
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 02 April 2024
Sec. Experimental Pharmacology and Drug Discovery
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1337282
 Parisa Ghasemiyeh1
Parisa Ghasemiyeh1 Rahil Fazlinejad2
Rahil Fazlinejad2 Mohammad Reza Kiafar2
Mohammad Reza Kiafar2 Shiva Rasekh2
Shiva Rasekh2 Mohammad Mokhtarzadegan3
Mohammad Mokhtarzadegan3 Soliman Mohammadi-Samani1,2*
Soliman Mohammadi-Samani1,2*Melasma is a chronic hyperpigmentation skin disorder that is more common in the female gender. Although melasma is a multifactorial skin disorder, however, sun-exposure and genetic predisposition are considered as the main etiologic factors in melasma occurrence. Although numerous topical and systemic therapeutic agents and also non-pharmacologic procedural treatments have been considered in melasma management, however, the commonly available therapeutic options have several limitations including the lack of sufficient clinical effectiveness, risk of relapse, and high rate of unwanted adverse drug reactions. Recruitment of nanotechnology for topical drug delivery in melasma management can lead to enhanced skin penetration, targeted drug delivery to the site of action, longer deposition at the targeted area, and limit systemic absorption and therefore systemic availability and adverse drug reactions. In the current review, first of all, the etiology, pathophysiology, and severity classification of melasma have been considered. Then, various pharmacologic and procedural therapeutic options in melasma treatment have been discussed. Afterward, the usage of various types of nanoparticles for the purpose of topical drug delivery for melasma management was considered. In the end, numerous clinical studies and controlled clinical trials on the assessment of the effectiveness of these novel topical formulations in melasma management are summarized.
Melasma is a common hyperpigmentation disorder, especially in the female gender, that is usually presented symmetrically as light brown to dark brown hyperpigmented spots in the face and also it can be exacerbated through prolonged sun exposure (Espósito et al., 2022). Melasma, as a multi-factorial disorder, is the most common pigmentary disorder and can be presented as centrofacial, malar, and mandibular skin patches (Guarneri, 2014) with possible inflammatory features (Noh et al., 2014). The prevalence of melasma can be varied from 1% in the normal population to 50% in the high-risk population. The difference in the prevalence of melasma in different nations can be attributed to ethnicity, genetics, and also the degree of sun exposure. As it has been reported, the prevalence of melasma is much higher in the Middle East and South East Asians, Hispanic Americans, Mediterranean Africans, and also Brazilian (Majid and Aleem, 2021). Melasma can affect all skin phototypes, however, it is more common in the middle (Fitzpatrick skin phototypes II, III, IV, and V) (Guarneri, 2014). The average age of onset of melasma can be varied from 20 to 40 years old according to reports from various nations. In addition, melasma is more predominant in the female gender and it has been reported that females are 9–10 times more prone to melasma disorder in comparison to males (Majid and Aleem, 2021). The prevalence of melasma in females during pregnancy is much higher and can be up to 63% in pregnant women. The most common risk factor for melasma in women is pregnancy, while the most common risk factors in men are sun exposure and positive family history of melasma (Majid and Aleem, 2021). Although melasma is a non-malignant skin disorder, however, if a proper, well-timed, and optimum therapeutic regimen is not considered, it can induce various psychological and emotional distress including feelings of frustration, unattractiveness, and embarrassment (Wu et al., 2021).
Numerous factors have been considered in melasma etiology. In this regard, genetic predisposition and gene polymorphisms, sun exposure and UV radiation, hormonal changes, underlying disorders including thyroid disorders, pregnancy, and also drug-induced melasma have critical roles in melasma induction. A list of drug-induced hyperpigmentation is summarized in Table 1.
As genetics has an important role in the incidence and prevalence of melasma in the patient population, the correlation between various gene polymorphisms and melasma occurrence has been considered. The correlation between Val92Met and Arg163Gln genotypes of Melanocortin-1 Receptor (MC1R) gene and the incidence of melasma was assessed in Indonesian women. In this study, 158 Indonesian women between 18 and 60 years old were included. The Val92Met genotype of the MC1R gene was significantly more common in melasma patients, however, the Arg163Gln genotype was not significantly associated with melasma incidence. In addition, the results of this study showed that the extent of sun exposure and also the positive family history of melasma were another risk factors for melasma occurrence in the Javanese population (Suryaningsih et al., 2019). Another study on the Egyptian population revealed the correlation between vitamin D receptor (VDR) gene polymorphism (TaqI) and melasma incidence. In this regard, 95 Egyptian women were included in this study and results revealed that there was a significant association between t allele and tt genotype with melasma occurrence. Therefore, TaqI polymorphism of the VDR gene was significantly associated with melasma in the Egyptian population (Seleit et al., 2017). Another study shows the correlation between estrogen receptor (ER) gene polymorphisms (PvuII and XbaI polymorphisms for the ERα gene and AluI and RsaI polymorphisms for the ERβ gene) and melasma incidence. In this study, 56 cases and 39 control patients were included. According to the results, there was a positive correlation between the ERα and ERβ genes overexpression and melasma occurrence in the case group. There was a significant association between XbaI polymorphism and melasma incidence. In addition, there was a significant association between AluI and RsaI polymorphisms and melasma. Based on the results patients with Xx, Aa, and RR genotype predominance are more prone to melasma (Bai, 2016).
As mentioned previously, the development of melasma can be affected and precipitated through the various factors including long-term sun-exposure and UV radiation, genetic predisposition, thyroid dysfunction, special drugs, oxidative stress, hormonal imbalance, and female gender especially the pregnant women and also those who are consuming oral contraceptives (OCPs) (Ortonne et al., 2009; Kwon and Park, 2014b; Katiyar and Yadav, 2022). In addition, the activity of various types of skin cells including keratinocytes, mast cells, endothelial cells, fibroblasts, and even sebocytes are associated with melasma incidence (KrupaShankar et al., 2014). Moreover, induction of inflammatory process in the dermis layer and activation of various enzymes including matrix metalloproteinase can cause melasma. Considering the remaining obstacles in melasma treatment, a deeper comprehension of its multifactorial pathogenesis can improve therapeutic outcome and overcome its high recurrence and resistance rates (Rajanala et al., 2019b; Artzi et al., 2021).
During the embryogenic phase, melanoblasts, which are melanocyte precursor cells, migrate, to reach the epidermal layer and hair follicle to create pigments. As listed below, various factors can affect the production of melanin:
a) Production of proopiomelanocortin (POMC) and its derivatives by skin cells.
b) Increased number of melanocortin-1 receptors (MC-1R) on the melanocyte surface.
c) Diacylglycerol (DAG) secretion which activates protein Kinase-C.
d) Nitric oxide (NO) release which triggers cGMP pathways.
e) Increased Cytokine and growth factor production by cytokines.
Hyperactivity or excess activation of each of these normal pathways can lead to enhanced melanin production in the dermis which in turn can induce irregular hyperpigmentation and melasma occurrence (da Cunha and da Silva Urzedo, 2022). It was shown that prolonged sun-exposure is an important risk factor for melasma development, and can have a crucial role in enhancing each of these melanogenesis paths (Passeron and Picardo, 2018; Artzi et al., 2021). Moreover, recent studies have indicated that the main radiations contributing to melasma incidence are high-energy visible light (HEVL) and long-wave UVA (UVA-1). The combination of these could have a synergic effect causing hyperpigmentation, inflammation, and erythema (Kohli et al., 2018; Kohli et al., 2019). Therefore, melasma can categorize as a photoaging disorder especially in patients with genetic vulnerability (Passeron and Picardo, 2018). The main pathomechanisms of melasma occurrence through the sun-exposure is depicted in Figure 1.
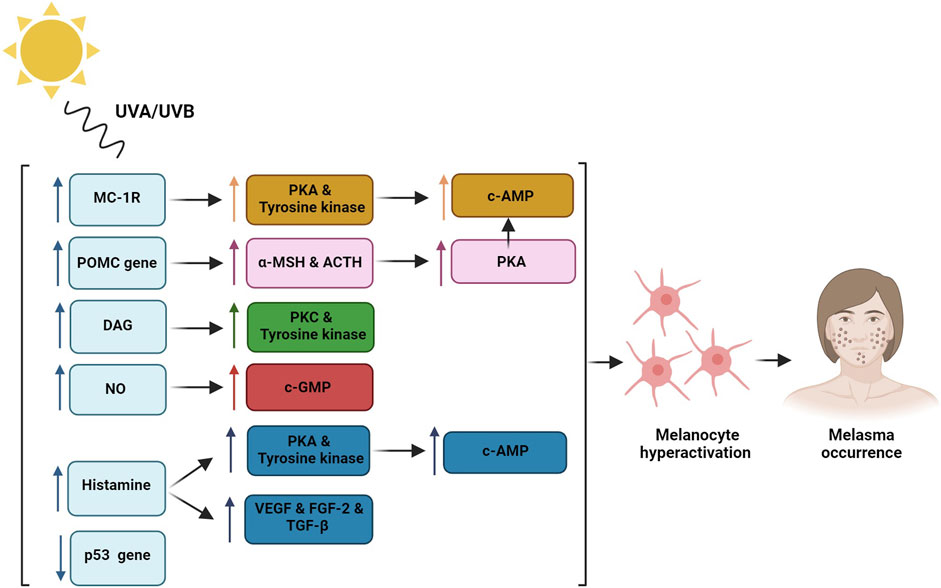
Figure 1. Sun exposure regulates Melanocortin-1 receptor (MC-1R) which induces protein kinase A (PKA) and tyrosine kinase activity that lead to c-AMP phosphorylation and melanocytes hyperactivation; Proopiomelanocortin (POMC) gene induction through the UV radiation enhances alpha-melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) production which cause increased PKA and melanogenesis process; Diacylglycerol (DAG) induction through the UV radiation activates protein kinase C (PKC) and tyrosine kinase activity; Nitric oxide (NO) release triggers cGMP pathway; Histamine release from the mast cells activates PKA and tyrosine kinase and induces hyper-vascularization through the induction of vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and transforming growth factor-beta (TGF-β) that induce melanogenesis process; p53 tumor suppressor gene damage through the UV radiation leads to enhanced melanin production.
In general, previous studies have found 5 main pathomechanisms associated with melasma occurrence (Kwon et al., 2016; Artzi et al., 2021; Morgado-Carrasco et al., 2022):
Although increase in melanin levels in melasma patients is a known fact, however, the exact mechanism of this increment is not fully understood. It is known that UV radiation can hyper activate the melanocytes through different ways including the regulation of melatonin-stimulating hormone receptors (MSHR), also known as Melanocortin-1 receptor (MC-1R), which can induce hormone binding and enhanced endogenous protein kinase A (PKA) and tyrosine kinase expression which can in turn lead to the phosphorylation of cAMP and control of melanogenesis that can induce higher melanin production. Moreover, UV radiation can activate the proopiomelanocortin (POMC) gene and thereby enhance the production of alpha-melanocyte stimulating hormone (α-MSH) and adrenocorticotropic hormone (ACTH) which can lead to increased PKA and melanogenesis (Im et al., 2002). Another mechanism would be an elevation in endogenous diacylglycerol (DAG) levels which can activate tyrosine kinase-inducing melanogenesis (Carsberg et al., 1995). In addition, p53 tumor suppressor gene damage through the UV radiation can lead to enhanced melanin production (Videira et al., 2013).
Mast cells are involved in several melasma pathomechanisms. Previous studies have shown that UV radiation can increase histamine release from the mast cells. Histamine binding to H2 receptors can activate PKA and induce tyrosinase pathway, and therefore melanogenesis process (Gilchrest et al., 1981; Malaviya et al., 1996; Yoshida et al., 2000). Moreover, histamine can induce melanocyte migration and proliferation (Kim and Lee, 2010). Furthermore, mast cells can cause hyper-vascularization by producing angiogenic factors including vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), and transforming growth factor-beta (TGF-β) (Crivellato et al., 2008). Therefore, mast cells have a crucial role in photoaging caused by prolonged UV exposure and are linked to solar elastosis, vascular dilation, and basal membrane obstruction which are all main features of melasma (Kwon et al., 2016).
Chronic exposure to UV radiation can induce unusual accumulation of elastic tissue which can lead to the photoaging process, called solar elastosis. It has been indicated that 83%–93% of melasma patients had varying ranges of solar elastosis including atypical elastic fibers which are thicker, more fragmented, and curled (Kang et al., 2002; Torres-Álvarez et al., 2011). UV radiations can elevate the production of tryptase enzyme in the mast cells. Tryptase in turn can cause solar elastosis by inducing elastin production in fibroblasts (Grimbaldeston et al., 2003). Mast cells can also induce elastin production through the cytokines (Hernández-Barrera et al., 2008) which can lead to the activation of mast cell metalloproteinase (MMP) that in turn induce the degradation of collagen type 4 and damage basement membrane (Bosset et al., 2003; Iddamalgoda et al., 2008).
In melasma patients 68.75% increase in skin vascularization was obvious in comparison to the normal population (Kim et al., 2007). As mentioned previously, mast cells and keratinocytes can induce vascularization through the increment in VEGF secretion, enhanced TGF-β and FGF-2, thus can create more, larger, and highly dilated vessels which are considered important targets in melasma treatment (Kim et al., 2005; Kwon and Park, 2014a; Kwon et al., 2016). Although VEGF is an important vascularization factor, there is insufficient evidence connecting it to melanogenesis process. Other reasons for hyper-vascularization are UV-induced solar elastosis and elevation of cytokines such as stem cell factor (SCF), inducible nitric oxide synthase (iNOS), and c-KIT (a strong melanogenic cytokine) (Kang et al., 2006; JO et al., 2009). In conclusion, it seems that anti-angiogenic agents would be a promising treatment for melasma (Kwon et al., 2016).
The disruption of the basement membrane is an important finding in melasma. The results of a recent study indicated that 83%–95% basement membrane disruption was obvious in melasma patients through different staining techniques (Torres-Álvarez et al., 2011). Prolonged UV exposure can induce matrix metalloproteinase-2 (MMP-2) and MMP-9 activation in basement membrane which can lead to collagen types 4 and 6 degradation and accumulation of more elastic fibers in melasma patients (Inomata et al., 2003). Moreover, Cadherin-11, an adhesion molecule expressed in fibroblasts, can activate MMP which in turn lead to more collagen deterioration and enhanced elastic fibers in the skin. In addition, Cadherin-11 can activate melanogenesis process in melanocytes (Kim et al., 2016). Furthermore, various factors including aging, iatrogenesis, and the environmental factors can lead to basement membrane damage and therefore easier migration of melanocytes and melanin to skin layers and their accumulation within the dermal layer. These accumulated cells in the dermis layer are called pendulous melanocytes that are usually observed in melasma patients (Kang et al., 2002; Torres-Álvarez et al., 2011). This phenomenon can induce a refractory response in melasma patients that can enhance the possibility of recurrence (Sanchez et al., 1981). In this regard, restoring the basement membrane and preventing the release of melanocytes and melanin into the dermis layer would be essential for long-term treatment of melasma. Consequently, any skin irritation with basement membrane disruption can deteriorate melasma condition and also induce persistence or recurrence type of the disease (Kwon et al., 2016).
Estrogen can be an important contributing factor in melasma development and its effects are mostly seen in post-puberty and pregnant women, and also in women using oral contraceptives. Moreover, an increase in estrogen and progesterone receptors were obvious in the dermis and epidermis layers of the melasma lesions, respectively. Through the estrogen binding to its receptor, tyrosinase and microphthalmia transcription factor (MITF) can be activated and therefore melanin would be increased (Cohen, 2017). Estrogen can also increase the expression of PDZ domain protein kidney-1 (PDZK-1) which can enhance melanogenesis and melanosomes transfer (Lee, 2015).
Long-term exposure to UV radiation can induce inflammation and enhanced skin fibroblasts which can produce SCF that can bind to its receptor (c-KIT receptor or more specifically m-KIT receptor) and therefore melanin production through the activation of tyrosine kinase paths in melanocytes (Yuan and Jin, 2018; Rajanala et al., 2019b). UV-induced inflammation is commonly caused by the increment in cyclooxygenase-2 (COX-2) and prostaglandin levels which in turn can enhance the tyrosinase pathway and stimulate melanocytes and melanogenesis process. Therefore, it seems that COX inhibitors would be promising drugs to alleviate melasma lesions (Kim et al., 2012; Rajanala et al., 2019b). In addition, an increase in superoxide dismutase and a notable reduction in glutathione levels, as the main cause of oxidative stress, were obvious in melasma patients (Kuthial et al., 2019).
Moreover, through the UVB radiation, the keratinocytes can increase the secretion of cytokines, growth factors, and hormones including iNOS which in turn induces the melanin production in melanocytes (IMOKAWA et al., 1998; Taraz et al., 2017).
It is interesting to know that, despite the whole face sun exposure, only some areas, specifically those with concentrated sebaceous glands including the forehead, cheeks, and upper lips are more commonly affected by melasma. It can be attributed to the ability of these skin regions in vitamin D, different cytokines, and various growth factors production (Abdel-Naser et al., 2012).
