- 1College of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Experimental Center, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Shandong Provincial Co-innovation Center of Classic TCM Formula, Jinan, China
Background: The Jiawei Kongsheng Zhenzhong pill (JKZP), a Chinese herbal prescription comprised of eight Chinese crude drugs, has been historically employed to treat neurological and psychological disorders. Nevertheless, the ambiguous material basis severely hindered its progress and application.
Purpose: The current study aimed to establish a rapid analytical method for identifying the chemical components of the JKZP aqueous extract and the components absorbed into the rat serum to investigate the quality markers (Q-markers) responsible for the neuroprotective effects of JKZP.
Methods: The qualitative detection of the chemical components, prototype components, and metabolites of the aqueous extracts of JKZP, as well as the serum samples of rats that were administered the drug, was performed using the ultra-performance liquid chromatography- quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS/MS) technology. This analysis combined information from literature reports and database comparisons. Moreover, the study was conducted to anticipate the potential Q-markers for the neuroprotective effects of JKZP based on the “five principles” of Q-marker determination.
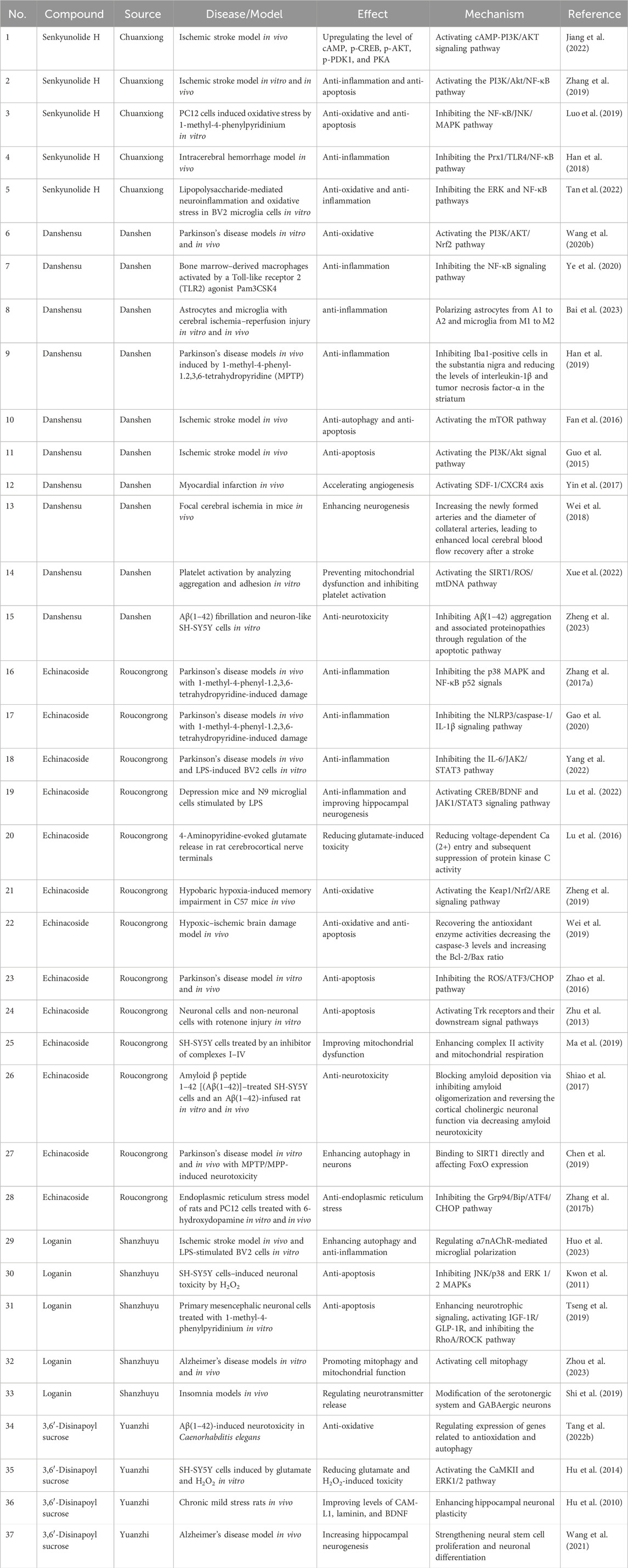
Results: A total of 67 compounds and 111 serum components (comprising 33 prototypes and 78 metabolites) were detected and identified. Combining the principles of quality transmission and traceability, compound compatibility environment, component specificity, effectiveness, and measurability, the study predicted that five key compounds, namely, senkyunolide H, danshensu, echinacoside, loganin, and 3,6′-disinapoyl sucrose, may serve as potential pharmacological bases for the neuroprotective effects of JKZP.
Conclusion: To summarize, the UPLC-Q-TOF-MS/MS technique can be employed to rapidly and accurately identify compounds in JKZP. Five active compounds have been predicted to be the Q-markers for the neuroprotective effects of JKZP. This discovery serves as a reference for improving quality, advancing further research and development, and utilizing Chinese herbal prescriptions.
1 Introduction
The Jiawei Kongsheng Zhenzhong pill (JKZP) was derived from the Kongsheng Zhenzhong pill as documented in the ancient medical classic “Thousand Golden Prescriptions.” The original prescription, consisting of Chinemys reevesii (Gray) (tortoise plastron; also named Guijia in Chinese), Os Draconis (Fossilia Ossia Mastodi) (dragon bones; also named Longgu in Chinese), Polygalae tenuifolia Willd (Radix Polygalae; also named Yuanzhi in Chinese), and Acorus tatarinowii Schott (Acorus Tatarinowii; also named Shichangpu in Chinese), manifested effects in tonifying the kidneys, tranquilizing the heart, enhancing wisdom, and soothing the mind. It was also esteemed as a conventional prescription for heightening wisdom and enlightenment. Consequently, its primary objective was to address conditions such as insomnia, amnesia, Alzheimer's disease, vascular cognitive impairment, depression, pediatric hyperactivity disorder, and similar ailments clinically (Rui and Yue, 2012; Hou et al., 2021; Chen et al., 2022). In accordance with traditional Chinese medicine (TCM) theory and clinical experience, Salvia miltiorrhiza Bunge (Salviae Miltiorrhizae; also named Danshen in Chinese), Ligusticum sinense ‘Chuanxiong’ (Chuanxiong Rhizoma; also named Chuanxiong in Chinese), Cornus officinalis Sieb. et Zucc. (Corni Fructus; also named Shanzhuyu in Chinese), and Cistanche deserticola Ma (Cistanches Herba; also named Roucongrou in Chinese) were incorporated into the original prescription to reinforce the effects of kidney and marrow tonification, blood circulation activation, blood stasis removal, and depression relief. Its principal application targeted symptoms associated with renal essence deficiency and blood stasis. What is more, it has demonstrated notable therapeutic effectiveness in addressing vascular cognitive impairment and post-stroke depression, exhibiting a commendable neuroprotective effect (Yu et al., 2013; Wu et al., 2015; Pang et al., 2016; Song, 2022; Yu et al., 2016; Zhou, 2022). However, the components of JKZP are relatively multifarious, and the precise pharmacological basis for its neuroprotective effect requires further elucidation. The compositions and proportions of JKZP are shown in Table 1.
Over the past few decades, an increasing number of studies have reported the components in the aqueous extract and the serum of the single plant drug in JKZP (Li et al., 2008; Yun et al., 2018; Hou, 2019; Qu et al., 2020; Yang et al., 2020; Zhao et al., 2020), which are aligned with prior research, and certain components that exhibit noteworthy neuroprotective activities (Feng et al., 2017; Wang et al., 2020a; Tang X. et al., 2022; Lu et al., 2022). Nevertheless, research reports regarding the analysis and identification of compounds in the whole prescription of JKZP require further elaboration. Quality markers (Q-markers) are compounds intricately linked to the pharmacological properties of herbs, particularly their effectiveness and measurability. They served for quality control in single herbs and Chinese herbal prescriptions, revealing potential pharmacological substance foundations, thus facilitating further research, development, and utilization (Jiang et al., 2023). The widely utilized technology for the qualitative detection of compounds in TCM is ultra-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry (UPLC-Q-TOF-MS/MS). This technology is distinguished by its robust separation capability, high sensitivity, and convenience. The components in the sample are ionized, resulting in ions with a certain charge and different mass numbers. Different ions have different motion behaviors in the electromagnetic field. The mass analyzer is used to separate ions according to the different mass-to-charge ratios (m/z), obtaining the mass spectra in the order of the mass-to-charge ratios and then comparing these with the database, which can be used for the identification of the properties of the compounds (Chen et al., 2023a).
Hence, this study conducted preliminary qualitative analyses and identifications of the aqueous extract of JKZP and JKZP-containing serum of rats using the UPLC-Q-TOF-MS/MS technique. Concurrently, leveraging the neuroprotective effect of JKZP, potential Q-markers were forecasted following the ‘five principles’ of Q-marker determination. This study sought to contribute insights and foundations for conducting fundamental research on the pharmacological substances of Chinese herbal prescriptions and improving the criteria for quality control.
2 Materials and methods
2.1 Apparatus
Waters H-Class UPLC (Waters, United States), AB Sciex Triple TOF® 4600 high-resolution mass spectrum (SCIEX, United States), KQ-300 BD ultrasonic cleaning instrument (Kunshan Ultrasonic Instrument, China), Sigma 3K15 high-speed centrifuge (Sigma, United States), LNG-T98 centrifugal concentration dryer (Taicang Huamei, China), and R583S small animal anesthesia machine (RWD, China) were used for this study.
2.2 Reagents and materials
The reagents and materials used for the study involved Chinese medicinal decoction pieces of JKZP (tortoise plastron, lot: 19081001; dragon bones, lot: 20200201; Radix Polygalae, lot: 20113001; Acorus Tatarinowii, lot: 20103001; Salviae Miltiorrhizae, lot: 20121401; Chuanxiong Rhizoma, lot: 20092103; Corni Fructus, lot: 20040801; Cistanches Herba, lot: 21021904), which were purchased from Shandong Bokang TCM Decoction Pieces Co. Ltd and identified as genuine, acetonitrile (MS pure, I1133829105, Merck company), methanol (MS pure, I1139035113, Merck company), formic acid (MS pure, Y6170039, CNW company), purified water (20221110C, Guangzhou Watsons Food and Beverage Co., Ltd.), and isoflurane (R510-22-10, Shenzhen Ruiwode Life Technology Co., Ltd.).
2.3 Preparation of the JKZP aqueous extract solution
To ensure the consistency of JKZP, the quality of each herb was evaluated before use, and the extraction of the decoction followed the standardized procedures specified in the “Pharmacopoeia of the People's Republic of China.” Chuanxiong Rhizoma (120 g) and Acorus Tatarinowii (90 g) were broken and soaked in a distillation flask for 30 min, and then volatile oil was extracted by distillation for 6 h. The volatile oil was taken out, and the filtrate and dregs were stored temporarily. After soaking for 60 min, the tortoise plastron (180 g) and dragon bones (180 g) were broken into pieces and decocted for 30 min and then added to the other medicines (Radix Polygalae, 90 g; Salviae Miltiorrhizae, 150 g; Corni Fructus, 150 g; and Cistanches Herba, 120 g) and dregs of Chuanxiong Rhizoma and Acorus Tatarinowii, and the entire mixture was then sequentially decocted for 45 min. The second decoction was made, and the filtrate was combined and filtered. The filtrate was concentrated by spinning in a water bath at 65°C, the volatile oil was combined, and the concentration of the drug aqueous extract solution was adjusted to 3.6 g mL−1 and stored at 4°C.
2.4 Animals, drug administration, and serum samples' pretreatment
Healthy male SD rats of SPF grade with a body weight of 250 ± 10 g were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., under animal production license no. SCXK (Beijing) 2021-0011. They were raised in an environment with a temperature of 20–26°C, relative humidity of 55 ± 10%, and light/darkness cycle of 12 h each, with ad libitum access to food and water. The animal handling during this experiment adhered to China's “Regulations on the Management of Laboratory Animals” and the relevant regulations of the Ethics Committee of the Laboratory Animal Center of Shandong University of Traditional Chinese Medicine. The study received ethical approval, with the number SDUTCM20221021004.
