- 1Department of Orthopedic, Hong-Hui Hospital, Xi’an Jiaotong University College of Medicine, Xi’an, China
- 2Department of Spinal Surgery, Qingdao Hospital, University of Health and Rehabilitation Sciences (Qingdao Municipal Hospital), Qingdao, China
Background: Several medications have been used for glucocorticoids-induced osteoporosis (GIO). However, the best therapeutic option for GIO is still controversial. A Bayesian network meta-analysis was conducted to compare the efficacy and safety of denosumab, teriparatide and bisphosphonates for patients with GIO.
Methods: Relevant randomized controlled trials published in PubMed, Embase, Cochrane Library and ClinicalTrials.gov up to August 2023 were searched. The following efficiency and safety outcomes were extracted for comparison: bone mineral density (BMD) percentage changes in lumbar spine, femur neck and total hip, and incidences of adverse events (AEs), serious adverse events (SAEs), vertebrae and non-vertebrae fracture. Bayesian random effects models were used for multiple treatment comparisons.
Results: 11 eligible RCTs involving 2,877 patients were identified. All the six medications including alendronate, risedronate, etidronate, zoledronate, teriparatide, and denosumab and were effective in increasing BMD. Teriparatide and denosumab were more effective in improving lumbar spine and femur neck BMD, and reducing vertebrae fracture. Alendronate and denosumab were more effective in improving total hip BMD. Alendronate and teriparatide had the lowest incidences of AEs and SAEs.
Conclusion: Teriparatide denosumab and the bisphosphonates are all effective in improving BMD for GIO patients. Based on this network meta-analysis, teriparatide and denosumab have higher efficiency in improving lumbar spine and femur neck BMD, and reducing vertebrae fracture.
Systematic Review Registration: 10.17605/OSF.IO/2G8YA, identifier CRD42023456305.
Introduction
Glucocorticoids are widely used for the treatment of chronic inflammatory and autoimmune diseases, such as rheumatoid arthritis, asthma and systemic lupus erythematosus (Pons-Estel et al., 2018). Long-term use of glucocorticoids can cause a series of side effects, including immune and cardiovascular disorders and osteoporosis (Waljee et al., 2017). Osteoporosis is defined as low bone quality, strength and elevated fracture risk. Primary osteoporosis is due to menopause-related bone demineralization or aging. Secondary osteoporosis is result from pathological conditions or medications other than menopause and aging, which lead to decrease of bone mass and increased fracture risk. Prolonged glucocorticoids use leads to secondary osteoporosis by facilitating osteoclast differentiation and inhibiting osteoblast proliferation, which can result in increased bone resorption (Laan et al., 1993; Wang et al., 2020).
Several treatment options have been proposed for glucocorticoids-induced osteoporosis (GIO). Bisphosphonates including alendronate, risedronate, etidronate and zoledronate are considered as the most common option for GIO (Reid et al., 2009; Buckley et al., 2017; Nasomyont et al., 2021). Numerous studies have proven the efficacy of bisphosphonates for GIO. Bisphosphonates are easily deposited on the surfaces of bone and suppress osteolysis by induction of inhibition and apoptosis of enzymes like farnesyl pyrophosphate synthase in the osteoclasts. However, potential side effects and inconvenient dosing regimens of bisphosphonate may lead to discontinuation of treatment. Other options for GIO include teriparatide and denosumab. Teriparatide is a parathormone analogue that can promote bone formation. Denosumab is a human monoclonal antibody binding to receptor activator of nuclear factor kappa-B ligand and inhibits osteoclastogenesis (Lipton and Goessl, 2011; Body, 2012; Lacey et al., 2012). The best therapeutic option for GIO, however, is still controversial. Several traditional pairwise meta-analysis have been performed to compare different treatments (Yanbeiy and Hansen, 2019; Yamaguchi et al., 2020; Jiang et al., 2022). However, network meta-analysis, having the advantages of indirect comparisons and ranking, is still in lack. Therefore, we conducted a Bayesian network meta-analysis with the aim to compare the efficacy and safety of denosumab, teriparatide and bisphosphonates for patients with GIO.
Materials and methods
This meta-analysis was performed in accordance with a registered protocol (CRD42023456305).
Search strategy
This network meta-analysis was performed according to the PRISMA extension statement for network meta-analysis (Hutton et al., 2015). We searched the Pubmed, Embase, Cochrane Library and ClinicalTrials.gov for relevant randomized controlled trials (RCTs) until the end of August 2023. The following search terms were used: osteoporosis, glucocorticoid-induced, steroids, bone mineral density (BMD), bisphosphonate, alendronate, risedronate, etidronate, zoledronate, teriparatide and denosumab. Additionally, references of selected articles were checked for studies that met the criteria.
Inclusion and exclusion criteria
Two authors screened the relevant studies independently, and disagreements were adjudicated by a third author. Original studies in English were eligible.
The inclusion criteria were: 1) studies that included patients with GIO; 2) double-blind or open-label RCTs lasting at least 12 months; 2) studies focusing on the comparisons among placebo, alendronate, risedronate, etidronate, zoledronate, teriparatide or denosumab; 3) studies reporting at least one of the following outcomes: BMD percentage changes in lumbar spine, total hip and femur neck. Incidences of adverse events (AEs), serious adverse events (SAEs), vertebrae and non-vertebrae fracture.
