
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 25 January 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1338951
This article is part of the Research Topic Preventive Potential of Antioxidants in Age-Related Diseases View all 7 articles
 Ting-Ting Deng1
Ting-Ting Deng1 Wen-Yu Ding2,3
Wen-Yu Ding2,3 Xi-Xue Lu4
Xi-Xue Lu4 Qing-Hao Zhang4
Qing-Hao Zhang4 Jin-Xin Du1
Jin-Xin Du1 Li-Juan Wang1,4
Li-Juan Wang1,4 Mei-Na Yang5*
Mei-Na Yang5* Ying Yin6*
Ying Yin6* Fan-Jie Liu4*
Fan-Jie Liu4*Osteoporosis (OP) is a bone disease associated with increasing age. Currently, the most common medications used to treat OP are anabolic agents, anti-resorptive agents, and medications with other mechanisms of action. However, many of these medications have unfavorable adverse effects or are not intended for long-term use, potentially exerting a severe negative impact on a patient’s life and career and placing a heavy burden on families and society. There is an urgent need to find new drugs that can replace these and have fewer adverse effects. Quercetin (Que) is a common flavonol in nature. Numerous studies have examined the therapeutic applications of Que. However, a comprehensive review of the anti-osteoporotic effects of Que has not yet been conducted. This review aimed to describe the recent studies on the anti-osteoporotic effects of Que, including its biological, pharmacological, pharmacokinetic, and toxicological properties. The outcomes demonstrated that Que could enhance OP by increasing osteoblast differentiation and activity and reducing osteoclast differentiation and activity via the pathways of Wnt/β-catenin, BMP/SMAD/RUNX2, OPG/RANKL/RANK, ERK/JNK, oxidative stress, apoptosis, and transcription factors. Thus, Que is a promising novel drug for the treatment of OP.
Osteoporosis (OP) is the most prevalent type of systemic bone disease and is characterized by decreased bone mass and damage to the microstructure of the bone tissue, leading to increased bone fragility (Kanis et al., 2019). Patients with OP are more prone to fractures. Over 200 million people worldwide have OP (Palacios, 2022). The lifetime risk of developing the disease in Caucasian women aged >50 years is 50%, and OP-induced fractures can result in substantial medical expenses (up to $25.3 billion by 2025) and impaired physical function (Armas and Recker, 2012).
Nearly all clinical medicine preparations are derived from natural compounds found in plants, animals, insects, marine organisms, and microbes (Harvey et al., 2015). Among them, flavonoids (Rodríguez et al., 2022), alkaloids (Lin et al., 2022), polysaccharides (Lei et al., 2021), quinones (Geng et al., 2019), terpenoids (Bellavia et al., 2021), lignans (Jang et al., 2022), saponins (Siddiqi et al., 2013), polyphenols (Tao et al., 2021), and amino acids (Ling et al., 2021) have been shown to have the ability to inhibit bone resorption and promote bone formation. Quercetin (Que) is a flavonoid compound widely found in traditional Chinese medicines, such as yam bean root, Scutellaria baicalensis, and Sophora japonica. Its chemical name is 3,3′,4′,5,7 pentahydroxyflavonoid, molecular formula is C15H10O7, and relative molecular mass is 302.236. The appearance is of a yellow acicular crystalline powder, slightly soluble in water, and readily soluble in an alkaline aqueous solution (Yang et al., 2020). Numerous therapeutic effects, such as osteoprotective (Xiao et al., 2023), neuroprotective (Fideles et al., 2023), antiallergic (Mlcek et al., 2016), anti-inflammatory (Hou et al., 2019), anticancer (Rauf et al., 2018), cardiovascular protection (Patel et al., 2018), antiviral (Di Petrillo et al., 2022), antidiabetic (Eid and Haddad, 2017), immunomodulatory (Wang et al., 2021), antihypertensive (Luo et al., 2017), and gastroprotective (Martín et al., 1998), have been reported. Que inhibits bone resorption and promotes bone formation. We reviewed the anti-osteoporosis effects of quercetin and its mechanisms, and summarized its application at the cellular level and in animal experiments in Table 1 and Table 2 respectively.
Que exists in the flowers, leaves, and fruits of many plants (Wang et al., 2016; Kandemir et al., 2022), mostly in the form of glycosides (Crespy et al., 2001), such as rutin (rutinoside), chrysin, and other plants with high content. Que is a bioactive flavonol found mainly in frequently consumed plant foods such as onions, apples, berries, and broccoli (David et al., 2016). Many herbs, including Ginkgo biloba, mulberry leaf, cuscuta, golden buckwheat, forsythia, Panax ginseng, and Fritillaria, contain Que (Shao et al., 2023). Que exists in the skin and leaves of the Iberian oak Quercus iberica of the family Crustacea, the red octopus of the family Berberidaceae, the red drought lily (Hunan forsythia) of the family Hypericum, and the red flax leaves of the family Oleaceae.
Que is a yellow needle-like crystal (Zou et al., 2021), mostly in the form of glycosides, and can be obtained by acid hydrolysis. Que dihydrate appears as yellow needle-like crystals (dilute ethanol), becomes anhydrous at 95°C–97°C, and has a melting point of 314°C (decomposition). Que is soluble in cold ethanol (1:290), hot ethanol (1:23), methanol, ethyl acetate, glacial acetic acid, pyridine, and acetone. However, it is insoluble in water, benzene, ethyl ether, chloroform, and petroleum ether. The alkaline aqueous solution is yellow and almost insoluble in water, and the ethanol solution tastes very bitter (Wang et al., 2016). The structure of Que is shown in Figure 1.
Biosynthesis of Que via the phenylpropyl metabolic pathway. In the biosynthesis of Que, phenylalanine derived from the mangiferolic acid pathway is used as the initial precursor to generate cinnamic acid by removing the amino group, catalyzed by phenylalanine ammonia-lyase. Cinnamic acid acquires a hydroxyl group through the catalytic action of cinnamate 4-hydroxylase to form p-coumaric acid. p-Coumaric acid undergoes a thioesterification reaction catalyzed by coumaryl 4-ligase to produce p-coumaroyl coenzyme A. Condensation of one molecule of p-coumaroyl coenzyme A and three molecules of malonyl coenzyme A catalyzed by chalcone synthase produces naringenin chalcone. Naringenin is catalyzed by flavanone 3-hydroxylase to form dihydrokaempferol. Flavanone 3′-hydroxylase is hydroxylated by dihydrokaempferol to form dihydroquercetin. Finally, dihydroquercetin is catalyzed by flavonol synthase for Que biosynthesis (Lakhanpal P and Rai, 2007; Alrawaiq and Abdullah, 2014; Nabavi et al., 2020). The biosynthetic pathway of Que is shown in Figure 2.
Osteoblasts (OBs) undergo four stages of bone formation: proliferation, extracellular matrix maturation, extracellular matrix mineralization, and apoptosis (Marcus and Majumder, 2001). They are affected by a variety of transcription factors and signaling pathways during various stages of development to ultimately complete normal bone formation. The factors and signaling pathways by which Que influences OB-mediated bone formation are outlined below. A diagram of the mechanisms involved in this part of the study are shown in Figure 3.
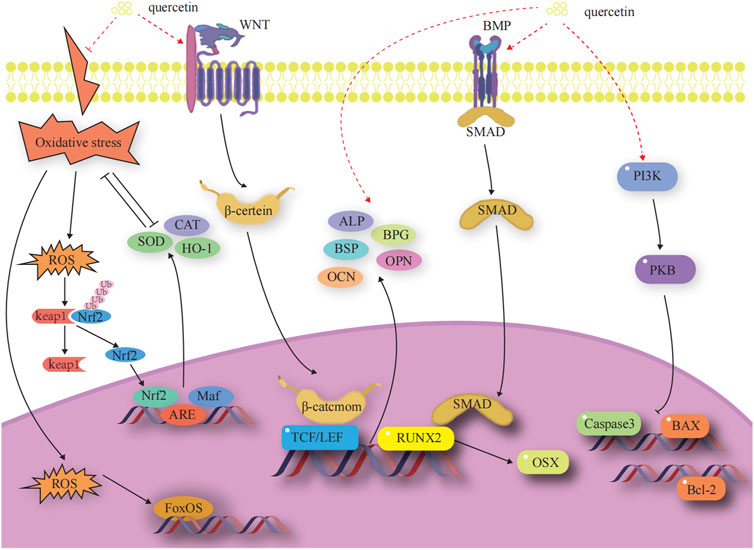
FIGURE 3. Effects and molecular mechanisms of quercetin induction on osteoblasts. (Little et al., 2002; Nakashima et al., 2002; Steitz et al., 2002; Zhang et al., 2003; Ohri et al., 2005; Kim et al., 2007; Chen et al., 2008; Nusse et al., 2008; Clevers et al., 2014; Jin et al., 2016; An and Shang, 2018; Gao et al., 2018; Zhang et al., 2019; Kim et al., 2021; Kimball et al., 2021; Li et al., 2021; Yu et al., 2021).
OB-specific Runt-associated transcription factor 2/core-binding factor alpha 1 and special protein 7 transcription factor (osterix) are required for the differentiation of mesenchymal stem cells (MSCs) to OBs and for the formation of functional OBs. The RUNX2 gene is the most critical transcription factor that regulates the differentiation of bone marrow mesenchymal stem cells (BMSCs) into OBs and the maturation of OBs in the process of bone development (Zhang et al., 2003). Expression of the RUNX2 gene is a marker for the onset of OB differentiation, making it the earliest and most specific gene in the process of bone formation. Deletion of this gene will result in hypoplasia or termination of bone development. Osterix acts as a downstream gene of RUNX2 to play an osteogenic role and is expressed exclusively in the cells of the bone tissue, which is required for the differentiation of OBs and for bone formation (Gao et al., 2018). (Figure 3)
Yang (2022) treated mouse bone marrow mesenchymal stem cells (mBMSCs) with Que and found that 5 μM Que significantly upregulates the expression levels of RUNX2 and SP7 mRNA. Xiao et al. (2023) used ferric ammonium citrate (FAC, 200 μM) to construct an iron overload environment. Iron deposition inhibits osteogenic differentiation of MC3T3-E1 cells and suppresses the expression of RUNX2 and Osterix. Iron-overloaded mice have reduced bone mass, loose trabeculae, and thinner bone marrow cavities. Que (2.5 or 5 μM) can rescue the proliferation inhibition of MC3T3-E1 cells induced by FAC and prevent the reduction of RUNX2 and Osterix expression. This action, in turn, rescues the dysfunction of osteogenic differentiation and attenuates the bone loss of MC3T3-E1 cells induced by iron overload.