A total of 20 rats were randomly allocated into a JKZP-containing serum group and a blank serum group, with 10 rats in each group. In the JKZP-containing serum group, rats received 56.7 g kg−1 d−1 of JKZP through gavage (calculated based on an adult body weight of 60 kg, and five times the equivalent clinical dose) for five consecutive days. The blank serum group received an equal volume of saline.
Within 1 h post final intragastric administration, continuous anesthesia was induced by inhaling 3% isoflurane. Blood was drawn from the abdominal aorta and left at room temperature for 2 h. Subsequently, serum separation was performed by centrifugation at 3000 rpm min−1 for 15 min. The complement was inactivated using a constant temperature water bath set at 56°C for 30 min. The serum from the same group was pooled to minimize individual variations, sub-packaged, and stored at −80°C for subsequent use.
2.5 Preparation of the sample solution
For the JKZP aqueous extract sample, 1 mL of the JKZP aqueous extract was placed in a centrifuge tube, and 2 mL of 20% methanol was added, then the supernatant was collected after centrifugation at 12,000 rpm min−1 for 15 min. For the serum sample, 2 mL of the rat blank serum and 2 mL of JKZP-containing serum were taken separately. Thrice the volume of methanol (mass spectrometry grade) was added to the precipitate proteins. The mixture was thoroughly mixed and stored at 4°C for 20 min, followed by centrifugation to obtain the supernatant. The supernatant was concentrated, dried by centrifugation, and stored at −80°C. The residue was dissolved in 200 μL of 50% methanol before analysis, thoroughly mixed, and then centrifuged to obtain the supernatant.
2.6 Chromatographic and mass spectrometry conditions
The chromatography analysis was performed using Waters® CORTECS® UPLC® T3 (1.6 µm, 2.1 mm × 100 mm) at 30°C. The mobile phase consisted of acetonitrile (A) and 0.1% formic acid in water (B). The gradient elution with the following program was carried out as follows: 0–3 min, 0% A; 3–7 min, 0%–5% A; 7–30 min, 5%–13% A; 30–55 min, 13%–25% A; 55–67 min, 25%–40% A; 67–72 min, 40%–95% A; 72–75 min, 95% A; 75–75.1 min, 95%–0% A; 75.1–78 min, 0% A. The flow rate was set at 0.3 mL min−1, the detection wavelength was 190–400 nm, and the injection volume was 2–5 μL. The MS detection was applied in an ESI-negative/positive ion mode.
2.7 Data analysis
Data analysis was conducted using the PeakView 1.2 software. MS data were preferentially matched with the Natural Products HR-MS/MS Spectral Library 1.0 database for identification by comparison with the controls. Compounds were initially screened based on the data of each peak and subsequently confirmed using the primary and secondary information of each peak. Subsequently, considering the preliminary analysis of the identified components using the UPLC-Q-TOF-MS/MS technique, potential Q-markers for the neuroprotective effect of JKZP were predicted following the five principles of Q-marker determination.
3 Results
3.1 Acquisition and identification of the UPLC-Q-TOF-MS/MS chromatogram of the JKZP aqueous extract
Under the aforementioned chromatographic and MS conditions, total ion flow diagrams of the JKZP aqueous extract were, respectively, collected in positive and negative ion modes. As illustrated in Figure 1A, B, the initial observation of the images revealed that the total ion flow map was clear, enabling data analysis. The results exhibited a high degree of reliability. More narrowly, the peak of gallic acid merged in 3.44 min, labeled as No. 4 in Figure 1A, while the peak of danshensu appeared in 7.05 min, described as No. 9 also in Figure 1A.
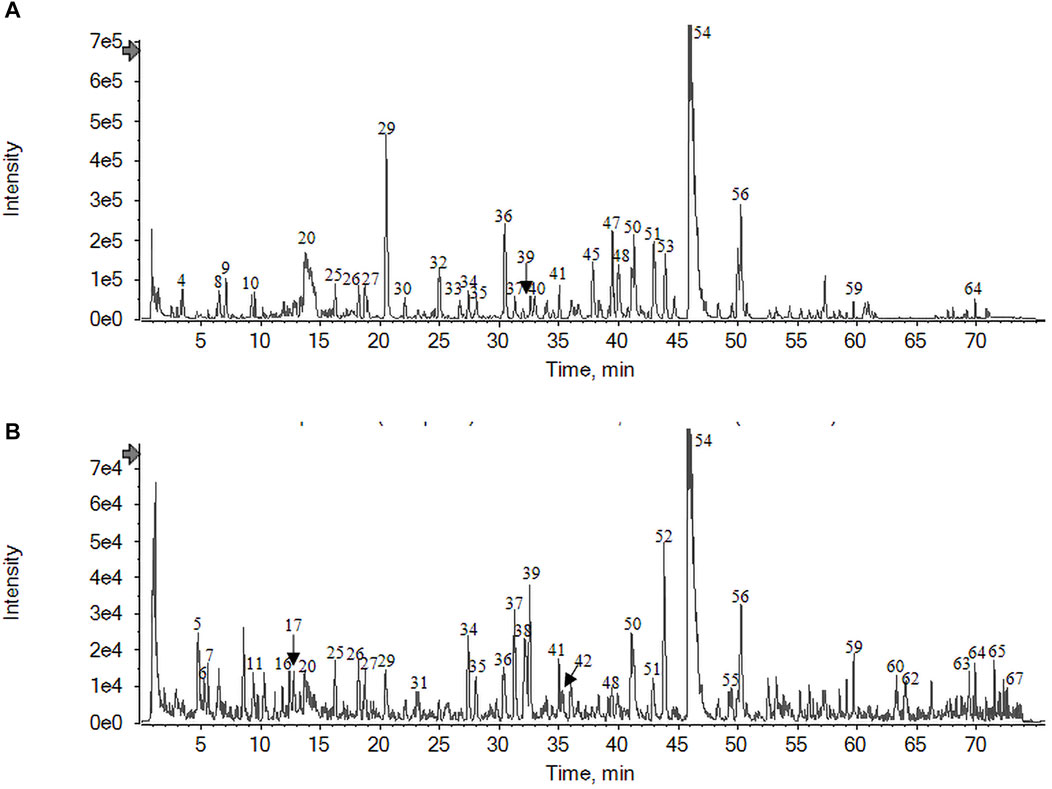
FIGURE 1. Total ion flow diagram of the JKZP aqueous extract by UPLC-HRMS: (A) negative ion modes; (B) positive ion modes.
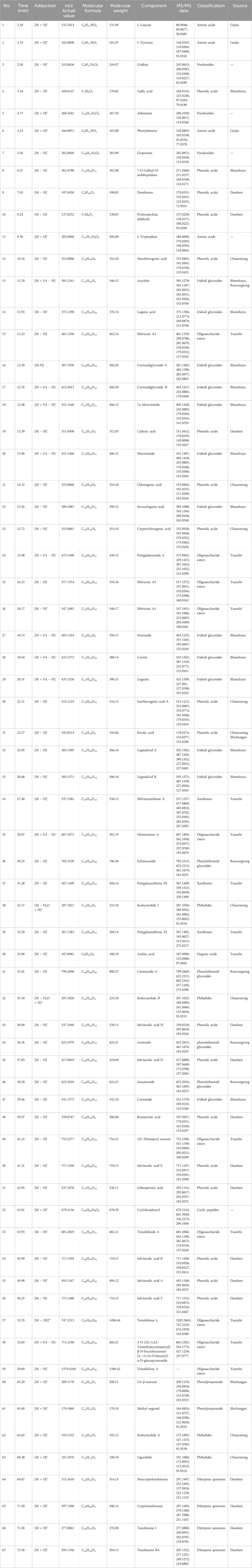
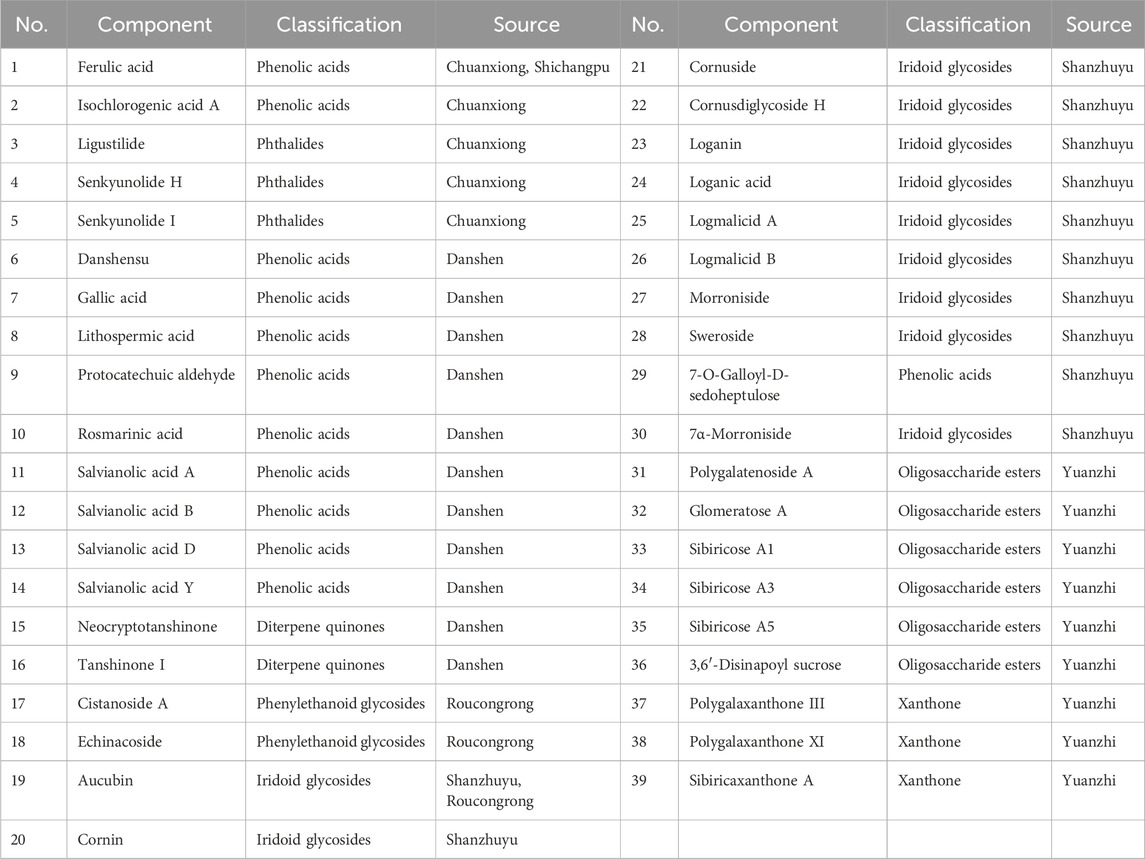
Utilizing the multistage MS information of the samples, coupled with the natural product high-resolution MS database and relevant literature, the ion peaks in the total ion flow diagram of the JKZP samples were identified. As depicted in Table 2, a total of 12 classes and 67 compounds were identified in the whole ion flow diagram of JKZP samples. These included 18 phenolic acids, 13 iridoid glycosides, 10 oligosaccharide esters, four each of amino acids, phenylethanoid glycosides, phthalides, and diterpene quinones, three each of xanthones and nucleosides, two phenylpropanoids, and one each of organic acid and cyclic peptide.