The exclusion criteria were: 1) duplicate studies for same population with different investigations; 2) studies including patients younger than 18 years old; 3) studies with patients taking medications that may have effect on BMD.
Data extraction and quality assessment
The following data was extracted from each included RCT by two authors independently: study characteristics (e.g., first author, publication date, location, sample size), antiosteoporosis medications (e.g., type of antiosteoporosis medicine, dosage regimen), patient characteristics (e.g., number of patients, age and sex), percentage changes in BMD, incidence of AEs, SAEs, vertebrae and non-vertebrae fracture.
Quality of the included RCTs were independently evaluated by two authors according to the Cochrane Collaboration’s risk of bias tool for RCTs (Higgins et al., 2011). Disagreements between the two authors were resolved through discussion.
Data synthesis and analysis
This Bayesian network meta-analysis was conducted by R (version 4.0.3) and gemtc package (van Valkenhoef et al., 2016; Shim et al., 2019). The outcomes of the network meta-analysis were presented by mean difference (MD) and 95% credibility intervals (95% CrI) for continuous data, and by odds ratio (OR) and 95% CrI for dichotomous data. The Markov chain Monte Carlo simulation technique within a Bayesian framework were used to perform the network meta-analysis. Random-effects and consistency models were used in this meta-analysis. The random model used four chains with 10,000 burn-ins and 50,000 iterations. Node-splitting analysis were performed between the direct and indirect evidence for consistency evaluation. Surface under cumulative ranking curve (SUCRA) was calculated to rank the outcomes of each treatment based on a Bayesian approach (Rücker and Schwarzer, 2015). A larger SUCRA value meant a higher rank of the intervention (Salanti et al., 2011; Shim et al., 2019). The publication bias was assessed using the funnel plot and Egger test.