Melasma can be clinically classified as centrofacial (with the highest incidence rate), malar and mandibular skin patches. In the centrofacial type, the forehead, cheeks, chin, nose, and upper lips are involved. While in malar melasma, cheeks and nose and in mandibular melasma, mandibular ramus are involved (Guarneri, 2014). In addition, melasma can be classified based on the depth of melanin. In this regard, through Wood’s lamp examination method, melasma has been categorized as epidermal, dermal, mixed, and intermediate types in which the epidermal melasma is the most common type among patients with melasma lesions. However, due to the discrepancies between the Wood’s lamp technique and in vivo histopathological results in melasma classification, reflectance confocal microscopy (RCM) was introduced (Liu et al., 2011). Based on the RCM imaging, melasma can be classified to epidermal and mixed types which is more compatible with the histopathological results (Kang and Bahadoran, 2012). RCM technique is a non-invasive, in vivo imaging technique that can be used in microscopic skin analysis with high resolution and without the need for skin biopsies preparation (Liu et al., 2011).
As shown in Figure 2, various therapeutic options have been considered in melasma management (Goel and Trivedi, 2023; Li et al., 2023). In this regard, most of them have proceeded through photo-protection, affecting melanocytes’ activity, regulating dermal and epidermal cells including endothelial cells, fibroblasts, mast cells, sebocytes, and keratinocytes which in turn can affect melanocytes activity and reversing abnormal tissue damage and photo aging process (Kwon et al., 2019). Hydroquinone as a topical depigmenting agent is among the most commonly used therapeutic option in melasma management. However, other topical depigmenting agents including niacinamide, azelaic acid, 4-n-butylresorcinol, kojic acid, resveratrol and ascorbic acid are considered in melasma management. In addition, antiaging agents have been added to the depigmenting agents to induce optimum clinical responses. In this regard, topical triple combination therapy containing hydroquinone 4%, tretinoin 0.05%, and fluocinolone 0.01% have received the Food and Drug Administration (FDA) approval in melasma management. Tretinoin have both hypopigmentary and antiaging characteristics. While, steroids including fluocinolone can inhibit inflammation-induced photo damage and melanogenesis. Besides various therapeutic and pharmacological treatment options, numerous procedural treatments including laser therapy, light therapy, and microneedling have been considered in melasma treatment (Kwon et al., 2019).
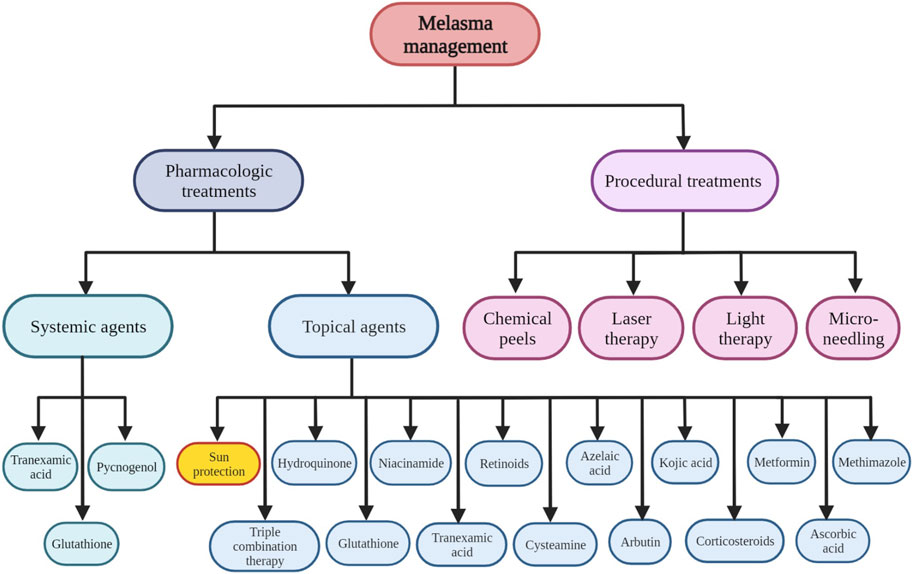
Figure 2. Various pharmacologic and non-pharmacologic therapeutic options considered in melasma management.
Although various therapeutic approaches including pharmacological and procedural treatments have been considered in melasma management, however, it is a chronic, recurrent, and relapsing skin disorder. Therefore, melasma treatment, especially in patients with dark skin types classified as Fitzpatrick types IV to VI, is challenging due to the possibility of relapse and also susceptibility to post-inflammatory hyperpigmentation (PIH) (Rodrigues and Pandya, 2015). Various topical and systemic therapeutic agents and also procedural treatments that are considered in melasma management are summarized as follows.
Topical route of administration has been considered as the main strategy for melasma treatment. A schematic view of the different therapeutic agents and their mechanism of actions in melasma management is represented in Figure 3.
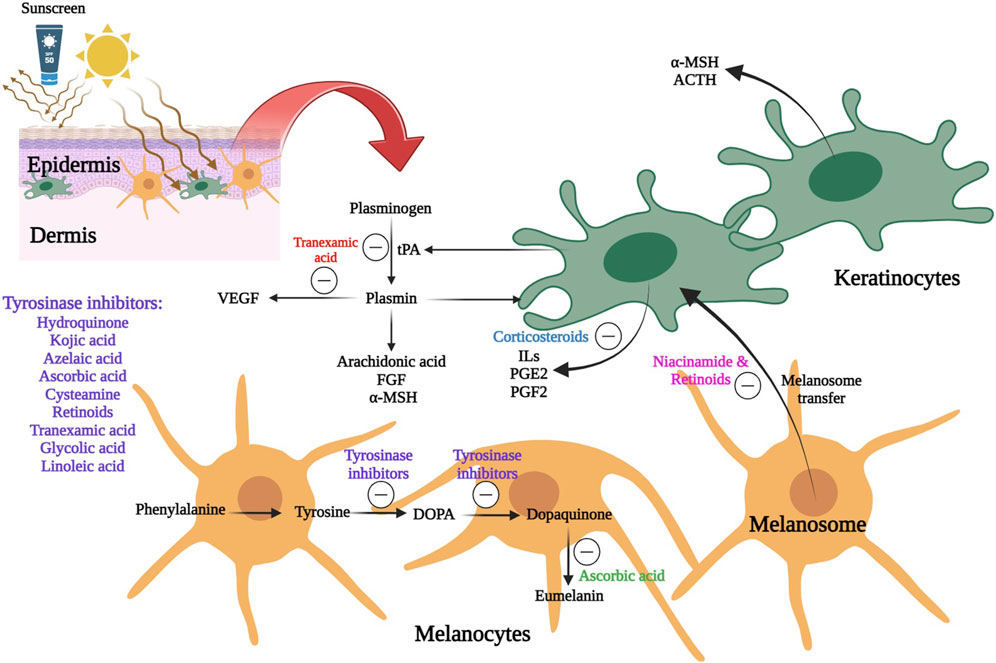
Figure 3. A schematic view of the different therapeutic agents and their mechanism of actions in melasma management 6. Line 562: Review the writing of the SLN abbreviation, since it was previously defined and was already being used as an abbreviation.
The key strategy for melasma management is consistent photoprotection through the application of broad-spectrum sunscreens with a notably high level of sun protection factor (SPF) of 50+ and persistent pigment darkening (PPD) of +++ or ++++) (Jansen et al., 2013). Solar elastosis is described as abnormal elastic tissue accumulation within the dermis layer due to the chronic sun exposure or photoaging process. High levels of solar elastosis have been observed in the skin of melasma patients. Furthermore, histological examination revealed that melasma skin is thicker, more curled, and contain more fragmented elastic fibers in comparison to normal skin (Rajanala et al., 2019a). It has been reported that prolonged exposure to both UV and visible light can result in the enhanced pigmentation in all skin types, especially darker ones (Mahmoud et al., 2010). Therefore, one of the most important approaches in melasma management and potentially mitigating its progression is photoprotection. Consequently, regular application of sunscreens along with other therapeutic agents can improved clinical outcome in melasma patients (Lakhdar et al., 2007).
Hydroquinone is a hydroxyphenolic natural substance that is widely used in melasma management (Gupta et al., 2006; Tse, 2010). Topical formulations of hydroquinone are commercially available in concentration ranges of 2%–4% and has been recommended as the golden standard for hyperpigmentation disorders including melasma (Boo, 2021). Hydroquinone can disrupt the architecture of melanocytes, and competitively inhibit tyrosinase (Adalatkhah and Sadeghi-Bazargani, 2015; McKesey et al., 2020; Suryantari et al., 2020), thereby it can inhibit the conversion of 1–3,4-dihydroxyphenylalanine to melanin (Tse, 2010) and further induce melanin necrosis, that can avoid the melanogenesis process. Although there are numerous evidences regarding the beneficial effects of topical hydroquinone in melasma treatment (Haddad et al., 2003; Nordlund et al., 2006a; Tirado-Sánchez et al., 2009; Khosravan et al., 2017), however it can induce various adverse reactions including itchiness, erythema, contact dermatitis, and ochronosis (Adalatkhah and Sadeghi-Bazargani, 2015; McKesey et al., 2020; Suryantari et al., 2020). Ochronosis can occur through the prolonged usage of hydroquinone in the absence of sufficient photoprotection (Bronzina et al., 2020). Moreover, leukoderma, permanent depigmentation, vitiligo-like hypochromia (Zhang et al., 2019), and possibility of cancer occurrence can develop due to hydroquinone byproducts (Briganti et al., 2003; Westerhof and Kooyers, 2005). However, the mentioned adverse reactions can be averted through the careful patient monitoring and restricting the duration of hydroquinone therapy (Nordlund et al., 2006b).
All trans retinoic acid (ATRA), isotretinoin, retinol, retinaldehyde, tazarotene, and adapalene are among retinoids used as depigmenting agents in melasma management. They can be administered with the purpose of the inhibition of tyrosinase activity, reduction of melanin transfer, acceleration of keratinocyte turnover, melanin dispersion, and enhanced skin permeation through the stratum corneum layer (Ortonne, 2006). Therefore, retinoids can be used simultaneously with other topical therapeutic agents to enhance their skin permeation (Ortonne, 2006; McKesey et al., 2020).
The application of tretinoin (ATRA) in melasma lesions in concentration ranges of 0.05%–0.1% could effectively decrease skin pigmentation. This depigmenting potential can be attributed to the inhibition of tyrosinase transcription and disruption of melanin synthesis (Rendon and Dryer, 2016; McKesey et al., 2020). Although the efficacy of tretinoin in melasma management has been established, however, a minimum treatment duration of 24 weeks is required to achieve the clinical effectiveness. Furthermore, topical application of retinoids on melasma lesions could potentially be associated with secondary hyperpigmentation as a result of drug-induced irritation (Piętowska et al., 2022). Topical retinoids can also induce erythema, dryness, flaking, and photosensitivity (Ball Arefiev and Hantash, 2012; Chandorkar et al., 2021). Moreover, sensations of burning and stinging were obvious in some patients. Nevertheless, these adverse reactions are predominantly well-tolerated (Rendon and Dryer, 2016; González-Molina et al., 2022).
Corticosteroids have a crucial role in inhibition of melanogenesis induced by UV-B radiation. Their potential mechanism of action would be the inhibition of prostaglandins and cytokines, including endothelin 1 and granulocyte macrophage colony-stimulating factor (GM-CSF), which are the main stimulators of melanin production (Ebanks et al., 2009). Corticosteroid as monotherapy depigmenting agents have demonstrated limited effectiveness and also could induce potential adverse reactions including epidermal atrophy, telangiectasia, acne, rosacea-like erythema, and perioral dermatitis (Menter, 2004). Therefore, it would be rational to administer corticosteroids simultaneously with other topical agents for melasma treatment (Menter, 2004; González-Molina et al., 2022).
The clinical effectiveness of triple combination (TC) therapy, also known as Kligman’s-Willi’s formulation, have been demonstrated in melasma patients. A modified triple combination cream consisting of hydroquinone, a retinoid, and a low-potency steroid have been considered as a therapeutic regimen for melasma management. Based on the previous studies, this triple combination therapy is well-tolerated and has sufficient clinical effectiveness. The addition of a steroid to a combination of hydroquinone and ATRA can efficiently suppress the secretory cytokines that are involved in melanocytes activation for melanin synthesis process. Moreover, recruitment of this triple combination therapy regimen can reduce the incidence of inflammation and skin irritation induced by hydroquinone or retinoids (Gupta et al., 2006).
Tri-Luma® cream containing hydroquinone 4%, tretinoin 0.05%, and fluocinolone acetonide 0.1% has been authorized by the FDA for melasma management and should be applied once daily on the affected facial areas for at least 8 weeks (Ferreira Cestari et al., 2007; Spierings, 2020). It has been reported that the variation in clinical effectiveness of triple combination cream and hydroquinone was more obvious in patients with darker skin tones and also in those with mixed type of melasma (Chan et al., 2008).
Azelaic acid can inhibit the mitochondrial enzyme and DNA synthesis in melanocytes, which might be cytotoxic. Tyrosinase inhibitory effects of azelaic acid can prevent DNA synthesis in melanoma cell lines without the risk of harmful adverse reactions including ochronosis (Baliña and Graupe, 1991; González-Molina et al., 2022). Previous studies have compared the clinical effectiveness of azelaic acid in comparison to hydroquinone in skin hyperpigmentation management. Based on the reports, azelaic acid was superior to hydroquinone in terms of hyperpigmentation treatment (Baliña and Graupe, 1991; Farshi, 2011a; Komal et al., 2021; Sobhan et al., 2023). Despite the promising therapeutic potential of azelaic acid in melasma, however due to limited water solubility and poor skin permeability, its conventional topical formulations have been fabricated in higher doses (10%–20%) to achieve the desired clinical outcome (Sieber and Hegel, 2014; Sobhan et al., 2023). The most common adverse reactions that have been reported with topical azelaic acid formulations are burning, itching, stinging, dryness, and erythema which are temporary and mild (Kirsch et al., 2019; Malik et al., 2019; Pekmezci, 2019; Searle et al., 2022).
The usage of tranexamic acid in melasma treatment was first documented in 1979. Tranexamic acid is an anti-plasmin substance that reduces the arachidonic acid formation, which in turn lowers the melanocyte-stimulating hormone (MSH) and pigment production (Kanechorn et al., 2012b). Moreover, tranexamic acid can inhibit the pigmentation induced by sun exposure and UV radiation (Shihab et al., 2020). Furthermore, it has been shown that endothelin-1 and VEGF, which are responsible for enhanced vascularity in melasma lesions, can be diminished by tranexamic acid administration. Tranexamic acid can be administered through either oral, intradermal, or topical route for melasma treatment (Shankar et al., 2014; Adalatkhah and Sadeghi-Bazargani, 2015; McKesey et al., 2020; Suryantari et al., 2020).
Administration of topical azelaic acid 20% and tranexamic acid 5% showed promising results in post-inflammatory hyperpigmentation management in patients diagnosed with acne vulgaris. Nevertheless, it seems that topical tranexamic acid would be safer in comparison to azelaic acid for hyperpigmentation treatment (Sobhan et al., 2023).
Kojic acid can be effective in melasma management through the inhibition of free tyrosinase synthesis (VIEIRA BRAZIL et al., 2023). Kojic dipalmitate which is the esterified derivative of kojic acid can underwent in situ hydrolysis in the different skin layers to release kojic acid. The released kojic acid can further inhibit the tyrosinase and melanin synthesis process. The main advantages of kojic dipalmitate over kojic acid would be its photostability, thermal stability, and stability at a wide range of pH in various topical formulations. However, due to the crystallinity nature, high lipophilicity, and low water solubility of kojic dipalmitate, its incorporations within the topical formulations would be more challenging (Zilles et al., 2023). Based on the previous studies, a combination of topical kojic acid and hydroquinone would be a promising depigmenting agent regimen. Kojic acid can also be used in contact dermatitis and erythema (Deo et al., 2013; Yenny, 2018). Previous studies have reported the favorable clinical efficacy outcomes of different concentrations of topical kojic acid formulations either alone or in conjunction with other therapeutic agents (Monteiro et al., 2013; Saeedi et al., 2019).
Cysteamine is a biosynthetic aminothiol that produced in mammalian cells, is widely known for its antioxidant properties (Besouw et al., 2013). In addition, numerous in vivo and in vitro studies have demonstrated its anti-carcinogenic and anti-mutagenic properties. Furthermore, cysteamine has been recognized as an efficient depigmenting agent for melasma treatment (Besouw et al., 2013). Although the exact mechanism of depigmenting potential of cysteamine is unclear, however, it has been reported that melanocytotoxicity is not involved (Qiu et al., 2000; Karrabi et al., 2021). In spite of promising results regarding the topical cysteamine therapy for melasma in terms of clinical efficacy and safety, however, its application is challenging due to its instability and also its unpleasant odor produced during the oxidation process (2013).
Ascorbic acid, also known as vitamin C, is an antioxidant that can bind to copper and successfully inhibit the tyrosinase enzyme. Therefore, it can suppress the oxidative polymerization of melanin intermediates. Consequently, melanin production in the melanogenesis process would be inhibited through the ascorbic acid administration (Ebanks et al., 2009; Telang, 2013). Based on the previous studies, ascorbic acid, as a depigmenting agent, could be well-tolerated with minimal risk of irritation in comparison to topical hydroquinone 4% (Espinal-Perez et al., 2004; Hwang et al., 2009). Various topical formulations of vitamin C with concentration range of 3.75%–30% have been considered in melasma, however, in most studies, vitamin C concentration was less than 10% for the purpose of skin photodamage and melasma treatment (Correia and Magina, 2023).
