
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 15 March 2024
Sec. Ethnopharmacology
Volume 15 - 2024 | https://doi.org/10.3389/fphar.2024.1321405
This article is part of the Research TopicReviews in Ethnopharmacology: 2023View all 31 articles
 Liangyu Cui1†
Liangyu Cui1† Xingfang Liu2†
Xingfang Liu2† Yukun Li1†
Yukun Li1† Tianyue Jing1
Tianyue Jing1 Dasheng Liu1
Dasheng Liu1 Cong Ren1
Cong Ren1 Tong Yin1
Tong Yin1 Yu Wang1
Yu Wang1 Zhiwei Zhao1
Zhiwei Zhao1 Jiaheng Wang3
Jiaheng Wang3 Xuejie Han1*
Xuejie Han1* Liying Wang1*
Liying Wang1*Backgroud: The co-administration of Chinese patent medicine with calcium channel blockers (CCBs) is a prevalent practice in China for treating essential hypertension (EH). However, robust evidence supporting the efficacy and safety of tailored combinations of different Chinese patent medicines with CCBs, according to individual patient conditions, is still limited. This study sought to elucidate the efficacy and safety of these combinations using a systematic review and network meta-analysis.
Materials and methods: Relevant studies were sourced from established databases, incorporating randomized controlled trials published up to 1 February 2023. The ROB2 tool from the Cochrane Collaborative Network was employed to independently assess and cross-verify the quality of the included literature. A network meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 and PRISMA-Network Meta-Analyses (PRISMA-NMA) guidelines. A Bayesian network meta-analysis was utilized to gauge the efficacy and safety of distinct integrations of Chinese patent medicine and CCBs. Primary outcomes were interpreted using a paired fixed-effect meta-analysis. Publication bias was appraised through Egger’s test and represented with funnel plots. All statistical analyses were executed within the R statistical framework.
Results: Following rigorous selection, data extraction, and bias evaluation, 36 articles were incorporated. Tianma Gouteng Granule, when combined with CCBs, displayed superior efficacy in reducing systolic blood pressure (SBP). In terms of diastolic blood pressure (DBP) reduction, Songling Xuemaikang Capsule combined with CCBs emerged as the most effective. Regarding enhancement of antihypertensive effective rates, Qinggan Jiangya Capsule paired with CCBs demonstrated optimal results. For diminishing Traditional Chinese Medicine syndrome scores, the Qiangli Dingxuan Tablet and CCBs combination proved most beneficial. When aiming to reduce total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) levels, Tianma Gouteng Granule and CCBs showcased superior results. In contrast, the combination of Songling Xuemaikang Capsule and CCBs was more effective in reducing LDL-C, tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6).
Conclusion: This study underscores variability in outcomes from combining Chinese patent medicine and CCBs for hypertension, emphasizing the importance of personalized medicinal combinations, especially Tianma Gouteng Granule and Songling Xuemaikang Capsule. The results offer robust evidence to inform clinical guidelines for essential hypertention and significantly aid clinician in seleting appropriate Chinese patent medicines for treatment.
Essential hypertension (EH) stands as the leading preventable risk factor for cardiovascular disease (CVD), contributing significantly to conditions such as coronary heart disease, heart failure, stroke, and cognitive impairment (Forouzanfar et al., 2017). The 2017 Global Burden of Disease Study revealed that high systolic blood pressure (SBP) is a primary risk factor, responsible for 10.4 million deaths and impacting 218 million people in terms of disability-adjusted life years (Stanaway, 2017). Among those aged 18 and above, the incidence rate of EH is 27.9%, with a pre-hypertension rate of 39.1% (Forouzanfar et al., 2015). Age plays a crucial role in EH prevalence: it is around 34.38% in middle-aged individuals and surges to nearly 90% in those over 80 years (Beaney et al., 2020), posing a substantial financial and psychological burden on societies and families.
While the primary treatment for EH centers around conventional medications known for their efficacy, ACE inhibitors (ACEI), angiotensin II receptor blockers (ARB), also referred to as sartans, Calcium Channel Blockers (CCBs), and thiazide diuretics are recognized as first-line antihypertensive drugs (James et al., 2014). However, specific challenges like particular contraindications and inadequate control in certain hypertension variants persist. For instance, ACEI and ARB are not advised for pregnant women due to heightened risks linked to renal teratogenicity (Mancia et al., 2013). CCBs target calcium channels situated at the plasma membrane, inducing cell depolarization (Godfraind, 2005). They cause vasodilation by blocking calcium entry, hence diminishing the active tone of vascular smooth muscle. This property has rendered CCBs especially beneficial for EH treatment (Morel and Godfraind, 1987). Nevertheless, despite the availability of such potent antihypertensive drugs, only one in every four women and one in five men with EH attain their treatment objectives (Ncd-RisC, 2021).
Traditional Chinese Medicine (TCM) offers a unique therapeutic perspective, especially with its holistic stance and syndrome differentiation-centric treatments. Leveraging antihypertensive Chinese patent medicine tailored to specific syndromes can efficiently regulate blood pressure and relieve related symptoms. For instance, Ilex hainanensis Merr., also known as Shan-Lv medicine, when paired with antihypertensive drugs, exhibits enhanced efficacy in blood pressure reduction than standalone drug treatments (Yang et al., 2018). Similarly, the Songling Xuemaikang Capsule combined with conventional medicine has demonstrated clear efficacy and safety for EH management (Meng et al., 2022). Over the years, pharmacological management of hypertension has advanced. In the management of EH, combining Chinese patent medicines with western medicines provides superior efficacy and safety compared to conventional treatment alone. Clinical studies have endorsed the blending of traditional Chinese medicine with conventional medicine in treating EH, underscoring mutual benefits, sustained pressure-relief effects, minimized adverse reactions, and fewer cardiovascular complications (Chen et al., 2013). CCBs play an important role in the treatment of EH as CCBs is one of the first recommended antihypertensive drugs. The combination of Chinese patent medicines with CCBs has been extensively utilized as an alternative treatment strategy for essential hypertension (EH) and dizziness in China. This strategy could improve the traditional Chinese medicines symptoms of EH patients, including dizziness, impetuosity, insomnia, tinnitus, etc., which is particularly important for improving the quality of life of EH patients (Lin et al., 2023).
Though numerous hypertension guidelines promote combination pharmacotherapy, solid evidence elucidating the clinical effectiveness and safety of Chinese patent medicine when integrated with CCBs for EH treatment remains scarce. Past meta-analyses have typically compared only two treatment methodologies, failing to provide a comprehensive overview of potential synergies between various Chinese patent medicines and CCBs (Yongcheng et al., 2022; Tong et al., 2023). An extant network meta-analysis did not distinctly categorize the conventional medicines, hindering its clinical application (Zhaochen et al., 2022).
Network meta-analysis (NMA) amalgamates both direct and indirect evidence, offering estimates for every treatment pair (Mavridis, 2019). It serves clinicians by ranking interventions based on their efficacy for each assessed outcome (Nikolakopoulou et al., 2020). This research aims to offer an exhaustive systematic review of RCTs focused on the synergy of Chinese patent medicine and CCBs in EH treatment, strictly adhering to the PRISMA 2020 and PRISMA-NMA guidelines (Hutton et al., 2015; Page et al., 2021). By employing the Bayes network meta-analysis, we aspire to gauge the efficacy and safety of different Chinese patent medicine-CCBs combinations, thereby offering robust evidence to guide clinical decisions.
All relevant randomized controlled trials (RCTs) including one or more interventions were identified through extensive searches of databases including PubMed, Cochrane Library, Web of Science, ClinicalTrials.gov, CNKI, WANFANG, VIP, and Sinomed. The timeline for the search spanned from the inception of each database up to 1 February 2023. To ensure a thorough examination of studies related to CCBs, the search began with a broad focus on RCTs that explored the integrative use of Chinese patent medicine with conventional medicine. The subsequent screening refined the results to spotlight studies specifically utilizing CCBs as the antihypertensive treatment. The primary search criteria were defined as: (subject = hypertension OR essential hypertension) AND (subject = Chinese and Western medicine OR Chinese patent medicine OR capsule OR tablet OR scatter OR pill OR ointment OR dan OR dropping pill OR granule OR oral liquid). For a more comprehensive retrieval of relevant studies, synonym expansion was also incorporated into the search formula.
We did meta-analysis and bayes network meta-analysis, which means the integration of direct and indirect comparisons, to compare 6 types of Chinese patent medicine for essential hypertension. Our analysis included studies of people with essential hypertension. Because multiple-treatments meta analysis require a reasonably homogeneous sample, we excluded RCTs done in patients with severe cardiovascular, cerebrovascular, and renal diseases; patients with special types of hypertension, such as gestational hypertension, menopausal hypertension, H-type hypertension, etc.
Studies selected were required to be peer-reviewed, published in either Chinese or English, and to employ a RCT design focusing on patients diagnosed with essential hypertension. Interventions assessed combinations of Chinese patent medicine and CCBs, while control groups received only CCBs. The literature we excluded including unable to obtain full text or incomplete information.
Outcomes were chosen based on the《Guidelines for clinical application of Chinese patent medicine in treating essential hypertension》and subsequently, RCTs were reviewed. Primary outcomes comprised Systolic and Diastolic Blood Pressure (SBP and DBP), antihypertensive effective rate, and adverse drug reaction events. If data from this scale were not available, SBP& DBP or antihypertensive effective rate is essential. Secondary outcomes were Traditional Chinese Medicine Syndrome Score (TCM syndrome score), Total Cholesterol (TC), Triglyceride (TG), Low-Density Lipoprotein Cholesterol (LDL-C), Quality of Life Score, Tumor Necrosis Factor-α (TNF-α), Interleukin-6 (IL-6), and Vascular Endothelin-1 (ET-1). Notably, outcomes such as high-density lipoprotein, cardiac function, and others were excluded owing to insufficient comparative data. Because bayes network meta-analysis requires reasonable homogeneity we focused on 8-week duration, and if this is not available, we used data from between 4 and 12 weeks (closest to 8 weeks).
Data from each study were meticulously extracted, encompassing study details (authorship and year of publication), participant demographics (group classifications, sample size, age), interventions (specific drug names and dosing durations), and a broad spectrum of outcomes including measures like SBP, DBP, TCM syndrome score, and various biochemical markers. Outcomes with continuous data were represented by both pre-intervention and post-intervention means and standard deviations, while dichotomous data were tabulated in 2 × 2 formats. To ensure accuracy and objectivity, two independent reviewers undertook the extraction. In cases of disagreement, resolution was sought with the intervention of a third reviewer. Subsequent to extraction, data were reformatted for compatibility with the R package’s requirements.