Results
Literature search and study characteristics
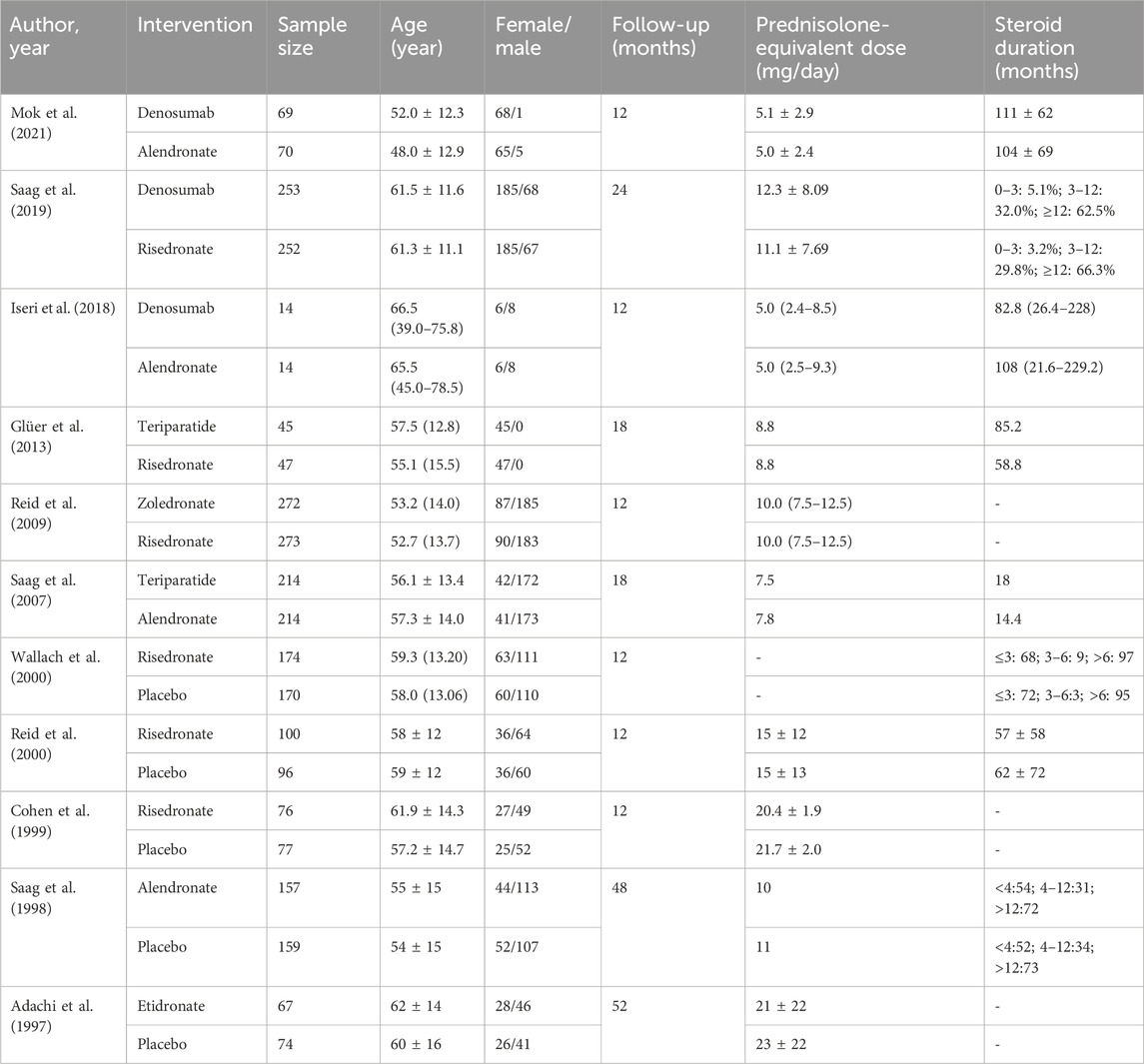
A total of 271 potentially relevant articles were identified in the database search. After reviewing the titles, abstracts and full texts, 259 articles were excluded. Eight studies (Langdahl et al., 2009; Losada et al., 2009; Burshell et al., 2010; Devogelaer et al., 2010; Eastell et al., 2010; Sambrook et al., 2012; Farahmand et al., 2013) were excluded for being subgroup analysis or post hoc study from previous data. Finally, 11 RCTs (Adachi et al., 1997; Saag et al., 1998; Cohen et al., 1999; Reid et al., 2000; Wallach et al., 2000; Saag et al., 2007; Reid et al., 2009; Glüer et al., 2013; Iseri et al., 2018; Saag et al., 2019; Mok et al., 2021) involving 2,877 patients were included for this meta-analysis. The sample size of the included RCTs ranged from 28 to 545, and the follow-up period ranged from 12 to 52 months. Figure 1 and Figure 2 presents the screening process and the network. Table 1 presents the baseline characteristics of the included RCTs. According to the Cochrane Collaboration’s risk of bias tool for RCTs, final risk of bias of the included studies was low (Supplementary Figure S1).
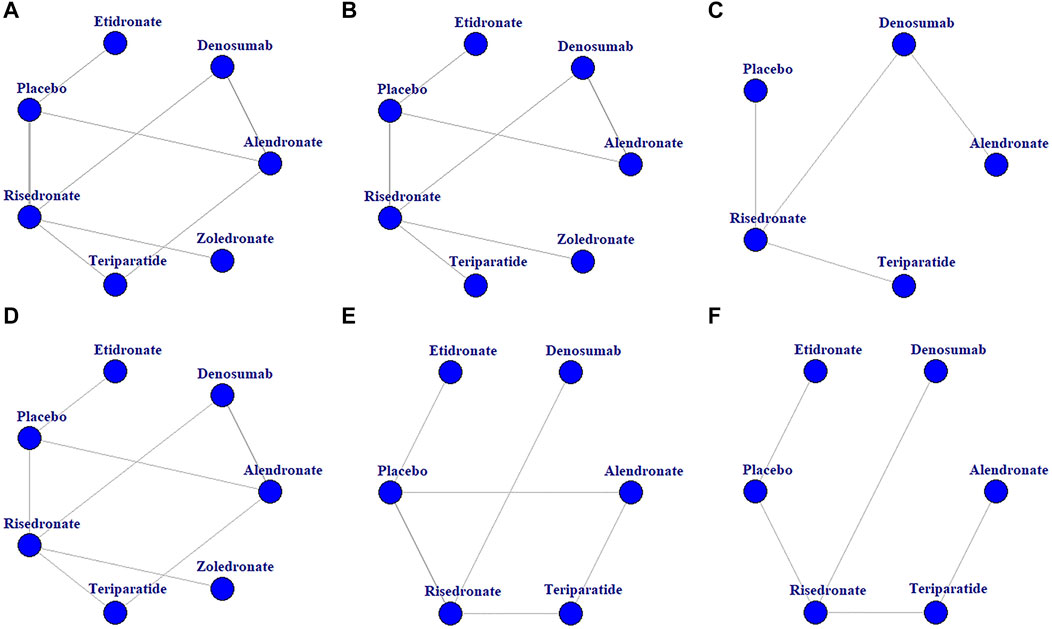
FIGURE 2. Network diagrams of the comparisons of treatments. BMD percentage changes in lumbar spine (A), femur neck (B), and total hip (C). Incidences of AEs (D), vertebrae fracture (E), and non-vertebrae fracture (F).
Efficiency and safety outcomes
At the end of the follow-up period, according to the network meta-analysis, all the six treatments (alendronate, risedronate, etidronate, zoledronate, teriparatide and denosumab) were significantly superior to placebo in improving lumbar spine BMD (p < 0.05) (Figure 3A; Table 2A). Based on the SUCRA values of various treatment strategies, teriparatide and denosumab were the best treatments for improving lumbar spine BMD, followed by zoledronate, etidronate, alendronate and risedronate (Figure 4A).