Glycolic acid, as an α-hydroxy acid (AHA), have a crucial rule in cell-adhesion disruption and further skin desquamation. In addition, glycolic acid can inhibit tyrosinase activity and therefore suppress the melanin production (Chaudhary and Dayal, 2013; Austin et al., 2019). Numerous studies have indicated that glycolic acid peels can augment the clinical effectiveness of other topical agents, particularly in individuals with darker skin tones (Ibrahim et al., 2015; Choi et al., 2019). The most common adverse reactions of topical glycolic acid would be mild to moderate erythema, pruritus, and inflammation. Administration of intense moisturizing agents would be helpful to alleviate these unfavorable adverse reactions (Ibrahim et al., 2015).
Niacinamide, also known as vitamin B3, is the active form of niacin (Farshi, 2011b; Rolfe, 2014) and can be used in melasma (Navarrete-Solís et al., 2011) and hyperpigmentation (Kimball et al., 2010) disorders through the regulation of melanosomes transfer from the melanocytes to the keratinocytes. Therefore, niacinamide can diminish melanin accumulation in the skin layers. In addition to its depigmenting potential, niacinamide showed anti-inflammatory and photoprotective characteristics against the solar degenerative alterations (Farshi, 2011b; Rolfe, 2014). In this regard, administration of topical niacinamide has been widely considered in melasma management. Prolonged use of topical niacinamide might be accompanied by some mild adverse reactions including mild burning, erythema, and pruritus (Taylor et al., 2003).
Salicylic acid, as a β-hydroxy acid (BHA), is commonly utilized in cosmetic products as a peeling agent for skin lightening purposes. This effect can be attributed to its keratolytic potential and its ability to dissolve lipids. In addition, salicylic acid has antibacterial and anti-inflammatory characteristics. Administration of topical salicylic acid in melasma treatment can result in some adverse reactions including erythema, burning sensation, irritation, peeling, blistering, or crusting (Dahl et al., 2013; González-Molina et al., 2022). The efficacy of salicylic acid peels in melasma treatment can be enhanced through the combination therapy with mandelic acid (Spierings, 2020).
Arbutin is an organic glucopyranoside that can inhibit tyrosinase activity and avoid melanocyte maturation without the risk of toxic effects. In addition, deoxy-arbutin is a dose-dependent tyrosine hydroxylase inhibitor which can further inhibit the melanogenesis process. Therefore, topical arbutin with skin-lightening potential would be promising for melasma and hyperpigmentation treatment (Raton, 2011; Searle et al., 2020).
Other topical agents including thiamidol, linoleic acid, phytic acid, yeast extract, mulberry extract, rucinol, undecylenoyl phenylalanine, and epidermal growth factors have been reported in previous studies as effective compounds in melasma management (Lee et al., 2002; Gupta et al., 2006; Khemis et al., 2007; Alvin et al., 2011; Katoulis et al., 2014; Lyons et al., 2018; Huerth et al., 2019; González-Molina et al., 2022).
Oral tranexamic acid has been shown to be effective as an adjuvant therapy for refractory cases of melasma or as a second or third-line of treatment (Kim et al., 2017). Tranexamic acid is usually administered in melasma at a dosage of 250 mg twice daily (Shin et al., 2013) either monotherapy or in combination with other therapeutic options (Karn et al., 2012; Kim et al., 2017; Bala et al., 2018). Numerous clinical trials have indicated that depigmenting effect of tranexamic acid was observed following a therapeutic course of at least 2–3 months (Lee et al., 2016a; Kim et al., 2017). The duration of tranexamic acid therapy should not be shortened due to the high risk of melasma relapse after discontinuation (Kim et al., 2017). The possible adverse reactions reported with oral tranexamic acid therapy are abdominal bloating, headache, and menstrual irregularities that are rare and transient (Lee et al., 2016a). The main concern regarding systemic tranexamic acid therapy would be the risk of thromboembolic events. In this regard, precise screenings for personal and family history of thromboembolic events, stroke, and heart disease should be considered prior to therapy initiation (Ball Arefiev and Hantash, 2012; Lee et al., 2016a).
Glutathione is a biosynthetic tripeptide consisting of glutamate, cysteine, and glycine, which is considered as one of the most potent endogenous antioxidants. The mechanism of skin lightening of glutathione can be attributed to the inhibition of tyrosinase and further alteration in the transformation of eumelanin to pheomelanin (Pillaiyar et al., 2017; Weschawalit et al., 2017; Grimes et al., 2019).
Carotenoids are naturally occurring pigments extracted from plants, algae, and photosynthetic bacteria. They are known with their anti-inflammatory, antioxidant, and photoprotective properties that can avert the photo aging process (Galasso et al., 2017).
Thiamidol is a potent tyrosinase inhibitor that can efficiently prevent UVB-induced hyperpigmentation (Vachiramon et al., 2021). The results of a randomized clinical trial revealed that the efficacy of thiamidol cream 0.2% was comparable with hydroquinone cream 4% (Vachiramon et al., 2021).
Various antioxidants including ascorbic acid and zinc have been frequently administered through the topical or oral routes for melasma management (Sarkar et al., 2012b; Yousefi et al., 2014). The application of topical vitamin C and zinc resulted in a notable amelioration of skin lesions among with minimal adverse reactions (Sharquie et al., 2008; Hwang et al., 2009). Other antioxidants including Korean red ginseng (Song et al., 2011), Petroselinum Crispum (Khosravan et al., 2017), and orchid extracts were also considered in melasma treatment and showed favorable efficacy and tolerability (Tadokoro et al., 2010).
Pycnogenol is a standardized herbal extract with high bioavailability, synergistic effects with other lightening agents, and low toxicity potential through the oral route of administration. Pycnogenol was resulted in reduced hyperpigmentation in melasma patients after 1 month of systemic therapy (Sarkar et al., 2012b; Babbush et al., 2021).
Chemical peels including glycolic acid, salicylic acid, or trichloroacetic acid have been considered in melasma management and have shown moderate clinical efficacy. However, chemical peels may potentially induce irritation, burning, and inflammation following treatment, and also may cause melasma relapse (Sheth and Pandya, 2011; Sarkar et al., 2012a).Glycolic acid is the most commonly used chemical peel for melasma treatment (Erbil et al., 2007). Based on the previous studies, either monotherapy or combination therapy of glycolic acid with hydroquinone and tretinoin was not associated with superior clinical outcomes and could induce more adverse reactions (Lim and Tham, 1997; Faghihi et al., 2011; Chaudhary and Dayal, 2013).
Laser and light-based therapies are modalities that are utilizing light energy to treat skin lesions (Piętowska et al., 2022). Laser and light therapy are considered as a third-line of therapeutic options in melasma management for those who are unresponsive to topical therapeutic agents and chemical peels. Laser and light therapies can accelerate the elimination of melanin (Trivedi et al., 2017). Intense pulsed light (IPL), Low-fluence Q-switched (LFQS) lasers, non-ablative fractional lasers (NAFL), and picosecond lasers are among the most frequently used light and laser-based therapies in melasma management (Goel et al., 2011). However, their response might be unpredictable and result in relapse hyperpigmentation (Hofbauer Parra et al., 2016; Piętowska et al., 2022).
Micro-needling procedure, which is commonly considered as a collagen induction treatment, involves repeated skin puncturing with sterile microneedles (Ball Arefiev and Hantash, 2012). This procedure can elicit a physiological reaction that can further facilitate the wound repairment process and collagen and elastin synthesis (Bailey et al., 2022). Micro-needling can be used to augment the dermal and transdermal delivery of active pharmaceuticals. Micro-needling can preserve the integrity of the epidermis layer while it can accelerate the healing process and reduce the risk of infection and scar formation (Cohen and Elbuluk, 2016; Saleh et al., 2019).
Various adverse drug reactions are associated with systemic treatment and also numerous challenges are existing regarding the skin penetration and clinical efficacy of the topical conventional formulations that are considered in melasma management. In this regard, in the recent years, new approaches including the recruitment of nanotechnology in targeted topical drug delivery have been considered to overcome these drawbacks and cause optimum clinical response (Salvioni et al., 2021) Therefore, different types of nanoparticles including lipid nanoparticles, nanoemulsion/microemulsion, vesicular nanocarriers, polymeric nanoparticles, nanocrystals, and metal nanoparticles have been used as topical drug delivery systems for melasma management.
Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), as the first and second generations of lipid nanoparticles, respectively, have promising characteristics for topical drug delivery purposes. In addition, SLNs and NLCs can accompany superior cosmetic and dermatological benefits, including increased skin elasticity, enhanced hydration, improved skin penetration and drug deposition, and drug protection against degradation (Hajare et al., 2014; Ghanbarzadeh et al., 2015b).
SLNs are colloidal drug delivery system that are consisted of lipid matrices that are solid at body temperature and also emulsifiers. These nanoparticles typically have an average diameter between 50 and 1,000 nm (Paliwal et al., 2020).
SLNs showed promising results for encapsulation of hydroquinone as a hydrophilic agent. In this regard, hydroquinone encapsulation within the SLNs was accompanied by higher drug stability against the oxidation process and enhanced skin penetration along with diminished systemic absorption. Results of previous studies on topical gel formulation of hydroquinone-loaded SLNs indicated a significantly higher drug deposition within the epidermis layer (46.5% ± 2.6%) in comparison to the conventional gel of hydroquinone (15.1% ± 1.8%) (Ghanbarzadeh et al., 2015b). In addition, the results of an in vitro permeation study on rat skin revealed that drug accumulation within the skin layers was approximately 3 times higher, while the drug flux into the receptor phase of Franz cells was about 6.5 times lower in hydroquinone-loaded SLNs in comparison to the hydroquinone gels which confirmed the reduced systemic absorption and further reduced adverse drug reactions through the encapsulation in SLNs (Wu et al., 2017).
Kojic acid encapsulation within the SLNs could significantly improve its dermal delivery. In this regard, kojic acid-loaded SLNs showed higher drug concentration within the skin layers, controlled drug release, and greater tyrosinase inhibition capability was seen in comparison to the conventional formulation (Khezri et al., 2020b).
Based on the results of previous studies, hydroquinone encapsulation within the NLCs was accompanied by enhanced drug stability, targeted drug delivery, and diminished skin irritation (Wu et al., 2019). Moreover, hydroquinone-loaded NLCs significantly improved skin penetration and UVA/UVB radiation protection in comparison to the hydroquinone conventional formulation (Wu et al., 2017).
According to the previous studies, azelaic acid-loaded NLCs was resulted in more occlusive properties, enhanced skin permeation, targeted drug delivery to the melanocytes with enhanced clinical efficacy (Kumari et al., 2015). Moreover, the sustained release capability of azelaic acid-loaded NLCs would be advantageous for topical drug delivery due to the prolonged localized drug deposition within the skin layers (Tangau et al., 2022).
Liposomes are vesicular nanoparticulate delivery systems that are composed of lipid bilayers of phospholipids and cholesterol. Liposomes are capable of encapsulating both hydrophobic and hydrophilic drugs and also have the potential of fusion with the cell membrane to modulate its fluidity and facilitate the skin penetration and distribution of the loaded drug (Sharma et al., 2018). Recruitment of liposomes as topical drug delivery systems would be promising due to their numerous advantages including enhanced skin penetration through the stratum corneum layer, skin moisturizing and restoring effects, controlled drug release, and biocompatibility and biodegradability properties (Rahimpour and Hamishehkar, 2012).
Results of a previous in vitro study indicated that arbutin-loaded liposomes had a slower drug flux and absorption rate along with higher and longer skin deposition in comparison to arbutin solution. Consequently, systemic absorption of the loaded drug was significantly reduced through the encapsulation within the liposomes (Wen et al., 2006b).
Patients with melasma were treated using liposomal serum that contained azelaic acid, 4-n-butylresorcinol, and retinol. Following the treatment course, the MASI score of the included patients was increased from 41.7% to 85%, however, the melasma severity scale (MSS) was decreased from moderate (score 2) to mild (score 1) during the treatment course (Kusumawardani et al., 2019).
The findings of a preliminary investigation indicated that liposomal hydroquinone, as a tyrosinase inhibitor, effectively enhance the therapeutic efficacy. Hydroquinone 4%-loaded niosomes were fabricated through the fusion method and characterized. The obtained MASI scores from this plot study indicated appreciable therapeutic effectiveness of liposomal hydroquinone in comparison to the conventional hydroquinone cream (Banihashemi et al., 2015a). Results of another study indicated that although the therapeutic effect of hydroquinone in melasma treatment was preserved after encapsulation in liposomes, however, no significant superiority was observed in comparison to the conventional cream (Taghavi et al., 2019b).
The incorporation of 4-n-butyl resorcinol into liposomes was resulted in enhanced drug stability, improved skin permeation, as well as increased tyrosinase inhibitory potential which in turns resulted in more efficient melanogenesis inhibition (Huh et al., 2010).
Niosomes are vesicular nanocarriers that are composed of nonionic surfactants with permeation enhancing potential. Niosomes are fabricated through the self-aggregation of nonionic surfactants in an aqueous environment usually through the either thin film hydration or solvent injection techniques (Rigano and Lionetti, 2016; Singh and Sharma, 2016).
Kojic acid and hydroquinone were simultaneously encapsulated within the noisomes. The prepared topical formulation exhibited prolonged drug release patterns (Divanbeygikermani et al., 2018).
Transfersomes are biocompatible vesicular nanocarriers with high deformability potential that are composed of lipids bilayer and membrane softeners (Chiranjeevi et al., 2013; Fadel et al., 2017). Transfersomes are promising in transdermal drug delivery due to their high deformability potential, ease of skin permeation through the stratum corneum layer, enhanced transepidermal drug flux, and longer skin deposition (Hatem et al., 2018).
The incorporation of niacinamide into the transfersomes was accompanied by enhanced skin permeation and improved depigmenting efficacy in comparison to the conventional liposomal niacinamide formulation (Lee et al., 2016b).
Arbutin encapsulation within the transfersomes was resulted in augmented skin permeation and enhanced depigmenting effectiveness of the loaded drug (Wen et al., 2006b).
Nanoemulsions are considered as suitable drug delivery systems to pass through the lipophilic barriers for dermal delivery purposes. Nanoemulsion and microemulsions are composed immiscible aqueous and organic phases that are stabilized through the incorporation of relatively larger amounts of suitable surfactants as emulsifiers. Nano and microemulsions are promising in the field of cosmeceutics and topical drug delivery systems with the main advantage of enhanced drug solubility, improve skin penetration through the stratum corneum barrier, and enhanced bioavailability (Ghosh and Murthy, 2006; Hatem et al., 2020).
Arbutin w/o/w nanoemulsions that were co-encapsulated with coumaric acid showed enhanced encapsulation efficiency, increased drug stability, sustained drug release, and improved skin delivery in comparison to the free drug (Wen et al., 2006a; Huang et al., 2019). Moreover, arbutin was co-encapsulated with lactic acid and niacinamide within the microemulsions in order to enhance drug stability, skin permeation, and whitening effect (Surini and Mellani, 2017b).
Results of a previous study on azelaic acid and hyaluronic acid-loaded nanoemulsion showed superior drug deposition within the skin layers, enhanced tyrosinase inhibition potential, and reduced cytotoxicity (Tomić et al., 2019b). Moreover, addition of hyaluronic acid to the azelaic acid nanoemulsions efficiently reduce melanin synthesis through the enhanced melanocyte-nanoemulsion interactions (Atrux-Tallau et al., 2014b).
Based on the results of a previous in vitro study, hydroquinone 4% microemulsions showed higher amounts of drug release, higher drug stability, and reduced skin irritation and skin layer disturbance in comparison to the conventional hydroquinone cream (Üstündağ Okur et al., 2019). Moreover, hydroquinone encapsulation within the microemulsion was accompanied by augmentation in skin permeability through the stratum corneum and also enhanced photostability potential (Tirnaksiz et al., 2012; Salimi and Hajiani, 2018).
Kojic monooleate, as a known tyrosinase inhibitor, was encapsulated in nanoemulsion and cytotoxicity results revealed a survival rate of 54.76% for 3T3 cells (Syed Azhar et al., 2018). Furthermore, kojic dipalmitate encapsulation within the nanoemulsions was accompanied by improved drug stability (Al-Edresi and Baie, 2009).
The incorporation of ascorbic acid within the microemulsions efficiently improve skin permeation of the loaded drug and also induce higher skin protection capabilities (Pakpayat et al., 2009).
Co-encapsulation of kojic acid and arbutin within the microemulasions was accompanied by higher photostability potential against the UVB radiation for both drugs in comparison to the aqueous solution of kojic acid and arbutin (Gallarate et al., 2004).
Gold nanoparticles dispersion in water with great stability, biocompatibility, and chemical inertness potential are suitable nanocarriers for topical drug delivery purposes (Khodakiya et al., 2012).
Arbutin and gold nanoparticles were mixed to fabricate a nanocomplex with higher skin lightening potential. The results of this study indicated that the prepared arbutin nanocomplex was accompanied by lower intracellular and extracellular melanin production, higher anti-inflammatory effects, and reduced toxicity potential in comparison to the free drug (Jiménez-Pérez et al., 2018; Park et al., 2019a).
Polymeric nanoparticles including nanospheres and nanocapsules can act as a matrix or reservoir system (Banihashemi et al., 2015b). Therefore, they can be used to encapsulate various therapeutic agents to control the drug release pattern and extend the drug deposition within the skin layers for topical delivery purposes (Guterres et al., 2007).