The classical Wnt signaling pathway is an important regulator of bone resorption and formation (Nusse et al., 2008; Clevers et al., 2014). The Wnt signaling pathway is activated when Wnt ligands bind to the heterodimeric receptor molecules of the frizzled protein family and low-density lipoprotein receptor-related protein 5 (LRP5). Subsequently, the Wnt protein binds to the cell surface receptors of the frizzled protein family, which activates the disheveled protein family and the downstream factor GSK-3β. This activation maintains β-catenin in a stable state in the cytoplasm. Eventually, β-catenin enters into the nucleus and interacts with the transcription factor Tcf/Lef to activate the expression of the target genes of Wnt signaling (Little et al., 2002; Nakashima et al., 2002). Dickkopf-1, a secreted glycoprotein, antagonizes Wnt/β-catenin signaling pathway activity by competitively binding to LRP5/6 receptors with Wnt proteins, thereby promoting bone destruction. (Figure 3).
Que promotes osteogenic differentiation of MC3T3-E1 cells by increasing β-catenin protein levels and activating the Wnt/β-catenin pathway (Guo et al., 2017). miR-625-5p expression upregulation or H19 expression downregulation suppresses β-catenin protein levels, and Que promotes the proliferation and osteogenic differentiation of BMSCs by targeting the H19/miR-625-5p axis to activate the downstream Wnt/β-catenin pathway (Bian et al., 2021). However, a high concentration of Que (10 μmol/L) inhibits the Wnt/β-catenin pathway. It downregulates the expression of the cyclin D1 gene involved in the G1/S cell cycle transition in stem cells. Additionally, it decreases the nuclear β-catenin level in undifferentiated MSCs as well as in MSCs induced to differentiate into OBs. The inhibition of the Wnt/β-catenin pathway results in the inhibition of OB formation in MSCs and promotes adipogenesis (Casado-Díaz et al., 2016).
The BMP-2/Smad signaling pathway affects OB differentiation and bone formation (Yu et al., 2021). After the release of BMP-2 in autocrine or paracrine forms, its monomer can form dimers through disulfide bonding. These dimers bind to BMP receptors, regulating the transcription of osteogenic genes through downstream Smad signaling. This process results in the upregulation of RUNX2 and OSX, promoting osteoclast (OC) proliferation and differentiation (Kim et al., 2021). (Figure 3).
Que (2 or 5 μM) increases the relative alkaline phosphatase (ALP) activity and matrix mineralization of mBMSCs and significantly upregulates the mRNA levels of ALP, RUNX2, BMP2, and other OB marker genes, thus promoting the osteogenic differentiation of the third-generation mBMSCs (Yang, 2022). Que enhances the activation of the BMP signaling pathway through the endoplasmic reticulum, upregulates the expression of downstream genes, such as OSX, RUNX2, and OPN, and promotes the proliferation and osteogenic differentiation of BMSCs; crosstalk between BMP-2 and estrogen receptor signaling pathways was experimentally confirmed. The upregulation of RUNX2, OSX, and OPN gene expression by Que and estrogen is inhibited after the addition of ICI182780, an estrogen receptor antagonist (Pang et al., 2018).
Apoptosis is a process by which cells respond to physiological and pathological stimulation signals from the environment, changes in environmental conditions, or palliative damage. The Bcl family plays a key role in promoting or inhibiting the intrinsic apoptotic pathway triggered by mitochondrial dysfunction (Zhang et al., 2019). Bcl-2 and Bax are antagonistic proteins involved in the regulation of apoptosis. When Bcl-2 is highly expressed, it promotes the formation of Bax/Bcl-2 heterodimers and inhibits the formation of Bax/Bax homodimers, preventing the release of pro-apoptotic factors, such as cytochrome c. When Bax proteins are highly expressed, they increase the formation of Bax/Bax homodimers and inhibit the formation of Bax/Bax heterodimers. This leads to the activation of the expression of downstream caspase family proteins, promoting the release of apoptotic factors and initiating apoptosis (Chen et al., 2008). (Figure 3).
Que can reduce FAC-induced apoptosis and reactive oxygen species (ROS) production, downregulate the expression of caspase-3 and Bax, and upregulate the expression of Bcl-2 (Xiao et al., 2023; Zhu et al., 2023). Que can attenuate chondrocyte apoptosis by modulating the Bcl-2/Bax-caspase-3 signaling pathway to reduce sodium nitroprusside-induced overproduction of intracellular ROS and restore mitochondrial membrane potential (Hu et al., 2019).
Oxidative stress is a pivotal factor that contributes to the functional uncoupling of OBs and OCs in OP. When a redox imbalance exists in cells, free Nrf2 binds to antioxidant response elements in the nucleus, thereby activating the expression of detoxification genes. Heme oxygenase 1 (HO-1), a well-known phase II detoxification enzyme, inhibits cytotoxicity originating from a variety of oxidative stresses and inflammatory responses, significantly balancing redox homeostasis (Jin et al., 2016). Under oxidative stress, cellular defense mechanisms against oxidative damage are enhanced by upregulating Nrf2 and HO-1 expression (An and Shang, 2018). Members of the forkhead box class O protein (FOXO) family are activated when intracellular reactive oxygen species are increased. FOXO binds β-catenin to shift transcription mediated by the transcription factor T cell factor/lymphocyte enhancer factor in the Wnt pathway toward FOXO transcription to increase the expression of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT), to attenuate oxidative stress damage, attenuate osteogenic differentiation of BMSCs, and reduce OB bone formation (Kimball et al., 2021). (Figure 3).
Que dose-dependently upregulates HO-1 mRNA expression in BMSCs (Papiez et al., 2008). It significantly enhances Nrf2 nuclear translocation in FAC-induced MC3T3-E1 cells and attenuates FAC-induced oxidative stress injury by activating the Nrf2/HO-1 signaling pathway (Xiao et al., 2023). Que downregulates ROS levels and upregulates the expression of antioxidant genes (Nrf2, CAT, SOD-1, and SOD-2) in BMSCs stimulated by H2O2 in vitro, maintaining the viability of BMSCs and OB differentiation (Lan et al., 2022).
Three non-collagenous proteins, namely, bone salivary protein (BSP), osteopontin (OPN), and osteocalcin (OCN), are essential for the formation and maturation of mineralized tissues. BSP is an effective nucleating agent for hyaluronic acid formation in stabilized agarose gel systems (Steitz et al., 2002). The presence of aspartic acid and phosphoserine in large quantities in the OPN molecule facilitates its binding to the surface of calcium-phosphorus crystals in mineralized tissues and inhibits the calcification and growth of these crystals (Ohri et al., 2005; Li et al., 2021). OCN is involved in the regulation of bone resorption and participates in matrix mineralization processes and OB differentiation. It is associated with bone turnover, maintains the normal rate of bone mineralization, inhibits the rate of cartilage mineralization, and inhibits the formation of abnormal hydroxyapatite crystals in the bone (Kim et al., 2007). (Figure 3).
Que increases BSP transcription in OB-like cells by targeting the reversed CCAAT and FRE elements in the proximal BSP gene promoter (Hunter and Goldberg, 1993). Treatment with Que significantly upregulates the expression of OCN and OPN mRNA in BMSCs, increases the number of mineralized nodules and the accumulation of mineralized matrix in BMSCs, and promotes the osteogenic differentiation of BMSCs (Zhang et al., 2020). Pretreatment with Que significantly restores bone mineralization and OCN mRNA and protein expression levels in lipopolysaccharide (LPS)-inhibited MC3T3-E1 cells in a dose-dependent manner (Guo et al., 2017).
OCs are multinucleated giant cells with bone resorption functions, formed by the fusion of bone marrow monocyte precursors (Boyce, 2013). They promote bone resorption and remodeling by secreting acids and enzymes that dissolve the bone matrix. When OCs are overactive or in excessive numbers, the equilibrium between OBs and OCs is disrupted, leading to excessive bone resorption, which can also lead to OP. When exercising the function of bone resorption, OCs initially adhere to the surface of the bone matrix to form a closed area, releasing integrins ɑ and β3 to facilitate adherence to the bone matrix. Subsequently, the H+ ion pump with the help of ATP6V0d2 pumps H+ into the closed area, forming an acidic microenvironment to degrade the bone matrix. Meanwhile, OCs secrete tartrate-resistant acid phosphatase (TRAP), matrix metalloproteinase 9 (MMP-9), and cathepsin K (CtsK), among others to dissolve the bone matrix (Soysa and Alles, 2016). The mechanisms underlying the Que regulation of OC-mediated bone resorption are shown in Figure 4.
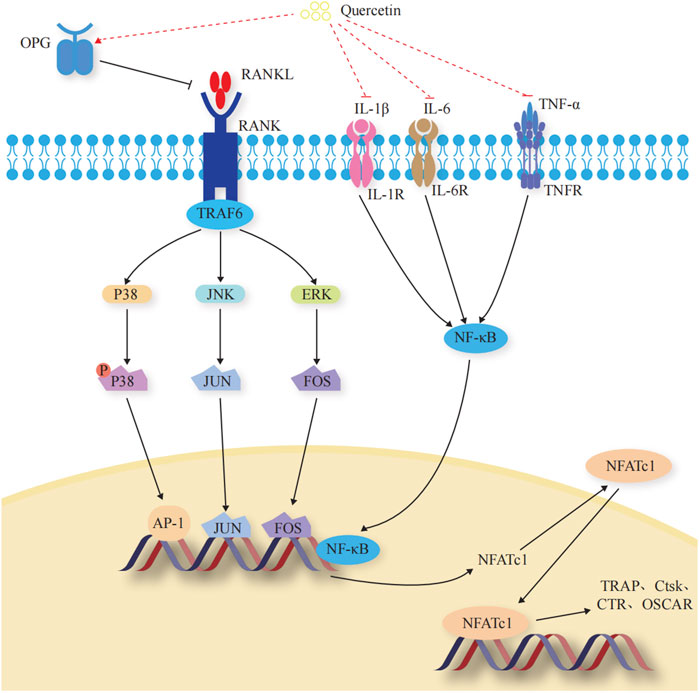
FIGURE 4. Effects and molecular mechanisms of quercetin induction on osteoclasts. (Takayanagi et al., 2002; Teitelbaum and Ross, 2003; Duplomb et al., 2008; Ikeda et al., 2008; Belibasakis and Bostanci, 2012; Ibrahim et al., 2016; Lee et al., 2016; Pinti et al., 2016; Soysa and Alles, 2016; Amarasekara et al., 2018; Lee et al., 2018; Luo et al., 2018; Noguchi et al., 2018; Ha and Choung, 2020; Kurotaki et al., 2020; Yang et al., 2020; Udagawa et al., 2021; Velletri et al., 2021; Yao et al., 2021).