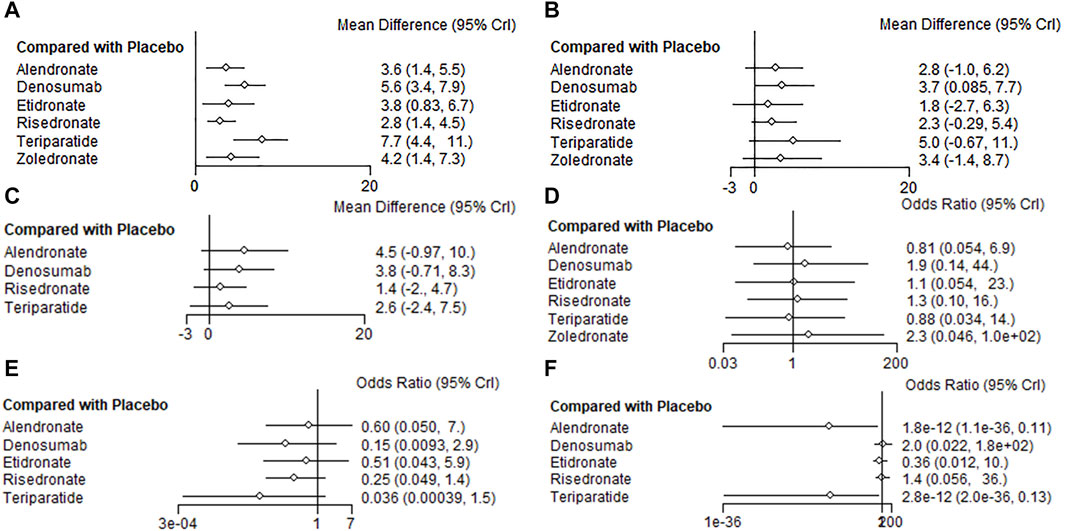
FIGURE 3. Forest plots of the outcomes. BMD percentage changes in lumbar spine (A), femur neck (B), and total hip (C). Incidences of AEs (D), vertebrae fracture (E), and non-vertebrae fracture (F).
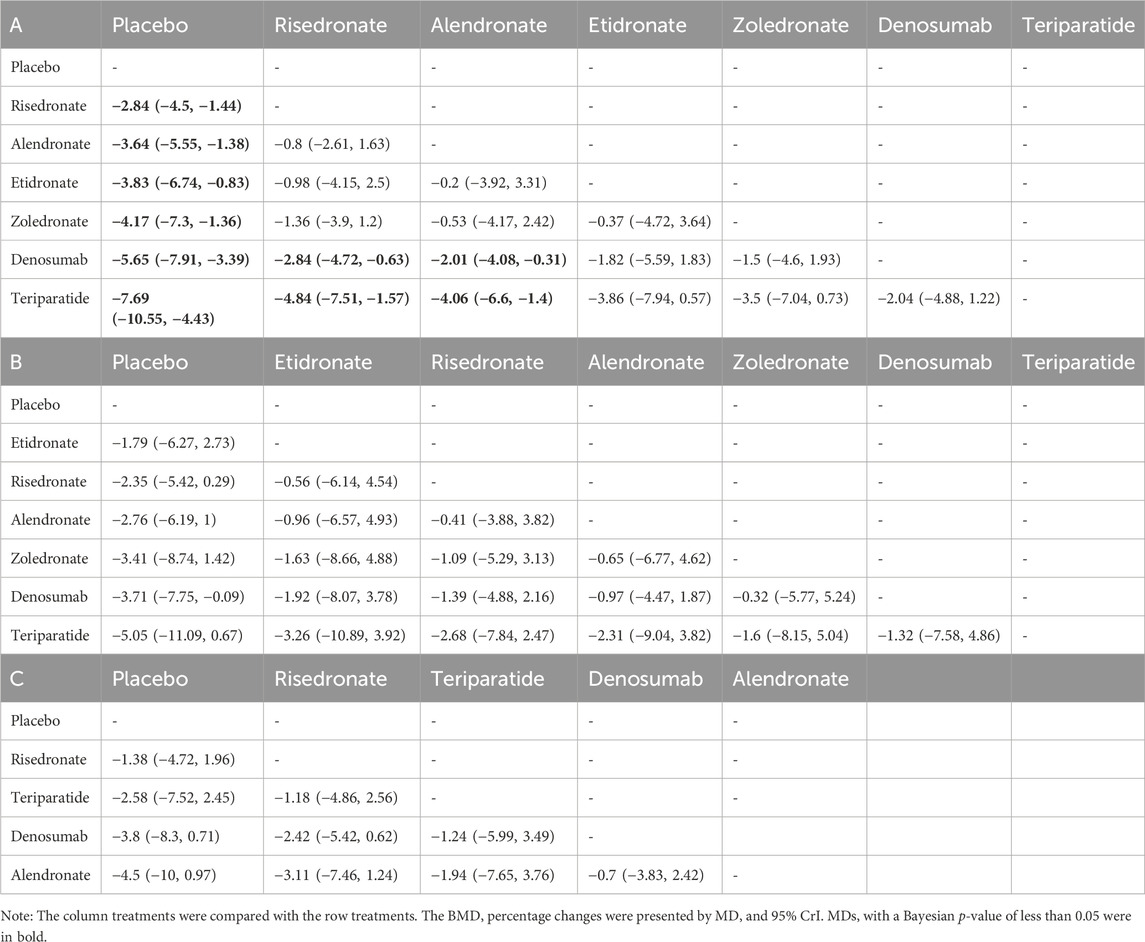
TABLE 2. Pooled estimates of the network meta-analysis. BMD percentage changes in lumbar spine (A), femur neck (B), and total hip (C).