Arbutin-loaded cross-linked amphiphilic guar gum nanoparticles were prepared and characterized. Based on the results, the prepared polymeric nanoparticles had a higher degree of hydrophobicity which lead to the improved skin penetration through the stratum corneum. In addition, the results of cytotoxicity on human keratinocyte (HaCaT cells) showed lower toxicity potential for the prepared arbutin-loaded polymeric nanoparticles (Bostanudin et al., 2021).
The application of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride as a liposome surface coating material was accompanied by enhanced kojic acid skin permeation and reduced melanin synthesis in comparison to the conventional liposomal kojic acid formulation (Wang et al., 2012a; Singh et al., 2023).
Encapsulation of ascorbic acid in ethyl cellulose nanoparticles was resulted in enhanced drug stability, increased anti-tyrosinase activity, and therefore improved skin whitening potential (Duarah et al., 2017b; Singh et al., 2023).
Nanocrystals with improved drug solubility, enhanced dissolution rate, and increased skin adhesiveness are promising for topical drug delivery purposes (Vidlářová et al., 2016; Malamatari et al., 2018).
Azelaic acid is a water soluble drug with two carboxylic acid groups in its structure, therefore, it has limited skin permeability. In this regard, fabrication of azelaic acid nanocrystals dispersed in Pluronic F127 and hyaluronic acid was accompanied by enhanced solubility, dissolution rate, drug stability, and skin permeability (Tomić et al., 2019b).
Fullerenes, also known as C60 carbon nanotube cylinder, are spherical nanoparticles with carbon in their structure (Jiménez-Pérez et al., 2018). The huge interior volume of the fullerenes are capable to load various biomolecules. In addition, the external surface of the fullerenes can be chemically modified for targeted topical drug delivery purposes (Koo et al., 2005).
In another study L-ascorbic acid and arbutin-loaded fullerenes incorporated within polyvinyl pyrrolidone. According to the results this delivery system were associated with enhanced anti-tyrosinase activity and reduced UVA-induced melanogenesis in comparison to L-ascorbic acid and arbutin solution (Xiao et al., 2007b).
In general, although topical treatment would be promising in melasma management, however, most of the available conventional topical formulations are challenging due to the limited skin permeability, low water solubility, low dermal bioavailability, and therefore insufficient clinical response. Therefore, as shown in Table 2, various types of nanoparticles including nanoemulsions/microemulsions, lipid nanoparticles, vesicular nanocarriers, polymeric nanoparticles, and gold nanoparticles were utilized to enhance skin permeation, improve drug solubility, increase photostability, control the drug release profile, induce longer drug deposition within the skin layers, and therefore enhance the clinical effectiveness of the therapeutic options for melasma treatment.
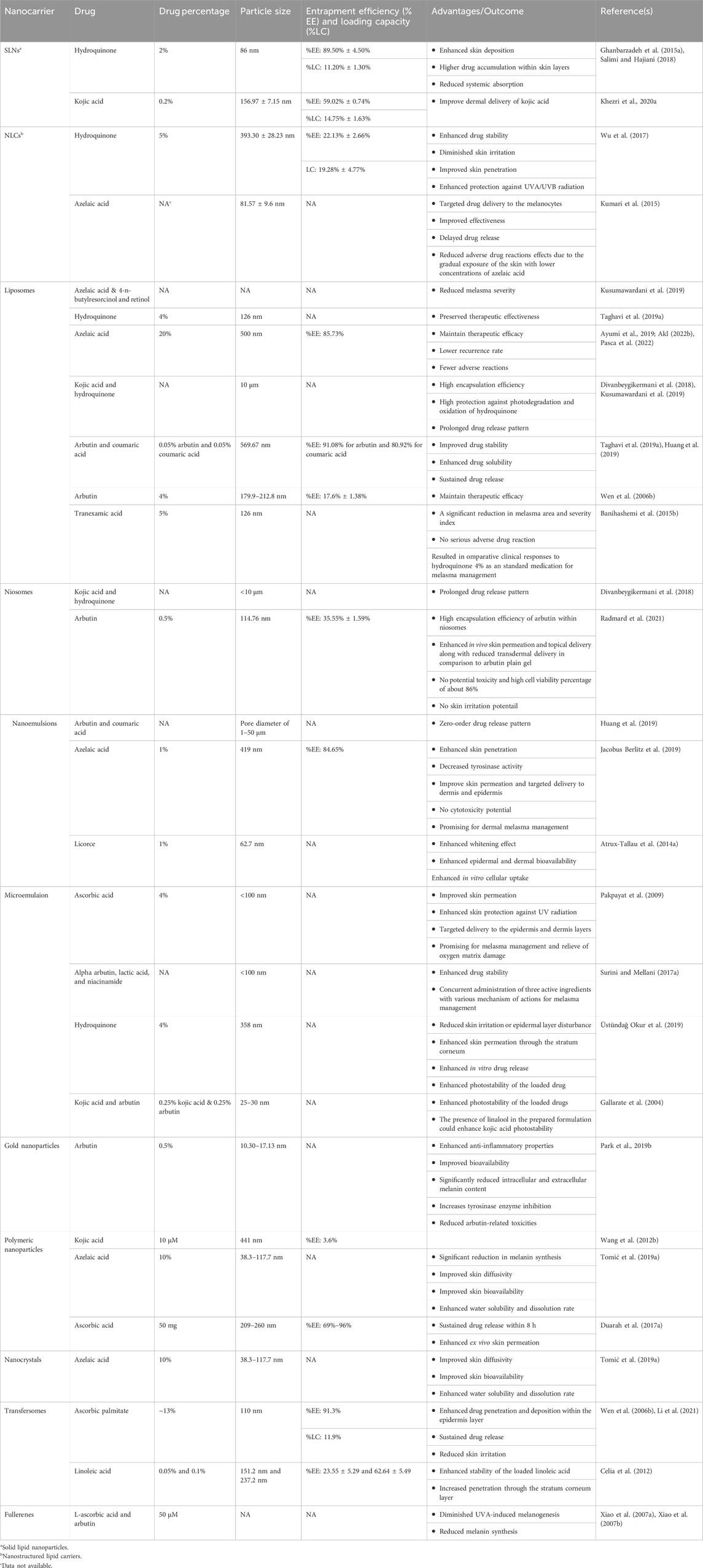
Table 2. A summary of different types of nanoparticles, the loaded cargo, particle size, and advantages of drug encapsulation in melsma management.
To date, numerous clinical studies including double- or single-blinded randomized clinical trials and split-face studies have been conducted to assess the therapeutic effect of various topical conventional and novel formulations in melasma management as summarized in Table 3.
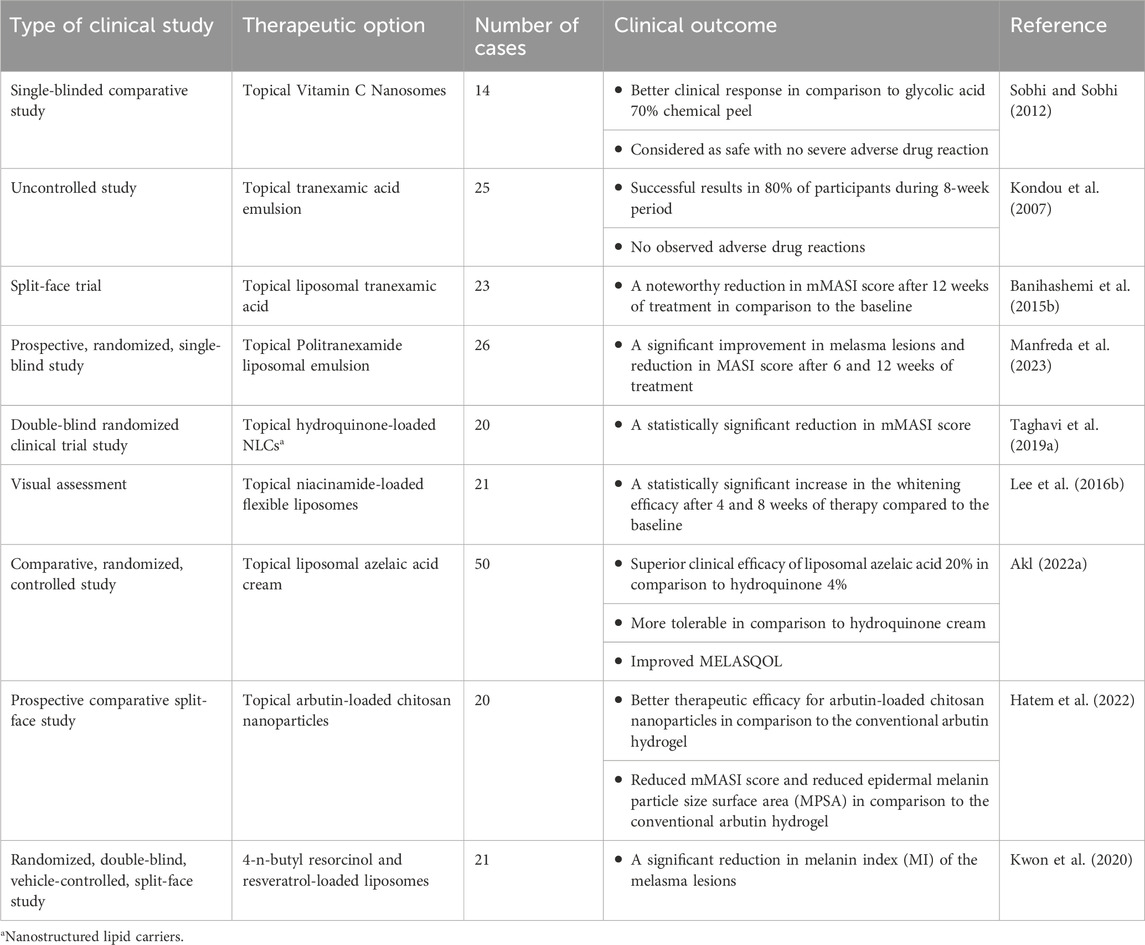
Table 3. A summary of clinical trials outcomes of different topical formulations in melasma management.
Topical liposomal tranexamic acid was designed and developed to minimize skin irritation and enhance whitening effects. Numerous studies have demonstrated that liposomal tranexamic acid was effective in approximately 80% of participants during an 8-week of treatment course (Manosroi et al., 2002). However, the results of a trial conducted in Thailand in 2012, showed that liposomal tranexamic acid had no additional benefit compared to conventional tranexamic acid formulation that would be due to the small sample size of this study (Kanechorn et al., 2012a). Nonetheless, the results of this study confirmed the clinical effectiveness of topical tranexamic acid with a significant reduction in average MASI scores after 12 weeks of treatment. Interestingly, these positive effects were persistent even 1 month after therapy discontinuation (Kanechorn et al., 2012a).
A previous study compared the efficacy and tolerability of two liposomal emulsions including Politranexamide® (sample A) and acetylglucosamine, ethyl linoleate, and phenylethylresorcinol (sample B) in facial melasma treatment. Based on the results of this report sample A was superior compared to sample B in melasma treatment after 6 and 12 weeks of patient monitoring. Moreover, Both treatment groups showed a noteworthy reduction in MASI score from the baseline (Manfreda et al., 2023).
A clinical study was conducted to assess the therapeutic efficacy of liposomal hydroquinone 4% in melasma management. The patients were randomly allocated to the conventional hydroquinone 4% and liposomal hydroquinone 4% groups and the melasma severity was assessed through the MASI score at defined time points. The findings of the study indicated that although the liposomal hydroquinone 4% was efficient in melasma management and could induce a significant reduction in MASI score, however, no superiority was obvious in comparison to the conventional hydroquinone cream (Taghavi et al., 2019a).
The clinical effectiveness of a liposomal serum containing azelaic acid, 4-n-butylresorcinol, and retinol was assessed in patients with malar pattern melasma. Following treatment, the MSS was improved from moderate to mild, and both the MASI score and the melasma quality of life scale (MELASQOL) had a significant improvement at the end of the therapy in these patients. Therefore, it seems that the prepared topical liposomal preparation was promising in melasma treatment (Kusumawardani et al., 2019).
The results of a double-blind, randomized clinical trial on melasma patients revealed a 32% improvement in the MASI score for the liposomal aloe vera gel group, while only a 10% improvement was obvious for the conventional aloe vera gel group (Ghafarzadeh and Eatemadi, 2017).
The safety and efficacy of topical vitamin C nanosome with iontophoresis was evaluated through a single-blind clinical study and results were compared with 70% glycolic acid chemical peel in patients with melasma. The results revealed that the vitamin C nanosome was superior to the glycolic acid peel hyperpigmentation management. Furthermore, vitamin C nanosomes was accompanied by reduced incidence of adverse drug reactions including skin burning, irritation, and dryness (Sobhi and Sobhi, 2012).
The results of a previous clinical study on liposomal 4-n-butyl resorcinol and resveratrol cream showed superior efficacy in melasma management. This liposomal formulation was accompanied by a significant reduction in melanin index (MI) of melasma lesions after 2 weeks of treatment, while the alteration in MI in the normal skin was not significant (Kwon et al., 2020; Shaw et al., 2022).
A prospective comparative split-face study was conducted in patients with melasma to assess the clinical efficacy of arbutin-loaded chitosan nanoparticles hydrogel in comparison to conventional arbutin gels during a 2-month treatment course. The results of this study revealed a better therapeutic efficacy for arbutin-loaded chitosan nanoparticles in terms of reduction in MASI score and reduction in epidermal melanin particle size surface area (MPSA) in comparison to the free drug (Hatem et al., 2022).
A clinical study was designed to assess the efficacy of niacinamide-loaded flexible liposomes with skin whitening potential in patients with melasma. M-values, as a measure of melanin estimated using the Mexameter, were assessed after 4 and 8 weeks of therapy initiation. The results revealed a significant improvement in M-values, 9.96% and 16.80% after 4 and 8 weeks of therapy, respectively, compared to the baseline values. Moreover, the visual assessment results and subjective evaluations reports revealed a statistically significant increase in the whitening efficacy of niacinamide after 4 and 8 weeks of therapy compared to the baseline (Lee et al., 2016b).
Another clinical study was conducted in females with melasma to assess the effectiveness of topical liposomal azelaic acid 20% cream in comparison to the conventional hydroquinone 4% cream during a 3-month treatment course. All participants were also received once daily oral doses of tranexamic acid during the treatment course. According to this study results a pronounced improvement in patients who were received liposomal azelaic acid cream in comparison to the conventional hydroquinone cream were seen. Moreover, the tolerability of liposomal azelaic acid was significantly superior to hydroquinone cream (Hagag and Allah, 2022).
In conclusion, melasma is a chronic skin disorder with numerous etiologies including sun exposure and geographic environment, genetic predisposition, and also drug-induced and disease-induced hyperpigmentary skin disorders. Although various therapeutic modality including systemic and topical pharmacological and procedural treatments are commonly available for melasma management, however, many of these treatments showed limited clinical response and accompany numerous unwanted adverse drug reactions. Moreover, due to the nature of melasma and other hyperpigmentary skin disorders, relapse and recurrence after therapy discontinuation is very common. Therefore, design and development of efficient topical therapeutic agents would be promising in melasma management not only to induce optimum clinical response, but also avoid unwanted adverse drug reactions and relapse through the enhance in water solubility, increase the skin permeability, augment the photostability, and improve the dermal bioavailability of the loaded drugs.
PG: Data curation, Methodology, Writing–original draft, Writing–review and editing, Investigation. RF: Data curation, Writing–original draft. MK: Data curation, Writing–original draft. SR: Data curation, Writing–original draft. MM: Data curation, Writing–original draft. SM-S: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to sincerely appreciate Iran’s National Elites Foundation and Vice Chancellor for Research of Shiraz University of Medical Sciences [Grant No. 27787] for their support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbasi, N. R., and Wang, N. (2008). Doxorubicin-induced hyperpigmentation. Dermatology Online J. 14, 18. doi:10.5070/d365m8x79p
Abdel Naser, M. B., Seltmann, H., and Zouboulis, C. C. (2012). SZ95 sebocytes induce epidermal melanocyte dendricity and proliferation in vitro. Exp. Dermatol. 21, 393–395. doi:10.1111/j.1600-0625.2012.01468.x
Adalatkhah, H., and Sadeghi-Bazargani, H. (2015). The first clinical experience on efficacy of topical flutamide on melasma compared with topical hydroquinone: a randomized clinical trial. Drug Des. Devel Ther. 9, 4219–4225. doi:10.2147/DDDT.S80713
Akl, E. M. (2022a). Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study. J. Dermatol. Treat. 33, 2008–2013. doi:10.1080/09546634.2021.1905765
Akl, E. M. (2022b). Liposomal azelaic acid 20% cream vs hydroquinone 4% cream as adjuvant to oral tranexamic acid in melasma: a comparative study. J. Dermatological Treat. 33, 2008–2013. doi:10.1080/09546634.2021.1905765
Al-Edresi, S., and Baie, S. (2009). Formulation and stability of whitening VCO-in-water nano-cream. Int. J. Pharm. 373, 174–178. doi:10.1016/j.ijpharm.2009.02.011
Alvin, G., Catambay, N., Vergara, A., and Jamora, M. J. (2011). A comparative study of the safety and efficacy of 75% mulberry (Morus alba) extract oil versus placebo as a topical treatment for melasma: a randomized, single-blind, placebo-controlled trial. J. Drugs Dermatol 10, 1025–1031.