3.2 Acquisition and identification of UPLC-Q-TOF-MS/MS chromatograms of the serum samples
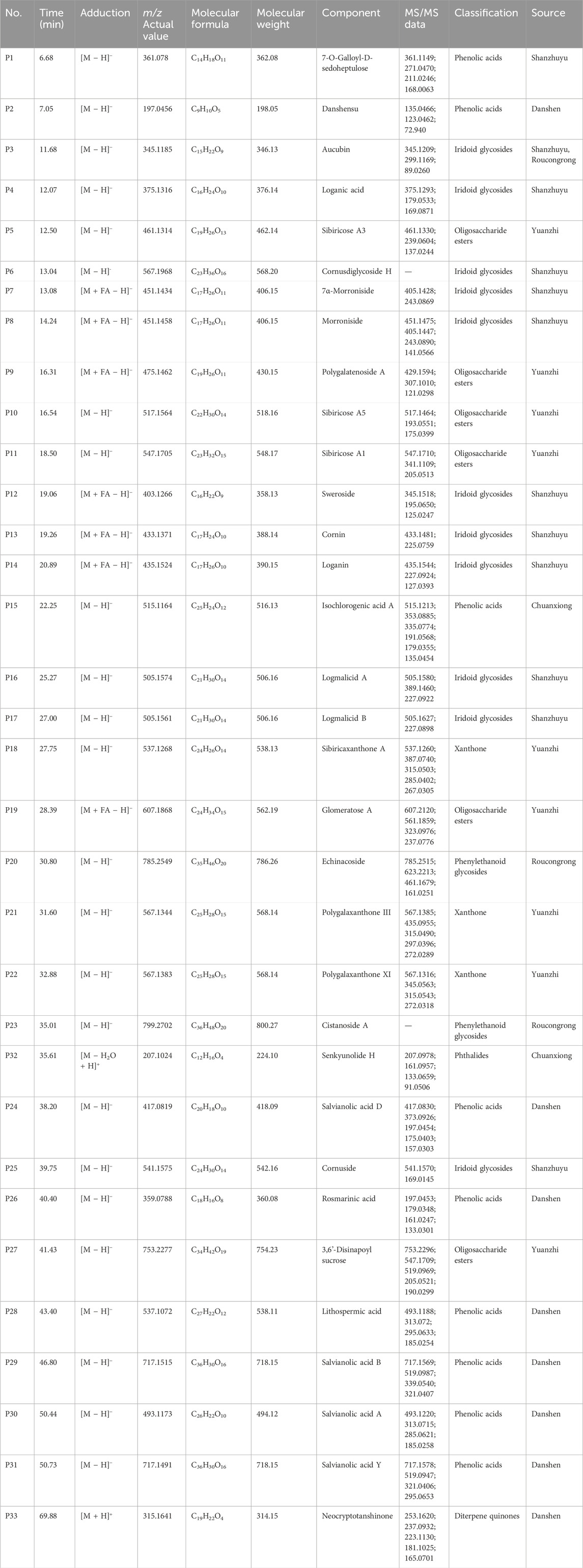
Total ion flow diagrams of the JKZP-containing serum and blank serum samples were individually collected in positive and negative ion modes (as displayed in Supplementary Figure S1A, B). According to the multistage MS information of the samples, retention time, cleavage pattern, characterization results of the components of the original prescription, and relevant literature, a comparative analysis between JKZP-containing serum and rat blank serum samples was conducted. A total of 111 components were identified from the JKZP-containing serum, which covered 33 prototypical components and 78 metabolites. Specifically speaking, as indicated in Table 3, these mainly included 11 iridoid glycosides (all from Corni Fructus), nine phenolic acids (seven from Salviae Miltiorrhizae, one from Corni Fructus, and one from Chuanxiong Rhizoma), six oligosaccharide esters (all from Radix Polygalae), three xanthones (all from Radix Polygalae), two phenylethanoid glycosides (all from Cistanches Herba), one phthalide (from Chuanxiong Rhizoma), and one diterpene quinone (from Salviae Miltiorrhizae). Additionally, as listed in Supplementary Table S1, 78 metabolites were authenticated in the JKZP-containing serum. These metabolites were all derived from 19 compounds and, then through various metabolic pathways, such as sulfation, methylation, deglycosylation, hydroxylation, dehydroxylation, and glucuronidation, were derivatized into the 78 metabolites. The prototypical components of the JKZP-containing serum are shown in Table 3.
In the present study, all absorption into the serum corresponded to the identified components of the JKZP aqueous extract. Out of all the components, four metabolites, which were found to remain after the intersection of the metabolites with the components in the JKZP-containing serum and JKZP aqueous extract samples, could also be traced back to the components in the aqueous extract of JKZP. Specifically, pyrogallic acid was derived from gallic acid, hydroxytyrosol was derived from echinacoside, and dihydroferulic acid and caffeic acid were both derived from ferulic acid (as exhibited in Figure 2). In this study, three amino acids (L-leucine, L-tyrosine, and phenylalanine) from the tortoise plastron were identified in both the JKZP aqueous extract and serum, nevertheless, considering that the blood of a normal creature contains a variety of amino acids, such as the three aforementioned amino acids (Fernstrom et al., 1987; Wang et al., 2020b). Then, the rat blank serum was considered as the negative control to eliminate the impact of endogenously occurring substances in rats on the analysis and identification of the JKZP-containing serum. This allowed for the accurate analysis and identification of absorption into the serum of JKZP to the greatest extent possible. The intersection of the components of each sample is revealed in Figure 2.
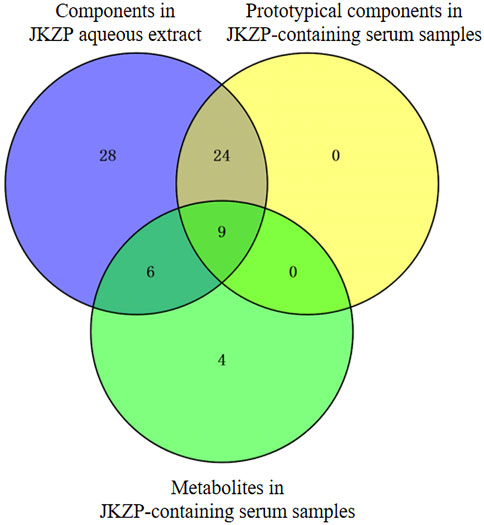
FIGURE 2. Components in the JKZP aqueous extract, prototypical components, and metabolites in JKZP-containing serum were intersected.
4 The current status of clinical and experimental studies on JKZP
The investigations proposed that JKZP exhibited neuroprotective properties. In the study by Xu (2006), 51 patients were diagnosed with vascular dementia (VD), with 30 patients were assigned to the JKZP treatment group and 21 to the Western medicine control group. The findings revealed that the JKZP therapy group achieved a total effective rate of 76.67%, resulting in considerable enhancements in the patient's MMSE score, primary clinical symptoms, and whole blood viscosity and erythrocyte aggregation patterns. Simultaneously, JKZP also improved the cognitive impairment and self-care skills of dementia patients, demonstrating definite clinical effectiveness. JKZP could also effectively perfect the sleep quality and Chinese medical symptoms of insomnia patients with heart and kidney deficiency type, which had high effectiveness and safety (Chen et al., 2023b). Regarding the molecular mechanism, it has been verified that JKZP possessed advantageous therapeutic effects in rats suffering from focal cerebral ischemia, vascular cognitive impairment, and post-stroke depression. This encompassed safeguarding neurons against programmed cell death, stimulating the growth of cerebral angiogenesis and augmenting the reorganization of synaptic remodeling, resulting in notable enhancements in neurological impairments, cognitive impairment, and depressive behavior (Yu et al., 2013; Wu et al., 2015; Pang et al., 2016; Yu et al., 2016; Song, 2022; Zhou, 2022). However, additional investigation is necessary to elucidate the detailed pharmacological material basis for its neuroprotective impact.
5 Q-marker prediction analysis of neuroprotective effects exerted by JKZP
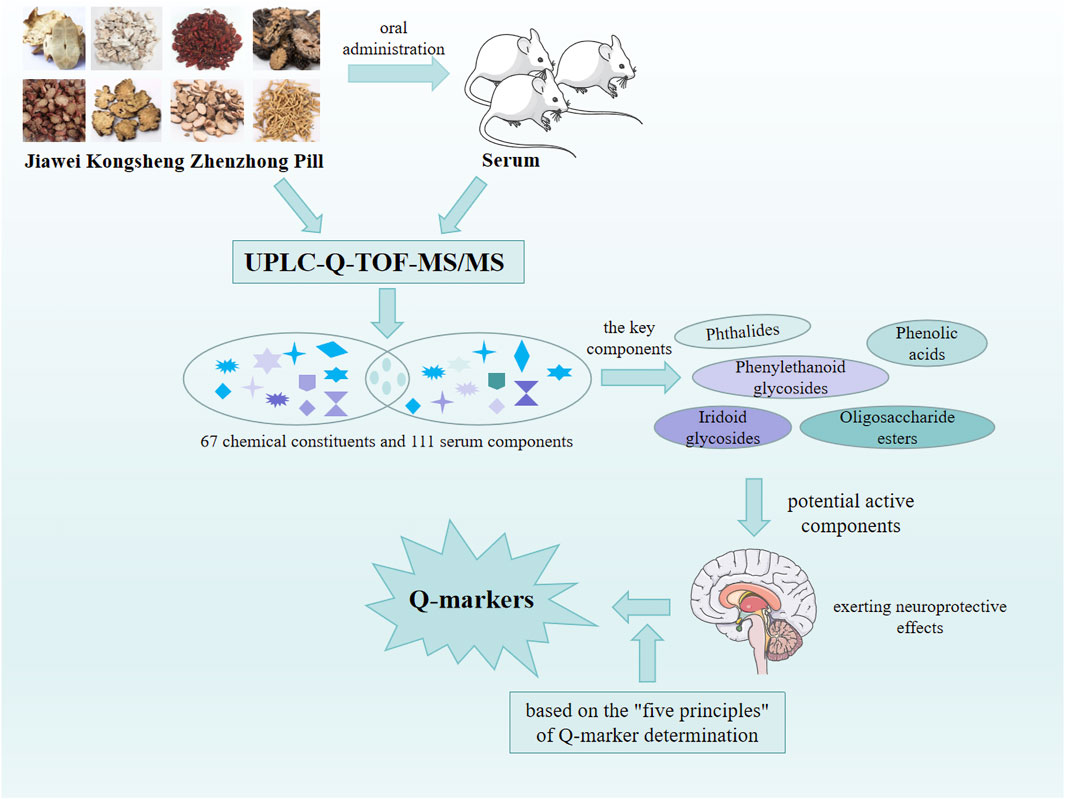
Changxiao Liu, the Chinese Academy of Engineering, proposed the innovative concept of the Chinese medicine Q-marker. This concept integrated the biological attributes, manufacturing process, and prescription theory of TCM. “Quality transmission and traceability,” “compound compatibility environment,” “component specificity,” “component effectiveness,” and “component measurability” comprised the five most crucial aspects of Q-markers. These aspects effectively facilitated quality control and contributed to enhance the quality of TCM prescriptions (Liu et al., 2016). Using the UPLC-Q-TOF-MS/MS technology and “five principles” of Q-marker determination, the potential Q-markers of JKZP with neuroprotective effects were predicted. This prediction aimed to offer guidance for enhancing the overall quality control and fostering more in-depth applied research and transformative achievements for JKZP. The research strategy is demonstrated in Figure 3.
5.1 Q-marker prediction based on quality transmission and traceability
A comprehensive total of 67 compounds, spanning 12 categories, which includes phenolic acids, iridoid glycosides, and oligosaccharide esters, were identified from the aqueous extract of the JKZP using the UPLC-Q-TOF-MS/MS technology. With the exclusion of nucleosides and cyclic peptides, specific attributions were identified for each compound. Among them, Salviae Miltiorrhizae contributed 15 components (comprising phenolic acids and diterpene quinones); Chuanxiong Rhizoma provided nine components (primarily phenolic acids and phthalides); Acorus Tatarinowii furnished three components (phenylpropanoids and phenolic acids); Radix Polygalae yielded 14 components (mainly oligosaccharide esters and phenylpropanoids); Corni Fructus contributed 15 components (phenolic acids and iridoid glycosides); Cistanches Herba added five components (iridoid glycosides and phenylethanoid glycosides); and tortoise plastron supplied three amino acids. The study failed to identify the pertinent components of the dragon bones. According to the report, the main constituents of the dragon bones were inorganic substances, such as calcium carbonate and calcium phosphate, as well as metal elements like iron, potassium, and sodium. Consequently, the relevant components were absent from this study (Chen et al., 2023c).