Transcription factors regulate the differentiation and activity of OCs (Kurotaki et al., 2020). Nuclear factor of activated T cell 1 (NFATc1) is the most critical regulator of OC differentiation (Takayanagi et al., 2002). It controls OC-specific genes, such as TRAP, Ctsk, calcitonin receptor, and OC-associated receptor, through the synergistic activation of microphthalmia-associated transcription factor (Mitf), nuclear factor kappa B (NF-κB), c-Fos, and c-jun, thus affecting OC activity and regulating bone resorption (Yao et al., 2021). (Figure 4).
Treatment with Que reduces the expression of the NFATc1 gene and protein through the TRAF6/c-fos/NFATc1 signaling pathway and inhibits the differentiation of RAW264.7 cells to OCs (Liu et al., 2022). Que-3-O-β-D-glucoside inhibits OC differentiation by suppressing RANKL-induced NFATc1 expression, thereby preventing OC differentiation and bone loss (Shim et al., 2015).
The OPG/RANKL/RANK signaling pathway is a key signaling axis during bone remodeling (Yang et al., 2020; Velletri et al., 2021). In the presence of M-CSF, RANK binds to the C-terminus of RANKL and recruits tumor necrosis factor receptor-associated factors to induce transcription and expression of downstream OC-specific genes. It ultimately activates key transcription factors, such as NF-κB, activator protein 1, cyclic adenosine monophosphate, cyclic adenosine monophosphate response element binding protein, and NFATc1, and induces OC markers, such as TRAP, β3 integrin, and CtsK, which regulate OC differentiation and induce bone resorption (Belibasakis and Bostanci, 2012; Udagawa et al., 2021). OPG secreted by OB is a high-affinity decoy-like receptor for RANKL. OPG reduces the binding of RANKL to RANK and competitively inhibits the blocking of signals from OBs to OCs, thereby inhibiting OC generation and maturation and reducing OC activity (Ibrahim et al., 2016). (Figure 4).
Que upregulates the expression of OPG and downregulates the expression of RANKL in the femoral bone tissues of ovariectomized rats at both high and medium doses, inhibits bone resorption, prevents OP, and improves the biomechanical properties of the femur (Wang, 2009). Que can directly stimulate OPG expression in OVX rBMSCs while concurrently inhibiting RANKL expression. This dual action leads to the indirect increase of the OPG/RANKL ratio, reestablishing the balance of the RANKL/OPG system and restoring the healing capacity of damaged bone in the state of OP (Zhou et al., 2017). When OB-OC-endothelial cells were inoculated in three cultures on Que-containing hydroxyapatite, the trend of OPG/RANKL levels was similar to the reduction in histone K levels, suggesting that the presence of Que has an inhibitory effect on OC viability (Forte et al., 2016).
Among the OC precursors, two ERK forms (ERK1/2) and three JNK isoforms (JNK1/2/3) are mainly involved in OC precursor proliferation and OC apoptosis, respectively (Teitelbaum and Ross, 2003; Ikeda et al., 2008; Lee et al., 2016). ERKs have a typical protein kinase structure and control osteoclastogenesis by phosphorylating c-Fos, NFATc1, MITF, TFE3, hedgehog-Gli, Egr2, RSK2, and MMP-9 (Lee et al., 2018). JNK signaling regulates downstream c-Jun, CaMK, c-Fos, NFATc1, and semaphorin 3D to control OC metabolism (Noguchi et al., 2018). (Figure 4).
Que-3-O-β-D-glucuronide significantly attenuates the activation of JNK and ERK in LPS-stimulated RAW264.7 macrophages. It inhibits the secretion of plasmatic NO and PGE, as well as the expression of iNOS and COX-2, thus exerting an anti-inflammatory activity in a concentration-dependent manner (Park et al., 2016). Que exerts its anti-apoptotic effects through the JNK-c-Jun/AP-1 and ERK-c-Fos/AP-1 pathways (Ishikawa and Kitamura, 2000).
Dynamic regulation of osteoclastogenic and anti-osteoclastogenic cytokines is essential for maintaining bone homeostasis (Amarasekara et al., 2018). During macrophage polarization, many inflammatory factors are involved in osteoclastogenesis through different pro-inflammatory and anti-inflammatory roles, further affecting the process of bone resorption and the development of OP (Pinti et al., 2016). Tumor necrosis factor-α (TNF-α) stimulates OC differentiation by upregulating RANK pro-inflammatory target genes through activating and inducing NF-κB nuclear translocation, disrupting the balance of the RANK-RANKL bio-axis, and increasing OC activity (Luo et al., 2018). Interleukin—1β (IL-1β) and IL-6 promote OC differentiation and maturation through a RANKL-independent mechanism, resulting in the occurrence of bone resorption (Duplomb et al., 2008; Ha and Choung, 2020). (Figure 4).
Que (2 or 5 μM) significantly reduces the accumulation of TNF-α and IL-6 in LPS-induced mouse RAW264.7 macrophages (Jung and Sung, 2004). Tsai et al. (2021) found that Que inhibits M1 polarization and significantly reduces the expression levels of M1 markers, such as IL-6, TNF-α, and IL-1β, in macrophages and microglia. Que significantly reduces the levels of TNF-α and IL-1β, inhibits OC activation, and attenuates bone destruction (Li et al., 2018).
Phytochemical treatments have been used to induce OB differentiation in in vitro models (Pan et al., 2005; Shakibaei et al., 2012; Zhou et al., 2015; Martiniakova et al., 2022; Shen et al., 2022; Zhou et al., 2023). The combination of Que with other bioactive ingredients has shown synergistic anti-osteoporotic effects (Rayalam et al., 2011). Giordani et al. (2023) found that a mixture of curcumin (1 M), polydatin (10 M), and Que (0.5 M) is a safe bioactive compound. This mixture has significant synergistic effects in promoting OB differentiation of MSCs and inhibiting inflammatory phenotypes associated with cellular senescence. It significantly reduces the expression of miR-21 and miR-146a, decreases the release of IL-8 and MCP-1, increases the expression of ALP mRNA, and efficiently reduces p38-MAPK and phosphorylated NF-κB. Lai et al. (2011) found that a combination of 2400 IU/kg of vitamin D, 400 mg/kg of resveratrol, 2,000 mg/kg of Que, and 1,040 mg/kg of genistein improves bone mineral density and trabecular structure and reduces postmenopausal bone loss in de-ovulated female rats. However, Ambati et al. (2018) found that the dietary intake of a combination of vitamin D, resveratrol, Que, and genistein, owing to their relatively low dose and synergistic properties, is not as effective as zoledronic acid in a postmenopausal rat model of OP. They reported that relatively low doses of phytochemicals may not have produced a sufficiently potent effect to prevent or reverse the dramatic loss of bone trabeculae induced by ovarian hormone deficiency. This lack of efficacy may be related to the prolonged duration of the study (16 weeks) and the dynamics of bone loss after estrogen withdrawal.
Researchers have used high-performance liquid chromatography to determine the concentration of Que in various organ tissues. They found that Que is mainly distributed in the gastrointestinal tract by gastrointestinal ease of diffusion and absorption, followed by the blood, liver, kidney, heart, lungs, and spleen, and has a very low distribution in the brain and muscle tissues of rats (Meng and Wang, 2000; Wu et al., 2008). Que metabolites are excreted through the kidneys, feces, and the respiratory system (Moon et al., 2000; Russo et al., 2012). Que is rapidly metabolized in the blood and has a short half-life. Que metabolites are detected in plasma 30 min after ingestion, with the major metabolites being Que-30-sulfate, Que-3-glucuronide, and Que-3-sulfate. The highest concentrations are observed at 0.8 and 0.6 h, but they are excreted in large quantities within 24 h (Mullen et al., 2006; Moon et al., 2008). Que-30-glucuronide, Que-diuronate, isorhamnetin-sulfate glucuronate, isorhamnetin-methylquercetin, and isorhamnetin-diuronate are the major urinary metabolites, with the highest concentrations observed at 4 h (Moon et al., 2008).
Que exhibits low in vitro bioavailability (5.9%) and demonstrates solubility of 235.5 g/mL in water and 2.3 × 104 g/mL in chloroform. It reaches a maximum concentration (Cmax) of 4.143 g/mL, an area under the curve of 12.015 g h/mL, and is encapsulated at a rate of 61%, with a drug loading capacity of 13 g/mg (Chen et al., 2019; Shao et al., 2019). Modern pharmaceutical scientists have improved the in vitro bioavailability of Que by applying delivery system technologies, such as particulate delivery systems, solid dispersions, encapsulation, phospholipid complexes, and hydrogels (Zhao et al., 2022). The amorphous solubility of Que-2-hydroxypropyl-β-cyclodextrin complex at small intestinal pH is at least 31 g/mL, with a Cmax value of 78.3 g/mL (Manta et al., 2020). The whey protein isolate-Que-lotus root branched chain amylose (LRA) hydrogel (whey protein isolate-Que-LRA) encapsulation rate was up to 92.4%, and Que was stable in the stomach and effectively released into the small intestine (Liu et al., 2020). The in vitro cumulative release of Que nanohybrids reached more than 65% at 30 min, which was significantly better than the cumulative release of Que APIs and physical mixtures at the same time (25.79% and 31.53%, respectively) (Liu et al., 2017). Shi et al. (2020) found a significant increase in the amount of Que passing through an artificial membrane after placing a Que/F68/HPMC 1/4/3 asd solution into the donor pool. Que shows a 12-fold increase in water solubility in the Que-phospholipid complex (from 3.44 μg/mL to 36.81 μg/mL) (Singh et al., 2012). Lee et al. (2020) loaded Que onto graphene planes by π-π stacking and weak hydrogen bonding. This loading method may result in a pH-responsive drug release mechanism in acidic environments, making it more suitable for targeted drug delivery in oncology therapy, with a maximum drug loading of 11 wt%. The oral administration of Que nanocapsules with triphenylphosphine cations as a matrix component with mitochondrial specificity results in high brain uptake and significant mitochondrial localization after cerebral ischemia/reperfusion. This effectively counteracts cerebral ischemia/reperfusion-induced cell death and neurodegenerative lesions in both young and aged rats (Ghosh et al., 2017).
