- 1Sichuan Provincial Ba-Yi Rehablitation Center (Sichuan Provincial Rehablitation Hospital), Chengdu, Sichuan, China
- 2Affiliated Rehabilitation Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Wenjiang Distrct People’s Hospital of Chengdu, Chengdu, Sichuan, China
- 4Department of Orthopaedics, The Affiliated Traditional Chinese Medicine, Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 5Southwest University Hospital, Chongqing, China
- 6School of Materials and Energy, Southwest University, Chongqing, China
Background: Denosumab is authorized to treat several diseases, including cancer and bone disorders. Nevertheless, its use in clinical practice has been affected by safety concerns. The work retrospectively investigated adverse events (AEs) of denosumab to better understand toxicities.
Methods: The FAERS data base data from Q1 of 2010 to Q3 of 2023 was chosen. The definition of Medical Dictionary for Regulatory Activities (MedDRA) was dependent on preferred terms (PTs) and system organ class (SOCs). Following the removal of duplicate reports, a disproportionality analysis was conducted to identify safety signals through the calculation of reporting odds ratios (ROR).
Results: During the reporting period, 130611 denosumab-related cases were identified; 670 pTs with a substantial disproportionality were retained. The connective and musculoskeletal tissue disorders, poisoning, injury, and procedural complications, as well as medical and surgical procedures, were among the important SOCs that satisfied the criteria. Reports at PT levels including off-label use, death, osteonecrosis of the jaw, arthralgia, and pain in extremities were determined. Severe consequences in terms of life-threatening injuries and death accounted for 841 and 19704 cases, respectively of the reported cases.
Conclusion: These findings underscore the critical importance of pharmacovigilance and are consistent with established clinical observations. Notably, osteonecrosis of the jaw, arthralgia, pain in extremities, back pain, myalgia, and bone pain were identified as the most prevalent risk signals associated with denosumab.
Introduction
The protein osteoprotegerin, discovered in 1997, serves as a “decoy” for receptor activators of nuclear factor kappa-B ligand (RANKL) to block bone resorption (Simonet et al., 1997). In response to this discovery, denosumab was developed, a fully human monoclonal antibody with a stronger antiresorptive activity and longer half-life which binds to RANKL to prevent RANK activation (Kendler et al., 2022). According to documented findings, the primary objective of the FREEDOM Extension study was to elucidate the long-term safety profile of denosumab, with a specific focus on the effects of prolonged inhibition of bone turnover on bone quality (Bone et al., 2017). No statistically significant variations were noted in the incidence of serious adverse events (SAEs) or adverse events (AEs) between the placebo-treated subjects and those treated with denosumab in the initial FREEDOM trial, except for SAEs related to cases of cellulitis and eczema, which were more common in the group receiving denosumab treatment (12 patients experienced cellulitis while receiving denosumab in contrast to only 1 on placebo) (Cummings et al., 2009). During the extension phase, the elderly study population exhibited a minimal incidence of adverse events, such as malignancies, cellulitis, and infections (Bone et al., 2017). In addition, previous studies have reported that multiple vertebral fractures are more likely to occur with the second dosage of denosumab (Lamy et al., 2019). Identifying and preventing osteoporosis and fragility fractures in individuals with chronic kidney disease (CKD), especially those with end-stage kidney disease (ESKD), is a complex process. The pronounced impact of denosumab-induced hypocalcemia on patients with end-stage kidney disease (ESKD) has been documented. Both high and low bone turnover, as well as lower baseline levels of 25 hydroxyvitamin D and blood calcium, are risk factors for hypocalcemia linked to denosumab usage in CKD (Gopaul et al., 2021). Therefore, it is essential to carefully select appropriate candidates for denosumab therapy, ensure adequate vitamin D and calcium supplementation, adjust calcium dialysate levels, and conduct thorough clinical monitoring of patients. FAERS, consisting of volunteered reports of adverse drug reactions (ADRs) associated with natural substances, drugs, medical devices and vaccines approved by the FDA, has been extensively employed in several studies. It serves as the benchmark approach for detecting “signals” and previously unreported ADRs (Zhang et al., 2016). There is a lack of data on FAERS analysis of RANK inhibitors, encompassing denosumab, to understand the safety of denosumab in the real world. This study aims to assess the adverse events associated with denosumab by utilizing data mining techniques on the FAERS.