In the JKZP-containing serum, a comprehensive analysis found a total of 111 blood-entry components, which comprised 33 prototypical components and 78 metabolites (as described in Table 3; Supplementary Table S1). The analysis revealed that the absorption into the serum was derived from a total of 39 original components. Specifically, Chuanxiong Rhizoma exhibited a dominance of phenolic acids and phthalides, Salviae Miltiorrhizae showcased phenolic acids and diterpene quinones, Cistanches Herba featured phenylethanoid glycosides, Corni Fructus presented iridoid glycosides, and Radix Polygalae manifested oligosaccharide esters and xanthones. Considering the aforementioned findings, it was hypothesized that phenolic acids, phthalides, phenylethanoid glycosides, iridoid glycosides, and oligosaccharide esters could potentially serve as the pivotal active components contributing to the pharmacological effects of the JKZP. All components absorbed into the serum are shown in Table 4 (after the exclusion of duplicates and endogenous components).
5.2 Q-marker prediction based on component specificity
Chuanxiong Rhizoma is the dried rhizome of the L. sinense Chuanxiong plant, which belongs to the Umbelliferae family. A study conducted by Liu (2016) revealed that Chuanxiong Rhizoma had six primary pharmacologic compounds: ferulic acid, senkyunolide A/I/H, ligustilide, and levistilide A. Phthalides and phenolic acids were commonly considered to be the active components in Chuanxiong Rhizoma. Therefore, senkyunolide H and ferulic acid can be identified as the specific components of Chuanxiong Rhizoma (Liu J. et al., 2022).
Salvia miltiorrhiza is the dried root and rhizome of S. miltiorrhiza Bunge, which belongs to the Labiatae family. Lai et al.'s (2022) study showed that danshensu, salvianolic acid A/B, lithospermic acid, and rosmarinic acid were the main phenolic acid active components of S. miltiorrhiza. As for danshensu presenting with species-origin specificity, it was regarded as a unique component of S. miltiorrhiza (Li et al., 2018).
Cistanches Herba refers to the dehydrated succulent stem, with leaf scales, of C. deserticola Ma, which belongs to the Orobanchaceae family. Yang et al. (2023) identified cistanoside analogs, such as echinacoside, geniposide, cistanoside A, acteoside, and isoacteoside, as the primary active components of Cistanches Herba. Additionally, echinacoside was considered one of the distinctive constituents found exclusively in Cistanches Herba.
Corni Fructus refers to the desiccated ripe sarcocarp of C. officinalis Sieb. et Zucc., which belongs to the Cornaceae family. In a study conducted by Liu (2022), it was found that the primary active components of Corni Fructus were iridoid glycosides and phenolic acid chemicals, and these included gallic acid, 5-HMF, protocatechuic acid, morroniside, sweroside, loganin, and cornuside I; particularly, loganin and morroniside were the peculiar constituents in Corni Fructus (Li et al., 2017).
Radix Polygalae refers to the dehydrated root of the Polygala tenuifolia Willd. or Polygala sibirica L. plant, which belongs to the Polygalaceae family. The medical properties of this substance may be attributed to its various components, such as xanthones, saponins, oligosaccharide esters, and lipids. One specific component, known as 3,6′-disinapoyl sucrose, was particularly distinctive of Radix Polygalae (Wu et al., 2018).
5.3 Q-marker prediction based on the compound compatibility environment
In this prescription, JKZP comprised tortoise plastron, dragon bones, Radix Polygalae, Acorus Tatarinowii, Salviae Miltiorrhizae, Chuanxiong Rhizoma, Corni Fructus, and Cistanches Herba in the proportions of 6:6:3:3:5:4:5:4. According to the theory of TCM, tortoise plastron excelled at tonifying essence and blood, nourishing the yin (阴), submerging the yang (阳), tonifying the heart, tranquilizing the mind, and removing stagnant blood (as per “the Classic of Materia Medica”), thereby establishing its role as the sovereignty herb. Concurrently, Acorus Tatarinowii served to eliminate phlegm for resuscitation and to facilitate the movement of qi (气), promoting the alleviation of depression. Dragon bones contributed to tranquillization with a potent prescription, while Chuanxiong Rhizoma was utilized for activating blood circulation, eliminating blood stasis, and facilitating the movement of qi (气), thereby promoting relief from depression. The trio of herbs functioned as ministerial herbs. Then, Cistanches Herba benefited essence and blood and tonified kidney yang; Salviae Miltiorrhizae activated blood circulation and eliminated blood stasis; Radix Polygalae stabilized the mind and strengthened the intellect; Corni Fructus consolidated yin(阴) and replenished essence. They also served as assistant herbs. Subsequent to this, Chuanxiong Rhizoma, proficient in clearing the way and ascending toward the head and eyes, was employed to guide the medicines to the site of illness, functioning as a courier herb. Overall, the combination of these herbs aimed to tonify the kidneys, benefit the essence, dissipate phlegm, eliminate blood stasis, tranquilize the mind, and alleviate depression.
Chinese medicines are predominantly utilized in clinical practice through the form of prescriptions. Contemporary pharmacological research has demonstrated that varied combinations or dosages of herbs would result in variations in the effectiveness and underlying pharmacodynamic properties. Hence, it is imperative to anticipate the distinctive quality indicators pertaining to the neuroprotective properties of JKZP based on the TCM prescriptions principles. The research conducted by Liu M. et al. (2022) has demonstrated a significant increase in the levels of danshensu sodium, protocatechuic aldehyde, rosmarinic acid, salvianolic acid A/B, cryptotanshinone, tanshinone Ⅰ, and tanshinone ⅡA in the extract of Salviae Miltiorrhizae and Chuanxiong Rhizoma when the two were combined. The combination of Chuanxiong Rhizoma and Salviae Miltiorrhizae with Pueraria lobata (Willd.) Ohwi for treating cardiac and cerebral diseases resulted in the presence of soy sapogenins, genistein, 3′-methoxy soy sapogenins, formononetin, and cryptotanshinone in the bloodstream. These compounds were closely associated with the targets AKR1B1, CA2, CA1, and ALDH2 (Tang Y. et al., 2022). It was found that the combination of Corni Fructus and Rehmannia glutinosa (Gaertn.) resulted in a notable increase in the dissolution of loganin, a bioactive compound found in Corni Fructus (Zhou and Cong, 2018). Lv et al. (2016) conducted a comprehensive analysis of the chemical components of Radix Polygalae before and after pairing it with Acorus Tatarinowii. The study found that the levels of the eight chemical constituents, such as tenuifoliside, sibiricose A5, and 3,6′-disinapoyl sucrose, remained unchanged. However, the concentration of the volatile constituent cis-β-asarone significantly decreased (Zhang et al., 2015). The role and therapeutic effect of Chinese herbal prescriptions could be attributed to the synergy between the individual drugs and active compounds. It was observed that when the same single drugs were combined in a compound environment, they exhibited different pharmacological material bases and mechanisms of action, resulting in varied therapeutic effects.
5.4 Q-marker prediction based on the association between components and effectiveness
The properties of the components determined the pharmacological effects, constituting the core element of the Q-marker, and were pivotal for the quality control in prescriptions. Synthesizing the above theories and analytical results, JKZP exhibited neuroprotective effects potentially related to the key absorption into the serum from Chuanxiong Rhizoma, Salviae Miltiorrhizae, Cistanches Herba, Corni Fructus, and Radix Polygalae.
5.4.1 Chuanxiong Rhizoma
The compounds of Chuanxiong Rhizoma, as recorded in the “Chinese Materia Medica,” encompassed senkyunolide, ferulic acid, and caffeic acid. Upon oral ingestion, the medicines entered the body and exerted their therapeutic effects either directly as basic components or after undergoing a series of metabolic processes. In the study by Liu Z. et al. (2022), rats were orally administered senkyunolide H at a dosage of 10 mg kg−1. After 24 h, the plasma, urine, bile, and feces samples were collected for analysis. A total of 32 metabolites were detected, with the primary metabolic reactions involving oxidation, hydrogenation, methylation, acetylation, dehydroxylation, glucuronidation, esterification, and cysteine binding.
Studies have indicated that senkyunolide H exhibited neuroprotective effects in animals with ischemia–reperfusion injury or by safeguarding neuronal cells from injury induced by oxygen–glucose deprivation and reperfusion (OGD/R) via the cAMP-PI3K/AKT signaling pathway or the PI3K/AKT/NF-κB signaling pathway. Additionally, senkyunolide H decreased the release of inflammatory factors in the brain tissues of mice with middle cerebral artery occlusion and enhanced the ability of neurons to resist apoptosis, leading to a decrease in neurological impairment, volume of brain tissue damage due to the lack of blood supply, and mortality of neurons, thereby demonstrating notable neuroprotective effects (Zhang et al., 2019). It bolstered the ability of neurons to withstand oxidative stress by reducing the generation of reactive oxygen species, mitigating the loss of mitochondrial membrane potential, restricting the release of cytochrome C, and decreasing the levels of malondialdehyde. Simultaneously, it augmented antioxidant enzyme activities, such as superoxide dismutase, catalase, and glutathione peroxidase (Luo et al., 2019). It also attenuated neuroinflammation by blocking the Prx1/TLR4/NF-kB, ERK and NF-κB signaling pathways (Han et al., 2018; Tan et al., 2022). These combined mechanisms contribute to its neuroprotective effects. Thus, it was further anticipated that senkyunolide H might function as a quality indicator for JKZP.
5.4.2 Salviae Miltiorrhizae
The primary active substances of Salviae Miltiorrhizae are phenolic acids and diterpene quinones (Xu et al., 2018). Danshensu and tanshinone ⅡA serve as the quality control indicators for the antioxidant and anti-apoptotic properties of Salviae Miltiorrhizae aqueous extracts, respectively (Zhou et al., 2012). The study conducted by Lai et al. (2022) analyzed the levels of six phenolic acids in a digested extract of S. miltiorrhiza using an artificial gastric fluid. The investigated components were danshensu, lithospermic acid, and salvianolic acid A/B, whose bioaccessibility followed the following order, from the highest to lowest: the percentages of danshensu (50.19%), salvianolic acid B (33.44%), lithospermic acid (27.34%), salvianolic acid A (21.71%), and rosmarinic acid (12.31%), respectively. A higher bioaccessibility indicated that the components could be readily metabolized and assimilated by the stomach and intestines, enabling them to efficiently deliver their therapeutic effect. Evidence has suggested that phenolic acids are more readily digested and released by artificial gastric juice. The experimental findings of Hu (2015) proved that when rats were administered phenolic acids intravenously via the tail vein, the presence and peak concentration of danshensu in rat plasma were significantly higher than those of salvianolic acid B, and the mean residence time and half-life of danshensu were significantly longer.
Numerous studies have demonstrated the significant effectiveness of danshensu in various aspects, which include its ability to restrain oxidative stress (Wang et al., 2020a), decrease neuroinflammation (Han et al., 2019; Ye et al., 2020; Bai et al., 2023), prevent apoptosis (Guo et al., 2015; Fan et al., 2016), promote angiogenesis (Yin et al., 2017), ameliorate neurogenesis (Wei et al., 2018), improve the mitochondrial function (Xue et al., 2022), and alleviate the toxic effects of Aβ proteins on the brain (Zheng et al., 2023). These findings suggest its potential therapeutic applications in neurodegenerative disorders (such as Parkinson's disease and Alzheimer's disease), cerebral ischemia or ischemia/perfusion injury, and other diseases related to the nervous system. Consequently, danshensu could be anticipated as one of the indicators of quality for JKZP.
5.4.3 Cistanches Herba
Cistanches Herba has been identified with more than 150 compounds (Song et al., 2021), and the “Pharmacopoeia of the People's Republic of China” has recorded echinacoside as its key active ingredient. In the study by Yan (2018), a thorough analysis was conducted on the plasma, urine, and feces of rats that were given Cistanches Herba extracts orally. The study found a total of 82 characteristic compounds, with echinacoside being one of the main components.