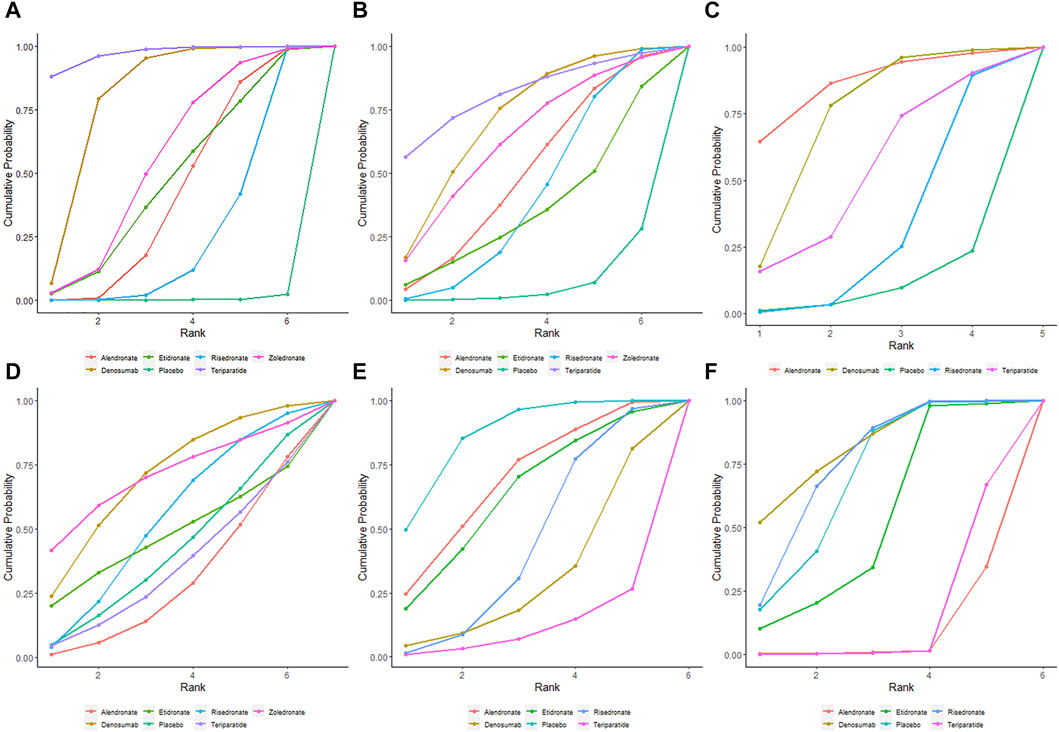
FIGURE 4. SUCRA indicating the probability ranks of outcomes. BMD percentage changes in lumbar spine (A), femur neck (B), and total hip (C). Incidences of AEs (D), vertebrae fracture (E), and non-vertebrae fracture (F).
Based on the network meta-analysis, all the six treatments had higher percentage changes in femur neck BMD than the placebo (Figure 3B; Table 2A). According to the SUCRA values, teriparatide and denosumab scored the highest, followed by zoledronate, alendronate, risedronate and etidronate (Figure 4B).
Concerning total hip BMD, four treatments were included in comparison. All the five treatments had higher BMD percentage changes than the placebo but without significance (p > 0.05) (Figure 3C; Table 2B). Regarding to SUCRA ranking analysis, alendronate and denosumab ranked highest, followed by teriparatide and risedronate (Figure 4C).
Regarding to the incidence of AEs, alendronate and teriparatide had the lowest incidence of AEs, followed by etidronate, risedronate, denosumab and zoledronate (Figure 4D). Teriparatide and alendronate had the lowest incidence of SAEs, followed by zoledronate, risedronate and denosumab.
With respect to the incidences of vertebrae fracture, teriparatide and denosumab had the lowest incidence of vertebrae fracture, followed by risedronate, etidronate and alendronate (Figure 4E). Alendronate and teriparatide had the lowest incidence of non-vertebrae fracture, followed by etidronate, risedronate and denosumab (Figure 4F).
Node-splitting analysis showed no significant heterogeneity or inconsistency occurred in direct and indirect evidence (p > 0.05) (Supplementary Figure S2). For comparisons with more than ten studies included, funnel plots and Egger’s test were used to assess publication bias. Regarding to comparisons of percentage changes in lumbar spine BMD, the funnel plots looked symmetric, and the Egger’s test showed no significant potential publication bias (p > 0.05) (Supplementary Figure S3).
Discussion
This present Bayesian network meta-analysis summarized the latest information and provided a ranking of various treatments (alendronate, risedronate, etidronate, zoledronate, teriparatide and denosumab) in treating GIO. A previous meta-analysis by Yuan et al. (2023) compared denosumab, teriparatide, and oral bisphosphonates (alendronate and risedronate) in the prevention of GIO, and noted that teriparatide and denosumab were similar or even superior to bisphosphonates. There are some differences between the meta-analysis by Yuan et al. (2023) and our study. Firstly, unlike the pairwise meta-analysis by Yuan et al. (2023), we conducted a Bayesian network meta-analysis. Network meta-analysis was performed to compensate for the lack of face-to-face comparisons among various treatments. Network meta-analysis has the advantages of indirect comparisons and providing ranking of different treatments. Secondly, in the meta-analysis by Yuan et al. (2023), bisphosphonates including alendronate and risedronate were compared with denosumab and teriparatide as a whole, while alendronate and risedronate, in fact, are different to some extent. Besides, the comparisons between denosumab and teriparatide were not performed in the meta-analysis by Yuan et al. (2023). Oppositely, in our network meta-analysis, alendronate, risedronate, etidronate, zoledronate, denosumab and teriparatide were compared with each other separately. Table 2 showed the comparison results of BMD percentage changes between every two treatments in detail.