While some controversy exists regarding the safety of Que, the majority of views support the idea that Que is not toxic. Experimental studies of chronic toxicity have shown that after 2 years of administration of 0.1%, 1%, and 4% Que (equivalent to 40, 400, and 1900 mg/(kg·d)) in experimental rats, focal epithelial hyperplasia of the renal tubules was observed in male rats. The incidence of chronic kidney disease slightly increased with an increase in Que intake, and the incidence of renal adenomas was increased by 1% and 4% Que doses (Dunnick and Hailey, 1992). A reduction in body mass, an increased incidence of non-neoplastic polyp hyperplasia of the cecum, hyperplasia of the parathyroid glands in male rats, and the detection of calcium oxalate crystals in the urine were observed in rats following Que ingestion (Divi and Doerge, 1996; Andres et al., 2018). Que shows significant mutagenicity in mammalian cells and is potentially genotoxic (Huan et al., 2010). However, a 2-year rodent carcinogenicity bioassay conducted by the National Toxicology Program did not reveal any adverse effects relevant to the safety assessment of orally administered Que in humans (NTP, 1992; Harwood et al., 2007). Feng (2013) found that Que is free of acute toxicity, mutagenicity, and chronic or subchronic toxicity in vivo and in vitro. This finding was based on the acute toxicity test, Ames test, mouse bone marrow cell micronucleus test, mouse spermatozoa aberration test, the 30-day feeding test in rats, and the 56-day feeding test in laying hens. The State Food and Drug Administration (2017) published the World Health Organization’s International Agency for Research on Cancer list of carcinogens, classifying Que as a Group 3 carcinogen due to its suspected carcinogenicity to humans, but with insufficient human or animal data. The in vitro test lacks a well-established regulatory mechanism in the animal body. Therefore, no accurate animal test data have been established to support the conclusion that there is insufficient evidence to suggest that Que poses a safety risk. Therefore, based on the current animal safety evaluation tests within a reasonable dose range, the safety of Que is considered high.
Que, as a polyphenolic compound, does have some limitations in in vitro studies. One of the important limitations is its interfering effect on many assays, leading to false positive results (Baell and Holloway, 2010). Since Que has strong antioxidant, anti-inflammatory, anticancer and many other biological activities (Singh et al., 2021), it may affect certain biochemical reactions and assay results in in vitro experiments. Because of these interfering effects of Que, the reliability of data in in vitro studies is somewhat compromised. In order to reduce this interference, researchers should fully consider the effects of Que when conducting in vitro experiments and take appropriate measures to avoid or reduce the interference. For example, choosing appropriate experimental conditions, optimising experimental methods and reagents, setting up control groups, adding blocking agents, etc. In addition, clinical and in vivo studies can provide a more realistic evaluation of drug action effects and safety. Therefore, the results of clinical studies and in vivo studies should be combined when assessing the pharmacological effects and safety of Que to obtain a more comprehensive and accurate understanding. Also, when studying the interaction of Que with other drugs or compounds, attention needs to be paid to this interfering effect in order to avoid misleading conclusions.
The clinical applications of Que in the treatment of other diseases, such as type 2 diabetes (Mazloom et al., 2014) and obesity (Pfeuffer et al., 2013), have been explored and evaluated. Therefore, more high-quality large-sample clinical observations are warranted to discover and validate a more detailed mechanism of action for Que in the treatment of OP and to determine the optimal dosage, which will help promote its development as a functional food and drug.
However, several aspects must be considered during the development process. As the low solubility and bioavailability of Que limit its applications, researchers can improve its bioavailability through chemical modifications and composite carriers. In addition, intestinal flora was found to be involved in the development of OP (Hao et al., 2019; Zhang et al., 2021). How Que participates in bone metabolism by regulating the intestinal flora is not yet clear and will provide a new avenue for Que research.
This article reviews advances in the mechanisms, pharmacokinetics, and toxicology of Que for the treatment of OP. Que promotes OB-mediated bone formation mainly by regulating transcription factors, the Wnt/β-catenin signaling pathway, the BMP-2/SMADs/RUNX2 signaling pathway, anti-apoptosis-mediated pathways, and oxidative stress-mediated pathways, and by promoting bone matrix formation and mineralization. It also inhibits OC-mediated bone resorption by regulating transcription factors, inflammatory factors, the OPG/RANKL/RANK signaling pathway, and the ERK1/2/JNK signaling pathway. Thus, Que is a potential drug for the prevention and treatment of OP.
T-TD: Writing–original draft. W-YD: Writing–review and editing. X-XL: Writing–review and editing. Q-HZ: Writing–review and editing. J-XD: Writing–review and editing. L-JW: Writing–review and editing. M-NY: Writing–review and editing. YY: Writing–review and editing. F-JL: Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Natural Science Foundation of China (No. 82004212), the Shandong Province Medical Health Science and Technology Development Plan Project (No. 202204070951) and the TCM Science and Technology Project of Shandong Province (No. 2021M175).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alrawaiq, N. S., and Abdullah, A. (2014). A review of flavonoid quercetin: metabolism, bioactivity and antioxidant properties. Int. J. PharmTech Res. 6 (3), 933–941.
Amarasekara, D. S., Yun, H., Kim, S., Lee, N., Kim, H., and Rho, J. (2018). Regulation of osteoclast differentiation by cytokine networks. Immune Netw. 18 (1), e8. doi:10.4110/in.2018.18.e8
Ambati, S., Miller, C. N., Bass, E. F., Hohos, N. M., Hartzell, D. L., Kelso, E. W., et al. (2018). Synergistic phytochemicals fail to protect AgainstOvariectomy induced bone loss in rats. J. Med. Food 21 (10), 1044–1052. doi:10.1089/jmf.2017.0113
An, X., and Shang, F. (2018). RA-XII exerts anti-oxidant and anti-inflammatory activities on lipopolysaccharide-induced acute renal injury by suppressing NF-κB and MAPKs regulated by HO-1/Nrf2 pathway. Biochem. Biophys. Res. Commun. 495 (3), 2317–2323. doi:10.1016/j.bbrc.2017.12.131
Andres, S., Pevny, S., Ziegenhagen, R., Bakhiya, N., Schäfer, B., Hirsch-Ernst, K. I., et al. (2018). Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 62 (1), 1700447. doi:10.1002/mnfr.201700447
Armas, L. A., and Recker, R. R. (2012). Pathophysiology of osteoporosis: new mechanistic insights. Endocrinol. Metab. Clin. North Am. 41 (3), 475–486. doi:10.1016/j.ecl.2012.04.006
Baell, J. B., and Holloway, G. A. (2010). New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 53 (7), 2719–2740. doi:10.1021/jm901137j
Baş, A., and Albeniz, I. (2022). Investigation of the effects of eugenol and quercetin on bone loss in STZ-NA induced diabetic rats utilizing micro CT. J. Diabetes Metab. Disord. 21 (1), 637–646. doi:10.1007/s40200-022-01026-y
Belibasakis, G. N., and Bostanci, N. (2012). The RANKL-OPG system in clinical periodontology. J. Clin. Periodontol. 39 (3), 239–248. doi:10.1111/j.1600-051X.2011.01810.x
Bellavia, D., Caradonna, F., Dimarco, E., Costa, V., Carina, V., De Luca, A., et al. (2021). Terpenoid treatment in osteoporosis: this is where we have come in research. Trends Endocrinol. Metab. 32 (11), 846–861. doi:10.1016/j.tem.2021.07.011
Bian, W., Xiao, S., Yang, L., Chen, J., and Deng, S. (2021). Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through the H19/miR-625-5p axis to activate the Wnt/β-catenin pathway. BMC Complement. Med. Ther. 21 (1), 243. doi:10.1186/s12906-021-03418-8
Bian, W., Yang, L., and Sun, H. (2016). Effects of quercetin on proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Pharmacol. Clin. Chin. Materia Medica 32 (05), 27–30. doi:10.13412/j.cnki.zyyl.2016.05.007
Bian, W., Zhu, H. Z., and Xiao, S. Q. (2022). Quercetin promotes bone marrow mesenchymal stem cell proliferation and osteogenic differentiation through inhibiting lncRNA DANCR expression. Shenzhen J. Integr. Traditional Chin. West. Med. 32 (11), 1–5. doi:10.16458/j.cnki.1007-0893.2022.11.001
Boyce, B. F. (2013). Advances in the regulation of osteoclasts and osteoclast functions. J. Dent. Res. 92 (10), 860–867. doi:10.1177/0022034513500306
Casado-Díaz, A., Anter, J., Dorado, G., and Quesada-Gómez, J. M. (2016). Effects of quercetin, a natural phenolic compound, in the differentiation of human mesenchymal stem cells (MSC) into adipocytes and osteoblasts. J. Nutr. Biochem. 32, 151–162. doi:10.1016/j.jnutbio.2016.03.005
Chandra, A., Lagnado, A. B., Farr, J. N., Schleusner, M., Monroe, D. G., Saul, D., et al. (2022). Bone marrow adiposity in models of radiation- and aging-related bone loss is dependent on cellular senescence. J. Bone Min. Res. 37 (5), 997–1011. doi:10.1002/jbmr.4537
Chen, D. C., and Wei, S. Q. (2002). Effects of quercetin on proliferation and apotosis of SD rat osteoclasts in vitro. West China J. Pharm. Sci. (01), 16–18. doi:10.13375/j.cnki.wcjps.2002.01.006
Chen, J. H., Cao, J. L., Chu, Y. L., Wang, Z. l., Yang, Z. t., and Wang, H. l. (2008). T-2 toxin-induced apoptosis involving Fas, p53, Bcl-xL, Bcl-2, Bax and caspase-3 signaling pathways in human chondrocytes. J. Zhejiang Univ. Sci. B 9 (6), 455–463. doi:10.1631/jzus.B0820013
Chen, L. P., Deng, M. T., and Du, C. (2014). A study of quercetin extracted from eucommia leaf promoting the proliferation of bone marrow derived mesenchymal stem cells through the phosphorylation of ERK. Lishizhen Med. Materia Medica Res. 25 (12), 2845–2847.
