(02)00537-1
Wild, D., Grove, A., Martin, M., Eremenco, S., McElroy, S., Verjee-Lorenz, A., et al. (2005). Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 8 (2), 94–104. doi:10.1111/j.1524-4733.2005.04054.x
Wille, N., Badia, X., Bonsel, G., Burström, K., Cavrini, G., Devlin, N., et al. (2010). Development of the EQ-5D-Y: a child-friendly version of the EQ-5D. Qual. Life Res. 19 (6), 875–886. doi:10.1007/s11136-010-9648-y
Williams, L. K., Peterson, E. L., Wells, K., Ahmedani, B. K., Kumar, R., Burchard, E. G., et al. (2011). Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J. Allergy Clin. Immunol. 128 (6), 1185–1191. doi:10.1016/j.jaci.2011.09.011
Yeatts, K. B., Stucky, B., Thissen, D., Irwin, D., Varni, J. W., DeWitt, E. M., et al. (2010). Construction of the pediatric asthma impact scale (PAIS) for the patient-reported outcomes measurement information System (PROMIS). J. Asthma 47 (3), 295–302. doi:10.3109/02770900903426997
Yildiz, F., Erbagci, A., Demirel, Y. S., Akcali, S. D., Ekici, A., Dursunoglu, N., et al. (2014). Importance of inhaler device use status in the control of asthma in adults: the asthma inhaler treatment study. Respir. Care 59 (2), 223–230. doi:10.4187/respcare.02478
Keywords: adherence, inhalation technique, pediatric asthma, health-related quality of life, asthma outcomes, asthma symptom control, asthma exacerbations
Citation: Lizano-Barrantes C, Garin O, Mayoral K, Dima AL, Pont A, Caballero-Rabasco MA, Praena-Crespo M, Valdesoiro-Navarrete L, Guerra MT, Bercedo-Sanz A and Ferrer M (2024) Impact of treatment adherence and inhalation technique on asthma outcomes of pediatric patients: a longitudinal study. Front. Pharmacol. 15:1340255. doi: 10.3389/fphar.2024.1340255
Received: 17 November 2023; Accepted: 23 February 2024;
Published: 13 March 2024.
Edited by:
Maria Teresa Herdeiro, University of Aveiro, PortugalReviewed by:
Alvaro Teijeiro, Pediatric Hospital of Cordoba, ArgentinaCopyright © 2024 Lizano-Barrantes, Garin, Mayoral, Dima, Pont, Caballero-Rabasco, Praena-Crespo, Valdesoiro-Navarrete, Guerra, Bercedo-Sanz and Ferrer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Montse Ferrer, bWZlcnJlckByZXNlYXJjaG1hci5uZXQ=; Olatz Garin, b2dhcmluQHJlc2VhcmNobWFyLm5ldA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.