American Academy of Dermatology (2013). Cysteamine cream as a new skin depigmenting product. J. Am. Acad. Dermatology 68, AB189. doi:10.1016/j.jaad.2012.12.781
Artzi, O., Horovitz, T., Bar-Ilan, E., Shehadeh, W., Koren, A., Zusmanovitch, L., et al. (2021). The pathogenesis of melasma and implications for treatment. J. Cosmet. Dermatology 20, 3432–3445. doi:10.1111/jocd.14382
Atrux-Tallau, N., Lasselin, J., Han, S.-H., Delmas, T., and Bibette, J. (2014a). Quantitative analysis of ligand effects on bioefficacy of nanoemulsion encapsulating depigmenting active. Colloids Surfaces B Biointerfaces 122, 390–395. doi:10.1016/j.colsurfb.2014.07.021
Atrux-Tallau, N., Lasselin, J., Han, S. H., Delmas, T., and Bibette, J. (2014b). Quantitative analysis of ligand effects on bioefficacy of nanoemulsion encapsulating depigmenting active. Colloids Surf. B Biointerfaces 122, 390–395. doi:10.1016/j.colsurfb.2014.07.021
Austin, E., Nguyen, J. K., and Jagdeo, J. (2019). Topical treatments for melasma: a systematic review of randomized controlled trials. J. Drugs Dermatol 18, S1545961619P1156X.
Ayumi, N. S., Sahudin, S., Hussain, Z., Hussain, M., and Samah, N. H. A. (2019). Polymeric nanoparticles for topical delivery of alpha and beta arbutin: preparation and characterization. Drug Deliv. Transl. Res. 9, 482–496. doi:10.1007/s13346-018-0508-6
Babbush, K. M., Babbush, R. A., and Khachemoune, A. (2021). Treatment of melasma: a review of less commonly used antioxidants. Int. J. Dermatol 60, 166–173. doi:10.1111/ijd.15133
Bailey, A. J. M., Li, H. O., Tan, M. G., Cheng, W., and Dover, J. S. (2022). Microneedling as an adjuvant to topical therapies for melasma: a systematic review and meta-analysis. J. Am. Acad. Dermatol 86, 797–810. doi:10.1016/j.jaad.2021.03.116
Bai, Z. (2016). The relationship between the incidence of melasma and estrogen gene polymorphism. Tianjin Med. J., 887–891.
Bala, H. R., Lee, S., Wong, C., Pandya, A. G., and Rodrigues, M. (2018). Oral tranexamic acid for the treatment of melasma: a review. Dermatol. Surg. 44, 814–825. doi:10.1097/DSS.0000000000001518
Baliña, L. M., and Graupe, K. (1991). The treatment of melasma. 20% azelaic acid versus 4% hydroquinone cream. Int. J. Dermatol 30, 893–895. doi:10.1111/j.1365-4362.1991.tb04362.x
Ball Arefiev, K. L., and Hantash, B. M. (2012). Advances in the treatment of melasma: a review of the recent literature. Dermatol Surg. 38, 971–984. doi:10.1111/j.1524-4725.2012.02435.x
Banihashemi, M., Zabolinejad, N., Jaafari, M. R., Salehi, M., and Jabari, A. (2015a). Comparison of therapeutic effects of liposomal Tranexamic Acid and conventional Hydroquinone on melasma. J. Cosmet. Dermatol 14, 174–177. doi:10.1111/jocd.12152
Banihashemi, M., Zabolinejad, N., Jaafari, M. R., Salehi, M., and Jabari, A. (2015b). Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J. Cosmet. dermatology 14, 174–177. doi:10.1111/jocd.12152
Ben Fadhel, N., Chaabane, A., Ammar, H., Ben Romdhane, H., Soua, Y., Chadli, Z., et al. (2019). Clinical features, culprit drugs, and allergology workup in 41 cases of fixed drug eruption. Contact Dermat. 81, 336–340. doi:10.1111/cod.13351
Besouw, M., Masereeuw, R., Van Den Heuvel, L., and Levtchenko, E. (2013). Cysteamine: an old drug with new potential. Drug Discov. Today 18, 785–792. doi:10.1016/j.drudis.2013.02.003
Blomberg, M., Zachariae, C., and Grønhøj, F. (2009). Hyperpigmentation of the face following adalimumab treatment. Acta dermato-venereologica 89, 546–547. doi:10.2340/00015555-0697
Boo, Y. C. (2021). Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants (Basel) 10, 1129. doi:10.3390/antiox10071129
Bosset, S., Bonnet-Duquennoy, M., Barre, P., Chalon, A., Kurfurst, R., Bonte, F., et al. (2003). Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatology 149, 826–835. doi:10.1046/j.1365-2133.2003.05456.x
Bostanudin, M. F., Salam, A., Mahmood, A., Arafat, M., Kaharudin, A. N., Sahudin, S., et al. (2021). Formulation and in-vitro characterisation of cross-linked amphiphilic guar gum nanocarriers for percutaneous delivery of arbutin. J. Pharm. Sci. 110, 3907–3918. doi:10.1016/j.xphs.2021.08.014
Braunstein, I., Wanat, K. A., Elenitsas, R., Xu, X., Frey, N., and Rosenbach, M. (2013). Eltrombopag-associated hyperpigmentation. JAMA dermatol. 149, 1112–1115. doi:10.1001/jamadermatol.2013.5107
Briganti, S., Camera, E., and Picardo, M. (2003). Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell. Res. 16, 101–110. doi:10.1034/j.1600-0749.2003.00029.x
Bronzina, E., Clement, A., Marie, B., Fook Chong, K. T., Faure, P., and Passeron, T. (2020). Efficacy and tolerability on melasma of a topical cosmetic product acting on melanocytes, fibroblasts and endothelial cells: a randomized comparative trial against 4% hydroquinone. J. Eur. Acad. Dermatol Venereol. 34, 897–903. doi:10.1111/jdv.16150
Calheiros, T., De Almeida, H. L., Jorge, V. M., De Almeida, A. L., and Motta, L. (2020). Light and electron microscopy of chlorpromazine-induced hyperpigmentation. J. Cutan. Pathology 47, 402–405. doi:10.1111/cup.13612
Carsberg, C. J., Ohanian, J., and Friedmann, P. (1995). Ultraviolet radiation stimulates a biphasic pattern of 1, 2-diacylglycerol formation in cultured human melanocytes and keratinocytes by activation of phospholipases C and D. Biochem. J. 305, 471–477. doi:10.1042/bj3050471
Celia, C., Cilurzo, F., Trapasso, E., Cosco, D., Fresta, M., and Paolino, D. (2012). Ethosomes® and transfersomes® containing linoleic acid: physicochemical and technological features of topical drug delivery carriers for the potential treatment of melasma disorders. Biomed. microdevices 14, 119–130. doi:10.1007/s10544-011-9590-y
Chandorkar, N., Tambe, S., Amin, P., and Madankar, C. (2021). Alpha arbutin as a skin lightening agent. A Rev. 13, 3502–3510. doi:10.31838/ijpr/2021.13.02.446
Chang, G.-C., Yang, T.-Y., Chen, K.-C., Yin, M.-C., Wang, R.-C., and Lin, Y.-C. (2004). Complications of therapy in cancer patients: case 1. Paronychia and skin hyperpigmentation induced by gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 22, 4646–4648. doi:10.1200/JCO.2004.02.168
Chan, R., Park, K. C., Lee, M. H., Lee, E. S., Chang, S. E., Leow, Y. H., et al. (2008). A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br. J. Dermatol 159, 697–703. doi:10.1111/j.1365-2133.2008.08717.x
Chaudhary, S., and Dayal, S. (2013). Efficacy of combination of glycolic acid peeling with topical regimen in treatment of melasma. J. Drugs Dermatol 12, 1149–1153.
Chiranjeevi, G., Muthukumaran, M., and Krishnamoorthy, B. (2013). A review on potency of vesicular systems in targeting drug delivery. Res. J. Pharm. Biol. Chem. Sci. 4, 156–170.
Chittari, K., Tagboto, S., and Tan, B. (2009). Cyclophosphamide-induced nail discoloration and skin hyperpigmentation: a rare presentation. Clin. Exp. dermatology 34, 405–406. doi:10.1111/j.1365-2230.2008.02896.x
Choi, F. D., Sung, C. T., Juhasz, M. L., and Mesinkovsk, N. A. (2019). Oral collagen supplementation: a systematic review of dermatological applications. J. Drugs Dermatol 18, 9–16.
Cohen, B. E., and Elbuluk, N. (2016). Microneedling in skin of color: a review of uses and efficacy. J. Am. Acad. Dermatol 74, 348–355. doi:10.1016/j.jaad.2015.09.024
Cohen, P. R. (2016). Paclitaxel-associated reticulate hyperpigmentation: report and review of chemotherapy-induced reticulate hyperpigmentation. World J. Clin. Cases 4, 390–400. doi:10.12998/wjcc.v4.i12.390
Cohen, P. R. (2017). Melasma treatment: a novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor. Med. Hypotheses 101, 1–5. doi:10.1016/j.mehy.2017.01.020
Connors, T. M., Restrepo, A., and Dao, H. (2018). Brown-gray hyperpigmentation in a photosensitive distribution after levofloxacin exposure. Dermatology Online J. 24. doi:10.5070/d3247040912
Correia, G., and Magina, S. (2023). Efficacy of topical vitamin C in melasma and photoaging: a systematic review. J. Cosmet. Dermatology 22, 1938–1945. doi:10.1111/jocd.15748
Crivellato, E., Nico, B., and Ribatti, D. (2008). Mast cells and tumour angiogenesis: new insight from experimental carcinogenesis. Cancer Lett. 269, 1–6. doi:10.1016/j.canlet.2008.03.031
Da Cunha, M. G., and Da Silva Urzedo, A. P. (2022). Melasma: a review about pathophysiology and treatment London, UK: Pigmentation Disorders.
Dahl, A., Yatskayer, M., Raab, S., and Oresajo, C. (2013). Tolerance and efficacy of a product containing ellagic and salicylic acids in reducing hyperpigmentation and dark spots in comparison with 4% hydroquinone. J. Drugs Dermatol 12, 52–58.
De Baat, C., Phoa, K., Zweers, P., Bolling, M., Rozema, F., and Vissink, A. (2020). Medicaments and oral healthcare. Hyperpigmentation of oral soft tissues due to afamelanotide. Nederl. Tijdschr. Voor Tandheelkd. 127, 237–243. doi:10.5177/ntvt.2020.04.19115
Deo, K. S., Dash, K. N., Sharma, Y. K., Virmani, N. C., and Oberai, C. (2013). Kojic acid vis-a-vis its combinations with hydroquinone and betamethasone valerate in melasma: a randomized, single blind, comparative study of efficacy and safety. Indian J. Dermatol 58, 281–285. doi:10.4103/0019-5154.113940
Di Tullio, F., Mandel, V. D., Scotti, R., Padalino, C., and Pellacani, G. (2018). Imatinib-induced diffuse hyperpigmentation of the oral mucosa, the skin, and the nails in a patient affected by chronic myeloid leukemia: report of a case and review of the literature. Int. J. Dermatology 57, 784–790. doi:10.1111/ijd.13931
Divanbeygikermani, M., Pardakhty, A., and Amanatfard, A. (2018). Kojic acid and hydroquinone non-ionic surfactant vesicles for topical application. Int. Pharm. Acta 1, 110. doi:10.22037/ipa.v1i1.20494
Duarah, S., Durai, R. D., and Narayanan, V. B. (2017a). Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 7, 750–760. doi:10.1007/s13346-017-0398-z
Duarah, S., Durai, R. D., and Narayanan, V. B. (2017b). Nanoparticle-in-gel system for delivery of vitamin C for topical application. Drug Deliv. Transl. Res. 7, 750–760. doi:10.1007/s13346-017-0398-z
Ebanks, J. P., Wickett, R. R., and Boissy, R. E. (2009). Mechanisms regulating skin pigmentation: the rise and fall of complexion coloration. Int. J. Mol. Sci. 10, 4066–4087. doi:10.3390/ijms10094066
Erbil, H., Sezer, E., Taştan, B., Arca, E., and Kurumlu, Z. (2007). Efficacy and safety of serial glycolic acid peels and a topical regimen in the treatment of recalcitrant melasma. J. dermatology 34, 25–30. doi:10.1111/j.1346-8138.2007.00211.x
Espinal-Perez, L. E., Moncada, B., and Castanedo-Cazares, J. P. (2004). A double-blind randomized trial of 5% ascorbic acid vs. 4% hydroquinone in melasma Int. J. Dermatol 43, 604–607. doi:10.1111/j.1365-4632.2004.02134.x
Espósito, A. C. C., Cassiano, D. P., Da Silva, C. N., Lima, P. B., Dias, J. A., Hassun, K., et al. (2022). Update on melasma—Part I: pathogenesis. Dermatology Ther. 12, 1967–1988. doi:10.1007/s13555-022-00779-x
Fadel, M., Samy, N., Nasr, M., and Alyoussef, A. A. (2017). Topical colloidal indocyanine green-mediated photodynamic therapy for treatment of basal cell carcinoma. Pharm. Dev. Technol. 22, 545–550. doi:10.3109/10837450.2016.1146294
Faghihi, G., Shahingohar, A., and Siadat, A. H. (2011). Comparison between 1% tretinoin peeling versus 70% glycolic acid peeling in the treatment of female patients with melasma. J. Drugs Dermatol 10, 1439–1442.
Farshi, S. (2011a). Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J. Cosmet. Dermatol 10, 282–287. doi:10.1111/j.1473-2165.2011.00580.x
Farshi, S. (2011b). Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J. Cosmet. dermatology 10, 282–287. doi:10.1111/j.1473-2165.2011.00580.x
Fathallah, N., Salem, C. B., Slim, R., Boussofara, L., Ghariani, N., and Bouraoui, K. (2011). Acetaminophen-induced cellulitis-like fixed drug eruption. Indian J. Dermatology 56, 206–208. doi:10.4103/0019-5154.80419
Ferreira Cestari, T., Hassun, K., Sittart, A., and De Lourdes Viegas, M. (2007). A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J. Cosmet. Dermatol 6, 36–39. doi:10.1111/j.1473-2165.2007.00288.x
Fiscus, V., Hankinson, A., and Alweis, R. (2014). Minocycline-induced hyperpigmentation. J. Community Hosp. Intern. Med. Perspect. 4, 24063. doi:10.3402/jchimp.v4.24063
Galasso, C., Corinaldesi, C., and Sansone, C. (2017). Carotenoids from marine organisms: biological functions and industrial applications. Antioxidants 6, 96. doi:10.3390/antiox6040096
Gallarate, M., Carlotti, M. E., Trotta, M., Grande, A. E., and Talarico, C. (2004). Photostability of naturally occurring whitening agents in cosmetic microemulsions. J. Cosmet. Sci. 55, 139–148.
Garcia, D., and Cohen, P. R. (2017). Dapsone-associated fixed drug eruption. Expert Rev. Clin. Pharmacol. 10, 717–725. doi:10.1080/17512433.2017.1322508
Geddes, E., and Cohen, P. R. (2010). Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South. Med. J. 103, 231–235. doi:10.1097/SMJ.0b013e3181ce0f5e
Genovese, G., Gelmetti, C., Spigariolo, C., and Colonna, C. (2020). Acetaminophen-induced generalized fixed drug eruption in a 5-year-old girl. Pediatr. Dermatol. 37, 756–758. doi:10.1111/pde.14161
Ghafarzadeh, M., and Eatemadi, A. (2017). Clinical efficacy of liposome-encapsulated Aloe vera on melasma treatment during pregnancy. J. Cosmet. Laser Ther. 19, 181–187. doi:10.1080/14764172.2017.1279329
Ghanbarzadeh, S., Hariri, R., Kouhsoltani, M., Shokri, J., Javadzadeh, Y., and Hamishehkar, H. (2015a). Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids surfaces B biointerfaces 136, 1004–1010. doi:10.1016/j.colsurfb.2015.10.041
Ghanbarzadeh, S., Hariri, R., Kouhsoltani, M., Shokri, J., Javadzadeh, Y., and Hamishehkar, H. (2015b). Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf. B Biointerfaces 136, 1004–1010. doi:10.1016/j.colsurfb.2015.10.041
Ghosh, P. K., and Murthy, R. S. (2006). Microemulsions: a potential drug delivery system. Curr. Drug Deliv. 3, 167–180. doi:10.2174/156720106776359168
Gilchrest, B. A., Soter, N. A., Stoff, J. S., and Mihm, M. C. (1981). The human sunburn reaction: histologic and biochemical studies. J. Am. Acad. Dermatology 5, 411–422. doi:10.1016/s0190-9622(81)70103-8
Goel, A., Krupashankar, D. S., Aurangabadkar, S., Nischal, K. C., Omprakash, H. M., and Mysore, V. (2011). Fractional lasers in dermatology--current status and recommendations. Indian J. Dermatol Venereol. Leprol. 77, 369–379. doi:10.4103/0378-6323.79732
Goel, A., and Trivedi, N. (2023). Current updates on melasma treatments. Cosmoderma 3, 79. doi:10.25259/csdm_84_2023
González-Molina, V., Martí-Pineda, A., and González, N. (2022). Topical treatments for melasma and their mechanism of action. J. Clin. Aesthet. Dermatol 15, 19–28.