Previous studies have indicated that echinacoside exerted a wide range of neuroprotective effects, such as anti-neuroinflammation (Zhang et al., 2017a; Gao et al., 2020; Lu et al., 2022; Yang et al., 2022), promotion of hippocampal neurogenesis (Lu et al., 2022), inhibition of glutamatergic excitotoxicity (Lu et al., 2016), reduction of oxidative stress (Zhao et al., 2016; Zheng et al., 2019), prevention of apoptosis (Zhu et al., 2013; Wei et al., 2019), enhancement of mitochondrial function (Ma et al., 2019), mitigation of β-amyloid neurotoxicity (Shiao et al., 2017), stimulation of autophagy (Chen et al., 2019), and suppression of endoplasmic reticulum stress (Zhang et al., 2017b). These effects have contributed to improving learning memory and cognitive disorders, as well as depressive behaviors. Consequently, echinacoside has extensive applications in neurological-related diseases.
5.4.4 Corni Fructus
Iridoid glycosides were the characteristic components of Corni Fructus, with 91 compounds of this class being isolated. The “Pharmacopoeia of the People's Republic of China” designated the total content of morroniside and loganin as a quality control index for Corni Fructus (Fan et al., 2020). A study conducted by Li (2007) examined the pharmacokinetic process of loganin in rats following the oral administration of a single dosage of loganin and Corni Fructus extract. Administering loganin (20 mg kg−1) and Corni Fructus extract (40 mg kg−1) orally to rats led to a notable elevation in their blood concentration. The blood concentration of loganin reached its highest level at 69 min, whereas that of the Corni Fructus extract peaked at 51 min. The compounds exhibited elimination half-lives of 93.6 min and 99.4 min, respectively.
Loganin exhibited anti-neuroinflammatory properties by boosting the polarization of M2 microglia, lowering the release of inflammation-related mediators, and playing a protective role in ischemic stroke mouse models (Huo et al., 2023). Loganin has also demonstrated effects such as anti-neuronal apoptosis (Kwon et al., 2011; Tseng et al., 2019), anti-oxidative stress (Kwon et al., 2011), improvement of mitochondrial function (Zhou et al., 2023), modulation of neurotransmitter release (Shi et al., 2019), reduction in neuronal damage, and alleviation of cognitive deficits in animal models. It shows potential for treating ischemic stroke and neurological illnesses.
5.4.5 Radix Polygalae
Modern pharmacological studies have indicated that extracts from Radix Polygalae attenuated neuronal cell damage and ameliorated cognitive impairments associated with learning and memory in various animal models of neurodegenerative disorders (Yuan et al., 2021). The compound 3,6′-disinapoyl sucrose was included in the “Pharmacopoeia of the People's Republic of China” as a quality control marker for Radix Polygalae. In the study by Xiong et al. (2023), rats were orally administered the Radix Polygalae extract at a dosage of 5 g kg−1. The UPLC-MS/MS approach was employed to ascertain the compounds present in the plasma. The findings indicated that the plasma blood concentration of 3,6′-disinapoyl sucrose peaked at 2 h, reaching a maximum concentration of 241.70 ± 15.18 ug·L−1.
The compound 3,6′-disinapoyl sucrose possesses the properties of anti-oxidative stress (Shi et al., 2015; Tang X. et al., 2022), counteracting glutamate excitotoxicity (Hu et al., 2014), enhancing neuroplasticity (Hu et al., 2010), and promoting neurogenesis (Wang et al., 2021). Therefore, it was frequently employed to treat conditions such as ischemic stroke, insomnia, amnesia, and depressive disorders.
5.5 Q-marker prediction based on component measurability
The composition of TCM prescriptions is intricate and diverse. Clarifying their key active components is crucial for elucidating the mechanism of their efficacy. Therefore, the Q-marker should be measurable. Studies by Qu et al. (2020) and Li et al. (2008) have detected danshensu in the plasma of rats gavaged with the Salviae Miltiorrhizae aqueous extract. The concentration of danshensu in the plasma ranged from 5 to 500 ng mL−1, demonstrating a linear relationship with its transformation. The study by Zhao et al. (2020) used the UHPLC-MS/MS technology to assess the pharmacokinetics and bioavailability of active components of Radix Polygalae in rat serum. The results have shown that the absolute bioavailability of sibiricose A5, A6, and 3,6′-disinapoyl sucrose was 3.25%, 2.95%, and 2.36%, respectively. The study by Yang et al. (2020) used the HPLC method to quantify the components of Cistanches Herba, such as echinacoside, cistanoside A, acteoside, and isoacteoside. This approach facilitated a comprehensive evaluation of the quality of the prepared slices. The study by Liu (2022) employed a traditional, reliable, and stable liquid-phase method to determine the quality of Corni Fructus, which included morroniside, sweroside, loganin, gallic acid, 5-HMF, protocatechuic acid, and cornuside I. The biological activity results indicated that these seven chemical ingredients could be used as Q-markers for evaluating the quality of Corni Fructus. Yalu et al.'s (2023) research identified the main components of Chuanxiong Rhizoma by liquid chromatography, encompassing senkyunolide H, chlorogenic acid, n-butylphenol, ligustrazine, ferulic acid, ligustilide, and others.
In conclusion, senkyunolide H, danshensu, echinacoside, loganic acid, and 3,6′-disinapoyl sucrose, with high proprietary and measurability, were predicted to be the key pharmacological bases for the neuroprotective effects of JKZP based on the “five principles” of Q-marker determination. The chemical structures of the Q-marker of JKZP are shown in Figure 4, the maps of peaks in the JKZP aqueous extract and JKZP-containing serum are revealed in Supplementary Figures S2, S3, and the mechanisms of neuroprotection are listed in Table 5.
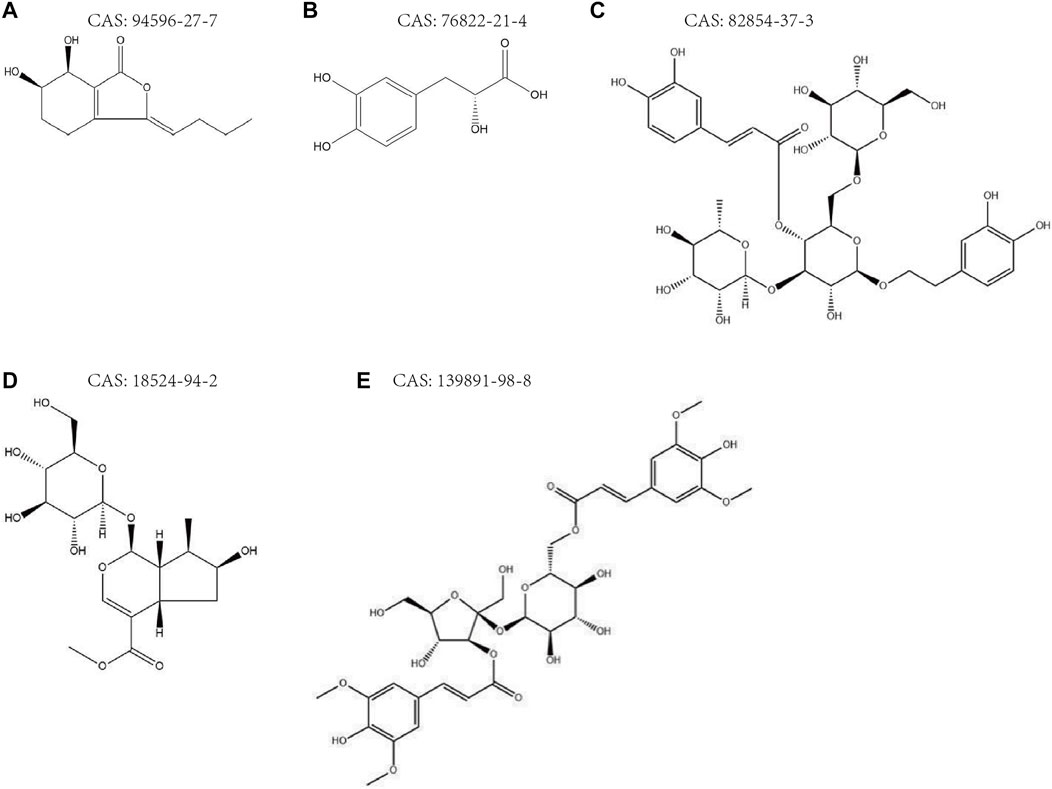
FIGURE 4. (A–E) Chemical structures of JKZP Q-markers. (A) Senkyunolide H; (B) danshensu; (C) echinacoside; (D) loganic acid; (E) 3,6′-disinapoyl sucrose.
6 Conclusion
JKZP exhibited the characteristics of tonifying the kidneys and marrow, promoting blood circulation, resolving stasis, and alleviating depression. Consequently, it has been utilized to address conditions such as stroke, post-stroke sequelae, vascular cognitive impairment, or dementia associated with kidney essence insufficiency, blood stasis obstruction, and blockage syndrome. It has had a notable impact on neuroprotection by inhibiting neuronal apoptosis, stimulating angiogenesis, and enhancing synaptic remodeling. Nevertheless, the lack of a distinct material foundation has severely hindered its progress and utilization. Hence, the article employed the UPLC-Q-TOF-MS/MS technology to examine and identify the compounds in the JKZP aqueous extract and JKZP-containing serum samples. This can serve as a source of information to further elucidate the pharmacological substance basis of JKZP.
The current study marked the first instance of analyzing and identifying the compounds of the aqueous extract of JKZP, along with the absorption into the serum. The aqueous extract contained a total of 12 chemical categories and 67 unique components identified. The JKZP-containing serum encompassed 111 components, comprising 33 prototype components and 78 metabolites. These components were derived from a pool of 39 original components. Iridoid glycosides, phenolic acids, oligosaccharide esters, phenylethanoid glycosides, and phthalides were posited as the potential key pharmacophore basis of JKZP. A comparison of the obtained compounds with the literature demonstrated the satisfactory results of this assay, with key compounds detected in all herbs except for dragon bones. The neuroprotective effects of JKZP were attributed to five specific components: senkyunolide H, danshensu, echinacoside, loganin, and 3,6
Ultimately, this work primarily examined the properties of JKZP compounds and their uptake into the rat serum through the utilization of UPLC-Q-TOF-MS/MS technology. Senkyunolide H, danshensu, echinacoside, loganin, and 3,6′-disinapoyl sucrose have been predicted as Q-markers for JKZP. These findings provide empirical evidence to support the assessment of the quality and application of JKZP, serving as a reliable foundation for the theoretical advancement of prescriptions. However, certain constraints endured, Acorus Tatarinowii and Chuanxiong Rhizoma in this prescription contained more volatile oil components, which brought out the effects of opening the mind, awakening the brain, and alleviating depression. The study did not specifically prioritize the detection and identification of these volatile oil components nor did it reconfirm the neuroprotective benefits of Q-markers on neurodegenerative and ischemic stroke illnesses. Subsequent studies may concentrate on a comprehensive examination of volatile oil components and perform trials both in vivo and in vitro to validate the neuroprotective benefits of JKZP's Q-markers with the aim to further enhance the fundamental research on the pharmacodynamic substances of JKZP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by the Laboratory Animal Center of Shandong University of Traditional Chinese Medicine. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QW: Writing–original draft, Writing–review and editing. CO: Data curation, Writing–original draft. JW: Conceptualization, Data curation, Writing–review and editing. XW: Conceptualization, Data curation, Formal Analysis, Writing–review and editing. ZG: Data curation, Writing–review and editing. YZ: Conceptualization, Data curation, Writing–review and editing. GL: Conceptualization, Writing–review and editing. ZW: Conceptualization, Formal Analysis, Writing–review and editing. HY: Conceptualization, Data curation, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81202641), the Natural Science Foundation of Shandong Province (ZR2020MH345), and Independent Innovation Team Project of Jinan (2020GXRC012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1328632/full#supplementary-material
References
Bai, M., Cui, N., Liao, Y., Guo, C., Li, L., Yin, Y., et al. (2023). Astrocytes and microglia-targeted Danshensu liposomes enhance the therapeutic effects on cerebral ischemia-reperfusion injury. J. Control. Release. 364, 473–489. doi:10.1016/j.jconrel.2023.11.002
Chen, C., Xia, B., Tang, L., Wu, W., Tang, J., Liang, Y., et al. (2019). Echinacoside protects against MPTP/MPP(+)-induced neurotoxicity via regulating autophagy pathway mediated by Sirt1. Metab. Brain Dis. 34 (1), 203–212. doi:10.1007/s11011-018-0330-3
Chen, D., Song, C., Song, S., Fan, Z., and Zhang, H. (2023). The dating of 9 batches of authentic Os Draconis and the correlation between the age range and the ingredients. Spectrosc. Spect. Anal. 43 (06), 1900–1904. Available at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=GUAN202306051&DbName=CJFQ2023.