Methods
The FDA has documented AEs from 2010 to 2023, which are cataloged in the FAERS database. The web-based analysis tool, AERSMine, was created to mine the FAERS data from Q1 of 2010 to Q3 of 2023. In accordance with FDA guidelines, a deduplication process was implemented. When identical CASEIDs were encountered, the record with the most recent FDA_DT was selected. In instances where both FDA_DT and CASEID were identical, the record with the higher PRIMARYID was chosen (Chen et al., 2021). Medical Dictionary for Regulatory Activities (MedDRA) (version 25.0) system organ class (SOC) and preferred term (PT) level were employed for categorizing the AEs.
Statistical analysis
From the first quarter of 2010 to the third quarter of 2023, FAERS reports listing “denosumab,” “Prolix,” “Kyprolia,” “Xgeva,” “Ranmark,” and “Pralia” as primary suspect drugs were analyzed after the removal of duplicate reports identified by the same ID number. Two researchers employed standardized MedDRA queries and Preferred Terms (PT) to classify AEs related to PARP inhibitors and extracted patient and drug information from the reports. We examined AEs brought on the study medications rather than illness states. To identify spontaneous signals, which were determined utilizing the case/non-case technique and indicating if there is a signal of a possible elevated risk of AE associated with the drug, a disproportionality analysis was carried out via the reporting odds ratio (ROR). Patients receiving medicine and reporting a certain AE were classified as “Cases,” with all other potential pairings being considered “non-cases.” To calculate the Reporting Risk Ratio (ROR), two-by-two contingency tables presenting counts of reported incidents for a given medicine relative to other drugs are utilized. ROR serves as a quantification of the likelihood that a particular outcome will occur in light of a specific exposure, thereby functioning as an indicator of the extent of correlation between the odds of a specific outcome and drug exposure (Rothman et al., 2004). A positive ROR signal was identified when the number of instances exceeded three, the Chi-square values surpassed four, the ROR value was greater than 2.0, and the lower limit of the 95% confidence interval (CI) was above 1.0 (Sakaeda et al., 2013). The count data were presented as frequencies (percentages), and intergroup comparisons of the count data were performed using the chi-square (χ2) test. Any serious AE that was found but was not mentioned in the FDA medication labeling was considered an unexpected AE. The R software was utilized for the entirety of the statistical analyses and data processing.
Results
Descriptive results
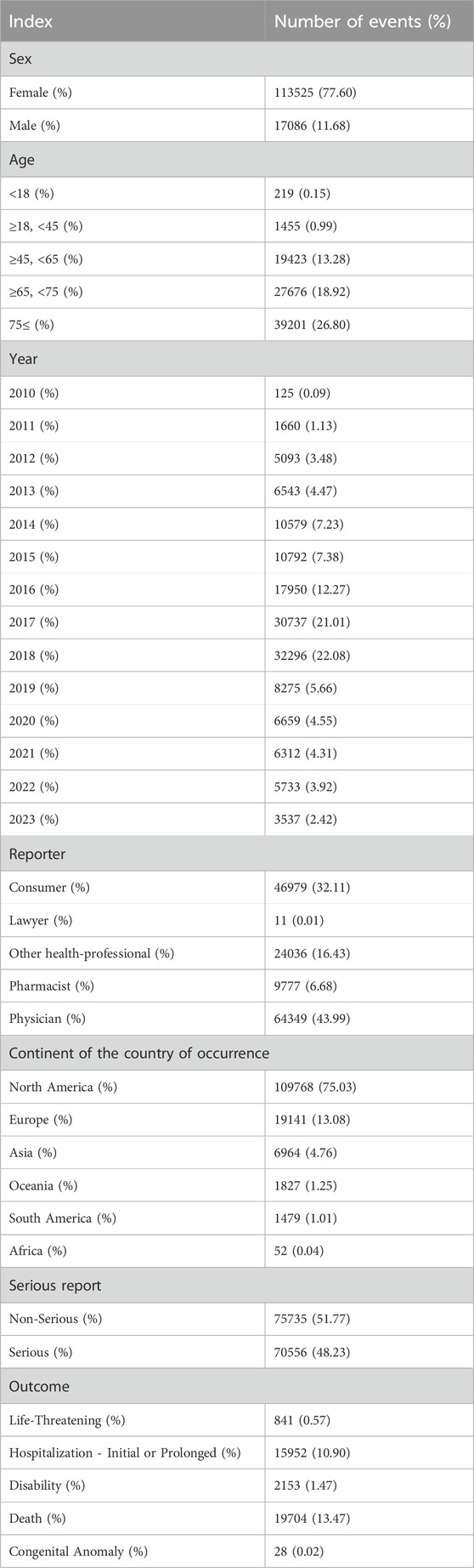
130611 reports on denosumab were submitted from the first quarter of 2010 through the third quarter of 2023 (i.e., the study period). Table 1 details the clinical features of denosumab-related incidents. Of all AEs, females made up a higher proportion (77.60%) than men. The majority of patients were over 65 years of age, a demographic significantly older than the median age typically observed in participants enrolled in clinical trials (Rothman et al., 2004). North America accounted for 75.03% of the reported AEs. Most of AEs were reported in 2018 (22.08%). The most commonly reported severe event was death (13.47%). Life-threatening events, disabilities, and hospitalizations occurred in 841 cases (0.57%), 2,153 cases (1.47%), and 15,952 cases (10.90%), respectively. The primary sources of reports were consumers and physicians, accounting for with 43.99% and 32.11%, respectively.