Chen, M., Li, M., Wei, Y., Xue, C., and Fei, Y. (2022). ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Biomaterials 291, 121878. doi:10.1016/j.biomaterials.2022.121878
Chen, W., Zou, M., Ma, X., Lv, R., Ding, T., and Liu, D. (2019). Co-encapsulation of EGCG and quercetin in liposomes for optimum antioxidant activity. J. Food Sci. 84 (1), 111–120. doi:10.1111/1750-3841.14405
Clevers, H., Loh, K. M., and Nusse, R. (2014). Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012. doi:10.1126/science.1248012
Crespy, V., Morand, C., Besson, C., Manach, C., Démigné, C., and Rémésy, C. (2001). Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J. Nutr. 131 (8), 2109–2114. doi:10.1093/jn/131.8.2109
David, A. V. A., Arulmoli, R., and Parasuraman, S. (2016). Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn. Rev. 10 (20), 84–89. doi:10.4103/0973-7847.194044
Deng, J., Zhang, J. L., and Zhou, G. P. (2020). Effect of quercetin on serum mineral content and bone metabolism in rats fed with low calcium and high magnesium diet. Chin. J. Osteoporos. 26 (07), 1039–1043.
Derakhshanian, H., Djalali, M., Djazayery, A., Nourijelyani, K., Ghadbeigi, S., Pishva, H., et al. (2013). Quercetin prevents experimental glucocorticoid-induced osteoporosis: a comparative study with alendronate. Can. J. Physiol. Pharmacol. 91 (5), 380–385. doi:10.1139/cjpp-2012-0190
Di Petrillo, A., Orrù, G., Fais, A., and Fantini, M. C. (2022). Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother. Res. 36 (1), 266–278. doi:10.1002/ptr.7309
Divi, R. L., and Doerge, D. R. (1996). Inhibition of thyroid peroxidase by dietary flavonoids. Chem. Res. Toxicol. 9 (1), 16–23. doi:10.1021/tx950076m
Dunnick, J. K., and Hailey, J. R. (1992). Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol. 19 (3), 423–431. doi:10.1016/0272-0590(92)90181-g
Duplomb, L., Baud'huin, M., Charrier, C., Berreur, M., Trichet, V., Blanchard, F., et al. (2008). Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology 149 (7), 3688–3697. doi:10.1210/en.2007-1719
Eid, H. M., and Haddad, P. S. (2017). The antidiabetic potential of quercetin: underlying mechanisms. Curr. Med. Chem. 24 (4), 355–364. doi:10.2174/0929867323666160909153707
Feng, J. J., Zou, H., and Zhu, H. Y. (2016). Effect of quercetin on serum osteocalcin levels and femur typeⅠcollagen protein levels in the ovariectomized rats. J. Traditional Chin. Orthop. Traumatology 28 (07), 10–13.
Feng, L., Yang, Z., Hou, N., Wang, M., Lu, X., Li, Y., et al. (2023). Long non-coding RNA Malat1 increases the rescuing effect of quercetin on tnfα-impaired bone marrow stem cell osteogenesis and ovariectomy-induced osteoporosis. Int. J. Mol. Sci. 24 (6), 5965. doi:10.3390/ijms24065965
Feng, X. A. (2013). Safety evaluation of quercetin as a feed additive. China: Northeast Agricultural University.
Fideles, S. O. M., de Cássia Ortiz, A., Buchaim, D. V., de Souza Bastos Mazuqueli Pereira, E., Parreira, M. J. B. M., de Oliveira Rossi, J., et al. (2023). Influence of the neuroprotective properties of quercetin on regeneration and functional recovery of the nervous system. Antioxidants (Basel) 12 (1), 149. doi:10.3390/antiox12010149
Forte, L., Torricelli, P., Boanini, E., Gazzano, M., Rubini, K., Fini, M., et al. (2016). Antioxidant and bone repair properties of quercetin-functionalized hydroxyapatite: an in vitro osteoblast-osteoclast-endothelial cell co-culture study. Acta Biomater. 32, 298–308. doi:10.1016/j.actbio.2015.12.013
Gao, Y., Xiao, F., Wang, C., Cui, P., and Zhang, X. (2018). Long noncoding RNA MALAT1 promotes osterix expression to regulate osteogenic differentiation by targeting miRNA-143 in human bone marrow-derived mesenchymal stem cells. J. Cell Biochem. 119 (8), 6986–6996. doi:10.1002/jcb.26907
Geng, Q., Gao, H., Yang, R., Guo, K., and Miao, D. (2019). Pyrroloquinoline quinone prevents estrogen deficiency-induced osteoporosis by inhibiting oxidative stress and osteocyte senescence. Int. J. Biol. Sci. 15 (1), 58–68. doi:10.7150/ijbs.25783
Ghosh, S., Sarkar, S., Choudhury, S. T., Ghosh, T., and Das, N. (2017). Triphenyl phosphonium coated nano-quercetin for oral delivery: neuroprotective effects in attenuating age related global moderate cerebral ischemia reperfusion injury in rats. Nanomedicine 13 (8), 2439–2450. doi:10.1016/j.nano.2017.08.002
Giordani, C., Matacchione, G., Giuliani, A., Valli, D., Scarpa, E. S., Antonelli, A., et al. (2023). Pro-osteogenic and anti-inflammatory synergistic effect of orthosilicic acid, vitamin K2, curcumin, polydatin and quercetin combination in young and senescent bone marrow-derived mesenchymal stromal cells. Int. J. Mol. Sci. 24 (10), 8820. doi:10.3390/ijms24108820
Guo, C., Yang, R. J., Jang, K., Zhou, X. L., and Liu, Y. Z. (2017). Protective effects of pretreatment with quercetin against lipopolysaccharide-induced apoptosis and the inhibition of osteoblast differentiation via the MAPK and wnt/β-catenin pathways in mc3t3-E1 cells. Cell Physiol. Biochem. 43 (4), 1547–1561. doi:10.1159/000481978
Ha, S. H., and Choung, P. H. (2020). MSM promotes human periodontal ligament stem cells differentiation to osteoblast and bone regeneration. Biochem. Biophys. Res. Commun. 528 (1), 160–167. doi:10.1016/j.bbrc.2020.05.097
Hao, M., Wang, G., Zuo, X., Qu, C. J., Yao, B. C., and Wang, D. L. (2019). Gut microbiota: an overlooked factor that plays a significant role in osteoporosis. J. Int. Med. Res. 47 (9), 4095–4103. doi:10.1177/0300060519860027
Harvey, A. L., Edrada-Ebel, R., and Quinn, R. J. (2015). The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 14 (2), 111–129. doi:10.1038/nrd4510
Harwood, M., Danielewska-Nikiel, B., Borzelleca, J. F., Flamm, G. W., Williams, G. M., and Lines, T. C. (2007). A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 45 (11), 2179–2205. doi:10.1016/j.fct.2007.05.015
Hou, D. D., Zhang, W., Gao, Y. L., Sun, Y. Z., Wang, H. X., Qi, R. Q., et al. (2019). Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 74, 105676. doi:10.1016/j.intimp.2019.105676
Hu, Y., Gui, Z., Zhou, Y., Xia, L., Lin, K., and Xu, Y. (2019). Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 145, 146–160. doi:10.1016/j.freeradbiomed.2019.09.024
Hu, Y., Yuan, W., Cai, N., Jia, K., Meng, Y., Wang, F., et al. (2022). Exploring quercetin anti-osteoporosis pharmacological mechanisms with in silico and in vivo models. Life (Basel) 12 (7), 980. doi:10.3390/life12070980
Huan, F., Cheng, J., and Jin, S. X. (2010). Genotoxicity effects of quercetin on mammalian cells in vitro. Chin. Prev. Med. 11 (08), 797–800. doi:10.16506/j.1009-6639.2010.08.011
Hunter, G. K., and Goldberg, H. A. (1993). Nucleation of hydroxyapatite by bone sialoprotein. Proc. Natl. Acad. Sci. U. S. A. 90 (18), 8562–8565. doi:10.1073/pnas.90.18.8562
Ibrahim, T., Ricci, M., Scarpi, E., Bongiovanni, A., Ricci, R., Riva, N., et al. (2016). RANKL: a promising circulating marker for bone metastasis response. Oncol. Lett. 12 (4), 2970–2975. doi:10.3892/ol.2016.4977
Ikeda, F., Matsubara, T., Tsurukai, T., Hata, K., Nishimura, R., and Yoneda, T. (2008). JNK/c-Jun signaling mediates an anti-apoptotic effect of RANKL in osteoclasts. J. Bone Min. Res. 23 (6), 907–914. doi:10.1359/jbmr.080211
Inoue, J., Choi, J. M., Yoshidomi, T., Yashiro, T., and Sato, R. (2010). Quercetin enhances VDR activity, leading to stimulation of its target gene expression in Caco-2 cells. J. Nutr. Sci. Vitaminol. (Tokyo) 56 (5), 326–330. doi:10.3177/jnsv.56.326
Ishikawa, Y., and Kitamura, M. (2000). Anti-apoptotic effect of quercetin: intervention in the JNK- and ERK-mediated apoptotic pathways. Kidney Int. 58 (3), 1078–1087. doi:10.1046/j.1523-1755.2000.00265.x
Jang, W. Y., Kim, M. Y., and Cho, J. Y. (2022). Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int. J. Mol. Sci. 23 (24), 15482. doi:10.3390/ijms232415482
Jin, C. H., So, Y. K., Han, S. N., and Kim, J. B. (2016). Isoegomaketone upregulates heme oxygenase-1 in RAW264.7 cells via ROS/p38 MAPK/Nrf2 pathway. Biomol. Ther. Seoul. 24 (5), 510–516. doi:10.4062/biomolther.2015.194
Jung, W. J., and Sung, M. K. (2004). Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors 21 (1-4), 113–117. doi:10.1002/biof.552210122
Kandemir, K., Tomas, M., McClements, D. J., and Capanoglu, E. (2022). Recent advances on the improvement of quercetin bioavailability. Trends Food Sci. Technol. 119, 192–200. doi:10.1016/j.tifs.2021.11.032