Chen, J., Yuan, M., and Hou, Z. (2022). Mechanistic analysis of Sagacious Confucius' Pillow Elixir in the treatment ofinsomnia and amnesia with concept of treating different diseases withsame method based on network pharmacology. Tianjin J. Traditional Chin. Med. 39 (10), 1335–1344. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7QA4U3gSMGC2nF_CbmxN6J4CUSoW_0mfi5C4mzBvUCeqTvoL7WgRQyv3DNjR9AFz0ddcpONmwpY2tm9F_q813NRhwPzGACRNYu7lwoeTwV9MnAN_4UxN4ebL0aEL3aVfuc=&uniplatform=NZKPT&language=CHS.
Chen, M., Zhu, W., Guan, Y., and Zhang, Y. (2023). Analysis of chemical constituents in puerariae lobatae Radix DispensinoGranules by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Traditional Med. Formulae 29 (19), 176–186. doi:10.13422/j.cnki.syfjx.20230762
Chen, Y., Wang, C., Pei, Q., and Zheng, Q. (2023). Clinical observation of Jiawei Kongsheng zhenzhong Pill decoction for the treatment of insomnia with heart-kidney deficiency type. J. Guangzhou Univ. Traditional Chin. Med. 40 (04), 833–837. doi:10.13359/j.cnki.gzxbtcm.2023.04.008
Fan, G., Yu, J., Asare, P. F., Wang, L., Zhang, H., Zhang, B., et al. (2016). Danshensu alleviates cardiac ischaemia/reperfusion injury by inhibiting autophagy and apoptosis via activation of mTOR signalling. J. Cell. Mol. Med. 20 (10), 1908–1919. doi:10.1111/jcmm.12883
Fan, Q., Chen, X., Rong, L., and Zhang, C. (2020). Research progress on chemical constituents,bioactivities, formula applications and quality control of Cornus officinalis. Nat. Prod. Res. Dev. 32 (07), 1244–1258. doi:10.16333/j.1001-6880.2020.7.021
Feng, S. Q., Aa, N., Geng, J. L., Huang, J. Q., Sun, R. B., Ge, C., et al. (2017). Pharmacokinetic and metabolomic analyses of the neuroprotective effects of salvianolic acid A in a rat ischemic stroke model. Acta Pharmacol. Sin. 38 (11), 1435–1444. doi:10.1038/aps.2017.114
Fernstrom, J. D., Fernstrom, M. H., and Grubb, P. E. (1987). Twenty-four-hour variations in rat blood and brain levels of the aromatic and branched-chain amino acids: chronic effects of dietary protein content. Metabolism 36 (7), 643–650. doi:10.1016/0026-0495(87)90147-8
Gao, M. R., Wang, M., Jia, Y. Y., Tian, D. D., Liu, A., Wang, W. J., et al. (2020). Echinacoside protects dopaminergic neurons by inhibiting NLRP3/Caspase-1/IL-1β signaling pathway in MPTP-induced Parkinson's disease model. Brain Res. Bull. 164, 55–64. doi:10.1016/j.brainresbull.2020.08.015
Guo, C., Yin, Y., Duan, J., Zhu, Y., Yan, J., Wei, G., et al. (2015). Neuroprotective effect and underlying mechanism of sodium danshensu [3-(3,4-dihydroxyphenyl) lactic acid from Radix and Rhizoma Salviae miltiorrhizae = Danshen] against cerebral ischemia and reperfusion injury in rats. Phytomedicine 22 (2), 283–289. doi:10.1016/j.phymed.2014.12.001
Han, B., Che, X., Zhao, Y., Li, C., He, J., Lu, Y., et al. (2019). Neuroprotective effects of Danshensu in Parkinson's disease mouse model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Behav. Pharmacol. 30 (1), 36–44. doi:10.1097/FBP.0000000000000412
Han, L., Liu, D. L., Zeng, Q. K., Shi, M. Q., Zhao, L. X., He, Q., et al. (2018). The neuroprotective effects and probable mechanisms of Ligustilide and its degradative products on intracerebral hemorrhage in mice. Int. Immunopharmacol. 63, 43–57. doi:10.1016/j.intimp.2018.06.045
Hornok, V., Amin, K., Kovács, A. N., Juhász, Á., Katona, G., Balogh, G. T., et al. (2022). Increased blood-brain barrier permeability of neuroprotective drug by colloidal serum albumin carriers. Colloids Surf. B Biointerfaces 220, 112935. doi:10.1016/j.colsurfb.2022.112935
Hou, Z. (2019). Metabolomics and system pharmacology-based approach for studying the mechanisms of sagacious confucius’ pillow elixir on mice with mild cognitive impairment. PhD dissertation. Harbin: Heilongjiang University of Traditional Chinese Medicine. doi:10.27127/d.cnki.ghlzu.2019.000467
Hou, Z., Song, X., Li, Y., Chen, J., and Shang, H. (2021). Mechanistic analysis of Kongsheng zhenzhong Pill in theTreatment of mild cognitive lmpairment based on network pharmacology. Liaoning Univ. Traditional Chin. Med. 23 (03), 73–80. doi:10.13194/j.issn.1673-842x.2021.03.017
Hu, S. (2015). Studies of Salvianolica acid metabolish and penetrate the blood-brain barrier. Masters thesis. Nanjing. Nanjing University of Chinese Medicine. Available at: https://kns.cnki.net/kcms2/article/abstract?v=pFbCq-yO4FAFUtjZx8oAXk7DrWOIsGr8gGzQXrVy4OWtImt7dzPzlaD24qHZUcX QrDsTZRc-nRQj9JiTTF7V14OEmnCV2z7V3_v-li-CeRRrDjOVoToSiWtG2HS3-XA1qHiBTSd90zY= &uniplatform=NZKPT&language=CHS.
Hu, Y., Liao, H. B., Dai-Hong, G., Liu, P., Wang, Y. Y., and Rahman, K. (2010). Antidepressant-like effects of 3,6'-disinapoyl sucrose on hippocampal neuronal plasticity and neurotrophic signal pathway in chronically mild stressed rats. Neurochem. Int. 56 (3), 461–465. doi:10.1016/j.neuint.2009.12.004
Hu, Y., Liu, M. Y., Liu, P., Dong, X., and Boran, A. D. (2014). Neuroprotective effects of 3,6'-disinapoyl sucrose through increased BDNF levels and CREB phosphorylation via the CaMKII and ERK1/2 pathway. J. Mol. Neurosci. 53 (4), 600–607. doi:10.1007/s12031-013-0226-y
Huo, K., Xu, J., Ma, K., Wang, J., Wei, M., Zhang, M., et al. (2023). Loganin attenuates neuroinflammation after ischemic stroke and fracture by regulating α7nAChR-mediated microglial polarization. Environ. Toxicol. 38 (4), 926–940. doi:10.1002/tox.23738
Jiang, Y., Chen, M., Gang, H., Li, X., Zhai, C., Feng, Z., et al. (2023). A funnel-type stepwise filtering strategy for identification of potential Q-markers of traditional Chinese medicine formulas. Front. Pharmacol. 14, 1143768. doi:10.3389/fphar.2023.1143768
Jiang, Y., Luo, Y., Chen, X., Liu, N., Hou, J., Piao, J., et al. (2022). Senkyunolide H protects PC12 cells from OGD/R-induced injury via cAMP-PI3K/AKT signaling pathway. J. Ethnopharmacol. 282, 114659. doi:10.1016/j.jep.2021.114659
Katila, N., Duwa, R., Bhurtel, S., Khanal, S., Maharjan, S., Jeong, J. H., et al. (2022). Enhancement of blood-brain barrier penetration and the neuroprotective effect of resveratrol. J. Control. Release. 346, 1–19. doi:10.1016/j.jconrel.2022.04.003
Kwon, S. H., Kim, J. A., Hong, S. I., Jung, Y. H., Kim, H. C., Lee, S. Y., et al. (2011). Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 58 (4), 533–541. doi:10.1016/j.neuint.2011.01.012
Lai, L., Jiang, Z., Feng, Y., Luo, G., Xie, Y., and Wang, S. (2022). Bioaccessibility analysis of active components in Salvia miltiorrhiza. Acta Pharm. Sin. 57 (08), 2435–2444. doi:10.16438/j.0513-4870.2022-0359
Li, C., Meng, Y., Du, H., and Jiang, H. (2017). Determination of morroniside and loganin in Cornus by UPLC. Northwest Pharm. J. 32 (04), 407–410. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7SJeE2Roc11YQG073ampKNg27DK8xH41DI4JY-04pePp66XcjwoLWR1E6bdstFpACfte9tRHqiAZ_i2jpKTPyazriHKHqxdM4vtOExc_k-C5LYxAxXK6dm4rk1bU28dkt6S_G4GbuKL_A==&uniplatform=NZKPT&language=CHS.
Li, W., Li, S., Li, T., Zhou, H., Luo, X., Chu, Y., et al. (2018). Quality marker research of monarch herb Salvia miltiorrhiza in compound danshen dripping pills. Chin. Traditional Herb. Drugs 49 (09), 2000–2006. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7RsQlt8mFjBGesFbc3knOlyteEOQwGsVSCkJj0IBGY5skw7eB2lPMRQ60fQ JIyhjHA-hAKpcnVc6VCAyBMohsFjOW4JQ6idRr4kOP38xZt8XTP_hC28qBSDPOK0U0a_e-o=&uniplatform=NZKPT&language=CHS.
Li, W., Li, Z. W., Han, J. P., Li, X. X., Gao, J., and Liu, C. X. (2008). Determination and pharmacokinetics of danshensu in rat plasma after oral administration of danshen extract using liquid chromatography/tandem mass spectrometry. Eur. J. Drug Metab. Pharmacokinet. 33 (1), 9–16. doi:10.1007/BF03191013
Li, X. (2007). Studies on isolation and pharmacokinetics of ChemicalConstituents from Fructus Corni. PhD dissertation. Shijiazhuang: Hebei Medical University. Available at: https://kns.cnki.net/kcms2/article/abstract?v=hqt_j-uEELF-_oPLW3Bpf7wVJDK8w6gXTWBX5MY98rbln2zRupbQbI-QbvDC4ZlAYbigHpL4YbYWCRVBigZV-88Z3ElpHP2rH_-fMbIJOSsofD488xV8ppGRIz23514FYOZm8q93C2N-IFgEf5IDxA==&uniplatform=NZKPT&language=CHS.
Liu, C., Liu, S., Liu, X., Zhang, T., Hou, W., and Liao, M. (2016). A new concept on quality marker of Chinese materia medica: quality control for Chinese medicinal products. Chin. Traditional Herb. Drugs 47 (09), 1443–1457. Available at: https://kns.cnki.net/kcms2/article/abstract?v=hqt_j-uEELFH16WMNqgstF2NLhb0rOcJEr9S5aWLXhZvAOd_1p2FEoSSk7rtdfR0P1lYiDEQ0xKiagDC6GtLHI5Bxv-ymh8IvaRgrX2yboI44eVj6yUu1yPiJR0jElpWmRBGQVKG82s8tySPsH1IbA==&uniplatform=NZKPT&language=CHS.
Liu, J. (2016). Establishment of quality evaluation method and standardized production of operation procedure of Ligusticum chuanxiong. PhD dissertation. Chengdu: Sichuan Agricultural University. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7RNFSiKS65_YEhqB3UhXhGE3xqqRcYPh4MK12vmd2LXkG_srS3WMJpEHtgSgoTAPLmWEKcB4GHOhsNgaKU4U9Ebnw9FX F5KRR5EaYx3aUfvFZJ6Ij2NNNuuztiW0XM1gxI=&uniplatform=NZKPT&language=CHS.