Signal values related to denosumab
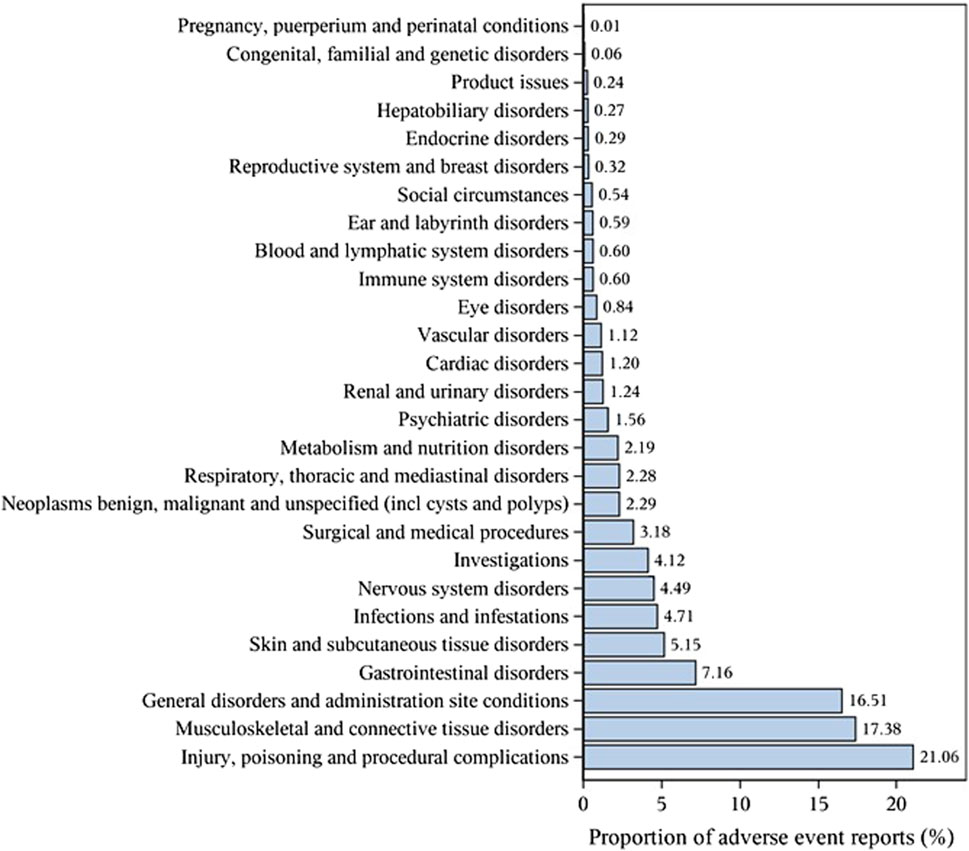
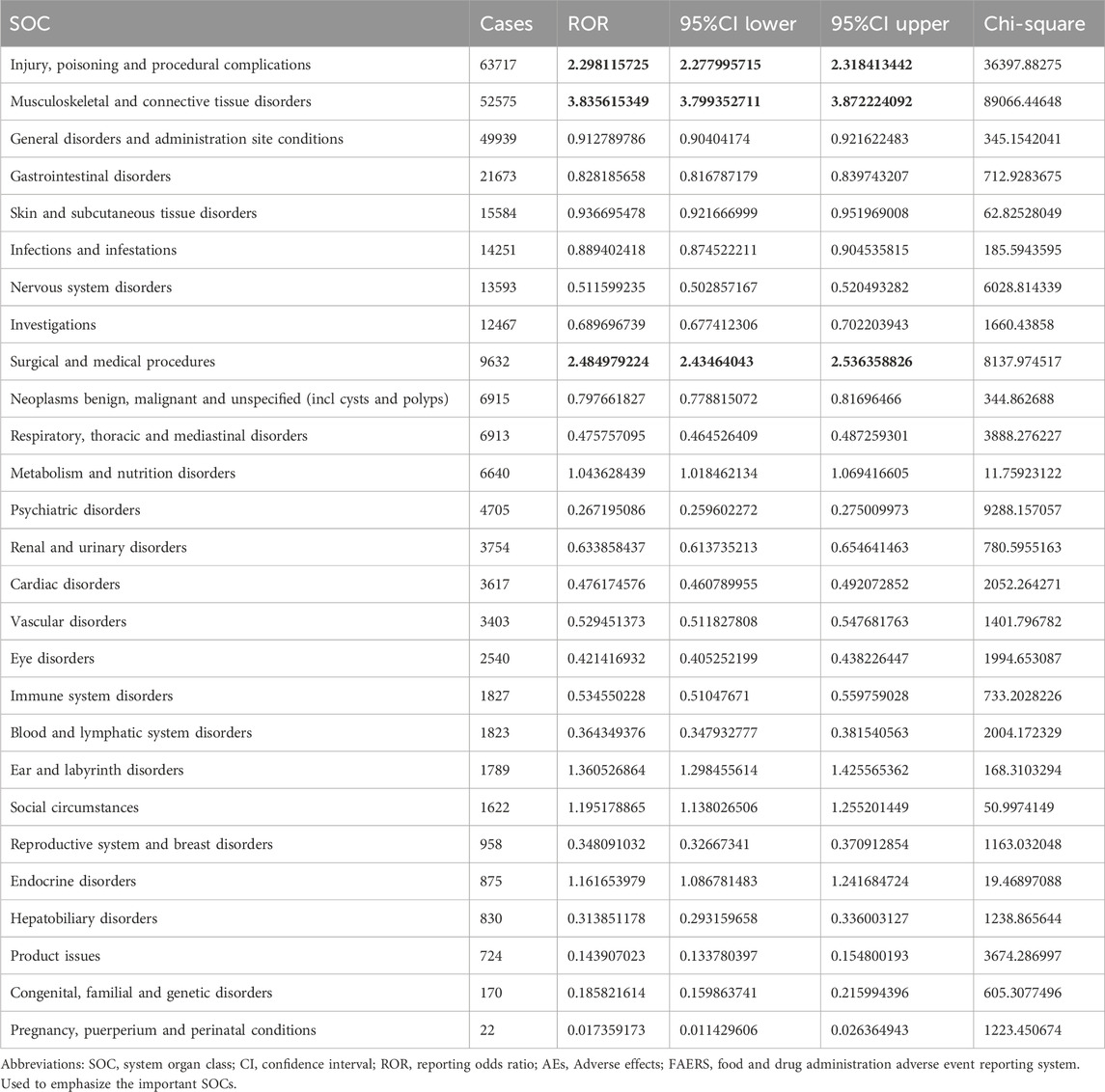
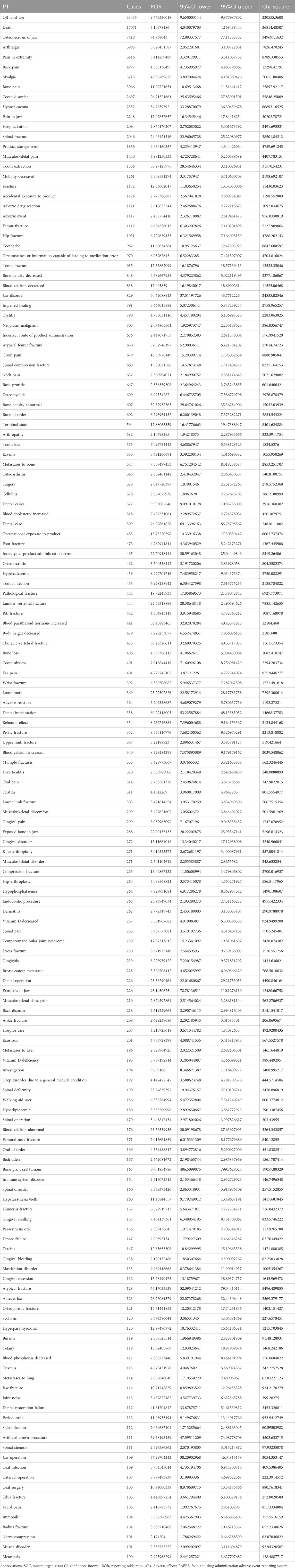
The important SOCs were ‘Musculoskeletal and connective tissue disorders’ (SOC: 10,028,395),’ ‘Surgical and medical procedures (SOC: 10,042,613)’ as well as ‘Injury, poisoning and procedural complications (SOC: 10,022,117)’ (Figure 1; Table 2). Table 3 lists important PTs. Significantly, the data mining process identified several terms, including ‘Off label use (PT: 10,053,762)', ‘Death (PT: 10,011,906)', ‘Osteonecrosis of jaw (PT: 10,064,658)', ‘Arthralgia (PT: 10,003,239)', ‘Pain in extremity (PT: 10,033,425)', ‘Back pain (PT: 10,003,988)', ‘Myalgia (PT: 10,028,411)', ‘Bone pain (PT: 10,006,002)', ‘Tooth disorder (PT: 10,044,034)', and ‘Hypocalcaemia (PT: 10,020,947)'. Events of osteonecrosis of jaw (PT: 10,064,658) and hypocalcaemia (PT: 10,020,947) were reported in patients with denosumab treatment, as noted in the