Kanis, J. A., Cooper, C., Rizzoli, R., and Reginster, J. Y. (2019). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 30 (1), 3–44. doi:10.1007/s00198-018-4704-5
Kim, D. S., Takai, H., Arai, M., Araki, S., Mezawa, M., Kawai, Y., et al. (2007). Effects of quercetin and quercetin 3-glucuronide on the expression of bone sialoprotein gene. J. Cell Biochem. 101 (3), 790–800. doi:10.1002/jcb.21233
Kim, J. H., Kim, M., Hong, S., Kim, E. Y., Lee, H., Jung, H. S., et al. (2021). Albiflorin promotes osteoblast differentiation and healing of rat femoral fractures through enhancing BMP-2/smad and wnt/β-catenin signaling. Front. Pharmacol. 12, 690113. doi:10.3389/fphar.2021.690113
Kimball, J. S., Johnson, J. P., and Carlson, D. A. (2021). Oxidative stress and osteoporosis. J. Bone Jt. Surg. Am. 103 (15), 1451–1461. doi:10.2106/JBJS.20.00989
Kurotaki, D., Yoshida, H., and Tamura, T. (2020). Epigenetic and transcriptional regulation of osteoclast differentiation. Bone 138, 115471. doi:10.1016/j.bone.2020.115471
Lai, C. Y., Yang, J. Y., Rayalam, S., Della-Fera, M. A., Ambati, S., Lewis, R. D., et al. (2011). Preventing bone loss and weight gain with combinations of vitamin D and phytochemicals. J. Med. Food 14 (11), 1352–1362. doi:10.1089/jmf.2010.0232
Lakhanpal, P., and Rai, D. K. (2007). Quercetin: a versatile flavonoid. Internet J. Med. Update 2 (2), 22–37. doi:10.4314/ijmu.v2i2.39851
Lan, D., Qi, S., Yao, C., Li, X., Liu, H., Wang, D., et al. (2022). Quercetin protects rat BMSCs from oxidative stress via ferroptosis. J. Mol. Endocrinol. 69 (3), 401–413. doi:10.1530/JME-22-0086
Lee, K., Chung, Y. H., Ahn, H., Kim, H., Rho, J., and Jeong, D. (2016). Selective regulation of MAPK signaling mediates RANKL-dependent osteoclast differentiation. Int. J. Biol. Sci. 12 (2), 235–245. doi:10.7150/ijbs.13814
Lee, K., Seo, I., Choi, M. H., and Jeong, D. (2018). Roles of mitogen-activated protein kinases in osteoclast biology. Int. J. Mol. Sci. 19 (10), 3004. doi:10.3390/ijms19103004
Lee, X. J., Lim, H. N., Gowthaman, N. S. K., Rahman, M. B. A., Che Abdullah, C. A., and Muthoosamy, K. (2020). In-situ surface functionalization of superparamagnetic reduced graphene oxide–Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 512, 145738. doi:10.1016/j.apsusc.2020.145738
Lei, S. S., Su, J., Zhang, Y., Huang, X. W., Wang, X. P., Huang, M. C., et al. (2021). Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: a review. Int. J. Biol. Macromol. 193 (Pt B), 1996–2005. doi:10.1016/j.ijbiomac.2021.11.030
Li, R., Zhu, X., Zhang, M., Zong, G., and Zhang, K. (2021). Association of serum periostin level with classical bone turnover markers and bone mineral density in Shanghai Chinese postmenopausal women with osteoporosis. Int. J. Gen. Med. 14, 7639–7646. doi:10.2147/IJGM.S335296
Li, X. Y., Yang, L., and Zhang, R. H. (2020). Research on quercetin regulating osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells through the estrogen receptor signaling pathway. China J. Traditional Chin. Med. Pharm. 35 (12), 6011–6014.
Li, Z., Zhang, J., Ren, X., Liu, Q., and Yang, X. (2018). The mechanism of quercetin in regulating osteoclast activation and the PAR2/TRPV1 signaling pathway in the treatment of bone cancer pain. Int. J. Clin. Exp. Pathol. 11 (11), 5149–5156.
Lin, B., Xu, P., Zheng, J., Deng, X., Ye, Q., Huang, Z., et al. (2022). Effects and mechanisms of natural alkaloids for prevention and treatment of osteoporosis. Front. Pharmacol. 13, 1014173. doi:10.3389/fphar.2022.1014173
Ling, C. W., Miao, Z., Xiao, M. L., Zhou, H., Jiang, Z., Fu, Y., et al. (2021). The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J. Clin. Endocrinol. Metab. 106 (10), e3852–e3864. doi:10.1210/clinem/dgab492
Little, R. D., Recker, R. R., and Johnson, M. L. (2002). High bone density due to a mutation in LDL-receptor-related protein 5. N. Engl. J. Med. 347 (12), 943–944. doi:10.1056/NEJM200209193471216
Liu, J., Liu, F., and Yan, C. (2020). Effect of quercetin supplementation on bone metabolism in elderly osteoporosis patients treated with bisphosphonates. Chin. J. Osteoporos. 26 (07), 1044–1048.
Liu, K., Zha, X. Q., Shen, W. D., Li, Q. M., Pan, L. H., and Luo, J. P. (2020). The hydrogel of whey protein isolate coated by lotus root amylopectin enhance the stability and bioavailability of quercetin. Carbohydr. Polym. 236, 116009. doi:10.1016/j.carbpol.2020.116009
Liu, X., Liu, J., Pang, J. Y., Shen, B. d., Shen, C. Y., Lian, W. Q., et al. (2017). Preparation of nanosuspension of quercetin with a miniaturized milling method. China J. Chin. Materia Medica 42 (15), 2984–2988. doi:10.19540/j.cnki.cjcmm.2017.0120
Liu, Z. M., Guan, Z. Y., and Jiang, T. P. (2022). To explore quercetin in Panax Notoginseng on autophagy regulation mechanism of osteoclast differentiation based on the theory of “treatment from stasis”. Chin. J. Osteoporos. 28 (12), 1740–1744.
Liu, Z. Q. (2022). Quercetin inhibits osteoclast formation and its mechanism. China: Anhui Medical University.
Luo, G., Li, F., Li, X., Wang, Z. G., and Zhang, B. (2018). TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Rep. 17 (5), 6605–6611. doi:10.3892/mmr.2018.8698
Luo, J., Zhang, C., Liu, Q., Ou, S., Zhang, L., and Peng, X. (2017). Combinative effect of sardine peptides and quercetin alleviates hypertension through inhibition of angiotensin I converting enzyme activity and inflammation. Food Res. Int. 100 (Pt 1), 579–585. doi:10.1016/j.foodres.2017.07.019
Ma, Z. Y., Zhang, B., and Zhang, Y. T. (2023). Effects of quercetin sustained release system on osteogenic properties of MC3T3-E1 cells. Chin. J. Tissue Eng. Res. 27 (12), 1870–1876.
Manta, K., Papakyriakopoulou, P., Chountoulesi, M., Diamantis, D. A., Spaneas, D., Vakali, V., et al. (2020). Preparation and biophysical characterization of quercetin inclusion complexes with β-cyclodextrin derivatives to be formulated as possible nose-to-brain quercetin delivery systems. Mol. Pharm. 17 (11), 4241–4255. doi:10.1021/acs.molpharmaceut.0c00672
Marcus, R., and Majumder, S. (2001). The nature of osteoporosis. Osteoporosis, 3–17. doi:10.1016/b978-012470862-4/50036-2
Martín, M. J., La-Casa, C., Alarcón-de-la-Lastra, C., Cabeza, J., Villegas, I., and Motilva, V. (1998). Anti-oxidant mechanisms involved in gastroprotective effects of quercetin. Z Naturforsch C J. Biosci. 53 (1-2), 82–88. doi:10.1515/znc-1998-1-215
Martiniakova, M., Babikova, M., Mondockova, V., Blahova, J., Kovacova, V., and Omelka, R. (2022). The role of macronutrients, micronutrients and flavonoid polyphenols in the prevention and treatment of osteoporosis. Nutrients 14 (3), 523. doi:10.3390/nu14030523
Masuhara, M., Tsukahara, T., Tomita, K., Furukawa, M., Miyawaki, S., and Sato, T. (2016). A relation between osteoclastogenesis inhibition and membrane-type estrogen receptor GPR30. Biochem. Biophys. Rep. 8, 389–394. doi:10.1016/j.bbrep.2016.10.013
Mazloom, Z., Abdollah, Z. S. M., and Dabbaghmanesh, M. H. (2014). The effect of quercetin supplementation on oxidative stress, glycemic control, lipid profile and insulin resistance in type 2 diabetes: a randomized clinical trial. J. Health Sci. Surveillance Syst. 2 (1), 8–14.
Meng, D. H., and Wang, S. L. (2000). Research progress of quercetin and its glycosides. China Pharm. (05), 42–43.
Messer, J. G., Hopkins, R. G., and Kipp, D. E. (2015). Quercetin metabolites up-regulate the antioxidant response in osteoblasts isolated from fetal rat calvaria. J. Cell Biochem. 116 (9), 1857–1866. doi:10.1002/jcb.25141
Min, J., Cheng, Q. F., and Zhang, Q. (2022). Effect of quercetin on osteoporosis in ovary castrated rats. Jiangxi Med. J. 57 (10), 1353–1356.
Mlcek, J., Jurikova, T., Skrovankova, S., and Sochor, J. (2016). Quercetin and its anti-allergic immune response. Molecules 21 (5), 623. doi:10.3390/molecules21050623
Moon, J. H., Nakata, R., Oshima, S., Inakuma, T., and Terao, J. (2000). Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279 (2), R461–R467. doi:10.1152/ajpregu.2000.279.2.R461
Moon, Y. J., Wang, L., DiCenzo, R., and Morris, M. E. (2008). Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 29, 205–217. doi:10.1002/bdd.605
Mu, L. Q., Du, J., and Hu, Y. Y. (2015). Effect of quercetin, geniposide, and aucubin in Eucommia ulmoides on proliferation and differentiation of osteoblast MC3T3-E1 in mice. Drug Eval. Res. 38 (02), 165–169.