To our knowledge, there is only one network meta-analysis performed by Migliorini et al. on antiresorptive treatments for GIO (Migliorini et al., 2022). In this network meta-analysis, the treatments included alendronate, zoledronate, risedronate, denosumab and etidronate, but not teriparatide. Teriparatide has the effect of promoting bone formation. Teriparatide has been proved effective in treating GIO and has been widely used (Solomon et al., 2017; Kaneko et al., 2018), so we conducted the present network meta-analysis including denosumab, teriparatide and bisphosphonates. Our study provided suggestions to help professionals and GIO patients make decisions on their treatment.
In the present network meta-analysis, teriparatide and denosumab ranked the first two in improving lumbar spine BMD, followed by the bisphosphonates. This result was consistent with previous RCTs. The study by Glüer et al. and Saag et al. suggested that teriparatide was more effective in increasing the BMD than the bisphosphonates (Saag et al., 2007; Glüer et al., 2013). And numerous studies have proved denosumab was superior in improving lumbar spine BMD than the bisphosphonates (Iseri et al., 2018; Saag et al., 2019; Mok et al., 2021). Similarly, in the meta-analysis by Yuan et al. (2023), teriparatide and denosumab were shown to be superior in improving BMD in lumbar spine than the bisphosphonates. In the network meta-analysis by Migliorini et al. (2022), denosumab and alendronate ranked the first two in improving lumbar spine BMD. However, teriparatide was not included in the study, whose limitation was compensated for by our study.
Based on the present network meta-analysis, teriparatide and denosumab ranked the first two in improving femur neck BMD, while alendronate and denosumab ranked the first two in improving total hip BMD. This difference in BMD improvement at various skeletal sites may be associated with different proportion of trabecular bone and different treatment-related changes in bone density and architecture (Choksi et al., 2018). Anti-resorptive medications (denosumab and bisphosphonates) decrease the endosteal diameter by increasing endosteal bone volume, but do not expand periosteal bone. On the other hand, anabolic agent (teriparatide) leads to increase in periosteal bone and increase in endosteal bone resorption simultaneously, which results in a bone without much cortical thickness change (Choksi et al., 2018). In the meta-analysis by Yuan et al. (2023), teriparatide was superior to bisphosphonates in increasing hip BMD. However, our network meta-analysis showed no significant differences between teriparatide and the bisphosphonates (Table 2), and alendronate ranked the first in increasing hip BMD (Figure 4). This result was consistent with the network by Migliorini et al. (2022), which noted alendronate was superior to other treatments. Further studies are needed to explore the efficacy of increasing hip BMD in various treatments.
With respect to the safety of treatments, alendronate and teriparatide had the lowest incidences of AEs and SAEs. Similarly, the network meta-analysis by Migliorini et al. noted the alendronate group had the lowest SAEs (Migliorini et al., 2022). Regarding to reducing the incidence of vertebrae fracture, teriparatide and denosumab ranked the first two, which was consistent with the result of lumbar spine BMD improvement.
There are several limitations in our study. First, patients included in the network meta-analysis had various backgrounds such as indications for glucocorticoid administration and the duration of therapy, which may lead to significant heterogeneity. Second, length of the follow-up varied among included studies, which may result in potential heterogeneity. Most of the included studies had a follow-up time of 12–24 months, while two studies (Adachi et al., 1997; Saag et al., 1998) had longer follow-up time of 48 and 52 months respectively. Third, the number of included studies and the sample size are relatively small.
Conclusion
For patients with GIO, teriparatide, denosumab and bisphosphonates are all effective in improving BMD. Based on this network meta-analysis, teriparatide and denosumab were superior in improving BMD in lumbar spine and femur neck, and reducing vertebrae fracture.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LD: Conceptualization, Writing–review and editing, Funding acquisition, Methodology, Supervision. LJ: Data curation, Formal Analysis, Methodology, Resources, Software, Writing–original draft. ZX: Data curation, Formal Analysis, Investigation, Resources, Writing–review and editing. XZ: Data curation, Formal Analysis, Investigation, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Natural Science Foundation of Shanxi Province (grant no 2022JM-546).