Grimbaldeston, M. A., Simpson, A., Finlay-Jones, J., and Hart, P. H. (2003). The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br. J. Dermatology 148, 300–306. doi:10.1046/j.1365-2133.2003.05113.x
Grimes, P. E., Ijaz, S., Nashawati, R., and Kwak, D. (2019). New oral and topical approaches for the treatment of melasma. Int. J. Womens Dermatol 5, 30–36. doi:10.1016/j.ijwd.2018.09.004
Guarneri, F. (2014). Etiopathogenesis of melasma. Pigment. Disord. 1, 2376–2427. doi:10.4172/2376-0427.S1-003
Gupta, A. K., Gover, M. D., Nouri, K., and Taylor, S. (2006). The treatment of melasma: a review of clinical trials. J. Am. Acad. Dermatol 55, 1048–1065. doi:10.1016/j.jaad.2006.02.009
Gupta, M., Gupta, H., and Gupta, A. (2015). Sorafenib induced acral pigmentation: a new entity. Avicenna J. Med. 5, 46–48. doi:10.4103/2231-0770.154199
Guterres, S. S., Alves, M. P., and Pohlmann, A. R. (2007). Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug target insights 2, 117739280700200–157. doi:10.1177/117739280700200002
Haddad, A. L., Matos, L. F., Brunstein, F., Ferreira, L. M., Silva, A., and Costa, D. (2003). A clinical, prospective, randomized, double-blind trial comparing skin whitening complex with hydroquinone vs. placebo in the treatment of melasma Int. J. Dermatol 42, 153–6. doi:10.1046/j.1365-4362.2003.01621.x
Hagag, M., and Allah, S. (2022). The effect of topical nano vitamin-C iontophoresis versus the effect of trichloroacetic acid 20% peel in treatment of melasma. Menoufia Med. J. 35, 489–495. doi:10.4103/mmj.mmj_237_21
Hajare, A., Mali, S., Ahir, A., Thorat, J., Salunkhe, S., Nadaf, S., et al. (2014). Lipid nanoparticles: a modern formulation approach in topical drug delivery systems. J. Adv. Drug Deliv. 1, 30–37.
Hatem, S., El Hoffy, N. M., Elezaby, R. S., Nasr, M., Kamel, A. O., and Elkheshen, S. A. (2020). Background and different treatment modalities for melasma: conventional and nanotechnology-based approaches. J. Drug Deliv. Sci. Technol. 60, 101984. doi:10.1016/j.jddst.2020.101984
Hatem, S., Elkheshen, S. A., Kamel, A. O., Nasr, M., Moftah, N. H., Ragai, M. H., et al. (2022). Functionalized chitosan nanoparticles for cutaneous delivery of a skin whitening agent: an approach to clinically augment the therapeutic efficacy for melasma treatment. Drug Deliv. 29, 1212–1231. doi:10.1080/10717544.2022.2058652
Hatem, S., Nasr, M., Elkheshen, S. A., and Geneidi, A. S. (2018). Recent Advances in antioxidant cosmeceutical topical delivery. Curr. Drug Deliv. 15, 953–964. doi:10.2174/1567201815666180214143551
Hernández-Barrera, R., Torres-Alvarez, B., Castanedo-Cazares, J., Oros-Ovalle, C., and Moncada, B. (2008). Solar elastosis and presence of mast cells as key features in the pathogenesis of melasma. Clin. Exp. dermatology 33, 305–308. doi:10.1111/j.1365-2230.2008.02724.x
Hofbauer Parra, C. A., Careta, M. F., Valente, N. Y., De Sanches Osório, N. E., and Torezan, L. A. (2016). Clinical and histopathologic assessment of facial melasma after low-fluence Q-switched neodymium-doped yttrium aluminium garnet laser. Dermatol Surg. 42, 507–12. doi:10.1097/DSS.0000000000000653
Huang, H., Belwal, T., Liu, S., Duan, Z., and Luo, Z. (2019). Novel multi-phase nano-emulsion preparation for co-loading hydrophilic arbutin and hydrophobic coumaric acid using hydrocolloids. Food Hydrocoll. 93, 92–101. doi:10.1016/j.foodhyd.2019.02.023
Huerth, K. A., Hassan, S., and Callender, V. D. (2019). Therapeutic insights in melasma and hyperpigmentation management. J. Drugs Dermatol 18, 718–729.
Huh, S. Y., Shin, J. W., Na, J. I., Huh, C. H., Youn, S. W., and Park, K. C. (2010). Efficacy and safety of liposome-encapsulated 4-n-butylresorcinol 0.1% cream for the treatment of melasma: a randomized controlled split-face trial. J. dermatology 37, 311–315. doi:10.1111/j.1346-8138.2010.00787.x
Hwang, S. W., Oh, D. J., Lee, D., Kim, J. W., and Park, S. W. (2009). Clinical efficacy of 25% L-ascorbic acid (C'ensil) in the treatment of melasma. J. Cutan. Med. Surg. 13, 74–81. doi:10.2310/7750.2008.07092
Ibrahim, Z. A., Gheida, S. F., El Maghraby, G. M., and Farag, Z. E. (2015). Evaluation of the efficacy and safety of combinations of hydroquinone, glycolic acid, and hyaluronic acid in the treatment of melasma. J. Cosmet. Dermatol 14, 113–23. doi:10.1111/jocd.12143
Ibrahimi, O. A., and Anderson, R. R. (2010). Images in clinical medicine. Bleomycin-induced flagellate hyperpigmentation. N. Engl. J. Med. 363, e36. doi:10.1056/NEJMicm1002334
Iddamalgoda, A., Le, Q. T., Ito, K., Tanaka, K., Kojima, H., and Kido, H. (2008). Mast cell tryptase and photoaging: possible involvement in the degradation of extra cellular matrix and basement membrane proteins. Archives dermatological Res. 300, 69–76. doi:10.1007/s00403-007-0806-1
Imokawa, G., Yada, Y., Morisaki, N., and Kimura, M. (1998). Biological characterization of human fibroblast-derived mitogenic factors for human melanocytes. Biochem. J. 330, 1235–1239. doi:10.1042/bj3301235
Im, S., Kim, J., and Kang, W. (2002). Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. Br. J. dermatology 146, 165–167. doi:10.1046/j.1365-2133.2002.4513_3.x
Inomata, S., Takada, K., Tsunenaga, M., Fukuda, M., Matsunaga, Y., Amano, S., et al. (2003). Possible involvement of gelatinases in basement membrane damage and wrinkle formation in chronically ultraviolet B-exposed hairless mouse. J. Investigative Dermatology 120, 128–134. doi:10.1046/j.1523-1747.2003.12021.x
Jacobus Berlitz, S., De Villa, D., Maschmann Inacio, L. A., Davies, S., Zatta, K. C., Guterres, S. S., et al. (2019). Azelaic acid-loaded nanoemulsion with hyaluronic acid–a new strategy to treat hyperpigmentary skin disorders. Drug Dev. industrial Pharm. 45, 642–650. doi:10.1080/03639045.2019.1569032
Jansen, R., Osterwalder, U., Wang, S. Q., Burnett, M., and Lim, H. W. (2013). Photoprotection: part II. Sunscreen: development, efficacy, and controversies. J. Am. Acad. Dermatology 69, 867. e1–14. doi:10.1016/j.jaad.2013.08.022
Jiménez-Pérez, Z. E., Singh, P., Kim, Y.-J., Mathiyalagan, R., Kim, D.-H., Lee, M. H., et al. (2018). Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. J. ginseng Res. 42, 327–333. doi:10.1016/j.jgr.2017.04.003
Jo, H. Y., Kim, C. K., Suh, I. B., Ryu, S. W., Ha, K. S., Kwon, Y. G., et al. (2009). Co-localization of inducible nitric oxide synthase and phosphorylated Akt in the lesional skins of patients with melasma. J. dermatology 36, 10–16. doi:10.1111/j.1346-8138.2008.00579.x
Kanechorn, N. A., Ayuthaya, P., Niumphradit, N., Manosroi, A., and Nakakes, A. (2012a). Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 14, 150–154. doi:10.3109/14764172.2012.685478
Kanechorn, N. A., Ayuthaya, P., Niumphradit, N., Manosroi, A., and Nakakes, A. (2012b). Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J. Cosmet. Laser Ther. 14, 150–4. doi:10.3109/14764172.2012.685478
Kang, H. Y., and Bahadoran, P. (2012). Application of in vivo reflectance confocal microscopy in melasma classification. J. Am. Acad. Dermatology 67, 157–158. doi:10.1016/j.jaad.2012.02.046
Kang, H., Hwang, J., Lee, J., Ahn, J., Kim, J. Y., Lee, E. S., et al. (2006). The dermal stem cell factor and c-kit are overexpressed in melasma. Br. J. dermatology 154, 1094–1099. doi:10.1111/j.1365-2133.2006.07179.x
Kang, W., Yoon, K., Lee, E. S., Kim, J., Lee, K., Yim, H., et al. (2002). Melasma: histopathological characteristics in 56 Korean patients. Br. J. dermatology 146, 228–237. doi:10.1046/j.0007-0963.2001.04556.x
Karn, D., Kc, S., Amatya, A., Razouria, E. A., and Timalsina, M. (2012). Oral tranexamic acid for the treatment of melasma. Kathmandu Univ. Med. J. (KUMJ) 10, 40–3. doi:10.3126/kumj.v10i4.10993
Karrabi, M., David, J., and Sahebkar, M. (2021). Clinical evaluation of efficacy, safety and tolerability of cysteamine 5% cream in comparison with modified Kligman’s formula in subjects with epidermal melasma: a randomized, double-blind clinical trial study. Skin Res. Technol. 27, 24–31. doi:10.1111/srt.12901
Katiyar, S., and Yadav, D. (2022). Correlation of oxidative stress with melasma: an overview. Curr. Pharm. Des. 28, 225–231. doi:10.2174/1381612827666211104154928
Katoulis, A., Alevizou, A., Soura, E., Mantas, N., Bozi, E., Gregoriou, S., et al. (2014). A double-blind vehicle-controlled study of a preparation containing undecylenoyl phenylalanine 2% in the treatment of melasma in females. J. Cosmet. Dermatol 13, 86–90. doi:10.1111/jocd.12089
Khemis, A., Kaiafa, A., Queille-Roussel, C., Duteil, L., and Ortonne, J. P. (2007). Evaluation of efficacy and safety of rucinol serum in patients with melasma: a randomized controlled trial. Br. J. Dermatol 156, 997–1004. doi:10.1111/j.1365-2133.2007.07814.x
Khezri, K., Saeedi, M., Morteza-Semnani, K., Akbari, J., and Rostamkalaei, S. S. (2020a). An emerging technology in lipid research for targeting hydrophilic drugs to the skin in the treatment of hyperpigmentation disorders: kojic acid-solid lipid nanoparticles. Artif. Cells, Nanomedicine, Biotechnol. 48, 841–853. doi:10.1080/21691401.2020.1770271
Khezri, K., Saeedi, M., Morteza-Semnani, K., Akbari, J., and Rostamkalaei, S. (2020b). An emerging technology in lipid research for targeting hydrophilic drugs to the skin in the treatment of hyperpigmentation disorders: kojic acid-solid lipid nanoparticles. Artif. Cells, Nanomedicine, Biotechnol. 48, 841–853. doi:10.1080/21691401.2020.1770271
Khodakiya, A. S., Chavada, J., Jivani, N., Patel, B. N., Khodakiya, M. S., and Ramoliya, A. P. (2012). Microemulsions as enhanced drug delivery carrier: an overview. Am. J. Pharmtech. Res., 206–226.
Khosravan, S., Alami, A., Mohammadzadeh-Moghadam, H., and Ramezani, V. (2017). The effect of topical use of Petroselinum Crispum (parsley) versus that of hydroquinone cream on reduction of epidermal melasma: a randomized clinical trial. Holist. Nurs. Pract. 31, 16–20. doi:10.1097/HNP.0000000000000186
Kim, B.-S., Won, T.-H., Seo, P.-S., and Park, S.-D. (2008). A case of generalized hyperpigmentation caused by antituberculosis drugs. Korean J. Dermatology, 1513–1516.
Kim, E. H., Kim, Y. C., Lee, E.-S., and Kang, H. Y. (2007). The vascular characteristics of melasma. J. dermatological Sci. 46, 111–116. doi:10.1016/j.jdermsci.2007.01.009
Kim, E. J., Park, H. Y., Yaar, M., and Gilchrest, B. A. (2005). Modulation of vascular endothelial growth factor receptors in melanocytes. Exp. Dermatol. 14, 625–633. doi:10.1111/j.0906-6705.2005.00345.x
Kim, H. J., Moon, S. H., Cho, S. H., Lee, J. D., and Kim, H. S. (2017). Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm. Venereol. 97, 776–781. doi:10.2340/00015555-2668
Kim, J. Y., Shin, J. Y., Kim, M. R., Hann, S. K., and Oh, S. H. (2012). siRNA-mediated knock-down of COX-2 in melanocytes suppresses melanogenesis. Exp. Dermatol. 21, 420–425. doi:10.1111/j.1600-0625.2012.01483.x
Kim, N.-H., Choi, S.-H., Lee, T. R., Lee, C.-H., and Lee, A.-Y. (2016). Cadherin 11 involved in basement membrane damage and dermal changes in melasma. Acta Dermato-Venereologica 96, 635–640. doi:10.2340/00015555-2315
Kim, N. H., and Lee, A. Y. (2010). Histamine effect on melanocyte proliferation and vitiliginous keratinocyte survival. Exp. Dermatol. 19, 1073–1079. doi:10.1111/j.1600-0625.2010.01133.x
Kimball, A. B., Kaczvinsky, J. R., Li, J., Robinson, L. R., Matts, P. J., Berge, C. A., et al. (2010). Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: results of a randomized, double-blind, vehicle-controlled trial. Br. J. Dermatol 162, 435–41. doi:10.1111/j.1365-2133.2009.09477.x
Kirsch, B., Hoesly, P. M., Jambusaria, A., Heckman, M. G., Diehl, N. N., and Sluzevich, J. C. (2019). Evaluating the efficacy, safety, and tolerability of the combination of tazarotene, azelaic acid, tacrolimus, and zinc oxide for the treatment of melasma: a pilot study. J. Clin. Aesthet. Dermatol 12, 40–45.