denosumab labeling. In our analysis, joint disorders such as osteoarthritis (PT: 10,057,178), arthritis (PT: 10,003,284), and arthralgia (PT: 10,023,226) were identified, which were associated with clinical trial outcomes.
Onset time of events
The onset times of AEs associated with denosumab were extracted from the database. After excluding false reports (n = 130,301, 89.07%), a total of 15,990 AEs with reported onset times were analyzed. The median onset time was 110 days, with an interquartile range (IQR) of 5–443 days. As illustrated in Table 1, the data suggest that the onset of denosumab-related AEs can span over a year. However, the majority of cases (n = 6,137, 4.2%) occurred within the first month following denosumab initiation. The incidence rates of AEs observed at 2 months (n = 886, 0.61%), 3 months (n = 632, 0.43%), 4 months (n = 516, 0.35%), 5 months (n = 453, 0.31%), and 6 months (n = 481, 0.33%) were comparable, indicating that AEs may occur at any point within the first year of treatment. Additionally, the data demonstrated that AEs occurred after 1 year of denosumab treatment at a rate of 3.26% (n = 4767).
Discussion
Denosumab is one of the human monoclonal antibodies targeting RANKL, a potent inhibitor of osteoclast activity and differentiation and is used in the treatment of osteoporosis and bone metastasis (Cummings et al., 2009; Delmas, 2008). Denosumab, as the first biologic agent utilized in the management of osteoporosis, has exhibited significant anti-resorptive properties and effectiveness in fracture prevention (Ferrari and Langdahl, 2023). Because the systematic review of AEs for denosumab is lacking, this work was conducted to investigate the AEs in patients following denosumab, and to provide reference for clinical safety applications. This study utilizes the FAERS pharmaceutical database to elucidate the various AEs linked to the use of denosumab and is one of the most extensive collections of such cases in history.