Mullen, W., Edwards, C. A., and Crozier, A. (2006). Absorption, excretion and metabolite profiling of methyl-glucuronyl-glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 96 (1), 107–116. doi:10.1079/bjn20061809
Nabavi, S. M., Šamec, D., Tomczyk, M., Milella, L., Russo, D., Habtemariam, S., et al. (2020). Flavonoid biosynthetic pathways in plants: versatile targets for metabolic engineering. Biotechnol. Adv. 38, 107316. doi:10.1016/j.biotechadv.2018.11.005
Nakashima, K., Zhou, X., Kunkel, G., Zhang, Z., Deng, J. M., Behringer, R. R., et al. (2002). The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108, 17–29. doi:10.1016/s0092-8674(01)00622-5
Noguchi, T., Ebina, K., Hirao, M., Otsuru, S., Guess, A. J., Kawase, R., et al. (2018). Apolipoprotein E plays crucial roles in maintaining bone mass by promoting osteoblast differentiation via ERK1/2 pathway and by suppressing osteoclast differentiation via c-Fos, NFATc1, and NF-κB pathway. Biochem. Biophys. Res. Commun. 503 (2), 644–650. doi:10.1016/j.bbrc.2018.06.055
NTP (1992). Toxicology and carcinogenesis studies of quercetin (CAS No. 117-39-5) in F344/N rats (feed study). NTP technical report series No. 409. Research Triangle Park (NC): National Toxicology Program.
Nusse, R., Fuerer, C., Ching, W., Harnish, K., Logan, C., Zeng, A., et al. (2008). Wnt signaling and stem cell control Cold. Spring Harb. Sym 73, 59–66. doi:10.1101/sqb.2008.73.035
Oh, J. H., Karadeniz, F., Seo, Y., and Kong, C. S. (2020). Effect of quercetin 3-O-β-D-Galactopyranoside on the adipogenic and osteoblastogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Int. J. Mol. Sci. 21 (21), 8044. doi:10.3390/ijms21218044
Ohri, R., Tung, E., Rajachar, R., and Giachelli, C. M. (2005). Mitigation of ectopic calcification in osteopontin-deficient mice by exogenous osteopontin. Calcif. Tissue Int. 76 (4), 307–315. doi:10.1007/s00223-004-0071-7
Oršolić, N., Goluža, E., Dikić, D., Lisičić, D., Sašilo, K., Rođak, E., et al. (2014). Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 53 (5), 1217–1227. doi:10.1007/s00394-013-0622-7
Palacios, S. (2022). Medical treatment of osteoporosis. Climacteric 25 (1), 43–49. doi:10.1080/13697137.2021.1951697
Pan, W., Quarles, L. D., Song, L. H., Yu, Y. H., Jiao, C., Tang, H. B., et al. (2005). Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J. Cell Biochem. 94 (2), 307–316. doi:10.1002/jcb.20308
Pandit, A. P., Omase, S. B., and Mute, V. M. (2020). A chitosan film containing quercetin-loaded transfersomes for treatment of secondary osteoporosis. Drug Deliv. Transl. Res. 10 (5), 1495–1506. doi:10.1007/s13346-020-00708-5
Pang, X. G., Cong, Y., Bao, N. R., Li, Y. G., and Zhao, J. N. (2018). Quercetin stimulates bone marrow mesenchymal stem cell differentiation through an estrogen receptor-mediated pathway. Biomed. Res. Int. 2018, 4178021. doi:10.1155/2018/4178021
Papiez, M. A., Cierniak, A., Krzysciak, W., Bzowska, M., Taha, H. M., Jozkowicz, A., et al. (2008). The changes of antioxidant defense system caused by quercetin administration do not lead to DNA damage and apoptosis in the spleen and bone marrow cells of rats. Food Chem. Toxicol. 46 (9), 3053–3058. doi:10.1016/j.fct.2008.06.006
Park, J. Y., Lim, M. S., Kim, S. I., Lee, H. J., and Kwon, Y. S. (2016). Quercetin-3-O-β-D-Glucuronide suppresses lipopolysaccharide-induced JNK and ERK phosphorylation in LPS-challenged RAW264.7 cells. Biomol. Ther. Seoul. 24 (6), 610–615. doi:10.4062/biomolther.2016.026
Patel, R. V., Mistry, B. M., Shinde, S. K., Syed, R., Singh, V., and Shin, H. S. (2018). Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 155, 889–904. doi:10.1016/j.ejmech.2018.06.053
Pfeuffer, M., Auinger, A., Bley, U., Kraus-Stojanowic, I., Laue, C., Winkler, P., et al. (2013). Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr. Metab. Cardiovasc Dis. 23 (5), 403–409. doi:10.1016/j.numecd.2011.08.010
Pinti, M., Appay, V., Campisi, J., Frasca, D., Fülöp, T., Sauce, D., et al. (2016). Aging of the immune system: focus on inflammation and vaccination. Eur. J. Immunol. 46 (10), 2286–2301. doi:10.1002/eji.201546178
Prouillet, C., Mazière, J. C., Mazière, C., Wattel, A., Brazier, M., and Kamel, S. (2004). Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem. Pharmacol. 67 (7), 1307–1313. doi:10.1016/j.bcp.2003.11.009
Rauf, A., Imran, M., Khan, I. A., Ur-Rehman, M., Gilani, S. A., Mehmood, Z., et al. (2018). Anticancer potential of quercetin: a comprehensive review. Phytother. Res. 32 (11), 2109–2130. doi:10.1002/ptr.6155
Rayalam, S., Della-Fera, M. A., and Baile, C. A. (2011). Synergism between resveratrol and other phytochemicals: implications for obesity and osteoporosis. Mol. Nutr. Food Res. 55 (8), 1177–1185. doi:10.1002/mnfr.201000616
Rodríguez, V., Rivoira, M., Picotto, G., de Barboza, G. D., Collin, A., and Tolosa de Talamoni, N. (2022). Analysis of the molecular mechanisms by flavonoids with potential use for osteoporosis prevention or therapy. Curr. Med. Chem. 29 (16), 2913–2936. doi:10.2174/0929867328666210921143644
Ruangsuriya, J., Charumanee, S., Jiranusornkul, S., Sirisa-Ard, P., Sirithunyalug, B., Sirithunyalug, J., et al. (2020). Depletion of β-sitosterol and enrichment of quercetin and rutin in Cissus quadrangularis Linn fraction enhanced osteogenic but reduced osteoclastogenic marker expression. BMC Complement. Med. Ther. 20 (1), 105. doi:10.1186/s12906-020-02892-w
Russo, M., Spagnuolo, C., Tedesco, I., Bilotto, S., and Russo, G. L. (2012). The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 83 (1), 6–15. doi:10.1016/j.bcp.2011.08.010
Shakibaei, M., Shayan, P., Busch, F., Aldinger, C., Buhrmann, C., Lueders, C., et al. (2012). Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS One 7 (4), e35712. doi:10.1371/journal.pone.0035712
Shao, X. M., Li, K., and Feng, S. (2023). Research progress on biological function and application of quercetin in animal production. Feed Ind. 44 (17), 29–35. doi:10.13302/j.cnki.fi.2023.17.005
Shao, Y., Yu, H., Yang, Y., Li, M., Hang, L., and Xu, X. (2019). A solid dispersion of quercetin shows enhanced Nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxid. Med. Cell Longev. 2019, 1479571. doi:10.1155/2019/1479571
Shen, D., Feng, Y., Zhang, X., Gong, L., Liu, J., Li, Y., et al. (2022). Antiosteoporosis studies of 20 medicine food homology plants containing quercetin, rutin, and kaempferol: TCM characteristics, in vivo and in vitro activities, potential mechanisms, and food functions. Evid. Based Complement. Altern. Med. 2022, 5902293. doi:10.1155/2022/5902293
Shi, X., Fan, N., Zhang, G., Sun, J., He, Z., and Li, J. (2020). Quercetin amorphous solid dispersions prepared by hot melt extrusion with enhanced solubility and intestinal absorption. Pharm. Dev. Technol. 25 (4), 472–481. doi:10.1080/10837450.2019.1709502
Shim, K. S., Ha, H., Kim, T., Lee, C. J., and Ma, J. Y. (2015). Orostachys japonicus suppresses osteoclast differentiation by inhibiting NFATc1 expression. Am. J. Chin. Med. 43 (05), 1013–1030. doi:10.1142/S0192415X15500585
Siddiqi, M. H., Siddiqi, M. Z., Ahn, S., Kang, S., Kim, Y. J., Sathishkumar, N., et al. (2013). Ginseng saponins and the treatment of osteoporosis: mini literature review. J. Ginseng Res. 37 (3), 261–268. doi:10.5142/jgr.2013.37.261
Siddiqui, J. A., Sharan, K., Swarnkar, G., Rawat, P., Kumar, M., Manickavasagam, L., et al. (2011). Quercetin-6-C-β-D-glucopyranoside isolated from Ulmus wallichiana planchon is more potent than quercetin in inhibiting osteoclastogenesis and mitigating ovariectomy-induced bone loss in rats. Menopause 18 (2), 198–207. doi:10.1097/gme.0b013e3181e84e67
Singh, D., Rawat, M. S., Semalty, A., and Semalty, M. (2012). Quercetin-phospholipid complex: an amorphous pharmaceutical system in herbal drug delivery. Curr. Drug Discov. Technol. 9 (1), 17–24. doi:10.2174/157016312799304507
Singh, P., Arif, Y., Bajguz, A., and Hayat, S. (2021). The role of quercetin in plants. Plant Physiol. Biochem. 166, 10–19. doi:10.1016/j.plaphy.2021.05.023
Soysa, N. S., and Alles, N. (2016). Osteoclast function and bone-resorbing activity: an overview. Biochem. Biophys. Res. Commun. 476 (3), 115–120. doi:10.1016/j.bbrc.2016.05.019
Steitz, S. A., Speer, M. Y., McKee, M. D., Liaw, L., Almeida, M., Yang, H., et al. (2002). Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 161 (6), 2035–2046. doi:10.1016/S0002-9440(10)64482-3
Sun, J., Pan, Y., Li, X., Wang, L., Liu, M., Tu, P., et al. (2022). Quercetin attenuates osteoporosis in orchiectomy mice by regulating glucose and lipid metabolism via the GPRC6A/AMPK/mTOR signaling pathway. Front. Endocrinol. (Lausanne) 13, 849544. doi:10.3389/fendo.2022.849544
Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., et al. (2002). Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3 (6), 889–901. doi:10.1016/s1534-5807(02)00369-6
Tao, H., Li, W., Zhang, W., Yang, C., Zhang, C., Liang, X., et al. (2021). Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol. Res. 174, 105967. doi:10.1016/j.phrs.2021.105967
Teitelbaum, S. L., and Ross, F. P. (2003). Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4 (8), 638–649. doi:10.1038/nrg1122
Tripathi, G., Raja, N., and Yun, H. S. (2015). Effect of direct loading of phytoestrogens into the calcium phosphate scaffold on osteoporotic bone tissue regeneration. J. Mater Chem. B 3 (44), 8694–8703. doi:10.1039/c5tb01574j
Tsai, C. F., Chen, G. W., Chen, Y. C., Shen, C. K., Lu, D. Y., Yang, L. Y., et al. (2021). Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients 14 (1), 67. doi:10.3390/nu14010067
Udagawa, N., Koide, M., Nakamura, M., Nakamichi, Y., Yamashita, T., Uehara, S., et al. (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab. 39 (1), 19–26. doi:10.1007/s00774-020-01162-6
Vakili, S., Zal, F., Mostafavi-Pour, Z., Savardashtaki, A., and Koohpeyma, F. (2021). Quercetin and vitamin E alleviate ovariectomy-induced osteoporosis by modulating autophagy and apoptosis in rat bone cells. J. Cell Physiol. 236 (5), 3495–3509. doi:10.1002/jcp.30087
Velletri, T., Huang, Y., Wang, Y., Li, Q., Hu, M., Xie, N., et al. (2021). Loss of p53 in mesenchymal stem cells promotes alteration of bone remodeling through negative regulation of osteoprotegerin. Cell Death Differ. 28 (1), 156–169. doi:10.1038/s41418-020-0590-4
Wang, N., Wang, L., Yang, J., Wang, Z., and Cheng, L. (2021). Quercetin promotes osteogenic differentiation and antioxidant responses of mouse bone mesenchymal stem cells through activation of the AMPK/SIRT1 signaling pathway. Phytother. Res. 35 (5), 2639–2650. doi:10.1002/ptr.7010
Wang, P., Xia, X. F., and Zuo, B. (2020). Preventive effect of quercetin on disused osteoporosis in rats and its effect on ERK1/2-MAPK signaling pathway. Med. Pharm. J. Chin. People's Liberation Army 32 (11), 6–10.