The quality of the selected literature was evaluated using the ROB2 tool from the Cochrane Collaborative Network. Two researchers independently carried out the assessment and cross-checked their evaluations. In cases of disagreement, a third researcher made the decision. The ROB2 tool focuses on assessing potential risks of bias in these areas: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selective reporting of outcomes (Sterne et al., 2019). Bias arising from the randomization process is about whether the allocation sequence was random, adequately concealed, or baseline differences between intervention groups suggest a problem with the randomization process. Bias due to deviations from intended interventions included whether participants, carers and people were aware of participants’assigned intervention during the trial. The purpose of this domain is also to address if applicable deviations from the intended intervention are a result of experimental context in addition to the effect of assignment to intervention. Bias due to missing outcome data is about whether data for this outcome were available for all, or nearly all, participants randomized, (if applicable) the result was not biased by missing outcome data; (if applicable) missingness in the outcome was likely to be influenced by its true value. Bias in measurement of the outcome is about whether the method of measuring the outcome was inappropriate; different intervention groups could used different mesurement or ascertainment for outcome; outcome assessors were aware of the intervention received by study participants; (if applicable) assessment of the outcome was likely to have been influenced by knowledge of intervention received. And at last, bias in selection of the report result addressed whether the trial was analysed according to a pre-specified plan which was finalized before unblinded outcome data were available for analysis; the numerical result being assessed may have been selected, on the basis of the results, from multiple outcome measurements within the outcome domain; the numerical result being assessed is likely to have been selected, on the basis of the results, from multiple analyses of the data. The response options are yes, probably yes, probably no, no, no information. Some signalling questions were logically related each other, i.e., it may be possible to skip next question because an option was selected for a previous signalling question; if a signalling question was skipped due to this logic setting, it was noted as not applicable (NA). If the risk of bias assessment results of all domains were “low risk”, then the overall risk of bias was “Low” risk. If the risk of bias evaluation results of some areas were “Some concers” risk and there was no area of “High” risk, then the overall risk of bias was “Some concers” risk. Whenever there was an area where the risk of bias was assessed as “High” risk, the overall risk of bias is “High” risk. Up-to-date information from the developers on RoB 2 and more detail is available via the Risk of Bias tools website: www.riskofbias.info and Cochrane Scientific Committee.
For each source of bias, studies were categorized as having a high, low, or some concerns risk based on, and summarise the answers to signalling questions (Higgins and Page, 2019).
This study conducted a NMA according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 and PRISMA-Network Meta-Analyses (PRISMA-NMA) guidelines (Hutton et al., 2015; Page et al., 2021). Bayes network meta-analysis was used to evaluate the efficacy and safety of various Chinese patent medicine combined with CCBs, aiming to inform clinical decisions.
The initial phase entailed the design of multiple network geometries to decipher the comparative dynamics between different medications, each integrated with distinct Chinese patent medicines. Subsequently, a consistency analysis juxtaposed direct and indirect therapeutic effect estimates. Given the absence of closed-loop networks in this research, only indirect comparative estimates apply. Anticipating study heterogeneity, we deploy a random-effect model.
We focused on constructing a Bayes model for an NMA concerning primary and secondary outcomes. We guided the model by four Markove chains on R software to perform NMA. When calculating the effect size, dichotomous data was expressed as odds ratio (OR), continuous variables were expressed as mean difference (MD), bayes network meta-analysis set 95% credible interval (CrI). Using I2 to test heterogeneity, and p < 0.05 was considered statistically significant.
Gelman-Rubin-Brooks plots were conducted to examine the convergence in diagnostic model combining interval-based graphical evaluation and quantitative analysis of PSRF. After n iterations, the curve was formed and observed whether the curve is fit with each other and kept stable. A Potential Scale Reduction Factor (PSRF) tended to 1.00 indicating that the degree of convergence was satisfactory (Jiahao et al., 2021), otherwise increase the number of iterations to achieve model convergence. When calculating the rank probability intervention the parameter preferred direction was equal to 1 means a higher value which indicates a better result in the antihypertensive effective rate and the quality of life score. Other outcomes took preferred direction equaling to −1 to indicate a lower value which means better results.
Trace plot was used to test whether the Markov chain reach stable and overlap during the caluation proces. It always showed the fluctuation process of Markove chain during the iterative computation process, which can be expressed in different forms depending on the number of iterations and preset distributions (Dodds and Vicini, 2004; Toft et al., 2007).
We conducted density plots to evaluate the consistent. Density plots were based on a predefined distribution, and after numerical simulation, the distribution of the a posteriori values is observed to see if it is consistent with the predefined distribution. The bandwidth value be used as a quantitative assessment. And the smaller the bandwidth, the smaller difference between distribution range of the parameter a posteriori values and the preset distribution range. After enough iterations, the Bandwidth tends to 0 and stabilizes (Harpole et al., 2014).
Then the efficacy of the interventions was compared, and then they were ranked. The Surface Under the Cumulative Ranking (SUCRA) score, visualized through a heatmap, offered a perspective on the probable efficacy of an intervention based on the ranking of all interventions. A SUCRA of x% meant that the drug achieves x% of the effectiveness of this imaginary drug, thus larger SUCRAs denoted more eff ective interventions (Leucht et al., 2013).
We conducted sensitivity analyses on the primary outcome to explore potential reasons for heterogeneity or inconsistency. Leave-one-out sensitivity analysis was used by metafor package in R software to perform a “leave-one-out” function, which examined the robustness of the results by repeatedly fitting the specified model, omitting one study at a time. The size of the forest plot squares was plotted in proportion to the sample size of each RCT to see which RCT carried more weight.
Subgroup analyses were used to examine whether there are significant differences in the effectiveness of interventions between different subgroups to explore which interventions are more effective. Contour-enhanced funnel plot was drawn to detect publication bias due to the suppression of non-significant findings (Peters et al., 2008). Plot of influence diagnostics was drawn to compute outlier and influential case diagnostics, which include externally standardized residuals, DFFITS values, Cook’s distances, covariance ratios, leave-one-out estimates of the amount of heterogeneity, leave-one-out values of the test statistics for heterogeneity, hat values, and weights (Viechtbauer and Cheung, 2010).
The network meta-analyses were facilitated by the netmeta and gemtc packages (Gert van Valkenhoef, 2021; Guido Schwarzer, 2023). All statistical evaluations are conducted in the R statistical environment (R 4.2.2, www.r-project.org).
An initial search of the literature database yielded 75,869 documents. After removing duplicates in Endnote, Prisma flow diagramand helped to identify RCTs for the remaining 70,756 records. 64,958 records were rejected as animal and cell experiment studies, and 5,798 papers were left. Upon reviewing the titles and abstracts, 173 articles that matched the study criteria were shortlisted. A thorough reading of the full text and further screening based on the inclusion criteria left 78 relevant Chinese papers. Studies with fewer than 2 outcomes were excluded, leaving 69 papers. Out of these, 36 articles focusing on the combination of Chinese patent medicine with CCBs were ultimately selected for this study. The detailed screening process is illustrated in Figure 1.
A total of 3,740 patients participated in studies involving six distinct types of Chinese patent medicine. These medicines include: Songling Xuemaikang Capsule (SLXM), Qiangli Dingxuan Tablet (QLDX), Tianma Gouteng Granule (TMGT), Qiju Dihuang Pill (QJDH), Qinggan Jiangya Capsule (QGJY), and Xinmaitong Capsule (XMT). The essential characteristics of the studies under consideration are detailed in Table 1. Exact information about Chinese patent medicines in our studies can be found in Supplementary Material S1. All the plant names have been checked with http://www. worldfloraonline.org mentioning on 25 May 2023.
Domain 1 assessed the bias stemming from the randomization process. Out of 36 articles, 10 (Gao and Jin, 2013; Zhou, 2013; Li, 2017; Yuan, 2017; Yu, 2019; Liu et al., 2020; Wang, 2020; Liu, 2021; Ye et al., 2021) used the term “random” to describe their stratified block randomization method without more details. Furthermore, one study (Hao et al., 2020) employed a stratified random method. Both these approaches were categorized as having a “Low” risk of bias. 8 (Jia and Liu, 2004; He et al., 2013; Han, 2014; Huang and Fu, 2014; Sun, 2015; Xin and Lin, 2016; Sun, 2019; Qiu et al., 2021) had “High” risk because of the bias arsing from the randomization process. Other 17 literature were “Some concerns” risk.
The assessment result of domain 2 showed that 17 (Du et al., 2009; Zhao, 2012; Gao and Jin, 2013; Liu, 2013; Zhou, 2013; Zhang et al., 2014a; Zhang, 2014; Hang et al., 2018; Huang et al., 2018; Jiao et al., 2018; Xiong, 2018; Liu et al., 2019; Wang, 2019; Yu, 2019; Hao et al., 2020; Ye et al., 2021; Wang et al., 2022) were “Low” risk, the other 17 literature (Jia and Liu, 2004; Wang, 2011; He et al., 2013; Han, 2014; Huang and Fu, 2014; Sun, 2015; Zhu et al., 2015; Li, 2017; Yan, 2017; Yuan, 2017; Chen and Qiu, 2018; Sun, 2019; Du et al., 2019; Liu et al., 2020; Wang, 2020; Liu, 2021; Qiu et al., 2021) were “Some concerns” risk, and the rest were “High” risk.
Assessments for the domain “bias due to missing outcome data” differed according to the integrity and missingness of the data, and the result showed that 33 (Du et al., 2009; Wang, 2011; Zhao, 2012; Gao and Jin, 2013; He et al., 2013; Liu, 2013; Zhou, 2013; Zhang et al., 2014a; Zhang et al., 2014b; Han, 2014; Huang and Fu, 2014; Zhang, 2014; Sun, 2015; Zhu et al., 2015; Xin and Lin, 2016; Li, 2017; Yan, 2017; Chen and Qiu, 2018; Hang et al., 2018; Huang et al., 2018; Jiao et al., 2018; Xiong, 2018; Du et al., 2019; Liu et al., 2019; Wang, 2019; Yu, 2019; Hao et al., 2020; Liu et al., 2020; Wang, 2020; Liu, 2021; Qiu et al., 2021; Ye et al., 2021; Wang et al., 2022)were “Low” risk, 3 (Jia and Liu, 2004; Yuan, 2017; Sun, 2019) were “Some concerns” risk.