About 48.23% of patients receiving denosumab treatment experienced significant intolerances (some of which were even fatal, accounting for 13.47%), which indicates that greater emphasis should be placed on addressing the safety concerns associated with denosumab, in addition to fatal complications, it can be seen that the drug has many different non-fatal AEs, which also plague patients. Furthermore, the study demonstrated that the most prevalent adverse events (AEs) in patients receiving denosumab included osteonecrosis of the jaw, arthralgia, pain in the extremities, back pain, myalgia, bone pain, tooth disorders, and hypocalcemia. Previous investigations have reported that osteonecrosis of the jaw is a common complication of denosumab therapy (Ahdi et al., 2023). Furthermore, the study indicated that drug-associated ONJ represents a significant adverse reaction observed in certain individuals administered commonly used medications for cancer and osteoporosis treatment, such as denosumab and anti-angiogenic agents. This condition is characterized by progressive bone destruction of the mandible or maxilla (Beth-Tasdogan et al., 2022). Our analysis also revealed that ONJ is top-ranked AE. A variety of antiremodeling or antiresorptive drugs, like monoclonal antibodies, bisphosphonates, hormonal replacement therapy, and are commonly administered to a multitude of patients. ONJ is a consequence of decreased bone turnover resulting from the administration of antiresorptive drugs (Uyanne et al., 2014). This reduction in bone turnover is ascribed to the antiresorptive characteristics of both denosumab and bisphosphonates since they impede the bone remodeling process by suppressing osteoclast activity and inducing cellular apoptosis (Zhang et al., 2016). One systematic review and meta-analysis (Boquete-Castro et al., 2016) comprising 7 randomized clinical trials in individuals with cancer treated by denosumab showed that the adverse effects of denosumab displayed that ONJ has an overall incidence of 1.7% [95% CI: 0.9%-3.1%]. Moreover, the study also revealed that pain in extremity, back pain, myalgia, and bone pain are common AEs for patients following denosumab drug use. Similar results have been reported by Vasiliki, et al. (Chatziravdeli et al., 2022). Yumie et al. demonstrated that the major AEs observed in more than 0.5% of patients were arthralgia (0.7%), dizziness (0.7%), myalgia (0.6%), and back pain (0.6%). Tomonori and his colleagues further demonstrated that 1.4% of patients experienced blindness, limb numbness, and diarrhea, while 4.3% of patients sustained new fractures (Kobayakawa et al., 2021). Furthermore, hypocalcemia was found to occur in 0.3% of postmenopausal Korean women with osteoporosis (Rhee et al., 2022). Huang et, al (Huang et al., 2020) reported that in both groups (zoledronic acid, denosumab), 194 patients who received a minimum of one dosage of the study medication had at least one treatment-emergent AE, and 18.6% of patients experienced bone pain in multiple myeloma. Furthermore, the study findings indicate that the prevailing AEs reported in both zoledronic acid and denosumab groups were pyrexia (38.2%, 41.3%), nausea (42.2%, 46.7%), and diarrhea (51.0%, 51.1%). However, our analysis did not identify these symptoms as statistically significant signals, emphasizing the need to enhance management strategies for these AEs (Huang et al., 2020). However, one study reported that denosumab has been displayed to reduce bone pain in individuals with multiple myeloma, breast as well as prostate cancer by avoiding skeletal-associated events, and the findings suggest that denosumab may confer benefits in pain prevention through the delay of bone pain onset, instead of producing direct analgesia (Porta-Sales et al., 2017). Furthermore, research has demonstrated that individuals with a history of mental illness experience recurrent episodes of acute respiratory complications and depressive relapse, which are often accompanied by heightened anxiety and psychomotor inhibition. These occurrences were observed in patients who received sequential administrations of denosumab, without any underlying calculus/phase imbalance, which is considered uncommon adverse events (Oteo-Álvaro et al., 2023), and based on our results, nervous system disorders were not significant signals.
Limitation
There were several limitations in the study. FAERS database, a spontaneous reporting system (SRS), the data mining method applied in this work did not improve it because of its inherent limitations. SRS utilized for signal detection relies on data from both clinical trials and post-marketing reports. Nevertheless, these systems are limited by the registration of only observed adverse events, leading to potential underreporting and reporting bias (Noguchi et al., 2021). The voluntary nature of adverse drug reaction (ADR) reporting within the FAERS system presents notable challenges, including under-reporting together with possible reporting biases like uneven information quality, false reporting, and inaccuracy. Furthermore, other biases including the weber effect, notoriety effects, masking effect or cloaking effect might be caused due to SRS which might have an impact on the results, thus, it is crucial to comprehend the range of biases present in adverse event reporting in order to accurately interpret data (Noguchi et al., 2021). Furthermore, although data mining techniques facilitated the identification of adverse reaction signals associated with denosumab, this alone does not establish a causal relationship. It is imperative to validate these findings through prospective studies. Future large-scale, population-based prospective studies are necessary to accurately determine the incidence of denosumab-related potential adverse events and to comprehensively elucidate the underlying biological mechanisms and risk factors, thereby enhancing risk management strategies. Furthermore, the utilization or exposure to multiple medications presents a challenge in discerning the specific etiology of adverse events, as well as determining the clinical indication for these pharmaceutical agents.