Liu, J., Feng, M., Yang, X., Li, Y., Jia, Z., and Xiao, H. (2022a). Chemical constituents of Ligusticum chuanxiong. J. Chin. Med. Mater. 45 (04), 848–852. doi:10.13863/j.issn1001-4454.2022.04.014
Liu, M., Zhang, A., Niu, L., and Feng, W. (2022b). Metabolite ldentification of senkyunolide H in rats by ultra-high performance LiquidChromatography quadrupole-time-of-flight mass spectrometry. Chin. Pharm. J. 57 (18), 1549–1556. Available at: https://kns.cnki.net/kcms2/article/abstract?v=pFbCq-yO4FCykkWrUNFADwTKC9XTs9Vr-yk-jQbFuNcL53s8TwEL64kvmwrZqzpCwmI8fYtvvsgkE4viEAEqzNAEynE6_vLDR_1MN5seEjKkUGcUFwupNw1pDevoPd6-0jeq1wVqc2MhJ7FK_w2BkA==&uniplatform=NZKPT&language=CHS.
Liu, Y. (2022). The research of quality marker and polysaccharides for Cornus officinalis. Masters thesis. Tianjin: Tianjin University of Science and Technology, 74. doi:10.27359/d.cnki.gtqgu.2022.000153
Liu, Z., Tan, X., Qiu, H., Qin, X., Sun, X., and Gao, H. (2022c). Study on the application of Salvia miltiorrhiza formulations based on effector component indices. Guid. J. Traditional Chin. Med. Pharm. 28 (05), 84–87+104. doi:10.13862/j.cn43-1446/r.2022.05.017
Lu, C. W., Lin, T. Y., Huang, S. K., and Wang, S. J. (2016). Echinacoside inhibits glutamate release by suppressing voltage-dependent Ca(2+) entry and protein kinase C in rat cerebrocortical nerve terminals. Int. J. Mol. Sci. 17 (7), 1006. doi:10.3390/ijms17071006
Lu, R., Zhang, L., Wang, H., Li, M., Feng, W., and Zheng, X. (2022). Echinacoside exerts antidepressant-like effects through enhancing BDNF-CREB pathway and inhibiting neuroinflammation via regulating microglia M1/M2 polarization and JAK1/STAT3 pathway. Front. Pharmacol. 13, 993483. doi:10.3389/fphar.2022.993483
Luo, Y., Li, X., Liu, T., Cao, Y., Zhang, J., Yaseen, A., et al. (2019). Senkyunolide H protects against MPP(+)-induced apoptosis via the ROS-mediated mitogen-activated protein kinase pathway in PC12 cells. Environ. Toxicol. Pharmacol. 65, 73–81. doi:10.1016/j.etap.2018.12.007
Lv, G., Wang, S., Hang, T., and Song, M. (2016). Qualitative and quantitative analysis of components of Radix Polygalae in herbal pair of Radix Polygalae and rhizoma acori tatarinowii. J. China Pharm. Univ. 47 (03), 329–336. Available at: https://kns.cnki.net/kcms2/article/abs tract?v=pFbCq-yO4FD0Dr76BwMM1VX5bGjZl1hdOjrDm4zoHiCZgn8TpXcjipRcba9slO41sCe_q0zEPOZZRuZRBtNHPMHBezzSOhcc4dPFpjtmC-Axrneaic16O9IDygEWAIMI1Bofcql6MpF86sNjGkvF4A==&uniplatform=NZKPT&language=CHS.
Ma, H., Liu, Y., Tang, L., Ding, H., Bao, X., Song, F., et al. (2019). Echinacoside selectively rescues complex I inhibition-induced mitochondrial respiratory impairment via enhancing complex II activity. Neurochem. Int. 125, 136–143. doi:10.1016/j.neuint.2019.02.012
Pang, X., Bi, C., Bi, H., Wu, B., Wu, Z., and Wang, Z. (2016). Impacts of Jiawei Kongsheng zhenzhong Pills on expression of VEGF and MVD in VD rats induced by chronic cerebral ischemia. Mod. J. Integr. Traditional Chin. West. Med. 25 (23), 2515–2518. Available at: https://kns.cnki.net/kcms2/article/abstract?v=hqt_j-uEELFB7eIPPe1s4h9Gacz252_IUmC2ChDCPAGEjtubOyNNU8jLrPy0MFcO4w5Pt20IYPhYuma4IB_ox88GqzA9iV642Fo_RKKRDwcSzo9NhJtkIZVNGn67NXZMS0YUjm_u6vY=&uniplatform=NZKPT&language=CHS.
Qu, C., Xu, D. Q., Yue, S. J., Shen, L. F., Zhou, G. S., Chen, Y. Y., et al. (2020). Pharmacodynamics and pharmacokinetics of Danshen in isoproterenol-induced acute myocardial ischemic injury combined with Honghua. J. Ethnopharmacol. 247, 112284. doi:10.1016/j.jep.2019.112284
Rui, H., and Yue, C. (2012). Current status of clinical and experimental research on Kongsheng zhenzhong Pill. J. Shaanxi Univ. Chin. 35 (04), 91–93. doi:10.13424/j.cnki.jsctcm.2012.04.034
Shi, Q., Chen, J., Zhou, Q., Lei, H., Luan, L., Liu, X., et al. (2015). Indirect identification of antioxidants in Polygalae Radix through their reaction with 2,2-diphenyl-1-picrylhydrazyl and subsequent HPLC-ESI-Q-TOF-MS/MS. Talanta 144, 830–835. doi:10.1016/j.talanta.2015.07.032
Shi, R., Han, Y., Yan, Y., Qiao, H. Y., He, J., Lian, W. W., et al. (2019). Loganin exerts sedative and hypnotic effects via modulation of the serotonergic system and GABAergic neurons. Front. Pharmacol. 10, 409. doi:10.3389/fphar.2019.00409
Shiao, Y. J., Su, M. H., Lin, H. C., and Wu, C. R. (2017). Echinacoside ameliorates the memory impairment and cholinergic deficit induced by amyloid beta peptides via the inhibition of amyloid deposition and toxicology. Food Funct. 8 (6), 2283–2294. doi:10.1039/c7fo00267j
Song, A. (2022). Study on the effect and mechanism of the Jiawei Kongsheng Zhenzhong Pill in influencing synaptic remodeling through modulation of Netrin-1/DCC signaling pathway to interfere with PSD. Masters thesis. Jinan: Shandong University of Traditional Chinese Medicine. doi:10.27282/d.cnki.gsdzu.2022.000920
Song, Y., Zeng, K., Jiang, Y., and Tu, P. (2021). Cistanches Herba, from an endangered species to a big brand of Chinese medicine. Med. Res. Rev. 41 (3), 1539–1577. doi:10.1002/med.21768
Tan, J., Li, W., Teng, Z., Wang, G., Li, Y., and Zhang, Y. (2022). Senkyunolide H inhibits activation of microglia and attenuates lipopolysaccharide-mediated neuroinflammation and oxidative stress in BV2 microglia cells via regulating ERK and NF-κB pathway. Kaohsiung J. Med. Sci. 38 (4), 378–384. doi:10.1002/kjm2.12477
Tang, X., Zhao, Y., Liu, Y., Liu, Y., Liu, Y., Niu, F., et al. (2022a). 3,6'-disinapoyl sucrose attenuates Aβ(1-42) - induced neurotoxicity in Caenorhabditis elegans by enhancing antioxidation and regulating autophagy. J. Cell. Mol. Med. 26 (4), 1024–1033. doi:10.1111/jcmm.17153
Tang, Y., Li, X., Guo, H., Yuan, C., Chen, S., Luo, S., et al. (2022b). Scientific connotation of ternary compatibility of danshen-chuanxiong-gegen inNaoxintongzhi based on the strategy of component in plasma-target-pathway. Pharmacol. Clin. Chin. Materia Medica 38 (06), 31–41. doi:10.13412/j.cnki.zyyl.2022.06.003
Tseng, Y. T., Lin, W. J., Chang, W. H., and Lo, Y. C. (2019). The novel protective effects of loganin against 1-methyl-4-phenylpyridinium-induced neurotoxicity: enhancement of neurotrophic signaling, activation of IGF-1R/GLP-1R, and inhibition of RhoA/ROCK pathway. Phytother. Res. 33 (3), 690–701. doi:10.1002/ptr.6259
Wang, Q., Shen, L., Fang, X., Hong, Y., Feng, Y., and Ruan, K. (2013). Shift of effective ingredients of Dachuanxiong Decoction along in vitro-plasma-cerebrospinal fluid brain tissue. WANG Qiang SHEN Lan Fang. Xin Hong. Yan-ong FENG Yi RUAN Ke-feng 35 (11), 2364–2371. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7QEetNPepUfWafo0HCrVnwXG_7PHSFKPtTERE7YmoOhjf1sXtkn5m0hT7DLRrbuSMZvutKlMRno8810SG__zGP9_NWZZGchJLxAbdGsSxLpUdAv1eKiwONg&uniplatform=NZKPT&language=CHS.
Wang, T., Li, C., Han, B., Wang, Z., Meng, X., Zhang, L., et al. (2020). Neuroprotective effects of Danshensu on rotenone-induced Parkinson's disease models in vitro and in vivo. BMC Complement. Med. Ther. 20 (1), 20. doi:10.1186/s12906-019-2738-7
Wang, W., Han, Z., Guo, D., and Xiang, Y. (2020). UHPLC-QTOFMS-based metabolomic analysis of serum and urine in rats treated with musalais containing varying ethyl carbamate content. Anal. Bioanal. Chem. 412 (27), 7627–7637. doi:10.1007/s00216-020-02900-5
Wang, X. F., Xiao, H. H., Wu, Y. T., Kong, L., Chen, J. C., Yang, J. X., et al. (2021). Active constituent of Polygala tenuifolia attenuates cognitive deficits by rescuing hippocampal neurogenesis in APP/PS1 transgenic mice. BMC Complement. Med. Ther. 21 (1), 267. doi:10.1186/s12906-021-03437-5
Wei, Z. Z., Chen, D., Liu, L. P., Gu, X., Zhong, W., Zhang, Y. B., et al. (2018). Enhanced neurogenesis and collaterogenesis by sodium danshensu treatment after focal cerebral ischemia in mice. Cell Transpl. 27 (4), 622–636. doi:10.1177/0963689718771889
Wei, W., Lan, X. B., Liu, N., Yang, J. M., Du, J., Ma, L., et al. (2019). Echinacoside alleviates hypoxic-ischemic brain injury in neonatal rat by enhancing antioxidant capacity and inhibiting apoptosis. Neurochem. Res. 44 (7), 1582–1592. doi:10.1007/s11064-019-02782-9
Wu, B., Wu, Z., Ji, X., Liu, G., Liu, H., and Yu, H. (2015). Lmpacts of modified Kongsheng Zhenzhong pills on apoptosis of neural cellsin MCAO rats. Chin. J. Gerontology 35 (22), 6325–6327. Available at: https://kns.cnki.net/kcms2/article/abstract?v=hqt_j-uEELFIvSWAhdj85bcf5uJARAxIOqYTSRIQgPhjVawlrFwJCoRWPrp-_fYNnpDkJLj1N-ByWgiy00ygsQkI_1aOX8XR7aXAtgVln5ubsZOuXutaDxGPGLxR6JCuGNK3nnCNhk0=&uniplatform=NZKPT&language=CHS.
Wu, L., Lin, Q., Tan, Z., and Wang, S. (2018). Study on HPLC fingerprint of Polygala glomerata lour. And Radix Polygalae. Lishizhen Med. Materia Medica Res. 29 (09), 2049–2052. Available at: https://kns.cnki.net/kcms2/article/abstract?v=lWc4gvQ5J17SQamZnsU6lHfyHfWO2vQT27CKzMvIlE1RhRPdMYBs gcTBnENK55jSIFJrInw5EfVHjhYLYLPX1zplP__oPsYg7b-1eCeIYC-_zcdKeVxdvShXYXzybnwWp-LCYy5Yh-QFNvPQWWYlgQ==&uniplatform=NZKPT&language=CHS.