The assignment to the measurement of the outcome showed 23 (Du et al., 2009; Wang, 2011; Zhao, 2012; Gao and Jin, 2013; Zhou, 2013; Zhang et al., 2014a; Han, 2014; Zhu et al., 2015; Li, 2017; Yan, 2017; Hang et al., 2018; Huang et al., 2018; Xiong, 2018; Du et al., 2019; Liu et al., 2019; Wang, 2019; Yu, 2019; Hao et al., 2020; Liu et al., 2020; Wang, 2020; Liu, 2021; Ye et al., 2021) with “Low” risk, 6 (Liu, 2013; Zhang et al., 2014a; Xin and Lin, 2016; Yuan, 2017; Chen and Qiu, 2018; Jiao et al., 2018) with “Some concerns” risk and 7 (Jia and Liu, 2004; He et al., 2013; Huang and Fu, 2014; Zhang, 2014; Sun, 2015; Sun, 2019; Qiu et al., 2021)with “High” risk.
Domain 5 was about the bias in selection of the reported result with 5 (Yan, 2017; Hao et al., 2020; Wang, 2020; Liu, 2021; Ye et al., 2021) “Low”, 29 (Jia and Liu, 2004; Du et al., 2009; Wang, 2011; Zhao, 2012; Gao and Jin, 2013; Liu, 2013; Zhou, 2013; Zhang et al., 2014a; Zhang et al., 2014b; Han, 2014; Huang and Fu, 2014; Sun, 2015; Zhu et al., 2015; Xin and Lin, 2016; Li, 2017; Yuan, 2017; Chen and Qiu, 2018; Hang et al., 2018; Huang et al., 2018; Jiao et al., 2018; Xiong, 2018; Du et al., 2019; Liu et al., 2019; Wang, 2019)"Some concerns” and 2 (He et al., 2013; Zhang, 2014) “High” risk. At last, overall bias with 10 (Gao and Jin, 2013; Zhou, 2013; Li, 2017; Yu, 2019; Hao et al., 2020; Liu et al., 2020; Wang, 2020; Liu, 2021; Ye et al., 2021; Wang et al., 2022) “Low” risk, 17 (Du et al., 2009; Wang, 2011; Zhao, 2012; Liu, 2013; Zhang et al., 2014a; Han, 2014; Zhu et al., 2015; Yan, 2017; Yuan, 2017; Chen and Qiu, 2018; Hang et al., 2018; Huang et al., 2018; Jiao et al., 2018; Xiong, 2018; Du et al., 2019; Liu et al., 2019; Wang, 2019)"Some concerns” risk and 9 (Jia and Liu, 2004; Zhang et al., 2014b; Huang and Fu, 2014; Zhang, 2014; Sun, 2015; Xin and Lin, 2016; Sun, 2019; Qiu et al., 2021)"High” risk which was an overall judgment for the result from the above 5 areas. More information was available in Supplementary Material.
Based on the evaluations across the aforementioned five domains, the results were determined in line with the ROB2 operational logic. Specifically, 30.56% of the studies exhibited low risk, 47.22% presented medium risk, and 22.22% had high risk. These findings were depicted in Figure 2. For a more detailed risk of bias assessment for the RCTs, please refer to Table 2 and Table 3.
A paired meta-analysis was conducted on the included studies using a fixed-effect model. The analysis indicated that the combination of Chinese patent medicine with CCBs yields greater efficacy in enhancing the antihypertensive effective rate compared to CCBs alone. This difference was statistically significant, as illustrated in Figure 3. To assess publication bias for the primary outcome’s effective rate, a funnel plot was generated. The plot revealed that most data points cluster around the center and top but display asymmetry, suggesting potential publication bias, as presented in Figure 4.
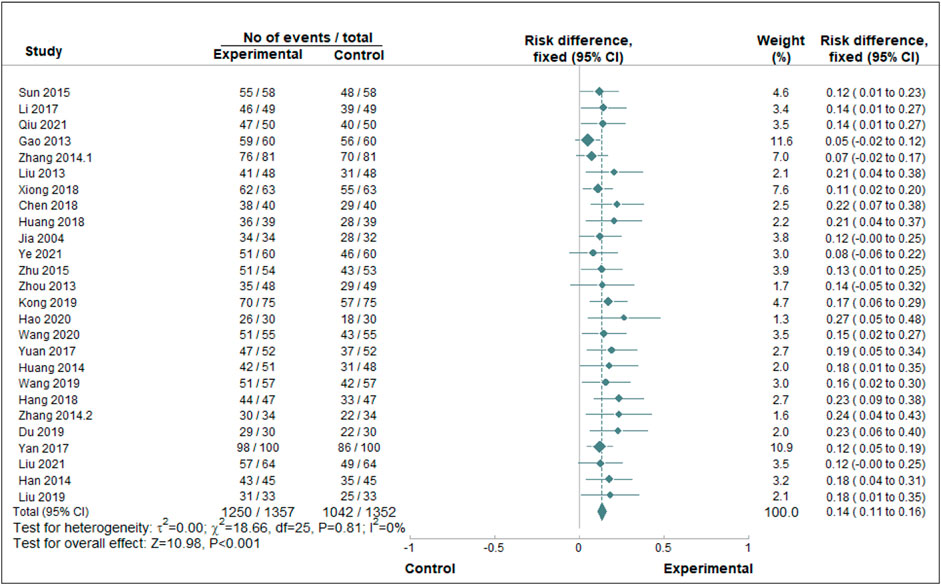
FIGURE 3. Paired meta-analysis of the antihypertensive effective rate. Notes: Chinse patent medicine + CCB vs. CCB.
An evidence network diagram was constructed to represent the 12 types of outcomes for 6 Chinese patent medicines combined with CCBs antihypertensive medications. Outcomes with consistent literature information were merged into a single diagram. Edges in the diagram were thicker when more RCTs are included. Points were larger when there’s a greater number of patients involved in those studies. All evidence networks and corresponding data are presented in Figure 5.
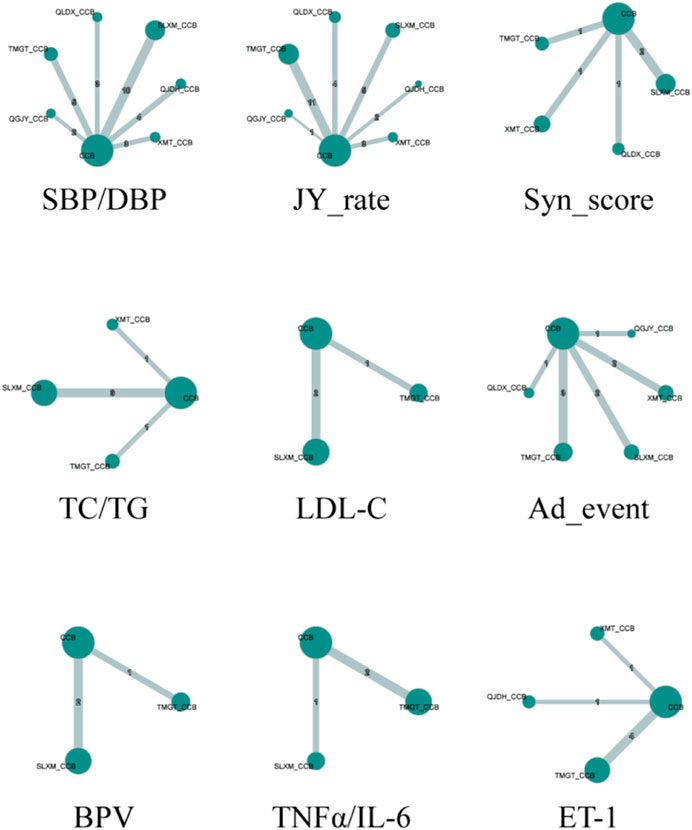
FIGURE 5. Network plot for the antihypertensive effective rate. Notes: SBP/DBP:Systolic blood pressure/Diastolic blood pressure; JY_rate: Rate of blood pressure decreases; Syn_score: TCM Syndrome score; TC/TG: Total cholesterol/Triglycerides; LDL-C: Low-density lipoprotein cholesterol; Ad_event: Adverse events; BPV: Blood pressure variability; TNFα/IL-6: tumor necrosis factor α/Interleukin- 6; ET-1: Endothelin-1.
Utilizing the gemtc package, we built a Bayesian network model with the antihypertensive effective rate reduction as our focal point. After 5,000 preliminary iterations, the observed fluctuations in each Markov chain were minimal, signifying satisfactory convergence of the model. Consequently, there was no necessity to augment the calculation coefficient. Post the 5,000 iterations, the PSRF for every group rapidly converged to 1. In conjunction with the gemtc software, the calculated PSRF for all groups equaled 1.00. This indicated that the computational outcomes between distinct chains are consistent. The level of convergence was deemed satisfactory, ensuring that the established Bayesian model can effectively forecast subsequent results. These findings are illustrated in Figures 6, 7; Supplementary Figure S7; Supplementary Figure S8.