Kleinegger, C. L., Hammond, H. L., and Finkelstein, M. W. (2000). Oral mucosal hyperpigmentation secondary to antimalarial drug therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90 (2), 189–194. doi:10.1067/moe.2000.106340
Kohli, I., Chaowattanapanit, S., Mohammad, T. F., Nicholson, C. L., Fatima, S., Jacobsen, G., et al. (2018). Synergistic effects of long-wavelength ultraviolet A1 and visible light on pigmentation and erythema. Br. J. Dermatology 178, 1173–1180. doi:10.1111/bjd.15940
Kohli, I., Zubair, R., Lyons, A. B., Nahhas, A. F., Braunberger, T. L., Mokhtari, M., et al. (2019). Impact of long-wavelength ultraviolet A1 and visible light on light-skinned individuals. Photochem. Photobiol. 95, 1285–1287. doi:10.1111/php.13143
Komal, S., Mashhood, A., Farooq, M., and Qayyum, N. (2021). A comparison of efficacy of intradermal tranexamic acid with topical 20% azelaic acid in the treatment of melasma. PAFMJ 71, 494–97. doi:10.51253/pafmj.v71i2.4528
Kondou, S., Okada, Y., and Tomita, Y. (2007). Clinical study of effect of tranexamic acid emulsion on melasma and freckles. Hifu no kagaku 6, 309–315. doi:10.11340/skinresearch.6.3_309
Koo, O. M., Rubinstein, I., and Onyuksel, H. (2005). Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine Nanotechnol. Biol. Med. 1, 193–212. doi:10.1016/j.nano.2005.06.004
Kounis, N. G., Frangides, C., Papadaki, P. J., Zavras, G. M., and Goudevenos, J. (1996). Dose-dependent appearance and disappearance of amiodarone-induced skin pigmentation. Clin. Cardiol. 19, 592–594. doi:10.1002/clc.4960190713
Kroumpouzos, G., Travers, R., and Allan, A. (2002). Generalized hyperpigmentation with daunorubicin chemotherapy. J. Am. Acad. Dermatology 46, S1–S3. doi:10.1067/mjd.2002.104509
Krupashankar, D. S. R., Somani, V. K., Kohli, M., Sharad, J., Ganjoo, A., Kandhari, S., et al. (2014). A cross-sectional, multicentric clinico-epidemiological study of melasma in India. Dermatology Ther. 4, 71–81. doi:10.1007/s13555-014-0046-1
Kumari, S., Pandita, D., Poonia, N., and Lather, V. (2015). Nanostructured lipid carriers for topical delivery of an anti-acne drug: characterization and ex vivo evaluation. Pharm. Nanotechnol. 3, 122–133. doi:10.2174/221173850302151116124757
Kusumawardani, A., Paramitasari, A., Dewi, S., and Betaubun, A. (2019). The application of liposomal azelaic acid, 4-n butyl resorcinol and retinol serum enhanced by microneedling for treatment of malar pattern melasma: a case series. Dermatol. Rep. 11. doi:10.4081/dr.2019.8075
Kuthial, M., Kaur, T., and Malhotra, S. (2019). Estimation of serum copper, superoxide dismutase and reduced glutathione levels in melasma: a case control study. Int. J. Sci. Res. 8, 25–27. doi:10.36106/ijsr
Kwon, S.-H., Hwang, Y.-J., Lee, S.-K., and Park, K.-C. (2016). Heterogeneous pathology of melasma and its clinical implications. Int. J. Mol. Sci. 17, 824. doi:10.3390/ijms17060824
Kwon, S. H., Na, J. I., Choi, J. Y., and Park, K. C. (2019). Melasma: updates and perspectives. Exp. Dermatol. 28, 704–708. doi:10.1111/exd.13844
Kwon, S. H., Yang, J. H., Shin, J. W., Park, K. C., Huh, C. H., and Na, J. I. (2020). Efficacy of liposome-encapsulated 4-n-butylresorcinol and resveratrol cream in the treatment of melasma. J. Cosmet. Dermatol 19, 891–895. doi:10.1111/jocd.13080
Kwon, S., Park, K., and Park, H. G. (2014b). Design of plasmonic cavities. J. Clin. Investig. Dermatol 2, 8. doi:10.1186/s40580-014-0008-4
Kwon, S., Park, K., Yoon, S. Y., Moon, J. Y., Choi, S. R., Choi, H. S., et al. (2014a). Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Pigment. Disord. 1, 2. doi:10.1186/1744-8069-10-2
Lakhdar, H., Zouhair, K., Khadir, K., Essari, A., Richard, A., Seité, S., et al. (2007). Evaluation of the effectiveness of a broad-spectrum sunscreen in the prevention of chloasma in pregnant women. J. Eur. Acad. Dermatol Venereol. 21, 738–42. doi:10.1111/j.1468-3083.2007.02185.x
Lee, A. Y. (2015). Recent progress in melasma pathogenesis. Pigment Cell. & melanoma Res. 28, 648–660. doi:10.1111/pcmr.12404
Lee, H. C., Thng, T. G., and Goh, C. L. (2016a). Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J. Am. Acad. Dermatol 75, 385–92. doi:10.1016/j.jaad.2016.03.001
Lee, M.-H., Kim, H.-J., Ha, D.-J., Paik, J.-H., and Kim, H.-Y. (2002). Therapeutic effect of topical application of linoleic acid and lincomycin in combination with betamethasone valerate in melasma patients. J. Korean Med. Sci. 17, 518–23. doi:10.3346/jkms.2002.17.4.518
Lee, M.-H., Lee, K.-K., Park, M.-H., Hyun, S.-S., Kahn, S.-Y., Joo, K.-S., et al. (2016b). In vivo anti-melanogenesis activity and in vitro skin permeability of niacinamide-loaded flexible liposomes (Bounsphere™). J. Drug Deliv. Sci. Technol. 31, 147–152. doi:10.1016/j.jddst.2015.12.008
Li, H. O.-Y., Pastukhova, E., and Dover, J. S. (2023). Update on melasma management. Adv. Cosmet. Surg. 6, 193–211. doi:10.1016/j.yacs.2022.10.003
Li, Y. M., Milikowski, C., Selvaggi, G., Abbo, L. M., Skiada, D., and Galimberti, F. (2020). Polymyxin B-induced skin hyperpigmentation. Transpl. Infect. Dis. 22, e13312. doi:10.1111/tid.13312
Li, J., Duan, N., Song, S., Nie, D., Yu, M., Wang, J., et al. (2021). Transfersomes improved delivery of ascorbic palmitate into the viable epidermis for enhanced treatment of melasma. Int. J. Pharm. 608, 121059. doi:10.1016/j.ijpharm.2021.121059
Lim, J. T., and Tham, S. N. (1997). Glycolic acid peels in the treatment of melasma among Asian women. Dermatol Surg. 23, 177–9. doi:10.1111/j.1524-4725.1997.tb00016.x
Liu, H., Lin, Y., Nie, X., Chen, S., Chen, X., Shi, B., et al. (2011). Histological classification of melasma with reflectance confocal microscopy: a pilot study in Chinese patients. Skin Res. Technol. 17, 398–403. doi:10.1111/j.1600-0846.2011.00517.x
Lyons, A., Stoll, J., and Moy, R. (2018). A randomized, double-blind, placebo-controlled, split-face study of the efficacy of topical epidermal growth factor for the treatment of melasma. J. Drugs Dermatol 17, 970–973.
Mahmoud, B. H., Ruvolo, E., Hexsel, C. L., Liu, Y., Owen, M. R., Kollias, N., et al. (2010). Impact of long-wavelength UVA and visible light on melanocompetent skin. J. Investig. Dermatol 130, 2092–7. doi:10.1038/jid.2010.95
Majid, I., and Aleem, S. (2021). Melasma: update on epidemiology, clinical presentation, assessment, and scoring. J. Skin Stem Cell. 8. doi:10.5812/jssc.120283
Malamatari, M., Taylor, K. M. G., Malamataris, S., Douroumis, D., and Kachrimanis, K. (2018). Pharmaceutical nanocrystals: production by wet milling and applications. Drug Discov. Today 23, 534–547. doi:10.1016/j.drudis.2018.01.016
Malaviya, R., Morrison, A. R., and Pentland, A. P. (1996). Histamine in human epidermal cells is induced by ultraviolet light injury. J. investigative dermatology 106, 785–789. doi:10.1111/1523-1747.ep12346356
Malik, F., Hanif, M. M., and Mustafa, G. (2019). Combination of oral tranexamic acid with topical 3% tranexamic acid versus oral tranexamic acid with topical 20% azelaic acid in the treatment of melasma. J. Coll. Physicians Surg. Pak 29, 502–504. doi:10.29271/jcpsp.2019.06.502
Manfreda, V., Eleonora, D. M., and Luca, B. (2023). Efficacy and safety of Politranexamide® liposomal emulsion on facial melasma: a comparative study. J. Cosmet. Dermatol 22, 1780–1785. doi:10.1111/jocd.15648
Manosroi, A., Podjanasoonthon, K., and Manosroi, J. (2002). Development of novel topical tranexamic acid liposome formulations. Int. J. Pharm. 235, 61–70. doi:10.1016/s0378-5173(01)00980-2
Mckesey, J., Tovar-Garza, A., and Pandya, A. G. (2020). Melasma treatment: an evidence-based review. Am. J. Clin. Dermatol 21, 173–225. doi:10.1007/s40257-019-00488-w
Menter, A. (2004). Rationale for the use of topical corticosteroids in melasma. J. Drugs Dermatol 3, 169–74.
Mir, A., Boyd, K. P., Meehan, S. A., and Mclellan, B. (2013). Hydroxycholoroquine-induced hyperpigmentation. Dermatology online J. 19, 20723. doi:10.5070/d31912020723
Mishra, S. N., Dhurat, R. S., Deshpande, D. J., and Nayak, C. S. (2013). Diagnostic utility of dermatoscopy in hydroquinone-induced exogenous ochronosis. Int. J. Dermatology 52, 413–417. doi:10.1111/j.1365-4632.2011.05305.x
Monteiro, R., Kishore, B., Bhat, R., Dandekeri, S., Martis, J., and Hundi, G. (2013). A comparative study of the efficacy of 4% hydroquinone vs 0.75% kojic acid cream in the treatment of facial melasma. Indian J. dermatology 58, 157. doi:10.4103/0019-5154.108070
Morgado-Carrasco, D., Piquero-Casals, J., Granger, C., Trullàs, C., and Passeron, T. (2022). Melasma: the need for tailored photoprotection to improve clinical outcomes. Photodermatol. Photoimmunol. Photomed. 38, 515–521. doi:10.1111/phpp.12783
Nahhas, A. F., Braunberger, T. L., and Hamzavi, I. H. (2019). An update on drug-induced pigmentation. Am. J. Clin. dermatology 20, 75–96. doi:10.1007/s40257-018-0393-2
Navarrete-Solís, J., Castanedo-Cázares, J. P., Torres-Álvarez, B., Oros-Ovalle, C., Fuentes-Ahumada, C., González, F. J., et al. (2011). A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol Res. Pract. 2011, 379173. doi:10.1155/2011/379173
Nelson, M., and Schiavone, M. (2004). Emtricitabine (FTC) for the treatment of HIV infection. Int. J. Clin. Pract. 58, 504–510. doi:10.1111/j.1368-5031.2004.00100.x
Noh, T. K., Choi, S. J., Chung, B. Y., Kang, J. S., Lee, J. H., Lee, M. W., et al. (2014). Inflammatory features of melasma lesions in Asian skin. J. Dermatology 41, 788–794. doi:10.1111/1346-8138.12573
Nordlund, J. J., Grimes, P. E., and Ortonne, J. P. (2006a). The safety of hydroquinone. J. Eur. Acad. Dermatology Venereol. 20, 781–787. doi:10.1111/j.1468-3083.2006.01670.x
Nordlund, J., Grimes, P., and Ortonne, J. (2006b). The safety of hydroquinone. J. Eur. Acad. Dermatology Venereol. 20, 781–787. doi:10.1111/j.1468-3083.2006.01670.x
Ortonne, J. P. (2006). Retinoid therapy of pigmentary disorders. Dermatol Ther. 19, 280–8. doi:10.1111/j.1529-8019.2006.00085.x
Ortonne, J., Arellano, I., Berneburg, M., Cestari, T., Chan, H., Grimes, P., et al. (2009). A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J. Eur. Acad. Dermatology Venereol. 23, 1254–1262. doi:10.1111/j.1468-3083.2009.03295.x
Pakpayat, N., Nielloud, F., Fortuné, R., Tourne-Peteilh, C., Villarreal, A., Grillo, I., et al. (2009). Formulation of ascorbic acid microemulsions with alkyl polyglycosides. Eur. J. Pharm. Biopharm. 72, 444–52. doi:10.1016/j.ejpb.2009.01.005
Paliwal, R., Paliwal, S. R., Kenwat, R., Kurmi, B. D., and Sahu, M. K. (2020). Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin. Ther. Pat. 30, 179–194. doi:10.1080/13543776.2020.1720649
Park, J. J., Hwang, S. J., Kang, Y. S., Jung, J., Park, S., Hong, J. E., et al. (2019a). Synthesis of arbutin–gold nanoparticle complexes and their enhanced performance for whitening. Archives Pharmacal Res. 42, 977–989. doi:10.1007/s12272-019-01164-7
Park, J., Hwang, S., Kang, Y., Jung, J.-S., Park, S., Hong, J., et al. (2019b). Synthesis of arbutin–gold nanoparticle complexes and their enhanced performance for whitening. Archives Pharmacal Res. 42, 977–989. doi:10.1007/s12272-019-01164-7
Pasca, P. M., Antonescu, A., Fritea, L., Banica, F., Vicas, S. I., Laslo, V., et al. (2022). Novel liposomal formulation with azelaic acid: preparation, characterization, and evaluation of biological properties. Appl. Sci. 12, 13039. doi:10.3390/app122413039
Passeron, T., and Picardo, M. (2018). Melasma, a photoaging disorder. Pigment Cell. & melanoma Res. 31, 461–465. doi:10.1111/pcmr.12684
Pekmezci, E. (2019). A novel triple combination in treatment of melasma: significant outcome with far less actives. J. Cosmet. Dermatol 18, 1700–1704. doi:10.1111/jocd.12904
Piętowska, Z., Nowicka, D., and Szepietowski, J. C. (2022). Understanding melasma-how can Pharmacology and cosmetology procedures and prevention help to achieve optimal treatment results? A narrative review. Int. J. Environ. Res. Public Health 19, 12084. doi:10.3390/ijerph191912084
Pillaiyar, T., Manickam, M., and Namasivayam, V. (2017). Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 32, 403–425. doi:10.1080/14756366.2016.1256882
Premjith, R., and Karthikeyan, K. (2022). Zidovudine induced hyperpigmentation. Indian Dermatology Online J. 13, 398–399. doi:10.4103/idoj.idoj_449_21
Qiu, L., Zhang, M., Sturm, R. A., Gardiner, B., Tonks, I., Kay, G., et al. (2000). Inhibition of melanin synthesis by cystamine in human melanoma cells. J. Investig. Dermatol 114, 21–7. doi:10.1046/j.1523-1747.2000.00826.x
Radmard, A., Saeedi, M., Morteza-Semnani, K., Hashemi, S. M. H., and Nokhodchi, A. (2021). An eco-friendly and green formulation in lipid nanotechnology for delivery of a hydrophilic agent to the skin in the treatment and management of hyperpigmentation complaints: arbutin niosome (Arbusome). Colloids Surfaces B Biointerfaces 201, 111616. doi:10.1016/j.colsurfb.2021.111616
Rahimpour, Y., and Hamishehkar, H. (2012). Liposomes in cosmeceutics. Expert Opin. drug Deliv. 9, 443–455. doi:10.1517/17425247.2012.666968
Rajanala, S., Maymone, M. B. C., and Vashi, N. A. (2019a). Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 25. doi:10.5070/d32510045810
Rajanala, S., Maymone, M. B. D. C., and Vashi, N. A. (2019b). Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatology Online J. 25. doi:10.5070/d32510045810
Raton, B. (2011). “Ethnic skin and pigmentation,” in Cosmetics and dermatologic problems and solutions.
Rendon, M., and Dryer, L. (2016). Investigator-blinded, single-center study to evaluate the efficacy and tolerability of a 4% hydroquinone skin care system plus 0.02% tretinoin cream in mild-to-moderate melasma and photodamage. J. Drugs Dermatol 15, 466–75.
Resnik, S. (1967). Melasma induced by oral contraceptive drugs. Jama 199, 601–605. doi:10.1001/jama.199.9.601
Reyes-Habito, C. M., and Roh, E. K. (2014). Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: part I. Conventional chemotherapeutic drugs. J. Am. Acad. Dermatology 71, 203. e1–203. doi:10.1016/j.jaad.2014.04.014
Rigano, L., and Lionetti, N. (2016). “Chapter 6 - nanobiomaterials in galenic formulations and cosmetics,” in Nanobiomaterials in galenic formulations and cosmetics. Editor A. M. GRUMEZESCU (Norwich, New York, USA: William Andrew Publishing).
Rodrigues, M., and Pandya, A. G. (2015). Melasma: clinical diagnosis and management options. Australas. J. Dermatol 56, 151–63. doi:10.1111/ajd.12290
Roji, M., Sebastian, M., Lucca, J. M., and Ranugha, P. (2018). Phenytoin induced oral Lichenoid eruption and melasma: a case report. Indian J. Pharm. Pract. 11, 55–57. doi:10.5530/ijopp.11.1.10
Rolfe, H. M. (2014). A review of nicotinamide: treatment of skin diseases and potential side effects. J. Cosmet. Dermatol 13, 324–8. doi:10.1111/jocd.12119
Saeedi, M., Eslamifar, M., and Khezri, K. (2019). Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 110, 582–593. doi:10.1016/j.biopha.2018.12.006
Saladi, R. N., Cohen, S. R., Phelps, R. G., Persaud, A. N., and Rudikoff, D. (2006). Diltiazem induces severe photodistributed hyperpigmentation: case series, histoimmunopathology, management, and review of the literature. Archives dermatology 142, 206–210. doi:10.1001/archderm.142.2.206
Saleh, F., Abdel-Azim, E., Ragaie, M., and Guendy, M. (2019). Topical tranexamic acid with microneedling versus microneedling alone in treatment of melasma: clinical, histopathologic, and immunohistochemical study. J. Egypt. Women's Dermatologic Soc. 16, 89. doi:10.4103/jewd.jewd_25_19
Salimi, A., and Hajiani, M. (2018). Enhanced stability and dermal delivery of hydroquinone using microemulsion-based system, 11.
Salvioni, L., Morelli, L., Ochoa, E., Labra, M., Fiandra, L., Palugan, L., et al. (2021). The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 293, 102437. doi:10.1016/j.cis.2021.102437
Sanchez, N. P., Pathak, M. A., Sato, S., Fitzpatrick, T. B., Sanchez, J. L., and Mihm, M. C. (1981). Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J. Am. Acad. Dermatology 4, 698–710. doi:10.1016/s0190-9622(81)70071-9
Sarkar, R., Bansal, S., and Garg, V. K. (2012a). Chemical peels for melasma in dark-skinned patients. J. Cutan. Aesthet. Surg. 5, 247–53. doi:10.4103/0974-2077.104912
Sarkar, R., Chugh, S., and Garg, V. K. (2012b). Newer and upcoming therapies for melasma. Indian J. Dermatol Venereol. Leprol. 78, 417–28. doi:10.4103/0378-6323.98071
Schallier, D., Decoster, L., and De Greve, J. (2011). Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 31, 1753–1755.