Conclusion
The current investigation, utilizing the FAERS database, has indicated potential safety concerns regarding the utilization of denosumab, specifically in relation to an elevated likelihood of experiencing osteonecrosis of the jaw, arthralgia, pain in extremities, back pain, myalgia, and bone pain events. Additionally, unforeseen adverse events such as nervous disorder may also manifest. Consequently, it is advised that vigilant monitoring and identification of these adverse events be implemented across all populations. To substantiate these findings and gain a more comprehensive understanding of denosumab’s safety profile, further research in the form of cohort studies and long-term clinical investigations is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Sichuan Bayi Rehabilitation Center (Sichuan Rehabilitation Hospital). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YH: Conceptualization, Data curation, Formal Analysis, Investigation, Writing–original draft. RZ: Investigation, Visualization, Writing–original draft. HS: Supervision, Validation, Writing–original draft. YL: Software, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project is sponsored by Natural Science Foundation of Chongqing, China (grant no. CSTB2023NSCQ-MSX0644), Sichuan Science and Technology Program (grant no. 2024NSFSC0570), Science and Technology Project of Sichuan Provincial Administration of Traditional Chinese Medicine: (grant no. 2024zd018) and Chinese Medicine Rehabilitation the Key Discipline Constructed by Chongqing Health Bureau (grant no. 2021-4322190044).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahdi, H. S., Wichelmann, T. A., Pandravada, S., and Ehrenpreis, E. D. (2023). Medication-induced osteonecrosis of the jaw: a review of cases from the Food and Arug administration adverse event reporting system (FAERS). BMC Pharmacol. Toxicol. 24 (1), 15. doi:10.1186/s40360-023-00657-y
Beth-Tasdogan, N. H., Mayer, B., Hussein, H., and Zolk, O. (2022). Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 7 (7), Cd012432. doi:10.1002/14651858.CD012432.pub2
Bone, H. G., Wagman, R. B., Brandi, M. L., Brown, J. P., Chapurlat, R., Cummings, S. R., et al. (2017). 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 5 (7), 513–523. doi:10.1016/S2213-8587(17)30138-9
Boquete-Castro, A., Gómez-Moreno, G., Calvo-Guirado, J. L., Aguilar-Salvatierra, A., and Delgado-Ruiz, R. A. (2016). Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants Res. 27 (3), 367–375. doi:10.1111/clr.12556
Chatziravdeli, V., Katsaras, G. N., Katsaras, D., Doxani, C., Stefanidis, I., and Zintzaras, E. (2022). A systematic review and meta-analysis of interventional studies of bisphosphonates and denosumab in multiple myeloma and future perspectives. J. Musculoskelet. Neuronal Interact. 22 (4), 596–621.