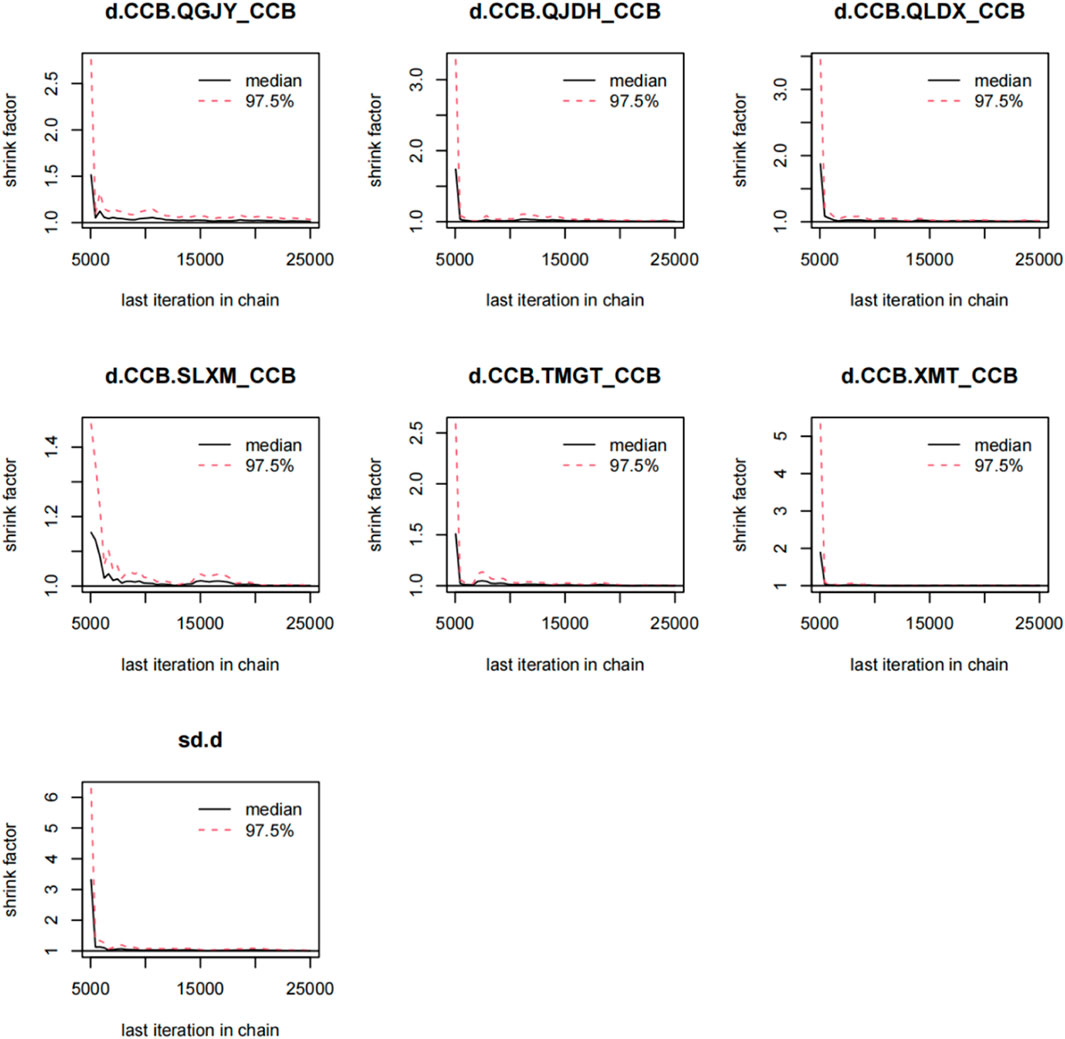
FIGURE 6. Gelman-Rubin-Brooks plot for rate of blood pressure decreases. Notes:Chinese patent drugs + CCB vs. CCB.
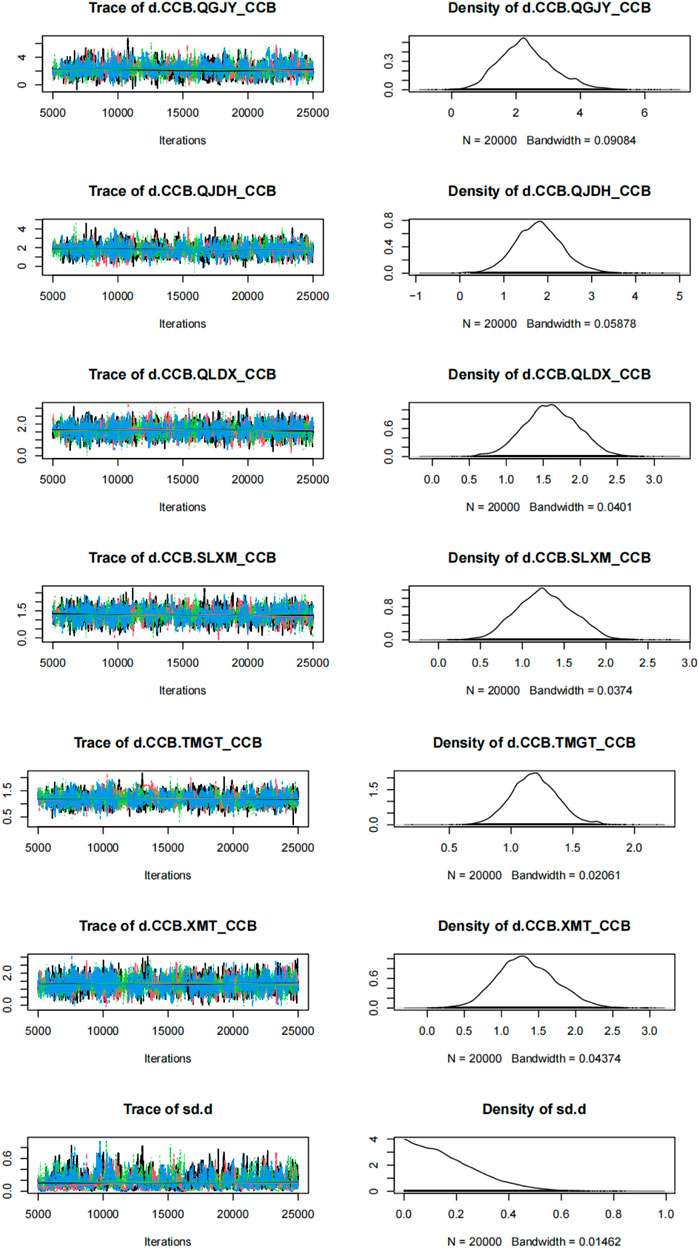
FIGURE 7. Time-series and density plots for rate of blood pressure decreases. Notes: Chinese patent drugs + CCB vs. CCB.
Our study, depicted in Figure 5, evaluated the impact of CCBs with various Chinese patent medicines on systolic blood pressure (SBP). The comprehensive results of the network meta-analysis are as follows:
QGJY [MD = −10.93, 95%CrI (−17.28,-4.62)], QJDH [MD = −7.3, 95%CrI (−11.91, −2.68)],QLDX [MD = −10.67,95%CrI(-16.06,-5.19)],SLXM [MD = −10.28,95%CrI(-13.25,−7.3)],TMGT [MD = −12.43,95%CrI(-16.86,−8.08)],XMT [MD = −6.99,95%CrI(-12.39,-1.56)]. These data are visualized in Figure 8. Each of the aforementioned combinations showcased a superior antihypertensive effect compared to the sole administration of CCBs, with statistical significance at p < 0.05.
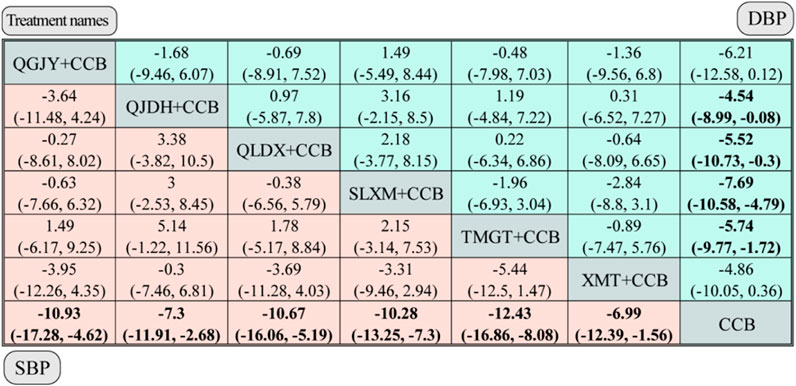
FIGURE 8. Network meta-analysis for SBP and DBP. Notes: different Chinese patent medicine + CCB vs. CCB. The comparison between different interventions is represented by MD and 95%CrI, and the ones in bold mean p < 0.05.
Further insights are provided by the Surface Under the Cumulative Ranking (SUCRA) scores, which indicate the probable efficacy of each combination in reducing SBP. Tianma Gouteng Granule combined with CCB (TMGT + CCB) emerged as the most likely superior intervention, boasting an 83.99% score. In terms of efficacy, the ranking of the Chinese patent medicine combined with CCB is as follows:
TMGT + CCB (83.99%) > QGJY + CCB (68.51%) > QLDX + CCB (66.73%) > SLXM + CCB (63.29%) > QJDH + CCB (34.06%)>XMT + CCB (33.26%). These comprehensive rankings and data are visualized in Figure 9 and Supplementary Material.
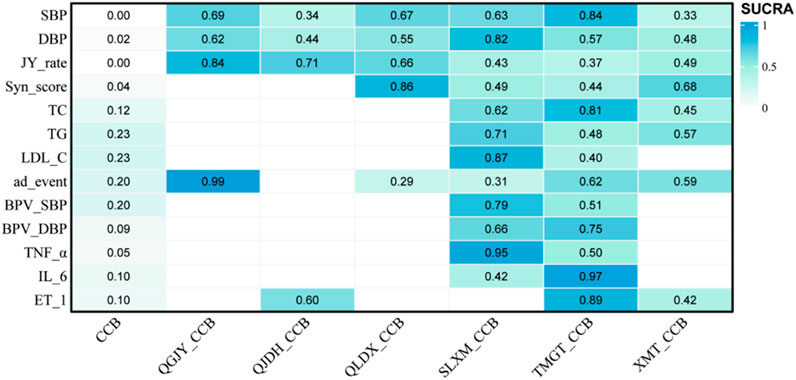
FIGURE 9. SUCRA score heat map of outcomes. Notes: SBP: systolic blood pressure; DBP: diastolic blood pressure; JY_rate: antihypertensive effect rate; Syn_score: TCM syndrome score; TC: total cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; ad_event: adverse reactions/events; BPV_SBP: systolic blood pressure variability; BPV_DBP: diastolic blood pressure variability; TNF_α: tumor necrosis factor-α; IL_6: interleukin-6; ET_1: vascular endothelin-1.
Table 4 shows combined treatments based on outcomes, analyzed in conjunction with SUCRA.
The network meta-analysis, depicted in Figure 5, assessed the impact of various Chinese patent medicines combined with CCBs on diastolic blood pressure (DBP). Here’s a breakdown of the findings: QJDH [MD = −4.54, 95%CrI(−8.99, −0.08)], QLDX [MD = −5.52, 95%CrI(-10.73, −0.3)], SLXM [MD = −7.69, 95%CrI (−10.58, −4.79)], TMGT [MD = −5.74, 95% CrI (−9.77, −1.72)]. These data are visualized in Figure 8.