Acknowledgments
We are grateful to the authors of the included studies and all the patients included in the studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1336075/full#supplementary-material
References
Adachi, J. D., Bensen, W. G., Brown, J., Hanley, D., Hodsman, A., Josse, R., et al. (1997). Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N. Engl. J. Med. 337 (6), 382–387. doi:10.1056/NEJM199708073370603
Body, J. J. (2012). Denosumab for the management of bone disease in patients with solid tumors. Expert Rev. anticancer Ther. 12 (3), 307–322. doi:10.1586/era.11.204
Buckley, L., Guyatt, G., Fink, H. A., Cannon, M., Grossman, J., Hansen, K. E., et al. (2017). 2017 American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis care and Res. 69 (8), 1095–1110. doi:10.1002/acr.23279
Burshell, A. L., Möricke, R., Correa-Rotter, R., Chen, P., Warner, M. R., Dalsky, G. P., et al. (2010). Correlations between biochemical markers of bone turnover and bone density responses in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone 46 (4), 935–939. doi:10.1016/j.bone.2009.12.032
Choksi, P., Jepsen, K. J., and Clines, G. A. (2018). The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin. Diabetes Endocrinol. 4, 12. doi:10.1186/s40842-018-0062-7
Cohen, S., Levy, R. M., Keller, M., Boling, E., Emkey, R. D., Greenwald, M., et al. (1999). Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheumatism 42 (11), 2309–2318. doi:10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K
Devogelaer, J. P., Adler, R. A., Recknor, C., See, K., Warner, M. R., Wong, M., et al. (2010). Baseline glucocorticoid dose and bone mineral density response with teriparatide or alendronate therapy in patients with glucocorticoid-induced osteoporosis. J. Rheumatology 37 (1), 141–148. doi:10.3899/jrheum.090411
Eastell, R., Chen, P., Saag, K. G., Burshell, A. L., Wong, M., Warner, M. R., et al. (2010). Bone formation markers in patients with glucocorticoid-induced osteoporosis treated with teriparatide or alendronate. Bone 46 (4), 929–934. doi:10.1016/j.bone.2009.12.021
Farahmand, P., Marin, F., Hawkins, F., Möricke, R., Ringe, J. D., Glüer, C. C., et al. (2013). Early changes in biochemical markers of bone formation during teriparatide therapy correlate with improvements in vertebral strength in men with glucocorticoid-induced osteoporosis. Osteoporos Int. 24 (12), 2971–2981. doi:10.1007/s00198-013-2379-5
Glüer, C. C., Marin, F., Ringe, J. D., Hawkins, F., Möricke, R., Papaioannu, N., et al. (2013). Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J. bone mineral Res. 28 (6), 1355–1368. doi:10.1002/jbmr.1870
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern. Med. 162 (11), 777–784. doi:10.7326/M14-2385
Iseri, K., Iyoda, M., Watanabe, M., Matsumoto, K., Sanada, D., Inoue, T., et al. (2018). The effects of denosumab and alendronate on glucocorticoid-induced osteoporosis in patients with glomerular disease: a randomized, controlled trial. PloS one 13 (3), e0193846. doi:10.1371/journal.pone.0193846
Jiang, L., Dong, J., Wei, J., and Liu, L. (2022). Comparison of denosumab and oral bisphosphonates for the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. BMC Musculoskelet. Disord. 23 (1), 1027. doi:10.1186/s12891-022-05997-0
Kaneko, T., Okamura, K., Yonemoto, Y., Okura, C., Suto, T., Tachibana, M., et al. (2018). Short-term daily teriparatide in patients with rheumatoid arthritis. Mod. Rheumatol. 28 (3), 468–473. doi:10.1080/14397595.2017.1362093
Laan, R. F., van Riel, P. L., van de Putte, L. B., van Erning, L. J., van’t Hof, M. A., and Lemmens, J. A. (1993). Low-dose prednisone induces rapid reversible axial bone loss in patients with rheumatoid arthritis. A randomized, controlled study. Ann. Intern. Med. 119 (10), 963–968. doi:10.7326/0003-4819-119-10-199311150-00001
Lacey, D. L., Boyle, W. J., Simonet, W. S., Kostenuik, P. J., Dougall, W. C., Sullivan, J. K., et al. (2012). Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 11 (5), 401–419. doi:10.1038/nrd3705
Langdahl, B. L., Marin, F., Shane, E., Dobnig, H., Zanchetta, J. R., Maricic, M., et al. (2009). Teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: an analysis by gender and menopausal status. Osteoporos. Int. 20 (12), 2095–2104. doi:10.1007/s00198-009-0917-y
Lipton, A., and Goessl, C. (2011). Clinical development of anti-RANKL therapies for treatment and prevention of bone metastasis. Bone 48 (1), 96–99. doi:10.1016/j.bone.2010.10.161
Losada, B. R., Zanchetta, J. R., Zerbini, C., Molina, J. F., De la Peña, P., Liu, C. C., et al. (2009). Active comparator trial of teriparatide vs alendronate for treating glucocorticoid-induced osteoporosis: results from the Hispanic and non-Hispanic cohorts. J. Clin. Densitom. 12 (1), 63–70. doi:10.1016/j.jocd.2008.10.002
Migliorini, F., Colarossi, G., Eschweiler, J., Oliva, F., Driessen, A., and Maffulli, N. (2022). Antiresorptive treatments for corticosteroid-induced osteoporosis: a Bayesian network meta-analysis. Br. Med. Bull. 143 (1), 46–56. doi:10.1093/bmb/ldac017