Chen, C., Chen, T., Liang, J., Guo, X., Xu, J., Zheng, Y., et al. (2021). Cardiotoxicity induced by immune checkpoint inhibitors: a pharmacovigilance study from 2014 to 2019 based on FAERS. Front. Pharmacol. 12, 616505. doi:10.3389/fphar.2021.616505
Cummings, S. R., San Martin, J., McClung, M. R., Siris, E. S., Eastell, R., Reid, I. R., et al. (2009). Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361 (8), 756–765. doi:10.1056/NEJMoa0809493
Delmas, P. D. (2008). Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J. Clin. Densitom. 11 (2), 325–338. doi:10.1016/j.jocd.2008.02.002
Ferrari, S., and Langdahl, B. (2023). Mechanisms underlying the long-term and withdrawal effects of denosumab therapy on bone. Nat. Rev. Rheumatol. 19 (5), 307–317. doi:10.1038/s41584-023-00935-3
Gopaul, A., Kanagalingam, T., Thain, J., Khan, T., Cowan, A., Sultan, N., et al. (2021). Denosumab in chronic kidney disease: a narrative review of treatment efficacy and safety. Arch. Osteoporos. 16 (1), 116. doi:10.1007/s11657-021-00971-0
Huang, S. Y., Yoon, S. S., Shimizu, K., Chng, W. J., Chang, C. S., Wong, R. S. M., et al. (2020). Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, randomized controlled phase 3 study-asian subgroup analysis. Adv. Ther. 37 (7), 3404–3416. doi:10.1007/s12325-020-01395-x
Kendler, D. L., Cosman, F., Stad, R. K., and Ferrari, S. (2022). Denosumab in the treatment of osteoporosis: 10 Years later: a narrative review. Adv. Ther. 39 (1), 58–74. doi:10.1007/s12325-021-01936-y
Kobayakawa, T., Miyazaki, A., Saito, M., Suzuki, T., Takahashi, J., and Nakamura, Y. (2021). Denosumab versus romosozumab for postmenopausal osteoporosis treatment. Sci. Rep. 11 (1), 11801. doi:10.1038/s41598-021-91248-6
Lamy, O., Stoll, D., Aubry-Rozier, B., and Rodriguez, E. G. (2019). Stopping denosumab. Curr. Osteoporos. Rep. 17 (1), 8–15. doi:10.1007/s11914-019-00502-4
Noguchi, Y., Tachi, T., and Teramachi, H. (2021). Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform 22 (6), bbab347. doi:10.1093/bib/bbab347
Oteo-Álvaro, Á., García, C. G., Sánchez, A. I., Santamaria, C. A., and de Diego-Adeliño, J. (2023). Neuropsychiatric adverse reactions in patients treated with denosumab: two case reports and a review of data from the FDA Adverse Event Reporting System (FAERS). Osteoporos. Int. 34 (10), 1799–1804. doi:10.1007/s00198-023-06838-z
Porta-Sales, J., Garzón-Rodríguez, C., Llorens-Torromé, S., Brunelli, C., Pigni, A., and Caraceni, A. (2017). Evidence on the analgesic role of bisphosphonates and denosumab in the treatment of pain due to bone metastases: a systematic review within the European Association for Palliative Care guidelines project. Palliat. Med. 31 (1), 5–25. doi:10.1177/0269216316639793
Rhee, Y., Chang, D. G., Ha, J., Kim, S., Lee, Y., Jo, E., et al. (2022). Real-world safety and effectiveness of denosumab in patients with osteoporosis: a prospective, observational study in South Korea. Endocrinol. Metab. Seoul. 37 (3), 497–505. doi:10.3803/EnM.2022.1427
Rothman, K. J., Lanes, S., and Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 13 (8), 519–523. doi:10.1002/pds.1001
Sakaeda, T., Tamon, A., Kadoyama, K., and Okuno, Y. (2013). Data mining of the public version of the FDA adverse event reporting system. Int. J. Med. Sci. 10 (7), 796–803. doi:10.7150/ijms.6048
Simonet, W. S., Lacey, D. L., Dunstan, C. R., Kelley, M., Chang, M. S., Lüthy, R., et al. (1997). Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89 (2), 309–319. doi:10.1016/s0092-8674(00)80209-3
Uyanne, J., Calhoun, C. C., and Le, A. D. (2014). Antiresorptive drug-related osteonecrosis of the jaw. Dent. Clin. North Am. 58 (2), 369–384. doi:10.1016/j.cden.2013.12.006
Keywords: denosumab, FAERS, adverse events, osteonecrosis of the jaw, RANKL (receptor activator for nuclear factor k B ligand)
Citation: He Y, Zhang R, Shen H and Liu Y (2024) A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for denosumab. Front. Pharmacol. 15:1339721. doi: 10.3389/fphar.2024.1339721
Received: 16 November 2023; Accepted: 20 August 2024;
Published: 30 August 2024.
Edited by:
Eli Ehrenpreis, Advocate Lutheran General Hospital, United StatesReviewed by:
Yoshihiro Noguchi, Gifu Pharmaceutical University, JapanJosef Yayan, University of Witten/Herdecke, Germany
Copyright © 2024 He, Zhang, Shen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingqi Liu, eWluZ3FpbGl1QGVtYWlsLnN3dS5lZHUuY24=
 Yue He
Yue He Rong Zhang3
Rong Zhang3 Yingqi Liu
Yingqi Liu