However, two combinations, namely, QGJY [MD = −6.21, 95%CrI (−12.58, 0.12)] and XMT [MD = −4.86, 95%CrI (−10.05, 0.36)], did not show a statistically significant improvement over using CCB alone. Despite this, most of these combinations indicated a statistically significant superior antihypertensive effect compared to using only CCB (p < 0.05).
A deep dive into the SUCRA scores provided insights into the probable efficacy of each combination in reducing DBP. The results spotlighted SLXM + CCB as the most promising intervention with a score of 82.44%. In terms of efficacy, the Chinese patent medicines combined with CCB are ranked as:
SLXM + CCB(82.44%)>QGJY + CCB(62.17%)>TMGT + CCB(57.41%)>QLDX + CCB(54.85%)>XMT + CCB(47.76%)>QJDH + CCB (43.56%). These comprehensive findings and rankings are visualized in Figure 9.
As presented in Figure 10, the network meta-analysis delved into the efficacy of various Chinese patent medicines when combined with calcium channel blockers (CCBs) to improve antihypertensive effective rates. Here are the key findings:
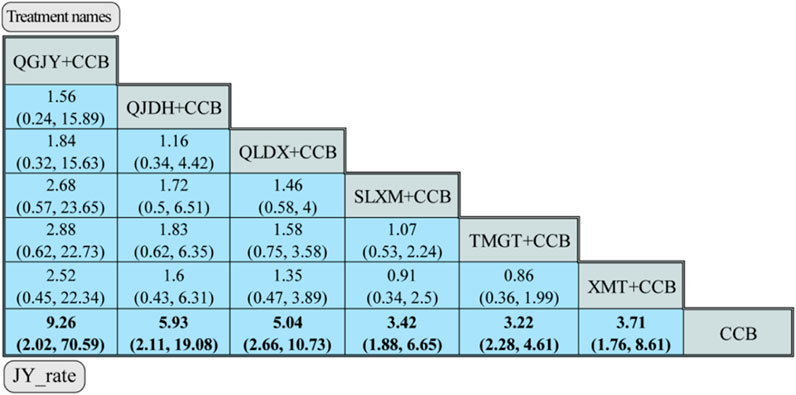
FIGURE 10. Network meta-analysis for JY_rate. Notes: different Chinese patent medicine + CCB vs. CCB. The comparison between different interventions is represented by MD and 95%CrI, and the ones in bold mean p < 0.05.
QGJY [OR = 9.26, 95% CrI (2.02, 70.59)], QJDH [OR = 5.93, 95% CrI (2.11, 19.08)], QLDX [OR = 5.04, 95%CrI (2.66, 10.73)], SLXM [OR = 3.42, 95%CrI (1.88, 6.65)], TMGT [OR = 3.22, 95%CrI (2.28, 4.61)], XMT [OR = 3.71, 95% CrI (1.76, 8.61)]. These combinations showcased a statistically significant advantage in antihypertensive efficacy when compared to using only CCB (p < 0.05).
The SUCRA scores further shed light on the probable efficiency of each combination in enhancing antihypertensive rates. Notably, QGJY + CCB emerged as the top contender with a remarkable score of 84.48%. Here’s the hierarchy based on efficacy:
QGJY + CCB (84.48%) >QJDH + CCB (70.89%) > QLDX + CCB (66.34%)>XMT + CCB (48.69%)>SLXM + CCB (42.62%)> TMGT + CCB (36.87%). These insightful findings are vividly portrayed in Figure 9.
See the secondary outcomes in Supplementary Material.
Safety is paramount when evaluating the efficacy of any medical treatment. In our analysis, 10 out of the total studies discussed potential adverse drug reactions/events. There was no significant difference in the rage of adverse events with no heterogeneity (Supplementary Figure S14). The most common adverse events included facial blushing, nauseous, palpitations, dizziness.
However, one of the challenges in consolidating these findings was the varied criteria each study used to determine and classify these adverse reactions/events. Due to these inconsistencies and to ensure clarity, we’ve opted for a descriptive analysis approach rather than a quantitative one.
For a detailed breakdown of the reported adverse reactions/events from each of these 10 studies, please refer to Table 5. This table provides insight into the type, frequency, and severity of reactions, giving both researchers and medical professionals a clearer picture of the safety profile of these treatments.
Using the leave-one-out sensitivity anlysis, we have revealed and examined that no single study altered the pooled effect of RR and MD. Most of the summary results remained the same after systematically excluding specific studies one bye one proving the robustness of our results. Additional results of sensitivity analyses of SBP, DBP, antihypertensive and adverse rate were provided in Supplement.
As the amount of data for subgroup analysis was insufficient, subgroup analysis in our study were conducted of SBP, DBP and antihypertensive effective rate. Specific of Chinese patent medicines included SLXM, TMGT, QLDX, QGJY, QJDH and XMT. Statistically significant differences were observed between subgroups, and heterogeneity was reduced in the subgroups (Supplementary Material), suggesting that the type of Chinese patent medicines may be the source of heterogeneity. Other results of the bias analysis were presented in Supplementary Material.
The global implications of elevated blood pressure are concerning. Among the 3.5 billion adults worldwide, blood pressure levels in many are below the optimal range. Alarmingly, about 874 million adults have systolic blood pressure exceeding 140 mmHg, translating to nearly one-quarter of the global adult population grappling with EH (1). The underpinnings of abnormal blood pressure can primarily be attributed to irregular blood pressure regulation. Ensuring physiological blood pressure requires the synchronized functioning of various elements within an intricate neurohumoral system. This includes factors such as the renin-angiotensin-aldosterone system, natriuretic peptides, endothelium, sympathetic nervous system, as well as inflammation and immune responses (Oparil et al., 2018).
Modern medicine has recognized the importance of vascular tone in regulating blood pressure and distributing blood flow within the body. Among the therapeutic arsenal, CCBs are particularly effective for hypertensive vessels, owing to their specificity to increased inactivated channels and the presence of a greater number of these channels (Morel and Godfraind, 1987). Beyond mere blood pressure control, CCBs also play a pivotal role in preventing the structural changes induced by EH in both the heart and arteries.
Complementing this modern understanding, TCM provides a unique perspective. While EH is not directly named in TCM, its clinical manifestations align with symptoms described as “dizziness” and “headache”. The TCM diagnosis points to various pathogenic factors, such as hyperactivity of liver yang, syndrome of static blood blocking collaterals, liver wind stirring, and kidney essence deficiency. Guided by its foundational principles of holistic treatment and syndrome differentiation, TCM emphasizes tailored treatments. Consequently, antihypertensive Chinese patent medicines, when prescribed in line with syndrome differentiation, have demonstrated efficacy in reducing blood pressure and alleviating clinical symptoms.
Recognizing and addressing EH is paramount, not just for individual health, but from a broader public health perspective. As supported by a 2009 meta-analysis, optimal management of EH, whether through modern antihypertensive drugs or TCM approaches, is instrumental in curtailing cardiovascular risks and fostering longevity in the global populace (Law et al., 2009).
Building on our understanding of the global prevalence of EH and the synergy of modern and traditional therapeutic approaches, it is essential to delve into the efficacy of combined treatments.
In the paired meta-analysis, our data presents compelling evidence supporting the enhanced therapeutic efficacy of combining Chinese patent medicine with CCBs in addressing EH. Specifically, when compared to the CCB-only group, the combined treatment approach exhibited a notably improved curative effect (RD = 0.14, 95%CI = 0.11, 0.16). Remarkably, this outcome demonstrated minimal heterogeneity (I2 = 0%).
Broadening our lens to the network meta-analysis, further differentiations emerge regarding the efficacy of various combinations:
SBP Reduction: Tianma Gouteng Granule combined with CCB stands out in its efficacy.
DBP Reduction: The combination of Songling Xuemaikang Capsule with CCB takes the lead.
Antihypertensive Efficacy: Qinggan Jiangya Capsule, when combined with CCB, showcases superior results.
TCM Syndrome Score: Patients treated with Qiangli Dingxuan Tablet combined with CCB observed the most significant reduction.
Lipid Profile Improvements: While Tianma Gouteng Granule and CCB excelled in reducing TC, the pairing of Songling Xuemaikang Capsule with CCB emerged as more potent in lowering LDL-C.
Inflammatory Marker Reduction: In terms of mitigating TNF-α and IL-6 levels, the combination of Songling Xuemaikang Capsule with CCB proved most effective.
It is crucial to note that the number of RCTs concerning secondary clinical outcomes was limited, which may impact the robustness of comparisons in those areas.
These insights underscore the importance of nuanced, individualized therapeutic strategies. The differential efficacy outcomes of the varied combinations offer clinicians a spectrum of options, allowing them to tailor EH management according to patient-specific needs, bridging the time-tested wisdom of TCM with the precision of modern pharmacology.
Having highlighted the comparative efficacy of combined treatments for EH, it becomes imperative to probe deeper into the mechanisms and metabolites of key TCM capsules that stood out in the meta-analysis. This can provide insights into how these traditional remedies potentiate the effects of modern drugs like CCBs.
Xinmaitong Capsule: This capsule is known for promoting blood circulation, clearing blood stasis, and nourishing the heart. This intricate formulation boasts a blend of medicinal botanical drugs, including but not limited to, Angelica sinensis (Oliv.) Diels, Salvia miltiorrhiza Bunge, Ilex pubescens Hook. and Arn., and Pueraria lobata (Willd.) Ohwi. Research by Wei Chengke (Wei et al., 2017) showed that this oil can protect vascular cells and help reduce blood pressure. Another significant metabolite, Tanshinone ⅡA from S. miltiorrhiza Bunge, aids in blood circulation (Li, 2017). Li Wendi’s study (Li and Sun, 2021)further highlighted its ability to regulate blood pressure, possibly by interacting with certain cellular pathways.
Tianma Gouteng Granule: Recognized for its calming and heat-clearing properties, its primary botanical drugss are Gastrodia elata Bl. and Uncaria rhynchophylla (Miq.) Miq., from Gastrodia, has potent antihypertensive effects (Chen et al., 2016). It has been shown to counteract certain vasoconstricting agents and promote vasodilation, leading to reduced blood pressure (Shan et al., 2017). Additionally, Cheng Xiankun’s research (Cheng et al., 2019) highlighted rhynchophylline’s capacity to optimize internal circulation and manage blood pressure in hypertensive model rats.