Mok, C. C., Ho, L. Y., Leung, S. M. T., Cheung, H. N., Chen, S. P. L., and Ma, K. M. (2021). Denosumab versus alendronate in long-term glucocorticoid users: a 12-month randomized controlled trial. Bone 146, 115902. doi:10.1016/j.bone.2021.115902
Nasomyont, N., Tian, C., Hornung, L., Khoury, J., Hochwalt, P. M., Tilden, J. C., et al. (2021). The effect of oral bisphosphonate therapy on vertebral morphometry and fractures in patients with Duchenne muscular dystrophy and glucocorticoid-induced osteoporosis. Muscle and nerve 64 (6), 710–716. doi:10.1002/mus.27416
Pons-Estel, B. A., Bonfa, E., Soriano, E. R., Cardiel, M. H., Izcovich, A., Popoff, F., et al. (2018). First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American group for the study of lupus (GLADEL, grupo latino Americano de Estudio del lupus)-pan-American league of associations of rheumatology (PANLAR). Ann. Rheum. Dis. 77 (11), 1549–1557. doi:10.1136/annrheumdis-2018-213512
Reid, D. M., Devogelaer, J. P., Saag, K., Roux, C., Lau, C. S., Reginster, J. Y., et al. (2009). Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373 (9671), 1253–1263. doi:10.1016/S0140-6736(09)60250-6
Reid, D. M., Hughes, R. A., Laan, R. F., Sacco-Gibson, N. A., Wenderoth, D. H., Adami, S., et al. (2000). Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J. bone mineral Res. 15 (6), 1006–1013. doi:10.1359/jbmr.2000.15.6.1006
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Saag, K. G., Emkey, R., Schnitzer, T. J., Brown, J. P., Hawkins, F., Goemaere, S., et al. (1998). Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N. Engl. J. Med. 339 (5), 292–299. doi:10.1056/NEJM199807303390502
Saag, K. G., Pannacciulli, N., Geusens, P., Adachi, J. D., Messina, O. D., Morales-Torres, J., et al. (2019). Denosumab versus risedronate in glucocorticoid-induced osteoporosis: final results of a twenty-four-month randomized, double-blind, double-dummy trial. Arthritis Rheumatology 71 (7), 1174–1184. doi:10.1002/art.40874
Saag, K. G., Shane, E., Boonen, S., Marín, F., Donley, D. W., Taylor, K. A., et al. (2007). Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 357 (20), 2028–2039. doi:10.1056/NEJMoa071408
Salanti, G., Ades, A. E., and Ioannidis, J. P. (2011). Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J. Clin. Epidemiol. 64 (2), 163–171. doi:10.1016/j.jclinepi.2010.03.016
Sambrook, P. N., Roux, C., Devogelaer, J. P., Saag, K., Lau, C. S., Reginster, J. Y., et al. (2012). Bisphosphonates and glucocorticoid osteoporosis in men: results of a randomized controlled trial comparing zoledronic acid with risedronate. Bone 50 (1), 289–295. doi:10.1016/j.bone.2011.10.024
Shim, S. R., Kim, S. J., Lee, J., and Rücker, G. (2019). Network meta-analysis: application and practice using R software. Epidemiol. health 41, e2019013. doi:10.4178/epih.e2019013
Solomon, D. H., Kay, J., Duryea, J., Lu, B., Bolster, M. B., Yood, R. A., et al. (2017). Effects of teriparatide on joint erosions in rheumatoid arthritis: a randomized controlled trial. Arthritis and rheumatology 69 (9), 1741–1750. doi:10.1002/art.40156
van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res. Synth. Methods 7 (1), 80–93. doi:10.1002/jrsm.1167
Waljee, A. K., Rogers, M. A., Lin, P., Singal, A. G., Stein, J. D., Marks, R. M., et al. (2017). Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 357, j1415. doi:10.1136/bmj.j1415
Wallach, S., Cohen, S., Reid, D. M., Hughes, R. A., Hosking, D. J., Laan, R. F., et al. (2000). Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif. tissue Int. 67 (4), 277–285. doi:10.1007/s002230001146
Wang, Y., Zhao, R., Gu, Z., Dong, C., Guo, G., and Li, L. (2020). Effects of glucocorticoids on osteoporosis in rheumatoid arthritis: a systematic review and meta-analysis. Osteoporos. Int. 31 (8), 1401–1409. doi:10.1007/s00198-020-05360-w
Yamaguchi, Y., Morita, T., and Kumanogoh, A. (2020). The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: a meta-analysis. Rheumatology Adv. Pract. 4 (1), rkaa008. doi:10.1093/rap/rkaa008
Yanbeiy, Z. A., and Hansen, K. E. (2019). Denosumab in the treatment of glucocorticoid-induced osteoporosis: a systematic review and meta-analysis. Drug Des. devel. Ther. 13, 2843–2852. doi:10.2147/DDDT.S148654
Keywords: osteoporosis, glucocorticoids, bone mineral density, fracture, network meta-analysis
Citation: Dong L, Jiang L, Xu Z and Zhang X (2024) Denosumab, teriparatide and bisphosphonates for glucocorticoid-induced osteoporosis: a Bayesian network meta-analysis. Front. Pharmacol. 15:1336075. doi: 10.3389/fphar.2024.1336075
Received: 10 November 2023; Accepted: 10 January 2024;
Published: 19 January 2024.
Edited by:
Stefano Bruscoli, University of Perugia, ItalyReviewed by:
Vladimira Vasileva Boyadzhieva, University Hospital St. Ivan Rilski, BulgariaAlessandra Bitto, University of Messina, Italy
Copyright © 2024 Dong, Jiang, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Dong, ZG9uZ2xpYW5nLTUyNkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Liang Dong
Liang Dong Lianghai Jiang2†
Lianghai Jiang2†