Wang, W., Sun, C., Mao, L., Ma, P., Liu, F., Yang, J., et al. (2016). The biological activities, chemical stability, metabolism and delivery systems of quercetin: a review. Trends Food Sci. Technol. 56 (56), 21–38. doi:10.1016/j.tifs.2016.07.004
Wang, Y. (2008). Effects of quercetin in ongenital system and bone mineral density in ovariectomized rats. China Pract. Med. (18), 22–24.
Wang, Y. (2009). Effects of quercetin on the expression of osteoprotegerin and receptor activator for NFκB ligand in femurs of ovariectomized rats. Her. Med. 28 (08), 999–1002.
Wang, Y., Che, L., Chen, X., He, Z., Song, D., Yuan, Y., et al. (2023). Repurpose dasatinib and quercetin: targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact. Mater 25, 13–28. doi:10.1016/j.bioactmat.2023.01.009
Wang, Y., Li, C., Wan, Y., Qi, M., Chen, Q., Sun, Y., et al. (2021). Quercetin-loaded ceria nanocomposite potentiate dual-directional immunoregulation via macrophage polarization against periodontal inflammation. Small 17 (41), e2101505. doi:10.1002/smll.202101505
Wattel, A., Kamel, S., Prouillet, C., Petit, J. P., Lorget, F., Offord, E., et al. (2004). Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. J. Cell Biochem. 92 (2), 285–295. doi:10.1002/jcb.20071
Wu, D. F., Zhang, L., and Zhang, S. P. (2008). Distribution of quercetin in plasma and tissues in rats. Chin. J. Hosp. Pharm. 28 (21), 1822–1824.
Xiao, J., Zhang, G., Chen, B., He, Q., Mai, J., Chen, W., et al. (2023). Quercetin protects against iron overload-induced osteoporosis through activating the Nrf2/HO-1 pathway. Life Sci. 322, 121326. doi:10.1016/j.lfs.2022.121326
Xing, L. Z., Ni, H. J., and Wang, Y. L. (2017). Quercitrin attenuates osteoporosis in ovariectomized rats by regulating mitogen-activated protein kinase (MAPK) signaling pathways. Biomed. Pharmacother. 89, 1136–1141. doi:10.1016/j.biopha.2017.02.073
Xing, X., Tang, Q., Zou, J., Huang, H., Yang, J., Gao, X., et al. (2023). Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. Acta Biomater. 157, 352–366. doi:10.1016/j.actbio.2022.11.056
Yang, B., Li, S., Chen, Z., Feng, F., He, L., Liu, B., et al. (2020). Amyloid β peptide promotes bone formation by regulating Wnt/β-catenin signaling and the OPG/RANKL/RANK system. FASEB J. 34 (3), 3583–3593. doi:10.1096/fj.201901550R
Yang, D., Wang, T., and Long, M. (2020). Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid. Med. Cell Longev. 2020, 8825387. doi:10.1155/2020/8825387
Yang, J. H. (2022). Evaluation on of the efficacy of open reduction and internal fixation in the treatment of femoral head fracture and the mechanism research of quercetin in promoting osteogenic differentiation. China: Zhengzhou University.
Yang, Y. J., Yang, Z. L., Wang, D. C., Xiao, X. c., and Li, P. (2006). Comparative study on effects of rutin and quercetin on metabolism in osteoblast cells. J. Chin. Med. Mater. 29 (05), 467–470. doi:10.13863/j.issn1001-4454.2006.05.024
Yao, Z., Getting, S. J., and Locke, I. C. (2021). Regulation of TNF-induced osteoclast differentiation. Cells 11 (1), 132. doi:10.3390/cells11010132
Yu, D., Huang, C., Jiang, C., and Zhu, H. (2021). Features of a simvastatin-loaded multi-layered co-electrospun barrier membrane for guided bone regeneration. Exp. Ther. Med. 22 (1), 713. doi:10.3892/etm.2021.10145
Yuan, Z., Min, J., and Wang, K. (2018). Effects of different pharmacological components of eucommia ulmoides flavonoids on postmenopausal osteoporosis. Chin. J. Osteoporos. 24 (02), 244–248.
Zhang, B., Hu, L. Y., and Gou, L. (2022). The effect of quercetin on the proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells and its molecular mechanism. Chin. J. Osteoporos. 28 (12), 1765–1769.
Zhang, Q., Chang, B., Zheng, G., Du, S., and Li, X. (2020). Quercetin stimulates osteogenic differentiation of bone marrow stromal cells through miRNA-206/connexin 43 pathway. Am. J. Transl. Res. 12 (5), 2062–2070.
Zhang, X., Aubin, J. E., and Inman, R. D. (2003). Molecular and cellular biology of new bone formation: insights into the ankylosis of ankylosing spondylitis. Curr. Opin. Rheumatol. 15 (4), 387–393. doi:10.1097/00002281-200307000-00004
Zhang, Y., Yang, X., Ge, X., and Zhang, F. (2019). Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. 109, 726–733. doi:10.1016/j.biopha.2018.10.161
Zhang, Y. W., Li, Y. J., Lu, P. P., Dai, G. C., Chen, X. X., and Rui, Y. F. (2021). The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain–gut–bone” axis. Food and Funct. 12 (13), 5703–5718. doi:10.1039/d0fo03468a
Zhao, X., Deng, Y., Xue, X., Liao, L., Zhou, M., Peng, C., et al. (2022). Research progress of quercetin delivery systems. Curr. Pharm. Des. 28 (9), 727–742. doi:10.2174/1381612828666220317141923
Zheng, H., Tang, W., and Jiao, J. L. (2017). Molecular mechanism of quercetin ameliorates on the castration osteoporosis rats by promoting osteogenetic differentiation. Pharmacol. Clin. Chin. Materia Medica 33 (05), 16–20. doi:10.13412/j.cnki.zyyl.2017.05.005
Zheng, Y., Zhao, L., Yi, J., and Cai, S. (2022). Effects and mechanisms of rhus chinensis mill. Fruits on suppressing RANKL-induced osteoclastogenesis by network pharmacology and validation in RAW264.7 cells. Nutrients 14 (5), 1020. doi:10.3390/nu14051020
Zhou, C., Shen, S., Zhang, M., Luo, H., Zhang, Y., Wu, C., et al. (2023). Mechanisms of action and synergetic formulas of plant-based natural compounds from traditional Chinese medicine for managing osteoporosis: a literature review. Front. Med. (Lausanne) 10, 1235081. doi:10.3389/fmed.2023.1235081
Zhou, Y., Wu, Y., Jiang, X., Zhang, X., Xia, L., Lin, K., et al. (2015). The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS One 10 (6), e0129605. doi:10.1371/journal.pone.0129605
Zhou, Y., Wu, Y., Ma, W., Jiang, X., Takemra, A., Uemura, M., et al. (2017). The effect of quercetin delivery system on osteogenesis and angiogenesis under osteoporotic conditions. J. Mater Chem. B 5 (3), 612–625. doi:10.1039/c6tb02312f
Zhu, F., Li, W., Wang, L., Dai, B., Liu, Z., Wu, H., et al. (2023). Study on the treatment of postmenopausal osteoporosis with quercetin in Liuwei Dihuang Pill based on network pharmacology. J. Orthop. Surg. Res. 18 (1), 21. doi:10.1186/s13018-022-03470-1
Zhu, X. J., and Wei, S. Q. (2005). Protective effect of quercetin on ovariectomy-induced bone loss in rats. Chin. J. Osteoporos. (04), 96–100.
Keywords: quercetin, antiosteoporosis, pharmacokinetics, toxicology, osteoblast, osteoclast
Citation: Deng T-T, Ding W-Y, Lu X-X, Zhang Q-H, Du J-X, Wang L-J, Yang M-N, Yin Y and Liu F-J (2024) Pharmacological and mechanistic aspects of quercetin in osteoporosis. Front. Pharmacol. 15:1338951. doi: 10.3389/fphar.2024.1338951
Received: 15 November 2023; Accepted: 10 January 2024;
Published: 25 January 2024.
Edited by:
Bee Ling Tan, Management and Science University, MalaysiaReviewed by:
Chiara Ciaccio, Università di Roma Tor Vergata, ItalyCopyright © 2024 Deng, Ding, Lu, Zhang, Du, Wang, Yang, Yin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan-Jie Liu, bGl1ZmoxOTgyMTFAMTI2LmNvbQ==; Ying Yin, NTYzMjk4MDk4QHFxLmNvbQ==; Mei-Na Yang, bWVpbmE4NjEwMTBAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.