Songling Xuemaikang Capsule: This capsule calms the liver and subsiding yang, tranquillizing with heavy prescription, promotes blood circulation, and removes blood stasis. Comprising mainly of Pinea Wolf and Pueraria lobata (Willd.) Ohwi, etc., clinical studies have shown its efficacy in treating mild hypertension, with the potential to replace control drugs (Lai et al., 2022). Its mechanism involves regulating the RAAS pathway (Liu et al., 2015), modulating gene expression (Zhao et al., 2013), and safeguarding vascular endothelial cells by inhibiting specific cellular pathways. It further mitigates oxidative stress injuries in rat aorta through modulating CAV1 and IGF1R gene expressions (Shi et al., 2018).
Qinggan Jiangya Capsule: Known for its liver-calming and heat-clearing effects, its main botanical drugs are Polygonum muliflorum Thunb. and S. miltiorrhiza Bunge. Wang Xiayun’s study (Wang et al., 2017) emphasized its role in vascular dilation and reducing specific vascular growth factors. Liang Yanfei (Liang et al., 2021) further showcased its ability to decrease blood pressure while ensuring liver, kidney, and heart functions remain intact.
Qiangli Dingxuan Tablet: This tablet is designed to calm the liver wind. Main botanical drugs include G. elata Blume and Eucommia ulmoides Oliv., which have numerous antihypertensive metabolites like lignans and phenylpropanoids. Research has indicated that these metabolites can work through various pathways to manage blood pressure, such as inhibiting specific enzymes and combating vasoconstriction (Taubert et al., 2002; Gu et al., 2011).
Past meta-analyses have compared Chinese patent medicines combined with western medicines in the treatment of EH, which showd significant reduction in both SBP and DBP compared to western medicines. There were significant beneficial effects in SLXM combined with antihypertensive drugs compared to the antihypertensive drugs using alone. However, no compelling evidence was found to demonstrate its superior efficacy compared to other Chinese patent medicines. RCTs about QLDX for the treatment of EH showed that SBP and DBP were significantly lower than in the placebo group (Yang et al., 2015; Ji et al., 2022; Zhang et al., 2022; Lin et al., 2023). While our results showed that TMGT and SLXM combined with CCB showed more effective in our NMA, and QLDX played an important role in reducing TCM Syndrome Score.
In furnishing healthcare professionals with a solid, evidence-based framework for treatment decision-making, the research challenges the prevailing one-size-fits-all medical paradigm, advocating for an alignment more intimately connected with the foundational doctrines of TCM. Based on our evidence, clinicians choose the most appropriate treatment plan based on the patient’s specific situation, enabling individualized medicine. The ideal integration of Chinese patent medicine and CCBs pivots on specific symptomatology, as discerned through the seasoned expertise of traditional Chinese medical practitioners. Grounded in the comprehensive principles of TCM and reflecting a tangible application in ethnopharmacological exploration, the findings accentuate the indispensability of personalized medical interventions.
Based on the results of our study, not only can provide high quality evidence for the formulation of clinical practice guidelines for EH, but also can guide clinical practice, and is of great significance for clinicians to choose proprietary Chinese patent medicines.
While this study offers the most updated review and network meta-analysis concerning Chinese patent medicine combined with CCB in the treatment of EH, several limitations merit attention. Firstly, the included RCTs did not retrieve the registration scheme, with the allocation concealment and blinding methods often omitted, potentially leading to biases. Furthermore, there’s a limited number of RCTs available for certain secondary outcomes, emphasizing the need for more comprehensive research and potentially undermining the credibility of some findings due to potential publication bias. And the number of studies and the patients in studies was limited, and many studies were not high quality and showed a degree of heterogeneity. There was an asymmetry in funnel plots, which is not necessarily a result of publication bias, but rather higher efficacy in small trials than in large trials for a variety of reasons.
It is also worth noting that all the included RCTs are exclusively Chinese literature, which means the study lacks data in other languages. This linguistic focus might introduce inherent biases or overlook crucial global perspectives. Additionally, potential heterogeneity in the clinical environments of the trials could introduce inconsistencies, although the consistency of the paired meta-analysis results of combined western medicine mitigates some of these concerns.
In addition, most of the patients in literature were middle-aged and elderly, and there were few studies on the efficacy of young EH patients which could lead to bias. Besides, whether the different duration of intervention causes the difference in efficacy should be studied which was absent in our study.
According to the SUCRA ranking in our study, different proprietary Chinese patent medicines had advantages in improving different outcomes, which may involve the simultaneous use of multiple drugs in clinical practice. Therefore, when the therapeutic effect is well established, economic evaluation such as cost-benefit analysis should be combined to provide valuable insights into the feasibility of these combined therapies. And for western doctors, treatment based on syndrome differentiation may be difficult, if the decision is only according to the outcomes, the syndrome will be ignored, and there may be wrong medication.
Looking forward, it is imperative to expand the linguistic and cultural scope of research. Future endeavors should incorporate studies from diverse linguistic and cultural backgrounds, ensuring a comprehensive global perspective. A deeper exploration into the molecular mechanisms through experimental research could elucidate the cellular interactions when blending these traditional medicines with western antihypertensives. Broadening clinical trials to target a wider variety of hypertensive patient profiles can pave the way to determine the best combinations for specific patient needs. Moreover, once therapeutic efficacy is well established, economic assessments such as cost-benefit analyses could offer valuable insights into the financial viability of these combined treatments, especially in settings where resources are limited.
However, the silver lining in this study is the innovative comparison of the curative effects of different Chinese patent medicines when combined with a specific type of antihypertensive western medicine. This provides clinicians with a more personalized approach to treatment, bolstered by our evidence-based analysis. As a beacon for future research, this study underscores the significant potential of integrating traditional practices with modern medicine, paving the way for more harmonized and effective healthcare solutions.
In conclusion, the results of our network meta-analysis provide evidence of the need for EH. This thorough review decisively underscores the nuanced therapeutic potentials and variances when integrating Chinese patent medicine with CCBs in the treatment of EH. The effectiveness of such combinations distinctly depends on the particular Chinese patent medicine and the unique clinical presentations of patients. Compared to traditional western therapy, the addition of Chinese patent medicine can simultaneously reduce cardiovascular risk factors, lower blood pressure, improve eradication rates and reduce side effects. Notably, Tianma Gouteng Granule and Songling Xuemaikang Capsule combined with CCB have the most prominent overall efficacy. The results of this study should be referenced by policymakers and the formulation of clinical practice guidelines, and should be applied in clinical practice in treatment of essential hypertension.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
LC: Writing–review and editing, Writing–original draft, Formal Analysis, Data curation, Conceptualization, XL: Writing–review and editing, Writing–original draft, Formal Analysis. YL: Writing–original draft, Data curation. TJ: Writing–original draft. DL: Writing–original draft. CR: Writing–original draft. TY: Writing–original draft. YW: Writing–original draft. ZZ: Writing–original draft. JW: Writing–original draft. XH: Writing–review and editing, Supervision, Methodology, Conceptualization. LW: Writing–review and editing, Supervision, Methodology, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Science and Technology Innovation Project of China Academy of Chinese Medical Sciences, under the project “Chinese Medicine Clinical Basic Discipline Innovation Team Project” (CI 2021B003) and “Research on Common Methodology for the Development of Key Technologies and Treatment Guidelines for the Whole Cycle of TCM Standardization” (CI 2021A00701-1). “Theoretical study on the dynamic spatio-temporal Chinese medicine diagnosis and treatment law of hypertension under the model of “cause-mechanism-symptom-treatment”” (CI2021A00105) and “The Seventh Batch of National Famous Elderly Chinese Medicine Han Xuejie Expert Academic Experience Inheritance Project” (Z0814).
We express our gratitude to Xing for his invaluable assistance with statistical analysis and R-based graphical plotting. His expertise significantly enhanced our research outcomes.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1321405/full#supplementary-material
Beaney, T., Schutte, A. E., Stergiou, G. S., Borghi, C., Burger, D., Charchar, F., et al. (2020). May measurement month 2019: the global blood pressure screening campaign of the international society of hypertension. Hypertension 76 (2), 333–341. doi:10.1161/HYPERTENSIONAHA.120.14874
Chen, H., Huang, T., and Liu, H. (2016). Effect on rats blood pressure changes and vascular protective effects of oxidative stress response. World Chin. Med. 11 (11), 2385–2388. doi:10.3969/j.issn.1673-7202.2016.11.047
Chen, Q., and Qiu, W. (2018). Study on the efficacy of Qiangli dingxuan tablet in combination with lacidipine tablets in the treatment of hypertension with hyperactivity of liver yang. Rural Health China 0 (24), 39.
Chen, Y., Fu, D. Y., Chen, Y., He, Y. M., Fu, X. D., Xu, Y. Q., et al. (2013). Effects of Chinese herbal medicine Yiqi Huaju Formula on hypertensive patients with metabolic syndrome: a randomized, placebo-controlled trial. J. Integr. Med. 11 (3), 184–194. doi:10.3736/jintegrmed2013031
Cheng, X., Wnag, S., and Jiang, H. (2019). Rhynchophylline improves renovascular hypertension-induced cardiac hypertrophy and its possible mechanisms in rats. J. Qiqihar Med. Coll. 40 (15), 1849–1853. doi:10.3969/j.issn.1002-1256.2019.15.001
Dodds, M. G., and Vicini, P. (2004). Assessing convergence of Markov chain Monte Carlo simulations in hierarchical Bayesian models for population pharmacokinetics. Ann. Biomed. Eng. 32 (9), 1300–1313. doi:10.1114/b:abme.0000039363.94089.08
Du, B., Huang, Z., and Kong, X. (2009). Effect of Qiju Dihuang pill combined with boydin on plasma ang II, ET and CGRP levels in hypertensive patients. Inf. Traditional Chin. Med. 26 (04), 53–54.
Du, J., Zhou, H., and Du, Y. (2019). Clinical efficacy observation of combination of Qiju Dihuang pill and sustainedrelease nifedipine on hypertension of liver-kidney yin deficiency type. J. Liaoning Univ. TCM 21 (06), 147–149.