Xiong, X., Lu, M., Shi, L., Zhong, L., and Ye, X. (2023). Effect of Jianchang stewing method on the pharmacokinetics and tissue distribution of 4components in Polygala tenuifolia in rats. Cent. South Pharm. 21 (05), 1149–1156. Available at: https://kns.cnki.net/kcms2/article/abstract?v=pFbCq-yO4FCvPPOJbra2nbzSVOAXVef3_7Tc2v1D_AU3jmQ_GNYwuJH_1HiCM3ua0kk7Ilk0Q8TNF_xK8kcd-M0byv6S6kIaVM8eDxI36E73Tax9GPhVbWHxmOlI6PH4Y_VYas45jmiYyvDOOndUtQ==&uniplatform=NZKPT&language=CHS.
Xu, J., Wei, K., Zhang, G., Lei, L., Yang, D., Wang, W., et al. (2018). Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: a review. J. Ethnopharmacol. 225, 18–30. doi:10.1016/j.jep.2018.06.029
Xu, R. (2006). The experimentcal and clinnical study of Jiawei Kongsheng pill on vascular dementia. Masters thesis. Ji’nan: Shandong University of Traditional Chinese Medicine. Available at: https://kns.cnki.net/kcms2/article/abstract?v=hqt_j-uEELHAz59Al5cHLqKtmdB6RYzbk2jdW6HTbFBdqQWUWrkzxyf3TnbIdq3f drHOINIBDc8kggyJ9KhT6TreTLBLJBWy_S7vlIWolAPPbsuVCh38yIvTUsJYlMAk&uniplatform=NZKPT&language=CHS.
Xue, Y., Zhang, L., Zhang, L., Sun, W., Fang, Z., Leng, Y., et al. (2022). Danshensu prevents thrombosis by inhibiting platelet activation via SIRT1/ROS/mtDNA pathways without increasing bleeding risk. Phytomedicine 104, 154271. doi:10.1016/j.phymed.2022.154271
Yalu, Q., Peng, G., Jiang, H., Qian, C., and Yan, W. (2023). Establishing the fingerprints of Ligusticum chuanxiong and effects of 60Co-γ irradiation on its effective components. J. Radiat. Res. Radiat. Process., 1–13. Available at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=FYFG20230705001&DbName=CAPJ2023.
Yan, Y. (2018). Analysis of the chemical components and in vivo components of the Chinese medicine Cistanches Herba. Masters thesis. Beijing: Minzu University of China. Available at: https://kns.cnki.net/kcms2/article/abstract?v=pFbCq-yO4FDDIFI-1Q5jEjO-92dz0XNFlot7VeX6yRBhHTWlIZsMuSGAY4zy4btU3869NX 7A4PPlsTNHsK1XdN5PC9ZHjTk2uIdhdmKiTo7fiIb1CauQdyz_MHCNQU8hE-4-s_yhCZeiCm5DGOz6dw==&uniplatform=NZKPT&language=CHS.
Yang, G., Han, L., Yu, Q., Liu, X., and Zhou, X. (2023). Effects of extraction and drying methods on effective components of Cistanche deserticola Y. C. Ma extract. Food Ferment. Industries 49 (18), 250–258. doi:10.13995/j.cnki.11-1802/ts.032559
Yang, G., Tc, B., Gp, T., and Al, E. (2020). Simultaneous determination of 8 components in Cistanches HerbaDecoction pieces by HPLC. China Pharm. 23 (07), 1442–1445. doi:10.13863/j.issn1001-4454.2020.07.027
Yang, J. (2019). Dynamics of the main components ofAcorus tatarinowiiSchott and Polygala tenuifolia Willd in vivo in Rats. Kunming: Kunming medical uniuersity. doi:10.27202/d.cnki.gkmyc.2019.000112
Yang, X., Yv, Q., Ye, F., Chen, S., He, Z., Li, W., et al. (2022). Echinacoside protects dopaminergic neurons through regulating IL-6/JAK2/STAT3 pathway in Parkinson's disease model. Front. Pharmacol. 13, 848813. doi:10.3389/fphar.2022.848813
Ye, T., Xiong, D., Li, Y., Gong, S., Zhang, L., Li, B., et al. (2020). Inhibition of nuclear factor kappa B as a mechanism of Danshensu during Toll-like receptor 2-triggered inflammation in macrophages. Int. Immunopharmacol. 83, 106419. doi:10.1016/j.intimp.2020.106419
Yin, Y., Duan, J., Guo, C., Wei, G., Wang, Y., Guan, Y., et al. (2017). Danshensu accelerates angiogenesis after myocardial infarction in rats and promotes the functions of endothelial progenitor cells through SDF-1α/CXCR4 axis. Eur. J. Pharmacol. 814, 274–282. doi:10.1016/j.ejphar.2017.08.035
Yu, H., Wu, Z., Liu, X., Liu, B., and Ji, X. (2016). Impacts of Jiawei Kongsheng zhenzhong pills on apoptosis of NeuralCells in callosum of VD rats induced by chronic cerebral lschemia. Liaoning J. Traditional Chin. Med. 43 (11), 2430–2432. doi:10.13192/j.issn.1000-1719.2016.11.067
Yu, H., Wu, Z., Zhao, Y., Liu, H., Wang, J., Wang, P., et al. (2013). Lmpacts of modified Kongsheng zhenzhong pills on neural behavior and VEGF expression in the rats of focal cerebral ischemia. World J. Integr. Traditional West. Med. 8 (12), 1211–1214. doi:10.13935/j.cnki.sjzx.2013.12.021
Yuan, J., Wang, H., Wang, Y., Wang, Z., Huo, Q., Dai, X., et al. (2021). Rapid identification of 3,6'-disinapoyl sucrose metabolites in Alzheimer's disease model mice using UHPLC-orbitrap mass spectrometry. Molecules 27 (1), 114. doi:10.3390/molecules27010114
Yun, W. J., Yao, Z. H., Fan, C. L., Qin, Z. F., Tang, X. Y., Gao, M. X., et al. (2018). Systematic screening and characterization of Qi-Li-Qiang-Xin capsule-related xenobiotics in rats by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1090, 56–64. doi:10.1016/j.jchromb.2018.05.014
Zhang, H., Wang, Y., and Hou, L. (2015). Analysis of volatilis components' variation in decoction before and after Acorus Tatarinowi Schottand and Polygala tenuifolia Willd matched-pairs. J. Pharm. Res. 34 (05), 269–271. doi:10.13506/j.cnki.jpr.2015.05.007
Zhang, J., Jiang, Y., Liu, N., Shen, T., Jung, H. W., Liu, J., et al. (2019). A network-based method for mechanistic investigation and neuroprotective effect on post-treatment of senkyunolid-H against cerebral ischemic stroke in mouse. Front. Neurol. 10, 1299. doi:10.3389/fneur.2019.01299
Zhang, J., Zhang, Z., Xiang, J., Cai, M., Yu, Z., Li, X., et al. (2017). Neuroprotective effects of echinacoside on regulating the stress-active p38MAPK and NF-κB p52 signals in the mice model of Parkinson's disease. Neurochem. Res. 42 (4), 975–985. doi:10.1007/s11064-016-2130-7
Zhang, Y., Long, H., Zhou, F., Zhu, W., Ruan, J., Zhao, Y., et al. (2017). Echinacoside's nigrostriatal dopaminergic protection against 6-OHDA-Induced endoplasmic reticulum stress through reducing the accumulation of Seipin. J. Cell. Mol. Med. 21 (12), 3761–3775. doi:10.1111/jcmm.13285
Zhang, Y. J., Wu, L., Zhang, Q. L., Li, J., Yin, F. X., and Yuan, Y. (2011). Pharmacokinetics of phenolic compounds of Danshen extract in rat blood and brain by microdialysis sampling. J. Ethnopharmacol. 136 (1), 129–136. doi:10.1016/j.jep.2011.04.023
Zhao, Q., Yang, X., Cai, D., Ye, L., Hou, Y., Zhang, L., et al. (2016). Echinacoside protects against MPP(+)-Induced neuronal apoptosis via ROS/ATF3/CHOP pathway regulation. Neurosci. Bull. 32 (4), 349–362. doi:10.1007/s12264-016-0047-4
Zhao, X., Xu, B., Wu, P., Zhao, P., Guo, C., Cui, Y., et al. (2020). UHPLC-MS/MS method for pharmacokinetic and bioavailability determination of five bioactive components in raw and various processed products of Polygala tenuifolia in rat plasma. Pharm. Biol. 58 (1), 969–978. doi:10.1080/13880209.2020.1818790
Zheng, H., Su, Y., Sun, Y., Tang, T., Zhang, D., He, X., et al. (2019). Echinacoside alleviates hypobaric hypoxia-induced memory impairment in C57 mice. Phytother. Res. 33 (4), 1150–1160. doi:10.1002/ptr.6310
Zheng, Y., Zheng, C., Tu, W., Jiang, Y., Lin, H., Chen, W., et al. (2023). Danshensu inhibits Aβ aggregation and neurotoxicity as one of the main prominent features of Alzheimer's disease. Int. J. Biol. Macromol. 245, 125294. doi:10.1016/j.ijbiomac.2023.125294
Zhou, H., and Cong, X. (2018). Effect of shanzhuyu-dihuang (Fructus corni-radices rehmanniae) compatibility on lridoid glycoside in Fructus Corni on dissolution. Chin. J. Traditional Med. Sci. Technol. 25 (04), 508–510. Available at: https://kns.cnki.net/kcms2/article/abstract?v=tc18asgQl7Rf14tFlap_8REvWSqUvIVW9mDc35d3HvsllfNlVwVYPdF3rVyWl_ECKeys6Moxdie5Jz4bnTZel040xem-oyzhyiPY-PbjeL2WTuGFTUCq8USn2cMMWsesCZkmuracjVM=&uniplatform=NZKPT&language=CHS.
Zhou, X., Chan, S. W., Tseng, H. L., Deng, Y., Hoi, P. M., Choi, P. S., et al. (2012). Danshensu is the major marker for the antioxidant and vasorelaxation effects of Danshen (Salvia miltiorrhiza) water-extracts produced by different heat water-extractions. Phytomedicine 19 (14), 1263–1269. doi:10.1016/j.phymed.2012.08.011
Zhou, Y. (2022). Study of Jiawei Kongsheng Zhenzhong pill regulates hippocampal synaptic plasticity via BDNF signaling pathway in MCI rats. Masters thesis. Ji’nan: Shandong University of Traditional Chinese Medicine. doi:10.27282/d.cnki.gsdzu.2022.000938
Zhou, Y., Luo, D., Shi, J., Yang, X., Xu, W., Gao, W., et al. (2023). Loganin alleviated cognitive impairment in 3×Tg-AD mice through promoting mitophagy mediated by optineurin. J. Ethnopharmacol. 312, 116455. doi:10.1016/j.jep.2023.116455
Keywords: Jiawei Kongsheng Zhenzhong Pill, compounds, Chinese herbal prescription, ultra-performance liquid chromatography quadrupole time-of-flight mass tandem spectrometry, quality marker, neuroprotective effect, pharmacodynamic material basis
Citation: Wu Q, Ou C, Wang J, Wu X, Gao Z, Zhao Y, Lu G, Wu Z and Yu H (2024) Jiawei Kongsheng Zhenzhong Pill: marker compounds, absorption into the serum (rat), and Q-markers identified by UPLC-Q-TOF-MS/MS. Front. Pharmacol. 15:1328632. doi: 10.3389/fphar.2024.1328632
Received: 27 October 2023; Accepted: 11 January 2024;
Published: 05 February 2024.
Edited by:
Dâmaris Silveira, University of Brasilia, BrazilReviewed by:
Jia-Wen Shou, The Chinese University of Hong Kong, ChinaFang Zhao, China Pharmaceutical University, China
Copyright © 2024 Wu, Ou, Wang, Wu, Gao, Zhao, Lu, Wu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhichun Wu, d3V6aGljaHVuQHNkdXRjbS5lZHUuY24=; Huayun Yu, eXVodWF5dW5maXNoQDE2My5jb20=
†These authors have contributed equally to this work and share the first authorship
 Qiaolan Wu
Qiaolan Wu Chunxue Ou1†
Chunxue Ou1† Jiayun Wang
Jiayun Wang Yue Zhao
Yue Zhao Zhichun Wu
Zhichun Wu