Searle, T., Ali, F. R., and Al-Niaimi, F. (2022). The versatility of azelaic acid in dermatology. J. Dermatological Treat. 33, 722–732. doi:10.1080/09546634.2020.1800579
Searle, T., Al-Niaimi, F., and Ali, F. R. (2020). The top 10 cosmeceuticals for facial hyperpigmentation. Dermatol Ther. 33, e14095. doi:10.1111/dth.14095
Seleit, I., Bakry, O. A., Masoud, E., and Nabil, S. (2017). Identification of genotypes and allelic frequencies of vitamin D receptor gene polymorphism (TaqI) in egyptian melasma patients. Indian Dermatology Online J. 8, 443–448. doi:10.4103/idoj.IDOJ_363_16
Shankar, K., Godse, K., Aurangabadkar, S., Lahiri, K., Mysore, V., Ganjoo, A., et al. (2014). Evidence-based treatment for melasma: expert opinion and a review. Dermatology Ther. 4, 165–186. doi:10.1007/s13555-014-0064-z
Sharma, D., Ali, A. A. E., and Trivedi, L. R. (2018). An Updated Review on: liposomes as drug delivery system. PharmaTutor 6, 50–62. doi:10.29161/pt.v6.i2.2018.50
Sharquie, K. E., Al-Mashhadani, S. A., and Salman, H. A. (2008). Topical 10% zinc sulfate solution for treatment of melasma. Dermatol. Surg. 34, 1346–1349. doi:10.1111/j.1524-4725.2008.34287.x
Shaw, T. K., Paul, P., and Chatterjee, B. (2022). Research-based findings on scope of liposome-based cosmeceuticals: an updated review. Future J. Pharm. Sci. 8, 46. doi:10.1186/s43094-022-00435-3
Sheth, V. M., and Pandya, A. G. (2011). Melasma: a comprehensive update: part II. J. Am. Acad. Dermatol 65, 699–714. doi:10.1016/j.jaad.2011.06.001
Shihab, N., Prihartono, J., Tovar-Garza, A., Agustin, T., Legiawati, L., and Pandya, A. G. (2020). Randomised, controlled, double-blind study of combination therapy of oral tranexamic acid and topical hydroquinone in the treatment of melasma. Australas. J. Dermatology 61, 237–242. doi:10.1111/ajd.13267
Shin, J. U., Park, J., Oh, S. H., and Lee, J. H. (2013). Oral tranexamic acid enhances the efficacy of low-fluence 1064-nm quality-switched neodymium-doped yttrium aluminum garnet laser treatment for melasma in Koreans: a randomized, prospective trial. Dermatol Surg. 39, 435–42. doi:10.1111/dsu.12060
Shkolnik, T. G., Feuerman, H., Didkovsky, E., Kaplan, I., Bergman, R., Pavlovsky, L., et al. (2014). Blue-gray mucocutaneous discoloration: a new adverse effect of ezogabine. JAMA dermatol. 150, 984–989. doi:10.1001/jamadermatol.2013.8895
Sieber, M. A., and Hegel, J. K. (2014). Azelaic acid: properties and mode of action. Skin. Pharmacol. Physiol. 27 (Suppl. 1), 9–17. doi:10.1159/000354888
Singh, T. G., and Sharma, N. (2016). “Chapter 7 - nanobiomaterials in cosmetics: current status and future prospects,” in Nanobiomaterials in galenic formulations and cosmetics. Editor A. M. GRUMEZESCU (Norwich, New York, USA: William Andrew Publishing).
Singh, S., Sharma, N., Zahoor, I., Behl, T., Antil, A., Gupta, S., et al. (2023). Decrypting the potential of nanotechnology-based approaches as cutting-edge for management of hyperpigmentation disorder. Molecules 28, 220. doi:10.3390/molecules28010220
Sobhan, M., Talebi-Ghane, E., and Poostiyan, E. (2023). A comparative study of 20% azelaic acid cream versus 5% tranexamic acid solution for the treatment of postinflammatory hyperpigmentation in patients with acne vulgaris: a single-blinded randomized clinical trial. J. Res. Med. Sci. 28, 18. doi:10.4103/jrms.jrms_443_22
Sobhi, R. M., and Sobhi, A. M. (2012). A single-blinded comparative study between the use of glycolic acid 70% peel and the use of topical nanosome vitamin C iontophoresis in the treatment of melasma. J. Cosmet. Dermatol 11, 65–71. doi:10.1111/j.1473-2165.2011.00599.x
Song, M., Mun, J. H., Ko, H. C., Kim, B. S., and Kim, M. B. (2011). Korean red ginseng powder in the treatment of melasma: an uncontrolled observational study. J. Ginseng Res. 35, 170–5. doi:10.5142/jgr.2011.35.2.170
Spierings, N. M. K. (2020). Melasma: a critical analysis of clinical trials investigating treatment modalities published in the past 10 years. J. Cosmet. Dermatol 19, 1284–1289. doi:10.1111/jocd.13182
Stähli, B., and Schwab, S. (2011). Amiodarone-induced skin hyperpigmentation. QJM Int. J. Med. 104, 723–724. doi:10.1093/qjmed/hcq131
Surini, S., and Mellani, T. (2017a). Formulation and physical evaluation of microemulsion and W/O/W multiple emulsions dosage forms with alpha arbutin, lactic acid, and niacinamide as skin-whitening cosmetics. Int. J. Appl. Pharm. 9, 67. doi:10.22159/ijap.2017.v9s1.39_45
Surini, S., and Mellani, T. (2017b). Formulation and physical evaluation of microemulsion and W/O/W multiple emulsions dosage forms with alpha arbutin, lactic acid, and niacinamide as skin-whitening cosmetics. Int. J. Appl. Pharm. 9, 67. doi:10.22159/ijap.2017.v9s1.39_45
Suryaningsih, B. E., Sadewa, A. H., Wirohadidjojo, Y. W., and Soebono, H. (2019). Association between heterozygote Val92Met MC1R gene polymorphisms with incidence of melasma: a study of Javanese women population in Yogyakarta. Clin. Cosmet. investigational dermatology 12, 489–495. doi:10.2147/CCID.S206115
Suryantari, S., Suweta, N. P. T., Veronica, E., Hartawan, I. G. N. B., and Karna, N. (2020). Systematic review of melasma treatments: advantages and disadvantages. Bali Dermatology Venereol. J. 3, 37–51. doi:10.15562/bdv.v3i2.41
Suvirya, S., Agrawal, A., and Parihar, A. (2014). 5-Fluorouracil-induced bilateral persistent serpentine supravenous hyperpigmented eruption, bilateral mottling of palms and diffuse hyperpigmentation of soles. Case Rep. 2014, bcr2014206793. doi:10.1136/bcr-2014-206793
Syed Azhar, S. N. A., Ashari, S. E., and Salim, N. (2018). Development of a kojic monooleate-enriched oil-in-water nanoemulsion as a potential carrier for hyperpigmentation treatment. Int. J. Nanomedicine 13, 6465–6479. doi:10.2147/IJN.S171532
Tadokoro, T., Bonté, F., Archambault, J. C., Cauchard, J. H., Neveu, M., Ozawa, K., et al. (2010). Whitening efficacy of plant extracts including orchid extracts on Japanese female skin with melasma and lentigo senilis. J. dermatology 37, 522–530. doi:10.1111/j.1346-8138.2010.00897.x
Taghavi, F., Banihashemi, M., Zabolinejad, N., Salehi, M., Jaafari, M. R., Marhamati, H., et al. (2019a). Comparison of therapeutic effects of conventional and liposomal form of 4% topical hydroquinone in patients with melasma. J. Cosmet. Dermatol 18, 870–873. doi:10.1111/jocd.12744
Taghavi, F., Banihashemi, M., Zabolinejad, N., Salehi, M., Jaafari, M. R., Marhamati, H., et al. (2019b). Comparison of therapeutic effects of conventional and liposomal form of 4% topical hydroquinone in patients with melasma. J. Cosmet. Dermatology 18, 870–873. doi:10.1111/jocd.12744
Takci, Z., and Ozoguz, P. (2012). Nail discoloration due to tinzaparin sodium. Cutan. Ocular Toxicol. 31, 332–334. doi:10.3109/15569527.2011.647178
Tangau, M. J., Chong, Y. K., and Yeong, K. Y. (2022). Advances in cosmeceutical nanotechnology for hyperpigmentation treatment. J. Nanoparticle Res. 24, 155. doi:10.1007/s11051-022-05534-z
Tansir, G., Gogia, A., and Gupta, R. (2022). Hydroxyurea-induced hyperpigmentation. Cancer Res. Statistics, Treat. 5, 385–386. doi:10.4103/crst.crst_72_22
Taraz, M., Niknam, S., and Ehsani, A. H. (2017). Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol. Ther. 30, e12465. doi:10.1111/dth.12465
Taylor, S. C., Torok, H., Jones, T., Lowe, N., Rich, P., Tschen, E., et al. (2003). Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis 72, 67–72.
Telang, P. S. (2013). Vitamin C in dermatology. Indian Dermatol Online J. 4, 143–6. doi:10.4103/2229-5178.110593
Thangaraju, P., Singh, H., Punitha, M., Giri, V., and Ali, M. S. (2015). Hyperpigmentation, a marker of rifampicin overuse in leprosy patient: an incidental finding. Sudan Med. Monit. 10, 25. doi:10.4103/1858-5000.157506
Tirado-Sánchez, A., Santamaría-Román, A., and Ponce-Olivera, R. M. (2009). Efficacy of dioic acid compared with hydroquinone in the treatment of melasma. Int. J. Dermatol 48, 893–5. doi:10.1111/j.1365-4632.2009.04105.x
Tirnaksiz, F., Kayiş, A., Çelebi, N., Adişen, E., and Erel, A. (2012). Preparation and evaluation of topical microemulsion system containing metronidazole for remission in rosacea. Chem. Pharm. Bull. (Tokyo) 60, 583–92. doi:10.1248/cpb.60.583
Tomić, I., Juretić, M., Jug, M., Pepić, I., Cetina Čižmek, B., and Filipović-Grčić, J. (2019a). Preparation of in situ hydrogels loaded with azelaic acid nanocrystals and their dermal application performance study. Int. J. Pharm. 563, 249–258. doi:10.1016/j.ijpharm.2019.04.016
Tomić, I., Juretić, M., Jug, M., Pepić, I., Cetina Čižmek, B., and Filipović-Grčić, J. (2019b). Preparation of in situ hydrogels loaded with azelaic acid nanocrystals and their dermal application performance study. Int. J. Pharm. 563, 249–258. doi:10.1016/j.ijpharm.2019.04.016
Torres-Álvarez, B., Mesa-Garza, I. G., Castanedo-Cázares, J. P., Fuentes-Ahumada, C., Oros-Ovalle, C., Navarrete-Solis, J., et al. (2011). Histochemical and immunohistochemical study in melasma: evidence of damage in the basal membrane. Am. J. Dermatopathol. 33, 291–295. doi:10.1097/DAD.0b013e3181ef2d45
Trivedi, M. K., Yang, F. C., and Cho, B. K. (2017). A review of laser and light therapy in melasma. Int. J. Womens Dermatol 3, 11–20. doi:10.1016/j.ijwd.2017.01.004
Tse, T. W. (2010). Hydroquinone for skin lightening: safety profile, duration of use and when should we stop? J. Dermatol. Treat. 21, 272–5. doi:10.3109/09546630903341945
Üstündağ Okur, N., Çağlar, E., Pekcan, A., Okur, M., and Ayla, S. (2019). Preparation, optimization and in vivo anti-inflammatory evaluation of hydroquinone loaded microemulsion formulations for melasma treatment, 23, 662–670.
Vachiramon, V., Kositkuljorn, C., Leerunyakul, K., and Chanprapaph, K. (2021). Isobutylamido thiazolyl resorcinol for prevention of UVB-induced hyperpigmentation. J. Cosmet. Dermatol 20, 987–992. doi:10.1111/jocd.13615
Videira, I. F. D. S., Moura, D. F. L., and Magina, S. (2013). Mechanisms regulating melanogenesis. An. Bras. Dermatol. 88, 76–83. doi:10.1590/s0365-05962013000100009
Vidlářová, L., Romero, G. B., Hanuš, J., Štěpánek, F., and Müller, R. H. (2016). Nanocrystals for dermal penetration enhancement - effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 104, 216–25. doi:10.1016/j.ejpb.2016.05.004
Vieira Brazil, R., Brasil Dos Santos, J., Bucco De Campos, V. E., Pereira Dos Santos, E., and Faria De Freitas, Z. (2023). Clinical application of kojic acid in the treatment of melasma: a scope review. J. Cosmet. Sci. 74.
Vignand-Courtin, C., Martin, C., Le Beller, C., Mateus, C., Barbault-Foucher, S., and Rieutord, A. (2012). Cutaneous side effects associated with sunitinib: an analysis of 8 cases. Int. J. Clin. Pharm. 34, 286–289. doi:10.1007/s11096-012-9615-5
Wang, Y.-W., Jou, C.-H., Hung, C.-C., and Yang, M.-C. (2012a). Cellular fusion and whitening effect of a chitosan derivative coated liposome. Colloids Surfaces B Biointerfaces 90, 169–176. doi:10.1016/j.colsurfb.2011.10.024
Wang, Y. W., Jou, C. H., Hung, C. C., and Yang, M. C. (2012b). Cellular fusion and whitening effect of a chitosan derivative coated liposome. Colloids Surf. B Biointerfaces 90, 169–76. doi:10.1016/j.colsurfb.2011.10.024
Wen, A.-H., Choi, M.-K., and Kim, D.-D. (2006a). Formulation of liposome for topical delivery of arbutin. Archives pharmacal Res. 29, 1187–1192. doi:10.1007/BF02969312
Wen, A. H., Choi, M. K., and Kim, D. D. (2006b). Formulation of liposome for topical delivery of arbutin. Arch. Pharm. Res. 29, 1187–92. doi:10.1007/BF02969312
Weschawalit, S., Thongthip, S., Phutrakool, P., and Asawanonda, P. (2017). Glutathione and its antiaging and antimelanogenic effects. Clin. Cosmet. Investig. Dermatol 10, 147–153. doi:10.2147/CCID.S128339
Westerhof, W., and Kooyers, T. (2005). Hydroquinone and its analogues in dermatology–a potential health risk. J. Cosmet. dermatology 4, 55–59. doi:10.1111/j.1473-2165.2005.40202.x
Wu, P. S., Li, Y. S., Kuo, Y. C., Tsai, S. J., and Lin, C. C. (2019). Preparation and evaluation of novel transfersomes combined with the natural antioxidant resveratrol. Molecules 24, 600. doi:10.3390/molecules24030600
Wu, P.-S., Lin, C.-H., Kuo, Y.-C., and Lin, C.-C. (2017). Formulation and characterization of hydroquinone nanostructured lipid carriers by homogenization emulsification method. J. Nanomater. 2017, 1–7. doi:10.1155/2017/3282693
Wu, M., Antony, R., and Mayrovitz, H. (2021). Melasma: a condition of asian skin. Cureus 13, e14398. doi:10.7759/cureus.14398
Xiao, L., Matsubayashi, K., and Miwa, N. (2007a). Inhibitory effect of the water-soluble polymer-wrapped derivative of fullerene on UVA-induced melanogenesis via downregulation of tyrosinase expression in human melanocytes and skin tissues. Arch. Dermatol Res. 299, 245–57. doi:10.1007/s00403-007-0740-2
Xiao, L., Matsubayashi, K., and Miwa, N. (2007b). Inhibitory effect of the water-soluble polymer-wrapped derivative of fullerene on UVA-induced melanogenesis via downregulation of tyrosinase expression in human melanocytes and skin tissues. Archives Dermatological Res. 299, 245–257. doi:10.1007/s00403-007-0740-2
Yenny, S. W. (2018). Comparison of the use of 5% methimazole cream with 4% kojic acid in melasma treatment. Turkish J. Dermatology 12, 167–171. doi:10.4274/tdd.3640
Yoshida, M., Takahashi, Y., and Inoue, S. (2000). Histamine induces melanogenesis and morphologic changes by protein kinase A activation via H2 receptors in human normal melanocytes. J. investigative dermatology 114, 334–342. doi:10.1046/j.1523-1747.2000.00874.x
Yousefi, A., Khani Khoozani, Z., Zakerzadeh Forooshani, S., Omrani, N., Moini, A. M., and Eskandari, Y. (2014). Is topical zinc effective in the treatment of melasma? A double-blind randomized comparative study. Dermatol Surg. 40, 33–7. doi:10.1111/dsu.12296
Yuan, X., and Jin, Z. (2018). Paracrine regulation of melanogenesis. Br. J. Dermatology 178, 632–639. doi:10.1111/bjd.15651
Zhang, Q., Tu, Y., Gu, H., Sun, D., Wu, W., Man, M. Q., et al. (2019). A cream of herbal mixture to improve melasma. J. Cosmet. Dermatol 18, 1721–1728. doi:10.1111/jocd.12938
Ziemer, M., Goetze, S., Juhasz, K., and Elsner, P. (2011). Flagellate dermatitis as a bleomycin-specific adverse effect of cytostatic therapy: a clinical-histopathologic correlation. Am. J. Clin. dermatology 12, 68–76. doi:10.2165/11537080-000000000-00000
Keywords: melasma, hyperpigmentation, topical drug delivery, nanoparticles, nanotechnology, skin permeation, clinical effectiveness
Citation: Ghasemiyeh P, Fazlinejad R, Kiafar MR, Rasekh S, Mokhtarzadegan M and Mohammadi-Samani S (2024) Different therapeutic approaches in melasma: advances and limitations. Front. Pharmacol. 15:1337282. doi: 10.3389/fphar.2024.1337282
Received: 12 November 2023; Accepted: 18 March 2024;
Published: 02 April 2024.
Edited by:
Jia-You Fang, Chang Gung University, TaiwanReviewed by:
Sergio Alberto Bernal Chávez, Universidad de las Américas Puebla, MexicoCopyright © 2024 Ghasemiyeh, Fazlinejad, Kiafar, Rasekh, Mokhtarzadegan and Mohammadi-Samani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soliman Mohammadi-Samani, c21zYW1hbmlAc3Vtcy5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.