Forouzanfar, M. H., Alexander, L., Anderson, H. R., Global, al. e., Bachman, V. F., Biryukov, S., et al. (2015). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386 (10010), 2287–2323. doi:10.1016/S0140-6736(15)00128-2
Forouzanfar, M. H., Liu, P., Roth, G. A., Ng, M., Biryukov, S., Marczak, L., et al. (2017). Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA 317 (2), 165–182. doi:10.1001/jama.2016.19043
Gao, M., and Jin, H. (2013). Clinical effect observation on nifedipine joint songling xuemaikang capsule in treatment of essential hypertension. Med. Recapitulate 19 (09), 1686–1687+90. doi:10.3969/j.issn.1006-2084.2013.09.053
Gert van Valkenhoef, J. K. (2021). Gemtc: network meta-analysis using bayesian methods: R package documentation. Available from: https://cran.r-project.org/web/packages/gemtc/gemtc.pdf.
Godfraind, T. (2005). Antioxidant effects and the therapeutic mode of action of calcium channel blockers in hypertension and atherosclerosis. Philos. Trans. R. Soc. Lond B Biol. Sci. 360 (1464), 2259–2272. doi:10.1098/rstb.2005.1774
Gu, J., Wang, J. J., Yan, J., Cui, C. F., Wu, W. H., Li, L., et al. (2011). Effects of lignans extracted from Eucommia ulmoides and aldose reductase inhibitor epalrestat on hypertensive vascular remodeling. J. Ethnopharmacol. 133 (1), 6–13. doi:10.1016/j.jep.2010.08.055
Guido Schwarzer, G. R. (2023). Network meta-analysis using frequentist methods: the comprehensive R archive network. Available from: https://github.com/guido-s/netmeta,https://link.springer.com/book/10.1007/978-3-319-21416-0.
Han, X. (2014). Clinical efficacy of Xinmaitong Capsule combined with amlodipine benzoate in the treatment of hypertension. Henan Tradit. Chin. Med. 34 (B06), 57–58.
Hang, L., Zhang, Y., and Tian, H. (2018). Effects of levamlodipine and Tianma Gouteng granule on the NT-ProBNP and carotid vascular intima thickness in patients with hypertension. Chin. J. Intergrative Med. Cardio-/Cerebrovascular Dis. 16 (10), 1327–1330. doi:10.12102/j.issn.1672-1349.2018.10.005
Hao, J., Zhang, H., Xiong, K., Ma, J., Zhang, D., Guan, Z., et al. (2020). Randomized controlled clinical trial of felodipine tablets combined used with Tianma Gouteng granule on treating hypertension. J. Pract. Traditional Chin. Intern. Med. 34 (11), 100–102. doi:10.13729/j.issn.1671-7813.Z20180392
Harpole, J. K., Woods, C. M., Rodebaugh, T. L., Levinson, C. A., and Lenze, E. J. (2014). How bandwidth selection algorithms impact exploratory data analysis using kernel density estimation. Psychol. Methods 19 (3), 428–443. doi:10.1037/a0036850
He, G., Jiang, Q., and Ma, Y. (2013). Clinical use of songling xuemaikang capsule. J. Med. Aesthet. Cosmetol. (9), 112–113.
Higgins, J. P. T. S. J., and Page, M. J. (2019). Sterne JAC on behalf of the RoB2 development group. Risk of bias tools. Available from: https://www.riskofbias.info/welcome/rob-2-0-tool.
Huang, C., Zhou, H., and Zhong, Z. (2018). Efficacy of Qiangli dingxuan tablet combined with lacidipine tablets in the treatment of hypertension with hyperactivity of liver yang. Zhejiang J. Integr. Traditional Chin. West. Med. 28 (3), 219–221. doi:10.3969/j.issn.1005-4561.2018.03.019
Huang, P., and Fu, X. (2014). Clinical effect of Tianma Gouteng Granule in the treatment of hypertension and its effect on the dyslipidemia of patients. Community Physicians China 30 (20), 105+7. doi:10.3969/j.issn.1007-614x.2014.20.63
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/M14-2385
James, P. A., Oparil, S., Carter, B. L., Cushman, W. C., Dennison-Himmelfarb, C., Handler, J., et al. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311 (5), 507–520. doi:10.1001/jama.2013.284427
Ji, Z. C., Lin, S. S., Hu, H. Y., Sheng, X. D., Yang, F. W., and Wang, X. L. (2022). Network Meta-analysis of efficacy and safety of oral Chinese patent medicines combined with conventional western medicine in treatment of hypertension. Zhongguo Zhong Yao Za Zhi 47 (7), 1955–1988. doi:10.19540/j.cnki.cjcmm.20211223.501
Jia, Q., and Liu, S. (2004). Clinical observation of 34 cases of hypertension treated with Tianma Gouteng granule and shihuida. Chin. Community Physicians Compr. Ed. (24), 52–54.
Jiahao, M., Xiangjun, Q., and Hongbin, X. (2021). Bayesian network Meta-analysis of Chinese medicine injections in treatment of chronic renal insufficiency. China J. Chin. Materia Medica 46 (02), 454–466. doi:10.19540/j.cnki.cjcmm.20200622.501
Jiao, J., Zeng, G., Wen, B., and Yang, R. (2018). Effect of amlodipine benzoate combined with Qiju Dihuang Pill on morning peak hypertension in elderly people with liver and kidney yin deficiency evidence. J. Integr. Traditional Chin. West. Med. 27 (19), 2151–2153. doi:10.3969/j.issn.1008-8849.2018.19.030
Lai, X., Dong, Z., Wu, S., Zhou, X., Zhang, G., Xiong, S., et al. (2022). Efficacy and safety of Chinese herbal medicine compared with losartan for mild essential hypertension: a randomized, multicenter, double-blind, noninferiority trial. Circ. Cardiovasc Qual. Outcomes 15 (3), e007923. doi:10.1161/CIRCOUTCOMES.121.007923
Law, M. R., Morris, J. K., and Wald, N. J. (2009). Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338, b1665. doi:10.1136/bmj.b1665
Leucht, S., Cipriani, A., Spineli, L., Mavridis, D., Orey, D., Richter, F., et al. (2013). Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382 (9896), 951–962. doi:10.1016/S0140-6736(13)60733-3
Li, W., and Sun, J. (2021). Effects of tanshinone ⅡA on spontaneous hypertensive rats and vasoactive substances based on Nrf2 pathway. Chin. J. Integr. Med. Cardio-/Cerebrovascuiar Dis. 19 (07), 1103–1107+12.
Li, Z. (2017). Efficacy analysis of songling xuemaikang capsule combined with I evamlodipine in the treatment of essential hypertension. Clin. J. traditional Chin. Med. 29 (04), 515–517. doi:10.16448/j.cjtcm.2017.0173
Liang, Y., Zhu, Y., and Li, J. (2021). Effectiveness of Qinggan Jiangya Capsules on treating essential hypertension and its effect on serum homocysteine and vascular endothelial growth factor levels. China Med. 16 (06), 837–840. doi:10.3760/j.issn.1673-4777.2021.06.009
Lin, J., Wang, Q., Zhong, D., Zhang, J., Yuan, T., Wu, H., et al. (2023). Efficacy and safety of Qiangli Dingxuan tablet combined with amlodipine besylate for essential hypertension: a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial. Front. Pharmacol. 14, 1225529. doi:10.3389/fphar.2023.1225529
Liu, H., Jin, F., Zhang, Z., Gao, Y., Liu, X., Li, Q., et al. (2020). Clinical effects of Songling Xuemaikang Capsule combined with benidipine hydrochloride on patients with essential hypertension. Chin. Tradit. Pat. Med. 42 (12), 3180–3184. doi:10.3969/j.issn.1001-1528.2020.12.012
Liu, J., Liu, F., and Ren, F. (2019). Clinical observation of Xinmaitong Capsule combined with levamlodipine in treatment of senile hypertension. Drugs and Clin. 34 (08), 2311–2316. doi:10.7501/j.issn.1674-5515.2019.08.013
Liu, K. (2013). Clinical observation of nifedipine controlled-release tablets combined with Qiangli dingxuan tablet on hypertension. Drugs and Clin. (3), 358–360. doi:10.7501/j.issn.1674-5515.2013.03.026
Liu, W., Wang, J., and Zhao, Y. (2015). Regulatory mechanism of Songling Xuemaikang Capsule on RAAS system in spontaneously hypertensive rats. China J. Traditional Chin. Med. Pharm. 30 (04), 1322–1324.
Liu, Y. (2021). Clinical study of Xinmaitong Capsule combined with verapamil in treatment of hypertension. Drugs and Clin. 36 (10), 2059–2062. doi:10.7501/j.issn.1674-5515.2021.10.012
Mancia, G., Fagard, R., Narkiewicz, K., Redon, J., Zanchetti, A., Bohm, M., et al. (2013). 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of hypertension (ESH) and of the European society of cardiology (ESC). J. Hypertens. 31 (7), 1281–1357. doi:10.1097/01.hjh.0000431740.32696.cc
Mavridis, D. (2019). Network meta-analysis in a nutshell. Evid. Based Ment. Health 22 (3), 100–101. doi:10.1136/ebmental-2019-300104
Meng, T., Wang, P., Xie, X., Li, T., Kong, L., Xu, Y., et al. (2022). Efficacy and safety of Songling Xuemaikang capsule for essential hypertension: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine 107, 154459. doi:10.1016/j.phymed.2022.154459
Morel, N., and Godfraind, T. (1987). Prolonged depolarization increases the pharmacological effect of dihydropyridines and their binding affinity for calcium channels of vascular smooth muscle. J. Pharmacol. Exp. Ther. 243 (2), 711–715.
Ncd-RisC, NCDRFC (2021). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 398 (10304), 957–980. doi:10.1016/S0140-6736(21)01330-1
Nikolakopoulou, A., Higgins, J. P. T., Papakonstantinou, T., Chaimani, A., Del, G. C., Egger, M., et al. (2020). CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 17 (4), e1003082. doi:10.1371/journal.pmed.1003082
Oparil, S., Acelajado, M. C., Bakris, G. L., Berlowitz, D. R., Cifkova, R., Dominiczak, A. F., et al. (2018). Hypertension. Nat. Rev. Dis. Prim. 4, 18014. doi:10.1038/nrdp.2018.14
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Peters, J. L., Sutton, A. J., Jones, D. R., Abrams, K. R., and Rushton, L. (2008). Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 61 (10), 991–996. doi:10.1016/j.jclinepi.2007.11.010
Qiu, S., Huo, C., and Li, X. (2021). Analysis of the efficacy of Songling Xuemaikang in the treatment of mild essential hypertension and its effect on the stability of blood pressure in patients. Health Guide (42), 23–24.
Shan, Y., Wang, Y., and Xu, G. (2017). Research development of rhizoma Gastrodiae for hypertension. World Chin. Med. 12 (12), 3182–3185. doi:10.3969/j.issn.1673-7202.2017.12.079
Shi, W., Yuan, R., Xin, Q., Xu, L., Teng, C., and Cong, W. (2018). Study on the protection mechanism of Songling Xuemaikang on blood vessels of spontaneously hypertensive rats based on oxidative stress. Cardiovasc. Dis. J. Integr. traditional Chin. West. Med. 6 (06), 39–41+3. doi:10.3969/j.issn.2095-6681.2018.06.023
Stanaway, Jd AAGE (2017). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390 (10100), 1345–1422. doi:10.1016/S0140-6736(17)32366-8
Sterne, J. A. C., Savovic, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, C. (2015). Experience of songling xuemaikang capsule combined with nifedipine controlled release tablets in the treatment of essential hypertension. Psychol. Dr. 21 (20), 120–121.
Sun, H. (2019). Clinical efficacy analysis of Tianma Gouteng Granule combined with western medicine in the treatment of hypertension in the elderly. Health Horiz. (2), 81–82.
Taubert, D., Berkels, R., Klaus, W., and Roesen, R. (2002). Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: essential structural features. J. Cardiovasc Pharmacol. 40 (5), 701–713. doi:10.1097/00005344-200211000-00008
Toft, N., Innocent, G. T., Gettinby, G., and Reid, S. W. (2007). Assessing the convergence of Markov chain Monte Carlo methods: an example from evaluation of diagnostic tests in absence of a gold standard. Prev. Vet. Med. 79 (2-4), 244–256. doi:10.1016/j.prevetmed.2007.01.003
Tong, Y., Xuejie, H., Liying, W., and Dasheng, L. (2023). Efficacy and safety of the method of removing phlegm andd removing blood stasis in the treatment of essential hypertension:a meta-analysis. Chin. J. Integr. Med. Cardio-cerebrovgascular Dis. 21 (02), 222–233+38. doi:10.12102/j.issn.1672-1349.2023.02.006
Viechtbauer, W., and Cheung, M. W. (2010). Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 1 (2), 112–125. doi:10.1002/jrsm.11
Wang, F. (2011). The efficacy of Songling Xuemaikang combined with amlodipine in reducing blood pressure variability in patients with essential hypertension. Jilin J. Chin. Med. 31 (02), 149–150. doi:10.13463/j.cnki.jlzyy.2011.02.045
Wang, G., Guo, Z., Huang, M., and Liao, H. (2022). The effect of Xinmaitong Capsule on vascular endothelial function and arterial elasticity function in elderly patients with hypertension. Chin. J. Gerontology 42 (05), 1035–1039. doi:10.3969/j.issn.1005-9202.2022.05.004
Wang, S. (2020). Clinical effect of Tianma Gouteng granule combined with nifedipine sustained-release tablets in the treatment of hypertension. Chin. Prim. Health Care 34 (10), 109–111. doi:10.3969/j.issn.1001-568X.2020.10.0033
Wang, W. (2019). Efficacy of Tianma Gouteng Granule combined with nifedipine in the treatment of hypertension in the elderly and the effects on vascular endothelial function and inflammatory cytokines. Chronic Pathematology J. 20 (02), 276–278. doi:10.16440/j.cnki.1674-8166.2019.02.045
Wang, X., Chen, M., Quan, S., Zhao, N., and Li, X. (2017). Effects of qinggan Jiangya capsules and irbesartan on serum levels of adiponectin,Hcy and VEGF in patients with hypertension. Prog. Mod. Biomed. 17 (11), 2068–2071. doi:10.13241/j.cnki.pmb.2017.11.017
Wei, C., Liu, B., and Li, Y. (2017). Protective effect of angelica volatile oil on lev-nitroarginine methyl ester-induced vascular endothelial injury in hypertensive rats. J. Chin. Med. Mater. 40 (04), 937–940. doi:10.13863/j.issn1001-4454.2017.04.041
Xin, D., and Lin, W. (2016). Clinical study on the treatment of essential hypertension with Songling Xuemaikang. all Health 10 (12), 155–156. doi:10.3969/j.issn.1009-6019.2016.06.219
Xiong, H. (2018). Clinical efficacy of nifedipine controlled-release tablets in combination with Qiangli Dingxuan Tablet in the treatment of hypertension. Chin. J. Clin. Ration. Drug Use 11 (30), 58–59. doi:10.15887/j.cnki.13-1389/r.2018.30.028
Yan, Y. (2017). Observation on the efficacy of nifedipine extended-release tablets combined with Qinggan Jiangya Capsule in the treatment of senile hypertension. China Pract. Med. 12 (16), 124–125. doi:10.14163/j.cnki.11-5547/r.2017.16.065
Yang, X., Yang, G., Li, W., Zhang, Y., and Wang, J. (2018). Therapeutic effect of Ilex hainanensis Merr. Extract on essential hypertension: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 9, 424. doi:10.3389/fphar.2018.00424
Yang, X. C., Xiong, X. J., Yang, G. Y., Wang, H. R., and Wang, J. (2015). Songling Xuemaikang Capsule for primary hypertension: a systematic review of randomized controlled trials. Chin. J. Integr. Med. 21 (4), 312–320. doi:10.1007/s11655-014-1709-6
Ye, Y., Zhang, J., Qiao, L., and Liang, Y. (2021). Effects of Tianma Gouteng Granule on blood pressure variability and vascular endothelial function in primary hypertension with hyperactive liver yang. Hebei J. Traditional Chin. Med. 43 (01), 76–79+83. doi:10.3969/j.issn.1002-2619.2021.01.019
Yongcheng, L., Ying, T., and Dong, G. (2022). Fufang Danshen Dripping Pills in treatment of essential hypertension: a systematic review and Meta-analysis. Chin. Traditional Herb. Drugs 53 (10), 3111–3124. doi:10.7501/j.issn.0253-2670.2022.10.022
Yu, Y. (2019). Effects of songling xuemaikang capsule and amlodipine besylate on plasma MDA,NOS,SOD activity and blood pressure variability in hypertensive patients with live-r yang hyperactivity syndrome. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 17 (07), 986–989. doi:10.12102/j.issn.1672-1349.2019.07.007
Yuan, F. (2017). Efficacy of Tianma Gouteng Granule combined with nifedipine in the treatment of elderly patients with hypertension and the effect on vascular endothelial function and inflammatory cytokines. Chin. J. Gerontology 37 (07), 1630–1632. doi:10.3969/j.issn.1005-9202.2017.07.026
Zhang, H., Li, W., and Bu, Z. (2014a). The effect of Songling Xuemaikang on blood lipids and blood rheology in patients with essential hypertension. Mod. J. Integr. Traditional Chin. West. Med. 23 (22), 2438–2440. doi:10.3969/j.issn.1008-8849.2014.22.017
Zhang, H., Li, W., Bu, Z., Guo, H., and Zhu, H. (2014b). The efficacy of Songling Xuemaikang combined with Amlodipine Benzoate in the treatment of essential hypertension. Mod. J. Integr. Traditional Chin. West. Med. 23 (23), 2550–2552. doi:10.3969/j.issn.1008-8849.2014.23.016
Zhang, H., Xie, W., Zhang, Y., Dong, X., Liu, C., Yi, J., et al. (2022). Oncolytic adenoviruses synergistically enhance anti-PD-L1 and anti-CTLA-4 immunotherapy by modulating the tumour microenvironment in a 4T1 orthotopic mouse model. Cancer Gene Ther. 29 (5), 456–465. doi:10.1038/s41417-021-00389-3
Zhang, J. (2014). Clinical study on the combination of Chinese and western medicine in the treatment of essential hypertension. J. Pract. Traditional Chin. Med. 30 (09), 830–831. doi:10.3969/j.issn.1004-2814.2014.09.029
Zhao, Y. (2012). Study of efficacies of nifedipine (I) and qingganjiangya capsule joint therapy on elderly hypertensive patients. Pract. J. Cardiac Cereb. Pneumal Vasc. Dis. 20 (04), 645–646. doi:10.3969/j.issn.1008-5971.2012.04.040
Zhao, Y., Liu, W., Cai, X., Xu, Q., Shi, H., Wang, W., et al. (2013). The regulatory mechanism of songling xuemaikang capsule on PPARgamma in spontaneously hypertensive rats: an experimental study. Chin. J. Integr. Traditional West. Med. 33 (09), 1236–1241. doi:10.7661/CJIM.2013.09.1236
Zhaochen, J., Shanshan, L., Haiyin, H., and Xiaodi, S. (2022). Network Meta-analysis of efficacy and safety of oral Chinese patent medicines combined with conventional western medicine in treatment of hypertension. China J. Chin. materia medica 47, 1955–1988. doi:10.19540/j.cnki.cjcmm.20211223.501
Zhou, J. (2013). Clinical observation on Tianma Gouteng granule combined with western medicine for treatment of senile hypertension. Chin. J. Exp. Traditional Med. Formulae 19 (07), 327–330. doi:10.11653/zgsyfjxzz2013070327
Zhu, J., Yang, L., and Fu, J. (2015). Effect of Tianma Gouteng Granule on vascular function in patients with primary hypertension. Shanghai J. Traditional Chin. Med. 49 (04), 52–54. doi:10.16305/j.1007-1334.2015.04.017
Keywords: ethnopharmacological research, calcium channel Blockers(CCBs), hypertension, network meta-analysis, review studies, therapeutic efficacy
Citation: Cui L, Liu X, Li Y, Jing T, Liu D, Ren C, Yin T, Wang Y, Zhao Z, Wang J, Han X and Wang L (2024) Chinese patent medicine combined with calcium channel blockers in the treatment of essential hypertension:a Bayes network meta-analysis and systematic review. Front. Pharmacol. 15:1321405. doi: 10.3389/fphar.2024.1321405
Received: 14 October 2023; Accepted: 21 February 2024;
Published: 15 March 2024.
Edited by:
Michel Frederich, University of Liège, BelgiumReviewed by:
Jieying Zhang, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaCopyright © 2024 Cui, Liu, Li, Jing, Liu, Ren, Yin, Wang, Zhao, Wang, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejie Han, eHVlamllaGFuQDEyNi5jb20=; Liying Wang, Y29sZG1vb25fbHlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.